Introduction
Fibroblasts, the main constituents of connective
tissue, are a well-known source of cells for use in regenerative
medicine owing to their ability to produce extracellular matrix
molecules and several bioactive factors (1,2).
Human fibroblasts are cultured in vitro for investigative
purposes and as a replacement therapy for damaged tissue, as they
can be reprogrammed into a pluripotent state that can differentiate
into different phenotypes (3,4).
Human skin is an accessible source of multipotent stromal cells
(MSCs) with self-renewal and multipotent capacities, which makes
them valuable for various MSC-based therapies (5). MSCs can differentiate into
adipocytes, osteocytes, and chondrocytes, and are characterized by
the expression of three surface markers, CD105, CD90, and CD73
(6). Dermal fibroblasts with
multipotent capacity express high levels of CD105, and
CD73− CD105+ fibroblasts have high
proliferation rates and adipocyte/osteocyte differentiation
potential (5,7).
Bone morphogenetic proteins (BMPs) are dimeric
proteins that bind to type I and type II BMP receptors, transducing
signals through small mothers against decapentaplegic
(Smad)-dependent and -independent pathways to regulate the
transcription of BMP target genes (8). BMP7 is a member of the transforming
growth factor-β (TGF-β) superfamily, which possesses a high
osteogenic capacity (9). BMPs
signal via the p38 class of mitogen-activated protein kinases
(MAPKs), and the activity of p38 MAPK is regulated by BMP signaling
(10). The role of BMP7 in the
osteogenic differentiation of human adipose-derived stem cells
(ADSCs) has been investigated extensively (11). In addition, BMP4 and the BMP2/7
heterodimer have been shown to induce the osteogenic
differentiation of mouse skin-derived fibroblasts (12). However, despite the potential
therapeutic application of human dermal-derived fibroblasts
(hDDFCs), the mechanisms underlying their osteogenic
differentiation and the role of BMP7 in this process remain to be
fully elucidated.
In the present study, human dermal-derived
CD105+ fibroblast cells (CD105+ hDDFCs) were
isolated to examine the role of BMP7 in the osteogenic
differentiation of dermal fibroblast populations with multipotent
stem cell capacity and investigate the underlying mechanisms.
Materials and methods
Immunomagnetic isolation of
CD105+ hDDFCs and cell culture
The present study was performed in accordance with
the Declaration of Helsinki for investigations involving human
subjects, and was approved by the Ethics Committee of Shanghai
Ninth People’s Hospital Affiliated to Shanghai Second Medical
University (Shanghai Ninth People’s Hospital, Shanghai Jiao Tong
University School of Medicine, Shanghai, China). Dermal fibroblasts
were isolated from residual skin during circumcision surgery in 5
older children (age, 6-9 years) between June and July 2009. All
patients provided written informed consent. The hDDFCs were
isolated as described previously (13). Immunomagnetic isolation of the
CD105+ cells was performed as described
previously (14). Briefly,
suspensions of hDDFCs obtained from human dermis were washed once
with 1X PBS and resuspended with magnetic cell sorting (MACS)
buffer (1X PBS containing 0.5% fetal bovine serum (FBS; cat. no.
SH30087.01; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
and 2 mM ethylenediamine tetraacetic acid, pH 7.2). A nylon mesh
was used to filter cell suspensions (30-µm pore). The cells
were resuspended in MACS buffer at 107 cells per 80
µl, mixed with 20 µl microbeads of directly
conjugated mouse anti-human CD105 antibody (1:200; cat. no.
MCA1557; Bio-Rad Laboratories, Inc., Hercules, CA, USA), and
incubated at 4°C for 15 min on a rotator in the dark. Following
washing in 1X PBS, the DDFCs-CD105 cells were resuspended in MACS
buffer and processed in an LS+/VS+ separation
column. The column was removed from the magnetic device, and the
cells were flushed out with MACS buffer. The CD105− and
CD105+ cells were recovered by centrifugation at 300 × g
for 10 min at 4°C for future use.
To determine the purity of the CD105+
cells, the cells were analyzed using the FACSCalibur device (BD
Biosciences, San Jose, CA). Aliquots containing 0.5×106
CD105+ cells were incubated with phycoerythrin
(PE)-conjugated anti-CD105 antibody (1:100; cat. no. FAB10971B,
R&D Systems, Inc., Minneapolis, MN, USA) on ice for 30 min,
washed three times, and resuspended in wash buffer. IgG-PE (1;200;
cat. no. SC-3756; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
was used as the isotype control. The hDDFCs were suspended basal
media, which consisted of DMEM (DMEM-HG; Invitrogen, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% FBS
(HyClone; GE Healthcare Life Sciences) and maintained in the
original culture medium at 37°C with 5% CO2.
Construction of recombinant
adenoviruses
The AdCMV-hBMP7 and AdCMV-LacZ viruses were
generated by Shanghai Key Laboratory of Tissue Engineering
(Shanghai, China) using the BD Adeno-Xä expression system (BD
Biosciences). In vitro ligation was used to incorporate a
mammalian expression cassette into a replication-incompetent
(∆E1/∆E3) human adenoviral type 5 (Ad5) genome. Full-length human
BMP7 (hBMP7) cDNA and pBlue-BMP7 (4.5 kb), obtained from the
American Tissue Culture Collection (Manassas, VA, USA) were cloned
into a shuttle vector via the KpnI and NotI sites.
The expression cassette was excised from the recombinant pShuttle2
plasmid using the PI-SceI and I-CeuI restriction
endonucleases and ligated into the BD Adeno-X viral DNA. The
recombinant linearized pAdeno-X DNA (PacI digestion) was
transfected into 293 cells (Type Culture Collection of the Chinese
Academy of Sciences, Shanghai, China) using FuGENE 6 (Roche
Diagnostics, Basel, Switzerland) and the virus was purified by
cesium chloride gradient ultracentrifugation at 100,000 × g for 2 h
at 4°C and stored in 10% glycerol in PBS. Titers were determined by
end-point dilution, yielding ~1.2×1010 plaque-forming
units (pfu)/ml. Recombinant adenoviruses expressing β-galactosidase
DNA (AdCMV-LacZ) were generated as controls.
Transduction of hDDFCs and treatment
The cultured CD105+ hDDFCs were harvested
and treated with virus at a multiplicity of infection of 200
pfu/cell in 1 ml DMEM-HG medium for 4 h at 37°C. Following culture
in DMEM with 10% FBS in a humidified atmosphere with 5%
CO2, the cells were harvested, counted, and resuspended
in an alginate hydrogel at the indicated concentrations.
Small interfering RNAs (siRNAs) against Smad4
(target sequence: 5′-GCC ATA GTG AAG GAC TGT T-3′) were synthesized
by GeneChem (Shanghai, China). The hDDFCs (1×105 cells)
infected with recombinant adenovirus were transfected with Smad4
siRNA (2.5 µg) for 48 h using Lipofectamine 2000
(Invitrogen, Thermo Fisher Scientific, Inc.). For inhibitor
treatment, the recombinant adenovirus-infected CD105+
hDDFCs were incubated with osteogenic medium (OM) consisting of
DMEM, 10% FBS, 1% antibiotics, 0.01 µM 1,25-dihydroxyvitamin
D3, 50 µM ascorbate-2-phosphate, and 10 mM
β-glycerophosphate (Sigma, EMD Millipore, Billerica, MA, USA) in
the presence or absence of the p38 inhibitor (SB203580; 10
µM; cat. no. tlrl-sb20; InvivoGen, San Diego, CA, USA) at
37°C for 24 h for western blot detection of p38 MAPK, 7 days for
osteogenesis-associated gene expression, alkaline phosphatase (ALP)
staining and ALP activity assay, and 21 days for Alizarin Red S
staining.
