Introduction
Vitiligo is a depigmentation disorder of the skin
clinically characterized by the appearance of disfiguring
circumscribed skin macules. It is hypothesized that the disease
primarily results from the destruction of melanocytes or
obstruction of the melanin synthesis pathway (1,2).
Melanin is an essential component in the pigmentary system of the
skin, which serves a vital role in the prevention from damage by
ultraviolet light (3).
Melanocytes synthesize melanin via a process termed melanogenesis
in the melanosome, a specialized organelle of melanocytes (4). Melanogenesis is a physiological
process leading to the production of melanin pigment and a crucial
step for the regulation of melanocyte functions, including
photoprotection (5).
Melanogenesis is regulated by melano-genic enzymes, including
tyrosinase (TYR), tyrosinase-related protein 1 (TRP 1) and
tyrosinase-related protein 2 (TRP 2) (6). TYR directly mediates the production
of melanin through the oxidation of melanogenesis-associated
substrates tyrosine (7).
Microphthalmia-associated transcription factor (MITF) is a master
regulator of the transcription of genes involved in melanin
synthesis (8).
Previous studies have identified signaling pathways
that mediate melanogenesis A summary obtained from Niu and Aisa
(9) is presented in Fig. 1. Among them, the p38
mitogen-activated protein kinase (p38 MAPK) pathway may upregulate
melanogenesis by increasing MITF expression (9,10).
The extracellular signal-regulated kinase mitogen-activated protein
kinase (ERK MAPK)-dependent MITF expression pathway is also
involved in melanogenesis (11).
In addition, the cyclic adenosine monophosphate (cAMP)/protein
kinase A (PKA) signal pathway is one of the primary pathways that
may increase the expression of MITF, which is also associated with
cAMP responsive element binding (CREB) and CREB binding protein
(CBP) (12). Previously, the
Wnt/β-catenin signal pathway was identified to serve an important
role in melanin synthesis through the nuclear mediator MITF
(13).
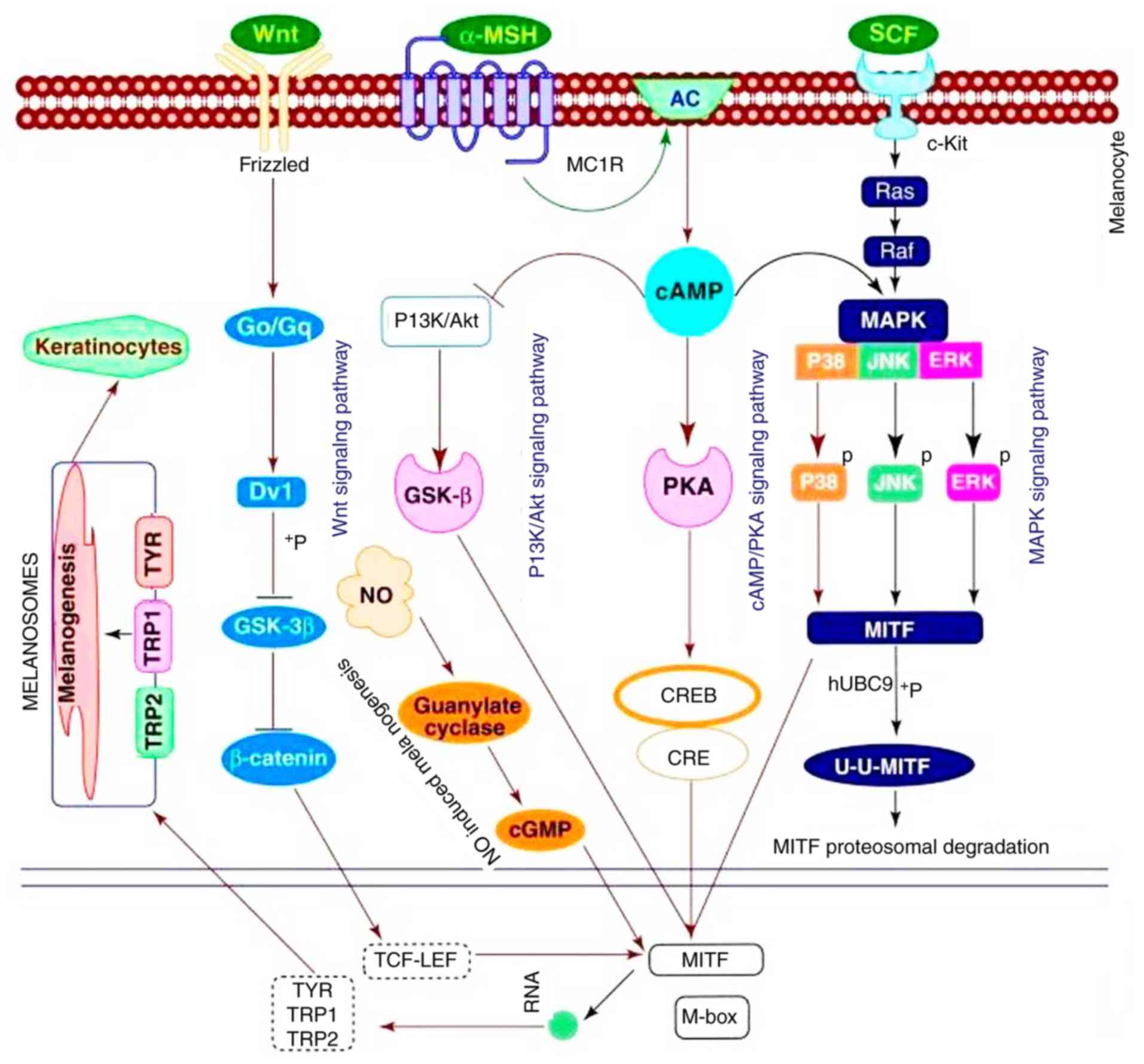 | Figure 1Melanogenesis. Obtained from Niu and
Aisa (9). TYR, tyrosinase; TRP-1,
tyrosinase-related protein 1; TRP-2, tyrosinase-related protein 2;
MITF, microphthalmia-associated transcription factor; MAPK,
mitogen-activated protein kinase; P38, p38 MAPK; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; cAMP,
intracellular cyclic adenosine monophosphate; Dvl, Dishevelled;
Go/Gq, G protein; GSK-3β, glycogen synthase kinase 3β; CRE,
cAMP-response element; CREB, cAMP-response element binding protein;
CBP, CREB binding protein; TCF-LEF, T cell factor-lymphoid enhancer
factor; PKA, protein kinase A; α-MSH, α melanocyte stimulating
hormone; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B;
NO, nitric oxide; MC1R, melanocortin 1 receptor; SCF, stem cell
factor; AC, adenylyl cyclase; c-Kit, proto-oncogene c-Kit. |
Vernonia anthelmintica (L.) Willd.
(Kaliziri), a member of the Asteraceae family, is an erect forb or
shrub that is broadly distributed in subtropical and tropical areas
throughout Asia and Africa (14).
It has been demonstrated that Kaliziri possesses a number of
pharmacological properties, including anti-inflammatory,
antibacterial, antioxidant, hypoglycemic and antithrombotic
activities (15). The seeds are
used in traditional therapy as treatment for skin diseases
including vitiligo (16).
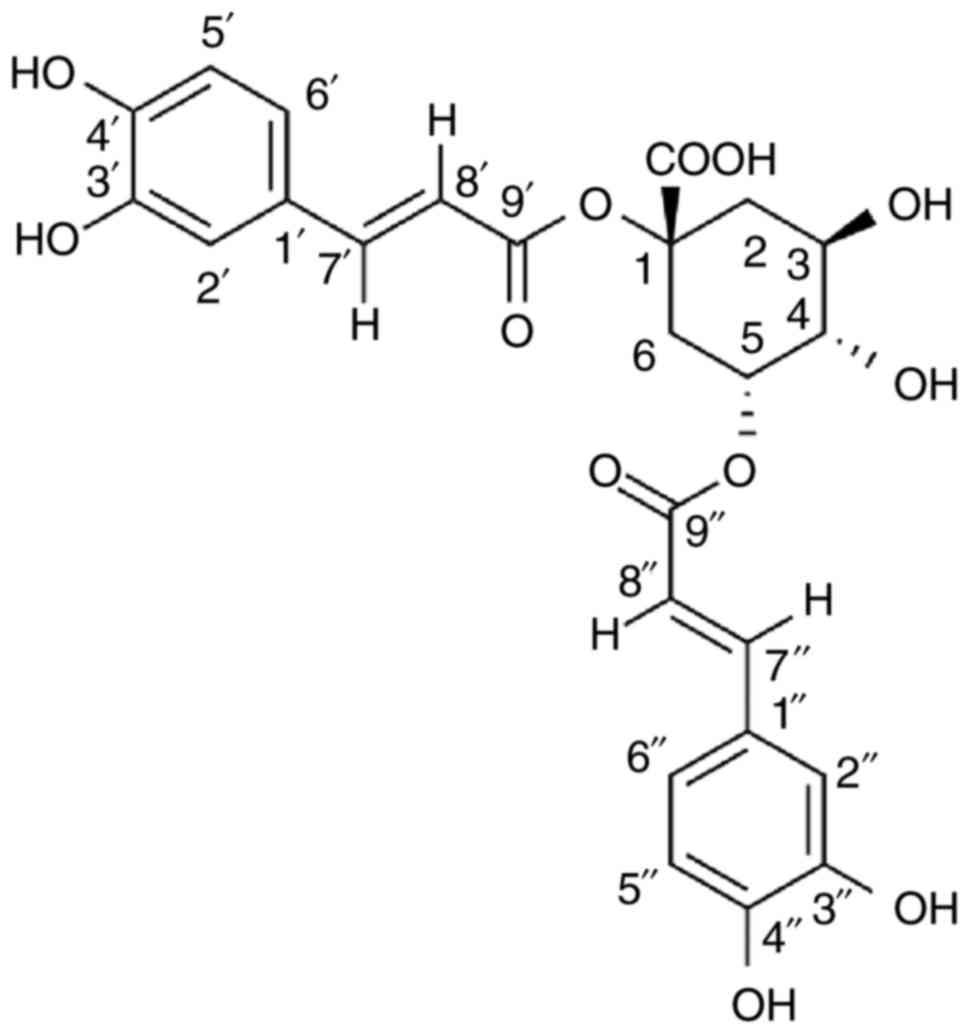
1,5-Dicaffeoylquinic acid (1,5-diCQA; Fig. 2) is a class of natural
polyphenolic compounds widely distributed in plants (17,18). Previously, it was also identified
that 1,5-diCQA exhibits antioxidant signal properties that
upregulate glutathione synthesis by stimulating the nuclear
factor-like 2 pathway in astrocytes and protects them from cell
death (19). 1,5-diCQA also
protects primary neurons from amyloid β 1-42-induced apoptosis via
the phosphoinositide 3-kinase/protein kinase B signal pathway
(20).
