Introduction
Obesity, a metabolic disease caused by excessive
nutrition primarily due to the consumption of high-fat diets (HFDs)
affects millions of individuals in developed countries and its
prevalence in developing countries is also gradually increasing. A
study in developing countries indicated that overweight and obesity
are increasing among women of reproductive age in urban Africa,
with obesity among this age group more than doubled or tripled in
12/24 countries surveyed (1),
whereas in developed countries such as the USA, data from 2015
estimate that at least one-third (36.5%) of US adults are obese
(2). This makes overweight and
obesity global public health problems. Obesity is also viewed as
the leading most preventable cause of mortality (2). There is evidence that the risks of
coronary heart disease, ischemic stroke, hypertension,
hyperlipidemia, liver disorders and Type 2 diabetes mellitus
increase steadily with overweight and obesity (3-6).
Oxidative stress serves a key function in obesity and its
associated complications. Obesity is able to induce systemic
oxidative stress through various biochemical mechanisms, such as
superoxide generation, oxidative phosphorylation, glyceraldehyde
auto-oxidation and protein kinase C activation (7). Other factors that contribute to
oxidative stress in obesity include hyperleptinemia, low
antioxidant defense, chronic inflammation and postprandial reactive
oxygen species generation (7).
Systemic oxidative stress and inflammation serve key functions in
the pathogenesis of obesity-associated diseases, including
atherosclerosis, insulin resistance, Type 2 diabetes and cancer
(8).
The current rate at which the prevalence of obesity
is increasing implies that diet control and exercise alone are
insufficient to prevent or control obesity. Anti-obesity
therapeutic agents such as orlistat, lorcaserin, liraglutide,
phentermine/topiramate and naltrexone/bupropion are currently in
use for treating obesity (9).
Although these agents have proven beneficial in managing weight
gain in obesity, they also led to marked side effects to patients
such as stomach ache, paresthesia, vomiting, insomnia,
constipation, headache and nausea. The majority of the drugs are
contraindicated in patients with cardiovascular diseases and those
with a high risk of cardiovascular diseases (9). For these reasons, there is a
requirement to develop other anti-obesity agents with fewer or less
marked side effects. Herbal medicines have been exploited and used
for weight control in many countries. A good example is Garcinia
cambogia with its main active compound, hydroxycitric acid,
which is widely becoming a popular natural product ingredient in
weight loss supplements and has no toxic effects. G.
cambogia with its hydroxycitric acid has been revealed to be
safe when taken orally as it did not lead to any abnormal changes
in hepatic and testicular lipid peroxidation, hematological, DNA
fragmentation or histopathological changes, and was identified to
be bioavailable in plasma following gas chromatography-mass
spectrometry analysis (10-12). Furthermore, the main active
compound of G. cambogia exhibits its anti-obesity properties
in weight management in animals (12), including humans (13,14), by promoting fat oxidation,
inhibiting ATP-citrate lyase, the building block for fat synthesis,
and also lowering leptin levels in obese subjects (12). Furthermore, phytochemicals from
plants as well as daily fermented foods have been identified to
prevent weight gain and decrease the incidence of metabolic
diseases by acting through several molecular mechanisms, such as
cell signaling and modulation of gene expression, decreasing
obesity-induced oxidative stress, production of inflammatory
molecules and lipid accumulation (15,16).
Muscat Bailey A (MBA) grape (Vitis labruscax
Vitis vinifera) is one of the principal grape varieties
grown in Korea. Its grape stalk is an organic waste produced in
marked amounts during the vinification of grape. Previous studies
have indicated that grape stalk is rich in bioactive phenolic
compounds and exhibits antioxidant and UV-protective activities in
in vivo studies in mice (17-19). Mattos et al (19) also suggested that the functional
properties of grape wastes which are rich in phenolic compounds may
be exploited to develop products ranging from medical to food
applications. Although other parts of the grape plant such as the
seed and fruits have been investigated for their anti-obesity
effects for which they exhibit anti-obesity potencies (20,21), to the best of our knowledge,
limited or no study has been performed to investigate the
anti-obesity effects of the grape stalk. Therefore, in order to
elucidate the effects of the grape stalk in obesity, a grape stalk
extract was investigated for its effects on adipocyte
differentiation in cell studies, and on HFD-induced obesity in mice
in vivo studies. We hypothesize that grape stalk may also
have anti-obesity potencies in vitroand in vivo. To
test this hypothesis, the antioxidant capacity and phytochemical
constituents of grape stalk harvested in different periods of the
year were investigated. Furthermore, the effects of Muscat Bailey A
grape stalk extracts (MGSE) were investigated on adipocyte
differentiation and in HFD-induced mice in vivo studies,
where a number of parameters associated with obesity were
investigated. In the present study, G. cambogia extract
(GCE) was used as a control, because of its main active compound,
hydroxycitric acid, to determine the effects of MGSE.
Materials and methods
Grape stalk extracts preparation
Muscat Bailey A grape stalks were separately
harvested in June, July, August and September 2016 from Jeongeup
(Korea). Each harvest was washed and dried at 40°C for 72 h and
then extracted (50 g) in 80% ethanol for 3 days. The extracts were
filtered through a 0.45-µm filter paper (Advantec; Toyo
Kaisha, Ltd., Japan), concentrated at decreased pressure and
freeze-dried to obtain the powder samples.
Total phenol and flavonoid contents, and
in vitro antioxidant activity
The total phenolic and flavonoid contents of the
various extracts harvested in the different time periods were
determined using methods described previously by Cho et al
(22). The total phenolic and
flavonoid contents were expressed as gallic acid and quercetin
equivalents, respectively. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)
radical-scavenging activities of the various extracts were
determined as described previously (22). Butylated hydroxytoluene and Trolox
were used as standards for the DPPH and ABTS scavenging activities,
respectively. All extracts were investigated in a set of three
separate experiments.
High-performance liquid chromatography
(HPLC) analysis
HPLC was performed using an Agilent 1200 series
instrument (Agilent Technologies, Inc., Santa Clara, CA, USA),
equipped with a binary pump delivery system, a degasser (G1379A),
an autosampler (G1313A) and a diode array detector (G1315B).
Compound separation was performed on an AegisPak C18
column (4.6×200 mm; 3 µm pore size) through a gradient
elution with 0.1% aqueous formic acid (A) and acetonitrile (B): 0
min, 20% B; 5 min, 20% B; 12 min, 30% B; 20 min, 60% B; 30 min, 80%
B; 34 min, 80% B; 37 min, 60% B; 40 min, 20% B. The mixture was
held for 10 min before returning to the initial conditions. The
flow rate of the mobile phase was 0.5 ml/min and the column
temperature was 35°C. The injection volume was 15 µl and UV
detection was monitored at 320 nm. All standards [(+)-catechin,
(−)-epicatechin, rutin, trans-resveratrol and quercetin]
were identified on the basis of retention times, and concentrations
were calculated on the basis of comparison with sample peak areas
obtained from various standards.
3T3-L1 cell culture, differentiation and
Oil Red O staining
3T3-L1 pre-adipocytes, from the American Type
Culture Collection (Manassas, VA, USA), were grown in Dulbecco’s
medium Eagle’s medium (DMEM; Hyclone; GE Healthcare; Logan, UT,
USA) supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare), 1% penicillin (10,000 U/ml) and 1% streptomycin (10
g/ml). To induce adipocyte differentiation, the 3T3-L1
pre-adipocytes were seeded in 6-mm cell culture dishes at a density
of 5×104 cells/ml. After 2 days when the cells had
reached 100% confluence, differentiation was induced by replacing
the medium with DMEM containing 10% FBS, 0.5 mM
3-isobutyl-1-methylxanthine (IBMX), 1 µM dexamethasone
(DEX), 1 µg/ml insulin and MGSE at 25, 50 and 100
µg/ml. After 3 days, the medium was replaced with DMEM
containing FBS, insulin and MGSE at 25, 50 and 100 µg/ml.
After 2 days, the medium was replaced with DMEM containing FBS,
insulin and MGSE at 25, 50 and 100 µg/ml. For Oil Red O
staining, cells in wells were fixed with 10% formalin for 1 h and
then washed with 60% propan-2-ol before being incubated in 5 ml Oil
Red O working solution for 5 min at room temperature. Following
incubation, the cells were immediately washed four times with
distilled water and images were captured under a Leica light
microscope. Then, Oil Red O was dissolved in 100% propan-2-ol and
absorbance was determined using a microplate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA) at 540 nm.
Triacylglycerol (TG) content in 3T3-L1
adipocytes
Total TG content in 3T3-L1 adipocytes was determined
using a commercial TG assay kit (Cayman Chemical Company, Ann
Arbor, MI, USA), according to the manufacturer’s protocol.
