Introduction
Gastric cancer is biologically aggressive and one of
the leading causes of cancer-related deaths (1). Although multiple therapeutic
modalities exist, including surgical excision, radiation therapy
and chemo therapy, the clinical results remain unsatisfactory due
to gastric cancer often being diagnosed in an advanced stage or
metastatic stage (2). Hence,
great research efforts have been explored to exploit novel
molecular markers and detailed molecular mechanisms contributing to
improving diagnostic and therapeutic management of gastric
cancer.
The molecular basis of gastric cancer is complicated
and involves the interactions between genetic and environmental
factors. Among these etiologies, phosphoinositide-3 kinase
(PI3K)/protein kinase B (Akt) pathway is believed to be important
in gastric cancer development (3–5).
Tian et al demonstrated that the expression of Akt and PI3K
were remarkably higher in tumor tissue than that in normal tissue
(3). In addition, Ye et al
found that PI3K/Akt signaling pathway plays a crucial role in the
formation and progression of gastric cancer (4). Simultaneously, phosphorylated-Akt
expression significantly correlated with a poor prognosis (5). Therefore, PI3K/Akt pathway may
represent a considerable therapeutic target for gastric cancer.
Deguelin, a natural component derived from
leguminous plants, has been reported to prevent breast cancer
(6), tobacco carcinogen-induced
lung carcinogenesis (7), prostate
cancer (8) and squamous cancer
(9) by blocking Akt activation.
Many studies have demonstrated that deguelin exerts its anticancer
effect by inhibiting cell viability, cell growth, migration and
invasion, inducing apoptosis, targeting cell cycle arrest and
anti-angiogenesis (7,10,11).
Therefore, deguelin may provide an alternative
potential approach for gastric cancer treatment. Here, we
investigated that deguelin not only inhibited the proliferation,
invasion, migration but also induced apoptosis in gastric cancer
MGC-803 and MKN-45 cells in vitro.
Materials and methods
Cell culture and reagents
Human gastric cancer cell lines MGC-803 and MKN-45
were purchased from Shanghai institute of Cell Biology, Chinese
Academy of Sciences and cultured in RPMI-1640 medium with 10% fetal
bovine serum (FBS) and 1% penicillin-streptomycin (Gibco, Grand
Island, NY, USA) under a humidified 5% CO2 atmosphere at
37°C. Deguelin (MedChem Express, Monmouth Junction, NJ, USA) was
dissolved in dimethyl sulfoxide (DMSO) at concentration of 10 mM as
stock solution and stored at −20°C. Before using, deguelin was
diluted directly to the desired dose with an identical final
concentration of DMSO.
Cell counting kit-8 (CCK-8) cell
proliferation assay
CCK-8 (Dojindo, Kumamoto, Japan) assay was used to
determine the inhibitory effect of deguelin on the proliferation of
two cancer cell types. The MGC-803 (3×103/well) and
MKN-45 (5×103/well) cells were plated in 96-well plates
with 200 µl of medium. Before the cells were treated with
various concentrations of deguelin (0, 1, 5, 10, 25 and 50
µM), they were starved in serum-free medium for 24 h to
allow for cell synchronization, and then tested at 72 h. At the
testing time-point, 10 µl of sterile CCK-8 solution was
added to each well and incubated for an additional 1.5 h at 37°C.
The optical density values at 450 nm were measured with a
microplate reader (Thermo Scientific, Waltham, MA, USA). The
inhibitory rate was calculated using the following formula:
Inhibitory rate (%) = (A450 of control
cells-A450 of treated cells)/(A450 of control
cells) ×100%. The assays were repeated using six cell samples. In
addition, for the cell viability test after treatment of deguelin,
the MGC-803 (1.5×103/well) and MKN-45
(2.5×103/well) cells were plated in 96-well plates with
the medium of 200 µl and incubated with various
concentrations of deguelin (0, 1, 10 and 25 µM), then tested
using CCK-8 assay at days 1, 2, 3 and 4.
Morphology observation
After treated with deguelin (0, 1, 10 and 25
µmol/l) for 48 h, MGC-803 and MKN-45 cells were observed
under an inverted phase contrast microscope to determine their
morphology changes.
Cell cycle analysis
After deguelin treatment for 48 h, cells were
collected, fixed in 70% ethanol and incubated at 4°C overnight.
Cells were then centrifuged and stained in ice-cold
phosphate-buffered saline (PBS) solution containing 100
µg/ml propidium iodide (PI), 0.1% Triton X-100, and 1 mg/ml
RNase for 0.5 h. After washing, the samples were analyzed by flow
cytometry (Beckman Coulter, Brea, CA, USA).
Annexin V/FITC-PI assay
The apoptotic assays were performed by an Annexin
V-FITC/PI apoptosis detection kit and detected as the
manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach,
Germany). Briefly, the 106 cells were washed using 500
µl binding buffer, centrifuged at 300 × g and stained with
10 µl Annexin V-FITC solution in room temperature for 30
min. Before testing, 5 µl PI solution was added to each
sample. The apoptosis rate was evaluated by flow cytometry. At
least 10,000 cells for each sample was analyzed. The analyses were
repeated in three cell samples.
Real-time quantitative PCR
Total RNAs from control and deguelin-treated cells
were extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
and reverse translated with AMV reverse transcriptase (Promega,
Madison, WI, USA). To synthesize complementary DNA, 2 µg
total RNA per sample was added to the reaction system of a final
volume of 20 µl containing 4 µl 5X buffer, 2
µl dNTP, 1 µl oligo(dT), 0.5 µl RNase
inhibitor, 0.5 µl AMV reverse transcriptase and
ddH2O to meet the final volume. After incubated at 30°C
for 10 min, 45°C for 60 min, 98°C for 5 min and 5°C for 5 min, the
amplified complementary DNA (cDNA) products were mixed with Power
SYBR-Green PCR master mix (Applied Biosystems, Foster City, CA,
USA). Quantitative polymerase chain reaction (qPCR) was conducted
using a real-time thermal cycler (Stratagene, La Jolla, CA, USA).
The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA
content was used to normalize with cDNA, and the comparative Ct
method was used to analyze data. The primers for real-time qPCR
analysis are shown in Table I.
Each assay was performed in triplicate and repeated in three cell
samples.
 | Table IPrimers used in quantitative PCR
analysis. |
Table I
Primers used in quantitative PCR
analysis.
| Gene | Primer sequence
(5′-3′)
temperature (°C) | Annealing size
(bp) | Product |
|---|
| Bax | Sense:
TGCTTCAGGGTTTCATCCAG | 58 | 170 |
| Antisense:
GGCGCCAATCATCCTCTG | | |
| Bcl-2 | Sense:
AATCAAACAGAGGTCGCATGCTGG | 58 | 192 |
| Antisense:
TTGTGGCCTTCTTTGAGTTCGGTG | | |
| Cyclin E1 | Sense:
AGTGGCGTTTAAGTCCCCTG | 58 | 326 |
| Antisense:
CAGTTTTGAGCTCCCCGTCT | | |
| p21 | Sense:
TCCAGCGACCTTCCTCATCCAC | 58 | 108 |
| Antisense:
TCCATAGCCTCTACTGCCACCATC | | |
| GAPDH | Sense:
TCACCATCTTCCAGGAGCG | 58 | 572 |
| Antisense:
CTGCTTCACCACCTTCTTGA | | |
Western blot analysis
Two different gastric cell lines were isolated after
6 h of incubation in the absence or different concentration of
deguelin. Cells were scraped in RIPA lysis buffer and the protein
content of cellular extracts were quantified by BCA assay. For
western blot analysis, the prepared protein was resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),
transferred to PVDF membranes and incubated with the appropriate
antibodies. For estimation of p-Akt/total-Akt protein, we used two
different specific antibodies: monoclonal anti-p-Akt and monoclonal
anti-total-Akt (both from Cell Signaling Technology, Beverly, MA,
USA). The blots were probed with β-actin antibody (Cell Signaling
Technology) for equal loading control. The secondary antibody was
goat anti-rabbit HRP-conjugated antibody (Jackson ImmunoResearch,
West Grove, PA, USA). Immunoreactive proteins were detected by the
enhanced chemiluminescent (ECL) protocol (Amersham Biosciences,
Piscataway, NJ, USA).
