Introduction
Androgenic alopecia (AGA) is a genetic disease that
affects men and is associated with the androgen steroid hormone. It
is characterized by patterned hair loss from the scalp, and is
recognized as a condition affecting physical and mental well-being
(1,2). The characteristic symptoms of AGA
include a gradual decrease in terminal hair density and a
simultaneous increase in the density of short and unpigmented hair.
This effect contributes to the miniaturization of hair follicles
and the reduction of hair diameter (3). With regard to the etiology of AGA,
the conversion of testosterone to dihydrotestosterone (DHT) is
catalyzed by 5α-reductase, and DHT then binds to the androgen
receptor of the dermal papilla cells (DPCs) in the hair follicles
to suppress the proliferation of DPCs and sustain the telogen
phase, thereby inhibiting the formation of new hair (4).
Finasteride is a 5α-reductase, acting as an
antiandrogen in the prostate gland and scalp (5,6).
Finasteride was originally developed to treat benign prostatic
hyperplasia; however, as hirsutism was observed as a side effect,
it was developed as a hair growth solution. Consequently,
finasteride alleviates AGA by suppressing the activity of
5α-reductase and reducing DHT levels (7). Studies have reported decreased DHT
levels in the serum and scalp following oral administration of
finasteride (8,9). A clinical trial reported that 1-mg
daily dosing of finasteride administered to men with AGA for 48
weeks delayed the progression of hair loss and increased hair
growth (10). Other clinical
trials in which 1,879 men were monitored for 5 years demonstrated
that oral finasteride treatment reduced hair loss (11,12), and that the daily administration
of 1 mg oral finasteride increased hair count and improved hair
appearance (13). However, to
maintain these therapeutic effects, oral finasteride should be
administered daily for a long time, and the inconvenience of daily
drug administration has been highlighted as a disadvantage.
Therefore, studies have been conducted to deliver finasteride in a
controlled manner causing fewer adverse effects. The polycarbonate,
poly(propylene carbonate maleate) microspheres loaded with
finasteride have been shown to exhibit continuous drug release up
to 5-6 weeks in vitro (14). Furthermore, the topical
application of chitosan-decorated polystyrene-b-poly(acrylic acid)
polymersomes with finasteride improves the drug penetration and
accumulation in the skin layer (15).
Polymer microspheres are widely used in the
pharmaceutical field due to their reduced toxicity, improved
efficacy, and patient convenience and compliance (16). Aliphatic polyester polymers,
including poly(L-lactide), poly(glycoside) and
poly(ε-caprolactone), represent a family of biodegradable materials
(17-19). In particular, biodegradable
polymers, such as poly(lactic-co-glycolic acid) (PLGA), have been
widely used due to their various properties including safety,
biodegradability, compatibility with different hydrophilic and
hydrophobic types of drugs, sustained drug release and targeting of
specific organs (20). PLGA
particles can generally be prepared via phase separation, spray
drying and solvent extraction-evaporation processes. The sustained
or controlled release of the drug from these particles is also
possible, depending on the rate of hydrolysis of the polymer
(21). The chemical structure of
PLGA provides various physicochemical properties depending on the
ratios of lactic and glycolic acids. Selecting the appropriate type
of PLGA is important in ensuring successful drug delivery (22). The use of extended-release
products also offers potential benefits, including sustained drug
levels in the blood, fewer adverse effects and improved patient
compliance. The preparation of controlled-release forms is
therefore important.
In the present study, finasteride-loaded
microspheres were used to enable the controlled release of
finasteride from PLGA microspheres in mice. The study aim was to
confirm the efficacy of a single injection of finasteride-loaded
microspheres on the alleviation of hair loss through an in
vivo experiment, by comparing its effects with those of daily
orally-applied finasteride treatment in mice in order to assess in
the future the possibility of administration in patients with
alopecia.
Materials and methods
Preparation of finasteride-loaded
microspheres
To produce finasteride-loaded microspheres, an
Inventage Lab Precision Particle Fabrication (IVL-PPF) method based
on micro-electromechanical systems was used. Briefly, finasteride
(Aurobindo Pharma, Ltd., Hyderabad, India) was dispersed in 20 ml
dichloromethane (DCM; J.T. Baker, Phillipsburg, NJ, USA) containing
the PLGA 7502A (PURAC Asia Pacific Pte. Ltd., Singapore)
biodegradable polymer for organic phase solutions, and the
surfactant polyvinyl alcohol (PVA; Merck KGaA, Darmstadt, Germany)
was dissolved in purified water at a concentration of 0.25% to
prepare a 250-ml water phase solution. The organic and water phase
solutions were introduced into the microchannels of the IVL-PPF
device and the finasteride-loaded microspheres were manufactured
and collected in a collecting bath. To obtain the pure
finasteride-loaded microspheres, stirring was continued for 3 h at
250 rpm on a magnetic stirrer at temperatures increasing from 17 to
25°C until the DCM had completely evaporated. This was followed by
washing in distilled water, freezing and lyophilizing using a
freeze-drier.
Characterization of finasteride-loaded
microspheres
The prepared microspheres were characterized
according to particle morphology, size, drug content and
microsphere production yield. The microspheres were visualized
using a scanning electron microscope (SEM; SNE-3000MS, SEC Co.,
Ltd., Suwon, Korea) to examine the morphology and the particle
surface structure. A laser-light particle size analyzer (S3550;
Microtrac, Inc., Montgomeryville, PA, USA) was used for particle
size analysis. With regard to drug content, 0.1 mg/ml of
finasteride test solution was prepared by dissolving 50 mg
microspheres in acetonitrile (Sigma-Aldrich; Merck KGaA) to obtain
100 ml solution, which was analyzed via high-performance liquid
chromatography (HPLC; ULTIMATE-3000, Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Animals
Male, 5-week-old C57BL/6 mice were supplied by
RaonBio, Inc. (Yongin, Korea). Solid feed (antibiotic-free) and
water were sufficiently supplied until the day of the experiment,
and the animals that were used for the experiment were acclimatized
for 1 week to a temperature of 23±2°C, humidity of 55±10% and 12-h
light/dark cycles. All experimental procedures were conducted based
on the principles of laboratory animal care of the National
Institute of Health (Bethesda, MA, USA) and were approved by the
Ethics Committee for Laboratory Animals at Chung-Ang University
(Seoul, Korea; no. 201800078).
