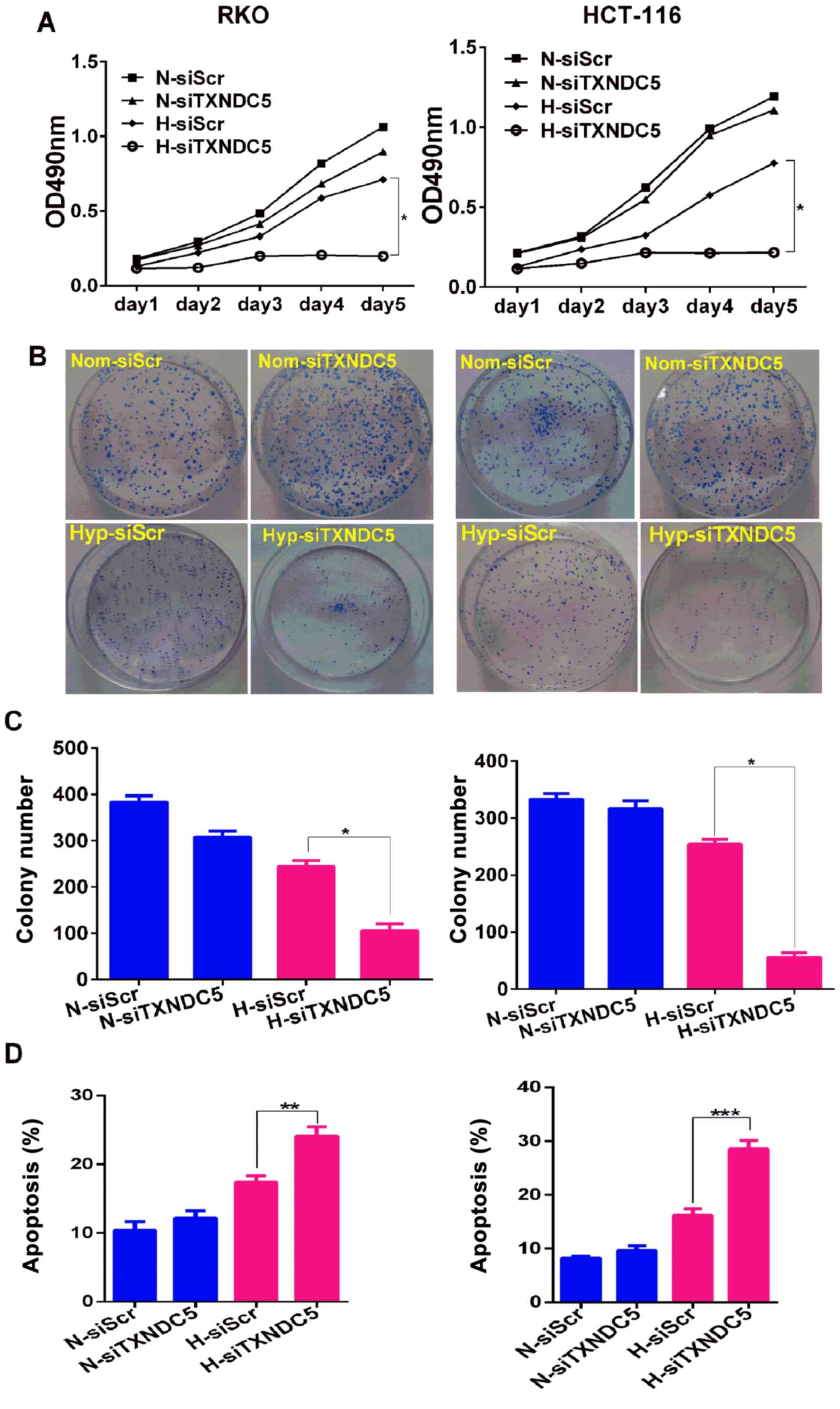
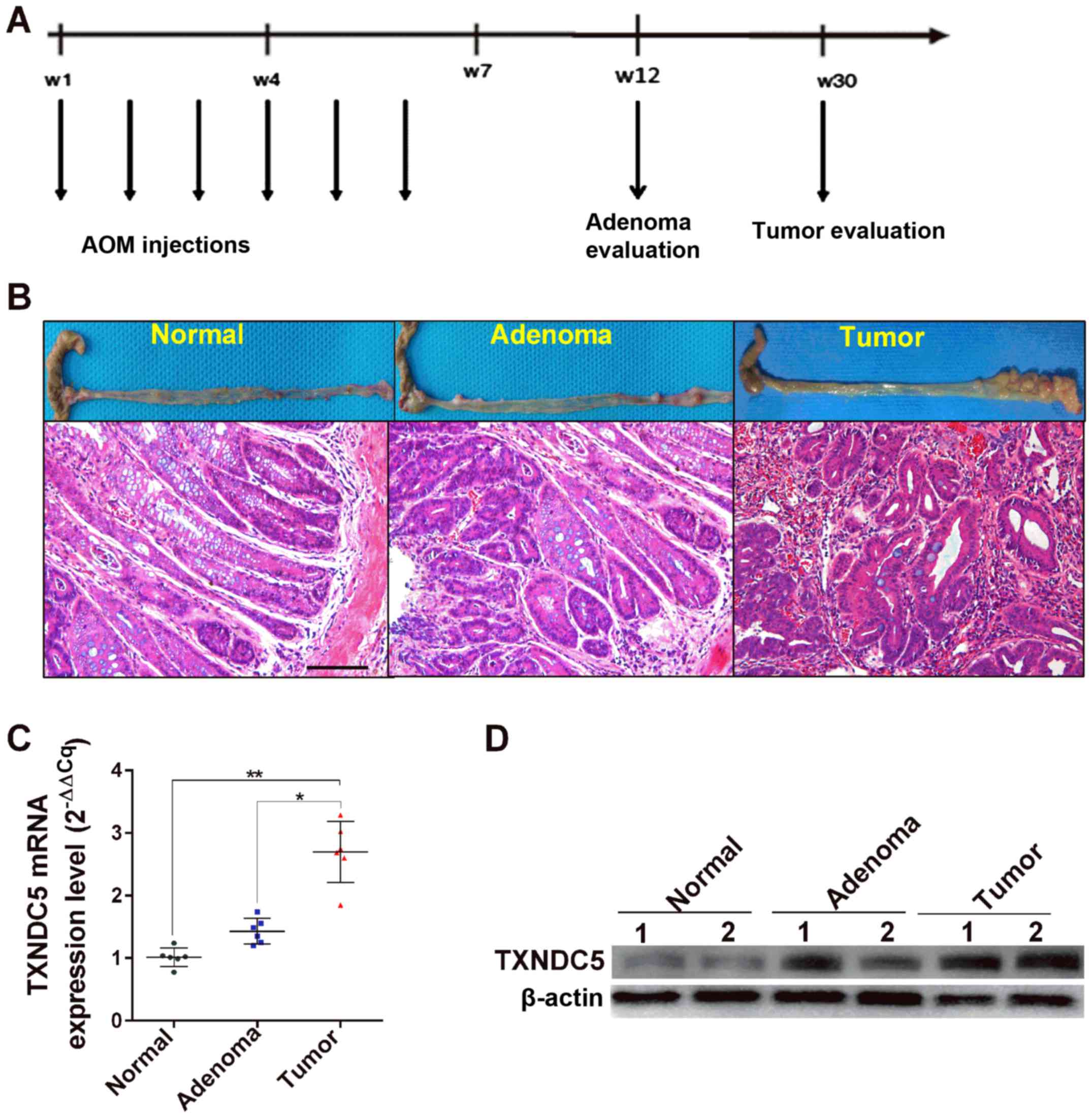
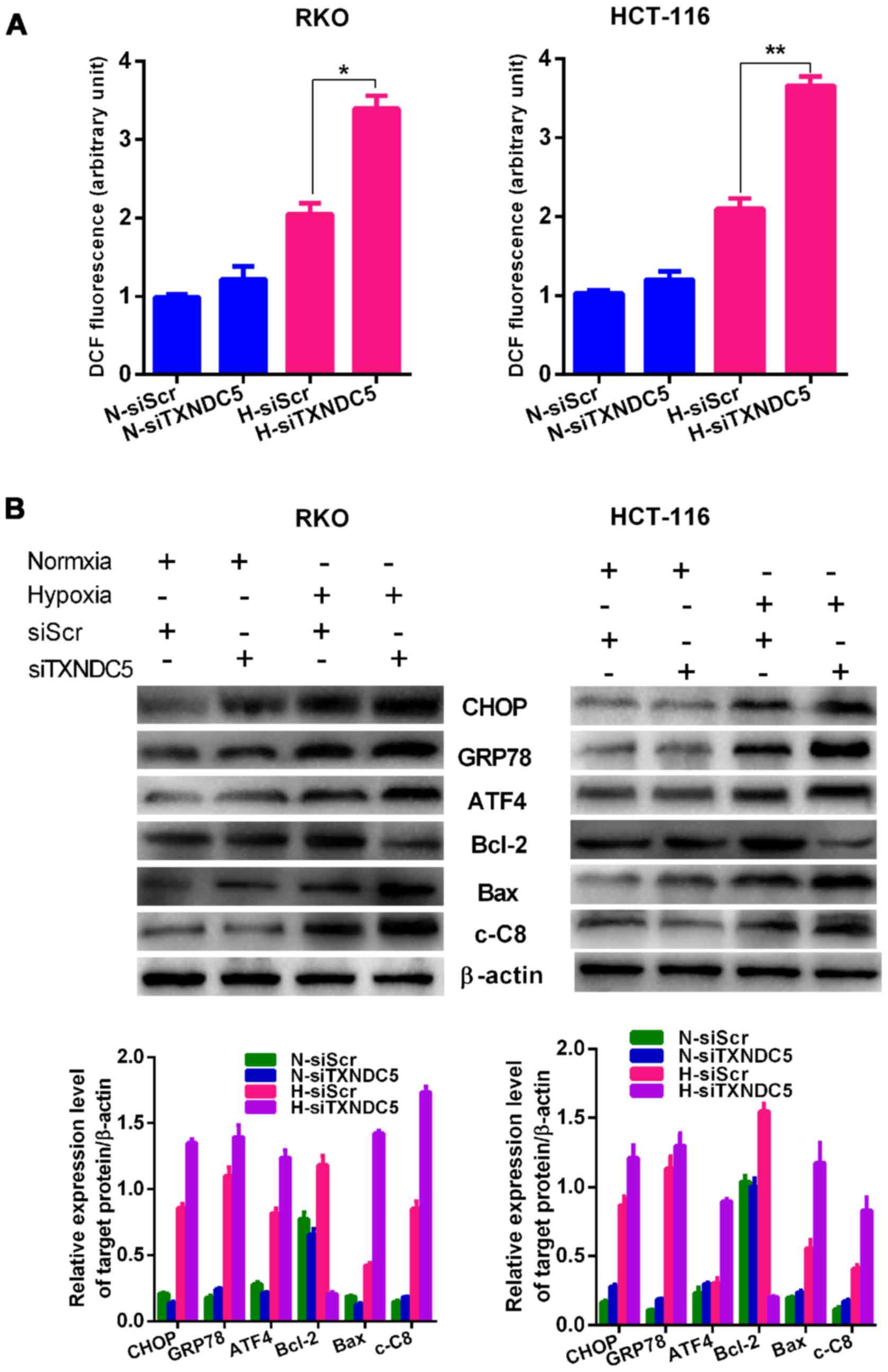
|
1
|
Zheng R, Zeng H, Zhang S, Chen T and Chen
W: National estimates of cancer prevalence in China, 2011. Cancer
Lett. 370:33–38. 2016. View Article : Google Scholar
|
|
2
|
Pesson M, Volant A, Uguen A, Trillet K, De
La Grange P, Aubry M, Daoulas M, Robaszkiewicz M, Le Gac G, Morel
A, et al: A gene expression and pre-mRNA splicing signature that
marks the adenoma-adenocarcinoma progression in colorectal cancer.
