Introduction
Oral cavity cancer is one of the most common types
of cancer worldwide, and oral squamous cell carcinoma (OSCC) is the
most prevalent type of tumour at this site, accounting for ~90% of
all cases (1,2). Despite previous advancements in
diagnostic techniques and therapeutic strategies, the prognosis of
OSCC has not improved, with an overall 5-year survival rate of ~50%
(2,3). This poor prognosis is primarily
attributed to the high rates of invasion and metastasis (4). Therefore, it is important to
understand the molecular events associated with tumour development
and to identify biomarkers for prognosis assessment in OSCC.
Epithelial-mesenchymal transition (EMT) is a complex
biological process that is important for embryogenesis, wound
healing and malignant tumour metastasis (5). During EMT, epithelial tumour cells
lose their polarity and adhesive phenotype, and they acquire
mesenchymal traits, including an increase in cell motility
potential and cytoskeletal remodelling (5). Alterations of adhesion molecules and
extracellular proteins are key events in tumour cells during EMT.
Epithelial cadherin (E-cadherin) and vimentin are two hallmarks of
EMT; one is a predominant cell-surface protein for adherens
junctions in epithelial cells, and the other is an intermediate
filament in mesenchymal cells that controls cell motility,
respectively (6). In primary head
and neck squamous cell carcinoma cancer, the loss of E-cadherin and
the gain of vimentin functions are associated with a higher
incidence of distant metastasis (7). In addition, zinc finger protein
SNAI1 (Snail) is one of the transcription factors involved in the
EMT process and regulates various aspects of the EMT phenotype,
including the upregulation of mesenchymal markers and the
suppression of epithelial markers (6).
The Notch signalling pathway is evolutionarily
conserved and serves important roles in numerous developmental
processes, including cell fate determination, proliferation and
differentiation (8). Notch
signalling is initiated when a Notch ligand binds to an adjacent
Notch receptor between two neighbouring cells. In mammals, the
Notch family consists of 4 transmembrane receptors (Notch-1-4) and
5 ligands [Delta-like protein (Delta-like) 1, Delta-like 3,
Delta-like 4, protein jagged (Jagged) 1 and Jagged-2] (9). Dysregulation of Notch signalling
results in human developmental anomalies, including Alagille
syndrome, cardiac disease and cancer development (10). Previous studies demonstrated that
the expression levels of certain Notch receptors and ligands were
dysregulated in human OSCC, which suggested that Notch signalling
serves a vital role in tumour development in OSCC (11-13).
Aberrant expression of EMT markers, including
downregulated E-cadherin and β-catenin and upregulated vimentin and
Neural cadherin, has been identified in OSCC tissues (14,15). The promotion of EMT in OSCC
involved signalling, including the phosphoinositide
3-kinase/protein kinase B and Wnt/β-catenin signalling pathways
(16). Notch signalling is also a
key regulator in the induction of EMT, but the role of this
signalling pathway and its association with EMT in cell motility in
OSCC remains unclear. Therefore, the present study used a
small-interfering RNA (siRNA) targeting Snail and a novel
γ-secretase inhibitor, DAPT, to examine the role of the Notch
signalling pathway and the EMT mechanism in the metastatic
potential of OSCC in vitro. The Tca8113 and CAL27 human OSCC
cell lines were adopted as cell models as they have been widely
used to study the role of Notch signalling and EMT in cancer
metastasis (11,17,18). The results of the present study
demonstrated that Snail-induced EMT may promote the migration of
OSCC cells, and that Notch signalling may mediate tumour metastasis
in OSCC cells via its association with EMT progression.
Materials and methods
Reagents
DAPT (GSI-IX) was purchased from Selleck Chemicals
(Houston, TX, USA). Dulbecco’s modified Eagle’s medium (DMEM),
RPMI-1640, foetal bovine serum (FBS) and trypsin-EDTA were supplied
by Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Primary
rabbit anti-human Notch1 intracellular domain (N1ICD; cat. no.,
ab83232) monoclonal antibodies were obtained from Abcam (Cambridge,
MA, USA). The rabbit anti-human Snail (cat. no., 3879), E-cadherin
(cat. no., 3195), and vimentin (cat. no., 5741) antibodies and the
GAPDH (cat. no., 5174) control antibody were all acquired from Cell
Signaling Technology (Danvers, MA, USA). The horseradish
peroxidase-labelled secondary antibody (cat. no., EK020) was
obtained from Xi’an Zhuangzhi Biotech Co., Ltd. (Xi’an, China).
Cell cultures
Established Tca8113 and CAL27 human OSCC cell lines
were purchased from CHI Scientific, Ltd., (Wuxi, China). TCA8113
cells were maintained in RPMI-1640 medium containing 20% FBS, 100
U/ml penicillin G and 100 µg/ml streptomycin, while CAL27
cells were maintained in DMEM containing 10% FBS, 100 U/ml
penicillin G and 100 µg/ml streptomycin. The 2 cell lines
were incubated in a humidified atmosphere of 5% CO2 at
37°C. Cells in the logarithmic growth phase were used in subsequent
experiments.
Cell viability assay
5×103 Tca8113 or CAL27 cells were seeded
in 96-well plates for 24 h, then cells were treated with 6
dilutions of DAPT in DMSO (1, 2, 5, 10, 20 and 40 µmol/l)
for 24, 48 and 72 h, and cell viability was determined by an MTT
assay. Following DAPT exposure, the medium was replaced with fresh
medium containing 20 µl (5 mg/ml PBS) MTT. After 4 h of
incubation at 37°C, the medium was removed, and 150 µl DMSO
was added to dissolve the formazan. Absorbance measurements were
made with an automatic ELISA reader (Infinite M200; Tecan Group,
Ltd., Männedorf, Switzerland) at 490 nm. Each experiment included 6
replicates and was repeated 3 times.
