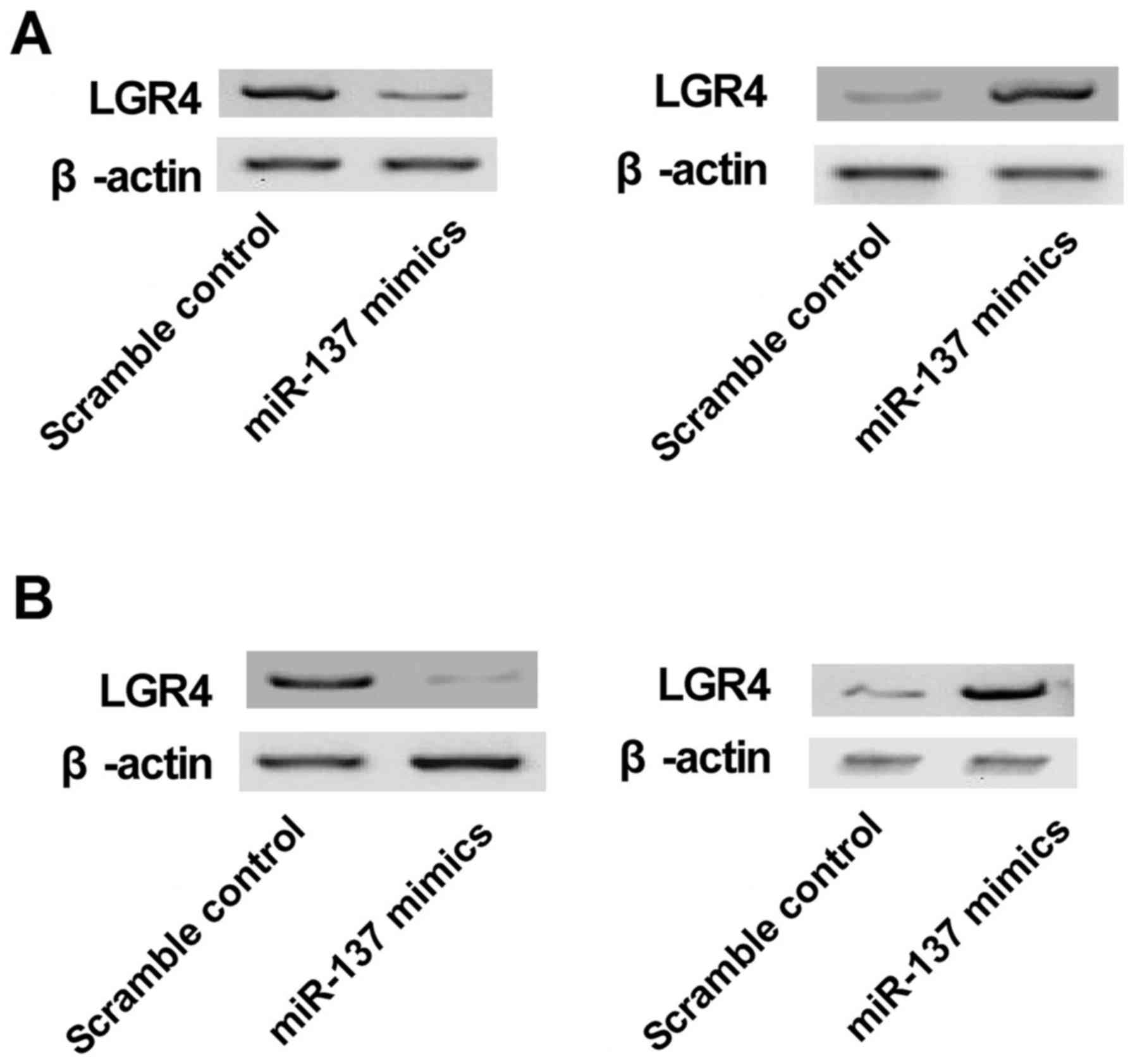
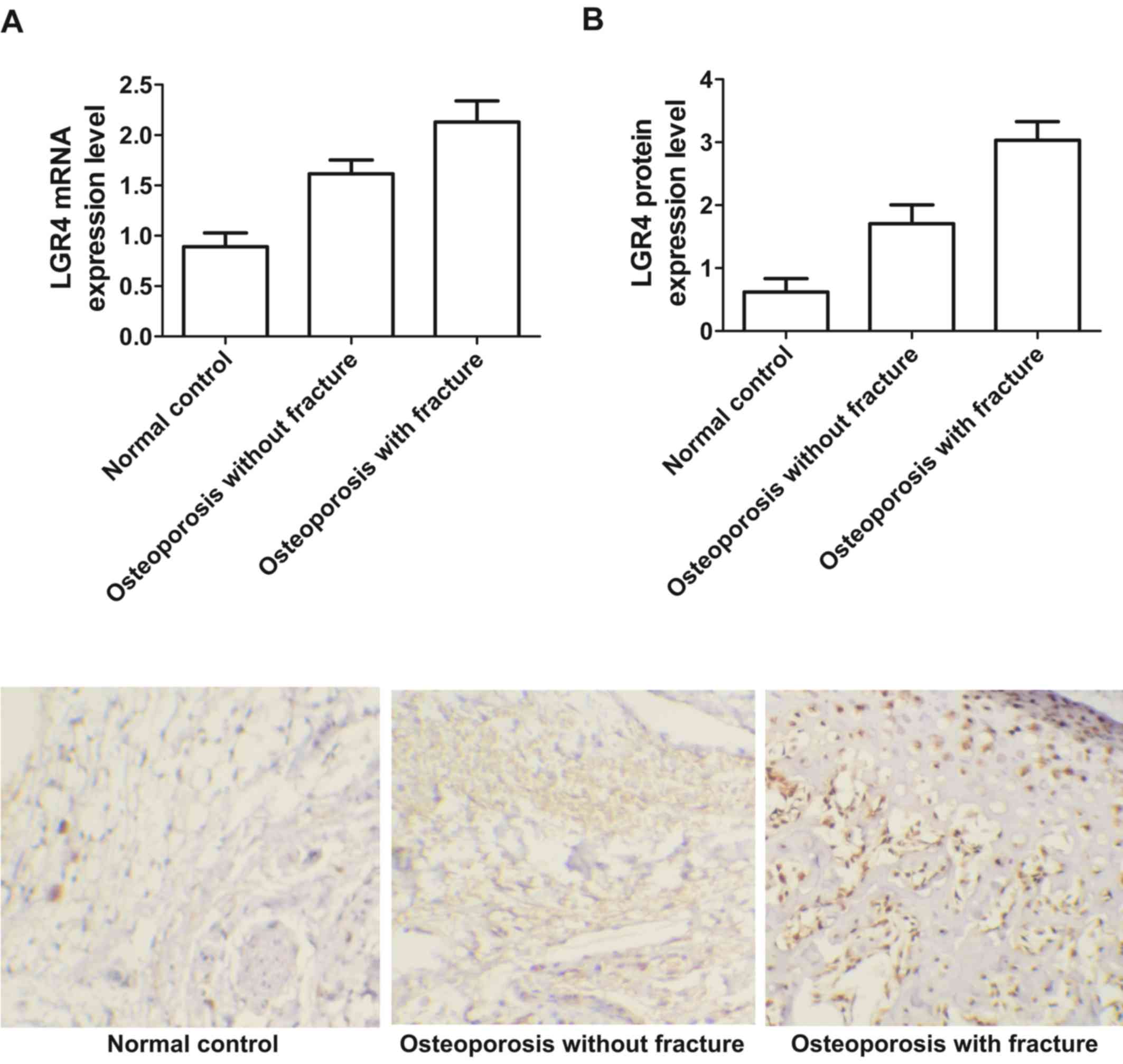
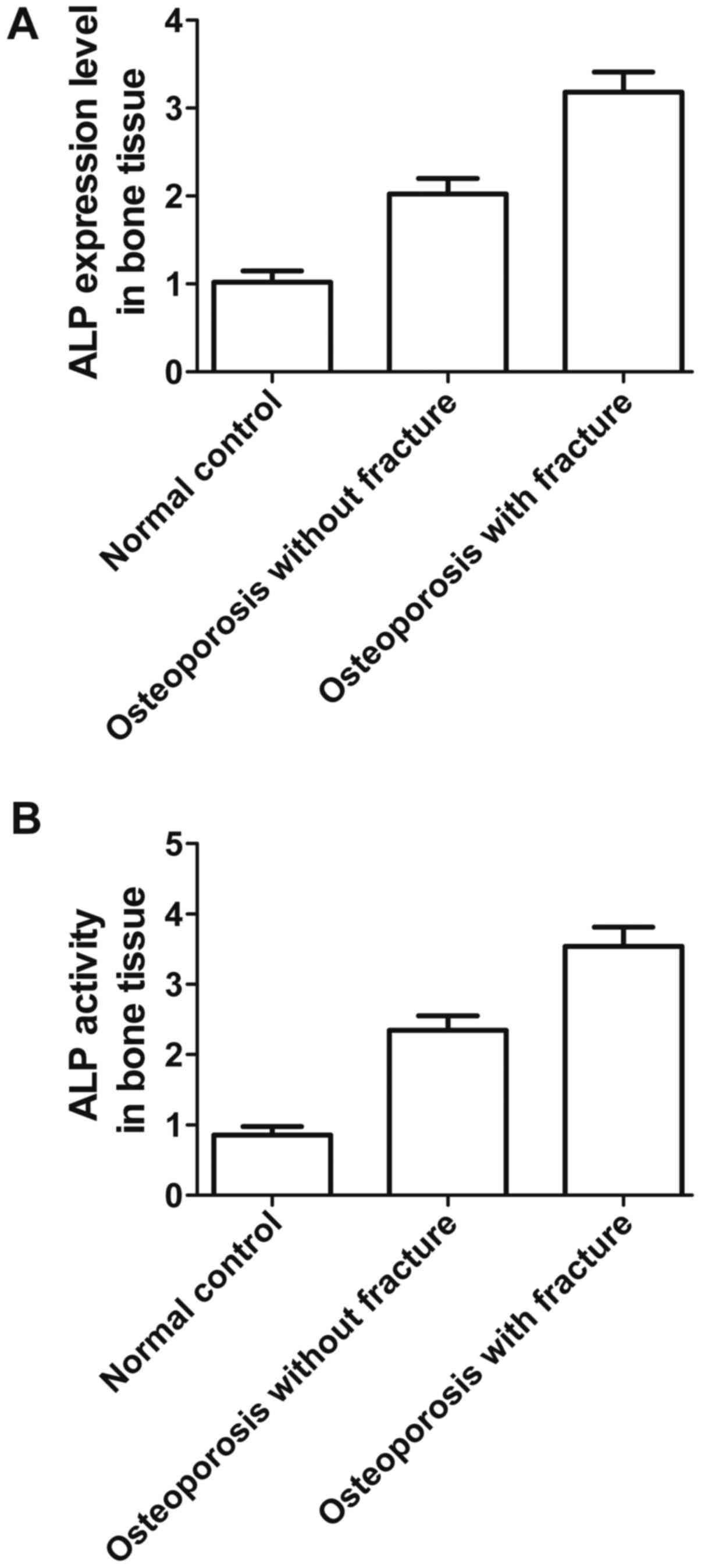
|
1
|
Bruyère O, Cooper C, Pelletier JP, Maheu
E, Rannou F, Branco J, Luisa Brandi M, Kanis JA, Altman RD,
Hochberg MC, et al: A consensus statement on the European Society
for Clinical and Economic Aspects of Osteoporosis and
Osteoarthritis (ESCEO) algorithm for the management of knee
osteoarthritis - From evidence-based medicine to the real-life
setting. Semin Arthritis Rheum. 45(Suppl 4): S3–S11. 2016.
View Article : Google Scholar
|
|
2
|
Melton LJ III, Gabriel SE, Crowson CS,
Tosteson AN, Johnell O and Kanis JA: Cost-equivalence of different
osteoporotic fractures. Osteoporos Int. 14:383–388. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adachi JD, Ioannidis G, Pickard L, Berger
C, Prior JC, Joseph L, Hanley DA, Olszynski WP, Murray TM,
Anastassiades T, et al: The association between osteoporotic
fractures and health-related quality of life as measured by the
Health Utilities Index in the Canadian Multicentre Osteoporosis
Study (CaMos). Osteoporos Int. 14:895–904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papaioannou A, Morin S, Cheung AM,
Atkinson S, Brown JP, Feldman S, Hanley DA, Hodsman A, Jamal SA,
Kaiser SM, et al Scientific Advisory Council of Osteoporosis
Canada: 2010 clinical practice guidelines for the diagnosis and
management of osteoporosis in Canada: summary. CMAJ. 182:1864–1873.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orimo H, Nakamura T, Hosoi T, Iki M,
Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, et al:
Japanese 2011 guidelines for prevention and treatment of
osteoporosis - executive summary. Arch Osteoporos. 7:3–20. 2012.