Adipogenic differentiation
Adipogenic differentiation was performed as
previously described (13).
Briefly, the cells were cultured at 37°C on cover slips at a
density of 3,000 cells/cm2 in adipogenic differentiation
medium consisting of low-glucose DMEM, 10% FBS, 1% antibiotics, 0.5
mM isobutylmethylxanthine, 1 µM dexamethasone, 10 µM
insulin, and 200 µM indomethacin (Sigma, EMD Millipore) for
3 weeks. To confirm differentiation, the cells were stained with
Oil Red O (Sigma, EMD Millipore) for 5 min following induction, and
photographed under an Axiovert inverted microscope (Zeiss AX10;
Carl Zeiss AG, Oberkochen, Germany).
Osteogenic differentiation
Osteogenic differentiation was examined as
previously described (13).
Briefly, the cells were seeded at a density of 3,000
cells/cm2 and cultured at 37°C in OM consisting of DMEM,
10% FBS, 1% antibiotics, 0.01 µM 1,25-dihydroxyvitamin D3,
50 µM ascorbate-2-phosphate, and 10 mM β-glycerophosphate.
Alizarin Red S staining was performed 4 weeks following induction
to confirm calcium deposition.
ALP staining was performed using the BCIP/NBT
Alkaline Phosphatase Color Development kit (Beyotime Institute of
Biotechnology, Jiangsu, China) following 7 days of osteogenic
induction. ALP activity was assessed using an Alkaline Phosphatase
Yellow (pNpp) Liquid Substrate system for ELISA (Sigma, EMD
Millipore) following 7 days of osteogenic induction.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the hDDFCs using TRIzol
reagent (Invitrogen, Thermo Fisher Scientific, Inc.). For the
synthesis of first strand cDNA, AMV reverse transcriptase (Promega,
Madison, WI, USA) was used. qPCR was then performed using the SYBR
Premix Ex Taq kit (Takara Biotechnology Co., Ltd., Dalian, China),
in a volume of 25 µl containing 2 µl cDNA, 12.5
µl SYBR Premix Ex Taq, 0.5 µl each of the forward and
reverse primers, and 9.5 µl RNase-free water, in an ABI 7900
sequence detection system under the following conditions: Initial
denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing at 60°C for 30 sec, and
extension at 72°C for 30 sec. The samples were then normalized to
the expression of GAPDH using the 2−ΔΔCq method
(15). The following primers were
used for qPCR: BMP7, sense 5′-GGG CTT CTC CTA CCC CTA CA-3′ and
antisense 5′-ACG TCT CAT TGT CGA AGC GT-3′; osteopontin (OPN),
sense 5′-AGG CCA AAA TAG AGC TGC CT-3′ and antisense 5′-GTG GTC ATG
GCT TTC GTT GG-3′; osteocalcin (OCN), sense 5′-CGT AGA AGC GCC GAT
AGG C-3′ and antisense 5′-ATG AGA GCC CTC ACA CTC CTC-3′; osterix
(OSX), sense 5′-CTC TGC GGG ACT CAA CAA CT-3′ and antisense 5′-ATG
GAT GCC TGC CTT GTA CC-3′; runt related transcription factor 2
(RUNX2), sense 5′-TCT GGC CTT CCA CTC TCA GT-3′ and antisense
5′-GTC CAC TCT GGC TTT GGG AA-3′; ALP, sense 5′-ACC GCT TCC CAT ATG
TGG CT-3′ and antisense 5′-GGT CTG GAA GTT GCC CTT GA-3′; GAPDH,
sense 5′-ACC ATC TTC CAG GAG CGA GA-3′ and antisense 5′-TGG TTC ACA
CCC ATG ACG AA-3′.
Western blot analysis
Protein was extracted from the cells using RIPA
buffer and protein concentration was quantified with a BCA kit
(Bio-Rad Laboratories, Inc., Richmond, CA, USA). Aliquots of cell
lysates containing equal quantities of protein (30 µg) were
separated by 12% SDS-PAGE and transferred onto nitrocellulose
membranes (Amersham, GE Healthcare Life Sciences, Chalfont, UK).
The membranes were blocked with 5% nonfat milk in TBST overnight,
and incubated with primary antibodies against p-Smad 1/5/8
(1:1,000; cat. no. 9511), Smad1 (1:1,000; cat. no. 9743),
extracellular signal-regulated kinase (ERK; 1:2,000; cat. no.
4696), phosphorylated (p)-ERK (1:1,000; cat. no. 9101), c-Jun
N-terminal kinase (JNK; 1:1,000; cat. no. 9252), and p-JNK
(1:1,000; cat. no. 9251) from Cell Signaling Technology, Inc.
(Beverly, MA, USA); p-p38 (1:500; cat. no. sc-7973), p38 (1:1,000;
cat. no. sc-7972), BMP7 (1:200; cat. no. sc-9305), runt-related
transcription factor 2 (RUNX2; 1:1,000; cat. no. sc-12488), and ALP
(1:200; cat. no. sc-15065) from Santa Cruz Biotechnology, Inc.);
and OSX (1:500; cat. no. ab94744), OPN (1:1,000; cat. no. ab8448),
OCN (1:500; cat. no. ab93876) and GAPDH (1:2,000; cat. no. ab22555)
from Abcam (Cambridge, UK) at 4°C overnight. The membranes were
then washed three times in TBST, and incubated with the
corresponding horseradish peroxidase-conjugated secondary
antibodies (1:5,000; cat. no. 31462 and 32230; both from Thermo
Fisher Scientific, Inc.) at 4°C for 1 h. Signals were detected
using the Pierce ECL western blotting substrate. The quantification
of western blot bands was conducted by comparison against the GAPDH
bands using ImageJ software (version 1.48; National Institutes of
Health, Bethesda, MD, USA).
Induction of bone formation by
hBMP7-transduced fibroblasts in vivo
A total of 10 immunodeficient C57BL/6 male mice aged
4-5 weeks (15±0.30 g) were obtained from Shanghai Second Medical
University Center of Laboratory Animals (Shanghai, China). All
experimental protocols were approved by the Animal Experiment
Committee of Shanghai Second Medical University. Mice had ad
libitum access to food and water and were maintained in a 12-h
light/dark cycle at 21±2°C with a relative humidity of 45±10%. The
mice were injected with CD105+ hDDFCs transfected with
Ad-LacZ (negative control) or Ad-BMP7. Alginate (Sigma, EMD
Millipore) was dissolved in PBS to a concentration of 2% (w/v), and
the adenovirus-infected CD105+ hDDFCs were added to the
alginate solution at a density of 2.5×107 cells/ml. The
cell-alginate preparation was mixed with excessive aqueous slurries
of 2M calcium chloride to produce hydrogels and washed with PBS.
The mice were anesthetized and injected subcutaneously with two 400
µl injections of cell-hydrogel mixture using an 18-gauge
needle into the dorsal panniculus carnosus. The Ad-BMP7-transduced
cells were injected into the right side and Ad-LacZ-transduced
cells were injected into the left side of mice. At 12 weeks
post-implantation, the mice were sacrificed by CO2
asphyxiation. Bone constructs were dissected and surrounding soft
tissue was removed. X-ray images were acquired and the bone
constructs were fixed in 4% paraformaldehyde for the histologic and
immunohistochemical analyses of bone formation.
