Introduction
Liver ischemia/reperfusion injury (LIRI) is a common
complication in liver surgery, and substantially influences the
surgical outcome and patient prognosis (1-3).
In previous years, the shortage of transplantation donors has
increased the prevalence of marginal donors that further increase
the risk of LIRI, and subsequently that of primary graft
insufficiency or post-transplantation dysfunction (4,5).
The mechanisms underlying LIRI pathology are highly complex and
include the excessive production of reactive oxygen species,
intracellular calcium overload, microvascular endothelial injury,
inflammation and autophagy (6).
It is essential to elucidate the exact molecular mechanism
underlying LIRI, in order to protect the hepatocytes against
ischemic injury.
Previous studies have revealed the involvement of
microRNAs (miRNAs/miRs) in LIRI-associated autophagy (7–9).
miRNAs are non-coding RNAs ~21–23 nucleotides long, and bind
specifically to the 3′-untranslated region (3′-UTR) of the target
mRNA, resulting in either mRNA degradation or a protein translation
block (10,11). miRNAs may additionally mediate the
upregulation of target mRNAs by direct activation and/or indirect
derepression to enhance mRNA stability and translational activation
(12). miRNAs have been
implicated in various cellular and molecular events, including
proliferation, differentiation and apoptosis, and the dysregulation
of miRNAs is the mechanistic basis of various pathophysiological
conditions (13,14). Previous studies have revealed a
notable function of miR-101 in modulating autophagy. For example,
Frankel et al (15)
identified that miR-101 inhibited autophagy in breast cancer cells,
whilst Valera et al (16)
demonstrated that miR-101 induced multiple system atrophy via
autophagy in the nervous system. In addition, Xu et al
(17) concluded that miR-101
inhibited autophagy and enhanced cisplatin-induced apoptosis in
hepatoma cells. The aim of the present study was to determine the
function of miR-101 in mediating autophagy in LIRI, in order to
identify a novel therapeutic target for LIRI.
Materials and methods
Animals and cell lines
A total of 60 male C57BL/6 mice (7–8 weeks old,
weighing 20–25 g) were obtained from the Experimental Animal Center
of Academy of Military Medical Sciences (Beijing, China). All
animals were maintained in an air-conditioned animal room at 25°C
with free access to water and food, and exposed to a 12-h
light/dark cycle. All animal experiments conformed to the National
Institute of Health guidelines (18,19), and the animals were treated
humanely. The study passed the ethical review of the Tianjin First
Center Hospital (Tianjin, China) for the use of experimental
animals, and the protocols were ethically approved by the ethics
committee of Tianjin First Central Hospital. The non-tumorigenic
mouse hepatocyte acute myeloid leukemia (AML)12 cell line was
purchased from the Shanghai Cell Bank of Chinese Academy of
Sciences (Shanghai, China).
Reagents and antibodies
Fetal bovine serum, 0.05%
trypsin-ethylenediaminetetraacetic acid and Dulbecco's modified
Eagle's medium (DMEM)/F12 medium were purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The miR-101
mimetics/inhibitor, miR-101 agomir/antagomir, miRNA negative
control (miR-NC), and RiboFECTTM CP Reagent were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Trizol and SYBR
Green reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) Master Mix were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.) and Beijing Transgen Biotech Co., Ltd. (Beijing,
China), respectively. The In Situ Cell Death Detection kit
was purchased from Roche Diagnostics GmbH (Mannheim, Germany). An
immunohistochemistry kit (cat. no. PV-9001) and DAB chromogenic kit
(cat. no. ZLI-9018) were purchased from OriGene Technologies, Inc.
(Beijing, China). The autophagy double-labeled adenovirus [m red
fluorescence protein (RFP)-green fluorescence protein (GFP)-LC3]
was acquired from Hanbio Biotechnology Co., Ltd. (Shanghai, China),
and 3-methyladenine (3-MA) from Selleck Chemicals (Houston, TX,
USA). Rapamycin (Rapa) and methylthiazole tetrazolium kit (MTT)
were acquired from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Antibodies against mechanistic target of rapamycin (mTOR; cat. no.
2972), phosphorylated (p-)mTOR (cat. no. 2971), caspase-3 (cat. no.
9662), sequestosome 1/p62 (cat. no. 16177), microtubule-associated
protein 1 light II (LC3II; cat. no. 3868), proliferating cell
nuclear antigen (PCNA; cat. no. 13110) and GAPDH (cat. no. 5174),
and the horseradish peroxidase (HRP)-conjugated anti-rabbit (cat.
no. 7074) and anti-mouse (cat. no. 7076) secondary antibodies were
all purchased from Cell Signaling Technology, Inc., (Danvers, MA,
USA).
Establishment of an in vivo model of
LIRI
This experiment established a segmental (70%) LIRI
model according to a previous study (20), with the arterial and portal venous
blood supply to the left and middle lobes interrupted using an
atraumatic clip. Following 90 min of local ischemia, the clip was
removed. Animals were sacrificed by dislocation of spine and
harvested after 2, 6, 12 or 24 h reperfusion. Sham-operated mice
underwent the same procedure, but without vascular occlusion as
previous described (20). The
mice were randomized into the following 10 groups (n=6/group): A
control/sham operated group, 4 untreated LIRI groups with different
reperfusion times (2, 6, 12 and 24 h) and 5 LIRI groups that were
administered an intravenous injection 24 h prior to ischemia and
harvested subsequent to 12 h reperfusion, with injections
consisting of the following: i) 10 nM miR-101 agomir, ii) 10 nM
miR-101 antagomir, iii) 10 nM miR-NC, iv) 5 mg/kg 3-MA and v)
miR-101 agomir plus 3-MA. The 3-MA was intraperitoneally
administered 1 h prior to ischemia.
Serological tests
Blood was collected from the mice from their
inferior vena cava and centrifuged (4°C, 15 min, 1,000 × g) to
collect the serum. The levels of serum aspartate aminotransferase
(AST) and alanine aminotransferase (ALT) were determined using
commercial assay kits (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) and according to the manufacturer's protocol.
Enzyme activity was expressed as international units per liter
(U/I).
Hematoxylin and eosin (H&E)
staining
The liver tissues were fixed in 4% formalin for 48 h
at 4°C, embedded in paraffin blocks and processed into
4-µm-thick sections. The slides were then dehydrated using
an ethanol gradient (100% for 10 min, 95% at 10 min and 80% for 10
min) and de-paraffinized using xylene. H&E staining was
performed according to a standard procedure (21), and the histopathological changes
were observed under a light microscope at a magnification of ×200.
IR-induced liver damage was quantified by measuring the Suzuki
score as presented in Table I and
a previous study (22).
 | Table ISuzuki scores for liver
ischemia/reperfusion injury. |
Table I
Suzuki scores for liver
ischemia/reperfusion injury.
| Numerical
assessment | Congestion | Vacuolization | Necrosis |
|---|
| 0 | None | None | None |
| 1 | Minimal (10%) | Minimal (10%) | Single-cell
necrosis |
| 2 | Mild (11-30%) | Mild (11-30%) | Mild (<30%) |
| 3 | Moderate
(31-60%) | Moderate
(31-60%) | Moderate
(31–60%) |
Immunohistochemistry
Paraffin sections of liver tissue were cut into
4-µm-thick sections and dehydrated, cleared using xylene and
heated to 95°C using 0.01 mol/l citrate buffer solution (pH 6.0) in
a water bath for antigen retrieval. Subsequent to blocking with 5%
goat serum (Solarbio Science & Technology Co., Ltd., Beijing,
China) for 1 h at room temperature, the sections were incubated
with rabbit anti-mouse PCNA and caspase-3 polyclonal antibodies
(both dilution, 1:1,000; Cell Signaling Technology, Inc.) overnight
at 4°C. Then sections were incubated with enzyme-labeled goat
anti-rabbit immunoglobulin G (IgG) polymer from the
immunohistochemistry kit for 1 h at room temperature. In total, 0.5
ml DAB staining solution A, 0.5 ml DAB staining solution B and 1 ml
DAB staining working solution were prepared and gently mixed. The
sections were incubated with the mixture at 20–25°C for 5 min, and
the sections were counterstained with 10% hematoxylin for 30 sec at
room temperature. The stained slides were washed thoroughly in
running tap water, dehydrated and mounted with cover slips.
Hepatocytes positively stained for PCNA and caspase-3 were examined
under a light microscope at a magnification of ×200, and ImageJ
1.48v software (National Institutes of Health, Bethesda, MD, USA)
was used to analyze the area occupied by the positively stained
cells.
Terminal uridine nick-end labeling
(TUNEL) assay
The In Situ Cell Death Detection kit (with
TMR red as the fluorescence marker) was used to detect TUNEL
positive apoptotic cells according to the manufacturer's protocol.
