Introduction
Lung cancer, a common form of cancer, is the leading
cause of mortality in China (1).
Chemotherapy remains one of the major therapies used to treat
advanced lung cancer. Cisplatin (Cis), one of the most effective
broad-spectrum anticancer drugs, is a first-line chemotherapeutic
drug for the treatment of lung cancer (2). However, Cis resistance seriously
influences the rate of success in the treatment of patients with
lung cancer (3). Therefore, it is
vital to examine less toxic and more effective drugs or
chemotherapy-sensitizing agents to overcome Cis resistance in
advanced lung cancer.
Pristimerin (Pris), a naturally occurring
triterpenoid quinone compound, is extracted from various plant
species of the Celastraceae and Hippocrateaceae families (4). Increasing evidence in previous years
has shown that Pris can act as a traditional medicine and possesses
marked anticancer properties in various cancer cell lines,
including esophageal squamous cell carcinoma cells (5), colorectal cancer cells (6), breast cancer cells (7), prostate cancer cells (8), melanoma cells (9), pancreatic cancer cells (10), ovarian cancer cells (11), glioma cells (12) and lung cancer cells (13). It has been reported that Pris
exerts anticancer activity via different mechanisms, including the
inhibition of nuclear factor (NF)-κB and Akt signaling pathways
(6,14), induction of cell cycle arrest
(15), mitochondrial dysfunction
and caspase activation (16). It
has also been reported that Pris enhances the chemosensitivity to
gemcitabine in pancreatic cancer cells by inhibiting the
gemcitabine-induced activation of NF-κB (17). Furthermore, Xie et al
(18) demonstrated that Pris
enhances the sensitivity of breast cancer cells to adriamycin
through suppressing Akt signaling. However, whether Pris can
enhance the sensitivity of lung cancer cells to Cis, and by what
mechanism this occurs, remain to be elucidated.
The present study aimed to investigate the potential
role of Pris in enhancing the anticancer effect of Cis in A549 and
NCI-H446 cells in vitro and in A549 cell-transplanted nude
mice in vivo. The mechanism underlying the anticancer
effects of Pris on enhancing the sensitivity of lung cancer cells
to Cis was also examined.
Materials and methods
Reagents and antibodies
Pris, Cis and 3-methyladenine (3-MA; an autophagy
inhibitor) were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). LY294002, a phosphatidylinositol-4,5-bisphosphate
3-kinase (PI3K) inhibitor were obtained from MedChem Express
(Monmouth Junction, NJ, USA). The primary antibodies against
microtubule-associated protein 1A/1B-light chain 3 (LC3B; cat. no.
4108; 1:2,000), beclin-1 (cat. no. 3738; 1:1,000), cyclin D1 (cat.
no. 2922; 1:1,000), phosphorylated (p-)AKT (cat. no. 4058;
1:2,000), AKT (cat. no. 9272; 1:3,000), glycogen synthase kinase 3β
(GSK-3β; cat. no. 12456; 1:1,000), p-GSK3β (Ser9; cat. no. 5558;
1:2,000), phosphatase and tensin homolog (PTEN; cat. no. 9559;
1:1,000), β-actin (cat. no. 4967; 1:4,000) and poly (ADP-ribose)
polymerase (PARP; cat. no. 9542; 1:1,000) were purchased Cell
Signaling Technology, Inc. (Danvers, MA, USA). The antibody against
p21 (cat. no. 195720; 1:1,000) was purchased from R&D Systems,
Inc. (Minneapolis, MN, USA). Dulbecco's modified Eagle's medium
(DMEM) and fetal bovine serum (FBS) were purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture and cell transfection
The A549 and NCI-H446 human lung carcinoma cell
lines were purchased from the Cell Bank of Shanghai Institute of
Biochemistry and Cell Biology (Shanghai, China). The cells were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin at 37°C with
5% CO2.
The microRNA (miR)-23a inhibitor (5′-GGA AAU CCC UGG
CAA UGU GAU-3′) and miR-23a negative control (NC, 5′-CAG UAC UUU
UGU GUA GUA CAA-3′) were purchased from Shanghai GenePharma Co.,
Ltd. (Shanghai, China). The A549 and NCI-H446 cells were seeded at
2×105 cells/ml in 6-well plates and transfected with
miR-23a inhibitor and miR-23a-NC using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Cell viability assay
Cell viability was detected using the Cell Counting
kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). The A549 and NCI-H446 cells were seeded into
96-well plates at a density of 1×104 cells/well and
cultured for 24 h. The cells were treated with indicated
concentrations of Pris (0, 10, 20, 40, 60 and 80 µM) and Cis
(0, 0.1, 0.25, 0.5, 1 and 2 µM) for 24 h. A total of 10
µl of CCK-8 solution was then added to each well for an
additional 4 h at 37°C. The absorbance at 450 nm was determined
using an ELISA Reader (Tecan Group Ltd., Männedorf, Switzerland).
This experiment was performed in triplicate.
Cell cycle assay
The A549 and NCI-H446 cells were seeded at
2×105 cells/ml in 6-well plates and treated with Pris
and Cis for 24 h. The cell cycle phase distribution was detected
using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA,
USA) and a Cell Cycle Detection kit (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China). In brief, the cells were trypsinized and
fixed with 75% ethanol overnight at 4°C. Propidium iodide (PI) was
used to stain the DNA of samples for 15 min, and flow cytometry was
used to determine cell cycle stage. All experiments were performed
at least three times. Data was analyzed with ModFit LT 3.0 (Verity
Software House, Topsham, ME, USA).
Cell apoptosis assay
The A549 and NCI-H446 cells were seeded at
2×105 cells/ml in 6-well plates and treated with Pris
and Cis for 24 h. Cell apoptosis was assessed using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(Nanjing KeyGen Biotech Co., Ltd.). Briefly, the cells were stained
with Annexin V-FITC and PI for 15 min at room temperature in the
dark. The apoptotic cells were then detected on a FACScalibur flow
cytometer (BD Biosciences) and analyzed with FlowJo 7.6 software
(FlowJo LLC, Ashland, OR, USA). Three independent experiments were
performed.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the A549 and NCI-H446
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA (2 µg) from the cell samples was reverse
transcribed using the Mir-X™ miRNA First-Strand Synthesis kit
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. The expression of miR-23a and U6 (Takara Bio, Inc.) was
determined using Power SYBR Green PCR Master mix (2X; Applied
Biosystems; Thermo Fisher Scientific, Inc.) on an ABI 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The forward
primer of miR-23a was used (5′-ATC ACA TTG CCA GGG ATT TCC-3′). The
primers of U6 were obtained from the Mir-X™ miRNA First-Strand
Synthesis kit, and U6 was used as a control for normalization. The
thermocycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 10
sec. The relative level of miR-23a was calculated using the
2−ΔΔCq method (19).
In vivo xenograft tumor model
Male BALB/c nude mice (8 weeks old; 18-20 g) were
obtained from the Animal Experiment Center of Xi'an Jiaotong
University (Xi'an, China). All mice were housed in a specific
pathogen free (SPF) animal at 20-26°C and 40-70% humidity with a 12
h light/dark cycle. Food and water were available ad
libitum. The experiments were approved by the Laboratory Animal
Care Committee of Xi'an Jiaotong University (approval no.
XJTULAC2018-527). The xenograft tumor model was performed as
previously described (20-22).
Briefly, A549 cells (5×106 cells/ml in 0.2 ml) were
subcutaneously injected into the right flanks of BALB/c nude mice.
The mice were divided into four groups (n=3 per group): Saline
control group, Pris (0.8 mg/kg) treatment group, Cis (2 mg/kg)
treatment group, and combined Pris + Cis treatment group. The
xenograft tumors were developed for 14 days post-injection.
Following this, the nude mice were treated with Pris (0.8 mg/kg)
and Cis (2 mg/kg) for 14 days. Tumor volume was calculated as
follows: Tumor volume (mm3) = long diameter of the tumor
× short diameter of the tumor2/2. On the last day of the
experiment (day 28), the tumor samples were collected and weighed.
Hematoxylin and eosin (H&E) staining and terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assays were used to examine morphology and cell apoptosis
in the xenografted lung tumors. Images were captured using a
Olympus BX53 microscope (Olympus Corporation, Tokyo, Japan).
