Introduction
Lithocholic acid (LCA) has been observed to kill rat
glioma cells, which implies that it has an antitumor effect via
mitochondrial outer membrane permeabilization (MOMP) (1). However, the exact anti-glioma
mechanism of LCA is at present unclear. MOMP brings about the
release of cytochrome c from the mitochondrial intermembrane space
into the cytosol (2–4), which indicates that MOMP is closely
related to the function of the mitochondrial inner membrane. The
presence of reactive oxygen species (ROS) can lead to severe damage
to cellular structures and their functions followed by cell death.
Proton leak, likely induced by lipid peroxidation, and backed into
the matrix of the mitochondria (5), and limited production of ROS
(6) results in the uncoupling of
oxidative phosphorylation. Uncoupling is brought about via the leak
of protons through downstream lipid peroxidation products other
than ATP synthase (7,8). Lipid peroxidation by ROS causes free
radical reactions resulting in various aldehyde products, including
trans-4-hydroxynonenal (4-HNE). 4-HNE is a toxic by-product of free
radical damage (9) and is also a
cell mediator acting as a signaling molecule. Lipid peroxidation
products and ROS are very active in DNA binding and usually cause
mutations that trigger oncogenesis (10).
The thiobarbituric acid (TBA) test was used to assay
lipid peroxidation (11), but
with other studies were different, focused on the effects of LCA on
glioma mitochondria. H2O2 was chosen as the
inducer of lipid peroxidation in this model and changes in UV peaks
caused by reactions between TBA and biologically active
α,β-unsaturated aldehydes (12)
were used as indicators of reaction. The effects of LCA on UV peaks
was investigated using a model of lipid peroxidation in
mitochondria induced by H2O2. Changes in UV
peaks corresponded to a variety of aldehydes as follows. 4-HNE
(13), a major peak at 530 nm and
shoulder peaks at 495 and 450 nm; trans, trans-muconaldehyde
(14), a major peak at 495 nm and
shoulder peaks at 460 and 530 nm; trans, trans-2,4-nonadienal,
which is a dehydration product of 4-HNE, a major peak at 532 nm and
shoulder peaks at 450 and 495 nm; acrolein (15), a major peak at 495 nm and shoulder
peaks at 460 and 530 nm; crotonaldehyde (16), a major peak at 495 nm and shoulder
peaks at 460 and 530 nm; malondialdehyde (MDA) (17), a major peak at 532 nm and a
shoulder peak at 495 nm; no peaks from propionaldehyde (18) were observed under any experimental
conditions.
Although MDA is not a specific indicator to detect
tumors, the presence of biologically active α,β-unsaturated
aldehydes (19) can be used to
detect glioma, especially in mitochondria. In the present study,
mitochondria were used to evaluate the correlation between LCA and
observed changes in the UV spectrum at 495, 532 and 450 nm. The
purpose of the study was to explore the anti-glioma mechanism of
LCA on mitochondria.
Materials and methods
Materials
The reagents potassium chloride (KCl),
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES),
magnesium chloride hexahydrate (MgCl2), ethylene diamine
tetraacetic acid (EDTA), albumin from bovine serum (BSA), sodium
chloride (NaCl), ferrous chloride (FeCl2),
2-thiobarbituric acid (TBA), glacial acetic
acid,1,1,3,3-tetramethoxypropane, trichloroacetic acid (TCA),
butylated hydroxytoluene (BHT) were all of analytical grade; 30%
hydrogen peroxide (H2O2) and lithocholic acid
were obtained from Aladdin Industrial Corporation, Shanghai, China.
Glioma mitochondria were obtained from glioma (Department of
Neurosurgery, First Hospital of Jilin University). Mitochondrial
extraction was performed on a Beckman Coulter Avanti J-26XPI. UV
detection was performed on a 2550 UV-visible spectrophotometer
(Beckman Coulter, Tokyo, Japan) and a PharmaSpec UV-1700 UV-Visible
spectrophotometer (Shimadzu Corp., Tokyo, Japan). The pH detection
used a PB-10pH/ATC electrode (Sartorius, Göttingen, Germany).
Samples were treated with a 3K15 desktop high-speed refrigerated
centrifuge (Sigma, Steinheim, Germany). Cold isolation medium
consisted of 120 mM KCl, 20 mM HEPES (pH 7.4), 2 mM
MgCl2, 1 mM EGTA, 5 mg/ml BSA, prepared in advance, and
cooled at 4°C.
Reagent setup
The 50 mM NaCl and 0.2 mM FeCl2 reagent
was freshly prepared by dissolving 730 mg NaCl and 9.94 mg
FeCl2 in 250 ml distilled water. The reagent must be
prepared fresh before use on the day, stored in a cool and dark
place, and used within 4 h.
The LCA reagent was prepared by dissolving 19.41 mg
97% LCA in 50 ml heated ethanol and measuring 0, 50, 100, 150, 200
and 300 µl aliquots of 1 mM LCA and bringing to a final
volume of 2 ml with distilled water, resulting in 0, 25, 50, 75,
100 and 150 µM solutions, respectively.
The 7.5% (W/V) TCA solution was prepared by weighing
7.50 g TCA in 100 ml distilled water.
The 46 mM TBA reagent was freshly prepared by
dissolving 1.3464 g TBA in 200 ml 99% glacial acetic acid, then
adding 6 ml of 2% butylated hydroxytoluene. The resulting solution
was kept in the dark.
The 2% butylated hydroxytoluene solution was
prepared by dissolving 120 mg of 99% butylated hydroxytoluene in 6
ml of 99% ethanol with heating.
Preparation of glioma mitochondria
With the approval of Institute Ethics Committee and
the informed consent signed by the glioma patients or their
guardians, the glioma samples were collected. Mitochondria derived
from glioma were prepared by a modification of the conventional
method of differential centrifugation (20). Briefly, 3.00 g of glioma tissue in
fresh was immediately excised and minced in 150 ml ice cold
isolation medium containing 120 mM KCl, 20 mM HEPES, pH 7.4, 2 mM
MgCl2, 1 mM EDTA and 5 mg/ml BSA. Minced blood-free
tissue was rinsed and suspended in the same fresh medium and
stirred. The sample was carefully homogenized with a tightly fitted
homogenizer: 10,000 revolutions, 10 sec each time, interval of 15
sec, glioma tissue homogenate three times. After homogenization,
isolation medium was added to the homogenate and centrifuged at 600
× g for 10 min. The resulting pellets were removed and the
supernatant suspension was again centrifuged at 17,000 × g for 10
min. The supernatant was decanted and the pellets were gently
resuspended in isolation medium and then centrifuged at 7,000 × g
for 10 min. The supernatant was discarded and the precipitated
pellets were resuspended in isolation medium, centrifuged at 3,500
× g for 10 min, and the supernatant containing the mitochondrial
fraction was collected. All mitochondrial isolation procedures were
performed at 0–4°C. The concentration of the mitochondrial protein
was estimated using Lowry's method (21) using BSA as the standard (50 mg
protein/ml). The mitochondrial suspensions were used within 4
h.
The establishment of a model using UV
peak changes to investigate aldehydes formed from lipid
peroxidation induced by H2O2 in glioma
mitochondria
A model relating to changes in UV peaks from
aldehydes after lipid peroxidation induced by
H2O2 in mitochondria was established using
the following procedures. To samples containing 2 ml 50 mM NaCl and
0.2 mM FeCl2 was added 0, 0.5, 1.0, 1.5, 2.0 and 2.5
mg/ml of glioma mitochondria and 1.76, 8.80, 17.60, 26.40, 35.20
and 70.40 µM of H2O2, respectively.
Samples of mitochondrial lipid peroxidation at 1, 5, 10, 20, 30 and
40 min were prepared for extraction of aldehydes. Extracted samples
(2 ml) were added to 4.3 ml of 7.5% (W/V) TCA, mixed and
centrifuged at 5,000 × g for 10 min, then kept at 36°C in a bath
for 10 min, centrifuged again at 5000 × g for 10 min and another
4.3 ml of 7.5% (W/V) TCA was added. The aldehyde extract of lipid
peroxidation in mitochondria was filtered using 12.50 mm medium
speed filter paper for determination. The reaction of the aldehydes
in lipid peroxidation with TBA and the formed adducts were
determined with spectrophotometric methods (12). The procedure was performed as
described by Ottino and Duncan (22) with some modifications. The 2 ml
sample extract and 1.0, 1.5, 2.0, 2.5, 3.0 and 4.0 ml of TBA
reagent were mixed in 4 ml test tubes and heated in a boiling water
bath for 15 min. The reaction mixture was chilled and centrifuged
at 5,000 × g for 10 min. The absorbance of samples over the
wavelength range 400–600 nm was measured using a 2550 UV-visible
spectrophotometer, focused on changes in absorbance at 450, 495 and
532 nm using distilled water as a blank, adjustable light
transmittance of 100%.