Apoptosis was observed under fluorescence microscope at a
magnification of ×200. The area occupied by the positive cells was
analyzed using Image J 1.48v software.
Cell culture and transfection
AML12 cells were plated at a density of
2×105 cells/ml in 6-well plates and divided into 4
treatment groups, as followings: i) A control untreated group; ii)
an ischemia/reperfusion (IR) group subjected to hypoxia for 1 h to
simulate ischemia and then cultured in DMEM/F12 for 12 h to
simulate reperfusion; iii) an miR-101 mimetics group transfected
with 50 nM miR-101 mimetics or miR-NC using RiboFECTTM for 48 h at
37°C, and subjected to hypoxia/reperfusion 48 h later; and iv)
miR-101 inhibitor group transfected with 50 nM miR-101 inhibitor or
miR-NC, and subjected to hypoxia/reperfusion 48 h later. To induce
hypoxia, the culture medium was removed 48 h after transfection and
replaced with 2 ml Hank's solution (Thermo Fisher Scientific,
Inc.), and the cells were placed in a low oxygen incubator. After
1.5 h, Hank's solution was removed and replaced with 2 ml DMEM/F12
medium, and the cells were cultured under normal oxygen tension
(with 5% CO2) for 12 h to simulate the reperfused
(re-oxygenated) state.
Confocal fluorescence microscopy
AML12 cells were plated in 24-well plates, and
cultured until they reached 60–70% confluence. The cells were
transduced with the GFP-RFP-LC3 adenovirus (3×107
PFU/well) for 48 h at 37°C according to the manufacturer's protocol
to induce autophagy and the resulting autophagosomes were observed
under a confocal microscope at a magnification of ×1,000. The
number of autophagosomes in each cell was counted, and the mean
number of autophagosomes of all cells was calculated to determine
the autophagic degree of each treatment group.
RT-qPCR
Total RNA was extracted from tissues or cells using
TRIzol total RNA isolation reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Subsequently, the PrimeScript RT reagent kit (Beijing Transgen
Biotech Co., Ltd.) was used for RT of the RNA into cDNA, according
to the manufacturer's protocol. SYBR-Green RT-qPCR Master Mix was
used as the flurophore. RT-qPCR was performed using specific
primers for miR-101 (forward, 5′-GTACAGTACTGTGATAACTGA-3′ and
reverse, 5′-TGCGTGTCGTGGAGTC-3′), mTOR (forward,
5′-TCGGTGCAAACCTACAGAAGC-3′ and reverse,
5′-TGCAGGTCGTATATGGACAGAG-3′) and GAPDH (forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′) used as the internal control. The
thermocycling conditions were as follows: Pre-denaturation at 94°C
for 30 sec, followed by 45 cycles of denaturation at 94°C for 5
sec, annealing at 60°C for 15 sec and extension at 72°C for 10 sec.
Each sample was tested in quadruplicates. The relative expression
of each gene was quantified using the comparative quantification
cycle method as follows: Copy number of target gene =
2−ΔΔCq, ΔCq = Cqtarget gene−Cqreference
gene, ΔΔCq = ΔCqexperimental group−ΔCqcontrol
group (23).
Immunofluorescence
AML12 cells were transfected with miR-101 mimetics
or miR-101 inhibitors using RiboFECTTM for 48 h at 37°C, according
to the reagent manufacturer's protocol. Following differentiation
and treatments, cells were fixed with 4% paraformaldehyde for 15
min at room temperature and permeabilized with 0.1% Triton X-100
for 5 min at room temperature. Cells were then incubated for 60 min
at room temperature with blocking solution (5% goat serum) followed
by overnight incubation at 4°C with anti-p62 antibodies (dilution,
1:800) Following washing with PBS, cells were incubated with
fluorescence-labeled secondary antibodies (Alexa Fluor®
594-conjugated goat polyclonal anti-rabbit; 1:500; cat. no. 8889;
Cell Signaling Technology, Inc.) for 1 h at room temperature in the
dark. In addition, DAPI (1:1,000; cat. no. D9564; Sigma-Aldrich;
Merck KGaA) was used to non-specifically stain the nuclei and
samples were incubated with 50 µl DAPI for 10 min at room
temperature. Immunostaining was visualized under a fluorescence
microscope at a magnification of ×400.
MTT bioassay
AML12 cells were seeded into 96-well plates
(5×104 cells/well) and after 24 h culturing, were
treated with Rapa or 3-MA for 2 h, or miR-101 inhibitor for 48 h at
37°C prior to reperfusion. Fresh medium was then added to each well
along with 20 µl MTT solution (5 mg/ml), and the cells were
incubated for another 4 h at 37°C. The medium was then removed, and
200 µl dimethylsulfoxide was added per well to stop the
reaction. The optical density of each well was determined at 490
nm.
Western blot analysis
Radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China) were used to
extract the total protein from AML12 cells and liver tissues. The
protein concentration was determined by Bicinchoninic Acid Protein
Assay kit (Solarbio Science & Technology Co., Ltd.). Equal
samples of protein (30 µg) were separated by SDS-PAGE on 12%
gels and transferred onto a polyvinylidene difluoride membrane. The
membrane was blocked with 5% skim milk for 1 h at room temperature.
The membrane was then incubated with mTOR, p-mTOR, GAPDH, LC3 II,
caspase-3 and p62 (all 1:1,000) primary antibodies overnight at
4°C. The membranes were then washed with TBS-Tween-20 at room
temperature (5 min/wash). Subsequently, the membrane was treated
with HRP-conjugated goat anti-rabbit and anti-mouse IgG secondary
antibodies (both 1:2,500), agitated and incubated at room
temperature for 1 h. The protein bands were visualized by using a
G:BOX imaging system (Gene Company, Ltd., Hong Kong, China). The
protein bands were measured with ImageJ 1.48v software and
normalized to the corresponding GAPDH bands. The relative density
of each target protein normalized to the control was used to
represent the changes in expression of target proteins.
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp., Armonk, NY, USA). Normally distributed data
were expressed as the mean ± standard deviation (x±s). A Student's
t test was used for comparing two groups, a one-way analysis of
variance for comparing multiple groups and the
Least-Significant-Difference method was used as a post-hoc test for
multiple comparisons between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
LIRI alters the expression of miR-101 and
mTOR in mouse liver
The expression levels of miR-101 and mTOR mRNA
initially significantly increased following reperfusion compared
with their respective sham groups (P<0.001), but significantly
decreased in the liver of the IR mouse model following reperfusion
at 6 and 12 h compared with the respective sham groups (P<0.001;
Fig. 1A).
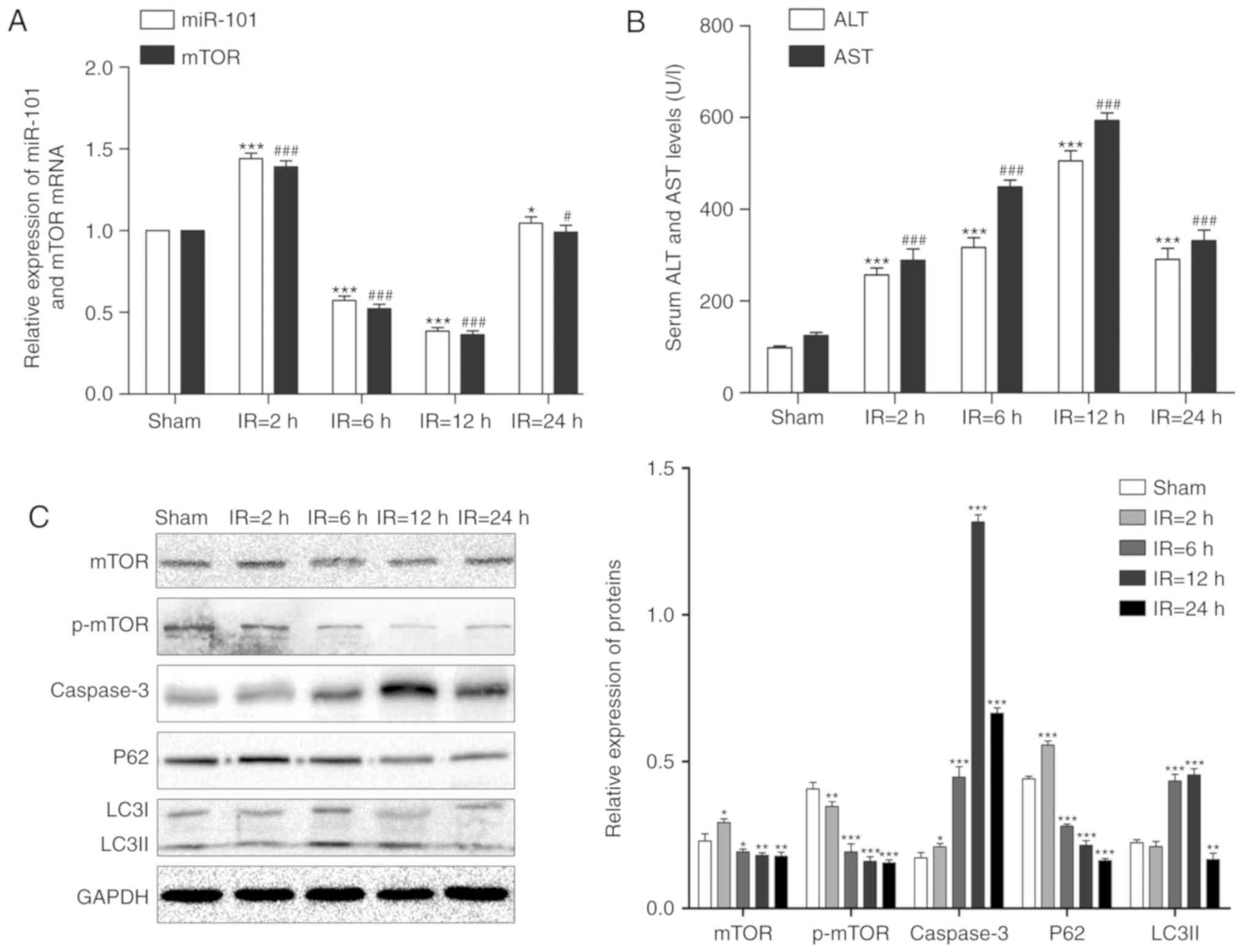 | Figure 1Alterations of miR-101 expression and
autophagy signaling in the liver IR injury mouse model. (A)
Relative expression levels of miR-101 and mTOR mRNA in mouse liver
at each reperfusion time point were detected using reverse
transcription-quantitative polymerase chain reaction.