Western blotting
Following treatment with Pris and Cis, the cells
were lysed in lysis buffer for western blot detection, as described
previously (23). Briefly, the
cell samples were lysed on ice with radioimmunoprecipitation assay
buffer containing protease inhibitors and the proteins were
quantified with Bicinchoninic Acid Protein Assay kit (Thermo Fisher
Scientific, Inc.). Proteins (40 µg/well) were separated on
6-12% gels using SDS-PAGE and protein was transferred onto
nitrocellulose membranes (Pall Life Sciences, Port Washington, NY,
USA) and the membranes were blocked with 5% nonfat milk for 2 h,
followed by incubation with anti-cyclin D1, anti-P21, anti-beclin1,
anti-LC3B, anti-p-AKT anti-AKT, anti-PTEN, anti-p-GSK3β,
anti-GSK-3β, anti-PARP and anti-β-actin antibodies at 4°C
overnight. Membranes were then incubated with horseradish
peroxidase (HRP)-conjugated anti-mouse IgG (cat. no. 7076;
1:20,000) and HRP-conjugated anti-rabbit IgG (cat. no. 7074;
1:20,000; both Cell Signaling Technology, Inc.) secondary
antibodies for 2 h at 25°C. The proteins were visualized using
SuperSignal West Pico Chemiluminescent Substrate (Pierce; Thermo
Fisher Scientific, Inc.) and exposed to X-ray film. Densitometry
was performed using ImageJ version 1.38× software (National
Institutes of Health, Bethesda, MD, USA) and the resulting data
analyzed using GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Data were analyzed using GraphPad Prism 6.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Differences between groups were
determined by one-way analysis of variance followed by Dunnett's or
Tukey's post hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
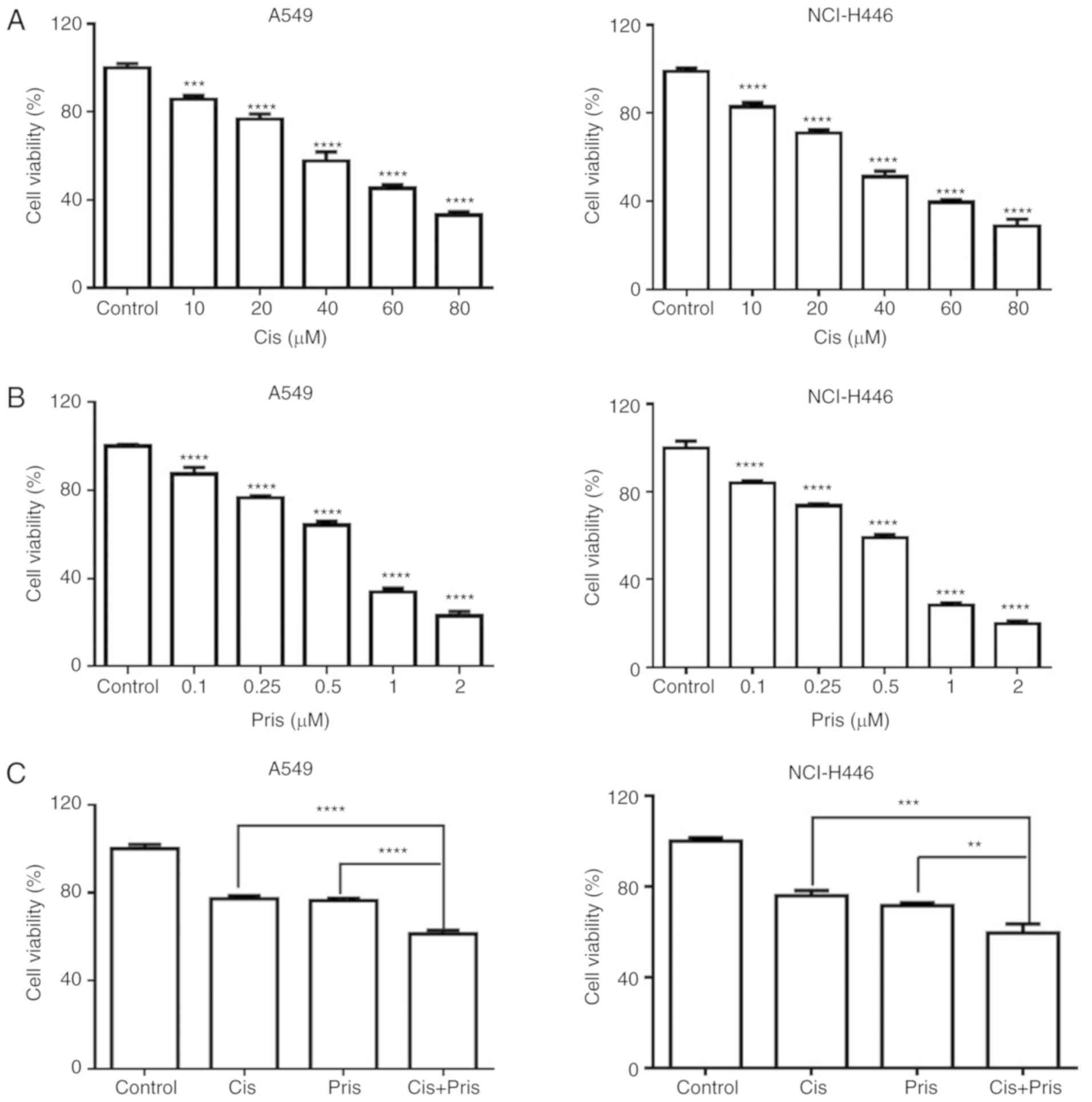
Pris and Cis inhibit cell growth in A549
and NCI-H446 cells
To investigate the effects of Pris and Cis on cell
proliferation, the A549 and NCI-H446 cells were exposed to
different concentrations of Pris or Cis for 24 h, and cell
viability was investigated using a CCK-8 assay. As shown in
Fig. 1A and B, Pris or Cis
significantly inhibited the growth of A549 and NCI-H446 cells in a
concentration-dependent manner. According to these results,
experiments were performed to investigate whether the combined
treatment of Pris + Cis significantly promoted the inhibition of
A549 and NCI-H446 cell viability in comparison with Pris or Cis
treatment alone. As shown in Fig.
1C, the combination treatment significantly enhanced the
inhibitory effect on cell viability compared with Pris or Cis
treatment alone in the A549 and NCI-H446 cell lines. These results
indicated that Pris may significantly increase the sensitivity to
Cis by inhibiting cell viability in A549 and NCI-H446 cells.
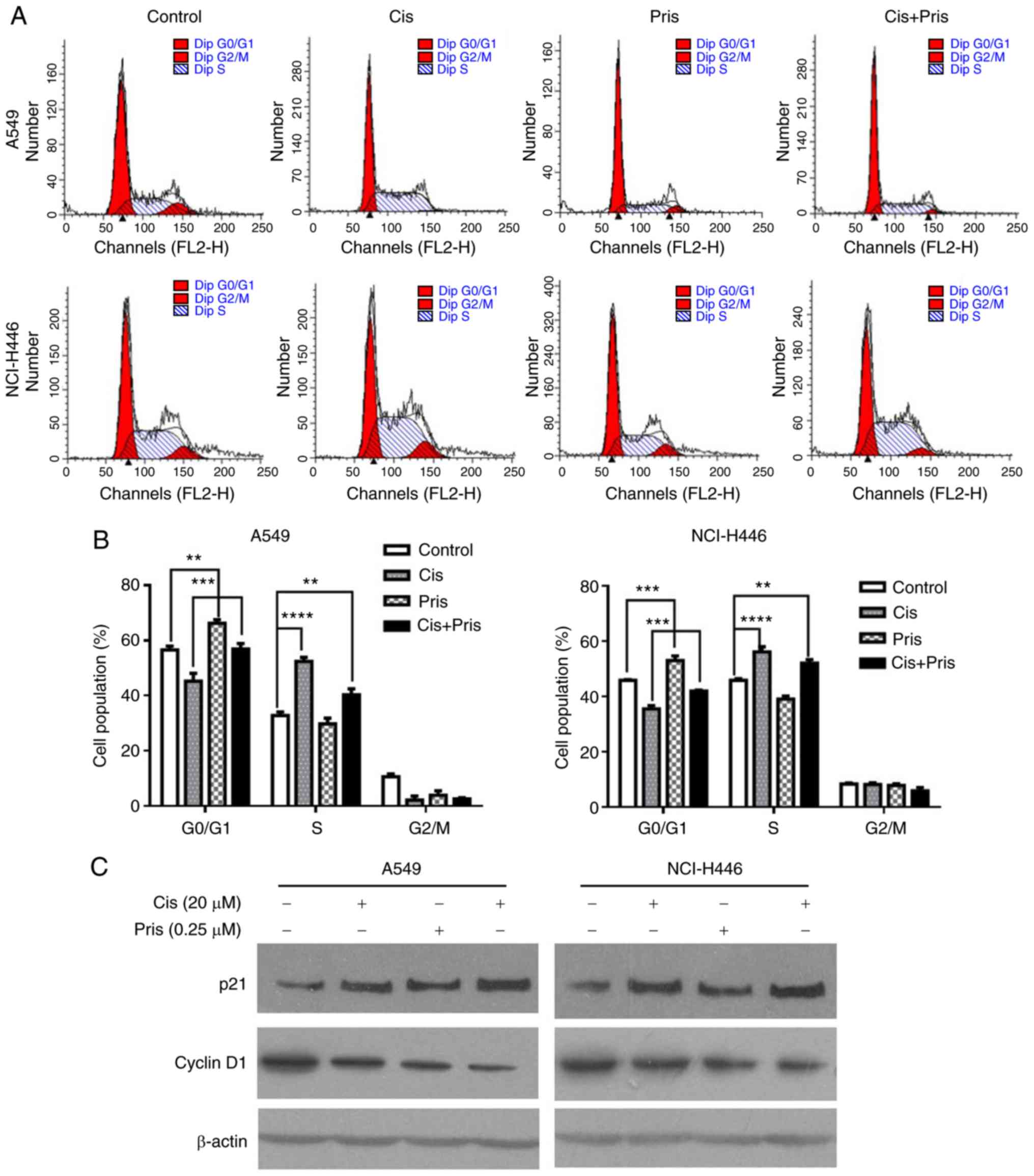
Pris and Cis induce cell cycle arrest in
A549 and NCI-H446 cells
It is well known that cell cycle arrest may lead to
cell growth inhibition. Therefore, the effect of Pris and Cis on
cell cycle arrest in A549 and NCI-H446 cells was detected using
flow cytometry. As shown in Fig. 2A
and B, the percentage of cells at G0/G1
phase significantly increased with Pris treatment and the
percentage of cells at S phase significantly increased with Cis
treatment in A549 and NCI-H446 cells. Furthermore, it was observed
that the combination treatment significantly elevated the
percentage of cells in the G0/G1 phase
compared with Cis treatment alone in the A549 and NCI-H446 cells
(Fig. 2A and B). These data
indicated that G0/G1 phase arrest may
contribute to enhancing cell growth inhibition induced by Cis.