Androgenic alopecia mouse model
Treatment with reagents was performed as described
previously with modifications (23). The hair on the back of the male
mice was removed with an electric clipper. A total of 60 mice were
randomly divided into four groups of 15 mice. All mice, with the
exception of those in the control group, were subcutaneously
injected with 0.1 ml of 5 mg/ml testosterone propionate (TP; Tokyo
Chemical Industry, Tokyo, Japan) once daily for 8 weeks. The mice
in the microspheres- and finasteride-loaded microspheres-treated
(15 mg/ml) groups were subcutaneously injected with 0.1 ml on the
first day of the experiment, and mice in the orally-applied
finasteride-treated group (0.1 mg/ml) were orally administered with
0.1 ml finasteride once daily for 56 days. Mice in the control
group were not treated with TP or finasteride. After 8 weeks, hair
growth was observed for a period of 2 weeks. The differences in
hair growth in each group were evaluated visually and recorded
weekly through image capture (Canon 3000D; Canon, Inc., Tokyo,
Japan). The effect of preventing androgenic alopecia was assessed
by measuring changes in the color of the mouse back skin, which
indicates hair cycle transitions. Mice with completely hair-covered
backs were counted as 'total' and partially hairy mice as
'partial'. Histological changes, including the number, length,
anagen/telogen ratio and diameter of hair follicles, were also
evaluated by hematoxylin and eosin (H&E) staining. The
experiment was performed over a total of 10 weeks. After 9 and 10
weeks, the mice were sacrificed, and their skins were
harvested.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from isolated skin tissues
using Tri-RNA reagent (Favorgen Biotech Corporation, Ping-Tung,
Taiwan). Single-stranded cDNA was synthesized from whole RNA
templates using Prime Script™ RT Master mix (Takara Bio, Inc.,
Tokyo, Japan). RT was performed after gently mixing the reaction
solution; the solution was then incubated at 37°C for 15 min and
85°C for 5 sec; cDNA was maintained at 4°C until qPCR. The
resulting cDNA was subjected to qPCR using qPCR 2X PreMIX SYBR
(Enzynomics, Seoul, Korea) with the CFX-96 system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The PCR used to amplify all
genes was performed over 40 cycles (95°C for 10 sec, 60°C for 15
sec and 72°C for 30 sec), following denaturation at 95°C for 10
min. The expression levels were calculated from the quantification
cycle (Cq) value using the ΔCq quantification method (24), and expression was normalized to
that of 60S acidic ribosomal protein P0 (Rplp0).
Amplification was performed using the following primers: Mouse
Wnt3a forward, 5′-CTCGCTGGCTACCCAATTTG-3′ and reverse,
5′-CTTCACACCTTCTGCTACGCT-3′; mouse dickkopf WNT signaling pathway
inhibitor 1 (Dkk1) forward, 5′-CTCATCAATTCCAACGCGATCA-3′ and
reverse, 5′-GCCCTCATAGAGAACTCCCG-3′; mouse alkaline phosphatase
(Alpl) forward, 5′-CCAACTCTTTTGTGCCAGAGA-3′ and reverse,
5′-GGCTACATTGGTGTTGAGCTTTT-3′; and mouse Rplp0 forward,
5′-AGATTCGGGATATGCTGTTGGC-3′ and reverse,
5′-TCGGGTCCTAGACCAGTGTTC-3′.
Immunoblot assay
The isolated skin tissues were dissolved in PRO-PREP
(Intron Biotechnology, Inc., Seongnam, Korea) and centrifuged at
14,000 × g for 20 min at 4°C. Protein content in the supernatants
was quantified using a BCA kit (Thermo Fisher Scientific, Inc.).
The proteins in the supernatant (total protein content, 30
µg) were heated for 5 min at 100°C. Each sample was
subjected to 12% sodium dodecyl sulfate-poly-acrylamide gel
electrophoresis, and the separated proteins were transferred onto a
polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica,
MA, USA). The PVDF membrane was blocked using 5% skim milk for 1 h
at room temperature and incubated with anti-transforming growth
factor (TGF)-β2 (1:1,000, cat. no. sc-90; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), caspase-3 (1:2,500, cat. no. 9662),
poly (ADP-ribose) polymerase (PARP; 1:2,500, cat. no. 9532S; both
Cell Signaling Technology Inc., Beverly, MA, USA), B-cell lymphoma
2 (Bcl-2; 1:1,000, cat. no. sc-492), Bcl-2-associated X protein
(Bax; 1:1,000, cat. no. sc-7480; both Santa Cruz Biotechnology,
Inc.), Wnt3a (1:2,500, cat. no. ab28472; Abcam, Cambridge, UK),
phospho (p)-glycogen synthase kinase 3β (GSK3β; 1:2,500, cat. no.
5558), GSK3β (1:2,500, cat. no. 9315), p-β-catenin (1:2,500, cat.
no. 4176S), β-catenin (1:2,500, cat. no. 8814S; all Cell Signaling
Technology, Inc.), and β-actin (1:1,000, cat. no. sc-47778; Santa
Cruz Biotechnology, Inc.) antibodies at 4°C for 12 h. After washing
with Tris-buffered saline with Tween®-20 (TBST),
horseradish peroxidase (HRP)-conjugated anti-mouse (1:10,000; cat.
no. PI-2000) or anti-rabbit (1:10,000; cat. no. PI-1000) secondary
antibodies (Vector Laboratories, Inc., Burlingame, CA, USA)
specific to the primary antibody was added and incubated at room
temperature for 1 h. After washing, the membranes were
color-developed using electrochemiluminescence solution (EMD
Millipore) and assessed using a ChemiDoc™ XRS+ system (Bio-Rad
Laboratories, Inc.).
Histological examination
The tissue slides were prepared according to methods
described previously (25), and
the tissues were sliced into 6-µm sections. Deparaffinized
skin sections were stained with H&E staining (hematoxylin for 5
min and eosin for 30 sec at room temperature). Following staining,
the tissues were dehydrated, sealed and examined using an optical
microscope (DM750; Leica Microsystems GmbH, Wetzlar, Germany). The
follicular length and diameter were evaluated using the
longitudinal section parallel to the direction of hair growth. The
follicular number, anagen follicles (A), and telogen follicles (T)
were measured, and the A/T ratio was calculated by analyzing the
transverse section.
Immunohistochemistry was performed using the
UltraVision LP Large Volume Detection System HRP Polymer kit
(Thermo Fisher Scientific, Inc.). Anti-β-catenin (1:500, cat. no.
8814S; Cell Signaling Technology, Inc.) and anti-TGF-β2 (1:500,
cat. no. sc-90; Santa Cruz Biotechnology, Inc.) primary antibodies
were used for incubation at 4°C for 18 h in a moist chamber. After
washing of the slides, a primary antibody enhancer was added for 10
min, and a HRP polymer solution was added for further incubation at
room temperature for 15 min. After washing, the slides were
color-developed using 3,3′-diaminobenzidine for 10-30 sec and were
dehydrated and sealed for observation following contrast staining
with Mayer's hematoxylin. After staining, tissues were observed via
optical microscopy (DM750; Leica Microsystems GmbH).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) staining
To evaluate the apoptosis of hair follicles, TUNEL
staining was performed using a DeadEnd™ Fluorometric TUNEL system
(Promega Corporation, Madison, WI, USA). The tissue slides were
deparaffinized and rehydrated, and the tissue sections were fixed
in 4% methanol-free formaldehyde solution in PBS for 15 min at room
temperature. After washing twice in PBS, each slide was incubated
with 20 µg/ml Proteinase K for 10 min at room temperature.
