Angiogenesis is a complex cellular mechanism
required for the formation of new blood vessels from the existing
vasculature or from bone marrow-derived endothelial progenitors,
allowing tumor growth and development at early stages of
carcinogenesis (1).
Neovascularization is a prerequisite for tumorigenesis when oxygen
and nutrient levels are insufficient to sustain cell proliferation
and tumor growth. During neovascularization, the tumor
microenvironment produces stimulatory signals that induce changes
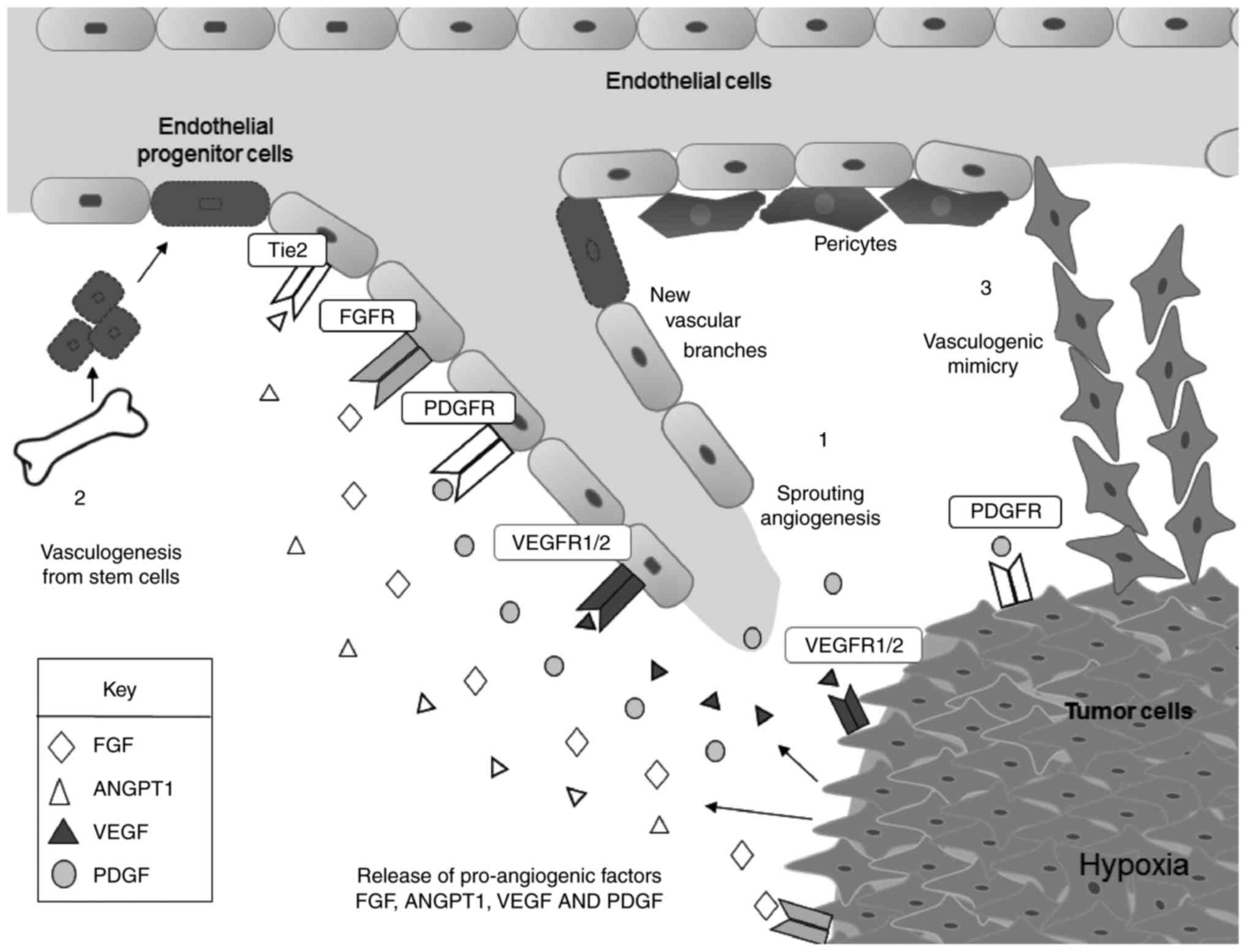
in diverse cell types (Fig. 1).
Pericytes detach from pre-existing vasculature disrupting the
integrity of mature blood vessels. Platelets are activated and
release stores of stimulatory factors into the tumor
microenvironment. In addition, new vascular branches may be
stimulated by bone marrow-derived endothelial progenitor cells
(EPCs). Tumor cells also participate in the formation of new
vessels through vascular mimicry, a novel angiogenesis-independent
mechanism in which highly aggressive and metastatic epithelial
tumor cells form vascular 3D channel-like structures resembling
classical endothelial blood vessels (2). All these cellular types secrete
soluble factors in the tumor microenvi-ronment enhancing
extracellular matrix (ECM) remodeling and inducing the production
of tortuous blood vessels (neovascularization). Notably, this
environment makes the tumor cells more invasive, allowing them to
intravasate into the vasculature and to disseminate to distant
tissues, resulting in metastasis.
The angiogenic switch that governs the tumor
neovascularization requires a change in the balance between pro-
and anti-angiogenic factors. Hypoxia is an important factor
required for activation of the angiogenesis program, as it
activates the expression of pro-angiogenic proteins from tumor and
stroma cells, such as vascular endothelial growth factor (VEGF),
transforming growth factor (TGF) α, fibroblast growth factor (FGF),
and platelet-derived growth factor (PDGF) among others. Hypoxia
inducible factor 1 (HIF1) acts a master regulator of the genetic
program leading to angiogenesis, mainly through activation of VEGF.
The HIF1 protein complex is a heterodimer consisting of the HIF1α
and HIF1β subunits (3). Under
normoxia conditions, HIF1α is rapidly hydroxylated on conserved
prolyl residues located within the oxygen-dependent degradation
domain, and then it binds to von Hippel-Lindau protein (pVHL),
which in turn targets HIF-1α for degradation through the
ubiquitin-proteasome pathway. By contrast, hypoxia inhibits the
hydroxylation of HIF1α prolyl residues 402 and 564, which in turn
inhibits both binding to pVHL and protein degradation. The HIF1
complex recognizes and binds to the hypoxia response sequence
element (5′-CGTG-3′) on the promoter regions of pro-angiogenic
genes, such as VEGF, PDGF, and TGF-α, activating them and resulting
in blood vessel remodeling and angiogenesis. In addition, growth
factors, cytokines and oncogenes, which stimulate the
mitogen-activated protein kinase (MAPK) and phosphoinositide
3-kinase (PI3K) pathways, enhance HIF-1α activity. Notably, the
genes responsible for the angiogenic switch may be regulated at the
post-transcriptional level by microRNAs (miRNAs). Understanding the
role of miRNAs is particularly relevant in aberrant angiogenesis in
human cancers. Thus, the crucial role of neovascularization to
tumor progression has rendered angiogenesis a particularly
interesting research field of drug development, as it provides
opportunities for clinical intervention.
miRNAs are conserved small non-coding RNAs of 21-25
nucleotides in length, which act as negative regulators of gene
expression. The canonical biogenesis of miRNAs initiates with
transcription of genes located in intergenic regions by RNA
polymerase II (RNA pol II) to generate hairpin-shaped long
transcripts, called primary miRNAs (pri-miRNA), with 5′-cap and
3′-end poly(A) tail (4).
Subsequently, these molecules are recognized by the DiGeorge
syndrome critical region protein 8 (DGCR8) which associates with
the RNAse III enzyme Drosha in a microprocessor complex that
cleaves pri-miRNA and liberates stem-loop structures, known as
precursor miRNAs (pre-miRNA) (5).
Alternatively, pre-miRNAs can be generated by the mRNA splicing
machinery from introns or pseudo-genes, without the participation
of the microprocessor complex (6). Pre-miRNA molecules have a 3′ end
two-nucleotide overhang that is recognized by the Ran-GTP dependent
export factor exportin 5 that facilitates their translocation to
the cytoplasm. In this cellular compartment, the RNAse III enzyme
Dicer interacting with the double-stranded (ds) RNA-binding protein
TRBP2 eliminates the loop, to produce an imperfect dsRNA duplex.
Although the transient strand miRNA* has previously been considered
as irrelevant, recent studies suggest that it is as functional as
the guide strand. These 19-25 bp RNA molecules, together with
Argonaute proteins, can be incorporated into the silencing complex
induced by RNA-induced silencing complex (RISC), to promote
recognition of the complementary sequence; predominantly in the 3′
untranslated region (3′-UTR) of target mRNAs (7). The fate of targeted transcripts
depends on the degree of complementarity between miRNA and mRNA. A
perfect interaction leads to messenger degradation, while imperfect
complementary binding induces translational repression (8). Both events occur in cytoplasmic foci
denoted as mRNA processing bodies (P-bodies), which represent mRNA
processing centers where non-translating transcripts are stored,
silenced or degraded (9).
Altered expression of miRNAs has been reported in
diverse types of human cancer, where they regulate the expression
of oncogenes and tumor suppressor genes, thus they have been dubbed
as oncomiRs. In many cases, aberrant expression of miRNAs
correlates with worst prognosis, low overall survival and
resistance to chemotherapy (10).