The G0/G1-related proteins
were examined by western blotting. As shown in Fig. 2C, Pris or Cis treatment alone
markedly upregulated the expression level of p21 but downregulated
the expression of cyclin D1 compared with the control group. The
combination treatment markedly upregulated the expression level of
p21 but downregulated the expression of cyclin D1 compared with
either Pris or Cis treatment alone in A549 and NCI-H446 cells.
These results indicated that Pris increased the antiproliferative
effect of Cis in A549 and NCI-H446 cells via upregulating p21 and
downregulating cyclin D1.
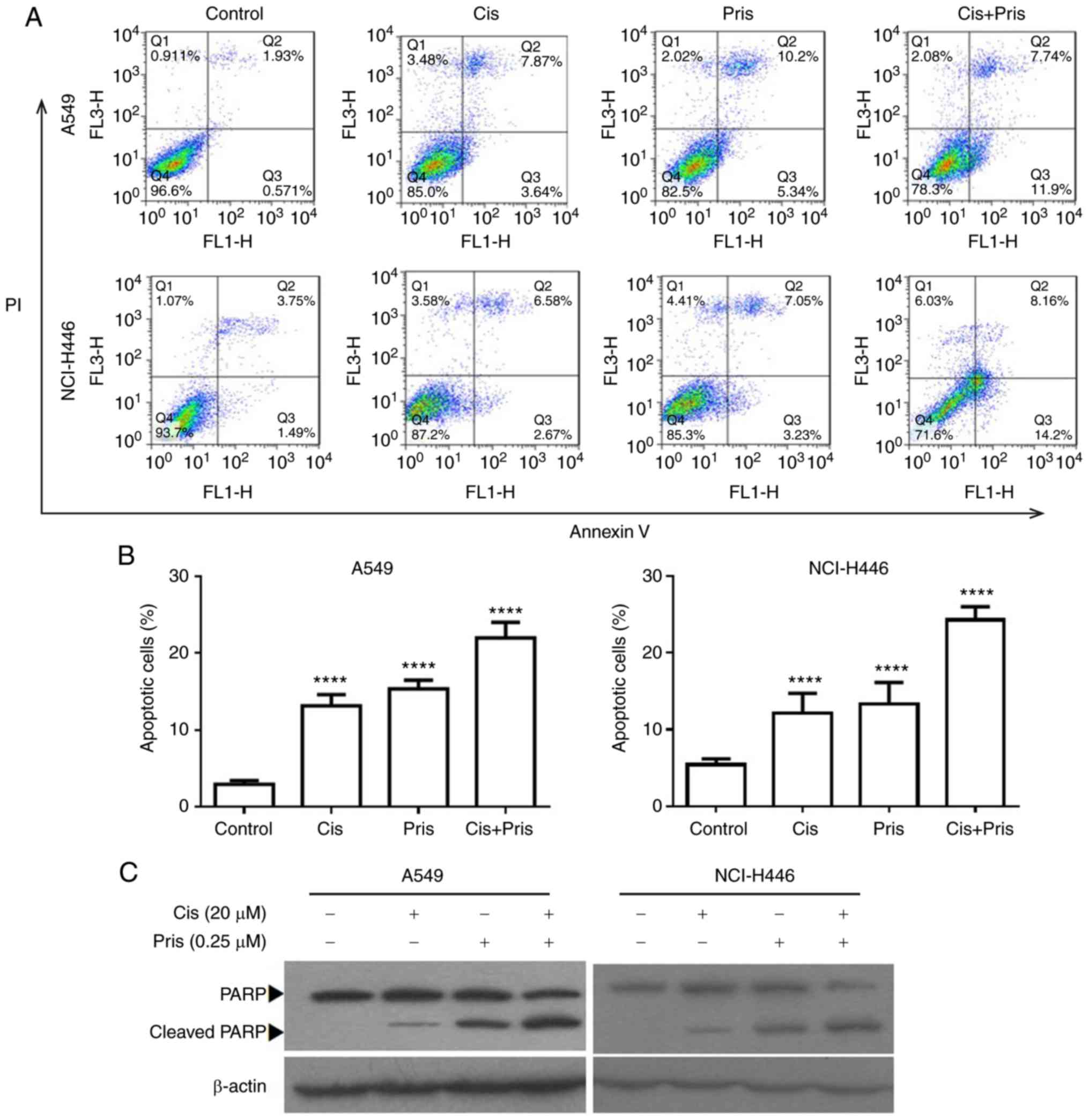
Pris and Cis induce cell apoptosis in
A549 and NCI-H446 cells
To further evaluate the effects of Pris and Cis on
cell growth inhibition, cell apoptosis was detected in A549 and
NCI-H446 cells using flow cytometry. As shown in Fig. 3A and B, Pris, Cis and the
combination treatment significantly induced cell apoptosis in the
A549 and NCI-H446 cells. Combination treatment with Pris and Cis
significantly increased the number of apoptotic cells compared with
either drug alone in the A549 and NCI-H446 cells (Fig. 3A and B). To further evaluate the
effect of apoptosis induced by Pris and Cis, the apoptosis-related
protein PARP was analyzed using western blotting. As shown in
Fig. 3C, Pris, Cis and the
combination treatment markedly upregulated the expression level of
cleaved PARP. Therefore, these results showed that Pris markedly
enhanced Cis-induced apoptosis in the A549 and NCI-H446 cells.
Pris enhances Cis-induced apoptosis by
suppressing the Akt/GSK3β signaling pathway in A549 and NCI-H446
cells
To further elucidate the molecular mechanism of Pris
and Cis-induced cell apoptosis in A549 and NCI-H446 cells, the
expression of proteins related to the Akt/GSK3 signaling pathway
were detected in A549 and NCI-H446 cells using western blotting. As
shown in Fig. 4A, the levels of
p-Akt and p-GSK3β were markedly inhibited by Pris, Cis and the
combination treatments. Furthermore, the combination treatment of
Pris + Cis markedly inhibited the phosphorylation of Akt and GSK3β
compared with either drug alone in the A549 and NCI-H446 cells
(Fig. 4A). LY294002, a type of
PI3K inhibitor, markedly inhibited the levels of p-GSK3β and p-Akt
in A549 and NCI-H446 cells (Fig.
4B). In addition, as shown in Fig. 4C, LY294002 in combination with Cis
enhanced the inhibitory effect on cell viability compared with Cis
alone in the A549 and NCI-H446 cell lines. These results indicated
that Pris enhanced the sensitivity of A549 and NCI-H446 cells to
Cis via suppressing Akt/GSK3β signaling.
