Introduction
Acute respiratory distress syndrome (ARDS) is a
common and devastating complication which is usually caused by
systemic inflammatory response syndrome (SIRS), severe trauma and
direct lung injury resulted from inhalation of toxic substances,
such as toxic gases and fluids (1). Among various kinds of accidental
aspiration, drowning has been recognized as a serious public health
problem, which was also the third most frequent causes for
accidental death and led to ~359,449 deaths in 2011 according to
the latest statistical data from the world health organization
(WHO) (2). Except for sudden
death on the spot where drowning happens, injuries and deaths
following near-drowning depend on both the composition and quantity
of water aspirated (3), as well
as the quality of following treatment (4). Compared with the injuries caused by
fresh water, previous studies have indicated that seawater
inhalation results in more infiltration of inflammatory cells, such
as neutrophils, monocytes and lymphocytes, and more secretion of
pro-inflammatory mediators including NO, COX-2, tumor necrosis
factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 (5). Besides, it has also been proven that
hypertonic stimulation as well as the concentration of inflammatory
cells could lead to excessive generation of reactive oxygen species
(ROS) (6), however, there is
little research showing the role of oxidative stress in seawater
inhalation-induced ARDS.
The excessive generation of ROS is the primary cause
for oxidative stress imbalance which is closely related to the
concentration of inflammatory cells and secretion of cytokines
(7,8). As one of the crucial aspects for
lung injuries, the generation of ROS is counterbalanced by
antioxidant systems. Among which, thioredoxin (Trx) system,
composed of NADPH, thioredoxin reductase (TrxR), Trx-1 and Prdxs,
is one of the most important systems. It is well known that
super-abundant ROS can evoke a series of antioxidant genes by
activating nuclear factor erythroid 2-related factor 2 (Nrf2),
which is a critical transcription factor regulating the expression
of major antioxidant enzymes and phase II detoxification enzymes
(9). There is also evidence
indicating that the expression of Nrf2 would subsequently induce
Trx-1 expression (10). While as
negative regulator, thioredoxin-interacting protein (Txnip) binds
to Trx-1 and suppresses Trx-1 mediated pro-survival signaling and
antioxidant process (11). Up to
now, there is little evidence revealing the regulation of Trx-1
axis on hypertonic stimulation induced ROS and there are also
limited material showing how Trx-1-related pathway participated in
seawater inhalation induced ARDS.
Resveratrol is a natural compound which can be found
in grapes, nuts and red wine, it is also one of most intensively
researched natural products for its multiple protecting effects.
Evidence show that resveratrol (Res) possesses anti-inflammation
property probably through interfering the activity of sirtuin 1
(SIRT1) and following inflammatory regulators (12,13). There are also studies indicating
that Res exhibited its anti-oxidative stress ability via varied
pathways (14). Although Res
possesses multiple biological and pharmacological activities, it
could not be adopted as a drug in clinic for its poor
pharmacokinetic and bio-availability properties (15). While as a prodrug of Res,
3,5,4′-tri-O-acetylresveratrol (AC-Res) prolongs the half-time of
Res and caused the accumulation of Res in lungs (16). Studies from our laboratory and
other teams have also revealed the biological benefits of AC-Res
(17); however, investigations
are still needed to further illustrate the pharmacological activity
of AC-Res.
Based on what is known, we hypothesized and
investigated, in the present study, seawater inhalation-caused
inflammation and oxidative stress in lungs, and abnormal expression
of Trx-1 pathway was found to cause the process. While
administration of AC-Res could protect lungs against seawater
exposure-induced ARDS by regulation the expression of Trx-1 axis
and following oxidative stress.
Materials and methods
Animals and reagents
Adult male Sprague-Dawley (SD) rats weighing 200–220
g were provided by the Animal center of the Fourth Military Medical
University (FMMU, Xi'an, China). Rats were captured in
air-filtered, temperature-controlled units with free access to food
and water. All experimental protocols were approved by the Animal
Care and Use Committee of the FMMU according to the Declaration of
the National Institutes of health guide for Care and use of
Laboratory Animals (publication no. 85–23, revised 1985).
Seawater was prepared according to the formula
provided by Chinese Ocean Bureau: osmolality 1,300 mOsm/l, pH 8.2,
SW 1.05, NaCl2 6.518 g/l, MgSO4 3.305 g/l,
MgCl2 2.447 g/l, CaCl2 1.141 g/l, KCl 0.725
g/l, NaHCO3 0.202 g/l, NaBr 0.083 g/l. Artificial
seawater was sterilized before experiments. Resveratrol
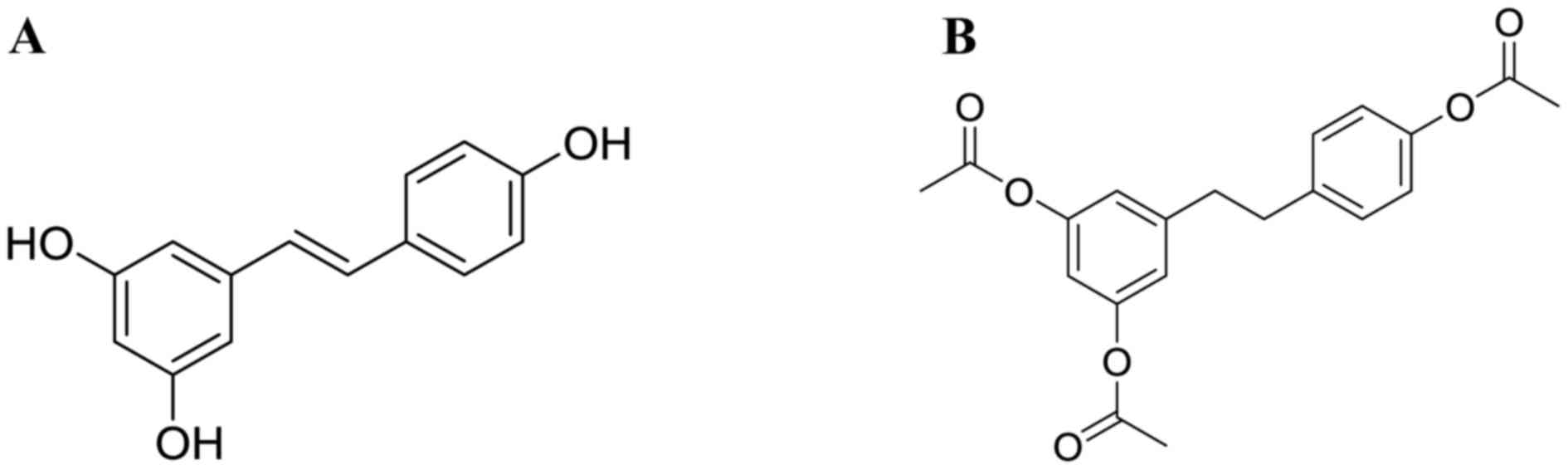
(3,5,4′-trihydroxystilbene, Res, structure is shown in
Fig. 1A), was purchased from
Xi'an grass Plant Technology Corp. (Xi'an, China) with purity
>98%. AC-Res, structure (Fig.
1B), was synthesized by the Pharmacy Department of Medicinal
Chemistry of FMMU with HPLC purity >99%. Enzyme-linked
immunosorbent assay (ELISA) kits for TNF-α and IL-1β were purchased
from the R&D Systems Inc. (Minneapolis, MN, USA). MDA and T-SOD
activity analyzing kits were purchased from Jiancheng
Bioengineering Institute (Nanjing, China). Antibodies against Nrf2,
Trx-1, Txnip and β-actin were purchased from the Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Modeling and grouping
SD rats were randomly assigned into 4 groups (n=8):
control (Ctl) group; seawater (SW) inhalation group; SW + AC-Res
group; AC-Res group. Rats from SW + AC-Res group and AC-Res group
were pretreated with AC-Res (50 mg/kg/day) for 7 days, and rats
from Ctl and SW group were treated with equal amount of normal
saline. Seawater was instilled into the trachea of rats from SW and
SW + AC-Res group 90 min after the last administration of normal
saline or AC-Res.