Determination of factors effecting the
impact of LCA on the reaction of aldehydes in lipid peroxidation in
glioma mitochondria
The effect of LCA on the reaction of aldehydes in
lipid peroxidation in glioma mitochondria was investigated at
different LCA concentrations (0, 25, 50, 75, 100 and 150
µM), reaction times (0, 5, 10, 15, 20 and 30 min), and micro
environmental pH (3.0, 7.0, 8.0).
Statistical treatment
Statistic software Origin 8.0 was used to perform
one-way analysis of variance for the experimental data.
Results
UV peaks change in the spectra of
aldehydes in lipid peroxidation induced by
H2O2 in glioma mitochondria
The influence of different mitochondrial
concentrations on UV peak changes from aldehydes in the lipid
peroxidation model is shown in Fig.
1A. Increasing concentrations of glioma mitochondria showed a
commensurate rise in lipid peroxidation. When the mitochondrial
concentrations were >1.50 mg/ml, a trend towards a decrease in
lipid peroxidation was observed. There are three peaks that can be
observed with a change in mitochondrial concentration; a major peak
at 495 nm and two shoulder peaks at 532 and 450 nm. The 532 nm peak
is much weaker than the 450 nm peak. All three peaks changed in a
concentration-dependent manner, this was seen especially in the
main peak at 495 nm. When the mitochondrial concentration was 1.5
mg/ml, all three peaks changed noticeably.
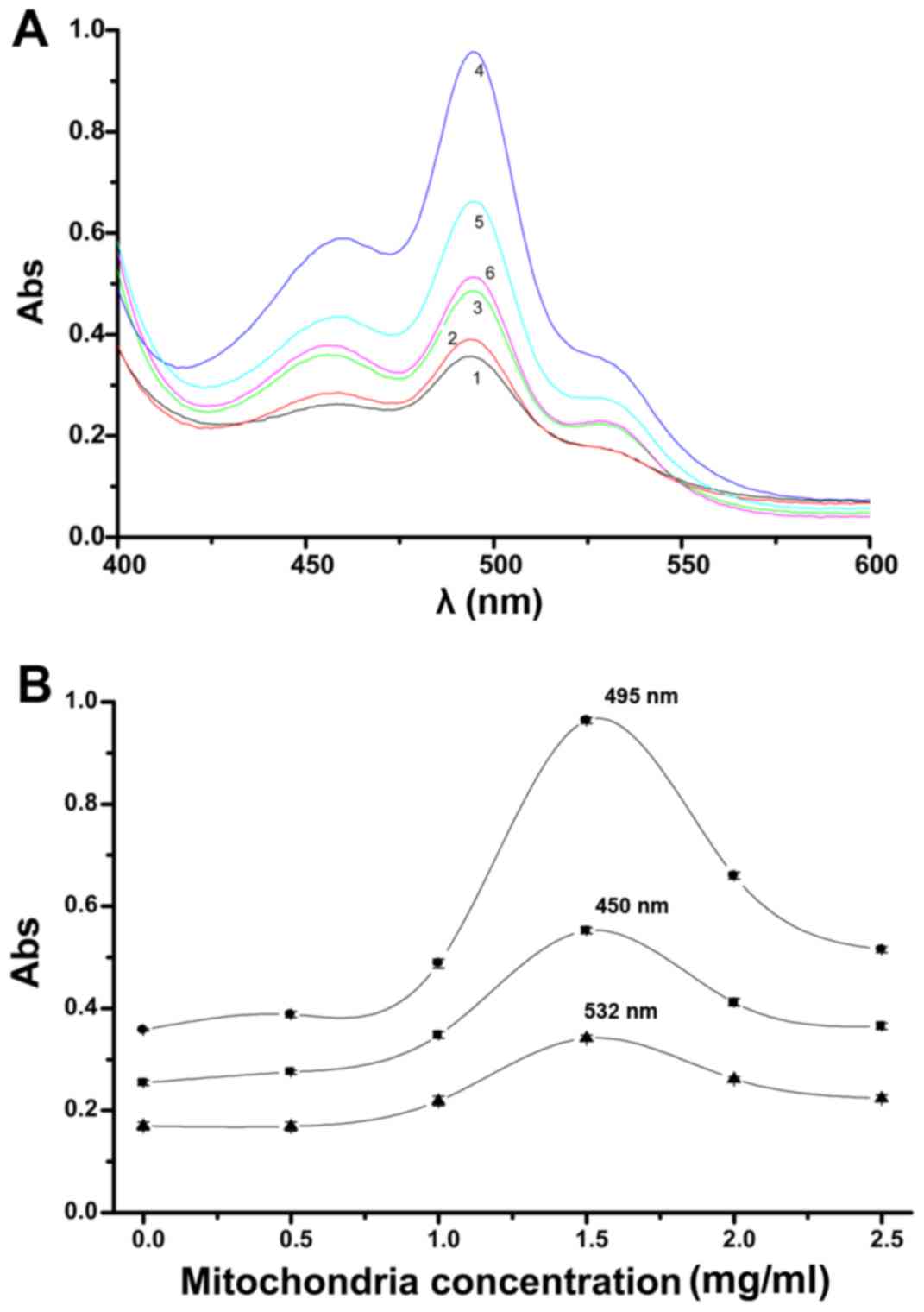 | Figure 1Impact of mitochondrial
concentrations deriving from glioma on UV peak changes from
aldehydes in the lipid peroxidation model (A). 1, 2, 3, 4, 5 and 6
indicate 0, 0.5, 1.0, 1.5, 2.0 and 2.5 mg/ml concentrations of
glioma mitochondria, respectively [20 min, 17.60 µM
H2O2, 4.0 ml thiobarbituric acid (TBA)]. The
absorbance curves at 495, 450 and 532 nm with changing
concentrations of the glioma mitochondria were observed separately
(B). |
The absorbance curves at 495, 450 and 532 nm with
changing concentrations of the glioma mitochondria are shown in
Fig. 1B. The 450 and 532 nm
curves were similar in shape, the change in the 450 nm peak was
greater than the change at 532 nm. The change in the curve at 495
nm, especially at a mitochondrial concentration of 1.5 mg/ml, was
much more pronounced than in the other two curves. The results
indicate that at a glioma mitochondrial concentration of 1.5 mg/ml,
the absorbances at 495, 450 and 532 nm were sensitive, prominent,
and significant, particularly the peak change at 495 nm.
The effect of H2O2
concentrations on the UV peaks from aldehydes in the lipid
peroxidation model are shown in Fig
2A. When concentrations were higher than 17.60 µM, the
three peaks decreased substantially, this is especially reflected
in the peaks at 495 and 532 nm. At low concentrations, the three
peaks show an obvious change. In the low concentration range with
increasing concentrations, the peak at 495 nm gradually became
larger, as did the peak at 450 nm to a lesser extent, and the peak
of 532 nm showed the least change. Results indicated that a
concentration of 17.60 µM H2O2 leads
to three substantial changes in UV peaks, but the appearance of the
peak of 532 nm was not as obvious. While at a concentration of 8.8
µM H2O2, the size of the three peaks
was substantially decreased, but three distinct peaks were
observed; therefore, this concentration is more appropriate for use
in the glioma mitochondrial lipid peroxidation model. When 8.80
µM is selected as the experimental concentration, the model
shows that the major peak at 450 nm changes the most, followed by
the peak at 495 nm, and the peak at 532 nm is relatively weak.
