Introduction
Intracranial aneurysms (IAs) are commonly observed
in general people with prevalence rate between 2 and 7% and
incidence rate between 1 and 5%. Subarachnoid bleeding due to a
ruptured IA leads to serious consequences resulting in marked
morbidity and substantial mortality rates. The mechanisms for the
development, progression and collapse of IAs remain largely
unknown, although a certain degree of progress has been made
recently in their diagnosis and treatment (1). In summary, cerebral infarction has a
substantial negative impact on the outcome after SAH. The main
potentially treatable factors associated with infarction were
symptomatic vasospasm and temperature greater than 38°C on day 8
(1). In the clinical setting,
systemic high blood pressure is correlated with the initiation of
an IA and subarachnoid hemorrhage due to aneurysmal collapse.
However, whether high blood pressure causes the initiation of an IA
or subarachnoid hemorrhage remains to be confirmed (2–4).
The hypothesis that inflammation is central to IA
genesis is strengthened by the flow-mediated dysfunction of the
endothelium and the subsequent regulation of smooth muscle cells
(SMCs) to a synthetic phenotype (5). The contractile phenotype produces a
pro-remodeling and pro-inflammatory milieu, and hence accounts for
the inflammation in a number of vascular diseases, which in turn
results in persistence and amplification of the recruitment of
immune cells and endothelial dysfunction. SMCs of the media
progressively transform to a pathophysiological synthetic phenotype
from a quiescent contractile phenotype during the development of
aneurysms, as observed on sections of human samples for
histological examination, and express matrix metalloproteinases and
pro-inflammatory cytokines (6,7).
These proteases have a role in the degradation of the extracellular
matrix and in inflammatory regulation, and are accepted to account
for the remodeling of IA lesions by the breaking down of the
cellular substrate, finally leading to a weakened vascular wall
(8,9). A variety of factors are involved in
the promotion of SMC apoptosis and increased cell death, and the
media of affected cerebral arteries become thinner with the
progression of the disease, developing into an aneurysmal cavity
(10). This process develops
collectively via the apoptosis or degeneration of SMCs, the
suppression of the proliferation of SMCs, and the suppression of
collagen generation and processing by SMCs, which has been noted in
rat IA models (11). However, the
major trigger for the remodeling of the media remains unclear.
MicroRNAs (miRNAs/miRs) are an important group of
small regulatory RNAs containing ~22 nucleotides, which are crucial
for gene control at the post-transcriptional level. miRNAs bind to
the 3′-untranslated regions (3′-UTRs) of mRNAs and either result in
message degradation or prevent translation through events mediated
by RNA-induced silencing complex (12). This family of small RNAs has been
commonly observed since they were first identified ~10 years ago.
Human microRNAs (n=2,578) are listed in the central database and
repository of miRNAs in its most recently updated version (v20.0)
at miRBase.org (13). miRNAs are located throughout the
genome, in polycistronic miRNA clusters consisting of 2–50 miRNAs,
with transcriptional units being predominantly intronic to
mRNAs.
There are correlations between altered levels of
miRNAs and numerous cardiovascular problems complicated with
diabetes (14), however, the
manner in which diabetes per se regulates the phenotype and
function of vascular SMC remains largely unknown. Gene expression
alteration and SMC aberrancies in individuals with type II diabetes
mellitus can be caused by dysregulated miRNAs triggered by the
metabolic milieu (15).
The first miRNA analysis in IA showed reduced levels
of 18 miRNAs in IA tissue (16).
A total of 681 target genes of the 18 miRNAs were investigated in
follow-up studies, including the analysis of the mRNA expression
profiles of IA and control samples. There is proliferation of
numerous cell types and enrichment in 'migration of phagocytes', as
indicated by functional classification of the target genes.
It has previously been demonstrated that miR-23b is
differentially expressed in SMCs collected from patients with IA,
and PTEN dysregulation has also been indicated to be involved in
the molecular mechanism of SMC apoptosis (16,17). By searching the online miRNA
database, it was found that PTEN is a virtual target of miR-23b.
The present study validated PTEN as a target of miR-23b, and
verified the involvement of miR-23b and PTEN in the development of
IA. The samples used in the present study were surgically removed
aneurysm tissues.
Materials and methods
Patients and tissue samples
The subjects were unrelated individuals assessed at
the Department of Interventional Radiology, The First Affiliated
Hospital of Zhengzhou University (Henan, China). For the case
group, the trial recruited 32 sporadic intact IA patients (mean
age, 48; age range, 39–65), while for the control group (mean age,
45; age range, 35–55), 17 individuals without any health problems
were recruited, between July 2013 and September 2014. The patients
with IA had been diagnosed by magnetic resonance angiography
digital subtraction angiography or three-dimensional computed
tomography angiography. The patients provided written signed
informed consent for participation in the study, and the study
protocol was approved by the Research Ethics Committee at the First
Affiliated Hospital of Zhengzhou University.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The total RNA, including miRNA, was isolated from
vascular SMCs (VSMCs; isolated from the vessel walls) and extracted
using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). RNA concentration and purity were determined using a NanoDrop
ND-1000 spectrophotometer (Nano Drop Technologies Inc., Rockland,
DE, USA), with a 260/280 value >1.8 considered acceptable.
RNA was extracted from pulmonary artery SMCs
(PASMCs) using TRIzol reagent (Thermo Fisher Scientific, Inc.) and
subsequently purified by column with RNeasy kits (Qiagen GmbH,
Hilden, Germany). For RT-qPCR of miR-23b-3p, a 7900HT Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used to reverse-transcribe 100 ng total RNA. The reverse
transcription kit used was mirVana™ qRT-PCR miRNA Detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.). For RT-qPCR of mRNAs,
a High Capacity cDNA Archive kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used to synthesize cDNA from 1 mg total RNA.
The SYBR Green assay (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used with the Applied Biosystems Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) to detect the
expression of PTEN and miR-23b-3p. The RT primer sequence used was:
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGGTAAT-3′
(forward, 5′-GGGATCACATTGCCAGGG-3′ and reverse,
5′-CAGTGCGTGTCGTGGAGT-3′). Mature sequence hsa-miR-23b-3p was used
as reference gene. The thermocycling conditions were as follows:
95°C for 10 min, 40 cycles at 95°C for 30 sec, 60°C for 30 sec and
72°C for 30 sec. The quantification method used was the
2−∆∆Cq method (18).