4′,6-Diamidino-2-phenylindole (DAPI)
staining
MGC-803 and MKN-45 cells were cultured as described,
and then treated with various concentrations of deguelin for 48 h.
Apoptosis was further determined by nucleus morphology using DAPI
staining (Sigma-Aldrich, St. Louis, MO, USA). After being fixed and
stained with DAPI, the cells were examined under a fluorescent
microscope (Olympus, Tokyo, Japan).
Cell migration assay
The MGC-803 and MKN-45 cells at a density of
2×105 cells/well were cultured in 6-well plates. When
reaching 90% confluence, the cells were scratched by a sterile
yellow 200 µl pipette tip and then washed three times with
PBS. The cells were then placed in fresh RPMI-1640 medium
containing 1% FBS with treatment of deguelin or DMSO for 24 h. Five
random fields were selected and photographed with an inverted
microscope, respectively.
Cell invasion assay
The ability of deguelin to inhibit cell invasion was
tested using Transwell assay. The Transwell is in 24-well plate
with an insert of 8-µm pore size polyethylene terephthalate
membrane (Corning Life Sciences, Tewksbury, MA, USA). The cells
were starved in serum-free medium overnight, then trypsinized and
resuspended in serum-free medium. The cells at a density of
6×104 cells/well were seeded in the upper Transwell
chamber which was coated with Matrigel (BD Biosciences, Bedford, MA
USA), then incubated with DMSO or deguelin (1 and 10 µM) for
24 h, and 500 µl of complete growth medium was added to the
bottom. Cells on the upper membrane were wiped off with a cotton
swab. Invaded cells on the lower membrane were fixed with 4%
paraformaldehyde, stained with DAPI and three random fields were
counted under a fluorescent inverted phase contrast microscope at
×200 magnification.
Statistical analysis
All data were expressed as mean ± standard deviation
(SD). Student's t test was used to analysis the difference between
the deguelin-treated and control groups. The analysis was conducted
with the statistical software SPSS 22 (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered statistically significant.
Results
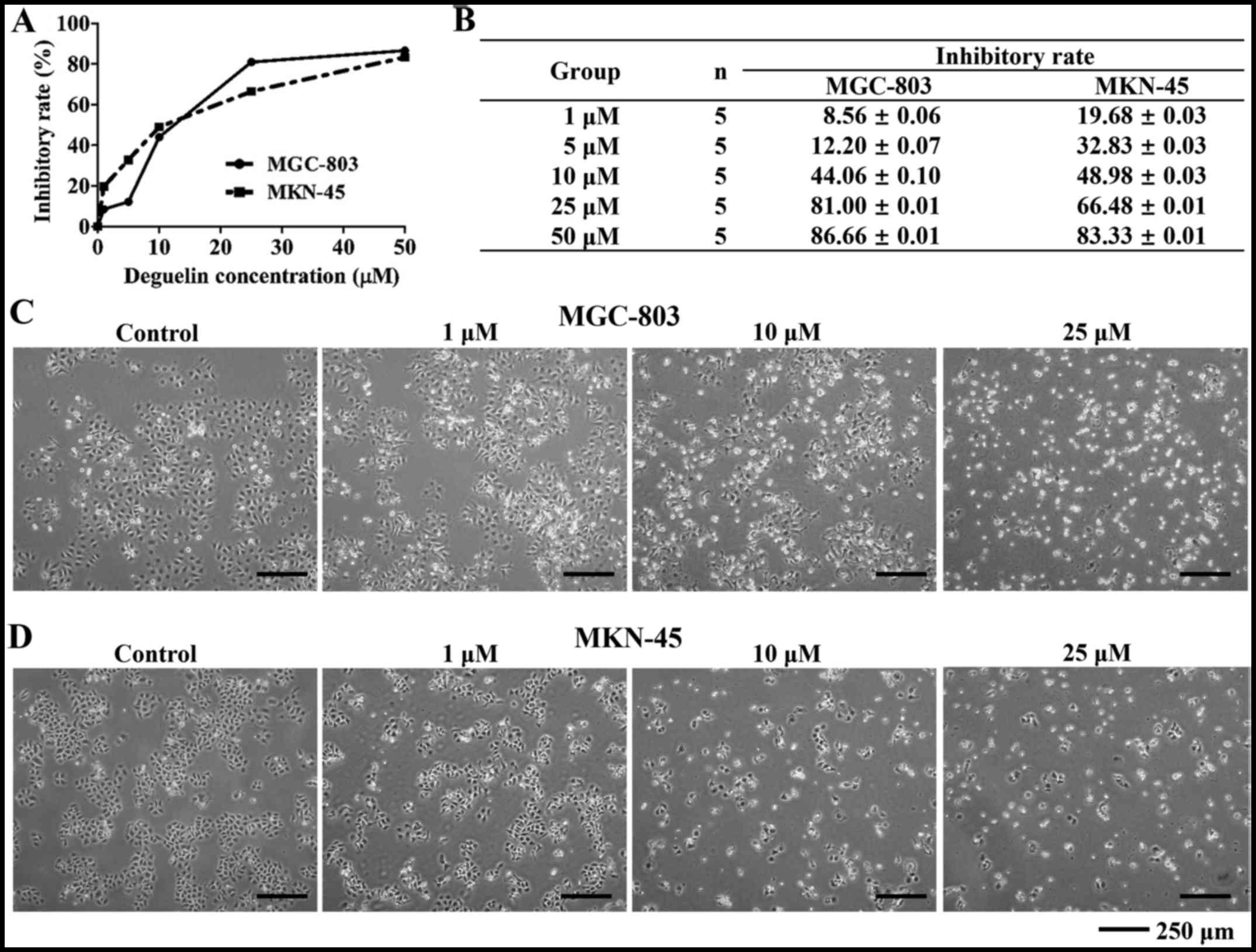
Inhibitory effect of deguelin on the
growth of MGC-803 and MKN-45 cells
CCK-8 assay showed inhibitory effect of deguelin on
the growth of MGC-803 and MKN-45 cells. As shown in Fig. 1A and B, after various doses (1, 5,
10, 25 and 50 µM) of deguelin treatment on MGC-803 and
MKN-45 cells for 72 h, the IR (inhibitory rate) of MGC-803 cells
was 8.56±0.06, 12.20±0.07, 44.06±0.10, 81.00±0.01 and 86.66±0.01%,
respectively and the IR of MKN-45 cells was 19.68±0.03, 32.83±0.03,
48.98±0.03, 66.48±0.01 and 83.33±0.01%, respectively. The
IC50 (72 h) value for MGC-803 and MKN-45 was 11.83 and
9.33 µM, respectively. As the dose of deguelin increased,
the IR of MGC-803 and MKN-45 cells improved gradually.
The effect of deguelin on the cell
morphology
As the IC50 described above, doses of 1,
10 and 25 µM deguelin were chosen to perform the rest of the
assays. As shown in Fig. 1C and
D, after incubated with different concentrations of deguelin
(1, 10 and 25 µM) for 48 h, the number of deguelin-treated
cells was less than that of blank control cells. In contrast to the
cells of control group which exhibited normal features with typical
adherent, membrane intact morphology, deguelin treatment groups
showed obvious apoptotic morphology with membrane distorted
morphology with dose-dependent severity.
Deguelin inhibited cell proliferation and
arrested the cell cycle of MGC-803 and MKN-45 cells
Deguelin treatment resulted in a dose- and
time-dependent decrease in cell viability (Fig. 2A and B). To address whether the
inhibitory effect of deguelin on the growth of cancer cells was
accompanied by blocking the cell cycle, FCM assay was performed.
