1. Introduction
In unstimulated tissues, numerous cellular
mechanisms contribute to the influx and efflux of Ca2+
to and from the cytoplasm in order to maintain homeostasis of
intracellular basal Ca2+ concentrations
[b(Ca2+)i], a phenomenon that
occurs in almost all cells (1-7).
In smooth muscle at rest, b[Ca2+]i
must be kept tightly within the range of 100 and 150 nM (8-15)
to maintain an equilibrium between contraction and relaxation. In
these cells, the processes of Ca2+ influx and efflux
preserve the myogenic tone, resting membrane potential and
sarcoplasmic reticulum (SR) Ca2+ refilling (1,10,16-18). It has been proposed that the
influx process involves entry of extracellular Ca2+
through L-type voltage dependent Ca2+ channels (L-VDCCs)
(10,19-22), receptor-operated Ca2+
channels (ROCCs) activated by agonists (23-28) and store-operated Ca2+
channels (SOCCs, capacitative Ca2+ entry) activated by
SR-Ca2+ depletion (10,29-33). An additional cytosolic
Ca2+ source is the SR, that is the main intracellular
Ca2+ store, activated via inositol 1,4,5-trisphosphate
(IP3) receptor channels (30,34-36) and ryanodine-receptor (RyR)
channels (35,37-40). Ca2+ extrusion from the
cytoplasm is accomplished via the action of membrane and
sarcoplasmic Ca2+ ATPases and
Na+/Ca2+ exchanger (NCX) in its forward mode
(41-49).
Pivotal work on basal Ca2+ influx
performed in aortic vascular smooth muscle cells using a
pharmacological approach, demonstrated two predominant mechanisms
of basal Ca2+ entry: One associated with L-VDCCs,
accounting for ~23-43% of the total Ca2+ entry, and
another associated with SOCCs, which contributed ~30% of the total
(50).
In a recent study on airway smooth muscle (ASM), the
present authors observed that the basal Ca2+ entry was
mediated by L-VDCCs and probably also a constitutively active
transient receptor potential canonical 3 (TRPC3) channel (18), which is described below. However,
the mechanisms that maintain their permeability to Ca2+
have yet to be elucidated.
In the present review, current knowledge regarding
different structures that maintain the
b[Ca2+]i in ASM, including those
involving L- and T-VDCCs, TRPC3, membrane and sarcoplasmic
Ca2+-ATPases, NCX in its forward mode, IP3
and RyRs, is discussed, including the most recent findings
associated with the phosphorylation of L- and T-VDCCs and the
dependence of TRPC3 on diacylglycerol (DAG).
For a better understanding of the participation of
each of these proteins in the
b[Ca2+]i regulation of ASM, novel
unpublished data from studies by our group have been included.
Firstly, Fig. 1A shows the
maximal reduction of intracellular Ca2+ concentration
([Ca2+]i) produced under Ca2+ free
medium. This maneuver allowed determination of the proportional
effect of each protein in the handling of
b[Ca2+]i.
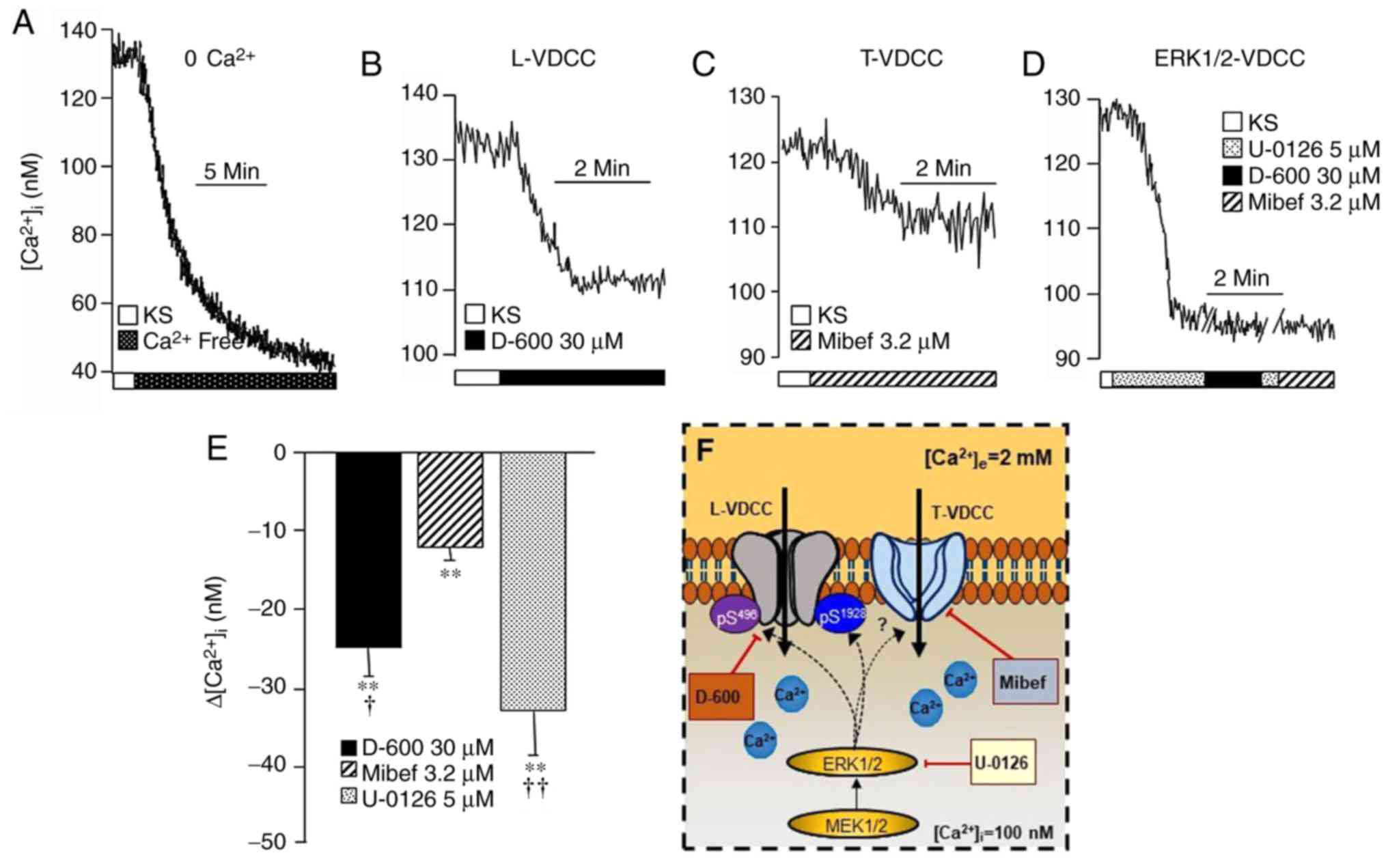 | Figure 1In guinea-pig airway myocytes at
rest, L-VDCC and T-VDCC contribute towards maintaining the
b[Ca2+]i, and apparently are
phosphorylated through the MEK-ERK1/2 pathway. Upper traces are
representative of the intracellular Ca2+ measurements
through fura-2AM in the different experimental protocols. (A)
Representative trace showing the amplitude of the reduction in the
b[Ca2+]i in the absence of
extracellular Ca2+. The addition of (B) D-600 (an L-VDCC
blocker; n=12) or (C) Mibef (a T-VDCC blocker; n=13) significantly
lowered the b[Ca2+]i to differing
extents. (D) Blockade of MEK-ERK1/2 kinase with U-0126 (n=12)
markedly diminished the b[Ca2+]i
and the administration of D-600 or Mibef did not lead to any
further decreases in the altered [Ca2+]i (n=6). (E) Bar
graph depicting the statistical analysis of the different
experimental protocols. Each bar represents the mean ± standard
error of the mean. **P<0.01 when compared with their
respective b[Ca2+]i values;
†P<0.05, ††P<0.01 with respect to the
Mibef group (according to the Student-Newman-Keuls multiple
comparison test). (F) Schematic representation of regulation of the
basal activity of the VDCCs. The MEK signaling pathway through
ERK1/2 phosphorylates the β2 Ser496
(pS496) and α1 Ser1928
(pS1928) sites, switching the L-VDCC and probably also
the T-VDCC between an open and closed state. D-600, Mibef or U-0126
diminished the b[Ca2+]i, (for
further details, see the ‘VDCCs’ section). These results suggest
that, under basal conditions, the two types of VDCC are
continuously phosphorylated through the MEK pathway, which is
responsible for their constitutive activity. L-VDCC, L-type
voltage-dependent channel; T-VDCC, T-type voltage dependent
Ca2+ channel; b[Ca2+]i,
intracellular basal Ca2+ concentration; MEK,
mitogen-activated protein kinase kinase; ERK1/2,
extracellular-signal-regulated kinase 1/2; Mibef, mibefradil; KS,
Krebs’ solution. |
2. VDCCs
L- and T-VDCCs have been described in different
types of smooth muscle (19,51,52); in particular, L-VDCC expression
has been abundantly reported in the ASM of different species,
including human (20,21,53-56). Opening of both types of channel is
dependent on membrane depolarization, allowing the entry of
Ca2+, which subsequently contributes to
contraction and SR Ca2+ refilling (9,10,19,20,57).
Several subunits for L-VDCC have been described:
CaV1.1, CaV1.2, CaV1.3 and
CaV1.4 (58). In ASM,
L-VDCC had generally been characterized by pharmacological and
electrophysiological methods (19). However, the presence of all the
subunits of this channel was recently reported in rat bronchial
smooth muscle (59).
Nevertheless, in bovine and guinea-pig tracheal myocytes, only
CaV1.2 and CaV1.2-CaV1.3,
respectively, were observed (21,60). As identified recently by the
present authors and shown in Fig. 1B
and E, in guinea-pig ASM, D-600 (methoxyverapamil
hydrochloride), a blocker of L-VDCC, significantly decreased the
b[Ca2+]i, corroborating that this
channel is constitutively active and contributes towards
maintaining the b[Ca2+]i (18). It is well known that this channel
is greatly dependent on the membrane voltage, and in canine ASM our
group observed that its membrane potential at rest is
approximately-59 mV, and is held steady. Furthermore, when the
tissue was stimulated with carbachol, a cholinergic agonist, its
membrane was depolarized, and when the depolarization reached-45
mV, it started oscillating (20).