Cell culture and transfection
The VSMCs were cultured at 37°C in a humidified
atmosphere of 5% CO2, and maintained in Dulbecco's
modified Eagle's medium (DMEM), supplemented with 10% fetal bovine
serum, 100 U/ml penicillin and 100 mg/ml streptomycin (all from
Thermo Fisher Scientific, Inc.). When 80% confluence was reached,
the VSMCs were transfected in suspension into 6-well plates
(5–6×105 cells/well) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. miR-23b-3p mimics, inhibitor or PTEN
siRNA were used to transfect the cultured cells, and VSMCs
transfected with a scramble control were used as a control
(transfection reagent, Lipofectamine 2000; concentration, 2
µl). The sequences used were as follows: miR-23b-3p mimics,
5′-ATCACATTGCCAGGGATTACC-3′ and miR-23b-3p inhibitor,
5′-GGTAATCCCTGGCAATGTGAT-3′. The growth medium was changed and the
total cellular protein or RNA was isolated for further experiments
4 h later. Western blot analysis and RT-PCR were performed to
determine the expression of PTEN and were performed according to
the methods stated below.
Vector construction and mutagenesis
The TargetScan public database (www.targetscan.org) was searched for the target gene
of miR-23b-3p, and PTEN was found to be the putative miR-23b-3p
binding site. The 3′-UTR of PTEN containing the binding site of
miR-23b-3p was amplified through PCR and inserted into the
psiCHECK-2 reporter vector (Promega Corporation, Madison, WI, USA).
PCR was performed according to the methods already stated.
Meanwhile, mutagenesis was performed by replacing the miR-23b-3p
binding site nucleotides and introduced into the control vector
(Ambion; Thermo Fisher Scientific, Inc.).
Cell proliferation assay
The effects of miR-23b-3p overexpression on VSMC
proliferation were assessed using Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan). The PASMC
proliferation reagent WST-1 was used to quantify proliferation
rates of PASMCs. Briefly, PASMCs (0.5×106 cells/well)
were transferred to 96-trawell plates and AZD5438, NU6140, and CPT
were used to treated the proliferating cells in serum containing
medium. Following treatment, the PASMCs were washed in DMEM without
L-glutamine and phenol red. WST-1 reagent was diluted (1:10 final
dilution) in DMEM without phenol red and with L-glutamine (all from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). This diluted
tetrazolium WST-1 reagent (200 µl)was then added to each
well and absorption was measured at 2, 4 and 6 h. Diluted WST-1
reagent was used for the background control. Absorbance was read at
440 nm using an ELISA plate reader.
Luciferase assay
The 3′-UTR of PTEN was cloned into the psiCHECK-2
vector, between the XhoI/NotI site, immediately
downstream of the firefly luciferase gene in the pGL3-control
vector (both Promega Corporation). For selected targets, inverse
PCR with non-overlapping primers carrying the mutated sequences was
used to introduce 3 point mutations into the 7-nt seed-binding
sequence (19). Lipofectamine
2000 (Invitrogen Life Technologies) was used to co-transfect the 10
ng of each psi-CHECK-2 construct and 10 nM miRNA duplexes or into
VSMCs in a 96-well plate. The cell extract was harvested 2 days
later and assayed using the Dual-Luciferase reporter system
(Promega Corporation) to measure Firefly and Renilla
luciferase activities according to the manufacturer's protocols.
Each experiment was analyzed in triplicate in three independent
experiments.
Western blot analysis
Ice-cold PBS and radio immunoprecipitation assay
lysis buffer were used to wash the cell monolayers in ice cold
extraction buffer [20 mM Tris-HCl (pH 7.5), 1 mM Na2
EDTA, 150 mM NaCl, 1% Triton X-100, 1 mM EGTA, 2.5 mM sodium
pyrophosphate, 1 mM Na3VO4 and 1
mM-glycerophosphate; Thermo Fisher Scientific, Inc.] containing
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) for 10 min
according to the manufacturer's protocols. Cell lysates were
centrifuged at 16,770 × g at 4°C for 10 min and proteins were
separated by 12% SDS-PAGE. The target proteins were transferred to
Hybond ECL nitrocellulose membrane (GE Healthcare, Chicago, IL,
USA), blocked using 5% skimmed dry milk and 0.1% Tween-20 for 2 h
at room temperature and detected with the indicated antibodies
[primary antibody, PTEN (cat. no. 9552S; dilution, 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) at 4°C for 12 h;
secondary antibody, HRP-linked antibody (cat. no. 7075S; dilution,
1:3,000; Cell Signaling Technology, Inc.) at 25°C for 2 h] in TBS
buffer (5% skimmed dry milk and 0.1% Tween-20; blotting grade;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The protein
determination method was BCA assay (Beyotime Institute of
Biotechnology, Haimen, China). Next, horseradish
peroxidase-conjugated secondary antibodies were used for 1 h at
room temperature according to the manufacturer's instructions. The
visualization reagent used was Amersham ECL Prime Western Blotting
Detection reagent (GE Healthcare).
Apoptosis analysis
Annexin V/propidium iodide staining with the
apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) was used to detect VSMC apoptosis. To measure the apoptosis
of VSMCs, flow cytometry was performed with Annexin V-FITC
(fluorescein isothiocyanate)/PI Apoptosis Detection kit
(Sigma-Aldrich; Merck KGaA). VSMCs were harvested following the
transfection with miR-23b-3p mimic after 48 h, and then washed
twice using PBS. Cells were resuspended at a final density of
4×105 cells per well with 1× binding buffer. After
treated with 5 µl PE Annexin V and 5 µl 7-AAD, 100
µl cell suspension was incubated in darkness for 15 min.
FACS (BD Biosciences; San Jose, CA, USA) was used to detect cell
apoptosis. The y-axis and x-axis were used to show plasma membrane
integrity and Annexin V immune fluorescence, respectively. Assays
were performed in three independent experiments, and the ratio of
dead cells/total cells was analyzed statistically.