Previous experiments suggested that a very high death rate occurred
in 25 µM deguelin-treated cells and therefore we chose 1 and
10 µM deguelin treatment group to conduct the cell cycle
assay. The assay indicated that deguelin altered the cell cycle
distribution after 48 h treatment in both tested cell lines. For
MGC-803 cells, deguelin induced cells cycle arrest at
G0/G1 phase (Fig. 2C and D). Compared with control
group (57.27±1.48%), the percentage of G0/G1
phase cells of deguelin treatment groups (67.53±1.72% at 1
µM, 72.1±4.10% at 10 µM) increased (P<0.05), and S
and G2/M phases decreased accordingly. For MKN-45 cells,
low dose of deguelin induced cell cycle arrest at S phase, while
high dose of deguelin induced cell cycle arrest at G2/M
phase (Fig. 2E and F). Compared
with control group (22.53±1.11% at S phase, 11.60±0.90% at
G2/M phase), the percentage of S phase cells of low
dose-treated group (34.27±1.31%) increased (P<0.05), and the
percentage of G2/M phase cells of high dose-treated
group (32.93±2.44%) increased (P<0.01).
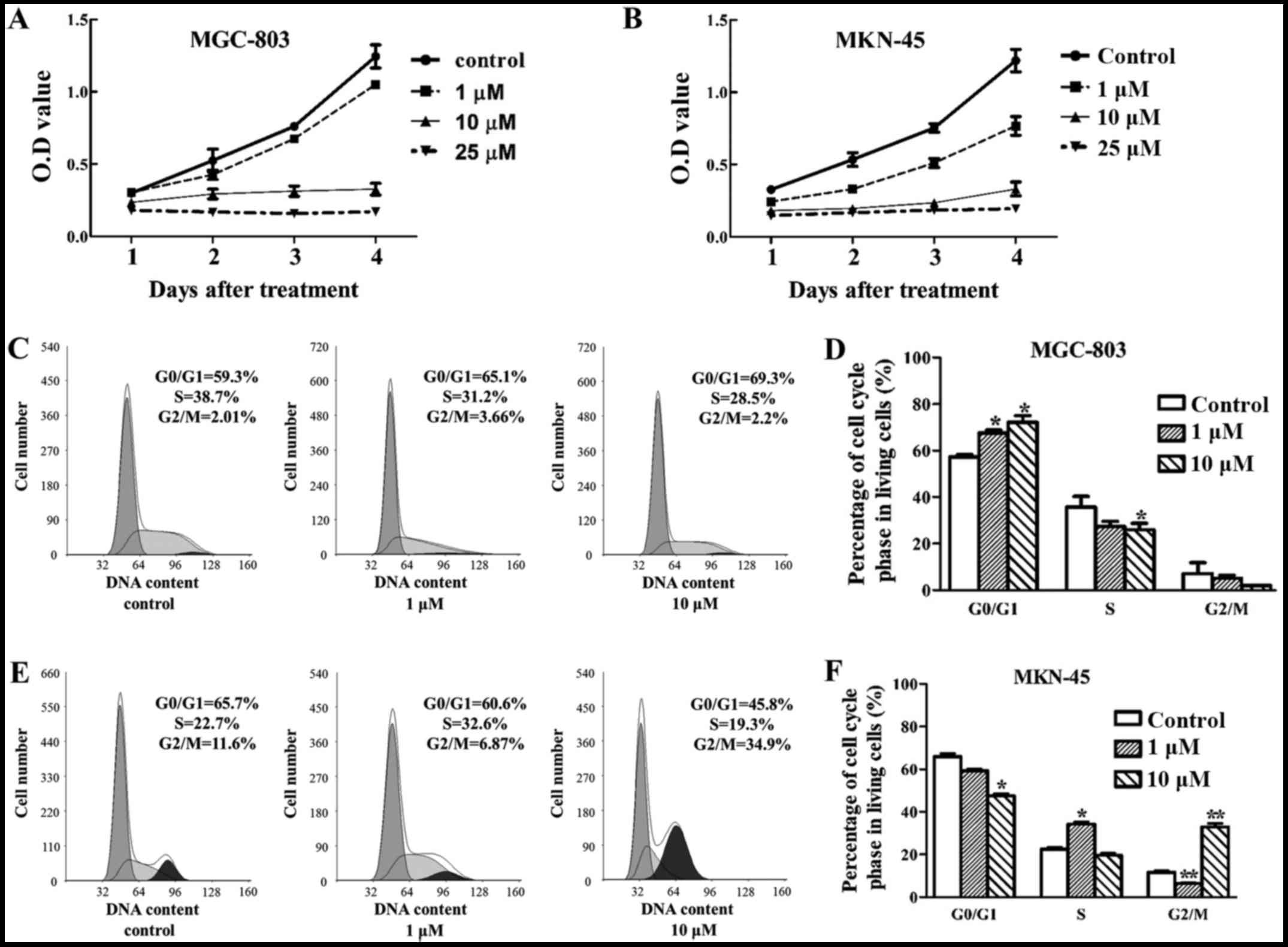 | Figure 2Treatment with deguelin inhibits
gastric cancer cell proliferation. (A) The cell viabilities of (A)
MGC-803 and (B) MKN-45 treated with or without deguelin (1, 10 and
25 µM) were measured with a cell counting kit-8 (CCK-8)
assay at days 1, 2, 3 and 4. After treatment with deguelin for 48
h, gastric cell lines were subjected to flow cytometry analysis. (C
and D) The effect of deguelin (1 and 10 µM) on the cell
cycle profiles of MGC-803 cell lines was further evaluated. (E and
F) The effect of deguelin (1 and 10 µM) on the cell cycle
profiles of MKN-45 cell lines was further evaluated. All the
experiments were repeated in three independent experiments.
G0/G1, gap between end of M phase and start
