Introduction
Lower back pain (LBP) is a common musculoskeletal
disorder that severely affects the quality of life of human beings
and imposes a substantial economic burden on society (1). Intervertebral disc (IVD)
degeneration (IDD) has been shown to be the major contributor to
LBP (2). IVD, composed of a
central nucleus pulposus (NP) surrounded by annulus fibrosus and
cartilaginous endplates, is an avascular organ. In healthy IVD, the
hydrated NP, which is mainly composed of NP cells and is rich in
the extracellular matrix (ECM), including type II collagen and
aggrecan, is important in the physiological function of the disc in
distributing the mechanical load acting on the spine, and in
multi-axial flexibility (3,4).
During the progression of IDD, the increased levels of
pro-inflammatory cytokines, elevated cell apoptosis, increased
production of ECM-catabolic proteinases, including matrix
metalloproteinases (MMPs) and a disintegrin and metalloproteinase
with thrombospondin motifs (ADAMTS), and decreased synthesis of
type II collagen and aggrecan have been observed within the NP
tissue (5-7). These cellular and molecular changes
lead to disruption of the physiological structure and function of
IVD and to spinal instability, which are the main triggers of LBP
(8).
The excessive production of proinflammatory
molecules secreted by NP cells, including tumor necrosis factor
(TNF)-α, interleukin (IL)-1α, IL-1β, IL-2 and IL-6, has been
demonstrated to serve critical roles in the initiation and
development of IDD (5,9). Among the above-mentioned
inflammatory cytokines, IL-1β is widely considered to be the
predominant cytokine that is expressed at high levels in
degenerative IVD tissues and cells and has been shown to be
involved in several pathological processes in NP cells, including
inflammatory responses, oxidative stress, apoptosis, and an
imbalance of ECM synthesis and degradation (10,11). Therefore, inhibiting the effect of
IL-1β on NP cells may postpone the progression of IDD.
Berberine (BBR) is an isoquinoline alkaloid that is
derived from several medicinal herbs, including Rhizoma Coptidis,
Cortex Phellodendri, and Mahonia bealei, which have long been used
in traditional Chinese medicine. BBR has been reported to possess
multiple pharmacological effects, including anti-oxidative,
anti-inflammatory and anti-apoptotic effects (12-14). Previous studies have found that
BBR has therapeutic effects on musculoskeletal disorders, including
rheumatoid arthritis and osteoarthritis, owing to its
anti-inflammatory properties (15,16). Zhao et al showed that BBR
treatment can protect articular cartilage from degeneration via
activating the Akt-p70S6K-S6 signaling pathway in IL-1β-stimulated
articular chondrocytes and in a rat osteoarthritis model (17). Hu et al reported that BBR
decreases glycosaminoglycan release and nitric oxide production in
IL-1β-stimulated chondrocytes (16). In addition, the administration of
BBR was found by Zhou et al to prevent nitric oxide-induced
chondrocyte apoptosis and cartilage degeneration in a rat model of
osteoarthritis (18). As the
morphology and avascular supply of NP cells are similar to those of
chondrocytes, and BBR has been reported to inhibit the effects of
oxidative stress in rat NP cells (19), it was hypothesized that BBR may
prevent the development of IDD by protecting NP cells from
IL-1β-induced degenerative effects. Therefore, the purpose of the
present study was to investigate the influence of BBR on
IL-1β-induced apoptosis and ECM degradation in human NP cells and
to elucidate the underlying molecular mechanism.
Materials and methods
Patient tissue samples
Between March and October 2017, human lumbar NP
tissues were collected from 10 patients (six women and four men;
mean age, 24.7 years; age range, 15-42 years) with idiopathic
scoliosis who underwent deformity correction surgery with the
approval of the Ethics Committee of Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China).
Written informed consent was obtained from all participants
involved in the study. The degrees of degeneration of the discs of
all participants were assessed using the modified Pfirrmann grading
system (20) and were classified
as grade II.
Human NP cell culture and treatment
Human NP cells were isolated using a method reported
previously by Kang et al (21), and were then cultured in DMEM/F12
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 15% of fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% of a penicillin-streptomycin solution at
37°C in a humidified atmosphere containing 5% CO2. The
cells were passaged twice for use in the following experiments. The
human NP cells were seeded in a six-well plate at a density of
105 cells/well. On reaching 80-90% confluence, the NP
cells were incubated with 25 µM BBR for 2 h prior to IL-1β
(10 ng/ml) treatment for 24 h at 37°C. The NP cells were harvested
for subsequent experiments. All experiments were conducted in
triplicate.
Cell viability analysis
Cell viability was evaluated using a Cell Counting
Kit-8 (CCK-8, Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Briefly, the human NP cells were seeded in a 96-well plate
(5×103 cells per well) and cultured as described above,
followed by treatment with various concentrations of BBR (5, 10,
15, 20 or 25 µM) or IL-1β (10 ng/ml) for 24 h at 37°C.
Subsequently, 10 µl of the CCK-8 dye was added into each
well, followed by incubation at 37°C for 2 h. The optical density
was measured at 450 nm on a microplate reader (Leica Microsystems
GmbH, Wetzlar, Germany).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from the human NP cells with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
mRNA expression levels of type II collagen, aggrecan, MMP-3,
MMP-13, ADAMTS-4 and ADAMTS-5 were quantified by RT-qPCR analysis
on a 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) under the cycling conditions recommended by the
manufacturer. qPCR was performed using a SYBR Prime Script™ RT-qPCR
kit (Takara Biotechnology Co., Ltd., Dalian, China). The reaction
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 30 sec and 60°C for 30 sec. The gene expression levels
were normalized to that of β-actin. Relative expression levels were
analyzed using the 2−ΔΔCq method (22). The primer sequences used for
RT-qPCR analysis were as follows: Type II collagen, forward 5′-AGA
ACT GGT GGA GCA GCA AGA-3′ and reverse 5′-AGC AGG CGT AGG AAG GTC
AT-3′; aggrecan, forward 5′-TGA GCG GCA GCA CTT TGA C-3′ and
reverse 5′-TGA GTA CAG GAG GCT TGA GG-3′; MMP-3, forward 5′-TTC CTT
GGA TTG GAG GTG AC-3′ and reverse 5′-AGC CTG GAG AAT GTG AGT GG-3′;
MMP-13, forward 5′-CCC AAC CCT AAA CAT CCA A-3′ and reverse 5′-AAA
CAG CTC CGC ATC AAC C-3′; ADAMTS-4, forward 5′-ACC CAA GCA TCC GCA
ATC-3′ and reverse 5′-TGC CCA CAT CAG CCA TAC-3′; ADAMTS-5, forward
5′-GAC AGT TCA AAG CCA AAG ACC-3′ and reverse 5′-TTT CCT TCG TGG
CAG AGT-3′; β-actin, forward 5′-AGC GAG CAT CCC CCA AAG TT-3′ and
reverse 5′-GGG CAC GAA GGC TCA TCA TT-3′.