The tissue sections were then fixed in 4% methanol-free
formaldehyde for 5 min at room temperature. After washing in PBS,
the slides were incubated with a nucleotide mix and recombinant
terminal deoxynucleotidyl transferase for 1 h at 37°C, avoiding
exposure to light. The reaction was terminated by the addition of a
stop solution for 15 min at room temperature. After washing in PBS,
the tissues were mounted in VECTASHEILD mounting medium with
4′,6-diamidino-2-phenylindole (DAPI; cat. no. H-1200; Vector
Laboratories, Inc.), and green fluorescence of the dead cells
(fluorescein-12-dUTP) was detected. Fluorescence images were
captured via confocal microscopy (LSM 700; Zeiss GmbH, Jena,
Germany).
Measurement of total serum testosterone
and DHT
The blood samples (1.5 ml) collected from mice were
centrifuged at 4°C and 1,000 × g for 30 min. Following
centrifugation, the supernatant was stored at -70°C until further
analysis. Serum testosterone (cat. no. CSB-E05101m) and DHT (cat.
no. CSB-E07880m) were measured via enzyme linked immunosorbent
assays (ELISA; Cusabio, Wuhan, China). Each sample (50 µl
each in duplicate) was placed in a 96-well plate, and 50 µl
of HRP-conjugate and 50 µl of antibodies (testosterone or
DHT) were added to each well, followed by incubation for 1 h at
37°C. After washing three times with wash buffer, 50 µl of
substrate A and 50 µl of substrate B were added and
incubated for 15 min at 37°C for color development. The reaction
was then terminated by the addition of 50 µl stop solution,
and the absorbance was measured at 450 nm using a Spectra Max i3x
Multi-Mode detection platform (Molecular Devices, LLC, Sunnyvale,
CA, USA).
Statistical analysis
All statistical analyses were performed using the
Statistical Package for the Social Sciences (IBM SPSS statistics
version 25, IBM Corp., Armonk, NY, USA). The experiment results are
expressed as the mean ± standard error of the mean, and statistical
significance was evaluated via one-way or two-way analysis of
variance for the levels of serum testosterone and DHT. Tukey's
honest significance test was performed post hoc, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of finasteride-loaded
microspheres
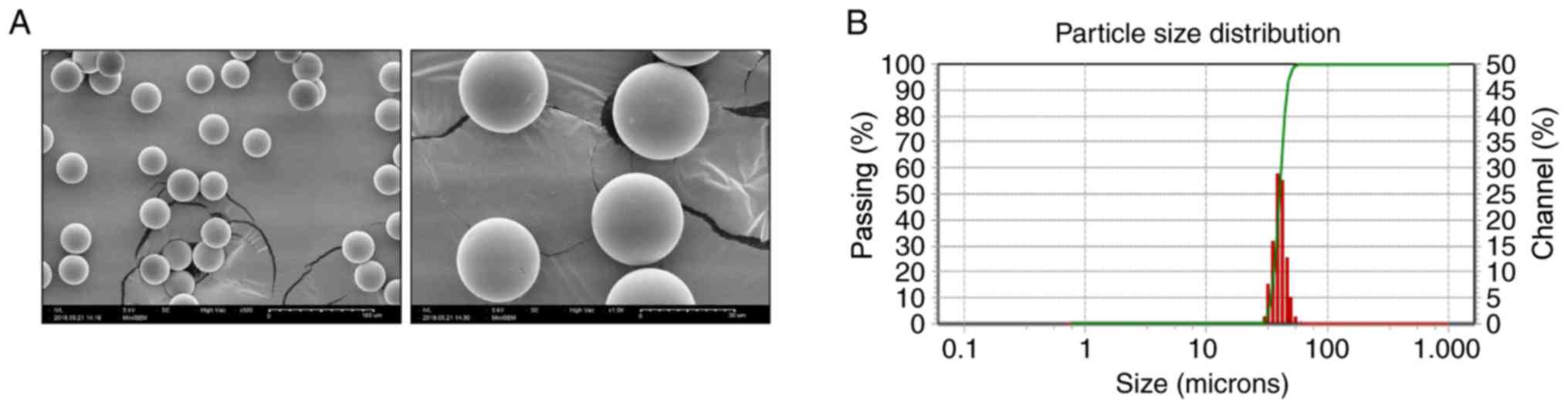
The morphology and size of microspheres containing
finasteride were analyzed. The SEM analysis of the prepared
finasteride-loaded microspheres revealed smooth and perfectly
spherical microspheres and the absence of collapsed spheres
(Fig. 1A). Finasteride was
encapsulated in the PLGA polymer and homogeneously prepared, and
the micrographs did not exhibit any pores on the microspheres. The
diameter of the prepared finasteride-loaded microspheres was ~30-50
µm as determined via SEM analysis. The particle size and
distribution of the finasteride-loaded microspheres were measured
using a laser diffraction particle size analyzer. The particle
diameter ranged between 30 and 50 µm with a median diameter
of 40 µm and a size distribution width of 9.29 µm.
That indicates the majority of particles were similar in size,
close to the median diameter, as shown in Fig. 1B; a narrower size distribution of
particles generally improves drug release characteristics.
The contents of the prepared finasteride-loaded
micro-spheres were confirmed via HPLC analysis, and the unique
finasteride standard peaks (retention time, 3.57 min; peak area,
24.40) and the drug peaks (retention time, 3.52 min; peak area,
24.20) were examined (Fig. 1C-E).
The drug content measured in the microspheres was 99.2%.
