Introduction
Chronic constipation, a gastrointestinal disorder,
presents symptoms such as hard stools, infrequent bowel movements,
incomplete bowel evacuation, difficulty during defecation, and the
need for excessive straining (1-3).
Often, this disorder is the result of insufficient dietary fiber
and fluid intakes, decreased physical activity, colorectal cancer
obstruction, hypothyroidism, and administration of certain drugs
(4). Medical treatments for
chronic constipation include the provision of bulk laxatives,
osmotic agents, stimulant laxatives, lubricating agents, and
neuromuscular agents (5,6). Commonly used stimulant laxatives
include senna, bisacodyl and docusate; however, their use may be
limited due to high costs and undesirable side effects including
hypernatremia, hypokalemia and protein-losing enteropathy (7). Stimulant laxatives enhance the
motility and secretion of the intestine through regulation of
electrolyte transport by the intestinal mucosa, which occurs when
smooth muscle, epithelial or nerve cells receive stimulation from
such substances (8). Bisacodyl, a
diphenylmethane derivative, has long been utilized as a first-line
stimulant laxative worldwide. Bisacodyl is a prokinetic with
hydrogogue effects that directly enhances motility, thereby
enhancing the water content and decreasing transit time of stool in
the large bowel (9,10).
Several plant extracts exhibit laxative activities
via their ability to enhance intestinal motility, ileum tension,
and the frequency and weight of stools. Leaf extracts of Aloe
ferox Mill., agarwood (Aquilaria sinensis, A.
crasna) and common fig (Ficus carica) paste are reported
to significantly increase total stool weight and intestinal
motility, and to normalize body weight in constipation rats treated
with loperamide (Lop) (11-13). In addition, an aqueous extract of
Liriope platyphylla (AEtLP) roots has been demonstrated to
increase the frequency and weight of stools, villus length, crypt
layer thickness, muscle thickness, mucin secretion, and
accumulation of lipid droplets in crypt enterocytes (14). In that previous study, marked
reductions in the levels of key factors in the muscarinic
acetylcholine receptor (mAChR) signaling pathway were observed in
the Lop+AEtLP-treated Sprague Dawley (SD) rats (14). Based on these findings, the
present study investigated whether red L. platyphylla
extract (EtRLP) has beneficial effects on factors associated
with laxative effects in experimental constipation SD rats. The
EtRLP used in the present study was produced by performing nine
repetitions of a two-step process (steaming of L.
platyphylla roots at 99°C for 3 h and air-drying at 70°C for 24
h). EtRLP has been considered a candidate for use in the
improvement of constipation because it has been demonstrated to be
useful in the treatment of various chronic conditions, including
diabetes, obesity, and neurodegenerative disease (1-4).
Additionally, although EtRLP is composed of crude protein,
carbohydrates, crude ash, crude fat, and moisture, it has been
reported to contain high concentration of total phenolic compounds,
total flavonoids, and 5-hydroxmethyl-2-furfural (15,16). However, there are no reports on
whether EtRLP has a therapeutic effect on constipation or on its
mechanism of action.
In the present study, the laxative effects and
molecular mechanism of EtRLP were investigated in constipation SD
rats treated with Lop. The results provide evidence that EtRLP may
be as effective as a commercial product containing bisacodyl,
sennoside calcium, and docusate sodium at alleviating constipation.
Additionally, the present study is the first to suggest that
EtRLP’s laxative effect may be correlated with control of the mAChR
signaling pathway and the endoplasmic reticulum (ER) stress
response. Finally, the role of spicatoside A, a key component in
EtRLP, as a laxative compound was elucidated.
Materials and methods
Preparation and analysis of EtRLP
Fresh roots of L. platyphylla were kindly
provided from the Miryang National Agricultural Cooperation
Federation at Miryang, Korea, and then dried in a heating dryer
(Ilshinbiobase, Seoul, Korea) at 60°C after washing them clean.
Voucher specimens of L. platy- phylla roots (WPC-11-010)
were deposited in the Functional Materials Bank of the Wellbeing
RIS Center (FMB-WRIS Center) at Pusan National University. The root
samples were confirmed to be L. platyphylla by Dr. Shin Woo
Cha, Division of Herbal Crop Research, National Institute of
Horticultural and Herbal Science (Eumseong, Korea).
EtRLP was prepared according to the method described
previously (15). Briefly, a
two-step process (steaming 200 g of dry root samples at 99°C for 3
h after air-drying at 70°C for 24 h) was repeated nine times to
prepare Red L. platyphylla (RLP) (Fig. 1A). Voucher specimens of RLP sample
(WPC-11-015) were deposited in the FMB-WRIS Center at Pusan
National University. Subsequently, the roots of RLP were completely
reduced to powder by using an electric blender (Fig. 1B). A mixture of 200 g RLP root
