Introduction
As the most common primary bone malignancy,
osteosarcoma is one of the major causes of cancer-associated
mortality in children and young adults (1,2).
In recent years, with the development of comprehensive treatments,
the 5-year survival rate for patients with osteosarcoma has
significantly improved (3–6).
However, patients that experience osteosarcoma recurrence still
have a low 5-year survival rate, as few clinical prognostic
biomarkers for recurrence have been identified (7,8).
Previous studies have characterized the cellular mechanisms
underlying osteosarcoma and have identified numerous potential
therapeutic targets (9–11). Wang et al reported that a
competitive endogenous RNA (ceRNA) may be a therapeutic target for
the molecular therapy of osteosarcoma (12). Furthermore, a growing number of
studies have demonstrated that ceRNAs regulate the processes of
osteosarcoma initiation and progression, including tumor cell
differentiation, proliferation, apoptosis and metastasis (13,14). These studies have implicated
ceRNAs in various biological processes in normal physiology and the
development of osteosarcoma; however, the prognostic role of ceRNAs
in recurrent osteosarcoma remains unclear. In particular, there are
currently no satisfactory biomarkers for the recurrence of
osteosarcoma following standard treatment. Therefore, it is
important to identify potential molecular markers associated with
the recurrence of osteosarcoma, which may provide valuable
information for the diagnosis and treatment of this disease.
The present study constructed a ceRNA-ceRNA network
for osteosarcoma (OSceNet) by repurposing publicly available
matched expression profiles for microRNA (miRNA/miR) and mRNA from
the Gene Expression Omnibus (GEO) database. The present analysis
identified two recurrence-free survival-associated modules that
were regulated by hsa-miR-335-5p, and determined that the
expression levels of the ceRNAs in each module could distinguish
patients with good and poor survival outcomes based on the risk
score model method. These findings demonstrated the association
between ceRNA modules and tumor recurrence in osteosarcoma,
potentially allowing for the detection of ceRNA biomarkers
associated with osteosarcoma recurrence.
Materials and methods
Data collection
miRNA and mRNA expression profiles for osteosarcoma
prior to and following treatment were obtained from the GEO
database (accession nos. GSE39040 and GSE39055, respectively;
https://www.ncbi.nlm.nih.gov/geo/)
(15). Probes were mapped to
genes; a gene with numerous probes was represented by the mean of
the expression values of the probes. Matching miRNA and mRNA
expression profiles were obtained for further analysis. Clinical
data for the patients with osteosarcoma were also obtained for the
two datasets, including age, sex, recurrence status and other
clinical information.
Human miRNA-mRNA target data was collected from
TarBase (16), miRTarBase
(17) and miRecords (18), which manually curate data
regarding high-quality, experimentally validated miRNA-mRNA target
interactions from published experiments. By integrating data from
these three databases, a total of 44,181 non-redundant miRNA-mRNA
target interactions were obtained.
Construction of the OSceNet
The OSceNet was constructed using R software
(v3.2.1; http://mirrors.tuna.tsinghua.edu.cn/CRAN/) according
to two principles: The two mRNAs were regulated by the same miRNA,
and the two mRNAs followed the same expression pattern. Firstly,
correlations between the previously validated miRNA-mRNA target
pairs in the matched miRNA and mRNA expression profiles were
evaluated by Pearson correlation coefficients. The pairs with a
significant negative correlation were regarded as relevant
miRNA-mRNA pairs in the context of osteosarcoma (R< −0.4,
adjusted P<0.05). Secondly, all mRNA-mRNA pairs that were both
targeted by at least one miRNA were listed as candidate ceRNA
pairs. Thirdly, for each candidate ceRNA pair, the Pearson
correlation coefficient between them was calculated. All candidate
ceRNA pairs with R>0.4 and adjusted P<0.05 were considered
ceRNA-ceRNA interactions. By assembling all the identified ceRNA
pairs, the OSceNet was constructed. Within the OSceNet, each node
represents an mRNA, and two nodes are connected if they are
co-regulated by at least one miRNA and are co-expressed in
osteosarcoma.
Network visualization
The networks were visualized using Cytoscape 3.3.0
(19), including the OSceNet and
the modules view.
Recurrence analysis using the risk score
model
The present study assessed the impact on the
recurrence-free prognosis for each module of the OSceNet using the
risk score model. The risk score model was constructed by
considering the power of each of the ceRNAs in the module, as
evaluated by univariate Cox regression analysis, and the relative
expression of each ceRNA, as follows:
Risk score=∑i=1ncoefi∗expi
where n is the number of ceRNAs in the module,
expi is the expression level of ceRNA i,
and coefi is the estimated regression coefficient
for ceRNA i from the univariate Cox regression analysis. The
median risk score value was selected as a cutoff to classify
patients into high- and low-risk groups. Recurrence-free survival
analyses were performed to assess the difference in recurrence
probability between the high- and low-risk groups, and the
statistical significance was determined by a log-rank test using
the R package ‘survival’ (
https://github.com/therneau/survival).
Statistical analysis
Random networks with the same architecture in
OSceNet were generated using R package ‘igraph’ (http://igraph.org). Modules in OSceNet were identified
using cFinder software (20).
Multivariate Cox regression analyses were performed using Cox
proportional hazards regression model to determine whether the
prognostic models were independent of other clinical variables,
adjusting for age, sex, primary disease site and chemosensitivity
as covariates. The time-dependent receiver operating characteristic
(ROC) curve analysis was performed using R software. All
statistical analyses were performed using R software (v3.2.1),
including Fisher’s exact test and Wilcoxon sum rank test. In the
present study, P<0.1 was considered to indicate a statistically
significant difference due to the small sample size (37
patients).