Diverse studies, focused on miRNA profiles impacting angiogenesis,
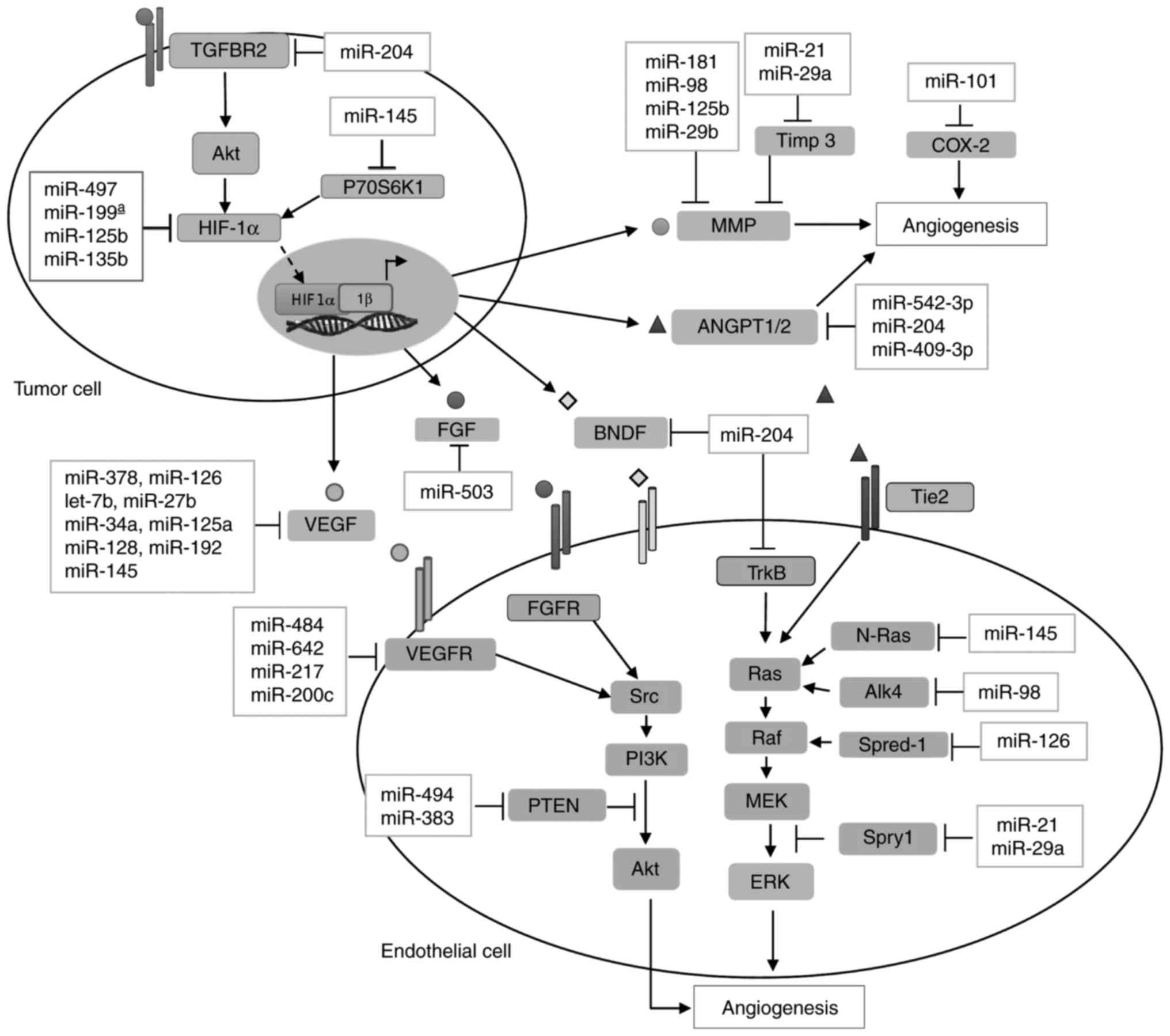
have been described in almost all human cancers (11,12). miRNAs controlling the angiogenic
mechanisms are collectively known as angiomiRs, as they regulate
this specialized process in both physiological and pathological
conditions (Fig. 2 and Table I) (13,14). The biological relevance of miRNAs
in angiogenesis was first uncovered by loss of function studies in
which Dicer, an endonuclease required for miRNA maturation, was
disrupted. Generation of Dicer1-deficient mice resulted in early
embryonic lethality and stem cell loss (15). In addition, mice carrying a
deletion corresponding to the first and second exons of the Dicer
gene exhibited severe vascular defects and had altered expression
of angiogenic regulators (16).
Since complete abrogation of Dicer in mice was embryonic lethal,
the specific role of miRNAs in angiogenesis was addressed by
generating endothelial-specific Dicer knockouts (17). The cell-specific inactivation of
Dicer resulted in the reduction of endothelial miRNAs and reduced
postnatal angiogenic response to exogenous VEGF, tumors, limb
ischemia, and wound healing. Furthermore, VEGF regulated the
expression of oncogenic miRNAs of the cluster miR-17-92. These data
indicated that endothelial miRNAs regulate postnatal angiogenesis
and VEGF upregulated the expression of miRNAs implicated in the
angiogenic response. Different laboratories have also demonstrated
that silencing Dicer in epithelial cells inhibits cell
proliferation, migration, and capillary sprouting under basal
conditions and in response to angiogenic factors (18,19). According to Kuehbacher et
al (18), depleting Dicer and
Drosha using siRNAs in endothelial cells reduced lef-7f and mir-27b
expression. In addition, inhibitors of let-7f and mir-27b reduced
sprout formation indicating that let-7f and mir-27b promote
angiogenesis by targeting antiangiogenic genes (18). By contrast, the knockdown of Dicer
in endothelial cells also altered the expression of regulators of
angiogenesis, including TEK receptor tyrosine kinase (also known as
Tie2), VEGFR2, Tie1, endothelial nitric oxide synthase and
interleukin (IL) 8. The global profiling of miRNAs revealed 25
upregulated miRNAs in endothelial cells and using miRNA mimicry,
miR-222/221 regulated nitric oxide synthase following Dicer
silencing (19). Although these
studies support the idea of miRNAs controlling vascular function
and angiogenesis, the contribution of additional non-canonical
functions of Dicer to the angiogenic process cannot be
excluded.
Further support for a role of miRNAs in the
vasculature came from studies that identified endothelial miRNAs
using microarrays (20-23). An early report identified 27
miRNAs highly expressed in human umbilical vein endothelial cells
(HUVECs), many of which had angiogenic factor receptors as their
predicted mRNA targets. Authors demonstrated that both miR-221 and
miR-222 specifically regulate stem cell factor (SCF)-induced
angiogenesis by targeting c-KIT (19). Likewise, McCall et al
(22) described a miRNAs
signature whose expression levels are generally consistent across
epithelial cells form different vascular locations with the
exception of miR-99b, miR-20b and let-7b. To date, close to 200
endothelial miRNAs have been described, though <20% of them have
been consistently found across different studies (22). Endothelial miRNA expression
profiles are also known to be modified in response to a wide array
of stimuli including hypoxia, VEGF and angiotensin II, providing
evidence of the plasticity of this system in fine-tuning vascular
function. For instance, miR-126, miR-210 and the miR17/92 cluster,
a polycistronic miRNA gene that encodes for miR-17, miR-18a,
miR-19a, miR-20a, miR-19b-1 and miR-92a, are an example of miRNAs
essential for maintaining vascular structure in vivo; but
many more have emerged as regulators of endothelial cell survival,
migration, proliferation and angiogenic signaling pathways
(22,23). Therefore, angiomiRs may be
promising targets and they may contribute to
anti-angiogenesis-based combined treatments of cancer (24).
Breast cancer is one of the most frequent carcinomas
and ranks second as a cause of cancer-related mortality in women
(25). Several research groups
have identified distinct miRNA expression profiles and individual
miRNAs relevant for angiogenesis, metastasis and overall survival
in breast cancer patients. For instance, the endothelial miR-126,
derived from the intron 7 of the EGF-like domain 7 (EGFL7)
(26), was found downregulated in
breast tumors and associated with poor overall metastasis-free
survival (27). Zhu et al
(28) demonstrated that miR-126
inhibits VEGF/PI3K/AKT signaling by targeting VEGFA and PI3K
regulatory subunit 2 (PIK3R2). Ectopic expression of miR-126
suppressed the expression of CD97, a G-coupled receptor that
promotes cell invasion and angiogenesis through integrin signaling
(29). miR-126 and miR-126* both
influence breast cancer metastasis by cell autonomous and non-cell
autonomous mechanisms involving angiogenesis (30). Of note, miR-126/miR-126* also
inhibited lung metastasis of breast cancer cells by suppressing the
recruitment of mesenchymal stem cells and inflammatory monocytes
into the tumor microenvironment in a stromal cell-derived factor 1
α (SDF1A)-dependent manner. Another study demonstrated that miR-126
regulates angiogenesis and metastasis by targeting the
pro-angiogenic insulin-like growth factor binding protein 2
(IGFBP2), phosphatidylinositol transfer protein cytoplasmic 1 and
c-Mer tyrosine kinase genes (31).
On the other hand, miR-497 reduces tumor growth and
angiogenesis in a mouse xenograft model (32). In addition, conditioned media
derived from miR-497-expressing cells, suppress endothelial cell
tube formation in vitro and reduce VEGF and HIF1α protein
levels. Tu et al (33)
reported that overexpression of miR-497 in 4T1 cells significantly
inhibited breast tumor growth, angiogenesis and VEGFR2 expression
when subcutaneously implanted in VEGFR2-luc transgenic mice. In
addition, miR-497 expression in HUVECs induces apoptosis and
inhibits cell proliferation by targeting AKT and extracellular
signal-regulated kinase (ERK) signaling pathways in a
VEGFR2-dependent way.
Tumors respond to low oxygen tension by activation
of HIF1α-dependent and hypoxia-induced genetic program involving
miRNAs (34). For instance,
recent studies reported that miR-155, miR-578 and miR-573 have key
roles in HIF1α-mediated angiogenesis, and their expression was
differentially modulated in BRCA1/2-related breast cancer (35,36). Kong et al (37) demonstrated that miR-155
overexpression in tumor cells promotes angiogenesis, proliferation
and proinflammatory cell recruitment in a mammary fat pad
xenotransplant model. In addition, miR-155 levels are inversely
correlated with von Hippel-Lindau (VHL), an E3 ubiquitin ligase
that targets HIF1 family members; this finding suggests that
miR-155 expression decreases HIF1α-mediated angiogenesis by
targeting VHL in breast tumors. By contrast, miR-578 and miR-573
are downregulated in BRCA1/2-related breast cancer and appear to
control angiogenesis by modifying VEGFA, focal adhesion kinase
(FAK), angiopoietin 2 (ANGPT2) and HIF1α expression through an
indirect mechanism, since they failed to bind to the 3′ UTR of the
aforementioned genes (38).
Additionally, miR-210, a hypoxia-inducible miRNA, is involved in
tumor growth, angiogenesis and activation of VEGF signaling in
breast cancer patients (39).