*P<0.05 and ***P<0.001 vs. miR-101
sham. #P<0.05 and ###P<0.001 vs. mTOR
sham. (B) Comparison of serum ALT and AST levels at different time
points. ***P<0.001 vs. ALT sham.
###P<0.001 vs. AST sham. (C) Western blots exhibiting
the levels of mTOR, p-mTOR, caspase-3, p62 and LC3II proteins in
the liver at different time points post-reperfusion. GAPDH was used
as the internal control. *P<0.05,
**P<0.01 and ***P<0.001 vs. the sham
treated group. miR, microRNA; mTOR, mechanistic target of
rapamycin; IR, ischemia/reperfusion; ALT, alanine aminotransferase;
AST, aspartate aminotransferase; p-, phosphorylated; LC3II,
microtubule-associated protein 1 light II. |
LIRI alters serum AST and ALT levels
The levels of serum AST and ALT were significantly
increased following reper-fusion in a time-dependent manner
compared with the sham groups (P<0.001). Compared with the
sham-treated group, the IR mice exhibited a gradual increase in the
serum AST and ALT levels that peaked at 12 h (P<0.001; Fig. 1B).
LIRI affects apoptosis and autophagy
As presented in Fig.
1C, LC3II expression levels significantly increased following 6
and 12 h reperfusion and peaked at 12 h compared with the sham
group (P<0.01) and the pro-apoptotic caspase-3 was also
significantly upregulated following reperfusion with maximum
expression at 12 h post-reperfusion compared with the sham group
(P<0.05). Although the expression levels of mTOR and p62
increased after 2 h reperfusion, the expression levels of mTOR,
p-mTOR and p62 significantly decreased steadily in a time-dependent
manner following reperfusion at 6, 12 and 24 h (P<0.05).
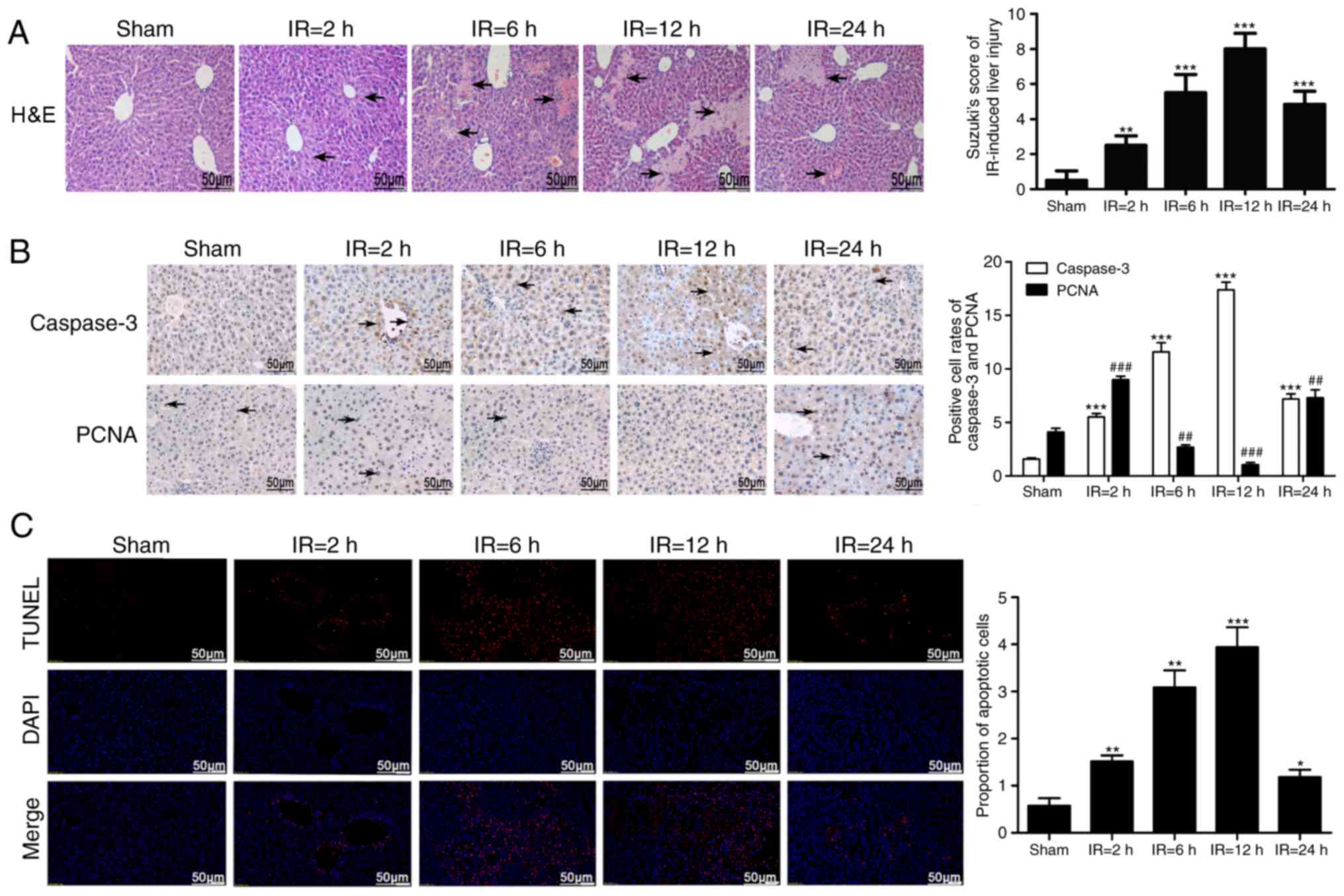
Histopathological changes in the liver
following IRI
The liver tissues of the IR mice demonstrated edema,
ballooning, steatosis, flaky necrosis, neutrophil infiltration and
congestion, in addition to the disappearance of the hepatic
sinusoidal structure in certain areas. These lesions were
substantially aggravated with time, with the severest injuries
observed at 12 h after reperfusion, but were relieved at 24 h
post-reperfusion. In addition, IR-induced liver damage was
quantified by measuring the Suzuki score, which gradually
significantly increased following reperfusion compared with the
sham group (P<0.01; Fig.
2A).
IRI alters proliferation and apoptosis of
liver cells
Compared with the sham-treated group (Fig. 2B), the intra-nuclear expression of
the proliferative marker PCNA significantly decreased following
reperfusion at the 6 and 12 h mark, despite initially increasing at
the 2 h mark and again increasing at the 24 h mark (P<0.01). The
cytoplasmic expression of caspase-3 gradually significantly
increased in the liver cells of IR mice with time compared with the
sham group (P<0.001) and a peak change was observed 12 h after
reperfusion. Additionally, as presented in Fig. 2C, the number of TUNEL-positive
apoptotic cells were also significantly higher in the IR groups
compared with the sham-treated group (P<0.05). The apoptotic
changes were time dependent, with peak alterations observed 12 h
after reperfusion.
miR-101 weakens LIRI by inhibiting
apoptosis
As presented in Fig.