PLoS One. 9:e877612014. View Article : Google Scholar :
|
|
3
|
Schell MJ, Yang M, Missiaglia E, Delorenzi
M, Soneson C, Yue B, Nebozhyn MV, Loboda A, Bloom G and Yeatman TJ:
A composite gene expression signature optimizes prediction of
colorectal cancer metastasis and outcome. Clin Cancer Res.
22:734–745. 2016. View Article : Google Scholar :
|
|
4
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar
|
|
5
|
Saito S, Lin YC, Tsai MH, Lin CS, Murayama
Y, Sato R and Yokoyama KK: Emerging roles of hypoxia-inducible
factors and reactive oxygen species in cancer and pluripotent stem
cells. Kaohsiung J Med Sci. 31:279–286. 2015. View Article : Google Scholar
|
|
6
|
Hotokezaka Y, van Leyen K, Lo EH, Beatrix
B, Katayama I, Jin G and Nakamura T: AlphaNAC depletion as an
initiator of ER stress-induced apoptosis in hypoxia. Cell Death
Differ. 16:1505–1514. 2009. View Article : Google Scholar
|
|
7
|
Pereira ER, Frudd K, Awad W and Hendershot
LM: Endoplasmic reticulum (ER) stress and hypoxia response pathways
interact to potentiate hypoxia-inducible factor 1 (HIF-1)
transcriptional activity on targets like vascular endothelial
growth factor (VEGF). J Biol Chem. 289:3352–3364. 2014. View Article : Google Scholar :
|
|
8
|
Chevet E, Hetz C and Samali A: Endoplasmic
reticulum stress-activated cell reprogramming in oncogenesis.
Cancer Discov. 5:586–597. 2015. View Article : Google Scholar
|
|
9
|
Yadav RK, Chae SW, Kim HR and Chae HJ:
Endoplasmic reticulum stress and cancer. J Cancer Prev. 19:75–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke HJ, Chambers JE, Liniker E and
Marciniak SJ: Endoplasmic reticulum stress in malignancy. Cancer
Cell. 25:563–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horna-Terrón E, Pradilla-Dieste A,
Sánchez-de-Diego C and Osada J: TXNDC5, a newly discovered
disulfide isomerase with a key role in cell physiology and
pathology. Int J Mol Sci. 15:23501–23518. 2014. View Article : Google Scholar
|
|
12
|
Sullivan DC, Huminiecki L, Moore JW, Boyle
JJ, Poulsom R, Creamer D, Barker J and Bicknell R: EndoPDI, a novel
protein-disulfide isomerase-like protein that is preferentially
expressed in endothelial cells acts as a stress survival factor. J
Biol Chem. 278:47079–47088. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kojima R, Okumura M, Masui S, Kanemura S,
Inoue M, Saiki M, Yamaguchi H, Hikima T, Suzuki M, Akiyama S, et
al: Radically different thioredoxin domain arrangement of ERp46, an
efficient disulfide bond introducer of the mammalian PDI family.
Structure. 22:431–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Funkner A, Parthier C, Schutkowski M,
Zerweck J, Lilie H, Gyrych N, Fischer G, Stubbs MT and Ferrari DM:
Peptide binding by catalytic domains of the protein disulfide
isomerase-related protein ERp46. J Mol Biol. 425:1340–1362. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang X, Xu B, Wang L, Wang Y, Wang Y and
Yan S: Investigating a pathogenic role for TXNDC5 in tumors. Int J
Oncol. 43:1871–1884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Ma Y, Lü B, Xu E, Huang Q and Lai
M: Differential expression of mimecan and thioredoxin
domain-containing protein 5 in colorectal adenoma and cancer: a
proteomic study. Exp Biol Med (Maywood). 232:1152–1159. 2007.
View Article : Google Scholar
|
|
17
|
Shen H, Huang J, Pei H, Zeng S, Tao Y,
Shen L, Zeng L and Zhu H: Comparative proteomic study for profiling
differentially expressed proteins between Chinese left- and
right-sided colon cancers. Cancer Sci. 104:135–141. 2013.