Transient transfection of Snail
siRNA
Viral transfer vectors encoding green fluorescent
protein and Snail siRNA, negative control (NC) siRNA, polybrene and
Enhanced Infection Solution (ENi.S) were constructed by Shanghai
Genechem Co., Ltd. (Shanghai, China). The target sequence for the
Snail siRNA was as follows: 5′-AGGACTCTAATCCAGAGTT-3′. Prior to
viral infection, Tca8113 and CAL27 cells were seeded into 6-well
plates at a density of 5×104 cells/well. Following 24 h
of incubation at 37°C, the cells were transiently trans-fected with
Snail siRNA or NC siRNA using viral transfer vectors when they
reached 40% confluence. Cell transfection was performed according
to the protocol of the manufacturer of the transfection kit. The
CAL27 virus infection solution contained 20 µl virus at
titer of 1E+8TU/ml, 880 µl ENi.S and 100 µl
phycoerythrin (enriched with 50 µg/ml polybrene ENi.S
solution), and the Tca8113 virus infection solution contained 120
µl virus at titer of 1E+8TU/ml, 780 µl ENi.S and 100
µl complete medium containing 50 µg/ml polybrene.
After 12 h of incubation at 37°C, the virus infection solution was
replaced with conventional culture medium to maintain cell growth
for 96 h prior to the subsequent experiments.
Migration and wound-healing assays
Cellular Transwell migration was assayed by
non-Matrigel-coated Transwell cell culture chambers (8-µm
pore size; Corning Incorporated, Corning, NY, USA). Briefly,
1×105 Tca8113 or CAL27 cells were seeded into the upper
chamber in 200 µl serum-free medium, and 600 µl
complete culture medium with 10% FBS for each cell line was placed
in the lower chamber as a chemoattractant. Cell migration assays
following Snail knockdown were conducted at 37°C for 24 h, and
subsequent to treatment with DAPT, the assays were conducted at
37°C for 24 and 48 h. Then, at room temperature, the cells were
fixed with 4% paraformaldehyde for 10 min and stained with 0.1%
crystal violet for 20 min. The unmigrated cells were removed from
the upper surface of the membrane using cotton-tipped swabs.
Finally, the migrated cells were counted at magnification, ×400
from 9 different randomly selected fields of each filter using an
inverted light microscope.
For the wound-healing assays, 5×105
Tca8113 and CAL27 cells were seeded in 6-well plates in the
aforementioned culture medium. When the cells reached 90%
confluence, wounds were made by scratching a line across the bottom
of the dish on a monolayer of confluent cells with a 10 µl
pipette tip. Then, the cells were incubated in serum-free media at
37°C. The gaps between the cells at 0, 24 and 48 h were imaged on a
microscope at a magnification, ×100 and then quantified using
ImageJ 1.8.0 software (National Institutes of Health, Bethesda, MD,
USA).
Real time-quantitative polymerase chain
reaction (RT-qPCR)
The total RNA from all experimental and control
cells was extracted using TRIzol® (Life Technologies;
Thermo Fisher Scientific, Inc.) according to the manufacturer’s
protocol. Reverse transcription was performed using the PrimeScript
RT Master Mix (Takara Bio, Inc., Otsu, Japan). The RT-qPCR was
performed using a CFX96 real-time PCR detection system with
SYBR® Premix Ex Taq II (Takara Bio, Inc.). Human GAPDH
was used as an internal control to normalize the amount of mRNA in
each sample using the 2−ΔΔCq method (19). The conditions for PCR were as
follows: 1 cycle of 95°C for 30 sec, followed by 40 cycles of 95°C
for 5 sec and 60°C for 30 sec. The primers used in the PCR
reactions were as follows: Vimentin forward (F),
5′-AACCTGGCCGAGGACATCA-3′; vimentin reverse (R),
5′-TCAAGGTCAAGACGTGCCAGA-3′; Snail F, 5′-GACCACTATGCCGCGCTCTT-3′;
Snail R, 5′-TCGCTGTAGTTAGGCTTCCGATT-3′; hes family bHLH
transcription factor 1 (Hes1) F, 5′-GTGTCAACACGACACCGGATAAAC-3′;
Hes1 R, 5′-CAGAATGTCCGCCTTCTCCAG-3′; E-cadherin F,
5′-AGGATGACACCCGGGACAAC-3′; E-cadherin R,
5′-TGCAGCTGGCTCAAGTCAAAG-3′; GAPDH F, 5′-GCACCGTCAAGGCTGAGAAC-3′;
GAPDH R, 5′-TGGTGAAGACGCCAGTGGA-3′.
Western blot analysis
The proteins were prepared using RIPA lysis buffer
(Thermo Fisher Scientific, Inc.), and protein concentrations were
determined using a BCA protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China). Each lane was loaded with 30
µg protein on 6-10% SDS-PAGE gels and then transferred onto
polyvinyl difluoride membranes (EMD Millipore, Billerica, MA, USA),
which were blocked with 5% non-fat dry milk/TBST (0.1% Tween-20)
for 1 h at 25°C to prevent non-specific binding. The membranes were
then incubated with primary antibodies (dilution, 1:1,000) in 5%
w/v BSA, 1X TBS and 0.1% Tween-20 at 4°C with gentle shaking
overnight. Following washing 3 times with TBST buffer (20 mM
Tris-HCl, 137 mM NaCl and 0.1% Tween-20; pH 7.6) for 5 min/wash,
the blots were incubated with a horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:5,000) for 1 h at room
temperature. Subsequently, the membrane was washed 3 times with
TBST for 5 min/wash, and the protein bands were detected with the
Millipore ECL luminous liquid (EMD Millipore). ImageJ 1.8.0
software was used to quantify the immunoblot bands.