View Article : Google Scholar :
|
|
6
|
de Almeida Pereira Coutinho M, Bandeira E,
de Almeida JM, Godoi ET, Vasconcelos G and Bandeira F: Low bone
mass is associated with increased carotid intima media thickness in
men with type 2 diabetes mellitus. Clin Med Insights Endocrinol
Diabetes. 6:1–6. 2013.PubMed/NCBI
|
|
7
|
Ralston SH: Genetics of osteoporosis. Rev
Endocr Metab Disord. 2:13–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng HW, Mahaney MC, Williams JT, Li J,
Conway T, Davies KM, Li JL, Deng H and Recker RR: Relevance of the
genes for bone mass variation to susceptibility to osteoporotic
fractures and its implications to gene search for complex human
diseases. Genet Epidemiol. 22:12–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kozomara A and Griffiths-Jones S: miRBase:
annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42(D1): D68–D73. 2014. View Article : Google Scholar :
|
|
12
|
Pauley KM and Cha S: miRNA-146a in
rheumatoid arthritis: a new therapeutic strategy. Immunotherapy.
3:829–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng P, Chen C, He HB, Hu R, Zhou HD, Xie
H, Zhu W, Dai RC, Wu XP, Liao EY, et al: miR-148a regulates
osteoclastogenesis by targeting V-maf musculoaponeurotic
fibrosarcoma oncogene homolog B. J Bone Miner Res. 28:1180–1190.
2013. View Article : Google Scholar
|
|
14
|
Wang Y, Li L, Moore BT, Peng XH, Fang X,
Lappe JM, Recker RR and Xiao P: miR-133a in human circulating
monocytes: a potential biomarker associated with postmenopausal
osteoporosis. PLoS One. 7:e346412012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seeliger C, Karpinski K, Haug AT, Vester
H, Schmitt A, Bauer JS and van Griensven M: Five freely circulating
miRNAs and bone tissue miRNAs are associated with osteoporotic
fractures. J Bone Miner Res. 29:1718–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garmilla-Ezquerra P, Sañudo C,
Delgado-Calle J, Pérez- Nuñez MI, Sumillera M and Riancho JA:
Analysis of the bone microRNome in osteoporotic fractures. Calcif
Tissue Int. 96:30–37. 2015. View Article : Google Scholar
|
|
17
|
Pawaputanon Na Mahasarakham C, Ezura Y,
Kawasaki M, Smriti A, Moriya S, Yamada T, Izu Y, Nifuji A,
Nishimori K, Izumi Y, et al: BMP-2 enhances Lgr4 gene expression in
osteoblastic cells. J Cell Physiol. 231:887–895. 2016. View Article : Google Scholar
|
|
18
|
Styrkarsdottir U, Thorleifsson G, Sulem P,
Gudbjartsson DF, Sigurdsson A, Jonasdottir A, Jonasdottir A,
Oddsson A, Helgason A, Magnusson OT, et al: Nonsense mutation in
the LGR4 gene is associated with several human diseases and other
traits. Nature. 497:517–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao J, Peng F, Yu C, Wang M, Li X, Li Z,
Jiang J and Sun C: microRNA-137 modulates pancreatic cancer cells
tumor growth, invasion and sensitivity to chemotherapy. Int J Clin
Exp Pathol. 7:7442–7450. 2014.
