Introduction
Ultraviolet (UV) radiation is an important
environmental factor; exposure of skin to UV irradiation may induce
various kinds of skin damage, including sunburn, premature aging,
cancer and inflammation. The effects of UV may be mediated by
various types of cellular-level changes, including autophagy, cell
cycle control and cell death, as well as molecular-level changes,
including signal transduction and gene expression. UV irradiation
may also serve a role as a broad activator of cell surface growth
factor receptors and cytokine receptors (1,2).
Activation of ligand-independent receptors stimulates various
downstream signaling pathways that control the expression of a
number of genes (1-3). Matrix metalloproteinases (MMPs) are
group of zinc-dependent endopeptidases that degrade extracellular
matrix proteins (4-6). Certain MMPs have been reported to be
induced by UV irradiation, which contributes to skin damage by UV
irradiation in vivo (2-4).
Gasdermin (GSDM)-C belongs to the Gasdermin
super-family, a novel group of genes that include GSDMA, GSDMB,
GSDMC and GSDMD, as well as the Gasdermin-related genes (GSDME and
pejvakin) in humans (5-7). GSDM family members were reported to
be differentially expressed in the epithelial cells of various
tissue types, including the skin (5,8).
Previous studies have suggested that GSDMC may serve a role in the
course of carcinogenesis, such as colorectal cancer cell
proliferation and increased metastatic potential in malignant
melanoma cells (9-11). However, the functions of GSDMC in
the skin remain poorly understood.
Our previous study reported that GSDMC is induced by
UV irradiation and contributes to MMP-1 expression through the
activation of extracellular signal-regulated kinase (ERK) and c-Jun
N-terminal kinase (JNK) pathways in human skin keratinocytes
(6). However, how UV modulates
GSDMC expression has remained unclear. In the present study, the
signaling pathways involved in UV-induced GSDMC expression in human
skin keratinocytes were examined by determining the role of
transient receptor potential cation channel subfamily V member 1
(TRPV1) on UV-induced GSDMC expression. TRPV1 is a capsaicin
receptor and functions as a non-selective cation channel that may
lead to calcium influx (12-14). TRPV1 and GSDMC have been
previously reported to serve crucial roles in UV-induced MMP-1
expression in human skin keratinocytes (6,12).
However, TRPV1 activation appears to occur at relatively early time
points (12), whereas GSDMC
expression increases at relatively late time points, following UV
irradiation (6). Therefore,
whether TRPV1 may serve any role in UV-induced GSDMC expression was
examined. The results demonstrated that TRPV1 serves an important
role in UV-induced GSDMC expression. Through additional studies,
UV-induced GSDMC expression was determined to be dependent on
calcium and calcineurin; it was also demonstrated that nuclear
factor of activated T-cells, cytoplasmic 1 (NFATc1) mediated
UV-induced GSDMC expression.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), was
purchased from Welgene, Inc. (Gyeongsan, Gyeongsangbuk, Korea).
Calcium-free DMEM was obtained from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Fetal Bovine Serum (FBS) was
purchased from HyClone (GE Healthcare Life Sciences, Logan, UT,
USA). Keratinocyte basal medium MCDB 153 was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and keratinocyte
growth medium was purchased from Clonetics Corp. (San Diego, CA,
USA). Antibiotics (penicillin and streptomycin) and TRIzol reagent
were obtained from Thermo Fisher Scientific, Inc. Capsazepine,
ruthenium red and capsaicin were purchased from Sigma-Aldrich;
Merck KGaA. Expression plasmids for the wild-type (pMX-NFATc1-WT)
or a constitutively active form of NFATc1 (pMSCV-NFATc1-CA) were
kindly provided by Dr Hong-Hee Kim (Department of Cell and
Developmental Biology, Seoul National University, Seoul,
Korea).
Cell culture and treatments
An immortalized human keratinocyte cell line, HaCaT,
was purchased from CLS Cell Lines Service GmbH (Eppelheim,
Germany). Primary human skin keratinocytes were cultured from
foreskin of healthy donors. HaCaT cells were cultured in DMEM
supplemented with glutamine (2 mM), penicillin (400 U/ml),
streptomycin (50 mg/ml) and 10% FBS in a humidified 5%
CO2 atmosphere at 37°C. Primary human skin keratinocytes
were cultured in keratinocyte growth medium supplemented with
penicillin (400 U/ml) and streptomycin (50 mg/ml) in a humidified
5% CO2 atmosphere at 37°C; cultured primary human skin
keratinocytes at passages 3-4 were used.
For treatments, HaCaT cells were cultured to 80%
confluence and serum-starved for 24 h in DMEM without FBS, and
primary human skin keratinocytes were serum-starved for 24 h in
MCDB 153. HaCaT cells and primary human skin keratinocytes were
washed with phosphate-buffered saline (PBS) two times;
subsequently, HaCaT cells were irradiated with UV at 60
mJ/cm2 and primary human skin keratinocytes were
irradiated with UV at 100 mJ/cm2 in PBS. UV irradiation
was performed with Philips TL 20W/12RS fluorescent sun lamps
(Philips Medical Systems B.V., Eindhoven, The Netherlands) with an
emission spectrum between 275 and 380 nm (peak, 310-315 nm); a
Kodacel filter TA401/407 (Kodak, Rochester, NY, USA) was used to
block UVC of wavelength below 290 nm. UV irradiation intensity was
measured with a Model 585100 UV meter from Herbert Waldmann GmbH
& Co. KG (Villingen-Schwenningen, Germany). Following UV
irradiation, PBS was removed and replaced with DMEM without FBS for
HaCaT cells and keratinocyte basal medium for primary human skin
keratinocytes, and cells were further incubated for 24 h. When
required, specific TRPV1 antagonist (capsazepine or ruthenium red)
or specific TRPV1 agonist (capsaicin) was added 30 min prior to UV
irradiation, and treated again with specific TRPV1 antagonist
(capsazepine or ruthenium red) for 24 h following UV irradiation;
calcineurin inhibitor (cyclo-sporine A) was added immediately
following UV irradiation and cells were incubated for 24 h. To
investigate the role of extracellular calcium in UV irradiation,
HaCaT cells were serum-starved for 24 h and cultured in either
calcium-free DMEM or calcium-containing DMEM for 30 min prior to UV
irradiation. Fresh corresponding culture medium was added, and the
cells were further incubated for 24 h. Each experiment was repeated
three times. The medical ethical committee at Seoul National
University approved the study protocol, and written informed
consent was received from the guardians of participants. The study
was conducted according to the Declaration of Helsinki
principles.
