Introduction
Hepatolithiasis is a prevalent disease in Southeast
Asia, and proliferative cholangitis (PC) serves an important role
in the pathogenesis of hepatolithiasis and is correlated with ~75%
of hepatolithiasis cases in Asia (1). Previously, it was confirmed that
chronic PC (CPC) is a pathological foundation and the key
contributor to the high recurrence rate of intrahepatic stones and
biliary restenosis in patients with hepatolithiasis (2,3).
CPC, as an active and long-term inflammation of stone-bearing bile
ducts with increased secretion of mucin-like glycoprotein, not only
facilitates the progression of hepatolithiasis, it may contribute
to biliary carcinogenesis (4).
CPC is induced by the formation of stones in the bile ducts and may
persist extensively within the bile ducts even following removal of
the stone (5), facilitating the
formation of new stones and leading to cholestasis (1). It has been shown that chemical
biliary duct embolization can uproot CPC and prevent the recurrence
of intrahepatic stones, however, its use is restricted as it
completely destroys the hepatic segment and related bile ducts
(6). Therefore, it is necessary
to identify more effective regimens for CPC.
Panitumumab (Pani), an immunoglobulin (Ig)G2
monoclonal antibody directed against epidermal growth factor
receptor (EGFR), is effective in 10-20% patients with unselected
metastatic colorectal cancer (mCRC) and promotes the
progression-free survival rate of patients with showing disease
progression of mCRC following standard chemotherapy (7-9).
Formerly known as ABX-EGF, Pani can be applied for the clinical
treatment of solid tumors and is directed against the extracellular
receptor, leading to the inhibition of critical downstream
signaling pathways that control apoptosis, proliferation and
differentiation in normal and tumor cells (10). The antitumor activity of Pani has
been verified in vivo and in vitro, and the
suppression of tumor growth has been investigated in various types
of cancer (10). Additionally,
Pani has a favorable tolerability profile when administered as a
monotherapy (11); Pani is
associated with dermatologic toxicity and appears to have a low
risk of immunogenicity, but is rarely a severe event associated
with EGFR inhibitors (12,13).
Furthermore, it has been reported that EGFR can serve as a target
in the treatment of proliferative cholangitis in hepatolithiasis
(14). Therefore, the present
study was performed to examine the effect of the EGFR monoclonal
antibody Pani on the excessive proliferation and stone-forming
potential of the bile duct mucosa in CPC, which may benefit the
development of promising treatment strategies for the treatment of
CPC.
Materials and methods
Ethics statement
All animal experiments performed in the present
study conformed to the management of local laboratory animal
guidelines and Medical Ethics Committee of Nanfang Hospital
(Guangzhou, China). All efforts were made to minimize the suffering
of animals during the experiment.
Establishment of CPC models and rat
grouping
A total of 50 male Sprague-Dawley rats of clean
grade (aged two months old; weighing 220-250 g) were purchased from
Shanghai Laboratory Animal Center of the Chinese Academy of
Sciences (Shanghai, China). The rats were maintained at a humidity
of 5-10% and at 22-25°C under a cycle of 12-h light and 12-h
darkness with free access to food and drinking water (acidified
water at pH=2.5-2.8). The rats were randomly assigned into five
groups according to different treatment approaches: Sham group, CPC
group (rats of the CPC model without any treatment), 2 mg/kg Pani
group (rats of the CPC model treated with 2 mg/kg of Pani), 4 mg/kg
Pani group (rats of the CPC model treated with 4 mg/kg of Pani) and
6 mg/kg Pani group (rats of the CPC model treated with 6 mg/kg of
Pani). The CPC model was established according to the following
protocol (1): The rats were
anesthetized with 10% chloral hydrate (300 mg/kg, no signs of
peritonitis were observed at this concentration), a 5-0 nylon
thread was inserted into the hepatic portal reversely from the
duodenal papilla to the common bile duct, and the poke hole at the
end of the suture was sutured. After 7 days of modeling, the bile
duct wall was thickened, and hyperplasia of the mucosa epithelium
and collagen fibers was observed by microscopy, indicating that the
modeling was successfully established. In the sham group, the
abdominal cavity of the rats was opened and then closed without any
other treatment. During the modeling, in the rats treated with
different doses of Pani, a No. 20 venous indwelling needle was
threaded with a nylon thread, and Pani (20 mg/ml) at a dose of 2, 4
or 6 mg/kg was injected into the common bile duct. The rats in each
group were intraperitoneally injected with 5-bromo-2′-deoxyuridine
(BrdU) at a dose of 100 mg/kg on days 4, 5 and 6 post-treatment. On
day 7 post-treatment, all rats were sacrificed by barbiturate
overdose (150 mg/kg pentobarbital sodium, i.v.) (15) and bile duct tissue specimens were
extracted by laparotomy for further experiments.