Statistical analysis
Each experiment was repeated at least 3 times. All
data are expressed as the mean ± standard deviation and calculated
by SPSS 19.0 software (SPSS, Inc., IBM Corp., Armonk, NY, USA). The
one-way analysis of variance followed by Fisher’s Least Significant
Difference test were used to compare the differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
EMT participates in the migration of OSCC
cells
To identify the role of EMT progression in the
migration of OSCC cells, an siRNA method was used to knockdown
Snail, a vital EMT transcriptional factor, in the OSCC Tca8113 and
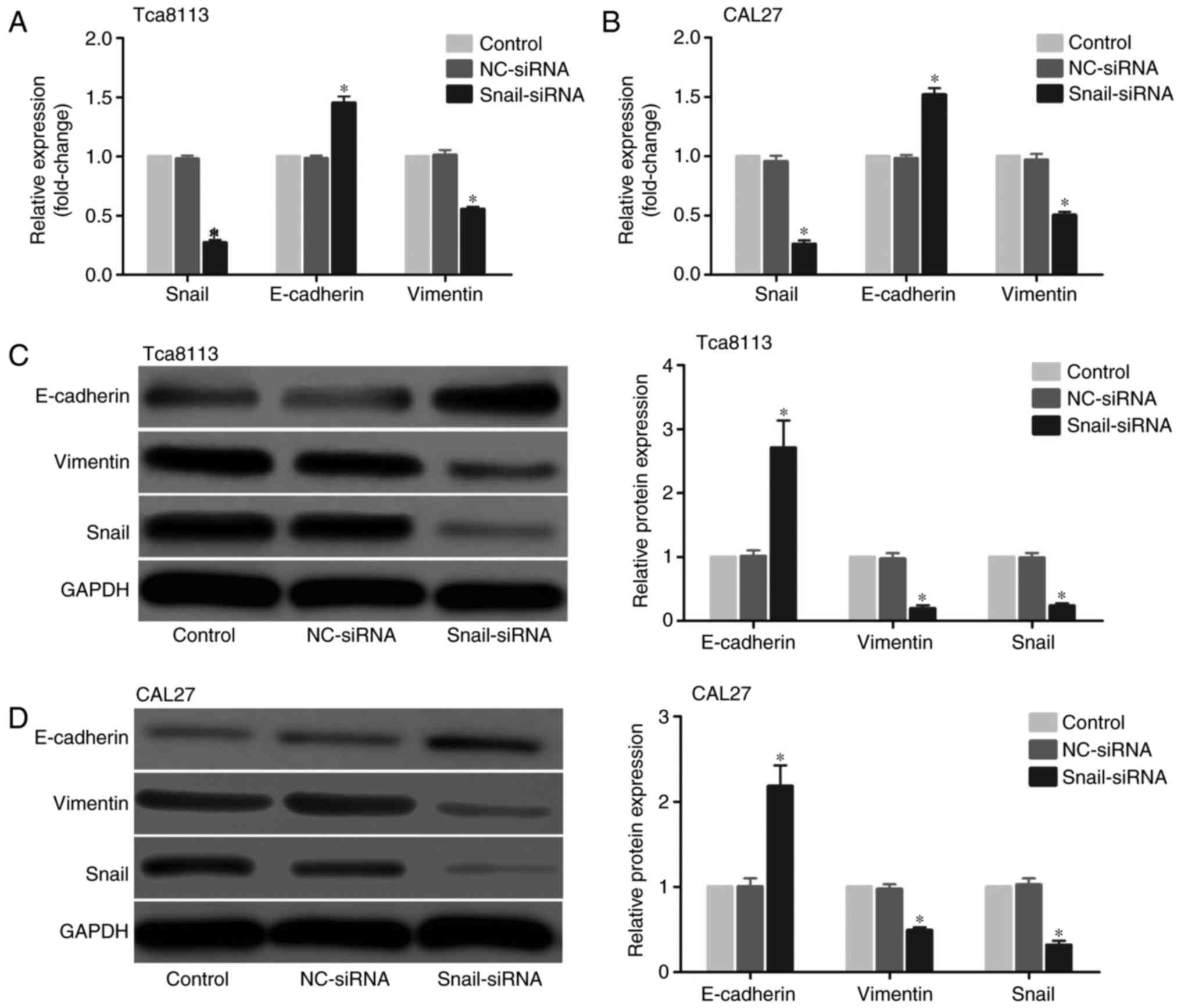
CAL27 cell lines. The mRNA level was first assessed using RT-qPCR,
and the results demonstrated that the Snail siRNA model effectively
suppressed Snail expression in OSCC cells. In addition, the
expression level of the mesenchymal marker vimentin was decreased,
while the level of the epithelial marker E-cadherin was increased
in the Tca8113 and CAL27 cells (Fig.
1A and B). Then, changes in protein expression were examined
with western blot analysis. In accordance with the mRNA results,
Snail and vimentin levels were decreased, while E-cadherin was
increased in the Tca8113 and CAL27 cells (Fig. 1C and D).
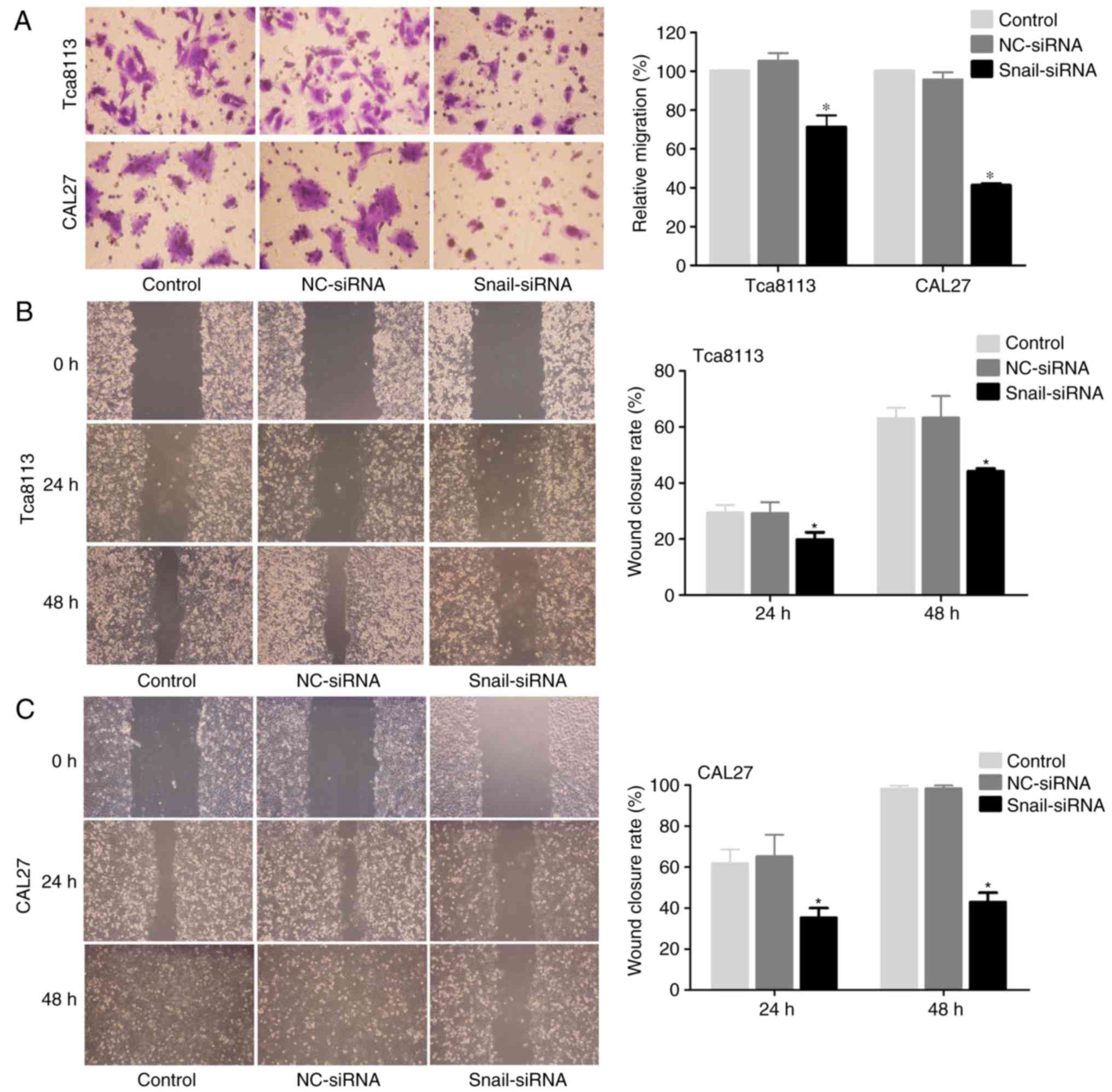