The seawater inhalation rat models were built
according to a previous study in our laboratory. Briefly, the
trachea was exposed after the rat was anesthetized with 3%
pentobarbital sodium (100 mg/kg body weight, i.p.). A 1 ml syringe
was gently stabbed into the trachea and 4 ml/kg body weight of
artificial seawater was instilled into both lungs within 4 min.
Rats were held in the supine position with the head elevated 30°
degree and maintained anesthesia with 25 mg/kg/h pentobarbital
sodium. Rats from all groups were sacrificed 4 h after
modeling.
Cell culture and treatment
The alveolar macrophage cell line NR8383 and
alveolar epithelial cell line A549 were cultured to evaluate the
protecting effects of Res, intermediate of AC-Res. NR8383 cells
were maintained in Ham's F12 and A549 cells in RPMI-1640 medium
containing 10% fetal calf serum at 37°C in a humidified atmosphere
containing 5% CO2 and 95% air. Cells within the
logarithmic growth phase were divided into four groups: control
(Ctl); 25% seawater treated only (SW); 25% seawater + 40
µg/ml resveratrol (SW + Res); 40 µg/ml Res. Cells
from SW and SW + Res group were stimulated with 25% seawater with
the presence of Res or not. All cells were collected after being
treated for 4 h.
Lung wet-to-dry (W/D) weight ratios
In order to quantify the magnitude of pulmonary
edema, W/D weight ratio of lung samples were calculated. Briefly,
lung tissues of the same lob from each rat in every group were
obtained and weighed immediately. After that, lung samples were
kept in an oven at 70°C for 72 h. Finally, samples were weighed
again and W/D was calculated by dividing the wet weight with the
dry.
Analysis of cytokines
Contents of TNF-α and IL-1β in lung tissues and
cells stimulated by seawater was measured to assess the degree of
lung injury. Lung tissues were homogenized in cold
phosphate-buffered saline (PBS) (lung tissue to PBS 1:5) and cells
were collected and homogenized with repeated freeze-thaw method.
Supernatants from tissues and cells were collected by centrifuging
at 12,000 rpm for 5 min at 4°C. Contents of TNF-α and IL-1β in
supernatant were measured according to the manufacturer's
instructions.
Evaluation of oxidative stress
T-SOD activity and MDA content were measured to
evaluate the status of oxidative stress in lung tissues stimulated
by seawater. The lung samples were homogenized in cold PBS (lung
tissue to PBS 1:10) and homogenate supernatant was collected by
centrifuging at 12,000 rpm for 5 min at 4°C. T-SOD activity and
content of MDA were measured at 550 and 523 nm, respectively.
Immunohistochemistry study of Trx-1 in
lungs
Immunohistochemistry was carried out to evaluate the
expression of Trx-1 in lungs stimulated by seawater or not, as well
as the effects of AC-Res on its expression. Briefly, slices of rat
lung tissues were deparaffinized, rehydrated in graded alcohol and
soaked in 0.3% H2O2 at room temperature for
30 min. Slices were blocked with goat serum albumin at 37°C for 30
min, and then incubated with the primary antibody against Trx-1
(1:100) at 4°C overnight. After that, sections were washed with PBS
and incubated in biotin-labeled secondary antibody derived from
goat at 37°C for 30 min. Then, slices were washed with PBS,
incubated in horseradish enzyme-labeled streptavidin working
solution and detained with diaminobenzidine (DAB). All slices were
checked under a light microscope (DMI6000; Leica, Wetzlar,
germany).
Immunofluorescence detection for Trx-1 in
A549 cells
Activity of Trx-1 was detected by immunofluorescence
in A549 cells challenged with or without seawater. Briefly,
confluent cells grown on coverslips were fixed with methanol at
room temperature for 20 min. After being blocked with 0.5% BSA,
cells were incubated with the primary antibody against Trx-1
(1:100) at 37°C for 1 h. Then, cells were incubated with CY3
conjugated secondary antibody. Then, nucleus was detained with DAPI
at room temperature for 5 min. Finally, cells were examined by
using an inverted fluorescence microscope (DMI6000B; Leica).
Western blot analysis
At the end of the experiment, lung and cell samples
from different groups were collected and total proteins were
extracted according to the manufacturer's instructions (Beyotime
Institute of Biotechnology, Jiangsu, China). Equal amount of
proteins from each group were separated on sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and
transferred to PVDF membranes by wet transfer method. Then,
membranes were blocked with 5% non-fat dry milk melted in
Tris-buffered saline with 0.1% Tween-20, and incubated with primary
antibodies overnight at 4°C against Txnip (1:200), Nrf2 (1:200) and
β-actin (1:2,000). After that, membranes were incubated with
secondary anti-body for 2 h at room temperature followed by 3 times
washing. Results of western blot analysis were examined by the
enhanced chemiluminescence (ECL) system (Amersham Pharmacia
Biotech, Arlington Heights, IL, USA).
Statistical analysis
Statistical analysis was performed with SPSS 17.0
for Windows. Numeric variables were expressed as means ± SD.
Differences between groups were performed by one-way analysis of
variance (ANOVA) followed by Dunnett's test. Statistical
significance was accepted as P<0.05.
Results
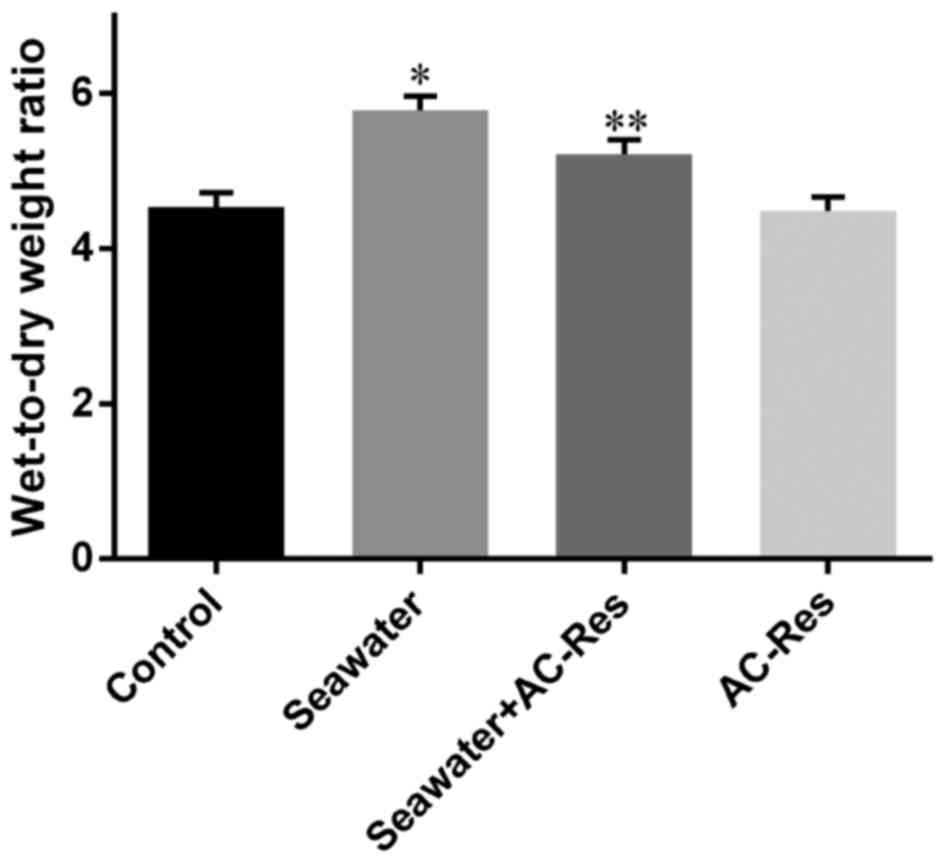
AC-Res alleviates seawater
inhalation-induced lung edema
Water content in lungs from each group was
manifested by W/D ratios. Seawater inhalation dramatically
increased the W/D ratios of lung tissues (P<0.05), while AC-Res
pretreatment significantly inhibited the increasing of water
content in lungs manifested by decreased W/D ratios compared with
that of seawater inhalation group (P<0.05) (Fig. 2). On the other hand,
administration of AC-Res alone did not affect the content of water
in lungs.