3A, the expression levels of mTOR in the miR-101 agomir group
were significantly increased compared with the mTOR miR-NC group
(P<0.001), while the expression levels of mTOR in the mir-101
antagomir group were significantly decreased compared with the mTOR
miR-NC group (P<0.01; Fig.
3A). The miR-101 antagomir significantly aggravated the
histopatho-logical changes in the liver and the corresponding
Suzuki scores induced by IR treatment, while miR-101 agomir
significantly alleviated these changes compared with the miR-101
miR-NC group (P<0.001; Fig.
3B). The overexpression of miR-101 significantly reduced the
expression of LC3II and caspase-3 compared with the miR-101 miR-NC
group (P<0.001) and significantly increased that of mTOR
compared with the miR-101 miR-NC group (P<0.01; Fig. 3C). IR-induced apoptosis was
significantly increased by miR-101 antagomir compared with the
miR-NC group (P<0.05) and alleviated by miR-101 agomir compared
with the miR-NC group (P<0.01; Fig. 3D).
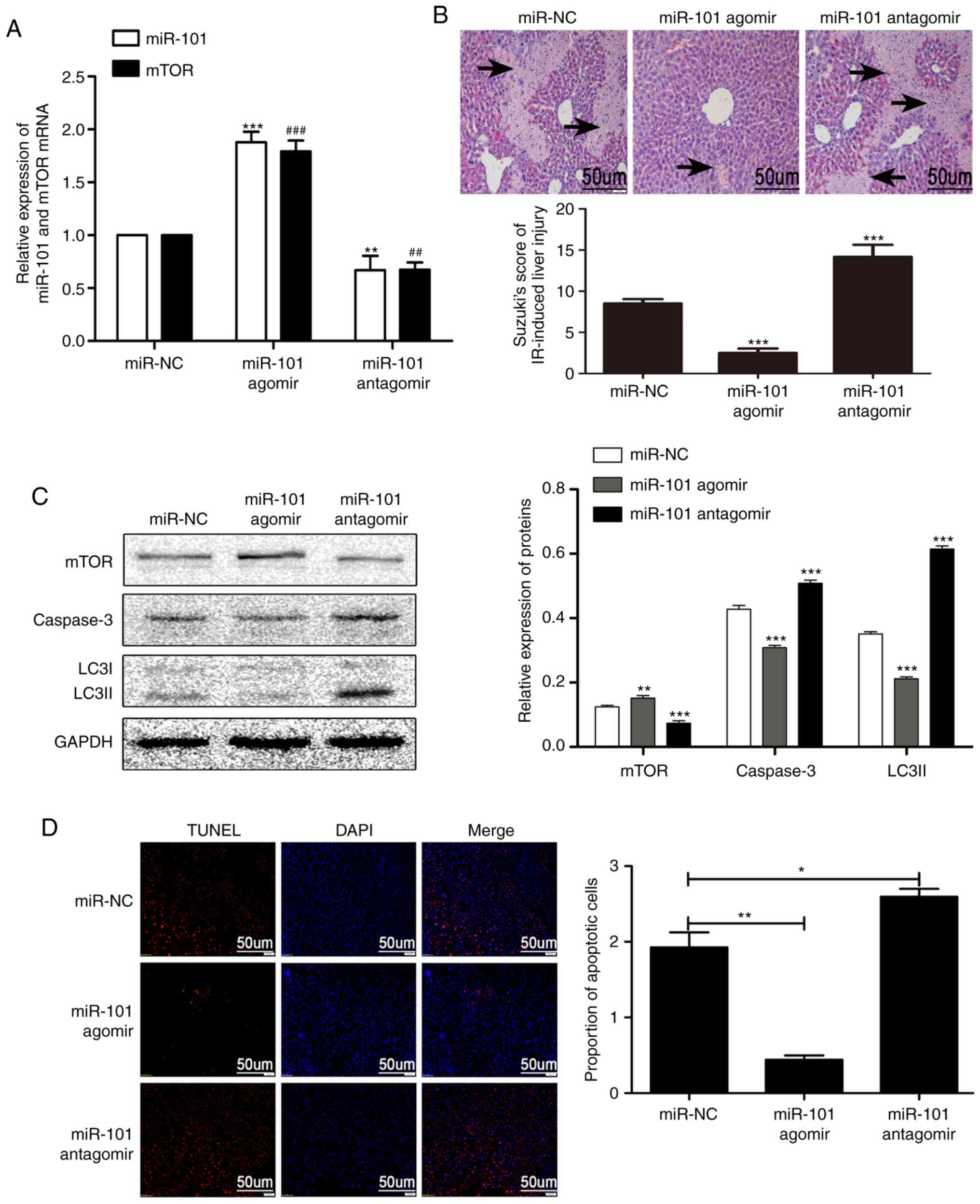 | Figure 3miR-101 ameliorates liver IR injury
by inhibiting apoptosis. (A) Relative expression levels of miR-101
and mTOR mRNA in response to miR-101 agomir/antagomir were detected
using reverse transcription-quantitative polymerase chain reaction.
**P<0.01 and ***P<0.001 vs. miR-101
miR-NC group. ##P<0.01 and ###P<0.001
vs. mTOR miR-NC group. (B) Representative images of haemotoxylin
and eosin-stained liver sections presenting histopathological
changes (×200 magnification; scale bars=50 µm) following
miR-101 agomir or antagomir injection. ***P<0.001 vs.
miR-NC group. (C) Western blots presenting mTOR, caspase-3 and
LC3II levels in the liver. **P<0.01 and
***P<0.001 vs. the miR-NC group. (D) Representative
images of TUNEL stained apoptotic nuclei (red) with DAPI
counterstaining (×200 magnification; scale bars=50 µm), and
comparison of the percentage of apoptotic cells analyzed using
Image J software. *P<0.05 and **P<0.01
with comparisons shown by lines. miR, microRNA; mTOR, mechanistic
target of rapamycin; NC, negative control; IR,
ischemia/reperfusion; LC3II, microtubule-associated protein 1 light
II; TUNEL, terminal uridine nick-end labeling; DAPI,
3,3′-diaminobenzidine. |
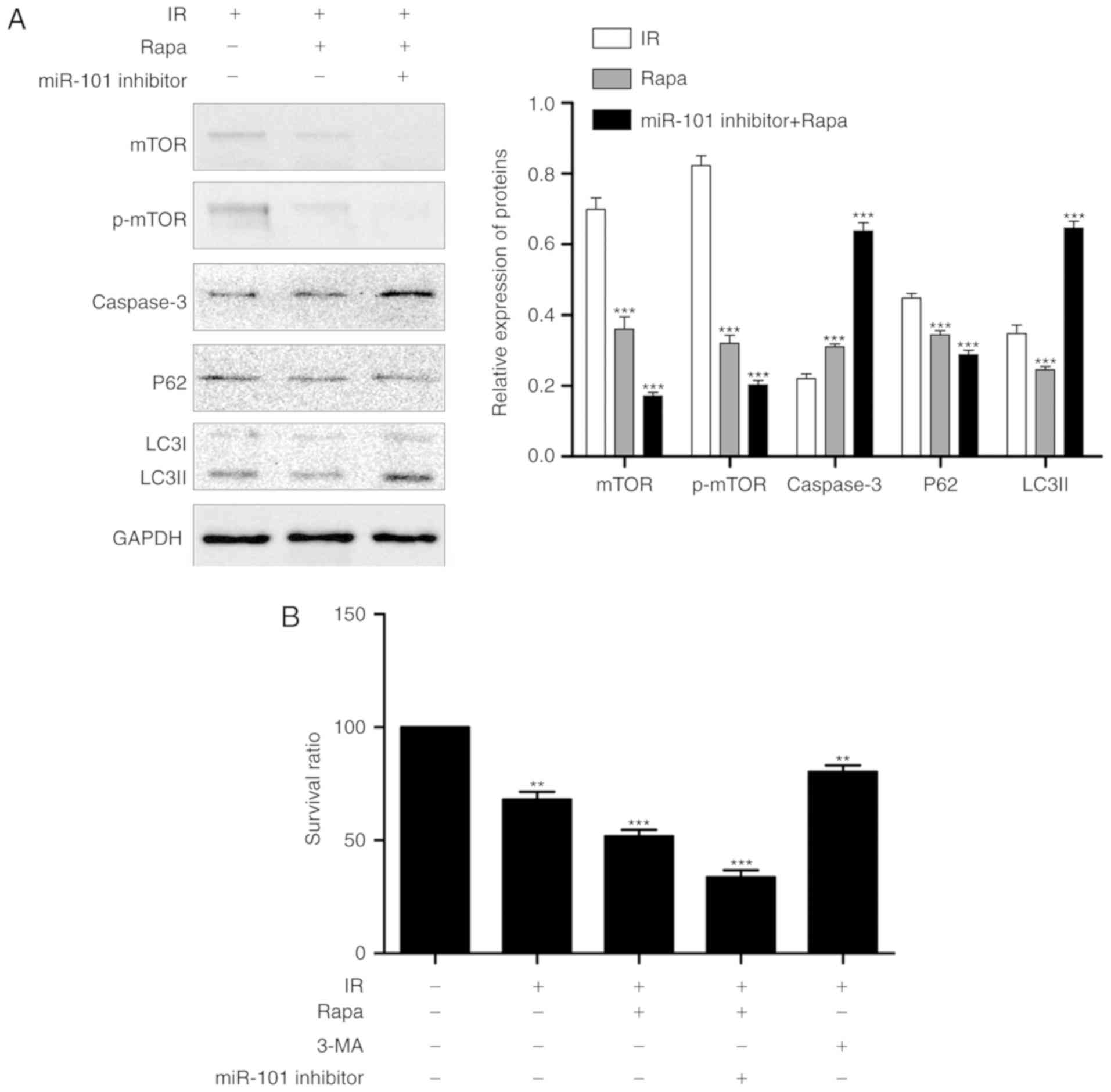
Inhibition of autophagy enhances the
protective effect of miR-101 on LIRI
Treatment of the IR mice with the autophagy
inhibitor 3-MA in addition to miR-101 transfection significantly
reduced the IR-induced histopathological changes and Suzuki scores
compared with the IR group (P<0.001; Fig. 4A) and significantly increased the
nuclear expression of PCNA compared with the untreated IR group
(P<0.001; Fig. 4B). In
addition, IR-induced apoptosis was significantly reduced in the
mice treated with miR-101+3-MA compared with the untreated IR group
(P<0.001; Fig. 4C) and
validated by the significantly lower expression levels of caspase-3
and LC3II and the upregulation in p62 levels compared with the IR
group (P<0.001; Fig. 4D).