To assess the effect of Snail knockdown on cell
migration in Tca8113 and CAL27 cells, a Transwell assay indicated
that Snail-silenced cells exhibited a significant decrease in
migration rate compared with the controls (Fig. 2A). Furthermore, it was observed
that Snail knockdown in OSCC cells significantly decreased the rate
of cell migration from the edge of the wound following scratching
(Fig. 2B and C).
Inhibition of Notch signalling with DAPT
inhibits EMT progression
To confirm the association between Notch signalling
and EMT progression, the γ-secretase DAPT was employed to block
Notch signalling and detect changes in the EMT mechanism in OSCC
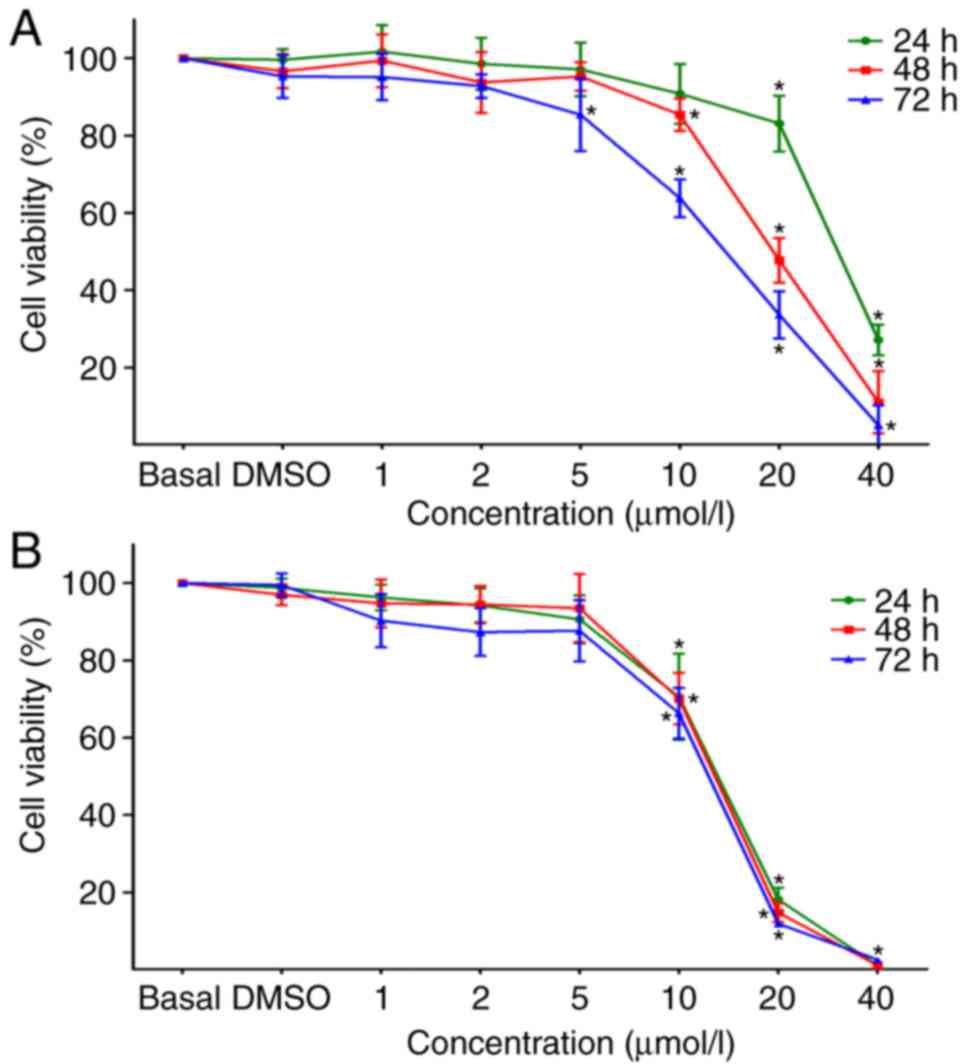
cells. Firstly, an MTT assay was used to examine the effects of
DAPT at different doses (1, 2, 5, 10, 20 and 40 µmol/l) on
the growth and viability of Tca8113 and CAL27 cells, and untreated
cells or cells treated with DMSO were used as controls. The results
demonstrated that the viabilities of Tca8113 and CAL27 cells were
markedly inhibited by DAPT in a time- and concentration-dependent
manner when the concentrations of DAPT were ≥10 µmol/l
(Fig. 3). Therefore, treatment
with DAPT at concentrations of 1 and 5 µmol/l for 24 and 48
h was used in subsequent studies, which had no marked effect on the
viability of Tca8113 and CAL27 cells.