These oscillations are nifedipine-sensitive, and therefore
corresponded to the opening and closing of the L-VDCC (61). Since the membrane potential at
rest is unchanging, it was highly improbable that the voltage was
influencing its opening at this stage.
Recently, a study in rat cardiomyocytes demonstrated
that extracellular signal-regulated kinases 1 and 2 (ERK1/2), the
mitogen-activated protein kinases (MAPKs), are able to
phosphorylate L-VDCC at two sites: On Ser496 of the
β2 subunit and Ser1928 of the α1
subunit. Phosphorylation on the β2 subunit or the
α1 subunit decreased or increased the L-VDCC activity,
respectively (62). Thus, it may
be hypothesized that in ASM, MAPK kinase (MEK)-ERK1/2 signaling may
be involved in the continual opening and closing of the channel
under basal conditions. This pathway may be associated with
receptor tyrosine kinases (RTKs), which are activated by basal
cyto-kines or growth factors. Our group previously demonstrated
that ERK1/2 are present in the phosphorylated state in
unstim-ulated bovine ASM (9).
Fig. 1D and E show that the
addition of U-0126, an inhibitor of ERK1/2, to guineapig tracheal
myocytes significantly diminished the [Ca2+ b ]i until
reaching a plateau. The addition of D-600 did not further modify
the [Ca2+]i, confirming that phosphorylation
of the L-VDCC through the MEK-ERK1/2 pathway is possibly involved
in its constitutive active mode. Therefore, the ERK1/2 signaling
pathway may be responsible for phosphorylating the β2
Ser496 and α1 Ser1928 sites,
serving to switch the L-VDCC between an open and closed state
(Fig. 1F).
Treatment with mibefradil, a T-VDCC blocker, also
signifi-cantly lowered [Ca2+ b ]i in the guinea-pig
tracheal myocytes, implying the participation of this channel in
sustaining [Ca2+ b ]i (Fig. 1C and E). The presence of T-VDCC
has been reported in this tissue (19), and the expression of
CaV3.1, CaV3.2 and CaV3.3 subunits
has been detected in ASM by immunohistochemistry (63). In this context, unexpectedly our
group found that the addition of mibefradil following U-0126 did
not further diminish b[Ca2+]i
(Fig. 1D). This finding suggested
that T-VDCC could also be regulated by the ERK1/2 signaling
pathway. Recent studies have shown that T-VDCC may be modified by
several serine/threonine protein kinase pathways, suggesting that
this channel is susceptible to undergo phosphorylation (64); however, further research is
required in this regard to determine the functional impact that
ERK1/2 signaling has on the T-VDCC. Notably, in sensitized
guinea-pigs that developed an airway inflammatory state, the
expression level of L-VDCC was not modified (60). This finding indicated that these
channels appear not to participate in the modification of
b[Ca2+]i that is observed in
inflammatory ailments, such as asthma (65).
3. TRPC channels
In smooth muscle, TRPC channel genes code for ROCC
and SOCC, which have an important role in intracellular
Ca2+ homeostasis, while recently transient receptor
potential vanilloid 1 (TRPV1) was revealed to be involved in the
modulation of ASM tone and Ca2+ handling during
agonist-induced contraction (66). In general, due to their ionic
permeability, all TRPC channels are considered to be non-selective
cation channels (NSCCs) (67,68). Thus far, all known TRPC channel
activity has been shown to be associated with a phospholipase C
(PLC) signaling pathway (69,70). In this context, it has been
proposed that certain TRPC channels, including TRPC1, -2 and -3,
are dependent on SR-Ca2+ depletion due to IP3
production [a process termed store-operated Ca2+ entry
(SOCE)] (36,71-75). On the other hand, ROCCs also
include TRPC channels (TRPC3, -4, -5, -6 and 7), although these are
activated by DAG, the other metabolite of PLC activity, and are
independent of SR-Ca2+ depletion (69,70,76). In this context, only TRPCs 3, 6
and 7 are directly activated by DAG not involving protein kinase C
(69,76), whereas TRPCs 4 and 5 are inhibited
by protein kinase C, since their activity may be observed when this
kinase is blocked (70).
In ASM, previous studies have reported the presence
of almost all TRPC channel subtypes (TRPC1, -2, -3, -4, -5 and -6),
with the exception of TRPC7 (67,68). Several TRPC channels have been
shown to be constitutively active in different types of tissue. For
example, TRPC1 and -4 were proposed to be continuously active in
C57 mice skeletal myocytes (77);
likewise, TRPC7 in human embryonic kidney cells (76), while TRPC3 was also observed to be
constitutively active in rabbit ear artery and mouse airway
myocytes (78,79). In this regard, our recent study
demonstrated that, in guinea-pig ASM, this channel was also
involved in maintaining the
b[Ca2+]i and preserving smooth
muscle basal tone (18). The role
of this channel in b[Ca2+]i is
illustrated in Fig. 2, where the
addition of 2-aminoethoxydiphenyl borate (2-APB), a blocker of the
TRPC3 channel (80), markedly
diminished the b[Ca2+]i (Fig. 2A and E). Furthermore, Pyr3,
another specific TRPC3 channel blocker (81), also lowered
b[Ca2+]i by a similar extent
(Fig. 2B and E). These results
suggested that TRPC3 is constitutively active in guineapig ASM,
even though the mechanism underlying this phenomenon has yet to be
fully elucidated.
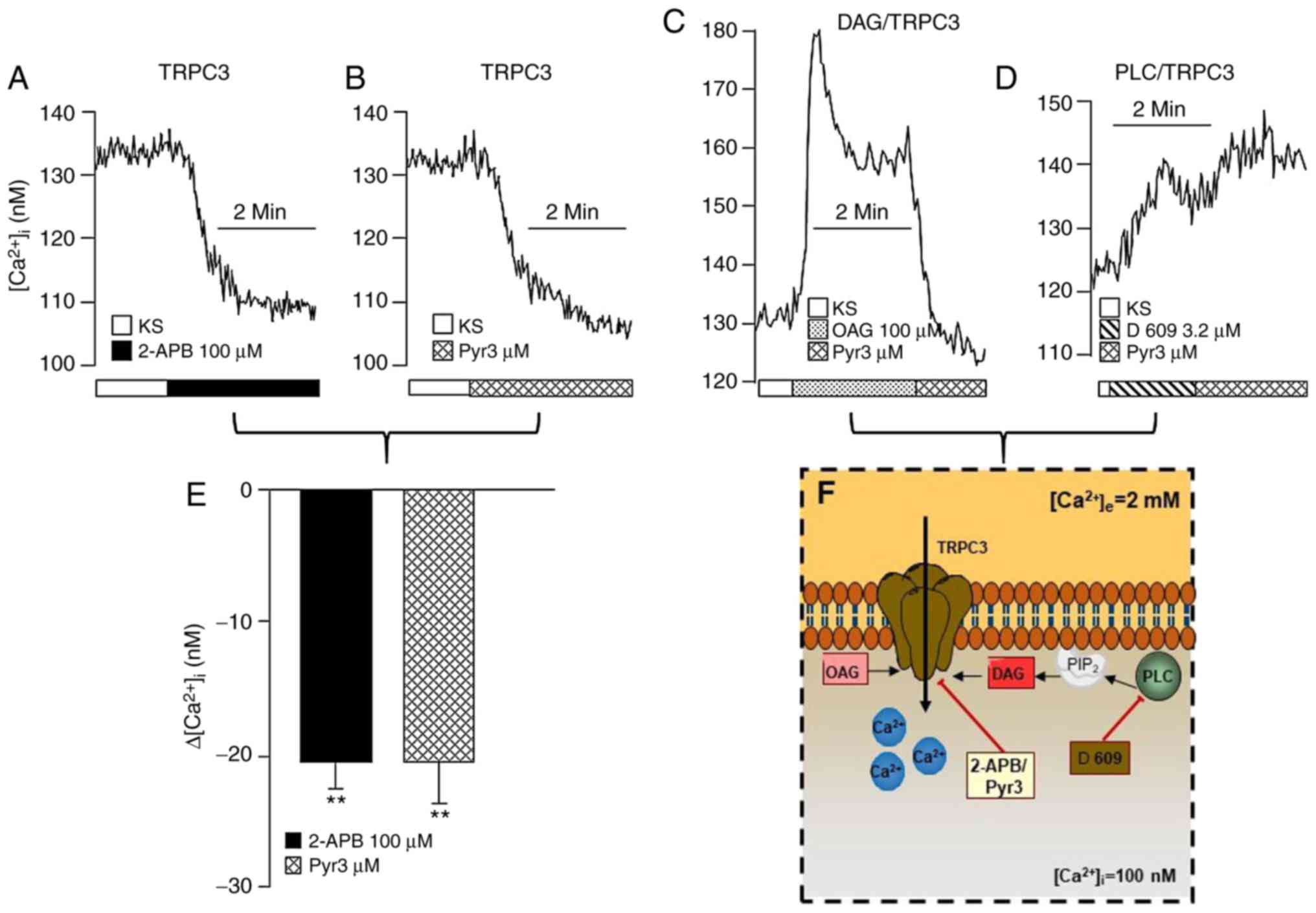 | Figure 2Membrane TRPC3 channel also
contributes to b[Ca2+]i in
guineapig airway smooth muscle. The upper traces shown are
representative of the different experimental protocols. The
addition of (A) 2-APB (a blocker of TRPC3; n=5) or (B) Pyr3 (a
specific TRPC3 blocker; n=5) lowered the
b[Ca2+]i. (C) The addition of OAG,
a DAG analog, induced a transient peak of the
[Ca2+]i, followed by a plateau. The
application of Pyr3 to the Ca2+ plateau returned
Ca2+ to its basal level, indicating that the main TRPC
channel functionally active in airway smooth muscle at rest is
TRPC3. (D) Incubation with D-609, an inhibitor of PLC, produced a
small incremental increase in the [Ca2+]i,
and the addition of Pyr3 no longer diminished the
b[Ca2+]i. (E) Bar graph
illustrating that the effects elicited by 2-APB and Pyr3 on
b[Ca2+]i are similar. Each bar
represents the mean ± standard error of the mean.
