Introduction
Ischemic stroke is a common cause of mortality
world-wide (1,2). The main pathogenetic mechanisms of
cerebral ischemia include intracellular calcium overload (3,4),
excitatory amino acid toxicity, increase in oxygen free radicals
(4), inflammatory responses
(4,5), autophagy and apoptosis (6). A complex interplay also exists among
these factors, for example, autophagy and apoptosis. Although a
suitable level of autophagy may provide neuroprotection, the
excessive activation of autophagy can induce cell death. Apoptosis
is essential in the pathogenesis and prognosis of different
cerebrovascular diseases, including chronic and acute ischemia. As
a result of hypoxia and lack of glucose, when cells suffer from
ischemic stimulation due to an increasing number of reactive oxygen
species (ROS) in the cytoplasm (7), including ·O2−,
H2O2 and ·OH, in addition to anti-apoptotic
protein B-cell lymphoma-2 (Bcl-2) and pro-apoptotic protein
Bcl-2-associated X protein (Bax) (8,9),
the mitochondrial membrane permeability transition pore is opened.
This leads to increased mitochondrial membrane permeability and
decreased mitochondrial membrane potential. Subsequently,
cytochrome c (cyt-c) is translocated from the mitochondrial
matrix to the cytoplasm, forming an apoptotic complex by combining
apoptotic protease activating factor 1 (apaf-1) and caspase-9.
Finally, the caspase-dependent apoptotic pathway is activated.
Furthermore, other transcription factors, including, p53, tumor
necrosis factor-α and nuclear factor-κB, may be involved in the
apoptotic process (10,11). Therefore, inhibiting autophagy and
apoptosis is a possible target for stroke treatment.
The hippocampus is a region of the brain sensitive
to changes in oxygen-glucose levels (12). Rat hippocampal slices are used in
in vitro experiments, as a mutual connection between neurons
and glial cells is maintained (13,14). Hippocampal slices have been used
to investigate the effects of anoxia, excitotoxicity and
depolarization (15-17). The excitability of CA1 neurons
increases when cerebral ischemia occurs. If oxygen-glucose supply
is not restored, or therapeutic windows exceed optimal levels,
neurons become excited until these cells die (18).
Dehydrocostuslactone (DHL) is a sesquiterpene
lactone derived from the medicinal plant Aucklandia lappa
Dence, belonging to the family Asteraceae (19) and is a major active ingredient of
Aucklandiae Radix. The metabolite of DHL can penetrate the
blood-brain barrier and exhibit various pharmacological activities,
including anti-inflammatory (20), anti-ulcer (19) and antimicrobial properties
(21), anti-apoptotic and
pro-apoptotic effects on different human cancer cell lines
(22,23), and oxidative and endoplasmic
reticulum stress responses (24).
In the present study, rat hippocampal slices were
subjected to oxygen-glucose deprivation and reoxygenation (OGD/R)
and used to investigate whether DHL reduces neuronal apoptosis and
autophagy in vitro.
Materials and methods
Animals
Male Sprague-Dawley rats (weighing 250-290 g, aged
8-10 weeks old) were purchased from the Experimental Animal Center
of Ningxia Medical University [Yinchuan, China; animal license no.
SCXK (Ning) 2011-0001]. All rats were maintained in a
well-ventilated environment with a 12 h/12 h light/dark cycle at
constant room temperature (25±2°C) and humidity (50–70%). The rats
were provided with free access to normal rodent food and water.
Reagents
DHL (cat. no. 34080) was purchased from Chengdu Pusi
Biological Technology Co., Ltd. (Chengdu, China). A stock solution
was prepared using dimethyl sulfoxide (DMSO) solution and diluted
with normal artificial cerebrospinal fluid (aCSF) containing 124 mM
NaCl, 3.00 mM KCl, 2.50 mM MgSO4·7H2O, 2.00
mM CaCl2, 26.00 NaHCO3, 1.25 mM
NaH2PO4 and 10.00 mM glucose, and
glucose-free aCSF (equal mole sucrose instead of glucose) to obtain
the required concentration. Low, middle and high DHL concentrations
were set at 1, 5 and 10 μM, respectively. Nimodipine
injection (cat. no. H20140301) was provided by Bayer Schering
Pharma AG (Shanghai, China). The nimodipine concentration was set
at 10 μM. 2,3,5-triphenyltetrazolium chloride (TTC; cat. no.
T8877; Sigma-Aldrich; EMD Millipore, Billerica, MA, USA) was
dissolved in aCSF and stored in the dark. The following substances
and kits were utilized in the present study: Lactate dehydrogenase
(LDH) assay kit (cat no. A020-2; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China); total protein extraction and BCA
protein quantification kits (Nanjing KeyGen Biotech, Co., Ltd.,
Nanjing, China); primary antibodies against Bcl-2 (cat. no. 2876),
Bax (cat. no. 2772), cyt-c (cat. no. 4272), apaf-1 (cat. no. 8723)
and β-actin (cat. no. 4970) were obtained from Cell Signaling
Technology, Inc., (Danvers, MA, USA); primary antibodies against
caspase-9 (cat. no. ab2013), caspase-7 (cat. no. ab25900) and
caspase-3 (cat. no. ab44976) were procured from Abcam (Cambridge,
UK); primary antibodies against sequestosome 1 (SQSTM1; cat. no.
bs-2951R) and microtubule-associated protein 1 light chain 3 (LC3;
cat. no. bs-8878R) were procured from BIOSS (Beijing, China);
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (cat. no. ZB-2301) was obtained from ZSGB-BIO (Beijing,
China); Z-VAD-FMK, a novel broad-spectrum caspase protease
inhibitor (cat. no. C1202) was obtained from Beyotime Institute of
Biotechnology (Nantong, China).
Rat hippocampal slice preparation
The rats were anesthetized with 7% chloral hydrate
(350 mg/kg, i.p.) and sacrificed by decapitation. Their brains were
rapidly excised and placed in an ice-cold bath filled with a 95%
O2/5% CO2 gas mixture of aCSF for 1 min. The
bilateral hippocampus was also rapidly excised. The hippocampal
slices were isolated, coronally trimmed, glued to the stage of a
vibration slicing machine (LEICA VT 1000S; Leica Microsystems,
Inc., Buffalo Grove, IL, USA), and coronally cut to a thickness of
400-μm at 0°C. The hippocampal slices were divided into
eight groups: Control, OGD, OGD+nimodipine (10 μM, dissolved
in aCSF and glucose-free aCSF), OGD+DHL (1, 5 and 10 μM
dissolved in DMSO, aCSF and glucose-free aCSF, respectively),
OGD+Z-VAD-FMK (20 μM), and OGD+Z-VAD-FMK (20 μM)+DHL
(10 μM). Each group contained 40 slices. All hippocampal
slices were immediately pipetted lightly into an incubator and then
recovered for 1 h in oxygenated aCSF at 37°C.
OGD/R injury model
Following the recovery period, the slices were
lightly washed with aCSF and transferred into a 24-well culture
plate with four slices per well. The OGD of the experimental groups
was induced by glucose-free aCSF. This dissection buffer was
bubbled with a 95% N2/5% CO2 gas mixture in a
24-well culture plate for 30 min. The control group was incubated
in aCSF equilibrated with 95% O2/5% CO2 for
30 min (OGD period). Subsequently, the slices were transferred into
a new 24-well culture plate containing oxygenated normal aCSF and
placed in a 95% O2/5% CO2 gas mixture
incubator. Finally, the slices were incubated for 1 h at 37°C. Five
treatment groups received nimodipine (10 μM), Z-VAD-FMK (20
μM), DHL (1, 5 and 10 μM), and Z-VAD-FMK (20
μM)+DHL (10 μM) within the OGD/R period. The drugs
were dissolved in aCSF and glucose-free aCSF.
LDH activity measurement
LDH is a cytosolic enzyme that can be released into
extracellular fluid when cell membranes are disrupted. Therefore,
this enzyme is used to evaluate the degree of injury. In the
present study, the viability of the rat hippocampal slices was
determined following the completion of reoxygenation by measuring
the concentration of LDH released into the culture supernatants
through spectrophotometry at 450 nm in an ELISA reader in
accordance with the manufacturer's protocol.
TTC staining
Following reoxygenation completion, the rat
hippocampal slices were transferred into small vials (50 ml)
containing 2% (w/v) TTC solution in aCSF aerated vigorously for 10
min at 37°C. The stained slices were subsequently removed and
rinsed twice with normal saline. The slices were dried and weighed.
An extraction buffer (DMSO and 100% ethanol prepared 1:1) was added
(20 μl/mg) and stored in the dark for 24 h at room
temperature. The quantity of the extracted formazan was then
measured through spectrophotometry at 490 nm using an ELISA
reader.