powder and 200 ml of distilled water was purified for 2 h at 100°C
by using circulating extractor (IKA Labortechnik, Staufen,
Germany). The supernatant of RLP was then concentrated in a
vertical tube of rotary evaporator (EYELA, Tokyo, Japan) to obtain
dry pellets of EtRLP, which were kept at −80°C until needed.
The composition and concentration of active
compounds in the obtained EtRLP were analyzed by using methods
described previously (15). The
EtRLP was mainly comprised of large carbohydrates (83.22%) and
small moisture (8.24%) with lesser amounts of proteins, fat, and
ash. The total phenol and total flavonoid concentrations increased
with an increasing number of steaming/drying episodes, but the
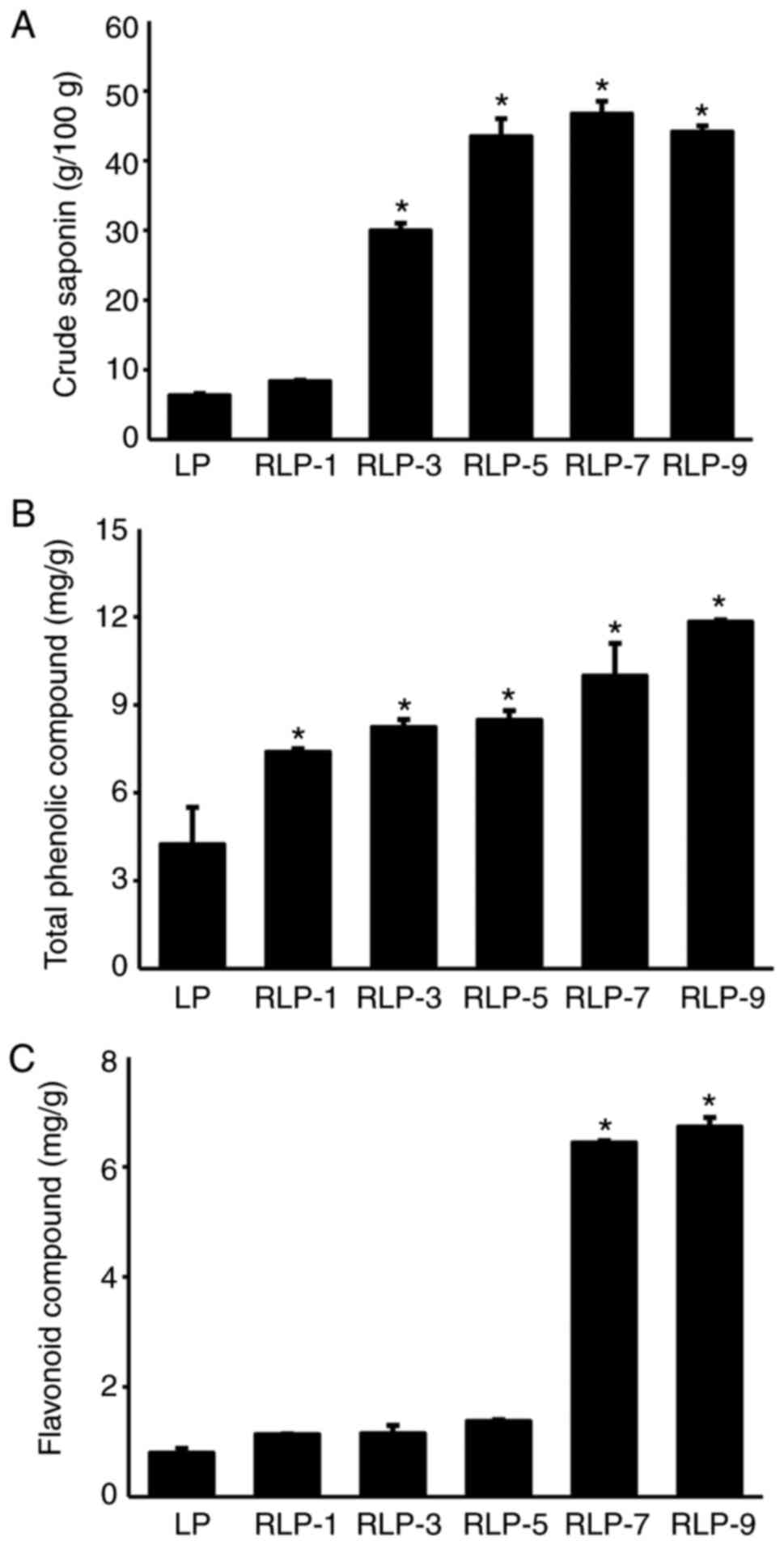
level of crude saponins was saturated after five repetitions
(Fig. 2). High-performance liquid
chromatography (HPLC) results revealed that the levels of
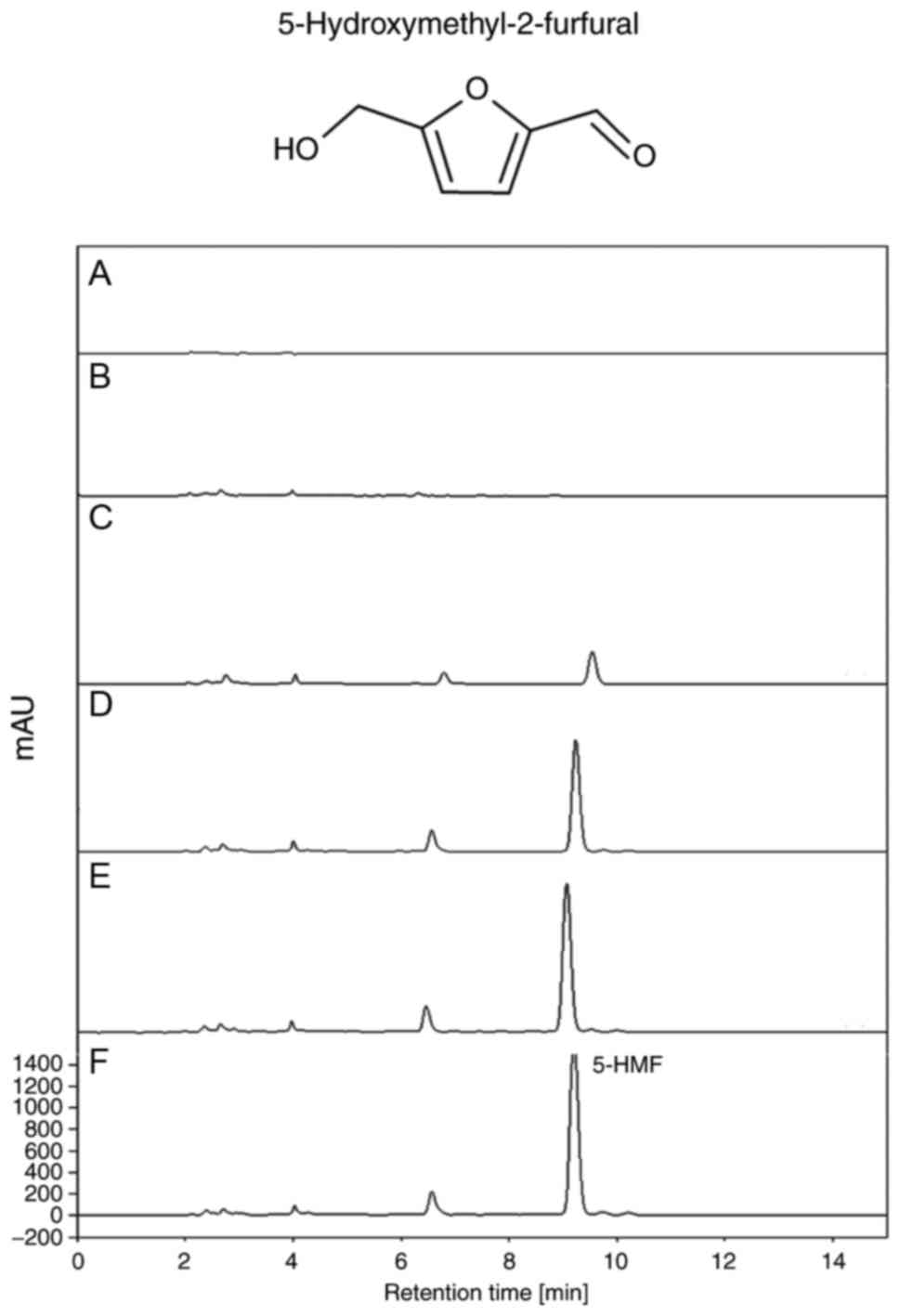
5-hydroxymethyl-2-furfural were markedly increased with the
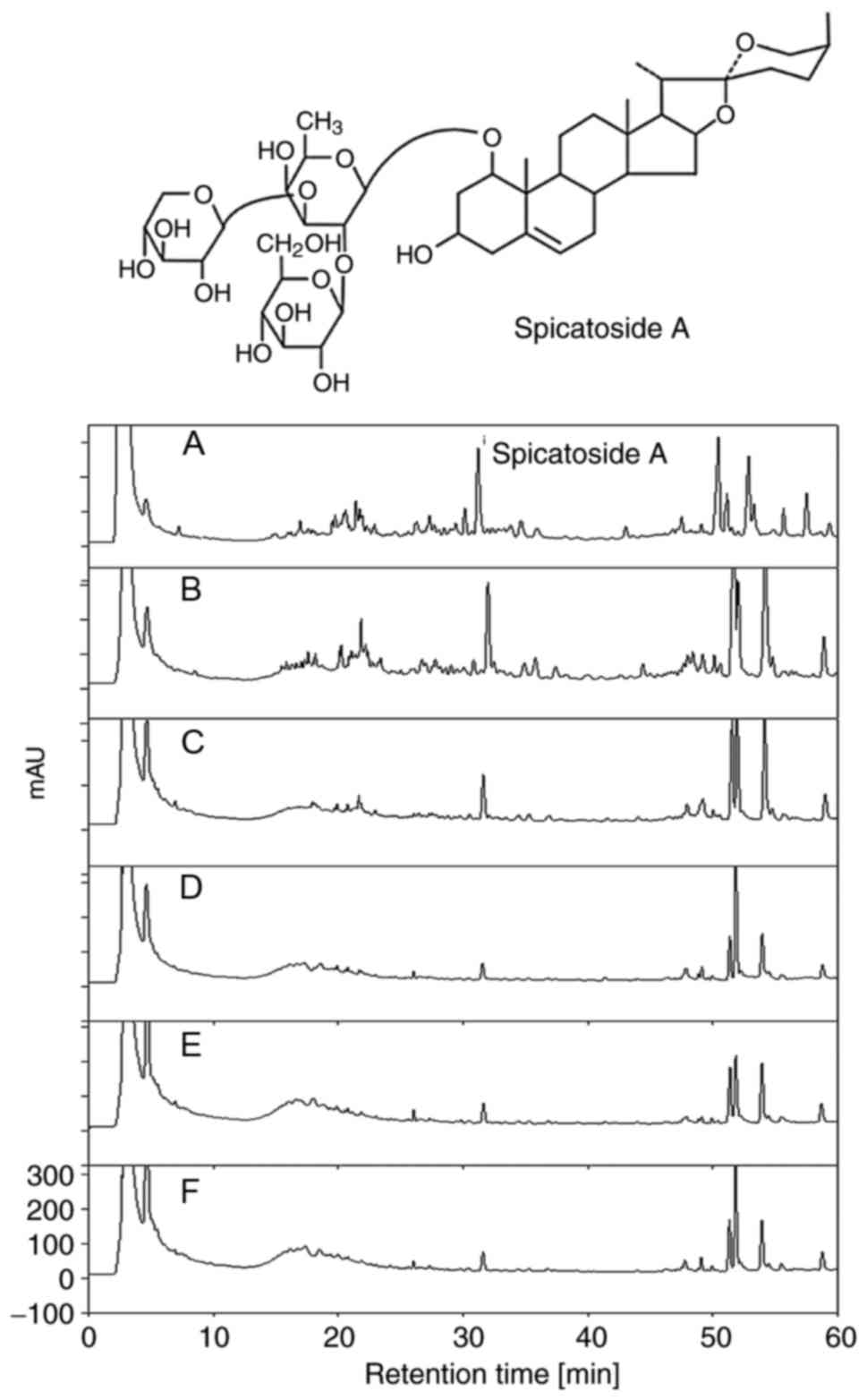
increase in number of steaming/drying repetitions (Fig. 3); however, a reverse pattern was
detected in the level of spicatoside A (Fig. 4).
Animal experiments
The experimental protocols involving the use of
animals was rfeviewed and approved based on the ethical and
scientific animal care procedures set by the Institutional Animal
Care and Use Committee of Pusan National University (approval no.
PNU-2012-0010). A total of 30 adult 8-week-old male SD rats (n=30)
were purchased from Samtako BioKorea Com., (Osan, Korea) and
handled in the Pusan National University, Laboratory Animal
Resources Center, which is accredited by the Korea Food and Drug
Administration (accredited unit no. 000231) and The Association for
Assessment and Accreditation of Laboratory Animal Care
International (accredited unit no. 001525). Rats were maintained in
a pathogen-free state under a 12-h light/dark cycle (lights on
between 06:00 h and 18:00 h) at a room temperature of 23±2°C and a
relative humidity of 50±10%. Standard irradiated chow (Purina
Mills, Seongnam, Korea) was provided ad libitum.
Constipation of SD rats was induced by subcutaneous
injection of Lop (4 mg/kg body weight) in 0.9% NaCl twice a day for
3 days as described in previous studies (11), while the non-constipation rats
received 0.9% NaCl alone. For the treatment experiments, 8-week-old
SD rats (n=30) were divided into either non-constipation (n=14) or
constipation (n=21) groups. The rats of non-constipation group were
subdivided further into Non-treated (n=7) and EtRLP-treated (n=7)
groups. The Non-treated group members were untreated throughout the
experimental period, while the EtRLP-treated group members received
a single treatment of 1,000 mg/kg body weight of EtRLP. SD rats of
the Lop-induced constipation group were subdivided further into
Lop+Vehicle-treated (n=7), Lop+EtRLP-treated (n=7), and
Lop+BisaC-treated (n=7) groups. Following constipation induction,
the Lop+Vehicle-treated group members received an equal amount of
water via oral administration, whereas the other cotreatment group
members received a single treatment of 1,000 mg/kg body weight of
EtRLP or 3.3 mg/kg body weight of Bisacodyl (BisaC). Primarily
comprised of BisaC, senno-side calcium, and docusate sodium, the
BisaC was purchased from Kolon Pharmaceuticals (Gyenggido, Korea).
At 24 h following EtRLP treatment, all SD rats were euthanized by
using compressed carbon dioxide (CO2) gas. Tissue
samples (transverse colon) were collected from animals and kept in
Eppendorf tubes at −80°C until use.
Measurement of excretion parameters
SD rats were maintained in metabolic cages during
the experimental procedure to avoid excretion sample
cross-contamination. Total excreted stools and urine were collected
at 10:00 am every day following Lop or EtRLP treatment. Stool
weight and number were determined three times by using a chemical
balance and a hand counter. Stool water content was defined as the
weight of water in stools and calculated dry weight from wet weight
as described in previous studies (11,14). Changes in urine volume were
measured three times by using a volumetric cylinder.
Western blot analysis
Total tissue proteins were collected from the
transverse colons of 5-6 rats from each of the five treatment
groups (Non-, EtRLP-, Lop+Vehicle-, Lop+EtRLP- and
Lop+BisaC-treated groups) and primary rat intestine smooth muscle
cells (pRISMCs) using Pro-Prep Protein Extraction Solution (Intron
Biotechnology Inc., Seongnam, Korea). The protein concentration of
tissue lysates was calculated by bicin-choninic acid assay (cat.
no. 23225; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer’s instructions. Following the
separation of total proteins (30 μg) by 4-20% SDS-PAGE,
these proteins were then transferred to nitrocellulose membranes
for 2-3 h at 40 V. Subsequently, the membranes were incubated with
primary antibodies targeting mAChR M2 (cat. no. AMR-002; 1:1,000;
Alomone Labs, Jerusalem, Israel), mAChR M3 (1:1,000, cat. no.