**P<0.01 compared with the respective
b[Ca2+]i value. (F) Schematic
representation of the basal activity regulation of the TRPC3
channel. The results suggest that, under basal conditions, TRPC3
may oscillate between an open and closed state in the plasma
membrane, i.e., these channels are constitutively active in this
tissue, and are regulated by PLC through DAG. See the ‘Transient
receptor potential canonical channels’ section for further details.
PIP2, phosphatidylinositol 4,5-bisphosphate; TRPC3,
transient receptor potential canonical-3; 2-APB,
2-aminoethoxydiphenyl borate; OAG, 1-oleoyl-2-acetyl-sn-glycerol;
DAG, diacylglycerol; PLC, phospholipase C. |
Since almost all TRPC channel subtypes are expressed
in ASM, in this review the DAG analog,
1-oleoyl-2-acetyl-sn-glic-erol (OAG), was used to investigate the
possible functional role of the channels present in this tissue.
Fig. 2C shows that the addition
of OAG to tracheal myocytes induced a transient peak in the
[Ca2+]i followed by a plateau. This response
could have been developed through TRPC3 and/or TRPC6 channels,
since these are both directly activated by DAG (69). However, after having reached the
Ca2+ plateau induced by OAG, the addition of Pyr3 led to
a return of [Ca2+]i to its basal level. This
finding indicated that the predominant TRPC channel that is
functionally active in guineapig ASM, is TRPC3. Our group has
postulated that TRPC3 is one of the channels involved in the
maintenance of b[Ca2+]i (18), probably in a DAG-dependent manner.
This lipid molecule is produced via the PLC or phospholipase D
(PLD) pathways. It has been reported in rabbit ear artery myocytes
that the PLD pathway produces DAG to sustain the constitutive
activity of TRPC3 that contributes to the resting membrane
potential (78,82). In ASM, protein kinase A was
reported to regulate PLD activity, and it has been postulated that
this phospholipase may be involved in the molecular mechanism
underlying cyclic adenosine 5′-phosphate (c-AMP)-mediated
relaxation in this tissue (83).
By contrast, PLC has been shown to be predominantly involved in the
IP3-Ca2+ signaling pathway and in contraction
(35). Therefore, in this review,
we investigated if PLC may participate in DAG production in ASM at
rest by using tricyclodecan-9-yl xanthogenate (D-609, a relatively
specific inhibitor of PLC) (84)
to inhibit this enzyme activity. It was observed that the addition
of Pyr3 following D-609 to tracheal myocytes did not result in any
further notable perturbations of the b[Ca2+]i
(Fig. 2D). Thus, these results
suggested that PLC generates DAG, which subsequently leads to the
activation of TRPC3 under basal conditions in order to maintain
b[Ca2+]i in ASM (Fig. 2F). Conceivably, the activity of
PLC may be regulated by endogenous ligands of RTKs, or by
G-protein-coupled receptors.
It has been demonstrated that the expression levels
and activity of the TRPC3 channel are greatly augmented in ASM
cells obtained from sensitized mice (79). This may lead to an increase in the
b[Ca2+]i, which could contribute
to airway hyperresponsiveness in asthma.
The TRPV receptors, which are other members of the
TRP family, have been implicated in mechanical stretch-induced
Ca2+ influx in human ASM (85). In this context, TRPV1 is expressed
in these cells, and was shown to be involved in Ca2+
oscillations and the maintenance of contraction by cholinergic
agonists (66). However, any role
in terms of maintaining the
b[Ca2+]i has not yet been
elucidated, and this requires further research.
4. Capacitative Ca2+ entry
SR-Ca2+ depletion mediated by
IP3 induces the established mechanism of capacitative
Ca2+ entry. The first studies on this were performed by
Putney (31) in non-excitable
cells. Capacitative Ca2+ entry also occurs in smooth
muscle via Ca2+ influx through diverse membrane channels
(32,86). One of these Ca2+ influx
mechanisms involves two types of protein associated with the SOCE
pathway: Stromal interaction molecules (STIMs) and Orai proteins
(87,88), both of which have been
characterized in vascular smooth muscle and ASM (89,90). Orai are plasma membrane proteins,
and three isoforms from different genes have been characterized:
Orai1, -2 and -3 (91). On the
other hand, two homologs of STIM have been identified: STIM1 and
STIM2, both of which are located in the SR membrane (88,92,93). Regarding the two protein groups,
Orai1 and STIM1 are the proteins that are chiefly expressed in ASM,
and are responsible for the capacitative Ca2+ entry
(89,94). Briefly, STIM1 on the SR functions
as a Ca2+ sensor, monitoring the organelle’s
Ca2+ content (95).
When the SR-Ca2+ store is depleted, STIM1 forms an
aggregate with other STIM1 molecules, thereby forming structures
designated as ‘puncta’, which interact with Orai1 plasma membrane
proteins to promote capacitative Ca2+ entry (89). Additionally, in several cell types
it has been postulated that STIM/Orai may interact with TRPC
channels, thereby establishing an alternative mechanism for
capacitative Ca2+ entry (89,96). It is noteworthy that, in ASM,
IP3 has been demonstrated to directly open membranal
TRPC3 channels. This recent finding implies that IP3
mediates SR-Ca2+ depletion (i.e., capacitative
Ca2+ entry) and also a direct, independent
Ca2+ influx by TRPC channels (36). In this context, in one of our
previous studies, we demonstrated that, in unstimulated airway
myocytes, capacitative Ca2+ entry was not activated
unless the SR Ca2+ content fell below 50% (8). However, it is well known that
capacitative Ca2+ entry is activated by contractile
agonists that act through the PLCβ-IP3
signaling cascade (32),
therefore providing no certainty that it does contribute to the
maintenance of b[Ca2+]i.
5. Na+/Ca2+
exchanger
The Na+/Ca2+ exchanger (NCX)
is a membrane Ca2+-handling protein that introduces
three Na+ ions to the cytoplasm, while extruding one
Ca2+ when in its forward mode. By contrast, in its
reverse mode, it introduces Ca2+ and extrudes
Na+ (42). To activate
the reverse mode (NCXREV), the entry of Na+
through an NSCC, and probably L-VDCC in proximity to the NCX, is
required (21,41,48,97). The NCX is encoded by three gene
isoforms, which generate NCX1, -2 and -3 (98-100). NCX1, extensively distributed in
mammalian cells, has 17 different splicing variants that are
tissue-specific and define the exchanger’s ionic sensitivity and
regulation (101). NCX2 has no
splicing variants and is located predominantly in the brain, spinal
cord, gastrointestinal and kidney tissues, whereas NCX3 has five
splice variants expressed in brain and skeletal muscle (101). In ASM, the NCX1.3 splicing
variant is the main isoform present (102).
In airway myocytes, it has been proposed that NCX
participates in the physiology of [Ca2+]i,
including SR-Ca2+ refilling (10,57), although it has been given a minor
role in Ca2+ homeostasis (43). In this context, we have observed
that NCX blockade with amiloride, a blocker of both the forward and
reverse NCX modes, or KB-R7943, a blocker of NCXREV, had
no noticeable effect on b[Ca2+]i,
indicating a minor role of this protein in terms of
b[Ca2+]i regulation (unpublished
data). Nevertheless, its participation in Ca2+
regulation, accomplished mainly through NCXREV, becomes
evident when b[Ca2+]i is increased
and acquires a new steady-state (Fig.
3A). In this context, in a murine chronic model of
allergen-induced airway hyperresponsiveness, it was shown that the
levels of NCX1 were significantly augmented, and that
NCXREV activity was increased (103). Furthermore, in human myocytes,
the addition of pro-inflammatory cytokines, including tumor
necrosis factor-α (TNFα) and interleukin (IL)-13, also increased
the expression of NCX1 and favored NCXREV activity
(104). These findings suggested
that, during inflammation, NCXREV could significantly
contribute to an increase in the
b[Ca2+]i, which would predispose
airway smooth muscle to hyperresponsiveness.