Western blot analysis
Western blot analysis was performed to detect the
relative levels of Bcl-2, Bax, cyt-c, apaf-1, caspase-9, caspase-7
and caspase-3. The slices were treated and grouped as indicated in
the previous section. In each group, four slices were collected as
one sample following administration of the respective treatments.
All samples were rapidly placed in an ice bath, and total protein
extraction was initiated using the total protein extraction kit.
The protein extraction fluid, containing lysis buffer (1 ml), PMSF
(10 μl), protein inhibitor (1 μl) and phosphatase
inhibitor (10 μl), was added in a volume of 0.5 ml to 100 mg
of tissue slice in a Pro homogenizer and homogenized 30 times. The
homogenate was transferred to a centrifuge tube and centrifuged at
12,000 × g for 10 min at 4°C to remove cellular debris. The BCA
protein assay reagent kit was used to determine the protein
concentration. The lysates from the slices were boiled for 5 min.
Equivalent quantities (80 μg) of protein samples were
subjected to 10% SDS-PAGE and electrically transferred onto PVDF
membranes using an electrophoretic transfer system (40D; Beijing
Liuyi Biotechnology Co., Ltd., Beijing, China). These membranes
were subdivided, and each target protein and loading control
protein were analyzed from a single transfer. Nonspecific binding
sites were blocked with PBST containing 5% nonfat dry powdered milk
for 1 h at room temperature. The membranes were then incubated with
primary antibodies against Bcl-2 (1:1,000), Bax (1:3,00), cyt-c
(1:1,000) apaf-1 (1:1,000), caspase-9 (1:1,000), caspase-7 (1:500),
caspase-3 (1:500), and β-actin (1:1,000) at 4°C overnight.
Immunostained β-actin served as the respective control. The
membranes were washed three times with PBST containing 0.1% Tween
and re-incubated with horseradish peroxidase-conjugated secondary
antibodies (1:2,000) for 2 h at room temperature. The bands were
then visualized using a Super Signal West Pico Chemiluminescence
kit and finally exposed to radiographic films. The resulting bands
were analyzed with Quantity One software (version 4.62; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The image intensities of
Bcl-2, Bax, caspase-9, caspase-7, caspase-3, SQSTM1 and LC3 were
quantified and normalized with β-actin protein band density.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). The results are presented as the
mean ± standard deviation. Differences among more than two groups
were assessed using one-way analysis of variance followed by the
least significant difference post hoc test. Differences between two
groups were analyzed using an unpaired t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
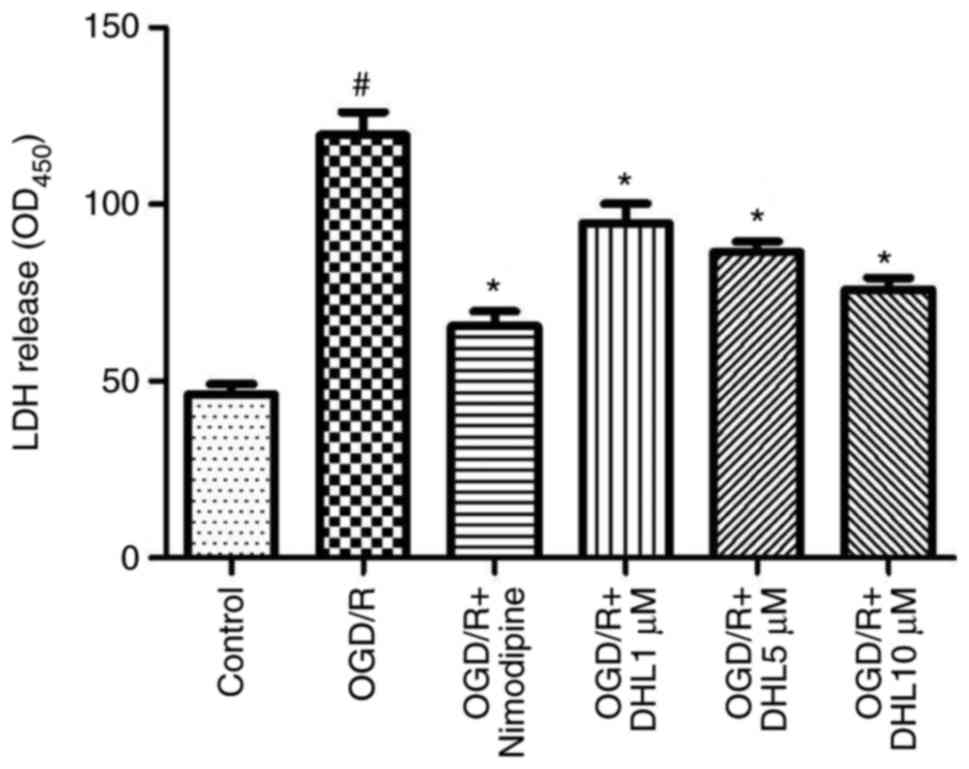
Effects of DHL on LDH release in
OGD/R-injured rat hippocampal slices
The degree of injury on the hippocampal slices
exposed to OGD/R was determined through the LDH assay. The OGD/R
group showed a significant increase in LDH catalytic activity
compared with that of the control group. The LDH concentrations of
the DHL-treated groups (1, 5 and 10 μM) were lower than that
of the OGD/R group (P<0.05), and this finding was
concentration-dependent. No significant differences in the levels
of LDH were observed between the nimodipine and DHL groups (1, 5
and 10 μM; P>0.05; Fig.
1).
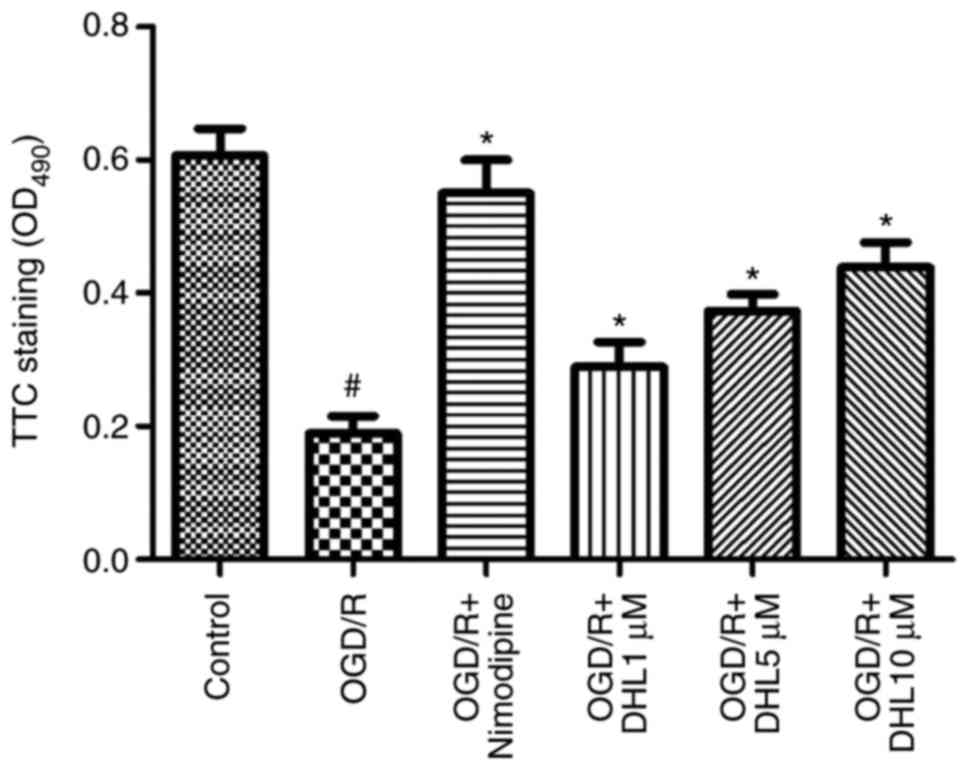
Effects of DHL on TTC staining of
OGD/R-injured hippocampal slices
The TTC staining results were quantified through
spectrophotometry at 490 nm using an ELISA reader. The
OD490 of TTC staining was obtained to evaluate the
degree of hippocampal slice injury. The results demonstrated that
the OD490 of the OGD/R group was significantly lower
than that of the control group, the DHL-treated groups, and the
nimodipine-treated group. The OD490 of the DHL (1, 5 and
10 μM) and nimodipine groups were significantly higher than
that of the OGD/R group (P<0.05), with no significant difference
in OD490 was observed between the nimodipine- and
DHL-treated groups (1, 5 and 10 μM; P>0.05; Fig. 2).