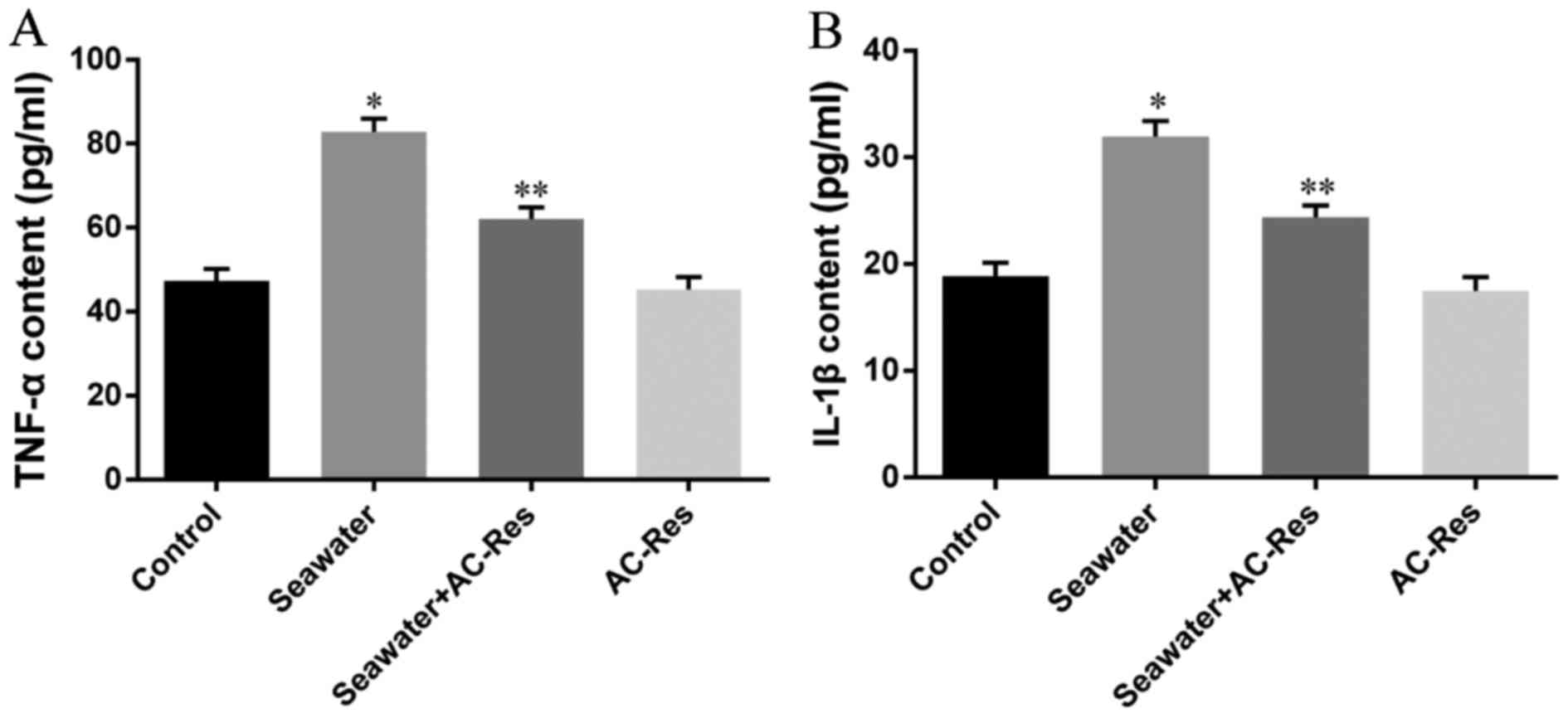
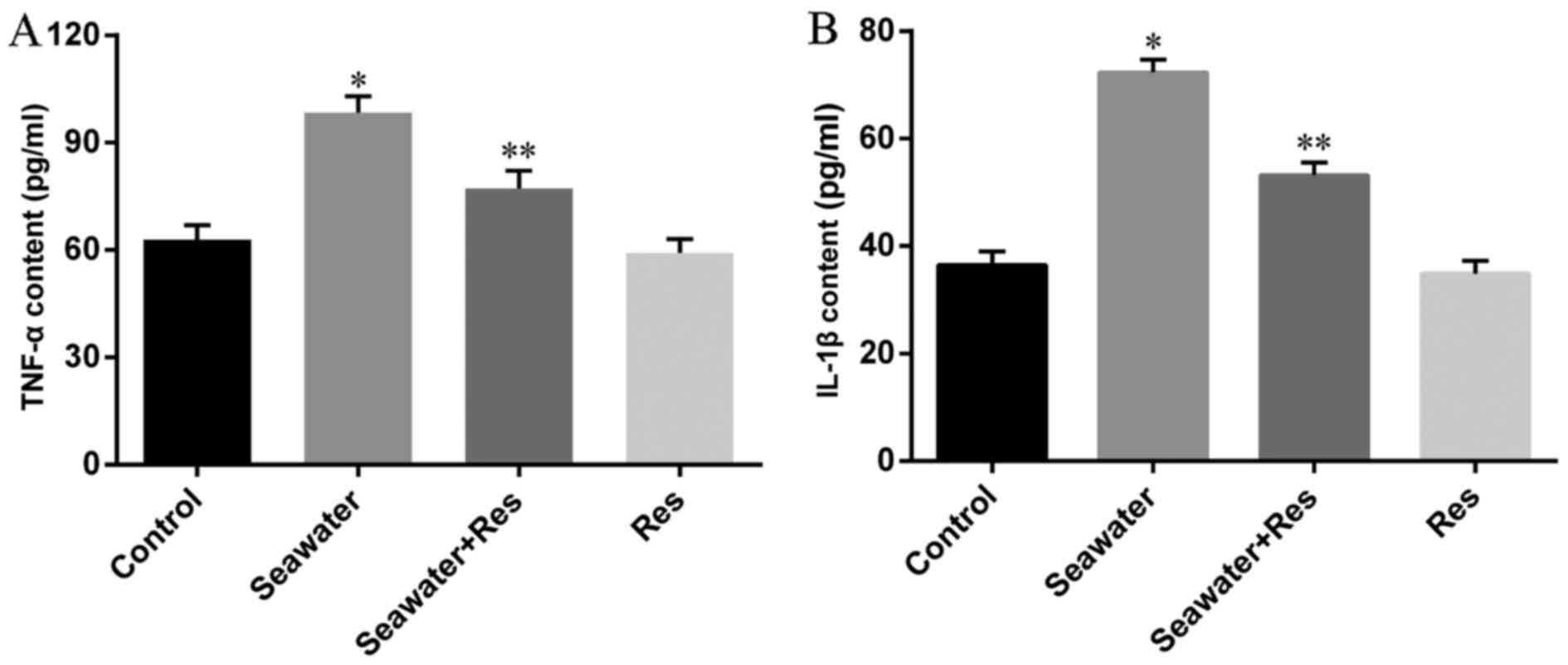
AC-Res decreased the content of
inflammatory cytokines in lungs
Content of inflammatory cytokines was taken as the
symbol of lung injury (Fig. 3)
the content of TNF-α (Fig. 3A)
and IL-1β (Fig. 3B) significantly
increased in lungs stimulated by seawater (P<0.05). While
pretreatment of AC-Res inhibited the formation and secretion of
TNF-α and IL-1β in lungs stimulated by seawater (P<0.05). Also,
treatment of AC-Res alone did not affect the secretion of cytokines
in lung.