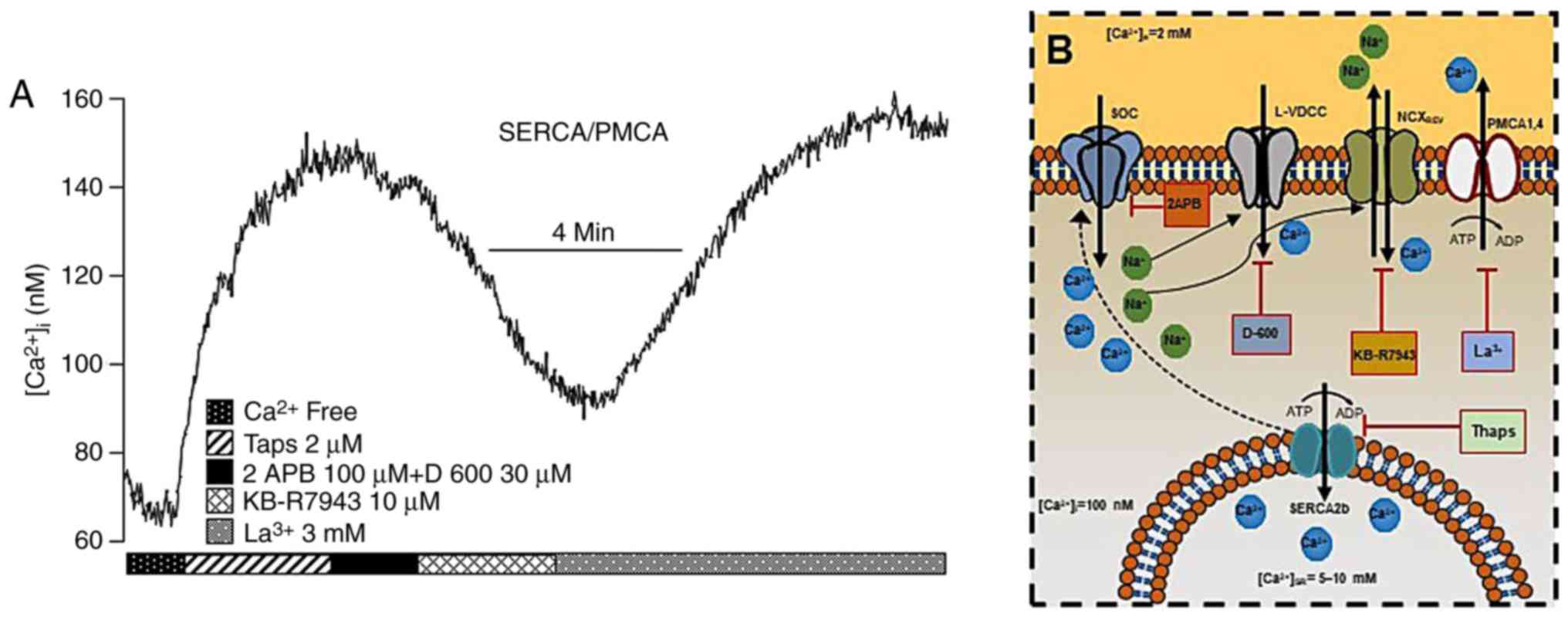 | Figure 3In guinea-pig airway smooth muscle,
SERCA and PMCA actively participate in maintaining the
b[Ca2+]i (A) The blockade of SERCA
with Thaps (n=6) increased the [Ca2+]i until
a new basal steady state was reached due to capacitative
Ca2+ entry involving L-VDCC and SOCC. At this point, the
NCX changes to its reverse mode, probably due to the entry of
Na+ through SOCC and L-VDCC, and thereby becomes the
main contributor to sustaining the Ca2+ plateau since
KB-R7943 brought [Ca2+]i to a new basal steady state.
The addition of lanthanum (La3+), a non-specific PMCA
blocker, led to large increase in [Ca2+]i, thus
indicating that the former new Ca2+ basal state was
maintained by PMCA activity. Note that all experimental protocols
were performed in Ca2+-containing Krebs solution, with
the exception of the first 1.5 min at the beginning of the
experiment. (B) Schematic representation of the roles of SERCA,
PMCA, NCX and NCXREV in maintaining
b[Ca2+]i. For further details, see the
‘Na+/Ca+ exchanger’ and the
‘Ca2+-ATPases in ASM’ sections. NCX,
Na+/Ca2+ exchanger; SERCA, sarcoplasmic
reticulum Ca2+-ATPase; PMCA, plasmalemmal
Ca2+-ATPase; Thaps, thapsigargin; L-VDCC, L-type
voltage-dependent channel; SOCC, store-operated Ca2+
channel. |
6. Ca2+-ATPases in ASM
Ca2+-ATPases form part of a large family
of membrane proteins defined as P-type ATPases, including the
plasmalemmal Ca2+-ATPase (PMCA) and the SR
Ca2+-ATPase (SERCA, or sarco/endoplasmic reticulum
Ca2+-ATPase) (105).
The PMCA extrudes Ca2+ against a high
concentration gradient to contribute to
b[Ca2+]i. It exists in a 1:1
relation-ship with ATP, is electroneutral via
H+/Ca2+ exchange, and its affinity for
Ca2+ and transport efficiency is increased by
calmodulin. PMCA1-4 are the products of four different genes with
several splice variants (105).
PMCA1 and -4 are ubiquitous, and have lower affinity for
calmodulin, whereas PMCA2 and PMCA3 have high calmodulin affinity
(105,106).
In ASM, the primordial function of PMCA in
Ca2+ homeostasis was demonstrated late in the 20th
century (43). Shortly
afterwards, the expression of this pump in canine ASM was reported
(107). More recently, in rat
bronchial myocytes, the presence of PMCA1 and PMCA4 was confirmed,
and the participation of these two isoforms in Ca2+
homeostasis was demonstrated (108).
On the other hand, SERCA is, in part, electrogenic,
since it introduces two Ca2+ ions to the SR, at the same
time releasing at least four H+ ions to the cytoplasm
(105). Additionally, it has
been demonstrated that SERCA transports two Ca2+ ions
for each hydrolyzed ATP molecule, and it appears to be the main
system for controlling [Ca2+]i in muscular
cells (105).
SERCA pumps are produced by three genes: SERCA1, -2
and -3. They are subjected to alternative splicing, resulting in
the isoforms, SERCA1a-b, SERCA2a-c and SERCA3a-f (105,109). In smooth muscle cells, the SERCA
isoforms predominantly present are 2a and 2b (109), whereas in ASM, SERCA2b is the
predominant isoform (110).
By measuring [Ca2+]i in the
absence of extracellular Ca2+, the addition of
thapsigargin, a SERCA blocker, to rat bronchial nmyocytes produced
a transient Ca2+ peak that returned to its basal value.
At this point, lanthanum, a PMCA blocker, induced a sustained
[Ca2+]i increment that promoted apoptosis
(108), demonstrating the
central functional role of the two pumps in Ca2+
handling in ASM. In this regard, it has been proposed that there is
a functional coupling between PMCA and SERCA to maintain
Ca2+ homeostasis (49). Under physiological conditions
(i.e., in the presence of extracellular Ca2+), we found
in guineapig tracheal myocytes that thapsigargin increased
[Ca2+]i until a plateau was reached (Fig. 3A). It is well known that, in ASM,
this Ca2+ increment is due to capacitative
Ca2+ entry (i.e., SOCE) predominantly via the TRPC3
channel, a process that also produces membrane depolarization due
to the entry of Na+ (79,111), consequently leading to L-VDCC
opening and further Ca2+ and Na+ entry
(10,18,21,36,79,112). At this stage, the NCX may change
to its reverse mode (i.e., NCXREV) due to the
Na+ entry, thereby becoming the main contributor towards
sustaining the Ca2+ plateau due to SERCA blockade. This
proposition was corroborated using an NCXREV-mode
blocker, KB-R7943, which brought [Ca2+]i to a
new basal Ca2+ steady state (Fig. 3A) that was maintained by the PMCA
activity. At this point, the addition of lanthanum, a non-specific
PMCA blocker, led to a marked increase in
[Ca2+]i, probably inducing cellular
apoptosis, as was suggested by a previous study (108). Taken together, these results
corroborated that, under physiological conditions, SERCA and PMCA
exert a primordial role in regulating [Ca2+]i
homeostasis, whereas NCXREV only participates when
b[Ca2+]i is modified and acquires a new
steady state (Fig. 3A and B).
Studies associated with the effects of
pro-inflammatory cytokines on the ASM SERCA have demonstrated that
over-night exposure of human airway myocytes to TNFα or IL-13
decreases the expression of SERCA that, in turn, diminishes the
reuptake of SR-Ca2+ (113). Notably, these authors also
revealed nthat, unlike other species, e.g., in porcine airways
(114), human ASM SERCA does not
express phospholamban, but is directly phosphorylated by
Ca2+/calmodulin-dependent protein kinase II (113). Thus, it is possible that in an
inflammatory process such as asthma, SR-ATPase activity is
decreased, which may lead to an increase in the
b[Ca2+]i to a new steady state,
favoring an augmented response to bronchoconstrictor agonists. The
same phenomenon may also be occurring as far as the PMCA is
concerned; however, further research is required in this field.
7. Ryanodine and IP3
receptors
RyR is a non-selective cation channel that releases
Ca2+ from the SR and, in mammals, its three isoforms,
RyR1, -2 and -3, are the products of different genes (115). All three isoforms are expressed
in smooth muscle, including ASM (115,116). Cyclic ADP-ribose (cADPR) is
considered to be their endogenous ligand in airway myocytes, which
is regulated by the membrane-bound protein, CD38 (117). This protein has ADP-ribosyl
cyclase and hydrolase activity, and is involved in the synthesis or
degradation of cADPR, respectively (118,119).
The IP3 receptor (ITPR) is another
non-selective cation channel that releases Ca2+ from the
SR via IP3 generated by the Gqα signaling
pathway (35). It has three
isoforms (ITPR1, -2 and -3) derived from different genes, which
share ~60-80% amino acid homology (120,121). These receptors have also been
identified in different smooth muscles types, including ASM
(36,122-124).
In 1993, Ca2+ ‘sparks’ were described in
heart muscle (125), and these
were associated with the Ca2+-induced Ca2+
release from RyRs (126). In
guineapig tracheal myocytes, the presence of spontaneous
Ca2+ sparks was observed for the first time in 1998
(127). Subsequently, in urinary
bladder smooth muscle, these Ca2+ sparks were
characterized as the elementary release of Ca2+ from
RyRs (128), and this finding
was later corroborated in mouse ASM, occurring predominantly
through RyR2 (116,129). In this context, studies on the
pulmonary artery revealed that Ca2+ sparks are activated
by Ca2+ released via ITPR (130), as well as in ASM (129). The physiological role of these
Ca2+ sparks in guineapig tracheal myocytes was well
established. Essentially, they produce spontaneous transient
outward currents caused by large-conductance
Ca2+-activated K+ channels; they also induce
spontaneous transient inward currents accomplished through
Ca2+-activated Cl-channels (127). Therefore, all these components
may serve an important role in the basal state regulation of the
ASM by stabilizing the membrane potential, the
b[Ca2+]i and the basal contractile
tone.