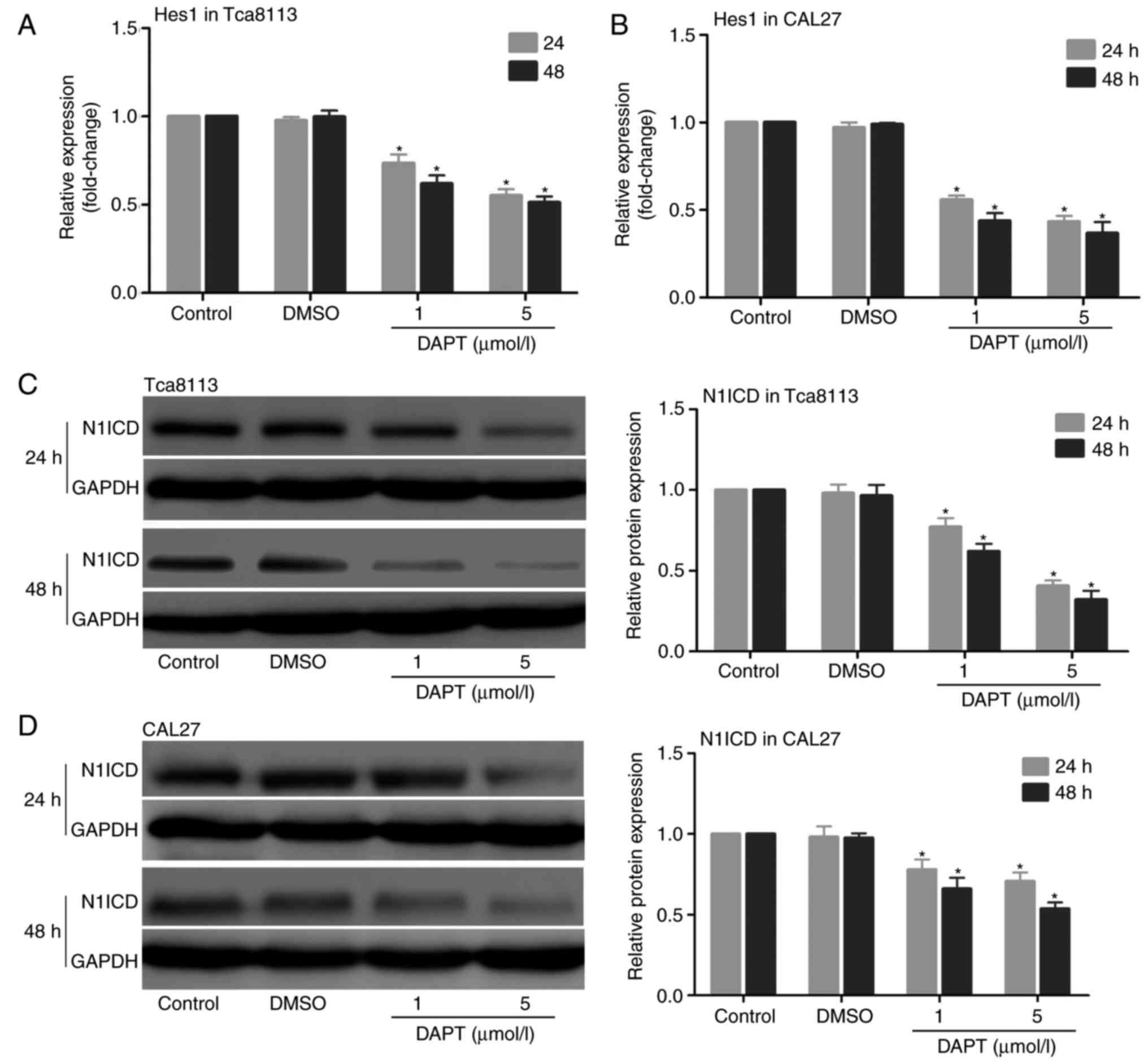
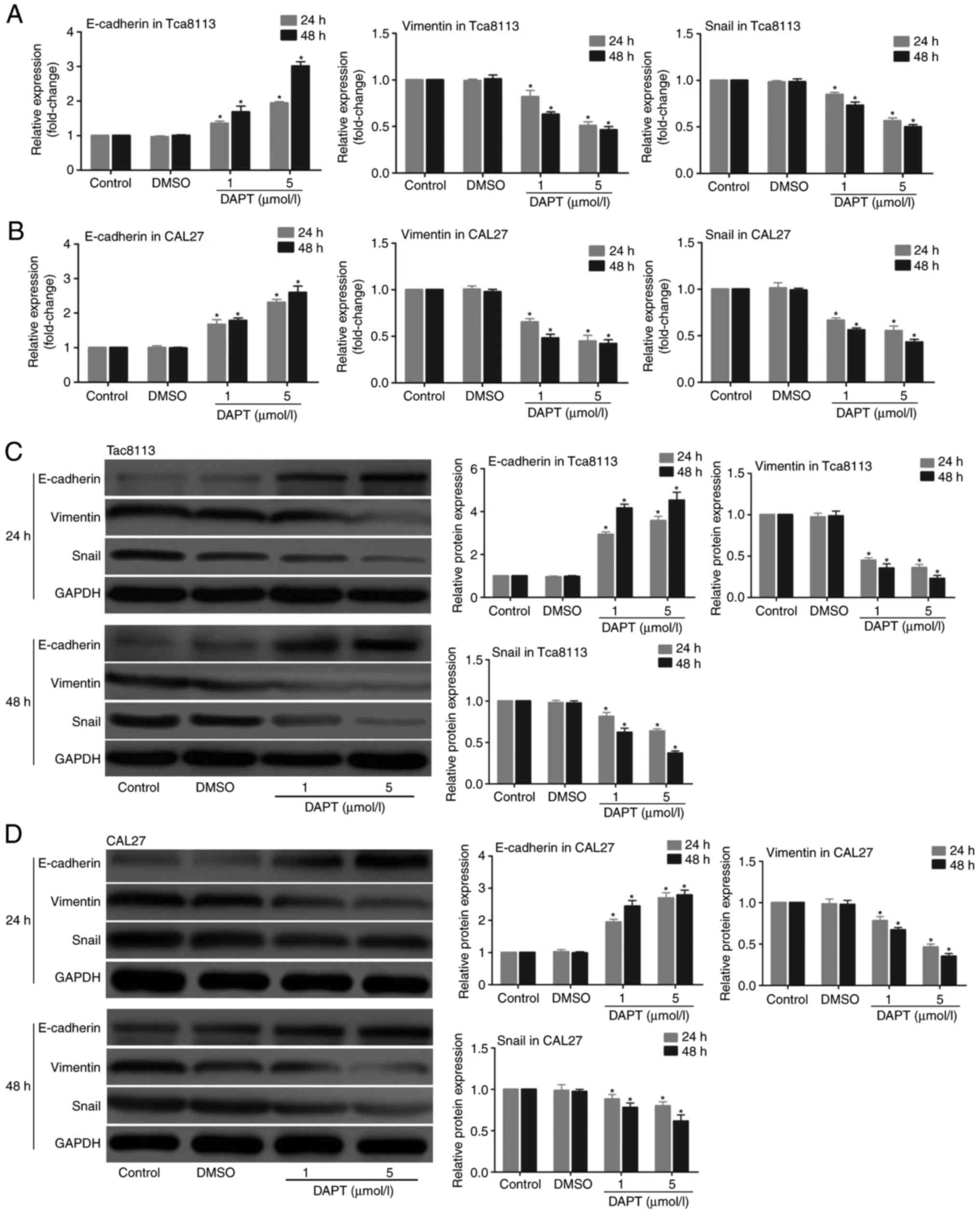
Then, the role of DAPT in the expression of the
Notch signalling pathway was examined in Tca8113 and CAL27 cells.