Transfection with NFATc1 small
interfering (si)RNA
For knockdown of NFATc1, cultured HaCaT cells were
seeded and maintain until approximately 80% confluency, and
subsequently transfected with the scrambled negative control siRNA
(siNC) or a NFATc1-specific siRNA (siNFATc1; 5′-CCA AGG UCA UUU UCG
UGG A-3′; Bioneer Corporation, Daejeon, Korea) at 100 pmol using
Lipofectamine® 2000 Reagent (Invitrogen: Thermo Fisher
Scientific, Inc.) in a humidified 5% CO2 atmosphere at
37°C for 6 h, according to manufacturer's instruction. The
concentration of siRNA primer pairs was determined by dose response
(data not shown). Following transfection, cells were serum-starved
for 24 h, treated with UV and incubated for an additional 24 h.
Cells were harvested for analysis of mRNA or protein. Each
experiment was repeated three times.
Transfection with mammalian NFATc1
overexpression vector
For overexpression of NFATc1, cultured HaCaT cells
were seeded and maintain until approximately 80% confluency, and
subsequently transfected with the control empty vector or the
mammalian expression vectors containing either pMX-NFATc1-WT or
pMSCV-NFATc1-CA using Lipofectamine 2000 Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at 2 µg in a humidified 5%
CO2 atmosphere at 37°C for 6 h, according to the
manufacturer's protocol. The concentration of vectors was
determined by dose response (data not shown). Following
transfection, cells were serum-starved for 24 h, treated with UV
and incubated for an additional 24 h. Cells were harvested for
analysis of mRNA or protein. Each experiment was repeated three
times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HaCaT cells at ~70%
confluency using the TRIzol method, according to the manufacturer's
protocol. The quality of isolated RNA samples were measured by
electrophoresis in 1% agarose gels (data not shown). Total RNA (1
µg) was used in a 20 µl reaction for first-strand cDNA synthesis
using First Strand cDNA Synthesis Kit (MBI Fermentas; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. cDNA
was subjected to amplification reactions using a 7500 Real-time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
SYBR Premix Ex Taq, Perfect Real-time (Takara Bio, Inc.,
Otsu, Japan), according to the manufacturer's protocol, with the
following primer pairs: 36B4, forward 5′-TGG GCT CCA AGC AGA
TGC-3′, reverse 5′-GGC TTC GCT GGC TCC CAC-3′; GSDMC, forward
5′-TGC TCC CTC GAG TTT CAA AT-3′, reverse 5′-GGC TCT GGA TCC AAC
AGT TT-3′. PCR thermocycling conditions were as follows: 50°C for 2
min and 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. Relative mRNA expression levels were normalized
to 36B4 and relative expression levels of the target gene were
calculated using the 2−ΔΔCq method (15). Each experiment was repeated three
times.
Western blotting
Western blot analysis was performed by extracting
proteins from HaCaT cells and primary human skin keratinocytes at
~70% confluency using Radioimmunoprecipitation Assay Lysis Buffer
(EMD Millipore, Billerica, MA, USA) mixed with protease inhibitor
mixture (Roche Applied Science, Penzberg, Germany) and phosphatase
inhibitor mixture (Sigma-Aldrich; Merck KGaA). Cell lysates were
centrifuged at 13,500 × g at 4°C for 15 min, and supernatants were
collected. The total cell extract protein concentration was
quantified by the Bicinchoninic Acid assay reagent (Sigma-Aldrich;
Merck KGaA). Equal amounts of protein, 20 µg per well, were
separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (Roche Applied Science). Following blocking
for 1 h in 5% skim milk diluted with Tris-buffered saline
containing 0.1% Tween-20, the membranes were incubated overnight
with primary antibodies (1:1,000) at 4°C with rabbit polyclonal
antibody against GSDMC (cat. no. STJ93220; St. John's Laboratory,
London, United Kingdom), mouse monoclonal antibody against NFATc1
(7A6; cat. no. sc-7294; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and goat polyclonal antibody against β-actin (I-19; cat. no.
sc-1616; Santa Cruz Biotechnology, Inc.); β-actin was used as a
loading control. The membranes were washed and incubated with
horseradish peroxidase-conjugated goat anti-rabbit (sc-2004), goat
anti-mouse (sc-2005) or mouse anti-goat (sc-2354) immunoglobulin G
(Santa Cruz Biotechnology, Santa Cruz, CA) as secondary antibodies
(1:5,000) for 1 h in room temperature. Immunoreactive bands were
visualized using the Enhanced Chemiluminescence Detection System
(Thermo Fisher Scientific, Inc.). Signal intensity was measured by
ImageJ software version 1.51w (National Institutes of Health,
Bethesda, MD). Protein expression levels were normalized to
β-actin. Each experiment was repeated three times.
Statistical analysis
Significance was determined using analysis of
variance followed by Tukey's multiple comparison test. Data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
TRPV1 serves an important role in
UV-induced GSDMC expression in human skin keratinocytes
A number of previous studies have reported that
TRPV1 or GSDMC serve important roles in UV-induced MMP-1 expression
(6,12,13). However, TRPV1 activation is
induced at relatively early time points (12), whereas GSDMC expression is induced
at relatively late time points, following UV irradiation (6). Therefore, whether early activation
of TRPV1 served a role in late induction of GSDMC expression
following UV irradiation was examined. Serum-starved HaCaT cells
were pre-treated with different TRPV1 inhibitors, either
capsazepine (a specific TRPV1 antagonist) or ruthenium red (a
non-selective TRPV1 antagonist), irradiated with UV and
subsequently treated again with capsazepine or ruthenium red. Cells
cultured with either TRPV1 inhibitor exhibited a reduction in
UV-induced expression of GSDMC in a dose-dependent manner (Fig. 1A and B). Furthermore, to confirm
whether TRPV1 was involved in UV-induced GSDMC expression, HaCaT
cells were treated with capsaicin (a specific TRPV1 agonist)
(16-18) and GSDMC expression was examined.
The results demonstrated that capsaicin treatment increased GSDMC
expression in a dose-dependent manner (Fig. 1C). In addition, whether the
induction of GSDMC expression in primary human skin keratinocytes
had similar effects s those obtained for HaCaT cells was examined;
similarly, capsazepine and ruthenium red treatments inhibited
UV-induced expression of GSDMC (Fig.
1D and E), whereas capsaicin treatment increased GSDMC
expression (Fig. 1F) in primary
human skin keratinocytes in a dose-dependent manner. Taken
together, these results suggested that TRPV1 may serve a crucial
role in UV-induced GSDMC expression in human skin
keratinocytes.