Western blotting
The western blotting procedure was performed to
analyze protein levels. The treated human NP cells were lysed with
RIPA lysis buffer, and protein concentration was measured using the
BCA Protein Assay kit (Beyotime Institute of Technology, Haimen,
China). The nuclear and cytoplasmic proteins were isolated with the
Nuclear/Cytosolic Fractionation kit (BioVision, Inc., Mountain
View, CA, USA). The concentration was measured using a BCA protein
assay. A total of 40 µg protein per lane was separated by
sodium dodecyl sulphate polyacrylamide gel electrophoresis on a 12%
gel and transferred onto a polyvinylidene fluoride membrane (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% non-fat
milk in TBST, the membranes were incubated with primary antibodies
against type II collagen (sc-7764; 142 kDa; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; 1:8,000), aggrecan (ab3778;
50-60 kDa; Abcam, Cambridge, UK; 1:100), MMP-3 (14351; 60 kDa; Cell
Signaling Technology, Inc., Danvers, MA, USA; 1:1,000), MMP-13
(ab39012; 54 kDa; Abcam; 1:4,000), ADAMTS-4 (ab185722; 90 kDa;
Abcam; 1:1,000), ADAMTS-5 (ab41037; 73 kDa; Abcam; 1:200), B-cell
lymphoma 1 (Bcl-2; ab32124; 26 kDa; Abcam; 1:1,000),
Bcl-2-associated X protein (Bax; ab32503; 20 kDa; Abcam; 1:1,000),
cleaved caspase3 (9664; 17 kDa; Cell Signaling Technology, Inc.;
1:1,000), nuclear factor (NF)-κB p65 (ab16502; 60 kDa; Abcam;
1:500), phosphorylated p65 (p-p65; ab86299; 60 kDa; Abcam;
1:1,000), inhibitor of κBα (IκBα; (9242; 39 kDa; Cell Signaling
Technology, Inc.; 1:1,000), β-actin (ab8227; 42 kDa; Abcam;
1:2,000) and lamin B1 (ab16048; 66 kDa; Abcam; 1:1,000) overnight
at 4°C, followed by incubation with the respective secondary
antibodies (BA1054; Boster Biological Technology, Pleasanton, CA,
USA, 1:5,000) at room temperature for 1 h. The protein bands were
detected using an enhanced chemiluminescence system and analyzed
quantitatively using BandScan software (version 4.30; BioMarin
Pharmaceutical Inc., UK). β-actin and lamin B1 served as loading
controls.
Flow cytometry
Following treatment with the different agents, the
human NP cells were harvested, washed with cold PBS, and stained
using the Annexin V-FITC Apoptosis Detection kit (Beyotime
Institute of Biotechnology, Haimen, China). The fluorescence of the
cells was determined immediately by flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA). The apoptotic rate was calculated as the
sum of the percentages of early (Annexin
V+/PI−) and late (Annexin
V+/PI+) apoptotic cells.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS v.18.0
software (SPSS, Inc., Chicago, IL, USA). Differences between groups
were evaluated by one-way analysis of variance followed by the
Tukey test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell viability of human NP cells
following treatment with BBR
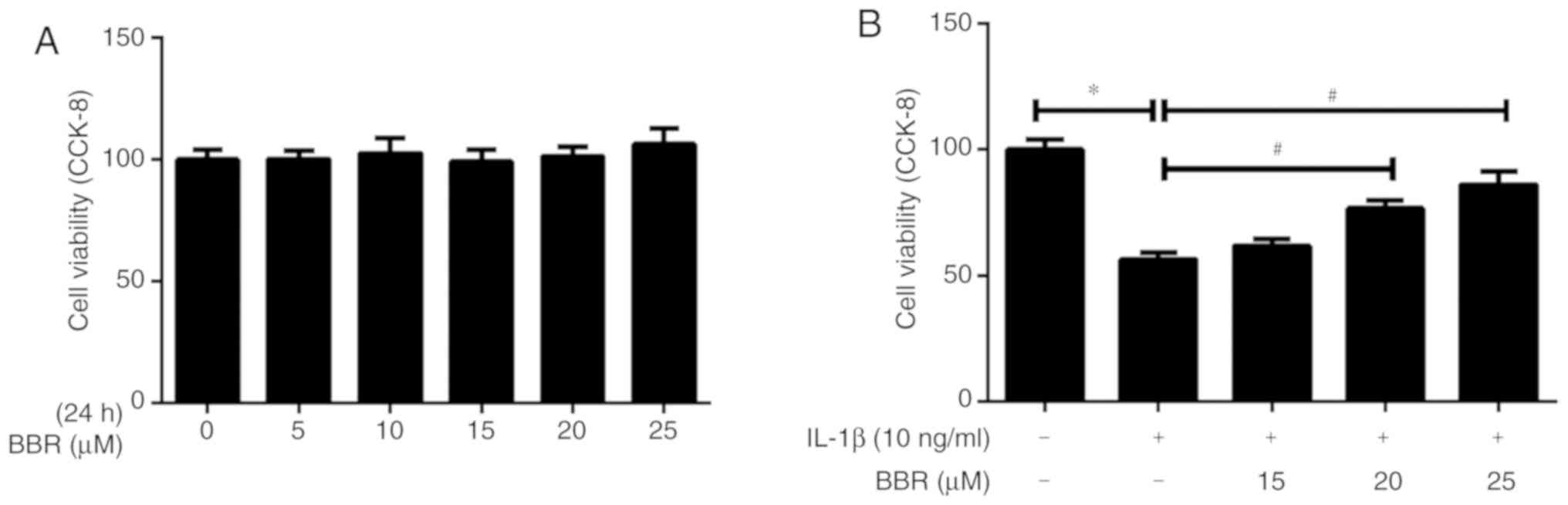
The present study evaluated the effect of BBR on the
viability of human NP cells at various concentrations (5, 10, 15,
20 or 25 µM) for 24 h using the CCK-8 assay. The results
indicated that BBR was not significantly toxic to human NP cells at
concentrations ranging between 5 and 25 µM (Fig. 1A). In addition, BBR was
administered to IL-1β-treated human NP cells at various
concentrations (15, 20 or 25 µM). Pretreatment with BBR had
a significant protective effect against IL-1β-induced cell
apoptosis, particularly at 25 µM (Fig. 1B). Therefore, 25 µM BBR was
selected for subsequent experiments.