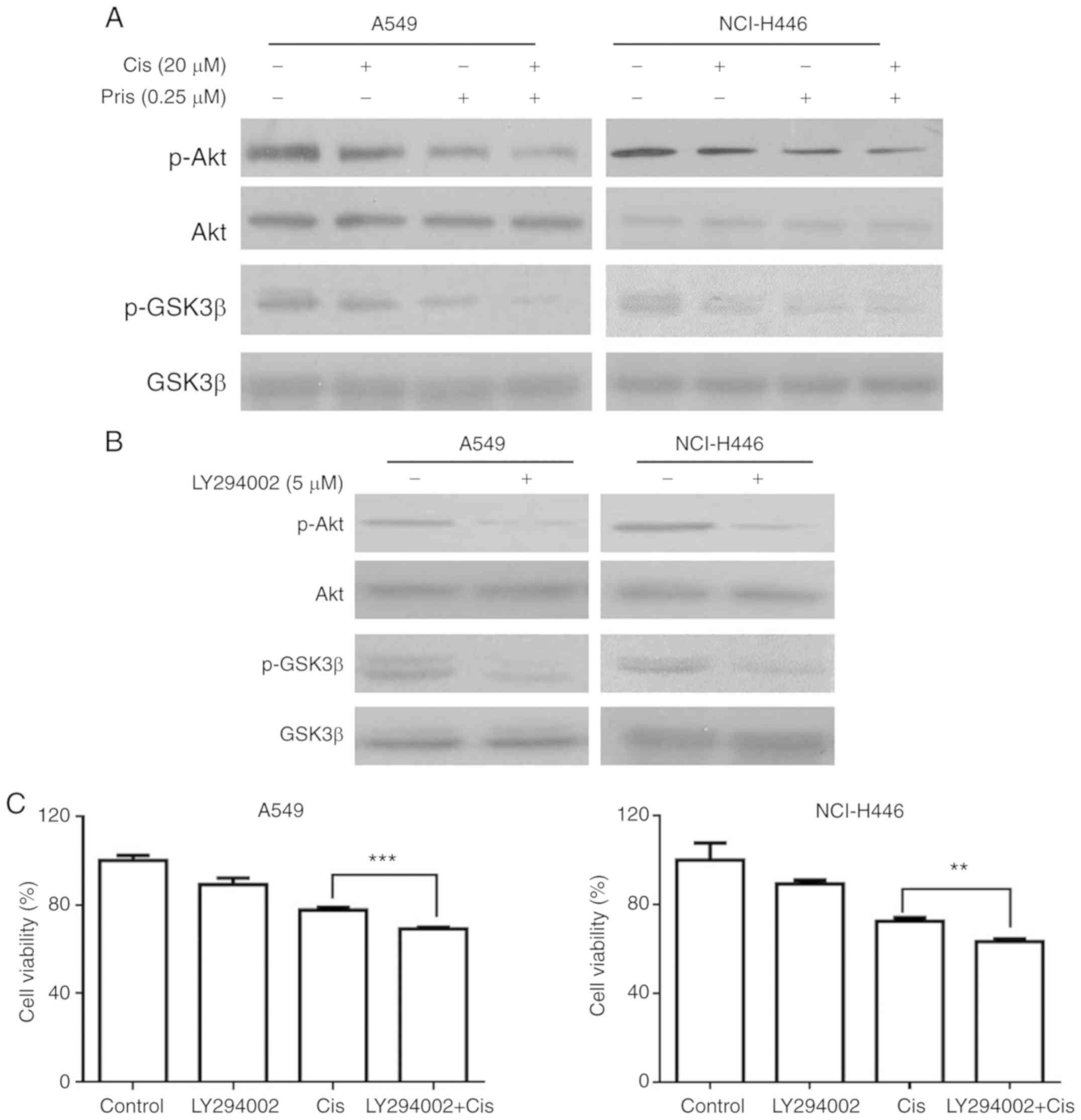 | Figure 4Pris enhances the apoptosis of
Cis-induced lung cancer cells via the inhibition of Akt/GSK3β
signaling. (A) A549 and NCI-H446 cells treated with Pris (0.25
µM) and/or Cis (20 µM) for 12 h. Western blotting was
used to detect the protein expression levels of p-Akt, Akt, p-GSK3β
and GSK3β. (B) A549 and NCI-H446 cells were treated with LY294002
(5 µM) for 12 h. The protein expression of p-Akt, Akt,
p-GSK3β and GSK3β were analyzed by western blotting. (C) A549 and
NCI-H446 cells were pretreated with LY294002 (5 µM) for 1 h,
followed by treatment with Pris (0.25 µM) and/or Cis (20
µm) for 24 h. Cell viability was measured using a Cell
Counting kit-8 assay. **P<0.01,
***P<0.001. Cis, cisplatin; Pris, pristimerin; GSK,
glycogen synthase kinase 3β; p-, phosphorylated. |
Pris enhances Cis-induced apoptosis
through the downregulation of miR-23a in A549 and NCI-H446
cells
miR-23a has been indicated to be as an important
regulator of PTEN/Akt signaling (24,25). Therefore, the present study
investigated whether miR-23a contributed to the sensitivity of A549
and NCI-H446 cells to Cis via the PTEN/Akt signaling pathway. As
shown in Fig. 5A, Pris
significantly downregulated the expression level of miR-23a in the
A549 and NCI-H446 cells. To further examine the effect of miR-23a
on the PTEN/Akt signaling pathway, the protein expression of GSK3β,
Akt and PTEN in A549 and NCI-H446 cells were examined using western
blotting. As shown in Fig. 5B,
the miR-23a inhibitor markedly upregulated the expression level of
PTEN and downregulated the phosphorylation of Akt and GSK3β
compared with that in the blank control cells. In addition, the
results of the flow cytometry revealed that combination treatment
with miR-23a inhibitor and Cis significantly increased the number
of apoptotic A549 and NCI-H446 cells compared with the Cis
treatment alone (Fig. 5C and D).
These results indicated that Pris enhanced the sensitivity of A549
and NCI-H446 cells to Cis via suppressing the miR-23a/PTEN/Akt
signaling pathway.
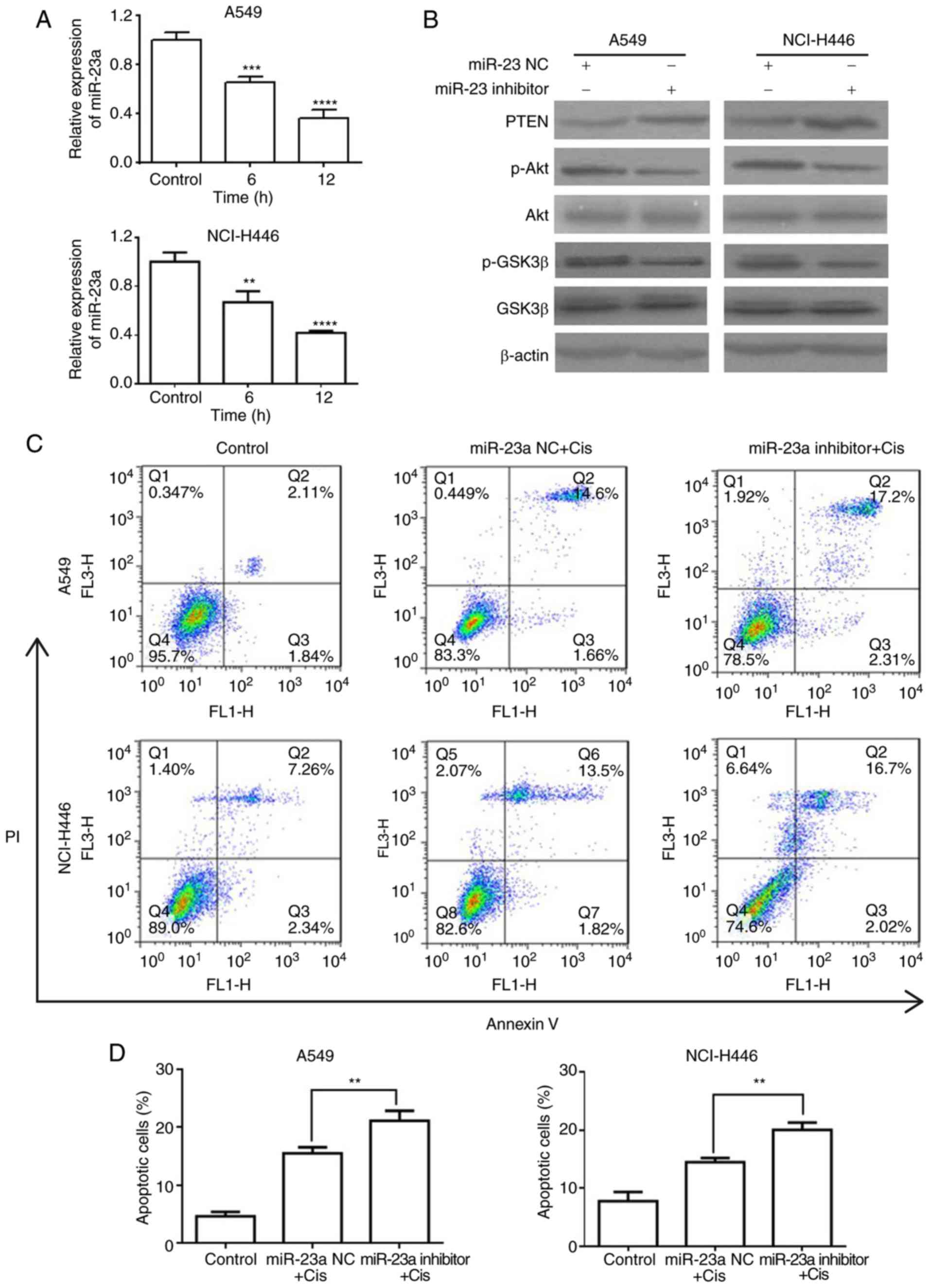 | Figure 5Pris enhances the apoptosis of
Cis-induced lung cancer cells via the downregulation of miR-23a.