View Article : Google Scholar
|
|
18
|
Affer M, Chesi M, Chen WD, Keats JJ,
Demchenko YN, Tamizhmani K, Garbitt VM, Riggs DL, Brents LA,
Roschke AV, et al: Promiscuous MYC locus rearrangements hijack
enhancers but mostly super-enhancers to dysregulate MYC expression
in multiple myeloma. Leukemia. 28:1725–1735. 2014. View Article : Google Scholar :
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
20
|
Dang DT, Chen F, Gardner LB, Cummins JM,
Rago C, Bunz F, Kantsevoy SV and Dang LH: Hypoxia-inducible
factor-1alpha promotes nonhypoxia-mediated proliferation in colon
cancer cells and xenografts. Cancer Res. 66:1684–1936. 2006.
View Article : Google Scholar
|
|
21
|
Shweta, Mishra KP, Chanda S, Singh SB and
Ganju L: A comparative immunological analysis of CoCl2
treated cells with in vitro hypoxic exposure. Biometals.
28:175–185. 2015. View Article : Google Scholar
|
|
22
|
Neufert C, Becker C and Neurath MF: An
inducible mouse model of colon carcinogenesis for the analysis of
sporadic and inflammation-driven tumor progression. Nat Protoc.
2:1998–2004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
George AL, Rajoria S, Suriano R, Mittleman
A and Tiwari RK: Hypoxia and estrogen are functionally equivalent
in breast cancer-endothelial cell interdependence. Mol Cancer.
11:802012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang L, Zheng Y, Xu H, Yan X and Chang X:
Investigate pathogenic mechanism of TXNDC5 in rheumatoid arthritis.
PLoS One. 8:e533012013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang X, Cui Y, Zong M, Zhao Y, Yan X,
Chen Y and Han J: Identification of proteins with increased
expression in rheumatoid arthritis synovial tissues. J Rheumatol.
36:872–880. 2009. View Article : Google Scholar
|
|
26
|
Vincent EE, Elder DJ, Phillips L, Heesom
KJ, Pawade J, Luckett M, Sohail M, May MT, Hetzel MR and Tavaré JM:
Overexpression of the TXNDC5 protein in non-small cell lung
carcinoma. Anticancer Res. 31:1577–1582. 2011.PubMed/NCBI
|
|
27
|
Mizukami Y, Kohgo Y and Chung DC: Hypoxia
inducible factor-1 independent pathways in tumor angiogenesis. Clin
Cancer Res. 13:5670–5674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagaraju GP, Bramhachari PV, Raghu G and
El-Rayes BF: Hypoxia inducible factor-1α: its role in colorectal
carcinogenesis and metastasis. Cancer Lett. 366:11–18. 2015.
View Article : Google Scholar
|
|
29
|
Zhang L, Hou Y, Li N, Wu K and Zhai J: The
influence of TXNDC5 gene on gastric cancer cell. J Cancer Res Clin
Oncol. 136:1497–1505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SO, Jin UH, Kang JH, Kim SB, Guthrie
AS, Sreevalsan S, Lee JS and Safe S: The orphan nuclear receptor
NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress
in pancreatic cancer cells. Mol Cancer Res. 12:527–538. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malhotra JD and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress: a vicious cycle or a
double-edged sword. Antioxid Redox Signal. 9:2277–2293. 2007.
View Article : Google Scholar
|
|
32
|
Clarke R, Cook KL, Hu R, Facey CO,
Tavassoly I, Schwartz JL, Baumann WT, Tyson JJ, Xuan J, Wang Y, et
al: Endoplasmic reticulum stress, the unfolded protein response,
autophagy, and the integrated regulation of breast cancer cell
fate. Cancer Res. 72:1321–1331. 2012.
|
|
33
|
Wang WA, Groenendyk J and Michalak M:
Endoplasmic reticulum stress associated responses in cancer.
Biochim Biophys Acta. 1843:2143–2149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gu MX, Fu Y, Sun XL, Ding YZ, Li CH, Pang
W, Pan S and Zhu Y: Proteomic analysis of endothelial lipid rafts
reveals a novel role of statins in antioxidation. J Proteome Res.
11:2365–2373. 2012. View Article : Google Scholar
|