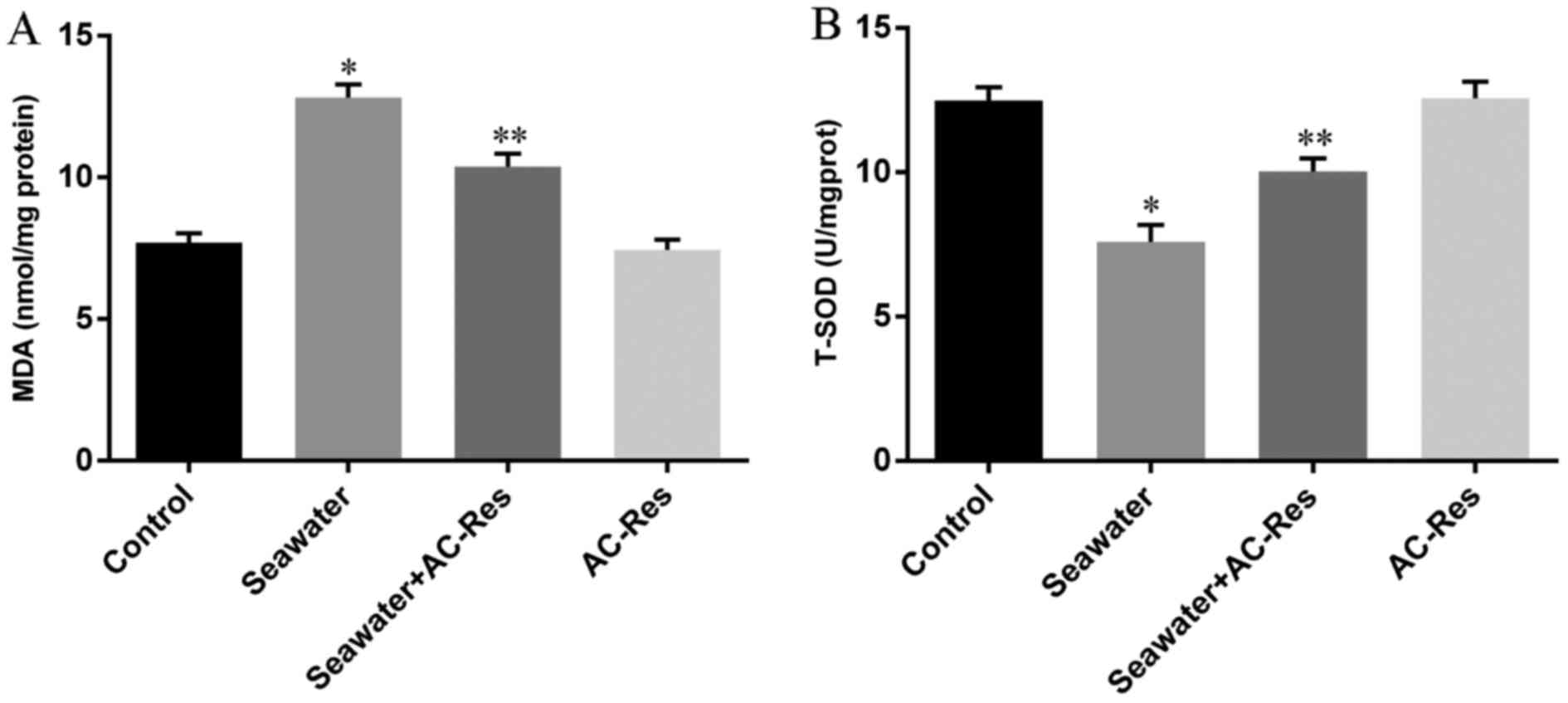
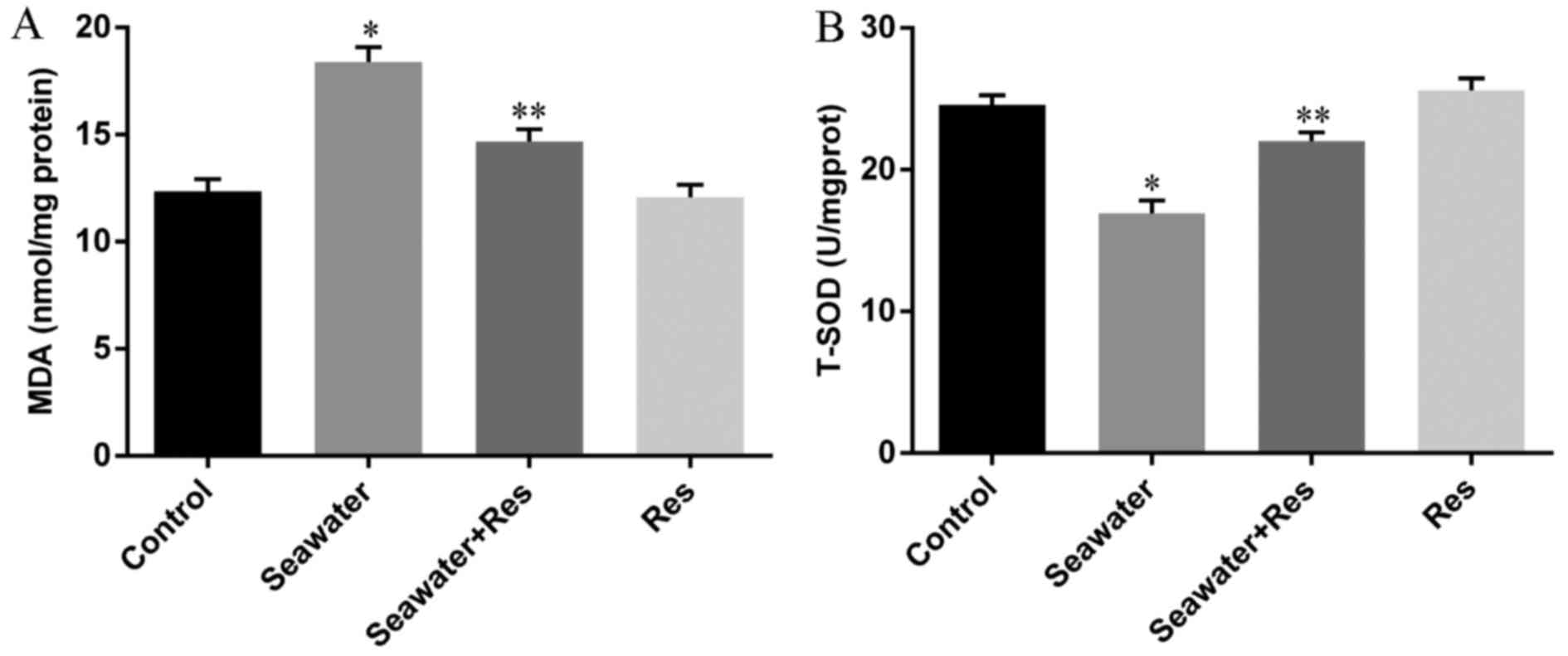
Effects of AC-Res on the oxidative stress
in lungs stimulated by seawater
In order to evaluate the oxidative stress in lungs
challenged by seawater and the protecting effects of AC-Res, The
content of MDA and activity of T-SOD were measured by TBA method
and hydroxylamine method, respectively. The results (Fig. 4) showed that seawater inhalation
led to increased content of MDA (Fig.