Oncogenic miR-21 has been identified as a potential molecular
prognostic marker for breast cancer progression, as its
over-expression correlates with advanced tumor stage, lymph node
metastasis and poor patient survival (40). In a VEGFR2-luc mouse model of
breast tumorigenesis, a miR-21 antagomir effectively suppressed
tumor growth and angiogenesis by targeting the VEGF/VEGFR2/HIF1α
axis (41). Notably, miR-21 and
miR-29a expression in macrophages promoted CD31+ vessel
growth in matrigel plugs and reduced the expression of
anti-angiogenic genes, such as collagen type IV α2 (COL4A2),
sprouty homolog 1 (SPRY1) and tissue inhibitor of
metalloproteinases-3 (TIMP3). These findings suggest that miR-21
and miR-29a facilitate a pro-angiogenic phenotype in
tumor-associated myeloid cells, which contributes to tumor
progression (42). In addition,
key modulators of angiogenesis and extracellular matrix remodeling,
like VEGFA, angiopoietin-like 4, PDGF, lysyl oxidase (LOX),
metallopeptidase (MMP) 2 and MMP9, contain functional
miR-29b-specific binding sites located in their 3′UTRs, suggesting
that miR-29b may act as a multi-target non-coding RNA to suppress
metastasis of cancer cells.
miR-542-3p levels inversely correlate with clinical
progression of breast cancer in patients with advanced stage
disease (43). Ectopic expression
of miR-542-3p reduced tumor growth, angiogenesis and metastasis in
a breast cancer mouse model (44). He et al (44) proposed a novel tumor-endothelial
cell-signaling pathway to explain the angiogenic inhibition induced
by miR-542-3p. In their model, tumor cell-derived angiogenin was
demonstrated to downregulate miR-542-3p in endothelial cells by
suppressing the CCAAT/enhancer-binding protein β (CEBPβ) and POU
class 2 homeobox 1 (POU2F1) transcription factors, while increasing
the expression of ANGPT2 protein (44).
miR-204 is a novel multi-target angiomiR in breast
cancer. Recently, we analyzed the miRNome of locally advanced
breast tumors and found a consistent and dramatic suppression of
miR-204 in patient tumors and breast cancer cell lines (47). Ectopic expression of miR-204
inhibited cell proliferation, anchorage-independent growth,
migration, and invasion. In vivo vascularization and
angiogenesis were also suppressed by miR-204 in a nu/nu mice model.
Transcriptome profiling of MDA-MB-231 cells expressing miR-204
indicated that expression of pro-angiogenic ANGPT1 and TGFβR2
proteins was suppressed by miR-204. Functional analysis confirmed
that ANGPT1 and TGFβR2 are novel targets of miR-204. In agreement,
an inverse correlation between miR-204 and ANGPT1/TGFβR2 expression
was evidenced in breast tumors, revealing a novel role for the
miR-204/ANGPT1/TGFβR2 axis in tumor angiogenesis (47). Recently, our group also reported
that miR-204 has a pivotal role in the formation of 3D
capillary-like networks by tumor cells, a cellular mechanism
denoted as vasculogenic mimicry (VM) in cancer cells. This
phenomenon was first described in melanoma cells as a novel blood
and oxygen supply event in which tumors can feed themselves. Of
note, VM operates simultaneously with angiogenesis. During VM,
tumor cells form patterned 3D channel-like structures which combine
with blood vessels (mosaic pattern), challenging the initial
assumption that angiogenesis is the only mechanism by which tumors
acquire nutrients and oxygen. These pseudo-3D channels contain
plasm, erythrocytes and blood flow with a hemodynamics resembling
angiogenesis (48). miR-204
targets multiple signaling transducers involved in VM and
angiogenesis in invasive triple negative MDA-MB-231 and Hs-578T
breast cancer cells. Ectopic restoration of miR-204 in MDA-MB-231
cells leads to a potent inhibition of hypoxia-induced VM and
reduction of number of branch points and capillary tubes (49). Finally, miR-204 reduces the
expression and phosphorylation of 13 proteins involved in PI3K/AKT,
RAF1/MAPK, VEGF, and FAK/SRC signaling. Functional studies
confirmed that miR-204 targets PI3Kα and c-SRC transducers,
indicating that miR-204 exerts a fine-tuning regulation of the
PI3K/AKT/FAK axis critical in VM formation and angiogenesis
(49).
Pancreatic cancer represents the fourth leading
cause of cancer-related deaths in the United States, and with a
5-year survival rate of only 7%, it has the worst outcome for
cancer patients (50). Pancreatic
ductal adenocarcinomas (PDACs) arise from the exocrine pancreas,
represent 75% of pancreatic tumors, and are usually diagnosed at
advanced stages (51). The
vasculature of these tumors appears to be highly disorganized and
hypoxic as a result of a characteristic desmoplasia, in which
excessive proliferation of activated fibroblasts and overproduction
of extracellular matrix proteins increase interstitial pressure and
cause vascular disruption (52).
Hypoxia-associated angiomiRs, such as miR-21, miR-200c and miR-199,
are known to be dysregulated in PDACs (53). Hypoxia promotes pancreatic cancer
cell migration, invasion and angiogenesis in vitro, and also
induces miR-21 expression (54).
miR-21 overexpression has been reported in pancreatic carcinoma
cell lines and tumors (55-57). Clinical studies have associated
miR-21 with poor clinical outcomes and resistance to chemotherapy;
miR-21 plasma levels also seem to correlate with advanced stage,
metastasis and shorter survival in patients with PDAC (57). Bao et al (58) recently reported that transfection
of a miR-21 antagomiR resulted in an increase of phosphatase and
tensin homolog (PTEN) expression in pancreatic cancer cells, a
potent suppression of AKT and ERK signaling pathways, and a
reduction of angiogenesis, as a result of HIF1α and VEGF
downregulation. Pancreatic cancer cells induce tumor-associated
fibroblasts to express miR-21 as a mechanism to enhance their own
invasiveness; and higher expression of stromal miR-21 correlates
with metastasis in PDAC patients (59). Increased levels of HIF1α are
associated with advanced tumor stages in PDAC patients (60). Notably, a HIF-1α single-nucleotide
polymorphism (SNPs), rs2057482, was reported as an important
genetic variant for PDAC risk and poor prognosis (61). This SNP was located near the
miR-199a seed-binding site in the 3′ UTR of HIF1α; and the presence
of a CC genotype decreased miR-199a-induced repression of HIF-1α.
Although the specific role of miR-199a in PDAC vasculature is
unknown, miR-199a modulates the ETS-1/MMP1 pathway in ECs and has
been described as an important regulator of angiogenic processes
(62).
Lung cancer represents the first cause of
cancer-related mortality in men and women. Approximately 80% of
lung tumors are non-small cell lung cancer (NSCLC) (69). Angiogenic factors are prognostic
indicators for tumor aggressiveness and survival in NSCLC, and
angiogenic inhibitors are currently being used as treatment with
varying results (70-72). A recent study by Chen et al
(73) highlighted the importance
of miRNAs in the development and maintenance of tumor vasculature
by subcutaneously injecting Dicer1−/− NSCLC cells into
flanks of nude mice. Furthermore, several angiomiRs have been
reported with altered expression levels in NSCLC, including
miR-126, miR-21, miR-210, miR-106a, miR-155, miR-182 and miR-424
(74). For instance, several
studies reported that miR-126 is downregulated in NSCLC tumors and
lung cancer cell lines, and high miR-126 expression has been
associated with lymph node status, poor survival and high VEGFA
expression (75,76). In addition, miR-126 emerged as
part of an angiogenic signature in a cohort of 335 NSCLC patients
(74). Liu et al (75), demonstrated that transducing
miR-126 into A549 tumor cells using a lentiviral vector produces
smaller tumor nodules through downregulation of its direct target
VEGFA and cell arrest induction. A possible anti-angiogenic role of
miR-126 and miRNA let-7b in lung cancer development was suggested
by a study in which miR-126 and let-7b downregulation correlated
with higher microvessel density (MVD) in tumor and surrounding
stroma when compared to non-tumor tissues (77). Notably, members from the let-7
family are known players in hypoxia-induced angiogenesis and their
downregulation has also been linked to poor survival outcomes of
lung cancer patients (78-80).
The cluster miR-132/212 is located within the same
intron of a non-coding gene on human chromosome 17, and its
deletion increases angiogenic responses in vivo (88). miR-132 expression is significantly
downregulated in NSCLC clinical specimens and cell lines, and
miR-212 silencing is frequent in lung cancer and closely correlates
with stage of disease in NSCLC patients (89,90). A study by Luo et al
(91) evaluated the effect of the
miR-132/212 cluster in subcutaneous xenografts of human lung cancer
H1299 cells in nude mice. The results demonstrated that miR-132/212
cluster expression inhibited tumor growth by increasing p21
expression, downregulating CyclinD1, and decreasing MVD. These
findings propose an important role of miR-132 and miR-212 in tumor
angiogenesis; however the exact mechanisms by which these miRNAs
reduce MVD remain unknown.
Tissue inhibitor of metalloproteinases-1 (TIMP1) has
emerged as a pro-angiogenic factor responsible for miR-210
upregulation in a CD63/PI3K/AKT/HIF1-dependent pathway in lung
adenocarcinoma cells (92).
Elevated TIMP-1 levels correlate with adverse prognosis in NSCLC
patients (93). Cui et al
(92) have reported that TIMP-1
overexpression in A549L cells increases angiogenesis in tumor
xenografts, results in exosomal miR-210 accumulation, and promotes
capillary tube formation in HUVECs. Additionally, fibroblast growth
factor receptor-like 1 (FGFRL1), E2F transcription factor 3 (E2F3),
vacuole membrane protein 1 (VMP1), Rad52 and succinate
dehydrogenase complex subunit D (SDHD) are miR-210 downstream
targets downregulated in the presence of TIMP-1, suggesting that
the pro-tumorigenic functions of TIMP-1 are partly mediated by
miR-210.
Several studies have demonstrated that miR-21 is
frequently overexpressed in serum and tumor tissues from CRC
patients (100-102). Since miR-21 is a negative
regulator of multiple tumor suppressor genes, including PTEN,
TIMP3, TPM1, maspin and programmed cell death protein 4 (PCDP4),
some research groups have focused on the effects of anti-miR-21
therapies in CRC (103).
Silencing of miR-21 in CRC cells using miR-21 antagomiRs affected
cell cycle and cell viability and activated apoptosis (104). In addition, anti-miR-21
treatment also inhibited capillary-like networks formation in
vitro. Bridge et al (105) reported a functional role for
miR-30 in the regulation of angiogenesis by targeting Delta-like 4
(DLL4), and demonstrated that introduction of exogenous miR-30 in
ECs or into zebrafish embryos promoted angiogenic sprouting. DLL4
is a membrane-bound ligand from the Notch signaling family,
restricted to tip cells, which regulates vessel sprouting and
branching in response to angiogenic factors during vascular
development and angiogenesis (106).
Thrombospondin 1 (TSP1), a protein mainly expressed
in tumor stroma, inhibits angiogenesis and tumor growth via the
TGFβ pathway in CRC (107).
Several reports have suggested that miR-182 and miR-194 (miR-17-92
cluster) contribute to angiogenesis through a mechanism that
represses TSP1 in CRC. The miR-17-92 cluster is upregulated in CRC
and correlates with progression from colorectal adenoma to
adenocarcinoma (108). According
to Dews et al (109),
K-RAS-transformed p53-null mouse colonocytes form poorly
vascularized tumors, which were reverted to highly vascularized
tumors with increased growth when transduced with a Myc-encoding
retrovirus. Behind these Myc-dependent effects was the upregulation
of miR-17-92 cluster, which appears to promote angiogenesis through
direct repression of TSP1 and connective tissue growth factor
(CTGF) by miR-18a and miR-19, respectively. In addition, miR-182
binds to the TSP1 3′UTR in CRC cells and decreases nuclear
translocation of early growth response 1 (EGR1) (110). Expression of miR-194 is known to
be gastrointestinal tract-specific and p53-dependent; loss of p53
in HCT116 cells significantly reduces miR-194 levels (111,112). A study by Sundaram et al
(112) described miR-194 as
intrinsically angiogenic. Furthermore, transient overexpression of
miR-194 in HCT116/THBS1 cells resulted in high angiogenesis in
vitro. Likewise, stable expression of miR-194 in RAS-induced
murine colon carcinomas, augmented MVD and vessel sizes. Notably,
these pro-angiogenic effects of miR-194 in vivo did not
translate into increased tumor growth, presumably due to regulation
of other miR-194 targets and its co-expression with miR-215, a
known inhibitor of the cell cycle (112,113).