|
|
20
|
Hao S, Luo C, Abukiwan A, Wang G, He J,
Huang L, Weber CE, Lv N, Xiao X, Eichmüller SB, et al: miR-137
inhibits proliferation of melanoma cells by targeting PAK2. Exp
Dermatol. 24:947–952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith AR, Marquez RT, Tsao WC, Pathak S,
Roy A, Ping J, Wilkerson B, Lan L, Meng W, Neufeld KL, et al: Tumor
suppressive microRNA-137 negatively regulates Musashi-1 and
colorectal cancer progression. Oncotarget. 6:12558–12573. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Liu T, Wu T, Wang Z, Rao Z and
Gao J: MicroRNA-137 functions as a tumor suppressor in human
non-small cell lung cancer by targeting SLC22A18. Int J Biol
Macromol. 74:111–118. 2015. View Article : Google Scholar
|
|
23
|
Cheng H, Jiang W, Phillips FM, Haydon RC,
Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, et al:
Osteogenic activity of the fourteen types of human bone
morphogenetic proteins (BMPs). J Bone Joint Surg Am. 85:1544–1552.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kozaki K, Imoto I, Mogi S, Omura K and
Inazawa J: Exploration of tumor-suppressive microRNAs silenced by
DNA hypermethylation in oral cancer. Cancer Res. 68:2094–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tarantino C, Paolella G, Cozzuto L,
Minopoli G, Pastore L, Parisi S and Russo T: miRNA 34a, 100, and
137 modulate differentiation of mouse embryonic stem cells. FASEB
J. 24:3255–3263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Silber J, Lim DA, Petritsch C, Persson AI,
Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello
JF, et al: miR-124 and miR-137 inhibit proliferation of
glioblastoma multiforme cells and induce differentiation of brain
tumor stem cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langevin SM, Stone RA, Bunker CH,
Lyons-Weiler MA, LaFramboise WA, Kelly L, Seethala RR, Grandis JR,
Sobol RW and Taioli E: MicroRNA-137 promoter methylation is
associated with poorer overall survival in patients with squamous
cell carcinoma of the head and neck. Cancer. 117:1454–1462. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhen G and Cao X: Targeting TGFβ signaling
in subchondral bone and articular cartilage homeostasis. Trends
Pharmacol Sci. 35:227–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Itkin T, Kaufmann KB, Gur-Cohen S, Ludin A
and Lapidot T: Fibroblast growth factor signaling promotes
physiological bone remodeling and stem cell self-renewal. Curr Opin
Hematol. 20:237–244. 2013.PubMed/NCBI
|
|
30
|
Du B, Luo W, Li R, Tan B, Han H, Lu X, Li
D, Qian M, Zhang D, Zhao Y, et al: Lgr4/Gpr48 negatively regulates
TLR2/4-associated pattern recognition and innate immunity by
targeting CD14 expression. J Biol Chem. 288:15131–15141. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mendive F, Laurent P, Van Schoore G,
Skarnes W, Pochet R and Vassart G: Defective postnatal development
of the male reproductive tract in LGR4 knockout mice. Dev Biol.
290:421–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kato S, Matsubara M, Matsuo T, Mohri Y,
Kazama I, Hatano R, Umezawa A and Nishimori K: Leucine-rich
repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is
essential for renal development in mice. Nephron Exp Nephrol.
104:e63–e75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kato S, Mohri Y, Matsuo T, Ogawa E,
Umezawa A, Okuyama R and Nishimori K: Eye-open at birth phenotype
with reduced keratinocyte motility in LGR4 null mice. FEBS Lett.
581:4685–4690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song H, Luo J, Luo W, Weng J, Wang Z, Li
B, Li D and Liu M: Inactivation of G-protein-coupled receptor 48
(Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation
through down-regulation of the ATF4 signaling pathway. J Biol Chem.
283:36687–36697. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mohri Y, Kato S, Umezawa A, Okuyama R and
Nishimori K: Impaired hair placode formation with reduced
expression of hair follicle-related genes in mice lacking Lgr4. Dev
Dyn. 237:2235–2242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamashita R, Takegawa Y, Sakumoto M,
Nakahara M, Kawazu H, Hoshii T, Araki K, Yokouchi Y and Yamamura K:
Defective development of the gall bladder and cystic duct in Lgr4-
hypomorphic mice. Dev Dyn. 238:993–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo J, Zhou W, Zhou X, Li D, Weng J, Yi Z,
Cho SG, Li C, Yi T, Wu X, et al: Regulation of bone formation and
remo-deling by G-protein-coupled receptor 48. Development.
136:2747–2756. 2009. View Article : Google Scholar : PubMed/NCBI
|