Results
Characterization of the topological
features and competitive interactions in the OSceNet
The matched miRNA and mRNA expression profiles of 37
patients with osteosarcoma were obtained from the GEO database,
after which the OSceNet was constructed. Three steps were followed
to generate the network: i) A total of 130 miRNA-mRNA pairs were
determined between 28 miRNAs and 122 mRNAs, which were
significantly negatively correlated in osteosarcoma (R< -0.4,
adjusted P<0.05). ii) A total of 1,826 candidate ceRNA-ceRNA
pairs were identified, which were co-regulated by at least one
miRNA. iii) A total of 156 candidate ceRNA-ceRNA pairs were
identified as positively correlated with each other (R>0.4,
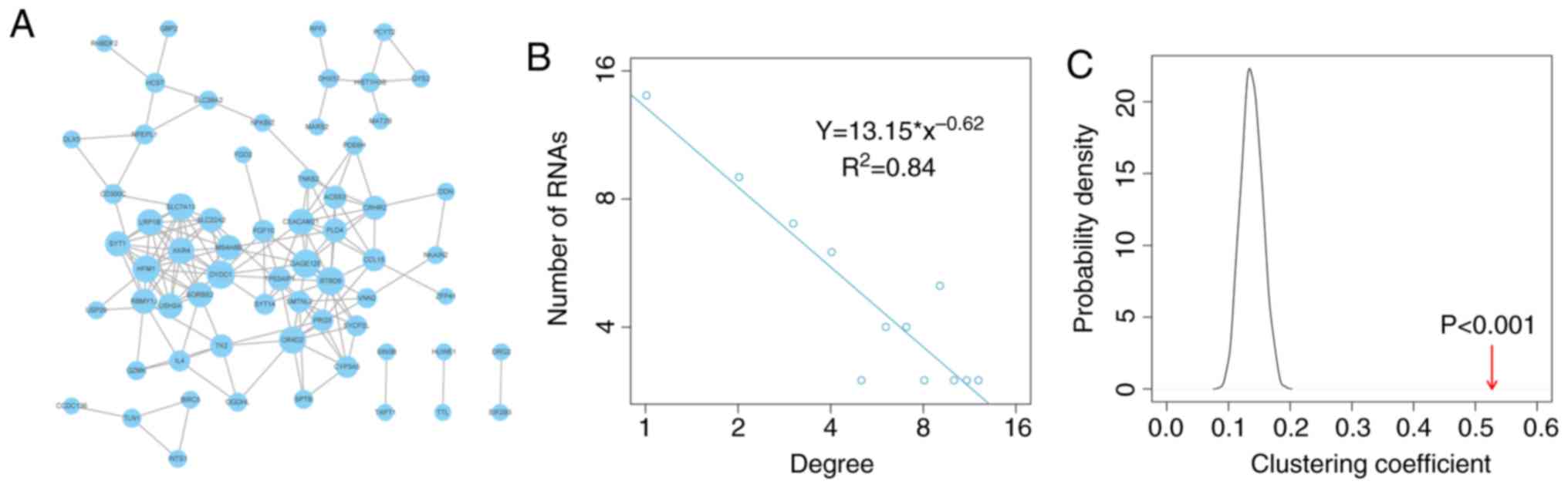
adjusted P<0.05), forming a network of 64 mRNAs (Fig. 1A).
To explore the structure and features of the
OSceNet, degree distribution analysis of the nodes in the network
was performed. The present study identified that most nodes had
relatively few interactions, whereas a small proportion of nodes
had several interactions, which fits with a power-law distribution,
thus suggesting that the network was scale-free and different from
randomly generated networks (Fig.
1B). Subsequently, 1,000 random networks were generated that
preserved the same number of nodes and edges, and the same degree
for each node as in OSceNet using the R package ‘igraph’. Comparing
the clustering coefficient between the OSceNet and the random
networks revealed strong, non-random competitive interactions
between the OSceNet ceRNA-ceRNA pairs (Fig. 1C; P<0.001).
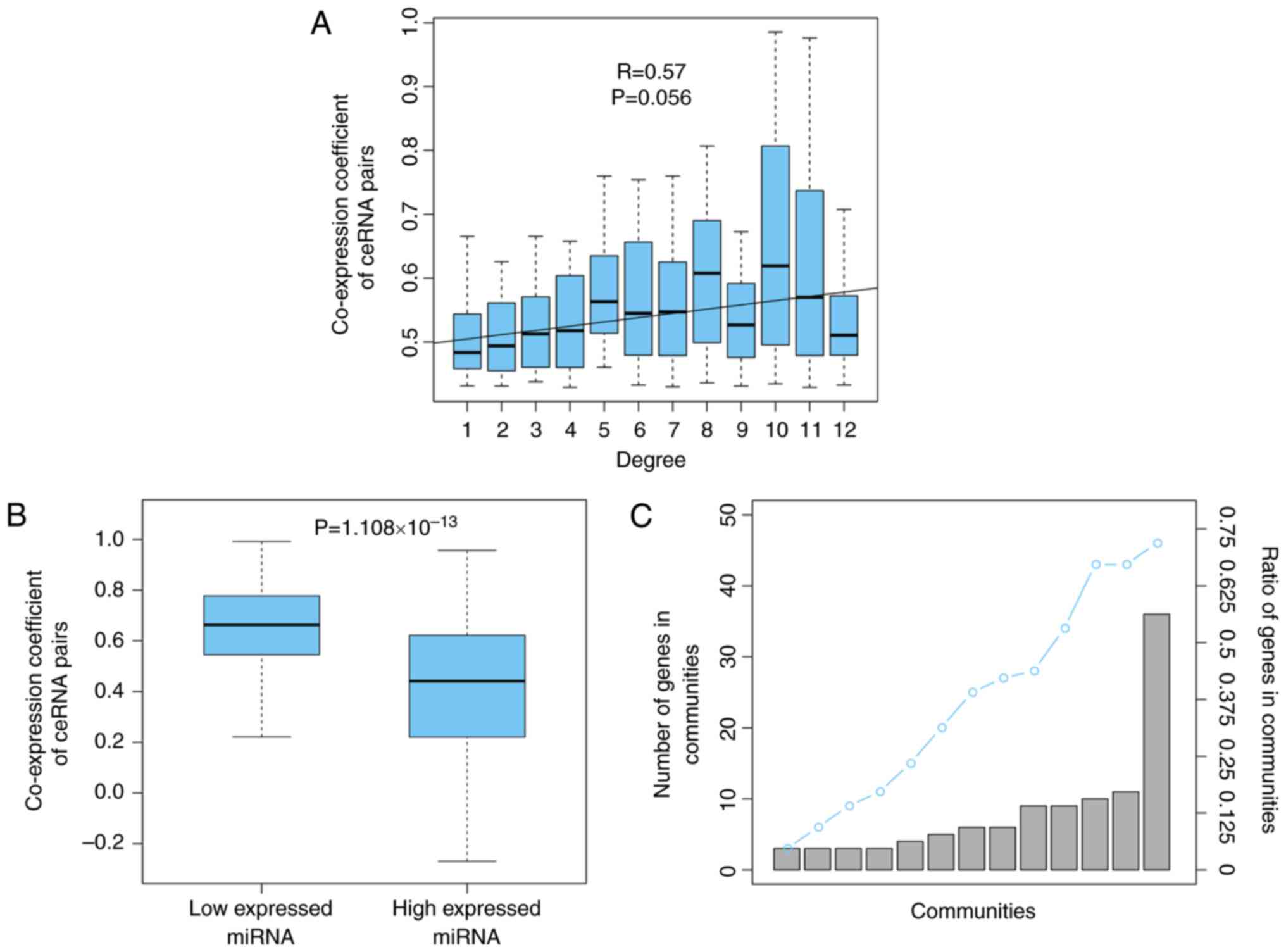
To analyze the competitive intensity among the ceRNA
interaction pairs in OSceNet, the present study demonstrated that
the nodes with a higher degree, indicating more competitors, were
more strongly regulated by their interacting neighbors (R=0.57,
P=0.056; Fig. 2A), which was
consistent with a previous study (21). In addition, the present study
investigated the effects of miRNA expression on ceRNA-ceRNA
interactions. For each miRNA shared by a ceRNA pair, the tumor
expression profiles were divided into high and low miRNA
expression, using the median value for miRNA expression as the
cutoff. By comparing the co-expression of the ceRNA pairs between
these two groups of tumor samples, it was revealed that the ceRNA
pairs displayed significantly increased competition in samples with
low miRNA expression (P=1.108×10−13; Fig. 2B). This is understandable, as
mRNAs will be fully suppressed when the interacting miRNA is
abundantly expressed; however, when the miRNA molecules are
relatively sparse, the targeted mRNAs will only be partially
suppressed, and the upregulation of one mRNA will further weaken
the miRNA suppression for its partner, resulting in strong
co-expression of the ceRNA pairs. Subsequently, the present study
analyzed the modular structure of the OSceNet. A total of 13
modules were identified using cFinder software (22). Analysis of the genes in the
modules showed that 72% of the nodes from the OSceNet were part of
at least one module, thus indicating that the ceRNA pairs tend to
interact with other pairs, as a module, in osteosarcoma (Fig. 2C).