Serum AST and ALT levels were also significantly lower in the
miR-101+3-MA group compared with the IR group (P<0.001; Fig. 4E).
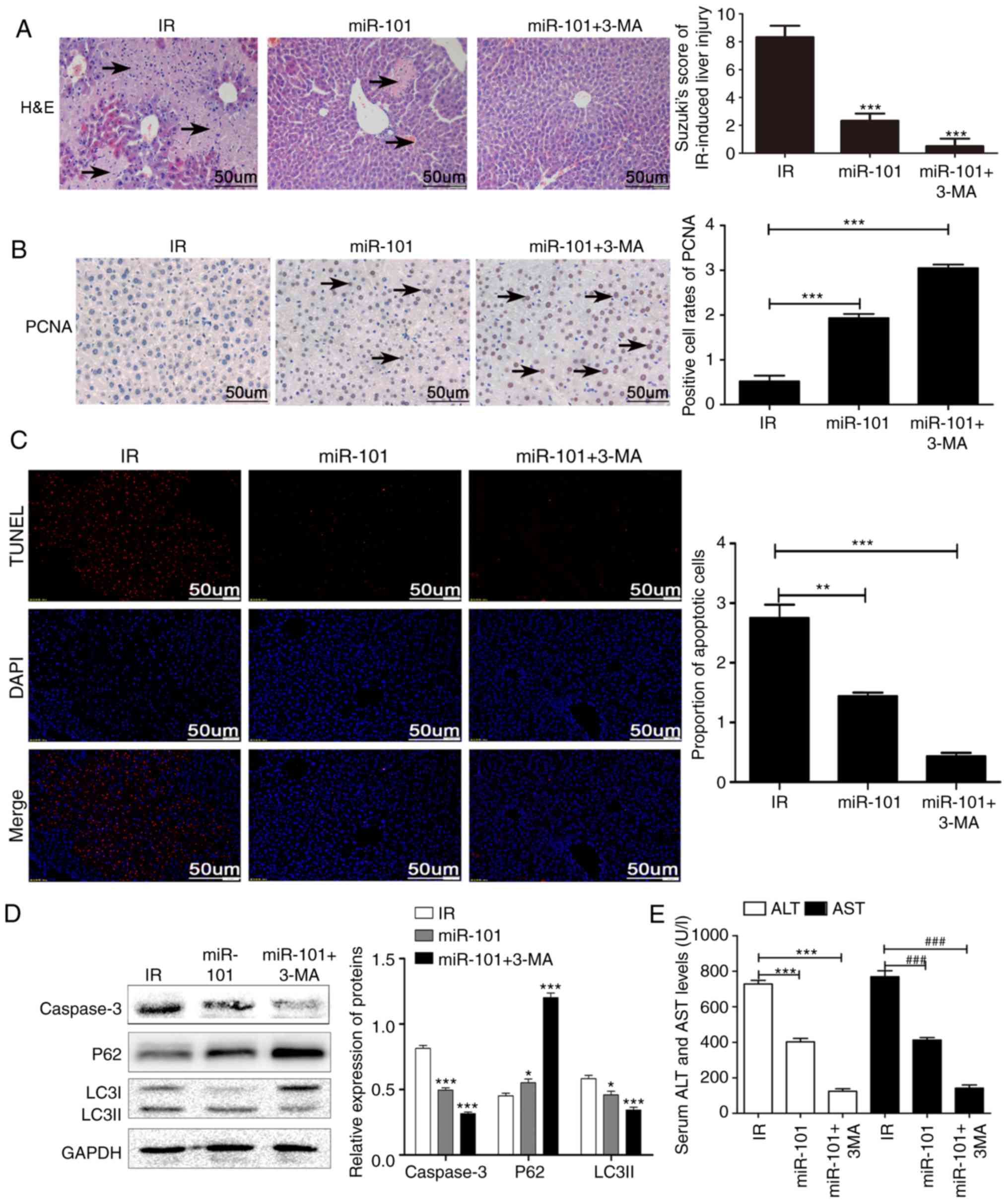 | Figure 4Inhibition of autophagy enhances the
protective effect of miR-101 on liver IR injury. (A)
Histopathological changes in liver tissue subsequent to treatment
with autophagy inhibitor (3-MA; ×200 magnification). Scale bars=50
µm. ***P<0.001 vs. IR group. (B)
Representative immunohistochemistry images of liver tissues
presenting nuclear staining of PCNA (×200 magnification; scale
bars=50 µm), and comparisons of the percentage of
PCNA-stained cells in different groups. ***P<0.001
with comparisons shown by lines. (C) Representative images of TUNEL
stained (red nuclei) apoptotic cells with DAPI counter-staining
(×200 magnification; scale bars=50 µm), and the percentage
of TUNEL positive cells analyzed using Image J software.
**P<0.01 and ***P<0.001 with
comparisons shown by lines. (D) Western blots presenting LC3II, p62
and caspase-3 protein levels. *P<0.05 and
***P<0.001 vs. the IR group. (E) Comparison of serum
ALT and AST in different groups. ***P<0.001 with
comparisons shown by lines. ###P<0.001 with
comparisons shown by lines. miR, microRNA; IR,
ischemia/reperfusion; 3-MA, 3-methyladenine; PCNA, proliferating
cell nuclear antigen; H&E, haemotoxylin and eosin; TUNEL,
terminal uridine nick-end labeling; DAPI, 3,3′-diaminobenzidine;
LC3II, microtubule-associated protein 1 light II; ALT, alanine
aminotransferase; AST, aspartate aminotransferase. |
miR-101 inhibits autophagy and weakens
LIRI by activating the mTOR pathway in vitro
As presented in Fig.
5, the expression levels of mTOR in the miR-101 mimetics group
were significantly increased compared with the mTOR NC group
(P<0.001), while the expression level of mTOR in the miR-101
inhibitor group was significantly decreased compared with the mTOR
NC group (P<0.01; Fig. 5A). In
addition, the overexpression of miR-101 significantly reduced the
expression of LC3II and caspase-3 compared with the NC group
(P<0.05; Fig. 5B). The
Ad-GFP-RFP-LC3 system was used to determine the potential function
of miR-101 in modulating autophagy subsequent to simulated-IR in
AML12 cells. The presence of co-localized GFP-LC3 or RFP-LC3
granules indicate the recruitment of the LC3 protein to
autophagosomes, which are formed when autophagy is triggered
(24). When autophagosomes fuse
with lysosomes and form autolysosomes, GFP but not RFP degrades in
the acidic environment, resulting in solely red granules (25). As presented in Fig. 5C, the number of autophagosomes in
the miR-101 mimetics group was significantly lower compared with
that in the IR group (P<0.001), indicating that miR-101 inhibits
autophagy. Furthermore, the number of autophagosomes significantly
increased upon miR-101 inhibition compared with that in the IR
group (P<0.05). In addition, p62 was upregulated in the miR-101
mimetics group and was downregulated in the miR-101 inhibitor group
compared with the NC group (Fig.