The cells were treated with DAPT for 24 and 48 h, and the mRNA
expression levels of Hes1 and the protein expression levels of
N1ICD were measured. The results indicated that the mRNA expression
levels of Hes1 and the protein expression levels of N1ICD were
decreased in the 2 cell lines following treatment with DAPT in a
time- and concentration-dependent manner (Fig. 4). Whether inhibition of the Notch
signalling pathway with DAPT affected the expression of EMT markers
in Tca8113 and CAL27 cells was also explored. The expression of
Snail, vimentin and E-cadherin were detected at the mRNA and
protein levels in the 2 cell lines. The results demonstrated that
the inhibition of the Notch signalling pathway with DAPT
downregulated the expression of Snail and vimentin and upregulated
the expression of E-cadherin at the mRNA (Fig. 5A and B) and protein levels
(Fig. 5C and D) in a time- and
concentration-dependent manner.
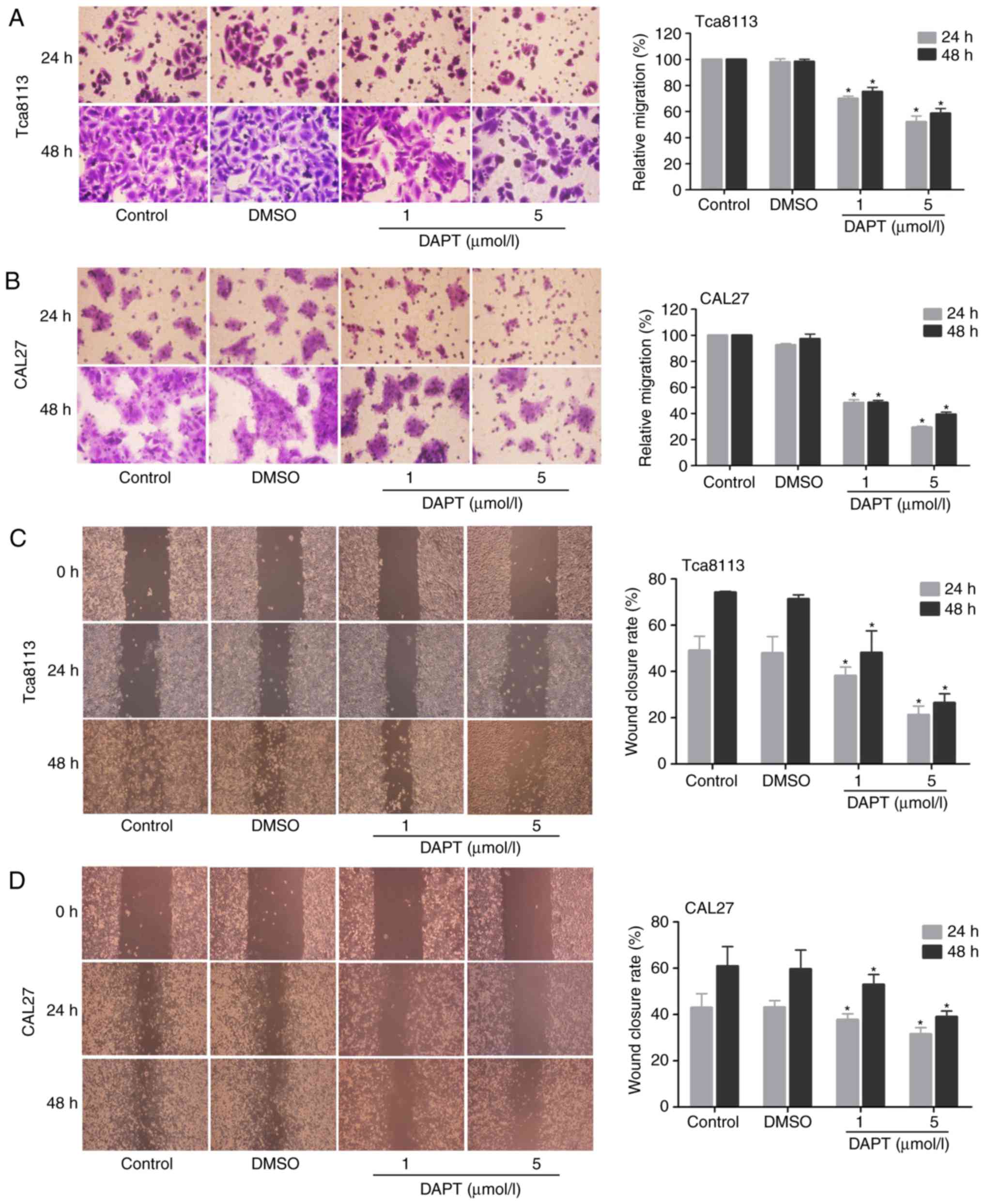
Inhibition of Notch signalling with DAPT
decreases OSCC cell migration
To determine whether Notch inhibition affects the
migration potential of OSCC cells, Transwell chamber migration and
wound-healing assays were performed. As indicated in Fig. 6A–D, the inhibition of Notch
signalling with DAPT markedly decreased the migratory capabilities
of Tca8113 and CAL27 cells in a time- and concentration-dependent
manner, and the number of migrating cells was significantly
decreased in the DAPT treatment groups compared with in the
controls.