Inhibitory effects of BBR on
IL-1β-induced ECM degradation by human NP cells
To determine whether BBR affects IL-1β-induced ECM
degradation by human NP cells, the present study assessed the mRNA
and protein expression levels of the main components of the NP ECM,
including type II collagen and aggrecan, and major NP ECM catabolic
proteinases MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 by RT-qPCR and
western blot analyses, respectively. The results revealed that
IL-1β treatment markedly decreased the mRNA expression levels of
type II collagen and aggrecan, whereas pretreatment with BBR
attenuated the downregulation induced by IL-1β (Fig. 2A and B). It was also found that
the mRNA expression levels of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5
in human NP cells were significantly increased following
stimulation with IL-1β (Fig.
2C-F). However, BBR pretreatment resulted in a statistically
significant attenuation of IL-1β-induced upregulation of these ECM
catabolic proteinases (Fig.
2C-F). The same results were observed for protein expression
levels (Fig. 2G-M)
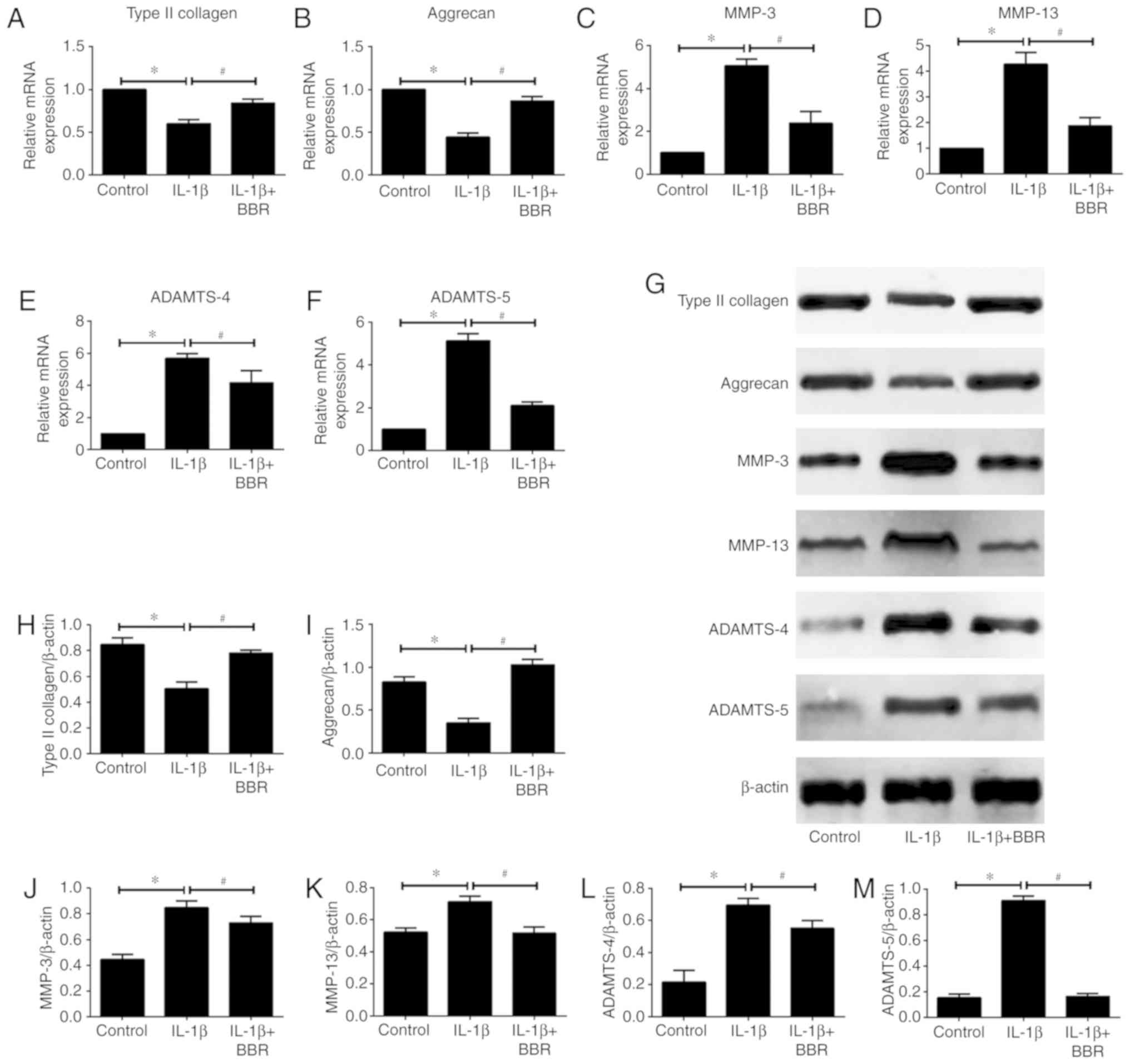 | Figure 2Effects of BBR on IL-1β-induced
extracellular matrix degradation by human NP cells. Following
treatment with BBR (25 µM) for 2 h, NP cells were stimulated
with IL-1β (10 ng/ml) for 24 h and harvested for RT-qPCR and
western blot analyses. mRNA expression of (A) type II collagen, (B)
aggrecan, (C) MMP-3, (D) MMP-13, (E) ADAMTS-4 and (F) ADAMTS-5, as
analyzed by RT-qPCR analysis. (G) Western blot analysis of the
protein levels of (H) type II collagen, (I) aggrecan, (J) MMP-3,
(K) MMP-13, (L) ADAMTS-4 and (M) ADAMTS-5. β-actin served as an
internal control. Data are presented as the mean ± standard
deviation. *P<0.05, vs. control group;
#P<0.05, vs. IL-1β group. NP, nucleus pulposus; BBR,
berberine; IL-1β, interleukin-1β; MMP, matrix metalloproteinase;
ADAMTS, a disintegrin and metalloproteinase with thrombospondin
motifs; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction. |
BBR protects human NP cells from
IL-1β-induced cell apoptosis
As NP cell apoptosis is important in the progression
of IDD, the present study investigated the influence of BBR on
IL-1β-induced human NP cell apoptosis. Flow cytometric analysis
revealed that IL-1β increased the rate of apoptosis compared with
that in the untreated control (Fig.
