Introduction
RNA interference (RNAi) has shown tremendous
potential in medicinal therapeutics development in past decade
(1–4). Now, it has been confirmed that RNAi
has great potential in treatment of various human diseases, such as
viral infections (5,6), cancers (7–10)
and orphan disease (11).
Recently, an attempt using a long double-strand siRNA was made, as
an alternative way, to carry on multiple interfering RNAs (iRNA)
for multiple gene suppression for treatment of many human diseases
(12–14), such as therapy for hepatocellular
carcinoma (HCC) (10). Currently
there are more than thirty types of siRNA drug candidates entered
into the stage of clinical trials (15–17). However, these synthetic
therapeutic long siRNA constructs in literature, including our
previously study (18), were all
based on a primary structure format (linearlized). It is a
well-known fact that the longer the RNA molecule is, the easer the
degradation occurs during RNA processing in circulation or in cell
level.
To stabilize long therapeutic RNA, in the study we
first described a group of long synthetic iRNA with multiple siRNA
targets in secondary structures (loop or cloverleaf) and applied
them to HCC cells for multiple gene suppression.
Materials and methods
Cell lines and cell culture
SMMC-7721 was cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 2 mM L-glutamine, 100 U/ml of penicillin and 100
µg/ml of streptomycin (all from Thermo Fisher Scientific,
Waltham, MA, USA). These cell lines were purchased from the
Institute of Cell Biology, Chinese Academy of Sciences, and cells
were maintained at 37°C with 5% CO2 and 95% air in a
humidified incubator.
Multi-target iRNA format design and
transfection
The gene sequence of survivin (accession no.
NM_001168) and B-cell lymphoma-2 (Bcl-2) (accession no.
NM_000633.2) were obtained from National Center of Biotechnology
Information (NCBI) GenBank (USA), and the single target siRNA (19
bp duplex with 2-nt 3′-overhangs) targeting either survivin or
Bcl-2 were screened in our precious studies (Table I) (18,19). A double-stranded RNA sequence with
no homology with human genes was designed as siRNA negative control
(NC_siR). All RNA oligonucleotides were synthesized by Biomics
Biotechnologies Co., Ltd. (Nantong, China). The multi-target iRNA
constructs targeting survivin and Bcl-2 were designed according to
the principle of Biomics Biotechnologies Co., Ltd. (Table II). The cells were transfected
in vitro with siRNAs using Lipofectamine® 2000
transfection reagent (Thermo Fisher Scientific) according to the
instructions of the manufacturer.
 | Table ISequences of the single target
siRNAs. |
Table I
Sequences of the single target
siRNAs.
| siRNAs | | Primer sequences
(5′-3′) |
|---|
| Survivin siRNA1 | S2 | Sense |
GACUUGGCCCAGUGUUUCUdTdT |
| Antisense |
AGAAACACUGGGCCAAGUCdTdT |
| Survivin siRNA2 | S38 | Sense |
UCCUUUCUGUCAAGAAGCAGUUdTdT |
| Antisense |
AACUGCUUCUUGACAGAAAGGAdTdT |
| Bcl-2 siRNA | B2 | Sense |
GGAUGACUGAGUACCUGAAdTdT |
| Antisense |
UUCAGGUACUCAGUCAUCCdTdT |
| Negative control
siRNA | NC_siR | Sense |
UUCUCCGAACGUGUCACGUdTdT |
| Antisense |
ACGUGACACGUUCGGAGAAdTdT |
 | Table IISequences of RT-qPCR primers. |
Table II
Sequences of RT-qPCR primers.
| Gene name | Primer sequences
(5′-3′) |
|---|
| Survivin | F:
CGACGTTGCCCCCTGCCTG |
| R:
AAGGAAAGCGCAACCGGACGA |
| Bcl-2 | F:
GGCTGGGATGCCTTTGTG |
| R:
GCCAGGAGAAATCAAACAGAGG |
| Ago2 | F:
GGCAGGAAGAATCTATACAC |
| R:
CTTGATGGACACCTTGAAG |
| OAS1 | F:
GTGAGCTCCTGGATTCTGCT |
| R:
TGTTCCAATGTAACCATATTTCTGA |
| IFIT1 | F:
AATAGACTGTGAGGAAGGATGG |
| R:
TCCAGGCGATAGGCAGAG |
| GAPDH | F:
GAAGGTGAAGGTCGGAGTC |
| R:
GAAGATGGTGATGGGATTTC |
Real-time quantitive PCR (RT-qPCR)
Total RNA of cells were extracted by RISO™ RNA
extraction reagent (Biomics Biotechnologies Co., Ltd.) and then
performed in a RT-qPCR reaction: 12.5 µl of 2X One-Step qPCR
Mix (Biomics Biotechnologies Co., Ltd.), 0.5 µl of each
forward and reverse primers (10 µM each; Biomics
Biotechnologies Co., Ltd.), 0.5 µl of 50X SYBR-Green I and 4
µl total RNA was then subjected to reverse transcription for
30 min at 42°C and initially denatured at 95°C for 5 min, and then
to 45 cycles of amplification with the condition of 95°C
denaturation for 20 sec, 55°C annealing for 30 sec, and 72°C
extension for 30 sec. Human glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) served as an internal control. The experiment
was performed in triplicate. All primer sequences are shown in
Table II. The results were
analyzed by 2−ΔΔCt method (20).