Discussion
The high rate of distant metastasis is a major
obstacle in improving the survival rates of patients with OSCC
(20). Therefore, an
understanding of the molecular mechanisms that regulate metastasis
may provide novel insights for the development of effective
treatments for OSCC, which in turn may decrease the rate of
metastasis and improve survival. The present study aimed to
understand the role of Notch signalling and its association with
EMT in metastasis during OSCC development using 2 cell lines,
Tca8113 and CAL27. Although STR detection identified that the
Tca8113 cell line was contaminated by HeLa cells (21), previous studies revealed that
contamination is unlikely to affect the outcomes of the present
study (13,17,22). Firstly, the results verified that
Snail regulates EMT and promotes tumour migration in OSCC cells,
and then it was demonstrated that the inhibition of Notch
signalling by DAPT decreased the migration of OSCC cells by
inhibiting the EMT mechanism. The present study suggests that Notch
signalling promotes EMT and is involved in tumour metastasis in
OSCC cells.
EMT is a dynamic cellular process during which
epithelial cells adopt characteristics of mesenchymal cells. EMT
itself is essential for tissue construction during normal
development and the development of metastatic disease (5). A number of in vitro and in
vivo studies have demonstrated that the cancer-associated EMT
process promotes cancer cell migration and invasion into the stroma
(23). A loss of epithelial
markers and cell-cell adhesion, and a gain of mesenchymal marker
expression, are key events in EMT for tumour cell metastasis and
invasion (24). In previous
studies, the downregulation of E-cadherin and matrix
metalloproteinase (MMP)-9 and the upregulation of vimentin and
MMP-2 were observed in OSCC cell lines (16), and a trend towards decreased
E-cadherin expression but increased vimentin expression was
associated with increased disease severity of OSCC (14). In addition, EMT markers, including
E-cadherin, β-catenin, adenomatous polyposis coli and vimentin,
have predictive value for the progression of multiple primary OSCC,
and the simultaneous downregulation of E-cadherin and β-catenin
exerted a significant prognostic effect in these patients (25). These data, combined with those
from the present study, suggest that the EMT process serves a
critical role in the progression of OSCC.
Snail is known as a pivotal mediator of EMT and
contributes to the repression of the transcription of E-cadherin
gene by binding to E-boxes during tumour progression (26). A number of studies have
demonstrated that Snail promotes tumour development and metastasis
through regulating EMT in a variety of types of cancer (27). A previous study demonstrated that
Snail knockdown in OSCC cells significantly inhibited cell
migration and invasion (28). An
additional study suggested that the overexpression of Snail induces
EMT and promotes cancer stem cell-like traits in OSCC SCC-9 cells
(29). The results of the present
study additionally confirmed that the knockdown of Snail
upregulated E-cadherin expression, downregulated vimentin
expression and decreased cell motility in Tca8113 and CAL27 cells.
All of these data suggest that Snail-induced EMT promotes tumour
metastasis in OSCC cells.
It is well-established that the EMT process is
stimulated and regulated by a number of signal transduction
pathways, including Transforming growth factor β, Wnt, Hedgehog and
Notch signalling (30). Emerging
evidence suggests that the Notch signalling pathway serves a vital
role in the regulation of EMT, resulting in tumour invasion and
metastasis (31,32), and the suppression of Notch
signalling with the γ-secretase inhibitor DAPT restricts the
growth, invasion and metastasis of gastric cancer by inhibiting EMT
(33). Previous
immunohistochemical examination demonstrated increased expression
levels of Notch1, Notch2, Jagged1, Hes1 and Hairy enhancer-of-split
related with YRPW motif protein 1 in oral tissues of OSCC compared
with those in the normal controls, suggesting that Notch signalling
is active in OSCC (13). However,
the roles of the Notch signalling pathway and the EMT process in
the metastatic potential of OSCC remains unclear. In the present
study, DAPT was used to inhibit the Notch signaling pathway in
Tca8113 and CAL27 cells. The results indicated that the mRNA
expression level of Hes1, a downstream target of Notch signalling,
and the protein expression level of the Notch1 intracellular domain
(N1ICD) were decreased following treatment with DAPT. These data
suggest that DAPT effectively inhibits the Notch pathway in OSCC
cell lines. In addition, the results suggested that the inhibition
of Notch signalling notably decreased Snail and vimentin and
increased E-cadherin at the mRNA and protein levels during the EMT
process. Furthermore, DAPT inhibition may markedly decrease the
migration ability of OSCC cells. Together, these observations
indicate that Notch signalling may be involved in the EMT-induced
metastasis of OSCC cells.
In summary, the results from the present study
confirmed that Snail-induced EMT promotes cell migration in OSCC
cells, and additionally demonstrated that Notch signalling mediates
tumour metastasis in OSCC cells through its association with EMT
progression. Therefore, targeting Notch signalling and its
association with EMT may provide novel insights into the mechanism
of invasion and metastasis in OSCC, and the inactivation of Notch
signalling or the inhibition of the EMT upstream molecule Snail may
be useful approaches for the development of therapeutic strategies
for OSCC.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Key Research
and Development Program of Hainan Province (grant no., ZDYF
2016113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JZ, GZ, QS and MY contributed to the study concept
and experimental design. JZ, LZ, QS, XW, PL and TW performed the
experiments. JZ, PL and TW contributed to the data acquisition and
analysis. JZ and MY drafted and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warnakulasuriya S: Living with oral
cancer: Epidemiology with particular reference to prevalence and
life-style changes that influence survival. Oral Oncol. 46:407–410.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta S, Kong W, Peng Y, Miao Q and
Mackillop WJ: Temporal trends in the incidence and survival of
cancers of the upper aerodigestive tract in Ontario and the United
States. Int J Cancer. 125:2159–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SY, Nam SY, Choi SH, Cho KJ and Roh
JL: Prognostic value of lymph node density in node-positive
patients with oral squamous cell carcinoma. Ann Surg Oncol.