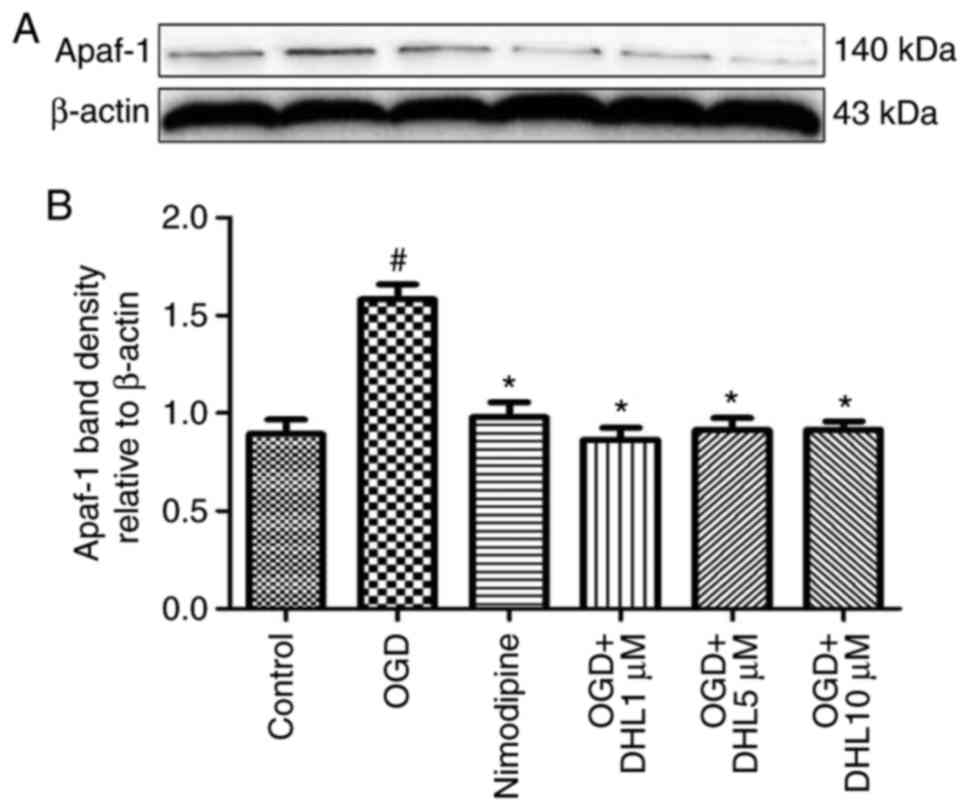
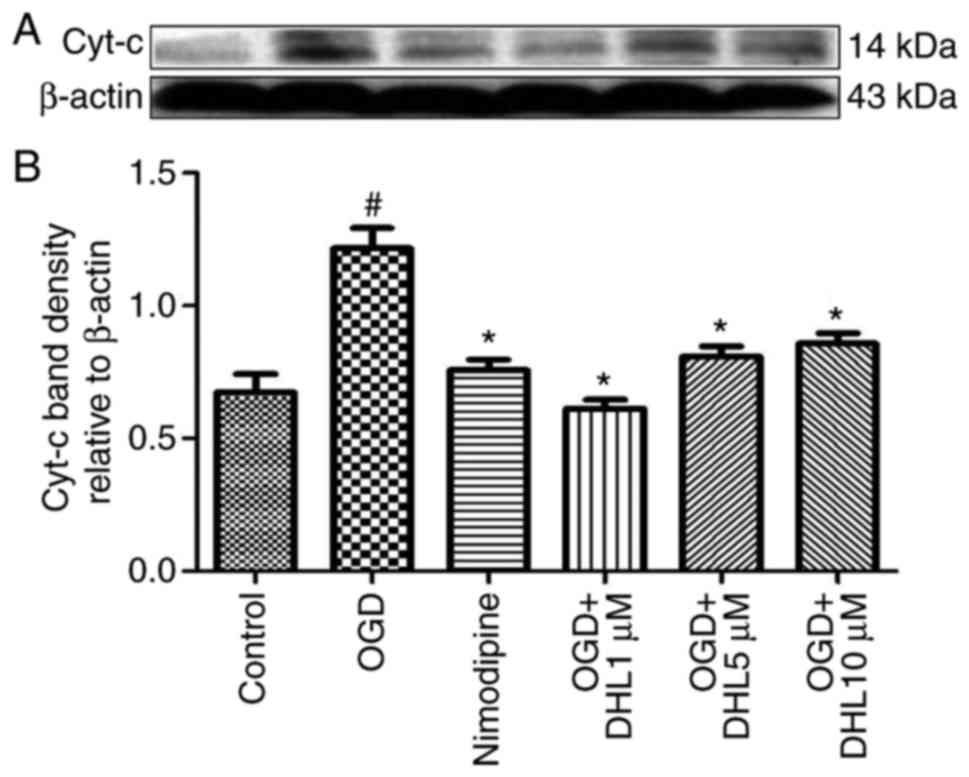
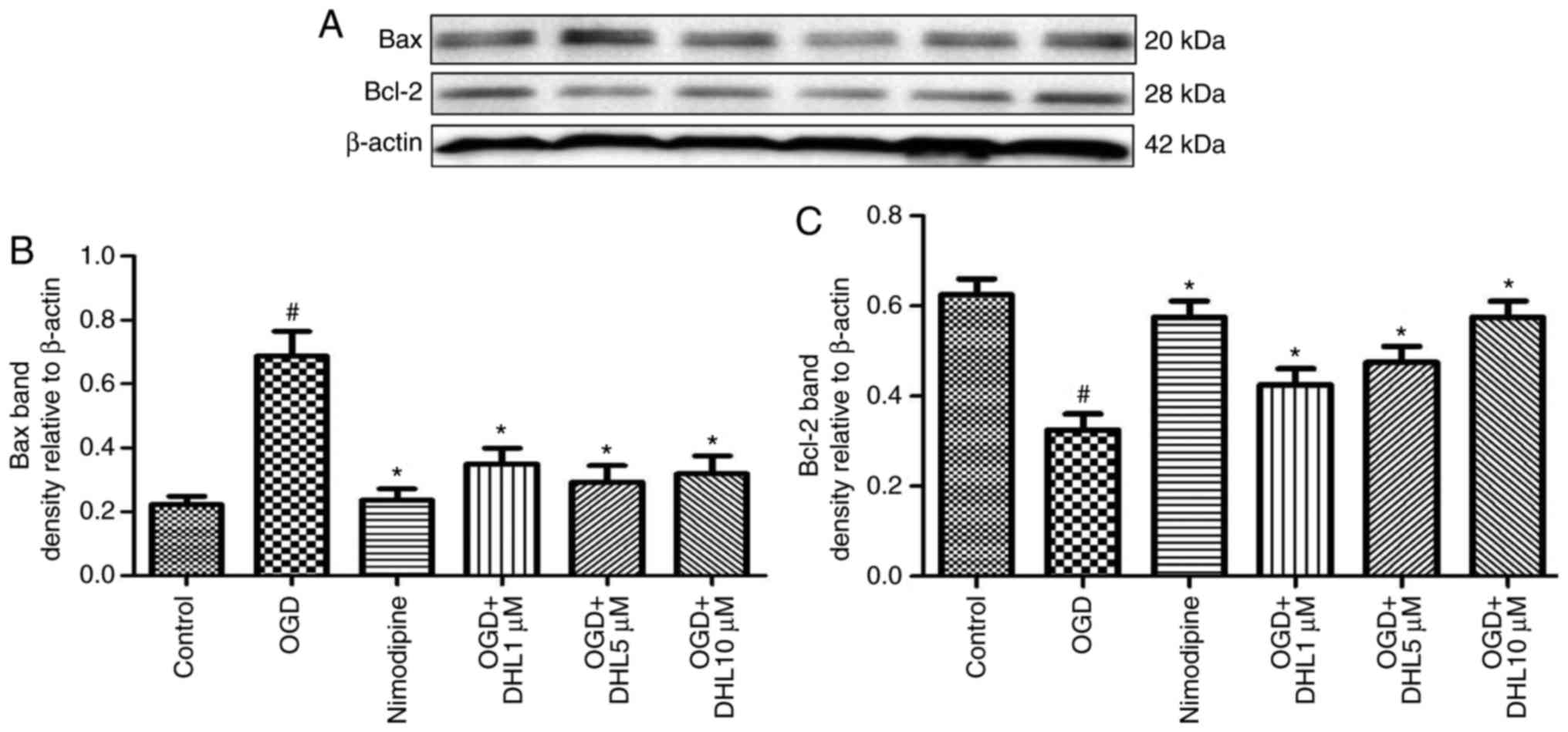
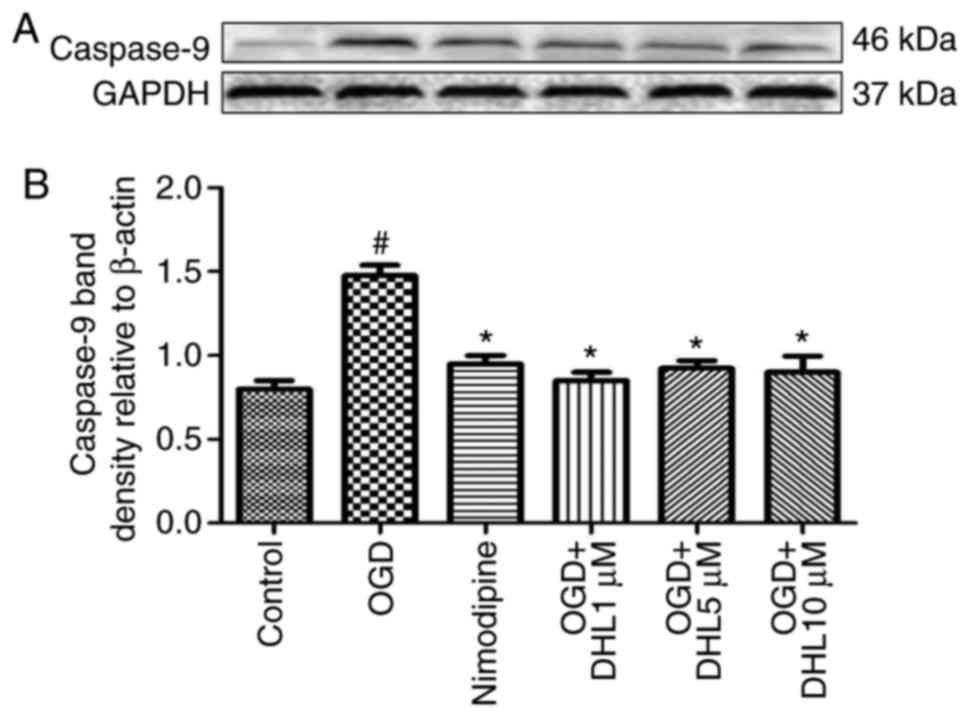
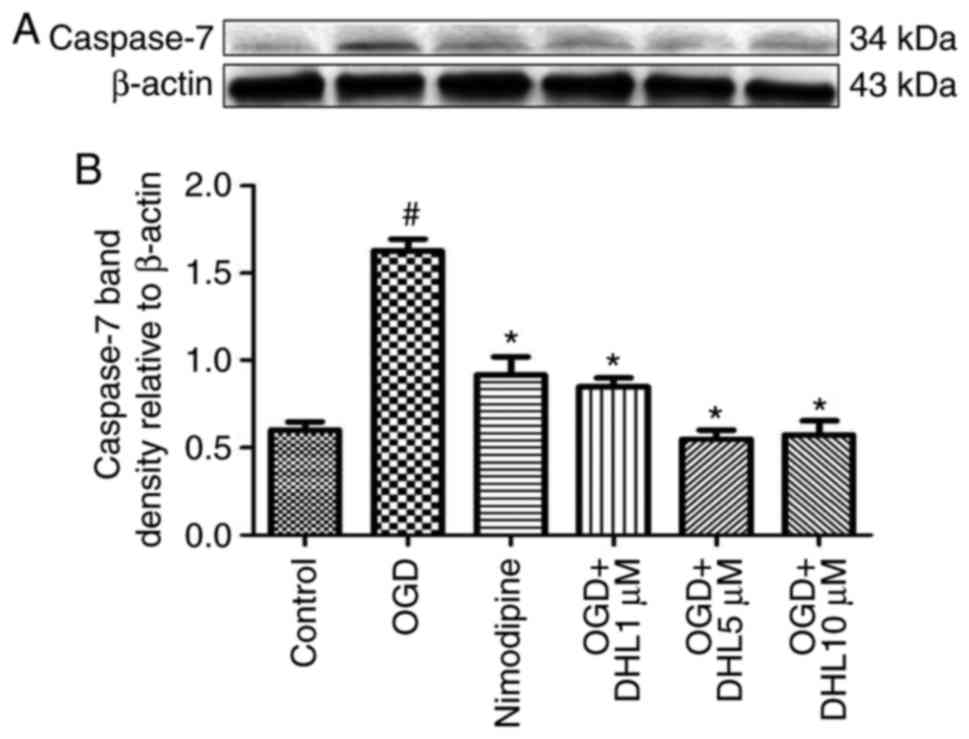
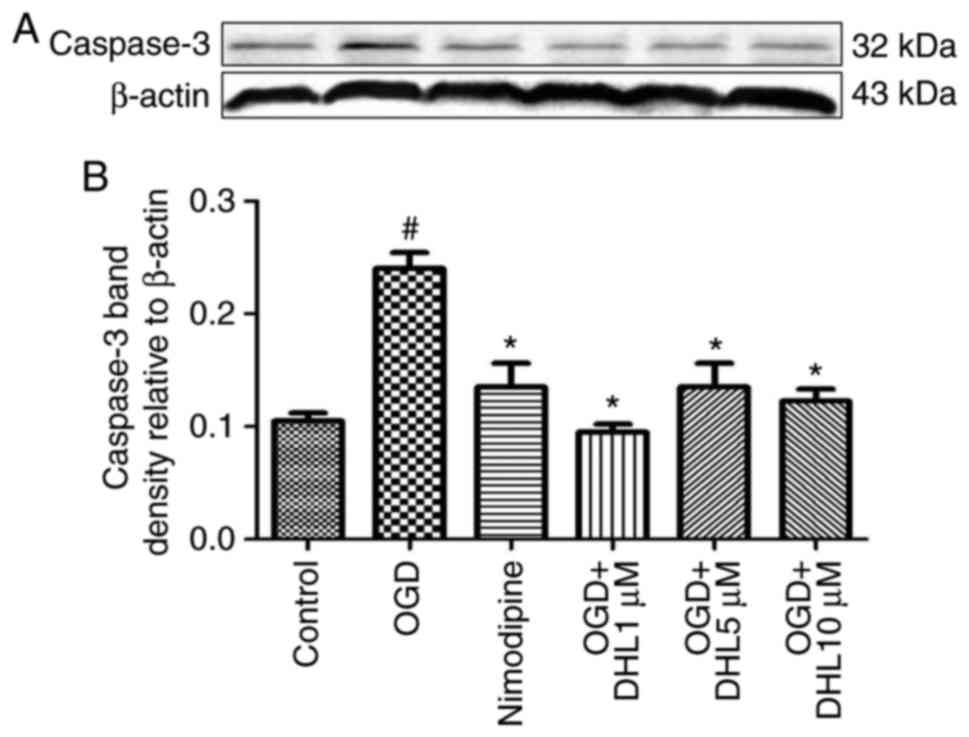
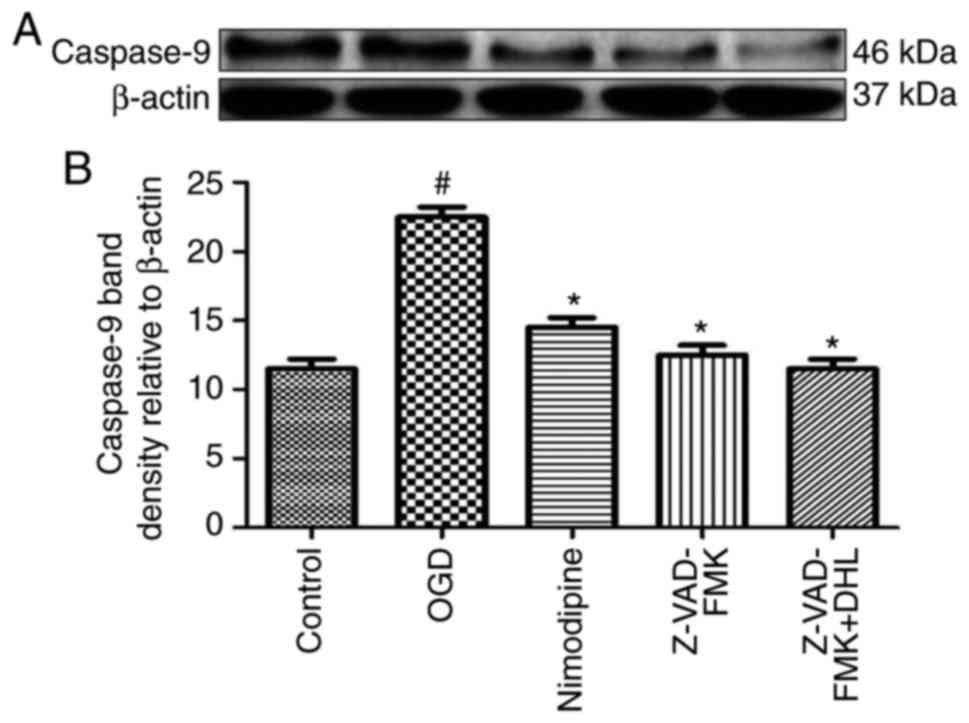
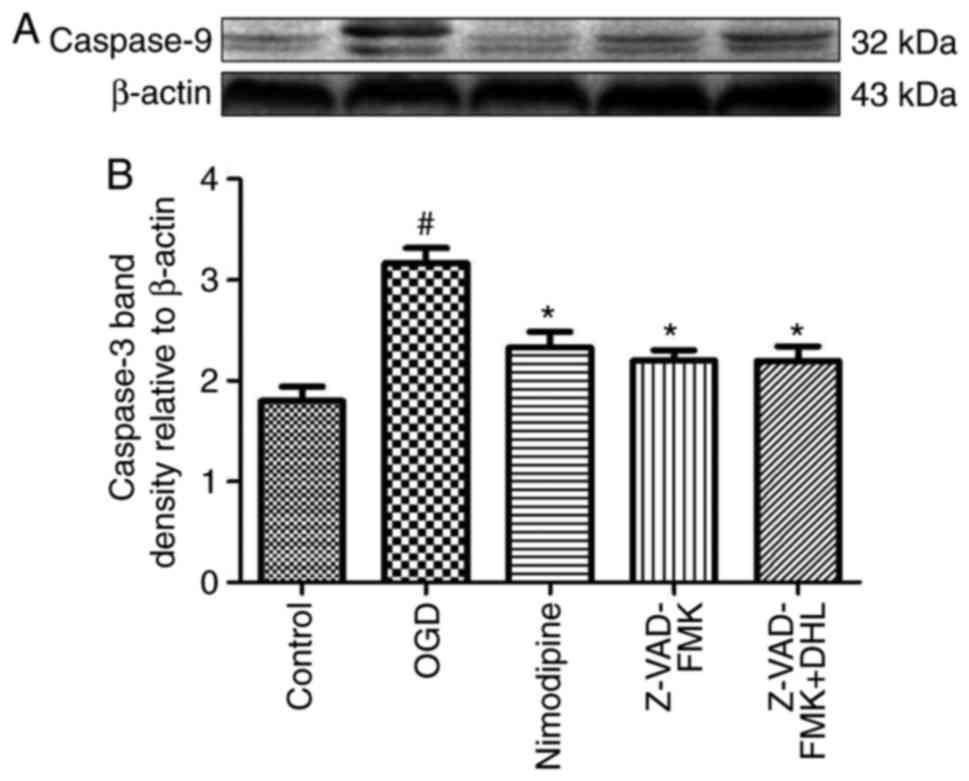
Effects of DHL on the expression of
Bcl-2, Bax, cyt-c, apaf-1, caspase-9, caspase-7 and caspase-3
To investigate the anti-apoptotic effects of DHL on
rat hippocampal slice injury induced by OGD/R, the present study
determined the expression levels of cyt-c (Fig. 3), apaf-1 (Fig. 4), Bcl-2, Bax (Fig. 5), caspase-9 (Fig. 6), caspase-7 (Fig. 7) and caspase-3 (Fig. 8), and used these as markers of
apoptosis in the mitochondria. The results of western blot analysis
revealed that the expression level of Bcl-2 in the OGD/R group was
significantly lower than that of the control group, whereas the
expression level of Bax in the OGD/R group was markedly higher than
that of the control group. Treatment with DHL (1, 5 and 10
μM) enhanced the expression of Bcl-2 and significantly
inhibited the expression of Bax. The expression levels of cyt-c,
apaf-1, caspase-9, caspase-7 and caspase-3 were also significantly