of S phase; S, DNA duplication phase; G2, gap between
end of S phase and start of M phase; M, mitosis.
*P<0.05, significant difference between control group
and the experiment group; **P<0.01, significant
difference between control group and the experiment group. |
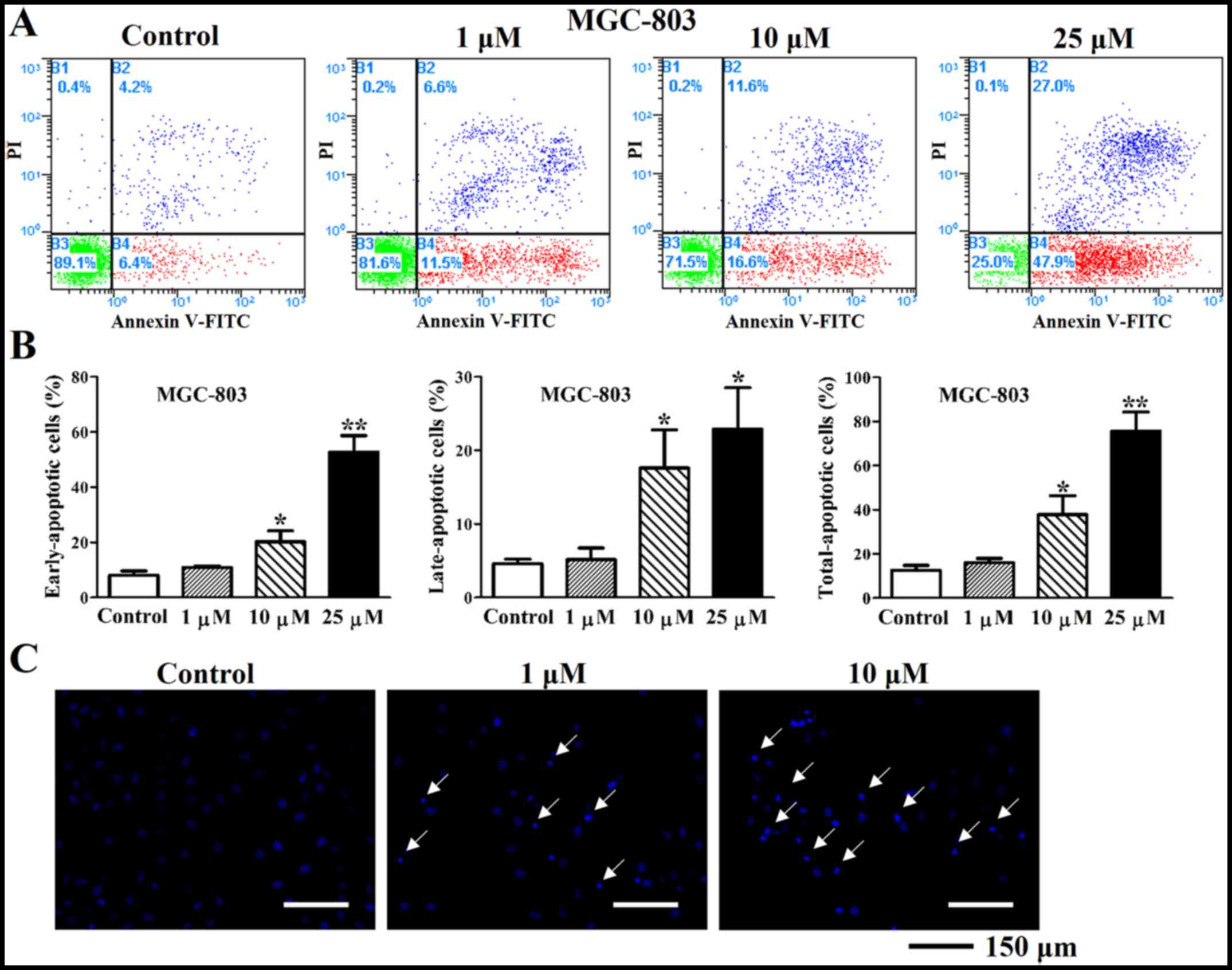
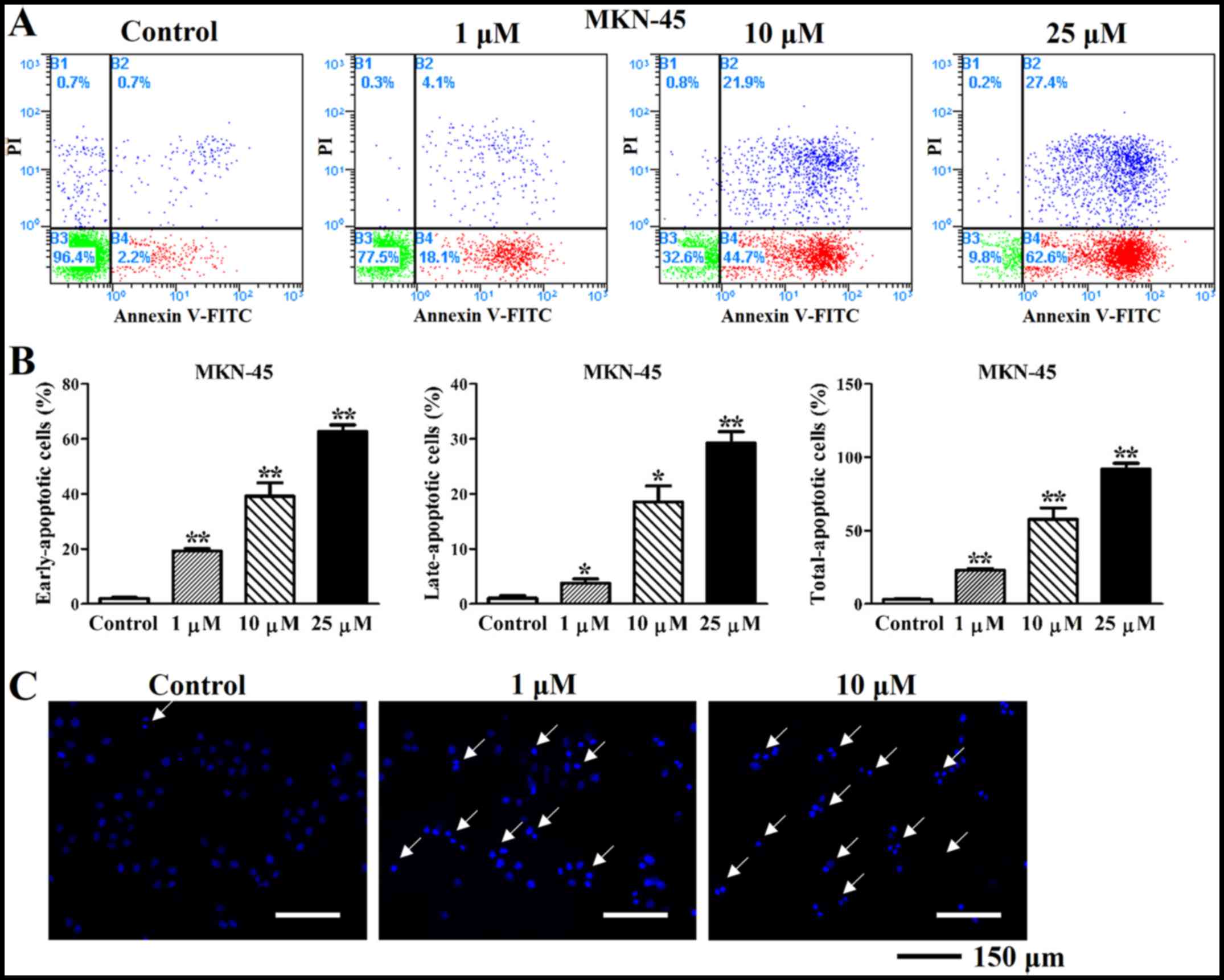
Deguelin promotes apoptotic effects on
cultured MGC-803 and MKN-45 cells
Apoptosis was assessed in MGC-803 and MKN-45 cells
after treatment with 1, 10 and 25 µM deguelin for 48 h. The
early (10.87±0.46% at 1 µM, 20.20±3.21% at 10 µM and
52.70±4.88% at 25 µM), late (5.17±1.28 at 1 µM,
17.60±4.25% at 10 µM and 22.90±4.58% at 25 µM) and
total (16.03±1.60% at 1 µM, 37.80±7.02% at 10 µM and
75.60±7.08% at 25 µM) apoptosis rates were increased
significantly in MGC-803 cells compared with control (8.03±1.31% of
early, 4.60±0.50% of late and 12.63±1.76% of total apoptosis rate)
(Fig. 3A and B). Similarly, the
early (19.20±0.79 at 1 µM, 39.13±4.01% at 10 µM and
62.63±2.00% at 25 µM), late (3.73±0.66 at 1 µM,
18.57±2.36% at 10 µM and 29.23±1.70% at 25 µM) and
total (22.93±0.90 at 1 µM, 57.70±6.33% at 10 µM and
91.87±3.37% at 25 µM) apoptosis rates were also increased in
MKN-45 cells compared with control (2.00±0.28% of early, 1.07±0.33%
of late and 3.07±0.46% of total apoptosis rate) (Fig. 4A and B). This suggested that
deguelin prominently induced apoptosis in gastric cancer cells.
DAPI staining showed that many apoptotic bodies with condensed
chromatin and apoptotic nucleus fragmentations were observed in
deguelin-treated cells (1 and 10 µM for 48 h), but almost
none in untreated cells (Fig. 3C
and 4C). Notably also, not only
did deguelin reduce the proliferation of MGC-803 and MKN-45 cells,
but also visibly induced apoptosis.