During hypoxia the miR-15-16 cluster is repressed by
c-Myc, which results in elevated tumor angiogenesis and metastasis
by inducing the expression of fibroblast growth factor 2 (FGF2)
protein (113,114). In addition, systemic delivery of
miR-15a/16-1 resulted in a significant reduction of tumor growth
and angiogenesis in colon cancer xenografts (115). Levels of miR-15a and miR-16-1 in
CRC cells inversely correlate with their target cyclin B1 (CCNB1),
a cell cycle regulatory protein associated with tumorigenic and
metastatic features of CRC cells (115,116).
Activation of the β-catenin/WNT signaling pathway is
a key event in the development of CRC and has been linked to
angiogenic processes in the tumor microenvironment (117). miR-29b targets transcription
factor 7-like 2 (TCF7L2), SNAIL and B-cell CLL lymphoma 9-like
protein (BCL9L) and it is associated with decreased translocation
of β-catenin to nuclei in SW-480 colorectal adenocarcinoma cells
(118,119). Ectopic expression of miR-29b
reduces the ability of SW-480 to induce tube formation in
vitro, suggesting that miR-29b participates in angiogenic
processes. Restoring miR-29b expression suppresses CRC tumor
invasion and metastasis by reversing epithelial-mesenchymal
transition (EMT) and by targeting MMP2 and T-cell lymphoma invasion
and metastasis 1 (TIAM1) (118-120). miR-130b is another angiomiR that
is overexpressed in advanced CRCs and promotes tumor growth through
induction of EMT and angiogenesis (121). Tumors derived from cells that
express high levels of miR-130b are highly vascularized. Notably, a
direct functional target of miR-130b is peroxisome
proliferator-activated receptor gamma (PPARγ), a CRC-independent
prognostic factor involved in cell differentiation and growth that
is highly expressed in tumor endothelium (121,122).
miR-192 inhibited metastasis by repressing key
pro-metastatic genes, including B-cell lymphoma 2 (BCL2), zinc
finger E-box binding homeobox 2 (ZEB2) and VEGFA in HCC (130). Further analysis of tumors from
CRC patients revealed an inverse correlation between miR-192
expression and advanced tumor stages. miR-192 expression in models
of CRC progression suppressed liver metastasis through VEGFA
repression which resulted in reduced vascularization of primary
tumors in vivo. In addition, VEGF expression and decreased
AKT activation by miR-145 have been reported in mouse CRC xenograft
tumors, and miR-145 expression inhibited tumor growth and
angiogenesis (129).
Furthermore, in CRC tissues, miR-145 levels inversely correlated
with two of its known targets: N-RAS and insulin receptor substrate
1 (IRS1) (131). Finally, it was
reported that miR-145 reduced HIF1α and VEGF levels, potentially
through repression of its upstream regulator p70S6K1 (132).
The insulin-like growth factor 1 receptor (IGFIR) is
a transmembrane protein that activates downstream effectors
involved in angiogenesis and tumorigenesis (133). miR-143 levels were significantly
decreased in plasma samples and CRC tissues, and inversely
correlated with IGF-IR levels in patients (134). miR-143 inhibited IGF-IR by
binding to its 3′UTR, and also inactivated AKT, HIF-1α and VEGF in
SW1116 cells. Additionally, when these miR-143-expressing cells
were subcutaneously injected into nude mice, they produced smaller
tumors with reduced VEGF expression. Ectopic expression of miR-143
in SW1116 cells significantly suppressed angiogenesis in a
chorioallantoic membrane system. Similarly, miR-23b, a repressor of
prometastatic genes frizzled class receptor (7FZD7) and
mitogen-activated protein kinase kinase kinase 1 (MAP3K1, was
downregulated in CRC. Using a genome-wide functional screening,
miR-23b was identified as an important suppressor of angiogenesis,
tumor growth and invasion (135).
Activation of TGFβ/SMAD signaling induces EMT, a
frequent event during cancer progression (136). Two angiomiRs, miR-885-3p and
miR-1246, both target the SMAD pathway in CRC. miR-885-3p
expression impairs the growth of HT-29 xenografts in nude mice and
suppresses angiogenesis by disrupting the bone morphogenetic
protein receptor type 1A (BMPR1A) and SMAD/ID1 signaling (137). miR-1246 was found in CRC-derived
microvesicles which induce proliferation, migration and tube
formation in HUVECs (138). The
angiogenic identity of these microvesicles was attributed to
activation of SMAD1/5/8 signaling in HUVECs by miR-1246 and TGF-β.
This study suggests that CRC-derived microvesicles contribute to
tumor angiogenesis.
Ovarian cancer (OC) is the second most common and
the most fatal gynecologic cancer in women (139). Several research groups have
identified distinct miRNAs involved in angiogenesis, tumorigenesis,
metastasis and chemoresistance of OC (140-142). For instance, miR-199a, miR-125b
and miR-145 have a key role in HIF1α-mediated angiogenesis in OC.
He et al (142)
demonstrated that miR-199a and miR-125b were downregulated in
epithelial ovarian carcinoma compared with normal tissues, and that
they repressed angiogenesis by directly targeting pro-angiogenic
factors HIF1α and VEGF, through AKT/p70S6K1/HIF1α signaling.
Similarly, miR-145 was downregulated in OC tissues and cell lines
and inhibited angiogenesis by targeting p70S6K. miR-145 inhibited
the expression of both HIF1α and VEGF (132). The expression of miR-497 was
lower in OC tissues in comparison with normal tissues. Restoration
of miR-497 resulted in decreased angiogenesis which was associated
to suppression of VEGFA via PI3K/AKT and MAPK/ERK signaling
pathways (143).
Another important mechanism of carcinogenesis
mediated by hypoxia is the acquisition of resistance to
chemotherapy. Several miRNAs, such as miR-484, miR-642 and miR-217,
have been demonstrated to modulate VEGFB and VEGFR2 and predict
tumor chemoresistance and increased angiogenesis in ovarian cancer
(11). Furthermore, expression of
miR-378 is decreased in OC cell lines and tumors compared with
normal tissues. miR-378 inhibits the expression of genes associated
with angiogenesis and apoptosis. Furthermore, the inhibition of
ALCAM and EHD1 by miR-378 reduces the expression of the multidrug
resistance gene (MDR) and is associated with progression-free
survival (PFS) in a subgroup of patients who received
anti-angiogenic therapy (144).
miRNAs may also modulate transcriptions factor
function in ovarian cancer. Restoration of miR-27a represses VEGF
expression, as well as COX2 and SP1 transcription factors (145). In addition, the miR-200 family,
including miR-200a, b and c, inhibit EMT by downregulating ZEB1 and
ZEB2 in diverse types of cancer, including ovarian tumors (146). Of note, miR-200 blocks
angiogenesis by targeting IL8 and CXCL1 secreted by the endothelial
cells, suggesting that miR-200 members can have therapeutic effects
on angiogenesis and EMT-driven metastasis in ovarian cancer
(147). Another study by Imam
et al (148) demonstrated
that genomic loci encoding miR-204 were frequently lost in multiple
malignancies, including ovarian cancer. The restoration of miR-204
levels in ovarian cancer cells reduced overall tumor growth, cell
proliferation and metastasis. In addition, the inhibition of
brain-derived neurotrophic factor (BDNF) by miR-204 reduced
angiogenesis and invasiveness, indicating that it acts as a tumor
suppressor (148).
A large number of anti-angiogenic agents are
currently being tested for the treatment of diverse types of human
malignancies. In the last decade, the Food and Drug Administration
(FDA) has approved various anti-angiogenetic agents for the
treatment of cancer, including monoclonal antibodies (e.g.
bevacizumab) and tyrosine kinase inhibitors (e.g. sunitinib,
sorafenib) (149). The
anti-angiogenic activity of these drugs and antibodies are derived
from their ability to block key angiogenic proteins, including
VEGFA, VEGFR1/2/3, PDGFR1/2, p38, MAPK and FGFR1.
Currently, other anti-angiogenic compounds are being
tested in clinical trials, although their approval might be
jeopardized by unexpected toxicity, and resistance developing with
molecular mechanisms poorly understood. The increasing research
evidence supports an important role of miRNAs in angiogenesis,
however this molecular knowledge needs to be properly translated
into improvement of clinical management for cancer patients. At the
pre-clinical level, several studies showed promising results in the
potential clinical applications of miRNAs targeting angiogenesis.
For instance, a previous report indicated that restoring miR-26
expression had dramatic effects in terms of tumor growth inhibition
in a mouse model of hepatocelular carcinoma without remarkable
toxicity (150). In addition, a
recent study demonstrated that miR-204-expressing breast cancer
cells suppressed angiogenesis in a nu/nu mice model (47). An in vivo analysis also
demonstrated that the interplay between HMOX1 and miR-378
significantly modulates NSCLC progression and angiogenesis;
miR-378-overexpressing tumors were larger, more vascularized and
more metastatic, suggesting that miR-378 may serve as a novel
therapeutic target (85). These
and others findings certainly suggest that modulating miRNA
expression could be a promising anti-angiogenic strategy in mouse
models of cancer, however, further evidence is still needed to
demonstrate successful application and low system toxicity in human
patients.
At the clinical level, several trials using miRNAs
as therapeutic agents are under development. For instance, the
first-in-human phase I study called MesomiR-1 used TargomiRs as 2nd
or 3rd line treatment for patients with recurrent malignant pleural
mesothelioma and non-small cell lung cancer (ClinicalTrials.gov identifier, NCT02369198). TargomiRs
are novel targeted minicells containing: A miR-16 mimic (the miR-16
family is a tumour suppressor with key roles in cell proliferation,
migration and angiogenesis in a multiple cancer types); an EnGeneIC
delivery vehicle [EDV; nonliving bacterial minicell carriers
(nanoparticles)]; and a moiety to targets EDVs to EGFR-expressing
cancer cells with an anti-EGFR bispecific antibody. For this study,
26 pleural mesothelioma patients were recruited at three major
cancer centers in Sydney (Australia), received at least one
Targomir dose (the maximum tolerated dose was 5×109
TargomiRs once weekly), the safety profile was acceptable, and 1 of
22 patients had an objective response that lasted 32 weeks. The
results were recently published by van Zandwijk et al
(151). The authors concluded an
acceptable safety profile and early signs of activity of TargomiRs
in patients, and highlighted the urgency for additional studies of
TargomiRs in combination with chemotherapy or immune checkpoint
inhibitors. Many of the side effects observed in this trial
consisted of inflammatory reactions, which strongly supports the
idea of an immunologic effect. In conclusion, although
TargomiRs-based therapy was an example of a successful
first-in-human use of a miRNA-based therapy for pleural
mesothelioma patients, concerns about toxic and immune effects
should be fully addressed.