Therefore, the present study evaluated the effect of
1,5-diCQA, which was isolated from Kaliziri seeds, on melanin
synthesis in murine B16 cells, and examined its molecular
mechanism.
Materials and methods
1,5-DiCQA extraction
1,5-DiCQA was extracted from Vernonia
anthelmintica (L.) Willd. Briefly, Vernonia
anthelmintica seeds (1 kg) were pulverized. Ethanol (40%) was
added and refluxed at 80°C three times, each time for 1 h. The
extracts were combined and concentrated. This extract was
subsequently purified by HPD-300 macroporous resin (diameter, 1:8;
loading volume, 6 BV; Cangzhou Bon Adsorber Technology Co., Ltd.,
Cangzhou, China). Following drying, a 60 g powder was obtained. The
powder was taken and dissolved in ethanol, and separated by medium
pressure preparative chromatography. At last, the fraction was
separated and purified by semi-preparative chromatography to obtain
1,5-diCQA, according to a previously described method (21).
Materials
The obtained 1,5-diCQA (white solid; purity, 98%)
was dissolved in dimethyl sulfoxide (DMSO; 100 mM) and stored at
−20 °C as a stock solution. ERK (cat. no. 4696), phosphorylated
(p)-ERK (Thr202/Tyr204; cat. no. 9106), p38 (cat. no. 9212), p-P38
(Thr180/Tyr182; cat. no. 5140), CREB (cat. no. 9104), p-CREB
(Ser133; cat. no. 9196), glycogen synthase kinase 3β (GSK-3β; cat.
no. 9832), p-GSK-3β (cat. no. 9323), β-catenin (cat. no. 8480) and
p-β-catenin (ser675; cat. no. 4176) antibodies were purchased from
Cell Signaling Technology, Inc., (Danvers, MA, USA). p-MITF (cat.
no. SAB4301514) antibody, phenyl methyl sulfonyl fluoride and the
components of the whole cell lysis buffer for western blot analysis
were obtained from Sigma-Aldrich, Merck KGaA (Darmstadt, Germany).
β-actin antibody (cat. no. MA1115), rabbit anti-goat secondary
antibody (cat. no. BA1060), goat anti-rabbit secondary antibody
(cat. no. BA1054) and goat anti-mouse secondary antibody (cat. no.
BA1050) were purchased from Wuhan Boster Biological Technology,
Ltd., (Wuhan, China). TYR (cat. no. sc-7833), MITF (cat. no.
sc-52938), TRP 1 (cat. no. sc-25543) and TRP 2 (cat. no. sc-25544)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The bicinchoninic acid (BCA) protein assay kit
was purchased from Beijing Biomed Gene Technology Co., Ltd.
(Beijing, China). The ERK (PD98059), JNK (SP600125) and p38 MAPK
inhibitors (SB203580) were purchased from Beyotime Institute of
Biotechnology (Haimen, China). The JNK inhibitor (SP600125) was
obtained from EMD Biosciences (EMD Millipore, Billerica, MA, USA)
and dissolved in DMSO. Deoxynucleotide triphosphates (dNTPs, cat.
no. BH7201B) were purchased from TAKARA BIO INC. (Yokohama, Japan).
The Recombinant RNasin Ribonuclease Inhibitor (cat. no. N2511) and
100 U Moloney murine leukemia virus (M-MLV) reverse transcriptase
(cat. no. M170A) were purchased from Promega Corporation (Madison,
WI, USA). Power SYBR™-Green PCR Master mix (cat. no. 4367659) was
purchased from Applied Biosystems (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). 8-Methoxypsoralen (8-MOP, cat. no. M3501) was
purchased from Sigma-Aldrich (Merck KGaA) and dissolved in DMSO (50
mM) for storage at −20°C as a stock solution.
Cell culture
B16 murine melanoma cells were obtained from the
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences, (Beijing, China). Cells were grown in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 µg/ml streptomycin and 100 U/ml
penicillin (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
15140-122) in a humidified atmosphere with 5% CO2 at
37°C.
Cell viability assay
To examine the effects of 1,5-diCQA on cell
viability, a TransDetect Cell Counting kit (CCK) assay (cat. no.
FC101; Beijing Transgen Biotech, Co., Ltd., Beijing, China) was
performed according to the manufacturer's protocol. Briefly, B16
cells were seeded in 96-well plates (5x103 cells/well)
and allowed to adhere at 37°C for 12 h. Cells were then treated
with 0, 5, 25, 50, 100, 200 or 400 µM 1,5-diCQA for 48 h.
Following treatment, 10 µl CCK solution was added into each
well and cells were incubated at 37°C for an additional 2 h.
Optical absorbance was determined at 450 nm with a Spectra Max M5
plate reader (Molecular Devices, LLC, Sunnydale, CA, USA). The
absorbance of cells without treatment was considered as 100% cell
survival. Each treatment was performed in quintuplicate, and each
experiment was repeated three times. To observe the cell
morphology, B16 cells were seeded in 6-well plates
(1x104 cells/well) and allowed to adhere at 37°C for 12
h. Cells were subsequently treated with 0, 5, 50 or 100 µM
1,5-diCQA for 48 h. Cell morphology was observed and images were
captured at room temperature using a LEICA DMI 8 fluorescence
inversion microscope (magnification, x200; Leica Microsystems GmbH,
Wetzlar, Germany).
Melanin content assay
The effect of 1,5-diCQA on melanogenesis in B16
cells was investigated according to a previously published
protocol, with certain modifications (22). More specifically, intracellular
melanin content was measured by using the NaOH dissolution method:
B16 cells were seeded in a 6-well plate at a density of
2x105 cells/well, and then allowed to attach for 12 h.
Then, cells were treated with 1,5-diCQA at 0, 5, 50, 100 µM
or 8-MOP at 50 µM for 48 h, and SB203580 (10 µM);
PD98059 (1 µM); SP600125 (10 µM) for 2 h prior to
1,5-diCQA application. Cells were washed with PBS and harvested
with 100 µl radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology) in 1.5 Eppendorf (Ep) tubes.
Samples were centrifuged at −4°C and 12,000 × g for 20 min, and the
supernatant was obtained to determine the protein concentrations.
Sediment was dissolved in 150 µl 1M NaOH (with 10% DMSO) for
1 h at 80°C, and solubilized melanin was measured at 405 nm. The
amount of melanin was calculated by normalizing the total melanin
values with the protein content.
Tyrosinase activity assay
Tyrosinase activity was estimated by measuring the
rate of L-DOPA oxidation as previously described, with certain
modifications (23). B16 cells
were seeded in a 6-well plate at a density of 3×105
cells/well and allowed to attach for 12 h. Then, cells were treated
with 1,5-diCQA at 0, 5, 50, 100 µM or 8-MOP at 50 µM
for 24 h; SB203580 (10 µM); PD98059 (1 µM); or
SP600125 (10 µM) for 2 h prior to 1,5-diCQA application.
Cells were then washed with ice-cold PBS twice, 100 µl lysis
buffer (1% sodium deoxycholate and 1% Triton X-100 in PBS) was
added and cells were collected with a cell scraper into an Ep tube.
All tubes were incubated at −20°C for 30 min, and then centrifuged
at −4°C and 12,000 × g for 20 min. Following centrifugation, the
supernatants were obtained for determining the protein
concentrations and tyrosinase activity assay. Protein
concentrations were determined using the BCA protein assay kit. A
reaction mixture containing 90 µl cell lysate and 10
µl 10 mM L-DOPA was added to each well of a 96-well plate
for each sample. Following a 20–60-min incubation (according to the
content of dopachrome formation) at 37°C in the dark, the
dopachrome product was detected at 490 nm by a multi-plate reader
(Spectra Max M5/M5e), The tyrosinase activity of each sample was
calculated and corrected for the concentrations of proteins, and
normalized with protein content levels.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was prepared from B16 cells using
TRIzol reagent® (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The quality of RNA
samples was assessed using 1.5% agarose gel electrophoresis, at 100
V for 20 min and was subsequently analyzed using Quantity One
version 3 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
concentration of total RNA was determined using a NanoDrop 2000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington DE, USA). cDNA was synthesized from 1.0 µg
total RNA using the Reverse Transcriptase M-MLV according to the
protocol of the manufacturer. The cDNA from each sample was used as
a template in the RT-qPCR to detect the mRNA level of the 1,5-diCQA
target genes. qPCR was conducted as previously described, with
certain modifications (24). qPCR
was performed in a 7300 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) in a final volume of 25 µl
[forward and reverse primers, 0.25 mol/l each; Power
SYBR®-Green PCR Master mix (cat. no. 4367659); and a 1
µl cDNA sample]. The thermoycling conditions were as
follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. PCR primers were as follows: TYR
forward, 5′GAG AAG CGA GTC TTG ATT AG3′ and reverse, 5′TGG TGC TTC
ATG CGC AAA ATC3′; TRP 1 forward, 5′GGC CTC TGA GGT TCT TTA
AT3′ and reverse, 5′AAT GAC AAA TTG AGG GTG AG3′; TRP 2
forward, 5′ATG AGA AAC TGC CAA CCT TA3′ and reverse, 5′AGG AGT GAG
GCC AAG TTA TGA3′; MITF forward, 5′AGT ACA GGA GCT GGA GAT
G3′ and reverse, 5′GTG AGA TCC AGA GTT GTC GT3′ (25); β-actin forward, 5′ATG AGA
AGG AGA TCA CTG C3′ and reverse, 5′CTG CGC AAG TTA GGT TTT GT3′
(26). Relative expression was
analyzed and determined by 2−ΔΔCq method, normalizing
the data to β-actin mRNA levels (27,28). Experiments were repeated in
triplicate.
