Introduction
Bladder cancer (BCa) is the most common malignancy
of the urinary system in China (1) and worldwide (2), and is the primary cause of mortality
in patients with urinary tract disease (3). Muscle-invasive BCa (MIBC) represents
25-40% of all BCa, and may spread from the bladder to the pelvic
lymph nodes and then to visceral organs (4). The 5-year mortality rate of MIBC
patients with lymph node (LN) metastasis (77.6%) is higher compared
with that of MIBC patients without LN metastasis (18.6%) even when
treatment with radical cystectomy was available (5,6).
Metastasis to LNs and distant organs is a complex multistep process
that involves dissemination of cancer cells to lymphatic vessels,
transport, settlement and colonization expansion of cancer cells
(7,8). However, the biological
characteristics and molecular mechanisms of BCa cell invasion and
metastasis remains largely unknown.
Long non-coding RNAs (lncRNAs) are a class of poorly
conserved endogenous RNAs >200 nucleotides that do not encode
proteins but regulate gene expression (9). On a functional level, lncRNAs are
involved in complex biological processes via diverse mechanisms.
These comprise, among others, gene regulation by titration of
transcription factors, alternative splicing, sponging of microRNAs
(miRNAs) and recruitment of chromatin modifying enzymes (10-13). Accumulating evidence indicates
that lncRNAs serve diverse roles in the initiation and progression
of human cancer. For example, lncRNAs HOX transcript antisense RNA,
upregulated in colorectal cancer liver metastasis and small
nucleolar RNA host gene 14 participate in the metastatic cascade by
regulating cell migration and invasion (14-16). lncRNAs urothelial cancer
associated 1 and H19 have been demonstrated to serve critical roles
in bladder cancer metastasis.
The lncRNA zinc finger E-box-binding homeobox 1
antisense 1 (ZEB1-AS1) derives from the promoter region of ZEB1, a
transcriptional factor that serves important roles in physiology
and tumorigenesis. As a well-known epithelial-mesenchymal
transition (EMT) promoter, ZEB1 serves important roles in cancer
metastasis, including BCa. Previously, Lin et al (17) demonstrated that lncRNA ZEB1-AS1
was associated with higher histopathological grade and promoted
tumorigenesis in BCa, indicating its oncogenic role in cancer
progression. However, the biological function and molecular
mechanism of lncRNA ZEB1-AS1 in BCa metastasis remains unknown.
The present study identified that lncRNA ZEB1-AS1
was significantly upregulated in BCa and closely associated with
poor prognosis. Through gain or loss of function, it was
demonstrated that ZEB1-AS1 promoted migration and invasion of BCa
cells in vitro, and enhanced tumor metastasis in
vivo. Mechanistically, ZEB1-AS1 guided heterogenous nuclear
ribonucleoprotein D0 (AUF1) to promote the translation of ZEB1
mRNA, contributing to an increase in ZEB1 protein expression.
Therefore, targeting ZEB1-AS1 may be a potential therapeutic
strategy leading to decreased rates of growth and metastasis in
BCa.
Materials and methods
Ethics statement and tissue samples
A total of 60 snap-frozen fresh BCa tissues [30 MIBC
and 30 non-MIBC (NMIBC)] and 60 normal adjacent tissues were
obtained with the written consent of patients who underwent surgery
at Peking Union Medical College Hospital (Beijing, China) between
January 2014 and January 2016. The diagnosis of recruited patients
was pathologically confirmed, and primary cancer tissues (no biopsy
samples) were collected prior to radiotherapy or chemotherapy. The
obtained tissue samples were immediately snap-frozen in liquid
nitrogen upon resection and then stored at -80°C until further use.
The present study was approved by Research Scientific Ethics
Committee of Peking Union Medical College Hospital. All
participants signed informed consent prior to use of the tissues
for scientific purposes.
Cell culture and reagents
The human BCa T24 and UM-UC-3 cell lines and normal
bladder epithelial SV-HUC-1 cell line were purchased from American
Tissue Culture Collection. UM-UC-3 and T24 cells were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.). Normal bladder epithelial SV-HUC-1 cells were grown in F-12K
medium (HyClone; GE Healthcare Life Sciences) containing 10% FBS
and 1% antibiotics. The cultures were incubated at 37°C in 5%
CO2. Cycloheximide (CHX) was purchased from
Sigma-Aldrich; Merck KGaA and used at the concentration of 20
µg/ml.
Vector construction and cell
transfection
Short interfering RNA (siRNA) oligonucleotides
targeting ZEB1-AS1, AUF1, ZEB1 and negative control siRNAs were
purchased from Shanghai GenePharma Co., Ltd. The siRNA sequences
are listed in Table IA. siRNA
transfections were performed using 75 nM siRNA and Lipofectamine
RNAimax (Thermo Fisher Scientific, Inc.). To establish stable
ZEB1-AS1-silencing cell lines, short hairpin RNA targeting ZEB1-AS1
(sh-ZEB1-AS1) were cloned into pCDH-CMV-MCS-EF1-Puro or pLKO.1-Puro
vectors and further loaded into a lentiviral vector (Shanghai
GeneChem Co., Ltd.). The infection concentration was
1×108 pfu/ml. The sequences of the siRNAs and shRNAs are
listed in Table IA.
 | Table IqPCR primer and siRNA sequences. |
Table I
qPCR primer and siRNA sequences.
A, siRNA sequences
|
|---|
| siRNAs | Sequence
(5′-3′) |
|---|
| si-ZEB1-AS1#1 |
GGACCAACTTTATGGAATA |
| si-ZEB1-AS1#2 |
GCTGAAGTCTGATGATTTA |
| si-ZEB1-AS1#3 |
GGAGCCATCTAGTGCATAA |
| si-AUF1 |
UCGACUAUCUGCUCCAAG |
| si-ZEB1 |
TGATCAGCCTCAATCTGCA |
| si-NC |
GACCTACAACTACCTATCA |
| sh-ZEB1-AS1 |
CUUCAAUGAGAUUGAACUUCA |
| sh-NC C |
AACAAGATGAAGAGCACCAA |
B, qPCR primer sequences
|
| Primers | Sequence
(5′-3′) |
|
| ZEB1-AS1 | F:
TCCCTGCTAAGCTTCCTTCAGTGT |
| ZEB1-AS1 | R:
GACAGTGATCACTTTCATATCC |
| ZEB1 | F:
CGCAGTCTGGGTGTAATCGTAA |
| ZEB1 | R:
GACTGCCTGGTGATGCTGAAA |
| GAPDH | F:
GCACCGTCAAGGCTGAGAAC |
| GAPDH | R:
ATGGTGGTGAAGACGCCAGT |
| U6 | F:
CTCGCTTCGGCAGCACA |
| U6 | R:
AACGCTTCACGAATTTGCGT |
| U1 | F:
GGGAGATACCATGATCACGAAGGT |
| U1 | R:
CCACAAATTATGCAGTCGAGTTTCCC |
Reverse transcription quantitative
polymerase chain reac- tion (RT-qPCR)
Total RNA was extracted from BCa tissues or cells
using the Qiagen RNeasy Mini kit according to the manufacturer's
protocol (Qiagen GbmH). RT and qPCR kits were used to evaluate the
expression of target RNAs. RT reactions (20 µl) were
performed using the PrimeScript® RT reagent kit (Takara
Biotechnology Co., Ltd.) and incubated for 30 min at 37°C followed
by 5 sec at 85°C. For qPCR, 2 µl diluted RT product was
mixed with 23 µl reaction buffer (Takara Biotechnology Co.,
Ltd.) to a final volume of 25 µl. All reactions were
performed using an Eppendorf Mastercycler EP Gradient S (Eppendorf)
under the following conditions: Initial denaturation at 95°C for 30
sec; and 45 cycles of denaturation (95°C for 5 sec), and annealing
and elongation (60°C for 30 sec). The transcript expression of
GAPDH was used for the normalization of detected RNAs using the
comparative 2-ΔΔCq method (18). The primer sequences for qPCR are
presented in Table IB.