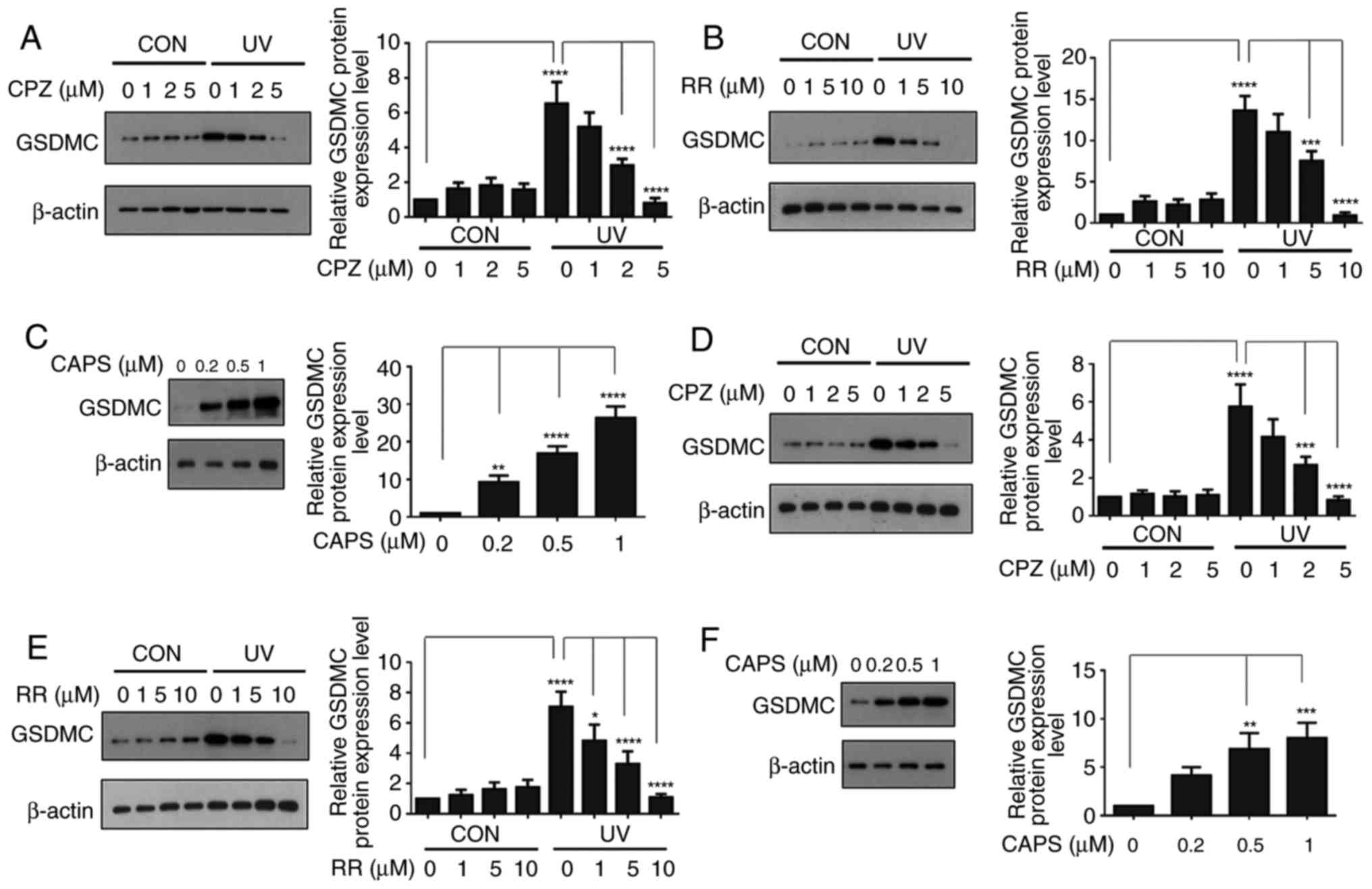 | Figure 1TRPV1 serves an important role in
UV-induced GSDMC expression in human skin keratinocytes. (A and B)
HaCaT cells were serum-starved for 24 h. Following pre-treatment
with (A) CPZ or (B) RR for 30 min, cells were irradiated with UV
and fresh media containing the corresponding inhibitor were added
and cells were incubated for an additional 24 h. (C) HaCaT cells
were serum-starved for 24 h and treated with CAPS at the various
concentrations for 24 h. (D and E) Primary human skin keratinocytes
were serum starved for 24 h. Following pretreatment with (D) CPZ or
(E) RR for 30 min, cells were irradiated with UV and fresh media
containing the corresponding inhibitor were added and cells were
incubated for an additional 24 h. (F) Primary human skin
keratinocytes were serum-starved for 24 h and treated with CAPS at
the various concentrations for 24 h. GSDMC protein expression was
analyzed by western blotting and relative protein levels were
quantified by ImageJ software; β-actin was used as a loading
control. Data are presented as the mean ± standard deviation; n=3;
*P<0.05, **P< 0.01,
***P<0.001 and ****P<0.0001. CAPS,
capsaicin; CPZ, capsazepine; CON, control non-UV irradiated; GSDMC,
gasdermin C; RR, ruthenium red; UV, ultraviolet irradiated. |
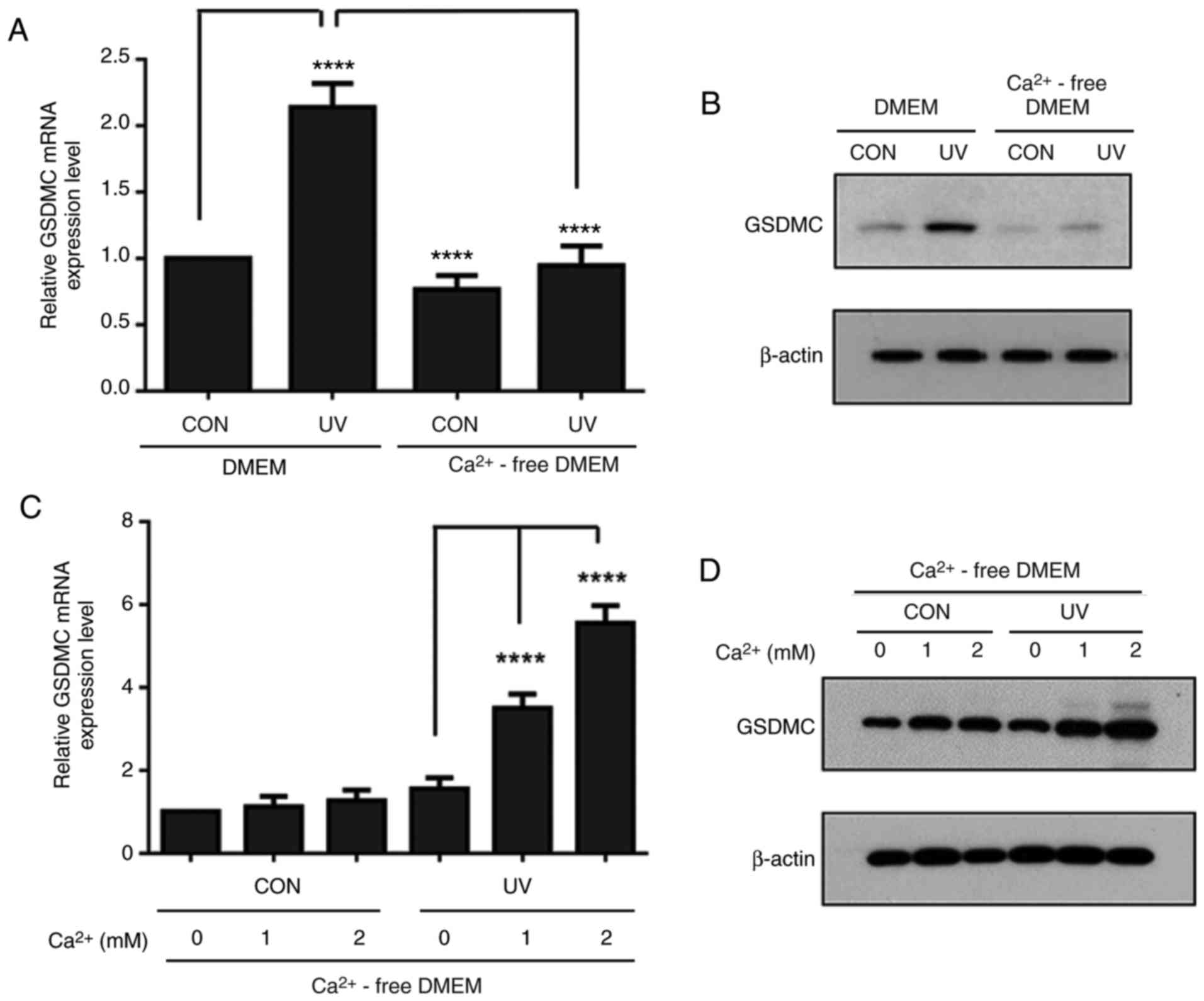
UV-induced GSDMC expression is
calcium-dependent in HaCaT cells
TRPV1 acts as a non-selective cation channel and the
activation of TRPV1 leads to calcium influx (12-14). As TRPV1 may be involved in