(A) A549 and NCI-H446 cells were treated with Pris (0.25 µM)
for 0, 6 and 12 h. Relative expression levels of miR-23a were
measured using reverse transcription-quantitative polymerase chain
reaction analysis. Data are presented as the mean ± standard
deviation of three independent experiments. **P<0.01,
***P<0.001, ****P<0.0001 vs. control.
(B) A549 and NCI-H446 cells were transfected with miR-23a NC or
inhibitor for 48 h. Western blotting was used to detect the protein
expression of PTEN, p-Akt, Akt, p-GSK3β and GSK3β. β-actin served
as a loading control. (C) A549 and NCI-H446 cells were transfected
with miR-23a NC or inhibitor for 48 h, followed by treatment with
or without Cis (20 µM) for 24 h. Cell apoptosis was analyzed
using flow cytometry. (D) Graphs showing results of flow cytometry.
Data are presented as the mean ± standard deviation of three
independent experiments. **P<0.01. Cis, cisplatin;
Pris, pristimerin; PTEN, phosphatase and tensin homolog; miR,
microRNA; NC, negative control; GSK, glycogen synthase kinase 3β;
p-, phosphorylated; PI, propidium iodide. |
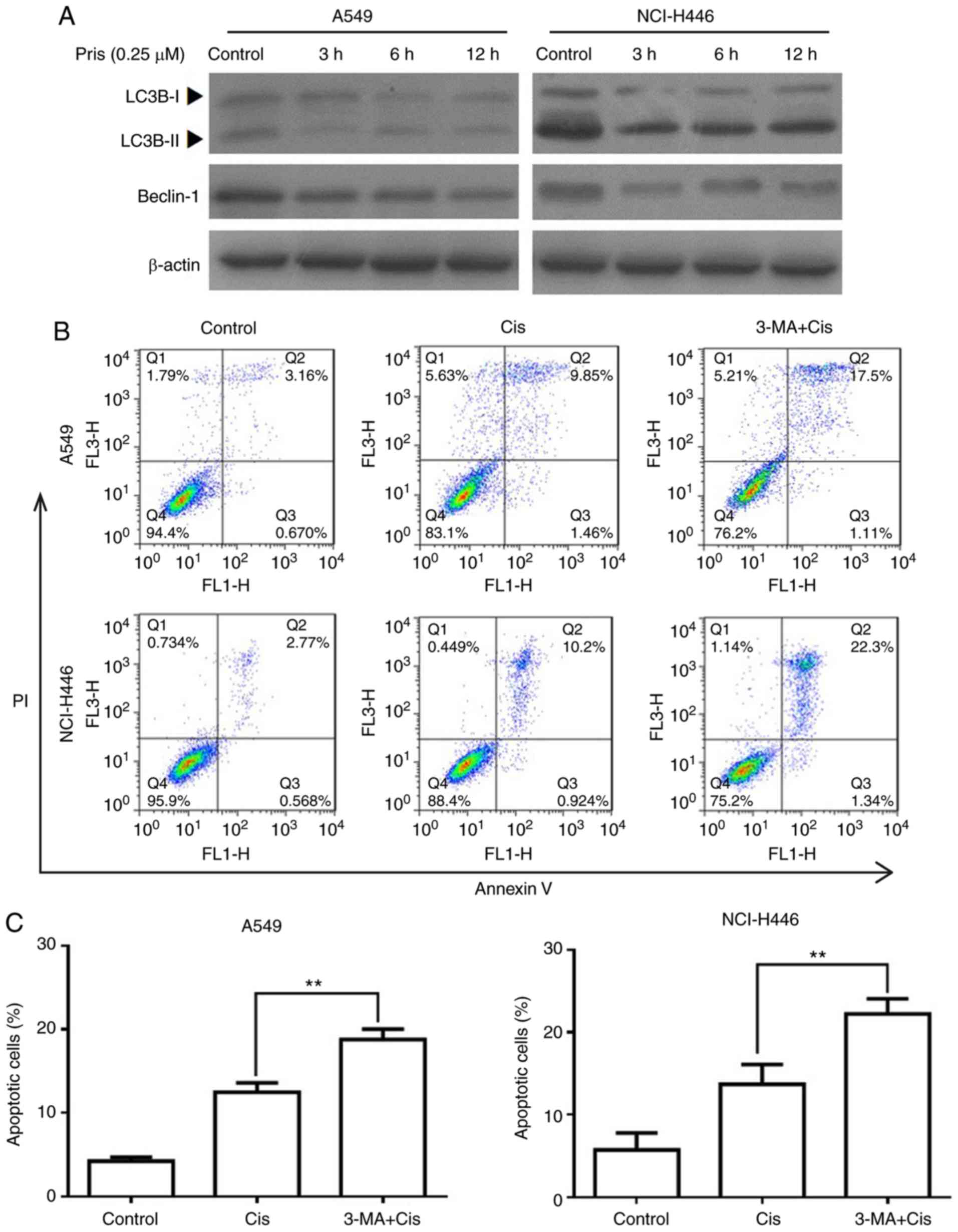
Pris enhances Cis-induced apoptosis via
suppressing autophagy in A549 and NCI-H446 cells
Numerous studies have demonstrated that autophagy is
associated with the regulation of cell apoptosis induced by
antitumor drugs (26-28). To evaluate whether autophagy is
involved in increasing the apoptosis of Cis-treated A549 and
NCI-H446 cells, the protein expression levels of LC3B and beclin-1
were detected by western blotting. As shown in Fig. 6A, the expression levels of LC3BII
and beclin-1 were markedly downregulated by Pris treatment with the
blank control cells. In addition, flow cytometry revealed that
combination treatment with 3-MA (an autophagy inhibitor) and Cis
significantly increased the number of apoptotic cells compared with
Cis treatment alone in the A549 and NCI-H446 cells (Fig. 6B and C). These results indicated
that Pris enhanced the sensitivity of A549 and NCI-H446 cells to
Cis via suppressing autophagy.
Pris enhances the anticancer activity of
Cis in vivo
To evaluate the synergistic effect of Pris and Cis,
an in vivo xenograft model was established. A549 cells were
injected into BALB/c nude mice. The xenograft tumors were developed
for 14 days post-injection and the nude mice were then treated with
Pris (0.8 mg/kg) and Cis (2 mg/kg) for a further 14 days. As shown
in Fig. 7A-D, the tumor volumes
and weights in the Pris treatment group, Cis treatment group and
combination treatment group were lower compared with those in the
control group. Furthermore, combination treatment significantly
attenuated tumor volume and weight compared with either drug alone.
However, no significant changes in body weight were observed among
the four experimental groups (Fig.
7E). The H&E staining and TUNEL analysis showed that
apoptotic cells in the tumor tissues were markedly increased
following Pris and Cis combination treatment compared with
treatment with either drug alone (Fig. 7F). In addition, western blotting
revealed that combination treatment with Pris and Cis markedly
inhibited the phosphorylation of Akt and GSK3β compared with
treatment with either drug alone in A549 tumor tissues (Fig. 7G-I). Taken together, the results
suggested that Pris and Cis acted synergistically against lung
cancer in vivo.