Cell migration and invasion assay
Cell migration ability was evaluated by performing
wound-healing assay. Cells were seeded onto 6-well plates at a
density of 5×105 cells/well. A total of 12 h after
transfection with the respective vectors, the cell layer was
scratched to form wounds using a sterile 20-µl pipette tip;
the non-adherent cells were washed away with culture medium, then
the cells were additionally incubated for 48 h and images were
captured to identify the wound size. Cell invasion was evaluated
using a Transwell invasion assay with Boyden chambers (BD
Biosciences) and membranes with 8 µm pores coated with
Matrigel (coated at 37°C for 1 h). Cells in serum-free media were
placed in the upper chamber of the insert. Medium containing 10%
FBS was added to the lower chamber. After 12 h of incubation, the
cells that had invaded through the membrane were fixed with 4%
paraformaldehyde at 4°C for 20 min, followed by staining with 0.1%
crystal violet for 10 min at room temperature. The images were
visualized using an inverted microscope (×20 magnification) (Leica
Microsystems GbmH).
In vivo lung-metastasis mice model
A total of 12 male BALB/c nude mice (19-22 g; 6
weeks old) were obtained from the Shanghai Laboratory Animal
Center, Chinese Academy of Science. They were randomly divided into
two groups of 6 mice in each, and housed with 3 mice/cage in a
suitable pathogen-free sterile environment at 28°C and 50% humidity
with a 12-12 h light-dark cycle, and were fed ad libitum
with sterile chow food and water. The experimental protocol was
approved by the Committee on the Ethics of Animal Experiments of
Peking Union Medical College Hospital. Experimental lung metastases
were induced by injections of single-cell suspension
(2×106 eGFP-luc2-marked UM-UC-3 cells in 100 µl)
into the mouse lateral tail vein. Cells were stably transfected
with sh-ZEB1-AS1 or control vectors, and all cell injections were
administered in a total volume of 500 µl PBS containing 0.1%
BSA over a duration of 60 sec, as described previously (19). A total of 5 weeks later, prior to
in vivo imaging, the mice were I.P. anaesthetized with
sodium phenobarbital (75 mg/kg). During anesthesia (duration 15 to
20 min) and while recovering, mice were kept warm under a red heat
lamp. The established lung metastases images were observed using
the LB983 NIGHTOWL II system (Berthold Technologies GmbH & Co.
KG).
Immunohistochemical (IHC) staining and
scoring analyses
IHC staining and score calculation were conducted as
described previously (20).
Anti-ZEB1 antibody (1:100; cat. no. ab203829; Abcam) was used to
detect their expression levels in mouse tumors. Images were
visualized using a Nikon ECLIPSE Ti (Nikon Corporation) inverted
microscope system (x20 magnification) and processed using Nikon
software (CaptureNX2, version 2.4.7).
Cytosolic/nuclear fractioning and RNA
florescence in situ hybridization (RNA FISH)
The cellular fraction was isolated to locate the
sublocation of ZEB1-AS1. Briefly, 1×107 cells were
harvested, resuspended in 1 ml ice-cold RNase-free PBS, 1 ml C1
buffer (1.28 M Sucrose, 40 mM Tris-HCl, pH 7.5, 20 mM
MgCl2 and 4% Triton X-100) and 3 ml RNase-free water,
and incubated for 15 min on ice. The cells were then centrifuged
for 15 min at 3,000 × g at 4°C, and the supernatant containing the
cytoplasmic constituents and the nuclear pellet were retained for
RNA extraction.
For RNA FISH, BCa cells were seeded into a 6-well
plate (1x105 cells/well) and fixed with 4%
paraformaldehyde for 15 min at 4°C and treated with 0.5% Triton in
PBS, followed by pre-hybridization. They were then hybridized with
the ZEB1-AS1 probe (5 µM) at 37°C overnight. The ZEB1-AS1
probes were synthesized by Sangon Biotech Co., Ltd. The cells were
visualized under a confocal microscope (x100 magnification; Carl
Zeiss Microscopy GmbH).
RNA pulldown, electrophoresis and mass
spectrometry
The RNA pulldown assay was performed using a
Magnetic RNA-Protein Pull-down kit (Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol. BCa cells (2x107)
were cross-linked for each hybridization reaction. The cell lysates
were hybridized with a mixture of biotinylated DNA probes for 4 h
at 37°C. The binding proteins were separated by electrophoresis. A
total of 25 µg proteins in each lane were loaded for
SDS-PAGE (10% gel) at 4°C. The concentrating voltage was 80 V and
separating voltage was 110 V. The binding proteins were also
identified by mass spectrometry to identify the potential binding
proteins (H. Wayen Biotechnology, Shanghai, China) as previously
described (21).
RNA immunoprecipitation (RIP) assay
The RIP assay was performed using the EZ-Magna RIP
kit (EMD Millipore) according to the manufacturer's protocol.
Briefly, 1×107 cells were lysed with RIP lysis buffer
using 1 freeze-thaw cycle. Cell extracts were coimmunoprecipitated
with anti-AUF1 (1:200; cat. no. ab61193; Abcam) antibody, and the
retrieved RNA was subjected to the aforementioned RT-qPCR analysis.
Normal IgG was used as a negative control. For RT-qPCR analysis,
GAPDH was used as the non-specific control.
Western blot analysis
Radioimmunoprecipitation assay lysis buffer
(Sigma-Aldrich; Merck KGaA) was used to lyse the cells to obtain
total protein lysates. Protein concentration was measured using the
bicinchoninic acid assay (Sigma-Aldrich; Merck KGaA). The
quantified protein (25 µg) was transferred onto
polyvinylidene fluoride membranes (Sigma-Aldrich; Merck KGaA)
following 10% SDS-PAGE gel electrophoresis. Then, the membranes
were blocked with 5% non-fat dry milk in TBS + Tween buffer (0.1%)
for 2 h at room temperature and incubated with anti-ZEB1 antibody
(1:1,000; cat. no. ab203829; Abcam) or anti-GAPDH antibody
(1:5,000; cat. no. PA1-987; Invitrogen; Thermo Fisher Scientific,
Inc.) at 4°C overnight, followed by horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no. ab7090;
Abcam) at room temperature for 1 h. Protein bands were detected
using ECL reagent (Amersham; GE Healthcare). Gray analysis by image
analysis was performed using the software Gel-Pro Analyzer (version
4.0; United States Biochemical) after scanning.