Another study in humans focuses in the assessment of
the potential therapeutic applications of tumor suppressor miR-34a,
a non-coding RNA that downregulates the expression of multiple
oncogenes across multiple signaling pathways, as well as genes
involved in tumor immune evasion, angiogenesis and metastasis in
endothelial cells and many malignancies (68,152-154). This
first-in-human, phase I study assessed the maximum tolerated dose
(MTD), safety, pharmacokinetics, and clinical activity of MRX34, a
liposomal miR-34a mimic, in patients with advanced solid tumors
(ClinicicalTrials.gov identifier,
NCT01829971). Adult patients with solid tumors refractory to
standard treatment were enrolled in a standard 3 + 3 dose
escalation trial. MRX34 was administered intravenously twice weekly
for three weeks in 4-week cycles. Forty-seven patients with various
solid tumors, including hepatocellular carcinoma were enrolled. The
authors concluded that MRX34 treatment with dexamethasone
premedication was associated with acceptable safety and exhibited
evidence of antitumor activity in a subset of patients with
refractory advanced solid tumors (155).
Basic research into the roles of miRNAs in tumor
angiogenesis has been increasing in the last decade. Although
definitive clinical evidence about the potential therapeutic
applications of angiomiRs in patients is still lacking, there is no
doubt that these small RNAs deserve attention as attractive targets
for development of novel anticancer drugs. An increased amount of
anti-angiogenic compounds is currently in preclinical and clinical
development for personalized cancer therapy. However, resistance to
angiogenesis inhibitors is real, and highlights the need to
identify alternative agents. Deciphering the molecular mechanisms
of angiogenesis inhibition by miRNAs is imperative, as the
successful translation of novel inhibitors to the clinic greatly
depends on an in-depth understanding of the biology and function of
miRNAs in tumor and endothelial cells. As chemotherapy is
angiogenesis-dependent, the implementation of angiogenesis
inhibitors to conventional therapies may have additional
advantages. For instance, miRNAs targeting endothelial cells may
have advantages over tumor-specific therapies, as they can overcome
drug resistance the least in preclinical models.
The present review has summarized the current
knowledge regarding the angiomiRs deregulation and functional
mechanisms in diverse types of human cancers, which may provide a
guide in their potential utilization as therapeutic targets in
aggressive tumors. Several miRNAs have anti-angiogenic properties
by targeting key angiogenic factors, including VEGF, HIF1α, PDGF,
FGF, EGF, as well as MAPK, PI3K and TGFβ signaling, which offers a
wide landscape of therapeutic opportunities. Finally,
first-in-humans studies derived from controlled clinical trials
showed an acceptable safety profile and antitumoral activity of
miRNAs in patients, and highlighted the urgency for additional
studies prior to potential routine clinical applications.
The authors acknowledge the Consejo Nacional de
Ciencia y Tecnología, Fondo de Investigación Científica Básica
(grant no. 222335), and Fondo SSA/IMSS/ISSSTE (grant no. 233370)
for financial support. YMSV received a CONACYT fellowship (grant
no. 588430). The authors also acknowledge Grupo Mexicano De
Investigacion En Cancer De Ovario Ac for financial support.
Not applicable.
YMSV, REZ and CLC reviewed the microRNA functions
and produced the majority of this review. LAM and CLC discussed the
roles of angiomiRs in lung and pancreatic cancer and the clinical
applications of angiomiRs. DGR and HAV reviewed the role of
angiomiRs in gynecological cancers. ERG discussed the role of
angiomiRs in colorectal cancer.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe’er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai X, Hagedorn CH and Cullen BR: Human
microRNAs are processed from capped, polyadenylated transcripts
that can also function as mRNAs. RNA. 10:1957–1966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han J, Lee Y, Yeom KH, Kim YK, Jin H and
Kim VN: The Drosha-DGCR8 complex in primary microRNA processing.
Genes Dev. 18:3016–3027. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruby JG, Jan CH and Bartel DP: Intronic
microRNA precursors that bypass Drosha processing. Nature.
448:83–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chendrimada TP, Gregory RI, Kumaraswamy E,
Norman J, Cooch N, Nishikura K and Shiekhattar R: TRBP recruits the
Dicer complex to Ago2 for microRNA processing and gene silencing.
Nature. 436:740–744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vasudevan S, Tong Y and Steitz JA:
Switching from repression to activation: microRNAs can up-regulate
translation. Science. 318:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sen GL and Blau HM: Argonaute 2/RISC
resides in sites of mammalian mRNA decay known as cytoplasmic
bodies. Nat Cell Biol. 7:633–636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vecchione A, Belletti B, Lovat F, Volinia
S, Chiappetta G, Giglio S, Sonego M, Cirombella R, Onesti EC,
Pellegrini P, et al: A microRNA signature defines chemoresistance
in ovarian cancer through modulation of angiogenesis. Proc Natl
Acad Sci USA. 110:9845–9850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Borges NM, do Vale Elias M, Fook-Alves VL,
Andrade TA, de Conti ML, Macedo MP, Begnami MD, Campos AH, Etto LY,
Bortoluzzo AB, et al: Angiomirs expression profiling in diffuse
large B-Cell lymphoma. Oncotarget. 7:4806–4816. 2016. View Article : Google Scholar :
|
|
13
|
Suárez Y and Sessa WC: MicroRNAs as novel
regulators of angiogenesis. Circ Res. 104:442–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki HI, Katsura A, Matsuyama H and
Miyazono K: MicroRNA regulons in tumor microenvironment. Oncogene.
34:3085–3094. 2015. View Article : Google Scholar
|
|
15
|
Bernstein E, Kim SY, Carmell MA, Murchison
EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV and Hannon
G: Dicer is essential for mouse development. Nat Genet. 35:215–217.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang WJ, Yang DD, Na S, Sandusky GE, Zhang
Q and Zhao G: Dicer is required for embryonic angiogenesis during
mouse development. J Biol Chem. 280:9330–9335. 2005. View Article : Google Scholar
|
|
17
|
Suárez Y, Fernández-Hernando C, Yu J,
Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager
M and Sessa WC: Dicer-dependent endothelial microRNAs are necessary
for postnatal angiogenesis. Proc Natl Acad Sci USA.
105:14082–14087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuehbacher A, Urbich C, Zeiher AM and
Dimmeler S: Role of Dicer and Drosha for endothelial microRNA
expression and angiogenesis. Circ Res. 101:59–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suárez Y, Fernández-Hernando C, Pober JS
and Sessa WC: Dicer dependent microRNAs regulate gene expression
and functions in human endothelial cells. Circ Res. 100:1164–1173.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poliseno L, Tuccoli A, Mariani L,
Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S and
Rainaldi G: MicroRNAs modulate the angiogenic properties of HUVECs.
Blood. 108:3068–3071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heusschen R, van Gink M, Griffioen AW and
Thijssen VL: MicroRNAs in the tumor endothelium: Novel controls on
the angioregulatory switchboard. Biochim Biophys Acta. 1805:87–96.
2010.
|
|
22
|
McCall M, Kent O, Yu J, Fox-Talbot K,
Zaiman A and Halushka M: MicroRNA profiling of diverse endothelial
cell types. BMC Med Genomics. 4:782011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen G, Li X, Jia Y, Piazza G and Xi Y:
Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol Sin.
34:336–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Beijnum J, Giovannetti E, Poel D,
Nowak-Sliwinska P and Griffioe A: miRNAs: micro-managers of
anticancer combination therapies. Angiogenesis. 20:269–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegel R, Miller K and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parker L, Schmidt M, Jin S, Gray A, Beis
D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier D, et al: The
endothelial-cell-derived secreted factor Egfl7 regulates vascular
tube formation. Nature. 428:754–758. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meister J and Schmidt M: miR-126 and
miR-126*: New players in cancer. ScientificWorld J. 10:2090–2100.
2010. View Article : Google Scholar
|
|
28
|
Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu
T, Bai Y, Shen Y, Yuan W, Jing Q and Qin Y: Endothelial-specific
intron-derived miR-126 is down-regulated in human breast cancer and
targets both VEGFA and PIK3R2. Mol Cell Biochem. 351:57–164. 2011.
View Article : Google Scholar
|
|
29
|
Lu YY, Sweredoski MJ, Huss D, Lansford R,
Hess S and Tirrell DA: Prometastatic GPCR CD97 is a direct target
of tumor suppressor microRNA-126. ACS Chem Biol. 9:334–338. 2014.
View Article : Google Scholar
|
|
30
|
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y,
Li C, Chong M, Ibrahim T, Mercatali L, et al: miR-126 and miR-126*
repress recruitment of mesenchymal stem cells and inflammatory
monocytes to inhibit breast cancer metastasis. Nat Cell Biol.
15:284–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Png KJ, Halberg N, Yoshida M and Tavazoie
SF: A microRNA regulon that mediates endothelial recruitment and
metastasis by cancer cells. Nature. 481:190–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Z, Cai X, Huang C, Xu J and Liu A:
miR-497 suppresses angiogenesis in breast carcinoma by targeting
HIF-1α. Oncol Rep. 35:1696–1702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tu Y, Liu L, Zhao D, Liu Y, Ma X, Fan Y,
Wan L, Huang T, Cheng Z and Shen B: Overexpression of miRNA-497
inhibits tumor angiogenesis by targeting VEGFR2. Sci Rep.
5:138272015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fox S, Generali D and Harris A: Breast
tumour angiogenesis. Breast Cancer Res. 9:2162017. View Article : Google Scholar
|
|
35
|
Chang S, Wang R, Akagi K, Kim K, Martin B,
Cavallone L; Kathleen Cuningham Foundation Consortium for Research
into Familial Breast Cancer (kConFab); Haines DC, Basik M, Mai P,
et al: Tumor suppressor BRCA1 epigenetically controls oncogenic
microRNA-155. Nat Med. 17:1275–1282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Danza K, De Summa S, Pinto R, Pilato B,
Palumbo O, Merla G, Simone G and Tommasi S: MiR-578 and miR-573 as
potential players in BRCA-related breast cancer angiogenesis.