Animals and diet
A total of 20 3-week-old C57BL/6N male mice weighing
19.5±1.0 g came from Orient Bio Inc. (Gwangju, Korea). The mice
were housed under standard environmental conditions with a
temperature of 22±2°C, humidity of 50-60% and a 12-h light/12-h
dark cycle. Mice were fed on a commercial standard laboratory diet
(AIN-76A; Research Diets, Inc., New Brunswick, NJ, USA) and water
ad libitum. After 1 week of acclimatization, mice were
divided into four groups (n=5) and fed for 16 weeks on either a
non-fat diet or an HFD (Rodent Diet with 60 kcal% fat; Research
Diets, Inc.) as follows: Group 1, mice were fed on non-fat diet
(ND) and orally given 0.2 ml distilled water daily; group 2, mice
were fed on HFD and orally given 0.2 ml distilled water daily;
group 3, mice were fed on HFD and orally administered with 200
mg/kg MGSE daily; group 4, mice were fed on HFD and orally
administered with 200 mg/kg GCE daily. The various extracts were
dissolved in distilled water. The animal protocols were performed
following approval from the Jeonju University Institutional Animal
Care and Use Committee (#JJU-IACUC-2016-011).
Weight and histochemical analysis
The weight of the mice in each group was determined
prior to and on the last day prior to sacrifice. Following
sacrifice and collection of blood samples, adipose and liver
tissues were removed and immediately weighed. For histological
analysis, the adipose and liver tissues were fixed in 10% neutral
formalin for 42 h, washed in several changes of PBS, dehydrated in
a series of graded ethanol, cleared in two changes of xylene and
embedded in three changes of paraffin wax. The tissues were blocked
and sectioned (5 µm thick) using a microtome. The tissues
sections were stained with hematoxylin and eosin stain.
Serum biochemical analysis
Blood samples were centrifuged at 2,000 × g for 15
min at 4°C, and the serum was separated and stored at −70°C for
subsequent experiments. Enzyme kits were employed to determine the
serum concentrations of total cholesterol (TC; cat. no. AM202-K),
high-density lipoprotein cholesterol (HDL-C; cat. no. AM203-K), TG
(cat. no. AM157S-K), aspartate transaminase (AST; cat. no. AM101-K)
and alanine transaminase (ALT; cat. no. AM101-K) (all Asan
Pharmaceutical Co., Ltd., Gyeonggi, Korea), leptin (cat. no. MOB00;
R&D Systems, Inc., Minneapolis, MN, USA) and insulin (cat. no.
80-INSMS-E01; Alpco Diagnostics, Windham, NH, USA). All experiments
were performed according to the manufacturers’ protocols and
absorbances (TC, 492 nm; HDL-C, 492 nm; TG, 540 nm; AST, 492 nm;
ALT, 492 nm; leptin, 450 nm; insulin, 450 nm) were determined using
a Tecan spectrophotometer (Tecan Group, Ltd., Männedorf,
Switzerland). Low-density lipoprotein cholesterol (LDL-C) was
calculated using the following formula, LDL-C = TC-HDL-C-(TG/5).
Atherogenic index and cardiac risk factor were calculated using the
following formulae: Atherogenic Index = (TC-HDL-C)/HDL-C; Cardiac
Risk Factor = TC/HDL-C.
Liver tissue analysis
Liver tissues (0.3 g) were homogenized in 0.5 ml PBS
and centrifuged at 2,000 × g for 15 min at 4°C to obtain the
supernatant. The supernatants were stored at −70°C for subsequent
experiments. The concentrations of catalase (cat. no. 707002),
superoxide dismutase (SOD; cat. no. 706002, Cayman Chemical
Company), malondialdehyde (MDA, cat. no. STA-330) and glutathione
(GSH, cat. no. STA-312) (Cell Biolabs, Inc., San Diego, CA, USA)
were determined using enzyme kits, following the manufacturers’
protocols. Absorbances (catalase, 540 nm; SOD, 450 nm; MDA, 532 nm;
GSH, 405 nm) were determined using a Tecan spectrophotometer.
Western blot analysis
Liver tissue samples (0.02-0.03 g) from each mice in
each group (n=5) were mixed and protein was extracted using
ice-cold lysis buffer (50 mM Tris/HCl, pH 7.4, 250 mM NaCl, 5 mM
EDTA, 2 mM Na3VO4, 1 mM NaF, 20 mM
Na4P2O7, 0.02% NaN3 and
proprietary detergent; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Protease inhibitor cocktail (cat. no. P2714; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and phosphatase inhibitor cocktail
(cat. no. P0044; Sigma-Aldrich; Merck KGaA) was added at 100-fold
dilution. To ensure equal loading of proteins, the protein
concentration in various samples were determined using Quick Start™
Bradford Protein Quantification reagent (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Proteins (80 µg) were loaded and
separated by SDS-PAGE (4-20% gel) and transferred onto
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.). The
membranes were blocked at room temperature with 5% (w/v) skimmed
milk (EMD Millipore, Billerica, MA, USA) in TBS-T [10 mM Tris/HCl
(pH 7.5), 150 mM NaCl and 0.1% (v/v) Tween-20]. The membranes were
then incubated on a shaker overnight at 4°C with the following
antibodies: Anti-peroxisome-proliferator-activated receptor γ
(PPARγ; 1:500 dilution; cat. no. MAB3632, EMD Millipore),
anti-CCAAT/enhancer-binding protein α (C/EBPα; 1:1,000 dilution;
cat. no. 2295S, Cell Signaling Technology, Danvers, MA, USA),
anti-adiponectin (1:1,000 dilution; cat. no. sc-136131; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-leptin (1:1,000
dilution; cat. no. AB3521; EMD Millipore), anti-inducible nitric
oxide synthase (iNOS; 1:2,000 dilution; cat. no. 610329; BD
Biosciences, San Jose, CA, USA) and anti-β-actin (1:5,000 dilution;
cat. no. 612657; BD Biosciences). Stripping buffer was used to
strip primary antibodies. The secondary antibodies (1:5,000
dilution) used were horseradish peroxidase-conjugated anti-goat
(cat. no. sc-2768; Santa Cruz Biotechnology, Inc.), anti-chicken
(cat. no. SA1-72004; Invitrogen; Thermo Fisher Scientific, Inc.),
anti-mouse (cat. no. 10004302, Cayman Chemical Company) or
anti-rabbit (cat. no. sc-2357; Santa Cruz Biotechnology, Inc.)
antibodies. Proteins were detected using the SuperSignal West Dura
Stable Peroxide solution (Thermo Fisher Scientific, Inc.) and
chemiluminescence detection systems (Alliance version 15.11;
UVITEC, Cambridge, UK).
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical analyses were performed using the SPSS
statistics program (version 22; IBM Corp., Armonk, NY, USA).
Differences between the variables were determined using one-way
analysis of variance with Duncan’s multiple range test. P<0.05
was considered to indicate a statistically significant
difference.
Results and Discussion
Obesity has been associated with several pathologies
such as the metabolic syndrome, hypertension, atherosclerosis,
liver diseases, oxidative stress and inflammation. In addition, the
majority of available therapies designated for the prevention
and/or treatment of obesity, and its associated abnormalities are
either insufficient or renders patients with severe side effects.
Therefore, a number of studies have focused on alternative
therapies from agricultural products because of the recently
uncovered biological actions of plant extracts and phytochemicals,
to be used for preventing and treating obesity, and a number of
previous studies have revealed the anti-obesity, antioxidative and
anti-inflammatory potentials of plant extracts and dietary
phytochemicals (17,20,21,23). In the present study, the
anti-obesity effects of MGSE (200 mg/kg) on HFD-induced obese
C57BL/6N mice were investigated for 16 weeks.
Initially, the time of harvest of the grape stalk
for which the polyphenol and flavonoid yield are highest, and also
exhibiting the greatest in vitro antioxidant activities, was
determined. The harvest time markedly affected the total polyphenol
and flavonoid contents of MGSE, with the June harvest yielding the
highest polyphenol content and the July harvest yielding the
highest flavonoid content (Table
I). Furthermore, the time of harvest affected the antioxidant
activities of MGSE as revealed using a DPPH and ABTS assay with the
highest antioxidant activity determined in the June sample
(Table II). HPLC analysis
indicated that (+)-catechin, (−)-epicatechin, rutin and quercetin
gradually decreased from June to September whereas
trans-resveratrol exhibited an increasing tendency from June
to September (Table III;
Fig. 1). The results clearly
indicated that the harvest period affects the polyphenol and
therefore the antioxidant activity of Muscat Bailey A grape. A
similar study, but on rabbiteye blueberry leaves concluded that
blueberry leaves from different seasons exhibited different
bioactive secondary metabolite content and different antioxidant
activities (24). Therefore, on
the basis of these results, grape stalk harvested in June was
selected for the investigation of its potential anti-obesity
effects.