Identification of two recurrence-free
survival-associated modules
To identify the modules with the most robust ability
to predict recurrence-free survival for patients with osteosarcoma,
the present study randomly divided the 37 samples into two groups:
The training dataset, which contained 19 samples, and the test
dataset, which consisted of 18 samples. To avoid the prognostic
noise of clinical factors, the division was balanced to obtain two
datasets with similar clinical information (Table I). For each of the 13 modules
identified, the associated recurrence-free survival prognosis was
evaluated using a module-specific risk score model (23,24). The risk score model was
constructed according to the linear combination of the expression
values of genes in the module weighted by the regression
coefficients derived from univariate Cox regression analysis
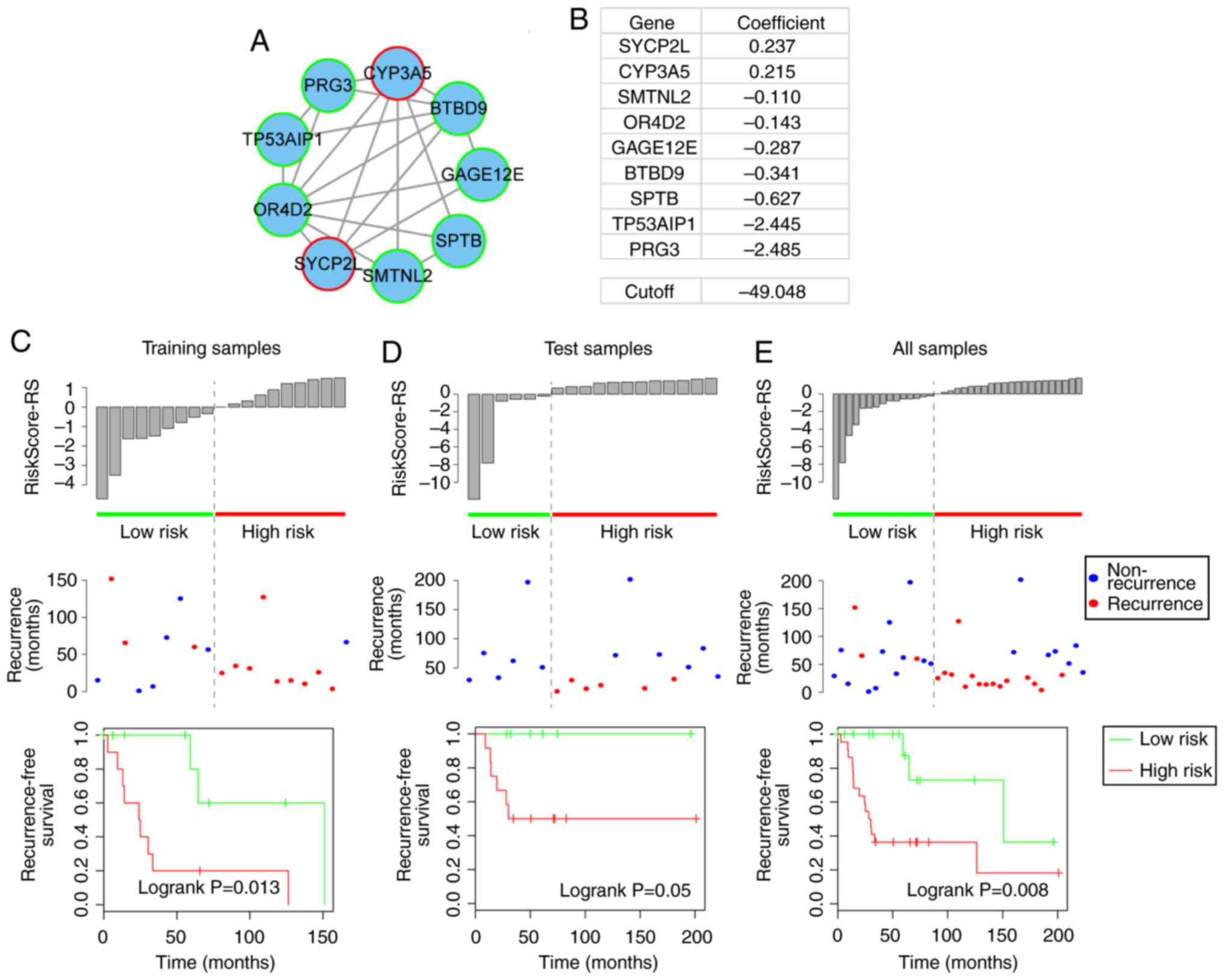
(25,26). For example, module 1 included two
risk-associated genes, which had positive regression coefficients,
and seven protective genes, which had negative regression
coefficients, as identified by univariate Cox regression analysis,
and the risk score model for module 1 was as follows: Risk
score=[0.237× expression value of synaptonemal complex protein
2-like (SYCP2L)]+[0.215× expression value of cytochrome P450 family
3 subfamily A member 5 (CYP3A5)]+[−0.11× expression value of
smoothelin-like 2 (SMTNL2)]+[−0.143× expression value of olfactory
receptor family 4 subfamily D member 2 (OR4D2)]+[−0.287× expression
value of G antigen 12E (GAGE12E)]+[−0.341× expression value of BTB
domain-containing 9 (BTBD9)]+[−0.627× expression value of spectrin
β, erythrocytic (SPTB)]+[−2.445× expression value of tumor protein
p53-regulated apoptosis-inducing protein 1 (TP53AIP1)]+[−2.485×
expression value of proteoglycan 3, pro eosinophil major basic
protein 2 (PRG3)]. The present study then calculated the risk score
of each module for each sample in the training dataset, and divided
the samples into a low-risk group (e.g. n=9 in module 1) and a
high-risk group (e.g. n=10 in module 1) with the median risk score
(e.g. risk score=−49.048 in module 1) as the cutoff. The prognosis
of each module was then validated in the test dataset and complete
datasets, using the same procedure with an unchanged risk score
model and cutoff. The two modules that most robustly predicted the
recurrence-free survival prognosis were then selected, as shown in
Figs. 3 and 4.
 | Table IClinical factors of patients in the
training and test datasets. |
Table I
Clinical factors of patients in the
training and test datasets.
