Introduction
The tumor-associated calcium signal transducer 2
(Trop-2) is a type-1 transmembrane protein. Although Trop-2 is
overexpressed in a large variety of human epithelial carcinomas
(1-5), its expression is minimal or
restricted in normal tissues. Notably, elevated expression of
Trop-2 is associated with more aggressive disease and poor
prognosis in several cancer types (6-8).
The study by Jiang et al (9) revealed that, in patients with
advanced non-small cell lung cancer (NSCLC), Trop-2 expression was
higher in tumor tissues in comparison with that in the
tumor-adjacent normal tissues. In addition, the study identified
that Trop-2 expression was correlated with the clinicopathological
features (9). These previous
studies investigating Trop-2 indicated that it is a novel tumor
antigen and may potentially be used as a lung cancer immunotherapy
target.
Virus-like particles (VLPs) contain the structure of
native virions, however, they are non-infectious due to lack of the
virus genome (10-12). Recently, recombinant VLP-based
vaccine strategies have been frequently used for novel vaccine
development. Various types of VLPs have been designed and produced
by utilizing the self-assemble ability of virus capsid and envelope
proteins (13-20). Commercialized VLP-based vaccines
have been successfully used in the prevention against hepatitis B
virus and human papillomavirus (21). The structural polyprotein Pr55gag
of the human immunodeficiency virus type I (HIV-1) gag gene directs
the viral assemble process, and the unprocessed gag precursor
itself is sufficient for the release of non-infectious VLPs. In
addition to their self-assembly abilities, Pr55gag-based VLPs are
able to deliver foreign polypeptides, and thus can be used both as
a direct immunogen and as a carrier for foreign antigens. Two
principal strategies have previously been established (22), involving the type I VLPs that are
constructed by integrating or fusing small epitopes with the
C-terminus of gag polyprotein, as well as the type II VLPs that are
constructed by incorporating foreign proteins at the outer particle
surface.
Although the use of VLPs is a promising strategy for
vaccine development, identifying approaches to enhance the
immunogenicity of VLPs is highly desirable. In the field of tumor
immunotherapy, the application of adjuvants is widely recognized. A
number of tumor necrosis factor superfamily ligands (TNFSFLs) have
been demonstrated to participate in the activation processes of
dendritic cells and T cells, and such molecules include the CD40
ligand (CD40L), CD70, OX40L, GITRL, RANKL, LIGHT, 4-1BBL and BAFF
(23,24). In addition, TNFSFLs as immune
adjuvants have previously been reported (25). As a member of the TNFSFL family,
CD40L is mainly expressed on the surface of activated
CD4+ T cells, and is able to enhance the humoral and
cellular immune responses (26,27). Furthermore, the CD40L/CD40
interaction serves a significant role in B-cell activation,
proliferation, differentiation and antibody production (28), whereas binding of CD40L to CD40
induces the expression of costimulatory molecules that reside on
antigen-presenting cells. Once these cells are activated, the
CD4+ T cell responses are augmented by increased
cytokine production, while the CD4+-dependent
CD8+ T cells are also activated (29). In the field of DNA vaccine design,
fusion of the immune stimulatory molecule CD40L genetically was
confirmed to be an effective way to obtain a robust T cell response
(30). In the field of VLP
development, Skountzou et al (31) also demonstrated that incorporation
of CD40L into VLPs resulted in enhanced immunogenicity of viral
antigens by stimulating T cell responses.
In the present study, the construction and
characterization of type II gag VLPs was reported. These VLPs were
produced in insect cells and constructed from full-length gag
molecules incorporating Trop-2 alone or a combination of Trop-2 and
CD40L adjuvant on the surface of VLPs. First, whether immunization
with either of these VLPs was able to generate specific humoral and
cellular immune responses against Trop-2 was examined, and whether
CD40L-incorporated VLPs generated a higher immune response compared
with Trop-2 only VLPs was also examined. Prevention and therapeutic
experiments were performed to examine whether VLP immunization may
result in reduced tumor growth and increased survival rate of
Lewis(Trop-2) tumor-bearing mice, and whether
CD40L-incorporated VLPs exhibited a more efficient tumor inhibition
effect when compared with Trop-2 only VLPs. Given the high
resistance of lung cancer to conventional treatments and
sensitivity to immunotherapy, the present study provided a
promising approach using VLPs as a carrier of the novel tumor
antigen Trop-2 and the immune adjuvant CD40L.
Materials and methods
Cells
Lewis murine lung adenocarcinoma cells were
purchased from American Type Culture Collection, (Manassas, VA,
USA; ATCC® no. 18188) and cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) (both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), at 37°C in an atmosphere of 5% CO2
and 95% humidified air. In addition, the normal insect cell line
TN5 was provided by Dr Ye Jing (The Fourth Military Medical
University, Xi'an, China) and were cultured in Sf-900 II (Gibco;
Thermo Fisher Scientific, Inc.) serum-free insect cell culture
medium. Full-length murine Trop-2 (mTrop-2) cDNA was cloned into
the pcDNA3.1 vector and termed plasmid (p-Trop-2). To generate
Lewis cells stably expressing mTrop-2, the p-Trop-2 was transfected
into Lewis cells using Lipfectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C. A total of 4-6 h later, the medium was
replaced with fresh DMEM and culturing was continued for 24 h at
37°C. Positive clones were selected using 700 µg/ml G418
(Invitrogen; Thermo Fisher Scientific, Inc.), incubated for 14 days
at 37°C, and the transfected cells presenting a stable expression
of mTrop-2 were termed as Lewis(Trop-2).
DNA constructs and preparation of
recombinant baculoviruses (rBVs)
Plasmids containing mouse CD40L or HIV gag gene were
kindly provided by Dr Ye Ling (Department of Microbiology and
Immunology, Emory University, Atlanta, Georgia). CD40L was
amplified by the polymerase chain reaction (PCR) method using the
following primers: CD40L forward (carrying an EcoRI site),
5′-ccggaattcgccacctaatgatagaaacatacagcc-3′,
and reverse (carrying an XhoI site), 5′-cccgctcgagtcagagtttgagtaagcc-3′,
with the underlined parts of the sequences indicating the
restriction enzyme recognition sites. In addition, gag gene
fragments were also obtained by PCR using the following primers:
Gag forward (EcoRI site), 5′-ccggattcatgggagatagggtggcagatgtaat-3′,
and reverse (HindIII site), 5′-cccaagcttttactaactggtctcctccaaagagagaat-3′.
The thermocycling conditions were as follows: 1 cycle for 30 s at
98°C; 30 cycles for 30 s at 65°C, and 1 min at 72°C; and 1 cycle
for 10 min at 72°C. PCR products were purified using the
PrimeSTAR® HS DNA Polymerase (Takara Bio Inc., Otsu,
Japan).
A newborn normal female mouse at 0-3 days of
age (weight, 1.5 g), housed under a controlled temperature (~22°C)
and relative humidity (40-60%) conditions with a 12-h light-dark
cycle and breast fed, was anesthetized and sacrificed using ether,
then the kidney was isolated, and the RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
full-length mTrop-2 cDNA was then amplified using the reverse
transcription-PCR method with the following primers: Trop-2 forward
(EcoRI site), 5′-ccggaattcatggcgaggggcttggatctagcac-3′,
and reverse (XhoI site), 5′-cccgctcgagctacaagctaggttcgcttctcatctcccc-3′.