Bone formation was monitored in vivo by
fixing bone constructs with 4% paraformaldehyde, followed by
decalcifi-cation in 10% formic acid. The samples were dehydrated in
a graded alcohol series, diaphonized in xylene, and embedded in
paraffin. The paraffin blocks were sectioned into 5-µm-thick
slides, deparaffinized, hydrated, stained with hematoxylin and
eosin (H&E) and photographed under an Axiovert inverted
microscope (Zeiss AX10).
Statistical analysis
The results are expressed as the mean ± standard
deviation. Student’s t-test was used for comparisons of two groups
and one-way analysis of variance was used for multiple comparisons.
Data were analyzed using SPSS software (version 14.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Isolation and in vitro differentiation of
CD105+ cells from hDDFCs
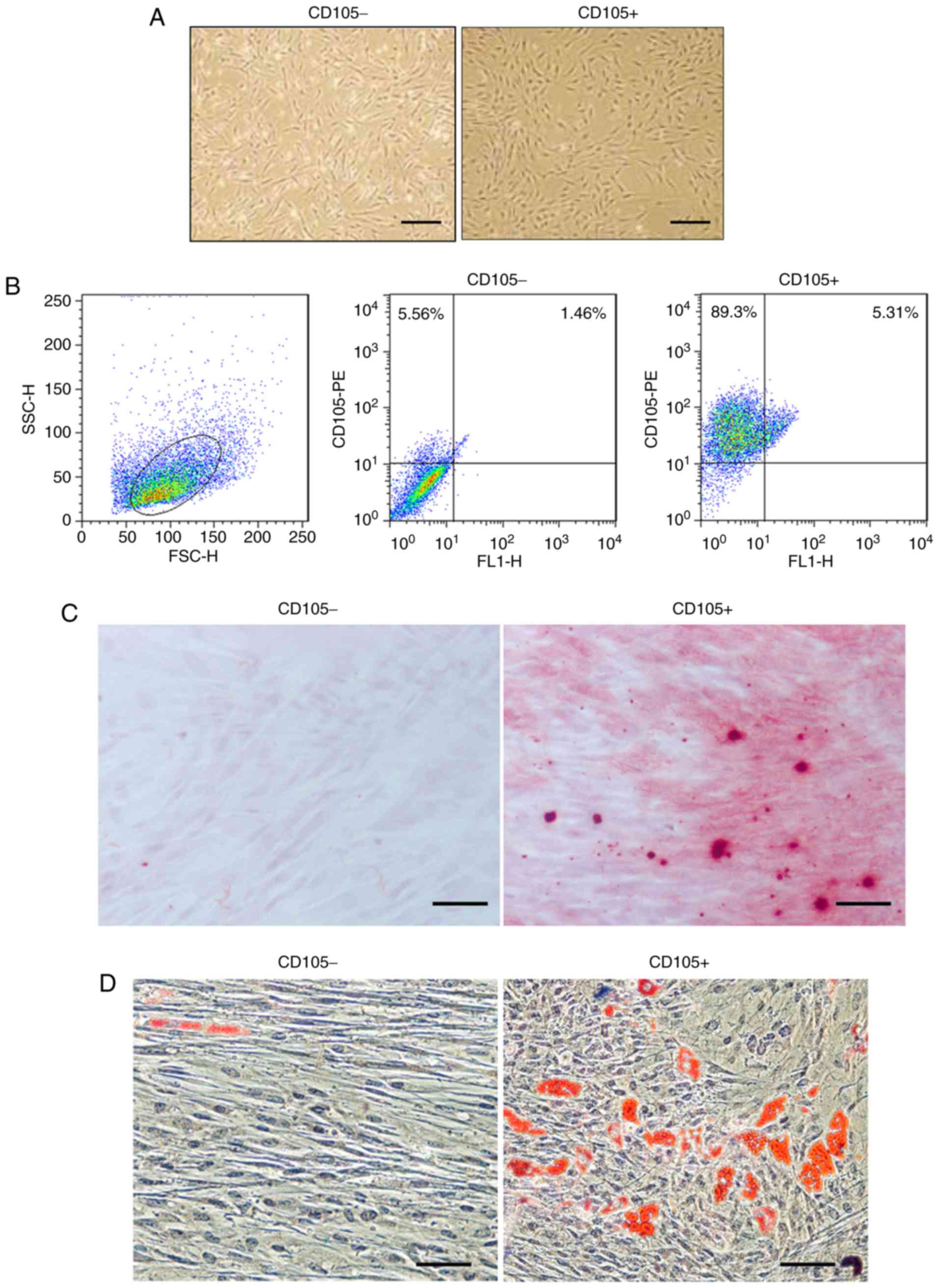
Primary CD105- hDDFCs exhibited an
extended and long narrow shape, whereas CD105+ hDDFCs
exhibited a spindle-like morphology following 12 h in culture
(Fig. 1A). Flow cytometric
analysis confirmed the expression of the surface marker CD105 in
isolated cells and the isolated CD105+ cells had a
purity of 95±2.5% (Fig. 1B). The
differentiation of hDDFCs into osteogenic and adipogenic lineages
was confirmed by Alizarin red staining and Oil-red-O staining 3-4
weeks following induction. Representative images of Alizarin red-
and Oil-red-O-stained CD105- and CD105+ cells
are shown in Fig. 1C and D.
BMP7 enhances the osteogenic
differentiation of CD105+ hDDFCs
To examine the effect of BMP7 on the osteogenic
differentiation potential of CD105+ hDDFCs, the cells
were infected with recombinant adenovirus expressing hBMP7 and
cultured in BM or OM. The expression of BMP7 and
osteogenesis-associated genes was assessed by RT-qPCR and western
blot analyses. The results showed that the expression levels of
RUNX2, OSX, OCN, OPN, and ALP were significantly upregulated by OM,
and BMP7 significantly enhanced this effect (Fig. 2A and B). Alizarin red staining at
21 days post-osteogenic induction showed that BMP7 enhanced the
intensity of staining, indicating increased calcium deposition
(Fig. 2C). BMP7 significantly
increased the expression and activity of ALP by ~1.5-fold in the
cells cultured in OM (Fig. 2D and
E). Taken together, these results indicated that BMP7 has a
potent effect on enhancing the osteogenic differentiation of
CD105+ hDDFCs.