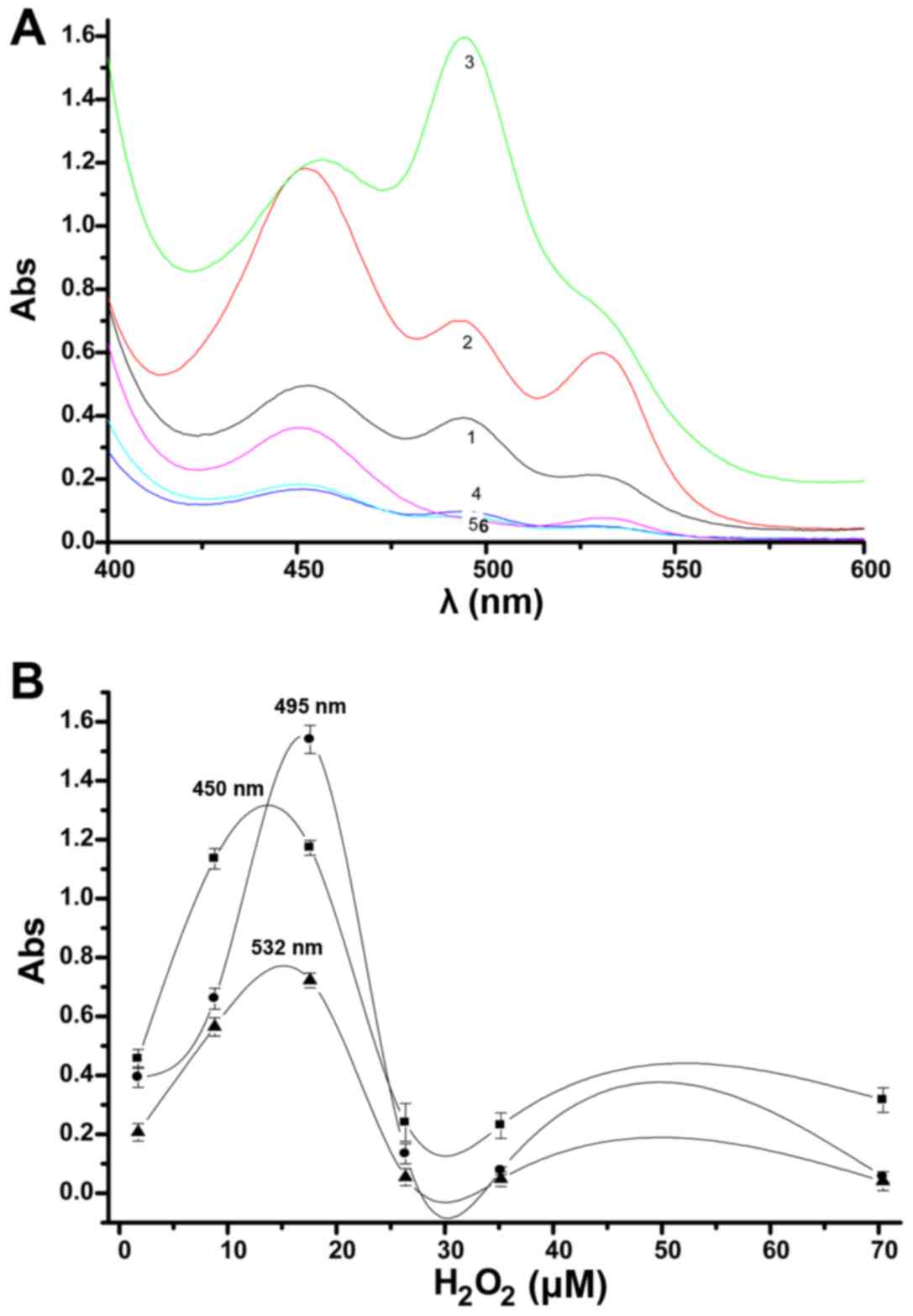 | Figure 2Effect of H2O2
on UV peak changes from aldehydes in the lipid peroxidation model
(A). 1, 2, 3, 4, 5 and 6 indicate 1.76, 8.80, 17.60, 26.40, 35.20
and 70.40 µM concentrations of H2O2,
respectively [20 min, 1.5 mg/ml mitochondria, 4.0 ml thiobarbituric
acid (TBA)]. The absorbance changes at 495, 450 and 532 nm with
H2O2 concentrations are shown (B). |
Changes in absorbance were accompanied by changes in
H2O2 concentration, as shown in Fig. 2B. When the
H2O2 concentration is low, the three peaks
were sensitive to changes in the concentration of
H2O2. The change in the 495 nm peak was the
most pronounced; changes in the 450 nm peak were similar to the 532
nm peak, with a greater change observed at 450 nm. Therefore, the
peak at 495 nm is the most important indicator to observe apoptosis
induction of glioma cells.
The effects over time of H2O2
on glioma mitochondria with regard to changes in the UV peaks from
aldehydes in the lipid peroxidation model can be seen in Fig. 3A. After H2O2
was added to the mitochondrial suspension, the establishment of
lipid peroxidation in glioma mitochondria required an appropriate
time. The experimental results show that three distinct peaks are
apparent, a major peak at 450 nm, a large shoulder peak at 495 nm
and a smaller shoulder peak at 532 nm. At different times, graphs
of the three peaks appear similar in shape. When the reaction time
is 30 min, the three peaks changed the most noticeably, over this
time, the peak change became small. In this case, detection of
absorbance at 450 and 495 nm was a sensitive indicator of the
effects.
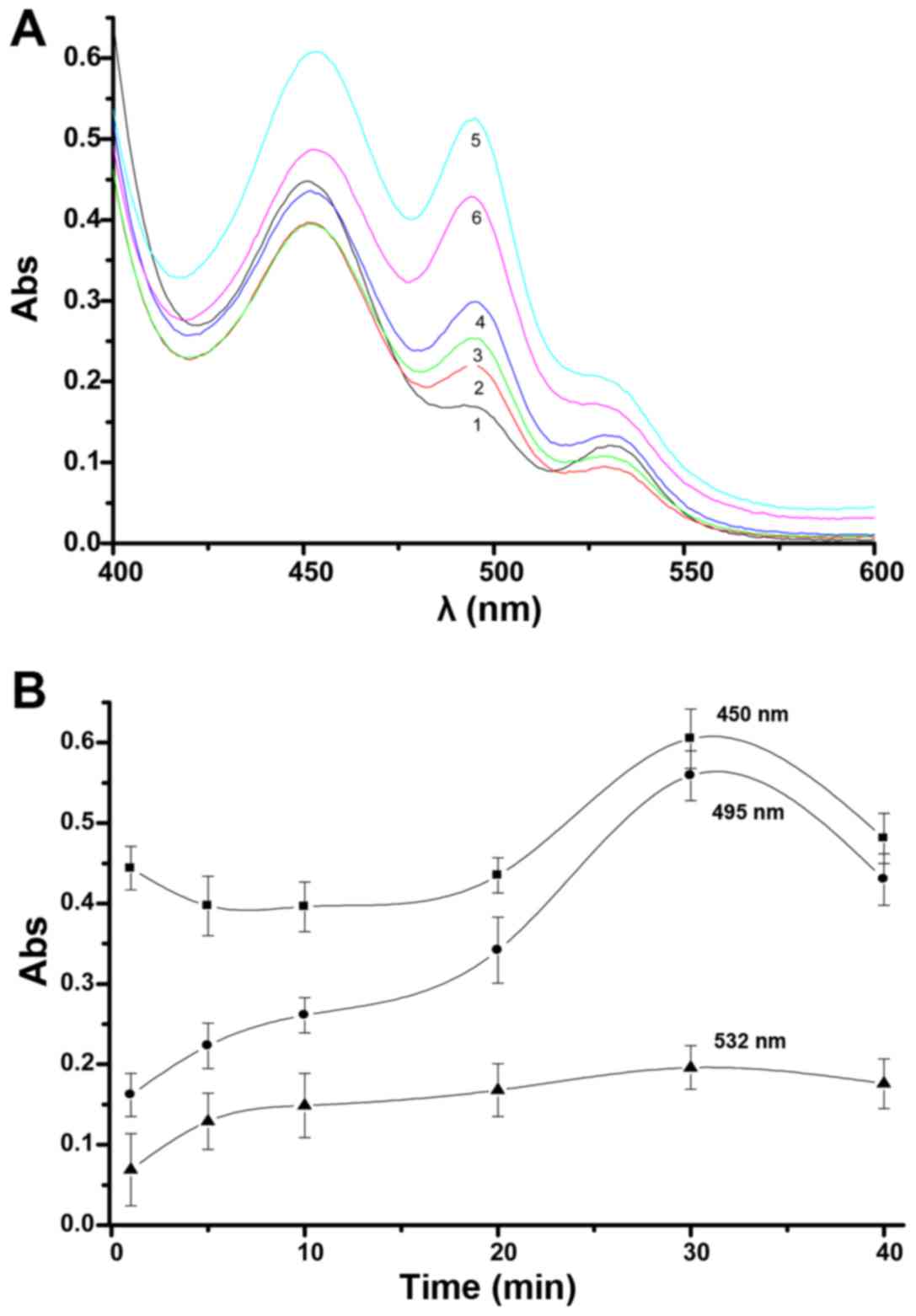 | Figure 3Time influence of
H2O2 on mitochondria with regard to UV peak
changes from aldehydes in the lipid peroxidation model (A). 1, 2,
3, 4, 5 and 6 indicate after 1, 5, 10, 20, 30 and 40 min,
respectively (8.8 µM H2O2, 1.5 mg/ml
mitochondria, 4.0 ml TBA). The absorbance curves at peaks of 495,
450 and 532 nm with changing of time were observed (B). |
In the initial few minutes after
H2O2 addition to glioma mitochondria, there
are some differences in the three absorbance curves, and after 30
min the curves at 450 and 495 nm displayed obvious changes, while
the curve at 532 nm was only gradually slightly elevated. After 30
min, the absorbance of the three peaks showed a downward trend, as
seen in Fig. 3B.