increased in the OGD/R group. Treatment with DHL (1, 5 and 10
μM) reduced the expression levels of apaf-1, cyt-c,
caspase-9, caspase-7 and caspase-3, however, no significant
difference was found in the expression levels of Bcl-2, Bax, cyt-c,
apaf-1, caspase-9, caspase-7 and caspase-3 between the nimodipine-
and DHL-treated groups (1, 5 and 10 μM; P>0.05).
Effects of Z-VAD-FMK and DHL on the
expression of cyt-c, apaf-1, caspase-9, caspase-7 and
caspase-3
To compare the anti-apoptotic effects of Z-VAD-FMK
and DHL on rat hippocampal slice injury induced by OGD/R, the
present study determined the expression levels of cyt-c, apaf-1,
caspase-7 (Fig. 9), caspase-9
(Fig. 10) and caspase-3
(Fig. 11). The results suggested
that Z-VAD-FMK downregulated the expression levels of caspase-9,
caspase-7 and caspase-3 (1, 5 and 10 μM; P<0.05).
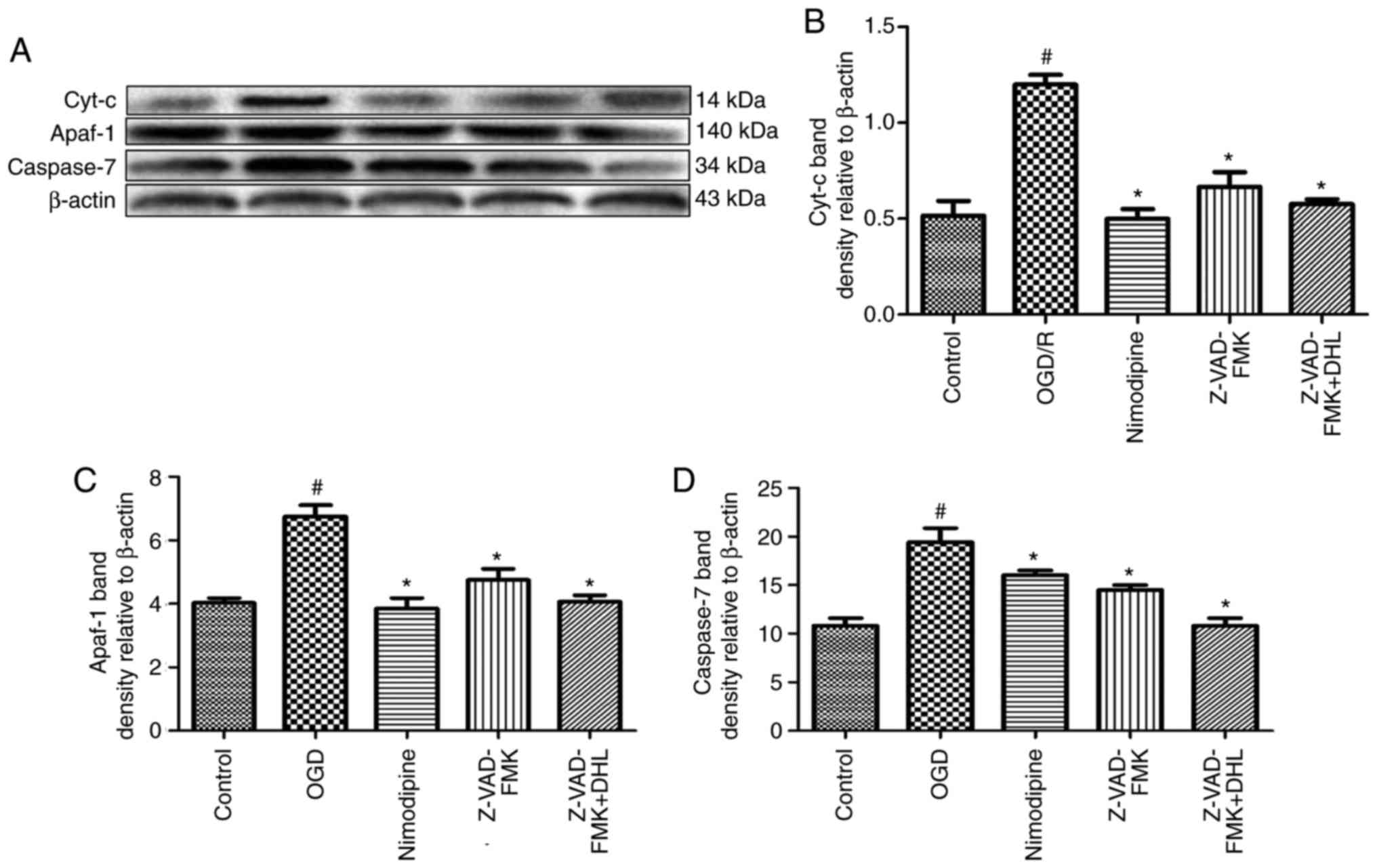 | Figure 9Effects of Z-VAD-FMK and DHL on the
expression of cyt-c, apaf-1 and caspase-7. (A) Western blot
analysis of f cyt-c, apaf-1 and caspase-7, with β-actin shown as a
loading control. Quantitative analysis of the expression of (B)
cyt-c, (C) apaf-1 and (D) caspase-7. Values are presented as the