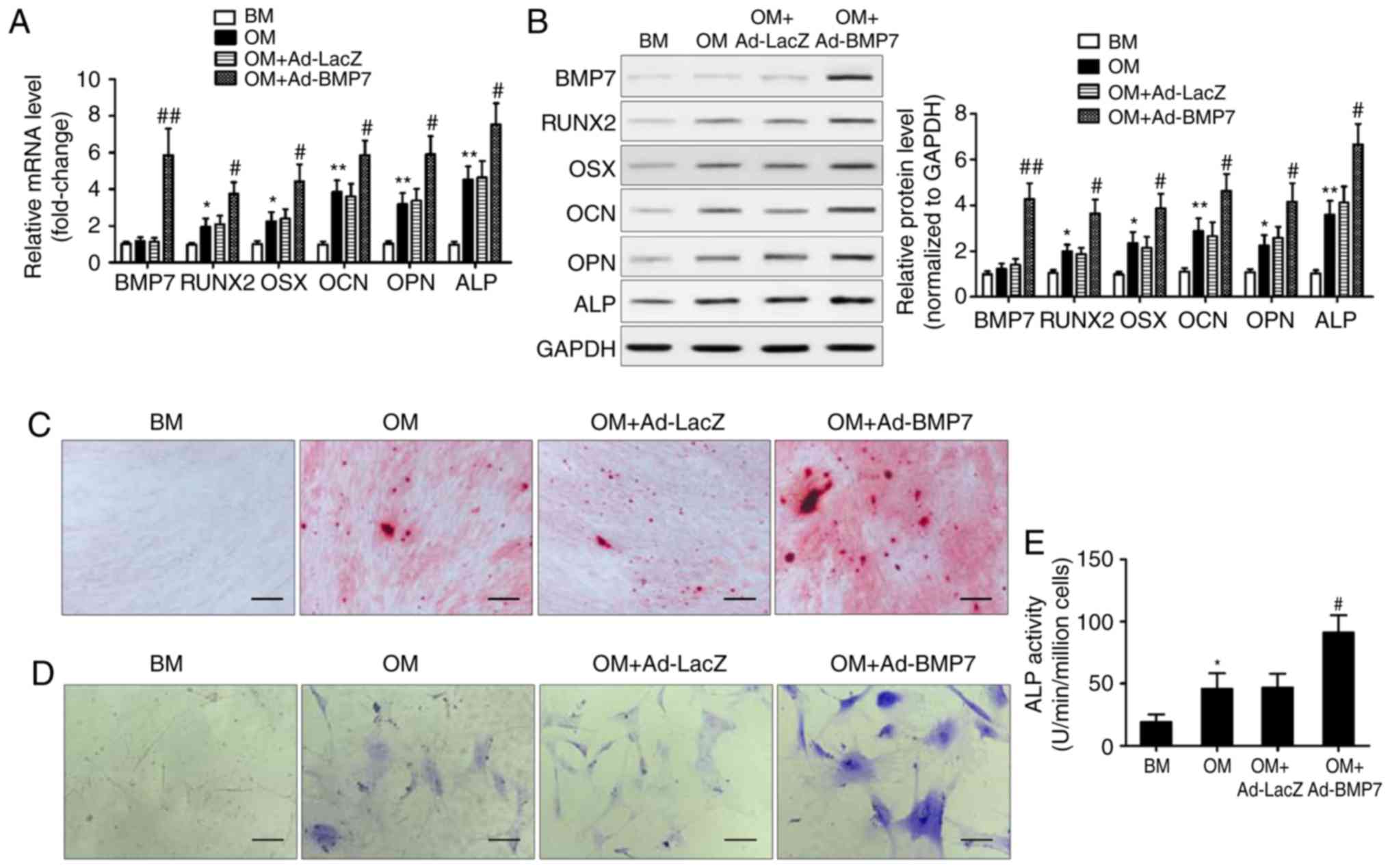 | Figure 2BMP7 promotes the osteogenic
differentiation of CD105+ hDDFCs in vitro.
CD105+ hDDFCs were infected with recombinant adenovirus
expressing human BMP7 for 48 h and cultured in BM or OM. Detection
of BMP7 and osteogenesis-associated gene expression by (A) reverse
transcription-quantitative polymerase chain reaction and (B)
western blot analyses at 7 days. Densitometric quantification of
the immunoblot normalized to GAPDH. *P<0.05 and
**P<0.01, vs. BM; #P<0.05 and
##P<0.01, vs. OM+Ad-LacZ. Osteogenic differentiation
of CD105+ hDDFCs infected with or without recombinant
adenovirus in the presence of BM or OM was determined by (C)
Alizarin Red S at 21 days, (D) ALP staining at 7 days, and an (E)
ALP activity assay at 7 days. Scale bar=200 µm.
*P<0.05, vs. BM; #P<0.05, vs.
OM+Ad-LacZ. hDDFCs, human dermal-derived fibroblast cells; BM,
basal medium; OM, osteogenic medium; BMP7, bone morphogenetic
protein 7; RUNX2, runt related transcription factor 2; OSX,
osterix; OCN, osteocalcin; OPN, osteopontin; ALP, alkaline
phosphatase. |
Activation of Smad and MAPK signaling in
CD105+ hDDFCs
The present study further examined the mechanisms
underlying the effect of BMP7 on the osteogenic differentiation of
CD105+ hDDFCs by assessing the expression of proteins
associated with the BMP canonical Smad-dependent and non-canonical
Smad-independent pathways. The results of the western blot analysis
and densitometric quantification showed that BMP7-expressing
adenovirus infection significantly enhanced the OM-induced
upregulation of p-Smad1/5/8, indicating the activation of Smad
signaling (Fig. 3A). OM treatment
induced the phosphorylation of ERK, p38 and JNK, and the
overexpression of BMP7 further upregulated the OM-induced
phosphorylated form of p38 (Fig.
3B). These results indicated the activation of p38/MAPK
signaling by BMP7 in the CD105+ hDDFCs.
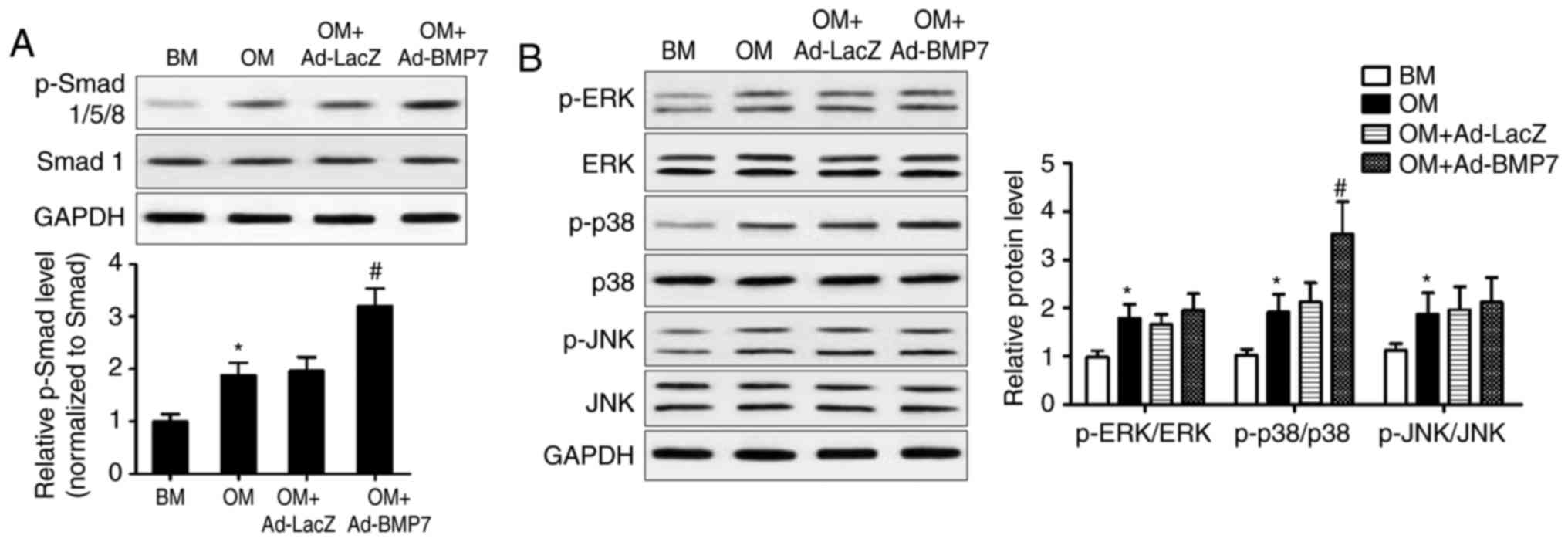 | Figure 3BMP7 activates Smad and MAPK pathways
in CD105+ hDDFCs cells. CD105+ hDDFCs or
BMP7-expressing adenovirus-infected CD105+ hDDFCs were
treated with BM or OM, and the phosphorylation of (A) Smad- and (B)
MAPK-related proteins was analyzed by western blot analysis
following 24 h of culture. Densitometric quantification of the
immunoblot normalized to total protein. *P<0.05, vs.