Bioinformatics analysis
The correlation between ZEB1-AS1 and ZEB1 mRNA
levels was analyzed by using The Cancer Genome Atlas BCa dataset
with the online database StarBase (http://starbase.sysu.edu.cn/panCancer.php).
Statistical analysis
The Kolmogorov-Smirnov test was applied to analyze
the distribution of data in each group. Data were presented as
median (interquartile range). Mann-Whitney U tests were performed
to compare the data between the two groups. The Kruskal-Wallis test
followed by Bonferroni correction post-hoc test was used for
evaluating the difference among multiple groups. Receiver operation
characteristic (ROC) analysis was performed to evaluate the
diagnostic performance of ZEB1-AS1. Spearman's correlation analysis
was performed to determine the correlation between variables. A
two-sided P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
GraphPad Prism 5 software (GraphPad Software, Inc.).
Results
Knockdown of ZEB1-AS1 inhibits migration
of BCa cells in vitro and metastasis in vivo
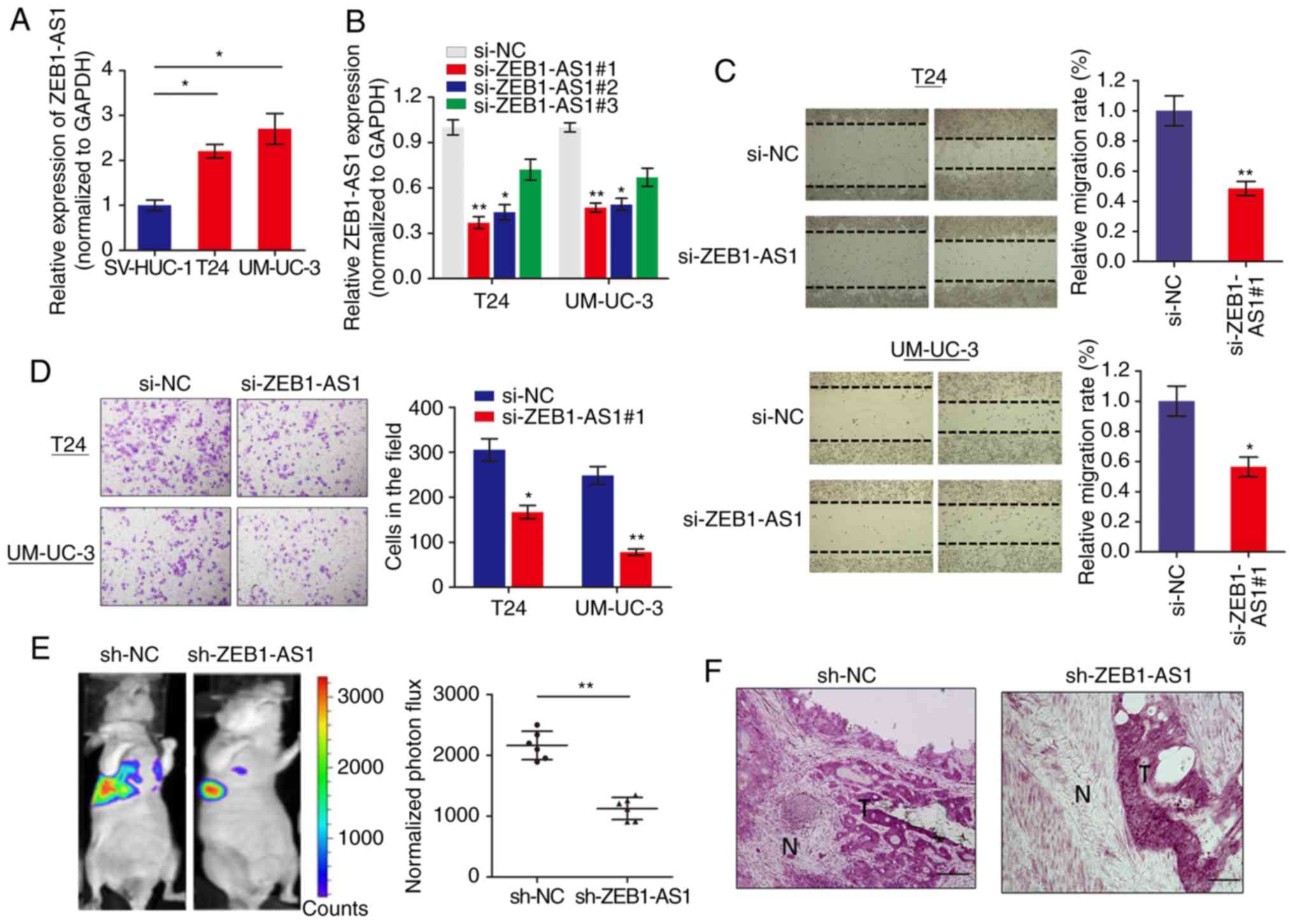
We measured the expression level of lncRNA ZEB1-AS1
in BCa cells via RT-qPCR and it was identified that ZEB1-AS1 was
significantly increased in BCa cells when compared with normal
SV-HUC-1 cells (Fig. 1A). To
investigate the role of ZEB1-AS1 in BCa progression, ZEB1-AS1
expression was silenced in BCa cells using 3 different siRNAs.
RT-qPCR analysis showed that ZEB1-AS1 was remarkably downregulated
in T24 and UM-UC-3 cells (Fig.
1B), and si-ZEB1-AS1#1 was selected for use in functional
experiments. The function of ZEB1-AS1 on cell motility and tumor
metastasis was investigated using wound healing and invasion
assays, respectively. Wound healing assays demonstrated that
downregulation of ZEB1-AS1 significantly decreased the migratory
ability of T24 and UM-UC-3 cells (Fig. 1C). In addition, ZEB1-AS1 knockdown
inhibited the invasive ability of BCa cells (Fig. 1D).
To additionally confirm the effects of ZEB1-AS1 in
BCa metastasis, an experimental lung metastases model was induced
by injections of single-cell suspension (2x106 UM-UC-3
cells in 100 µl) into the lateral tail vein of the mice. The
luciferase flux count of lung metastases was significantly less in
ZEB1-AS1-knockdown group compared to control group (Fig. 1E). Notably, it was observed that
the tumors formed by the ZEB1-AS1-knockdown BCa cells grown in the
nude mice exhibited sharp edges, while the control tumors exhibited
spike-like structures that invaded the surrounding muscle tissues
(Fig. 1F), which additionally
supported the hypothesis that knockdown of ZEB1-AS1 suppressed BCa
metastasis. Collectively, these results indicated that ZEB1-AS1
regulates BCa cells migration and invasion in vitro, and
metastasis in vivo.