UV-induced GSDMC expression, the role of extracellular calcium on
UV-induced GSDMC expression was examined. Serum-starved HaCaT cells
were pre-incubated in either calcium-containing DMEM or
calcium-free DMEM, irradiated with UV and further incubated for 24
h in the corresponding media. UV-induced GSDMC mRNA and protein
expression levels were notably reduced in calcium-free DMEM,
compared with expression in calcium-containing DMEM (Fig. 2A and B). However, calcium
supplementation into calcium-free DMEM led to increased GSDMC mRNA
and protein expression levels following UV irradiation in a
dose-dependent manner (Fig. 2C and
D). These results indicated that UV-induced GSDMC expression
may be calcium-dependent.
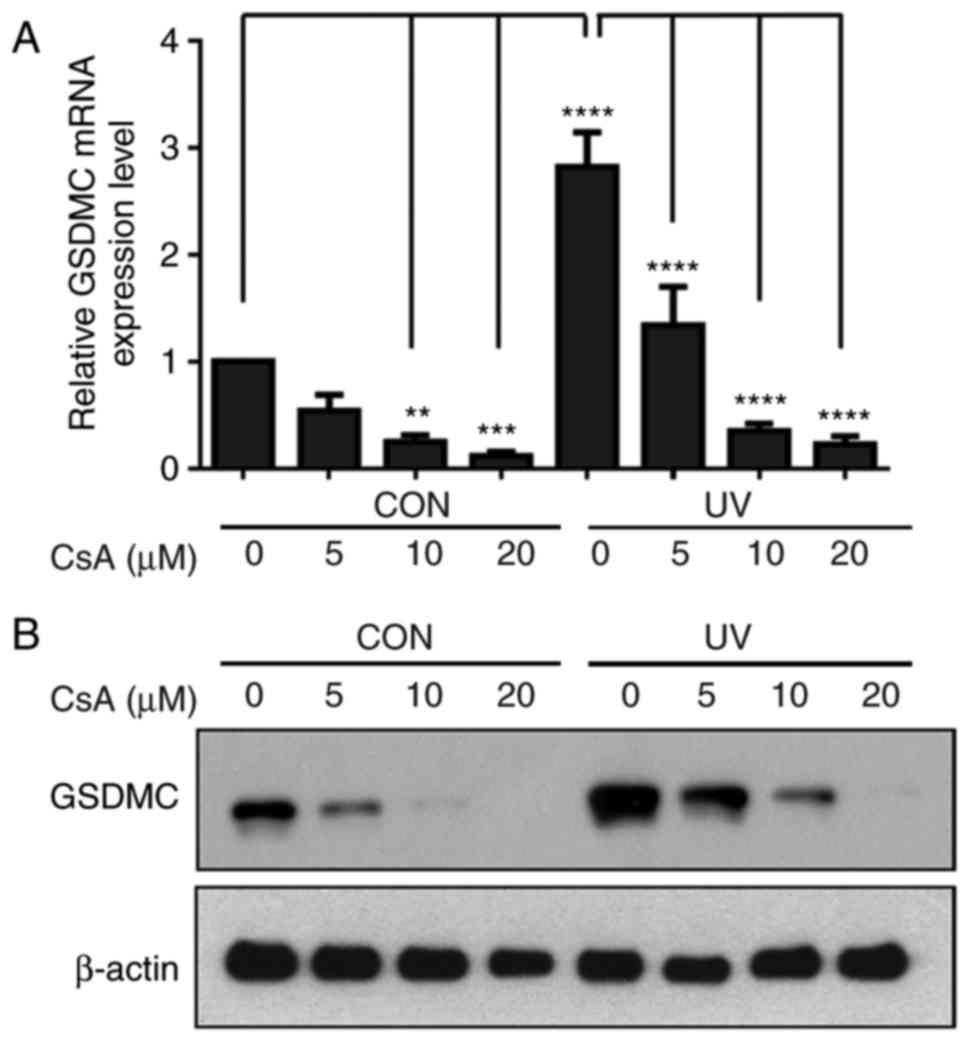
Calcineurin pathway serves a crucial role
in UV-induced GSDMC expression in HaCaT cells
Calcineurin is a calcium and calmodulin-dependent
serine/threonine protein phosphatase (19,20). As the present results indicated
that UV-induced GSDMC expression may be calcium-dependent, whether
calcineurin was involved in UV-induced GSDMC expression was
examined. HaCaT cells were irradiated with UV and subsequently
treated with cyclosporine A (a calcineurin inhibitor) at the
various concentrations (0, 5, 10 or 20 µM) for 24 h. UV-induced
GSDMC mRNA and protein expression levels were notably inhibited by
cyclosporine A in a dose-dependent manner, in control
non-UV-irradiated cells and in UV-irradiated cells (Fig. 3A and B). These results indicated
that the calcineurin pathway may serve an important role in
UV-induced GSDMC expression.