| Variable | Training
set
(n=19) | Test set
(n=18) | P-value |
|---|
| Sex | | | |
| Female | 8 | 9 | 0.746a |
| Male | 11 | 9 | |
| Site | | | |
| Left | 11 | 13 | 0.495a |
| Right | 8 | 5 | |
|
Chemosensitivity | | | |
| Favorable | 7 | 7 | 1a |
| Unfavorable | 12 | 11 | |
| Recurrence | | | |
| Yes | 12 | 6 | 0.103a |
| No | 7 | 12 | |
| Age (mean ±
SD) | 13.32±6.87 | 13.67±14.91 | 0.445b |
| Recurrence time
(mean ± SD) | 46.89±45.11 | 59.28±55.55 | 0.328b |
Prognostic module 1
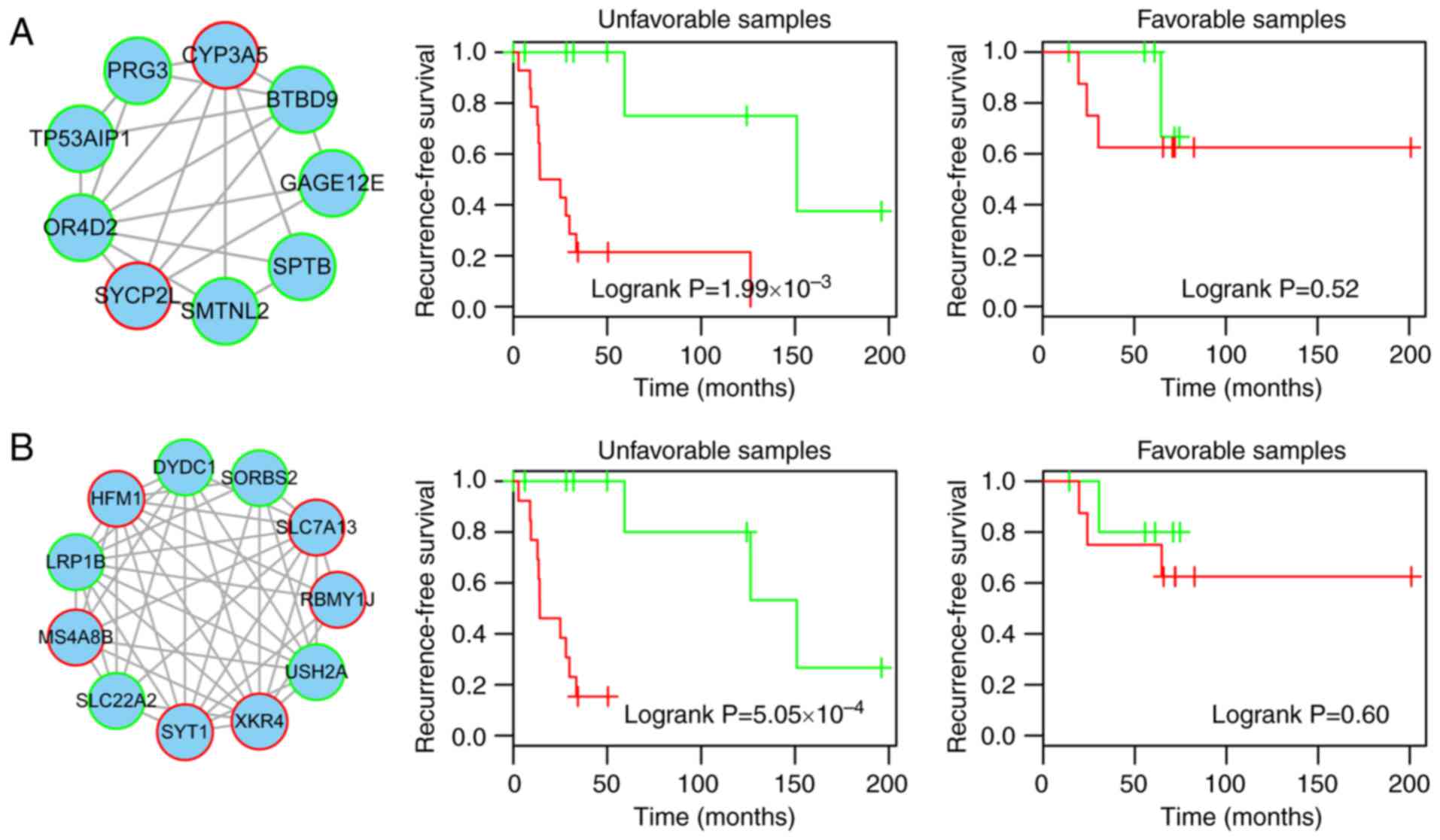
Prognostic module 1 consisted of two risk genes
(SYCP2L and CYP3A5) and seven protective genes (SMTNL2, OR4D2,
GAGE12E, BTBD9, SPTB, TP53AIP1 and PRG3) (Fig. 3A and B). Of these, previous
studies also revealed CYP3A5 to be a risk gene in osteosarcoma. For
example, Dhaini et al reported that the expression levels of
CYP3A5 were significantly increased in primary biopsies from
patients who developed distant metastatic disease compared with
biopsies from patients with nonmetastatic disease (27). Furthermore, Trujillo-Paolillo
et al also reported that CYP genes serve an important role
in osteosarcoma tumorigenesis at primary and metastatic sites, as
well as in treatment response (28). The present study revealed a novel
potential mechanism by which CYP3A5 may promote osteosarcoma
tumorigenesis via its ceRNA activity.
Compared with the low-risk samples in the training,
test and complete datasets, the high-risk samples, as defined by
module 1 exhibited a higher rate of recurrence (training, 33.3 vs.
90%; test, 0 vs. 50%; total, 20 vs. 68.2%) and a significantly
reduced recurrence-free survival time (log-rank P=0.013, 0.05 and
0.008, respectively; Fig.
3C–E).