Oncotarget. 6:471–483. 2015. View Article : Google Scholar :
|
|
37
|
Kong W, He L, Coppola M, Guo J, Esposito
NN, Coppola D and Cheng JQ: MicroRNA-155 regulates cell survival,
growth, and chemosensitivity by targeting FOXO3a in breast cancer.
J Biol Chem. 285:17869–17879. 2016. View Article : Google Scholar
|
|
38
|
Kong W, He L, Richards EJ, Challa S, Xu
CX, Permuth-Wey J, Lancaster JM, Coppola D, Sellers TA, Djeu JY and
Cheng JQ: Upregulation of miRNA-155 promotes tumour angiogenesis by
targeting VHL and is associated with poor prognosis and
triple-negative breast cancer. Oncogene. 33:679–689. 2014.
View Article : Google Scholar :
|
|
39
|
Foekens J, Sieuwerts A, Smid M, Look M, de
Weerd V, Boersma A, Klijn J, Wiemer E and Martens J: Four miRNAs
associated with aggressiveness of lymph node-negative, estrogen
receptor-positive human breast cancer. Proc Natl Acad Sci USA.
105:13021–13026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao D, Tu Y, Wan L, Bu L, Huang T, Sun X,
Wang K and Shen B: In vivo monitoring of angiogenesis inhibition
via down-regulation of mir-21 in a VEGFR2-luc murine breast cancer
model using bioluminescent imaging. PLoS One. 8:e714722013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mathsyaraja H, Thies K, Taffany D, Deighan
C, Liu T, Yu L, Fernandez S, Shapiro C, Otero J, Timmers C, et al:
CSF1-ETS2-induced microRNA in myeloid cells promote metastatic
tumor growth. Oncogene. 34:3651–3661. 2015. View Article : Google Scholar
|
|
43
|
He T, Qi F, Jia L, Wang S, Song N, Guo L,
Fu Y and Luo Y: MicroRNA-5423 pinhibits tumour angiogenesis by
targeting angiopoietin-2. J Pathol. 232:499–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
He T, Qi F, Jia L, Wang S, Wang C, Song N,
Fu Y, Li L and Luo Y: Tumor cell-secreted angiogenin induces
angiogenic activity of endothelial cells by suppressing miR-542-3p.
Cancer Lett. 368:115–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Leidner RS, Li L and Thompson CL:
Dampening enthusiasm for circulating microRNA in breast cancer.
PLoS One. 8:e578412013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li JT, Wang LF, Zhao YL, Yang T, Li W,
Zhao J, Yu F, Wang L, Meng YL, Liu NN, et al: Nuclear factor of
activated T cells 5 maintained by Hotair suppression of miR-568
upregulates S100 calcium binding protein A4 to promote breast
cancer metastasis. Breast Cancer Res. 16:4542014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Flores-Pérez A, Marchat L,
Rodríguez-Cuevas S, Bautista-Piña V, Hidalgo-Miranda A, Ocampo E,
Martínez M, Palma-Flores C, Fonseca-Sánchez M, Astudillo-de la Vega
H, et al: Dual targeting of ANGPT1 and TGFBR2 genes by miR-204
controls angiogenesis in breast cancer. Sci Rep. 6:345042016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: Vasculogenic mimicry in
tumor cells: Diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–3272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Salinas-Vera YM, Marchat LA,
García-Vázquez R, González de la Rosa CH, Castañeda-Saucedo E, Tito
NN, Flores CP, Pérez-Plasencia C, Cruz-Colin JL, Carlos-Reyes Á, et
al: Cooperative multi-targeting of signaling networks by
angiomiR-204 inhibits vasculogenic mimicry in breast cancer cells.
Cancer Lett. 432:17–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
American Cancer Society: Cancer facts and
figures 2015. Atlanta: American Cancer Society; pp. 1–52. 2015
|
|
51
|
Abramson MA, Jazag A, van der Zee JA and
Whang EE: The molecular biology of pancreatic cancer. Gastrointest
Cancer Res. 1(4 Suppl 2): S7–S12. 2007.PubMed/NCBI
|
|
52
|
Carr RM and Fernandez-Zapico ME:
Pancreatic cancer microenvironment, to target or not to target?
EMBO Mol Med. 8:80–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Khan S, Ansarullah, Kumar D, Jaggi M and
Chauhan SC: Targeting microRNAs in pancreatic cancer: Microplayers
in the big game. Cancer Res. 73:6541–6547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mace TA, Collins AL, Wojcik SE, Croce CM,
Lesinski GB and Bloomston M: Hypoxia induces the overexpression of
microRNA-21 in pancreatic cancer cells. J Surg Res. 184:855–860.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Volinia S, Calin G, Liu C, Ambs S, Cimmino
A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner
MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ and Schmittgen
TD: Expression profiling identifies microRNA signature in
pancreatic cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar
|
|
57
|
Moriyama T, Ohuchida K, Mizumoto K, Yu J,
Sato N, Nabae T, Takahata S, Toma H, Nagai E and Tanaka M:
MicroRNA-21 modulates biological functions of pancreatic cancer
cells including their proliferation, invasion, and chemoresistance.
Mol Cancer Ther. 8:1067–1074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bao B, Ali S, Kong D, Sarkar S, Wang Z,
Banerjee S, Aboukameel A, Padhye S, Philip P and Sarkar F:
Anti-tumor activity of a novel compound-CDF is mediated by
regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS
One. 6:e178502011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kadera BE, Li L, Toste PA, Wu N, Adams C,
Dawson DW and Donahue TR: MicroRNA-21 in pancreatic ductal
adenocarcinoma tumor-associated fibroblasts promotes metastasis.
PLoS One. 8:e719782013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hoffmann A, Mori R, Vallbohmer D,
Brabender J, Klein E, Drebber U, Baldus S, Cooc J, Azuma M, Metzger
R, et al: High expression of HIF1a is a predictor of clinical
outcome in patients with pancreatic ductal adenocarcinomas and
correlated to PDGFA, VEGF, and bFGF. Neoplasia. 10:674–679. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang X, Ren H, Zhao T, Ma W, Dong J, Zhang
S, Xin W, Yang S, Jia L and Hao J: Single nucleotide polymorphism
in the microRNA-199a binding site of HIF1A gene is associated with
pancreatic ductal adenocarcinoma risk and worse clinical outcomes.
Oncotarget. 7:13717–1329. 2016.PubMed/NCBI
|
|
62
|
Chan YC, Roy S, Huang Y, Khanna S and Sen
C: The microRNA miR-199a-5p down-regulation switches on wound
angiogenesis by derepressing the v-ets erythroblastosis virus E26
oncogene homolog 1-matrix metalloproteinase-1 pathway. J Biol Chem.
287:41032–41043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Morton J, Timpson P, Karim S, Ridgway R,
Athineos D, Doyle B, Jamieson N, Oien K, Lowy A, Brunton V, et al:
Mutant p53 drives metastasis and overcomes growth arrest/senescence
in pancreatic cancer. Proc Natl Acad Sci USA. 107:246–251. 2010.
View Article : Google Scholar
|
|
64
|
Frampton A, Krell J, Jamieson N, Gall T,
Giovannetti E, Funel N, Mato Prado M, Krell D, Habib N, Castellano
L, et al: microRNAs with prognostic significance in pancreatic
ductal adenocarcinoma: A meta-analysis. Eur J Cancer. 51:1389–1404.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Alemar B, Izetti P, Gregório C, Macedo GS,
Castro MA, Osvaldt AB, Matte U and Ashton-Prolla P: miRNA-21 and
miRNA-34a are potential minimally invasive biomarkers for the
diagnosis of pancreatic ductal adenocarcinoma. Pancreas. 45:84–92.
2016. View Article : Google Scholar
|
|
66
|
Chang T, Wentzel E, Kent O, Ramachandran
K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M,
Lowenstein C, et al: Transactivation of miR-34a by p53 broadly
influences gene expression and promotes apoptosis. Mol Cell.
26:745–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vogt M, Munding J, Grüner M, Liffers ST,
Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A and Hermeking H:
Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG
methylation in colorectal, pancreatic, mammary, ovarian,
urothelial, and renal cell carcinomas and soft tissue sarcomas.
Virchows Arch. 458:313–322. 2011. View Article : Google Scholar
|
|
68
|
Zhao T, Li J and Chen AF: MicroRNA-34a
induces endothelial progenitor cell senescence and impedes its
angiogenesis via suppressing silent information regulator 1. Am J
Physiol Endocrinol Metab. 299:E110–E116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bremnes RM, Camps C and Sirera R:
Angiogenesis in non-small cell lung cancer: The prognostic impact
of neoangiogenesis and the cytokines VEGF and bFGF in tumours and
blood. Lung Cancer. 51:143–158. 2006. View Article : Google Scholar
|
|
71
|
Korpanty G, Smyth E and Carney D: Update
on anti-angiogenic therapy in non-small cell lung cancer: Are we
making progress? J Thorac Dis. 3:19–29. 2011.
|
|
72
|
Al Farsi A and Ellis P: Anti-angiogenic
therapy in advanced non-small cell lung carcinoma (NSCLC): Is there
a role in subsequent lines of therapy? J Thorac Dis. 7:214–216.
2015.PubMed/NCBI
|
|
73
|
Chen S, Xue Y, Wu X, Le C, Bhutkar A, Bell
L, Zhang F, Langer R and Sharp PA: Global microRNA depletion
suppresses tumor angiogenesis. Genes Dev. 28:1054–1067. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Donnem T, Fenton CG, Lonvik K, Berg T,
Eklo K, Andersen S, Stenvold H, Al-Shibli K, Al-Saad S, Bremnes RM
and Busund LT: MicroRNA signatures in tumor tissue related to
angiogenesis in non-small cell lung cancer. PLoS One. 7:e296712012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Donnem T, Lonvik K, Eklo K, Berg T, Sorbye
SW, Al-Shibli K, Al-Saad S, Andersen S, Stenvold H, Bremnes RM and
Busund LT: Independent and tissue-specific prognostic impact of
miR-126 in nonsmall cell lung cancer: Coexpression with vascular
endothelial growth factor-A predicts poor survival. Cancer.
117:3193–3200. 2011. View Article : Google Scholar
|
|
77
|
Jusufović E, Rijavec M, Keser D, Korošec
P, Sodja E, Iljazović E, Radojević Z and Košnik M: le7 and miR-126
are down-regulated in tumor tissue and correlate with microvessel
density and survival outcomes in non-small-cell lung cancer. PLoS
One. 7:e455772012. View Article : Google Scholar
|
|
78
|
Chen Z, Lai TC, Jan YH, Lin FM, Wang WC,
Xiao H, Wang YT, Sun W, Cui X, Li YS, et al: Hypoxia-responsive
miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest.