3A and B). When the human NP cells were cotreated with BBR and
IL-1β, the results showed a significant decrease in the rate of
apoptosis (Fig. 3A and B).
Furthermore, the expression levels of apoptosis-related proteins
(Bcl-2, Bax and cleaved caspase3) were assessed. The protein levels
of Bax (pro-apoptotic) and cleaved caspase3 (pro-apoptotic) were
increased and the protein level of Bcl-2 (anti-apoptotic) was
decreased in the IL-1β-treated human NP cells (Fig. 3C-G). These trends were reversed by
BBR pretreatment (Fig. 3C-G).
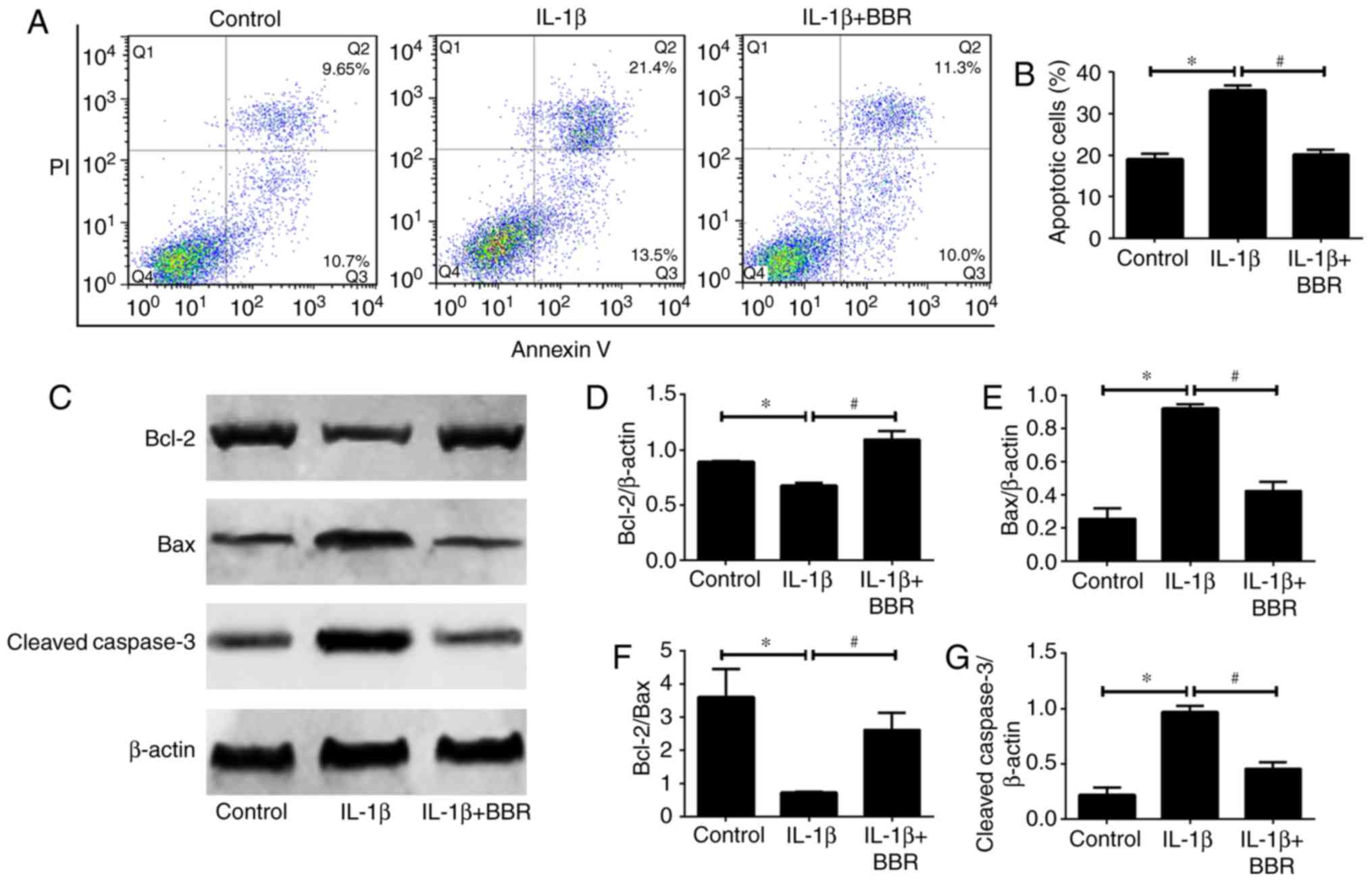 | Figure 3Impact of BBR on IL-1β-induced human
NP cells apoptosis. Following treatment with BBR (25 µM) for
2 h, NP cells were stimulated with IL-1β (10 ng/ml) for 24 h and
harvested for flow cytometry and western blotting. (A) Flow
cytometry was used to determine the (B) rates of apoptosis of human
NP cells. (C) Western blot analysis and quantification of protein
levels of (D) Bcl-2, (E) Bax, (F) Bcl-2/Bax and (G) cleaved caspase
3. β-actin served as an internal control. Data are presented as the
mean ± standard deviation. *P<0.05, vs. control
group; #P<0.05, vs. IL-1β group. NP, nucleus
pulposus; BBR, berberine; IL-1β, interleukin-1β; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein. |
Suppressive effects of BBR on the