Western blot analysis
Protein samples that were collected during the
melanin content assay was used for western blot analysis. The
lysates were denatured in SDS-PAGE protein loading buffer 5X (cat.
no. AR1112; Wuhan Boster Biological Technology, Ltd.) separated on
10% SDS-PAGE at 80 V, and transferred onto polyvinylidene fluoride
membranes for 2 h at 400 A. Membrane blocking was performed with 5%
skim milk dissolved in TBS with 1% Tween-20 (TBST) at room
temperature for 1 h and the membrane was incubated with primary
antibodies at dilutions of 1:1,000 at 4°C overnight. Subsequent to
washing in TBST, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies at a dilution of 1:2,000
for 1 h at room temperature. The membranes were then washed with
TBST. Proteins were visualized by enhanced chemiluminescent western
blotting detection reagents (GE Healthcare, Chicago, IL, USA).
Densitometry analysis was performed using Quantity One version 3
(Bio-Rad Laboratories, Inc.).
Determination of intracellular cAMP
levels
B16 melanoma cells were treated with 1,5-diCQA at 0,
5, 50 or 100 µM at 37°C for 12 h. Intracellular cAMP levels
were measured using a cAMP ELISA kit (cat. no. STA-500; Cell
Biolabs, Inc., San Diego, CA, USA) following the manufacturer's
protocol.
Statistical analysis
All data are expressed as mean ± standard deviation.
Statistical analysis was performed by one-way analysis of variance
followed by Tukey's post-hoc test for multiple comparisons using
SPSS 19.0 (IBM Corp., Armonk, NY, USA) and Graphpad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of 1,5-diCQA on B16 cell
viability
The total melanin content in the skin is determined
by the number of melanocytes and the amount of melanin synthesized
by single cells (29). In order
to avoid the possibility that effect of melanin synthesis was due
to the cell viability, the cytotoxicity of 1,5-diCQA to B16 cells
was first determined. B16 cells treated at various concentrations
of 1,5-diCQA were incubated for 48 h and compared with the
1,5-diCQA untreated cells. Cell viability was measured using a CCK
assay. The results indicated that 1,5-diCQA exhibited no
cytotoxicity at concentration ranges of 0–400 µM (Fig. 3A). Concurrently, 1,5-diCQA did not
induce any change in cell morphology when compared with the control
cells at the concentration of 0–100 µM (Fig. 3B). Accordingly, it was determined
that 1,5-diCQA is not cytotoxic to melanoma cells.
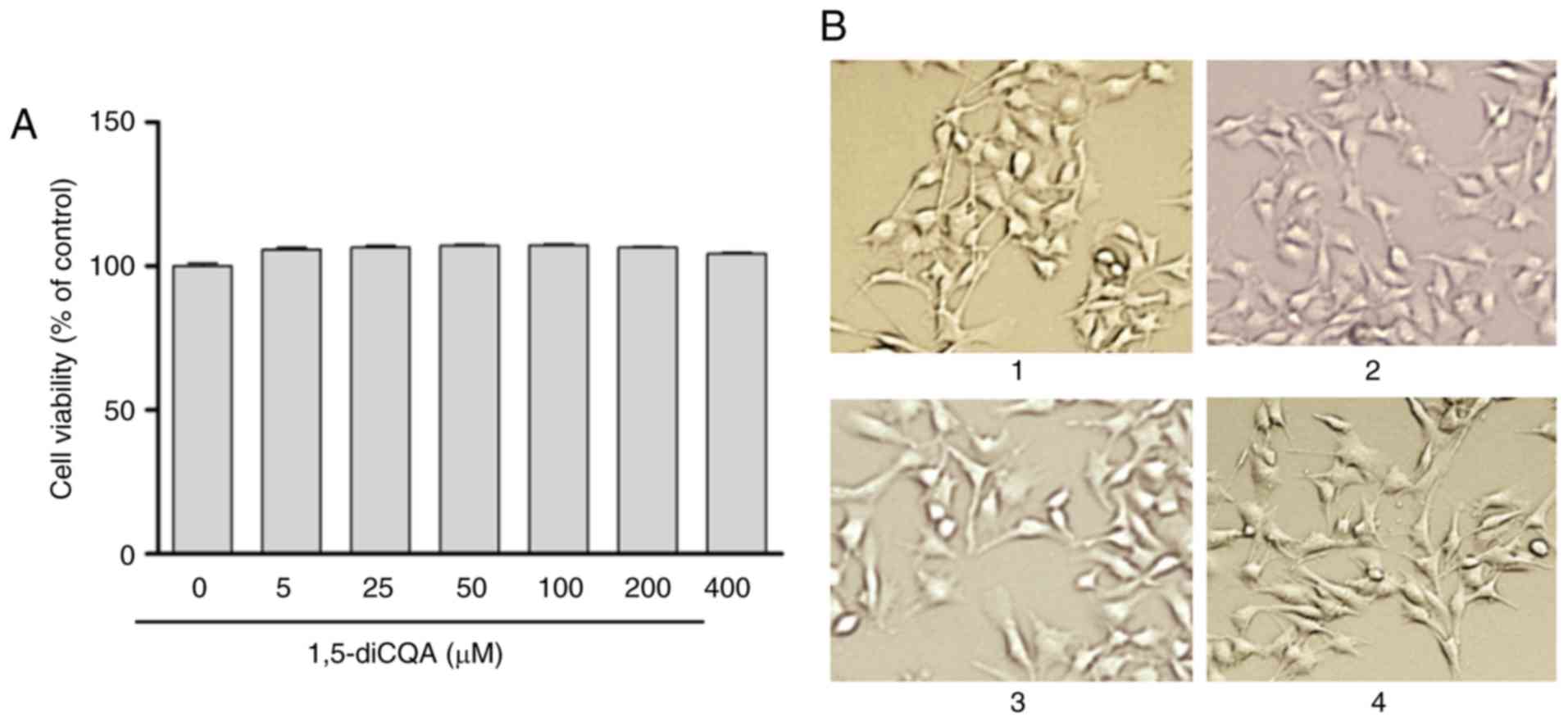 | Figure 3Effects of 1,5-diCQA on B16 cell
viability and morphology. (A) B16 cells were treated with 0.1%
dimethyl sulfoxide as a vehicle control, or with 1,5-diCQA at 5,
25, 50, 100, 200 and 400 µM for 48 h and cell viability was
measured by CCK assay. (B) Cell morphology was observed under a
microscope at magnification, ×200. (1) control; (2) 5; (3) 50; and (4) 100 µM 1,5-diCQA. Values are
expressed as the mean ± standard deviation of three separate
independent experiments. 1,5-diCQA, 1,5-dicaffeoylquinic acid. |
Effect of 1,5-diCQA on melanin formation
and Tyr activity
The effect of 1,5-diCQA on melanin production in B16
cells was determined by a melanin content assay. As indicated in
Fig. 4A and B, intracellular
melanin levels increased in response to 1,5-diCQA treatment in a
dose- and time-dependent manner, which suggests that 1,5-diCQA may
promote melanin synthesis in B16 cells. Fig. 4C denotes the cell precipitation
following centrifugation and visual observation. It indicates that
cells became darker following the addition of 1,5-diCQA. Studies
investigating pigmentary disorders have primarily focused on
tyrosinase activity, as it is the most important enzyme in melanin
biosynthesis in the melanocytes (30). The results also suggested that
1,5-diCQA promotes intracellular Tyr activity in B16 cells in a
dose-dependent manner after 24 h of treatment (Fig. 4D).