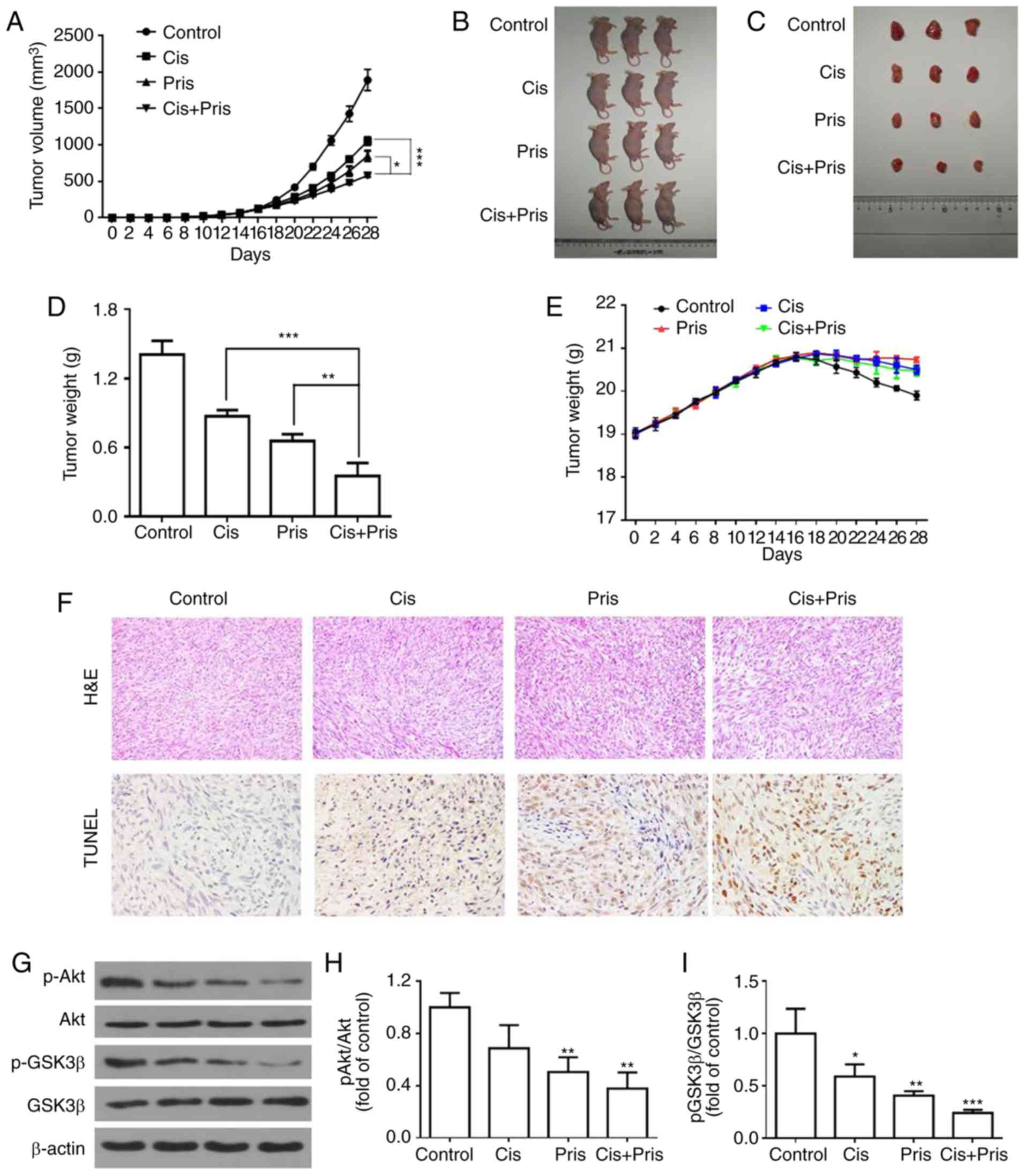 | Figure 7Pris combined with Cis inhibits lung
cancer xenograft growth in vivo. (A) Tumor volumes in
the Pris-, Cis- and combination-treated BALB/c nude mice were
measured every 2 days for 28 days. (B) Mice and (C) tumors of the
tumor xenograft models of the four groups. A549 cells were injected
into BALB/c nude mice. At 14 days post-tumor injection, nude mice
were treated with Pris (0.8 mg/kg) and Cis (2 mg/kg) for 14 days.
(D) Tumor weights for each group were measured on the last day of
the experiment (day 28). (E) Body weights of the Pris-, Cis- and
combination-treated BALB/c nude mice were measured once every 2
days for 28 days. (F) Tumor samples of each group were subjected to
H&E staining (magnification, ×200) and TUNEL assays
(magnification, ×400) on the last day of the experiment (day 28).
(G) Western blotting was used to examine the effect of Pris and/or
Cis on the protein expression levels of (H) p-Akt and Akt, and (I)
p-GSK3β and GSK3 β in the tumor samples (day 28). Data are
presented as the mean ± standard deviation (n=3).
*P<0.05, **P<0.01,
***P<0.001 vs. control. Cis, cisplatin; Pris,
pristimerin; H&E, hematoxylin and eosin; TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling; GSK,
glycogen synthase kinase 3β; p-, phosphorylated. |
Discussion
Lung cancer is one of the most common malignancies
worldwide, and mortality rates in China are the highest globally.
Although Cis is a broad-spectrum anticancer drug that is often used
in the treatment of lung cancer, chemoresistance critically limits
the efficacy of treatment. Therefore, the development of novel
anticancer drugs to improve the chemosensitivity of lung cancer
cells to Cis has become a necessity. In the present study, the role
of Pris in the sensitization of A549 and NCI-H446 cells to
Cis-induced cell death was investigated. The results showed that
A549 and NCI-H446 cells treated with a combination of Pris and Cis
enhanced cell growth inhibition and cell apoptosis compared with
cells treated with either drug alone and untreated cells. It was
further validated that Pris enhanced tumor growth inhibition and
cell apoptosis in combination with Cis in an in vivo
xenograft model, which was consistent with the findings in
vitro. In addition, it was found that combination treatment
significantly induced G0/G1 phase arrest
compared with Cis treatment alone. It was also demonstrated that
Pris enhanced the sensitivity of A549 and NCI-H446 cells to Cis
through suppressing autophagy and inhibiting miR-23a/Akt/GSK3β
signaling.
Numerous studies have shown that cell cycle arrest
induced by anticancer drugs is an effective strategy for inhibiting
cancer cell proliferation (29,30). A previous study showed that Cis
may inhibit cell proliferation via triggering S phase arrest in
A549 cells (31,32). Pris, a potential anticancer drug,
has been reported to enhance the chemosensitivity of pancreatic
cancer cells to gemcitabine via inducing
G0/G1 phase arrest (17). Yousef et al (15) also reported that Pris exerted
anticancer activity in colorectal cancer cells by inducing
G0/G1 phase arrest. The results of the
present study demonstrated that Pris or Cis significantly induced
G0/G1 phase arrest or S phase arrest in A549
and NCI-H446 cells. Compared with Cis alone, the combination
treatment of Pris and Cis significantly increased
G0/G1 phase arrest in the A549 and NCI-H446
cells. Notably, the cell cycle is regulated by multiple molecular
processes, including cyclin-dependent kinase (CDK)-regulated
processes. Previous results have demonstrated that a reduction in
the protein expression of cyclin D1 may inhibit the
G0/G1 to S phase transition (33,34). Additionally, it has been reported
that p21, a crucial CDK inhibitor, may promote
G0/G1 phase arrest by downregulating the
expression of CDK complexes (35,36). In the present study, it was found
that Pris treatment alone markedly upregulated the expression level
of p21 but downregulated the expression of cyclin D1 compared with
the control group. Furthermore, combination treatment markedly
upregulated the expression level of p21 but downregulated the
expression of cyclin D1 compared with Cis treatment alone in the
A549 and NCI-H446 cell lines. These data suggested that the
downregulation of cyclin D1 and upregulation of p21 may be
potential mechanisms that contributes to Pris enhancing Cis-induced
cell growth inhibition in A549 and NCI-H446 cells.
Anticancer drug-induced apoptosis has been reported
as an effective strategy in anticancer therapy (37). Cis is a broad-spectrum anticancer
drug that can induce cell apoptosis in a variety of cancer cells.
Furthermore, increasing evidence has demonstrated that Pris
can induce the apoptosis of cells in various types of cancer,
including breast cancer (7),
colorectal cancer (15),
pancreatic cancer (17) and
prostate cancer (38). In the
present study, it was observed that Pris, Cis and combination
treatments significantly induced the apoptosis of A549 and NCI-H446
cells and in the in vivo xenograft model. The combination
treatment of Pris and Cis significantly increased the number of
apoptotic cells compared with either drug alone in vitro and
in vivo. The results of the western blotting also showed
that the Pris, Cis and the combination treatment markedly
upregulated the expression level of cleaved PARP in the A549 and
NCI-H446 cells. Combination treatment with Pris and Cis markedly
increased the expression of cleaved PARP compared with either drug
alone in A549 and NCI-H446 cells. These results indicated that the
upregulation of cleaved PARP contributed to Pris enhancing
Cis-induced cell apoptosis.
Cell apoptosis is induced by multiple signaling
pathways, including the Akt signaling pathway which has a central
role in cell apoptosis. Therefore, anticancer drugs often induce
cancer apoptosis through inhibiting the AKT signaling pathway
(39,40). It has been reported that Pris is a
potential anticancer drug that can induce apoptosis in pancreatic
cancer cells and colorectal cancer cells (15,41). Bi et al (42) reported that metformin
synergistically enhances Cis-induced apoptosis via increasing the
inhibition of Akt activity mediated by cisplatin. Liao et al
(43) also revealed that matrine
enhances the pro-apoptotic ability of Cis in urothelial bladder
cancer cells through increasing the inhibition of Akt activity
mediated by Cis (43). In the
present study, Pris, Cis and the combination treatment markedly
inhibited the phosphorylation of Akt, and the combination treatment
markedly inhibited the phosphorylation of Akt compared with either
drug alone. To further evaluate whether the Akt signaling pathway
is involved in enhancing Cis-induced apoptosis, the A549 and
NCI-H446 cells were treated with LY294002 and Cis. The effect of
Cis combined with LY294002 on the viability of A549 and NCI-H446
cells was similar to that of Pris combined with Cis. These results
confirmed that Pris enhanced Cis-induced apoptosis through
inhibiting the AKT signaling pathways.