ZEB1-AS1 regulates BCa metastasis via the
upregulation of ZEB1 protein
The subcellular localization of a lncRNA is
associated closely with its biological mechanism. Cellular
fractionation and RNA-FISH assays demonstrated that ZEB1-AS1 was
primarily distributed in the cytoplasm in BCa cells (Fig. 2A and B). lncRNAs located in the
cytoplasm are usually associated with post-transcriptional
regulation (15,22). As ZEB1 is a critical transcription
factor in cancer metastasis, we hypothesized that the antisense
transcript ZEB1-AS1 may regulate the expression of ZEB1 and thereby
induce the metastatic effect in BCa. Western blot analysis
indicated that ZEB1 was downregulated by ZEB1-AS1 knockdown in BCa
cells (Fig. 2C). IHC analysis of
the tumor tissues grown in nude mice also suggested that ZEB1
expression was inhibited in the ZEB1-AS1-silencing group compared
with the sh-NC group (Fig. 2D).
However, when the expression of ZEB1 mRNA was analyzed using
RT-qPCR, knockdown of ZEB1-AS1 demonstrated no effect on ZEB1 mRNA
expression in BCa cells (Fig.
2E). Then, the functional role of ZEB1 in ZEB1-AS1-mediated BCa
metastasis was examined by overexpression of ZEB1-AS1 and knockdown
of ZEB1 (Fig. 2F and G). As
indicated in Fig. 2H and I,
enhanced expression of ZEB1-AS1 promoted BCa cell migration and
invasion, however, this effect was significantly reversed by
co-expression of siZEB1 in the ZEB1-AS1 overexpressing cells. These
data demonstrated that ZEB1-AS1 regulates BCa metastasis via the
upregulation of ZEB1 protein.
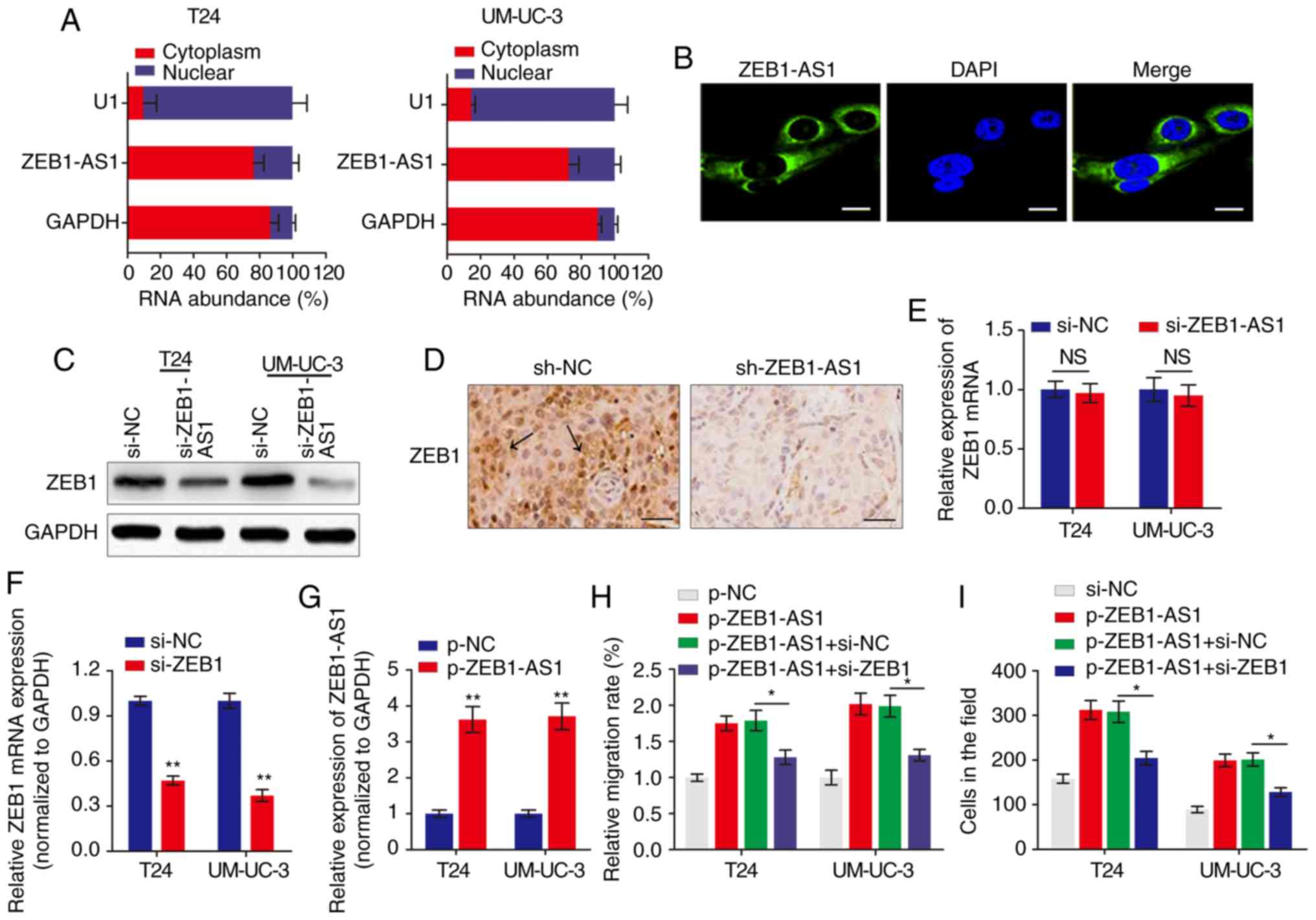 | Figure 2ZEB1 is a functional target of
ZEB1-AS1 in BCa. (A) The relative expression level of ZEB1-AS1 in
the nucleus and cytoplasm of BCa cells was measured by RT-qPCR. U1
(retained in the nucleus) and GAPDH (exported to cytoplasm) were
used as controls. (B) The subcellular distribution of ZEB1-AS1 was
visualized by RNA fluorescence in situ hybridization in
UM-UC-3 cells. Scale bars, 10 µm. (C) Western blot analysis
of ZEB1 protein levels in BCa cells with ZEB1-AS1 knockdown. (D)
Immunohistochemistry assay was used to measure the levels of ZEB1
expression in tumor tissues grown in mice. The arrows indicate the
ZEB1-rich area. Scale bars, 50 µm. (E) RT-qPCR was performed
to evaluate the effect of ZEB1-AS1 knockdown on ZEB1 mRNA level. (F
and G) The effects of (F) ZEB1 knockdown and (G) ZEB1-AS1
overexpression were verified using RT-qPCR. **P<0.01.
(H) Wound-healing assays and (I) Transwell invasion assays were
performed to detect the effect of ZEB1 knockdown and ZEB1-AS1
overexpression on BCa cell migration and invasion, respectively.