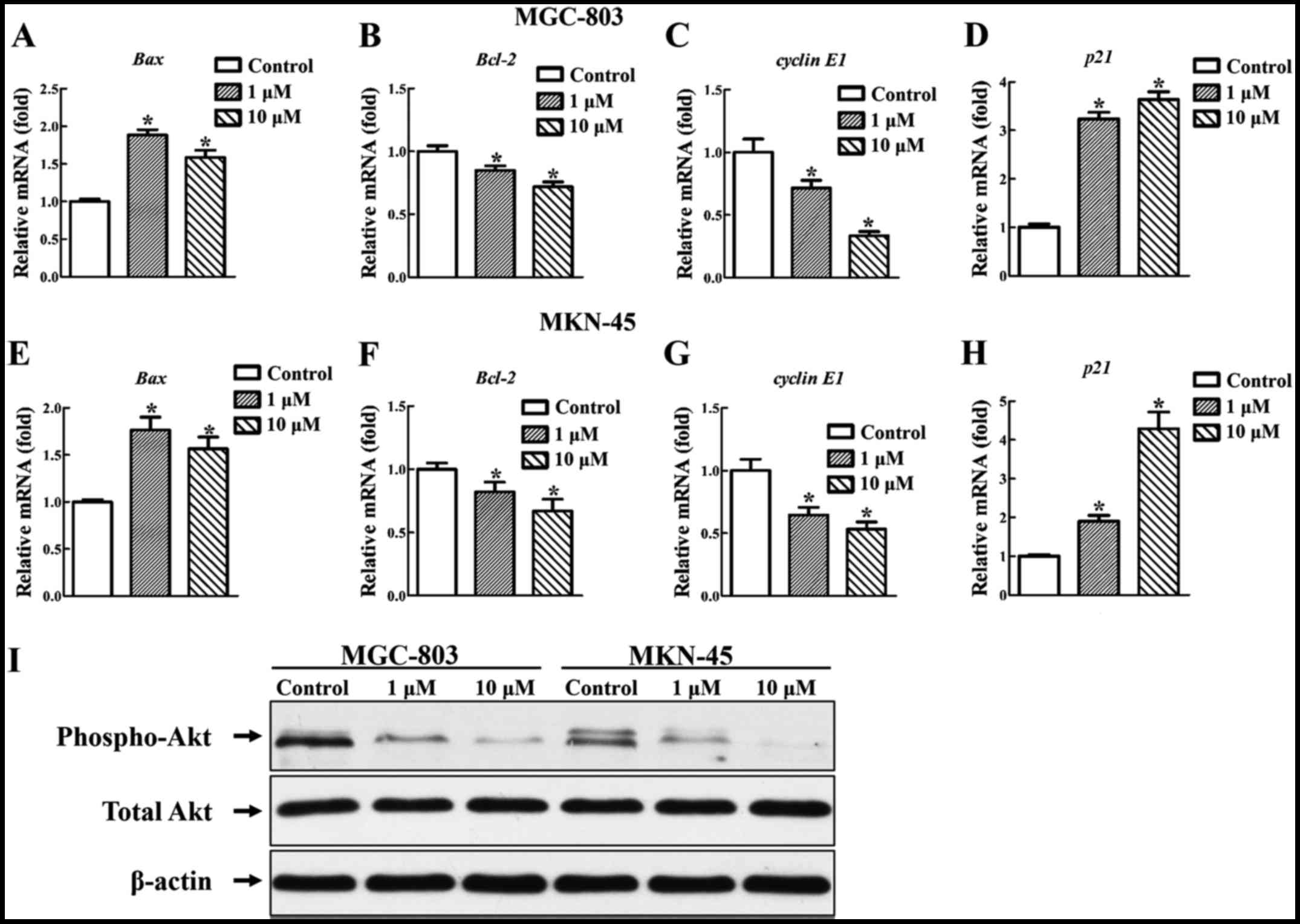
Relative gene and protein expression
under the treatment of deguelin
Deguelin exerted its antitumor activity by inducing
cell cycle arrest, apoptosis and inhibiting cell proliferation.
Further experiments revealed the effect of deguelin on the
apoptotic and proliferative gene expression. After treated in
vitro with 1 and 10 µM deguelin for 48 h. Deguelin
upregulated the gene expression of Bax (Fig. 5A and E) and downregulated that of
Bcl-2 (Fig. 5B and F) of
MGC-803 and MKN-45 cells. The expression of Bax and
Bcl-2 in MGC-803 and MKN-45 cells showed significant
difference from the control cells (P<0.05 for all) (Fig. 5A, B, E and F). The gene expression
of cyclin E1 was downregulated and that of p21 was
upregulated dramatically in a dose-dependent manner. The expression
of cyclin E1 and p21 of MGC-803 and MKN-45 cells
showed significant difference from the control cells (P<0.05 for
all) (Fig. 5C, D, G and H).
Demonstration of deguelin-induced
apoptosis of cancer cells by mediating Akt signal pathway
To test Akt inhibition by deguelin, MGC-803 and
MKN-45 cells were treated in vitro with 1 and 10 µM
deguelin for 6 h. Consistent with previous studies in other tumor
cells (6,12,13), deguelin downregulated the
expression of p-Akt (Fig. 5I).
This result implied that deguelin induced apoptosis in gastric
cells by downregulating Akt activity.
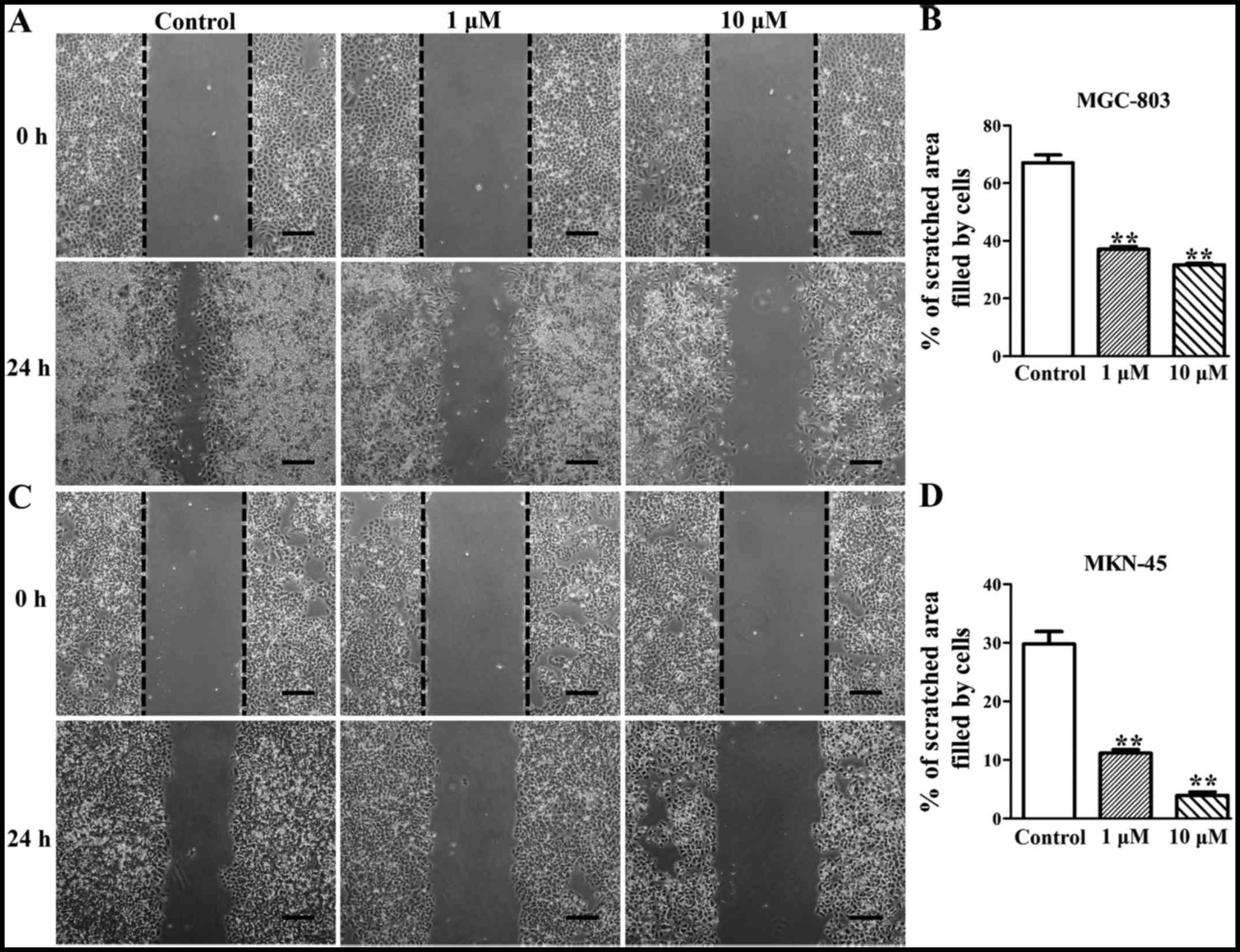
Deguelin inhibits the migration of
MGC-803 and MKN-45 cells in vitro
To evaluate the effect of deguelin on a migration
ability of MGC-803 and MKN-45 cells, wounded scratch assay was
performed on both cell lines. The result showed that 24 h after
scratching the cell migration of both cell lines was significantly
inhibited by deguelin treatment (Fig.