Western blotting
Cells were plated in a 6-well plate at
1×106 cells/well and grown for 24 h until ~70–80%
confluence. After cells were treated as described above for 48 h,
they were harvested and lysed in RIPA buffer (Pierce, Rockford, IL,
USA). Total protein was quantified by bicinchoninic acid (BCA)
assay (Promega, Madison, WI, USA), and then ~20 µg of
protein was separated by polyacrylamide gel electrophoresis (PAGE)
and electroblotted onto polyvinylidine difluoride filter (PVDF)
membranes (Millipore, Billerica, MA, USA), followed by blocking
with 5% skim milk in TBST (20 mM Tris, 150 mM NaCl, 0.05% Tween-20,
pH 7.5) buffer for 2 h at room temperature, then incubated with
rabbit anti-human survivin antibody (1:500 dilution) and mouse
anti-human Bcl-2 (1:500 dilution), mouse anti-human β-actin
antibody (1:500 dilution) (all from Abcam, Cambridge, MA, USA) as
internal control. The membranes were washed in TBST and incubated
with a goat anti-rabbit or goat anti-mouse HRP-conjugated secondary
antibody (1:1,000 dilution; Jackson Immunoresearch, West Grove, PA,
USA) at room temperature for 2 h. Then, the specific proteins were
detected with ECL chemiluminescence reagent (Beyotime, Beijing,
China); the membranes were exposed to film (Kodak, Rochester, NY,
USA).
Cell viability assay
MTT assay was used to measure the viability of
cells. Cells were plated into a 96-well plate at 5×103
cells/well and grown for 24 h, then treated as above for 0, 24, 48,
72 and 96 h, 10 µl MTT (Beyotime) were added to each well of
96-well plate containing 100 µl DMEM medium and incubated at
37°C for 4 h, then 150 µl/well DMSO was added and incubation
was continue at 37°C for 10 min. The optical density (OD) was
measured at 490 nm using a Microplate Reader (Bio-Rad, Hercules,
CA, USA).
Cell invasion assay
Cells were seeded into a 24-well plate at
2×104 cells/well and grown for 24 h, then treated as
described above, post 48 h treament, cells were suspended in DMEM
medium at the density of 1×106 cells/ml. Briefly,
Transwell chambers (Corning, Inc., Corning, NY, USA) and treated
with DMEM for 1 h before treatments; an 8 µm pore
polycarbonate membrane was coated with 50 µl of 0.5 mg/ml
Matrigel (BD Biosciences, San Jose, CA, USA) and used to separate
the upper and lower chambers. Cell suspension (100 µl) was
added into each upper chamber and 600 µl DMEM containing 10%
FBS or conditioned medium which was the cell supernatant with
siRNAs post-transfected for 48 h. The cells on the top surface of
the membrane were carefully removed at 24 h post treatments. Cells
on the Transwell chambers were fixed for 30 sec in 10%
formaldehyde, and then stained by 0.5% crystal violet, after
washing by phosphate-buffered saline (PBS), the cells on the top
surface of the membrane were carefully removed again. The cells on
the bottom surface of the membrane were counted in 3–5 random
fields under a microscope (magnification, ×100).
Cell apoptosis assay
Annexin V-FITC/PI double staining and flow cytometry
(FCM) analysis method was used to determine cell apoptosis.
Briefly, 1×106 cells/well in a 6-well plate treated with
different treatments as above for 48 h were harvested and washed in
PBS, cells were stained by using Annexin V-FITC apoptosis detection
kit (Sigma-Aldrich, St. Louis, MO, USA), then detected by FCM
analysis (BD Biosciences).
Statistical analysis
All experiments were performed independently three
times, the results are shown as mean ± standard deviation (SD), and
statistical analyses were performed using SPSS 19.0 software. The
differences were compared using Student's t-test and one way ANOVA
followed by post hoc test to assess statistical significance. All
P-values were based on a two-sided statistical analysis and
P<0.05 was considered to indicate statistical significance.