4A) together with the decreased activity of T-SOD (Fig. 4B), while pretreatment of AC-Res
increased the activity of T-SOD (P<0.05) and inhibited the
formation of MDA (P<0.05) compared with those in lungs
stimulated by seawater. In addition, administration of AC-Res alone
did not affect the content of MDA and activity of T-SOD in
lungs.
Effects of AC-Res on the expression of
Trx-1 axis in lungs stimulated by seawater
In order to explore the mechanisms of oxidative
stress in lungs stimulated by seawater and to illustrate how AC-Res
inhibited the oxidative stress, we further examined the expression
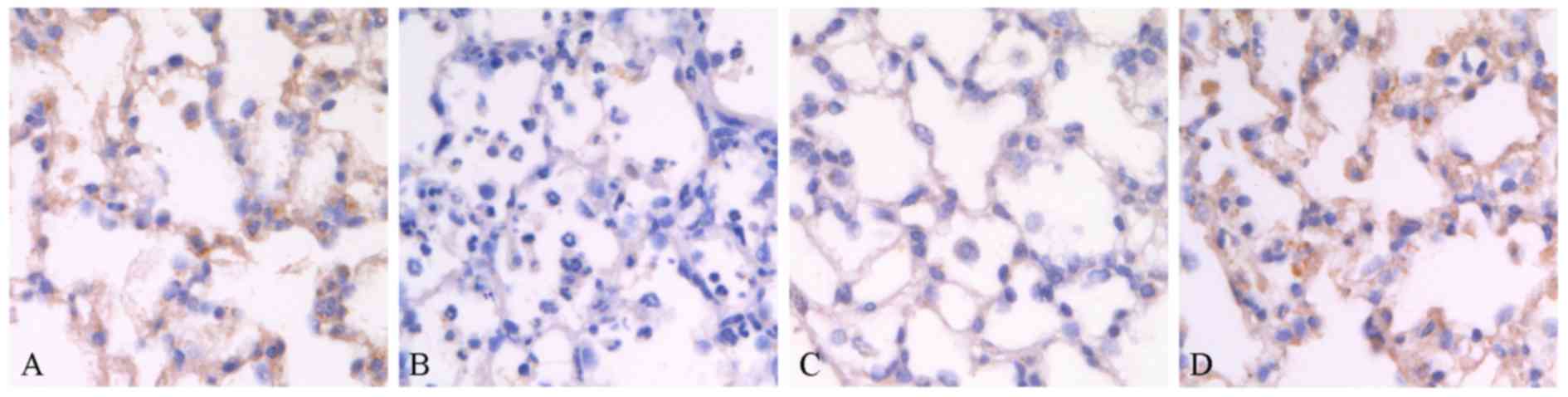
of Trx-1 axis in lungs. As shown in Fig. 5A, immunohistochemistry staining
revealed that Trx-1 expressed abundant alveoli epithelium from
normal rat lungs, while the expression of Trx-1 dramatically
decreased (Fig. 5B) when lungs
were challenged by seawater. However, pre-administration of AC-Res
strikingly maintained the relative high level expression of Trx-1
in lungs when exposed to seawater (Fig. 5C). Administration of AC-Res alone
did not affect the expression of Trx-1 in lungs.
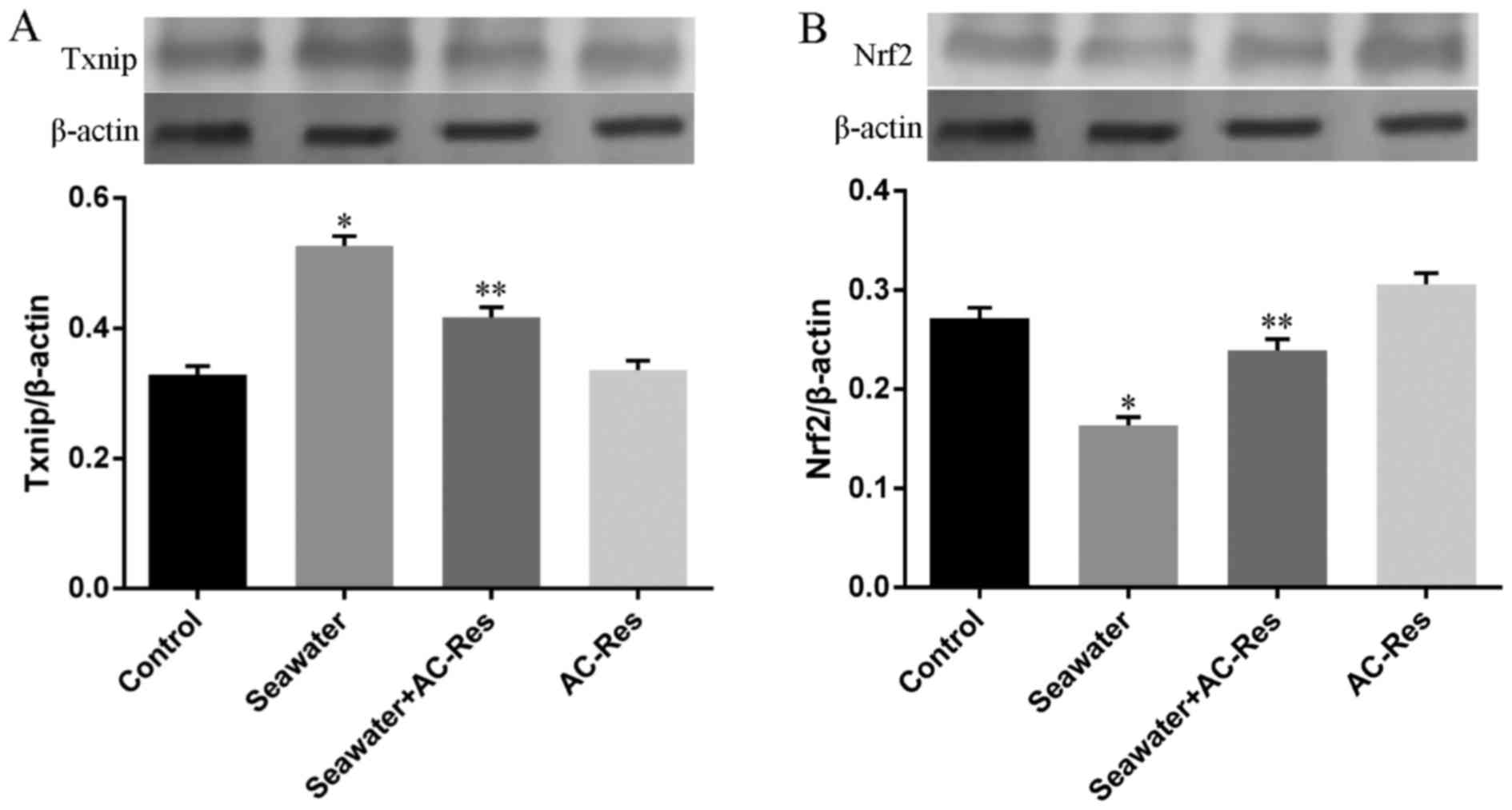
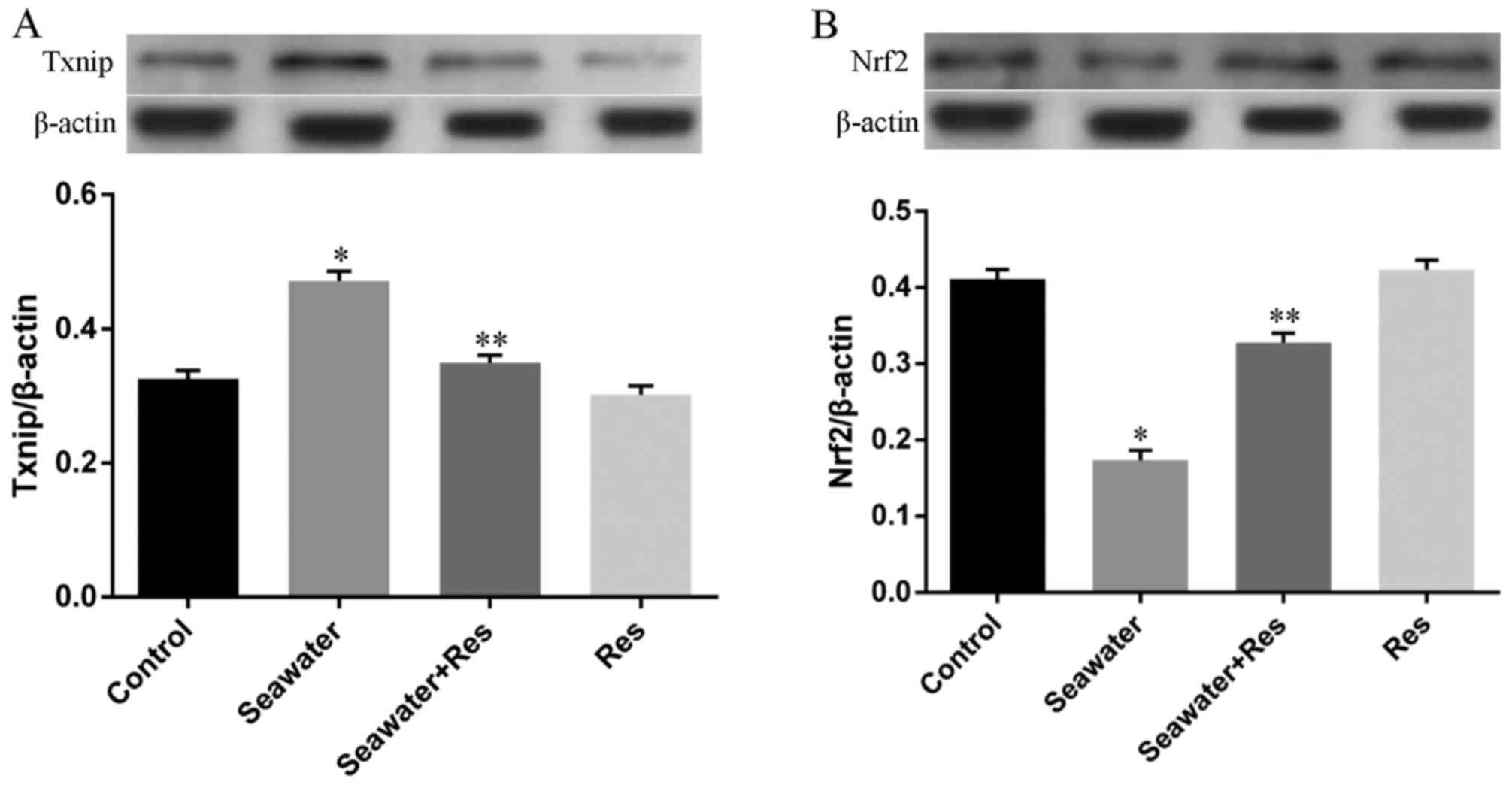
Besides, the expression of key regulators for Trx-1,
Nrf2 and Txnip, has also been measured by western blot analysis. As
shown in Fig. 6, seawater
inhalation upregulated the expression of Txnip (Fig. 6A) and downregulated the expression
of Nrf2 (Fig. 6A) (P<0.05).
While pretreatment of AC-Res reversed this trend by inhibiting the
express of Txnip increasing Nrf2 expression when lungs were
stimulated by seawater (P<0.05). AC-Res administration alone did
not markedly influenced the expression of the two regulators.