6). MGC-803 and MKN-45 cells in the control group efficiently
migrated into the scratched area (Fig. 6A and C). MGC-803 cells of the
control group migrated 67.07±4.67% of the scratched area, whereas
the 1 µM deguelin-treated MGC-803 cells migrated 37.12±1.53%
of the area (P<0.05) and the 10 µM deguelin-treated
MGC-803 cells migrated 31.69±0.81% of the area (P<0.05)
(Fig. 6A and B). Blank control
MKN-45 cells migrated 29.78±3.67% of the area, whereas the MKN-45
cells of the 1 µM and the 10 µM deguelin-treated
group, respectively, migrated 11.14±1.09 and 3.96±1.03% of the area
(P<0.05), and the migration rate was significantly lower in
deguelin-treated group than that in blank control groups
(P<0.05) (Fig. 6C and D).
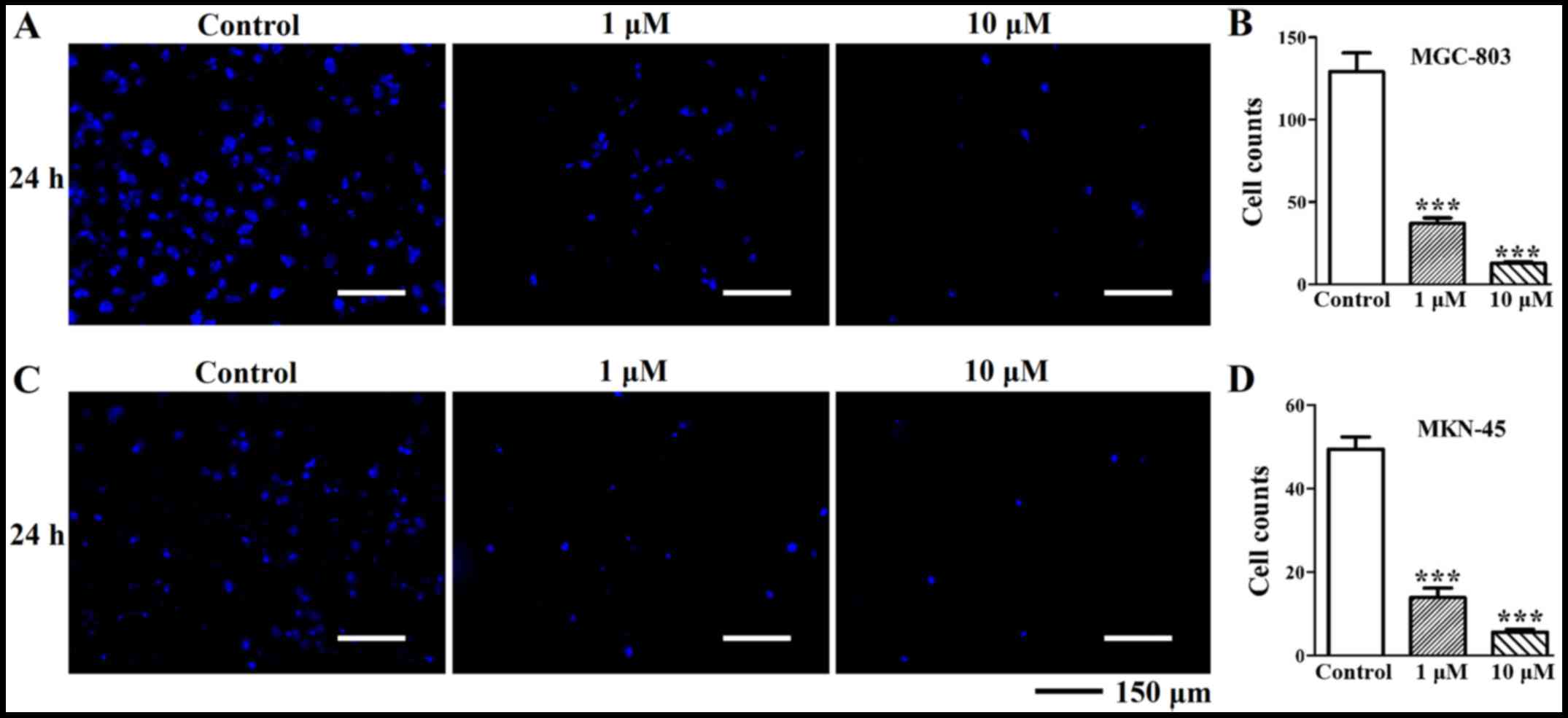
Deguelin inhibits the invasion of MGC-803
and MKN-45 cells in vitro
Because the enhanced invasion properties of gastric
cancer cells are the critical parameters in the development of
gastric cancer, we wondered if deguelin would affect the cell
behavior of MGC-803 and MKN-45 cells in vitro. Transwell
chamber invasion assay results showed that, when compared with the
control group (129.1±32.38), the invasion of the 1 µM
deguelin-treated MGC-803 cells (36.89±9.67) and the 10 µM
deguelin-treated MGC-803 cells (12.67±2.62) was significantly
inhibited in a dose dependent manner (P<0.05) (Fig. 7A and B). Deguelin (1 and 10
µM)-treated MKN-45 cells exhibited much lower invasion
ability compared to the blank control as evidenced by decreasing
the number of cells migrated through the Matrigel (Fig. 7C and D). The invasion ability of
MKN-45 cells treated with deguelin was dose-dependently decreased
compared to that of DMSO treated MKN-45 cells (13.90±6.76 at 1
µM and 5.60±1.85 at 10 µM). These results indicated
that deguelin was able to inhibit invasive ability of gastric
cancer cells.
Discussion
Gastric cancer is a highly mortal malignancy with
few effective therapies. Resulted from both genetic and
environmental risk factors, such as gene mutations, cigarette
smoking, helicobacter pylori and intake of salty and smoked
food (14), gastric cancer is a
heterogeneous and multifactorial disease. Most patients with
aggressive gastric cancer fail to respond to surgery and
radiotherapy, but they are sensitive to systemic chemotherapy as
palliative care (15,16). Therefore, the exploitation of
potential alternative chemotherapy drug for gastric cancer is
highly encouraging. Deguelin, a natural component of the flavonoid
family products, has been used as a promising chemopreventive and
therapeutic agent against various cancer cells (13,17,18).
Deguelin has been reported to inhibit the
proliferation of different cancer cells, including breast cancer
cells, prostate cancer cells and lung squamous cell cancer cells
(6,8,9).
This study revealed that proliferation of two different gastric
cancer MGC-803 and MKN-45 cell lines were inhibited in a time- and
dose-dependent manner by deguelin treatment (Fig. 1A and B). Some previous studies
demonstrated the anti-proliferative effect of deguelin in different
cancer cells was related to G0/G1 phase, S
phase or G2/M phase arrest (10,19,20). Murillo et al found that
deguelin promoted cell cycle arrest at G0/G1
phase in colon cancer cells (10). Our observations were in accord
with an overall efficacy of deguelin in inducing a
G0/G1 arrest in MGC-803 cells (Fig. 2C and D). In another study,
premalignant and malignant human HBE cells treated with deguelin
were observed to arrest at G2/M phase (7). Deguelin treatment of MKN-45 cells
resulted in S phase arrest at lower dose but G2/M phase
arrest at higher dose (Fig. 2E and
F). Indeed, more future studies are needed to identify the
underlying mechanisms responsible for the action of deguelin to
fully understand the seemingly puzzling role of this compound.
Abnormalities of cell cycle checkpoint regulators
have been recognized as critical factors in the development of
human cancers. Cyclin-dependent kinase (CDK) inhibitor p21 is a
significant element in this regulatory cascade (21), and is shown to be associated with
the prognosis of gastric cancer (22). p21 is a negative regulator of cell
cycle progression (21).