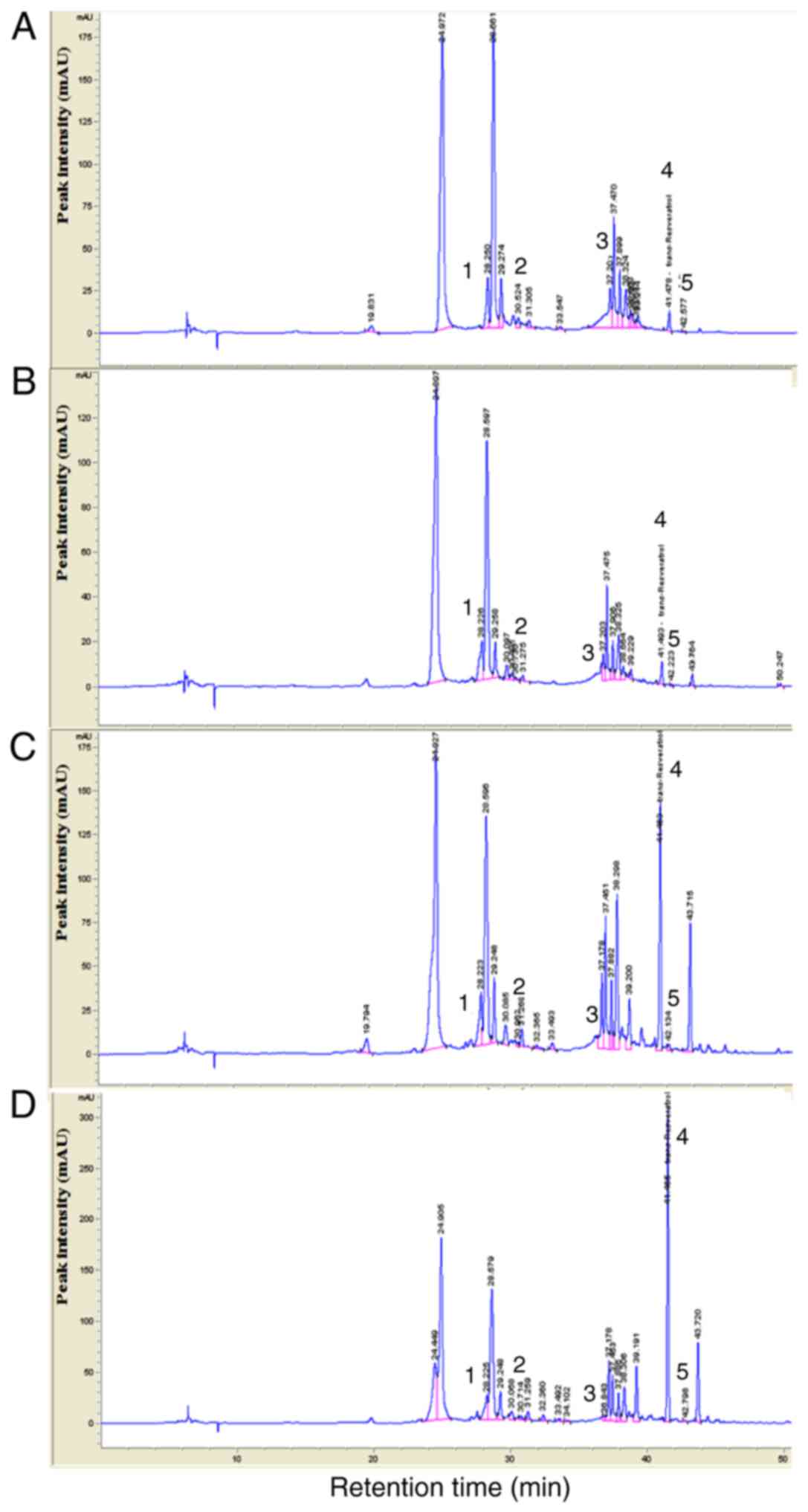 | Figure 1HPLC chromatograms of MGSE. Each MGSE
collected in (A) June, (B) July, (C) August and (D) September 2016
was profiled using HPLC at 320 nm and compared with the
conventional compounds using HPLC-diode array detector. Peak number
represents the standard compound 1, (+)-catechin; 2,
(-)-epicatechin; 3, rutin; 4, trans-resveratrol; 5,
quercetin. HPLC, high-performance liquid chromatography; MGSE,
Muscat Bailey A grape stalk extract; mAU, micro-absorbance
units. |
 | Table ITotal polyphenol and flavonoid
contents of Muscat Bailey A grape stalk extracts. |
Table I
Total polyphenol and flavonoid
contents of Muscat Bailey A grape stalk extracts.
| Sample | Total polyphenol
content (mg·gallic acid/g) | Total flavonoid
content (mg·quercetin/g) |
|---|
| June | 282.87±0.81 | 10.74±0.06 |
| July | 189.27±0.61 | 16.04±0.17 |
| August | 158.08±0.98 | 10.34±0.85 |
| September | 115.50±0.38 | 5.69±0.23 |
 | Table IIAntioxidant activity of Muscat Bailey
A grape stalk extracts. |
Table II
Antioxidant activity of Muscat Bailey
A grape stalk extracts.
| Sample | DPPH
IC50 | ABTS
IC50 |
|---|
| June | 29.10±0.67 | 101.47±0.99 |
| July | 45.26±0.71 | 107.84±0.39 |
| August | 63.29±0.85 | 148.90±0.49 |
| September | 88.49±2.39 | 238.74±1.46 |
| BHT | 75.96±1.27 | - |
| Trolox | - | 61.49±0.25 |
 | Table IIIContents (µg/g) of flavonoids
from Muscat Bailey A grape stalk extracts determined using
high-performance liquid chromatography-diode array detector. |
Table III
Contents (µg/g) of flavonoids
from Muscat Bailey A grape stalk extracts determined using
high-performance liquid chromatography-diode array detector.
| Sample
collected | Final results, mg/g
|
|---|
| 1 | 2 | 3 | 4 | 5 |
|---|
| June | 39.98 | 19.07 | 15.71 | 0.11 | 0.17 |
| July | 22.64 | 9.49 | 6.17 | 0.09 | 0.05 |
| August | 17.51 | 9.56 | 3.21 | 0.46 | 0.09 |
| September | 17.94 | 0.59 | 0.74 | 0.91 | 0.08 |
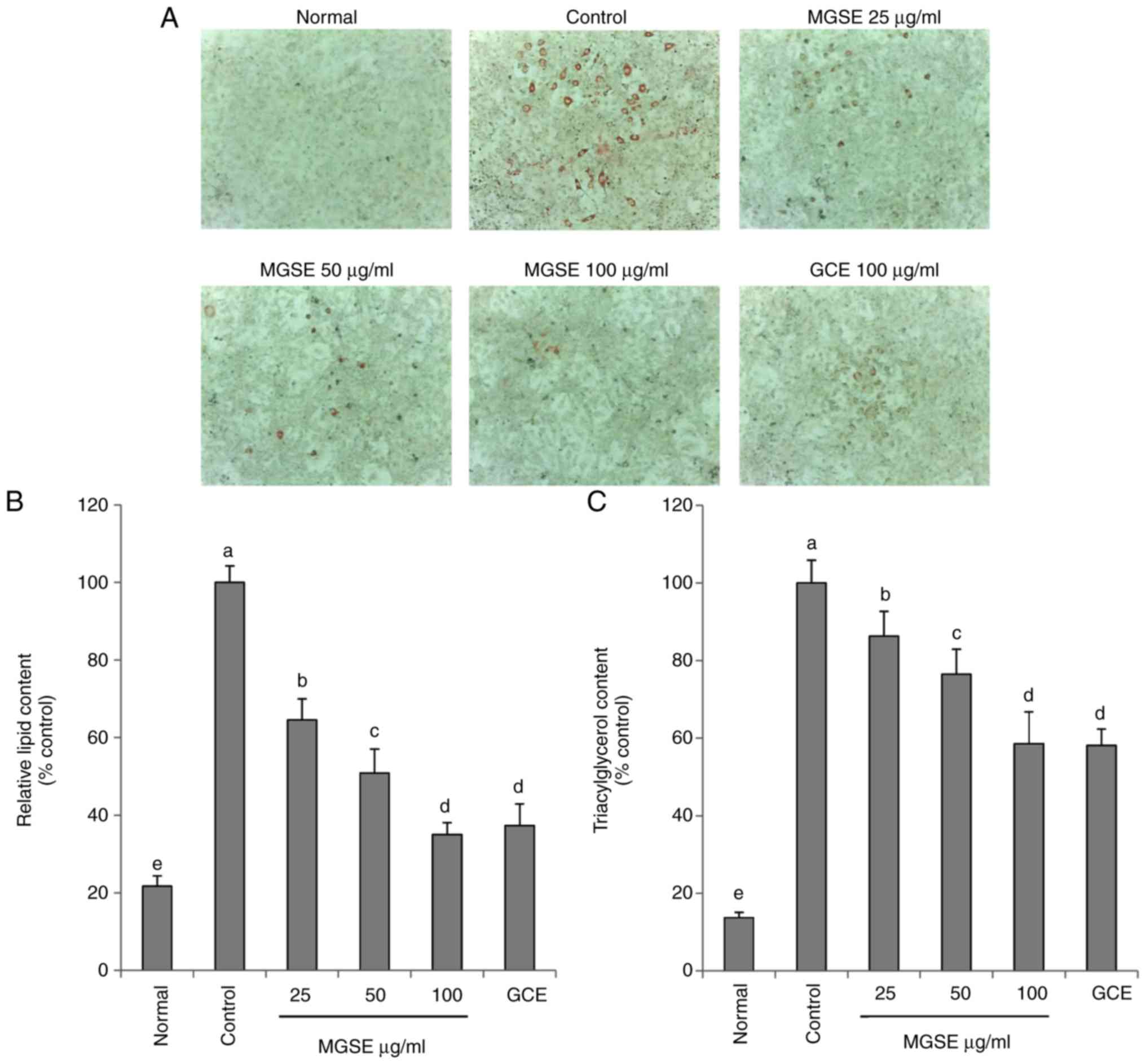
The 3T3-L1 cell line is the most widely used cell
line for evaluating anti-obesity activities of a number of agents
including plant extracts. This cell line can be stimulated by
metabolic-regulating hormones such as IBMX, DEX and insulin to
accumulate intracellular lipid droplets (25). A dose-dependent study was
performed to investigate the effects of MGSE on fat and TG
accumulation in the 3T3-L1 adipocyte cells. The results indicated
that MGSE dose-dependently decreased lipid accumulation and TG
content in 3T3-L1 adipocyte cells (Fig. 2). Similar previous studies but on
other parts of the grape plant such as the skin and the seeds also
revealed the anti-adipogenic effects of these parts of grape in
3T3-L1 adipocytes at the induction of differentiation and,
primarily, on the PPARγ signaling pathway (26,27).