BM; #P<0.05, vs. OM+Ad-LacZ. hDDFCs, human
dermal-derived fibroblast cells; BMP7, bone morphogenetic protein
7; BM, basal medium; OM, osteogenic medium; Smad, small mothers
against decapentaplegic; MAPK, mitogen-activated protein kinase;
ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; p-, phosphorylated. |
Smad4 knockdown impairs the promotion of
osteogenic differentiation of CD105+ hDDFCs induced by
BMP7
To further elucidate the involvement of the Smad
pathway in the effect of BMP7, the present study examined the
effect of Smad4 knockdown on the enhancing effect of BMP7 on the
OM-induced osteogenic differentiation of CD105+ hDDFCs.
The silencing of Smad eliminated the BMP7-induced upregulation of
RUNX2, OSX, OCN, OPN and ALP in the presence of OM, compared with
that in the si-Control transfected cells (Fig. 4A). Consistent with these results,
Smad knockdown reversed the BMP7-induced increase in Alizarin red
and ALP staining (Fig. 4B) and
ALP activity (Fig. 4C),
indicating that the effect of BMP7 on promoting the osteogenic
differentiation of CD105+ hDDFCs occurred via a
Smad-dependent pathway. The expression of p38 MAPK (p38/p-p38) was
also measured and the results showed that it was not altered by
siR-Smad4 (data not shown).
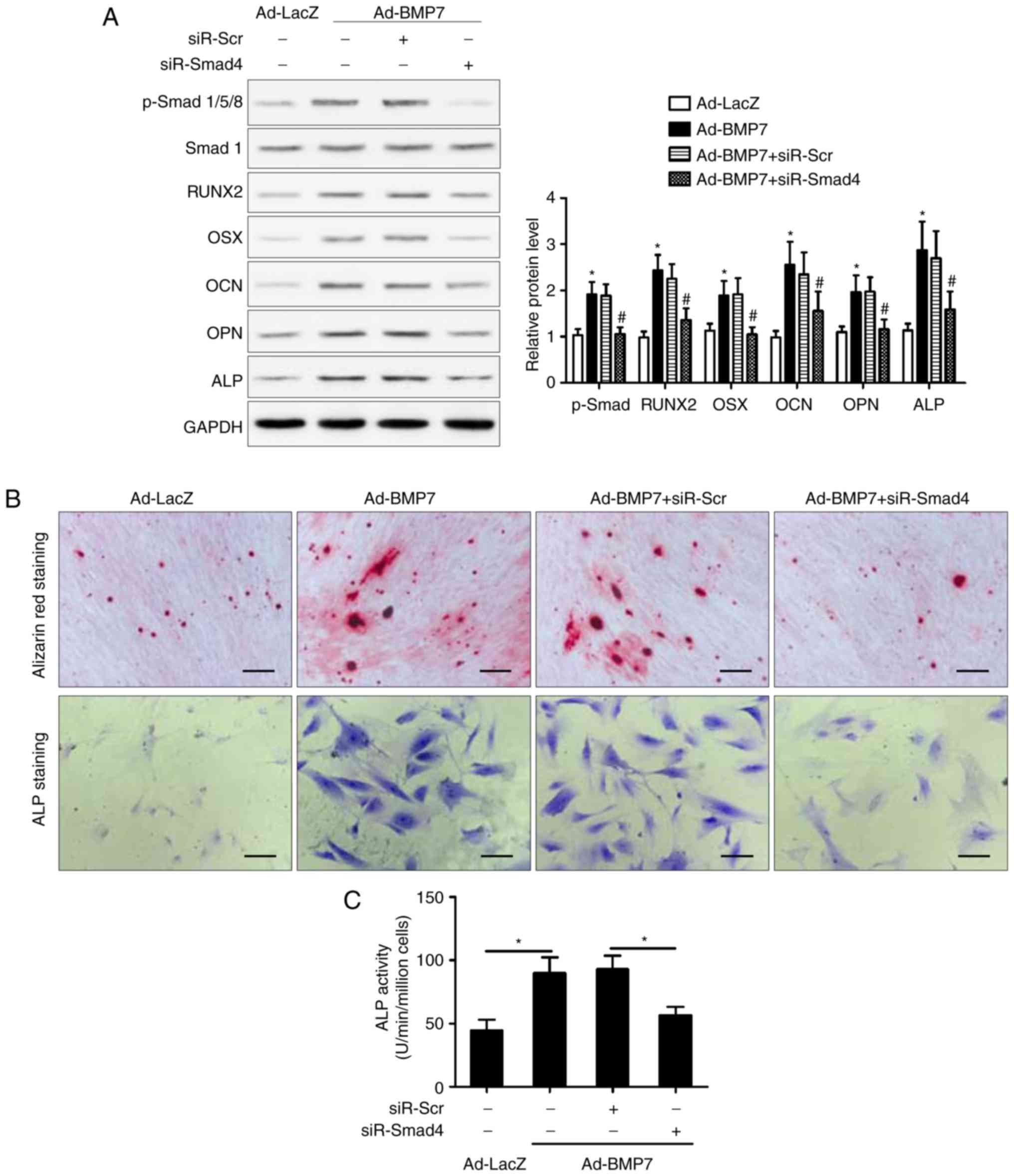 | Figure 4SMAD4 knockdown attenuates the
BMP7-induced promotion of CD105+ hDDFCs osteogenic
differentiation. (A) Recombinant adenovirus-infected
CD105+ hDDFCs transfected with siRNA for Smad4 or
control were subjected to western blot analysis for the detection
of Smad/p-Smad and osteogenesis- associated gene expression 7 days
following osteogenic induction. Densitometric quantification of
p-Smad was normalized to total Smad; densitometric quantification
of other proteins was normalized to GAPDH. *P<0.05,
vs. Ad-LacZ; #P<0.05, vs. Ad-BMP7+siR-Scr. Osteogenic
differentiation of CD105+ hDDFCs infected with or
without recombinant adenovirus in presence of osteogenic medium was
determined by (B) Alizarin Red S and ALP staining and an (C) ALP
activity assay 7 or 21 days following osteogenic induction. Scale
bar=200 µm. *P<0.05. hDDFCs, human
dermal-derived fibroblast cells; Smad, small mothers against
decapentaplegic; siR, small interfering RNA; Scr, scramble; BMP7,
bone morphogenetic protein 7; RUNX2, runt related transcription
factor 2; OSX, osterix; OCN, osteocalcin; OPN, osteopontin; ALP,
alkaline phosphatase; p-, phosphorylated. |
Inhibition of p38/MAPK attenuates the
osteogenic differentiation of CD105+ hDDFCs induced by
BMP7
To determine the role of the p38 MAPK pathway, the
cells were treated with the p38 inhibitor SB203580 and incubated in
OM in the presence or absence of BMP7. The results of the western
blot analysis showed that inhibition of p38 attenuated the
BMP7-induced upregulation of OSX, OCN, OPN, and ALP, whereas the
expression of RUNX2 was not affected by the inhibition of p38
(Fig. 5A). The Alizarin red and
ALP staining showed that the inhibition of p38 attenuated the
effect of BMP7 on enhancing the OM-induced osteogenic
differentiation of CD105+ hDDFCs (Fig. 5B). In addition, treatment with
SB203580 reversed the BMP7-induced increase in ALP activity
(Fig. 5C). Taken together, these
results indicated that the p38 MAPK signaling pathway was involved
in the enhancement of the OM-induced osteogenic differentiation of
CD105+ hDDFCs by BMP7. The results also showed that p38
inhibitor SB203580 suppressed BMP7-induced Smad1/5/8
phosphorylation (data not shown).