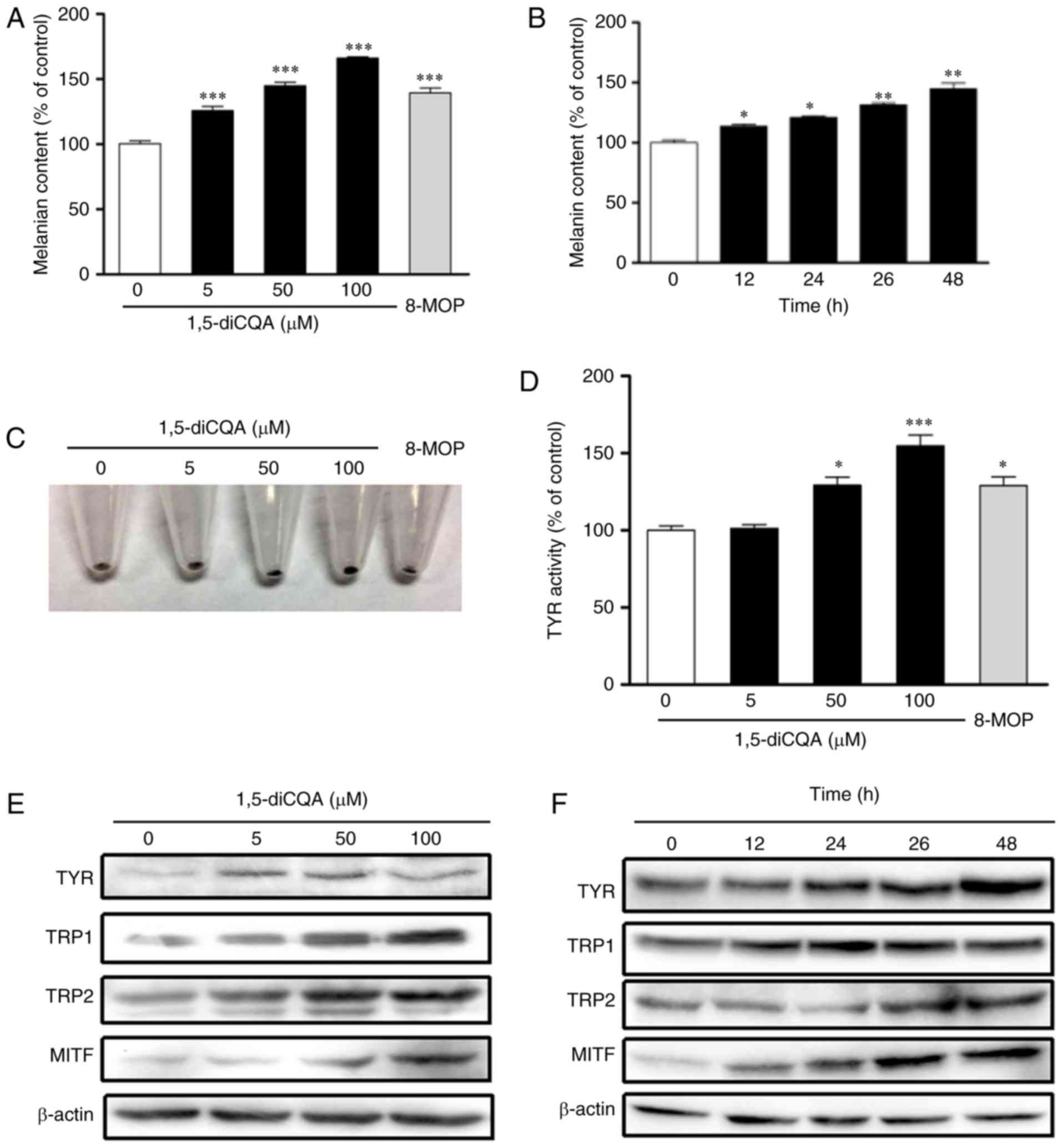 | Figure 4Effects of 1,5-diCQA on the melanin
contents in B16 cells. Melanin content was measured in dose- and
time-dependent manners. (A) B16 cells were treated with 0.1%
dimethyl sulfoxide as a blank control, 8-MOP (50 µM) as a
positive control or 1,5-diCQA at 5, 50 and 100 µM for 48 h.
(B) Melanin content was assayed in a time-dependent manner
following treatment with 1,5-diCQA at 50 µM. (C) B16 cells
were treated with 1,5-diCQA at 5, 50 and 100 µM for 48 h,
and cell precipitation following centrifugation is indicated. (D)
B16 cells were treated with 1,5-diCQA at 5, 50 and 100 µM
for 24 h, and TYR activity was measured. (E) B16 cells were treated
with 1,5-diCQA at 5, 50 and 100 µM for 48 h. TYR, TRP1, TRP2
and MITF protein expression levels were detected by western blot
analysis, and β-actin was used as a loading control. (F) B16 cells
were treated with 100 µM 1,5-diCQA for 0,12, 24, 36 and 48
h. TYR, TRP1, TRP2 and MITF protein expression levels were detected
by western blot analysis, and β-actin was used as a loading
control. Values are expressed as the mean ± standard deviation of
three separate independent experiments. Each percentage value in
the treated cells was calculated with respect to that in the
control cells. *P<0.05, **P<0.01 and
***P<0.001 vs. control group. TYR, tyrosinase; TRP-1,
tyrosinase-related protein 1; TRP-2, tyrosinase-related protein 2;
MITF, microphthalmia-associated transcription factor; 1,5-diCQA,
1,5-dicaffeoylquinic acid. |
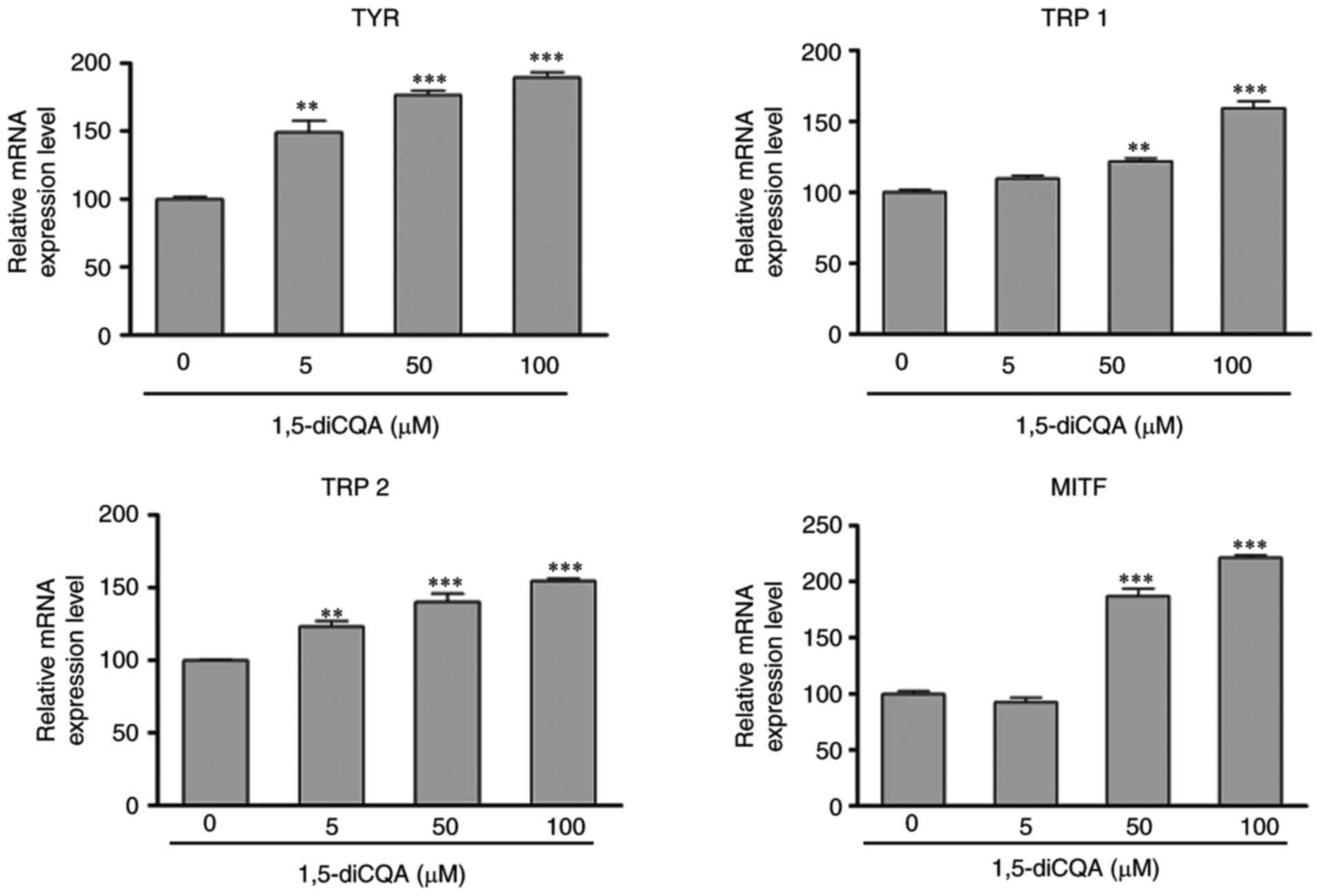
Effects of 1,5-diCQA on the expression of
melanogenesis-associated genes
The effect of 1,5-diCQA on the expression of
melanogenic genes was additionally explored by western blot
analysis and RT-qPCR. Western blot analysis indicated that
1,5-diCQA significantly increased the protein expression levels of
melanogenic genes TYR, TRP 1, TRP 2 and
transcription factor MITF in a dose- and time-dependent
manner (Fig. 4E and F). In order
to confirm whether the changes in these protein expressions were
due to the changes in RNA levels, the effects of 1,5-diCQA on mRNA
levels was also detected, and RNA levels of MITF and its
downstream genes TYR, TRP 1, and TRP 2. The results
demonstrated that the transcriptional levels of MITF,
TYR, TRP 1, and TRP 2 were significantly
increased in B16 cells in the presence of 1,5-diCQA in a
dose-dependent manner (Fig.
5).