mean ± standard deviation (n=10). #P<0.05, compared
with the control group; *P<0.05, compared with the
OGD/R group. cyt-c, cytochrome c; apaf-1, apoptotic protease
activating factor 1; DHL, dehydrocostuslactone; OGD/R,
oxygen-glucose deprivation/reoxygenation. |
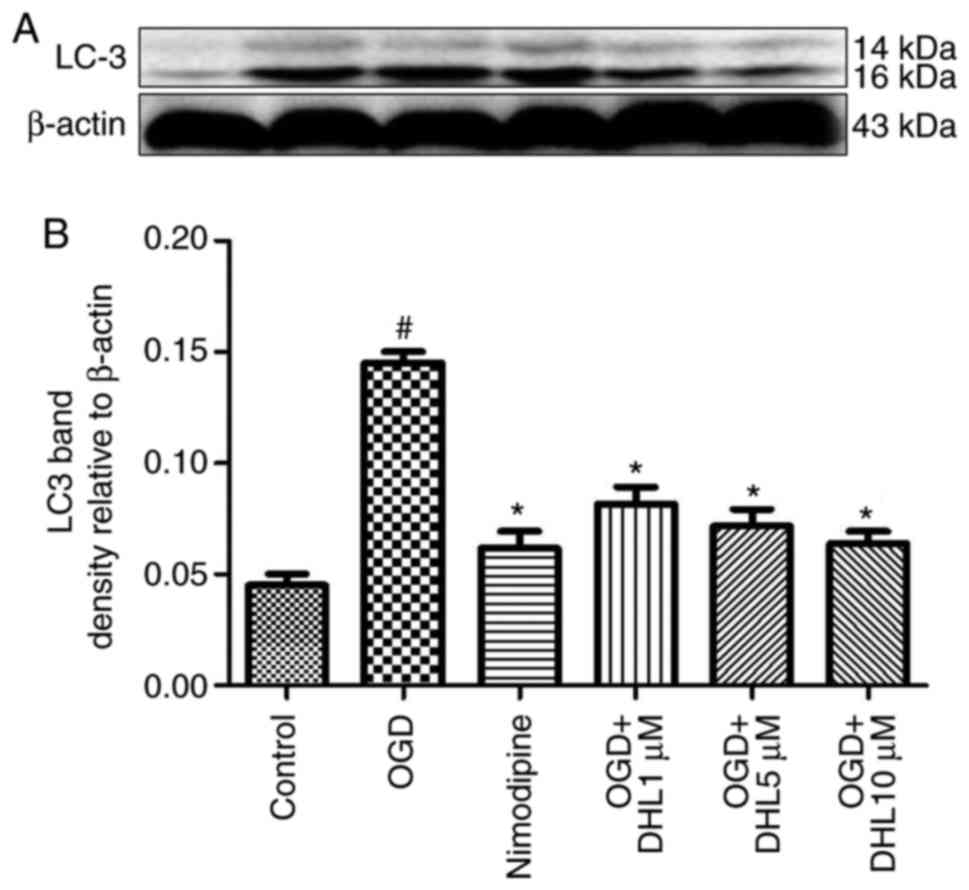
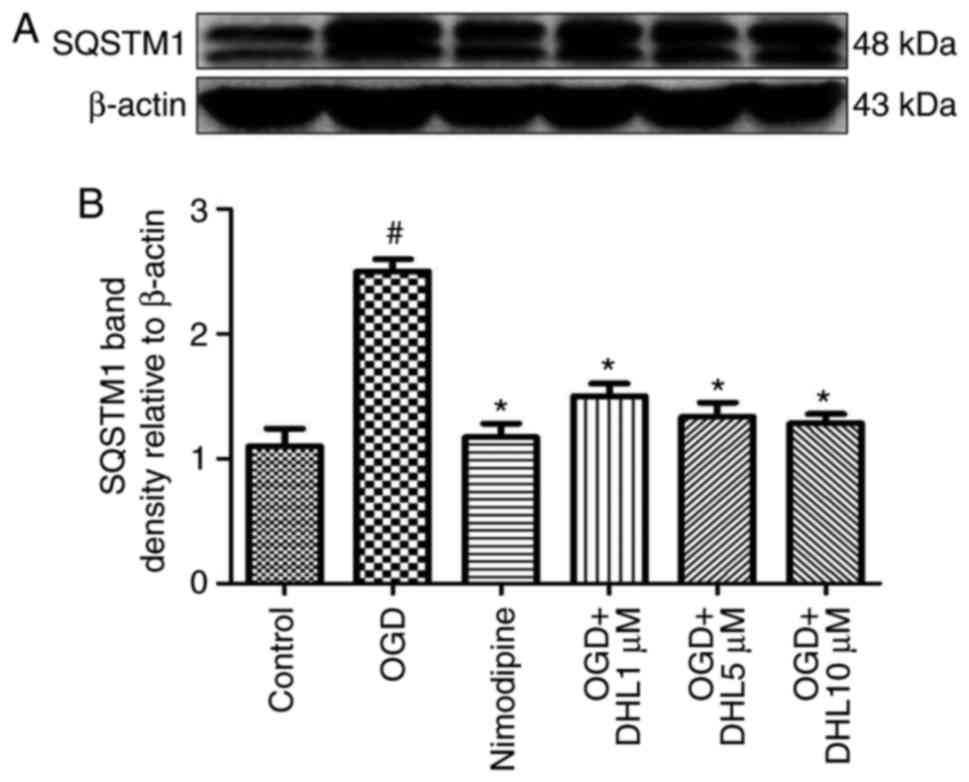
Effects of DHL on the expression of
SQSTM1 and LC3-II
To investigate the anti-autophagy effects of DHL on
rat hippocampal slice injury induced by OGD/R, the present study
determined the expression levels of LC3-II (Fig. 12) and SQSTM1 (Fig. 13). The expression levels of
LC3-II and SQSTM1 were markedly increased in the OGD/R group
compared with that of the control group. Treatment with DHL (1, 5
and 10 μM) inhibited the expression of LC3-II and SQSTM1
(P<0.05).
Discussion
In the present study, rat hippocampal slices were
used to establish an OGD/R model, which is an in vitro model
of ischemia (25,26), and ischemic stroke was simulated
to investigate the protective effects and mechanisms of DHL.
Changes in the quality and quantity of LDH directly
affect the energy metabolism of the body. LDH can be released into
extracellular spaces through a damaged cell membrane if external
stimuli, including hypoxia-ischemia, are present (27). Therefore, an LDH assay can be used
to quantify the effects of OGD (28). Cechetti et al (29) performed an LDH assay and examined
the effects of treadmill exercise on cell damage in rat hippocampal
slices subjected to oxygen and glucose deprivation. The LDH assay
can also be utilized to evaluate membrane integrity loss and
pathological necrosis (30). In
addition, TTC staining has been used to examine brain injuries
quantitatively (31). In this
method, TTC is oxidized only by metabolically active mitochondrial
dehydrogenases and converted into red formazan. The quantity of
formazan is directly proportional to cell activity (32). For example, TTC staining has been
used to measure the area of cerebral infarcts in brain slices, and
TTC assays have also been performed to assess cell and tissue
viability (33,34). In the present study, the level of
released LDH was significantly increased, and the OD490
of TTC staining was significantly reduced when the cells were
exposed to OGD/R; this change demonstrated that the hippocampal
slices were more vulnerable to OGD/R injury (26). DHL treatment markedly decreased
the release of LDH and increased the OD490 of TTC
staining.