Subcutaneous administration of
finasteride-loaded microspheres induces hair follicle growth via
the inhibition of 5α-reductase in a testosterone-induced AGA mouse
model
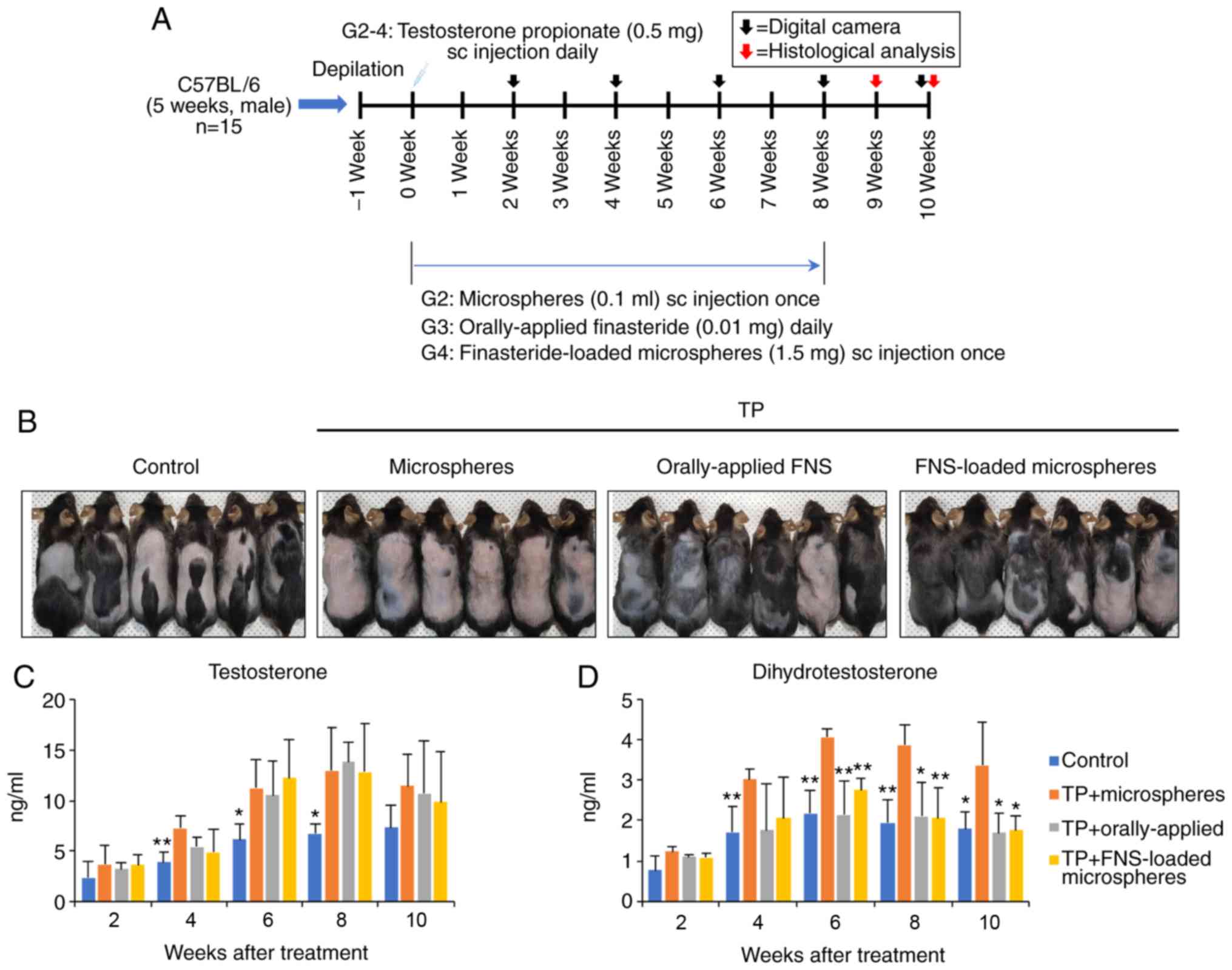
To investigate the effect of finasteride-loaded
microspheres on AGA in the murine model, hair loss was induced in
the dorsal skin of C57BL/6 mice via testosterone treatment
(Fig. 2A). In the control mice,
hair growth was observed from week 4 and in 80% of the mice (total
hair growth, 46.6%; partial hair growth, 33.3%). However, hair
began to grow from week 6 in the orally-applied finasteride and the
finasteride-loaded micro-spheres treatment groups. At week 10, hair
growth was observed in 86.7% of mice (total, 60%; partial, 26.7%)
in the orally-applied finasteride-treated group, and 93.3% of mice
(total, 60%; partial, 33.3%) in the finasteride-loaded
microspheres-treated group. In the microspheres-treated group, hair
grew in 33.3% of the mice (total, 6.7%; partial, 26.7%) (Figs. 2B and S1).
The levels of serum testosterone and DHT in the
TP-induced mice were significantly higher than those in the control
group in the first 8 weeks (Fig. 2C
and D). However, the DHT levels were significantly lower in the
orally-applied finasteride- and finasteride-loaded
microspheres-treated groups than in the microspheres-treated group
after 6 weeks. There was no difference between the orally-applied
finasteride- and finaste-ride-loaded microspheres-treated groups.
These results suggest that the 5α-reductase inhibitory effect of
the finasteride-loaded microspheres may last for ~4-8 weeks
following a single injection, and that treatment with
finasteride-loaded microspheres exerts similar effects to daily
orally-applied finasteride.
Finasteride-loaded microspheres reduce
testosterone-induced catagen in C57BL/6 AGA mice
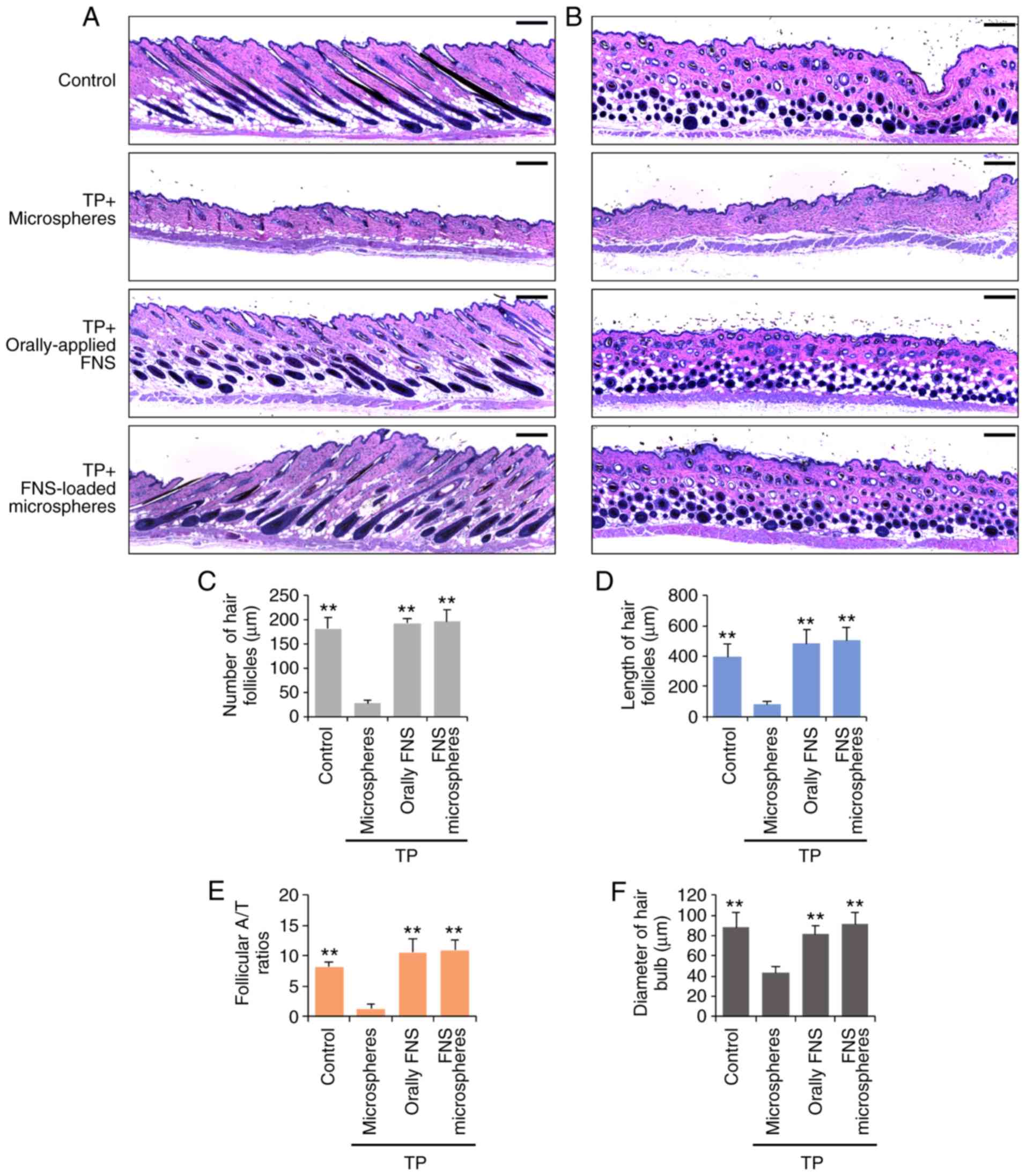
The tissues observed in the longitudinal and
transverse sections revealed that the hair follicle tissue was in
the anagen phase (Figs. 3A and B
and S2). The miniaturization of
hair follicles was observed in the skin sections of mice treated
with TP and the microspheres, however, the number and length of
hair follicles was markedly increased in the orally-applied
finasteride- and finasteride-loaded microspheres-treated groups
(Figs. 3C and D, S2C and D). At weeks 9 and 10, the
orally-applied finasteride- and finasteride-loaded
microspheres-treated mice exhibited significantly higher numbers of
anagen hair follicles (A/T ratio), and the hair bulb diameters were
larger than those in the microspheres-treated mice (Figs. 3C and S2C). In addition, the expression of
Wnt3a increased, the protein kinase B (Akt)/GSK-3β/β-catenin
signaling pathway was activated and the expression of β-catenin was
increased in the newly developed hair follicles (Fig. 4A-C). Taken together, these results
indicate that a single injection of finasteride-loaded
micro-spheres results in marked hair growth-promoting activity and
catagen-induction preventative effects, similar to those of
orally-applied finasteride.