Results
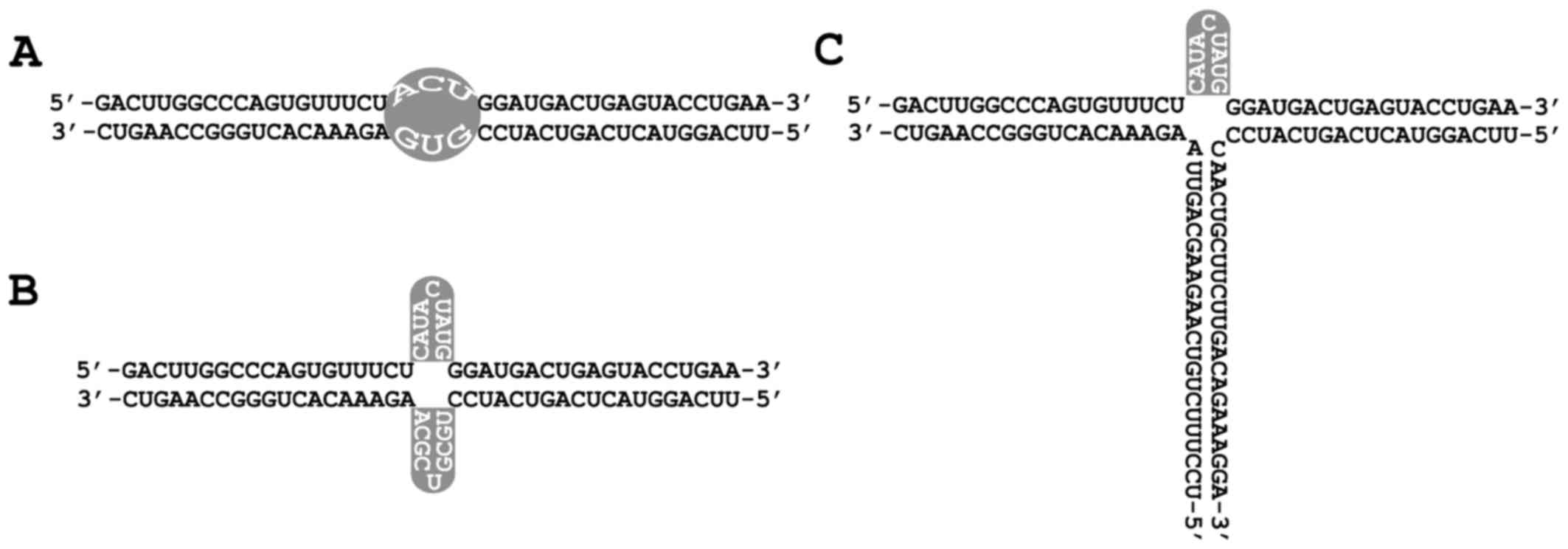
Designing different multi-target
iRNAs
Multi-target iRNAs targeting survivin and Bcl-2 were
designed according to different structure formats (Fig. 1). The sense and antisense strands
of iRNAs were synthesized by Biomics Biotechnologies Co., Ltd. and
iRNAs were obtained after annealing of both strands (Table II and Fig. 1).
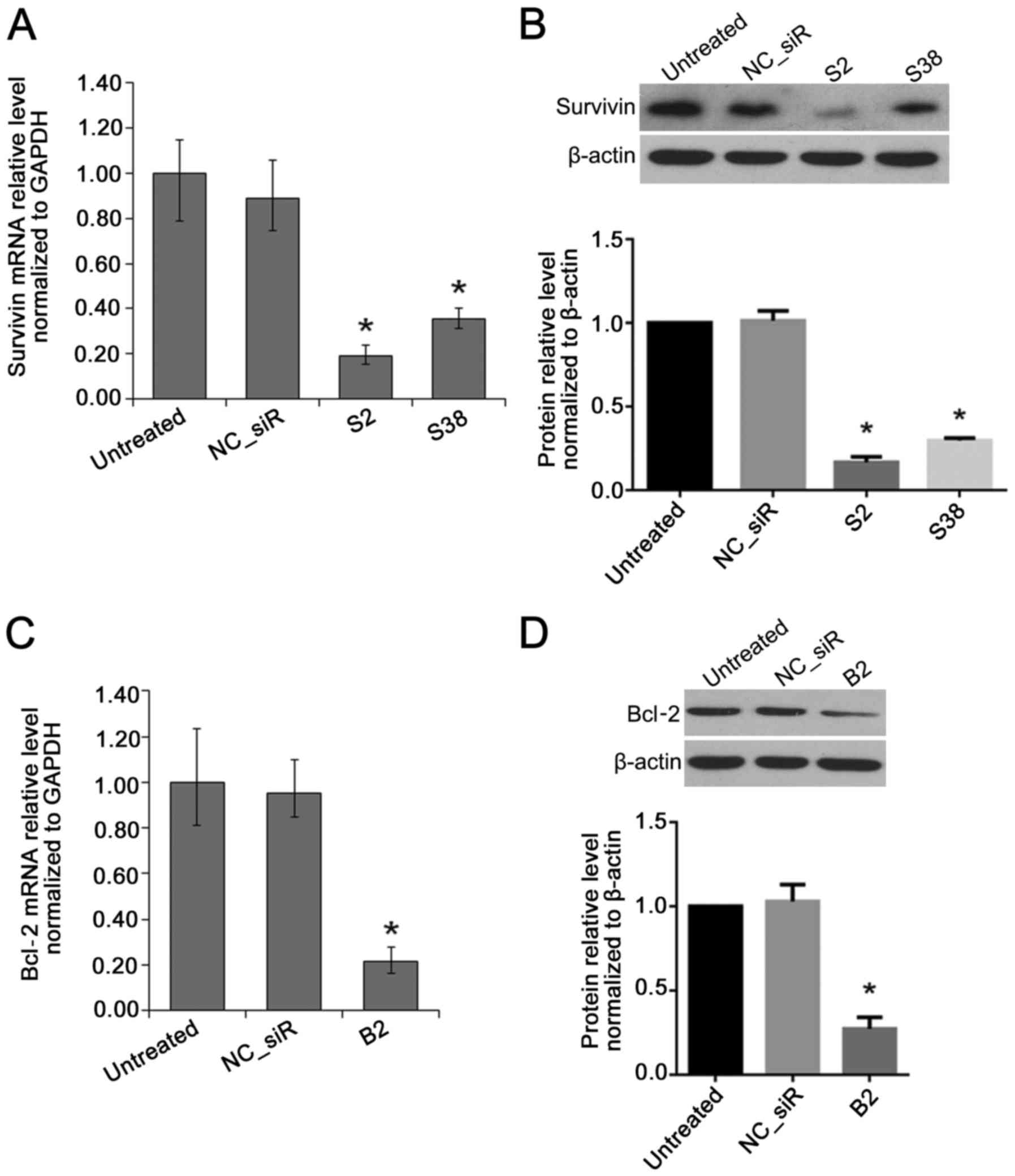
Inhibition effects of target genes in HCC
cells by multi-target iRNAs
The result of RT-qPCR and western blot analysis
showed that, compared with untreated cell, the mRNA and protein
level of survivin were both inhibited by survivin siRNA (S2 or
S38). The mRNA and protein level of Bcl-2 were both inhibited by
Bcl-2 siRNA (B2) (P<0.05) (Fig.