To enhance our understanding of the mechanisms
underlying the action of OGD/R, the present study elucidated the
levels of apoptosis-related proteins as factors that may be
involved in DHL-induced neuroprotection against rat hippocampal
slice OGD/R damage, as Bcl-2 inhibits programmed cell death without
affecting cellular proliferation (35). Bcl-2 was the first anti-apoptotic
protein identified with a protective activity against ischemic
injury (36). Furthermore, Bax, a
pro-apoptotic protein found in the cytosol of healthy cells,
promotes apoptosis when cells are stimulated by ischemia (37,38). In addition, Bax can combine with
Bcl-2 to form polymers, increase the permeability of the
mitochondrial membrane, activate the caspase-apoptosis pathway, and
trigger cell apoptosis. The expression levels of Bcl-2 are
decreased, and the expression levels of Bax and caspase-3 are
increased in rats with cerebral ischemia injury (39,40). Cysteine-requiring aspartate
proteases are a family of proteases that are involved in apoptosis
in vivo and in vitro (41,42). Based on its functionality, the
upstream pro-apoptotic factor caspase-9 becomes activated when a
cell suffers from injury; the downstream factor caspase-7 and
apoptosis of the executive factor caspase-3 are further stimulated,
and various cells consequently undergo apoptosis (43,44). Secondary messengers, including
Ca2+, Bcl-2 and Bax, are also activated when an
apoptotic signal is delivered to the mitochondria (45). As a result, cyt-c is released from
the mitochondria, and apaf-1 and caspase-9 combine to form a
complex (46). This activates
caspase-9 and other downstream effector factors, including
caspases-3, caspase-6 and caspase-7; cell apoptosis is induced as a
result (45). Consistent with
previous reports, the present study demonstrated that the protein
expression levels of Bcl-2 were markedly decreased, and the protein
expression levels of Bax, cyt-c, apaf-1, caspase-9, caspase-7 and
caspase-3 were increased when the cells were exposed to OGD/R.
Treatment with DHL upregulated the expression levels of Bcl-2, and
downregulated the expression levels of Bax, cyt-c, apaf-1,
caspase-9, caspase-7 and caspase-3. Z-VAD-FMK, a widely
irreversible caspase inhibitor, was used in the present study, and
the results suggested that Z-VAD-FMK downregulated the expression
levels of caspase-9, caspase-7 and caspase-3. DHL treatment also
downregulated caspase-9, caspase-7 and caspase-3 to a greater
extent than Z-VAD-FMK treatment.
Autophagy is a programmed and physiologically
conserved self-degradation process involved in focal cerebral
infarction (47,48). However, whether autophagy promotes
neuronal death in response to OGD/R remains to be elucidated. In
the present study, LC3 and SQSTM1, which are specific markers of
autophagosomes, were probed to determine whether autophagic
activity is induced by DHL treatment. LC3 is used as an
autophagosome marker. Upon the induction of autophagy, LC3-I
becomes converted to LC3-II, which then translocates to
autophagosome membranes. The ratio of LC3-II to LC3-I is increased
in mice subjected to middle cerebral artery occlusion (49). SQSTM1 (p62) is a ubiquitin-binding
protein involved in autophagy. SQSTM1 binds to the autophagosomal
membrane protein LC3 and carries SQSTM1-containing protein
aggregates to autophagosomes. The level of SQSTM1 is used to
monitor autophagic flux (46,50). In the present study, LC3-II and
SQSTM1 were markedly increased in the rat hippocampal slices
injured by OGD/R. This result suggested that autophagy was
activated in the rat hippocampal slices exposed to OGD/R, and this
change was reversed by DHL treatment.
In the present study, the desired results were not
obtained in paraffinized sections, frozen sections or flow
cytometry. The results in the paraffinized sections were
unsatisfactory due to the smaller and thinner hippocampal sample
size (400 μm), and there were more ice crystals in the
frozen sections. Based on these reasons, it was not possible not
evaluate the region or degree of hippocampal slice injury with
hematoxylin and eosin staining, immunohistochemistry or TUNEL
detection. Finally, the present study aimed to evaluate the degree
of damage in the hippocampal slices using flow cytometry; the
Annexin V/PI staining showed that the majority of cells were dead,
and this cell death may have been induced by the preparation
process of producing a single-cell suspension from the hippocampal
slice.
Despite the limitations of the present study, the
results revealed that DHL provided protective effects against
hippocampal slice injury induced by OGD/R through anti-apoptotic
and anti-autophagic effects. However, further investigations are
required to determine other underlying mechanisms.
Funding
The authors would like to acknowledge the financial
support provided by the National Natural Science Foundation of
Ningxia (grant no. NZ16078), the Ningxia College First-Class
Discipline Construction Project (Chinese Medicine) Funded Project
(grant no. NXYLXK2017A06) and the National Natural Science
Foundation of China (grant nos. 81660700 and 81260679).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author upon reasonable
request.
Authors' contributions
QZ, AC and XW established the hippocampal slice
injury model and performed western blotting analysis. YuZ and YH
analyzed and interpreted the data. ZZ performed lactate
dehdrogenase activity measurement and tetrazolium chloride
staining, and wrote the manuscript. YaZ and SR designed the study,
supervised the research group and revised the manuscript critically
for important intellectual content. The final version of the
manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Ningxia Medical University (Yinchuan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Dávalos A, Toni D, Iweins F, Lesaffre E,
Bastianello S and Castillo J: Neurological deterioration in acute
ischemic stroke: Potential predictors and associated factors in the
European cooperative acute stroke study. Stroke. 30:2631–2636.
1999. View Article : Google Scholar
|
|
2
|
Wang Q, Tang XN and Yenari MA: The
inflammatory response in stroke. J Neuroimmunol. 184:53–68. 2007.
View Article : Google Scholar :
|
|
3
|
Vssuvanish K, Gopalakrishnan A, Nazıroğlu
M and Rajanikant GK: Calcium ion-the key player in cerebral
ischemia. Curr Med Chem. 21:2065–2075. 2014. View Article : Google Scholar
|
|
4
|
Doyle KP, Simon RP and Stenzel-Poore MP:
Mechanisms of ischemic brain damage. Neuropharmacology. 55:310–318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barone FC and Feuerstein GZ: Inflammatory
mediators and stroke: New opportunities for novel therapeutics. J
Cereb Blood Flow Metab. 19:819–834. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li D, Liu M, Tao TQ, Song DD, Liu XH and
Shi DZ: Panax quinquefolium saponin attenuates cardiomyocyte
apoptosis and opening of the mitochondrial permeability transition
pore in a rat model of ischemia/reperfusion. Cell Physiol Biochem.
34:1413–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
8
|
Hengardner MO: Insight review articles:
The biochemistry of apoptosis. Nature. 207:770–776. 2000.
View Article : Google Scholar
|
|
9
|
Wu KJ, Huang JM, Zhong HJ, Dong ZZ,
Vellaisamy K, Lu JJ, Chen XP, Chiu P, Kwong DWJ, Han QB, et al: A
natural product-like JAK2/STAT3 inhibitor induces apoptosis of
malignant melanoma cells. PLoS One. 12:e01771232017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leung CH, He HZ, Liu LJ, Wang MD, Chan DSH
and Ma DL: Metal complexes as inhibitors of transcription factor
activity. Coord Chem Rev. 257:3139–3151. 2013. View Article : Google Scholar
|
|
11
|
Wu KJ, Zhong HJ, Li G, Liu C, Wang HD, Ma
DL and Leung CH: Structure-based identification of a
NEDD8-activating enzyme inhibitor via drug repurposing. Eur J Med
Chem. 143:1021–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirino T, Tamura A and Sano K: Selective
vulnerability of the hippocampus to ischemia-reversible and
irreversible types of ischemic cell damage. Prog Brain Res.
63:39–58. 1985. View Article : Google Scholar
|
|
13
|
Obeidat AS, Jarvis CR and Andrew RD:
Glutamate does not mediate acute neuronal damage after spreading
depression induced by O2/glucose deprivation in the
hippocampal slice. J Cereb Blood Flow Metab. 20:412–422. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhan RZ1, Qi S, Wu C, Fujihara H, Taga K
and Shimoji K: Intravenous anesthetics differentially reduce
neurotransmission damage caused by oxygen-glucose deprivation in
rat hippocampal slices in correlation with N-methyl-D-aspartate
receptor inhibition. Crit Care Med. 29:808–813. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sick TJ, Solow EL and Roberts EL:
Extracellular potassium ion activity and electrophysiology in the
hippocampal slice: Paradoxical recovery of synaptic transmission
during anoxia. Brain Res. 418:227–234. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schurr A, Payne RS, Heine M and Rigor BM:
Hypoxia, excitotoxicity, and neuroprotection in the hippocampal
slice preparation. J Neurosci Methods. 59:129–138. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schurr A, Payne RS and Rigor BM:
Protection by MK-801 against hypoxia-, excitotoxin-, and
depolarization-induced neuronal damage in vitro. Neurochem Int.