Interestingly, further lines of research have
demonstrated that pro-inflammatory cytokines (predominantly TNFα),
promote the augmentation of CD38-cADPR signaling and increase
Ca2+ responses to agonists (117,131), a phenomenon that is probably
mediated by an augmentation of
b[Ca2+]i. Furthermore, TNFα also
enhances Gqα protein expression, thereby increasing the
ASM response to carbachol (132). However, upregulation of the
IP3-Ca2+ signaling pathway and any consequent
modification of the b[Ca2+]i in an
inflammatory context, such as in asthma, has not readily been
identified, and this requires further research.
8. Conclusion
The current review has discussed how several
Ca2+ handling mechanisms are finely tuned to regulate
the b[Ca2+]i, summarized in
Fig. 4. It is conceivable that
alterations in any of these processes could render ASM susceptible
to developing the type of hyperresponsiveness that is commonly
observed in ailments such as asthma, and this warrants further
study.
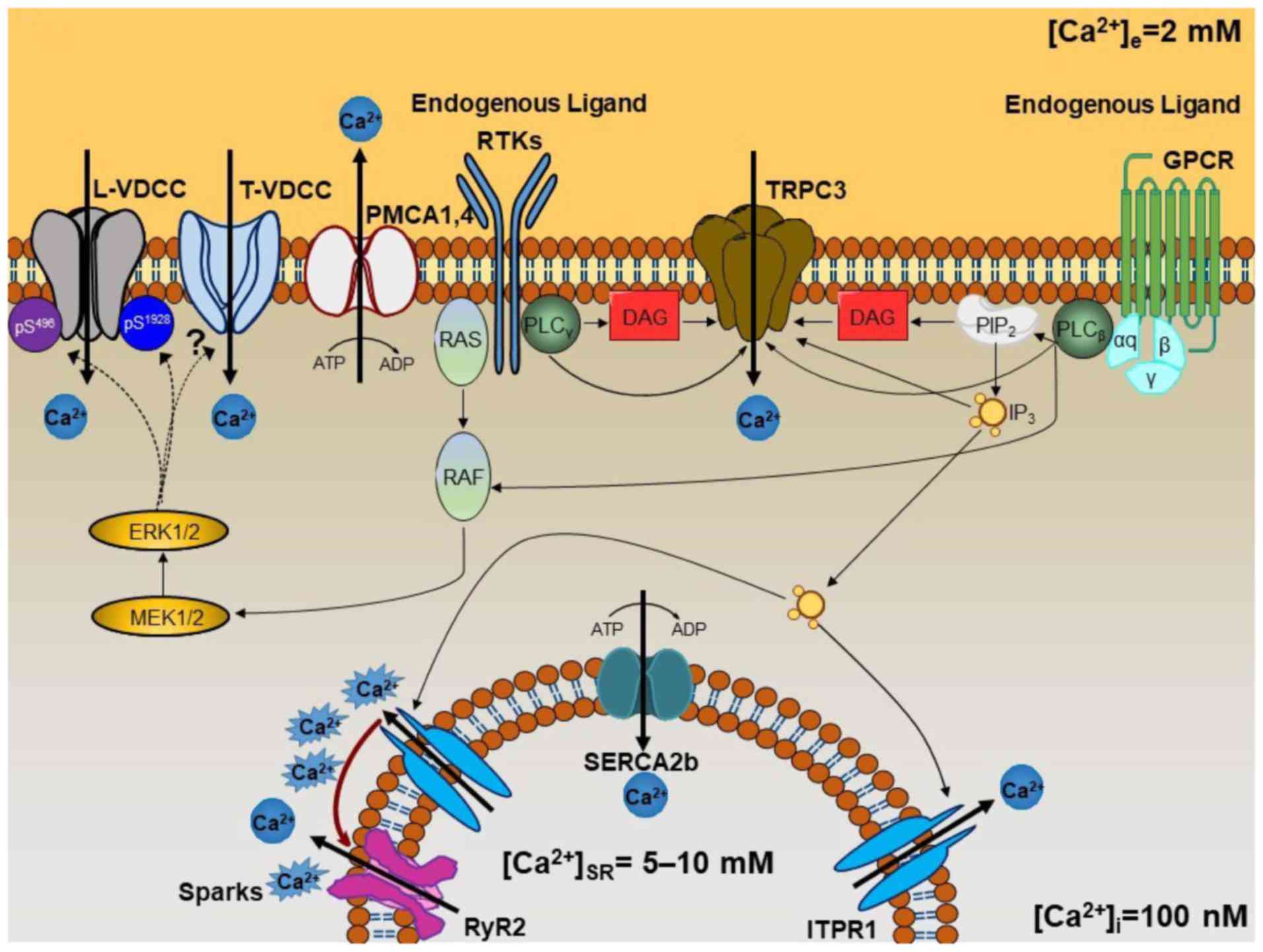 | Figure 4Schematic representation of the
mechanisms involved in the maintenance of
b[Ca2+]i. Membranal Ca2+ channels,
such as L-VDCC, T-VDCC and TRPC3, appear to be constitutively
active under basal conditions through different signaling pathways.
The two types of voltage-dependent Ca2+ channel may be
modulated by phosphorylation processes mediated by
mitogen-activated protein kinase ERK1/2 signaling. This signaling
pathway can be activated by GPCRs through the αq subunit
when the endogenous ligand is present under basal conditions (i.e.,
acetylcholine, histamine, leukotrienes, etc.). It may also be
stimulated when RTKs are occupied by the appropriate ligand
(cytokines, growth factors, etc.). ERK1/2 phosphorylates L-VDCC on
Ser496 of the β2 subunit and
Ser1928 of the α1 subunit, decreasing or
increasing the channel activity, respectively, enabling it to
switch between an open and closed state. T-VDCC is probably also
phosphorylated by ERK1/2, but further research is needed to
identify the phosphorylation sites (see Fig. 1D). TRPC3 is directly activated by
DAG and IP3 arising from PLCβ or
PLCγ, the first coupled to the αq subunit of
GPCR, and the second to RTKs. Constitutive IP3
production induces SR-Ca2+ release through ITPR1. This
Ca2+ induces Ca2+-induced Ca2+
release through the RyR2 (designated as Ca2+ ‘sparks’).
Finally, [Ca2+]i is efficiently regulated by
the SERCA2b and PMCA1 or PMCA4. L-VDCC, L-type voltage-dependent
channel; T-VDCC, T-type voltage dependent Ca2+ channel;
TRPC3, transient receptor potential canonical-3; ERK1/2,
extracellular-signal-regulated kinase 1/2; GPCR, G-protein-coupled
receptor; RTK, receptor tyrosine kinase; DAG, diacylglycerol;
IP3, inositol 1,4,5-trisphosphate; PLC, phospholipase C;
SR, sarcoplasmic reticulum; ITPR, IP3 receptor; RyR,
ryanodine receptor; SERCA, sarcoplasmic reticulum
Ca2+-ATPase; PMCA, plasmalemmal
Ca2+-ATPase. |
Funding
The present study was partly supported by grants
from Consejo Nacional de Ciencia y Tecnología, Ciudad de México,
México (grant no. 219859) and Dirección General de Asuntos del
Personal Académico (DGAPA), Universidad Nacional Autónoma de México
(grant no. IN201216) to LMM.
Availability of data and materials
The datasets presented in the current review are
available from the corresponding author on reasonable request.
Authors’ contributions
With particular regard to the previously
unpublished work presented herein, the contribution of each author
was as follows. JRG and ACG performed the assays of intracellular
Ca2+ levels. EFS performed enzymatic isolation of
tracheal myocytes, participated in the assays of intracellular
Ca2+ levels and data analysis, and provided critical
ideas during the writing of the manuscript. BS contributed to the
data analysis and writing of the manuscript. LMM contributed to the
design and global supervision of the study, data analysis and
writing of the manuscript, and was responsible for submitting the
paper for publication. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Acknowledgments
Not applicable.
References
|
1
|
Albert AP, Piper AS and Large WA:
Properties of a constitutively active Ca2+-permeable
non-selective cation channel in rabbit ear artery myocytes. J
Physiol. 549:143–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Demirel E, Laskey RE, Purkerson S and van
Breemen C: The passive calcium leak in cultured porcine aortic
endothelial cells. Biochem Biophys Res Commun. 191:1197–1203. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fayazi AH, Lapidot SA, Huang BK, Tucker RW
and Phair RD: Resolution of the basal plasma membrane calcium flux
in vascular smooth muscle cells. Am J Physiol. 270:H1972–H1978.
1996.PubMed/NCBI
|
|
4
|
Hodgkin AL and Keynes RD: Movements of
labelled calcium in squid giant axons. J Physiol. 138:253–281.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holland WC and Sekul A: Influence of
potassium and calcium ions on the effect of ouabain on
Ca45 entry and contracture in rabbit atria. J Pharmacol
Exp Ther. 133:288–294. 1961.PubMed/NCBI
|
|
6
|
Rutter GA, Hodson DJ, Chabosseau P,
Haythorne E, Pullen TJ and Leclerc I: Local and regional control of
calcium dynamics in the pancreatic islet. Diabetes Obes Metab.
19(Suppl 1): S30–S41. 2017. View Article : Google Scholar
|
|
7
|
Wu X, Weng L, Zhang J, Liu X and Huang J:
The plasma membrane calcium ATPases in calcium signaling network.
Curr Protein Pept Sci. 19:813–822. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bazan-Perkins B, Flores-Soto E,
Barajas-Lopez C and Montaño LM: Role of sarcoplasmic reticulum
Ca2+ content in Ca2+ entry of bovine airway
smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol.
368:277–283. 2003. View Article : Google Scholar
|
|
9
|
Carbajal V, Vargas MH, Flores-Soto E,
Martinez-Cordero E, Bazán-Perkins B and Montaño LM: LTD4
induces hyperresponsiveness to histamine in bovine airway smooth
muscle: Role of SR-ATPase Ca2+ pump and tyrosine kinase.
Am J Physiol Lung Cell Mol Physiol. 288:L84–L92. 2005. View Article : Google Scholar
|
|
10
|
Flores-Soto E, Reyes-Garcia J, Sommer B
and Montaño LM: Sarcoplasmic reticulum Ca2+ refilling is
determined by L-type Ca2+ and store operated
Ca2+ channels in guinea pig airway smooth muscle. Eur J
Pharmacol. 721:21–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Montaño LM and Bazán-Perkins B: Resting
calcium influx in airway smooth muscle. Can J Physiol Pharmacol.