The selection of the optimum amount of TBA for the
reaction with the extracted aldehydes to assess the UV peak changes
from aldehydes in the lipid peroxidation model is shown in Fig. 4A. When the amount of TBA
increased, three peaks gradually appeared in the UV spectra, first
a peak at 450 nm, then 495 nm and finally at 532 nm. When the
amount of TBA was low, the three peaks appeared gradually in order,
but with low absorbance. When the amount of TBA reached 2.5 ml, the
three peaks appeared at the maximum absorbance value and when the
amount of TBA was further increased, the peaks tended to become
smaller with decreased amplitude. The changes in the three peaks
were significant, stable, and sensitive with amounts of TBA from
2.5 to 3.0 ml, but when the amount of TBA reached 2.5 ml, the 532
nm peak was not obvious. All three peaks decreased substantially
when the amount of TBA was 4.0 ml.
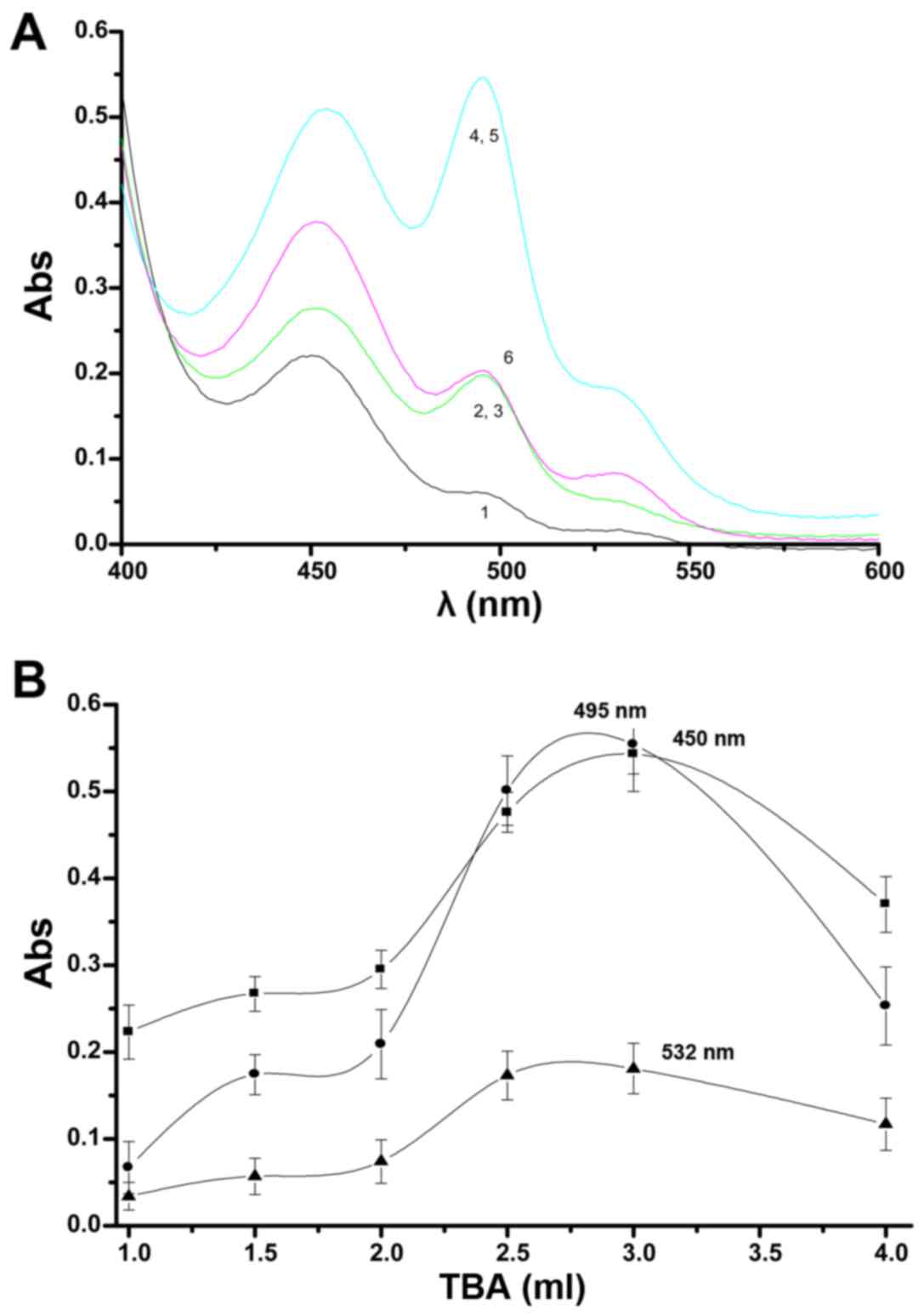 | Figure 4The optimum amount of thiobarbituric
acid (TBA) for reaction with the extracted aldehydes. Changes in
the UV peaks from aldehydes in the lipid peroxidation model (A). 1,
2, 3, 4, 5 and 6 indicate 1.0, 1.5, 2.0, 2.5, 3.0 and 4.0 ml TBA
reagent, respectively. The absorbance peaks at 495, 450 and 532 nm
with changing amount of TBA are shown (B). |
Changes in the peaks with differing amounts of TBA
can be seen in Fig. 4B. No curves
changed greatly when the amount of TBA was 2.0 ml or less. Further
increases in the amount of TBA resulted in curves with prominent
and sensitive characteristics. The curves reached a maximum when
the amount of TBA was 3.0 ml, the peaks were especially high at 495
and 450 nm. Above 3.0 ml TBA, each curve declines, but the decline
in the 532 peak was not as obvious. Therefore, detection would be
most effective when using 4.0 ml TBA.
In conclusion, the conditions established for the
glioma mitochondrial lipid peroxidation model were a concentration
of glioma mitochondria of 1.5 mg/ml, H2O2
concentration of 8.80 µM, a time of 30 min for the reaction
of H2O2 with glioma mitochondria, and an
amount of 46 mM TBA for optimum reaction with the extracted
aldehydes of 4.0 ml. The indices for evaluation in the model were
the peak changes at 495, 450 and 532 nm.