Statistical analysis
All data are presented as the mean ± standard
deviation. Each experiment was analyzed for three times, with three
samples for each. The results of western blot analyses from three
experiments are shown. One-way analysis of variance and
Student-Newman-Keuls post hoc test determined the significance of
the differences between the means. P<0.05 was considered to
indicate a statistically significance.
Results
PTEN is a virtual target of
miR-23b-3p
miR-23b-3p has been reported to be involved in
numerous diseases (20),
including non-small cell lung cancer and gastric cancer, by acting
on different signaling pathways. The present study aimed to
increase our understanding of the association between miR-23b-3p
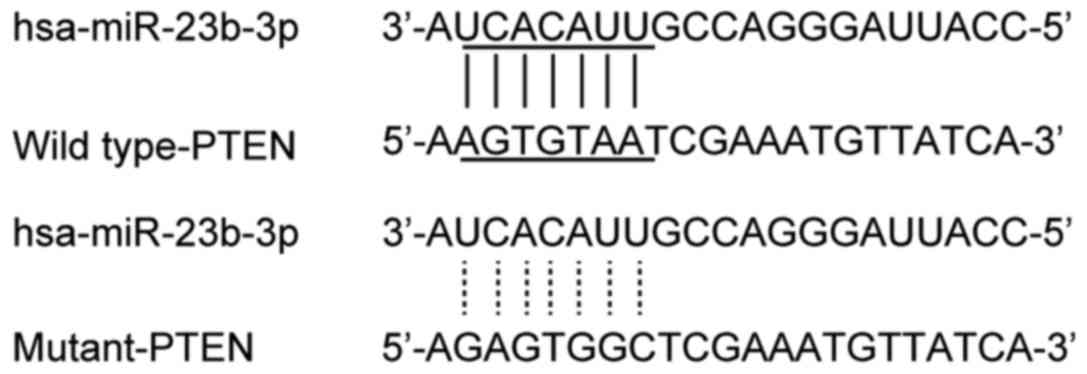
level and IA. Online miRNA target prediction tools were used to
search for the regulatory gene of miR-23b-3p, and consequently PTEN
was identified in cells with the 'seed sequence' in the 3′-UTR
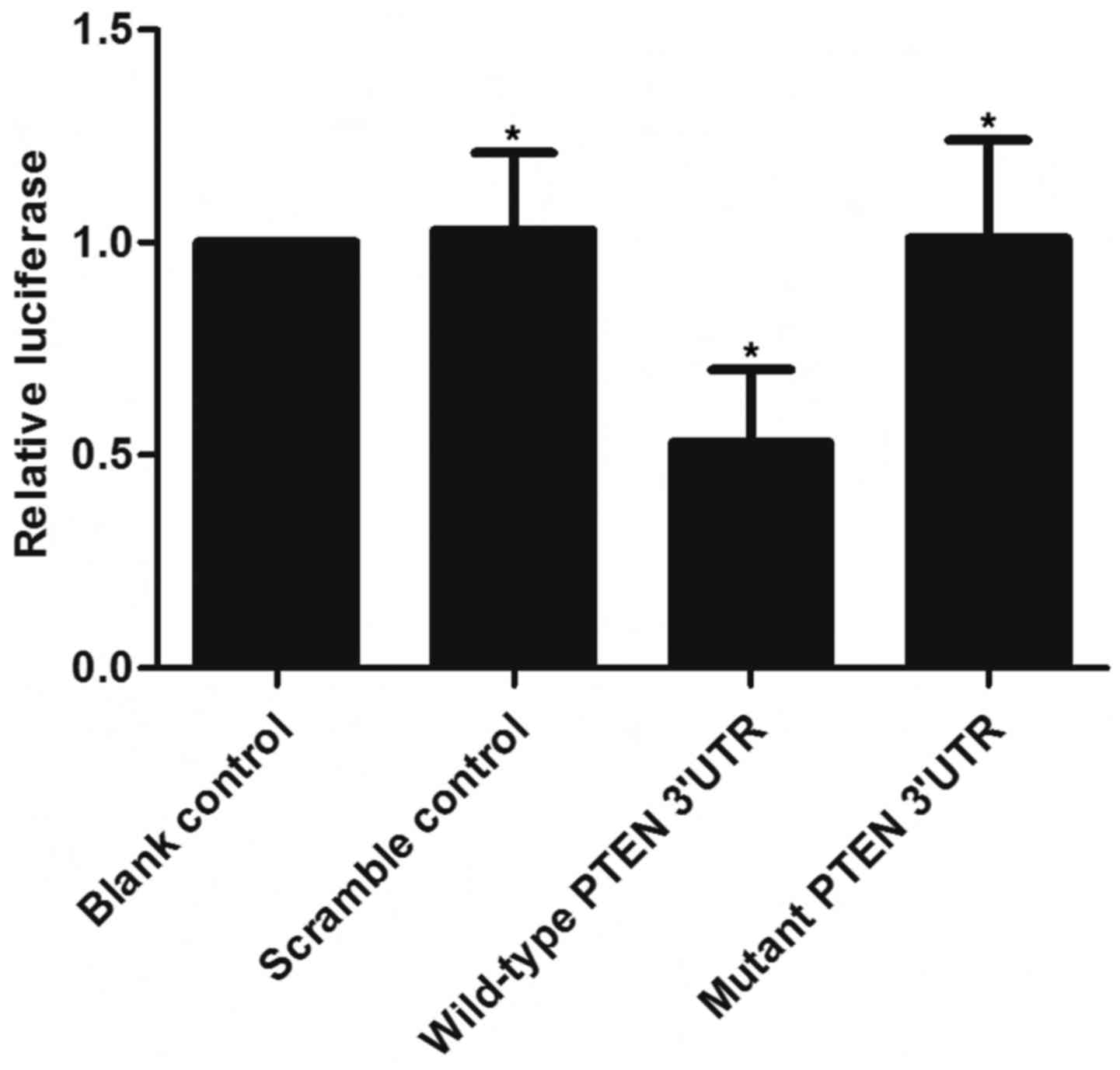