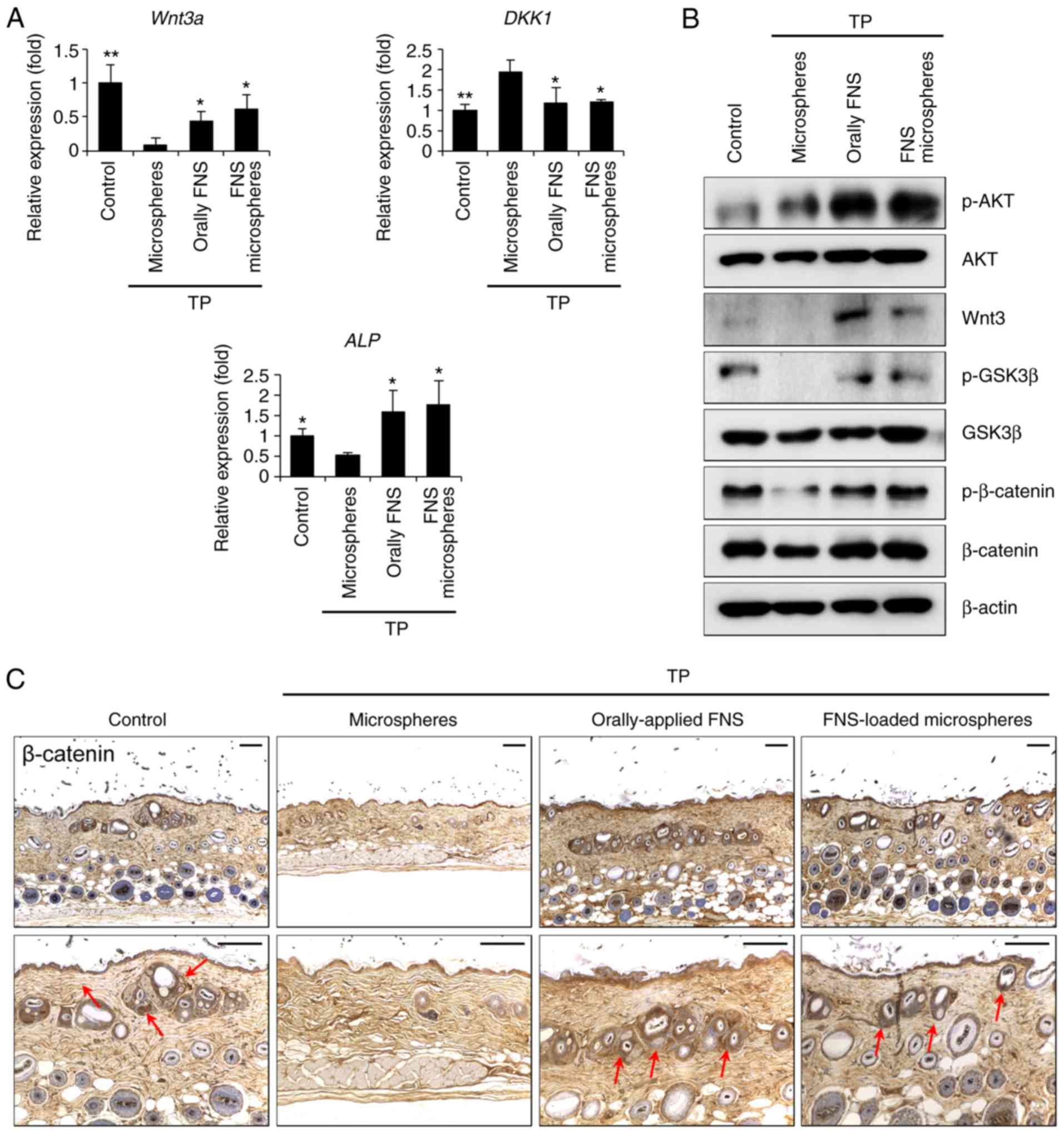 | Figure 4FNS-loaded microspheres efficiently
induce entry into the anagen phase in a testosterone-induced
androgenic alopecia mouse model. Mice were sacrificed and skin
biopsies were obtained 9 weeks after the start of treatment. (A)
Following treatment with testosterone and orally-applied FNS or
FNS-loaded microspheres, Wnt3a, Dkk1 and Alp
transcripts were detected via reverse transcription-quantitative
polymerase chain reaction analysis. (B) Dorsal skin lysates were
immunoblotted using anti-p-Akt, anti-Wnt3a, anti-p-GSK-3β,
anti-p-β-catenin and anti-β-actin antibodies. (C) Comparison of
histological images (β-catenin indicated by red arrows). Scale bar,
100 µm. All data are presented as the mean ± standard error
of the mean from three independent experiments.