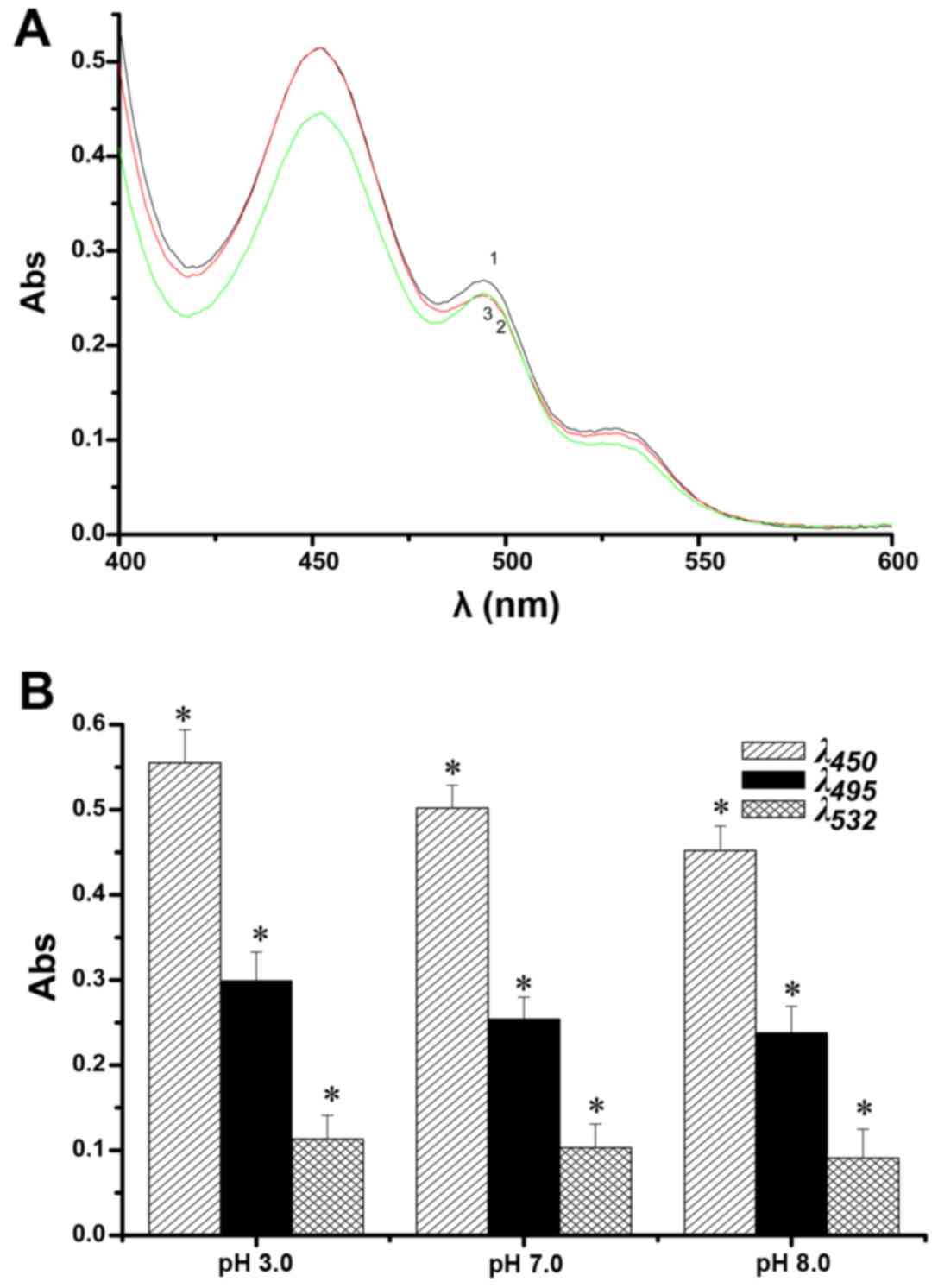
Using the above-mentioned conditions for the lipid
peroxidation model, the contribution of pH on the changes in the UV
peaks from aldehydes in the lipid peroxidation model are displayed
in Fig. 5A. In addition to the
main peak at 450 nm, there was a large shoulder peak at 495 nm and
a smaller shoulder peak at 532 nm. The absorbance of the three
peaks at different pH microenvironments is shown in Fig. 5B. With a decrease in pH, the three
peaks gradually increased, in particular the peaks at 450 and 495
nm. The results indicate that the three peaks in acidic and
alkaline microenvironments are quite prominent, especially in the
former. In short, an acidic microenvironment greatly promoted cell
apoptosis.
Effect of LCA on changes in aldehyde
concentration after lipid peroxidation in glioma mitochondria
Using the above mentioned conditions for the lipid
peroxidation model, the effect of LCA concentrations on changes in
the UV peaks from aldehydes was investigated and the results are
given in Fig. 6A. Under
conditions of ≤100 µM LCA, increases in LCA increased the
peaks at 495, 450 and 532 nm, in a concentration-dependent manner,
especially the peak at 495 nm. A concentration of >100
µM, led to the peak at 495 nm decreasing, as seen in
Fig. 6B. These results show that
in the model of mitochondrial lipid peroxidation via measuring an
increase in aldehydes, addition of LCA caused further rapid
peroxide production to achieve an anti-glioma effect. The LCA
antitumor contribution is reflected in the changes in the peak at
495 nm, followed by the peak at 450 nm and with less contribution
from the peak at 532 nm. As determined from the peaks from the
different aldehydes, LCA increased the amounts of 4-HNE, trans,
trans-muconaldehyde, trans, trans-2,4-nonadienal, acrolein,
crotonaldehyde and MDA to produce an antitumor effect.
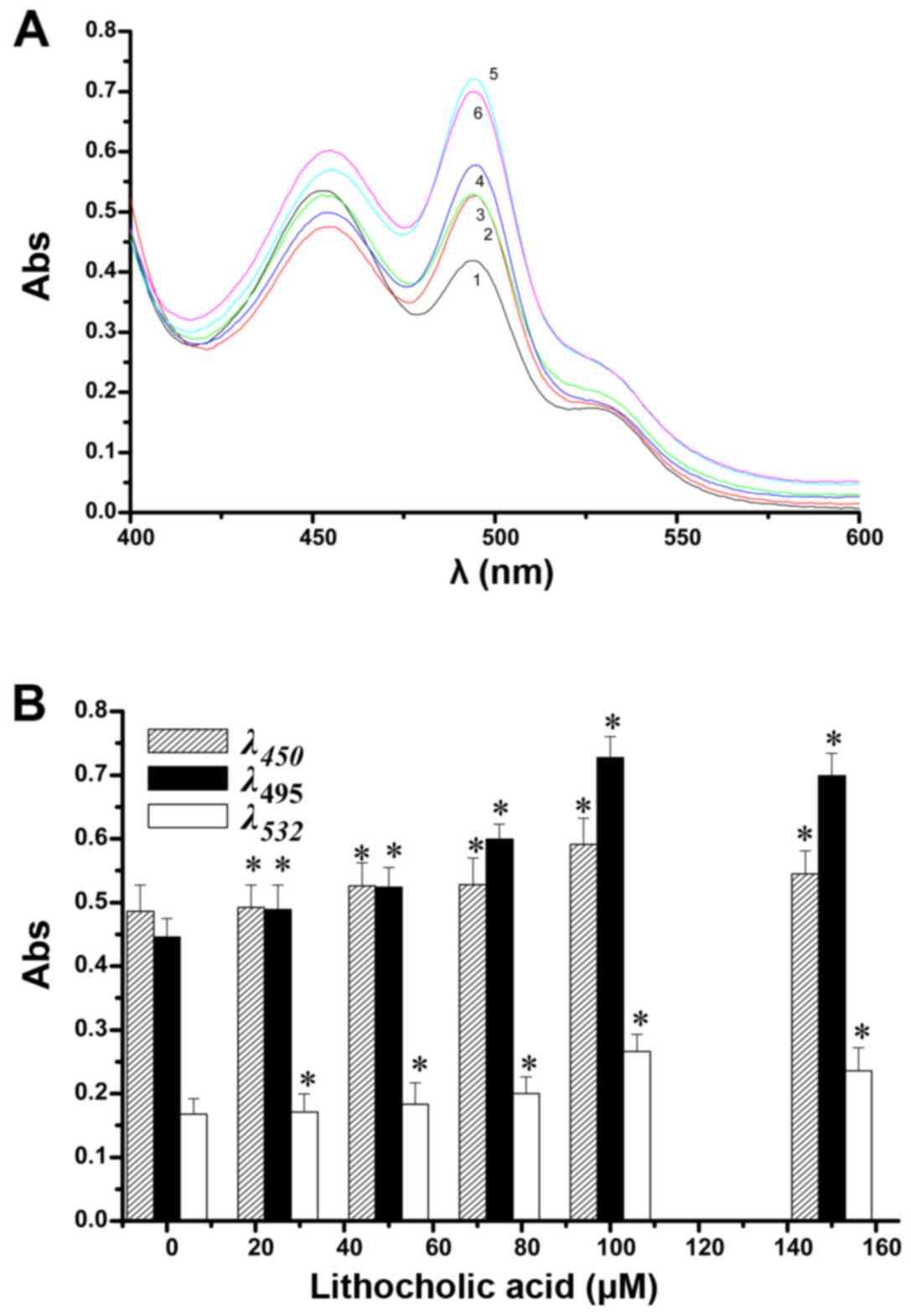 | Figure 6Effect of lithocholic acid
concentrations on changes in UV peaks from aldehydes in the lipid
peroxidation model (A). 1, 2, 3, 4, 5 and 6 indicate 0, 25, 50, 75,
100 and 150 µM lithocholic acid (LCA), respectively. The
absorbance peaks at 495, 450 and 532 nm with changing
concentrations of lithocholic acid are shown (B). Compared with the
normal control group, statistically significant difference,
*p<0.05. (20 min, pH 7.4). |
The effects over time of LCA addition on the change
in the UV peaks from aldehydes in the lipid peroxidation model can
be seen in Fig. 7A. At ≤30 min,
LCA elicits three UV absorption peaks, at 495, 450 and 532 nm. The
main peak was at 495 nm, followed by a large shoulder peak at 450
nm and a small shoulder peak at 532 nm. The intensity of the three
peaks first increased with increasing time and then decreased. As
shown in Fig. 7B, after ≤15 min
and with time of operation of LCA prolonged, the peaks changed
significantly, especially the peaks at 495 and 450 nm. After >15
min, a downward trend in the peaks can be clearly observed. The
results show that when the reaction time of LCA was 15 min, the
three peaks displayed the most sensitive and pronounced change,
particularly the peaks at 495 and 450 nm, while the change in the
peak at 532 nm showed relatively poor sensitivity.