(Fig. 1). A luciferase activity
reporter assay was conducted in the VSMCs to determine the
interactions, and only the luciferase activity from the miR-23b-3p
and wild-type PTEN 3′-UTR cotransfected cells decreased
significantly (Fig. 2). The
activity of cells with miR-23b-3p and mutant PTEN 3′-UTR
cotransfection was similar to that of the scramble control
(Fig. 2). The results
demonstrated that PTEN was a validated target of miR-23b-3p in the
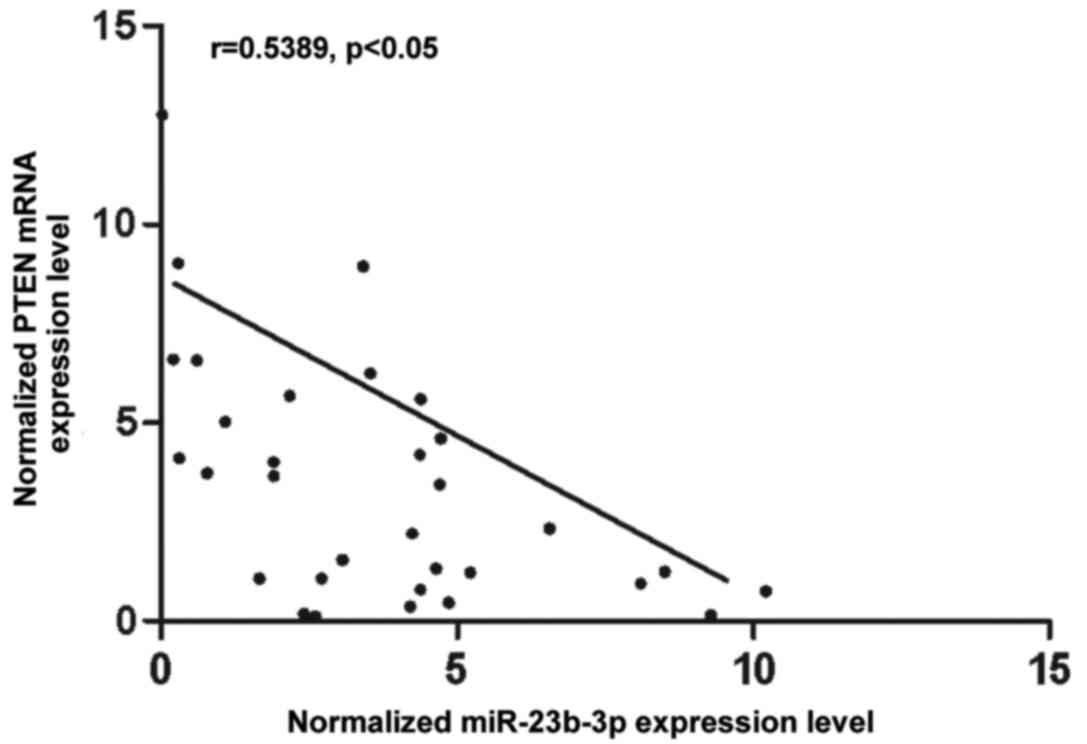
VSMCs. To further understand the interactions between miR-23b-3p
and PTEN, the correlation between the expression level of
miR-23b-3p and PTEN mRNA among the tissues was analyzed (n=32), and
a negative regulatory correlation was found (Fig. 3).
Determination of expression patterns of
miR-23b-3p and PTEN in tissues of different groups
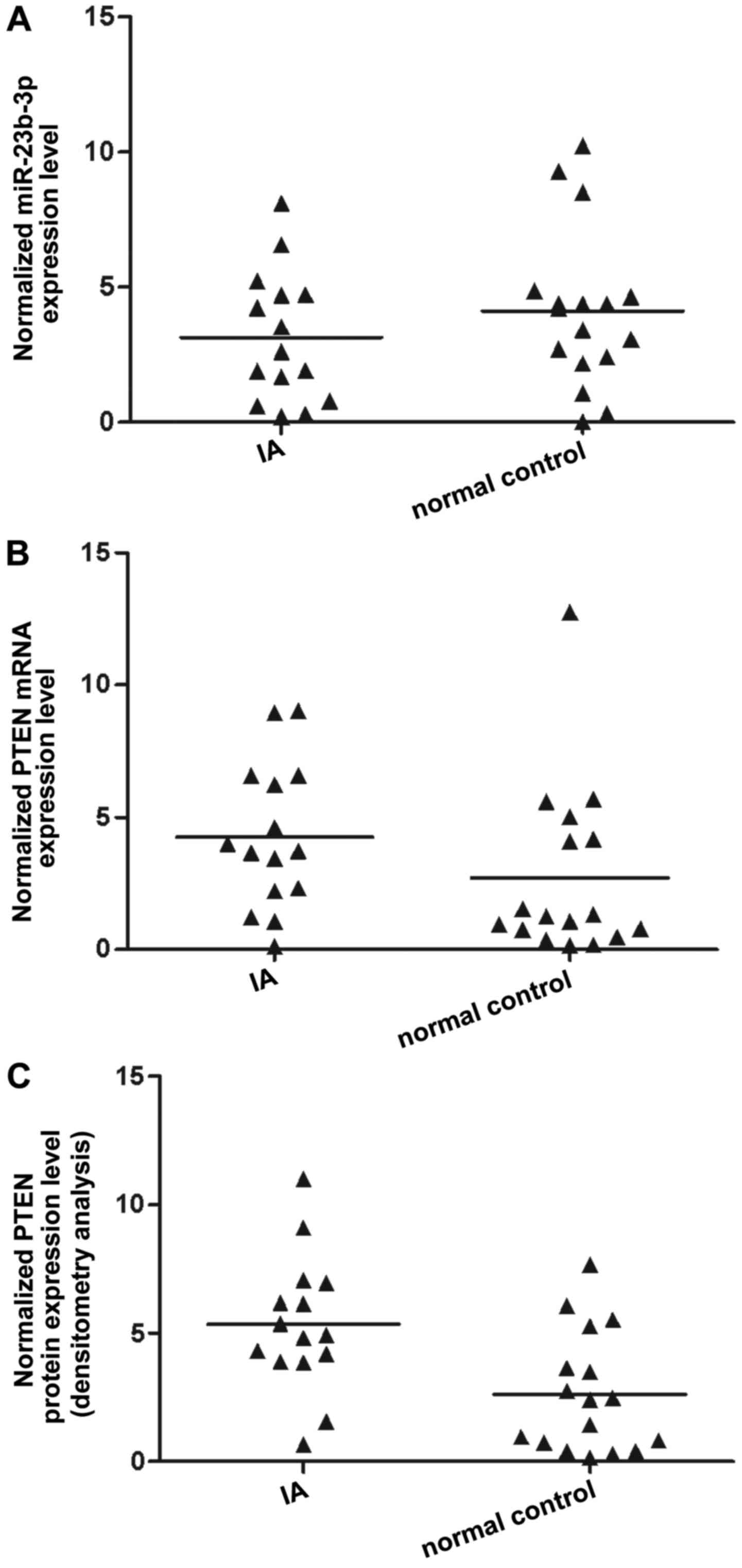
The tissues of two different groups (IA, n=32;
control, n=17) were used to further investigate the impact on the
interaction between miR-23b-3p and the PTEN 3′-UTR. qPCR was