UV-induced GSDMC expression is mediated
by NFATc1 in HaCaT cells
Whether NFATc1 was involved in UV-induced GSDMC
expression was examined, as calcineurin is known to activate the
NFATc family members by dephosphorylating them, and NFATc1 was
reported to be expressed in HaCaT cells (21-24). HaCaT cells were transfected with
either siNC or siNFATc1, serum-starved for 24 h, treated with UV
and further incubated for 24 h. NFATc1 protein expression levels
were increased by UV irradiation in the siNC- and in the
siNFATc1-treated cells (Fig. 4A),
which was consistent with previous reports indicting that UV
induces NFATc1 expression (25).
However, the knockdown of NFATc1 expression notably reduced the
basal and the UV-induced levels of GSDMC protein and mRNA
expression (Fig. 4A and B). In
addition, HaCaT cells were transfected with a control empty vector
or with either a mammalian expression vectors containing a
wild-type or a constitutively active form of NFATc1 gene. The
vector-transfected HaCaT cells were serum-starved for 24 h, treated
with UV and further incubated for 24 h. Overexpression of the
wild-type of NFATc1 notably increased UV-induced GSDMC expression
and the overexpression of a constitutively active form of NFATc1
also notably increased both basal levels and UV-induced levels of
GSDMC mRNA and protein expression (Fig. 4C and D). Taken together, these
results indicated that UV-induced GSDMC expression may be mediated
through NFATc1 in HaCaT cells.
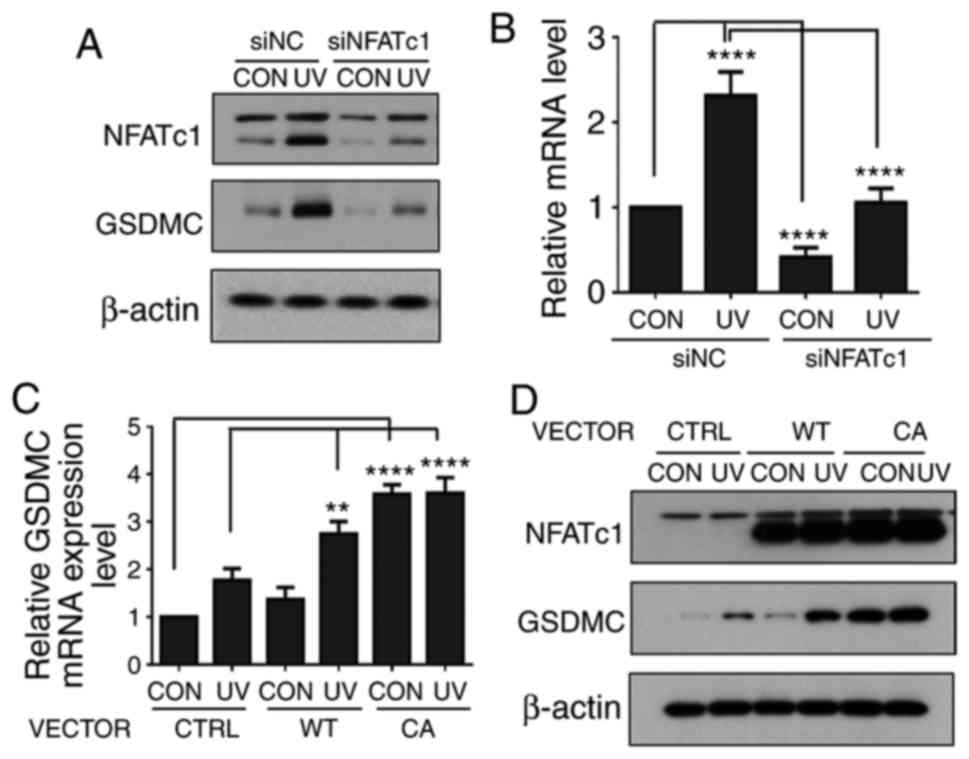 | Figure 4UV-induced GSDMC expression is
mediated through NFATc1 in HaCaT cells. (A and B) HaCaT cells were
transfected with siNC or siNFATc1, serum-starved, irradiated UV and
harvested at 24 h following UV irradiation. (A) NFATc1 and GSDMC
protein expression levels were analyzed by western blotting;
β-actin was used as a loading control; n=3. (B) GSDMC mRNA
expression levels were analyzed by reverse
transcription-quantitative polymerase chain reaction. (C and D)
HaCaT cells were transfected with the CTRL empty vector, NFATc1-WT
or NFATc1-CA overexpression vectors. The cells were serum-starved,
irradiated with UV and harvested at 24 h following UV irradiation.
(C) GSDMC mRNA expression levels were analyzed by reverse
transcription-quantitative polymerase chain reaction. (D) NFATc1
and GSDMC protein expression level were analyzed by western
blotting; β-actin was used as a loading control; n=3. mRNA
expression data were normalized to 36B4 and are presented mean ±
standard deviation; n=3; **P<0.01 and
****P<0.0001. CA, constitutively active NFATc1
expression vector; CON, control non-UV irradiated; CTRL, empty
vector control; GSDMC, gasdermin C; NFATc1, nuclear factor of
activated T-cells, cytoplasmic 1; si, small interfering RNA; siNC,
negative control siRNA; UV, ultraviolet irradiated; WT, wild-type
NFATc1 expression vector. |
Discussion
UV radiation is a major environmental factor that
affects human health. The skin is the largest organ of the body,
through which humans interact with their environment. Consequently,
skin is frequently exposed to UV radiation. Exposure of skin to UV
is known to induce a variety of skin damages, including premature
skin aging and skin carcinogenesis, through multiple and complex
molecular signaling pathways (1-3,14).