The thermocycling conditions were 1 cycle for 30 s at 98°C; 30
cycles for 2 min at 68°C; and 1 cycle for 10 min at 72°C. PCR
products were purified using PrimeSTAR® HS DNA
Polymerase (Takara Bio Inc.).
Gag, CD40L and Trop-2 genes were cloned into
multiple cloning sites of the pFastBacI vector (Invitrogen; Thermo
Fisher Scientific, Inc.), respectively. The pFastBacI plasmids
containing the gag, Trop-2 or CD40L genes were introduced by
transformation into DH10Bac™ E. coli at 37°C for 48 h (Invitrogen;
Thermo Fisher Scientific, Inc.). Following verification of DH10Bac
with recombinant bacmid, recombinant bacmid DNA was isolated. The
rBVs expressing the gag, Trop-2 or CD40L proteins were generated by
transfecting TN5 insect cells with recombinant Trop-2 or CD40L
bacmids using Cellfectin II Reagent (Thermo Fisher Scientific,
Inc.) at 27°C for 4 days, and the culture supernatant containing
the rBv was collected 4 days post transfection.
VLP production and purification
TN5 cells were infected with rBV gag at a
multiplicity of infection (MOI) of 2 in order to release gag VLPs.
Next, VLPs containing Trop-2 were produced by co-infecting TN5
cells with rBV gag and rBV Trop-2 at a MOI ratio of 1:5. Chimeric
VLPs containing both Trop-2 and CD40L were also produced by
coinfecting TN5 cells with rBV gag, Trop-2 and CD40L at a MOI ratio
of 1:5:2. The medium containing the VLPs was collected at 72 h post
infection, and the cell debris was clarified by high-speed
centrifugation (3,000 × g, 30 min, 4°C). Subsequently, the
supernatant containing the VLPs was concentrated with a
QSM-03SP/50quixstand™ Benchtop System (GE Healthcare Life Sciences,
Little Chalfont, UK) and further purified by ultra-centrifugation
at 100,000 × g for 2.5 h at 4°C in an SW-41 rotor (Beckman Coulter,
Inc., Brea, CA, USA) through a 15-50% discontinuous sucrose
gradient. The layer containing the purified VLPs was then
suspended, washed with phosphate-buffered saline (PBS),
ultra-centrifuged at 28,000 rpm for 30 min and re-suspended in PBS.
A BCA assay kit (Thermo Fisher Scientific, Inc. CA, USA) was
subsequently used to detect the protein concentration of the
VLPs.
Western blot analyses of VLPs
Purified VLPs were characterized by western blot
analysis for the presence of Trop-2 and CD40L proteins. Briefly,
the VLPs were quantified using a BCA assay kit (cat. no. BCA02;
Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China)
according to the manufacturer's protocol, samples of 5, 2, and 1
µg were loaded per lane and were subjected to 10% SDS-PAGE,
and transferred to a nitrocellulose membrane (Whatman GmbH, Dassel,
Germany). The membrane was blocked using 5% nonfat milk in PBS with
20% Tween-20 at 4°C overnight, and then incubated with polyclonal
rabbit anti-HIV gag p24 (1:2,000 dilution; cat. no. ab32352; Abcam,
Cambridge, MA, USA), mouse anti-Trop-2 (1:1,000 dilution; cat. no.
sc-376181; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
monoclonal mouse anti-CD40L (1:2,000 dilution; cat. no. MAB4401;
R&D Systems, Inc., Minneapolis, MN, USA) primary antibodies
overnight at 4°C. Then, the membrane was washed three times with
PBST for 10 min each. Subsequently, the samples were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit (1:5,000;
cat. no. ZDR-5306) or HRP-conjugated goat anti-mouse IgG (1:5,000;
cat. no. ZDR-5307) secondary antibodies (both from OriGene
Technologies, Inc., Beijing, China) for 1 h at room temperature.
The protein bands were detected with an enhanced chemiluminescence
developing reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Protein bands were quantified by densitometry using ImageQuant TL
7.0 Image Analysis Software (GE Healthcare Life Sciences).
Transmission electron microscopy
(TEM)
VLPs were further examined by TEM, which was
performed as previously described (32). Briefly, the Trop-2 or Trop-2-CD40L
VLPs prepared as described above were negatively stained with 1%
potassium phosphotungstate (cat. no. GZ02536; Beijing Zhongjingkeyi
Technology Co., Ltd, Haidian, China) at room temperature for 1 min,
washed with PBS for 1 min at room temperature and then examined
using a Hitachi-H7500 TEM system (Hitachi, Ltd., Tokyo, Japan).
Characterization of CD40L incorporated
into VLPs by B-cell activation
To measure the B-cell activation, one female C57BL/6
mouse (aged 6 weeks; weight, 20 g), housed under a controlled
temperature (~22°C) and relative humidity (40-60%) conditions with
a 12-h light-dark cycle and provided ad libitum access to a
standard laboratory diet of food and water was sacrificed by
cervical dislocation. The spleens were removed and processed into
single-cell suspensions in RPMI 1640 supplemented with 2 mM
L-glutamine (Invitrogen; Thermo Fisher Scientific, Inc.), 10% fetal
bovine serum (HyClone Systems, Inc., Logan, UT, USA), 100 U of
penicillin per ml and 100 µg/ml streptomycin. The isolated
spleen cells were cultured in 48-well cell culture plates in
triplicate, with or without 2 µg/ml VLP stimulation. The
cells were then collected 4 days post stimulation, and
106 cells were stained with phycoerythrin-conjugated
anti-CD69 (1:2,000 dilution; cat. no. MA1-10276; eBioscience;
Thermo Fisher Scientific, Inc.) and peridinin chlorophyll
protein-conjugated anti-B220 (1:2,000 dilution; cat. no.
45-0452-82; eBioscience; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h in the dark. Cells were subsequently washed
with PBS, fixed with 1% paraformaldehyde and analyzed by flow
cytometry (Cytomics FV 500; Beckman Coulter, Brea, CA, USA).
Immunization
A total of 24 pathogen-free female C57BL/6 mice
(age, 6-8 weeks old, weight, 20-23 g) kept in pathogen-free
environments were purchased from The Fourth Military Medical
University Animal Center (Xi'an, China). Mice were housed under a
controlled temperature (~22°C) and relative humidity (40-60%)
conditions with a 12-h light-dark cycle and provided ad
libitum access to a standard laboratory diet of food and water.
Animal studies were performed according to the current animal
experimental protocols approved by the Institutional Review Board
of Tangdu Hospital at the Fourth Military Medical University
(assigned no. TDLL-201611-25). In order to examine the effect of
VLP immunization, female C57BL/6 mice (6-8-week-old) were randomly
divided into four groups (6 mice/group), and each mouse was
immunized twice with a 2-week interval by subcutaneous injection
with 40 µg gag VLPs, Trop-2 VLPs, Trop-2-CD40L VLPs or 100
µl PBS, respectively. Retro-orbital bleeding was used to
collect blood samples at 2 weeks post each immunization. The serum
samples were inactivated at 56°C for 30 min and then stored at
−80°C. At 1 week after the last vaccination, mice were challenged
subcutaneously in the right flank with 1×106
Lewis(Trop-2) tumor cells, and the tumor growth was
monitored twice a week for up to 65 days.