performed to detect miR-23b-3p and PTEN mRNA expression, with
results revealing that compared with that of the normal control,
miR-23b-3p expression was decreased in the IA group (Fig. 4A). PTEN mRNA expression (Fig. 4B) was increased in the IA group
compared with the normal control, as was PTEN protein expression,
according to densitometry analysis (Fig. 4C). To further validate the
hypothesis of the negative regulatory association between
miR-23b-3p and PTEN, PTEN mRNA expression in SMCs was investigated,
with the SMCs being transfected with scramble control, miR-23b-3p
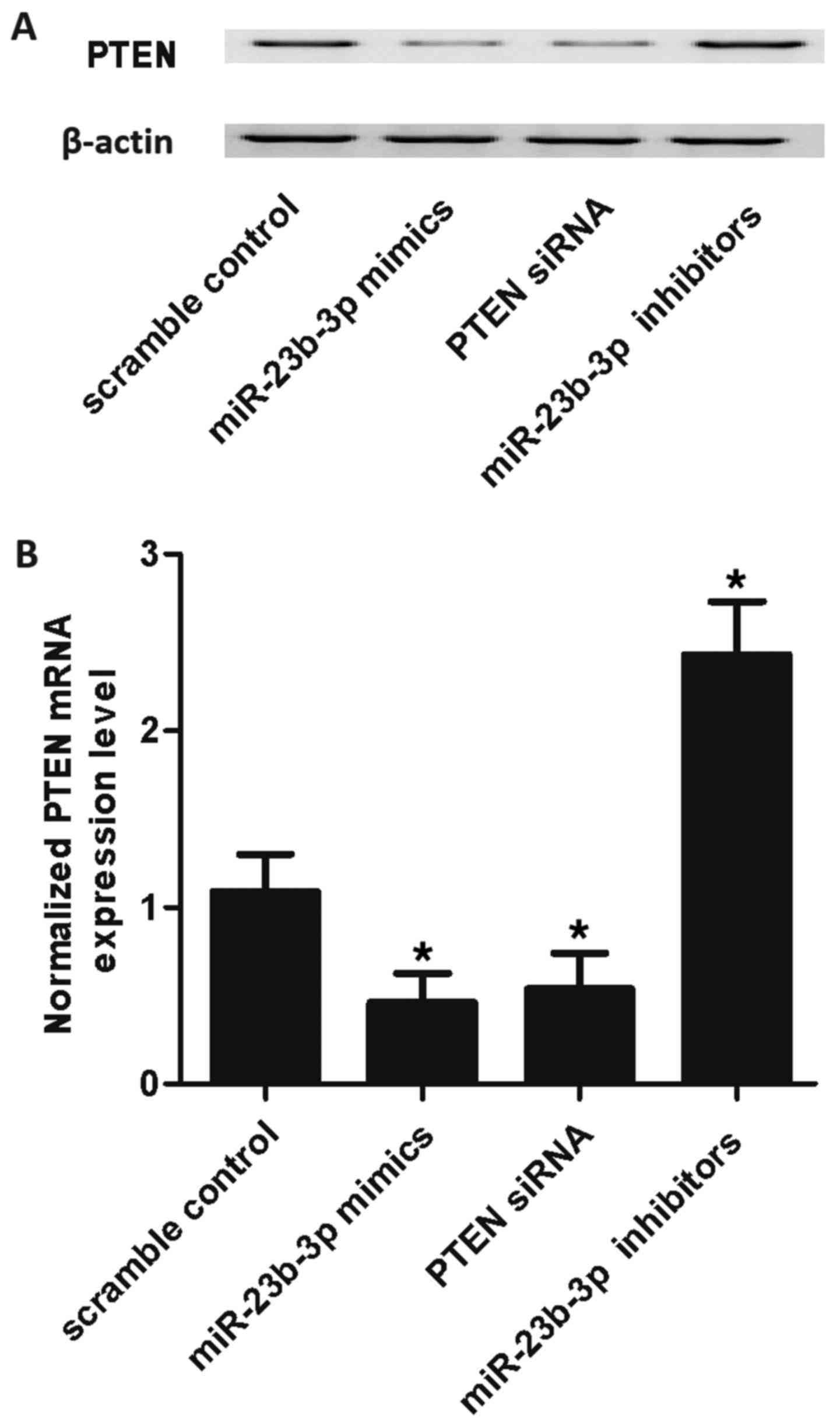
mimics, PTEN siRNA and miR-23b-3p inhibitors. As shown in Fig. 5, the PTEN mRNA expression level of
SMCs treated with miR-23b-3p mimics and PTEN siRNA were markedly
lower than those of the scramble control, while the expression of
cells treated with miR-23b-3p inhibitors was markedly higher than
that of the scramble control, validating the negative regulatory
association between miR-23b-3p and PTEN.