Following the anti-adipogenic effects of MGSE on
lipid accumulation in 3T3-L1 adipocytes, the focus was turned to
elucidating the anti-obesity, anti-diabetic, antioxidative,
anti-inflammatory and liver protective effects of MGSE in
HFD-induced obesity in in vivo mouse studies. Obesity was
induced in mice by feeding them on an HFD for 16 weeks. When the
mice fed on the HFD were orally administered daily with 200 mg/kg
MGSE or GCE, a decrease in body, liver and epididymal fat weights
was observed (Table IV),
compared with the mice fed on only HFD. The efficacy of grape stalk
in the prevention of lipid accumulation in 3T3-L1 adipocytes was
confirmed in the in vivo studies as MGSE treatment decreased
epididymal fat and body weight of the mice. Blood serum analysis
further revealed that total cholesterol, TG and LDL concentrations
were significantly decreased in the mice fed on the HFD and
administered with MGSE or GCE compared with the HFD-fed group. On
the other hand, the high-density lipoprotein concentrations
increased in the HFD-fed mice that were administered with MGSE or
GCE (Fig. 3A–D). However, the
differences were not statistically significant. Abdominal fat
distribution is associated with increased serum TG, cholesterol and
LDL-C (28). In addition,
increased concentrations of TG lead to accumulation of TC, and
LDL-C can build up on artery walls, thereby increasing the risk of
heart disease (29). However,
increased HDL-C levels attenuate the accumulation of LDL-C and
protect against heart disease by transporting LDL-C, TC and TG from
the arteries (30). The results
of the present study indicated that MGSE and GCE have the potential
to decrease TG, TC and LDL-C in obese mice, thereby decreasing or
even preventing the risk of heart disease. The atherogenic index
and cardiac risk factors were significantly decreased when obese
mice were administered with MGSE or GCE (Fig. 3E and F).
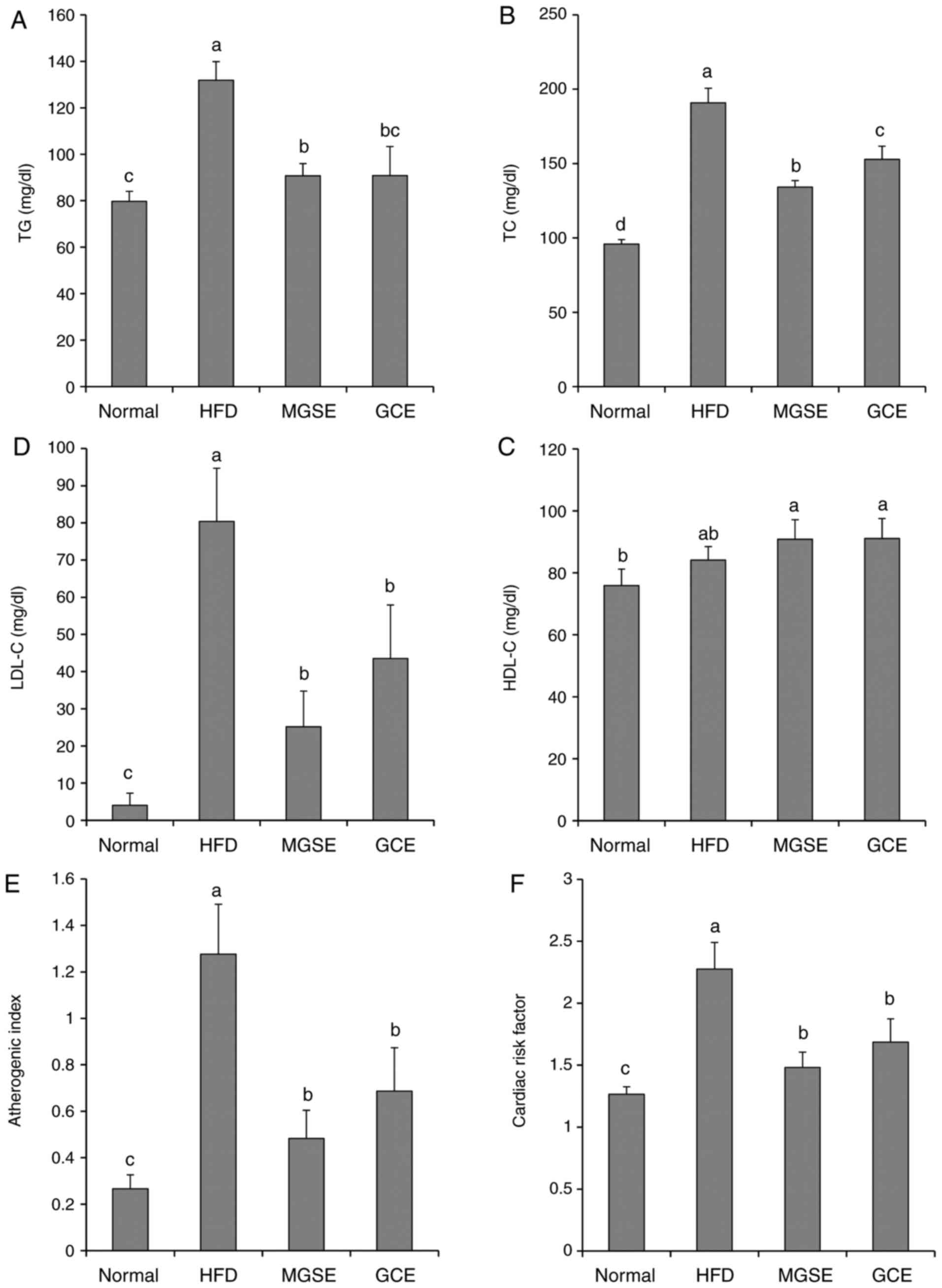 | Figure 3Effects of MGSE on serum lipid
profiles of (A) TG, (B) TC, (C) LDL-C and (D) HDL-C, (E)
atherogenic index, (F) cardiac risk factor, (G) insulin and (H)
leptin, and (I) the expression of leptin and adiponectin in
HFD-induced obesity in mice. Results are presented as the mean ±
standard deviation. Results were significantly different
(P<0.05) from all other results, unless labelled with the same
letter. Normal, mice fed on the control diet (negative control);
HFD, mice fed on the HFD (positive control); MGSE, mice fed on the
HFD and administered with 200 mg/kg/day MGSE; GCE, mice fed on the
HFD and administered with 200 mg/kg/day GCE. MGSE, Muscat Bailey A
grape stalk extract; GCE, Garcinia cambogia extract; TG,
triacylglycerol; TC, total cholesterol; LDL-C, low-density
lipoprotein cholesterol; HDL-C, high-density lipoprotein
cholesterol; HFD, high-fat diet. |
 | Table IVEffect of MGSE on body weight, weight
gain, liver and epididymal adipose weight in HFD-induced obese
mice. |
Table IV
Effect of MGSE on body weight, weight
gain, liver and epididymal adipose weight in HFD-induced obese
mice.
| Parameter | Normal | HFD control | MGSE | GCE |
|---|
| Initial body
weight, g |
21.18±0.74a |
21.72±0.63a |
21.56±0.62a |
21.70±0.87a |
| Final body weight,
g |
30.12±2.20a |
47.98±1.61b |
45.28±0.88c |
44.94±1.50c |
| Weight gain, g |
8.94±2.29a |
26.27±1.72b |
23.72±0.62c |
23.24±1.68c |
| Liver weight,
g |
1.10±0.10a |
1.96±0.24b |
1.57±0.24c |
1.55±0.30c |
| Epididymal adipose
weight, g |
1.19±0.18a |
2.33±0.24b |
1.80±0.23c |
2.01±0.21b,c |
Leptin, a key hormone, predominantly produced by
adipose cells, regulates energy balance, appetite and adiposity,
and its secretion levels are positively associated with TG stores
in adipose tissue (31). Diabetes
in obesity has been associated with changes in insulin secretion.
Several risk factors in subjects with hypertension are associated
with insulin resistance, including low HDL-C, high TG levels and
glucose intolerance (32).
Adipocytes contribute to insulin resistance via adipocytokine and
hormone secretion, where retinol-binding protein 4, adiponectin,
leptin, interleukin (IL)-6, IL-1β and tumor necrosis factor α
(TNF-α) have been observed (33).
Noteworthy, the results from the MGSE-treated group indicated
significantly decreased insulin (Fig.