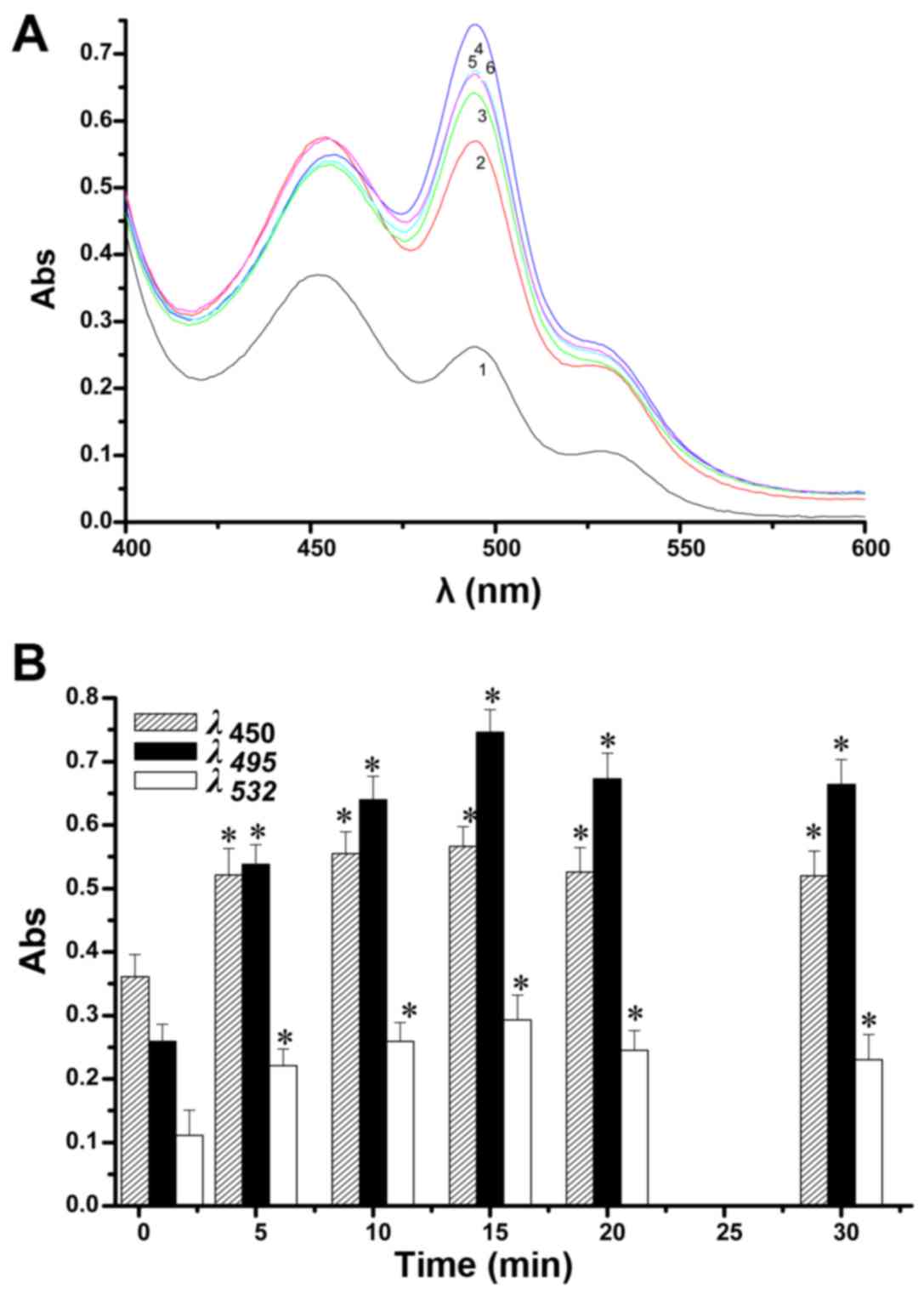 | Figure 7The effects over time of lithocholic
acid (LCA) addition on the change in the UV peaks from aldehydes in
the lipid peroxidation model (A). 1, 2, 3, 4, 5 and 6 indicate 0,
5, 10, 15, 20 and 30 min reaction times, respectively. The
absorbance changing curves at 495, 450 and 532 nm with changing
reaction times were observed separately (B). Compared with the
normal control group, statistically significant difference,
*p<0.05. (150 µM LCA, pH 7.4). |
Discussion
LCA is a kind of bile acid. Recently, low
concentrations of LCA have been shown to make BE(2)-m17 and SK-n-MCIXC cells sensitive to
H2O2-induced death of apoptotic cells, which
was regulated by mitochondria. Certain concentrations could make
primary cultures of human neurons resistant to death. In the
treatment of glioma, research into efficient, hypotoxic drugs which
can induce glioma cell apoptosis is of current importance. While
the antitumor effect of LCA is just corresponding to it. However,
the molecular biological mechanisms of the antitumor effect of LCA
is not clear at present. Therefore, research into the antitumor
mechanisms of LCA is of great importance to the research and
development of anti-glioma drugs. In a recent study, the oxygen
radical has been determined to be the most important radical
affecting the body (23). Besides
direct damage to the body, the oxygen radical mostly can make the
cells, especially the polyunsaturated fatty acids of mitochondrial
membrane peroxide, to damage the cytomembrane, as well as affecting
the structure and function of the mitochondrial membrane. These
effects could lead to energy metabolism disturbance causing damage
to the cell functions (24).
Lipid peroxidation can generate unsaturated aldehydes, which are
the signaling molecules of the mitochondria. Lipid peroxidation may
also activate cell apoptosis pathways, such as Fas/FasL, to induce
cell apoptosis (25).
Biologically active α,β-unsaturated aldehydes can be used to detect
effects on glioma after development of an appropriate glioma
mitochondria model.
A glioma mitochondrial lipid peroxidation model has
been successfully established, in which three small peaks from
aldehydes appear in the UV spectra after reaction with TBA. This
model enables the exploration of the LCA anti-glioma mechanism.
Small molecule aldehydes generated in the lipid oxidation process
are closely linked to tumorigenesis, development and outcome. Such
small molecule aldehydes can lead to the demise of glioma tissues
and may have an important research value. After reaction with TBA,
these small molecule aldehydes show three characteristic UV
absorption peaks at 495, 450 and 532 nm (18). These results are in accordance
with our study and indicate that small molecule aldehydes are the
major reaction products in the lipid peroxidation model in glioma
mitochondria.
The basis of the glioma mitochondrial model is cell
death as a consequence of lipid peroxidation.
H2O2 generated in an aerobic environment
leads to toxic levels of DNA damage (26–29). Cells are more damaged when exposed
to H2O2 in low concentrations for a long time
than for a short time. The mechanism of cell death caused by
H2O2 is through regulation of iron in the
mitochondrial inner membrane because cell-permeable iron chelators
effectively prevent H2O2-mediated DNA damage
in cells (30–32). The role of small molecule
aldehydes, such as 4-HNE, in H2O2-induced
cell death remains to be elucidated.