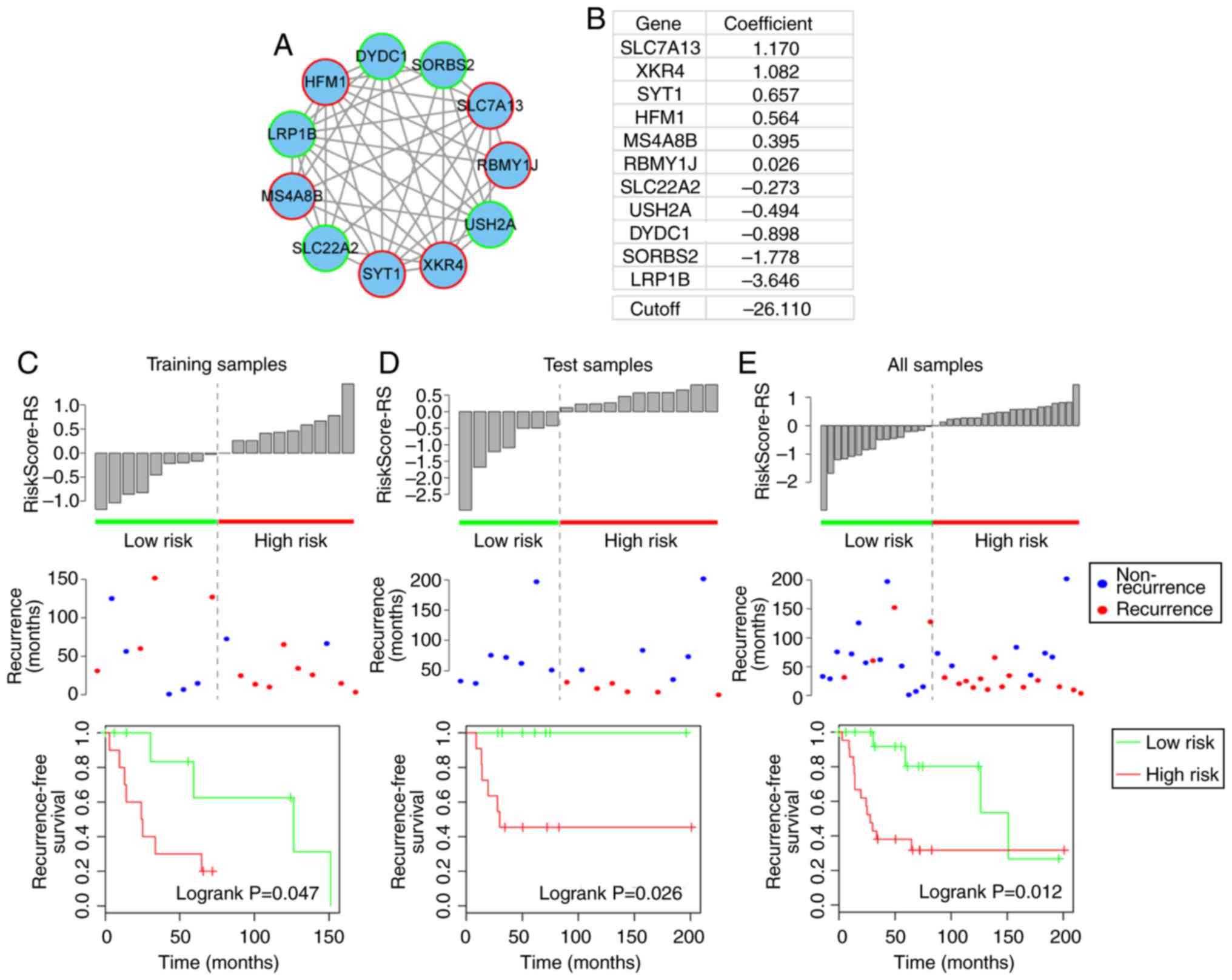
Prognostic module 2
Prognostic module 2, as shown in Fig. 4A, contained 11 genes, including
six risk genes [solute carrier family 7 member 13 (SLC7A13),
XK-related 4 (XKR4), synaptotagmin 1, HFM1, ATP-dependent DNA
helicase homolog (HFM1), membrane spanning 4-domains A8 and
RNA-binding motif protein, Y-linked, family 1, member J] and five
protective genes [solute carrier family 22 member 2, usherin
(USH2A), DPY30 domain-containing 1 (DYDC1), sorbin and SH3
domain-containing 2 and low-density lipoprotein (LDL)
receptor-related protein 1B (LRP1B)]. From this module, LRP1B,
which is a member of the LDL receptor family, is particularly
notable, as another member of the LDL receptor family (LRP1/LRP1A)
is significant in osteoblast behavior (29). In addition, the present study
revealed that LRP1B is a protective gene in osteosarcoma (Fig. 4B). The high- and low-risk samples
defined by module 2 also exhibited significantly different
associated recurrence-free survival prognosis in the training, test
and complete datasets (log-rank P=0.047, 0.026 and 0.012,
respectively) (Fig. 4C–E).
Effects of the two identified modules on
recurrence-free prognosis are independent of clinical factors
To determine whether the association of the two
modules with recurrence-free prognosis was independent and robust,
a multivariate Cox regression analysis was conducted, including
risk score defined by the modules, age, sex, primary disease site
and chemosensitivity as covariates. The results indicated that risk
score was independently significant and the most valuable
prognostic factor (HR=9.03, P=0.0045 for module 1; HR=13.42,
P=0.0014 for module 2; Table
II). In addition, chemosensitivity of the samples was also
associated with recurrence-free prognosis (P=0.03 for module 1,
P=0.0068 for module 2; Table
II). Therefore, the present study subsequently inspected the
ability of the risk score to predict recurrence-free prognosis in
the samples stratified by chemosensitivity into favorable (n=14)
and unfavorable (n=23) strata. The patients within each
chemosensitivity stratum were then classified into high and low
risk scores based on modules 1 and 2, respectively. For the
unfavorable samples, the results demonstrated that the risk score
variables derived from modules 1 or 2 had significant prognostic
value for recurrence-free survival (log-rank P=1.99×10−3
and 5.05×10−4, respectively, Fig. 5). For the favorable
chemosensitivity stratum, the prognostic impact of the modules was
reduced (Fig. 5). These results
indicated that the prognostic power of the module-based risk score
was also chemosensitivity-independent, particularly for patients
with unfavorable chemosensitivity. For patients with unfavorable
chemosensitivity, early diagnosis may be possible using the risk
score. In addition, combining chemosensitivity and the risk score
may provide more accurate prognostic information for patients.
 | Table IIMultivariate Cox regression analysis
of the risk score defined by the two modules and recurrence-free
survival for patients with osteosarcoma. |
Table II
Multivariate Cox regression analysis
of the risk score defined by the two modules and recurrence-free