83:717–723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Ma R and Gong J: Investigation of
testosterone-mediated non-transcriptional inhibition of
Ca2+ in vascular smooth muscle cells. Biomed Rep.
4:197–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Braunstein TH, Inoue R, Cribbs L, Oike M,
Ito Y, Holstein-Rathlou NH and Jensen LJ: The role of L- and T-type
calcium channels in local and remote calcium responses in rat
mesenteric terminal arterioles. J Vasc Res. 46:138–151. 2009.
View Article : Google Scholar
|
|
14
|
Wakle-Prabagaran M, Lorca RA, Ma X,
Stamnes SJ, Amazu C, Hsiao JJ, Karch CM, Hyrc KL, Wright ME and
England SK: BKCa channel regulates calcium oscillations induced by
alpha-2-macroglobulin in human myometrial smooth muscle cells. Proc
Natl Acad Sci USA. 113:E2335–E2344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aguilar HN and Mitchell BF: Physiological
pathways and molecular mechanisms regulating uterine contractility.
Hum Reprod Update. 16:725–744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asano M, Nomura Y, Hayakawa M, Ito KM,
Uyama Y, Imaizumi Y and Watanabe M: Increased Ca2+ influx in the
resting state maintains the myogenic tone and activates
charyb-dotoxin-sensitive K+ channels in femoral arteries from young
SHR. Clin Exp Pharmacol Physiol Suppl. 22(Suppl): S225–S227. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bae YM, Park MK, Lee SH, Ho WK and Earm
YE: Contribution of Ca2+-activated K+
channels and non-selective cation channels to membrane potential of
pulmonary arterial smooth muscle cells of the rabbit. J Physiol.
514:747–758. 1999. View Article : Google Scholar
|
|
18
|
Flores-Soto E, Reyes-García J,
Carbajal-García A, Campuzano-González E, Perusquía M, Sommer B and
Montaño LM: Sex steroids effects on guinea pig airway smooth muscle
tone and intracellular Ca2+ basal levels. Mol Cell
Endocrinol. 439:444–456. 2017. View Article : Google Scholar
|
|
19
|
Janssen LJ: T-type and L-type
Ca2+ currents in canine bronchial smooth muscle:
Characterization and physiological roles. Am J Physiol.
272:C1757–C1765. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montaño LM, Barajas-Lopez C and Daniel EE:
Canine bronchial sustained contraction in Ca2+-free
medium: Role of intracellular Ca2+. Can J Physiol
Pharmacol. 74:1236–1248. 1996. View Article : Google Scholar
|
|
21
|
Sommer B, Flores-Soto E, Reyes-García J,
Diaz-Hernández V, Carbajal V and Montaño LM: Na+
permeates through L-type Ca2+ channel in bovine airway
smooth muscle. Eur J Pharmacol. 782:77–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Worley JF III and Kotlikoff MI:
Dihydropyridine-sensitive single calcium channels in airway smooth
muscle cells. Am J Physiol. 259:L468–L480. 1990.PubMed/NCBI
|
|
23
|
Bolton TB: Mechanisms of action of
transmitters and other substances on smooth muscle. Physiol Rev.
59:606–718. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godin N and Rousseau E: TRPC6 silencing in
primary airway smooth muscle cells inhibits protein expression
without affecting OAG-induced calcium entry. Mol Cell Biochem.
296:193–201. 2007. View Article : Google Scholar
|
|
25
|
Hallam TJ and Rink TJ: Receptor-mediated
Ca2+ entry: Diversity of function and mechanism. Trends
Pharmacol Sci. 10:8–10. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinsen A, Dessy C and Morel N:
Regulation of calcium chan-nels in smooth muscle: New insights into
the role of myosin light chain kinase. Channels (Austin).
8:402–413. 2014. View Article : Google Scholar
|
|
27
|
McFadzean I and Gibson A: The developing
relationship between receptor-operated and store-operated calcium
channels in smooth muscle. Br J Pharmacol. 135:1–13. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murray RK and Kotlikoff MI:
Receptor-activated calcium influx in human airway smooth muscle
cells. J Physiol. 435:123–144. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ay B, Prakash YS, Pabelick CM and Sieck
GC: Store-operated Ca2+ entry in porcine airway smooth
muscle. Am J Physiol Lung Cell Mol Physiol. 286:L909–L917. 2004.
View Article : Google Scholar
|
|
30
|
Bazan-Perkins B, Carbajal V, Sommer B,
Macías-Silva M, González-Martínez M, Valenzuela F, Daniel EE and
Montaño LM: Involvement of different Ca2+ pools during
the canine bronchial sustained contraction in Ca2+-free
medium: Lack of effect of PKC inhibition. Naunyn Schmiedebergs Arch
Pharmacol. 358:567–573. 1998. View Article : Google Scholar
|
|
31
|
Putney JW Jr: A model for
receptor-regulated calcium entry. Cell Calcium. 7:1–12. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sweeney M, McDaniel SS, Platoshyn O, Zhang
S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA and Yuan JX: Role of
capacitative Ca2+ entry in bronchial contraction and
remodeling. J Appl Physiol 1985. 92:1594–1602. 2002. View Article : Google Scholar
|
|
33
|
Avila-Medina J, Mayoral-González I,
Domínguez-Rodriguez A, Gallardo-Castillo I, Ribas J, Ordoñez A,
Rosado JA and Smani T: The complex role of store operated calcium
entry pathways and related proteins in the function of cardiac,
skeletal and vascular smooth muscle cells. Front Physiol.
9:2572018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baron CB, Cunningham M, Strauss JF III and
Coburn RF: Pharmacomechanical coupling in smooth muscle may involve
phosphatidylinositol metabolism. Proc Natl Acad Sci USA.
81:6899–6903. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berridge MJ: Inositol trisphosphate and
calcium signalling. Nature. 361:315–325. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song T, Hao Q, Zheng YM, Liu QH and Wang
YX: Inositol 1,4,5-trisphosphate activates TRPC3 channels to cause
extracellular Ca2+ influx in airway smooth muscle cells.
Am J Physiol Lung Cell Mol Physiol. 309:L1455–L1466. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bazan-Perkins B, Sánchez-Guerrero E,
Carbajal V, Barajas-López C and Montaño LM: Sarcoplasmic reticulum
Ca2+ depletion by caffeine and changes of
[Ca2+]i during refilling in bovine airway
smooth muscle cells. Arch Med Res. 31:558–563. 2000. View Article : Google Scholar
|
|
38
|
Sieck GC, Kannan MS and Prakash YS:
Heterogeneity in dynamic regulation of intracellular calcium in
airway smooth muscle cells. Can J Physiol Pharmacol. 75:878–888.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuki K, Kato D, Takemoto M, Suzuki Y,
Yamamura H, Ohya S, Takeshima H and Imaizumi Y: Negative regulation
of cellular Ca2+ mobilization by ryanodine receptor type
3 in mouse mesenteric artery smooth muscle. Am J Physiol Cell
Physiol. 315:C1–C9. 2018. View Article : Google Scholar
|
|
40
|
Zhao C, Wu AY, Yu X, Gu Y, Lu Y, Song X,
An N and Shang Y: Microdomain elements of airway smooth muscle in
calcium regulation and cell proliferation. J Physiol Pharmacol.
69:2018.
|
|
41
|
Blaustein MP and Lederer WJ:
Sodium/calcium exchange: Its physiological implications. Physiol
Rev. 79:763–854. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eisner DA and Lederer WJ: Na-Ca exchange:
Stoichiometry and electrogenicity. Am J Physiol. 248:C189–C202.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Janssen LJ, Walters DK and Wattie J:
Regulation of [Ca2+]i in canine airway smooth muscle by
Ca2+-ATPase and Na+/Ca2+ exchange
mechanisms. Am J Physiol. 273:L322–L330. 1997.PubMed/NCBI
|
|
44
|
Lipskaia L, Bobe R, Chen J, Turnbull IC,
Lopez JJ, Merlet E, Jeong D, Karakikes I, Ross AS, Liang L, et al:
Synergistic role of protein phosphatase inhibitor 1 and
sarco/endoplasmic reticulum Ca2+-ATPase in the
acquisition of the contractile phenotype of arterial smooth muscle
cells. Circulation. 129:773–785. 2014. View Article : Google Scholar
|
|
45
|
Liu B, Zhang B, Huang S, Yang L, Roos CM,
Thompson MA, Prakash YS, Zang J, Miller JD and Guo R:
Ca2+ Entry through reverse mode
Na+/Ca2+ Exchanger contributes to store
operated channel-mediated neointima formation after arterial
injury. Can J Cardiol. 34:791–799. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mazur II, Veklich TO, Shkrabak OA, Mohart
NA, Demchenko AM, Gerashchenko IV, Rodik RV, Kalchenko VI and
Kosterin SO: Selective inhibition of smooth muscle plasma membrane
transport Ca2+, Mg2+-ATPase by calixarene
C-90 and its activation by IPT-35 compound. Gen Physiol Biophys.