4-HNE, the most cytotoxic aldehyde (13), is generated in lipid peroxidation
by degradation of omega-3-polyunsaturated fatty acids (33). 4-HNE along with other small
molecule aldehydes possesses the capacity to block cell
proliferation and lead to cell death in <60 min (9). In addition, DNA polymerase, a thiol
enzyme (34), was blocked by the
sulfhydryl reactive agents, HNE and acrolein (35). HNE-GSH adducts display a feedback
inhibition on GSH transferases. Unlike ROS, which have been
associated with cell proliferation and differentiation (36,37), aldehydes in lipid peroxidation
linger a comparatively long time and can diffuse from the initial
site to reach distant targets. These kinds of small molecule
aldehydes react with nucleic acids, contributing to mutagenesis and
carcinogenesis (38). Aldehydes
are detected based on the formation of aldehydes in lipid
peroxidation. The basic process leading to the production of
aldehydes is via the β-cleavage reaction of lipid hydroperoxides,
more accurately described as lipid alkoxy-radicals (33). 4-HNE and its dehydration product
trans, trans-nonadienal react with TBA to form chromogens absorbed
maximally at 530 and 532 nm. Other biologically active
α,β-unsaturated aldehydes, including E-2-butenal, acrolein,
crotonaldehyde, trans-muconaldehyde also react with TBA, absorbing
maximally at 495 nm (18). These
characteristic absorption peaks appear to provide a strong support
for the detection of biologically active α,β-unsaturated
aldehydes.
These aldehydes bind stably with thiols under acidic
conditions, leading to the condensation of the small molecule
aldehydes, and participation in the death of glioma (33,39). Of course, glutathione easily
reacts in a neutral environment with α,β-unsaturated aldehydes
(40). The reaction of
α,β-unsaturated aldehydes with thiols in an environment of pH ≥7.0
can proceed up to hundred times faster than in an acidic
environment, which explains the results observed with LCA in an
acidic environment. A concentration of <0.1 µM HNE may be
regarded as a normal physiological level, but at a range of 0.1–20
µM, HNE may attack target thiol proteins and inhibit DNA and
protein synthesis within the lipid bilayer (34). In addition, the effects
accompanying cell death in the process of lipid peroxidation
include the rapid depletion of glutathione (41), a decrease in protein thiols
(42), inhibition of DNA, RNA and
protein synthesis (43), and
morphological changes. Therefore, the reaction of α,β-unsaturated
aldehydes with the thiols in proteins has important significance
(44).
The reaction of MDA with nucleosides has been shown
to be promoted by an acidic environment and acrolein, which was the
strongest electrophile and showed the highest reactivity with
thiols can be formed during lipid peroxidation (45).
Acrolein, which is highly cytotoxic to cells,
reacted approximately over a hundred times faster with GSH than
crotonal or HNE (40). The
mechanism of the reaction of acrolein and crotonal with GSH is
basically the same as HNE. When acrolein causes rapid depletion of
thiols, the thiols in proteins become progressively modified
(46) and then acrolein-treated
DNA does not act as substrate for DNA-methylase (47). Crotonal has much lower toxicity
than acrolein. MDA, a low molecular aldehyde is produced from ROS
attacking polyunsaturated fatty acids.
In conclusion, the optimum conditions for a lipid
peroxidation model using glioma mitochondria have been investigated
according to changes in UV peaks at 495, 450 and 532 nm after
reaction between TBA and biologically active α,β-unsaturated
aldehydes. The optimal conditions determined for the lipid
peroxidation model in glioma mitochondria were a mitochondrial
concentration of 1.5 mg/ml, H2O2
concentration of 0.3 mg/ml, a duration of action of 30 min, and
addition of 4.0 ml of 46 mM TBA. The model indicators were changes
in the peaks at 495, 450 and 532 nm related to aldehydes formed
during lipid peroxidation of glioma mitochondria induced by
H2O2. The effect of LCA on the peaks at 450,
495 and 532 nm was greatest when the conditions were an LCA
concentration of 100 µM, a duration of action of 15 min, and
an acidic microenvironment, according to the mitochondria model.
The mechanism of LCA anti-glioma action may be interpreted as
regulation and control of the aldehydes represented by changes in
peaks at 450, 495 and 532 nm, and the use of the glioma
mitochondrial model may be conducive to in-depth research in this
topic.
In conclusion, the impact of LCA on glioma was
closely related to the production of aldehydes from lipid
peroxidation in the glioma mitochondria. The optimum conditions
have been confirmed according to changes in the peaks at 495, 450
and 532 nm using a lipid peroxidation model in glioma mitochondria,
by means of reactions between TBA and biologically active
α,β-unsaturated aldehydes. Mitochondrial modeling in glioma lipid
peroxidation was successfully established and the optimal
conditions were determined to be a glioma mitochondrial
concentration of 1.5 mg/ml, a H2O2
concentration of 0.3 mg/ml, a duration of action of 30 min, and
addition of 4.0 ml of 46 mM TBA. The model indicators were changes
in the peaks at 495, 450 and 532 nm related to aldehydes formed
during lipid peroxidation of glioma mitochondria induced by
H2O2. The effect of LCA on the peaks at 450,
495 and 532 nm was greatest when the conditions were an LCA
concentration of 100 µM, a duration of action of 15 min and
an acidic microenvironment according to the newly created
mitochondria model. LCA caused increases in peaks at 450, 495 and
532 nm. These three peaks, especially the peak at 495 nm, represent
the formation of α,β-unsaturated aldehydes, which could induce
glioma cell apoptosis by several pathways. We believe that LCA may
induce glioma cell apoptosis by increasing the concentration of
α,β-unsaturated aldehydes from lipid peroxidation production in
glioma mitochondria. In addition, the glioma mitochondrial model
will be conducive to further in-depth research.
Acknowledgments
The authors would like to express their gratitude to
all those who helped them during the experiment. Their deepest
gratitude goes first and foremost to Professor Zhengqiang Li who is
the leader of Key Laboratory for Molecular Enzymology and
Engineering, Jilin University. He supported the authors with
equipments and technical guidance through all the stages of the
experiment. Without his consistent and selfless support, this
experiment could not be finished successfully.
Notes
[1]
Funding
This study was supported by the development plan
project of Jilin Province Science and Technology, China (no.
20120946), the doctoral fund project, Jilin Institute of Chemical
Technology, China (no. 2012121), the National Natural Science
Foundation of China (no. 81201980), the Natural Science Foundation
of Jilin Province, China (nos. 20130522028JH and 20130101149JC) and
the Youth Science Foundation of First Hospital of Jilin University,
China (no. JDYY72016010).
[2] Availability
of data and material
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
YanbinS analyzed and interpreted the experiment
data. BZ designed the study, collected the samples and interpreted
the data. DW and LB were involved in drafting the manuscript and
revising it critically for important intellectual content. YanwenS
and HX performed the UV detection. FZ was a major contributor in
specimen processing and mitochondrial collection. All authors read
and approved the final manuscript.
[4] Ethics
approval and consent to participate
The drug clinical trial and research was approved by
the Ethics Committee of the First Hospital of Jilin University
(2012)Trial NO(2012-105).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Goldberg AA, Beach A, Davies GF, Harkness
TA, Leblanc A and Titorenko VI: Lithocholic bile acid selectively
kills neuroblastoma cells, while sparing normal neuronal cells.
Oncotarget. 2:761–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jourdain A and Martinou JC: Mitochondrial
outer-membrane permeabilization and remodelling in apoptosis. Int J
Biochem Cell Biol. 41:1884–1889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parsons MJ and Green DR: Mitochondria in
cell death. Essays Biochem. 47:99–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miwa S and Brand MD: Mitochondrial matrix
reactive oxygen species production is very sensitive to mild
uncoupling. Biochem Soc Trans. 31:1300–1301. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parker N, Vidal-Puig A and Brand MD:
Stimulation of mitochondrial proton conductance by hydroxynonenal
requires a high membrane potential. Biosci Rep. 28:83–88. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papa S and Skulachev V: Reactive oxygen
species, mitochondria, apoptosis and aging. Detection of
Mitochondrial Diseases. Gellerich FN and Zierz S: Springer; pp.