In order to investigate the therapeutic effect of
the VLPs, mice (6 mice for each group) were initially challenged
with 1×106 Lewis(Trop-2) cells subcutaneously
in the right flank on day 0. Next, on days 4 and 18, challenged
mice were inoculated with 40 µg of each kind of VLPs or with
100 µl PBS, respectively. Mice were monitored twice a week
and the survival rate was recorded.
Total anti-Trop-2 IgG measurement
Trop-2-specific IgG titers in the mouse sera were
detected by ELISA. Briefly, a 96-well plate was coated overnight at
4°C with purified Trop-2 protein, which was expressed and purified
using Ni-NTA Agarose (Invitrogen; Thermo Fisher Scientific; cat.
no. R90101) at a concentration of 0.8 µg/well. Subsequent to
three washes with 200 µl PBST, the plate was blocked with
100 µl 2% bovine serum albumin for 1 h in a 37°C water bath.
Following a further three washes with PBST, the serum samples were
series diluted and added into the wells (100 µl/well) for
incubation at 37°C for 1 h. A horseradish peroxidase-conjugated
goat anti-mouse IgG second antibody with a dilution of 1:5,000
(cat. no. ZDR-5307; Origene Technologies, Inc.) was then added.
After three further washes, the color was developed using
3,3′,5,5′-tetramethylbenzidine/hydrochloride (Sigma-Aldrich; Merck
KGaA; cat. no. ALDR-ICH-860336), and the optical density at 450 nm
was recorded.
Cytokine detection by ELISA
In order to detect the cytokines produced following
immunization, the splenocytes of each immunized mice were isolated
and mixed. Next, CD4+ T cells or CD8+ T cells
were isolated using the corresponding
CD4+/CD8a+ T cell Isolation kit (Miltenyi
Biotec GmbH, Bergisch Gladbach, Germany). Cells were then cultured
in 96-well microplates at a density of 2×104 cells/well
(EMD Millipore, Billerica, MA, USA) with 100 µl RPMI 1640
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS and
stimulated with Trop-2 peptide (ProSci, Inc., Poway, CA, USA).
Subsequent to incubation at 37°C for 48 h, the plate was
centrifuged at 300 × g for 5 min, and the cell culture supernatant
was carefully collected and assayed using ELISA kits for the
production of interferon (IFN)-γ (cat. no. 88-8314-88), interleukin
(IL)-29 (cat. no. 88-7024-88), IL-10 (cat. no. BMS614-2) and IL-4
(cat. no. BMS613TWO) using cytokine ELISA kits (all Invitrogen;
Thermo Fisher Scientific, Inc.).
Statistical analysis
Results are expressed as the mean ± standard error
of the mean, and were analyzed by one-way analysis of variance.
P-values of <0.05 were considered to indicate differences that
were statistically significant.
Results
Trop-2 and CD40L are effectively
incorporated onto VLPs
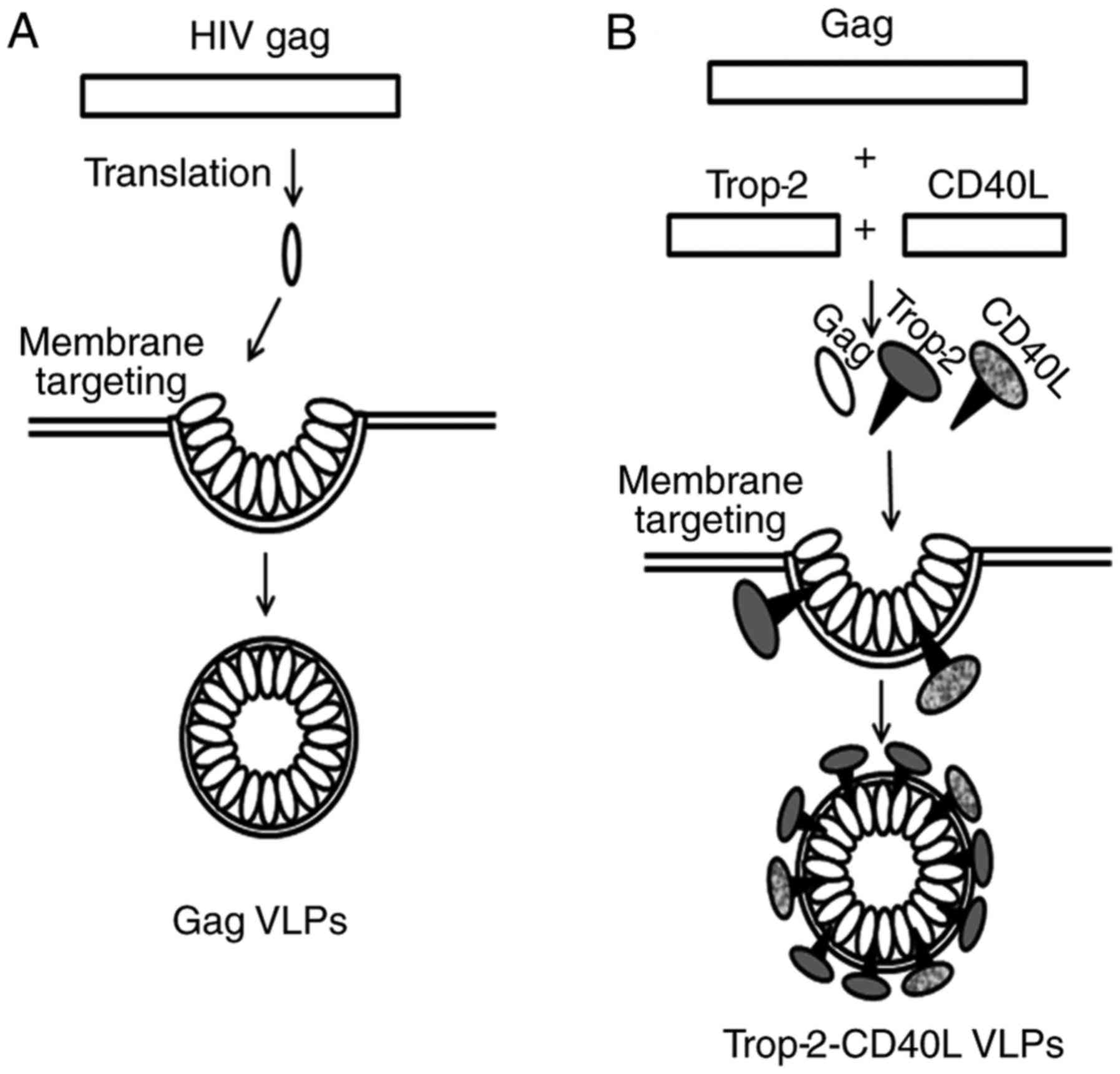
HIV gag can be modified to form type II VLPs with
heterologous antigens on the surface of their membrane envelope. A
model describing the formation of gag VLPs and chimeric
Trop-2-CD40L VLPs is shown in Fig.