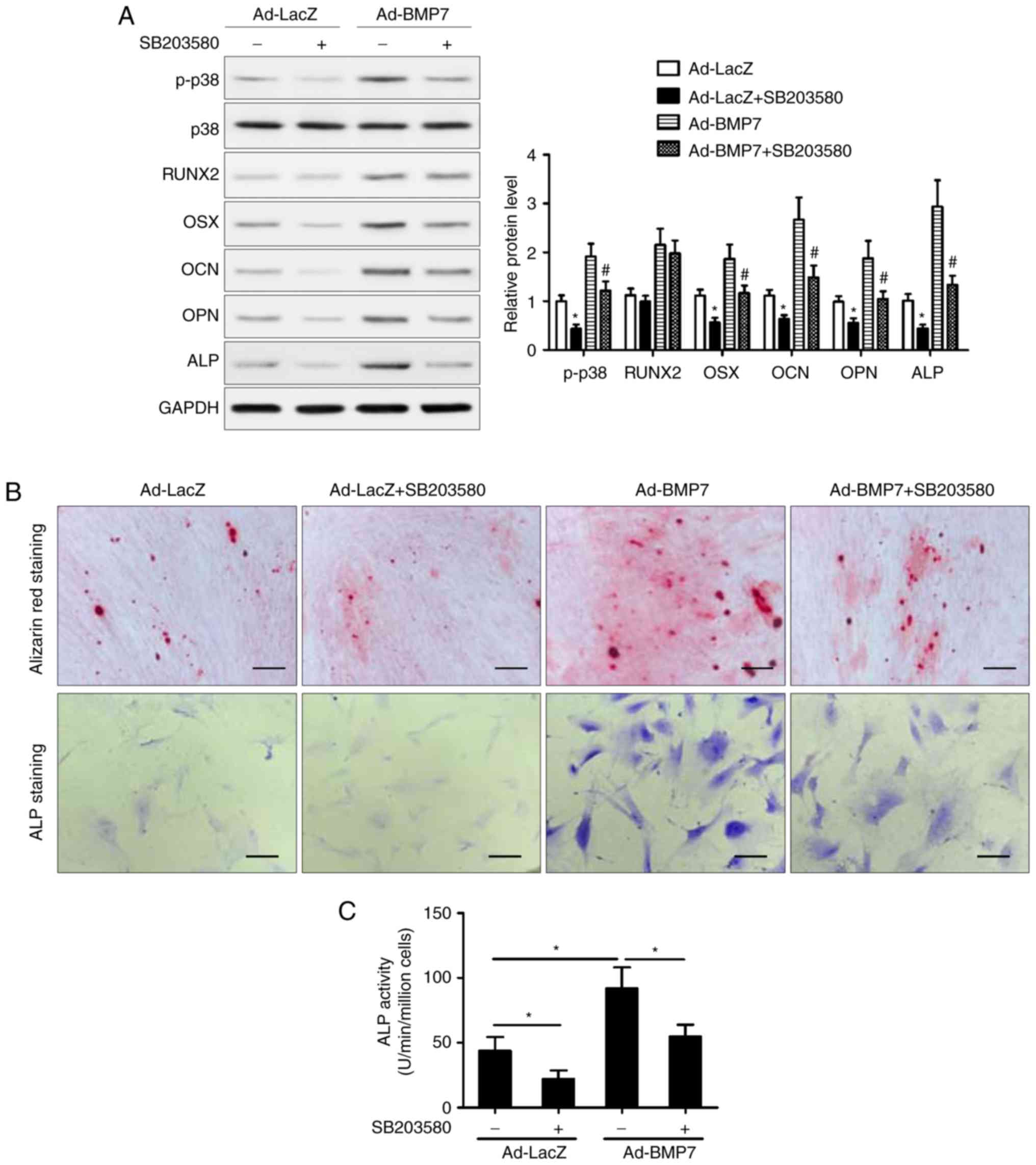 | Figure 5Inhibition of p38 MAPK attenuates the
BMP7-induced promotion of CD105+ hDDFCs osteogenic
differentiation. Recombinant adenovirus infected CD105+
hDDFCs were incubated with OM in the presence or absence of the p38
inhibitor (SB203580, 10 µM). (A) Western blot detection of
p38 MAPK following 24 h of culture in OM, and
osteogenesis-associated gene expression 7 days following osteogenic
induction. Densitometric quantification of p-p38 was normalized to
total p38; densitometric quantification of other proteins was
normalized to GAPDH. *P<0.05, vs. Ad-LacZ;
#P<0.05, vs. Ad-BMP7. Osteogenic differentiation of
CD105+ hDDFCs infected with or without recombinant
adenovirus in the presence of BM or OM was determined by (B)
Alizarin Red S and ALP staining, and an (C) ALP activity assay 7 or
21 days following osteogenic induction. Scale bar=200 µm.
*P<0.05. hDDFCs, human dermal-derived fibroblast
cells; BMP7, bone morphogenetic protein 7; BM, basal medium; OM,
osteogenic medium; Smad, small mothers against decapentaplegic;
BMP7, bone morphogenetic protein 7; RUNX2, runt related
transcription factor 2; OSX, osterix; OCN, osteocalcin; OPN,
osteopontin; ALP, alkaline phosphatase; p-, phosphorylated. |
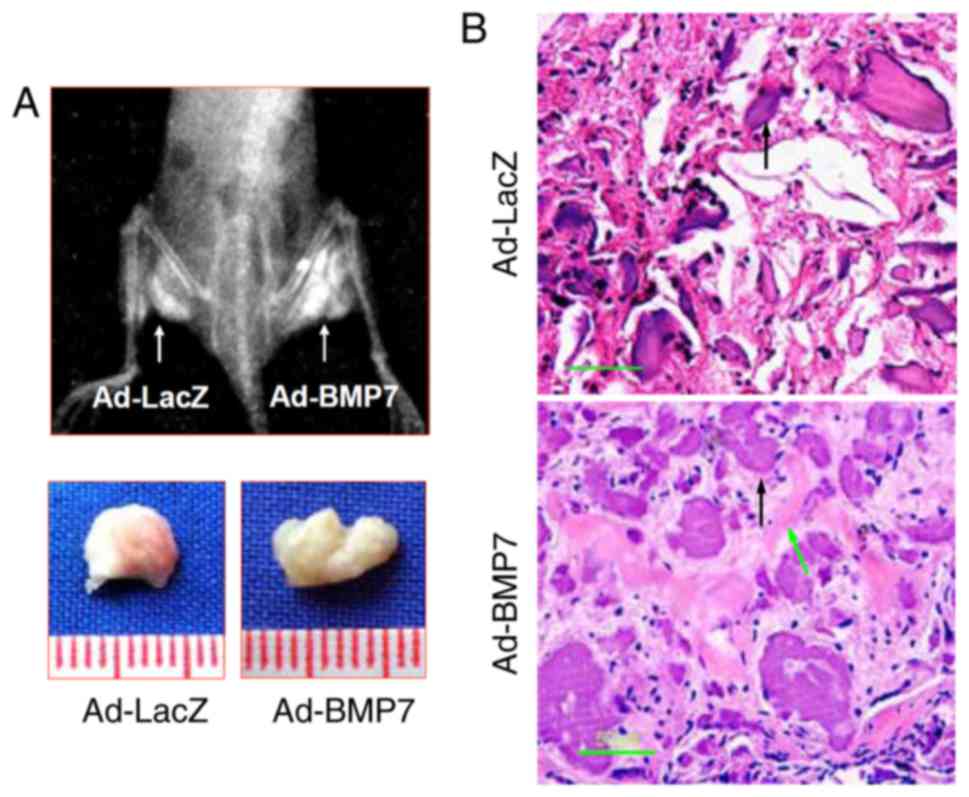
BMP7 enhances bone formation of
CD105+ hDDFCs in vivo
The effects of BMP7 on osteogenic differentiation
were examined in nude mice injected intramuscularly with adenovirus
infected CD105+ hDDFCs. Representative X-ray images and
bone volumes from mice are shown in Fig. 6A, indicating enhanced bone
formation by BMP7 in vivo. The H&E staining of tissue
sections showed enhanced trabecular bone formation in Ad-BMP7
sections compared with Ad-LacZ sections (Fig. 6B). These results confirmed the
effect of BMP7 on promoting the osteogenic differentiation of
CD105+ hDDFCs in an in vivo model.