Alternations in miR-23b-3p and PTEN
affect the viability of SMCs
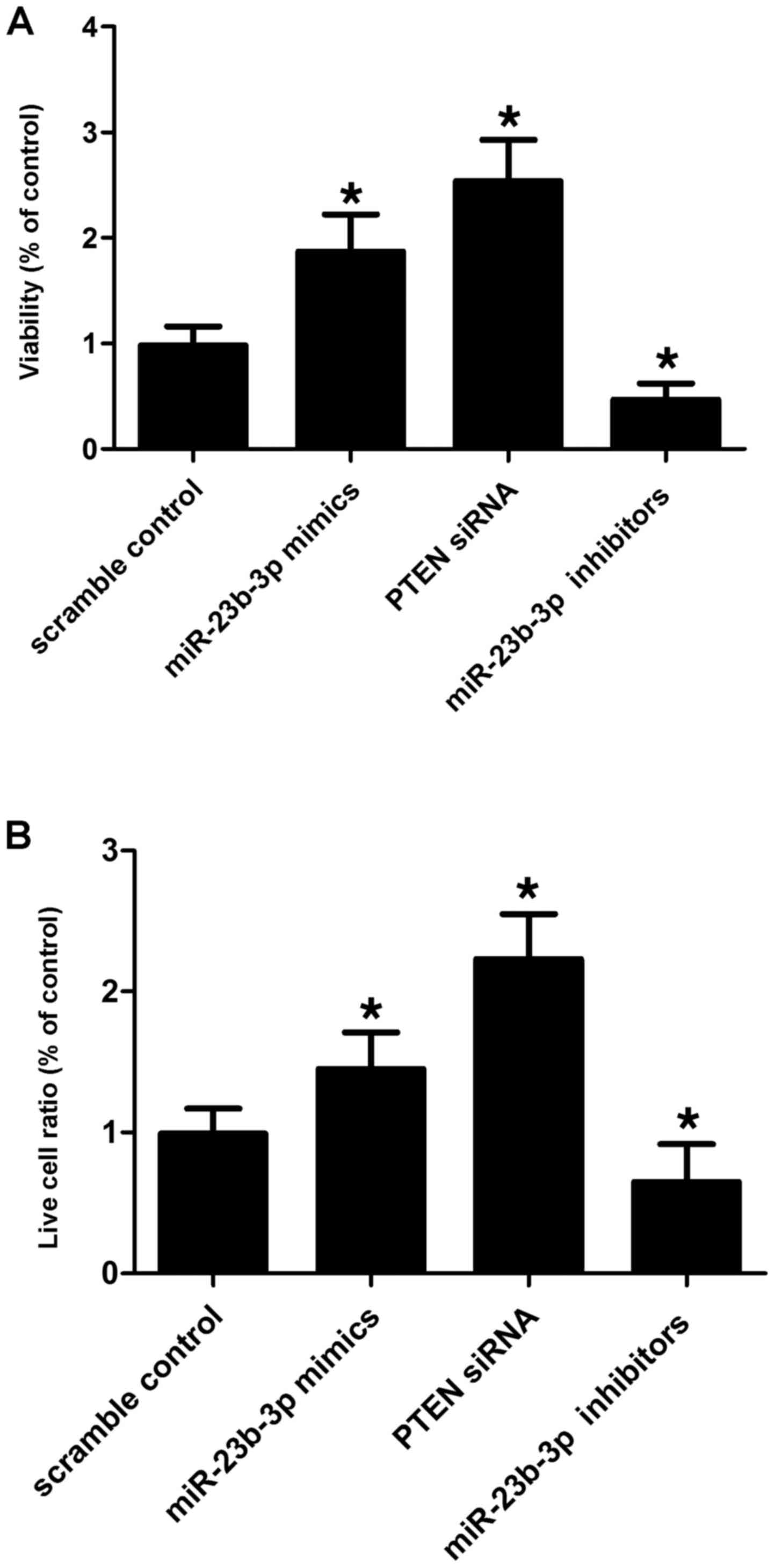
VSMCs were transfected with scramble control,
miR-23b-3p mimics, PTEN siRNA and miR-23b-3p inhibitors to evaluate
the effect of alternations in miR-23b-3p on the relative viability
and apoptosis of the cells. miR-23b-3p inhibitor transfection
suppressed the viability of the SMCs (Fig. 6A) by promoting their apoptosis
(Fig. 6B) when compared with the
scramble controls, while miR-23b-3p mimic and PTEN siRNA
transfection enhanced the viability of the VSMCs (Fig. 6A) by inducing apoptosis (Fig. 6B). This indicates that miR-23b-3p
negatively affects the viability of SMCs, while PTEN positively
affects the viability of cells.
Discussion
miR-23b-3p is a member of the miR-23b/27b/24-1
cluster, the dysregulation of which has been demonstrated in a
variety of cancer types. In human colon cancer, downregulation of
miR-23b-3p is observed, regulating MAP3k1 or FZD7 to trigger the
multiple processes of metastasis in vivo. Moreover,
miR-23b-3p acts as an oncogenic miRNA to suppress PTEN in renal
cell carcinoma (21). Also,
thymic lymphoma induced by radiation has been demonstrated to
exhibit upregulated expression of miR-23b-3p (22). miR-23b-3p was also found to induce
the sensitivity of GC cells to chemotherapy (23). Furthermore, our previous study
showed that miR-23b-3p has two directly functional targets,
including high-mobility group box 2 and autophagy-related gene 12,
which are overexpressed in GC multidrug-resistant cells and
positively associated with either chemoresistance or autophagy
(24). It has been previously
shown that miR-23b is differentially expressed in SMCs collected
from patients diagnosed with IA (16). In the present study, tissues were
collected from patients with IA (n=32) or unaffected controls
(n=17), and qPCR was used to determine the expression of
miR-23b-3p. This revealed that the miRNA was substantially
downregulated in the IA samples. Furthermore, it was found that
PTEN is a virtual target of miR-23b by using an online miRNA
database.