AMR-006; Alomone Labs), phosphoinositide 3-kinase (PI3K, 1:1,000,
cat. no. 4292S; Cell Signaling Technology, Inc., Danvers, MA, USA),
phosphorylated (p-) PI3K (1:1,000, cat. no. 4228S; Cell Signaling
Technology, Inc.), protein kinase C (PKC, 1:1,000, cat. no. 2058S;
Cell Signaling Technology, Inc.), p-PKC (1:1,000, cat. no. 9376S;
Cell Signaling Technology, Inc.), inositol-requiring enzyme (IRE)
1α (1:1,000, cat. no. ab37073; Abcam, Cambridge, UK), IRE1β
(1:1,000, cat. no. SC-10511; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), p-IRE1 (1:1,000, cat. no. 48187; Abcam), c-Jun
N-terminal kinase (JNK, 1:1,000, cat. no. 9252; Cell Signaling
Technology, Inc.), p-JNK (1:1,000, cat. no. 9251; Cell Signaling
Technology, Inc.), guanine nucleotide binding protein (G) α
(1:1,000, cat. no. ab128900; Abcam), eukaryotic initiation factor
(eIF) 2α (1:1,000, cat. no. 9722; Cell Signaling Technology, Inc.),
p-eIF2α (1:1,000, cat. no. 9721; Cell Signaling Technology, Inc.)
or β-actin (1:3,000, cat. no. A5316; Sigma-Aldrich Co.; Merck KGaA,
Darmstadt, Germany) overnight at 4°C. The membranes were then
treated with washing buffer solution (137 mM NaCl, 10 mM
Na2HPO4, 2 mM KH2PO4,
2.7 mM KCl, and 0.05% Tween 20) and incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin (Ig) G
antibody (1:1,000, cat. no. G21234; Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 2 h. Finally, proteins on
the membrane blot were detected with the Chemiluminescence Reagent
Plus kit (Pfizer Inc., Gladstone, NJ, USA) and results were
analyzed using SoftMax Pro 5.2 (Molecular Devices, LLC, Sunnyvale,
CA, USA).
Histological analysis
Briefly, transverse colon samples were collected
from 5-6 SD rats from each of the five treatment groups and fixed
with 10% formalin for 48 h. The fixed samples were then embedded in
paraffin blocks, cut into 4 μm thick sections, and stained
with hematoxylin and eosin (H&E) solution (Sigma-Aldrich Co.;
Merck KGaA). Their morphological features, including villus length,
crypt layer thickness, and muscle thickness were measured by using
Leica Application Suite software (Leica Microsystems, GmbH,
Wetzlar, Germany).
For detection of mAChR M2 and M3 via
immunofluores-cence staining analysis, transverse colon tissues
were fixed in 10% formalin for 48 h, embedded in paraffin blocks,
and sliced into 4 μm thick sections. Sections (n=5) were
then deparaf-finized with xylene, rehydrated with different
concentrations of EtOH, and pretreated with blocking buffer
containing 10% goat serum (cat. no. 94010; Vector Laboratories,
Inc., Burlingame, CA, USA) in PBS solution for 30 min at room
temperature. The pretreated sections were then incubated with
anti-mAChR M2 (1:200, cat. no. AMR-002; Alomone Labs) or anti-mAChR
M3 (1:200, cat. no. AMR-006; Alomone Labs) antibodies diluted
1:1,000 in blocking buffer. After thorough washing in PBS solution,
the sections were incubated with goat fluorescein isothiocyanate
(FITC)-labeled anti-rabbit IgG (1:200, cat. no. A11008; Invitrogen;
Thermo Fisher Scientific, Inc.) for 45 min, washed thrice in PBS
for 30 min each, and mounted with vector shield mounting medium.
Finally, green fluorescence intensity on the tissue section of
transverse colon were detected using a Motic AE31 Inverted Phase
Contrast Fluorescence Microscope (Motic Incoporation, Ltd.,
Causeway Bay, Hong Kong).
For mucin staining, transverse colon samples
collected from 5-6 rats from each of the five treatment groups were
fixed with 10% formalin solution for 48 h, embedded in paraffin
blocks, and sectioned into slices with 4 μm thickness, which
were subsequently deparaffinized with xylene and rehydrated with
different concentrations of EtOH. The sections (n=5) were then
washed with distilled water (dH2O) and stained with
alcian blue staining solution (IHC WORLD, Woodstock, MD, USA).
Finally, blue dots indicated mucin levels were observed in the
stained colon tissue sections using light microscopy (Leica
Microsystems, GmbH; DM500).
Transmission electron microscopy (TEM)
analysis
Transverse colon tissues collected from 5-6 rats
from each of the five treatment groups were fixed in 2.5%
glutaraldehyde solution, rinsed with PBS solution, dehydrated with
ascending concentrations of EtOH solution, postfixed in 1% osmium
tetroxide (OsO4) for 1-2 h at room temperature, and
embedded in Epon 812 media (Polysciences, Hirschberg an der
Bergstrasse, Germany). Subsequently, ultra-thin sections of colon
tissue (70 nm thick) were placed on holey formvar-carbon coated
grids and then made the negative stain grids using uranyl acetate
and lead citrate. Morphological features of tissues were examined
by TEM (Hitachi, Ltd., Tokyo, Japan).
HPLC analysis
Spicatoside A in EtRLP was detected by using an
Agilent 1100 HPLC system (Agilent Technologies, Inc., Santa Clara,
CA, USA) comprised of an autosampler, a degasser, a diode array
detector, an automatic thermostatic column compartment, and a
quaternary pump. For spicatoside A analysis, a Shiseido CAPCELL PAK
C18 MG column (Shiseido, Tokyo, Japan; 150x4.6 mm inside diameter,
5 mm particle size) was used with the following eluents: (A) 0.025%
formic acid in water and (B) acetonitrile. A gradient programmer
was used to apply the following HPLC program: 0-7 min (B: 8-12%, C:
10%), 7-23 min (B: 18-60% C: 10%), 23-35 min (B: 60%, C: 10%),
35-45 min (B: 60-90%, C: 10%), and 45-60 min (B: 90%, C: 10%).
During analysis, the flow rate was 0.8 ml/min and the column
temperature was 30°C. Flow rate and pressure were maintained at
1.53 l/min and 35±2 pound per square inch (psi), respectively. The
output signals were detected at 254 nm and recorded with Clarity
chromatography software (DataApex, Prague, Czech Republic).
Treatment of pRISMCs with spicatoside
A
The pRISMCs used in the present study were prepared
using a method described in previous studies, with slight
modification (17). After the
euthanasia of infant rats (3 days old) using a chamber filled with
CO2 gas, their small intestines were collected from 1 cm
below the pyloric ring to the cecum and excised along the
mesenteric border. Gut luminal contents were removed from intestine
by washing with calcium-free Hanks solution (5.36 mmol/l KCl, 125
mmol/l NaCl, 0.34 mmol/l NaOH, 0.44 mmol/l
Na2HCO3, 10 mmol/l glucose, 2.9 mmol/l
sucrose, and 11 mmol/l HEPES, pH 7.4). The opened intestine was
pinned to the base plate of a silicon-covered Petri dish, and the
mucosa layer was removed by sharp dissection. Small tissue strips
of the circular and longitudinal muscles (~3-5 cm) were incubated
in digestion mixture solution [1 mg/ml collagenase (Worthington
Biochemical, Lakewood, NJ, USA), 0.5 mg/ml trypsin inhibitor
(Sigma-Aldrich Co.; Merck KGaA), and 1 mg/ml bovine serum albumin
(Sigma-Aldrich Co.; Merck KGaA)] at 37°C for 30 min. Digested
tissue was centrifuged at 94 x g for 10 min and the pellet
containing pRISMCs was harvested. The isolated cells were cultured
in Dulbecco’s Modified Eagle’s Medium (DMEM; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) with 10% fetal bovine serum (FBS;
Welgene, Daegu, Korea), then incubated in a culture incubator under
a humidified atmosphere with 5% CO2 and 95% air.
The spicatoside A, used as a key compound, was
kindly provided by the National Development Institute of Korean
Medicine (Daegu, Korea). To investigate the effects of spicatoside
A, pRISMCs were seeded into culture dishes (100 mm diameter) at a
density of 107 cells in 10 ml, then incubated with 20
μM Lop for 12 h at 37°C. Subsequently, the culture media
containing Lop were removed and the cells were incubated with 10
μM spicatoside A or PBS for a further 12 h, then collected
by centrifugation at 848 x g for 10 min. The harvested pRISMCs were
used to determine inositol triphos-phate (IP3) concentration and to
measure the expression levels of specific proteins.
Determination of IP3 concentration
IP3 levels in pRISMCs were measured by ELISA
(Cusabio Biotech, Wuhan, China; CSB-E13004r), based on the
manufacturer’s manual. The pRISMCs (2x107) harvested
from each of the five treatment groups were homogenized in ice-cold
PBS (pH 7.2-7.4) by using a homogenizer (Sigma-Aldrich Co.; Merck
KGaA). The supernatant from total cell lysate after centrifugation
at 5,000 x g for 5 min at 4°C was then collected for IP3 analysis.
A specific antibody for IP3 was added to the supernatant and the
mixture was incubated at 37°C for 1 h, followed by the addition of
substrate solution at 37°C for 15 min. The reaction was terminated
by the addition of stop solution into the plate, and the optical
density was measured at 450 nm with a VERSA max plate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
Statistical analyses were performed with SPSS for
Windows, release 10.10, standard version (SPSS, Inc., Chicago, IL,
USA). One-way analysis of variance followed by Tukey’s post hoc
test for multiple comparisons was performed to identify significant
differences between groups. Data were presented as mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of EtRLP on feeding and excretion
behavior of SD rats with Lop-induced-constipation
To investigate whether EtRLP treatment affects the
feeding and excretion behavior of SD rats with Lop-induced
constipation, the daily food intake, daily water consumption, and
daily stool excretion were determined in each of the three
treatment groups. No significant difference was observed in body
weight and daily food intake between the Lop+Vehicle-treated and
Lop+EtRLP-treated groups, while daily water consumption in the
Lop+EtRLP-treated group was at a similar level to the Non-treated
group (Table I). In addition, the
decreased stool numbers and urine volumes were recovered in the
Lop+EtRLP- and Lop+BisaC-treated groups to similar levels to the
Non-treated group. Indeed, the water content and weight of stool in
the Lop+EtRLP-treated group were significantly higher compared with
the Non-treated group (Table I).