IL-1β-induced activation of NF-κB in human NP cells
The NF-κB pathway has been found to be aberrantly
activated in IDD and has been demonstrated to be an important
mediator of the IL-1β-induced degenerative effects in human NP
cells. Therefore, to further examine the underlying mechanism of
BBR-induced anti-degenerative effects, the present study determined
whether BBR regulates the NF-κB pathway in IL-1β-treated human NP
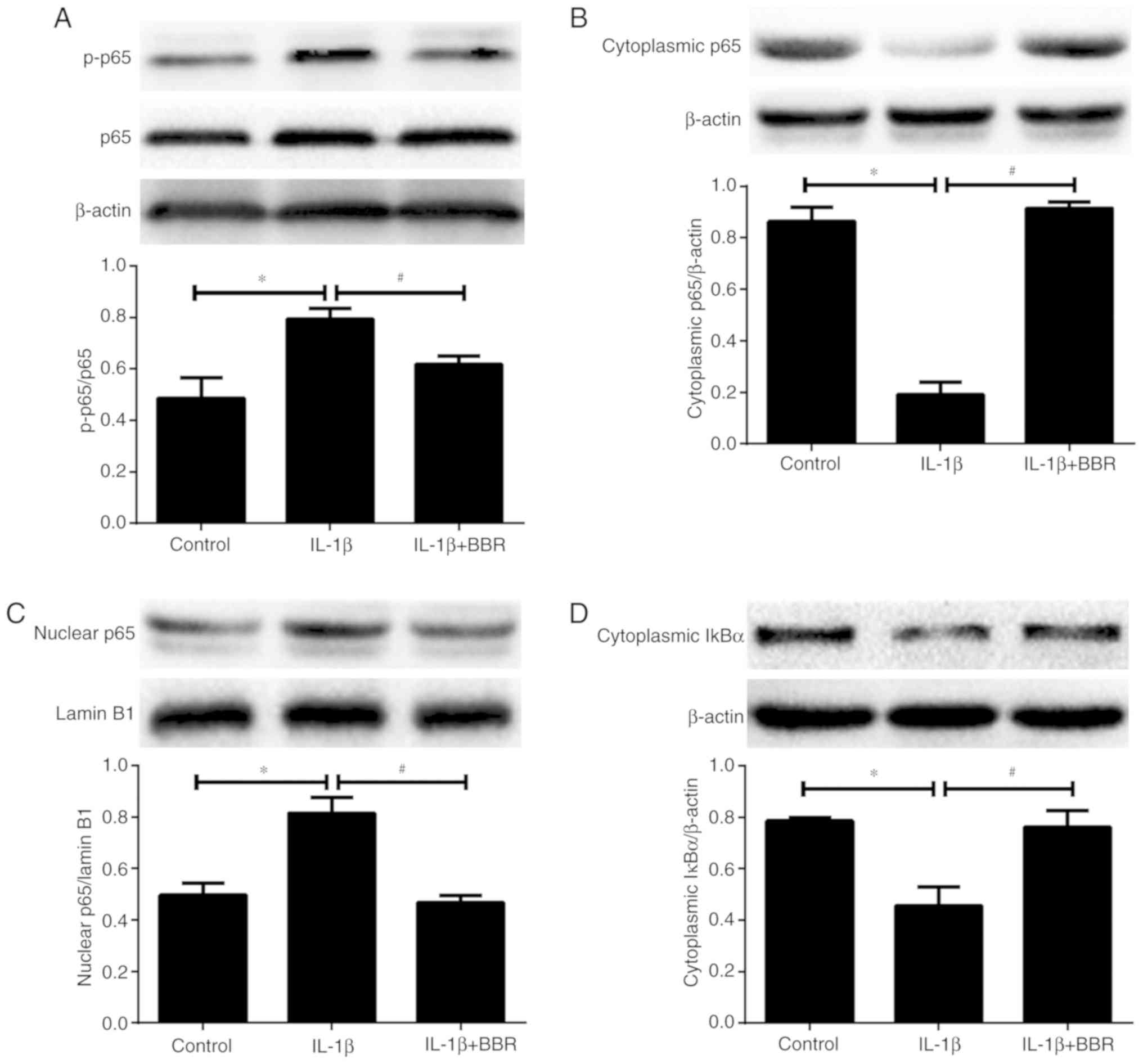
cells. The extent of NF-κB p65 phosphorylation and the
intracellular distribution of p65 were first evaluated by western
blotting. Compared with the untreated group, a higher ratio
p-p65/p65 and nuclear translocation of p65 from the cytoplasm were
observed following IL-1β stimulation (Fig. 4A-C), indicating activation of the
NF-κB signaling pathway in the IL-1β-treated human NP cells.
However, pretreatment with BBR markedly suppressed this
IL-1β-induced phosphorylation and translocation of p65 (Fig. 4A-C). IκBα is an inhibitor protein
for p65, the phosphorylation of IκBα and its subsequent degradation
induce the translocation of p65 to the nucleus. Therefore, the
level of cytoplasmic IκBα was analyzed by western blotting in human
NP cells. IL-1β treatment induced the degradation of cytoplasmic
IκBα, whereas this effect was attenuated by BBR pretreatment
(Fig. 4D).