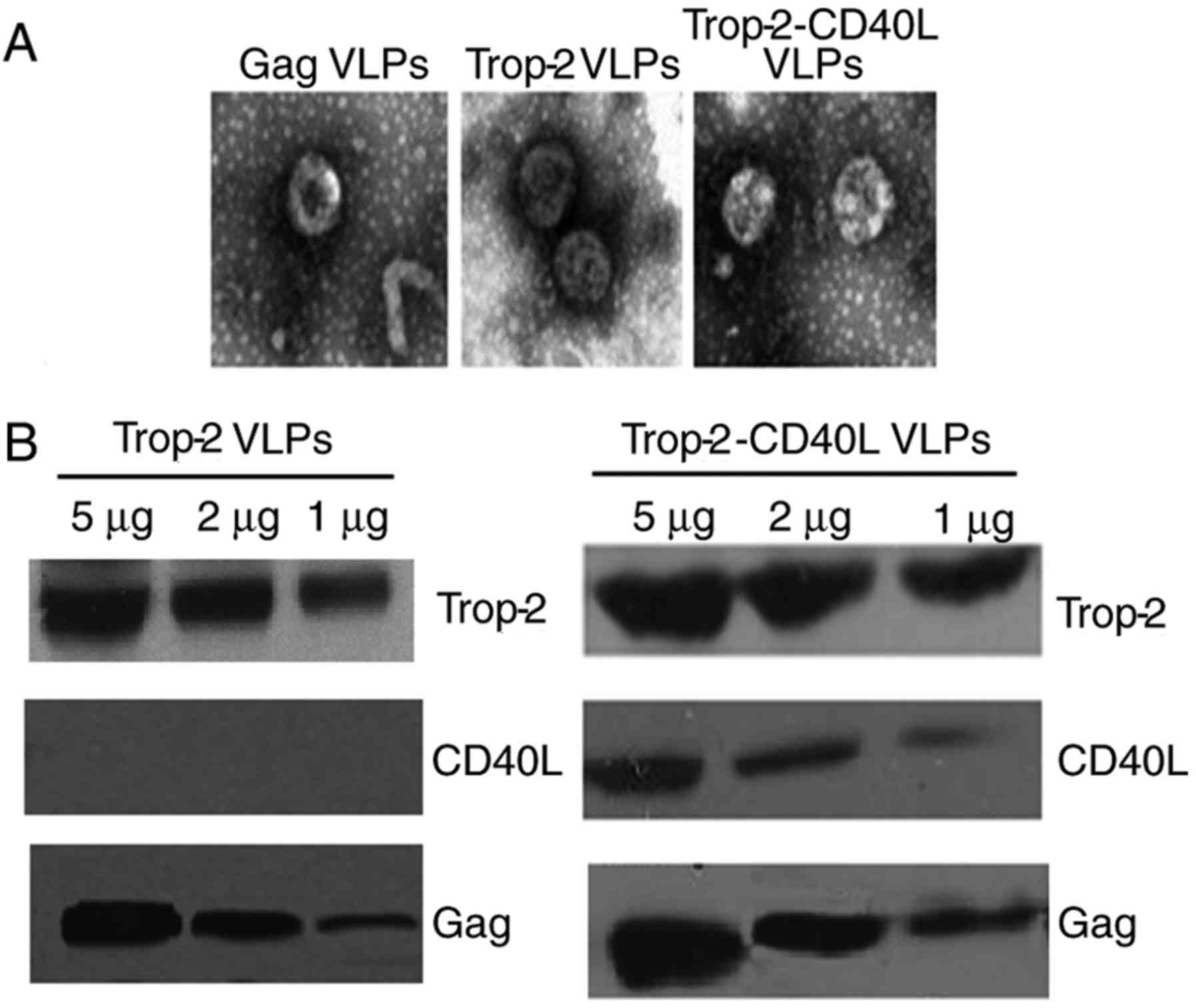
1. The morphology of VLPs was detected under an electron
microscope. As shown in Fig. 2A,
Trop-2 and Trop-2-CD40L VLPs exhibited a similar morphology
compared with gag VLPs, with a diameter of ~110 nm. VLPs were also
characterized by immunoblotting using monoclonal antibodies
specifically recognizing mTrop-2, CD40L or HIV gag p24. As shown in
Fig. 2B, Trop-2 protein was
detected in Trop-2 and Trop-2-CD40L VLPs; however, CD40L was only
expressed in Trop-2-CD40L VLPs. Therefore, these data strongly
suggested that rBv infection successfully released mTrop-2 VLPs and
mTrop-2-CD40L VLPs.
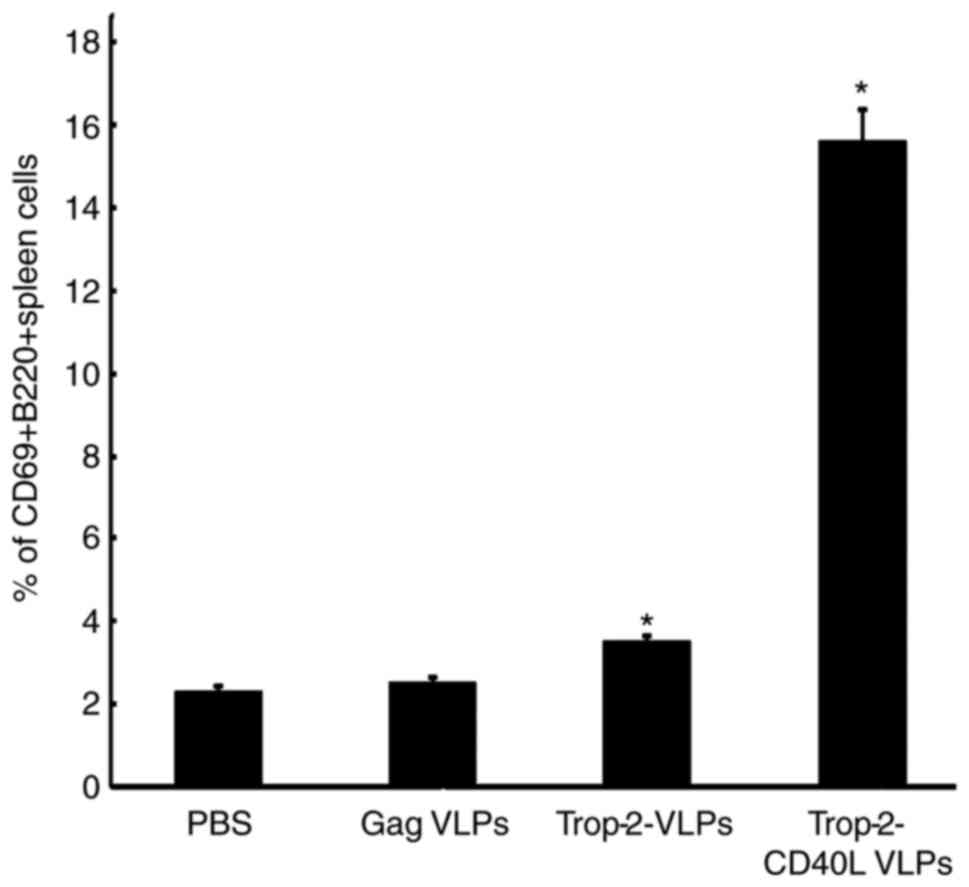
CD40L-incorporated VLPs activate
B-cells
As CD40L serves a critical role in the B-cell
activation process, splenocytes were cultured from naïve mice in
the presence of Trop-2-CD40L or Trop-2 VLPs. After 4 days, the
expression of the B-cell activation marker CD69 was assessed to
analyze the B-cell activation (P<0.01; Fig. 3). It was observed that when
splenocytes were cultured with chimeric Trop-2-CD40L VLPs,
increased numbers of activated CD69+B220+
cells were detected, whereas less CD69+B220+
B-cells were detected post Trop-2 VLPs stimulation. Overall,
incorporation of CD40L increased the number of
CD69+B220+ cells by nearly a 4-fold.