*P<0.05 and **P<0.01, vs.
microspheres-treated group. TP, testosterone propionate; FNS,
finasteride; DKK1, dickkopf WNT signaling pathway inhibitor 1; ALP,
alkaline phosphatase; AKT, protein kinase B; GSK-3β, glycogen
synthase kinase 3β; p-, phosphorylated. |
Finasteride-loaded microspheres suppress
hair follicle apoptosis in a testosterone-induced AGA mouse
model
DHT leads hair follicles into the catagen phase,
which leads to increased levels of TGF-β2 and the apoptosis of hair
follicle cells (26). The
increase in TGF-β2 alters the regulation of Bcl-2 and caspase-3,
thereby affecting the apoptosis of hair cells. The expression of
Bcl-2 in the finasteride-loaded microspheres-treated group was
increased, whereas the expression levels of cleaved-PARP,
cleaved-caspase-3, TGF-β2 and Bax were lower than those in the
microspheres-treated group (Fig. 5A
and B). In addition, TGF-β2 was expressed and TUNEL staining,
which indicated DNA damage by apoptosis, was observed in the hair
follicles of the testosterone-treated mice, in which the catagen
and telogen phases were maintained. However, the expression of
TGF-β2 and TUNEL staining were reduced in the orally-applied
finasteride- and finasteride-loaded microspheres-treated groups, in
which hair follicle growth was induced in the anagen phase
(Fig. 5C and D). These results
suggest that a single injection of finasteride-loaded micro-spheres
interferes with the expression of apoptotic factors in
testosterone-exposed hair follicles.
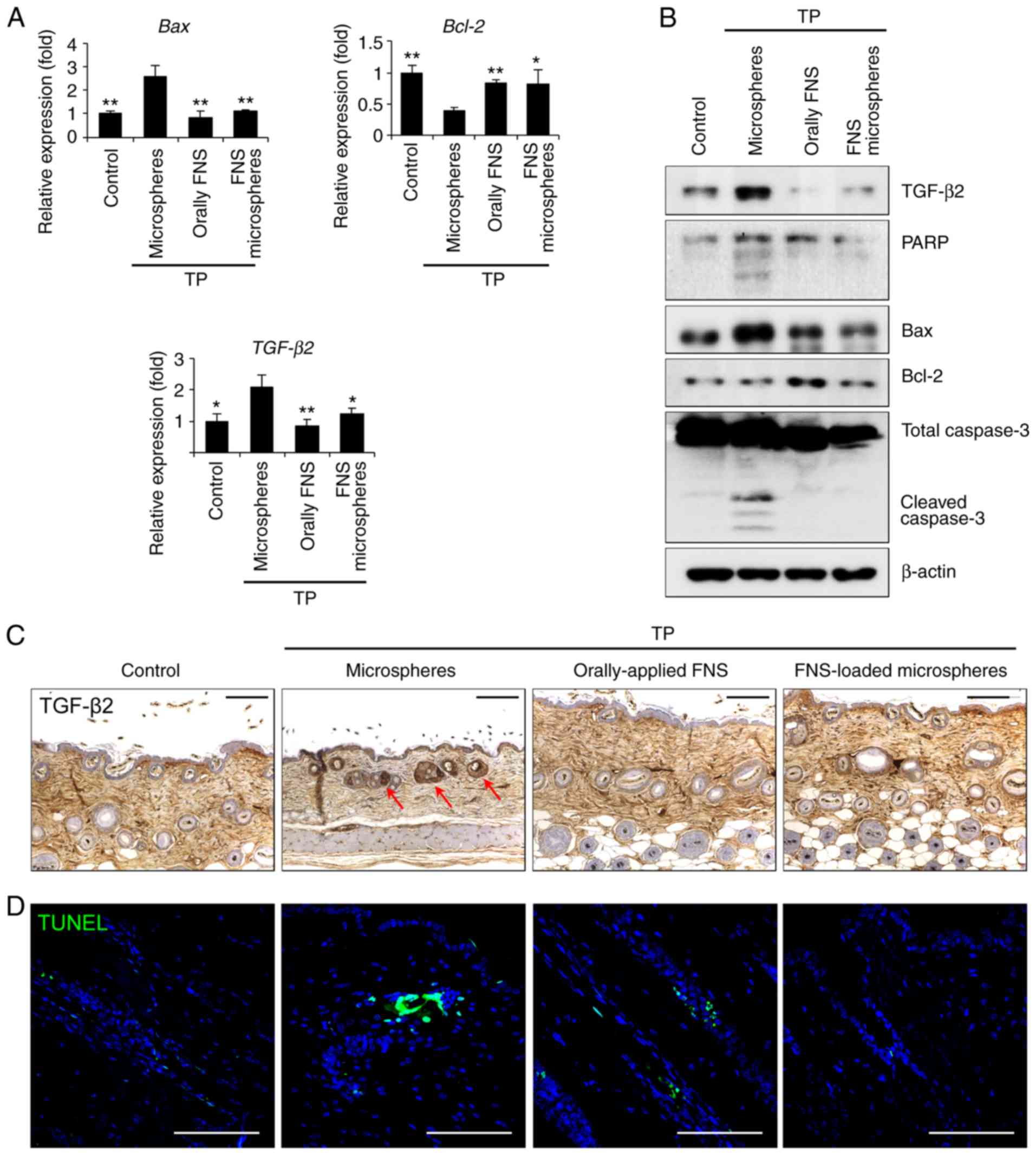 | Figure 5FNS-loaded microspheres hinder
testosterone-induced apoptosis in mouse hair follicles. Mice were
sacrificed and skin biopsies were obtained 9 weeks after the start
of treatment. (A) Following treatment with testosterone and
orally-applied FNS or FNS-loaded microspheres, Bcl-2, Bax
and TGF-β2 transcripts were detected via
reverse-transcription-quantitative polymerase chain reaction
analysis. (B) Dorsal skin lysates were immunoblotted using
anti-TGF-β2, anti-PARP, anti-Bcl-2, anti-Bax, anti-cleaved
caspase-3 and anti-β-actin antibodies. (C) Comparison of
histological images (TGF-β2 indicated by red arrows). (D) Positive
staining of damaged cell nucleus with TUNEL stain (green
fluorescence). Scale bar, 100 µm. All data are presented as
the mean ± standard error of the mean from three independent
experiments. *P<0.05 and **P<0.01, vs.
microspheres-treated group. TP, testosterone propionate; FNS,
finasteride; TGF-β2, transforming growth factor-β2, PARP, poly (ADP
ribose) polymerase; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated
X-protein; TUNEL, terminal deoxynucleotidyl transferase dUTP nick
end labeling. |
Discussion
The present study was performed to investigate the
effectiveness of one-off subcutaneous dosing of finasteride-loaded
microspheres (finasteride:PLGA=1:4) on the inhibition of
5α-reductase compared with that of repeated oral dosing of
finasteride, and to evaluate the anagen entry time in a TP-induced
AGA mouse model over 10 weeks. Hair shafts began to form in some
mice at week 4 in the control group, and at week 6 in the
orally-applied finasteride- and the subcutaneous finasteride-loaded
microspheres-treated groups. The hair growth effects (5α-reductase
inhibitory effects) exerted by orally-applied finasteride- and
finasteride-loaded microspheres were similar. In addition, serum
DHT was significantly decreased in the orally-applied finasteride-
and finasteride-loaded microspheres-treated groups compared with
that in the microspheres-treated group, indicating similar
5α-reductase inhibitory effects after 8 weeks. The activity of DHT
is ~10 times more potent than that of testosterone, and suppresses
hair growth by acting on the hair root (27). Androgen receptors are present only
in DPCs, which are important in hair follicle formation, and not in
hair follicular matrix cells derived from epidermal cells. DHT
binds to DPCs and either inhibits the secretion of substances that
stimulate hair follicular matrix growth or secretes substances that
suppress hair growth (28).