To advance the development of strategies and reagents for the
prevention or treatment of skin damage, it is important to
understand UV-induced skin damage at the molecular level and to
elucidate how UV regulates the expression of a number of genes,
including MMPs, such as MMP-1, MMP-3 and MMP-9, which have been
reported to be important factors for the destruction of
extracellular matrix proteins in the skin (26-28). MMP-1 is a major protease
responsible for initiating cleavage within the central triple helix
of native collagen fibrils, typically types I and III, in the skin
(28). Our previous study
reported that GSDMC expression is increased by UV irradiation,
which contributes to the induction of MMP-1 expression through the
activation of ERK and JNK pathways in human skin keratinocytes
(6). However, how UV affects the
regulation of GSDMC expression has not been studied yet.
The present study aimed to identify the signaling
pathways that may be involved in UV-induced GSDMC expression in
HaCaT immortalized human keratinocyte cell line and in primary
human skin keratinocytes. TRPV1 and GSDMC were reported to serve
important roles in UV-induced MMP-1 expression in human skin
keratinocytes; however, TRPV1 activation occurs at relatively early
time points, whereas GSDMC expression increases at relatively late
time points, following UV irradiation (6,12).
Therefore, it was hypothesized that TRPV1 may serve a role in
UV-induced GSDMC expression. The results indicated that inhibition
of TRPV1 activity suppressed UV-induced GSDMC expression. In
addition, direct activation of TRPV1 by capsaicin increases GSDMC
expression. These results indicated that TRPV1 may serve an
important role in GSDMC expression. It has been reported that TRPV1
acts as a non-selective cation channel and that the activation of
TRPV1 may lead to calcium influx (12-14). As the results from the present
study indicated that TRPV1 may be involved in UV-induced GSDMC
expression, the role of extracellular calcium on UV-induced GSDMC
expression was examined and it was demonstrated that UV-induced
GSDMC expression was calcium-dependent. Calcium is known to
modulate several proteins, such as calcium-binding protein
calmodulin, kinases and phosphatases (21,29,30). Previous studies reported that
calcineurin is a calcium and calmodulin-dependent serine/threonine
protein phosphatase (19,20). The present study results
demonstrated that UV-induced GSDMC expression was
calcium-dependent; therefore, whether calcineurin may be involved
in UV-induced GSDMC expression was investigated. The data revealed
that the calcineurin pathway may serve an important role in
UV-induced GSDMC expression.
Calcineurin is known to activate NFATc family
members by dephosphorylating them (21-24). A previous study demonstrated that
UV is a strong inducer for NFATc1 transactivation and that UV
induces NFATc1 by activating calcium/calcineurin pathway in skin
(31). UV induces transcriptional
activity and nuclear translocation of NFATc1 in human skin
keratinocytes (32). Furthermore,
UV is known to enhance NFATc1 binding activity to DNA. NFATc1 binds
DNA cooperatively with other transcription factors to increase
transcription of certain genes (33). These results indicated that NFATc1
may be activated by UV in human skin keratinocytes. Therefore, the
present study aimed to determine whether NFATc1 was involved in
UV-induced GSDMC expression. The results demonstrated that
UV-induced GSDMC expression may be mediated through NFATc1. Taken
together, the present study results indicated that the
TRPV1/calcium/calcineurin/NFATc1 signaling pathway may be involved
in UV-induced GSDMC expression in human skin keratinocytes.
The present study findings may help us to not only
identify the molecular mechanisms involved in UV-induced GSDMC
expression, but also to better understand the signaling pathways
involved in UV-induced MMP-1 expression (Fig. 5). The induction of MMP-1
expression by UV may be regulated by various factors and signaling
pathways. The activation of cell surface receptors including EGFR
by UV induces signal transduction cascades and activates various
signaling pathways, such as ERK and JNK, that are known to be
important for MMP-1 expression (34,35). In addition, TRPV1 may also be
activated by UV and serves a crucial role in UV-induced MMP-1
expression (12,13). Even though the mechanism through
which TRPV1 is activated by UV remains unclear, it has been
reported that Src kinase mediates UV-induced TRPV1 trafficking from
a vesicle inside cytoplasm to cell membrane within 15 min following
UV irradiation, which was suggested to be an important step in
TRPV1 activation (36). The
activation of TRPV1 by UV turns on the calcium/calcineurin/NFATc1
signaling pathway, which increases GSDMC expression. The increase
of GSDMC expression activates ERK and JNK pathways, leading to the
induction of MMP-1 expression.