These results suggest that EtRLP treatment can improve the
excretion parameters of SD rats with Lop-induced constipation.
 | Table IConstipation parameters following
EtRLP treatment in experimental rats. |
Table I
Constipation parameters following
EtRLP treatment in experimental rats.
| Category | Non-treated | EtRLP | Loperamide
|
|---|
| Vehicle | EtRLP | BisaC |
|---|
| Body weight
(g) | 292.15±7.29 | 294.65±12.22 | 306.09±14.39 | 297.21±24.34 | 279.15±21.24 |
| Food intake
(g/day) | 29.07±4.38 | 28.05±3.72 | 28.94±3.46 | 28.42±3.67 | 27.13±3.81 |
| Water consumption
(ml) | 21.80±2.49 | 23.00±2.00 | 27.40±1.94a | 23.00±2.23b | 24.00±3.80b |
| Stool number
(ea) | 41.00±7.80 | 46.00±6.50 | 32.00±5.30a | 50.00±5.20b | 47.00±6.80b |
| Stool weight
(g) | 4.33±0.58 | 4.43±0.50 | 2.17±0.29a | 4.5±0.50b | 4.17±0.76b |
| Water content
(%) | 49.30±2.12 | 77.7±2.72a | 27.62±1.58a |
74.04±3.33a,b |
68.78±2.4a,b |
| Urine volume
(ml) | 10.20±2.31 | 11.35±3.70 | 14.95±3.17a | 12.75±5.36b | 11.22±5.08b |
Recovery effect of EtRLP on histological
alterations of the transverse colon
To investigate the beneficial effects of EtRLP
treatment on the recovery of constipation-affected histological
structures of the transverse colon, the villus length, thickness of
crypt layer and muscle, and mucin secretion were measured in the
rats with Lop-induced constipation. Transverse colon sampled from
the Lop+Vehicle-treated group exhibited short length of the villus
layer compared with the Non-treated group. Following Lop+EtRLP or
Lop+BisaC treatments, the villus length increased by 110-120%
relative to the Lop+Vehicle-treated group (Fig. 5 and Table II). Furthermore, the alterations
in crypt layer and muscle thickness were similar to those in villus
length, although crypt layer thickness only increased by 30-35%
relative to the Non-treated group (Fig. 5 and Table II). The histological structure
recovery was greater in the Lop+EtRLP-treated rats compared with
the Lop+BisaC-treated rats. In addition, following Lop+EtRLP or
Lop+BisaC treatments, the total mucin levels were markedly
increased (by 90-110%) compared with the Non-treated group
(Fig. 5). These findings
indicated that EtRLP treatment improved the abnormal histological
structure of the transverse colon in SD rats with Lop-induced
constipation.
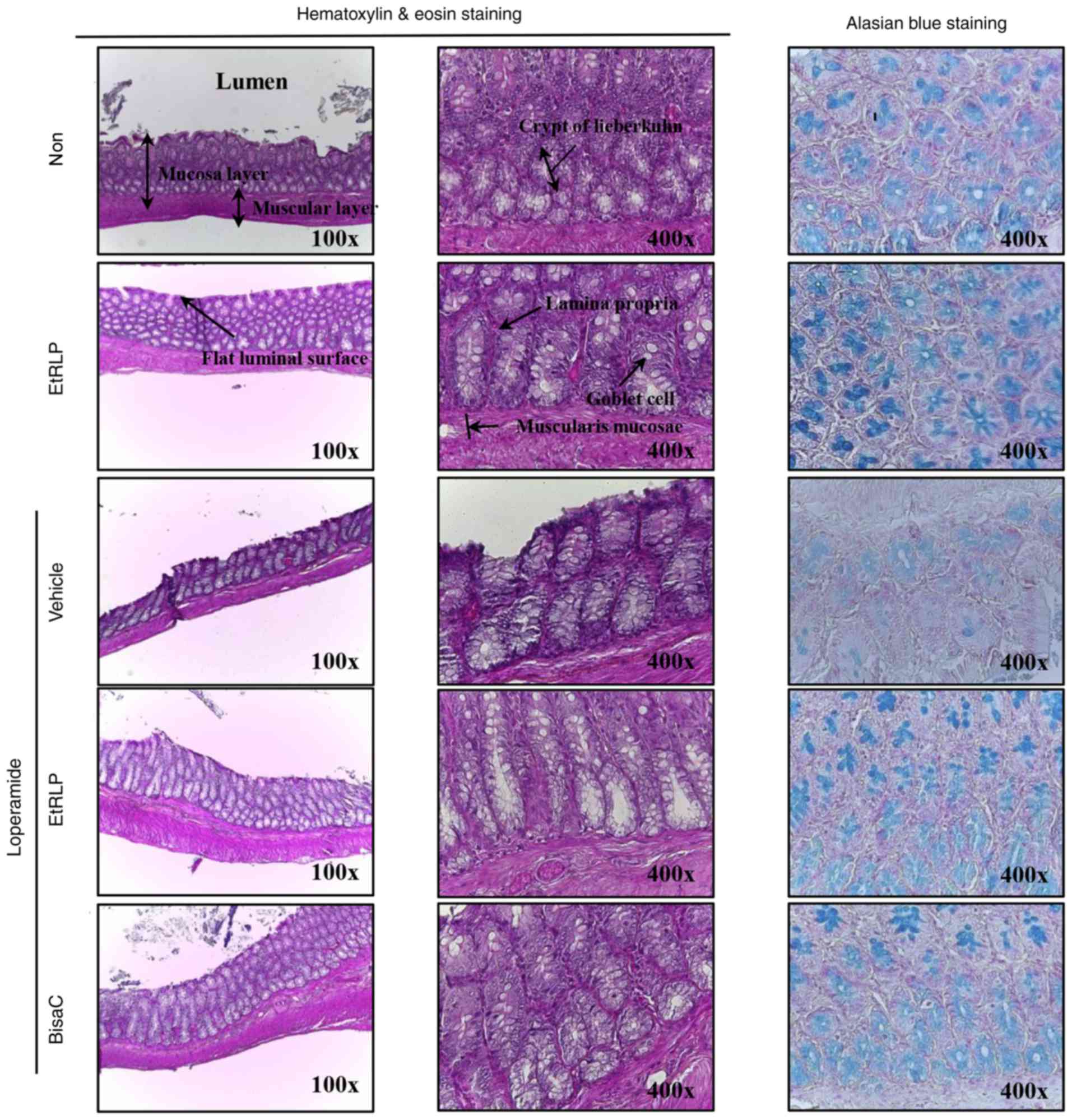 | Figure 5Histological structure of transverse
colon collected from Lop-induced constipated rats. Following the
collection of transverse colons from the Non-, EtRLP-,
Lop+Vehicle-, Lop+EtRLP- and Lop+BisaC-treated groups, H&E
stained sections of these tissues were observed at two
magnifications via light microscopy (left and middle column). Mucin
on the tissue sections was stained with alcian blue (right column).
Crypt layer thickness, muscle thickness, and villus length in
transverse colon were measured in H&E stained images with the
Leica Application Suite software. EtRLP, Red Liriope
platyphylla extract; Lop, loperamide; BisaC, bisacodyl;
H&E, hematoxylin and eosin. |
 | Table IIHistological parameters of
experimental rats with Lop-induced constipation. |
Table II
Histological parameters of
experimental rats with Lop-induced constipation.
| Categories | Non-treated | EtRLP | Loperamide
|
|---|
| Vehicle | EtRLP | BisaC |
|---|
| Mucosa thickness
(μm) | 257.5±34.4 | 264.3±25.1 | 167.9±18.3a | 219.2±11.1b | 225.3±9.8b |
| Muscle thickness
(μm) | 152.0±14.6 | 123.3±10.1 | 80.8±9.0a | 189.4±11.1b | 172.8±27.2b |
| Flat luminal
surface thickness (μm) | 35.7±3.0 | 36.6±4.2 | 15.3±3.3a | 33.4±1.9b | 31.7±1.7b |
| Number of goblet
cell (ea) | 284.8±13.8 | 282.4±8.8 | 164.0±23.9a | 299.6±107b | 219.8±13.6b |
| Number of crypt of
lieberkuhn (ea) | 33.8±3.7 | 26.4±5.7 | 11.2±1.9a | 22.2±2.4b | 24.0±2.9b |
Improvement effect of EtRLP treatment on
transverse colon ultrastructure
To determine if the recovering effect of EtRLP on
transverse colon histological structure is accompanied by
significant changes in its ultrastructure, organelles and cell
microstructures were evaluated by TEM analysis. In the Non-treated
group, crypts of Lieberkuhn formed a round structure, in which
goblet cells, enterocytes and paneth cells surrounded a central
lumen. In the EtRLP-treated group, the crypt structure was very
similar to that in the Non-treated group, even though the
EtRLP-treated group had an increased number of lipid droplets.
Following Lop treatment, the ultra-structure of the crypt changed
markedly in various parts of cell (Fig. 6). Specifically, there was a
decreased diameter of the crypt lumen and an increased number of
lipid droplets in the Lop+Vehicle-treated rats compared with the
Non-treated rats. In the latter group, the affluence of lipid
droplets and granules enhanced notably in enterocytes and paneth
cells, while the levels in goblet cells were constant. However, in
the Lop+EtRLP- and Lop+BisaC-treated groups, lipid droplets and
granules were not distributed in enterocytes and paneth cells
(Fig. 6). These results indicate
that EtRLP treatment effectively restored Lop-altered transverse
colon ultrastructure by enhancing the affluence of lipid droplets
and granules in the crypts of Lieberkuhn in Lop-constipated
rats.