Hematoxylin and eosin (H&E)
staining
The bile duct tissues of each group were immersed in
10% neutral formaldehyde solution, and paraffin sections were
prepared. The specimens were fixed with 10% neutral formaldehyde
solution, dehydrated in alcohol, cleared twice with xylene,
immersed in wax, embedded in paraffin and cut into 4-µm
sections. Thereafter, the paraffin sections were sliced
continuously and placed in an oven at 80°C for 1 h. Following
cooling, the sections were dehydrated in conventional gradient
alcohol, cleared with xylene and then washed with PBS. The sections
were stained with hematoxylin (H8070; 5 g; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) for 3 min, removed and
washed. The sections were then differentiated with hydrochloric
acid alcohol for 10 sec, washed and soaked for 5 min, and
reconverted to blue with ammonia for 10 min, followed by staining
with eosin solution (cat. no. PT001; Shanghai Bogoo Biological
Technology Co., Ltd., Shanghai, China) for a few seconds.
Thereafter, the sections were dehydrated with gradient alcohol for
1 min/time and cleared twice with xylene (1 min/time). In the
ventilator, following mounting of the sections with neutral gum,
pathological changes were observed under an optical microscope
(DMM-300D; Shanghai Caikon Optical Instrument Co., Ltd., Shanghai,
China).
Masson staining
The bile duct tissue sections were dewaxed
conventionally, washed with distilled water, stained with Ponceau
(cat. no. RTD6301; Real-Times Biotechnology Co., Ltd., Beijing,
China) for 2 min and mordanted with 5% phosphomolybdate solution
(cat. no. P1910-100G; Shanghai Regal Biology Technology Co., Ltd.,
Shanghai, China) for 2 min. Following staining with methyl green
(cat. no. BI005; Shanghai Regal Biology Technology Co., Ltd.) for 3
min, the sections were color-separated with 95% alcohol, dehydrated
with 100% alcohol, cleared with xylene and mounted with neutral
gum. Subsequently, the sections were observed under a polarized
light microscope (XPT-480; Shanghai Zhongheng Co., Ltd., Shanghai,
China) and analyzed by Image Pro-6 software (Media Cybernetics
Inc., Bethesda, MD, USA). The collagen fibers were stained bluish
green, muscle fibers and cellulose were stained pink, and cells
were stained orange red.
Vitoria blue Ponceau S (VBPS)
staining
The bile duct tissue sections were dewaxed
conventionally, washed with 70% ethanol solution for 2 min,
immersed in Vitoria blue B staining solution (YML5840; Shanghai
Youyu Biological Technology Co., Ltd., Shanghai, China) for 2 h,
stained with 95% ethanol solution for 2 sec, washed with distilled
water for 2 min, and stained with Ponceau S staining solution (cat.
no. C0039; Shanghai Baoman Biological Technology Co., Ltd.,
Shanghai, China) for 4 min. Following drying with absorbent paper,
the sections were immediately soaked twice with absolute ethyl
alcohol, dried directly in air, cleared with xylene and mounted
with neutral gum following air drying in the ventilator. The
collagen fibers stained with red were observed under an optical
microscope for quantitative analysis (DMM-300D; Shanghai Caikon
Optical Instrument Co., Ltd.).