In another study, we previously demonstrated that
there was an association between miR-23b-3p level and survival in
patients with renal carcinoma (22). As with miR-21, all patients
exhibited low or moderate expression of miR-23b-3p, but only half
of the patients with high miR-23b-3p expression achieved 5-year
survival following surgery. miR-23b-3p-knockdown in renal cancer
cells contributed to an elevated quantity of apoptotic cells, and
reduced cell migration and invasion. Tumor inhibitor PTEN was not
detected in tumor samples with high expression of miR-23b-3p and
was augmented in miR-23b-3p-knockdown cells. miR-23b-3p directly
targeted the 3′-UTR of PTEN, demonstrating that PTEN is involved in
the apoptotic mechanism induced by miR-23b-3p. GEN treatment
reduced the expression of miR-23b-3p, revealing that downregulated
miR-23b-3p expression could trigger the pro-apoptotic role of GEN
on renal cancer cells (22). In
the present study, a luciferase activity reporter assay was
conducted in the VSMCs, and it was revealed that only the
luciferase activity from the miR-23b-3p and wild-type PTEN 3′-UTR
cotransfected cells significantly decreased (Fig. 2), while cells with miR-23b-3p and
mutant PTEN 3′-UTR cotransfection showed results similar to that of
the scramble control (Fig. 2).
This supported PTEN as a validated target of miR-23b-3p in
VSMCs.
PTEN is a lipid phosphatase and protein that serves
an inhibitory role in numerous pathways implicated in
proliferation, cell survival and inflammation (25). The effect of PTEN has been well
established in cancer, but its effects in vascular disorders are
less well characterized. Several studies have demonstrated that the
inactivation of PTEN contributes to elevated SMC proliferation and
reduced cell death (26,27). In addition, vascular injury
accounts for inactivation of SMC PTEN and is correlated with
elevated formation of neointima (28). SMC-specific-knockout of PTEN in
vivo leads to a larger neointima responding to injury of the
vessel wall caused by recruitment of inflammatory cells and
elevated proliferation (29). An
inflammatory SMC phenotype can be promoted by in vitro
shRNA-dependent depletion of PTEN, characterized by elevated
production of chemokines and inflammatory cytokines, reduced
SM-gene expression and elevated proliferation (17). Moreover, a study by Ji et
al revealed that miR-21 directly targets PTEN and that miRs
such as miR-21 are differentially expressed by SMC when vascular
injury occurs (30). In addition,
neointima formation is regulated by altered miR-21 in
vivo.
In humans, germline mutations of PTEN are correlated
with Lhermitte-Duclos disease, Bannayan-Zonana syndrome and Cowden
disease (31,32). Patients have common pathological
characteristics, such as benign hyperplasias or tumors of different
tissue origins, and initiation of hamartomas. A variety of tumors
and hyperplasias are developed in mice that are heterozygous for
Pten genes during their lifetime (33). Notably, Freeman et al
showed that PtenΔ5/+ mice exhibited vascular
abnormalities resembling hemangiomas or angiomatosis, and that the
genetic background was the basis of the initiation and spectrum of
tumor development (34).
Moreover, vascular malformation is observed in transgenic murine
models with maintained activation of Akt. Abnormal blood vessel
formation can be caused by expression of myristoylated-Akt1 in
endothelial cells (35,36). It is unclear why endothelial cells
are particularly prone to tumor development correlated with loss of
three of the four Pten alleles, and this therefore requires
further study in
ptena+/−ptenb−/− and
ptena−/−ptenb+/− fish. In the
present study, SMCs were transfected with scramble control,
miR-23b-3p mimics, PTEN siRNA and miR-23b-3p inhibitors to evaluate
the effect of alternation of miR-23b-3p on relative cell viability
and apoptosis. It was found that cells transfected with miR-23b-3p
inhibitors suppressed the viability of SMCs by promoting the
apoptosis of the cells when compared with the scramble controls,
while cells transfected with miR-23b-3p mimics and PTEN siRNA
enhanced the viability of VSMCs by inducing apoptosis, indicating
that miR-23b-3p negatively affected the viability of the cells,
while PTEN positively affected the viability of the cells.
In conclusion, PTEN was virtual target of miR-23b-3p
and a negative regulatory association existed between miR-23b-3p
and PTEN in the present study. miR-23b-3p and PTEN affected the
viability and apoptosis of SMCs.
Competing interests
Authors declare they have no competing interest.
References
|
1
|
Fergusen S and Macdonald RL: Predictors of
cerebral infarction in patients with aneurysmal subarachnoid
hemorrhage. Neurosurgery. 60:658–667; discussion 667. 2007.
View Article : Google Scholar
|
|
2
|
Schievink WI: Intracranial aneurysms. N
Engl J Med. 336:28–40. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Connolly ES Jr, Choudhri TF, Mack WJ,
Mocco J, Spinks TJ, Slosberg J, Lin T, Huang J and Solomon RA:
Influence of smoking, hypertension, and sex on the phenotypic
expression of familial intracranial aneurysms in siblings.
Neurosurgery. 48:64–68; discussion 68–69. 2001.PubMed/NCBI
|
|
4
|
Bonita R: Cigarette smoking, hypertension
and the risk of subarachnoid hemorrhage: a population-based
case-control study. Stroke. 17:831–835. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaźmierski M, Michalewska-Włudarczyk A and
Krzych LJ: Intima-media thickness and flow-mediated dilatation in
the diagnosis of coronary artery disease in perimenopausal women.
Pol Arch Med Wewn. 120:181–188. 2010.
|
|
6
|
Nakajima N, Nagahiro S, Sano T, Satomi J
and Satoh K: Phenotypic modulation of smooth muscle cells in human
cerebral aneurysmal walls. Acta Neuropathol. 100:475–480. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoki T, Kataoka H, Morimoto M, Nozaki K
and Hashimoto N: Macrophage-derived matrix metalloproteinase-2 and
-9 promote the progression of cerebral aneurysms in rats. Stroke.
38:162–169. 2007. View Article : Google Scholar
|
|
8
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parks WC, Wilson CL and López-Boado YS:
Matrix metallo-proteinases as modulators of inflammation and innate
immunity. Nat Rev Immunol. 4:617–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng H, Metaxa E, Gao L, Liaw N, Natarajan
SK, Swartz DD, Siddiqui AH, Kolega J and Mocco J: Progressive
aneurysm development following hemodynamic insult. J Neurosurg.