*P<0.05. ZEB1-AS1, zinc finger E-box-binding homeobox
1-antisense 1; BCa, bladder cancer; RT-qPCR, reverse transcription
quantitative polymerase chain reaction; si, small interfering; sh,
short hairpin; NC, negative control; NS, not significant. |
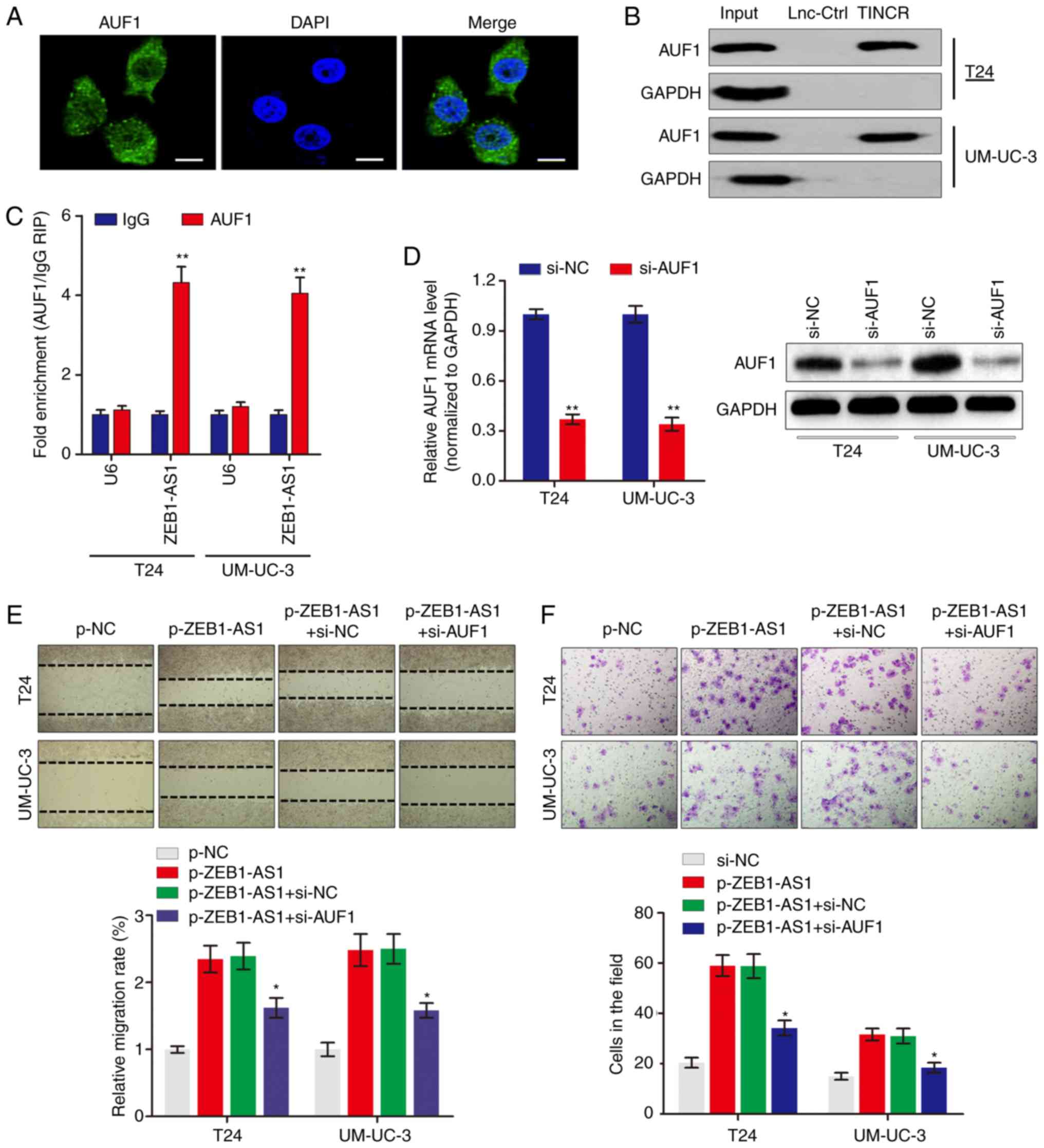
ZEB1-AS1 directly interacts with AUF1 in
BCa
To examine the potential mechanism by which ZEB1-AS1
regulates the protein levels of ZEB1, RNA-pulldown experiments were
performed followed by mass spectrometry to identify
ZEB1-AS1-interacting proteins. The data identified a number of
potential ZEB1-AS1-interacting proteins (Table II), among which AUF1 was
identified. AUF1 is able to bind to (A + U)-rich elements within
3'-untranslated region (3'-UTR) of target mRNA and promote
translation without affecting the mRNA level (23). Consistent with ZEB1-AS1
localization, AUF1 was also primarily distributed in the cytoplasm
in BCa cells (Fig. 3A). The
significance of the interaction between ZEB1-AS1 and AUF1 was
confirmed using RNA pulldown (Fig.
3B) and RIP (Fig. 3C) assays.
To explore the role of the ZEB1-AS1-AUF1 association in the
function of ZEB1-AS1, ZEB1-AS1 was overexpressed, and then AUF1
expression was knocked down in BCa cells (Fig. 3D). Notably, AUF1 knockdown
eliminated the ZEB1-AS1-mediated enhancement of the migration
(Fig. 3E) and invasion (Fig. 3F) of BCa cells in vitro.
These data suggested that the direct interaction between ZEB1-AS1
and AUF1 may be important for the ZEB1-AS1-induced BCa
metastasis.
 | Table IIIdentification of ZEB1-AS1 binding
proteins by mass spectrometry. |
Table II
Identification of ZEB1-AS1 binding
proteins by mass spectrometry.
| Protein | Beads | ZEB1-AS1 | Ratio
(ZEB1-AS1/Beads) |
|---|
| AUF1 | 0 | 3 | NA |
| LAS1L | 0 | 3 | NA |
| STT3B | 0 | 3 | NA |
| PCH2 | 1 | 3 | 3 |
| MRP1 | 0 | 3 | NA |
| ARF6 | 1 | 3 | 3 |
| AKAP8 | 0 | 3 | NA |
| GSTO1 | 0 | 3 | NA |
| AP1B1 | 0 | 3 | NA |
| DPM1 | 0 | 3 | NA |
| PSDE | 1 | 3 | 3 |
| PLST | 0 | 3 | NA |
| PSAL | 0 | 3 | NA |
| TTL12 | 0 | 3 | NA |