Periodic acid Schiff (PAS) staining
The bile duct tissues of each group were collected
and treated with acetylaminofluorene solution, Carnoy's solution or
at low temperature in the refrigerator. The sections were
dehydrated with absolute ethyl alcohol, cleared with xylene,
immersed in wax and embedded in paraffin, cut into 5-µm
sections and dehydrated again. Subsequently, the sections were
oxidized with 0.5-1% periodic acid for 5-10 min. The ambient
temperature did not exceed 20°C in order to prevent reduction in
the oxidation time. Thereafter, the sections were washed under
running water for 5 min, rinsed twice with distilled water, stained
with Schiff solution protected from light and rinsed twice with
0.5% sodium metabisulfite (1-2 min/time for differentiation). The
sections were then washed under running water for 5-10 min, washed
with distilled water, stained with Harris hematoxylin for 2-5 min,
washed under running water, differentiated using l% hydrochloric
acid, rinsed under running water and reconverted to blue with l%
ammonia. Finally, the sections were rinsed under running water,
conventionally dehydrated, cleared with xylene and mounted using
neutral gum. Mucin-like glycoprotein stained purplish red was
observed under an optical microscope (DMM-300D; Shanghai Caikon
Optical Instrument Co., Ltd.) for quantitative analysis with the
Image Pro-6 software.
Immunohistochemistry
The bile duct tissue sections were conventionally
dewaxed and then incubated in 3% hydrogen peroxide at room
temperature for 15 min to eliminate endogenous peroxidase. The
antigen was repaired in 0.01 mol/l citric acid buffer solution (pH
6.0) in a microwave for 10 min and was naturally cooled down.
Subsequently, the sections were sealed in 5% normal goat serum
(Abcam, Cambridge, MA, USA) at room temperature for 15 min and were
incubated with rabbit monoclonal primary antibody specific to EGFR
(cat. no. ab52894; 1:100) overnight at 4°C. Following incubation at
room temperature for 40 min, the sections were incubated with
horseradish peroxidase (HRP)-labeled goat-anti rabbit
immunoglobulin G (IgG; cat. no. ab205718; 1:2,000) for 1 h at 37°C
and HRP-labeled streptavidin for 15 min. During the intervals of
the above steps, the sections were washed twice with 0.01 mol/l PBS
(pH 7.4) for 5 min/time. The above antibodies were purchased from
Abcam. Thereafter, the sections were stained with diaminobenzidine
(DAB) for 3-5 min, fully washed with double-distilled water;
counterstained with hematoxylin for 1-3 min, dehydrated
conventionally, cleared and sealed using neutral gum. The results
were observed and images were captured using a Primo Star digital
microscope (Motic China Group Co., Ltd., Guangzhou, China).
Following random grouping and design, EGFR-positive expression was
observed as a yellow-stained cytoplasm and cell membrane
(magnification, ×100). Each slice was continuously observed for
five high-power fields. In each field, 200 cells were counted in
total, and the number of positive cells and negative cells in each
high-power field was counted. Finally, the mean cell numbers were
calculated. The SP staining kit was purchased from Shanghai Blue
Gene for Life Science Biotechnology Co., Ltd. (Shanghai, China),
and the DAB chromogenic kit was purchased from Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd. (Beijing, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA of bile duct tissues of each group was
extracted with TRIzol RNA extract (Invitrogen; Thermo Fisher
Scientific, Inc. Waltham, MA, USA). Reverse transcription of cDNA
was conducted according to the instructions of the Primescript™ RT
reagent kit (cat. no. RRO37A; Takara Biotechnology, Co., Ltd.,
Dalian, China). Following successful transcription, the target
genes and internal reference genes were amplified by fluorescence
qPCR (ABI 7500; Applied Biosystems; Thermo Fisher Scientific, Inc.)
with glyceralde-hyde-3-phosphate dehydrogenase (GAPDH) used as the
internal reference. The reaction conditions were indicated as
follows: Pre-denaturation for 10 min at 95°C, and 40 cycles of 10
sec at 94°C (denaturation), 20 sec at 60°C (annealing) and 34 sec
at 72°C (extension). The mRNA levels of EGFR and mucin 5AC (MUC5AC)
were calculated using the 2-ΔΔCq method (16). The formula was as follows:
ΔΔCq=ΔCqexperimental group−ΔCqcontrol group,
and ΔCq=Cqtarget gene−CqGAPDH. Cq refers to
the amplified cycle number when the real-time fluorescence
intensity of the reaction reaches the set threshold. The experiment
was conducted three times, and the primer sequences are presented
in Table I.