GSK3β is an important downstream target of AKT
involved in regulating cell apoptosis (44,45). It has been reported that Akt can
inactivate GSK3β via the phosphorylation of Ser9 (45,46). Therefore, the present study
detected the phosphorylation of GSK3β at Ser 9 in A549 and NCI-H446
cells. Pris, Cis and the combination treatment mediated the
dephosphorylation of GSK3β. In addition, the combination treatment
markedly inhibited the phosphorylation of GSK3β at Ser 9 compared
with either drug alone. Notably, treatment of the cells with
LY294002 alone markedly attenuated the phosphorylation of GSK3β at
Ser 9. It was also observed that the combination treatment of Pris
and Cis markedly inhibited the phosphorylation of Akt and GSK3β
compared with either drug alone in vivo. Therefore, these
results indicated that Pris may enhance Cis-induced apoptosis
through inhibiting AKT/GSK3β signaling. Additional studies have
demonstrated that miRs are associated with apoptosis in a variety
of cancer cells (47-49). In the present study, it was
observed that Pris significantly downregulated the expression level
of miR-23a in A549 and NCI-H446 cells. Han et al (24) reported that the inhibition of
miR-23a enhanced erlotinib-mediated lung cancer stem cell apoptosis
through the PTEN/PI3K/AKT signaling (24). To confirm the association between
miR-23a and PTEN/AKT signaling, western blotting was performed in
the present study and the results demonstrated that the
downregulation of miR-23a markedly increased the expression of
PTEN, but decreased the expression of p-AKT. In addition, the
effect of Cis combined with the miR-23a inhibitor on the apoptosis
of A549 and NCI-H446 cells was similar to that of Pris combined
with Cis. Taken together, these data indicated that Pris enhanced
the pro-apoptotic ability of Cis in A549 and NCI-H446 cells through
inhibiting miR-23a/PTEN/Akt signaling.
Autophagy is often considered as type II-programmed
cell death, which has a dual role in regulating the homeostasis of
cells (50,51). Increasing evidence suggests that
autophagy induced by anticancer drugs can promote cell survival or
autophagic cell death in various types of cancer (52-54). In the present study, the results
demonstrated Pris markedly inhibited autophagy through
downregulating the expression levels of LC3BII and beclin-1 in A549
and NCI-H446 cells. To confirm whether autophagy was involved in
cell apoptosis, the A549 and NCI-H446 cells were treated with 3-MA
and Cis. The results showed that inhibiting autophagy significantly
enhanced Cis-mediated cell apoptosis. These results indicated that
Pris enhanced Cis-induced apoptosis in A549 and NCI-H446 cells
through inhibiting autophagy.
In conclusion, the results of the present study
showed that the combination of Cis and Pris synergistically
inhibited cell proliferation, induced cell cycle arrest and
promoted apoptosis in A549 and NCI-H446 cells. The findings
indicated that Pris enhanced the sensitivity of A549 and NCI-H446
cells to Cis through inhibiting miR-23a/Akt/GSK3β signaling and
suppressing autophagy. Therefore, these observations indicate that
the combination of Cis and Pris may be a potential therapeutic
strategy for overcoming lung cancer.
Funding
The present study was supported by grants from the
Shaanxi National Science Foundation (grant no. 2011JM4037), the
Scientific Research Program Funded by Shaanxi Provincial Education
Department (grant no. 16JK1761) and the National Key Research and
Development Program (grant no. 2016YFC0905001).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and JW conducted the experiments and analyzed the
data. LS made substantial contributions to the design of the study
and prepared the manuscript. BH, WS, BL, FS, SC and LC performed
the western blotting and analyzed the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Laboratory
Animal Care Committee of Xi'an Jiaotong University (approval no.
XJTULAC2018-527).
Patient consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Costa PM, Ferreira PM, Bolzani Vda S,
Furlan M, de Freitas Formenton Macedo Dos Santos VA, Corsino J, de
Moraes MO, Costa-Lotufo LV, Montenegro RC and Pessoa C:
Antiproliferative activity of pristimerin isolated from Maytenus
ilicifolia (Celastraceae) in human HL-60 cells. Toxicol In Vitro.
22:854–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tu Y, Tan F, Zhou J and Pan J: Pristimerin
targeting NF-κB pathway inhibits proliferation, migration, and
invasion in esophageal squamous cell carcinoma cells. Cell Biochem
Funct. 36:228–240. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yousef BA, Hassan HM, Zhang LY and Jiang
ZZ: Pristimerin exhibits in vitro and in vivo anticancer activities
through inhibition of nuclear factor-small ka, CyrillicB signaling
pathway in colorectal cancer cells. Phytomedicine. 40:140–147.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cevatemre B, Erkisa M, Aztopal N, Karakas
D, Alper P, Tsimplouli C, Sereti E, Dimas K, Armutak EII, Gurevin
EG, et al: A promising natural product, pristimerin, results in
cytotoxicity against breast cancer stem cells in vitro and
xenografts in vivo through apoptosis and an incomplete autopaghy in
breast cancer. Pharmacol Res. 129:500–514. 2018. View Article : Google Scholar
|
|
8
|
Liu YB, Gao X, Deeb D, Arbab AS and Gautam
SC: Pristimerin induces apoptosis in prostate cancer cells by
down-regulating Bcl-2 through ROS-dependent ubiquitin-proteasomal
degradation pathway. J Carcinog Mutagen. (Suppl 6): 0052013.
|
|
9
|
Zhang B, Zhang J and Pan J: Pristimerin
effectively inhibits the malignant phenotypes of uveal melanoma
cells by targeting NFkappaB pathway. Int J Oncol. 51:887–898. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deeb D, Gao X, Liu Y, Pindolia K and
Gautam SC: Inhibition of hTERT/telomerase contributes to the
antitumor activity of pristimerin in pancreatic ductal
adenocarcinoma cells. Oncol Rep. 34:518–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao X, Liu Y, Deeb D, Arbab AS and Gautam
SC: Anticancer activity of pristimerin in ovarian carcinoma cells
is mediated through the inhibition of prosurvival
Akt/NF-kappaB/mTOR signaling. J Exp Ther Oncol. 10:275–283.
2014.
|
|
12
|
Yan YY, Bai JP, Xie Y, Yu JZ and Ma CG:
The triterpenoid pristimerin induces U87 glioma cell apoptosis
through reactive oxygen species-mediated mitochondrial dysfunction.
Oncol Lett. 5:242–248. 2013. View Article : Google Scholar
|
|
13
|
Chang FR, Hayashi K, Chen IH, Liaw CC,
Bastow KF, Nakanishi Y, Nozaki H, Cragg GM, Wu YC and Lee KH:
Antitumor agents. 228. five new agarofurans, Reissantins A-E, and
cytotoxic principles from Reissantia buchananii. J Nat Prod.
66:1416–1420. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JH and Kim JK: Pristimerin, a
naturally occurring triterpenoid, attenuates tumorigenesis in
experimental colitis-associated colon cancer. Phytomedicine.
42:164–171. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yousef BA, Guerram M, Hassan HM, Hamdi AM,
Zhang LY and Jiang ZZ: Pristimerin demonstrates anticancer
potential in colorectal cancer cells by inducing G1 phase arrest
and apoptosis and suppressing various pro-survival signaling
proteins. Oncol Rep. 35:1091–1100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR
and Wu YC: Pristimerin induces caspase-dependent apoptosis in
MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer
Ther. 4:1277–1285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Zhou Y, Zhou H, Jia G, Liu J, Han
B, Cheng Z, Jiang H, Pan S and Sun B: Pristimerin causes G1 arrest,
induces apoptosis, and enhances the chemosensitivity to gemcitabine
in pancreatic cancer cells. PLoS One. 7:e438262012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie G, Yu X, Liang H, Chen J, Tang X, Wu S
and Liao C: Pristimerin overcomes adriamycin resistance in breast
cancer cells through suppressing Akt signaling. Oncol Lett.
11:3111–3116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Zhang K, Xu H, Jia X, Chen Y, Ma M, Sun L
and Chen H: Ultrasound-triggered nitric oxide release platform
based on energy transformation for targeted inhibition of
pancreatic tumor. ACS Nano. 10:10816–10828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang K, Li P, He Y, Bo X, Li X, Li D,
Chen H and Xu H: Synergistic retention strategy of RGD active
targeting and radiofrequency-enhanced permeability for intensified
RF & chemotherapy synergistic tumor treatment. Biomaterials.