114:1095–1103. 2011. View Article : Google Scholar
|
|
11
|
Aoki T, Kataoka H, Ishibashi R, Nozaki K,
Morishita R and Hashimoto N: Reduced collagen biosynthesis is the
hallmark of cerebral aneurysm: contribution of interleukin-1beta
and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol.
29:1080–1086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Griffiths-Jones S: The microRNA registry.
Nucleic Acids Res. 32:D109–D111. 2004. View Article : Google Scholar :
|
|
14
|
Pandey AK, Agarwal P, Kaur K and Datta M:
MicroRNAs in diabetes: tiny players in big disease. Cell Physiol
Biochem. 23:221–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porter KE and Riches K: The vascular
smooth muscle cell: a therapeutic target in type 2 diabetes? Clin
Sci (Lond). 125:167–182. 2013. View Article : Google Scholar
|
|
16
|
Jiang Y, Zhang M, He H, Chen J, Zeng H, Li
J and Duan R: MicroRNA/mRNA profiling and regulatory network of
intra-cranial aneurysm. BMC Med Genomics. 6:362013. View Article : Google Scholar
|
|
17
|
Furgeson SB, Simpson PA, Park I, Vanputten
V, Horita H, Kontos CD, Nemenoff RA and Weiser-Evans MC:
Inactivation of the tumour suppressor, PTEN, in smooth muscle
promotes a pro-inflammatory phenotype and enhances neointima
formation. Cardiovasc Res. 86:274–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
19
|
Hartl DL and Ochman H: Inverse polymerase
chain reaction. Methods Mol Biol. 58:293–301. 1993.
|
|
20
|
Chen D, Wu X, Xia M, Wu F, Ding J, Jiao Y,
Zhan Q and An F: Upregulated exosomic miR-23b-3p plays regulatory
roles in the progression of pancreatic cancer. Oncol Rep.
38:2182–2188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin
S, Sun C, Ma M, Huang Y and Xi JJ: Genome-wide functional screening
of miR-23b as a pleiotropic modulator suppressing cancer
metastasis. Nat Commun. 2:5542011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaman MS, Thamminana S, Shahryari V,
Chiyomaru T, Deng G, Saini S, Majid S, Fukuhara S, Chang I, Arora
S, et al: Inhibition of PTEN gene expression by oncogenic
miR-23b-3p in renal cancer. PLoS One. 7:e502032012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li B, Sun M, Gao F, Liu W, Yang Y, Liu H,
Cheng Y, Liu C and Cai J: Up-regulated expression of miR-23a/b
targeted the pro-apoptotic Fas in radiation-induced thymic
lymphoma. Cell Physiol Biochem. 32:1729–1740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
An Y, Zhang Z, Shang Y, Jiang X, Dong J,
Yu P, Nie Y and Zhao Q: miR-23b-3p regulates the chemoresistance of
gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis.
6:e17662015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oudit GY, Sun H, Kerfant BG, Crackower MA,
Penninger JM and Backx PH: The role of phosphoinositide-3 kinase
and PTEN in cardiovascular physiology and disease. J Mol Cell
Cardiol. 37:449–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang J, Niu XL, Pippen AM, Annex BH and
Kontos CD: Adenovirus-mediated intraarterial delivery of PTEN
inhibits neointimal hyperplasia. Arterioscler Thromb Vasc Biol.
25:354–358. 2005. View Article : Google Scholar
|
|
27
|
Mitra AK, Jia G, Gangahar DM and Agrawal
DK: Temporal PTEN inactivation causes proliferation of saphenous
vein smooth muscle cells of human CABG conduits. J Cell Mol Med.
13:177–187. 2009. View Article : Google Scholar :
|
|
28
|
Mourani PM, Garl PJ, Wenzlau JM, Carpenter
TC, Stenmark KR and Weiser-Evans MC: Unique, highly proliferative
growth phenotype expressed by embryonic and neointimal smooth
muscle cells is driven by constitutive Akt, mTOR, and p70S6K
signaling and is actively repressed by PTEN. Circulation.
109:1299–1306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nemenoff RA, Horita H, Ostriker AC,
Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S and
Weiser-Evans MC: SDF-1α induction in mature smooth muscle cells by
inactivation of PTEN is a critical mediator of exacerbated
injury-induced neointima formation. Arterioscler Thromb Vasc Biol.
31:1300–1308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNA expression signature and
antisense-mediated depletion reveal an essential role of microRNA
in vascular neointimal lesion formation. Circ Res. 100:1579–1588.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nelen MR, van Staveren WC, Peeters EA,
Hassel MB, Gorlin RJ, Hamm H, Lindboe CF, Fryns JP, Sijmons RH,
Woods DG, et al: Germline mutations in the PTEN/MMAC1 gene in
patients with Cowden disease. Hum Mol Genet. 6:1383–1387. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marsh DJ, Dahia PL, Zheng Z, Liaw D,
Parsons R, Gorlin RJ and Eng C: Germline mutations in PTEN are
present in Bannayan-Zonana syndrome. Nat Genet. 16:333–334. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suzuki A, de la Pompa JL, Stambolic V,
Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A,
Khoo W, et al: High cancer susceptibility and embryonic lethality
associated with mutation of the PTEN tumor suppressor gene in mice.
Curr Biol. 8:1169–1178. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Freeman D, Lesche R, Kertesz N, Wang S, Li
G, Gao J, Groszer M, Martinez-Diaz H, Rozengurt N, Thomas G, et al:
Genetic background controls tumor development in PTEN-deficient
mice. Cancer Res. 66:6492–6496. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun JF, Phung T, Shiojima I, Felske T,
Upalakalin JN, Feng D, Kornaga T, Dor T, Dvorak AM, Walsh K, et al:
Microvascular patterning is controlled by fine-tuning the Akt
signal. Proc Natl Acad Sci USA. 102:128–133. 2005. View Article : Google Scholar
|
|
36
|
Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah
G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I,
Nagy JA, et al: Pathological angiogenesis is induced by sustained
Akt signaling and inhibited by rapamycin. Cancer Cell. 10:159–170.
2006. View Article : Google Scholar : PubMed/NCBI
|