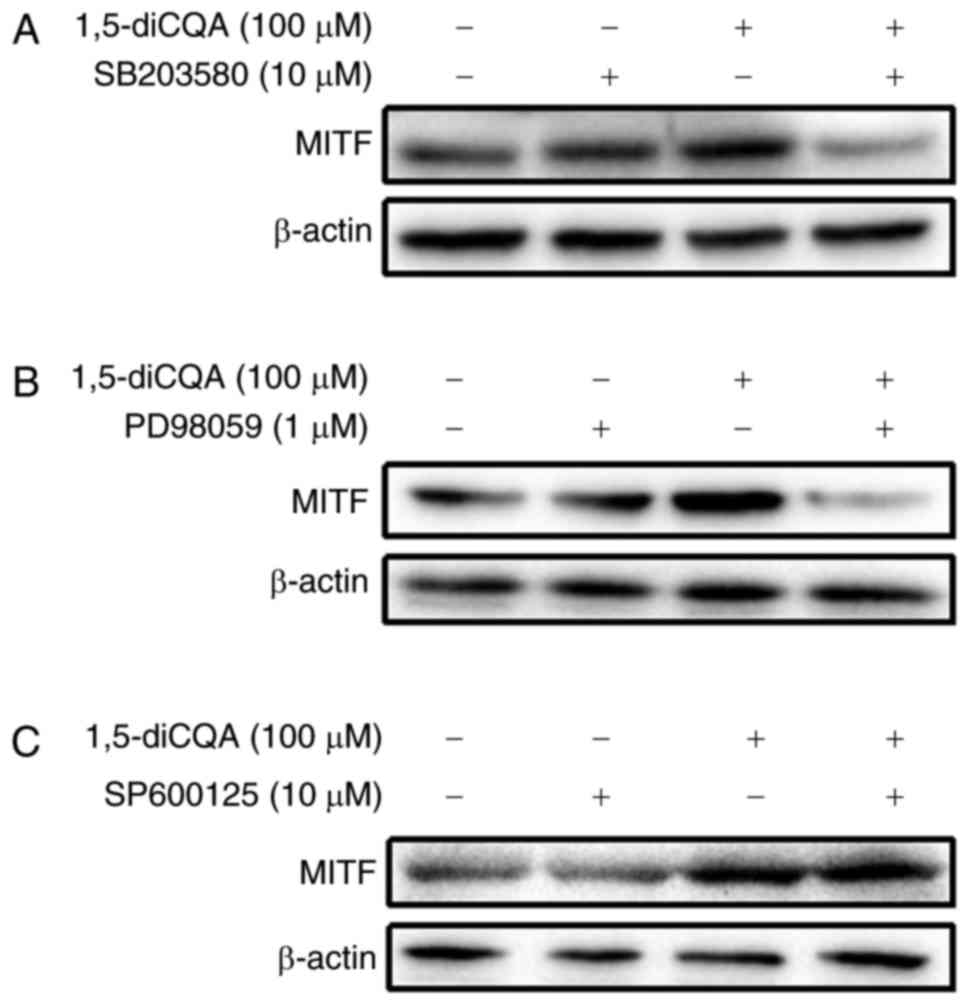
Effects of 1,5-diCQA on the MAPK and Wnt
signaling pathways
It has been demonstrated that the phosphorylation of
MAPK and signaling cascades of ERK, JNK and p38 regulate melanin
production (31). As the results
of the present study indicated that 1,5-diCQA increased melanin
production via the induction of the melanogenic enzyme and
pigmentation-associated transcription factor MITF, the
melanin-associated signal pathways involved were additionally
explored. To investigate the involvement of the MAPK signal pathway
in 1,5-diCQA-promoted melanin synthesis, western blot analysis was
performed on B16 cells following 1,5-diCQA treatment. The results
demonstrated that the phosphorylation levels of p38 and ERK, but
not JNK, were significantly increased following 30 min treatment
with different concentrations of 1,5-diCQA (Fig. 6). These data indicated that
1,5-diCQA may increase melanin synthesis by increasing the levels
of p38 and ERK phosphorylation. Therefore, inhibitors of p38
(SB203580), ERK (PD98059) and JNK (SP600125) were applied to verify
our prior hypothesis. B16 cells were pre-treated with different
inhibitors for 2 h prior to the addition of 1,5-diCQA (100
µM). Then, melanin content and Tyr activities were measured.
The results indicated that SB203580 and PD98059 may reverse the
1,5-diCQA effects on melanin content and TYR activities (Fig. 7A–D). However, no effects from the
JNK inhibitor (SP600125) were observed (Fig. 7E and F), which was consistent with
the western blotting results. In addition, MITF expression was
measured by western blot analysis following treatment with
SB203580, PD98059, SP600125 and 1,5-diCQA (100 µM) for 48 h
(Fig. 8). The results also
indicate that 1,5-diCQA increased melanin content through the
phosphorylation of p38 and ERK, but not JNK.
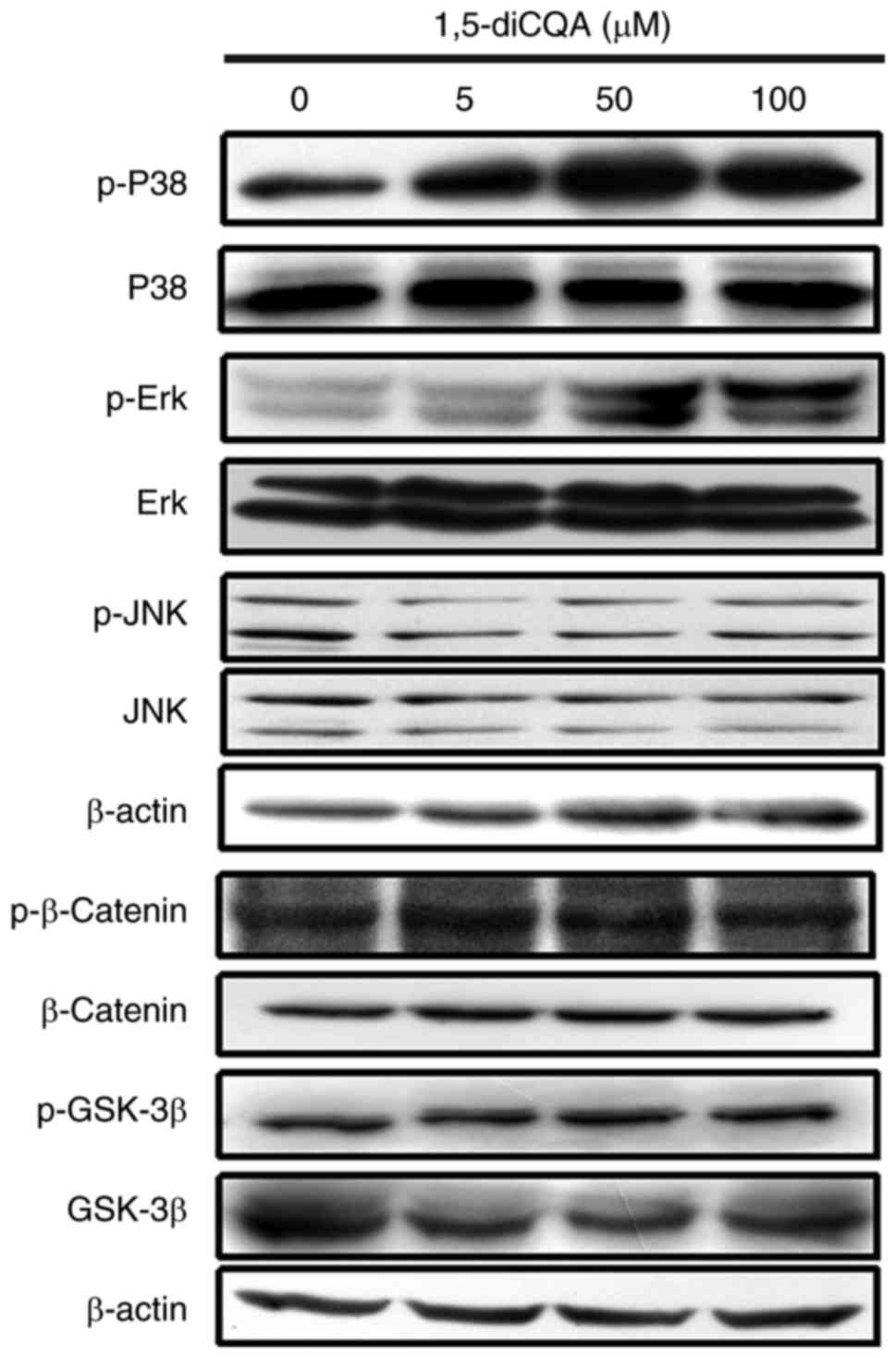 | Figure 6Effects of 1,5-diCQA on the MAPK and
Wnt signal pathways. B16 cells were treated with 5, 50 and 100
µM 1,5-diCQA for 30 min, and total and phosphorylated forms
of p38, ERK and JNK was measured by western blot analysis. Levels
of total and phosphorylated forms of β-catenin and GSK-3β were
measured following treatment with 1,5-diCQA for 48 h. p,
phos-phorylated; MAPK, mitogen-activated protein kinase; P38, p38
MAPK; ERK, extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase; GSK-3β, glycogen synthase kinase 3β; 1,5-diCQA,
1,5-dicaffeoylquinic acid. |
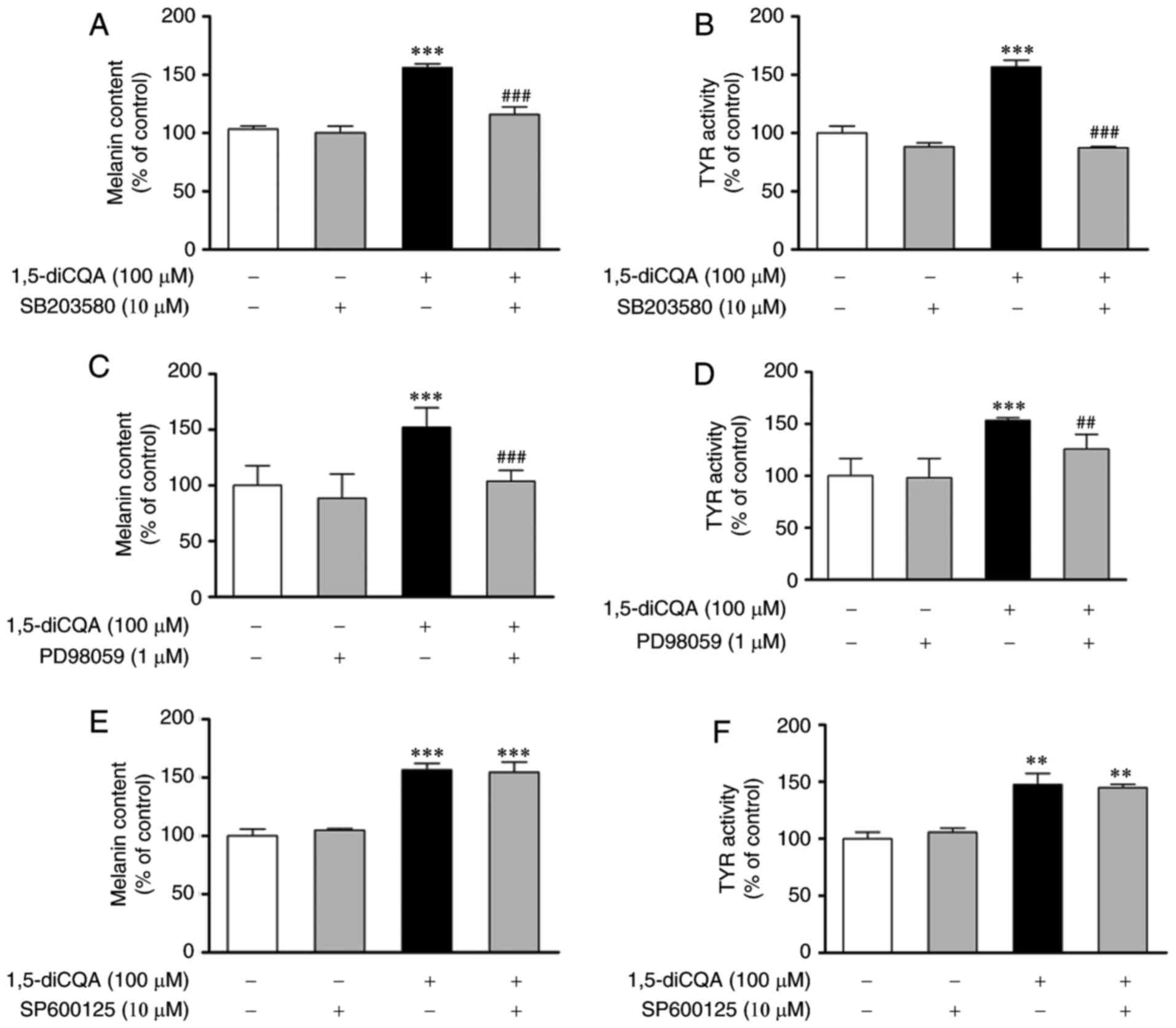 | Figure 7Effects of p38, ERK and JNK
inhibitors on 1,5-diCQA-induced melanin synthesis. B16 cells were
pre-incubated with SB203580 (10 µM) for 2 h prior to the
addition of 1,5-diCQA (100 µM), and then (A) incubated for
48 h for the measurement of melanin content or (B) incubated for 24
h for the measurement of TYR activity. (C) B16 cells were
pre-incubated with PD98059 (10 µM) for 2 h prior to the
addition of 1,5-diCQA (100 µM), and then (C) incubated for
48 h for the measurement of melanin content or (D) incubated for 24
h for the measurement of TYR activity. B16 cells were pre-incubated
with SP600125 (10 µM) for 2 h prior to the addition of
1,5-diCQA (100 µM), and then (E) incubated for 48 h for the
measurement of melanin content or (F) incubated for 24 h for the
measurement of TYR activity. Each percentage value in the treated
cells was calculated with respect to that in the control cells.
Values are expressed as the mean ± standard deviation of three