survival for patients with osteosarcoma.
| Variable | Module 1
| Module 2
|
|---|
| HR | 95% CI of HR | P-value | HR | 95% CI of HR | P-value |
|---|
| Risk score | 9.03 | (1.98–41.25) |
4.5×10−3 | 13.42 | (2.73–66.07) |
1.4×10−3 |
| Age | 1.03 | (0.98–1.08) | 0.22 | 1.00 | (0.96–1.06) | 0.76 |
| Sex | | | | | | |
| Female | | | 1 (reference) | | |
| Male | 1.84 | (0.68–4.98) | 0.23 | 3.34 | (1.13–9.90) | 0.03 |
| Site | | | | | | |
| Left | | | 1 (reference) | | |
| Right | 2.76 | (0.95–8.03) | 0.06 | 1.47 | (0.52–4.19) | 0.47 |
|
Chemosensitivity | | | | | | |
| Favorable | | | 1 (reference) | | |
| Unfavorable | 3.73 | (1.14–12.17) | 0.03 | 6.04 | (1.64–22.20) |
6.8×10−3 |
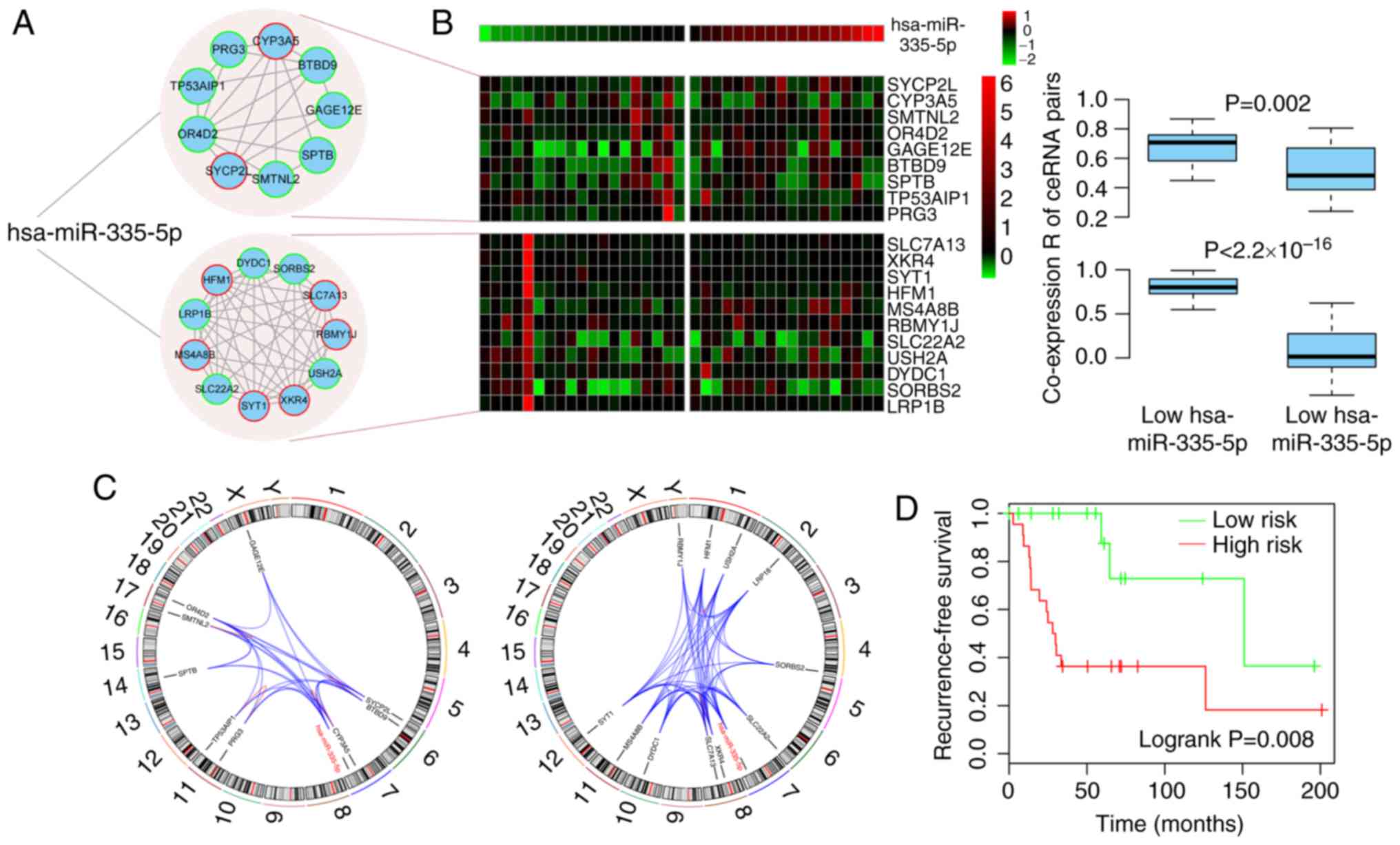
Two prognostic modules are regulated by
hsa-miR-335-5p
The present study revealed that the two modules were
independent prognostic factors for recurrence-free survival in
osteosarcoma. No common genes existed common between the two
modules. When considering upstream miRNAs that regulate the two
modules, the same miRNA, hsa-miR-335-3p, was shared by all nodes in
modules 1 and 2 (Fig. 6A). Using
the median relative expression value for hsa-miR-335-3p across all
samples, the samples were divided into high and low hsa-miR-335-3p
expression groups. In the two modules, the co-expression
coefficients for ceRNA pairs in the low group were significantly
higher than those in the high group (P=0.002,
<2.2×10−16, respectively), thus suggesting that the
two modules are highly regulated by hsa-miR-335-3p (Fig. 6B).
When analyzing the genomic location of the ceRNAs in
the modules, it was revealed that the majority of the ceRNA pairs
regulated each other across chromosomes, as only three out of 21
and two out of 45 ceRNA pairs were located on the same chromosome
for module 1 and module 2 (SYCP2L and BTBD9 on chromosome 6,
TP53AIP1 and PRG3 on chromosome 11, OR4D2 and SMTNL2 on chromosome
17 for module 1; HFM1 and USH2A on chromosome 1, XKR4 and SLC7A13
on chromosome 8 for module 2), respectively, which is consistent
with previous studies (21,30). In addition, the miRNA-mRNA
regulation had a similar result, as only one miRNA-mRNA pair
(hsa-miR-335-5p and CYP3A5) was located on the same chromosome in
module 1 (Fig. 6C). By combining
the expression levels of the genes from the two modules and
hsa-miR-335-5p, a novel prognostic model for recurrence-free
survival was generated with enhanced power. In this novel risk
score model, samples in the high-risk group had a significantly
reduced recurrence-free survival time compared with those in the
low-risk group (median recurrence-free survival, 28.9 vs. 151.0
months, log-rank P=0.008; Fig.
6D).
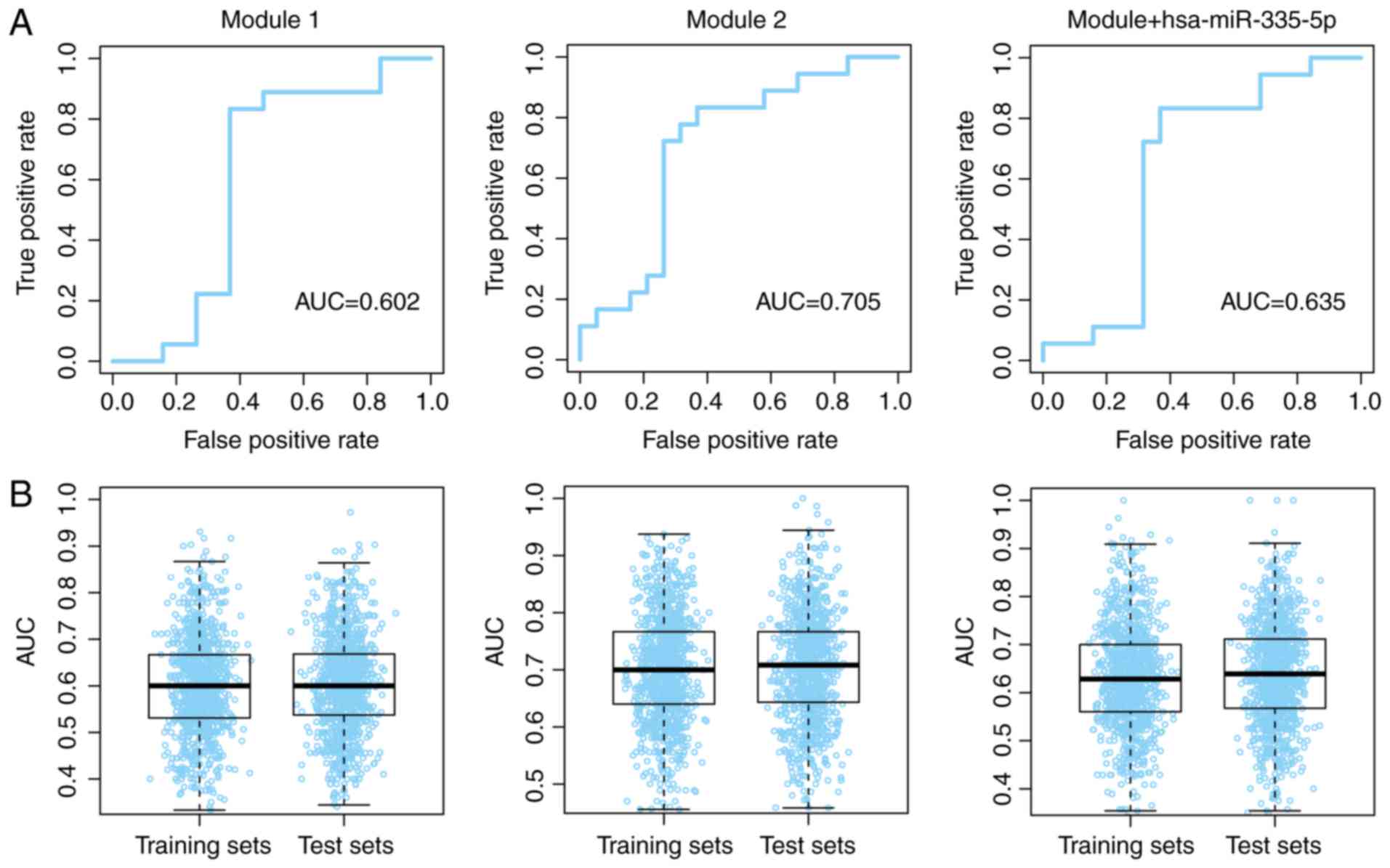
The prognostic value for modules 1 and 2, and the
combination of the modules with hsa-miR-335-5p, was further
validated using ROC curves. The patients with and without
recurrence were used as true positive and true negative data-sets,
respectively. Good area under the curve (AUC) values were obtained
(Fig. 7A). To avoid model
overfitting to the training and test datasets, the group division
process was repeated with 1,000 iterations, and the AUC values for
the three models were evaluated in each training and test dataset.