Discussion
The adult mammalian dermis contains a subpopulation
of precursor cells that possess the capacity to differentiate into
different lineages (16-18). These fibroblastic MSCs have
attracted attention for their plasticity and, therefore, their
potential therapeutic applications, including in transplantation
for bone formation (19). In the
present study, the role of BMP7 in the osteogenic differentiation
of CD105+ hDDFCs was examined in vitro and in
vivo, and the underlying Smad-dependent and -independent
mechanisms were identified.
Conflicting reports exist on the differentiation
potential of dermal fibroblasts, with certain studies suggesting
limited potential and others demonstrating adipocytic, osteocytic
and chondrocytic differentiation capacities (20-23). One reason for these controversial
results is the heterogeneity of isolated dermal fibroblasts, which
include populations with different differentiation capacities
(5,13). Although dermal fibroblasts have a
surface antigen profile similar to that of MSCs and ADSCs, human
foreskin-derived dermal fibroblasts do not always have the
potential to differentiate into adipogenic or osteogenic lineages.
This is a result of only a fraction of dermal-derived fibroblasts
being positive for CD105, and the expression of CD105 determines
the properties of MSCs (5,24).
In the present study, a CD105+ subpopulation of hDDFCs
was isolated from human foreskin specimens and it was shown that
they possessed the capacity to differentiate into osteogenic and
adipogenic lineages by incubation in the corresponding induction
medium. Furthermore, the overexpression of BMP7 via adenovirus
infection enhanced the BM-induced osteogenic differentiation of
CD105+ hDDFCs. In a previous study, rat dermal
fibroblasts transduced with an adenovirus vector engineered to
express BMP7 were transplanted into immunocompromised mice. These
transduced dermal fibroblasts formed bone and repaired skeletal
defects, indicating the role of BMP7 in promoting bone formation
in vivo (25). Similarly,
BMP7-transduced dermal fibroblasts repaired segmental defects in
rat femurs when injected subcutaneously, and only BMP7-transduced
fibroblasts formed bone in diffusion chambers, suggesting that BMP
induces osteoblastic differentiation of fibroblasts (22). These studies support the findings
of present study and suggest that further investigations is
warranted to assess the potential of BMP7-expressing
CD105+ hDDFCs for in vivo applications.
To investigate the mechanisms underlying the effect
of BMP7 on the osteogenic differentiation of CD105+
hDDFCs, the present study examined Smad-dependent and -independent
pathways. The results showed that the overexpression of BMP7
enhanced the OM-induced activation of Smad and MAPK signaling.
Furthermore, Smad4 knockdown or the inhibition of p38 MAPK
signaling suppressed the effect of BMP7 on enhancing the OM-induced
osteogenic differentiation of CD105+ hDDFCs, indicating
the involvement of the two signaling pathways. The role of the
BMP/Smad pathways in regulating osteoblast differentiation has been
investigated extensively (26).
TGF-β signals are transmitted through the formation of type I and
type II serine/threonine kinase receptor complexes (27). The conserved canonical TGF-β/BMP
signaling cascade depends on cell surface BMP receptors that
mediate the phosphorylation of Smad proteins, which translocate
into the nucleus to regulate the transcription of specific genes
(28). In the non-canonical
Smad-independent signaling pathway, the activation of p38 MAPK
mediates the differentiation of mesenchymal precursor cells
(29). In response to BMP
induction, the Smad and p38 MAPK pathways converge at the Runx2
gene to control MSC differentiation, and the activity of Runx2 and
BMP-activated Smads is essential for bone formation. The results of
the present study showed that the expression of Runx2 was not
affected by p38 MAPK inhibition; further experiments are required
to determine the involvement of Runx2 in the BMP7-induced
osteogenic differentiation of CD105+ hDDFCs. However,
the present study demonstrated the involvement of the two pathways
in the effect of BMP7. BMP2-induced osteogenic differentiation and
increased bone formation have previously been shown to be mediated
by the activation of Smad and p38 signaling pathways in MC3T3-E1
preosteoblasts (30). Crosstalk
between these two pathways and other signaling pathways, including
Wnt, Hedgehog and Notch, are key in BMP signaling; therefore,
understanding the mechanisms underlying their effect on the
induction of osteoblastogenesis and bone formation is essential for
their clinical application (31-35).
In the present study, an in vivo ectopic bone
formation model was used to confirm that the ectopic expression of
BMP7 enhanced bone formation in nude mice injected intramuscularly
with adenovirus-infected CD105+ hDDFCs. The increasing
use of BMP-containing osteogenic implants for the treatment of
bone-related diseases requires the development of effective
delivery systems and cell sources. The present study provides a
rationale for the use of skin-derived precursors with the potential
to differentiate into an osteogenic lineage and demonstrated their
efficacy in vivo. This provides the basis for further
investigations to establish systems for the use of subpopulations
of hDDFCs as a cellular source for tissue engineering.
In conclusion, the present study showed that a
subpopulation of dermal fibroblasts with positive expression of
CD105 offer potential for osteogenic differentiation, which is
enhanced by the induction of BMP7and mediated by the activation of
Smad and p38/MAPK signaling. These data indicate that adenoviral
BMP7 gene transfer in CD105+ hDDFCs may be an effective
tool for bone tissue engineering.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81272126).
Availability of data and materials
All data used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
FC made substantial contributions to the design of
this study and wrote the manuscript. DB and CC performed
experiments. SM, YL and KC performed data analysis. DB, CC, SM, YL
and KC revised the critically. All authors read and approved the
final manuscript and agreed to the publication of the final
manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki for investigations involving human
subjects, and was approved by the Ethics Committee of Shanghai
Ninth People’s Hospital Affiliated to Shanghai Second Medical
University (Shanghai Ninth People’s Hospital, Shanghai Jiao Tong
University School of Medicine). All patients provided written
informed consent. All anima experiment protocols were approved by
the Animal Experiment Committee of Shanghai Second Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sorrell JM and Caplan AI: Fibroblasts-a
diverse population at the center of it all. Int Rev Cell Mol Biol.
276:161–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang F, Ni K, Cai Y, Ye Z, Shang J, Shen S
and Xiong C: Biological characters of human dermal fibroblasts
derived from foreskin of male infertile patients. Tissue Cell.