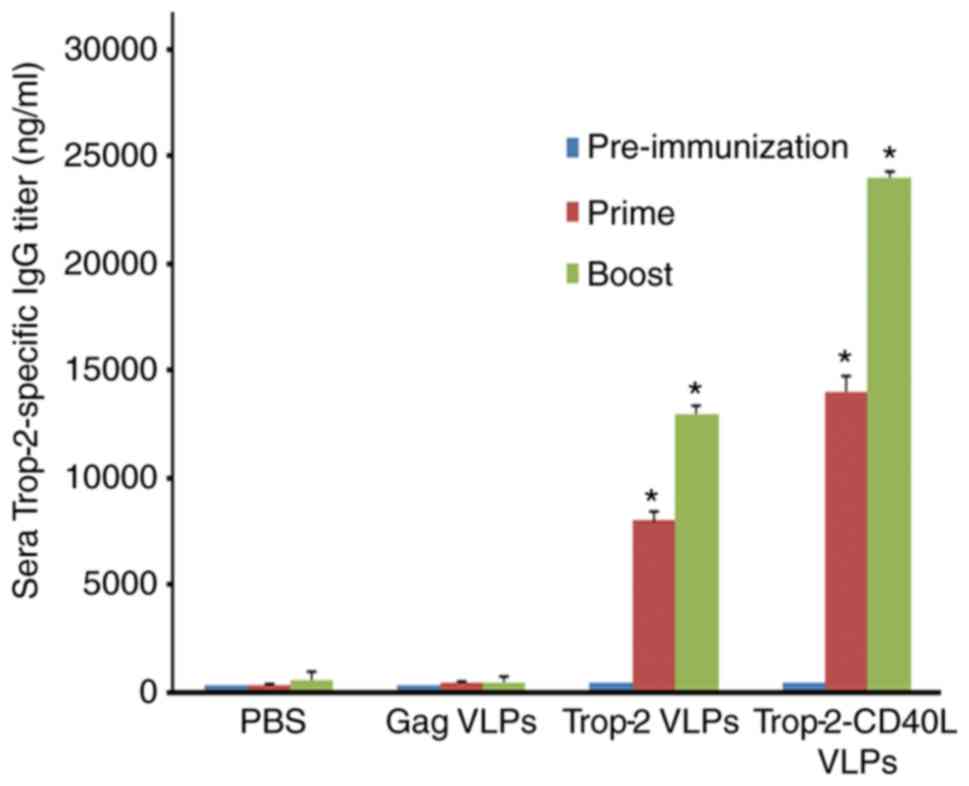
Trop-2-CD40L VLPs enhance anti-Trop-2 IgG
production in immunized mice
ELISA was performed to determine whether
Trop-2-CD40L VLP immunization was able to induce a higher level of
anti-Trop-2 antibody when compared with Trop-2 VLP immunization. As
shown in Fig. 4, Trop-2 specific
IgG was nearly undetectable in the serum of gag VLP-immunized mice.
By contrast, high levels of Trop-2-specific IgG were detected from
the serum of Trop-2-CD40L or Trop-2 VLP-immunized mice.
Furthermore, compared with Trop-2 VLPs, Trop-2-CD40L VLPs induced a
nearly 2-fold higher number of IgG titer (P<0.01). Therefore,
incorporation of CD40L onto VLPs was able to efficiently enhance
the immunogenicity of Trop-2 VLPs.
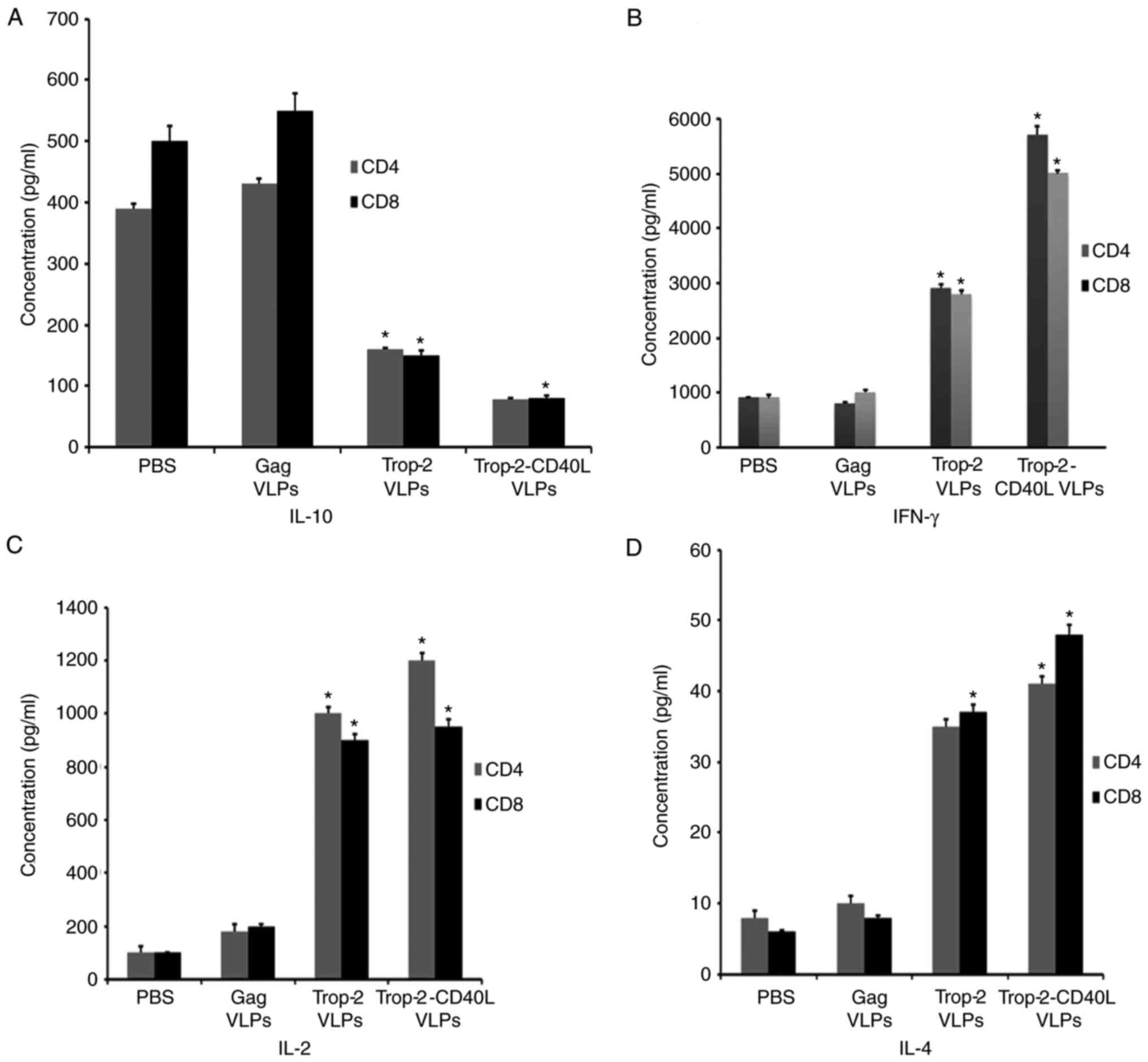
Trop-2-CD40L VLPs enhance cellular
responses more effectively
Cytokine ELISA was performed to detect the CD4 and
CD8 T cell immune responses. Spleens from immunized mice were
isolated, sorted and stimulated with Trop-2 peptide to measure the
production of IFN-γ, IL-4, IL-2 and IL-10 by Trop-2-specific CD4
and CD8 T cells (Fig. 5).