Finasteride is widely used in AGA, which
significantly improves hair growth and slows hair loss compared
with placebo (11,29). However, finasteride is not
recommended for use in women due to the risk of birth defects in
women of childbearing age and its high transdermal absorption. In
men, the risk of developing sexual dysfunction and the
inconvenience of long-term daily administration of oral finasteride
have increased. The US Food and Drug Administration (FDA) announced
a specific label change on April 11, 2012 to include libido,
erectile, ejaculatory and orgasmic disorders as side effects for
Propecia (finasteride 1 mg) and Proscar (finasteride 5 mg), which
have been reported to continue following discontinuation of the
drug (30). Studies have shown
that sexual arousal occurs at a rate of 3.4-15.8%, erectile
dysfunction is the most common side effect, and ejaculatory
disorder and decreased libido occur at the beginning of treatment
(10,11,31-34). These effects returned to normal on
stopping or in time following continuous use of the drug (10,32). Current evidence from finasteride
indicates safety, although concerns regarding sexual side effects
are increasing. Therefore, investigations on the development of a
systemic delivery system in controlled release form with fewer
adverse effects is important (14,35,36). Dutasteride, as another therapeutic
agent, is an inhibitor of type I and type II 5α-reductase, whereas
finasteride inhibits only the type II enzyme. Dutasteride is
approximately three times and 100 times more potent than
finasteride for type I and type II, respectively (37). However, ~50% of participants
treated with dutasteride for 48 weeks have reported one or more
adverse effects, including nasopharyngitis and erectile dysfunction
(38,39). Furthermore, studies involving
6,460 patients concluded that dutasteride was effective for
improving benign prostate hyperplasia, but was associated with an
increased risk of sexual dysfunction (40,41). In addition, dutasteride has been
demonstrated to be effective in several trials in AGA, but it is
not approved by the US FDA for AGA due to possible adverse effects,
including reduced sperm count, gynecomastia and drug-drug
interactions (42).
A single dose of finasteride-loaded microspheres
effectively alleviated hair loss for >4 weeks, and its
effectiveness for up to 8 weeks was confirmed. The total 30-day
dose administered was based on orally-applied finasteride dosage,
and it was suggested that the effects of a lower dose administered
over a longer period may be similar to those of orally-applied
finasteride daily for 8 weeks. Furthermore, a single injection can
reduce the inconvenience of daily administration and the
requirement for drug storage.
In conclusion, the present study demonstrated the
inhibitory effect of finasteride-loaded microspheres on
5α-reductase. Finasteride-loaded microspheres improved
testosterone-induced hair loss, and its effect in a single dose on
hair follicle tissue growth was similar to that of daily
orally-applied finasteride for up to 8 weeks. This suggests that
the effect of finasteride-loaded microspheres does not appear
immediately following administration, but rather that there is a
delayed onset of drug efficacy following the initial dosing. The
present study showed that the growth period, length and number of
hairs following orally-applied finasteride- and finasteride-loaded
microspheres administration were similar, although the injection
dose was lower. Therefore, the evaluation of a novel dosage form
demonstrated the possibility of an effective novel dose and
administration route for finasteride. However, the present study
included only a short-term experiment and did not investigate the
intra-body distribution of finasteride-loaded microspheres.
Therefore, long-term studies in mice are required for the
acceptance of dosage and periodicity of injections in the scalp,
followed by long-term experiments assessing the distribution and
permanence of finasteride-loaded microspheres by injection in
further investigations.
Supplementary Materials
Funding
The present study was supported by Inventage Lab,
Inc. (Seongnam, Korea; grant no. 20171134).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HSL, BJK, JHK and JN designed experiments; JHK, JN,
DHB, BCL, EL, MJC, CHR, and SL performed experiments; HSL, BJK,
JHK, JN, CHR, SL, SKM and BCP analyzed and interpreted data; JHK,
JN and DHB prepared figures; HSL, BJK, JHK and JN wrote the
manuscript. HSL and BJK had primary responsibility for final
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Chung-Ang University
(2018-00078).
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Sinclair R: Male pattern androgenetic
alopecia. BMJ. 317:865–869. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cash TF: The psychology of hair loss and
its implications for patient care. Clin Dermatol. 19:161–166. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whiting DA: Possible mechanisms of
miniaturization during androgenetic alopecia or pattern hair loss.
J Am Acad Dermatol. 45(Suppl 3): S81–S86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urysiak-Czubatka I, Kmieć ML and
Broniarczyk-Dyła G: Assessment of the usefulness of
dihydrotestosterone in the diagnostics of patients with
androgenetic alopecia. Postepy Dermatol Alergol. 31:207–215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aggarwal S, Thareja S, Verma A, Bhardwaj
TR and Kumar M: An overview on 5alpha-reductase inhibitors.
Steroids. 75:109–153. 2010. View Article : Google Scholar
|
|
6
|
Azzouni F, Godoy A, Li Y and Mohler J: The
5 alpha-reductase isozyme family: A review of basic biology and
their role in human diseases. Adv Urol. 2012:5301212012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McConnell JD, Wilson JD, George FW, Geller
J, Pappas F and Stoner E: Finasteride, an inhibitor of 5
alpha-reductase, suppresses prostatic dihydrotestosterone in men
with benign prostatic hyperplasia. J Clin Endocrinol Metab.
74:505–508. 1992.PubMed/NCBI
|
|
8
|
Gormley GJ, Stoner E, Bruskewitz RC,
Imperato-McGinley J, Walsh PC, McConnell JD, Andriole GL, Geller J,
Bracken BR, Tenover JS, et al: The effect of finasteride in men
with benign prostatic hyperplasia. The Finasteride Study Group. N
Engl J Med. 327:1185–1191. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drake L, Hordinsky M, Fiedler V, Swinehart
J, Unger WP, Cotterill PC, Thiboutot DM, Lowe N, Jacobson C,
Whiting D, et al: The effects of finasteride on scalp skin and
serum androgen levels in men with androgenetic alopecia. J Am Acad
Dermatol. 41:550–554. 1999.PubMed/NCBI
|
|
10
|
Kaufman KD, Olsen EA, Whiting D, Savin R,
DeVillez R, Bergfeld W, Price VH, Van Neste D, Roberts JL,
Hordinsky M, et al: Finasteride in the treatment of men with
androgenetic alopecia. Finasteride Male Pattern Hair Loss Study
Group. J Am Acad Dermatol. 39:578–589. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Finasteride Male Pattern Hair Loss Study
Group: Long-term (5-year) multinational experience with finasteride
1 mg in the treatment of men with androgenetic alopecia. Eur J
Dermatol. 12:38–49. 2002.PubMed/NCBI
|
|
12
|
Whiting DA, Olsen EA, Savin R, Halper L,
Rodgers A, Wang L, Hustad C and Palmisano J; Male Pattern HairLoss
Study Group: Efficacy and tolerability of finasteride 1 mg in men
aged 41 to 60 years with male pattern hair loss. Eur J Dermatol.