| ERLN1 | 0 | 3 | NA |
| NSF | 0 | 3 | NA |
| KTN1 | 1 | 3 | 3 |
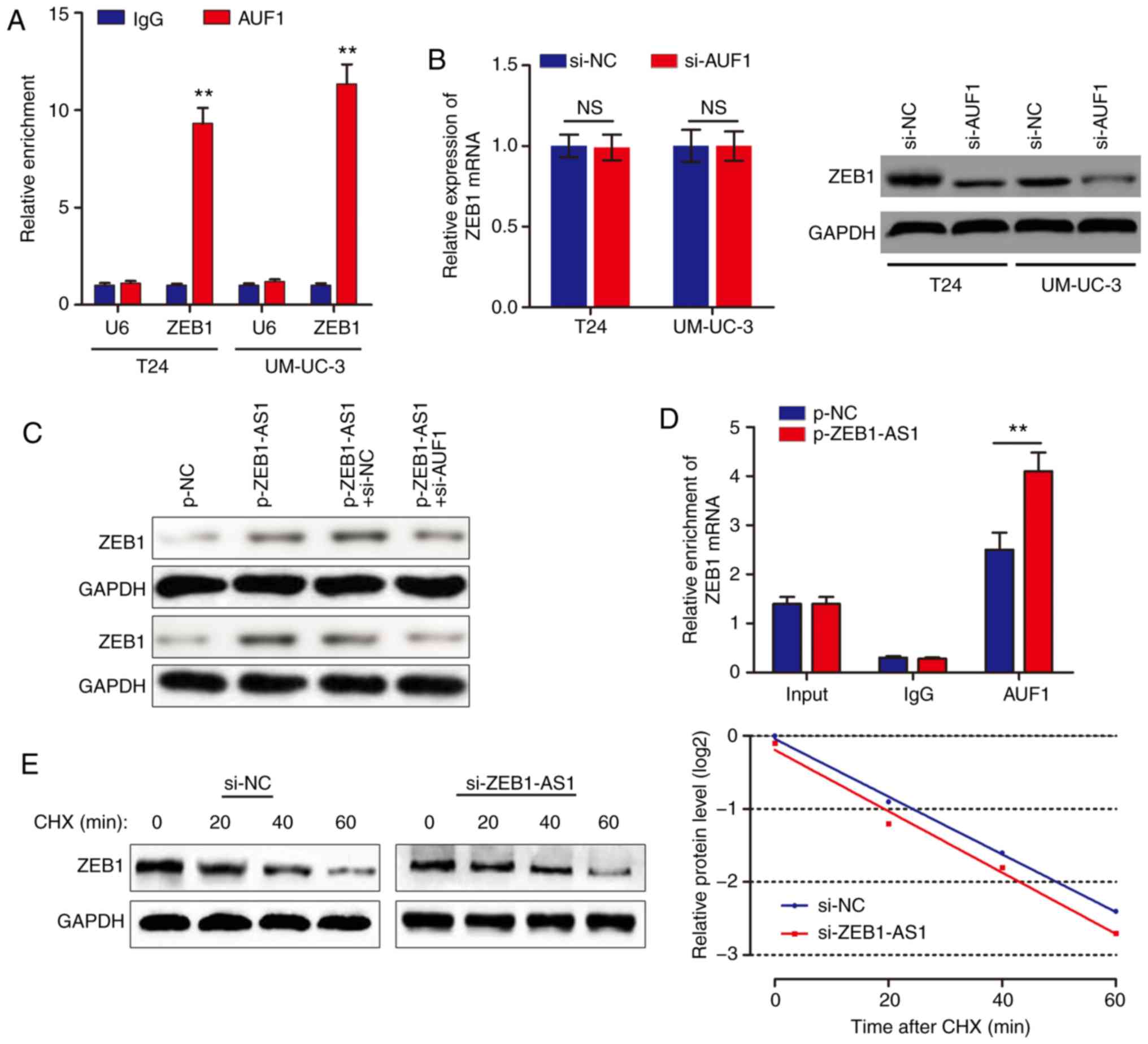
ZEB1-AS1 activates the translation of
ZEB1 mRNA via recruiting AUF1
Based on the above results, we hypothesized that
ZEB1-AS1 promoted ZEB1 mRNA translation by binding with AUF1. To
examine this hypothesis, a RIP assay was performed, and it was
identified that ZEB1 was enriched in AUF1 precipitates (Fig. 4A). AUF1 knockdown decreased ZEB1
protein level without affecting the ZEB1 mRNA level (Fig. 4B). In addition, knockdown of AUF1
eliminated the ZEB1-AS1-mediated increases in ZEB1 protein levels
(Fig. 4C). As ZEB1-AS1 and AUF1
regulate ZEB1 expression synergistically, we hypothesized that
ZEB1-AS1 may increase ZEB1 protein level through recruiting AUF1 to
bind to the ZEB1 3'-UTR. In support of this hypothesis, ZEB1-AS1
overexpression promoted endogenous AUF1 binding to ZEB1 mRNA by RIP
(Fig. 4D). In addition, UM-UC-3
cells were treated with CHX, which enabled the measurement of the
degradation of pre-existing proteins. The data indicated that
knockdown of ZEB1-AS1 or AUF1 had no effect on half-life of ZEB1
protein in BCa cells (Fig. 4E).
Taken together, these results indicated that ZEB1-AS1 assists AUF1
in binding to ZEB1 mRNA, activating its translation without
affecting the mRNA level.
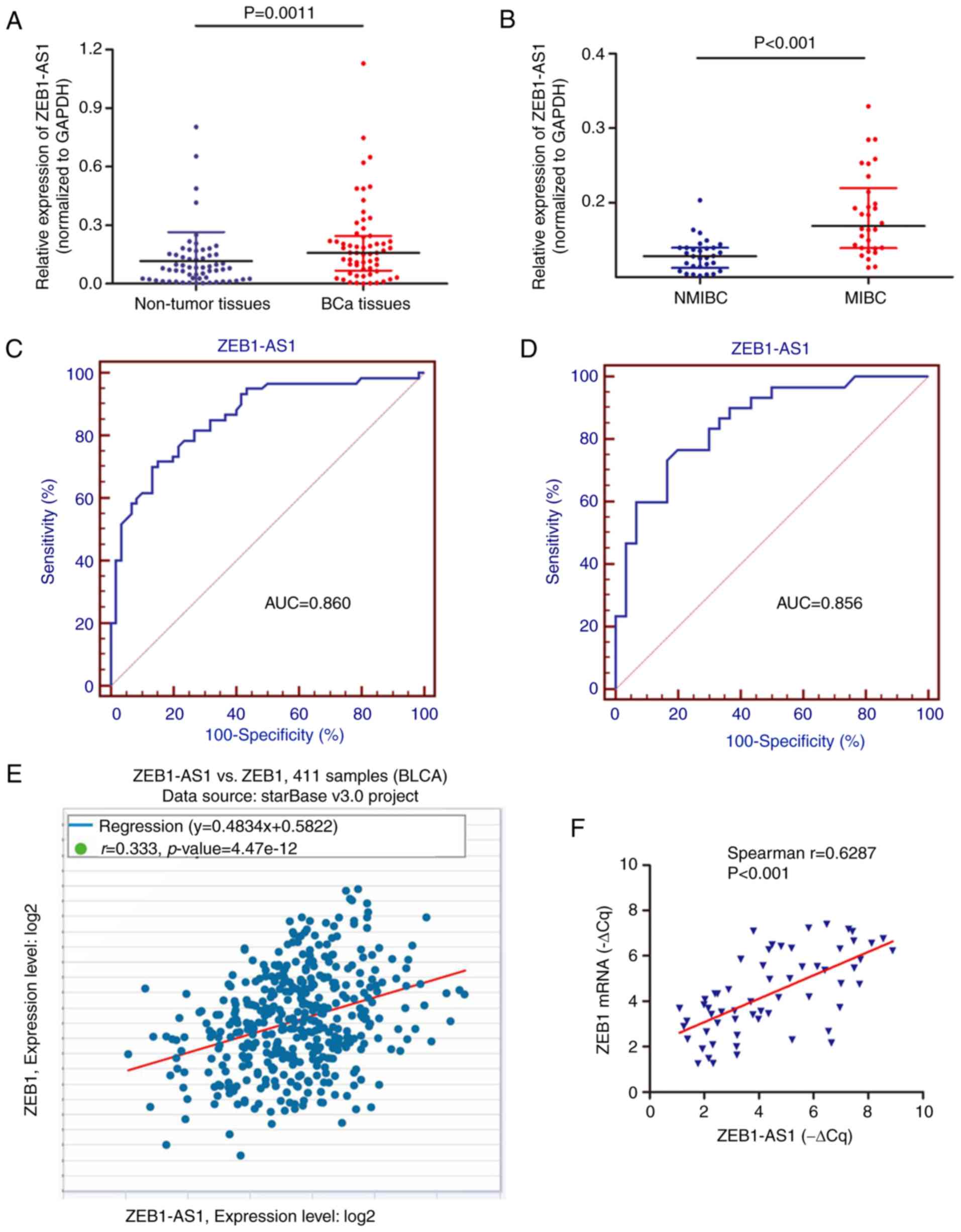
ZEB1-AS1 expression is associated with
metastasis in patients with BCa
A preliminary study was performed to identify the
clinical role of ZEB1-AS1 using 60 BCa tissues (30 MIBC and 30
NMIBC) and paired non-cancer tissues from patients with primary
BCa. RT-qPCR analysis showed that ZEB1-AS1 was upregulated in BCa
tissues in contrast to non-cancer tissues (Fig. 5A). In addition, the expression
level of ZEB1-AS1 in MIBC tissues was significantly increased
compared with that in NMIBC tissues (Fig. 5B). ROC curve analysis was then
used to investigate the predictive value of ZEB1-AS1 in
differentiating patients with cancer from the noncancerous
population. As demonstrated in Fig.