5D). Altogether, the overexpression of miR-101 inhibited the
formation of autophagosomes and autolysosomes, and thus attenuated
autophagy.
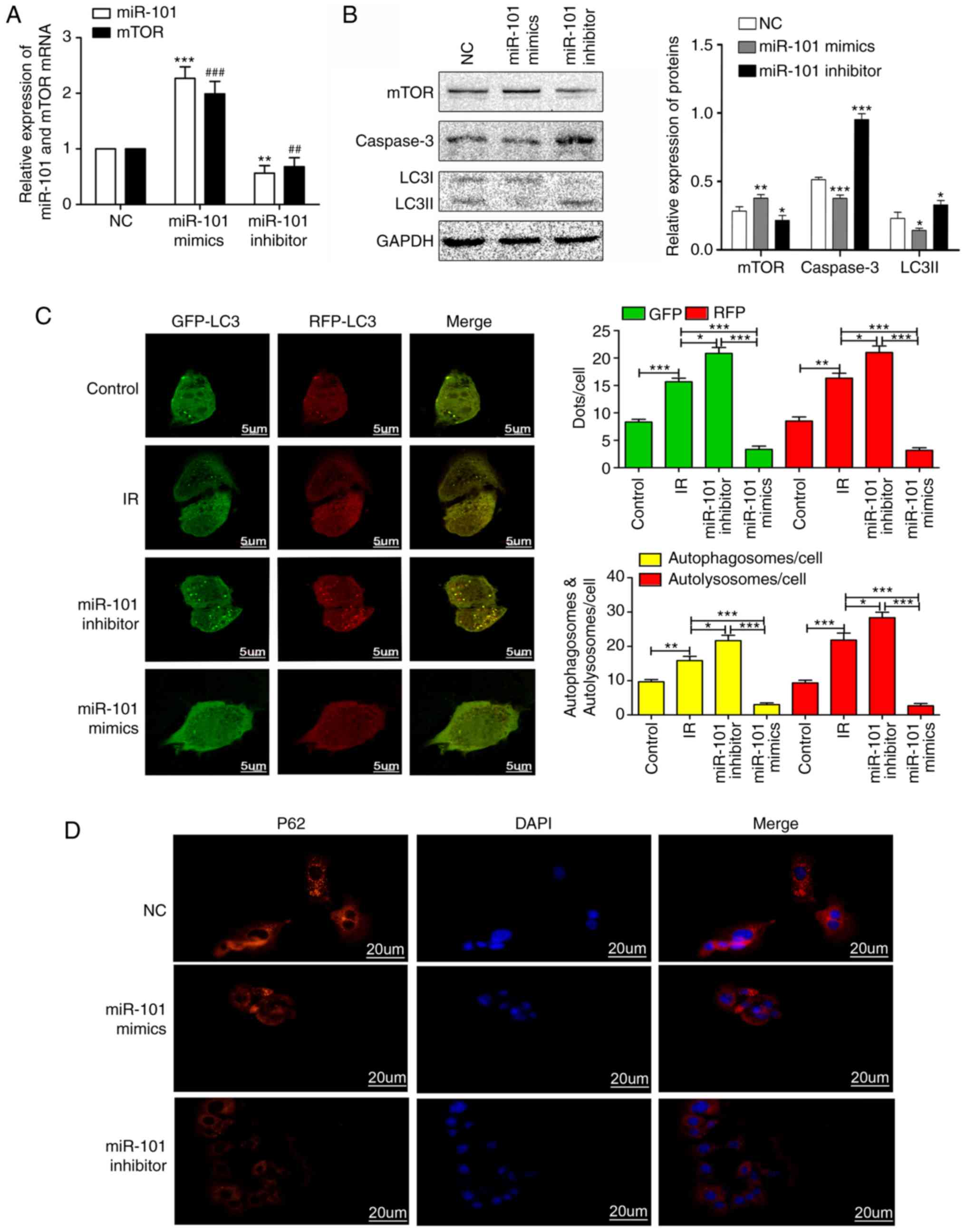 | Figure 5miR-101 inhibits autophagy and
weakens IR injury by activating the mTOR pathway in vitro.
(A) Relative expression levels of miR-101 and mTOR mRNA in response
to miR-101 mimetics/inhibitors. **P<0.01 and
***P<0.001 vs. the miR-101 NC group.
##P<0.01 and ###P<0.001 vs. the mTOR NC
group. (B) Western blots presenting mTOR, caspase-3 and LC3II
levels in AML12 cells. *P<0.05,
**P<0.01 and ***P<0.001 vs. NC group.
(C) Representative confocal images of immunofluorescent GFP-RFP-LC3
expression in AML12 cells. Yellow dots indicate the autophagosomes
with GFP and RFP merging, and the red dots represent the
autolysosomes with degraded GFP due to the acidic environment.
Scale bars=5 µm. *P<0.05,
**P<0.01 and ***P<0.001 with
comparisons shown by lines. (D) Representative immunofluorescence
images presenting the expression of p62 in AML12 at ×400
magnification. Scale bars=20 µm. miR, microRNA; mTOR,
mechanistic target of rapamycin; NC, negative control; LC3II,
microtubule-associated protein 1 light II; IR,
ischemia/reperfusion; GFP, green fluorescence protein; RFP, red
fluorescence protein; DAPI, 3,3′-diaminobenzidine. |
Inhibition of miR-101 and mTOR expression
aggravates LIRI
Reperfusion was established following the
pre-treatment of AML12 cells with miR-101 inhibitor and mTOR
inhibitor rapamycin. The expression of LC3II and caspase-3 were
significantly increased compared with the IR group (P<0.001;
Fig. 6A), and the percentage of
viable cells was significantly decreased following co-suppression
compared with the IR group (P<0.001; Fig. 6B).
Discussion
Liver transplantation is the only treatment option
currently available for end-stage liver disease. Unfortunately, it
is associated with various complications, including LIRI, which is
a common pathophysiological consequence of liver surgery (26). The mechanism of LIRI is complex,
and is closely associated with inflammation, metabolic disorders,
oxidative stress and autophagy. In addition, each of these factors
may be mutually antagonistic or synergistic (27).
Autophagy is an intracellular self-digestion pathway
present in the majority of eukaryotic cells, which helps in
organelle recycling and fulfils cellular metabolic requirements
under stress conditions (28).
The autophagy-related genes (Atgs) induce the detachment of bilayer
membrane structures from the rough endoplasmic reticulum, which
then encapsulate organelles and other cytoplasmic contents to form
autophagosomes. The latter then fuse with lysosomes to form
autolysosomes, and the intra-vesicular contents are degraded by the
lysosomal enzymes (29). LC3/Atg8
is a marker of the autophagosome membrane, and the conversion of
LC3I to LC3II is used as a measure of autophagosome formation
(30). One previous study has
demonstrated that hepatic autophagy is notably enhanced in LIRI
models (31), but the underlying
mechanism is not fully understood. Autophagy functions as a
double-edged sword in hepatic IR and influences cell survival and
apoptosis (32). In moderate IRI,
the autophagosomes digest damaged organelles and provide energy to
the cells. However, severe reperfusion injury results in excessive
autophagy, which may trigger cell death (33). Therefore, targeting the autophagy
pathway may effectively protect against IRI (34).
In previous years, studies have focused on the
function of miRNAs in hepatic IRI, particularly their involvement
in autophagy (35–37). miRNAs are able to regulate
autophagy by inhibiting the expression of target genes, which in
turn affect IRI (13). Previous
studies have demonstrated that miR-17 upregulated autophagy and
aggravated the degree of LIRI by inhibiting Stat3 expression in
LIRI (31), while miR-30b reduced
autophagy and protected against LIRI by inhibiting Atg12-Atg5
binding (38). Studies have
additionally demonstrated that the inhibition of miR-34a enhanced
sirtuin 1 expression, which downregulated autophagy and
subsequently protected the liver from p65/p53 deacetylation-induced
damage (39,40). Therefore, the miRNAs regulating
autophagy in LIRI may be potential therapeutic targets. miRNAs
typically function by causing mRNA degradation through interacting
with the 3′-UTR of the target mRNAs, resulting in mRNA degradation
and/or translational repression (12). Conversely, the miRNA-mediated
upregulation of target mRNAs may be elucidated by direct activation
and/or indirect derepression to enhance mRNA stability and
translational activation (41).