18:2310–2317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L and Li W: Epithelial-mesenchymal
transition in human cancer: Comprehensive reprogramming of
metabolism, epigenetics, and differentiation. Pharmacol Ther.
150:33–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scanlon CS, Van Tubergen EA, Inglehart RC
and D’Silva NJ: Biomarkers of epithelial-mesenchymal transition in
squamous cell carcinoma. J Dent Res. 92:114–121. 2013. View Article : Google Scholar :
|
|
7
|
Nijkamp MM, Span PN, Hoogsteen IJ, van der
Kogel AJ, Kaanders JH and Bussink J: Expression of E-cadherin and
vimentin correlates with metastasis formation in head and neck
squamous cell carcinoma patients. Radiother Oncol. 99:344–348.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto S, Schulze KL and Bellen HJ:
Introduction to Notch signaling. Methods Mol Biol. 1187:1–14. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bray SJ: Notch signalling: A simple
pathway becomes complex. Nat Rev Mol Cell Biol. 7:678–689. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang TH, Liu HC, Zhu LJ, Chu M, Liang YJ,
Liang LZ and Liao GQ: Activation of Notch signaling in human tongue
carcinoma. J Oral Pathol Med. 40:37–45. 2011. View Article : Google Scholar
|
|
12
|
Osathanon T, Nowwarote N and Pavasant P:
Expression and influence of Notch signaling in oral squamous cell
carcinoma. J Oral Sci. 58:283–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hijioka H, Setoguchi T, Miyawaki A, Gao H,
Ishida T, Komiya S and Nakamura N: Upregulation of Notch pathway
molecules in oral squamous cell carcinoma. Int J Oncol. 36:817–822.
2010.PubMed/NCBI
|
|
14
|
Chaw SY, Majeed AA, Dalley AJ, Chan A,
Stein S and Farah CS: Epithelial to mesenchymal transition (EMT)
biomarkers-E-cadherin, beta-catenin, APC and Vimentin - in oral
squamous cell carcinogenesis and transformation. Oral Oncol.
48:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Angadi PV, Patil PV, Angadi V, Mane D,
Shekar S, Hallikerimath S, Kale AD and Kardesai SG:
Immunoexpression of epithelial mesenchymal transition proteins
E-cadherin, β-catenin, and N-cadherin in oral squamous cell
carcinoma. Int J Surg Pathol. 24:696–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krisanaprakornkit S and Iamaroon A:
Epithelial-mesenchymal transition in oral squamous cell carcinoma.
ISRN Oncol. 2012:6814692012.PubMed/NCBI
|
|
17
|
Xie SM, Lu ZY, Lin YZ, Shen LJ and Yin C:
Upregulation of PTEN suppresses invasion in Tca8113 tongue cancer
cells through repression of epithelial-mesenchymal transition
(EMT). Tumor Biol. 37:6681–6689. 2016. View Article : Google Scholar
|
|
18
|
Zhao XP, Zhang H, Jiao JY, Tang DX, Wu YL
and Pan CB: Overexpression of HMGA2 promotes tongue cancer
metastasis through EMT pathway. J Transl Med. 14:262016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
20
|
Pu Y, Wang L, Wu H, Feng Z, Wang Y and Guo
C: High MMP-21 expression in metastatic lymph nodes predicts
unfavorable overall survival for oral squamous cell carcinoma
patients with lymphatic metastasis. Oncol Rep. 31:2644–2650. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye F, Chen C, Qin J, Liu J and Zheng C:
Genetic profiling reveals an alarming rate of cross-contamination
among human cell lines used in China. FASEB J. 29:4268–4272. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou X, Liu S, Cai G, Kong L, Zhang T, Ren
Y, Wu Y, Mei M, Zhang L and Wang X: Long Non Coding RNA MALAT1
promotes tumor growth and metastasis by inducing
epithelial-mesenchymal transition in oral squamous cell carcinoma.
Sci Rep. 5:159722015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe? Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
da Silva SD, Morand GB, Alobaid FA, Hier
MP, Mlynarek AM, Alaoui-Jamali MA and Kowalski LP:
Epithelial-mesenchymal transition (EMT) markers have prognostic
impact in multiple primary oral squamous cell carcinoma. Clin Exp
Metastas. 32:55–63. 2015. View Article : Google Scholar
|
|
26
|
Giroldi LA, Bringuier PP, de Weijert M,
Jansen C, van Bokhoven A and Schalken JA: Role of E boxes in the
repression of E-cadherin expression. Biochem Bioph Res Commun.
241:453–458. 1997. View Article : Google Scholar
|
|
27
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of Snail in EMT and tumorigenesis. Curr Cancer Drug Tar.
13:963–972. 2013. View Article : Google Scholar
|
|
28
|
Li YY, Zhou CX and Gao Y: Snail regulates
the motility of oral cancer cells via RhoA/Cdc42/p-ERM pathway.
Biochem Bioph Res Commun. 452:490–496. 2014. View Article : Google Scholar
|
|
29
|
Zhu LF, Hu Y, Yang CC, Xu XH, Ning TY,
Wang ZL, Ye JH and Liu LK: Snail overexpression induces an
epithelial to mesenchymal transition and cancer stem cell-like
properties in SCC9 cells. Lab Invest. 92:744–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang ZW, Li YW, Kong DJ and Sarkar FH: The
role of Notch signaling pathway in epithelial-mesenchymal
transition (EMT) during development and tumor aggressiveness. Curr
Drug Targets. 11:745–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li YM, Ma J, Qian XJ, Wu Q, Xia J, Miele
L, Sarkar FH and Wang ZW: Regulation of EMT by Notch signaling
pathway in tumor progression. Curr Cancer Drug Tar. 13:957–962.
2013. View Article : Google Scholar
|
|
33
|
Li LC, Peng Y, Liu YM, Wang LL and Wu XL:
Gastric cancer cell growth and epithelial-mesenchymal transition
are inhibited by γ-secretase inhibitor DAPT. Oncol Lett.
7:2160–2164. 2014. View Article : Google Scholar : PubMed/NCBI
|