separate independent experiments. **P<0.01 and
***P<0.001 vs. untreated control group.
##P<0.01 and ###P<0.001 vs. single drug
treatment group. P38, p38 mitogen-activated protein kinase; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; 1,5-diCQA, 1,5-dicaffeoylquinic acid. |
The Wnt signal pathway was demonstrated to be
closely associated with melanin synthesis (32). In order to investigate whether the
Wnt signal pathway was involved in the effects of 1,5-diCQA on
melanogenesis, the changes in β-catenin and GSK-3β in B16 cells
following treatment with 1,5-diCQA were measured by western blot
analysis. B16 cells were treated with 5, 50 and 100 µM
1,5-diCQA for 48 h, and total and phosphorylated forms of β-catenin
and GSK-3β were measured. The results indicated that neither total
nor phosphorylated β-catenin and GSK-3β were altered following
1,5-diCQA treatment (Fig. 6).
Effects of 1,5-diCQA on the PKA signaling
pathway
It is well-known that the PKA signaling pathway is
involved in melanogenesis. Increased cellular cAMP levels may
activate PKA. In turn, activated PKA may activate CREB, leading to
the upregulation of MITF transcription (33). Therefore, to determine whether the
effects of 1,5-diCQA on melanogenesis were also mediated by the PKA
signal pathway, a range of experiments using the PKA inhibitor H89
were conducted to identify if PKA affected this process. B16 cells
were pre-incubated with H89 (10 µM) for 2 h prior to the
addition of 1,5-diCQA (100 µM), and then incubated for 48 h
for the measurement of melanin content and expression of MITF, or
incubated for 24 h for the measurement of TYR activity. The results
indicated that the effects of 1,5-diCQA on melanogenesis were
inhibited following pre-incubation with H89 (Fig. 9A and B). MITF expression was also
decreased in the samples that were treated with H89 (10 µM)
and 1,5-diCQA (100 µM) (Fig.
9F). In addition, cAMP content of the B16 cells following
treatment with 1,5-diCQA was measured. The results confirmed that
cAMP content was also significantly increased in response to
1,5-diCQA treatment in a dose-dependent manner (Fig. 9C). The levels of CREB induced by
PKA, which may activate MITF transcription levels, were also
investigated using western blot analysis. Phosphorylation of CREB
was significantly increased following treatment with 1,5-diCQA in
time- and dose-dependent manners. (Fig. 9D and E).
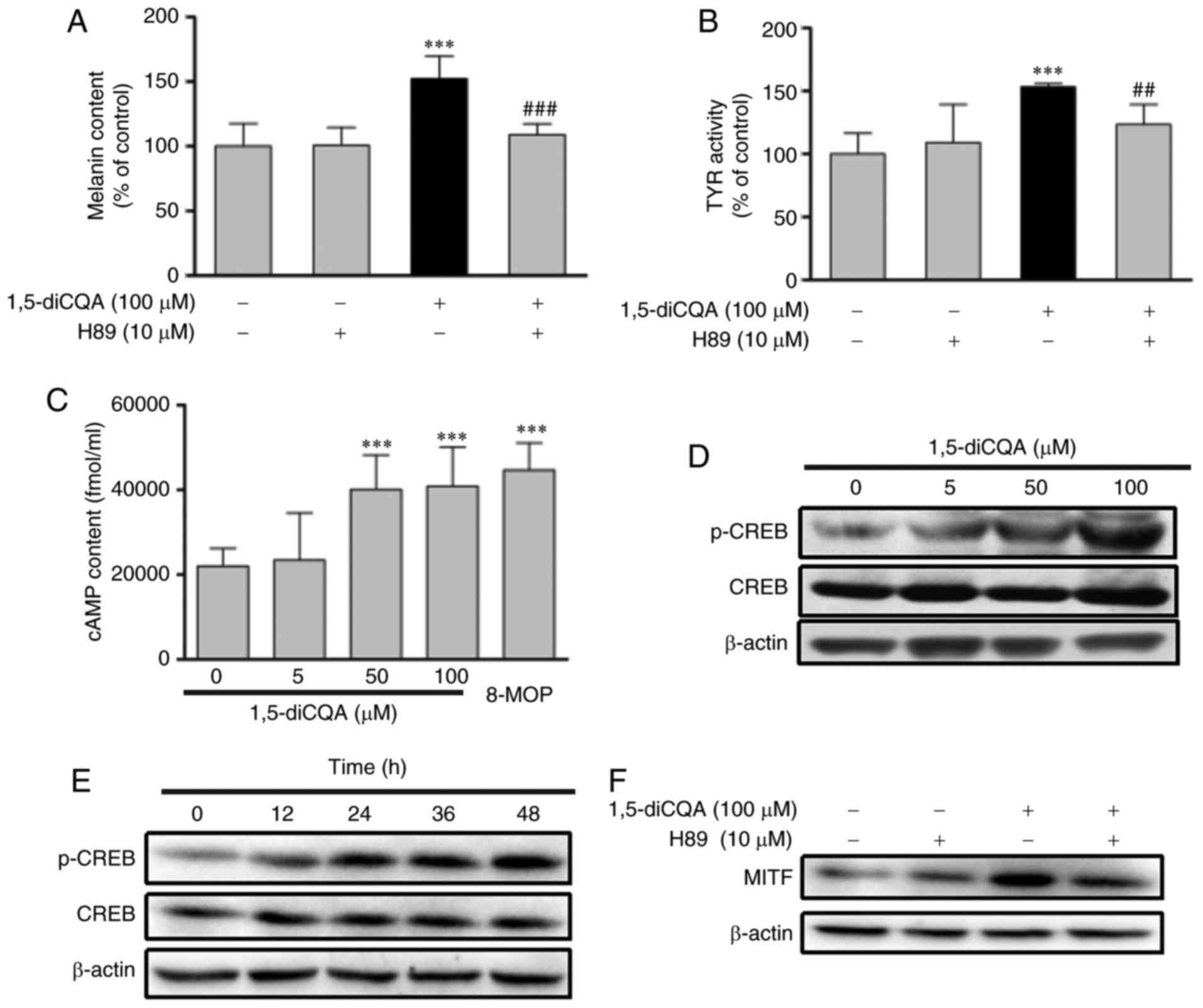 | Figure 9Effects of 1,5-diCQA on the PKA
signal pathway. B16 cells were pre-incubated with H89 (10
µM) for 2 h prior to the addition of 1, 5-diCQA (100
µM), and then (A) incubated for 48 h for the measurement of
melanin content or (B) incubated for 24 h for the measurement of
TYR activity. (C) B16 cells were treated with 0.1% dimethyl
sulfoxide and 8-MOP as positive controls or 1,5-diCQA at 5, 50 and
100 µM for 12 h, and then cAMP content was measured by a
cAMP-ELISA kit. (D) B16 cells were treated with 5, 50 and 100
µM 1,5-diCQA for 48 h, and levels of total and
phosphorylated CREB were measured by western blot analysis. (E) B16
cells were treated with 1,5-diCQA (100 µM) for 0, 12, 24, 36
and 48 h, and levels of total and phosphorylated CREB were measured
by western blot analysis. (F) B16 cells were pre-incubated with H89
(10 µM) for 2 h prior to the addition of 1,5-diCQA (100
µM), and then incubated for 48 h. MITF expression levels
were the measured by western blot analysis. **P<0.01
and ***P<0.001 vs. untreated control group.