Discussion
LBP affects 80% of the global population, and IDD is
widely recognized as the main cause of LBP (2). The prevention or reversal of IDD is
a potential treatment of LBP; however, the pathological basis and
mechanisms underlying IDD remain to be fully elucidated. Current
evidence suggests that the excessive inflammatory response, an
increase in the proportion of apoptotic NP cells, and the imbalance
between anabolism and catabolism of NP ECM are closely associated
with the development of IDD (7,8,23);
therefore, targeting these pathological processes is a novel
strategy for the treatment of IDD. Medical treatments of LBP caused
by IDD mainly include conservative approaches and, rarely, surgical
procedures. Conservative approaches, including non-steroidal
anti-inflammatory drugs, are prescribed to inhibit inflammation and
to relieve the symptom of back pain temporarily; however, the
widespread and long-term use of non-steroidal anti-inflammatory
drugs results in considerable adverse effects (24). Surgical procedures, including
spine fusion and discectomy (25), which cannot preserve the function
of IVD, imposes a financial burden on the patient and is not an
optimal choice. Therefore, investigating novel drug treatments that
can promote endogenous repair of degenerative IVD and prevent IDD
development is crucial, particularly for early-stage IDD. In
previous years, plant-derived compounds have attracted the
attention of those investigating IDD treatment owing to their
anti-inflammatory effects and few adverse effects (10,26). BBR, an isoquinoline alkaloid
extracted from Coptidis Rhizoma and Cortex Phellodendri, has been
shown to have potent therapeutic potential against inflammatory
diseases (12,27). The present study provides the
first evidence, to the best of our knowledge, that BBR has
pharmacological inhibitory effects on ECM degradation and apoptosis
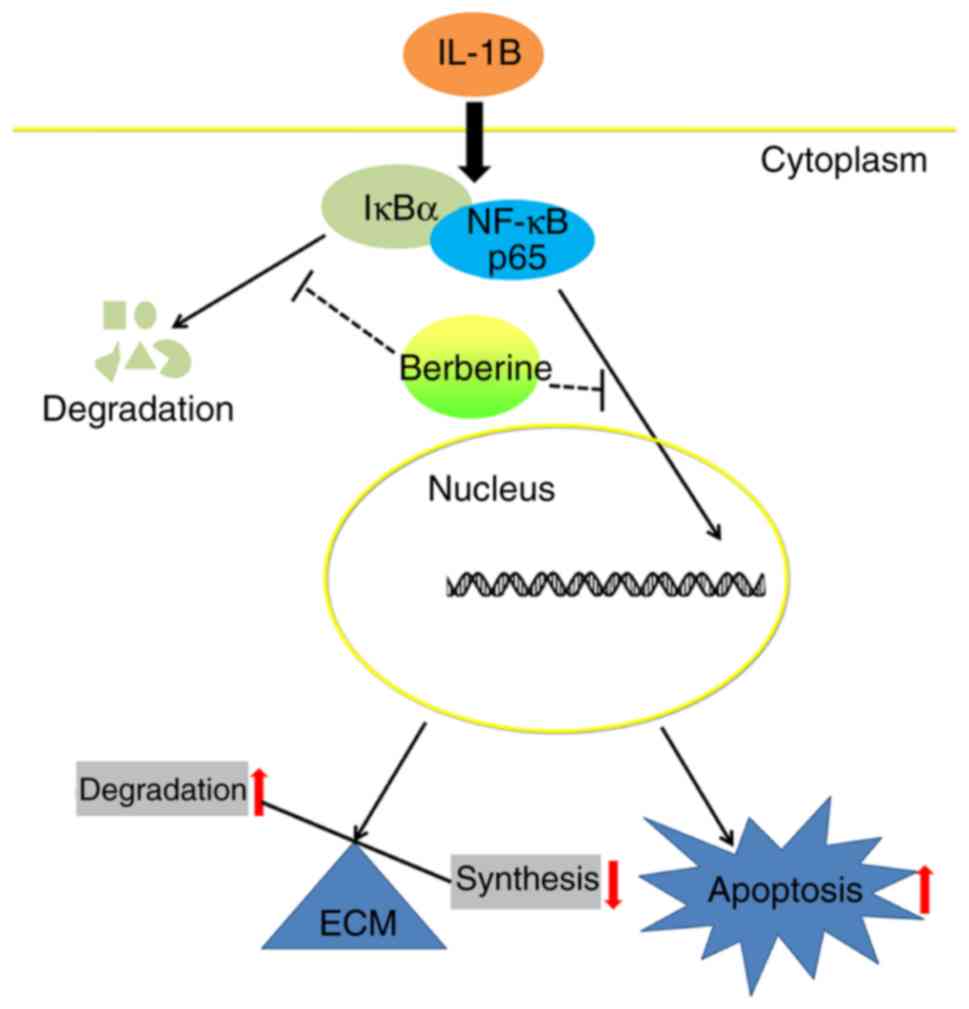
in IL-1β-stimulated human NP cells. The results also showed that
BBR inhibits the IL-1β-induced activation of the NF-κB pathway in
human NP cells (Fig. 5).
IL-1β is a major risk factor of IDD, with potent
proinflammatory activity involving stimulation of the production of
multiple proinflammatory mediators (28). It has been demonstrated that IL-1β
is involved in multiple pathological processes related to the
development of IDD (5,10,29). The progressive degradation of the
NP ECM, which mainly consists of type II collagen and aggrecan
(performing a vital function in the maintenance of the
physiological function of IVD), is the most prominent feature of
IDD. MMPs and ADAMTSs are the major ECM-degrading enzymes in IDD,
and their functions in IDD have been investigated extensively
(30). MMP-3 and MMP-13 have been
identified as the main collagenases that lead to degradation of
type II collagen in NP, whereas ADAMTS-4 and ADAMTS-5 are the
primary aggrecanases due to their potent and specific activity in
cleaving aggrecan. The expression of all these enzymes, which are
often regarded as the catabolic markers of IDD, has been found to
be upregulated in degenerative NP (31). The inhibition of MMPs and ADAMTSs
has a therapeutic effect on IDD in vitro and in vivo.
There is substantial evidence that, in NP cells, IL-1β can promote
the production of ECM-degrading enzymes that degrade type II
collagen and aggrecan in NP ECM (5,10).
In the present study, the results showed that IL-1β treatment
resulted in the upregulation of MMP-3, MMP-13, ADAMTS-4 and
ADAMTS-5 and the downregulation of type II collagen and aggrecan in
human NP cells, as expected, and that BBR reversed these changes
induced by IL-1β. These results suggest that BBR has the potential
to control the progression of IDD by restoring the balance between
ECM anabolism and catabolism in human NP cells.