3G), leptin (Fig. 3H and I),
IL-6, IL-1β, TNF-α and iNOS levels (Fig. 4) compared with those of the
HFD-fed group. However, the adiponectin level was increased in the
MGSE-treated group (Fig. 3I).
This increase in adiponectin level in the liver can be beneficial
as a decrease in circulating adiponectin in Type 2 diabetes
contributes to an abnormal increase in glucose production. In
addition, low adiponectin signaling and high insulin levels
indicate an insulin resistance state (34). These results suggested that MGSE
might have a beneficial effect in Type 2 diabetes by ameliorating
insulin resistance in HFD-induced obesity that leads to diabetes.
This test, to the best of our knowledge, was performed on
MGSE-treated HFD mice for the first time and also confirms the
anti-inflammatory effects of MGSE in obesity. A similar effect was
observed in the GCE-treated HFD mice. The beneficial
anti-inflammatory effects of MGSE may be associated with the high
levels of phenolic compounds, which are known for their
anti-inflammatory potential.
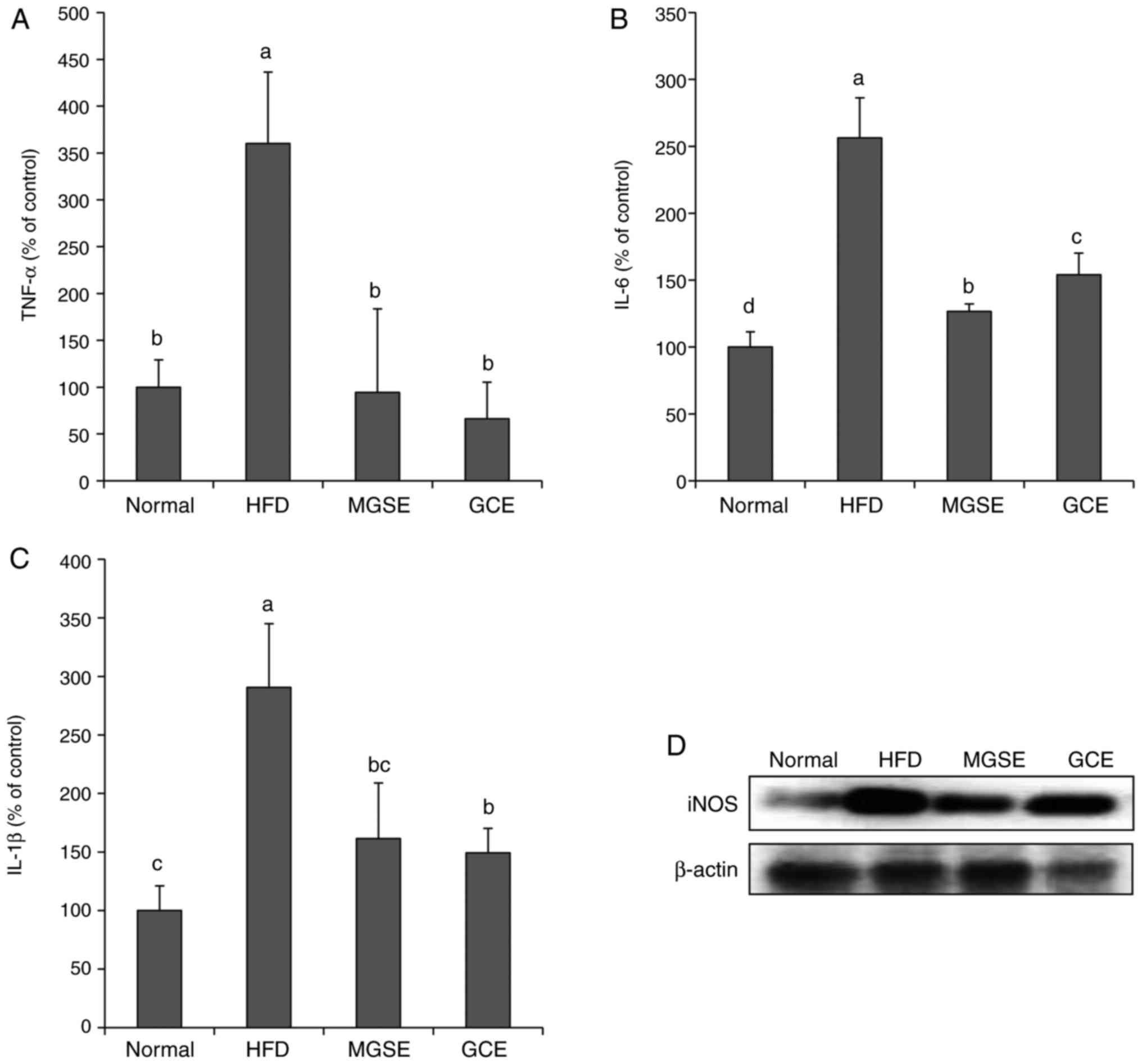 | Figure 4Effects of MGSE on the inflammatory
response induced by HFD in obese mice. Levels of the
pro-inflammatory cytokines (A) TNF-α, (B) IL-6 and (C) IL-1β were
determined in serum samples of mice. Results are presented as the
mean ± standard deviation. Results were significantly different
(P<0.05) from all other results, unless labelled with the same
letter. (D) Effects of MGSE on the expression of iNOS. Liver tissue
samples (0.02-0.03 g) from each mice in each group (n=5) were mixed
and the expression levels of the indicated proteins was determined
by western blotting. Normal, mice fed on the control diet (negative
control); HFD, mice fed on the HFD (positive control); MGSE, mice
fed on the HFD and administered with 200 mg/kg/day MGSE; GCE, mice
fed on the HFD and administered with 200 mg/kg/day GCE. MGSE,
Muscat Bailey A grape stalk extract; GCE, Garcinia cambogia
extract; HFD, high-fat diet; TNF-α, tumor necrosis factor α; IL,
interleukin; iNOS, inducible nitric oxide synthase. |
AST and ALT serve important functions in the
formation of amino acids in the liver, and their activity increases
with HFD-induced liver damage. Fatty liver disease is a disease in
which abnormally large numbers of lipid droplets, primarily
composed of TG, accumulate in liver cells. Most importantly, the
non-alcoholic fatty liver disease caused by excessive caloric
consumption induces chronic inflammation (hepatic steatosis) in the
presence of oxidative stress that may result in cancer (35). Therefore, decreasing serum levels
of AST, ALT and pro-inflammatory cytokines as well as upregulating
the antioxidants defense system will definitely prevent fatty liver
diseases in obesity. The results of the present study indicated
that MGSE decreased serum levels of the liver enzymes AST and ALT
(Fig. 5); decreased serum IL-6,
IL-1β and TNF-α, and iNOS expression in the liver (Fig. 4); prevented lipid peroxidation in
the liver; and also upregulated GSH, SOD and catalase in liver
tissues (Fig. 6). Increases in
GSH, SOD and catalase levels explain the decrease in lipid
peroxidation exhibited by MDA levels, and further explain the
significantly decreased serum levels of AST and ALT observed in
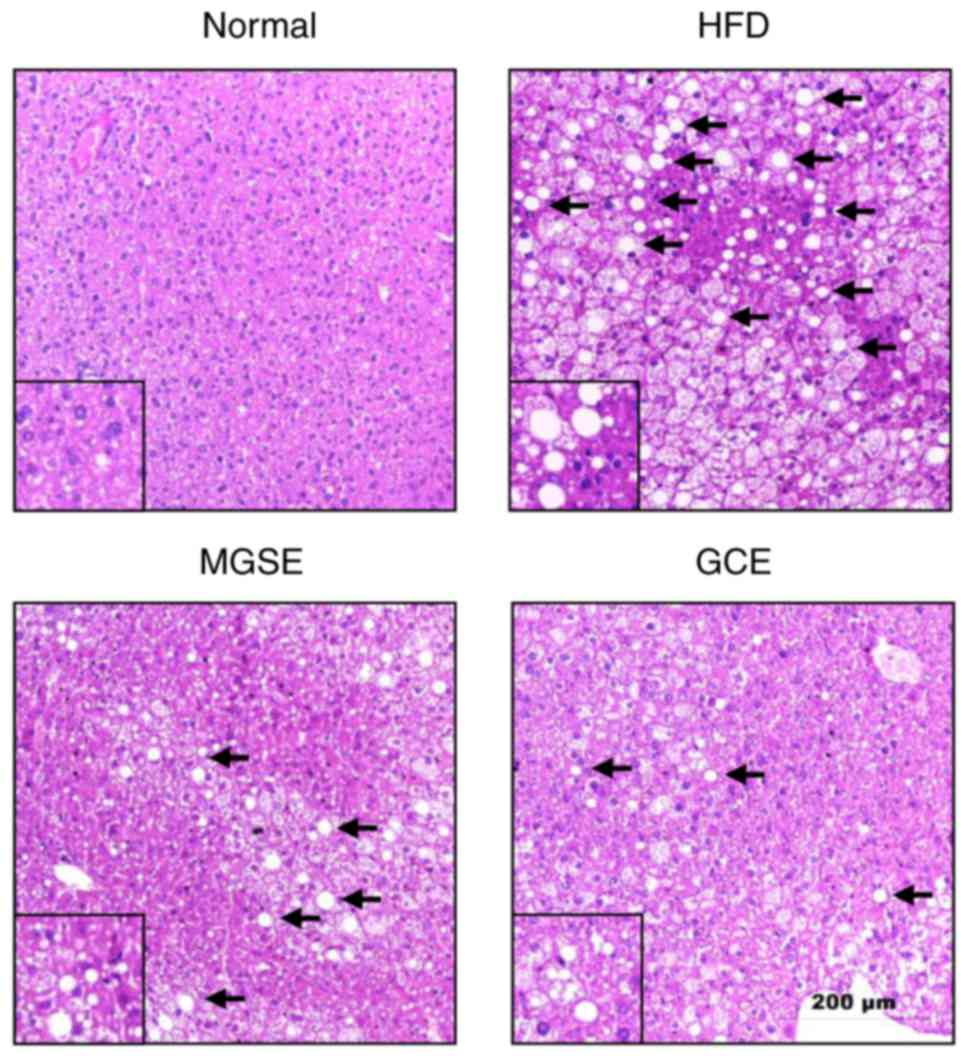
MGSE-treated mice. Furthermore, photomicrographs of liver samples
stained with H&E revealed that the livers of mice fed on a
normal diet exhibited no pathological abnormalities (Fig. 7). However, in livers of mice fed
on the HFD, the hepatocytes exhibited a number of large vacuoles
within a number of lipid droplets (Fig. 7). Treatment with MGSE attenuated
the hepatic steatosis. The GCE-treated group exhibited a similar
effect. These results therefore indicated that MGSE treatment
exerts hepatoprotective functions in obesity and liver damage via
regulation of chronic inflammation and oxidative stress.