##P<0.01 and ###P<0.001 vs. single
treatment group. PKA, protein kinase A; TYR, tyrosinase; MITF,
microphthalmia-associated transcription factor; 1,5-diCQA,
1,5-dicaffeoylquinic acid; p-phosphorylated; CREB, cAMP-response
element binding protein. |
Discussion
Melanin serves a pivotal role in solar UV
irradiation-induced skin injury. Lack of melanin is involved in the
development of skin diseases, including vitiligo and albinism
(34). At present, a number of
studies have focused on the specific mechanisms, including the
induction of melanin biosynthesis and functional melanocytes, with
the aim of developing novel therapeutic agents for vitiligo
(35–37). Kaliziri is a plant that only grows
in high-altitude areas of southern Xinjiang (China) and small
regions of Pakistan and India. The seeds of Kaliziri have
historically been used for treating skin diseases including
vitiligo in Traditional Chinese medicine (16). Our study group have focused on
identifying novel compounds in Kaliziri, and have isolated
1,5-diCQA using high performance liquid chromatography (HPLC) and
preparative HLPC, which may be the active ingredient (38,39). Previous studies suggested that
methyl 3,5-dicaffeoylquinate and 3,5-dicaffeoylquinic acid, two
different structural analogues of 1,5-diCQA, induce melanin
synthesis (40,41). Therefore, we hypothesized that
1,5-diCQA may have effects on melanin synthesis. In the present
study, the effects of 1,5-diCQA on skin pigmentation induction and
its underlying mechanism were investigated. The results indicated
that 1,5-diCQA increased melanin production by the induction of the
pigmentation-associated transcription factor MITF and melanogenic
enzymes TYR, TRP 1 and TRP 2. Then, the present study attempted to
elucidate the molecular mechanism of melanogenesis induction by
1,5-diCQA.
There are certain melanin-associated signal pathways
involved in this process, through affecting the
pigmentation-associated transcription factor MITF (42). It was demonstrated that p38 MAPK
activation contributes to melanin production by activating CREB,
which in turn activates MITF expression (43). In addition, the ERK MAPK pathway
is also involved in melanogenesis (44). Therefore, the present study
examined the effect of 1,5-diCQA on the MAPK signal pathway. It was
identified that 1,5-diCQA upregulated the phosphorylation levels of
ERK MAPK and p38 MAPK, while levels of JNK MAPK phosphorylation
remained the same. This suggested that the p38 MAPK and ERK MAPK
pathways may contribute to the stimulation of melanin
production.
1,5-DiCQA is a structural analogs of isochlorogenic
acid A, which stimulates melanin synthesis via the Wnt signaling
pathway (41). Therefore, the
present study examined the effect of 1,5-diCQA on the Wnt signal
pathway. However, the levels of total and phosphorylated GSK-3β and
β-catenin, which are key proteins in the Wnt signal pathway
(13), were demonstrated to be
similar prior and subsequent to 1,5-diCQA treatment. This indicates
that the Wnt signaling pathway is not a major factor of
1,5-diCQA-induced melanin synthesis.
It has been demonstrated that cAMP up-regulation
activates MAPK in B16 cells and normal human melanocytes (45). The cAMP signal pathway is
modulated via the MAPK and PKA pathways during melanin synthesis
(46). Once intracellular cAMP is
accumulated, it activates PKA that subsequently phosphorylates
CREB. Then CREB binds to the CRE motif of the MITF promoter and
activates MITF transcription (12). Therefore, the effects of 1,5-diCQA
on the PKA signal pathway was additionally examined. The results
indicated that 1,5-diCQA effects may activate the PKA signaling
pathway through the accumulation of cAMP content. In turn,
activated PKA activates CREB, sequentially leading to the
activation of MITF transcription. Eventually, MITF
upregulates melanogenic genes, including TYR, TRP 1
and TRP 2 (Fig. 10).
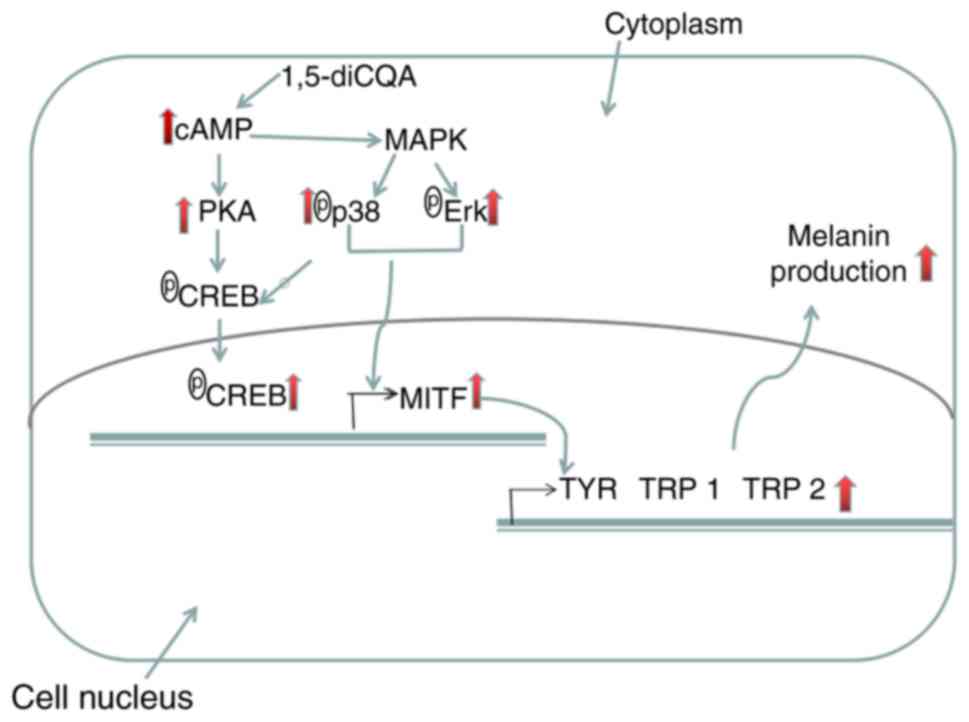 | Figure 10Potential mechanisms through which
1,5-diCQA functions in promoting melanin content in B16 cells.
1,5-diCQA increases the levels of p38 MAPK and ERK MAPK
phosphorylation, and therefore increases the activation of MITF.
1,5-diCQA increase the cAMP content, which activates PKA that
subsequently phosphorylates CREB. CREB binds to the CRE motif of
the MITF promoter and activates MITF transcription TYR, tyrosinase;
TRP-1, tyrosinase-related protein 1; TRP-2, tyrosinase-related
protein 2; MITF, microphthalmia-associated transcription factor;
1,5-diCQA, 1,5-dicaffeoylquinic acid; MAPK, mitogen-activated
protein kinase; P38, p38 MAPK; ERK, extracellular signal-regulated
kinase; cAMP, intracellular cyclic adenosine monophosphate; CREB,
cAMP-response element binding protein; PKA, protein kinase A. |
In conclusion, the present study suggested that the
1,5-diCQA purified from Kaliziri promoted melanogenesis in B16
cells by activating the p38 MAPK, ERK MAPK and PKA signaling
pathways. Taken together, the present study suggested that
1,5-diCQA may be a useful agent for treating hypopigmentation skin
disorders.
Funding
The present study was supported by grants from the
Key Research and Development Project of Xinjiang Autonomous Region
(grant no. 2016B03038-3) and Projects of International Science and
Technology Cooperation of the Xinjiang Uyghur Autonomous Region
(grant no. 20146020).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HAA and NM conceived and designed the experiments
and wrote the paper; NM and XYL performed the experiments; MK
analyzed the data; HAA revised the paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Boniface K, Seneschal J, Picardo M and
Taïeb A: Vitiligo: Focus on clinical aspects, immunopathogenesis,
and therapy. Clin Rev Allergy Immunol. 54:52–67. 2018. View Article : Google Scholar
|
|
2
|
Schallreuter KU, Bahadoran P, Picardo M,
Slominski A, Elassiuty YE, Kemp EH, Giachino C, Liu JB, Luiten RM,
Lambe T, et al: Vitiligo pathogenesis: Autoimmune disease, genetic
defect, excessive reactive oxygen species, calcium imbalance, or
what else? Exp Dermatol. 17:139–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta AK, Gover MD, Nouri K and Taylor S:
The treatment of melasma: A review of clinical trials. J Am Acad
Dermatol. 55:1048–1065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamaguchi Y and Hearing VJ: Melanocytes
and their diseases. Cold Spring Harb Perspect Med. 4:a0170462014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pillaiyar T, Manickam M and Jung SH:
Recent development of signaling pathways inhibitors of
melanogenesis. Cell Signal. 40:99–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rad HH, Yamashita T, Jin HY, Hirosaki K,
Wakamatsu K, Ito S and Jimbow K: Tyrosinase-related proteins
suppress tyrosinase-mediated cell death of melanocytes and melanoma
cells. Exp Cell Res. 298:317–328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YJ and Uyama H: Tyrosinase inhibitors
from natural and synthetic sources: Structure, inhibition mechanism
and perspective for the future. Cell Mol Life Sci. 62:1707–1723.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levy C, Khaled M and Fisher DE: MITF:
Master regulator of melanocyte development and melanoma oncogene.