5C, the area under the curve (AUC) was 0.860, with the
diagnostic sensitivity and specificity measuring 71.7 and 85.0%
with the cut-off value of 0.134, respectively. Notably, the ROC
curve using the ZEB1-AS1 level to differentiate MIBC from NMIBC
demonstrated the AUC, sensitivity and specificity values were
0.856; 76.7; and 80.0%, respectively (Fig. 5D). By analyzing The Cancer Genome
Atlas BCa dataset with the online database StarBase, positive
associations between the RNA expression of ZEB1-AS1 and ZEB1 mRNA
were identified in BCa (Fig. 5E).
This positive association was additionally verified in the 60
cancer tissues (Fig. 5F), which
supports the regulatory mechanism of ZEB1-AS1 and ZEB1 in BCa
metastasis.
Discussion
Numerous previous studies have assisted in gaining
an improved understanding of the molecular mechanisms during cancer
progression and chemoresistance. However, the specific regulatory
model remains largely unknown in cancer, including BCa. Therefore,
it is of importance to identify new molecular signatures which may
be useful for cancer prevention and therapy. In the present study,
it was demonstrated that over-expression of ZEB1-AS1 in BCa
promoted the migration and metastasis of BCa cells in vitro
and in vivo. Mechanistically, ZEB1-AS1 increased ZEB1
protein expression by guiding AUF1 to activate the translation of
ZEB1 mRNA. In addition, ZEB1-AS1 expression was upregulated in BCa
tissues and associated with metastasis in patients with BCa.
lncRNAs have been demonstrated to regulate
biological functions via diverse mechanisms: Guider; decoy;
scaffold effect on DNA, RNA or protein; and post-transcriptional
effects (24). The expression of
ZEB1-AS1 has been described, and it has been identified as an
oncogene in several cancer types, including gastric (25), prostate (26) and colorectal cancer (27), and glioma (28). Lin et al (17) demonstrated that lncRNA ZEB1-AS1
serves an oncogenic role in BCa through the promotion of cell
growth, migration and the inhibition of apoptosis in BCa 5637 and
SW780 cell lines. Consistent with the data from Lin et al
(17), the present study showed
that ZEB1-AS1 enhanced metastasis and invasion in vitro, and
promoted metastasis in vivo, confirming that ZEB1-AS1 may
serve as a oncogene in BCa.
The subcellular localization of a lncRNA is closely
associated with its biological mechanism. The results from the
present study showed that ZEB1-AS1 was primarily distributed in the
cytoplasm in BCa cells, indicating it may regulate cancer
progression at the post-transcriptional level. ZEB1-AS1 is a
noncoding antisense transcript generated from ZEB1 promoters and
located in physical contiguity with ZEB1. It is well known that
ZEB1 is a transcription factor that promotes tumor invasion and
metastasis by inducing EMT in carcinoma cells. Emerging studies
have indicated that overexpression of ZEB1-AS1 increased ZEB1
levels and promoted tumor progression in different kinds of
malignancies (26,27,29-31). However, the regulatory interaction
between lncRNA ZEB1-AS1 and ZEB1 has not been described in BCa.
Notably, the present study verified that ZEB1 was positively
regulated by ZEB1-AS1 at protein level but not at the mRNA level,
which suggests that ZEB1-AS1 may regulate ZEB1 expression without
affecting mRNA level.
As ZEB1-AS1 affects ZEB1 protein level but not mRNA
level, we hypothesized that this regulatory model may occur at the
post-transcriptional level. To uncover the underlying mechanism by
which ZEB1-AS1 regulates ZEB1 protein level, the
ZEB1-AS1-interacting proteins were verified. It was identified that
AUF1 was associated with ZEB1-AS1 and may serve as an adaptor
protein that cooperates with ZEB1-AS1 to bind to ZEB1 mRNA. AUF1 an
RNA-binding protein that produces 4 transcript variants following
alternative pre-messenger RNA (pre-mRNA) splicing, with canonical
roles in controlling the stability or translation of mRNA targets
based on recognition of AU-rich sequences within 3'-UTR of target
mRNA (32). As ZEB1-AS1 affected
ZEB1 protein level without affecting the mRNA, it was assumed that
AUF1 regulated the translation of ZEB1 mRNA. The results of the RIP
assay verified the direct interaction between AUF1 and ZEB1. In
addition, ZEB1-AS1 showed no effect on the ZEB1 protein stability,
which confirmed our hypothesis. Notably, Li et al (33) suggested that AUF1 could bind to
the 3'-UTR of ZEB1 mRNA and affect the mRNA stability in thyroid
cancer. Therefore, the regulatory mechanism of AUF1 for ZEB1 in
cancer requires additional study. In addition, whether miRNAs are
regulated by ZEB1-AS1 in BCa progression also requires
verification, as there is a close association between lncRNAs and
miRNAs.
Take a step further, the present study also
investigated the clinical role of ZEB1-AS1 expression in patients
with BCa. It was observed that ZEB1-AS1 was upregulated in BCa
tissues, and expressed at significantly increased levels in MIBC
tissues. ROC curve analysis clearly demonstrated a high predictive
value of ZEB1-AS1 in differentiating patients with MIBC from
patients with NMIBC, indicating a high predictive value for BCa
metastasis. These data validated the experimental conclusion
derived from the in vitro and in vivo studies.
In summary, the present study demonstrated that
ZEB1-AS1 functionally and clinically participated in the metastasis
and progression of BCa, based on an AUF1-mediated translation
activation of ZEB1 mRNA. Identification of the precise role of
ZEB1-AS1 in the progression of BCa will not only improve the
understanding of lncRNA-induced tumorigenesis and metastasis, but
also enable the development of novel therapeutic strategies to
treat BCa.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and ZJ acquired the data and created a draft of
the manuscript; YD and JZ collected clinical samples and performed
the experimental assays; DW and GL analyzed and interpreted the
data, and performed statistical analysis; XZ, DW and ZJ reviewed
the manuscript, figures and tables. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Clinical
Research Ethics Committee of Peking Union Medical College Hospital,
and the experimental protocols for the animal model was approved by
the Committee on the Ethics of Animal Experiments of Peking Union
Medical College Hospital. Written informed consent was obtained
from each participant prior to tissue collection.