37:223–231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nishiyama K, Azuma YT, Morioka A, Yoshida
N, Teramoto M, Tanioka K, Kita S, Hayashi S, Nakajima H, Iwamoto T
and Takeuchi T: Roles of Na+/Ca2+ exchanger
isoforms NCX1 and NCX2 in motility in mouse ileum. Naunyn
Schmiedebergs Arch Pharmacol. 389:1081–1090. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sommer B, Flores-Soto E and González-Avila
G: Cellular Na+ handling mechanisms involved in airway
smooth muscle contraction (Review). Int J Mol Med. 40:3–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang WB and Kwan CY: Pharmacological
evidence that potentiation of plasmalemmal
Ca2+-extrusion is functionally coupled to inhibition of
SR Ca2+-ATPases in vascular smooth muscle cells. Naunyn
Schmiedebergs Arch Pharmacol. 389:447–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Poburko D, Lhote P, Szado T, Behra T,
Rahimina R, McManus B, Van Breemen C and Ruegg UT: Basal calcium
entry in vascular smooth muscle. Eur J Pharmacol. 505:19–29. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bean BP: Classes of calcium channels in
vertebrate cells. Annu Rev Physiol. 51:367–384. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu J and Bose R: Calcium channels in
smooth muscle. Gastroenterology. 100:1448–1460. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Green KA, Small RC and Foster RW: The
properties of voltage-operated Ca2+-channels in bovine
isolated trachealis cells. Pulm Pharmacol. 6:49–62. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hisada T, Kurachi Y and Sugimoto T:
Properties of membrane currents in isolated smooth muscle cells
from guineapig trachea. Pflugers Arch. 416:151–161. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kotlikoff MI: Calcium currents in isolated
canine airway smooth muscle cells. Am J Physiol. 254:C793–C801.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Marthan R, Martin C, Amedee T and
Mironneau J: Calcium channel currents in isolated smooth muscle
cells from human bronchus. J Appl Physiol (1985). 66:1706–1714.
1989. View Article : Google Scholar
|
|
57
|
Hirota S and Janssen LJ: Store-refilling
involves both L-type calcium channels and reverse-mode
sodium-calcium exchange in airway smooth muscle. Eur Respir J.
30:269–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Catterall WA, Perez-Reyes E, Snutch TP and
Striessnig J: International union of pharmacology. XLVIII.
Nomenclature and structure-function relationships of voltage-gated
calcium channels. Pharmacol Rev. 57:411–425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Du W, McMahon TJ, Zhang ZS, Stiber JA,
Meissner G and Eu JP: Excitation-contraction coupling in airway
smooth muscle. J Biol Chem. 281:30143–30151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Reyes-Garcia J, Flores-Soto E,
Solis-Chagoyan H, Sommer B, Diaz-Hernandez V, Garcia-Hernandez LM
and Montaño LM: Tumor necrosis factor alpha inhibits L-type Ca2+
channels in sensitized guinea pig airway smooth muscle through ERK
1/2 pathway. Mediators Inflamm. 2016.5972302:2016.
|
|
61
|
Janssen LJ and Daniel EE: Depolarizing
agents induce oscillations in canine bronchial smooth muscle
membrane potential: Possible mechanisms. J Pharmacol Exp Ther.
259:110–117. 1991.PubMed/NCBI
|
|
62
|
Xu KY, Zhu W and Xiao RP:
Serine496 of β2 subunit of L-type
Ca2+ channel participates in molecular crosstalk between
activation of (Na++K+)-ATPase and the
channel. Biochem Biophys Res Commun. 402:319–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang Y, Sun J, Jin R, Liang Y, Liu YY and
Xu YD: Influence of acupuncture on expression of T-type calcium
channel protein in airway smooth muscle cell in airway remodeling
rats with asthma. Zhongguo Zhen Jiu. 32:534–540. 2012.In Chinese.
PubMed/NCBI
|
|
64
|
Blesneac I, Chemin J, Bidaud I, Huc-Brandt
S, Vandermoere F and Lory P: Phosphorylation of the Cav3.2 T-type
calcium channel directly regulates its gating properties. Proc Natl
Acad Sci USA. 112:13705–13710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wylam ME, Gungor N, Mitchell RW and Umans
JG: Eosinophils, major basic protein, and polycationic peptides
augment bovine airway myocyte Ca2+ mobilization. Am J
Physiol. 274:L997–L1005. 1998.
|
|
66
|
Yocum GT, Chen J, Choi CH, Townsend EA,
Zhang Y, Xu D, Fu XW, Sanderson MJ and Emala CW: Role of transient
receptor potential vanilloid 1 in the modulation of airway smooth
muscle tone and calcium handling. Am J Physiol Lung Cell Mol
Physiol. 312:L812–L821. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dietrich A, Chubanov V, Kalwa H, Rost BR
and Gudermann T: Cation channels of the transient receptor
potential superfamily: Their role in physiological and
pathophysiological processes of smooth muscle cells. Pharmacol
Ther. 112:744–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ong HL, Brereton HM, Harland ML and
Barritt GJ: Evidence for the expression of transient receptor
potential proteins in guinea pig airway smooth muscle cells.
Respirology. 8:23–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hofmann T, Obukhov AG, Schaefer M,
Harteneck C, Gudermann T and Schultz G: Direct activation of human
TRPC6 and TRPC3 channels by diacylglycerol. Nature. 397:259–263.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Storch U, Forst AL, Pardatscher F,
Erdogmus S, Philipp M and Gregoritza M: Dynamic NHERF interaction
with TRPC4/5 proteins is required for channel gating by
diacylglycerol. Proc Natl Acad Sci USA. 114:E37–E46. 2017.
View Article : Google Scholar
|
|
71
|
Li SW, Westwick J and Poll CT:
Receptor-operated Ca2+ influx channels in leukocytes: A
therapeutic target. Trends Pharmacol Sci. 23:63–70. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zitt C, Zobel A, Obukhov AG, Harteneck C,
Kalkbrenner F, Luckhpoff A and Schultz G: Cloning and functional
expression of a human Ca2+-permeable cation channel
activated by calcium store depletion. Neuron. 16:1189–1196. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu SZ and Beech DJ: TrpC1 is a
membrane-spanning subunit of store-operated Ca2+
channels in native vascular smooth muscle cells. Circ Res.
88:84–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wu X, Babnigg G and Villereal ML:
Functional significance of human trp1 and trp3 in store-operated
Ca2+ entry in HEK-293 cells. Am J Physiol Cell Physiol.
278:C526–C536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gailly P and Colson-Van Schoor M:
Involvement of trp-2 protein in store-operated influx of calcium in
fibroblasts. Cell Calcium. 30:157–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Okada T, Inoue R, Yamazaki K, Maeda A,
Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, et
al: Molecular and functional characterization of a novel mouse
transient receptor potential protein homologue TRP7.
Ca2+-permeable cation channel that is constitutively
activated and enhanced by stimulation of G protein-coupled
receptor. J Biol Chem. 274:27359–27370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Vandebrouck C, Martin D, Colson-Van Schoor
M, Debaix H and Gailly P: Involvement of TRPC in the abnormal
calcium influx observed in dystrophic (mdx) mouse skeletal muscle
fibers. J Cell Biol. 158:1089–1096. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Albert AP, Pucovsky V, Prestwich SA and
Large WA: TRPC3 properties of a native constitutively active
Ca2+-permeable cation channel in rabbit ear artery myocytes. J
Physiol. 571:361–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xiao JH, Zheng YM, Liao B and Wang YX:
Functional role of canonical transient receptor potential 1 and
canonical transient receptor potential 3 in normal and asthmatic
airway smooth muscle cells. Am J Respir Cell Mol Biol. 43:17–25.
2010. View Article : Google Scholar :
|
|
80
|
Trebak M, Bird GS, McKay RR and Putney JW
Jr: Comparison of human TRPC3 channels in receptor-activated and
store-operated modes. Differential sensitivity to channel blockers
suggests fundamental differences in channel composition. J Biol
Chem. 277:21617–21623. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kiyonaka S, Kato K, Nishida M, Mio K,
Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, et
al: Selective and direct inhibition of TRPC3 channels underlies
biological activities of a pyrazole compound. Proc Natl Acad Sci
USA. 106:5400–5405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Albert AP, Piper AS and Large WA: Role of
phospholipase D and diacylglycerol in activating constitutive
TRPC-like cation channels in rabbit ear artery myocytes. J Physiol.
566:769–780. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Mamoon AM, Smith J, Baker RC and Farley
JM: Activation of protein kinase A increases phospholipase D
activity and inhibits phospholipase D activation by acetylcholine
in tracheal smooth muscle. J Pharmacol Exp Ther. 291:1188–1195.
1999.PubMed/NCBI
|
|
84
|
Monick MM, Carter AB, Gudmundsson G,
Mallampalli R, Powers LS and Hunninghake GW: A
phosphatidylcholine-specific phospholipase C regulates activation
of p42/44 mitogen-activated protein kinases in
lipopolysaccharide-stimulated human alveolar macrophages. J
Immunol. 162:3005–3012. 1999.PubMed/NCBI
|
|
85
|
Ito S, Kume H, Naruse K, Kondo M, Takeda
N, Iwata S, Hasegawa Y and Sokabe M: A novel Ca2+ influx
pathway activated by mechanical stretch in human airway smooth
muscle cells. Am J Respir Cell Mol Biol. 38:407–413. 2008.
View Article : Google Scholar
|
|
86
|
Leung FP, Yung LM, Yao X, Laher I and
Huang Y: Store-operated calcium entry in vascular smooth muscle. Br
J Pharmacol. 153:846–857. 2008. View Article : Google Scholar
|
|
87
|
Prakriya M, Feske S, Gwack Y, Srikanth S,
Rao A and Hogan PG: Orai1 is an essential pore subunit of the CRAC
channel. Nature. 443:230–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Roos J, DiGregorio PJ, Yeromin AV, Ohlsen
K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD,
et al: STIM1, an essential and conserved component of
store-operated Ca2+ channel function. J Cell Biol.
169:435–445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Peel SE, Liu B and Hall IP: ORAI and
store-operated calcium influx in human airway smooth muscle cells.