13:150–160. 2003.PubMed/NCBI
|
|
13
|
Mella JM, Perret MC, Manzotti M, Catalano
HN and Guyatt G: Efficacy and safety of finasteride therapy for
androgenetic alopecia: A systematic review. Arch Dermatol.
146:1141–1150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng D, Huang K, Liu Y and Liu S:
Preparation of novel polymeric microspheres for controlled release
of finasteride. Int J Pharm. 342:82–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caon T, Porto LC, Granada A, Tagliari MP,
Silva MA, Simões CM, Borsali R and Soldi V: Chitosan-decorated
polystyrene-b-poly(acrylic acid) polymersomes as novel carriers for
topical delivery of finasteride. Eur J Pharm Sci. 52:165–172. 2014.
View Article : Google Scholar
|
|
16
|
Freiberg S and Zhu X: Polymer microspheres
for controlled drug release. Int J Pharm. 282:1–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nojehdehian H and Ekrami M: Loading of
gentamicin sulfate into poly (lactic-co-glycolic acid)
biodegradable microspheres. Shahid Beheshti University. J Dent Sch.
33:145–151. 2015.
|
|
18
|
Sinha V, Bansal K, Kaushik R, Kumria R and
Trehan A: Poly-ϵ-caprolactone microspheres and nanospheres: An
overview. Int J Pharma. 278:1–23. 2004. View Article : Google Scholar
|
|
19
|
Burns SA, Hard R, Hicks WL Jr, Bright FV,
Cohan D, Sigurdson L and Gardella JA Jr: Determining the protein
drug release characteristics and cell adhesion to a PLLA or PLGA
biodegradable polymer membrane. J Biomed Mater Res A. 94:27–37.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Danhier F, Ansorena E, Silva JM, Coco R,
Le Breton A and Préat V: PLGA-based nanoparticles: An overview of
biomedical applications. J Control Release. 161:505–522. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Corrigan OI and Li X: Quantifying drug
release from PLGA nanoparticulates. Eur J Pharm Sci. 37:477–485.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mu L and Feng S: A novel controlled
release formulation for the anticancer drug paclitaxel (Taxol):
PLGA nanoparticles containing vitamin E TPGS. J Control Release.
86:33–48. 2003. View Article : Google Scholar
|
|
23
|
Wang ZD, Feng Y, Ma LY, Li X, Ding WF and
Chen XM: Hair growth promoting effect of white wax and policosanol
from white wax on the mouse model of testosterone-induced hair
loss. Biomed Pharmacother. 89:438–446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Kim YJ, Choi MJ, Bak DH, Lee BC, Ko EJ,
Ahn GR, Ahn SW, Kim MJ, Na J and Kim BJ: Topical administration of
EGF suppresses immune response and protects skin barrier in
DNCB-induced atopic dermatitis in NC/Nga mice. Sci Rep.
8:118952018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hibino T and Nishiyama T: Role of
TGF-beta2 in the human hair cycle. J Dermatol Sci. 35:9–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen W, Zouboulis CC and Orfanos CE: The
5-alpha reductase system and its inhibitors. Recent development and
its perspective in treating androgen-dependent skin disorders.
Dermatology. 193:177–184. 1996. View Article : Google Scholar
|
|
28
|
Thornton MJ, Messenger AG, Elliott K and
Randall VA: Effect of androgens on the growth of cultured human
dermal papilla cells derived from beard and scalp hair follicles. J
Invest Dermatol. 97:345–348. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato A and Takeda A: Evaluation of
efficacy and safety of finasteride 1 mg in 3,177 Japanese men with
androgenetic alopecia. J Dermatol. 39:27–32. 2012. View Article : Google Scholar
|
|
30
|
Mysore V and Shashikumar B: Guidelines on
the use of finasteride in androgenetic alopecia. Indian J Dermatol
Venereol Leprol. 82:128–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirshburg JM, Kelsey PA, Therrien CA,
Gavino AC and Reichenberg JS: Adverse effects and safety of 5-alpha
reductase inhibitors (finasteride, dutasteride): A systematic
review. J Clin Aesthet Dermatol. 9:56–62. 2016.PubMed/NCBI
|
|
32
|
Wessells H, Roy J, Bannow J, Grayhack J,
Matsumoto AM, Tenover L, Herlihy R, Fitch W, Labasky R, Auerbach S,
et al: Incidence and severity of sexual adverse experiences in
finasteride and placebo-treated men with benign prostatic
hyperplasia. Urology. 61:579–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moinpour CM, Darke AK, Donaldson GW,
Thompson IM Jr, Langley C, Ankerst DP, Patrick DL, Ware JE Jr, Ganz
PA, Shumaker SA, et al: Longitudinal analysis of sexual function
reported by men in the prostate cancer prevention trial. J Natl
Cancer Inst. 99:1025–1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carbone D Jr and Hodges S: Medical therapy
for benign prostatic hyperplasia: Sexual dysfunction and impact on
quality of life. Int J Impot Res. 15:299–306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peng D, Huang K, Liu Y, Liu S, Wu H and
Xiao H: Preparation of carbon dioxide/propylene
oxide/ε-caprolactone copolymers and their drug release behaviors.
Polymer Bull. 59:117–125. 2007. View Article : Google Scholar
|
|
36
|
Ahmed OA, Hussein AK and Mady FM:
Optimisation of microstructured biodegradable finasteride
formulation for depot parenteral application. J Microencapsul.
33:229–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clark RV, Hermann DJ, Cunningham GR,
Wilson TH, Morrill BB and Hobbs S: Marked suppression of
dihydrotestosterone in men with benign prostatic hyperplasia by
dutasteride, a dual 5α-reductase inhibitor. J Clin Endocrinol
Metab. 89:2179–2184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai TF, Choi GS, Kim BJ, Kim MB, Ng CF,
Kochhar P, Jasper S, Brotherton B, Orban B and Lulic Z: Prospective
randomized study of sexual function in men taking dutasteride for
the treatment of androgenetic alopecia. J Dermatol. 45:799–804.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fertig R, Shapiro J, Bergfeld W and Tosti
A: Investigation of the plausibility of 5-alpha-reductase inhibitor
syndrome. Skin Appendage Disord. 2:120–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wilt TJ, MacDonald R, Hagerty K,
Schellhammer P, Tacklind J, Somerfield MR and Kramer BS:
5-α-reductase inhibitors for prostate cancer chemoprevention: An
updated Cochrane systematic review. BJU Int. 106:1444–1451. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu XJ, Zhi Y, Zheng J, He P, Zhou XZ, Li
WB and Zhou ZS: Dutasteride on benign prostatic hyperplasia: A
meta-analysis on randomized clinical trials in 6,460 patients.
Urology. 83:539–543. 2014. View Article : Google Scholar
|
|
42
|
Arif T, Dorjay K, Adil M and Sami M:
Dutasteride in androge-netic alopecia: An update. Curr Clin
Pharmacol. 12:31–35. 2017. View Article : Google Scholar
|