Overexpression of p21 has been identified as a crucial element
resulting in cell cycle arrest at G0/G1, S
and G2/M phase (23).
Radhakrishnan et al found that p21 was specifically
associated with cyclin E, rather than cyclin D1, cyclin A, CDK4 or
PCNA (23). Their finding are
consistent with our results of increased expression of p21
and decreased that of cyclin E1 after deguelin treatment in
gastric cancer cells (Fig. 5C, D, G
and H). These results suggested that p21-mediated inhibition of
cyclin E could be one of the factors that is responsible for the
proliferation inhibition and cell cycle arrest of gastric cancer
with deguelin treatment.
Deguelin induced apoptosis in a wide array of cancer
cell types in vitro, including lung squamous cell carcinoma
cells, colon cancer cells and head and neck squamous cell cancer
cell lines (9,10,12). In the present study, we found that
deguelin could induce apoptosis of gastric cancer MGC-803 and
MKN-45 cell lines in vitro in a dose-dependent manner
(Figs. 3 and 4), which is consistent with the effect
of deguelin performed in other cancer cells (9,11,12). The induction effect of apoptosis
could be mediated by the suppression of various molecular pathways,
such as PI3K-Akt, IKK-IκBα-NF-κB, EMT, HSP90 and AMPK-mTOR-survivin
pathways (11,24,25). Among all the target pathways
suggested for deguelin, the PI3K/Akt pathways have the strongest
support for inducing cell apoptosis (7,26–29). As shown in Fig. 5, compared to the control group,
phosphorylation of Akt was dramatically decreased by treatment with
deguelin in a dose-dependent manner in both cell lines, suggesting
that Akt pathway was responsible for deguelin-induced cell
apoptosis inhibition. To further corroborate the induced-apoptosis
effects of deguelin, investigation on Akt and other pathways is
still necessary.
In addition, based on the references of related
literatures (38,39), we assumed that another mechanism
of deguelin induced-apoptosis in gastric cancer cells may be
related to the mitochondria-mediated (also called the intrinsic)
apoptosis pathways. Mitochondria-dependent apoptosis is mediated by
the proteins of Bcl-2 family (30,31), including antiapoptotic and
proapoptotic proteins such as Bcl-2/xL and Bax/Bak (32–37). Bcl-2 family proteins change the
mitochondrial membrane permeability required for the release of
cytochrome c (38)
(39,40). Bcl-2 blocks the release of
cytochrome c, whereas Bax localizes to mitochondria and
enhances the release of cytochrome c, promotes nuclear
fragmentation and induces cell death (41). Therefore, we measured the gene
expression of Bcl-2 and Bax to clarify the probable
mechanism. Gene expression study by RT-qPCR has shown that deguelin
persuaded apoptosis through intrinsic pathway by upregulating the
pro-apoptotic gene Bax and down-regulating the
anti-apoptotic gene Bcl-2 in a dose-dependent manner
(Fig. 5A, B, E and F). In
conclusion, deguelin induced gastric cancer cell lines apoptosis
via two pathways including the regulation of Bax/Bcl-2 ratio and
inhibition of Akt activation.
The motility of gastric cancer cells plays a
critical role in development of metastasis. A number of reported
studies have demonstrated the effect of deguelin on the migration
and invasion of different cancer types (25,42–44). Hu et al found that the
treatment of deguelin inhibited lung cancer migration via
downregulating Akt and the MAPK pathway (25). In another study, deguelin
inhibited the migration and invasion of human osteosarcoma cells
via the inhibition of MMP-2/9 in vitro by inhibiting the
expression of GRB2, FAK and RhoA (44). However, the effects of deguelin on
motility of gastric cancer MGC-803 and MKN-45 cell lines have not
been reported. As shown in Figs.
6 and 7, the results showed
that deguelin reduced cell migration and invasion of gastric cancer
MGC-803 and MKN-45 cell lines in vitro. Therefore, deguelin
would be a useful candidate to suppress cancer progression and
metastasis. Based on the literature, deguelin suppressed the
migration and invasion of cancer cells by complicated mechanisms
such as reducing the expression and inactivation of FAK, RhoA-ROCK,
MAPK and Akt. The mechanism that occurred in the effect of deguelin
on the migration and invasion of gastric cancer cells need further
investigation.
In conclusion, this study demonstrated that deguelin
exhibited anticancer effect by inhibiting cell proliferation,
causing cell cycle arrest, inducing apoptosis and suppressing cell
migration and invasion of human gastric cancer in vitro. To
provide a guide for further clinical trials, more studies on the
precise mechanism of deguelin treatment of gastric cancer, however,
are still needed. Since a number of reported animal studies have
proved the antitumor activity of deguelin at doses of 3-5 mg/kg
using xenograft models of different cancer types in vivo
(24,43,45). These bionic models as well as our
study warrant further in vivo investigations of deguelin
efficacy. Collectively, deguelin may represent a promising
anticancer agent capable of combating the progression and
metastasis of gastric cancer.
Acknowledgments
The authors would like to thank the Shanghai Key
Laboratory of Tissue Engineering for its technical assistance.
Notes
[1]
Funding
This study was supported by grants from the
Scientific Research Foundation of Traditional Chinese Medicine of
Shanghai (2014JP013A, to PW) and the Research Innovation Program of
Shanghai Municipal Education Commission (09yz79, to SG).
[2] Availability
of data and material
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
WK and XZ performed the experiments and wrote the
manuscript. SG and PW designed the experimental plan and analyzed
the data. All authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
2
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian WY, Chen WC, Li R and Liu L: Markers
CD40, VEGF, AKT, PI3K and S100 correlate with tumor stage in
gastric cancer. Onkologie. 36:26–31. 2013.
|
|
4
|
Ye B, Jiang LL, Xu HT, Zhou DW and Li ZS:
Expression of PI3K/AKT pathway in gastric cancer and its blockade
suppresses tumor growth and metastasis. Int J Immunopathol
Pharmacol. 25:627–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sukawa Y, Yamamoto H, Nosho K, Kunimoto H,
Suzuki H, Adachi Y, Nakazawa M, Nobuoka T, Kawayama M, Mikami M, et
al: Alterations in the human epidermal growth factor receptor
2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer.
World J Gastroenterol. 18:6577–6586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu W, Hai Y, Chen L, Liu RJ, Han YX, Li
WH, Li S, Lin S and Wu XR: Deguelin-induced blockade of I3K/protein
kinase B/MAP kinase signaling in zebrafish and breast cancer cell
lines is mediated by downregulation of fibroblast growth factor
receptor 4 activity. Pharmacol Res Perspect. 4:e002122016.
View Article : Google Scholar
|
|
7
|
Chun KH, Kosmeder JW II, Sun S, Pezzuto
JM, Lotan R, Hong WK and Lee HY: Effects of deguelin on the
phosphatidylinositol 3-kinase/Akt pathway and apoptosis in
premalignant human bronchial epithelial cells. J Natl Cancer Inst.
95:291–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thamilselvan V, Menon M and Thamilselvan
S: Anticancer efficacy of deguelin in human prostate cancer cells
targeting glycogen synthase kinase-3β/β-catenin pathway. Int J
Cancer. 129:2916–2927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan B, Zhao D, Yao Y, Bao Z, Lu G and Zhou
J: Deguelin induces the apoptosis of lung squamous cell carcinoma
cells through regulating the expression of galectin-1. Int J Biol
Sci. 12:850–860. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murillo G, Salti GI, Kosmeder JW II,
Pezzuto JM and Mehta RG: Deguelin inhibits the growth of colon
cancer cells through the induction of apoptosis and cell cycle
arrest. Eur J Cancer. 38:2446–2454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Ma W and Zheng W: Deguelin, a
novel antitumorigenic agent targeting apoptosis, cell cycle arrest
and anti-angiogenesis for cancer chemoprevention. Mol Clin Oncol.