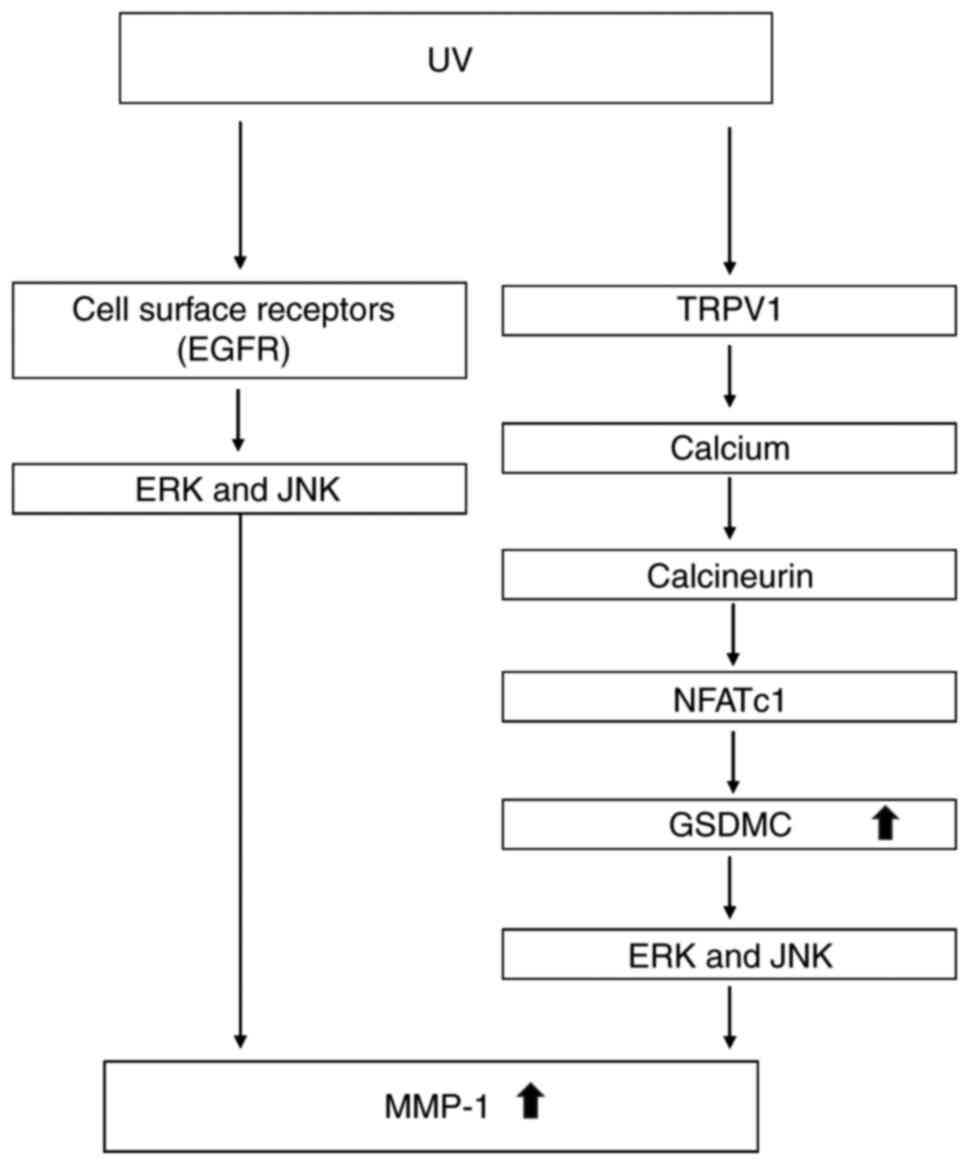 | Figure 5Schematic model of the signaling
pathways involved in UV-induced GSDMC and MMP-1 expression.
UV-induced expression of MMP-1 may be regulated through various
factors and signaling pathways. The activation of cell surface
receptors, including EGFR, by UV induces signal transduction
cascades and activates various signaling pathways, such as ERK and
JNK pathways, that are known to be important for MMP-1 expression.
In addition to these cell surface receptors, TRPV1 is also be
activated by UV, which turns on the calcium/calcineurin/NFATc1
signaling pathway and leads to increased GSDMC expression. The
increase of GSDMC expression activates ERK and JNK pathways,
ultimately inducing MMP-1 expression. EGFR, epidermal growth factor
receptor; ERK, extracellular signal-regulated kinase; GSDMC,
gasdermin C; JNK, c-Jun N-terminal kinase; MMP-1, matrix
metalloproteinase 1; NFATc1, nuclear factor of activated T-cells,
cytoplasmic 1; TRPV1, transient receptor potential cation channel
subfamily V member 1; UV, ultraviolet irradiation. |
In addition, the involvement of the EGFR pathway in
UV-induced GSDMC expression in HaCaT cells was also examined in the
present study; however, the inhibition of EGFR by the chemical EGFR
inhibitor (AG1478) did not affect UV-mediated GSDMC induction (data
not shown). These results indicated that UV-induced GSDMC
expression may occur independent of the EGFR pathway, and may be
through the TRPV1 pathway. However, it has been reported that EGFR
serves a critical role in UV-induced MMP-1 expression (2,26,37). Therefore, these previous and
present results further support our hypothesis (Fig. 5), which suggested that several
signaling pathways are involved in UV-induced MMP-1 expression.
In conclusion, TRPV1 may serve an important role in
the induction of GSDMC expression by UV and that UV-induced GSDMC
expression is mediated via the calcium/calcineurin/NFATc1 signaling
pathway. These results may help us to better understand UV-induced
signal transduction pathways and associated gene expression at the
molecular level.
Funding
This study was supported by a grant from The
National Research Foundation of Korea funded by the Ministry of
Science, ICT & Future Planning (grant no.
2014M3C9A2064536).
Availability of data and material
The data sets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
NK and CHP designed the study and performed the
experiments. DHL was involved in drafting the article and in the
analysis of the data. HSY provided the preliminary data. CHP and
JHC had full access to all the data and take full responsibility
for the integrity of data and the accuracy of data analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The medical ethical committee at Seoul National
University approved the study protocol, and all the guardians of
participants gave their written informed consent. The study was
conducted according to the Declaration of Helsinki principles.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
UV
|
ultraviolet
|
|
GSDMC
|
gasdermin C
|
|
NFATc1
|
nuclear factor of activated T-cells,
cytoplasmic 1
|
|
TRPV1
|
transient receptor potential cation
channel subfamily V member 1
|
Acknowledgments
Not applicable.
References
|
1
|
Rittie L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu YR and Fisher GJ: Ultraviolet (UV)
light irradiation induced signal transduction in skin photoaging. J
Dermatol Sci. S12005.
|
|
3
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kahari VM and Saarialho-Kere U: Matrix
metalloproteinases in skin. Exp Dermatol. 6:199–213. 1997.
View Article : Google Scholar
|
|
5
|
Saeki N, Kuwahara Y, Sasaki H, Satoh H and
Shiroishi T: Gasdermin (Gsdm) localizing to mouse Chromosome 11 is
predominantly expressed in upper gastrointestinal tract but
significantly suppressed in human gastric cancer cells. Mamm
Genome. 11:718–724. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kusumaningrum N, Lee DH, Yoon HS, Kim YK,
Park CH and Chung JH: Gasdermin C is induced by ultraviolet light
and contributes to MMP-1 expression via activation of ERK and JNK
pathways. J Dermatol Sci. 90:180–189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saeki N and Sasaki H: Gasdermin
superfamily: A novel gene family functioning in epithelial cells.
Endothelium and Epithelium: Composition, Functions and Pathology.