Am J Respir Cell Mol Biol. 38:744–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Potier M, Gonzalez JC, Motiani RK,
Abdullaev IF, Bisaillon JM, Singer HA and Treback M: Evidence for
STIM1- and Orai1-dependent store-operated calcium influx through
ICRAC in vascular smooth muscle cells: Role in proliferation and
migration. FASEB J. 23:2425–2437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shuttleworth TJ: Orai3-the ‘exceptional’
Orai. J Physiol. 590:241–257. 2012. View Article : Google Scholar
|
|
92
|
Liou J, Kim ML, Heo WD, Jones JT, Myers
JW, Ferrel JE Jr and Meyer T: STIM is a Ca2+ sensor
essential for Ca2+-store-depletion-triggered
Ca2+ influx. Curr Biol. 15:1235–1241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Prakriya M and Lewis RS: Store-operated
calcium channels. Physiol Rev. 95:1383–1436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Peel SE, Liu B and Hall IP: A key role for
STIM1 in store operated calcium channel activation in airway smooth
muscle. Respir Res. 7:1192006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck
TJ, Ellisman MH, Stauderman KA and Cahalan MD: STIM1 is a
Ca2+ sensor that activates CRAC channels and migrates
from the Ca2+ store to the plasma membrane. Nature.
437:902–905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liao Y, Erxleben C, Yildirim E, Abramowitz
J, Armstrong DL and Birnbaumer L: Orai proteins interact with TRPC
channels and confer responsiveness to store depletion. Proc Natl
Acad Sci USA. 104:4682–4687. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Dai JM, Kuo KH, Leo JM, van Breemen C and
Lee CH: Mechanism of ACh-induced asynchronous calcium waves and
tonic contraction in porcine tracheal muscle bundle. Am J Physiol
Lung Cell Mol Physiol. 290:L459–L469. 2006. View Article : Google Scholar
|
|
98
|
DiPolo R and Beaugé L: Sodium/calcium
exchanger: Influence of metabolic regulation on ion carrier
interactions. Physiol Rev. 86:155–203. 2006. View Article : Google Scholar
|
|
99
|
Philipson KD and Nicoll DA: Sodium-calcium
exchange: A molecular perspective. Annu Rev Physiol. 62:111–133.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lytton J: Na+/Ca2+
exchangers: Three mammalian gene families control Ca2+
transport. Biochem J. 406:365–382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Khananshvili D: The SLC8 gene family of
sodium-calcium exchangers (NCX)-structure, function, and regulation
in health and disease. Mol Aspects Med. 34:220–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
A lga ra-Sua rez P, Mejia-Elizondo R, Sims
SM, Saavedra-Alanis VM and Espinosa-Tanguma R: The 1.3 isoform of
Na+-Ca2+ exchanger expressed in guinea pig
tracheal smooth muscle is less sensitive to KB-R7943. J Physiol
Biochem. 66:117–125. 2010. View Article : Google Scholar
|
|
103
|
Rahman M, Inman M, Kiss L and Janssen LJ:
Reverse-mode NCX current in mouse airway smooth muscle:
Na+ and voltage dependence, contributions to
Ca2+ influx and contraction, and altered expression in a
model of allergen-induced hyperresponsiveness. Acta Physiol (Oxf).
205:279–291. 2012. View Article : Google Scholar
|
|
104
|
Sathish V, Delmotte PF, Thompson MA,
Pabelick CM, Sieck GC and Prakash YS: Sodium-calcium exchange in
intracellular calcium handling of human airway smooth muscle. PLoS
One. 6:e236622011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Brini M and Carafoli E: Calcium pumps in
health and disease. Physiol Rev. 89:1341–1378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Carafoli E: Calcium pump of the plasma
membrane. Physiol Rev. 71:129–153. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Darby PJ, Kwan CY and Daniel EE: Caveolae
from canine airway smooth muscle contain the necessary components
for a role in Ca2+ handling. Am J Physiol Lung Cell Mol
Physiol. 279:L1226–L1235. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen YF, Cao J, Zhong JN, Chen X, Cheng M,
Yang J and Gao YD: Plasma membrane Ca2+-ATPase regulates
Ca2+ signaling and the proliferation of airway smooth
muscle cells. Eur J Pharmacol. 740:733–741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bobe R, Bredoux R, Corvazier E, Andersen
JP, Clausen JD, Dode L, Kovács T and Enouf J: Identification,
expression, function, and localization of a novel (sixth) isoform
of the human sarco/endoplasmic reticulum Ca2+ATPase 3
gene. J Biol Chem. 279:24297–24306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Mahn K, Hirst SJ, Ying S, Holt MR,
Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, et
al: Diminished sarco/endoplasmic reticulum Ca2+ ATPase
(SERCA) expression contributes to airway remodelling in bronchial
asthma. Proc Natl Acad Sci USA. 106:10775–10780. 2009. View Article : Google Scholar
|
|
111
|
Helli PB and Janssen LJ: Properties of a
store-operated nonse-lective cation channel in airway smooth
muscle. Eur Respir J. 32:1529–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Perusquia M, Flores-Soto E, Sommer B,
Campuzano-González E, Martinez-Villa I, Martinez-Banderas AI and
Montaño LM: Testosterone-induced relaxation involves L-type and
store-operated Ca2+ channels blockade, and
PGE2 in guinea pig airway smooth muscle. Pflugers Arch.
467:767–777. 2015. View Article : Google Scholar
|
|
113
|
Sathish V, Thompson MA, Bailey JP,
Pabelick CM, Prakash YS and Sieck GC: Effect of proinflammatory
cytokines on regulation of sarcoplasmic reticulum Ca2+
reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol
Physiol. 297:L26–L34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sathish V, Leblebici F, Kip SN, Thompson
A, Pabelick CM, Prakash YS and Sieck GC: Regulation of sarcoplasmic
reticulum Ca2+ reuptake in porcine airway smooth muscle.
Am J Physiol Lung Cell Mol Physiol. 294:L787–L796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Guerrero-Hernandez A, Ávila G and Rueda A:
Ryanodine receptors as leak channels. Eur J Pharmacol. 739:26–38.
2014. View Article : Google Scholar
|
|
116
|
Liu QH, Zheng YM, Korde AS, Yadav VR,
Rathore R, Wess J and Wang YX: Membrane depolarization causes a
direct activation of G protein-coupled receptors leading to local
Ca2+ release in smooth muscle. Proc Natl Acad Sci USA.
106:11418–11423. 2009. View Article : Google Scholar
|
|
117
|
Deshpande DA, Walseth TF, Panettieri RA
and Kannan MS: CD38/cyclic ADP-ribose-mediated Ca2+
signaling contributes to airway smooth muscle hyper-responsiveness.
FASEB J. 17:452–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Rusinko N and Lee HC: Widespread
occurrence in animal tissues of an enzyme catalyzing the conversion
of NAD+ into a cyclic metabolite with intracellular
Ca2+-mobilizing activity. J Biol Chem. 264:11725–11731.
1989.PubMed/NCBI
|
|
119
|
White TA, Johnson S, Walseth TF, Lee HC,
Graeff RM, Munshi CB, Prakash YS, Sieck GC and Kannan MS:
Subcellular localization of cyclic ADP-ribosyl cyclase and cyclic
ADP-ribose hydrolase activities in porcine airway smooth muscle.
Biochim Biophys Acta. 1498:64–71. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Ross CA, Danoff SK, Schell MJ, Snyder SH
and Ullrich A: Three additional inositol 1,4,5-trisphosphate
receptors: Molecular cloning and differential localization in brain
and peripheral tissues. Proc Natl Acad Sci USA. 89:4265–4269. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Taylor CW, Genazzani AA and Morris SA:
Expression of inositol trisphosphate receptors. Cell Calcium.
26:237–251. 1999. View Article : Google Scholar
|
|
122
|
Narayanan D, Adebiyi A and Jaggar JH:
Inositol trisphosphate receptors in smooth muscle cells. Am J
Physiol Heart Circ Physiol. 302:H2190–H2210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wang YX, Zheng YM, Mei QB, Wang QS,
Collier ML, Fleischer S, Xin HB and Kotlikoff MI: FKBP12.6 and
cADPR regulation of Ca2+ release in smooth muscle cells.
Am J Physiol Cell Physiol. 286:C538–C546. 2004. View Article : Google Scholar
|
|
124
|
Montaño LM, Flores-Soto E, Reyes-Garcia J,
Diaz Hernández V, Carbajal-Garcia A, Campuzáno González E,
Ramirez-Salinas GL, Velasco-Velázquez M and Sommer B: Testosterone
induces hyporesponsiveness by interfering with IP3
receptors in guinea pig airway smooth muscle. Mol Cell Endocrinol.
473:17–30. 2018. View Article : Google Scholar
|
|
125
|
Cheng H, Lederer WJ and Cannell MB:
Calcium sparks: Elementary events underlying excitation-contraction
coupling in heart muscle. Science. 262:740–744. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Fabiato A: Calcium-induced release of
calcium from the cardiac sarcoplasmic reticulum. Am J Physiol.
245:C1–C14. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
ZhuGe R, Sims SM, Tuft RA, Fogarty KE and
Walsh JV Jr: Ca2+ sparks activate K+ and
Cl- channels, resulting in spontaneous transient
currents in guineapig tracheal myocytes. J Physiol. 513:711–718.
1998. View Article : Google Scholar
|
|
128
|
Collier ML, Ji G, Wang Y and Kotlikoff MI:
Calcium-induced calcium release in smooth muscle: Loose coupling
between the action potential and calcium release. J Gen Physiol.
115:653–662. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu QH, Zheng YM and Wang YX: Two distinct
signaling pathways for regulation of spontaneous local
Ca2+ release by phospholipase C in airway smooth muscle
cells. Pflugers Arch. 453:531–541. 2007. View Article : Google Scholar
|
|
130
|
Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li
WH and Sham JS: ET-1 activates Ca2+ sparks in PASMC:
Local Ca2+ signaling between inositol trisphosphate and
ryanodine receptors. Am J Physiol Lung Cell Mol Physiol.
285:L680–L690. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Jude JA, Solway J, Panettieri RA Jr,
Walseth TF and Kannan MS: Differential induction of CD38 expression
by TNF-α in asthmatic airway smooth muscle cells. Am J Physiol Lung
Cell Mol Physiol. 299:L879–L890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hotta K, Emala CW and Hirshman CA: TNF-α
upregulates Giα and Gqα protein expression and function in human
airway smooth muscle cells. Am J Physiol. 276:L405–L411.
1999.PubMed/NCBI
|


