1:215–219. 2013. View Article : Google Scholar
|
|
12
|
Baba Y, Fujii M, Maeda T, Suzuki A, Yuzawa
S and Kato Y: Deguelin induces apoptosis by targeting both EGFR-Akt
and IGF1R-Akt pathways in head and neck squamous cell cancer cell
lines. BioMed Res Int. 2015:6571792015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mehta RR, Katta H, Kalra A, Patel R, Gupta
A, Alimirah F, Murillo G, Peng X, Unni A, Muzzio M, et al: Efficacy
and mechanism of action of Deguelin in suppressing metastasis of
4T1 cells. Clin Exp Metastasis. 30:855–866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karimi P, Islami F, Anandasabapathy S,
Freedman ND and Kamangar F: Gastric cancer: Descriptive
epidemiology, risk factors, screening, and prevention. Cancer
Epidemiol Biomarkers Prev. 23:700–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clarke JS, Cruze K, El Farra S and
Longmire WP Jr: The natural history and results of surgical therapy
for carcinoma of the stomach. An analysis of 250 cases. Am J Surg.
102:143–152. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janunger KG, Hafström L, Nygren P and
Glimelius B; SBU-group: Swedish council of technology assessment in
health care: A systematic overview of chemotherapy effects in
gastric cancer. Acta Oncol. 40:309–326. 2001. View Article : Google Scholar
|
|
17
|
Zhao H, Jiao Y and Zhang Z: Deguelin
inhibits the migration and invasion of lung cancer A549 and H460
cells via regulating actin cytoskeleton rearrangement. Int J Clin
Exp Pathol. 8:15582–15590. 2015.
|
|
18
|
Yi S, Wen L, He J, Wang Y, Zhao F, Zhao J,
Zhao Z, Cui G and Chen Y: Deguelin, a selective silencer of the
NPM1 mutant, potentiates apoptosis and induces differentiation in
AML cells carrying the NPM1 mutation. Ann Hematol. 94:201–210.
2015. View Article : Google Scholar
|
|
19
|
Murillo G, Peng X, Torres KE and Mehta RG:
Deguelin inhibits growth of breast cancer cells by modulating the
expression of key members of the Wnt signaling pathway. Cancer Prev
Res (Phila). 2:942–950. 2009. View Article : Google Scholar
|
|
20
|
Xiong JR and Liu HL: Regulatory effects of
deguelin on proliferation and cell cycle of Raji cells. J Huazhong
Univ Sci Technolog Med Sci. 33:491–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okuyama T, Maehara Y, Kabashima A,
Takahashi I, Kakeji Y and Sugimachi K: Combined evaluation of
expressions of p53 and p21 proteins as prognostic factors for
patients with gastric carcinoma. Oncology. 63:353–361. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Radhakrishnan SK, Feliciano CS, Najmabadi
F, Haegebarth A, Kandel ES, Tyner AL and Gartel AL: Constitutive
expression of E2F-1 leads to p21-dependent cell cycle arrest in S
phase of the cell cycle. Oncogene. 23:4173–4176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehta R, Katta H, Alimirah F, Patel R,
Murillo G, Peng X, Muzzio M and Mehta RG: Deguelin action involves
c-Met and EGFR signaling pathways in triple negative breast cancer
cells. PLoS One. 8:e651132013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu J, Ye H, Fu A, Chen X, Wang Y, Chen X,
Ye X, Xiao W, Duan X, Wei Y, et al: Deguelin - an inhibitor to
tumor lymphangiogenesis and lymphatic metastasis by downregulation
of vascular endothelial cell growth factor-D in lung tumor model.
Int J Cancer. 127:2455–2466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng XH, Karna P, O'Regan RM, Liu X,
Naithani R, Moriarty RM, Wood WC, Lee HY and Yang L: Downregulation
of inhibitor of apoptosis proteins by deguelin selectively induces
apoptosis in breast cancer cells. Mol Pharmacol. 71:101–111. 2007.
View Article : Google Scholar
|
|
27
|
Chen Y, Wu Q, Cui GH, Chen YQ and Li R:
Deguelin blocks cells survival signal pathways and induces
apoptosis of HL-60 cells in vitro. Int J Hematol. 89:618–623. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bortul R, Tazzari PL, Billi AM, Tabellini
G, Mantovani I, Cappellini A, Grafone T, Martinelli G, Conte R and
Martelli AM: Deguelin, A PI3K/AKT inhibitor, enhances
chemosensitivity of leukaemia cells with an active PI3K/AKT
pathway. Br J Haematol. 129:677–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu ZH, Liang XH, Zhou XL, Huang RF, Zhan
Q and Jiang JW: Effects of deguelin on proliferation and apoptosis
of MCF-7 breast cancer cells by phosphatidylinositol 3-kinase/Akt
signaling pathway. Zhong Xi Yi Jie He Xue Bao. 9:533–538. 2011.In
Chinese. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiu TH, Lan KY, Yang MD, Lin JJ, Hsia TC,
Wu CT, Yang JS, Chueh FS and Chung JG: Diallyl sulfide promotes
cell-cycle arrest through the p53 expression and triggers induction
of apoptosis via caspase- and mitochondria-dependent signaling
pathways in human cervical cancer Ca Ski cells. Nutr Cancer.
65:505–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu J, Yang J, Liu Q, Wu S, Ma H and Cai Y:
Lanthanum induced primary neuronal apoptosis through mitochondrial
dysfunction modulated by Ca2+ and Bcl-2 family. Biol
Trace Elem Res. 152:125–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Gurp M, Festjens N, van Loo G, Saelens
X and Vandenabeele P: Mitochondrial intermembrane proteins in cell
death. Biochem Biophys Res Commun. 304:487–497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scorrano L and Korsmeyer SJ: Mechanisms of
cytochrome c release by proapoptotic BCL-2 family members. Biochem
Biophys Res Commun. 304:437–444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuwana T, Mackey MR, Perkins G, Ellisman
MH, Latterich M, Schneiter R, Green DR and Newmeyer DD: Bid, Bax,
and lipids cooperate to form supramolecular openings in the outer
mitochondrial membrane. Cell. 111:331–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crompton M: Bax, Bid and the
permeabilization of the mitochondrial outer membrane in apoptosis.
Curr Opin Cell Biol. 12:414–419. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim R, Emi M and Tanabe K: Role of
mitochondria as the gardens of cell death. Cancer Chemother
Pharmacol. 57:545–553. 2006. View Article : Google Scholar
|
|
38
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghribi O, Herman MM, Spaulding NK and
Savory J: Lithium inhibits aluminum-induced apoptosis in rabbit
hippocampus, by preventing cytochrome c translocation, Bcl-2
decrease, Bax elevation and caspase-3 activation. J Neurochem.
82:137–145. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rossé T, Olivier R, Monney L, Rager M,
Conus S, Fellay I, Jansen B and Borner C: Bcl-2 prolongs cell
survival after Bax-induced release of cytochrome c. Nature.
391:496–499. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu YP, Lee JJ, Lai TC, Lee CH, Hsiao YW,
Chen PS, Liu WT, Hong CY, Lin SK, Ping Kuo MY, et al: Suppressive
function of low-dose deguelin on the invasion of oral cancer cells
by downregulating tumor necrosis factor alpha-induced nuclear
factor-kappa B signaling. Head Neck. 38(Suppl 1): E524–E534. 2016.
View Article : Google Scholar
|
|
43
|
Boreddy SR and Srivastava SK: Deguelin
suppresses pancreatic tumor growth and metastasis by inhibiting
epithelial-to-mesenchymal transition in an orthotopic model.
Oncogene. 32:3980–3991. 2013. View Article : Google Scholar
|
|
44
|
Shang HS, Chang JB, Lin JH, Lin JP, Hsu
SC, Liu CM, Liu JY, Wu PP, Lu HF, Au MK, et al: Deguelin inhibits
the migration and invasion of U-2 OS human osteosarcoma cells via
the inhibition of matrix metalloproteinase-2/-9 in vitro.
Molecules. 19:16588–16608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang YL, Ji C, Bi ZG, Lu CC, Wang R, Gu B
and Cheng L: Deguelin induces both apoptosis and autophagy in
cultured head and neck squamous cell carcinoma cells. PLoS One.
8:e547362013. View Article : Google Scholar : PubMed/NCBI
|