2).
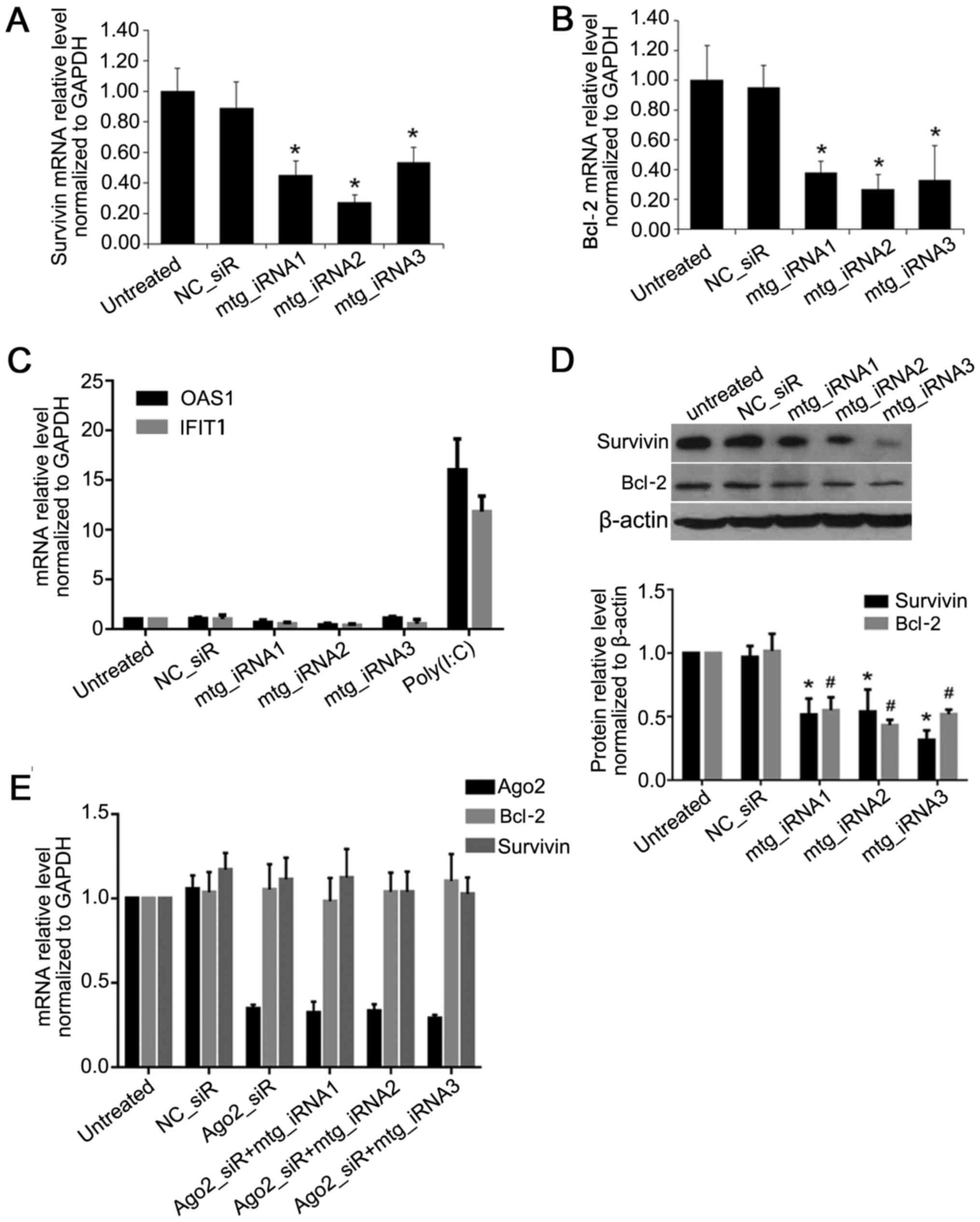
Compared to untreated group, the mRNA level of
survivin was inhibited by multi-target interfering RNAs
(mtg_iRNA1), mtg_iRNA2 and mtg_iRNA3 up to 45, 27 and 53%
(P<0.05) (Fig. 3A); the mRNA
level of Bcl-2 was inhibited up to 38, 27 and 33% (P<0.05)
(Fig. 3B); and there was no
difference between NC_siRNA and untreated ones. Moreover,
mtg_iRNA1, mtg_iRNA2 and mtg_iRNA3 showed no interferon response
(Fig. 3C). The protein level of
survivin was inhibited by mtg_iRNA1, mtg_iRNA2 and mtg_iRNA3 up to
48, 46 and 68%, respectively (P<0.05); the protein level of
Bcl-2 was inhibited up to 45, 57 and 48% (P<0.05), and there was
no difference between NC_siR and untreated group (P>0.05)
(Fig. 3D).