Effects of Res on the secretion of
cytokines in seawater stimulated NR8383 cells
Based on the findings that seawater inhalation
resulted in pulmonary edema and lung inflammation, and
pre-administration of AC-Res could alleviate lung injuries by
interfering with the Trx-1 axis in lungs. We further investigated
the protecting effects of Res, intermediate of AC-Res, by in
vitro experiments. As shown in Fig. 7, formation of TNF-α (Fig. 7A) and IL-1β (Fig. 7B) in NR8383 cells increased when
stimulated by 25% seawater (P<0.05), while co-incubation of Res
decreased the generation of TNF-α and IL-1β (P<0.05) in seawater
stimulated cells. In addition, Res treatment alone did not affect
the secretion of cytokines in cells.
Effects of Res on the oxidative stress in
seawater-stimulated NR8383 cells
Activity of T-SOD and content of MDA were measured
to manifest the oxidative stress in NR8383 cells stimulated by
seawater. Results (Fig. 8) showed
that seawater stimulation increased the content of MDA (Fig. 8A) and inhibited the activity of
T-SOD (Fig. 8B) in NR8383 cells
(P<0.05), while Res co-incubation decreased the content of MDA
and increased the activity of T-SOD in cells (P<0.05), which was
also similar to the effects of AC-Res on seawater stimulated
lungs.
Effects of Res on the expression of Trx-1
axis in seawater stimulated cells
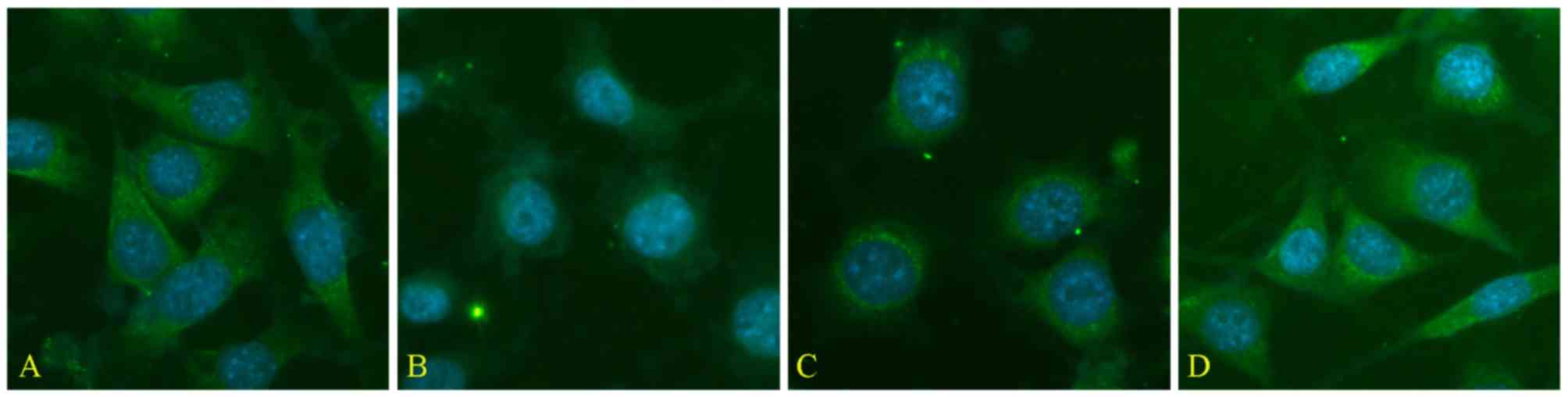
The expression of Trx-1 in seawater-stimulated A549
cells were measured by immunofluorescence detection, and results
(Fig. 9B) showed that the
expression of Trx-1 decreased in A549 cells 4 h after seawater
exposure while treatment of Res dramatically inhibited the decrease
of Trx-1 expression manifested by the relatively higher
fluorescence intensity (Fig.