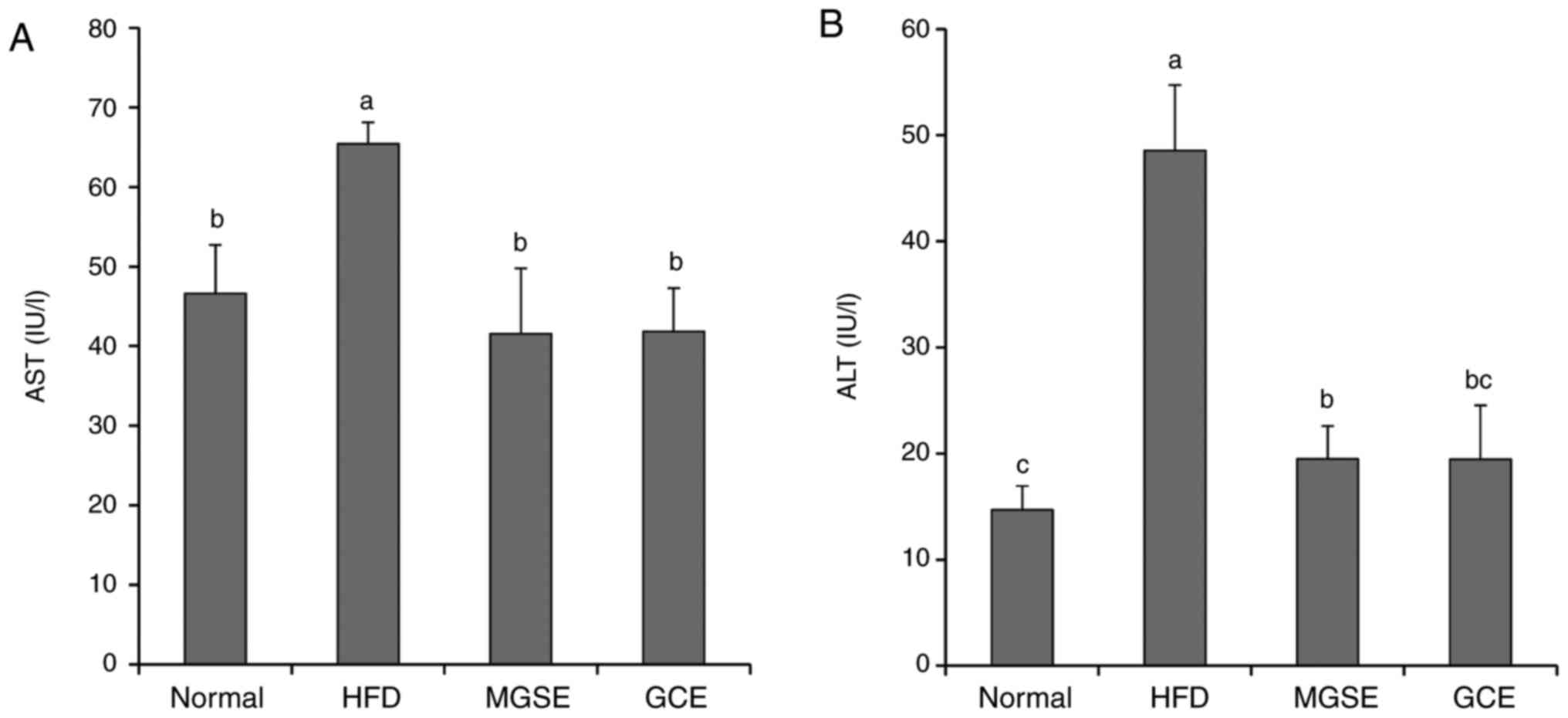 | Figure 5Effects of MGSE on (A) AST and (B)
ALT levels in HFD-induced obesity in mice. Results are presented as
the mean ± standard deviation. Results were significantly different
(P<0.05) from all other results, unless labelled with the same
letter. Normal, mice fed on the control diet (negative control);
HFD, mice fed on the HFD (positive control); MGSE, mice fed on the
HFD and administered with 200 mg/kg/day MGSE; GCE, mice fed on the
HFD and administered with 200 mg/kg/day GCE. MGSE, Muscat Bailey A
grape stalk extract; GCE, Garcinia cambogia extract; AST,
aspartate aminotransferase; ALT, alanine aminotransferase; HFD,
high-fat diet. |
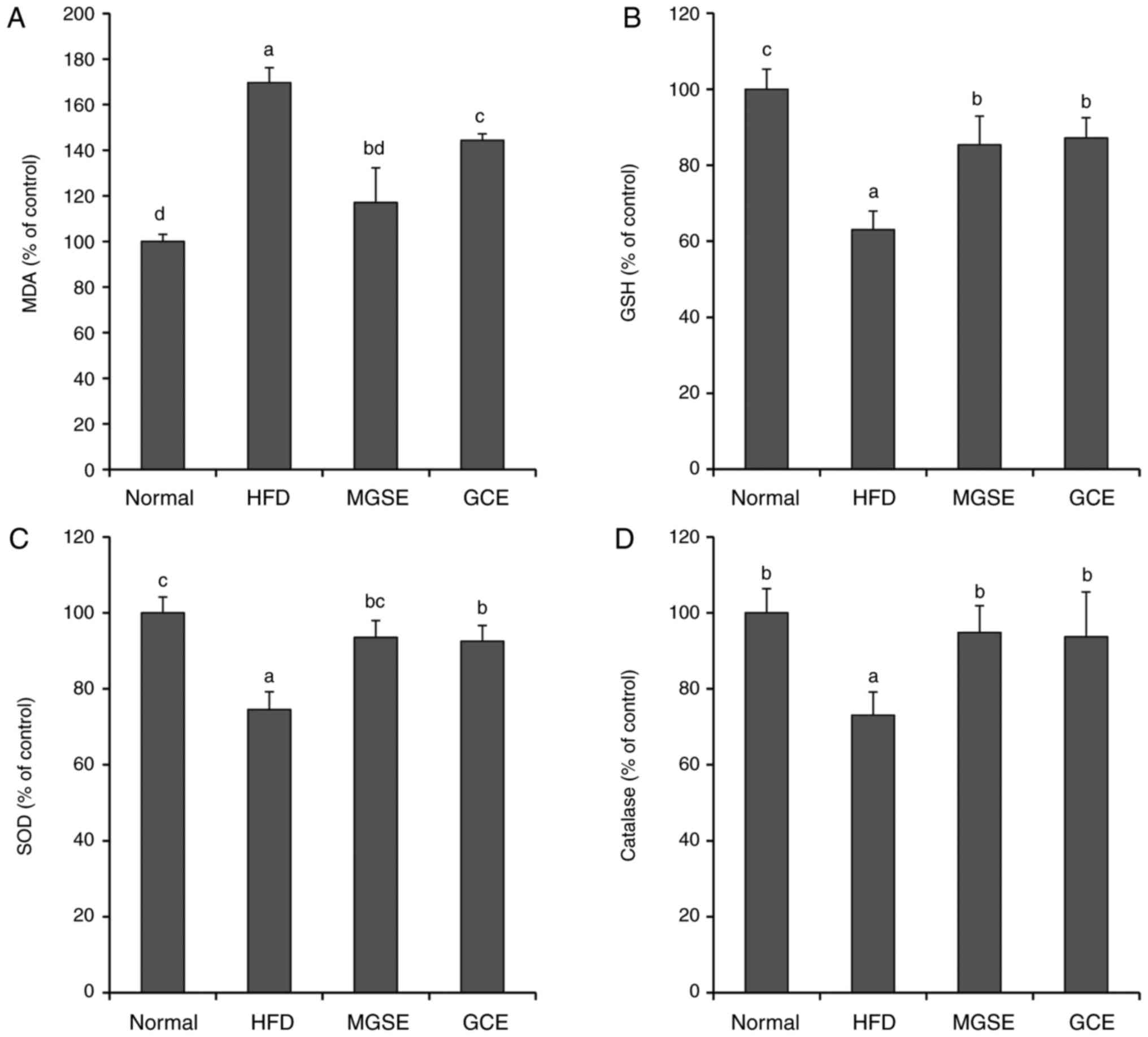 | Figure 6Effects of MGSE on (A) lipid
peroxidation, (B) GSH concentration, (C) SOD and (D) catalase
activity in liver tissues of mice. Lipid peroxidation was
quantified as a level of MDA. Results are presented as the mean ±
standard deviation. Results were significantly different
(P<0.05) from all other results, unless labelled with the same
letter. Normal, mice fed on the control diet (negative control);
HFD, mice fed on the HFD (positive control); MGSE, mice fed on the
HFD and administered with 200 mg/kg/day MGSE; GCE, mice fed on HFD
and administered with 200 mg/kg/day GCE. MGSE, Muscat Bailey A
grape stalk extract; GCE, Garcinia cambogia extract; HFD,
high-fat diet; MDA, malondialdehyde; GSH, glutathione; SOD,
superoxide dismutase. |
To determine the molecular mode-of-action of MGSE in
obesity, the protein expression levels of C/EBPα and PPARγ in the
mice liver tissue were determined. PPARγ and C/EBPα co-regulate the
transcriptional pathway of adipogenesis. In the early phase of
adipogenesis, C/EBPβ and C/EBPδ are expressed, but PPARγ and C/EBPα
are induced. C/EBPα binds to the PPARγ promoter, leading to the
expression of PPARγ. PPARγ serves a dominant function in the
differentiation and maturation of adipocytes (36,37). Previous studies have identified
that an HFD induces the expression of adipogenic genes such as
those encoding PPARγ and C/EBPα (38-40). This was also true in the present
study as mice fed on an HFD exhibited an increase in the expression
of phosphorylated PPARγ and C/EBPα protein. However, when the mice
fed on the HFD were administered with MGSE, the expression of
phosphorylated PPARγ and C/EBPα were downregulated (Fig. 8). The GCE-treated group exhibited
a similar effect. These results may provide a clue to the
understanding of the mechanism of action of MGSE on obesity in
mouse models. However, in-depth molecular studies are required.