Gene knockdown by mtg_iRNAs in an
Ago2-dependent manner
Long double-stranded RNA was reported with gene
knockdown effect in an Ago2-dependent manner (21). To verify the manner of the effect
of mtg_iRNAs, the Ago2 siRNA (sense, 5′-AAUCUCUUCUUGCCGAUCGdTdT-3′
and antisense, 5′-CGAUCGGCAAGAAGAGAUUdTdT-3′) was used to knockdown
the expression of Ago2, and we further investigated whether
mtg_iRNAs could downregulate the target genes. As shown in Fig. 3, compared with untreated cells,
the expression of Ago2 was inhibited efficiently by Ago2_siR, when
Ago2 was downregulated, target gene survivin or Bcl-2 could not be
inhibited by mtg_iRNAs, and thus gene knockdown by mtg_iRNAs was
Ago2-dependent (Fig. 3E).
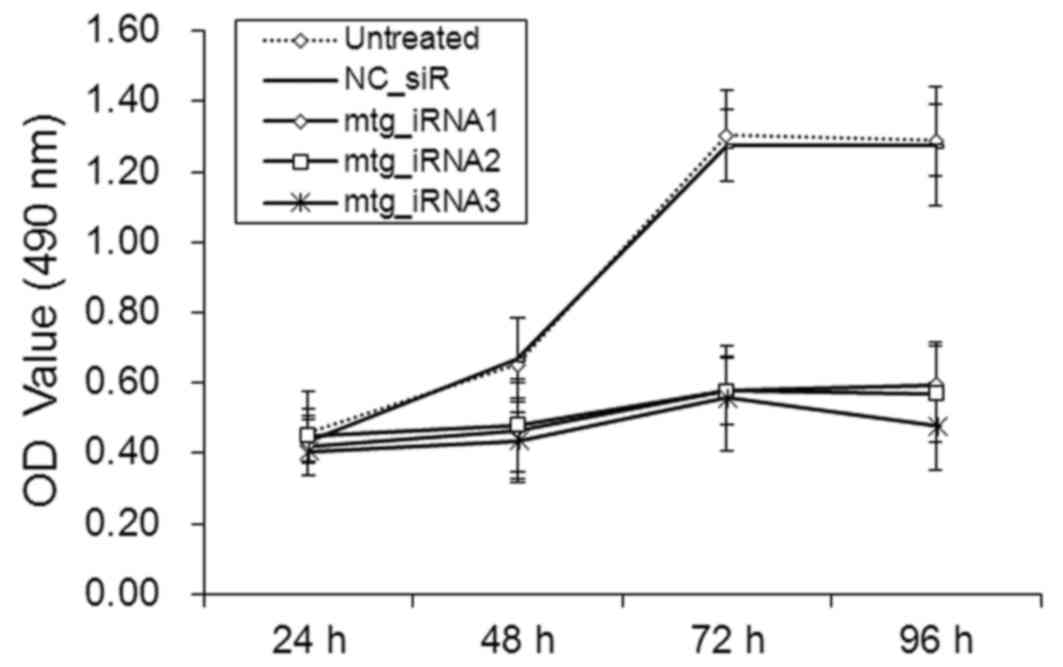
Inhibition effects on cell viability by
multi-target siRNAs
The viability of SMMC-7721 cells was measured by MTT
assay. The absorbance values (490 nm) of the cells at 48, 72 and 96
h post-transfection with mtg_iRNA1, mtg_iRNA2 and mtg_iRNA3 were
significantly lower than those of NC_siR treated and untreated
cells, respectively (P<0.05) (Fig.
4). There was no significant difference between NC_siR treated
and untreated cells (P>0.05).