9C).
Also, we detected the expression of Nrf2 and Txnip
in seawater-stimulated NR8383 cells. The results showed that
seawater stimulation increased the expression of Txnip (Fig. 10A) and decreased Nrf2 expression
(Fig. 10B) compared with that of
control, while Res co-incubation upregulated the expression of Nrf2
(P<0.05) and inhibited the expression of Txnip (P<0.05) in
NR8383 cells when stimulated by seawater.
Discussion
In the present study, we demonstrated that seawater
inhalation resulted in pulmonary edema, inflammation and oxidative
stress in lungs, further exploration also revealed that abnormal
expression of Trx-1 axis was deeply involved in seawater induced
inflammation and oxidative stress imbalance. Based on these
findings, we evaluated the effects of AC-Res on seawater-induced
oxidative stress and lung injury, and the results showed that
AC-Res together with its intermediate, Res, inhibited
seawater-induced oxidative stress and lung injuries by interfering
the expression of Trx-1 related pathways in vitro and in
vivo.
There are basically two different outcomes for
drowning victims, one is to die on the drowning spot from
suffocation, and the other one is to survive the initial process.
However, those who survived the near-drowning process would
probably suffer from varied degrees of lung injuries, and maybe
ARDS (4,18). Although several pharmacological
compounds and therapies have been adopted in clinic for ARDS
patients, none have dramatically decreased the motility of ARDS.
However, on the bright side the studies have demonstrated that
controlling on inflammation and oxidative stress is beneficial for
the outcomes of ARDS patients (19,20).
As known, Res possesses protecting effects on
different diseases, such as cardiovascular disorders (21,22), different kinds of cancers
(23,24), inflammation (25,26), oxidative stress (14,27) and nervous system disease (28,29). However, Res has never been adopted
as a clinical drug due to its poor pharmacokinetic and
bio-availability properties (16,30). While as an analog for Res, AC-Res
could overcome some of those shortages by extending the biological
half-time and inducing the accumulation of Res in lungs (16). Importantly, results from our team
and other studies showed that AC-Res possessed anti-inflammation
and anti-oxidative stress property which could decrease radiation
resulted death and seawater resulted inflammation (31). The present study was designed to
further the protecting effects of AC-Res as an antioxidant on
seawater induced ARDS.
Seawater inhalation may cause severe pulmonary edema
since local high permeability would drive fluid from blood vessels
into pulmonary alveoli and lung tissue spaces. Furthermore,
neutrophils and macrophages concentrate in lung tissue and secret
pro-inflammatory factors, such as NO, COX-2, TNF-α, IL-1β and IL-6
(5). Besides, accumulation of
inflammatory cells in lung tissues leads to inflammatory responses
and oxidative stress imbalance (32). Excessive cytokines and ROS have
been recognized to be closely related with the occurrence of ARDS
(33). In the present study, it
was found that seawater inhalation increased water contents in
lungs, enhanced the secretion of TNF-α and IL-1β and inhibited the
antioxidant ability of lung tissues. While pretreatment of AC-Res
inhibited the infiltration of water from blood vessels into
alveolar, decreased the secretion of inflammatory cytokines and
rebuilt the antioxidant ability of lung tissues.
We further explored the mechanisms underlying the
protecting effects of AC-Res on seawater inhalation induced lung
injury and oxidative stress. It is known that the generation of ROS
is counterbalanced by antioxidant systems and there are two
ROS-scavenging systems in the body: glutathione (GSH) and
thioredoxin (Trx) system. As one of the crucial members in the
Trx-1 systems, Trx-1 is a 12 kDa protein which provides electrons
to a large range of enzymes and plays a major role in keeping the
intracellular redox balance. Trx-1 exhibits protective effects
against oxidative stress by scavenging ROS and cooperating with
peroxiredoxin (Prdx) (33). In
the present study we have found that seawater stimulation inhibited
the expression of Trx-1 followed by deregulated T-SOD activity and
increased MDA content, while pretreatment of AC-Res in vivo
and co-incubation of Res in vitro annihilated the inhibition
effects of seawater on activities of Trx-1 followed by upregulated
T-SOD activity and decreased MDA content in lungs and cells,
respectively.
Besides, it is well known that superabundant of ROS
evokes a series of antioxidant genes by activating Nrf2, including
the expression of Trx-1 (34).
Based on those knowledge, we checked the expression of Nrf2 in
seawater stimulated lungs and cells, and the results revealed that
seawater exposure inhibited the expression of Nrf2 both in
vivo and in vitro, while AC-Res and Res treatment
annihilated the effects of seawater and maintained the expression
of Nrf2 at a relative high level. Besides, Txnip has been confirmed
to combine with Trx-1 and suppress Trx-1 mediated pro-survival
signaling and antioxidant process (11,35). Therefore, we evaluated the
activity of this negative regulator in the present study, and
results show that seawater stimulation enhanced the expression of
Txnip in lungs and cells, while AC-Res and Res treatment inhibited
the effects of seawater on Txnip expression both in vitro
and in vivo.
In conclusion, the results from the present study
revealed that abnormal expression of Trx-1 pathway is blamed for
the ARDS induced by seawater inhalation. Besides, it was
demonstrated that AC-Res pretreatment in vivo and Res
co-incubation in vitro inhibited pulmonary inflammation and
oxidative stress by interfering with the expression of the Trx-1
axis in lung and cell lines stimulated by seawater. Those results
provide scientific evidence for AC-Res as the potential agent for
seawater inhalation induced ARDS although further investigations
are needed in the clinic.
Acknowledgments
This study was supported by grants from the Military
Key Projects in the 12th Five-year Plan of China (project no.
CWS13J043).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Koh Y: Update in acute respiratory
distress syndrome. J Intensive Care. 2:22014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Organisation, World Health: Disease and
injury regional mortality estimates for 2000-2011. Global summary
estimates. 2015-1-20. 2013.
|
|
3
|
Simcock AD: Treatment of near drowning - a
review of 130 cases. Anaesthesia. 41:643–648. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gregorakos L, Markou N, Psalida V,
Kanakaki M, Alexopoulou A, Sotiriou E, Damianos A and Myrianthefs
P: Near-drowning: Clinical course of lung injury in adults. Lung.
187:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Zhang B, Xu DQ, Li WP, Xu M, Li
JH, Xie XY, Fan QX, Liu W, Mu DG, et al: Tanshinone IIA attenuates
seawater aspiration-induced lung injury by inhibiting macrophage
migration inhibitory factor. Biol Pharm Bull. 34:1052–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang T, Zhang A, Honeggar M, Kohan DE,
Mizel D, Sanders K, Hoidal JR, Briggs JP and Schnermann JB:
Hypertonic induction of COX-2 in collecting duct cells by reactive
oxygen species of mitochondrial origin. J Biol Chem.