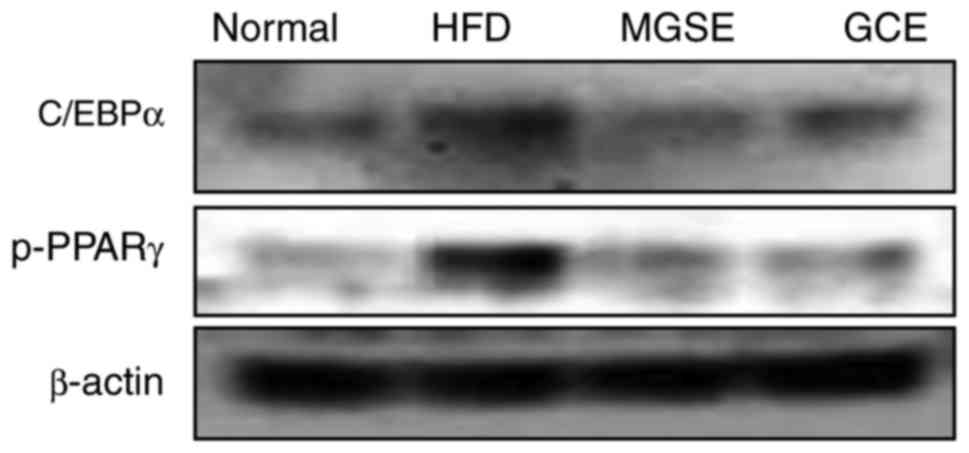 | Figure 8Effects of MGSE on the expression of
C/EBPα and phosphorylated PPARγ. Liver tissue samples (0.02-0.03 g)
from each mice in each group (n=5) were mixed and the expression
levels of the indicated proteins was determined by western
blotting. Normal, mice fed on the control diet (negative control);
HFD, mice fed on the HFD (positive control); MGSE, mice fed on the
HFD and administered with 200 mg/kg/day MGSE; GCE, mice fed on the
HFD and administered with 200 mg/kg/day GCE. MGSE, Muscat Bailey A
grape stalk extract; GCE, Garcinia cambogia extract; HFD,
high-fat diet; C/EBPα, CCAAT/enhancer-binding protein α; PPARγ,
peroxisome-proliferator-activated receptor γ; p-, phospho-. |
A number of studies have indicated that polyphenol
compounds such as resveratrol, rutin and quercetin attenuate
HFD-induced obesity in rodents (41-43). It was previously identified that
catechin and epicatechin-rich grape seed extract suppressed
HFD-induced obesity in mice (44). Rahman et al (45) demonstrated that Cosmos
caudatus Kunth leaf extract including catechin, epicatechin,
quercetin and rutin exhibited an anti-obesity effect in HFD-fed
rats. Rodriguez Lanzi et al (46) also identified that grape pomace
extract contained various compounds including resveratrol,
catechin, epicatechin, quercetin and rutin, inhibited
differentiation of 3T3-L1 cells and decreased white adipose fat
weight in rats. In addition, it has been identified that
resveratrol suppresses differentiation of 3T3-L1 adipocytes
(47). The results of the present
study indicated that MGSE decreased the lipid and TG content of
3T3-L1 cells, and also that catechin, epicatechin, rutin and
resveratrol attenuated lipid content in 3T3-L1 cells (data not
shown). Therefore, the results of the present study suggest that
the anti-obesity effect of MGSE may be associated with polyphenol
compounds such as catechin, epicatechin, rutin and resveratrol.
In conclusion, the results of the present study
indicated that the harvest period affects secondary metabolite
content and therefore the antioxidant abilities of the grape stalk.
Furthermore, it has been identified that MBA grape stalk harvested
in June has beneficial effects in HFD-induced obesity that can lead
to Type 2 diabetes, hepatic steatosis, cardiovascular disorders,
inflammation and oxidative stress. MGSE led to no evident
hepatotoxicity while it improved liver test results. Detailed
mechanisms of action of MGSE remain to be investigated further.
However, these results suggested that MBA grape stalk may be a
potential nutraceutical for the prevention/treatment of obesity and
obesity-associated disorders.
Acknowledgments
The authors thank Mr. Denis Nchang Che (Department
of Food Science and Technology, Chonbuk National University,
Jeonju, Republic of Korea), Mr. Jae Young Shin (Department of
Health Management, Jeonju University, Jeonju, Republic of Korea)
and Miss Hyun Ju Kang (Ato Q&A Co. Ltd., Jeonju, Republic of
Korea) for animal care, and Mr. Sang Jun Kim (Jeonju
AgroBio-Materials Institute, Jeonju, Republic of Korea) for HPLC
analysis.
Funding
The present study was supported financially by the
Ministry of Trade, Industry, and Energy, Korea, under the Regional
Specialized Industry Development Program (grant no. R0006168)
supervised by the Korea Institute for Advancement of
Technology.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
BMK, BOC and SIJ designed the research. BMK and BOC
performed the research. BMK, BOC and SIJ analyzed the data and
wrote the manuscript. SIJ supervised the research project. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal protocols were performed following
approval from Jeonju University Institutional Animal Care and Use
Committee (#JJU-IACUC-2016-011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Amugsi DA, Dimbuene ZT, Mberu B, Muthuri S
and Ezeh AC: Prevalence and time trends in overweight and obesity
among urban women: An analysis of demographic and health surveys
data from 24 African countries. BMJ Open. 7:e0173442017. View Article : Google Scholar
|
|
2
|
Ogden CL, Carroll MD, Fryar CD and Flegal
KM: Prevalence of Obesity Among Adults and Youth: United States,
2011-2014. NCHS Data Brief. 219:1–8. 2015.
|
|
3
|
World Health Organization, Obesity
Situation and trends: Global health observatory (GHO) data.
http://www.who.int/gho/ncd/risk_factors/obesity_text/en/2016
accessed Feb 1, 2017.
|
|
4
|
Mokdad AH, Ford ES, Bowman BA, Dietz WH,
Vinicor F, Bales VS and Marks JS: Prevalence of obesity, diabetes,
and obesity-related health risk factors, 2001. JAMA. 289:76–79.
2003. View Article : Google Scholar
|
|
5
|
Clain DJ and Lefkowitch JH: Fatty liver
disease in morbid obesity. Gastroenterol Clin North Am. 16:239–252.
1987.PubMed/NCBI
|
|
6
|
Fabbrini E, Sullivan S and Klein S:
Obesity and nonalcoholic fatty liver disease: Biochemical,
metabolic, and clinical implications. Hepatology. 51:679–689. 2010.
View Article : Google Scholar
|
|
7
|
Manna P and Jain SK: Obesity, oxidative
stress, adipose tissue dysfunction, and the associated health
risks: Causes and therapeutic strategies. Metab Syndr Relat Disord.
13:423–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bryan S, Baregzay B, Spicer D, Singal PK
and Khaper N: Redox-inflammatory synergy in the metabolic syndrome.