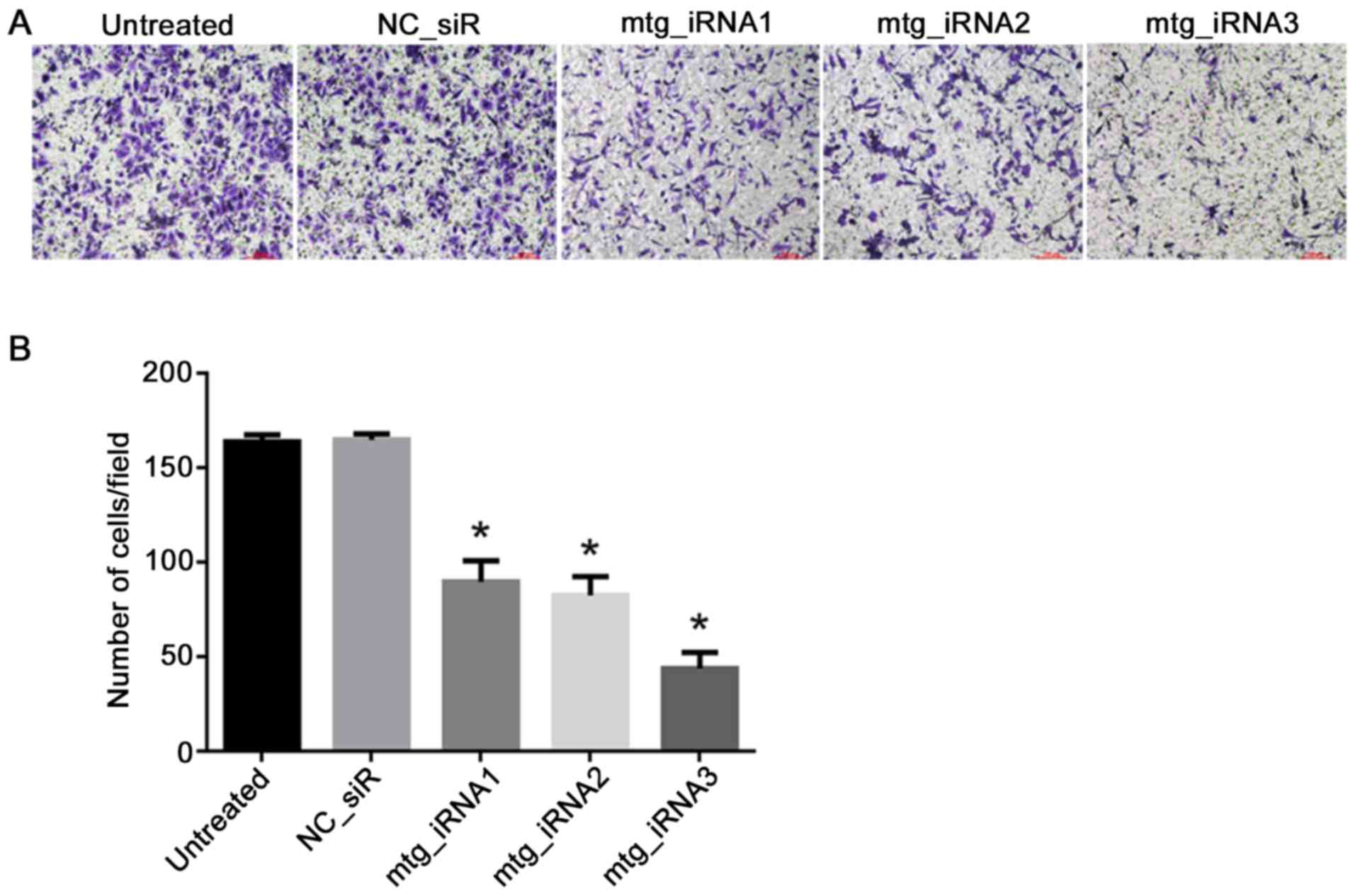
Inhibition effects on cell invasion by
multi-target siRNAs
The cell invasion abilities with different
treatments were detected by Transwell assay, the result showed that
the invasion of the cells treated with mtg_iRNA1, mtg_iRNA2 and
mtg_iRNA3 were inhibited significantly post 48 h transfection
(P<0.05). There was no significant difference of inhibition
abilities between NC_siR and untreated cells (P>0.05) (Fig. 5).
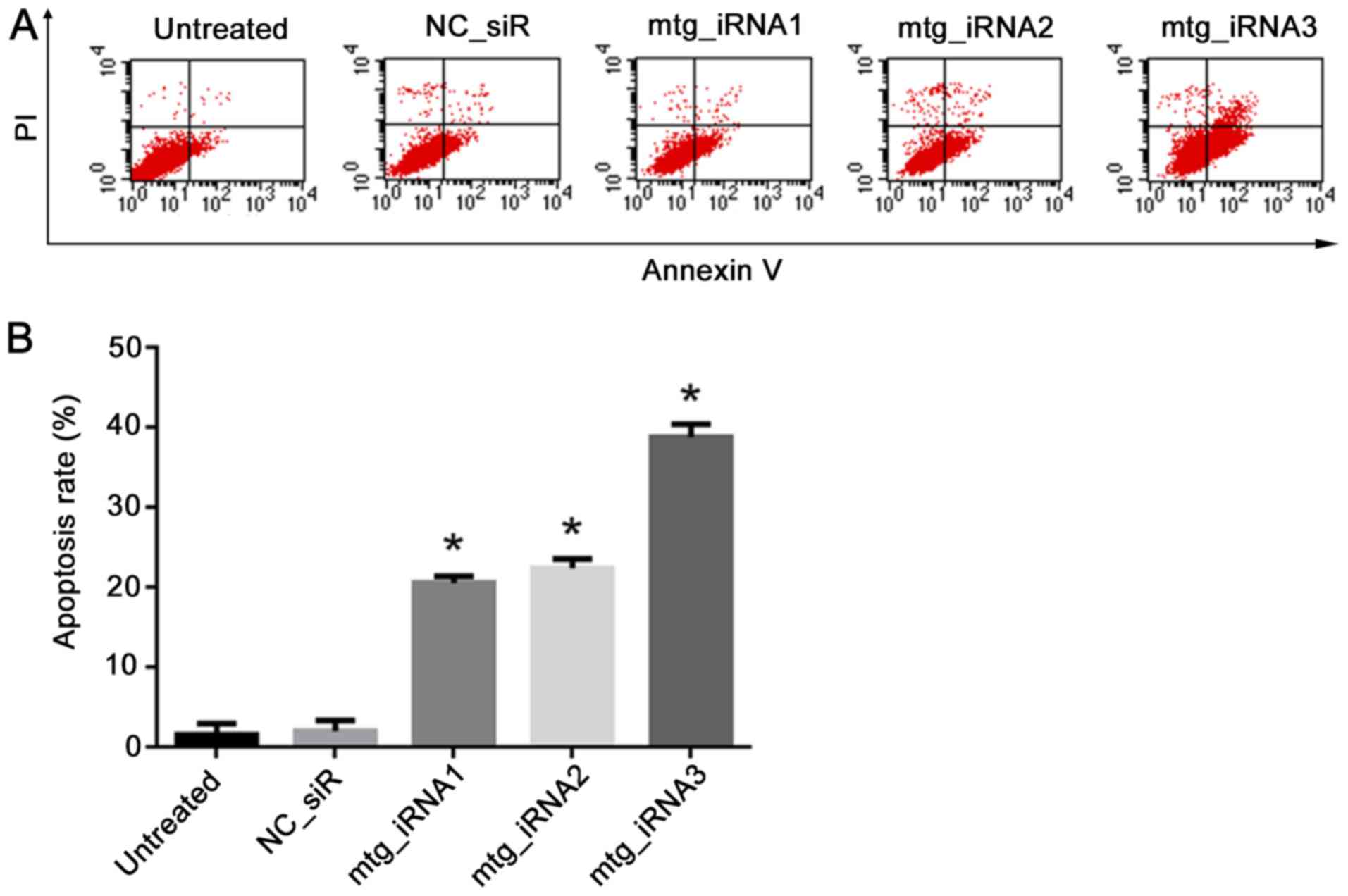
Cell apoptosis induces multi-target
siRNAs
Annexin V-FITC/PI double staining and FCM analysis
were used to detect the ability of mtg_iRNAs on inducing SMMC-7721
cell apoptosis. The result showed that cells treated with
mtg_iRNA1, 20.52±0.81%, mtg_iRNA2, 22.36±1.15% and mtg_iRNA3,
38.79±1.63% resulted in a significant increase of apoptosis
compared with that of NC_siR treated cells, 1.96±1.35% and
untreated cells, 1.52±1.41% (P<0.05) (Fig. 6).
Discussion
The present study is the first attempt to use long
synthetic iRNA formats (loop or cloverleaf) to carry multiple siRNA
targets. Survivin and Bcl-2 were used as HCC therapeutic targets in
our study for iRNA therapeutics development. survivin is an
apoptosis inhibitor that is expressed during the G2/M phase of the
cell cycle and it is a member of the inhibitor of apoptosis (IAP)
gene family, survivin encodes negative regulatory proteins that
prevent apoptotic cell death (25). Bcl-2 is specifically considered as
an important anti-apoptotic oncogene, and it is the founding member
of the Bcl-2 family of regulator proteins that regulate cell death
(26,27).
The efficiency of gene co-inhibition by long
secondary structure iRNA was demonstrated (Fig. 3A, B and D), in which all three
mtg_iRNAs inhibited target genes (survivin or Bcl-2) effectively in
both mRNA and protein level.
The side-effect of undesired interferon response
caused long double-stranded RNA (dsRNA) is a major concern. It was
addressed by a previous study indicating that specific gene
knockdown via RNAi triggered by long dsRNA could induce interferon
response (28), thus the
expression of OAS1 and IFIT1 were detected to monitor the