49:56–63. 2017. View Article : Google Scholar
|
|
3
|
Bussmann BM, Reiche S, Marí-Buyé N,
Castells-Sala C, Meisel HJ and Semino CE: Chondrogenic potential of
human dermal fibroblasts in a contractile, soft, self-assembling,
peptide hydrogel. J Tissue Eng Regen Med. 10:E54–E62. 2016.
View Article : Google Scholar
|
|
4
|
Guerreiro SG, Oliveira MJ, Barbosa MA,
Soares R and Granja PL: Neonatal human dermal fibroblasts
immobilized in RGD-alginate induce angiogenesis. Cell Transplant.
23:945–957. 2014. View Article : Google Scholar
|
|
5
|
Lee SB, Shim S, Kim MJ, Shin HY, Jang WS,
Lee SJ, Jin YW, Lee SS and Park S: Identification of a distinct
subpopulation of fibroblasts from murine dermis: CD73(-) CD105(+)
as potential marker of dermal fibroblasts subset with multipotency.
Cell Biol Int. 40:1008–1016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesen-chymal stromal cells. The International Society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar
|
|
7
|
Lorenz K, Sicker M, Schmelzer E, Rupf T,
Salvetter J, Schulz-Siegmund M and Bader A: Multilineage
differentiation potential of human dermal skin-derived fibroblasts.
Exp Dermatol. 17:925–932. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar :
|
|
10
|
Lee KS, Hong SH and Bae SC: Both the Smad
and p38 MAPK pathways play a crucial role in Runx2 expression
following induction by transforming growth factor-beta and bone
morphogenetic protein. Oncogene. 21:7156–7163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Guo J, Wu G and Zhou Y: Effects
of heterodimeric bone morphogenetic protein-2/7 on osteogenesis of
human adipose-derived stem cells. Cell Prolif. 48:650–660. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Myllylä RM, Haapasaari KM, Lehenkari P and
Tuukkanen J: Bone morphogenetic proteins 4 and 2/7 induce
osteogenic differentiation of mouse skin derived fibroblast and
dermal papilla cells. Cell Tissue Res. 355:463–470. 2014.
View Article : Google Scholar
|
|
13
|
Chen FG, Zhang WJ, Bi D, Liu W, Wei X,
Chen FF, Zhu L, Cui L and Cao Y: Clonal analysis of nestin(−)
vimentin(+) multi-potent fibroblasts isolated from human dermis. J
Cell Sci. 120:2875–2883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aslan H, Zilberman Y, Kandel L, Liebergall
M, Oskouian RJ, Gazit D and Gazit Z: Osteogenic differentiation of
noncultured immunoisolated bone marrow-derived CD105+
cells. Stem Cells. 24:1728–1737. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Crigler L, Kazhanie A, Yoon TJ, Zakhari J,
Anders J, Taylor B and Virador VM: Isolation of a mesenchymal cell
population from murine dermis that contains progenitors of multiple
cell lineages. FASEB J. 21:2050–2063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toma JG, Akhavan M, Fernandes KJ,
Barnabé-Heider F, Sadikot A, Kaplan DR and Miller FD: Isolation of
multipotent adult stem cells from the dermis of mammalian skin. Nat
Cell Biol. 3:778–784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
French MM, Rose S, Canseco J and
Athanasiou KA: Chondrogenic differentiation of adult dermal
fibroblasts. Ann Biomed Eng. 32:50–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirata K, Tsukazaki T, Kadowaki A,
Furukawa K, Shibata Y, Moriishi T, Okubo Y, Bessho K, Komori T,
Mizuno A and Yamaguchi A: Transplantation of skin fibroblasts
expressing BMP-2 promotes bone repair more effectively than those
expressing Runx2. Bone. 32:502–512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brendel C, Kuklick L, Hartmann O, Kim TD,
Boudriot U, Schwell D and Neubauer A: Distinct gene expression
profile of human mesenchymal stem cells in comparison to skin
fibroblasts employing cDNA microarray analysis of 9600 genes. Gene
Expr. 12:245–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeney F, Bazsó-Dombi E, Oravecz K, Szabó J
and Nagy IZ: Cytochemical studies on the fibroblast-preadipocyte
relationships in cultured fibroblast cell lines. Acta Histochem.
102:381–389. 2000. View Article : Google Scholar
|
|
22
|
Rutherford RB, Moalli M, Franceschi RT,
Wang D, Gu K and Krebsbach PH: Bone morphogenetic
protein-transduced human fibroblasts convert to osteoblasts and
form bone in vivo. Tissue Eng. 8:441–452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizuno S and Glowacki J: Low oxygen
tension enhances chondroinduction by demineralized bone matrix in
human dermal fibroblasts in vitro. Cells Tissues Organs.
180:151–158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lysy PA, Smets F, Sibille C, Najimi M and
Sokal EM: Human skin fibroblasts: From mesodermal to
hepatocyte-like differentiation. Hepatology. 46:1574–1585. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krebsbach PH, Gu K, Franceschi RT and
Rutherford RB: Gene therapy-directed osteogenesis: BMP-7-transduced
human fibroblasts form bone in vivo. Hum Gene Ther. 11:1201–1210.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao CY, Wang YG, Zhang X, Zheng XY, Tang
TT and Lu EY: Double-edged-sword effect of IL-1beta on the
osteogenesis of periodontal ligament stem cells via crosstalk
between the NF-kappaB, MAPK and BMP/Smad signaling pathways. Cell
Death Dis. 7:e22962016. View Article : Google Scholar
|
|
27
|
Moustakas A, Souchelnytskyi S and Heldin
CH: Smad regulation in TGF-beta signal transduction. J Cell Sci.
114:4359–4369. 2001.
|
|
28
|
Guo X and Wang XF: Signaling cross-talk
between TGF-beta/BMP and other pathways. Cell Res. 19:71–88. 2009.
View Article : Google Scholar
|
|
29
|
Beederman M, Lamplot JD, Nan G, Wang J,
Liu X, Yin L, Li R, Shui W, Zhang H, Kim SH, et al: BMP signaling
in mesenchymal stem cell differentiation and bone formation. J
Biomed Sci Eng. 6:32–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi H, Jeong BC, Kook MS and Koh JT:
Betulinic acid synergically enhances BMP2-induced bone formation
via stimulating Smad 1/5/8 and p38 pathways. J Biomed Sci.
23:452016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horikiri Y, Shimo T, Kurio N, Okui T,
Matsumoto K, Iwamoto M and Sasaki A: Sonic hedgehog regulates
osteoblast function by focal adhesion kinase signaling in the
process of fracture healing. PLoS One. 8:e767852013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reichert JC, Schmalzl J, Prager P, Gilbert
F, Quent VM, Steinert AF, Rudert M and Nöth U: Synergistic effect
of Indian hedgehog and bone morphogenetic protein-2 gene transfer
to increase the osteogenic potential of human mesenchymal stem
cells. Stem Cell Res Ther. 4:1052013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JH, Liu X, Wang J, Chen X, Zhang H,
Kim SH, Cui J, Li R, Zhang W, Kong Y, et al: Wnt signaling in bone
formation and its therapeutic potential for bone diseases. Ther Adv
Musculoskelet Dis. 5:13–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bessa PC, Casal M and Reis RL: Bone
morphogenetic proteins in tissue engineering: The road from the
laboratory to the clinic, part I (basic concepts). J Tissue Eng
Regen Med. 2:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rahman MS, Akhtar N, Jamil HM, Banik RS
and Asaduzzaman SM: TGF-β/BMP signaling and other molecular events:
Regulation of osteoblastogenesis and bone formation. Bone Res.
3:150052015. View Article : Google Scholar
|