Studies have demonstrated that in miRNA-mediated upregulation,
micro-ribonucleoprotein (miRNP) trans-expression promotes the
expression of its target mRNA, which is similar to miRNA-mediated
downregulation (42-44). MRNA expression may be activated
directly by miRNP and/or alleviated indirectly from miRNA-mediated
inhibition by eliminating the inhibitory effect of miRNP (45).
The present study investigated whether miR-101 was
able to affect autophagy and serve a function in LIRI through the
mTOR pathway. In previous studies, miR-101 was able to inhibit
tumor growth by inhibiting autophagy (46–48). mTOR is a notable serine-threonine
protein kinase downstream of phosphoinositide-3-kinase
(PI3K)/protein kinase B (Akt) (49) and inhibits autophagy during tumor
growth and progression (17,47). Li et al (50) revealed that octreotide is able to
upregulate the expression of miR-101 and inhibit autophagy by
inactivating AMP-activated protein kinase (AMPK) and activating the
mTOR pathway, thereby reducing the incidence of intestinal
mucositis following anticancer treatment. However, little is known
regarding the function of the miR101/mTOR axis in LIRI.
The present study revealed that IRI induced a number
of pathological, functional and molecular changes in the liver,
including increased serum levels of ALT and AST, the downregulation
of miR-101, mTOR mRNA and p62, increased levels of LC3II and
caspase-3, decreased intra-nuclear PCNA and extensive tissue
necrosis and apoptosis. PCNA is closely associated with cellular
DNA synthesis and therefore a good indicator of cell proliferation
status (51,52). Caspase-3 is a necessary terminal
cleavage enzyme in the intrinsic apoptotic pathway, and an
established indicator of apoptosis (53).
Based on the results of the present study, miR-101
was negatively associated with autophagy. Furthermore, the
over-expression of miR-101 reduced apoptosis, increased mTOR
expression and decreased LC3II and caspase-3 levels, whilst the
inhibition of miR-101 had the reverse effects. In addition, the
inhibition of autophagy by 3-MA augmented the protective effects of
miR-101 overexpression against LIRI.
To assess the hypothesis that miR-101 regulates the
mTOR signaling pathway, the present study investigated the function
of miR-101 in the regulation of the mTOR signaling pathway. The
overexpression of miR-101 in LIRI-mimicking AML12 cells increased
mTOR expression, decreased the number of autophagosomes and
increased p62 expression. miR-101 inhibition exhibited the reverse
effects on mTOR expression and autophagosome formation.
Subsequently, miR-101 and mTOR were co-inhibited in AML12 cells,
and it resulted in an increase in autophagy and cell death. These
results indicate that miR-101 protects hepatocytes against IR
injury by inhibiting autophagy via activation of mTOR signaling
pathway.
The present study identified the inhibitory effect
of miR-101 on autophagy in LIRI by regulating the expression of
mTOR. But, a number of limitations of the present study should be
taken into consideration. For example, the underlying mechanisms of
the inductory effect of miR-101 on mTOR should be further studied.
However, microRNA regulation is multi-directional. Nikoonahad et
al (54) revealed that
miR-101 inhibits the growth of AML cancer cells by directly
upregulating the expression of the pro-apoptotic gene Bcl2 like11
(BIM). Different diseases and different conditions may cause miRNAs
to exhibit distinct regulatory mechanisms. In addition, as one of
the substrates of Akt, the P13K/Akt/mTOR regulatory pathway has
been confirmed to serve a necessary function in cell growth and
regulation (55). At present, the
function of miR-101 in LIRI through the P13K/Akt/mTOR regulatory
pathway remains to be further verified. AMPK is a cellular energy
receptor, and a number of studies have demonstrated that AMPK is a
negative regulator of the mTOR pathway (56,57). Studies have also revealed that
miR-101 may inhibit the action of AMPK by directly targeting the
3′-UTR region of AMPK (58,59). Therefore, miR-101 is likely to
regulate the mTOR signaling pathway and serve a function in LIRI by
affecting the expression of AMPK. Whether miR-101 affects the
expression of mTOR by regulating AMPK in LIRI and whether there are
other regulatory objectives and mechanisms has yet to be further
studied.
In conclusion, the present study revealed that
miR-101 attenuates LIRI by activating the mTOR pathway and
inhibiting autophagy. Further studies are required to further
dissect the association between autophagy and miRNAs in hepatocytes
following IR in order to develop novel therapies.
Funding
The present study was supported by the Tianjin
Clinical Research Center for Organ Transplantation Project (grant
no. 15ZXLCSY00070).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and JZ conceived and designed the experiments.
HS, CD and XW performed the experiments. HS, JZ and ZS analyzed the
data. HS and CD wrote the paper. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The use and care of the animal were in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health (18). The research protocols were
approved by the Ethics Committee of Tianjin First Center Hospital
(Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Cursio R, Colosetti P and Gugenheim J:
Autophagy and liver ischemia-reperfusion injury. Biomed Res Int.
2015:4175902015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun P, Zhang P, Wang PX, Zhu LH, Du Y,
Tian S, Zhu X and Li H: Mindin deficiency protects the liver
against ischemia/reperfusion injury. J Hepatol. 63:1198–1211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clavien PA: How far can we go with
marginal donors? J Hepatol. 45:483–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halazun KJ, Quillin RC, Rosenblatt R,
Bongu A, Griesemer AD, Kato T, Smith C, Michelassi F, Guarrera JV,
Samstein B, et al: Expanding the margins: High volume utilization
of marginal liver grafts among ≥2000 liver transplants at a single
institution. Ann Surg. 266:441–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lentsch AB, Kato A, Yoshidome H, McMasters
KM and Edwards MJ: Inflammatory mechanisms and therapeutic
strategies for warm hepatic ischemia/reperfusion injury.
Hepatology. 32:169–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M and
Lu L: Exosomes derived from miR-181-5p-modified adipose-derived
mesenchymal stem cells prevent liver fibrosis via autophagy
activation. J Cell Mol Med. 21:2491–2502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang B, Bao N, He G and Wang J: Long
noncoding RNA HOTAIR regulates autophagy via the miR-20b-5p/ATG7
axis in hepatic ischemia/reperfusion injury. Gene. 686:56–62. 2019.
View Article : Google Scholar
|
|
9
|
Chen J, Yu Y, Li S, Liu Y, Zhou S, Cao S,
Yin J and Li G: MicroRNA-30a ameliorates hepatic fibrosis by
inhibiting beclin1-mediated autophagy. J Cell Mol Med.
21:3679–3692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim LP, Glasner ME, Yekta S, Burge CB and
Bartel DP: Vertebrate microRNA genes. Science. 299:15402003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weiss JB, Eisenhardt SU, Stark GB, Bode C,
Moser M and Grundmann S: MicroRNAs in ischemia-reperfusion injury.
Am J Cardiovasc Dis. 2:237–247. 2012.PubMed/NCBI
|
|
12
|
Valinezhad Orang A, Safaralizadeh R and
Kazemzadeh-Bavili M: Mechanisms of miRNA-mediated gene regulation
from common downregulation to mRNA-specific upregulation. Int J
Genomics. 2014:9706072014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y and Liang C: MicroRNAs: An emerging
player in autophagy. ScienceOpen Res. 2015:14293/S2199–1006.
2015.
|
|
14
|
Bartel DP: Metazoan MicroRNAs. Cell.