NP cells are important for maintaining the
structural integrity of IVD by generating the molecular components
of ECM. Recently, an apoptosis-associated decrease in the NP cell
number was proposed as an important mechanism underlying the
development of IDD (32,33). Therefore, treatment of IDD not
only depends on the NP ECM homeostasis but also on inhibiting the
apoptosis of NP cells. Several studies have demonstrated that IL-1β
is an important regulator of the apoptosis of NP cells (34,35). The majority of rabbit NP cells
were shown to undergo apoptosis following IL-1β stimulation and
exhibit morphological changes related to apoptosis, whereas a
combined treatment with insulin-like growth factor 1 and IL-1β
significantly reduced IL-1β-mediated apoptosis (35). Shen et al (34) reported that IL-1β induces the
mitochondrial pathway in NP cells by increasing the expression
ratio Bax/Bcl-2 and by releasing cytochrome c from the
mitochondria to the cytoplasm, subsequently activating downstream
caspases 9 and 3 to complete the apoptotic process. In addition,
Chen et al (19) found
that BBR may mitigate oxidative-stress-induced apoptosis through
the mitochondrial pathway. The results of flow cytometric analysis
in the present study revealed that BBR effectively prevented
IL-1β-induced apoptosis. The data also indicated that BBR
attenuated the downregulation of Bcl-2 and the upregulation of Bax
and cleaved caspase 3 at the protein level in IL-1β-treated human
NP cells. Taken together, these results suggest that BBR protects
human NP cells from IL-1β-induced apoptosis.
Various intracellular signaling pathways are
activated in response to inflammatory stimulation associated with
IDD, thereby mediating the increase in the production of a
downstream effector that is closely involved in the progression of
IDD (36). As one of the most
critical intracellular signaling proteins, NF-κB can regulate the
expression of genes associated with ECM degradation and cell
apoptosis in IL-1β-treated human NP cells (21,37). Inhibiting the activation of NF-κB
is regarded as a potential therapeutic strategy against IDD. Under
normal conditions, NF-κB is located in the cytoplasm bound to an
inhibitory protein, IκB, which prevents NF-κB from entering the
nucleus. Upon stimulation by IL-1β, the IκB protein is
phosphorylated and degraded, resulting in the translocation of
NF-κB from the cytoplasm to the nucleus. Subsequently, NF-κB
facilitates gene transcription by binding to specific sequences in
the promoter region of NF-κB-responsive genes, which upregulate the
production of catabolic enzymes, inflammatory mediators and
cyto-kines (5,10). To further elucidate the molecular
mechanism underlying the inhibitory effect of BBR on ECM
degradation and apoptosis in IL-1β-treated NP cells, the present
study assessed the influence of BBR on the IL-1β-induced activation
of NF-κB in human NP cells. The results revealed that BBR
significantly inhibited the IL-1β-induced upregulation of the
phosphorylation of NF-κB p65 and its nuclear translocation in human
NP cells. In addition, the IL-1β-induced decrease in the level of
cytoplasmic IκBα was reversed by BBR, indicating that treatment
with BBR inhibited the degradation of IκBα, thereby maintaining
NF-κB in an inactive state. Taken together, these results suggest
that the therapeutic action of BBR on IDD may be mediated by
suppression of the NF-κB signaling pathway.
However, as human NP cells cultured in vitro
and human NP cells in vivo may respond differently to BBR
treatment, whether BBR is effective as a IDD treatment in
vivo remains to be fully elucidated. Therefore, animal
experiments that can truly mimic IDD pathogenesis are urgently
required to confirm the protective effect of BBR against IDD and to
advance current understanding of the molecular mechanisms
underlying this effect.
In conclusion, the results of the present study
suggest that BBR exerts potent anti-ECM catabolic and
anti-apoptotic actions by inhibiting the IL-1β-induced activation
of NF-κB in human NP cells, indicating that BBR may be validated as
an effective therapeutic agent for IDD in the future.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
JW and ZY designed the study. LL wrote the
manuscript. LL, JH, and QW conducted the experiments. LL, JH, QW,
YA, and WC collected the data. JW, ZY, and LL analyzed the data. JW
and ZY reviewed and revised the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tongji Medical College, Huazhong University of Science
and Technology (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Martin BI, Deyo RA, Mirza SK, Turner JA,
Comstock BA, Hollingworth W and Sullivan SD: Expenditures and
health status among adults with back and neck problems. JAMA.
299:656–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luoma K, Riihimaki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine (Phila Pa 1976).
25:487–492. 2000. View Article : Google Scholar
|
|
3
|
Pattappa G, Li Z, Peroglio M, Wismer N,
Alini M and Grad S: Diversity of intervertebral disc cells:
Phenotype and function. J Anat. 221:480–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakai D and Grad S: Advancing the cellular
and molecular therapy for intervertebral disc disease. Adv Drug
Deliv Rev. 84:159–171. 2015. View Article : Google Scholar
|
|
5
|
Yang W, Yu XH, Wang C, He WS, Zhang SJ,
Yan YG, Zhang J, Xiang YX and Wang WJ: Interleukin-1β in
intervertebral disk degeneration. Clin Chim Acta. 450:262–272.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu B, Meng C, Wang H, Jia C and Zhao Y:
Changes of proteoglycan and collagen II of the adjacent
intervertebral disc in the cervical instability models. Biomed
Pharmacother. 84:754–758. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Li K, Han X, Mao C, Zhang K, Zhao T
and Zhao J: The imbalance between TIMP3 and matrix-degrading
enzymes plays an important role in intervertebral disc
degeneration. Biochem Biophys Res Commun. 469:507–514. 2016.
View Article : Google Scholar
|
|
8
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar
|
|
9
|
Yamamoto J, Maeno K, Takada T, Kakutani K,
Yurube T, Zhang Z, Hirata H, Kurakawa T, Sakai D, Mochida J, et al:
Fas ligand plays an important role for the production of
pro-inflammatory cytokines in intervertebral disc nucleus pulposus
cells. J Orthop Res. 31:608–615. 2013. View Article : Google Scholar
|
|
10
|
Chen J, Xuan J, Gu YT, Shi KS, Xie JJ,
Chen JX, Zheng ZM, Chen Y, Chen XB, Wu YS, et al: Celastrol reduces
IL-1β induced matrix catabolism, oxidative stress and inflammation
in human nucleus pulposus cells and attenuates rat intervertebral
disc degeneration in vivo. Biomed Pharmacother. 91:208–219. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar
|
|
12
|
Wang Z, Chen Z, Yang S, Wang Y, Huang Z,
Gao J, Tu S and Rao Z: Berberine ameliorates collagen-induced
arthritis in rats associated with anti-inflammatory and
anti-angiogenic effects. Inflammation. 37:1789–1798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Z, Geng YN, Jiang JD and Kong WJ:
Antioxidant and anti-inflammatory activities of berberine in the
treatment of diabetes mellitus. Evid Based Complement Alternat Med.