123:1057–1067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens M, Okamoto A, Yokota J, Tanaka T, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hu J, Cheng Y, Li Y, Jin Z, Pan Y, Liu G,
Fu S, Zhang Y, Feng K and Feng Y: microRNA-128 plays a critical
role in human non-small cell lung cancer tumourigenesis,
angiogenesis and lymphangiogenesis by directly targeting vascular
endothelial growth factor-C. Eur J Cancer. 50:2336–2350. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tejero R, Navarro A, Campayo M, Viñolas N,
Marrades M, Cordeiro A, Ruíz-Martínez M, Santasusagna S, Molins L,
Ramirez J and Monzó M: miR-141 and miR-200c as markers of overall
survival in early stage non-small cell lung cancer adenocarcinoma.
PLoS One. 9:e1018992014. View Article : Google Scholar :
|
|
83
|
Owen S and Souhami L: The management of
brain metastases in non-small cell lung cancer. Front Oncol.
4:2482014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen LT, Xu SD, Xu H, Zhang JF, Ning JF
and Wang SF: MicroRNA-378 is associated with non-small cell lung
cancer brain metastasis by promoting cell migration, invasion and
tumor angiogenesis. Med Oncol. 9:1673–1680. 2012. View Article : Google Scholar
|
|
85
|
Skrzypek K, Tertil M, Golda S, Ciesl M,
Weglarczyk K, Collet G, Guichard A, Kozakowska M, Boczkowski J, Was
H, et al: Interplay between heme oxygenase-1 and miR-378 affects
non-small cell lung carcinoma growth, vascularization, and
metastasis. Antioxid Redox Signal. 9:644–660. 2013. View Article : Google Scholar
|
|
86
|
Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin
L, Liu X and Wang N: Tumor-derived microRNA-494 promotes
angiogenesis in non-small cell lung cancer. Angiogenesis.
18:373–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao WY, Wang Y, An ZJ, Shi CG, Zhu GA,
Wang B, Lu MY, Pan CK and Chen P: Downregulation of miR-497
promotes tumor growth and angiogenesis by targeting HDGF in
non-small cell lung cancer. Biochem Biophys Res Commun.
435:466–447. 2013. View Article : Google Scholar
|
|
88
|
Kumarswamy R, Volkmann I, Beermann J, Napp
LC, Jabs O, Bhayadia R, Melk A, Ucar A, Chowdhury K, Lorenzen JM,
et al: Vascular importance of the miR-212/132 cluster. Eur Heart J.
35:3224–3231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
You J, Li Y, Fang N, Liu B, Zu L, Chang R,
Li X and Zhou Q: MiR-132 suppresses the migration and invasion of
lung cancer cells via targeting the EMT regulator ZEB2. PLoS One.
9:e918272014. View Article : Google Scholar
|
|
90
|
Incoronato M, Urso L, Portela A, Laukkanen
MO, Soini Y, Quintavalle C, Keller S, Esteller M and Condorelli G:
Epigenetic regulation of miR-212 expression in lung cancer. PLoS
One. 6:e277222011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Luo J, Meng C, Tang Y, Zhang S, Wan M, Bi
Y and Zhou X: miR-132/212 cluster inhibits the growth of lung
cancer xenografts in nude mice. Int J Clin Exp Med. 7:4115–4122.
2014.
|
|
92
|
Cui H, Seubert B, Stahl E, Dietz H,
Reuning U, Moreno-Leon L, Ilie M, Hofman P, Nagase H, Mari B and
Krüger A: Tissue inhibitor of metalloproteinases-1 induces a
pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells
and their exosomes. Oncogene. 34:3640–3650. 2015. View Article : Google Scholar
|
|
93
|
Pesta M, Kulda V, Kucera R, Pesek M,
Vrzalova J, Liska V, Pecen L, Treska V, Safranek J, Prazakova M, et
al: Prognostic significance of TIMP-1 in non-small cell lung
cancer. Anticancer Res. 31:4031–4038. 2011.PubMed/NCBI
|
|
94
|
American Cancer Society: Cancer facts and
figures 2016. Atlanta, Ga: American Cancer Society; 2016
|
|
95
|
Hur K, Toiyama Y, Schetter AJ, Okugawa Y,
Harris CC, Boland CR and Goel A: Identification of a
metastasis-specific MicroRNA signature in human colorectal cancer.
J Natl Cancer Inst. 107:dju4922015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yamaguchi T, Iijima T, Wakaume R,
Takahashi K, Matsumoto H, Nakano D, Nakayama, Y Mori T, Horiguchi S
and Miyaki M: Underexpression of miR-126 and miR-20b in hereditary
and nonhereditary colorectal tumors. Oncology. 87:58–66. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang Y, Wang X, Xu B, Wang B, Wang Z,
Liang Y, Zhou J, Hu J and Jiang B: Epigenetic silencing of miR-126
contributes to tumor invasion and angiogenesis in colorectal
cancer. Oncol Rep. 30:1976–1984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hansen TF, Andersen CL, Nielsen BS,
Spindler KL, Sørensen FB, Lindebjerg J, Brandslund I and Jakobsen
A: Elevated microRNA-126 is associated with high vascular
endothelial growth factor receptor 2 expression levels and high
microvessel density in colorectal cancer. Oncol Lett. 2:1101–1106.
2011. View Article : Google Scholar
|
|
99
|
Hansen TF, Christensen Rd, Andersen RF,
Sørensen FB, Johnsson A and Jakobsen A: MicroRNA-126 and epidermal
growth factor-like domain 7-an angiogenic couple of importance in
metastatic colorectal cancer. Results from the Nordic ACT trial Br
J Cancer. 109:1243–1251. 2013.
|
|
100
|
Yu W, Wang Z, Shen LI and Wei Q:
Circulating microRNA-21 as a potential diagnostic marker for
colorectal cancer: A meta-analysis. Mol Clin Oncol. 4:237–244.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Slaby O, Svoboda M, Fabian P, Smerdova T,
Knoflickova D, Bednarikova M, Nenutil R and Vyzula R: Altered
expression of miR-21, miR-31, miR-143 and miR-145 is related to
clinico-pathologic features of colorectal cancer. Oncology.
72:397–402. 2007. View Article : Google Scholar
|
|
102
|
Nielsen S, Jørgensen S, Fog JU, Søkilde R,
Christensen IJ, Hansen U, Brünner N, Baker A, Møller S and Nielsen
HJ: High levels of microRNA-21 in the stroma of colorectal cancers
predict short disease-free survival in stage II colon cancer
patients. Clin Exp Metastasis. 28:27–38. 2011. View Article : Google Scholar :
|
|
103
|
Nagao Y, Hisaoka M, Matsuyama A, Kanemitsu
S, Hamada T, Fukuyama T, Nakano R, Uchiyama A, Kawamoto M,
Yamaguchi K and Hashimoto H: Association of microRNA-21 expression
with its targets, PDCD4 and TIMP3, in pancreatic ductal
adenocarcinoma. Mod Pathol. 25:112–121. 2012. View Article : Google Scholar
|
|
104
|
Song MS and Rossi JJ: The anti-miR21
antagomir, a therapeutic tool for colorectal cancer, has a
potential synergistic effect by perturbing an
angiogenesis-associated miR30. Front Genet. 4:3012014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bridge G, Monteiro R, Henderson S, Emuss
V, Lagos D, Georgopoulou D, Patient R and Boshoff C: The
microRNA-30 family targets DLL4 to modulate endothelial cell
behavior during angiogenesis. Blood. 120:5063–5072. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hellström M, Phng LK, Hofmann JJ, Wallgard
E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N,
et al: Dll4 signalling through Notch1 regulates formation of tip
cells during angiogenesis. Nature. 445:776–780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Miyanaga K, Kato Y, Nakamura T, Matsumura
M, Amaya H, Horiuchi T, Chiba Y and Tanaka K: Expression and role
of thrombospondin-1 in colorectal cancer. Anticancer Res.
22:3941–3948. 2002.
|
|
108
|
Diosdado B, van de Wiel A, Terhaar Sive
Droste S, Mongera S, Postma C, Meijerink WJ, Carvalho B and Meijer
GA: MiR-17-92 cluster is associated with 13q gain and c-myc
expression during colorectal adenoma to adenocarcinoma progression.
Br J Cancer. 101:707–714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Dews M, Homayouni A, Yu D, Murphy D,
Sevignani C, Wentzel E, Furth E, Lee M, Enders H, Mendell T and
Thomas-Tikhonenko A: Augmentation of tumor angiogenesis by a
Myc-activated microRNA cluster. Nat Genet. 38:1060–1065. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Amodeo V, Bazan V, Fanale D, Insalaco L,
Caruso S, Cicero G, Bronte G, Rolfo C, Santini D and Russo A:
Effects of anti-miR-182 on TSP-1 expression in human colon cancer
cells: There is a sense in antisense? Expert Opin Ther Targets.
17:1249–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liang Y, Ridzon D, Wong L and Chen C:
Characterization of microRNA expression profiles in normal human
tissues. BMC Genomics. 8:1662007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sundaram P, Hultine S, Smith LM, Dews M,
Fox JL, Biyashev D, Schelter JM, Huang Q, Cleary MA, Volpert OV and
Thomas-Tikhonenko A: p53-responsive miR-194 inhibits
thrombospondin-1 and promotes angiogenesis in colon cancers. Cancer
Res. 71:7490–7501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Braun CJ, Zhang X, Savelyeva I, Wolff S,
Moll UM, Schepeler T, Ørntoft TF, Andersen CL and Dobbelstein M:
p53-responsive micrornas 192 and 215 are capable of inducing cell
cycle arrest. Cancer Res. 68:10094–10104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Xue G, Yan HL, Zhang Y, Hao LQ, Zhu XT,
Mei Q and Sun SH: c-Myc-mediated repression of miR-15-16 in hypoxia
is induced by increased HIF-2α and promotes tumor angiogenesis and
metastasis by upregulating FGF2. Oncogene. 34:1393–1406. 2015.
View Article : Google Scholar
|
|
115
|
Dai L, Wang W, Zhang S, Jiang Q, Wang R,
Dai L, Cheng L, Yang Y, Wei YQ and Deng HX: Vector-based
miR-15a/16-1 plasmid inhibits colon cancer growth in vivo. Cell
Biol Int. 36:765–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fang Y, Liang X, Jiang W, Li J, Xu J and
Cai X: Cyclin b1 suppresses colorectal cancer invasion and
metastasis by regulating e-cadherin. PLoS One. 10:e01268752015.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Planutis K, Planutiene M and Holcombe F: A
novel signaling pathway regulates colon cancer angiogenesis through
Norrin. Sci Rep. 4:56302014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang B, Li W, Liu H, Yang L, Liao Q, Cui
S, Wang H and Zhao L: miR-29b suppresses tumor growth and
metastasis in colorectal cancer via downregulating Tiam1 expression
and inhibiting epithelial-mesenchymal transition. Cell Death Dis.