99:34–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang K, Li P, Chen H, Bo X, Li X and Xu
H: Continuous cavitation designed for enhancing radiofrequency
ablation via a special radiofrequency solidoid vaporization
process. ACS Nano. 10:2549–2558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung D, Khurana A, Roy D, Kalogera E,
Bakkum-Gamez J, Chien J and Shridhar V: Quinacrine upregulates
p21/p27 independent of p53 through autophagy-mediated
downregulation of p62-Skp2 axis in ovarian cancer. Sci Rep.
8:24872018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han Z, Zhou X, Li S, Qin Y, Chen Y and Liu
H: Inhibition of miR-23a increases the sensitivity of lung cancer
stem cells to erlotinib through PTEN/PI3K/Akt pathway. Oncol Rep.
38:3064–3070. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li ZW, Zhao L, Han QC and Zhu X: CXCL13
inhibits microRNA-23a through PI3K/AKT signaling pathway in adipose
tissue derived-mesenchymal stem cells. Biomed Pharmacother.
83:876–880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X, Xue X, Wang L, Wang W, Han J, Sun X,
Zhang H, Liu Y, Che X, Yang J and Wu C: Suppressing autophagy
enhances disulfiram/copper-induced apoptosis in non-small cell lung
cancer. Eur J Pharmacol. 827:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han C, Xing G, Zhang M, Zhong M, Han Z, He
C and Liu X: Wogonoside inhibits cell growth and induces
mitochondrial-mediated autophagy-related apoptosis in human colon
cancer cells through the PI3K/AKT/mTOR/p70S6K signaling pathway.
Oncol Lett. 15:4463–4470. 2018.PubMed/NCBI
|
|
28
|
Sun CY, Zhu Y, Li XF, Wang XQ, Tang LP, Su
ZQ, Li CY, Zheng GJ and Feng B: Scutellarin increases
cisplatin-induced apoptosis and autophagy to overcome cisplatin
resistance in non-small cell lung cancer via ERK/p53 and c-met/AKT
signaling pathways. Front Pharmacol. 9:922018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Czarnomysy R, Surazynski A, Muszynska A,
Gornowicz A, Bielawska A and Bielawski K: A novel series of
pyrazole-platinum(II) complexes as potential anti-cancer agents
that induce cell cycle arrest and apoptosis in breast cancer cells.
J Enzyme Inhib Med Chem. 33:1006–1023. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ling Z, Guan H, You Z, Wang C, Hu L, Zhang
L, Wang Y, Chen S, Xu B and Chen M: Aloperine executes antitumor
effects through the induction of apoptosis and cell cycle arrest in
prostate cancer in vitro and in vivo. Onco Targets Ther.
11:2735–2743. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmad M, Hahn IF and Chatterjee S: GRP78
up-regulation leads to hypersensitization to cisplatin in A549 lung
cancer cells. Anticancer Res. 34:3493–3500. 2014.PubMed/NCBI
|
|
32
|
Sivalingam KS, Paramasivan P, Weng CF and
Viswanadha VP: Neferine potentiates the antitumor effect of
cisplatin in human lung adenocarcinoma cells via a
mitochondria-mediated apoptosis pathway. J Cell Biochem.
118:2865–2876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang LS, Chen SJ, Zhang JF, Liu MN, Zheng
JH and Yao XD: Anti-proliferative potential of Glucosamine in renal
cancer cells via inducing cell cycle arrest at G0/G1 phase. BMC
Urol. 17:382017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SH, Gong X, Zhang Y, Van Horn RD, Yin
T, Huber L, Burke TF, Manro J, Iversen PW, Wu W, et al: RAF
inhibitor LY3009120 sensitizes RAS or BRAF mutant cancer to CDK4/6
inhibition by abemaciclib via superior inhibition of phospho-RB and
suppression of cyclin D1. Oncogene. 37:821–832. 2018. View Article : Google Scholar
|
|
35
|
Stivala LA, Cazzalini O and Prosperi E:
The cyclin-dependent kinase inhibitor p21CDKN1A as a target of
anti-cancer drugs. Curr Cancer Drug Targets. 12:85–96. 2012.
View Article : Google Scholar
|
|
36
|
Starostina NG and Kipreos ET: Multiple
degradation pathways regulate versatile CIP/KIP CDK inhibitors.
Trends Cell Biol. 22:33–41. 2012. View Article : Google Scholar :
|
|
37
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang H, Landis-Piwowar KR, Lu D, Yuan P,
Li L, Reddy GP, Yuan X and Dou QP: Pristimerin induces apoptosis by
targeting the proteasome in prostate cancer cells. J Cell Biochem.
103:234–244. 2008. View Article : Google Scholar
|
|
39
|
Liu D, You P, Luo Y, Yang M and Liu Y:
Galangin Induces Apoptosis in MCF-7 human breast cancer cells
through mitochondrial pathway and phosphatidylinositol 3-Kinase/Akt
Inhibition. Pharmacology. 102:58–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin W, Xie J, Xu N, Huang L, Xu A, Li H,
Li C, Gao Y, Watanabe M, Liu C and Huang P: Glaucocalyxin A induces
G2/M cell cycle arrest and apoptosis through the PI3K/Akt pathway
in human bladder cancer cells. Int J Biol Sci. 14:418–426. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deeb D, Gao X, Liu YB, Pindolia K and
Gautam SC: Pristimerin, a quinonemethide triterpenoid, induces
apoptosis in pancreatic cancer cells through the inhibition of
pro-survival Akt/NF-κB/mTOR signaling proteins and anti-apoptotic
Bcl-2. Int J Oncol. 44:1707–1715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bi T, Zhu A, Yang X, Qiao H, Tang J, Liu Y
and Lv R: Metformin synergistically enhances antitumor activity of
cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway.
Cytotechnology. 70:439–448. 2018. View Article : Google Scholar :
|
|
43
|
Liao XZ, Tao LT, Liu JH, Gu YY, Xie J,
Chen Y, Lin MG, Liu TL, Wang DM, Guo HY and Mo SL: Matrine combined
with cisplatin synergistically inhibited urothelial bladder cancer
cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer
Cell Int. 17:1242017. View Article : Google Scholar
|
|
44
|
Zhang R, Li G, Zhang Q, Tang Q, Huang J,
Hu C, Liu Y, Wang Q, Liu W, Gao N and Zhou S: Hirsutine induces
mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in
human lung cancer cells. Cell Death Dis. 9:5982018. View Article : Google Scholar
|
|
45
|
Xue M, Ji X, Xue C, Liang H, Ge Y, He X
and Zhang L, Bian K and Zhang L: Caspase-dependent and
caspase-independent induction of apoptosis in breast cancer by
fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro.
Biomed Pharmacother. 94:898–908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Zhao M, Liu J, Sun Z, Ni J and Liu
H: miRNA-125b regulates apoptosis of human non-small cell lung
cancer via the PI3K/Akt/GSK3β signaling pathway. Oncol Rep.
38:1715–1723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li Z, Lin C, Zhao L, Zhou L, Pan X, Quan
J, Peng X, Li W, Li H, Xu J, et al: Oncogene miR-187-5p is
associated with cellular proliferation, migration, invasion,
apoptosis and an increased risk of recurrence in bladder cancer.
Biomed Pharmacother. 105:461–469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu X, Wang Y, Liang H, Fan Q, Zhu R, Cui
J, Zhang W, Zen K, Zhang CY, Hou D, et al: miR-23a/b promote tumor
growth and suppress apoptosis by targeting PDCD4 in gastric cancer.
Cell Death Dis. 8:e30592017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Farooqi AA, Qureshi MZ, Coskunpinar E,
Naqvi SK, Yaylim I and Ismail M: MiR-421, miR-155 and miR-650:
Emerging trends of regulation of cancer and apoptosis. Asian Pac J
Cancer Prev. 15:1909–1912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ryter SW, Mizumura K and Choi AM: The
impact of autophagy on cell death modalities. Int J Cell Biol.
2014:5026762014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Keta O, Bulat T, Golic I, Incerti S, Korac
A, Petrovic I and Ristic-Fira A: The impact of autophagy on cell
death modalities in CRL-5876 lung adenocarcinoma cells after their
exposure to gamma-rays and/or erlotinib. Cell Biol Toxicol.
32:83–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheng X, Feng H, Wu H, Jin Z, Shen X,
Kuang J, Huo Z, Chen X, Gao H, Ye F, et al: Targeting autophagy
enhances apatinib-induced apoptosis via endoplasmic reticulum
stress for human colorectal cancer. Cancer Lett. 431:105–114. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bai XY, Liu YG, Song W, Li YY, Hou DS, Luo
HM and Liu P: Anticancer activity of tetrandrine by inducing
pro-death apoptosis and autophagy in human gastric cancer cells. J
Pharm Pharmacol. 70:1048–1058. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu X, Lin H, Wang Y, Lv W, Zhang S, Qian
Y, Deng X, Feng N, Yu H and Qian B: d-limonene exhibits antitumor
activity by inducing autophagy and apoptosis in lung cancer. Onco
Targets Ther. 11:1833–1847. 2018. View Article : Google Scholar : PubMed/NCBI
|