280:34966–34973. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu HL, Zhao TF, Wu H, Pan YZ, Zhang Q,
Wang KL, Zhang CC and Jin YP: Pinellia ternata lectin exerts a
pro-inflammatory effect on macrophages by inducing the release of
pro-inflammatory cytokines, the activation of the nuclear factor-κB
signaling pathway and the overproduction of reactive oxygen
species. Int J Mol Med. 36:1127–1135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lortz S, Gurgul-Convey E, Lenzen S and
Tiedge M: Importance of mitochondrial superoxide dismutase
expression in insulin-producing cells for the toxicity of reactive
oxygen species and proinflammatory cytokines. Diabetologia.
48:1541–1548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Qiu J, Wang Z, You W, Wu L, Ji C
and Chen G: Dimethylfumarate alleviates early brain injury and
secondary cognitive deficits after experimental subarachnoid
hemorrhage via activation of Keap1-Nrf2-ARE system. J Neurosurg.
123:915–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niso-Santano M, González-Polo RA,
Bravo-San Pedro JM, Gómez-Sánchez R, Lastres-Becker I, Ortiz-Ortiz
MA, Soler G, Morán JM, Cuadrado A and Fuentes JM; Centro de
Investigación Biomédica en Red sobre Enfermedades
Neurodegenerativas (CIBERNED): Activation of apoptosis
signal-regulating kinase 1 is a key factor in paraquat-induced cell
death: Modulation by the Nrf2/Trx axis. Free Radic Biol Med.
48:1370–1381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogata FT, Batista WL, Sartori A, Gesteira
TF, Masutani H, Arai RJ, Yodoi J, Stern A and Monteiro HP:
Nitrosative/oxidative stress conditions regulate
thioredoxin-interacting protein (TXNIP) expression and
thioredoxin-1 (TRX-1) nuclear localization. PLoS One. 8:e845882013.
View Article : Google Scholar :
|
|
12
|
Zhang C, Li Q, Kang L, Lei X, Zhai X, Zhao
S, Zhang C and Dong W: Resveratrol inhibits hyperxia-induced cell
apoptosis through up-regulating SIRT1 expression in HPAECs. Xi Bao
Yu Fen Zi Mian Yi Xue Za Zhi. 31:590–595. 2015.In Chinese.
PubMed/NCBI
|
|
13
|
Kuno A, Tanno M and Horio Y: The effects
of resveratrol and SIRT1 activation on dystrophic cardiomyopathy.
Ann NY Acad Sci. 1348:46–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tvrda E, Kovacik A, Tusimova E, Massanyi P
and Lukac N: Resveratrol offers protection to oxidative stress
induced by ferrous ascorbate in bovine spermatozoa. J Environ Sci
Health A Tox Hazard Subst Environ Eng. 50:1440–1451. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr
and Walle UK: High absorption but very low bioavailability of oral
resveratrol in humans. Drug Metab Dispos. 32:1377–1382. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang L, Liu X, Wang Q, Cheng S, Zhang S
and Zhang M: Pharmacokinetics, tissue distribution and excretion
study of resveratrol and its prodrug 3,5,4′-tri-O-acetylresveratrol
in rats. Phytomedicine. 20:558–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma L, Zhao Y, Li B, Wang Q, Liu X, Chen X,
Nan Y, Liang L, Chang R, Liang L, et al:
3,5,4′-Tri-O-acetylresveratrol attenuates seawater
aspiration-induced lung injury by inhibiting activation of nuclear
factor-kappa B and hypoxia-inducible factor-1α. Respir Physiol
Neurobiol. 185:608–614. 2013. View Article : Google Scholar
|
|
18
|
Ibsen LM and Koch T: Submersion and
asphyxial injury. Crit Care Med. 30(Suppl 11): S402–S408. 2002.
View Article : Google Scholar
|
|
19
|
Janz DR and Ware LB: Biomarkers of
ALI/ARDS: Pathogenesis, discovery, and relevance to clinical
trials. Semin Respir Crit Care Med. 34:537–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ware LB: Autopsy in ARDS: Insights into
natural history. Lancet Respir Med. 1:352–354. 2013. View Article : Google Scholar
|
|
21
|
Tang PC, NG YF, Ho S, Gyda M and Chan SW:
Resveratrol and cardiovascular health - promising therapeutic or
hopeless illusion? Pharmacol Res. 90:88–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu DG: Regulation of redox signalling and
autophagy during cardiovascular diseases-role of resveratrol. Eur
Rev Med Pharmacol Sci. 19:1530–1536. 2015.PubMed/NCBI
|
|
23
|
Ma L, Li W, Wang R, Nan Y, Wang Q, Liu W
and Jin F: Resveratrol enhanced anticancer effects of cisplatin on
non-small cell lung cancer cell lines by inducing mitochondrial
dysfunction and cell apoptosis. Int J Oncol. 47:1460–1468. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Empl MT, Albers M, Wang S and Steinberg P:
The resveratrol tetramer r-viniferin Induces a cell cycle arrest
followed by apoptosis in the prostate cancer cell line LNCaP.
Phytother Res. 29:1640–1645. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kjær TN, Thorsen K, Jessen N, Stenderup K
and Pedersen SB: Resveratrol ameliorates imiquimod-induced
psoriasis-like skin inflammation in mice. PLoS One.
10:e01265992015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimbrough CW, Lakshmanan J, Matheson PJ,
Woeste M, Gentile A, Benns MV, Zhang B, Smith JW and Harbrecht BG:
Resveratrol decreases nitric oxide production by hepatocytes during
inflammation. Surgery. 158:1095–1101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo S, Yao Q, Ke Z, Chen H, Wu J and Liu
C: Resveratrol attenuates high glucose-induced oxidative stress and
cardiomyocyte apoptosis through AMPK. Mol Cell Endocrinol.
412:85–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ates O, Cayli SR, Yucel N, Altinoz E,
Kocak A, Durak MA, Turkoz Y and Yologlu S: Central nervous system
protection by resveratrol in streptozotocin-induced diabetic rats.
J Clin Neurosci. 14:256–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Venturini CD, Merlo S, Souto AA, Fernandes
MC, Gomez R and Rhoden CR: Resveratrol and red wine function as
antioxidants in the nervous system without cellular proliferative
effects during experimental diabetes. Oxid Med Cell Longev.
3:434–441. 2010. View Article : Google Scholar
|
|
30
|
Chen X, He H, Wang G, Yang B, Ren W, Ma L
and Yu Q: Stereospecific determination of cis- and
trans-resveratrol in rat plasma by HPLC: Application to
pharmacokinetic studies. Biomed Chromatogr. 21:257–265. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koide K, Osman S, garner AL, Song F, Dixon
T, Greenberger JS and Epperly MW: The use of
3,5,4′-tri-O-acetylresveratrol as a potential pro-drug for
resveratrol protects mice from γ-irradiation-induced death. ACS Med
Chem Lett. 2:270–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Forsgren P, Modig J, Gerdin B, Axelsson B
and Dahlbäck M: Intrapulmonary deposition of aerosolized Evans blue
dye and liposomes in an experimental porcine model of early ARDS.
Ups J Med Sci. 95:117–136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Zhang D, Zhao B and Zhao J:
Superoxide anion, the main species of ROS in the development of
ARDS induced by oleic acid. Free Radic Res. 38:1281–1287. 2004.
View Article : Google Scholar
|
|
34
|
Kovac S, Angelova PR, Holmström KM, Zhang
Y, Dinkova-Kostova AT and Abramov AY: Nrf2 regulates ROS production
by mitochondria and NADPH oxidase. Biochim Biophys Acta.
1850:794–801. 2015. View Article : Google Scholar :
|
|
35
|
Yu M, Geiger B, Deeb N and Rothschild MF:
Investigation of TXNIP (thioredoxin-interacting protein) and TRX
(thioredoxin) genes for growth-related traits in pigs. Mamm genome.
18:197–209. 2007. View Article : Google Scholar : PubMed/NCBI
|