Splenocytes from Trop-2 or Trop-2-CD40L VLP-injected mice
demonstrated a 4- to 6-fold decrease of IL-10-producing CD4 and CD8
T cells as compared with the gag VLP-immunized mice. The lowest
IL-10 secretion level was observed in the Trop-2-CD40L
VLP-immunized group (Fig. 5A). In
the same experiment, the highest IFN-γ secretion level was observed
in the Trop-2-CD40L VLP-immunized group (Fig. 5B). By contrast, the Trop-2-CD40L
VLP immunization exhibited a reduced effect on IL-4 and IL-2
secretion compared with the Trop-2 VLP immunization (P=0.0008;
Fig. 5C and D). These results
demonstrated that incorporation of CD40L onto Trop-2 VLPs may
enhance the number of IFN-γ-producing T cells and reduce the
IL-10-producing T cells more potently in comparison with Trop-2
VLPs.
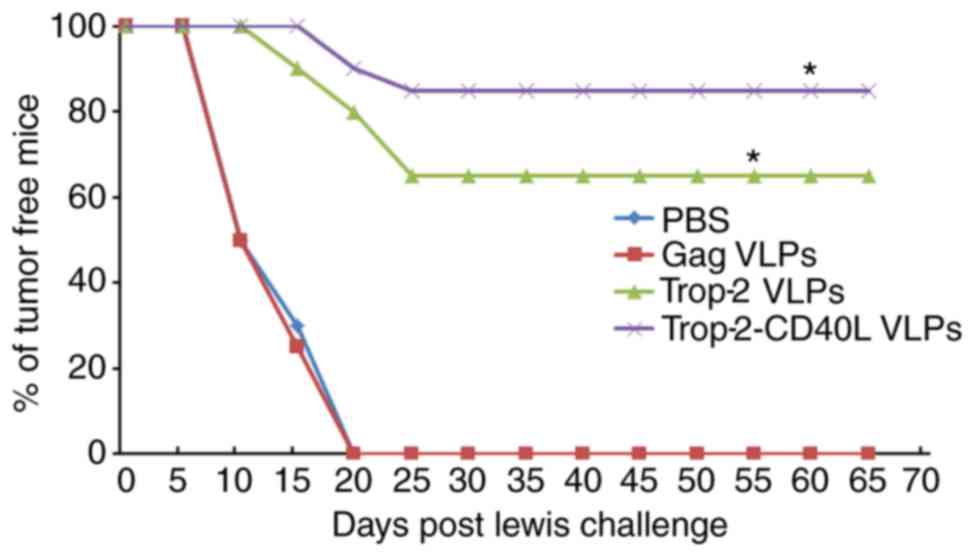
Preventive effect of Trop-2-CD40L VLP
immunization
In vivo tumor prevention tests were conducted
to determine whether immunization with Trop-2-CD40L or Trop-2 VLPs
induced preventive antitumor effects against lung cancer. Female
C57BL/6 mice were immunized twice with 40 µg gag,
Trop-2-CD40L or Trop-2 VLPs. Each mouse was then challenged with
1×106 Lewis cells and monitored for tumor formation.
Fig. 6A describes a brief
immunization schedule. As shown in Fig. 6B, at 65 days after the Lewis cell
challenge, nearly 85% of mice immunized with Trop-2-CD40L VLPs
remained tumor-free, while the percentage of tumor-free mice in the
Trop-2 VLP-immunized group was <70%. However, 100% of the mice
immunized with PBS or gag VLPs developed tumors (P<0.01 compared
with the PBS immunized group).
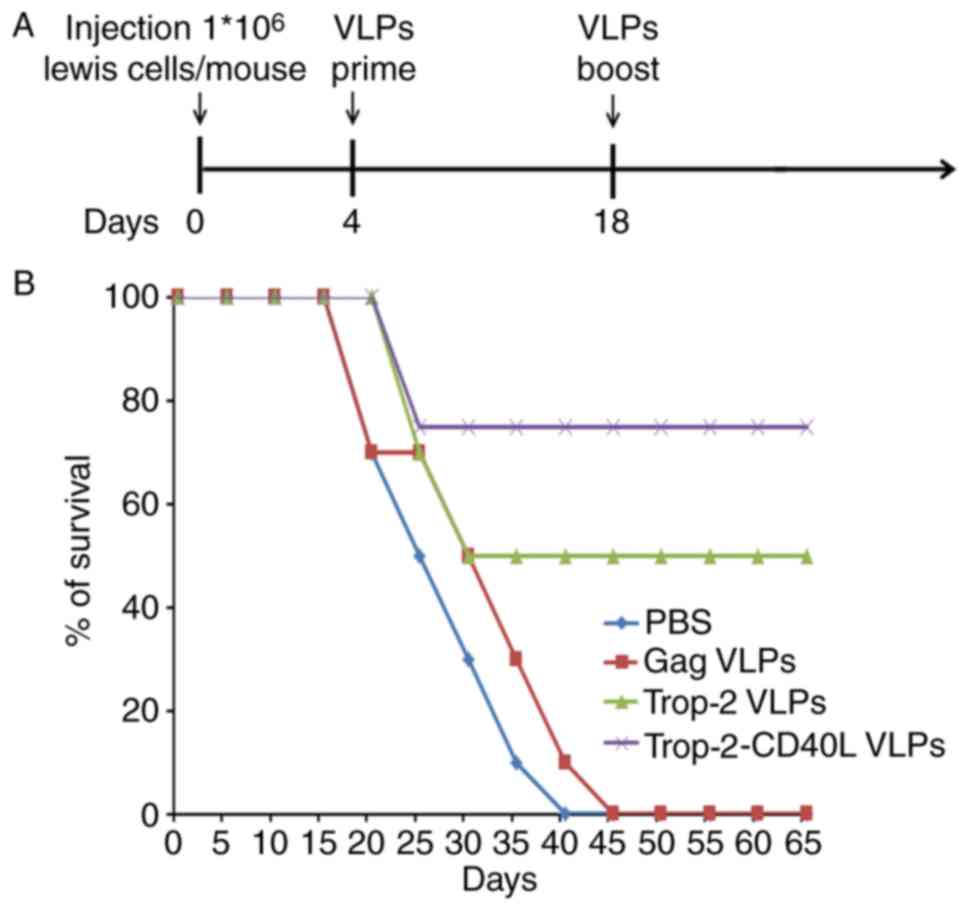
Trop-2-CD40L VLP immunization increases
the survival of tumor-bearing mice more effectively
The present study also investigated whether
Trop-2-CD40L or Trop-2 VLP immunization increased the survival of
tumor-bearing mice. In this experiment, mice were initially
challenged subcutaneously with Lewis (Trop-2) cells,
followed by VLP injection in each corresponding group. Fig. 7A describes a brief immunization
schedule. As shown in Fig. 7B,
all mice in the PBS or gag VLP-immunized groups succumbed within 40
days, whereas the Trop-2 and Trop-2-CD40L VLP-immunized mice
demonstrated significantly higher survival rates (P<0.001).