173:20–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Frankel LB, Wen J, Lees M, Høyer-Hansen M,
Farkas T, Krogh A, Jäättelä M and Lund AH: microRNA-101 is a potent
inhibitor of autophagy. EMBO J. 30. pp. 4628–4641. 2011, View Article : Google Scholar
|
|
16
|
Valera E, Spencer B, Mott J, Trejo M,
Adame A, Mante M, Rockenstein E, Troncoso JC, Beach TG, Masliah E
and Desplats P: MicroRNA-101 modulates autophagy and
oligodendroglial alpha-synuclein accumulation in multiple system
atrophy. Front Mol Neurosci. 10:3292017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y, An Y, Wang Y, Zhang C, Zhang H,
Huang C, Jiang H, Wang X and Li X: miR-101 inhibits autophagy and
enhances cisplatin-induced apoptosis in hepatocellular carcinoma
cells. Oncol Rep. 29:2019–2024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US); Washington, DC:
1996
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. National
Academies Press (US); Washington, DC: 2011
|
|
20
|
Ji H, Shen X, Gao F, Ke B, Freitas MC,
Uchida Y, Busuttil RW, Zhai Y and Kupiec-Weglinski JW: Programmed
death-1/B7-H1 negative costimulation protects mouse liver against
ischemia and reperfusion injury. Hepatology. 52:1380–1389. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coleman MC, Olivier AK, Jacobus JA,
Mapuskar KA, Mao G, Martin SM, Riley DP, Gius D and Spitz DR:
Superoxide mediates acute liver injury in irradiated mice lacking
sirtuin 3. Antioxid Redox Signal. 20:1423–1435. 2014. View Article : Google Scholar :
|
|
22
|
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ
and Cejalvo D: Neutrophil infiltration as an important factor in
liver ischemia and reperfusion injury. modulating effects of FK506
and cyclosporine. Transplantation. 55:1265–1272. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Vergne I, Roberts E, Elmaoued RA, Tosch V,
Delgado MA, Proikas-Cezanne T, Laporte J and Deretic V: Control of
autophagy initiation by phosphoinositide 3-phosphatase jumpy. EMBO
J. 28:2244–2258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou C, Zhong W, Zhou J, Sheng F, Fang Z,
Wei Y, Chen Y, Deng X, Xia B and Lin J: Monitoring autophagic flux
by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3)
reveals that high-dose rapamycin impairs autophagic flux in cancer
cells. Autophagy. 8:1215–1226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu C, Yu C and Li Y: Current studies on
therapeutic approaches for ischemia/reperfusion injury in steatotic
livers. Hepatol Res. 38:851–859. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li CX, Ng KT, Shao Y, Liu XB, Ling CC, Ma
YY, Geng W, Qi X, Cheng Q, Chung SK, et al: The inhibition of
aldose reductase attenuates hepatic ischemia-reperfusion injury
through reducing inflammatory response. Ann Surg. 260:317–328.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohsumi Y: Historical landmarks of
autophagy research. Cell Res. 24:9–23. 2014. View Article : Google Scholar :
|
|
29
|
Ohsumi Y: Molecular dissection of
autophagy: Two ubiquitin-like systems. Nat Rev Mol Cell Biol.
2:211–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weidberg H, Shpilka T, Shvets E, Abada A,
Shimron F and Elazar Z: LC3 and GATE-16 N termini mediate membrane
fusion processes required for autophagosome biogenesis. Dev Cell.
20:444–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Zhang J, Wang Z, Wang T, Yu Y, He J,
Zhang H, Yang T and Shen Z: MicroRNA-17 regulates autophagy to
promote hepatic ischemia/reperfusion injury via suppression of
signal transductions and activation of transcription-3 expression.
Liver Transpl. 22:1697–1709. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schneider JL and Cuervo AM: Liver
autophagy: Much more than just taking out the trash. Nat Rev
Gastroenterol Hepatol. 11:187–200. 2014. View Article : Google Scholar :
|
|
33
|
Shin CS and Huh WK: Bidirectional
regulation between TORC1 and autophagy in saccharomyces cerevisiae.
Autophagy. 7:854–862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu A, Huang L, Guo E, Li R, Yang J, Li A,
Yang Y, Liu S, Hu J, Jiang X, et al: Baicalein pretreatment reduces
liver ischemia/reperfusion injury via induction of autophagy in
rats. Sci Rep. 6:250422016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan L, Jiang W, Lu A, Cai H and Kong L:
miR-155 aggravates liver ischemia/reperfusion injury by suppressing
SOCS1 in mice. Transplant Proc. 50:3831–3839. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao Q, Ye QF, Wang W, Fu BQ, Xia ZP, Liu
ZZ, Zhang XJ and Wang YF: Mild hypothermia pretreatment protects
hepatocytes against ischemia reperfusion injury via down-regulating
miR-122 and IGF-1R/AKT pathway. Cryobiology. 75:100–105. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang W, Chen J, Meng Y, Chen Z and Yang J:
Novel targets for treating ischemia-reperfusion injury in the
liver. Int J Mol Sci. 19:E13022018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li SP, He JD, Wang Z, Yu Y, Fu SY, Zhang
HM, Zhang JJ and Shen ZY: miR-30b inhibits autophagy to alleviate
hepatic ischemia-reperfusion injury via decreasing the Atg12-Atg5
conjugate. World J Gastroenterol. 22:4501–4514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim HJ, Joe Y, Yu JK, Chen Y, Jeong SO,
Mani N, Cho GJ, Pae HO, Ryter SW and Chung HT: Carbon monoxide
protects against hepatic ischemia/reperfusion injury by modulating
the miR-34a/SIRT1 pathway. Biochim Biophys Acta. 1852:1550–1559.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang G, Yao J, Li Z, Zu G, Feng D, Shan W,
Li Y, Hu Y, Zhao Y and Tian X: miR-34a-5p inhibition alleviates
intestinal ischemia/reperfusion-induced reactive oxygen species
accumulation and apoptosis via activation of SIRT1 signaling.
Antioxid Redox Signal. 24:961–973. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vasudevan S, Tong Y and Steitz JA:
Switching from repression to activation: microRNAs can up-regulate
translation. Science. 318:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carthew RW and Sontheimer EJ: Origins and
Mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siomi H and Siomi MC: On the road to
reading the RNA-interference code. Nature. 457:396–404. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin CC, Liu LZ, Addison JB, Wonderlin WF,
Ivanov AV and Ruppert JM: A KLF4-miRNA-206 autoregulatory feedback
loop can promote or inhibit protein translation depending upon cell
context. Mol Cell Biol. 31:2513–2527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vasudevan S and Steitz JA:
AU-rich-element-mediated upregulation of translation by FXR1 and
argonaute 2. Cell. 128:1105–1118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moshiri F, Salvi A, Gramantieri L,
Sangiovanni A, Guerriero P, De Petro G, Bassi C, Lupini L, Sattari
A, Cheung D, et al: Circulating miR-106b-3p miR-101-3p and miR-1246
as diagnostic biomarkers of hepatocellular carcinoma. Oncotarget.
9:15350–15364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu L, Beckebaum S, Iacob S, Wu G, Kaiser
GM, Radtke A, Liu C, Kabar I, Schmidt HH, Zhang X, et al:
MicroRNA-101 inhibits human hepatocellular carcinoma progression
through EZH2 downregulation and increased cytostatic drug
sensitivity. J Hepatol. 60:590–598. 2014. View Article : Google Scholar
|
|
48
|
Zhang S, Wang M, Li Q and Zhu P: MiR-101
reduces cell proliferation and invasion and enhances apoptosis in
endometrial cancer via regulating PI3K/Akt/mTOR. Cancer Biomark.
21:179–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saxton RA and Sabatini DM: mTOR signaling
in growth, metabolism, and disease. Cell. 168:960–976. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Wang S, Gao X, Zhao Y, Li Y, Yang B,
Zhang N and Ma L: Octreotide alleviates autophagy by up-regulation
of MicroRNA-101 in intestinal epithelial cell line caco-2. Cell
Physiol Biochem. 49:1352–1363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cox LS: PCNA tightens its hold on the
nucleus. Cell Cycle. 14:2727–2728. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Melo RM, Martins YS, Luz RK, Rizzo E and
Bazzoli N: PCNA and apoptosis during post-spawning ovarian
remodeling in the teleost oreochromis niloticus. Tissue Cell.
47:541–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ and
Los M: Apoptosis and cancer: Mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nikoonahad Lotfabadi N, Mohseni
Kouchesfahani H, Sheikhha MH and Kalantar SM: In vitro transfection
of anti-tumor miR-101 induces BIM, a pro-apoptotic protein,
expression in acute myeloid leukemia (AML). EXCLI J. 16:1257–1267.
2017.
|
|
55
|
Guertin DA and Sabatini DM: An expanding
role for mTOR in cancer. Trends Mol Med. 11:353–361. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim J, Kim YC, Fang C, Russell RC, Kim JH,
Fan W, Liu R, Zhong Q and Guan KL: Differential regulation of
distinct Vps34 complexes by AMPK in nutrient stress and autophagy.
Cell. 152:290–303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu D, Tang H, Li XY, Deng MF, Wei N, Wang
X, Zhou YF, Wang DQ, Fu P, Wang JZ, et al: Targeting the
HDAC2/HNF-4A/miR-101b/AMPK pathway rescues tauopathy and dendritic
abnormalities in alzheimer's disease. Mol Ther. 25:752–764. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu P, Ye F and Xie X, Li X, Tang H, Li S,
Huang X, Song C, Wei W and Xie X: mir-101-3p is a key regulator of
tumor metabolism in triple negative breast cancer targeting AMPK.
Oncotarget. 7:35188–35198. 2016.PubMed/NCBI
|