The median AUC values ranged between 0.60 and 0.71 across the
different dataset divisions (Fig.
7B). These results indicated that the modules may possess a
robust ability to predict the recurrence-free survival of patients
with osteosarcoma.
Discussion
Osteosarcoma is the most commonly occurring primary
bone malignancy in children and young adults; however diagnostic
accuracy and the effectiveness of treatment are far from
satisfactory, particularly for patients experiencing osteosarcoma
recurrence (31,32). Previous studies have demonstrated
that ceRNAs serve a crucial role in the development of osteosarcoma
(12,31,33); however, it remains unclear whether
ceRNAs are implicated in tumor recurrence. Therefore, the present
study aimed to investigate the potential role of ceRNAs in
osteosarcoma recurrence, and to identify potential biomarkers.
The present study identified two modules (modules 1
and 2), with no common ceRNAs between them, with prognostic power
for predicting the recurrence-free survival outcome for patients
with osteosarcoma. Either module 1 or 2 could effectively divide
patients with osteosarcoma into high- and low-risk groups, as
defined by recurrence-free survival, through the relative
expression levels of genes in each module. When comparing the
results of the patients with osteosarcoma between the two modules,
it was revealed that 32 out of 37 patients were classified into the
same group by modules 1 and 2. Considering the mutually exclusive
ceRNAs in the two modules, these consistent predictive results
indicated the accuracy of modules 1 and 2 in predicting the
prognosis for patients with osteosarcoma.
Numerous studies have identified
osteosarcoma-associated ceRNAs, and have demonstrated their value
in predicting prognosis for survival or recurrence, including small
nucleolar RNA host gene 1 and X inactive specific transcript
(non-protein coding) (10,34,35).
However, the majority of these studies focus on just one
ceRNA-ceRNA pair, instead of taking a global view of the
ceRNA-ceRNA network and modules. In the prognostic modules 1 and 2
identified in the present study, no single gene exhibited a
significant prognostic ability for the recurrence-free survival of
patients with osteosarcoma, with the exception of TP53AIP1, which
was somewhat associated with prognosis (P=0.08). This may be the
reason why these ceRNAs have not been identified in previous
studies; therefore, it may be hypothesized that the ceRNAs in the
modules affect osteosarcoma development as a whole, rather than by
acting individually. In addition, the results revealed that
hsa-miR-335-5p may influence the recurrence of osteosarcoma by
regulating these two ceRNA interaction modules, rather than by
itself (P=0.717, univariate Cox regression analysis). These results
indicated that hsa-miR-335-5p may be considered a potential novel
therapeutic target, as the regulator of these two modules. The
present identification of recurrence-free survival-associated
factors at the level of modules opens novel avenues for leveraging
publicly available data to identify prognostic biomarkers for
patients with osteosarcoma.
In conclusion, the present study integrated
confirmed experimental interactions between miRNAs and mRNAs, and
used gene expression data for osteosarcoma from the GEO database to
construct a ceRNA-ceRNA interaction network. Two modules were
subsequently identified, and further survival analysis demonstrated
that these modules were significantly associated with the
recurrence-free survival of patients with osteosarcoma. The ability
of these two modules to predict recurrence-free prognosis was
independent of other clinical factors. Furthermore, hsa-miR-335-3p
regulated all of the ceRNAs from the two modules, and the vast
majority of the ceRNA pairs regulated each other across
chromosomes, as determined by analyzing their genomic locations.
These findings may provide additional useful data for the
development of clinical therapeutic targets against osteosarcoma
recurrence.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The full network containing 64 mRNAs is available in the
bilab repository (http://shiyabshi230.j2eeall.com).
Authors’ contributions
ZB and YC conceived and designed the experiments.
YC, QC, JZ and YZ analyzed the data. ZB and YC wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no conflicts of
interest.
References
|
1
|
Lee L, Fei L, Pope J and Wagner LM: Early
lymphocyte recovery and outcome in osteosarcoma. J Pediatr Hematol
Oncol. 39:179–183. 2017. View Article : Google Scholar
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cam M, Gardner HL, Roberts RD, Fenger JM,
Guttridge DC, London CA and Cam H: ∆Np63 mediates cellular survival
and metastasis in canine osteosarcoma. Oncotarget. 7:48533–48546.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao L, Wang J and Wang PQ: MiR-326 is a
diagnostic biomarker and regulates cell survival and apoptosis by
targeting Bcl-2 in osteosarcoma. Biomed Pharmacother. 84:828–835.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tu B, Zhu J, Liu S, Wang L, Fan Q, Hao Y,
Fan C and Tang TT: Mesenchymal stem cells promote osteosarcoma cell
survival and drug resistance through activation of STAT3.
Oncotarget. 7:48296–48308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Y, Lu Q, Xu J, Yan R, Zhu J, Xu J,
Jiang X, Li J and Wu F: The effect of pathological fractures on the
prognosis of patients with osteosarcoma: A meta-analysis of 14
studies. Oncotarget. 8:73037–73049. 2017.PubMed/NCBI
|
|
7
|
Li YJ, Yao K, Lu MX, Zhang WB, Xiao C and
Tu CQ: Prognostic value of the C-reactive protein to albumin ratio:
A novel inflammation-based prognostic indicator in osteosarcoma.