Furthermore, the highest survival rate reached 75% in the
Trop-2-CD40L VLP-immunized group.
Discussion
As the leading cause of cancer-associated mortality
in China (33), lung cancer has
been demonstrated to be immunogenic. Inhibitors of PD-L1 have been
approved for use in the treatment of squamous and non-squamous lung
cancer; however, growing populations of NSCLC patients do not
respond to PD-1/PD-L1 inhibition (34). Thus, novel immunotherapy targets
or immunomodulatory agents need to be explored. Trop-2 is a
recently identified cell surface glycoprotein, and has been
considered to be a promising target for cancer immunotherapy. A
study by Cubas et al (35)
on pancreatic cancer revealed that mTrop-2 was able to be
effectively incorporated on the membrane envelope of SIV gag-based
VLPs. The study also indicated that mTrop-2 VLP immunization
activated humoral immune responses, as well as cellular immune
response, while mTrop-2 VLPs demonstrated an improved effect when
combined with the chemotherapy agent gemcitabine (35). However, whether Trop-2 may be used
as an immunotherapy target against lung cancer has not yet been
examined.
As a well-known immune-adjuvant, CD40L is widely
used in DNA vaccine development (36,37). In the field of VLPs design, CD40L
was successfully incorporated into Hantaan VLPs (29) and SIV VLPs, and whether it is an
effective adjuvant in lung cancer VLPs based vaccine development is
yet to be determined.
In the present study, using a HIV gag backbone-based
VLP strategy, Trop-2 VLPs were constructed, while the immune
adjuvant CD40L was also incorporated onto the surface of the
mTrop-2 VLPs. The results of the present study revealed that a HIV
gag VLP-based Trop-2-CD40L vaccine was successfully prepared.
Trop-2 and Trop-2-CD40L VLPs were then used to immunize C57BL/6
mice, and the serum samples of the immunized mice were examined.
The Trop-2-specific IgG level was markedly increased at 2 weeks
post boost immunization as measured by ELISA in the Trop-2 and
Trop-2-CD40L VLP-immunized groups. In addition, the Trop-2-CD40L
VLPs immunization induced a higher IgG titer in comparison with the
Trop-2 VLPs immunization. Furthermore, the present study used a
cytokine ELISA method to detect the cellular immune responses. The
results revealed that Trop-2-CD40L VLPs immunization stimulated
Trop-2-specific cellular immune responses more effectively in
comparison with Trop-2 VLPs immunization, which was evidenced by
the elevated IFN-γ and reduced IL-10 secretion in Trop-2
peptide-stimulated splenocytes. This result was consistent with Lin
et al's (30) study that
the incorporation of CD40L into HTNV VLPs significantly enhanced
their immunogenicity by enhancing cellular immune responses. In the
prevention and therapeutic experiments conducted in the present
study, tumor-bearing mice were prepared by Lewis(Trop-2)
injection, and tumor growth was monitored. The tumor growth was
suppressed more efficiently in the Trop-2-CD40L VLP-immunized group
as compared with that in the Trop-2 VLP-immunized group. Therefore,
addition of the CD40L immune adjuvant onto Trop-2 VLPs provided a
more powerful capacity to induce antitumor immunity.
In conclusion, the results of the present study
demonstrate that immunization with either Trop-2 or Trop-2-CD40L
VLPs generated specific humoral and cellular immune responses
against Trop-2, whilst Trop-2-CD40L VLPs generated a higher immune
response. In the prevention and therapeutic experiments, the
Trop-2-CD40L VLPs exhibited a more efficient tumor inhibition
effect when compared with the Trop-2 only VLPs. The results of the
present study validated that Trop-2 has the potential to be used as
an immunotherapeutic target for lung cancer; the current result
also validated the adjuvant activity of CD40L when used in
VLP-based tumor vaccines. Trop-2-CD40L VLPs, due to their
immunogenic properties and effects on the immune system, therefore
represent a promising approach to lung cancer immunotherapy.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by grants from the China
Natural Sciences Foundation (grant nos. 81572974 and 81372836) and
the Shaanxi Science and Technology Innovation Project (grant no.
2016KTZDSF01-07).
[2] Availability
of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
ZHZ conceived and designed the study. DK and LM
performed the animal experiments. WL performed the cell culture
experiments; ZJH provided the Molecular Biology Experiments; LF
performed the VLPs production and identification part; GZW and ZZ
detected the antibody responses and cellular responses post
immunization; WX wrote the paper. CX reviewed and edited the
manuscript. All authors read and approved the manuscript.
[4] Ethics
approval and consent to participate
The present study was approved by the Institutional
Review Board of Tangdu Hospital of the Fourth Military Medical
University (assigned no. TDLL-201611-25).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests as defined by Molecular Medicine, or other interests that
might be perceived to influence the results and discussion reported
in this paper.
References
|
1
|
Fong D, Moser P, Krammel C, Gostner JM,
Margreiter R, Mitterer M, Gastl G and Spizzo G: High expression of
TROP2 correlates with poor prognosis in pancreatic cancer. Br J
Cancer. 99:1290–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simms A, Jacob RP, Cohen C and Siddiqui
MT: TROP-2 expression in papillary thyroid carcinoma: Potential
diagnostic utility. Diagn Cytopathol. 44:26–31. 2016. View Article : Google Scholar
|
|
3
|
Trerotola M, Ganguly KK, Fazli L, Fedele
C, Lu H, Dutta A, Liu Q, De Angelis T, Riddell LW, Riobo NA, et al:
Trop-2 is up-regulated in invasive prostate cancer and displaces
FAK from focal contacts. Oncotarget. 6:14318–14328. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Day R, Dong Y, Weintraub SJ and
Michel L: Identification of Trop-2 as an oncogene and an attractive
therapeutic target in colon cancers. Mol Cancer Ther. 7:280–285.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stepan LP, Trueblood ES, Hale K, Babcook
J, Borges L and Sutherland CL: Expression of Trop-2 cell surface
glycoprotein in normal and tumor tissues: Potential implications as
a cancer therapeutic target. J Histochem Cytochem. 59:701–710.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambrogi F, Fornili M, Boracchi P,
Trerotola M, Relli V, Simeone P, La Sorda R, Lattanzio R, Querzoli
P, Pedriali M, et al: Trop-2 is a determinant of breast cancer
survival. PLoS One. 9:e969932014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bignotti E, Zanotti L, Calza S, Falchetti
M, Lonardi S, Ravaggi A, Romani C, Todeschini P, Bandiera E, Tassi
RA, et al: Trop-2 protein overexpression is an independent marker
for predicting disease recurrence in endometrioid endometrial
carcinoma. BMC Clin Pathol. 12:222012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bignotti E, Todeschini P, Calza S,
Falchetti M, Ravanini M, Tassi RA, Ravaggi A, Bandiera E, Romani C,
Zanotti L, et al: Trop-2 overexpression as an independent marker
for poor overall survival in ovarian carcinoma patients. Eur J
Cancer. 46:944–953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang A, Gao X, Zhang D, Zhang L and Lu H:
Expression and clinical significance of the Trop-2 gene in advanced
non-small cell lung carcinoma. Oncol Lett. 6:375–380. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pushko P, Tretyakova I, Hidajat R, Zsak A,
Chrzastek K, Tumpey TM and Kapczynski DR: Virus-like particles
displaying H5, H7, H9 hemagglutinins and N1 neuraminidase elicit
protective immunity to heterologous avian influenza viruses in
chickens. Virology. 501:176–182. 2017. View Article : Google Scholar :
|
|
11
|
Hill BD, Zak A, Khera E and Wen F:
Engineering virus-like particles for antigen and drug delivery.