interferon response and a synthetic dsRNA analog-poly(I:C) was used
as positive control (29). The
designed long secondary structure iRNAs here had no obvious
interferon response in mammalian cells (Fig. 3C). RNAi effects can be triggered
by different structural modifications of siRNA, also long
multi-target based siRNA structures designed as Dicer substrates
have been developed in therapeutic research (19), and these siRNAs were all
Ago2-dependent. In the present study, also our mtg_iRNAs were all
Ago2-dependent by the method of Ago2 downregulation first (Fig. 3E). Furthermore, we demonstrated
that the proliferation and invasion of HCC cells were inhibited,
also cell apoptosis was promoted by mtg_iRNAs effectively (Fig. 4Figure 5–6). The results of this study
demonstrated that the designed mtg_iRNAs construct had RNAi
activities on knockdown target genes simultaneously. The results
showed that, mtg_iRNAs may be a preferred strategy for multigenic
disease therapy, especially in cancer.
In conclusion, the long iRNA with different formats
(loop and cloverleaf) showed initial multi-gene co-inhibition
effects without showing side-effect of interferon response. The
design could be as an alternative way to open its new applications
in RNAi based gene therapy and drug development.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by the National High
Technology Research and Development Program of China (863 Program,
no. 2012AA022501); the National Science and Technology Major
Project of China (no. 2013ZX09301303-005).
[2] Availability
of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
TL and YYZ designed the study. YYZ designed the
different structures of multi-target interfering RNAs. TL, YJ and
SZ performed the RT-qPCR, western blot analysis, MTT. TL performed
the cell invasion assay and cell apoptosis assay. TL was a major
contributor in writing the manuscript; YYZ revised the manuscript
and supervised the study. All authors read and approved the final
manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chendrimada TP, Gregory RI, Kumaraswamy E,
Norman J, Cooch N, Nishikura K and Shiekhattar R: TRBP recruits the
Dicer complex to Ago2 for microRNA processing and gene silencing.