Onco Targets Ther. 10:5255–5261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang XX, Liao Y, Hong L, Zeng Z, Yuan TB,
Xia X and Qin J: Tissue microarray staining reveals PLD1 and Sp1
have a collaborative, pro-tumoral effect in patients with
osteosarcomas. Oncotarget. 8:74340–74347. 2017.PubMed/NCBI
|
|
9
|
Chen YM, Shen QC, Gokavarapu S, Ong HS,
Cao W and Ji T: Osteosarcoma of the Mandible: A site-specific study
on survival and prognostic factors. J Craniofac Surg. 27:1929–1933.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Dou P, Liu T and He S: Application
of long Noncoding RNAs in osteosarcoma: Biomarkers and therapeutic
targets. Cell Physiol Biochem. 42:1407–1419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simpson S, Dunning MD, de Brot S,
Grau-Roma L, Mongan NP and Rutland CS: Comparative review of human
and canine osteosarcoma: Morphology, epidemiology, prognosis,
treatment and genetics. Acta Vet Scand. 59:712017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Zhang Y, Yang T, Zhao W, Wang N,
Li P, Zeng X and Zhang W: Long non-coding RNA MALAT1 for promoting
metastasis and proliferation by acting as a ceRNA of miR-144 3p in
osteosarcoma cells. Oncotarget. 8:59417–59434. 2017.PubMed/NCBI
|
|
13
|
Gao S, Cheng C, Chen H, Li M, Liu K and
Wang G: IGF1 3’UTR functions as a ceRNA in promoting angiogenesis
by sponging miR-29 family in osteosarcoma. J Mol Histol.
47:135–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Yang T, Zhang Z, Lu M, Zhao W,
Zeng X and Zhang W: Long non-coding RNA TUG1 promotes migration and
invasion by acting as a ceRNA of miR-335-5p in osteosarcoma cells.
Cancer Sci. 108:859–867. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly AD, Haibe-Kains B, Janeway KA, Hill
KE, Howe E, Goldsmith J, Kurek K, Perez-Atayde AR, Francoeur N, Fan
JB, et al: MicroRNA paraffin-based studies in osteosarcoma reveal
reproducible independent prognostic profiles at 14q32. Genome Med.
5:22013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vlachos IS, Paraskevopoulou MD, Karagkouni
D, Georgakilas G, Vergoulis T, Kanellos I, Anastasopoulos IL,
Maniou S, Karathanou K, Kalfakakou D, et al: DIANA-TarBase v7.0:
Indexing more than half a million experimentally supported miRNA:
mRNA interactions. Nucleic Acids Res. 43(Database issue):
D153–D159. 2015. View Article : Google Scholar
|
|
17
|
Hsu SD, Tseng YT, Shrestha S, Lin YL,
Khaleel A, Chou CH, Chu CF, Huang HY, Lin CM, Ho SY, et al:
miRTarBase update 2014: An information resource for experimentally
validated miRNA-target interactions. Nucleic Acids Res. 42(Database
issue): D78–D85. 2014. View Article : Google Scholar :
|
|
18
|
Xiao F, Zuo Z, Cai G, Kang S, Gao X and Li
T: miRecords: An integrated resource for microRNA-target
interactions. Nucleic acids Res. 37(Database issue): D105–D110.
2009. View Article : Google Scholar
|
|
19
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palla G, Derényi I, Farkas I and Vicsek T:
Uncovering the overlapping community structure of complex networks
in nature and society. Nature. 435:814–818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer
A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J
and Califano A: An extensive microRNA-mediated network of RNA-RNA
interactions regulates established oncogenic pathways in
glioblastoma. Cell. 147:370–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adamcsek B, Palla G, Farkas IJ, Derényi I
and Vicsek T: CFinder: Locating cliques and overlapping modules in
biological networks. Bioinformatics. 22:1021–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alizadeh AA, Gentles AJ, Alencar AJ, Liu
CL, Kohrt HE, Houot R, Goldstein MJ, Zhao S, Natkunam Y, Advani RH,
et al: Prediction of survival in diffuse large B-cell lymphoma
based on the expression of 2 genes reflecting tumor and
microenvironment. Blood. 118:1350–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lossos IS, Czerwinski DK, Alizadeh AA,
Wechser MA, Tibshirani R, Botstein D and Levy R: Prediction of
survival in diffuse large-B-cell lymphoma based on the expression
of six genes. N Engl J Med. 350:1828–1837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou M, Wang X, Shi H, Cheng L, Wang Z,
Zhao H, Yang L and Sun J: Characterization of long non-coding
RNA-associated ceRNA network to reveal potential prognostic lncRNA
biomarkers in human ovarian cancer. Oncotarget. 7:12598–12611.
2016.PubMed/NCBI
|
|
26
|
Zhou M, Xu W, Yue X, Zhao H, Wang Z, Shi
H, Cheng L and Sun J: Relapse-related long non-coding RNA signature
to improve prognosis prediction of lung adenocarcinoma. Oncotarget.
7:29720–29738. 2016.PubMed/NCBI
|
|
27
|
Dhaini HR, Thomas DG, Giordano TJ, Johnson
TD, Biermann JS, Leu K, Hollenberg PF and Baker LH: Cytochrome P450
CYP3A4/5 expression as a biomarker of outcome in osteosarcoma. J
Clin Oncol. 21:2481–2485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trujillo-Paolillo A, Tesser-Gamba F,
Petrilli AS, de Seixas Alves MT, Garcia Filho RJ, de Oliveira R and
de Toledo SRC: CYP genes in osteosarcoma: Their role in
tumorigenesis, pulmonary metastatic microenvironment and treatment
response. Oncotarget. 8:38530–38540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niemeier A, Kassem M, Toedter K, Wendt D,
Ruether W, Beisiegel U and Heeren J: Expression of LRP1 by human
osteoblasts: A mechanism for the delivery of lipoproteins and
vitamin K1 to bone. J Bone Miner Res. 20:283–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tay Y, Kats L, Salmena L, Weiss D, Tan SM,
Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, et al:
Coding-independent regulation of the tumor suppressor PTEN by
competing endogenous mRNAs. Cell. 147:344–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cong C, Wang W, Tian J, Gao T, Zheng W and
Zhou C: Identification of serum miR-124 as a biomarker for
diagnosis and prognosis in osteosarcoma. Cancer Biomark.
21:449–454. 2018. View Article : Google Scholar
|
|
32
|
Jin H, Luo S, Wang Y, Liu C, Piao Z, Xu M,
Guan W, Li Q, Zou H, Tan QY, et al: miR-135b stimulates
osteosarcoma recurrence and lung metastasis via Notch and
Wnt/β-catenin signaling. Mol Ther Nucleic Acids. 8:111–122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sha B, Gao W, Han Y, Wang S, Wu J, Xu F
and Lu T: Potential application of titanium dioxide nanoparticles
in the prevention of osteosarcoma and chondrosarcoma recurrence. J
Nanosci Nanotechnol. 13:1208–1211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Cao L, Wu J and Wang Q: Long
non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326
and promotes tumorigenesis in osteosarcoma. Int J Oncol. 52:77–88.
2018.
|
|
35
|
Zhang R and Xia T: Long non-coding RNA
XIST regulates PDCD4 expression by interacting with miR-21-5p and
inhibits osteosarcoma cell growth and metastasis. Int J Oncol.
51:1460–1470. 2017. View Article : Google Scholar : PubMed/NCBI
|