Can J Physiol Pharmacol. 91:22–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Savini I, Catani MV, Evangelista D,
Gasperi V and Avigliano L: Obesity-associated oxidative stress:
Strategies finalized to improve redox state. Int J Mol Sci.
14:10497–10538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loe YC, Bergeron N, Rodriguez N and
Schwarz JM: Gas chromatography/mass spectrometry method to quantify
blood hydroxycitrate concentration. Anal Biochem. 292:148–154.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shara M, Ohia SE, Schmidt RE, Yasmin T,
Zardetto-Smith A, Kincaid A, Bagchi M, Chatterjee A, Bagchi D and
Stohs SJ: Physico-chemical properties of a novel (−)-hydroxycitric
acid extract and its effect on body weight, selected organ weights,
hepatic lipid peroxidation and DNA fragmentation, hematology and
clinical chemistry, and histopathological changes over a period of
90 days. Mol Cell Biochem. 260:171–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shara M, Ohia SE, Yasmin T, Zardetto-Smith
A, Kincaid A, Bagchi M, Chatterjee A, Bagchi D and Stohs SJ: Dose-
and time-dependent effects of a novel (−)-hydroxycitric acid
extract on body weight, hepatic and testicular lipid peroxidation,
DNA fragmentation and histopathological data over a period of 90
days. Mol Cell Biochem. 254:339–346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mattes RD and Bormann L: Effects of
(−)-hydroxycitric acid on appetitive variables. Physiol Behav.
71:87–94. 2000. View Article : Google Scholar
|
|
14
|
Preuss HG, Bagchi D, Bagchi M, Rao CV, Dey
DK and Satyanarayana S: Effects of a natural extract of
(-)-hydroxycitric acid (HCA-SX) and a combination of HCA-SX plus
niacin-bound chromium and Gymnema sylvestre extract on weight loss.
Diabetes Obes Metab. 6:171–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujioka K: Current and emerging
medications for overweight or obesity in people with comorbidities.
Diabetes Obes Metab. 17:1021–1032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eo H, Jeon YJ, Lee M and Lim Y: Brown alga
Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis,
oxidative stress, and inflammation by activation of AMPK and SIRT1
in high-fat diet-induced obese mice. J Agric Food Chem. 63:349–359.
2015. View Article : Google Scholar
|
|
17
|
González-Castejón M and Rodriguez-Casado
A: Dietary phytochemicals and their potential effects on obesity: A
review. Pharmacol Res. 64:438–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cárcel JA, García-Pérez JV, Mulet A,
Rodríguez L and Riera E: Ultrasonically assisted antioxidant
extraction from grape stalks and olive leaves. Phys Procedia.
3:147–152. 2010. View Article : Google Scholar
|
|
19
|
Mattos GN, Tonon RV, Furtado AA and Cabral
LM: Grape by-product extracts against microbial proliferation and
lipid oxidation: A review. J Sci Food Agric. 97:1055–1064. 2017.
View Article : Google Scholar
|
|
20
|
Arora P, Ansari S and Nazish I: Study of
antiobesity effects of ethanolic and water extracts of grapes
seeds. J Complement Integr Med. 8:1553–3840.1510. 2011.
|
|
21
|
Fujioka K, Greenway F, Sheard J and Ying
Y: The effects of grapefruit on weight and insulin resistance:
Relationship to the metabolic syndrome. J Med Food. 9:49–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho BO, Nchang Che D, Yin HH and Jang SI:
Enhanced biological activities of gamma-irradiated persimmon leaf
extract. J Radiat Res. 58:647–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azab A, Nassar A and Azab AN:
Anti-inflammatory activity of natural products. Molecules.
21:E13212016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu L, Liu X, Tan J and Wang B: Influence
of harvest season on antioxidant activity and constituents of
rabbiteye blueberry (Vaccinium ashei) leaves. J Agric Food Chem.
61:11477–11483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pinent M, Bladé MC, Salvadó MJ, Arola L,
Hackl H, Quackenbush J, Trajanoski Z and Ardévol A: Grape-seed
derived procyanidins interfere with adipogenesis of 3T3-L1 cells at
the onset of differentiation. Int J Obes (Lond). 29:934–941. 2005.
View Article : Google Scholar
|
|
27
|
Jeong YS, Hong JH, Cho KH and Jung HK:
Grape skin extract reduces adipogenesis- and lipogenesis-related
gene expression in 3T3-L1 adipocytes through the peroxisome
proliferator-activated receptor-γ signaling pathway. Nutr Res.
32:514–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zwiauer K, Widhalm K and Kerbl B:
Relationship between body fat distribution and blood lipids in
obese adolescents. Int J Obes. 14:271–277. 1990.PubMed/NCBI
|
|
29
|
Kwon OJ, Kim MY and Roh SS: Improving
effect of extract of Ganoderma lucidum in atherosclerosis from LDL
receptor knockout mouse. Korea J Herbol. 31:17–23. 2016. View Article : Google Scholar
|
|
30
|
Sacks FM, Tonkin AM, Craven T, Pfeffer MA,
Shepherd J, Keech A, Furberg CD and Braunwald E: Coronary heart
disease in patients with low LDL-cholesterol: Benefit of
pravastatin in diabetics and enhanced role for HDL-cholesterol and
triglycerides as risk factors. Circulation. 105:1424–1428. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reaven GM: Banting lecture 1988 Role of
insulin resistance in human disease. Diabetes. 37:1595–1607. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Modan M, Halkin H, Almog S, Lusky A,
Eshkol A, Shefi M, Shitrit A and Fuchs Z: Hyperinsulinemia A link
between hypertension obesity and glucose intolerance. J Clin
Invest. 75:809–817. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rabe K, Lehrke M, Parhofer KG and Broedl
UC: Adipokines and insulin resistance. Mol Med. 14:741–751. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Combs TP and Marliss EB: Adiponectin
signaling in the liver. Rev Endocr Metab Disord. 15:137–147. 2014.
View Article : Google Scholar :
|
|
35
|
Dowman JK, Tomlinson JW and Newsome PN:
Pathogenesis of non-alcoholic fatty liver disease. QJM. 103:71–83.
2010. View Article : Google Scholar :
|
|
36
|
Rosen ED, Hsu CH, Wang X, Sakai S, Freeman
MW, Gonzalez FJ and Spiegelman BM: C/EBPalpha induces adipogenesis
through PPARgamma: A unified pathway. Genes Dev. 16:22–26. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Imai T, Takakuwa R, Marchand S, Dentz E,
Bornert JM, Messaddeq N, Wendling O, Mark M, Desvergne B, Wahli W,
et al: Peroxisome proliferator-activated receptor gamma is required
in mature white and brown adipocytes for their survival in the
mouse. Proc Natl Acad Sci USA. 101:4543–4547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jo YH, Choi KM, Liu Q, Kim SB, Ji HJ, Kim
M, Shin SK, Do SG, Shin E, Jung G, et al: Anti-obesity effect of
6,8-diprenylge-nistein, an isoflavonoid of Cudrania tricuspidata
fruits in high-fat diet-induced obese mice. Nutrients.
7:10480–10490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim Y, Kwon MJ, Choi JW, Lee MK, Kim C,
Jung J, Aprianita H, Nam H and Nam TJ: Anti-obesity effects of
boiled tuna extract in mice with obesity induced by a high-fat
diet. Int J Mol Med. 38:1281–1288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sung YY, Kim DS, Kim SH and Kim HK:
Anti-obesity activity, acute toxicity, and chemical constituents of
aqueous and ethanol Viola mandshurica extracts. BMC Complement
Altern Med. 17:2972017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seo S, Lee MS, Chang E, Shin Y, Oh S, Kim
IH and Kim Y: Rutin increases muscle mitochondrial biogenesis with
AMPK activation in high-fat diet-induced obese rats. Nutrients.
7:8152–8169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao L, Zhang Q, Ma W, Tian F, Shen H and
Zhou M: A combination of quercetin and resveratrol reduces obesity
in high-fat diet-fed rats by modulation of gut microbiota. Food
Funct. 8:4644–4656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Milton-Laskibar I, Gómez-Zorita S, Aguirre
L, Fernández- Quintela A, González M and Portillo MP:
Resveratrol-induced effects on body fat differ depending on feeding
conditions. Molecules. 22:E20912017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ohyama K, Furuta C, Nogusa Y, Nomura K,
Miwa T and Suzuki K: Catechin-rich grape seed extract
supplementation attenuates diet-induced obesity in C57BL/6J mice.
Ann Nutr Metab. 58:250–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rahman HA, Sahib NG, Saari N, Abas F,
Ismail A, Mumtaz MW and Hamid AA: Anti-obesity effect of ethanolic
extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat
diet. BMC Complement Altern Med. 17:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rodriguez Lanzi C, Perdicaro DJ, Landa MS,
Fontana A, Antoniolli A, Miatello RM, Oteiza PI and Vazquez Prieto
MA: Grape pomace extract induced beige cells in white adipose
tissue from rats and in 3T3-L1 adipocytes. J Nutr Biochem.
56:224–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen S, Li Z, Li W, Shan Z and Zhu W:
Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via
activation of AMPK. Can J Physiol Pharmacol. 89:793–799.
2011.PubMed/NCBI
|