Patient consent for publication
Written informed consent was obtained from each
participant prior to tissue collection.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Batavia J, Yamany T, Molotkov A, Dan
H, Mansukhani M, Batourina E, Schneider K, Oyon D, Dunlop M, Wu XR,
et al: Bladder cancers arise from distinct urothelial
sub-populations. Nat Cell Biol. 16:982–991. 1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youssef RF and Raj GV: Lymphadenectomy in
management of invasive bladder cancer. Int J Surg Oncol.
2011:7581892011.
|
|
5
|
Wu XR: Urothelial tumorigenesis: A tale of
divergent pathways. Nat Rev Cancer. 5:713–725. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hautmann RE, de Petriconi RC, Pfeiffer C
and Volkmer BG: Radical cystectomy for urothelial carcinoma of the
bladder without neoadjuvant or adjuvant therapy: Long-term results
in 1,100 patients. Eur Urol. 61:1039–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao Y: Opinion: Emerging mechanisms of
tumour lymphan-giogenesis and lymphatic metastasis. Nat Rev Cancer.
5:735–743. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karaman S and Detmar M: Mechanisms of
lymphatic metastasis. J Clin Invest. 124:922–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loewen G, Jayawickramarajah J, Zhuo Y and
Shan B: Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol.
7:902014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar
|
|
11
|
Nakagawa S and Kageyama Y: Nuclear lncRNAs
as epigenetic regulators-beyond skepticism. Biochim Biophys Acta.
1839:215–222. 2014. View Article : Google Scholar
|
|
12
|
Kornienko AE, Guenzl PM, Barlow DP and
Pauler FM: Gene regulation by the act of long non-coding RNA
transcription. BMC Biol. 11:592013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ni W, Zhang Y, Zhan Z, Ye F, Liang Y,
Huang J, Chen K, Chen L and Ding Y: A novel lncRNA uc.134 represses
hepatocellular carcinoma progression by inhibiting CUL4A-mediated
ubiquiti-nation of LATS1. J Hematol Oncol. 10:912017. View Article : Google Scholar
|
|
14
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen DL, Lu YX, Zhang JX, Wei XL, Wang F,
Zeng ZL, Pan ZZ, Yuan YF, Wang FH, Pelicano H, et al: Long
non-coding RNA UICLM promotes colorectal cancer liver metastasis by
acting as a ceRNA for microRNA-215 to regulate ZEB2 expression.
Theranostics. 7:4836–4849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu G, Ye Z, Zhao X and Ji Z: SP1-induced
up-regulation of lncRNA SNHG14 as a ceRNA promotes migration and
invasion of clear cell renal cell carcinoma by regulating N-WASP.
Am J Cancer Res. 7:2515–2525. 2017.
|
|
17
|
Lin J, Zhan Y, Liu Y, Chen Z, Liang J, Li
W, He A, Zhou L, Mei H, Wang F and Huang W: Increased expression of
ZEB1-AS1 correlates with higher histopathological grade and
promotes tumorigenesis in bladder cancer. Oncotarget.
8:24202–24212. 2017.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Gu P, Xie R, Han J, Liu H, Wang B,
Xie W, Xie W, Zhong G, Chen C, et al: Heterogeneous nuclear
ribonucleoprotein K is associated with poor prognosis and regulates
proliferation and apoptosis in bladder cancer. J Cell Mol Med.
21:1266–1279. 2017. View Article : Google Scholar :
|
|
21
|
Gong X, Du X, Xu Y and Zheng W: LINC00037
inhibits proliferation of renal cell carcinoma cells in an
epidermal growth factor receptor-dependent way. Cell Physiol
Biochem. 45:523–536. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu QC, Rui ZH, Guo ZL, Xie W, Shan S and
Ren T: LncRNA-DANCR contributes to lung adenocarcinoma progression
by sponging miR-496 to modulate mTOR expression. J Cell Mol Med.
22:1527–1537. 2018. View Article : Google Scholar
|
|
23
|
Moore AE, Chenette DM, Larkin LC and
Schneider RJ: Physiological networks and disease functions of
RNA-binding protein AUF1. Wiley Interdiscip Rev RNA. 5:549–564.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution, and mechanisms. Cell. 154:26–46. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu TP, Wang YF, Xiong WL, Ma P, Wang WY,
Chen WM, Huang MD, Xia R, Wang R, Zhang EB, et al: E2F1 induces
TINCR transcriptional activity and accelerates gastric cancer
progression via activation of TINCR/STAU1/CDKN2B signaling axis.
Cell Death Dis. 8:e28372017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su W, Xu M, Chen X, Chen N, Gong J, Nie L,
Li L, Li X, Zhang M and Zhou Q: Long noncoding RNA ZEB1-AS1
epigenetically regulates the expressions of ZEB1 and downstream
molecules in prostate cancer. Mol Cancer. 16:1422017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong WC, Han N, Wu N, Zhao KL, Han C,
Wang HX, Ping GF, Zheng PF, Feng H, Qin L and He P: Interplay
between long noncoding RNA ZEB1-AS1 and miR-101/ZEB1 axis regulates
proliferation and migration of colorectal cancer cells. Am J Transl
Res. 10:605–617. 2018.PubMed/NCBI
|
|
28
|
Lv QL, Hu L, Chen SH, Sun B, Fu ML, Qin
CZ, Qu Q, Wang GH, He CJ and Zhou HH: A long noncoding RNA ZEB1-AS1
promotes tumorigenesis and predicts poor prognosis in glioma. Int J
Mol Sci. 17:E14312016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng R, Li N, Yang S, Liu L and Han S:
Long non-coding RNA ZEB1-AS1 promotes cell invasion and epithelial
to mesenchymal transition through inducing ZEB1 expression in
cervical cancer. Onco Targets Ther. 11:7245–7253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu C and Lin J: Long noncoding RNA-ZEB1
AS1 acts as an oncogene in osteosarcoma by epigenetically
activating ZEB1. Am J Transl Res. 8:4095–4105. 2016.
|
|
31
|
Qu R, Chen X and Zhang C: LncRNA
ZEB1-AS1/miR-409-3p/ZEB1 feedback loop is involved in the
progression of non-small cell lung cancer. Biochem Biophys Res
Commun. 507:450–456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi YJ, Yoon JH and Chang JH: Crystal
structure of the N-Terminal RNA recognition motif of mRNA decay
regulator AUF1. Biomed Res Int. 2016:32861912016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Zhang HY, Du ZX, Li C, An MX, Zong
ZH, Liu BQ and Wang HQ: Induction of epithelial-mesenchymal
transition (EMT) by Beclin 1 knockdown via posttranscriptional
upregulation of ZEB1 in thyroid cancer cells. Oncotarget.
7:70364–70377. 2016.PubMed/NCBI
|