26:519–525. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krnjević K: Electrophysiology of cerebral
ischemia. Neuropharmacology. 55:319–333. 2008. View Article : Google Scholar
|
|
19
|
Zheng H, Chen Y, Zhang J, Wang L, Jin Z,
Huang H, Man S and Gao W: Evaluation of protective effects of
costunolide and dehydrocostuslactone on ethanol-induced gastric
ulcer in mice based on multi-pathway regulation. Chem Biol
Interact. 250:68–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park EJ, Park SW, Kim HJ, Kwak JH, Lee DU
and Chang KC: Dehydrocostuslactone inhibits LPS-induced
inflammation by p38MAPK-dependent induction of hemeoxygenase-1 in
vitro and improves survival of mice in CLP-induced sepsis in vivo.
Int Immunopharmacol. 22:332–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HK, Song HE, Lee HB, Kim CS, Koketsu
M, Ngan LT and Ahn YJ: Growth inhibitory, bactericidal, and
morphostructural effects of dehydrocostus lactone from Magnolia
sieboldii Leaves on antibiotic-susceptible and -resistant strains
of Helicobacter pylori. PLoS One. 9:e955302014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin X, Peng Z and Su C: Potential
anti-cancer activities and mechanisms of costunolide and
dehydrocostuslactone. Int J Mol Sci. 16:10888–10906. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park HJ, Kwon SH, Han YN, Choi JW,
Miyamoto K, Lee SH and Lee KT: Apoptosis-Inducing costunolide and a
novel acyclic monoterpene from the stem bark of Magnolia sieboldii.
Arch Pharm Res. 24:342–348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hung JY, Hsu YL, Ni WC, Tsai YM, Yang CJ,
Kuo PL and Huang MS: Oxidative and endoplasmic reticulum stress
signaling are involved in dehydrocostuslactone-mediated apoptosis
in human non-small cell lung cancer cells. Lung Cancer. 68:355–365.
2010. View Article : Google Scholar
|
|
25
|
Zhang H, Schools GP, Lei T, Wang W,
Kimelberg HK and Zhou M: Resveratrol attenuates early pyramidal
neuron excitability impairment and death in acute rat hippocampal
slices caused by oxygen-glucose deprivation. Exp Neurol. 212:44–52.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang XJ, Li Q, Zhang YP, Lü Q, Guo LJ,
Huang L and He Z: Neuroprotective effects of cactus polysaccharide
on oxygen and glucose deprivation induced damage in rat brain
slices. Cell Mol Neurobiol. 28:559–568. 2008. View Article : Google Scholar
|
|
27
|
Huang XJ, Li Q, Zhang YP, Lü Q, Guo LJ,
Huang L and He Z: Neuroprotective effects of cactus polysaccharide
on oxygen and glucose deprivation induced damage in rat brain
slices. Brain Res Bull. 87:521–525. 2012.
|
|
28
|
Tagliari B, Zamin LL, Salbego CG, Netto CA
and Wyse AT: Hyperhomocysteinemia increases damage on brain slices
exposed to in vitro model of oxygen and glucose deprivation:
Prevention by folic acid. Dev Neurosci. 24:285–291. 2006.
View Article : Google Scholar
|
|
29
|
Cechetti F, Rhod A, Simão F, Santin K,
Salbego C, Netto CA and Siqueira IR: Effect of treadmill exercise
on cell damage in rat hippocampal slices submitted to oxygen and
glucose deprivation. Brain Res. 1157:121–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Renic M, Kumar SN, Gebremedhin D, Florence
MA, Gerges NZ, Falck JR, Harder DR and Roman RJ: Protective effect
of 20-HETE inhibition in a model of oxygen-glucose deprivation in
hippocampal slice cultures. Am J Physiol Heart Circ Physiol.
302:H1285–H1293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Preston E and Webster J:
Spectrophotometric measurement of experimental brain injury. J
Neurosci Methods. 94:187–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mathews KS, McLaughlin DP, Ziabari LH,
Toner CC, Street PC, Hisgrove E, Bezzina EL and Stamford JA: Rapid
quantification of ischemic injury and cerebroprotection in brain
slices using densitometric assessment of 2,3,5-triphenyltetrazolium
chloride staining. J Neurosci Methods. 102:43–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang ZJ, Li GM, Nie BM, Lu Y and Yin M:
Neuroprotective effect of the stearic acid against oxidative stress
via phosphatidylinositol 3-kinase pathway. Chem Biol Interact.
160:880–887. 2006. View Article : Google Scholar
|
|
34
|
Zhao Q, Cheng X, Wang X, Wang J, Zhu Y and
Ma X: Neuroprotective effect and mechanism of Mu-Xiang-You-Fang on
cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol.
192:140–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lalonde CC and Mielke JG: Selective
vulnerability of hippocampal sub-fields to oxygen-glucose
deprivation is a function of animal age. Brain Res. 1543:271–279.
2014. View Article : Google Scholar
|
|
36
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar
|
|
37
|
Kitagawa K, Matsumoto M, Tsujimoto Y,
Ohtsuki T, Kuwabara K, Matsushita K, Yang G, Tanabe H, Martinou JC,
Hori M and Yanagihara T: Amelioration of hippocampal neuronal
damage after global ischemia by neuronal overexpression of BCL-2 in
transgenic mice. Stroke. 29:2616–2621. 1998. View Article : Google Scholar
|
|
38
|
Misao J, Hayakawa Y, Ohno M, Kato S,
Fujiwara T and Fujiwara H: Expression of bcl-2 protein, an
inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in
ventricular myocytes of human hearts with myocardial infarction.
Circulation. 94:1506–1512. 1996. View Article : Google Scholar
|
|
39
|
van Delft MF and Huang DC: How the Bcl-2
family of proteins interact to regulate apoptosis. Cell Res.
16:203–213. 2006. View Article : Google Scholar
|
|
40
|
Wang GH, Lan R, Zhen XD, Zhang W, Xiang J
and Cai DF: An-Gong-Niu-Huang Wan protects against cerebral
ischemia induced apoptosis in rats: Up-regulation of Bcl-2 and
down-regulation of Bax and caspase-3. J Ethnopharmacol.
154:156–162. 2014. View Article : Google Scholar
|
|
41
|
Cao G, Minami M, Pei W, Yan C, Chen D,
O'Horo C, Graham SH and Chen J: Intracellular Bax translocation
after transient cerebral ischemia: Implications for a role of the
mitochondrial apoptotic signaling pathway in ischemic neuronal
death. J Cereb Blood Flow Metab. 21:321–333. 2001. View Article : Google Scholar
|
|
42
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View Article : Google Scholar
|
|
43
|
García de la Cadena S and Massieu L:
Caspases and their role in inflammation and ischemic neuronal
death. Focus on caspase-12. Apoptosis. 21:763–777. 2016. View Article : Google Scholar
|
|
44
|
Zhu L, Yuan H, Guo C, Lu Y, Deng S, Yang
Y, Wei Q, Wen L and He Z: Zearalenone induces apoptosis and
necrosis in porcine granulosa cells via a caspase-3- and
caspase-9-dependent mitochondrial signaling pathway. J Cell
Physiol. 227:1814–1820. 2012. View Article : Google Scholar
|
|
45
|
Bhuiyan MS and Fukunaga K: Inhibition of
HtrA2/Omi ameliorates heart dysfunction following
ischemia/reperfusion injury in rat heart in vivo. Eur J Pharmacol.
557:168–177. 2007. View Article : Google Scholar
|
|
46
|
Li Y, Zhang J, Chen L, Xing S, Li J, Zhang
Y, Li C, Pei Z and Zeng J: Ebselen reduces autophagic activation
and cell death in the ipsilateral thalamus following focal cerebral
infarction. Neurosci Lett. 600:206–212. 2015. View Article : Google Scholar
|
|
47
|
Solá S, Morgado AL and Rodrigues CM: Death
receptors and mitochondria: Two prime triggers of neural apoptosis
and differentiation. Biochim Biophys Acta. 1830:2160–2166. 2013.
View Article : Google Scholar
|
|
48
|
Xing S, Zhang Y, Li J, Zhang J, Li Y, Dang
C, Li C, Fan Y, Yu J, Pei Z and Zeng JS: Beclin 1 knockdown
inhibits autophagic activation and prevents the secondary
neurodegenerative damage in the ipsilateral thalamus following
focal cerebral infarction. Autophagy. 8:63–76. 2012. View Article : Google Scholar
|
|
49
|
Zhang J, Zhang Y, Li J, Xing S, Li C, Li
Y, Dang C, Fan Y, Yu J, Pei Z and Zeng JS: Autophagosomes
accumulation is associated with β-amyloid deposits and secondary
damage in the thalamus after focal cortical infarction in
hypertensive rats. J Neurochem. 120:564–573. 2012. View Article : Google Scholar
|
|
50
|
Jiang P and Mizushima N: LC3- and
p62-based biochemical methods for the analysis of autophagy
progression in mammalian cells. Methods. 75:13–18. 2015. View Article : Google Scholar
|