Curr Protein Pept Sci. 19:112–127. 2018.
|
|
12
|
Zdanowicz M and Chroboczek J: Virus-like
particles as drug delivery vectors. Acta Biochim Pol. 63:469–473.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu X, Fang Y, Zhou P, Lu Y, Zhang Q, Xiao
S, Dong Z, Pan L, Lv J, Zhang Z, et al: Chimeric virus-like
particles elicit protective immunity against serotype O
foot-and-mouth disease virus in guinea pigs. Appl Microbiol
Biotechnol. 101:4905–4914. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong H and Seong BL: Exploiting
virus-like particles as innovative vaccines against emerging viral
infections. J Microbiol. 55:220–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohan T, Berman Z, Luo Y, Wang C, Wang S,
Compans RW and Wang BZ: Chimeric virus-like particles containing
influenza HA antigen and GPI-CCL28 induce long-lasting mucosal
immunity against H3N2 viruses. Sci Rep. 7:402262017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robert MA, Lytvyn V, Deforet F, Gilbert R
and Gaillet B: Virus-like particles derived from HIV-1 for delivery
of duclear proteins: Improvement of production and activity by
protein engineering. Mol Biotechnol. 59:9–23. 2017. View Article : Google Scholar
|
|
17
|
Feng Q, He Y and Lu J: Virus-like
larticles lroduced in lichia lastoris induce protective immune
responses against Coxsackievirus A16 in mice. Med Sci Monit.
22:3370–3382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan Q, Wu L, Chen L, Qin Y, Pan Z and Chen
M: Vesicular stomatitis virus-based vaccines expressing EV71
virus-like particles elicit strong immune responses and protect
newborn mice from lethal challenges. Vaccine. 34:4196–4204. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Xiao X, Zhao M, Liu W, Pang L, Sun
X, Cen S, Yang BB, Huang Y, Sheng W and Zeng Y: EV71 virus-like
particles produced by co-expression of capsid proteins in yeasT
cells elicit humoral protective response against EV71 lethal
challenge. BMC Res Notes. 9:422016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alshaikhahmed K and Roy P: Generation of
virus-like particles for emerging epizootic haemorrhagic disease
virus: Towards the development of safe vaccine candidates. Vaccine.
34:1103–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ludwig C and Wagner R: Virus-like
particles-universal molecular toolboxes. Curr Opin Biotechnol.
18:537–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deml L, Speth C, Dierich MP, Wolf H and
Wagner R: Recombinant HIV-1 Pr55gag virus-like particles: Potent
stimulators of innate and acquired immune responses. Mol Immunol.
42:259–277. 2005. View Article : Google Scholar
|
|
23
|
Figgett WA, Vincent FB, Saulep-Easton D
and Mackay F: Roles of ligands from the TNF superfamily in B cell
development, function, and regulation. Semin Immunol. 26:191–202.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bazzoni F and Beutler B: The tumor
necrosis factor ligand and receptor families. N Engl J Med.
334:1717–1725. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta S, Termini JM, Kanagavelu S and
Stone GW: Design of vaccine adjuvants incorporating TNF superfamily
ligands and TNF superfamily molecular mimics. Immunol Res.
57:303–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song I, Kim J, Kwon K, Koo S and Jo D:
Expression of CD154 (CD40L) on stimulated T lymphocytes in patients
with idopathic thrombocytopenic purpura. Hematology. 21:187–192.
2016. View Article : Google Scholar
|
|
27
|
Quezada SA, Jarvinen LZ, Lind EF and
Noelle RJ: CD40/CD154 interactions at the interface of tolerance
and immunity. Annu Rev Immunol. 22:307–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tripp RA, Jones L, Anderson LJ and Brown
MP: CD40 ligand (CD154) enhances the Th1 and antibody responses to
respiratory syncytial virus in the C57BL/6 mouse. J Immunol.
164:5913–5921. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng LF, Wang F, Zhang L, Yu L, Ye W, Liu
ZY, Ying QK, Wu XA, Xu ZK and Zhang FL: Incorporation of GM-CSF or
CD40L enhances the immunogenicity of Hantaan virus-like particles.
FronT cell Infect Microbiol. 6:1852016. View Article : Google Scholar
|
|
30
|
Stone GW, Barzee S, Snarsky V, Kee K,
Spina CA, Yu XF and Kornbluth RS: Multimeric soluble CD40 ligand
and GITR ligand as adjuvants for human immunodeficiency virus DNA
vaccines. J Virol. 80:1762–1772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Skountzou I, Quan FS, Gangadhara S, Ye L,
Vzorov A, Selvaraj P, Jacob J, Compans RW and Kang SM:
Incorporation of glycosylphosphatidylinositol-anchored
granulocyte-macrophage colony-stimulating factor or CD40 ligand
enhances immunogenicity of chimeric simian immunodeficiency
virus-like particles. J Virol. 81:1083–1094. 2007. View Article : Google Scholar
|
|
32
|
Daniel RA and Errington J: Control of cell
morphogenesis in bacteria: two distinct ways to make a rod-shaped
cell. Cell. 113:767–776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen W, Zheng R, Zeng H and Zhang S:
Epidemiology of lung cancer in China. Thorac Cancer. 6:209–215.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Somasundaram A and Burns TF: The next
generation of immunotherapy: Keeping lung cancer in check. J
Hematol Oncol. 10:872017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cubas R, Zhang S, Li M, Chen C and Yao Q:
Chimeric Trop-2 virus-like particles: A potential immunotherapeutic
approach against pancreatic cancer. J Immunother. 34:251–263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Wang YM, Wang Y, Zheng G, Zhang
GY, Zhou JJ, Tan TK, Cao Q, Hu M, Watson D, et al: DNA vaccine
encoding CD40 targeted to dendritic cells in situ prevents the
development of Heymann nephritis in rats. Kidney Int. 83:223–232.
2013. View Article : Google Scholar
|
|
37
|
Kwa S, Sadagopal S, Shen X, Hong JJ,
Gangadhara S, Basu R, Victor B, Iyer SS, LaBranche CC, Montefiori
DC, et al: CD40L-adjuvanted DNA/modified vaccinia virus Ankara
simian immunodeficiency virus (SIV) vaccine enhances protection
against neutralization-resistant mucosal SIV infection. J Virol.
89:4690–4695. 2015. View Article : Google Scholar : PubMed/NCBI
|