Nature. 436:740–744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hammond SM, Bernstein E, Beach D and
Hannon GJ: An RNA-directed nuclease mediates post-transcriptional
gene silencing in Drosophila cells. Nature. 404:293–296. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elbashir SM, Lendeckel W and Tuschl T: RNA
interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev.
15:188–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Shi X, Zhang X, Wang L, Luo J,
Xing G, Deng R, Yang H, Li J, Wang A, et al: Porcine reproductive
and respiratory syndrome virus (PRRSV) inhibits RNA-mediated gene
silencing by targeting Ago-2. Viruses. 7:5539–5552. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikitenko NA, Speiseder T, Lam E, Rubtsov
PM, Tonaeva KhD, Borzenok SA, Dobner T and Prassolov VS: Regulation
of human adenovirus replication by RNA interference. Acta Naturae.
7:100–107. 2015.PubMed/NCBI
|
|
7
|
Tai W, Qin B and Cheng K: Inhibition of
breast cancer cell growth and invasiveness by dual silencing of
HER-2 and VEGF. Mol Pharm. 7:543–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shahzad MM, Lu C, Lee JW, Stone RL, Mitra
R, Mangala LS, Lu Y, Baggerly KA, Danes CG, Nick AM, et al: Dual
targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther.
8:1027–1034. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim DH, Behlke MA, Rose SD, Chang MS, Choi
S and Rossi JJ: Synthetic dsRNA Dicer substrates enhance RNAi
potency and efficacy. Nat Biotechnol. 23:222–226. 2005. View Article : Google Scholar
|
|
10
|
Tabernero J, Shapiro GI, LoRusso PM,
Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR,
Alsina M, et al: First-in-humans trial of an RNA interference
therapeutic targeting VEGF and KSP in cancer patients with liver
involvement. Cancer Discov. 3:406–417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coelho T, Adams D, Silva A, Lozeron P,
Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, et
al: Safety and efficacy of RNAi therapy for transthyretin
amyloidosis. N Engl J Med. 369:819–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zimmermann GR, Lehár J and Keith CT:
Multi-target therapeutics: When the whole is greater than the sum
of the parts. Drug Discov Today. 12:34–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li T, Wu M, Zhu YY, Chen J and Chen L:
Development of RNA interference-based therapeutics and application
of multi-target small interfering RNAs. Nucleic Acid Ther.
24:302–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyapalle S, Xu W, Raulji P, Mohapatra S
and Mohapatra SS: A multiple siRNA-based anti-HIV/SHIV microbicide
shows protection in both in vitro and in vivo models. PLoS One.
10:e01352882015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeshita F and Ochiya T: Therapeutic
potential of RNA interference against cancer. Cancer Sci.
97:689–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aigner A: Applications of RNA
interference: Current state and prospects for siRNA-based
strategies in vivo. Appl Microbiol Biotechnol. 76:9–21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davidson BL and McCray PB Jr: Current
prospects for RNA interference-based therapies. Nat Rev Genet.
12:329–340. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng W, Chen J, Qin Y, Yang Z and Zhu YY:
Long double-stranded multiplex siRNAs for dual genes silencing.
Nucleic Acid Ther. 23:281–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li T, Zhu YY, Chen L, Sun Y, Yuan J,
Graham M and French P: Size unbiased representative enzymatically
generated RNAi (SURER) library and application for RNAi therapeutic
screens. Nucleic Acid Ther. 25:35–46. 2015. View Article : Google Scholar :
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Chang CI, Kang HS, Ban C, Kim S and Lee
DK: Dual-target gene silencing by using long, synthetic siRNA
duplexes without triggering antiviral responses. Mol Cells.
27:689–695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rice RR, Muirhead AN, Harrison BT,
Kassianos AJ, Sedlak PL, Maugeri NJ, Goss PJ, Davey JR, James DE
and Graham MW: Simple, robust strategies for generating
DNA-directed RNA interference constructs. Methods Enzymol.
392:405–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin D, Lee H, Kim SI, Yoon Y and Kim M:
Optimization of linear double-stranded RNA for the production of
multiple siRNAs targeting hepatitis C virus. RNA. 15:898–910. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aviñó A and Ocampo SM: Perales and JC
Eritja R: Branched RNA: A new architecture for RNA interference. J
Nucleic Acids. 5869352011.
|
|
25
|
Verdecia MA, Huang H, Dutil E, Kaiser DA,
Hunter T and Noel JP: Structure of the human anti-apoptotic protein
survivin reveals a dimeric arrangement. Nat Struct Biol. 7:602–608.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsujimoto Y, Finger LR, Yunis J, Nowell PC
and Croce CM: Cloning of the chromosome breakpoint of neoplastic B
cells with the t(14;18) chromosome translocation. Science.
226:1097–1099. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cleary ML, Smith SD and Sklar J: Cloning
and structural analysis of cDNAs for bcl-2 and a hybrid
bcl-2/immunoglobulin transcript resulting from the t(14;18)
translocation. Cell. 47:19–28. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palchetti S, Starace D, De Cesaris P,
Filippini A, Ziparo E and Riccioli A: Transfected poly(I:C)
activates different dsRNA receptors, leading to apoptosis or
immunoadjuvant response in androgen-independent prostate cancer
cells. J Biol Chem. 290:5470–5483. 2015. View Article : Google Scholar : PubMed/NCBI
|