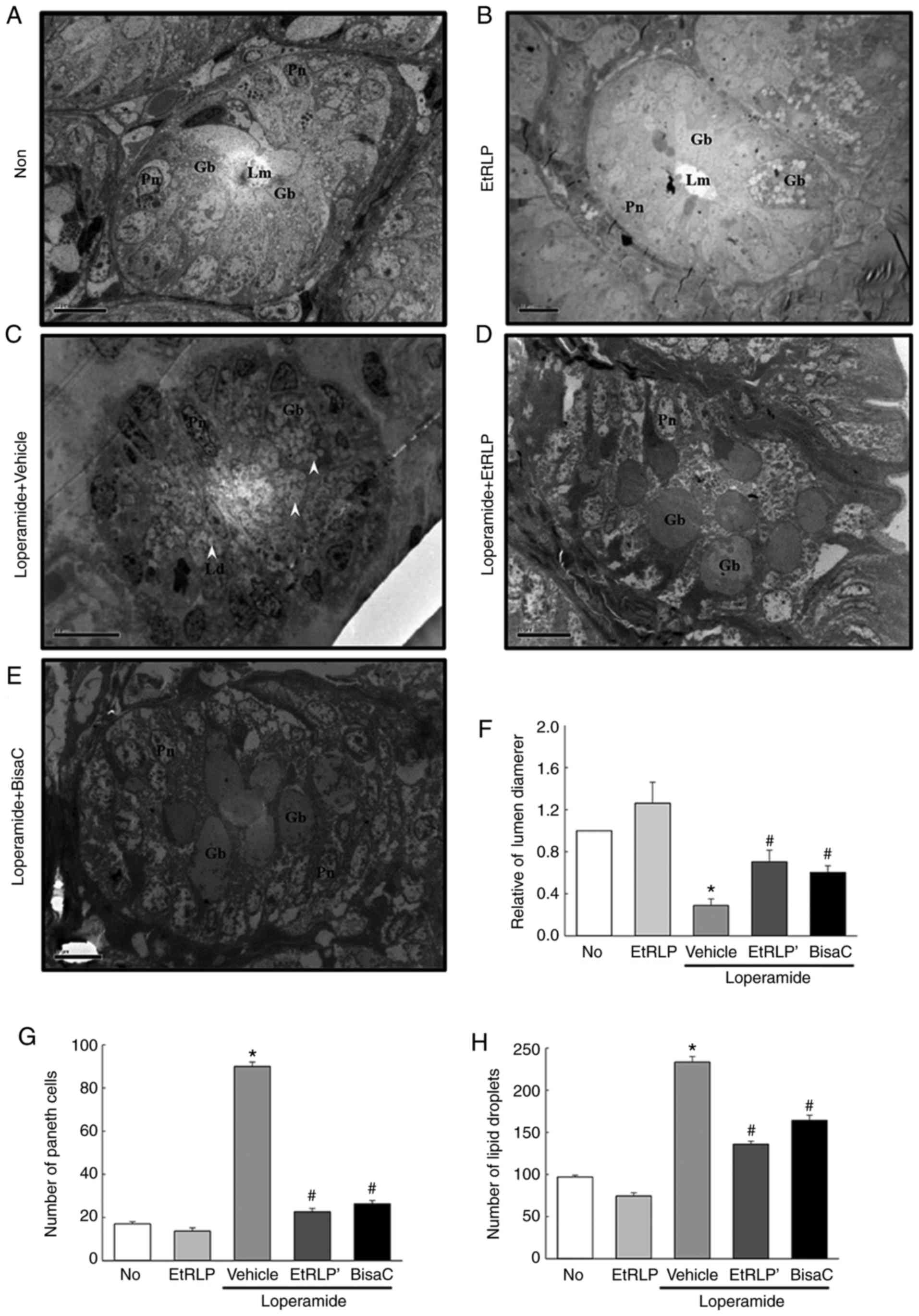 | Figure 6Representative TEM images of rat
transverse colons. Crypt ultrastructure of transverse colon in the
(A) Non-treated, (B) EtRLP, (C) Lop+Vehicle, (D) Lop+EtRLP, and (E)
Lop+BisaC-treated groups were observed by TEM at 1,800x
magnification. (F) Crypt lumen diameter, (G) number of paneth
cells, and (H) number of lipid droplets were determined with Leica
Application Suite software. TEM analysis was performed in samples
from five to six rats from each treatment group in triplicate. Data
are presented as mean ± standard deviation from three replicates.
*P<0.05 compared with the Non-treated group;
#P<0.05 compared with the Lop+Vehicle-treated group.
TEM, transmission electron microscopy; EtRLP, Red Liriope
platyphylla extract; Lop, loperamide; BisaC, bisacodyl; Gb,
goblet cells; Lm, crypt lumen; Gr, granule cells; Pn, paneth cells;
Ld, lipid droplets. |
Correlation between EtRLP treatment and
the mAChR downstream signaling pathway
To determine if the laxative effects of EtRLP were
accompanied by alterations in mAChRs signaling, the expression
levels of mAChR-related proteins were detected in the three
Lop-treated groups. The expression patterns of two mAChR proteins
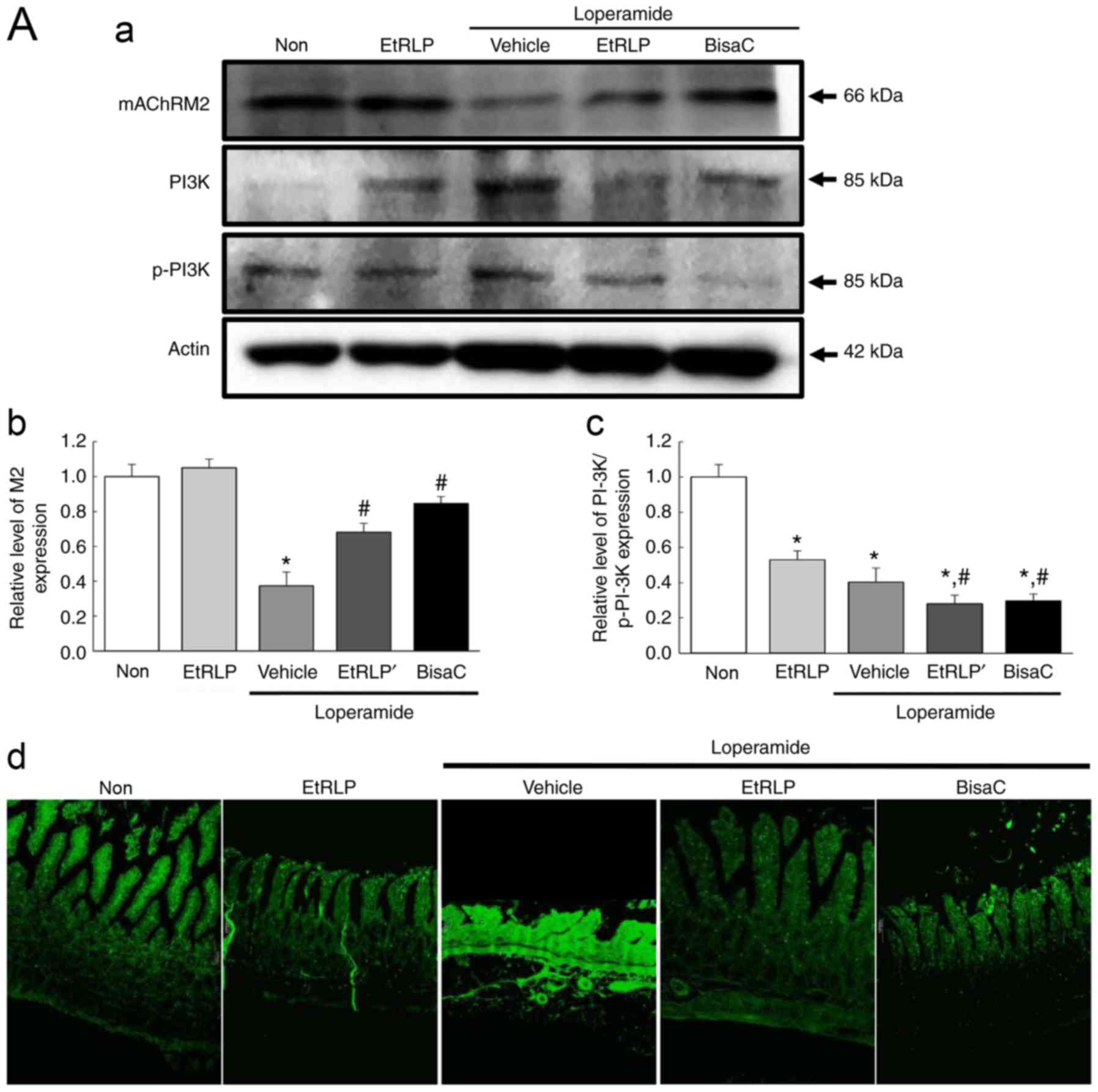
(M2 and M3) were similar among the experimental groups (Fig. 7). The lowest expression levels of
those two receptors were observed in the Lop+Vehicle-treated group
(Fig. 7). Following Lop+EtRLP or
Lop+BisaC treatment, M2 and M3 levels were significantly increased,
to levels similar to the Non-treated group (Fig. 7). The protein levels of mAChR M2
and M3 in the Lop+EtRLP-treated group increased by 184 and 286%
respectively, relative to the Lop+Vehicle-treated group (Fig. 7).
However, the phosphorylation pattern of PI3K, a key
signaling protein downstream of mAChR M2, was different from that
of PKC, a key signaling protein downstream of mAChR M3, in all
experimental. The phosphorylation level of PKC was recovered in
Lop+EtRLP and Lop+BisaC-treated group, while that of PI3K was
further decreased in the same groups (Fig. 7A-c and B-c). In addition,
immunofluorescence assays revealed local distributions of M2 and M3
proteins in slide sections of the transverse colon. The observed
fluorescence intensities for M2 and M3 supported the results
obtained via western blot assays (Fig. 7A-d and B-d). Taken together, these
results suggested that the laxative effects of EtRLP may be due to
the downregulation of mAChR expression and its downstream signaling
in the transverse colons of Lop-constipated SD rats.
Effects of EtRLP on the ER stress
response
To investigate the beneficial effects of EtRLP
treatment on the ER stress response in SD rats with Lop-induced
constipation, alterations in ER stress biomarkers, including
related protein levels and ultra-structure of the transverse colon,
were examined by western blot and TEM analysis. The phosphorylation
levels of IRE1α and IRE1β were increased in the Lop+Vehicle-treated
SD rats compared with the Non-treated group. Following Lop+EtRLP or
Lop+BisaC treatments, their levels almost recovered to the levels
of the Non-treated group (Fig.