 | Table IPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Sequence |
|---|
| EGFR | Forward:
5′-CCCACTCATGCTCTACAACC-3′ |
| Reverse:
5′-GCCGGTATGATTTCTAGGT-3′ |
| MUC5AC | Forward:
5′-AGCACAGTTGCCTCAAGTCC-3′ |
| Reverse:
5′-CTCGGCTACAGGTCCATCC-3′ |
| GAPDH | Forward:
5′-CGTCTTCACCACCATGGAGA-3′ |
| Reverse:
5′-GCCAGTAGACTCCACGACAT-3′ |
Western blot analysis
The total protein in the bile duct tissues was
extracted using a protein lysis buffer (cat. no. C0481;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and the protein was
quantified using the bicinchoninic acid method. Subsequently, 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis was
performed. To each well, 20 µg of protein loading sample was
added, which was then mixed with sample loading buffer solution and
boiled for 5 min at 100°C. Following cooling in an ice bath and
centrifugation at 258 × g for 20 min at 4°C, the sample was loaded
into each lane equally by a microinjector for electrophoretic
separation. Following electrophoresis, the protein on the gel was
transferred onto a nitrocellulose membrane. The membrane was
blocked with 5% skimmed milk powder and incubated with rabbit
monoclonal antibody specific to EGFR (1 µg/ml; cat. no
ab52894) and Ki-67 (1 µg/ml; cat. no. ab16667), rabbit
polyclonal antibody specific to type I collagen (1 µg/ml;
cat. no. ab34710), rabbit polyclonal antibody specific to mammalian
target of rapamycin (mTOR; 0.5 µg/ml; cat. no. ab2732), and
rabbit monoclonal antibody specific to phosphorylated (p-)mTOR (1
µg/ml; cat. no. ab137133) overnight at 4°C. The following
day, the membrane was washed three times with Tris-buffered saline
containing 1% Tween 20 (TBST) for 5 min/time and was incubated with
HRP-labeled goat-anti rabbit antibody specific to IgG (0.5
µg/ml; cat. no. ab205718) at room temperature for 1.5 h. The
above antibodies were purchased from Abcam. The membrane was washed
with TBST again, and protein expression was observed using 1 ml
chemiluminescence reagent (prepared in accordance with
SuperSignal®West DuraExtended Duration Substrate). The
excess liquid was discarded, and the membrane was wrapped with a
fresh film. In a dark room, the membrane was exposed to X-ray film
for 5-10 min, following which the membrane was developed and fixed.
GAPDH served as the internal reference. The relative expression of
EGFR, Ki-67 and type I collagen was calculated by the ratio of the
optical density (OD) value of EGFR, Ki-67 and type I collagen to
the average gray value of the developing imaging of the GAPDH band,
and gray value analysis was performed using ImageJ software
(version 1.8.0; National Institutes of Health, Bethesda, MD, USA).
The experiment was repeated three times.
Immunofluorescence assay
The frozen sections of the bile duct wall were
incubated with rabbit polyclonal antibody specific to BrdU (1:100;
cat. no. ab152095) overnight at 4°C. The sections were washed with
PBS three times and were then incubated with goat-anti rabbit
antibody specific to IgG (1:200; cat. no. ab150077) labeled with
Alexa Fluor 488, and then were incubated at 37°C, washed three
times with PBS protected from light, and mounted. The above
antibodies were purchased from Abcam. A laser scanning confocal
microscope (Carl Zeiss AG, Oberkochen, Germany) was used to observe
the results. The results showed that the emission wavelength of
Alexa Fluor 488 was 519 nm using NIS-Elements Viewer software
(v4.2.0; Nikon Instruments, Inc., Melville, NY, USA). The
BrdU-positive cells showed blue fluorescence staining.
Determination of β-glucuronidase (β-G)
activity
Using β-G phenolphthalein as the substrate, the
activity of β-G was quantitatively determined under the condition
of pH 4.95 using the modified Fisherman method (17). The experiment was repeated three
times.