305–319. 1997, View Article : Google Scholar
|
|
8
|
Brand MD: Uncoupling to survive? The role
of mitochondrial inefficiency in ageing. Exp Gerontol. 35:811–820.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esterbauer H, Schaur RJ and Zollner H:
Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and
related aldehydes. Free Radic Biol Med. 11:81–128. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YS and Wurster RD: Potentiation of
anti-proliferative effect of nitroprusside by ascorbate in human
brain tumor cells. Cancer Lett. 78:19–23. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halliwell B and Chirico S: Lipid
peroxidation: Its mechanism, measurement, and significance. Am J
Clin Nutr. 57(Suppl 5): 715S–725S. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Draper HH and Hadley M: Malondialdehyde
determination as index of lipid peroxidation. Methods Enzymol.
186:421–431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benedetti A, Comporti M and Esterbauer H:
Identification of 4-hydroxynonenal as a cytotoxic product
originating from the peroxidation of liver microsomal lipids.
Biochim Biophys Acta. 620:281–296. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Witz G, Rao GS and Goldstein BD:
Short-term toxicity of trans, trans-muconaldehyde. Toxicol Appl
Pharmacol. 80:511–516. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patton S and ParkKurtz GW: A note on the
thiobarbituric acid test for milk lipid oxidation. J Dairy Sci.
38:9011955. View Article : Google Scholar
|
|
16
|
Esterbauer H, Cheeseman KH, Dianzani MU,
Poli G and Slater TF: Separation and characterization of the
aldehydic products of lipid peroxidation stimulated by
ADP-Fe2+ in rat liver microsomes. Biochem J.
208:129–140. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sinnhuber RO and Yu TC and Yu TC:
Characterization of the red pigment formed in the 2-thiobarbituric
acid determination of oxidative rancidity. J Food Sci. 23:626–634.
1958. View Article : Google Scholar
|
|
18
|
Witz G, Lawrie NJ, Zaccaria A, Ferran HE
Jr and Goldstein BD: The reaction of 2-thiobarbituric acid with
biologically active alpha, beta-unsaturated aldehydes. J Free Radic
Biol Med. 2:33–39. 1986. View Article : Google Scholar
|
|
19
|
Inci S, Özcan OE and Kilinç K: Time-level
relationship for lipid peroxidation and the protective effect of
α-tocopherol in experimental mild and severe brain injury.
Neurosurgery. 43:330–335; discussion 335-336. 1998. View Article : Google Scholar
|
|
20
|
Nulton-Persson AC and Szweda LI:
Modulation of mitochondrial function by hydrogen peroxide. J Biol
Chem. 276:23357–23361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
22
|
Ottino P and Duncan JR: Effect of
alpha-tocopherol succinate on free radical and lipid peroxidation
levels in BL6 melanoma cells. Free Radic Biol Med. 22:1145–1151.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malecki EA: Manganese toxicity is
associated with mitochondrial dysfunction and DNA fragmentation in
rat primary striatal neurons. Brain Res Bull. 55:225–228. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hartley A, Stone JM, Heron C, Cooper JM
and Schapira AH: Complex I inhibitors induce dose-dependent
apoptosis in PC12 cells: Relevance to Parkinson's disease. J
Neurochem. 63:1987–1990. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugiyama A and Sun J: Immunochemical
detection of lipid hydroperoxide-and aldehyde-modified proteins in
diseases. Lipid Hydroperoxide-Derived Modification of Biomolecules.
Kato Y: Springer; pp. 115–125. 2014, View Article : Google Scholar
|
|
26
|
Park S, You X and Imlay JA: Substantial
DNA damage from submicromolar intracellular hydrogen peroxide
detected in Hpx- mutants of Escherichia coli. Proc Natl Acad Sci
USA. 102:9317–9322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Demple B, Halbrook J and Linn S:
Escherichia coli xth mutants are hypersensitive to hydrogen
peroxide. J Bacteriol. 153:1079–1082. 1983.PubMed/NCBI
|
|
28
|
Carlsson J and Carpenter VS: The
recA+ gene product is more important than catalase and
superoxide dismutase in protecting Escherichia coli against
hydrogen peroxide toxicity. J Bacteriol. 142:319–321.
1980.PubMed/NCBI
|
|
29
|
Imlay JA and Linn S: Bimodal pattern of
killing of DNA-repair-defective or anoxically grown Escherichia
coli by hydrogen peroxide. J Bacteriol. 166:519–527. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imlay JA, Chin SM and Linn S: Toxic DNA
damage by hydrogen peroxide through the Fenton reaction in vivo and
in vitro. Science. 240:640–642. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mello Filho AC, Hoffmann ME and Meneghini
R: Cell killing and DNA damage by hydrogen peroxide are mediated by
intracellular iron. Biochem J. 218:273–275. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bar-Or D and Winkler JV: Copper is
involved in hydrogen-peroxide-induced DNA damage. Free Radic Biol
Med. 32:197–199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Kuijk FJ, Holte LL and Dratz EA:
4-Hydroxyhexenal: A lipid peroxidation product derived from
oxidized docosahexaenoic acid. Biochim Biophys Acta. 1043:116–118.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petersen DR and Doorn JA: Reactions of
4-hydroxynonenal with proteins and cellular targets. Free Radic
Biol Med. 37:937–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wawra E, Zollner H, Schaur RJ, Tillian HM
and Schauenstein E: The inhibitory effect of 4-hydroxy-nonenal on
DNA-polymerases alpha and beta from rat liver and rapidly dividing
Yoshida ascites hepatoma. Cell Biochem Funct. 4:31–36. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldkorn T, Balaban N, Matsukuma K, Chea
V, Gould R, Last J, Chan C and Chavez C: EGF-Receptor
phosphorylation and signaling are targeted by
H2O2 redox stress. Am J Respir Cell Mol Biol.
19:786–798. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang RP, Peng A, Golard A, Hossain MZ,
Huang R, Liu YG and Boynton AL: Hydrogen peroxide promotes
transformation of rat liver non-neoplastic epithelial cells through
activation of epidermal growth factor receptor. Mol Carcinog.
30:209–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Petruzzelli S, Hietanen E, Bartsch H,
Camus AM, Mussi A, Angeletti CA, Saracci R and Giuntini C:
Pulmonary lipid peroxidation in cigarette smokers and lung cancer
patients. Chest. 98:930–935. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Esterbauer H and Zollner H: Methods for
determination of aldehydic lipid peroxidation products. Free Radic
Biol Med. 7:197–203. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Esterbauer H, Zollner H and Scholz N:
Reaction of glutathione with conjugated carbonyls. Z Naturforsch C.
30:466–473. 1975.PubMed/NCBI
|
|
41
|
Poot M, Verkerk A, Koster JF, Esterbauer H
and Jongkind JF: Influence of cumene hydroperoxide and
4-hydroxynonenal on the glutathione metabolism during in vitro
ageing of human skin fibroblasts. Eur J Biochem. 162:287–291. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dogterom P, Mulder GJ and Nagelkerke JF:
Lipid peroxidation-dependent and -independent protein thiol
modifications in isolated rat hepatocytes: Differential effects of
vitamin E and disulfiram. Chem Biol Interact. 71:291–306. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Poot M, Verkerk A, Koster JF, Esterbauer H
and Jongkind JF: Reversible inhibition of DNA and protein synthesis
by cumene hydroperoxide and 4-hydroxy-nonenal. Mech Ageing Dev.
43:1–9. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Esterbauer H: Kinetics of the reaction of
sulfhydryl compounds with alpha-beta-unsaturated aldehydes in
aqueous system. Monatsh Chem. 101:782–810. 1970. View Article : Google Scholar
|
|
45
|
Witz G: Biological interactions of
α,β-unsaturated aldehydes. Free Radic Biol Med. 7:333–349. 1989.
View Article : Google Scholar
|
|
46
|
Grafström RC, Dypbukt JM, Willey JC,
Sundqvist K, Edman C, Atzori L and Harris CC: Pathobiological
effects of acrolein in cultured human bronchial epithelial cells.
Cancer Res. 48:1717–1721. 1988.PubMed/NCBI
|
|
47
|
Cox R, Goorha S and Irving CC: Inhibition
of DNA methylase activity by acrolein. Carcinogenesis. 9:463–465.
1988. View Article : Google Scholar : PubMed/NCBI
|