8A). Furthermore, the pattern of change in the phosphorylation
levels of eIF2α was similar to the level changes in the
phosphorylation of IRE1 in all treated groups. A similar
phosphorylation pattern was also observed in JNK and IRE1 in the
Non, Lop+Vehicle and Lop+EtRLP-treated groups. However, the level
of JNK was not suppressed in Lop+BisaC-treated SD rats (Fig. 8A). Similar results were detected
in the alteration patterns of ER stress protein levels detected by
TEM analysis. We focused on alterations of paneth cells in the
crypts of Lieberkuhn because they have been associated with ER
stress, through PERK, eIF2α, and ATF4 (18). Compared to the secretory granule
decrease in the Lop+Vehicle group, the number of secretory granules
was enhanced in the Lop+EtRLP-treated group, while that in the
Lop+BisaC-treated group remained at a similarly low level as that
in the Lop+Vehicle group. In addition, the ER membrane sack was
closely stacked in the cytoplasm of paneth cells in the
Lop+Vehicle-treated group, while an intact ER sack was observed in
the Non-treated group. Following Lop+EtRLP or Lop+BisaC treatment,
the ER structure was recovered to be similar to the Non-treated
group; however, the changes were not as clear in the
Lop+EtRLP-treated group as in the Lop+BisaC group (Fig. 8B). These results indicate that the
Lop-induced ER stress response in paneth cells may be improved by
EtRLP treatment.
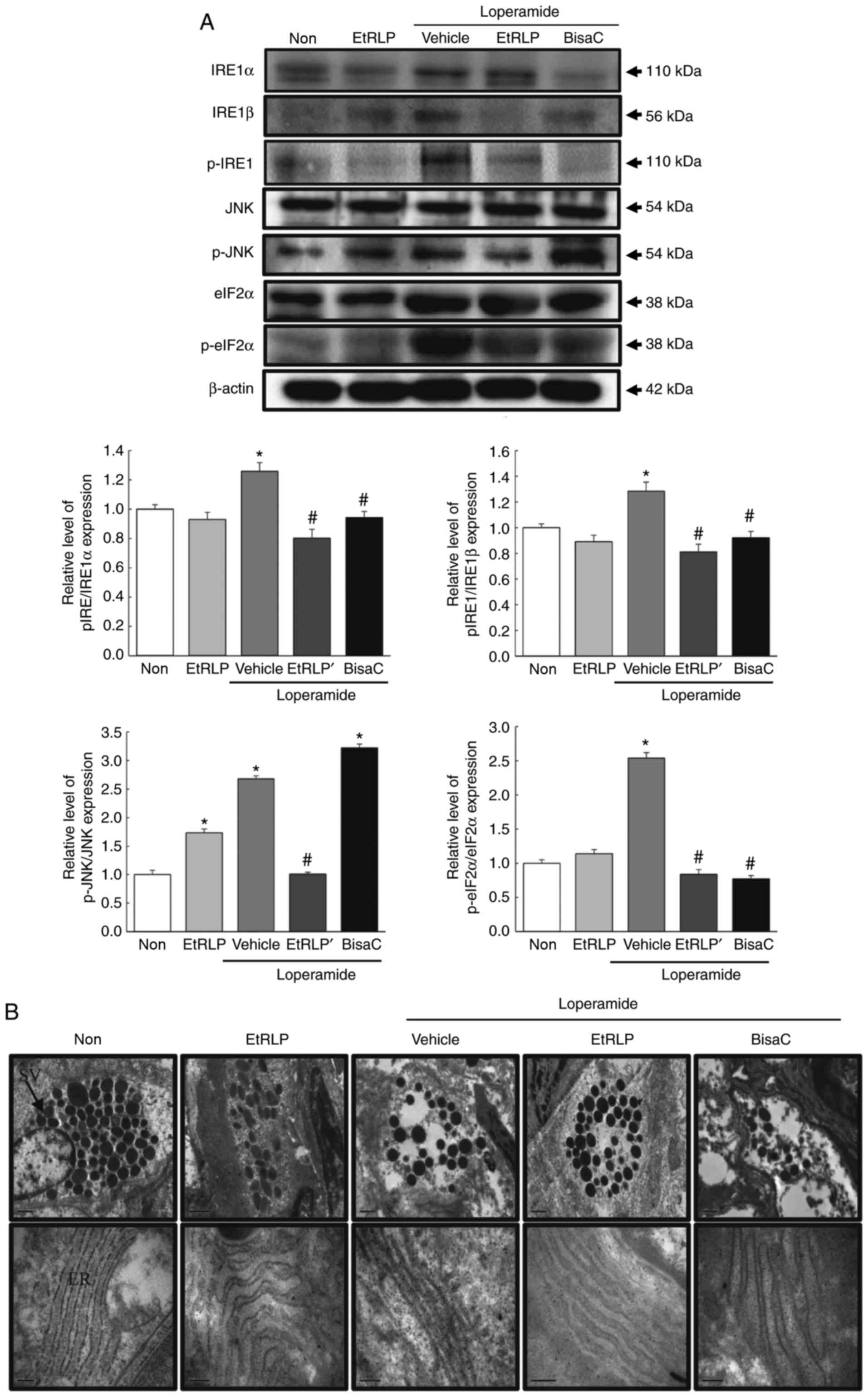 | Figure 8Detection of the ER stress response.
(A) Expression levels of ER stress-related proteins IRE1α, IRE1β,
p-IRE1, JNK, p-JNK, eIF2α and p-eIF2α were measured by western blot
analysis. Band intensities were evaluated relative to the intensity
of the actin bands. Data are presented as mean ± standard deviation
from three replicates (N=5-6 rats per treatment group).
*P<0.05 compared with the Non-treated group;
#P<0.05 compared with the Lop+Vehicle-treated group.
(B) Ultrastructure of the secretory vesicle and ER in paneth cells
were evaluated by transmission electron microscopy at 4,000x
magnification. ER, endoplasmic reticulum; IRE, inositol-requiring
enzyme; JNK, c-Jun N-terminal kinase; eIF2α, eukaryotic initiation
factor 2α; p-, phosphorylated; EtRLP, Red Liriope
platyphylla extract; Lop, loperamide; BisaC, bisacodyl. |
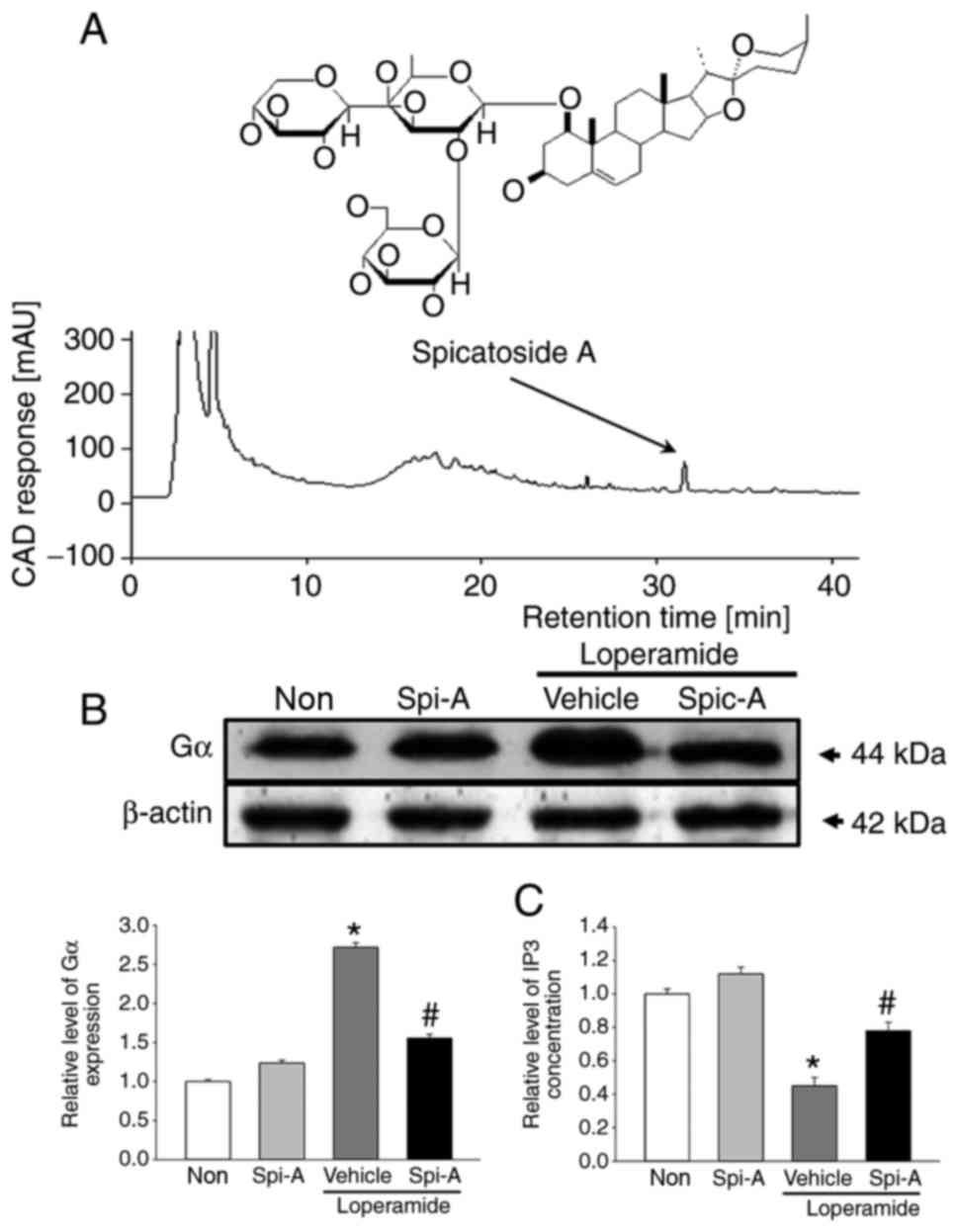
Identification of spicatoside A as a key
component
Spicatoside A was selected as a potential compound
for the treatment of constipation, because it is a main compound of
EtRLP, it can stimulate epithelial cells, and it has been linked to
neuronal cell function. Briefly, we confirmed the existence of
spicatoside A in EtRLP by performing HPLC. As illustrated in
Fig. 9A, spicatoside A was
detected in the HPLC chromatogram of EtRLP after a retention time
of 32 min. Then, the effects of spicatoside A as a laxative
compound were investigated. To accomplish this, alterations in the
mAChR downstream signaling pathway were measured in pRISMCs
cotreated with Lop and spicatoside A. The expression levels of Gα
protein were increased by 165% in the Lop+Vehicle-treated group
compared with the Non-treated group (Fig. 9B). However, the levels in the
Lop+spicatoside A-treated group were similar to those in the
Non-treated or the spicatoside A-treated groups (Fig. 9B). Next, the IP3 concentrations
were examined. The increased levels of IP3 concentration in the
Lop+Vehicle-treated group were significantly lower following
spicatoside A treatment (Fig.