17:e13352014. View Article : Google Scholar
|
|
119
|
Subramanian M, Rao SR, Thacker P,
Chatterjee S and Karunagaran D: MiR-29b downregulates canonical Wnt
signaling by suppressing coactivators of β-catenin in human
colorectal cancer cells. J Cell Biochem. 115:1974–1984.
2014.PubMed/NCBI
|
|
120
|
Ding Q, Chang CJ, Xie X, Xia W, Yang Y,
Wang SC, Wang Y, Xia J, Chen L, Cai C, et al: APOBEC3G promotes
liver metastasis in an orthotopic mouse model of colorectal cancer
and predicts human hepatic metastasis. J Clin Invest.
121:4526–4536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Colangelo T, Fucci A, Votino C, Sabatino
L, Pancione M, Laudanna C, Binaschi M, Bigioni M, Maggi A, Parente
D, et al: MicroRNA-130b promotes tumor development and is
associated with poor prognosis in colorectal cancer. Neoplasia.
15:1086–1099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Panigrahy D, Singer S, Shen LQ,
Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing
S, Fletcher C, et al: PPARgamma ligands inhibit primary tumor
growth and metastasis by inhibiting angiogenesis. J Clin Invest.
110:923–932. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Urbich C, Kaluza D, Frömel T, Knau A,
Bennewitz K, Boon RA, Bonauer A, Doebele C, Boeckel JN,
Hergenreider E, et al: MicroRNA-27a/b controls endothelial cell
repulsion and angiogenesis by targeting semaphorin 6A. Blood.
119:1607–1616. 2012. View Article : Google Scholar
|
|
124
|
Veliceasa D, Biyashev D, Qin G, Misener S,
Mackie AR, Kishore R and Volpert OV: Therapeutic manipulation of
angiogenesis with miR-27b. Vasc Cell. 24:62015. View Article : Google Scholar
|
|
125
|
Chintharlapalli S, Papineni S, Abdelrahim
M, Abudayyeh A, Jutooru I, Chadalapaka G, Wu F, Mertens-Talcott S,
Vanderlaag K, Cho D, et al: Oncogenic microRNA-27a is a target for
anticancer agent methyl
2-cyano-3,11-dioxo-18beta-olean-1,12-dien-30-oate in colon cancer
cells. Int J Cancer. 125:1965–1974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Pathi S, Jutooru I, Chadalapaka G,
Sreevalsan S, Anand S, Thatcher GR and Safe S: GT-094, a NO-NSAID,
inhibits colon cancer cell growth by activation of a reactive
oxygen species-microRNA-27a: ZBTB10-specificity protein pathway.
Mol Cancer Res. 9:195–202. 2011. View Article : Google Scholar
|
|
127
|
Colangelo T, Polcaro G, Ziccardi P, Pucci
B, Muccillo L, Galgani M, Fucci A, Milone MR, Budillon A,
Santopaolo M, et al: Proteomic screening identifies calreticulin as
a miR-27a direct target repressing MHC class I cell surface
exposure in colorectal cancer. Cell Death Dis. 25:e21202016.
View Article : Google Scholar
|
|
128
|
Bao Y, Chen Z, Guo Y, Feng Y, Li Z, Han W,
Wang J, Zhao W, Jiao Y, Li K, et al: Tumor suppressor microRNA-27a
in colorectal carcinogenesis and progression by targeting SGPP1 and
Smad2. PLoS One. 9:e1059912014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z,
Chen Z, Qiu F, Xu J and Huang J: miRNA-27b targets vascular
endothelial growth factor C to inhibit tumor progression and
angiogenesis in colorectal cancer. PLoS One. 8:e606872013.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Geng L, Chaudhuri A, Talmon G, Wisecarver
JL, Are C, Brattain M and Wang J: MicroRNA-192 suppresses liver
metastasis of colon cancer. Oncogene. 33:5332–5340. 2014.
View Article : Google Scholar :
|
|
131
|
Yin Y, Yan ZP, Lu NN, Xu Q, He J, Qian X,
Yu J, Guan X, Jiang BH and Liu LZ: Downregulation of miR-145
associated with cancer progression and VEGF transcriptional
activation by targeting N-RAS and IRS1. Biochim Biophys Acta.
1829:239–247. 2013. View Article : Google Scholar
|
|
132
|
Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li
D, Lai L and Jiang BH: MiR-145 directly targets p70S6K1 in cancer
cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res.
40:761–774. 2012. View Article : Google Scholar :
|
|
133
|
Bustin SA, Dorudi S, Phillips SM, Feakins
RM and Jenkins PJ: Local expression of insulin-like growth factor-I
affects angiogenesis in colorectal cancer. Tumour Biol. 23:130–138.
2002. View Article : Google Scholar
|
|
134
|
Qian X, Yu J, Yin Y, He J, Wang L, Li Q,
Zhang LQ, Li CY, Shi ZM, Xu Q, et al: MicroRNA-143 inhibits tumor
growth and angiogenesis and sensitizes chemosensitivity to
oxaliplatin in colorectal cancers. Cell Cycle. 12:1385–1394. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin
S, Sun C, Ma M, Huang Y and Xi JJ: Genome-wide functional screening
of miR-23b as a pleiotropic modulator suppressing cancer
metastasis. Nat Commun. 2:5542011. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lin RL and Zhao LJ: Mechanistic basis and
clinical relevance of the role of transforming growth factor-β in
cancer. Cancer Biol Med. 12:385–393. 2015.
|
|
137
|
Xiao F, Qiu H, Cui H, Ni X, Li J, Liao W,
Lu L and Ding K: MicroRNA-885-3p inhibits the growth of HT-29 colon
cancer cell xenografts by disrupting angiogenesis via targeting
BMPR1A and blocking BMP/Smad/Id1 signaling. Oncogene. 34:1968–1978.
2015. View Article : Google Scholar
|
|
138
|
Yamada N, Tsujimura N, Kumazaki M,
Shinohara H, Taniguchi K, Nakagawa Y, Naoe T and Akao Y: Colorectal
cancer cell-derived microvesicles containing microRNA-1246 promote
angiogenesis by activating Smad 1/5/8 signaling elicited by PML
down-regulation in endothelial cells. Biochim Biophys Acta.
1839:1256–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wang Y, Kim S and Kim IM: Regulation of
metastasis by microRNAs in ovarian cancer. Front Oncol. 4:1432014.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Dwivedi SK, Mustafi SB, Mangala LS, Jiang
D, Pradeep S, Rodriguez-Aguayo C, Ling H, Ivan C, Mukherjee P,
Calin GA, et al: Therapeutic evaluation of microRNA-15a and
microRNA-16 in ovarian cancer. Oncotarget. 7:15093–15104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Nusrat O, Belotte J, Fletcher NM, Memaj I,
Saed MG, Diamond MP and Saed GM: The role of angiogenesis in the
persistence of chemoresistance in epithelial ovarian cancer. Reprod
Sci. 23:1484–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
He J, Jing Y, Li W, Qian X, Xu Q, Li FS,
Liu LZ, Jiang BH and Jiang Y: Roles and mechanism of miR-199a and
miR-125b in tumor angiogenesis. PLoS One. 8:e566472013. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor a through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chan JK, Kiet TK, Blansit K, Ramasubbaiah
R, Hilton JF, Kapp DS and Matei D: MiR-378 as a biomarker for
response to anti-angiogenic treatment in ovarian cancer. Gynecol
Oncol. 133:568–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Lai Y, Zhang X, Zhang Z, Shu Y, Luo X,
Yang Y, Wang X, Yang G, Li L and Feng Y: The microRNA-27a:
ZBTB10-specificity protein pathway is involved in follicle
stimulating hormone-induced VEGF, Cox2 and survivin expression in
ovarian epithelial cancer cells. Int J Oncol. 42:776–784. 2013.
View Article : Google Scholar
|
|
146
|
Korpal M and Kang Y: The emerging role of
miR-200 family of microRNAs in epithelial-mesenchymal transition
and cancer metastasis. RNA Biol. 5:115–119. 2008. View Article : Google Scholar
|
|
147
|
Pecot C, Rupaimoole R, Yang D, Akbani R,
Ivan C, Lum C, Wu S, Han HD, Shah MY, Rodriguez-Aguayo C, et al:
Tumour angiogenesis regulation by the miR-200 family. Nat Commun.
4:24272013. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Imam JS, Plyler JR, Bansal H, Prajapati S,
Bansal S, Rebeles J, Chen HI, Chang YF, Panneerdoss S, Zoghi B, et
al: Genomic loss of tumor suppressor miRNA-204 promotes cancer cell
migration and invasion by activating AKT/mTOR/Rac1 signaling and
actin reorganization. PLoS One. 7:e523972012. View Article : Google Scholar
|
|
149
|
Zhu AX, Duda DG, Sahani DV and Jain RK:
HCC and angiogenesis: Possible targets and future directions. Nat
Rev Clin Oncol. 8:292–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Kota J, Chivukula RR, O’Donnell KA,
Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P,
Torbenson M, Clark KR, et al: Therapeutic microRNA delivery
suppresses tumorigenesis in a murine liver cancer model. Cell.
137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
van Zandwijk N, Pavlakis N, Kao SC, Linton
A, Boyer MJ, Clarke S, Huynh Y, Chrzanowska A, Fulham MJ, Bailey
DL, et al: Safety and activity of microRNA-loaded minicells in
patients with recurrent malignant pleural mesothelioma: A
first-in-man, phase 1, open-label, dose-escalation study. Lancet
Oncol. 18:1386–1396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Kumar B, Yadav A, Lang J, Teknos TN and
Kumar P: Dysregulation of microRNA-34a expression in head and neck
squamous cell carcinoma promotes tumor growth and tumor
angiogenesis. PLoS One. 7:e376012012. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Yu G, Yao W, Xiao W, Li H, Xu H and Lang
B: MicroRNA-34a functions as an anti-metastatic microRNA and
suppresses angiogenesis in bladder cancer by directly targeting
CD44. J Exp Clin Cancer Res. 33:7792014. View Article : Google Scholar
|
|
154
|
Arunachalam G, Lakshmanan AP, Samuel SM,
Triggle CR and Ding H: Molecular interplay between microRNA-34a and
Sirtuin1 in hyperglycemia-mediated impaired angiogenesis in
endothelial cells: Effects of metformin. J Pharmacol Exp Ther.
356:314–323. 2016. View Article : Google Scholar
|
|
155
|
Beg MS, Brenner AJ, Sachdev J, Borad M,
Kang YK, Stoudemire J, Smith S, Bader AG, Kim S and Hong DS: Phase
I study of MRX34, a liposomal miR-34a mimic, administered twice
weekly in patients with advanced solid tumors. Invest New Drugs.
35:180–188. 2017. View Article : Google Scholar
|