Trends Mol Med. 12:406–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu C and Aisa HA: Upregulation of
melanogenesis and tyrosinase activity: Potential agents for
vitiligo. Molecules. 22:E13032017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamaguchi Y, Brenner M and Hearing VJ: The
regulation of skin pigmentation. J Biol Chem. 282:27557–27561.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu WJ, Ma HJ, Zhao G, Yuan XY, Zhang P,
Liu W, Ma LJ and Lei XB: Additive effect of heat on the UVB-induced
tyrosinase activation and melanogenesis via ERK/p38/MITF pathway in
human epidermal melanocytes. Arch Dermatol Res. 306:583–590. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buscà R and Ballotti R: Cyclic AMP a key
messenger in the regulation of skin pigmentation. Pigment Cell Res.
13:60–69. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schepsky A, Bruser K, Gunnarsson GJ,
Goodall J, Hallsson JH, Goding CR, Steingrimsson E and Hecht A: The
microph-thalmia-associated transcription factor Mitf interacts with
beta-catenin to determine target gene expression. Mol Cell Biol.
26:8914–8927. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin R and Chen YL: Compositae. Flora of
China. 4. Science Press; Beijing: pp. 5–8. 1985
|
|
15
|
Jamil S, Khan RA, Ahmed S and Fatima S:
Evaluation of anti-inflammatory and anti-oxidant potential of seed
extracts o Vernonia anthelmintica. Pak J Pharm Sci. 30:755–760.
2017.PubMed/NCBI
|
|
16
|
Tuerxuntayi A, Liu YQ, Tulake A, Kabas M,
Eblimit A and Aisa HA: Kaliziri extract upregulates tyrosinase,
TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC
Complement Altern Med. 14:1662014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang B, Meng Z, Dong J, Yan L, Zou L, Tang
Z and Dou G: Metabolic profile of 1,5-dicaffeoylquinic acid in
rats, an in vivo and in vitro study. Drug Metab Dispos. 33:930–936.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McDougall B, King PJ, Wu BW, Hostomsky Z,
Reinecke MG and Robinson WE Jr: Dicaffeoylquinic and
dicaffeoyltartaric acids are selective inhibitors of human
immunodeficiency virus type 1 integrase. Antimicrob Agents
Chemother. 42:140–146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao X, Xiao H, Zhang Y, Zou L, Chu Y and
Chu X: 1-5-Dicaffeoylquinic acid-mediated glutathione synthesis
through activation of Nrf2 protects against
OGD/reper-fusion-induced oxidative stress in astrocytes. Brain Res.
1347:142–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao HB, Cao X, Wang L, Run XQ, Su Y, Tian
C, Sun SG and Liang ZH: 1-5-dicaffeoylquinic acid protects primary
neurons from amyloid β 1-42-induced apoptosis via PI3K/Akt
signaling pathway. Chin Med J (Engl). 124:2628–2635. 2011.
|
|
21
|
Zheng Z, Wang X, Liu P, Li M, Dong H and
Qiao X: Semi-preparative separation of 10 caffeoylquinic acid
derivatives using high speed counter-current chromatogaphy combined
with semi-preparative HPLC from the roots of burdock (Arctium lappa
L.). Molecules. 23:E4292018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SY, Jin ML, Kim YH, Kim Y and Lee SJ:
Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by
inactivating CREB and MITF signaling pathways. Arch Dermatol Res.
303:737–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou J, Ren T1, Li Y, Cheng A, Xie W, Xu
L, Peng L, Lin J, Lian L, Diao Y, et al: Oleoylethanolamide
inhibits α-melanocyte stimulating hormone-stimulated melanogenesis
via ERK, Akt and CREB signaling pathways in B16 melanoma cells.
Oncotarget. 8:56868–56879. 2017.PubMed/NCBI
|
|
24
|
Hout DR, Schweitzer BL, Lawrence K, Morris
SW, Tucker T, Mazzola R, Skelton R, McMahon F, Handshoe J,
Lesperance M, et al: Performance of a RT-PCR assay in comparison to
fish and immunohistochemistry for the detection of ALK in non-small
cell lung cancer. Cancers (Basel). 9:E992017. View Article : Google Scholar
|
|
25
|
Zhu PY, Yin WH, Wang MR, Dang YY and Ye
XY: Andrographolide suppresses melanin synthesis through
Akt/GSK3β/β-catenin signal pathway. J Dermatol Sci. 79:74–83. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng MF, Shen SY and Huang WD: DCA
increases the antitumor effects of capecitabine in a mouse B16
melanoma allograft and a human non-small cell lung cancer A549
xenograft. Cancer Chemother Pharmacol. 72:1031–1041. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–428. 2001.
View Article : Google Scholar
|
|
28
|
Lu XY, Li JQ, Liu XN, Li XB and Ma J:
Characterization and expression analysis of six chitinase genes
from the desert beetle microdera punctipennis in response to low
temperature. Cryo Letters. 35:438–448. 2014.PubMed/NCBI
|
|
29
|
Smit NP, Kolb RM, Lentjes EG, Noz KC, van
der Meulen H, Koerten HK, Vermeer BJ and Pavel S: Variations in
melanin formation by cultured melanocytes from different skin
types. Arch Dermatol Res. 290:342–349. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramsden CA and Riley PA: Tyrosinase: The
four oxidation states of the active site and their relevance to
enzymatic activation, oxidation and inactivation. Bioorg Med Chem.
22:2388–2395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen T, Heo SI and Wang MH: Involvement of
the p38 MAPK and ERK signaling pathway in the anti-melanogenic
effect of methyl 3,5-dicaffeoyl quinate in B16F10 mouse melanoma
cells. Chem Biol Interact. 199:106–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hart MJ, de los Santos R, Albert IN,
Rubinfeld B and Polakis P: Downregulation of beta-catenin by human
axin and its association with the APC tumor suppressor,
beta-catenin and GSK3 beta. Curr Biol. 8:573–581. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim DS, Cha SB, Park MC, Park SA, Kim HS,
Woo WH and Mun YJ: Scopoletin stimulates melanogenesis via cAMP/PKA
pathway and partially p38 activation. Biol Pharm Bull.
14:2068–2074. 2017. View Article : Google Scholar
|
|
34
|
Kaidbey KH, Agin PP, Sayre RM and Kligman
AM: Kligman, hotoprotection by melanin-a comparison of black and
caucasian skin. J Am Acad Dermatol. 1:249–260. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato K, Ando R, Kobayashi H and Nishio T:
2-Ethoxybenzamide stimulates melanin synthesis in B16F1 melanoma
cells via the CREB signaling pathway. Mol Cell Biochem. 423:39–52.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee KM, Lee KY, Choi HW, Cho MY, Kwon TH,
Kawabata S and Lee BL: Activated phenoloxidase from Tenebrio
molitor larvae enhances the synthesis of melanin by using a
vitellogenin-like protein in the presence of dopamine. Eur J
Biochem. 267:3695–3703. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar R, Parsad D, Rani S, Bhardwaj S and
Srivastav N: Glabrous lesional stem cells differentiated into
functional mela-nocytes: New hope for repigmentation. J Eur Acad
Dermatol Venereol. 30:1555–1560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maimaiti Z, Turak A and Aisa HA: Two new
compounds from the seeds o Vernonia anthelmintica. J Asian Nat Prod
Res. 19:862–868. 2017. View Article : Google Scholar
|
|
39
|
Turak A and Aisa HA: Three new
elemanolides from the seeds o Vernonia anthelmintica. J Asian Nat
Prod Res. 20:313–320. 2018. View Article : Google Scholar
|
|
40
|
Kim HJ, Kim JS, Woo JT, Lee IS and Cha BY:
Hyperpigmentation mechanism of methyl 3,5-dicaffeoylquinate through
activation of p38 andMITF induction of tyrosinase. Acta Biochim
Biophys Sin (Shanghai). 47:548–556. 2015. View Article : Google Scholar
|
|
41
|
Mamat N, Dou J, Lu X, Eblimit A and Haji
Akber A: Isochlorogenic acid A promotes melanin synthesis in B16
cell through the β-catenin signal pathway. Acta Biochim Biophys Sin
(Shanghai). 49:800–807. 2017. View Article : Google Scholar
|
|
42
|
Pei T, Zheng C, Huang C, Chen X, Guo Z, Fu
Y, Liu J and Wang Y: Systematic understanding the mechanisms of
vitiligo pathogenesis and its treatment by Qubaibabuqi formula. J
Ethnopharmacol. 190:272–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahn JH, Jin SH and Kang HY: LPS induces
melanogenesis through p38 MAPK activation in human melanocytes.
Arch Dermatol Res. 300:325–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yanase H, Ando H, Horikawa M, Watanabe M,
Mori T and Matsuda N: Possible involvement of ERK 1/2 in
UVA-induced melanogenesis in cultured normal human epidermal
melanocytes. Pigment Cell Res. 14:103–109. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Buscà R, Abbe P, Mantoux F, Aberdam E,
Peyssonnaux C, Eychène A, Ortonne JP and Ballotti R: Ras mediates
the cAMP-dependent activation of extracellular signal-regulated
kinases (ERKs) in melanocytes. EMBO J. 19:2900–2910. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jung HG, Kim HH, Paul S, Jang JY, Cho YH,
Kim HJ, Yu JM, Lee ES, An BJ, Kang SC and Bang BH: Quercetin
-3-O-β-D-g lucopyranosyl-(1→6)-β-D-glucopyranoside suppresses
melanin synthesis by augmenting p38 MAPK and CREB signaling
pathways and subsequent cAMP down-regulation in murine melanoma
cells. Saudi J Biol Sci. 22:706–713. 2015. View Article : Google Scholar : PubMed/NCBI
|