9C). The present results indicated that spicatoside A can be
considered as a laxative and a potential candidate to improve the
symptoms of chronic constipation.
Discussion
Recently, many herbal plants and medicinal foods
have received research attention as novel therapeutic strategies
for use in the treatment of chronic constipation and its related
conditions, because they may effectively improve a variety of
conditions without significant adverse side effects (12,15,19). In an effort to assess the effects
of plant-derived drugs for the treatment of constipation, the
present study conducted an efficacy study of EtRLP produced from
L. platyphylla root via a steaming process on an
experimental model of Lop-induced constipation. The results clearly
demonstrated that EtRLP treatment had a laxative effect, leading to
increased stool and urine volumes, recovery of histological and
cell ultrastructure changes in colon, and enhanced mucin secretion.
Furthermore, the results are the first to demonstrate that the
laxative function of EtRLP is closely associated with regulation of
the mAChR signaling pathway and the ER stress response in the
transverse colon of SD rats.
Extracts of L. platyphylla and RLP roots
contain a great variety of functional components, but elucidation
of the correlation between their properties and therapeutic effects
requires further analyses. Carbohydrates (6.89 g/100 g) and sodium
(6.32 g/100 g) are the predominant components of dried roots of
L. platyphylla, with proteins, fats, and sugars also present
but at much lower levels, while the level of trans-fat, saturated
fat, and cholesterol are not detected (20). Previously, AEtLP was reported to
contain saponins (1.73%), oligosaccharides (6.54%), succinic acid
(111.48 mg/100 g), hydroxyproline (1,290 μg/100 g), and
potassium (151.35 μg/100 g) (21). In addition, an ethanolic extract
of L. platyphylla was examined, leading to the purification
of five novel compounds, (-)-liriopein A/B, (+)platyphyllarin A/B,
and ethyltributanoate, along with 21 previously reported secondary
metabolites (22). However, very
few reports have described the composition and bioactive compounds
of EtRLP, a steam-processed extract of the L. platyphylla
root. Previous reports have identified the total phenolic
compounds, total flavonoid compounds, and the presence of diosgenin
and 5-hydroxmethyl-2-furfural in EtRLP (15,16). However, further studies are needed
to compare the concentrations of bioactive components between L.
platyphylla and EtRLP to identify the key components with
therapeutic effects against specific diseases. L.
platyphylla, the raw material from which EtRLP was derived, has
also been reported to effectively improve the symptoms of
Lop-induced constipation (14).
However, the rate of improvement in key factors was greater in the
EtRLP-treated group compared with the L. platyphylla-treated
group. In that previous study (14), stool weight, water content, and
lumen diameter were higher in the EtRLP-treated group than in the
L. platyphylla-treated group; however, the numbers of stools and
paneth cells were similar in both groups. Furthermore, differences
in the level of water consumption and food intake were detected in
both groups, although body weights were similar. In the present
study, water consumption was significantly lower in the
Lop+EtRLP-treated group compared with the Lop+Vehicle group and the
Lop+BisaC-treated group. In addition, food intake remained at a
constant level in these groups even though their levels were lower
than the Non-treated group. Such differences between studies can be
attributed to differences in composition and concentration of
several compounds contained in the treatment materials.
The function of gut epithelial cell is regulated by
mAChRs, which serve a role in the digestion process of foods and in
absorption of nutrients, electrolytes and water, through
acetylcholine action (23).
Although mAChRs are expressed on neuronal and non-neuronal cells in
the gastrointestinal system, subtype-specific expressions have not
been fully described in the different tissues of rat intestine.
Indeed, only a few studies have investigated whether M1, M2, and M3
are expressed in the epithelial cells of the ileum, or examined M4
expression in colon nerve fibers (1,24,25). In the present study, mAChR M2 and
M3 proteins were detected in the transverse colons of normal and
Lop-constipated SD rats. Additionally, alterations in PKC and PI3K
phosphorylation levels in the mAChR downstream signaling pathway
were observed in the transverse colon, and Gα and IP3 were measured
in pRISMCs isolated from the transverse colon (Figs. 7 and 9). Although the present results suggest
the importance of the mAChR signaling pathway in constipation,
further studies are required to understand the role of other
factors in the regulation of this mechanism.
Protein misfolding within the ER can be enhanced by
a variety of disturbances, including viral infection (26), abnormal calcium regulation
(27), mutation (28), and high fat intake (29). An accumulation of unfolded
proteins in the ER lumen leads to ER stress response. During ER
stress, glucose-regulated protein 78 (GRP78) initiates an unfolded
protein response (URP) signaling cascade (30). This signal is propagated via three
ER-localized transmembrane proteins: double-stranded RNA-dependent
protein kinase-like ER kinase (PERK), IRE1α and activating
transcription factor 6 (ATF6) (31,32). The expression of biomarkers, such
as GPR78, IRE1, ATF6, and PERK, is decreased in response to
Wolfberry water-soluble phytochemicals in Jurkat cells (33). In the present study, the levels of
IRE1 and JNK phosphorylation were significantly lower in the
Lop+EtRLP-treated group. The present results are in agreement with
previous reports (31,32), although the investigated models
differed. The ER stress response is also observed in various
diseases or abnormal conditions, including cancer,
neurodegenerative disease, diabetes and ischemia (34-36). However, evidence of a correlation
between the responses of ER stress and constipation, as well as
between EtRLP’s laxative effects and ER stress, has not been
presented to date. In the present study, the results from western
blot and TEM analyses indicated that ER stress may be a key marker
of clinical symptoms in SD rats with Lop-induced constipation. In
addition, the results of the present study demonastrated that ER
stress was lowered by EtRLP treatment. Therefore, it is worth
studying the laxative effects of EtRLP in an experimental model
with constipation in order to elucidate the mechanism associated
with the response of ER stress.
In the present study, spicatoside A was selected as
a candidate laxative treatment to improve chronic constipation.
This compound was first isolated as a novel monoterpenoid from
whole Mentha spicata L. and exhibits anti-inflammatory and
hemostatic activities (37).
Notably, it has also been isolated from L. platyphylla
(38). Few studies have
investigated the role of spicatoside A in biological processes to
date. Spicatoside A induced neurite outgrowth via TrkA activation
and enhanced memory consolidation via an increase in hippocampal
mBDNF levels (38,39). This compound has also been
described as a therapeutic drug that can inhibit cartilage
breakdown in joints (40).
Furthermore, spicatoside A has been reported to enhance mucin
production and secretion by directly stimulating airway epithelial
cells (39). Based on these
results, spicatoside A was selected as a candidate compound that
may have laxative activity. The present results suggested that
spicatoside A can be considered a laxative.
Taken together, the results of the present study
suggested that EtRLP recovered several key excretion parameters,
including stool number, stool water content and urine quantity, in
Lop-induced constipated SD rats. Furthermore, EtRLP treatment
recovered villus length, thickness of crypt layer and muscle, and
the secretion of lipid droplets in Lop-constipated SD rats. In
addition, EtRLP treatment enhanced the expression of several genes,
including mAChRs and mucins, through the regulation of PKC, PI3K
and IP3 levels, while it may inhibit chaperons and inflammatory
genes through the suppression of X-box binding protein 1 (XBP1),
p-JNK and p-eIF2α activation (Fig.
10). The present findings provide evidence that the laxative
effects of spicatoside A-containing EtRLP may be mediated by
regulation of the mAChR signaling pathway and the ER stress
response.
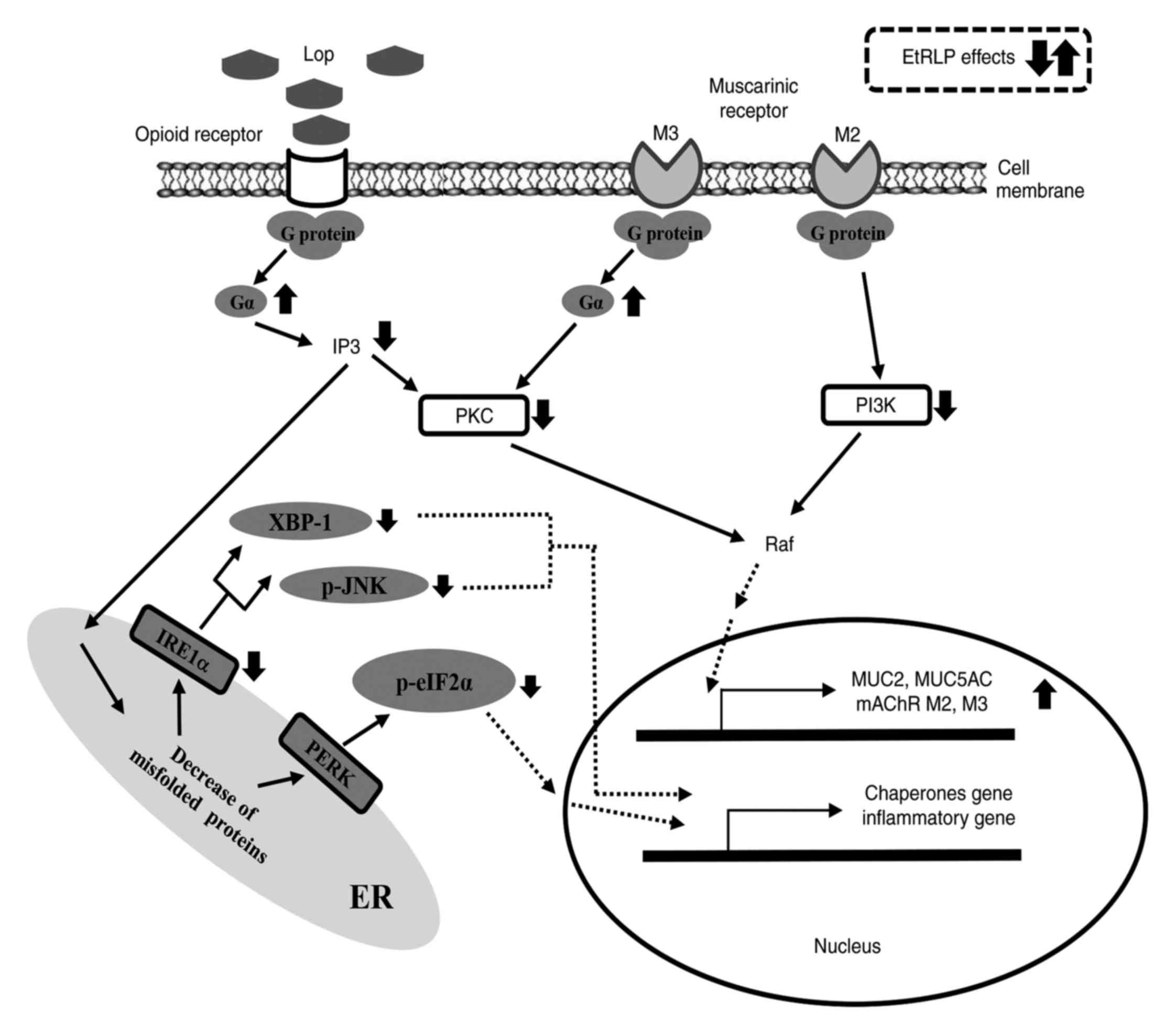 | Figure 10Suggested mechanism of action of
EtRLP in Lop-induced constipated rats. The effects of EtRLP in
suppressing the ER stress response are hypothesized to be exerted
via the inhibition of XBP-1, p-JNK and p-eIF2α. The effects of
EtRLP in recovering the transcription of mAChRs and mucin genes are
hypothesized to be mediated by the activation of signaling proteins
downstream of the opioid receptor and mAChR. EtRLP, Red Liriope
platyphylla extract; Lop, loperamide; ER, endoplasmic
reticulum; XBP-1, X-box binding protein 1; p-phosphorylated; JNK,
c-Jun N-terminal kinase; eIF2α, eukaryotic initiation factor 2α;
mAChR, muscarinic acetylcholine receptor; PI3K, phosphoinositide
3-kinase; PKC, protein kinase C; IRE1α, inositol-requiring enzyme
1α; PERK, double-stranded RNA-dependent protein kinase-like ER
kinase. |
Acknowledgments
We thank the animal technician, Jin Hyang Hwang, for
directing the care and management of animals at the Laboratory
Animal Resources Center.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant nos. 2014R1A1A2058360
and 2017R1D1A3B03032631).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JEK, JG, HSL and DYH participated in the design of
the study, sample preparation, animal experiments and data
analyses. JTH helped with data analysis and manuscript preparation.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All protocols involving animals were approved by the
Pusan National University Institutional Animal Care and Use
Committee (approval no. PNU-2012-0010).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walia R, Mahajan L and Steffen R: Recent
advances in chronic constipation. Curr Opin Pediatr. 21:661–666.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCallum IJ, Ong S and Mercer-Jones M:
Chronic constipation in adults. BMJ. 338:b8312009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Emmanuel AV, Tack J, Quigley EM and Talley
NJ: Pharmacological management of constipation. Neurogastroenterol
Motil. 21(Suppl 2): S41–S54. 2009. View Article : Google Scholar
|
|
4
|
Leung FW: Etiologic factors of chronic
constipation: Review of the scientific evidence. Dig Dis Sci.