2014.289264:2014.
|
|
14
|
Liang Y, Huang M, Jiang X, Liu Q, Chang X
and Guo Y: The neuroprotective effects of Berberine against amyloid
β-protein-induced apoptosis in primary cultured hippocampal neurons
via mitochondria-related caspase pathway. Neurosci Lett. 655:46–53.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, He X, Zhang CF, Guo CR, Wang CZ
and Yuan CS: Anti-arthritic effect of berberine on adjuvant-induced
rheumatoid arthritis in rats. Biomed Pharmacother. 89:887–893.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu PF, Chen WP, Tang JL, Bao JP and Wu LD:
Protective effects of berberine in an experimental rat
osteoarthritis model. Phytother Res. 25:878–885. 2011. View Article : Google Scholar
|
|
17
|
Zhao H, Zhang T, Xia C, Shi L, Wang S,
Zheng X, Hu T and Zhang B: Berberine ameliorates cartilage
degeneration in interleukin-1β-stimulated rat chondrocytes and in a
rat model of osteoarthritis via Akt signalling. J Cell Mol Med.
18:283–292. 2014. View Article : Google Scholar
|
|
18
|
Zhou Y, Liu SQ, Yu L, He B, Wu SH, Zhao Q,
Xia SQ and Mei HJ: Berberine prevents nitric oxide-induced rat
chondrocyte apoptosis and cartilage degeneration in a rat
osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis.
20:1187–1199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Zheng Z, Wang J, Tang C, Khor S,
Chen J, Chen X, Zhang Z, Tang Q, Wang C, et al: Berberine
suppresses apoptosis and extracellular matrix (ECM) degradation in
nucleus pulposus cells and ameliorates disc degeneration in a
rodent model. Int J Biol Sci. 14:682–692. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar
|
|
21
|
Kang L, Hu J, Weng Y, Jia J and Zhang Y:
Sirtuin 6 prevents matrix degradation through inhibition of the
NF-κB pathway in intervertebral disc degeneration. Exp Cell Res.
352:322–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Purmessur D, Walter BA, Roughley PJ,
Laudier DM, Hecht AC and Iatridis J: A role for TNFα in
intervertebral disc degeneration: A non-recoverable catabolic
shift. Biochem Biophys Res Commun. 433:151–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roelofs PD, Deyo RA, Koes BW, Scholten RJ
and van Tulder MW: Nonsteroidal anti-inflammatory drugs for low
back pain: An updated Cochrane review. Spine (Phila Pa 1976).
33:1766–1774. 2008. View Article : Google Scholar
|
|
25
|
Phillips FM, Reuben J and Wetzel FT:
Intervertebral disc degeneration adjacent to a lumbar fusion. An
experimental rabbit model. J Bone Joint Surg Br. 84:289–294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Xu L, Zhuo N and Shen J:
Resveratrol protects against mitochondrial dysfunction through
autophagy activation in human nucleus pulposus cells. Biochem
Biophys Res Commun. 493:373–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chandirasegaran G, Elanchezhiyan C, Ghosh
K and Sethupathy S: Berberine chloride ameliorates oxidative
stress, inflammation and apoptosis in the pancreas of
Streptozotocin induced diabetic rats. Biomed Pharmacother.
95:175–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jimbo K, Park JS, Yokosuka K, Sato K and
Nagata K: Positive feedback loop of interleukin-1beta upregulating
production of inflammatory mediators in human intervertebral disc
cells in vitro. J Neurosurg Spine. 2:589–595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnson ZI, Schoepflin ZR, Choi H, Shapiro
IM and Risbud MV: Disc in flames: Roles of TNF-α and IL-1β in
intervertebral disc degeneration. Eur Cells Mater. 30:104–116;
discussion 116–117. 2015. View Article : Google Scholar
|
|
30
|
Wang WJ, Yu XH, Wang C, Yang W, He WS,
Zhang SJ, Yan YG and Zhang J: MMPs and ADAMTSs in intervertebral
disc degeneration. Clin Chim Acta. 448:238–246. 2015. View Article : Google Scholar
|
|
31
|
Kang L, Yang C, Yin H, Zhao K, Liu W, Hua
W, Wang K, Song Y, Tu J, Li S, et al: MicroRNA-15b silencing
inhibits IL-1β-induced extracellular matrix degradation by
targeting SMAD3 in human nucleus pulposus cells. Biotechnol Lett.
39:623–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han Y, Li X, Yan M, Yang M, Wang S, Pan J,
Li L and Tan J: Oxidative damage induces apoptosis and promotes
calcification in disc cartilage endplate cell through
ROS/MAPK/NF-κB pathway: Implications for disc degeneration. Biochem
Biophys Res Commun. Mar 23–2017.Epub ahead of print. View Article : Google Scholar
|
|
33
|
Cheng X, Zhang L, Zhang K, Zhang G, Hu Y,
Sun X, Zhao C, Li H, Li YM and Zhao J: Circular RNA VMA21 protects
against intervertebral disc degeneration through targeting miR-200c
and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis.
77:770–779. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen J, Xu S, Zhou H, Liu H, Jiang W, Hao
J and Hu Z: IL-1β induces apoptosis and autophagy via mitochondria
pathway in human degenerative nucleus pulposus cells. Sci Rep.
7:410672017. View Article : Google Scholar
|
|
35
|
Zhang CC, Zhou JS, Hu JG, Wang X, Zhou XS,
Sun BA, Shao C and Lin Q: Effects of IGF-1 on IL-1β-induced
apoptosis in rabbit nucleus pulposus cells in vitro. Mol Med Rep.
7:441–444. 2013. View Article : Google Scholar
|
|
36
|
Wuertz K, Vo N, Kletsas D and Boos N:
Inflammatory and catabolic signalling in intervertebral discs: The
roles of NF-κB and MAP kinases. Eur Cell Mater. 23:103–119;
discussion 119–120. 2012.
|
|
37
|
Li Z, Wang X, Pan H, Yang H, Li X, Zhang
K, Wang H, Zheng Z, Liu H and Wang J: Resistin promotes CCL4
expression through toll-like receptor-4 and activation of the
p38-MAPK and NF-κB signaling pathways: Implications for
intervertebral disc degeneration. Osteoarthritis Cartilage.
25:341–350. 2017. View Article : Google Scholar
|