Nova Science Publishers, Inc.; New York: pp. 193–211. 2012
|
|
8
|
Tamura M, Tanaka S, Fujii T, Aoki A,
Komiyama H, Ezawa K, Sumiyama K, Sagai T and Shiroishi T: Members
of a novel gene family, Gsdm, are expressed exclusively in the
epithelium of the skin and gastrointestinal tract in a highly
tissue-specific manner. Genomics. 89:618–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watabe K, Ito A, Asada H, Endo Y,
Kobayashi T, Nakamoto K, Itami S, Takao S, Shinomura Y, Aikou T, et
al: Structure, expression and chromosome mapping of MLZE, a novel
gene which is preferentially expressed in metastatic melanoma
cells. Jpn J Cancer Res. 92:140–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saeki N, Usui T, Aoyagi K, Kim DH, Sato M,
Mabuchi T, Yanagihara K, Ogawa K, Sakamoto H, Yoshida T and Sasaki
H: Distinctive expression and function of four GSDM family genes
(GSDMA-D) in normal and malignant upper gastrointestinal
epithelium. Genes Chromosomes Cancer. 48:261–271. 2009. View Article : Google Scholar
|
|
11
|
Miguchi M, Hinoi T, Shimomura M, Adachi T,
Saito Y, Niitsu H, Kochi M, Sada H, Sotomaru Y, Ikenoue T, et al:
Gasdermin C Is upregulated by inactivation of transforming growth
factor β receptor type II in the presence of mutated Apc, promoting
colorectal cancer proliferation. PLoS One. 11:e01664222016.
View Article : Google Scholar
|
|
12
|
Lee YM, Kim YK, Kim KH, Park SJ, Kim SJ
and Chung JH: A novel role for the TRPV1 channel in UV-induced
matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J Cell
Physiol. 219:766–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li WH, Lee YM, Kim JY, Kang S, Kim S, Kim
KH, Park CH and Chung JH: Transient receptor potential vanilloid-1
mediates heat-shock-induced matrix metalloproteinase-1 expression
in human epidermal keratinocytes. J Invest Dermatol. 127:2328–2335.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahern GP, Brooks IM, Miyares RL and Wang
XB: Extracellular cations sensitize and gate capsaicin receptor
TRPV1 modulating pain signaling. J Neurosci. 25:5109–5116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Rami HK, Thompson M, Stemp G, Fell S,
Jerman JC, Stevens AJ, Smart D, Sargent B, Sanderson D, Randall AD,
et al: Discovery of SB-705498: A potent, selective and orally
bioavailable TRPV1 antagonist suitable for clinical development.
Bioorg Med Chem Lett. 16:3287–3291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Knotkova H, Pappagallo M and Szallasi A:
Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or
revival? Clin J Pain. 24:142–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Wang S, Chuang AY, Cohen BE and
Chuang HH: Activity-dependent targeting of TRPV1 with a
pore-permeating capsaicin analog. Proc Natl Acad Sci USA.
108:8497–8502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilkins BJ and Molkentin JD:
Calcium-calcineurin signaling in the regulation of cardiac
hypertrophy. Biochem Biophys Res Commun. 322:1178–1191. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schulz RA and Yutzey KE: Calcineurin
signaling and NFAT activation in cardiovascular and skeletal muscle
development. Dev Biol. 266:1–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Crabtree GR: Calcium, calcineurin, and the
control of transcription. J Biol Chem. 276:2313–2316. 2001.
View Article : Google Scholar
|
|
22
|
Crabtree GR: Generic signals and specific
outcomes: Signaling through Ca2+, calcineurin, and
NF-AT. Cell. 96:611–614. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Daraji WI, Grant KR, Ryan K, Saxton A
and Reynolds NJ: Localization of calcineurin/NFAT in human skin and
psoriasis and inhibition of calcineurin/NFAT activation in human
keratinocytes by cyclosporin A. J Invest Dermatol. 118:779–788.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smit NP, Van Rossum HH, Romijn FP, Sellar
KJ, Breetveld M, Gibbs S and Van Pelt J: Calcineurin activity and
inhibition in skin and (epi)dermal cell cultures. J Invest
Dermatol. 128:1686–1690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang E, Ngo HTT, Seo SA, Park B, Zhang M,
Gao W and Yi TH: Urtica thunbergiana prevents UVB-induced premature
skin aging by regulating the transcription factor NFATc1: An in
vitro and in vivo study. J Funct Foods. 36:162–177. 2017.
View Article : Google Scholar
|
|
26
|
Brenneisen P, Sies H and
Scharffetter-Kochanek K: Ultraviolet-B irradiation and matrix
metalloproteinases: From induction via signaling to initial events.
Ann NY Acad Sci. 973:31–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bootman MD, Collins TJ, Peppiatt CM,
Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT,
Berridge MJ, et al: Calcium signalling-an overview. Semin Cell Dev
Biol. 12:3–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bootman MD: Calcium signaling. Cold Spring
Harb Perspect Biol. 4:a0111712012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang C, Mattjus P, Ma WY, Rincon M, Chen
NY, Brown RE and Dong Z: Involvement of nuclear factor of activated
T cells activation in UV response. Evidence from cell culture and
transgenic mice. J Biol Chem. 275:9143–9149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Flockhart RJ, Diffey BL, Farr PM, Lloyd J
and Reynolds NJ: NFAT regulates induction of COX-2 and apoptosis of
keratinocytes in response to ultraviolet radiation exposure. FASEB
J. 22:4218–4227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maziere C, Morliere P, Louandre C, Conte
MA, Gomilla C, Santus R, Antonicelli F, Hornebeck W and Mazière JC:
Low UVA doses activate the transcription factor NFAT in human
fibroblasts by a calcium-calcineurin pathway. Free Radic Biol Med.
39:1629–1637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y, Voorhees JJ and Fisher GJ: Epidermal
growth factor receptor is a critical mediator of ultraviolet B
irradiation-induced signal transduction in immortalized human
keratinocyte HaCaT cells. Am J Pathol. 169:823–830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Y, Shao Y, Voorhees JJ and Fisher GJ:
Oxidative inhibition of receptor-type protein-tyrosine phosphatase
kappa by ultraviolet irradiation activates epidermal growth factor
receptor in human keratinocytes. J Biol Chem. 281:27389–27397.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han S, Kang SM, Oh JH, Lee DH and Chung
JH: Src kinase mediates UV-induced TRPV1 trafficking into cell
membrane in HaCaT keratinocytes. Photodermatol Photoimmunol
Photomed. 34:214–216. 2018. View Article : Google Scholar
|
|
37
|
Di Girolamo N, Coroneo M and Wakefield D:
Epidermal growth factor receptor signaling is partially responsible
for the increased matrix metalloproteinase-1 expression in ocular
epithelial cells after UVB radiation. Am J Pathol. 167:489–503.
2005. View Article : Google Scholar : PubMed/NCBI
|