52:313–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel JD and Di Palma JA: Medical
treatment constipation. Clin Colon Rectal Surg. 18:76–80. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Portalatin M and Winstead N: Medical
management of constipation. Clin Colon Rectal Surg. 25:12–19. 2012.
View Article : Google Scholar :
|
|
7
|
Tzavella K, Riepl RL, Klauser AG,
Voderholzer WA, Schindbeck NE and Müller-Lissner SA: Decreased
substance P levels in rectal biopsies from patients with slow
transit constipation. Eur J Gastroenterol Hepatol. 8:1207–1211.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schiller LR: Review article: The therapy
of constipation. Aliment Pharmacol Ther. 15:749–763. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ewe K: Effect of bisacodyl on intestinal
electrolyte and water net transport and transit. Perfusion studies
in men. Digestion. 37:247–253. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kienzle-horn S, Vix JM, Schuijt C, Peil H,
Jordan CC and Kamm MA: Efficacy and safety of bisacodyl in the
acute treatment of constipation: A double-blind, randomized,
placebo-controlled study. Aliment Pharmacol Ther. 23:1479–1488.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wintola OA, Sunmonu TO and Afolayan AJ:
The effect of Aloe ferox Mill. in the treatment of
loperamide-induced constipation in Wistar rats. BMC Gastroenterol.
10:952010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kakino M, Izuta H, Ito T, Tsuruma K, Araki
Y, Shimazawa M, Oyama M, Iinuma M and Hara H: Agarwood induced
laxative effects via acetylcholine receptors on loperamide-induced
constipation in mice. Biosci Biotechnol Biochem. 74:1550–1555.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HY, Kim JH, Jeung HW, Lee CU, Kim DS,
Li B, Lee GH, Sung MS, Ha KC, Back HI, et al: Effects of Ficus
carica paste on loperamide-induced constipation in rats. Food Chem
Toxicol. 50:895–902. 2012. View Article : Google Scholar
|
|
14
|
Kim JE, Lee YJ, Kwak MH, Ko J, Hong JT and
Hwang DY: Aqueous extracts of Liriope platyphylla induced
significant laxative effects on loperamide-induced constipation of
SD rats. BMC Complement Altern Med. 13:3332013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HR, Kim JE, Goo JS, Choi SI, Hwang IS,
Lee YJ, Son HJ, Lee HS, Lee JS and Hwang DY: Red Liriope
platyphylla contains a large amount of polyphenolic compounds which
stimulate insulin secretion and suppress fatty liver formation
through the regulation of fatty acid oxidation in OLETF rats. Int J
Mol Med. 30:905–913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JS, Kim SH, Choi DW, Lee HS, Soon HJ
and Hwang DY: Red Liriopis tuber production by steam and drying
process of Liriopis tuber and development of its functional food
products. Lee JS: Ministry of Agriculture, Food and +Rural Affairs;
Sejong: pp. 285–289. 2013
|
|
17
|
Kim JE, Koh EK, Song SH, Sung JE, Lee HA,
Lee HG, Choi YW and Hwang D: Effects of five candidate laxatives
derived from Liriope platyphylla on the 5-HT receptor signaling
pathway in three cell types present in the transverse colon. Mol
Med Rep. 15:431–441. 2017. View Article : Google Scholar
|
|
18
|
Adolph TE, Tomczak MF, Niederreiter L, Ko
HJ, Böck J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M,
Hartwig J, Hosomi S, et al: Paneth cells as a site of origin for
intestinal inflammation. Nature. 503:272–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Méité S, Bahi C, Yéo D, Datté JY, Djaman
JA and N’guessan DJ: Laxative activities of Mareya micrantha
(Benth.) Müll. Arg. (Euphorbiaceae) leaf aqueous extract in rats.
BMC Complement Altern Med. 10:72010. View Article : Google Scholar
|
|
20
|
Shahbazian A, Heinemann A, Schmidhammer H,
Beubler E, Holzer-Petsche U and Holzer P: Involvement of mu- and
kappa-, but not delta-, opioid receptors in the peristaltic motor
depression caused by endogenous and exogenous opioids in the
guinea-pig intestine. Br J Pharmacol. 135:741–750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wingate D, Phillips SF, Lewis SJ,
Malagelada JR, Speelman P, Steffen R and Tytgat GN: Guidelines for
adults on self-medication for the treatment of acute diarrhoea.
Aliment Pharmacol Ther. 15:773–782. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huighebaert S, Awouters F and Tytgat GN:
Racecadotril versus loperamide: Antidiarrheal research revisited.
Dig Dis Sci. 48:239–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirota CL and McKay DM: Cholinergic
regulation of epithelial ion transport in the mammalian intestine.
Br J Pharmacol. 149:463–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levey AI: Immunological localization of
m1-m5 muscarinic acetylcholine receptors in peripheral tissues and
brain. Life Sci. 52:441–448. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anini Y, Hansotia T and Brubaker PL:
Muscarinic receptors control postprandial release of glucagon-like
peptide-1: In vivo and in vitro studies in rats. Endocrinology.
143:2420–2426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Isler JA, Skalet AH and Alwine JC: Human
cytomegalovirus infection activates and regulates the unfolded
protein response. J Virol. 79:6890–6899. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mattson MP: Calcium and neurodegeneration.
Aging Cell. 6:337–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YM, Zhou Y, Go G, Marmerstein JT,
Kikkawa Y and Miner JH: Laminin β2 gene missense mutation produces
endoplasmic reticulum stress in podocytes. J Am Soc Nephrol.
24:1223–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deldicque L, Cani PD, Philp A, Raymackers
JM, Meakin PJ, Ashford ML, Delzenne NM, Francaux M and Baar K: The
unfolded protein response is activated in skeletal muscle by
high-fat feeding: Potential role in the down regulation of protein
synthesis. Am J Physiol Endocrino Metab. 299:E695–E705. 2010.
View Article : Google Scholar
|
|
30
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang Y, Zhang Y, Wark L, Ortiz E, Lim S,
He H, Wang W, Medeiros D and Lin D: Wolfberry water soluble
phytochemicals down-regulate ER stress biomarkers and modulate
multiple signaling pathways leading to inhibition of proliferation
and induction of apoptosis in jurkat cells. J Nutr Food Sci.
S2:pii: 0012011.
|
|
34
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida H: ER stress and diseases. FEBS J.
274:630–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niederreiter L and Kaser A: Endoplasmic
reticulum stress and inflammatory bowel disease. Acta Gastroenterol
Belg. 74:330–333. 2011.PubMed/NCBI
|
|
37
|
Zheng J, Wu LJ, Zheng L, Wu B and Song AH:
Two new mono-terpenoid glycosides from Mentha spicata L. J Asian
Nat Prod Res. 5:69–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hur J, Lee P, Moon E, Kang I, Kim SH, Oh
MS and Kim SY: Neurite outgrowth induced by spicatoside A, a
steroidal saponin, via the tyrosine kinase A receptor pathway. Eur
J Pharmacol. 620:9–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park SH, Lee HJ, Ryu J, Son KH, Kwon SY,
Lee SK, Kim YS, Hong JH, Seok JH and Lee CJ: Effects of
ophiopogonin D and spicatoside A derived from Liriope tuber on
secretion and production of mucin from airway epithelial cells.
Phytomedicine. 21:172–176. 2014. View Article : Google Scholar
|
|
40
|
Lim H, Min DS, Kang Y, Kim HW, Son KH and
Kim HP: Inhibition of matrix metalloproteinase-13 expression in
IL-1β-treated articular chondrocytes by a steroidal saponin,
spicatoside A, and its cellular mechanisms of action. Arch Pharm
Res. 38:1108–1116. 2015. View Article : Google Scholar : PubMed/NCBI
|