Statistical analysis
The data were analyzed using SPSS 21.0 software (IBM
Corp, Armonk, NY, USA). The measurement data are presented as the
mean ± standard deviation, and all data were tested for the
normality of distributions and homogeneity of variance. Differences
among groups were analyzed by one-way analysis of variance, and
pairwise comparisons among multiple groups were checked using
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
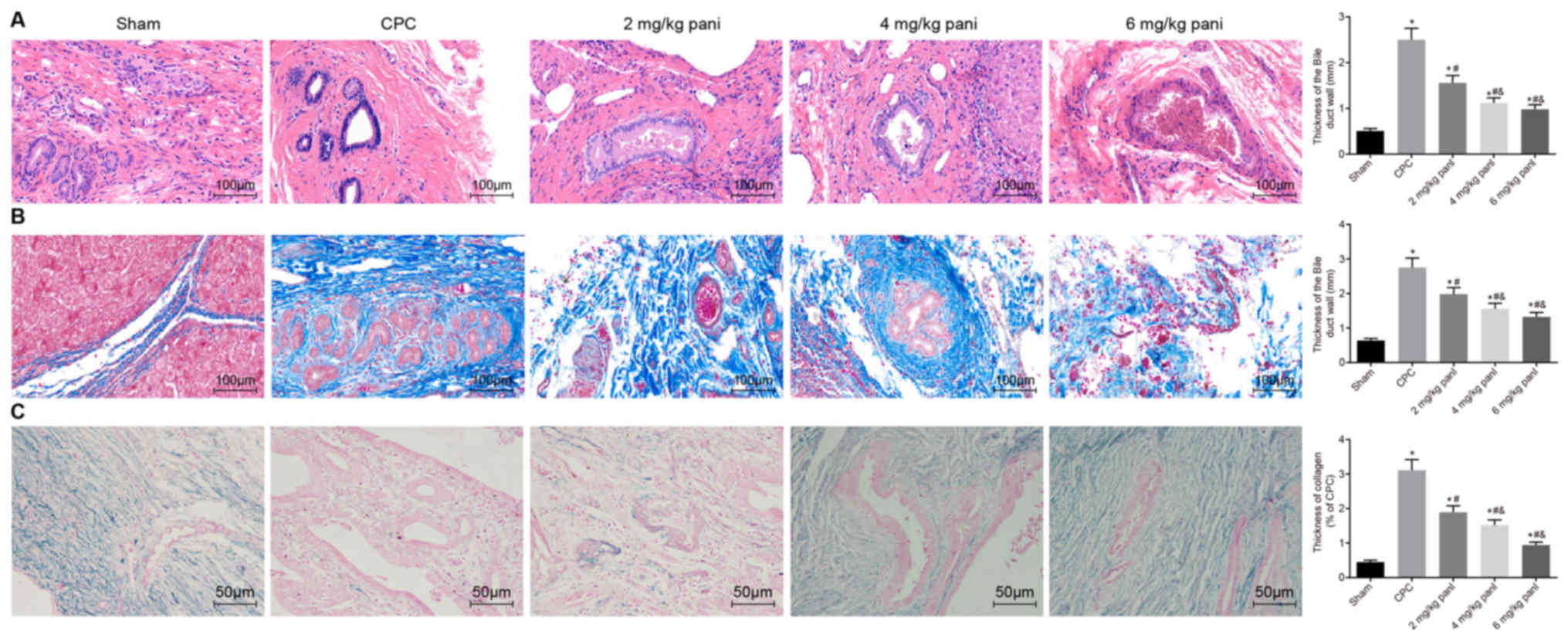
Pathological observation of bile duct
tissues in each group following CPC modeling
Following model construction, the rats of the CPC
model were treated with 2, 4 or 6 mg/kg Pani, respectively. Bile
duct specimens were collected to observe the pathological
characteristics and the diameter of the bile duct in each group was
measured. Pathological observation of the cholangitis tissues
extracted from the sham-operated and CPC rats with or without Pani
treatment was performed by H&E staining (Fig. 1A), Masson staining (Fig. 1B) and VBPS staining (Fig. 1C). Compared with the sham group,
the pathological changes of the cholangitis tissues in the CPC
group were as follows: H&E staining revealed hyperplasia of the
mucosa epithelium appearing villous in the lumen of the bile duct.
The microtissue of the hyperplastic bile duct wall encapsulated the
gland of the bile duct wall, mucus stagnation and expansion of the
adenoid cavity. Masson staining showed that the bile duct walls and
perivascular collagen fibers were proliferated in large numbers.
Hyperplasia of the collagen fibers wrapped the submucosal glands.
VBPS staining showed a significant increase in collagen fiber
thickness. Compared with the CPC group, a shorter diameter of the
bile duct, thinner bile duct wall, less fibrous tissues and
submucosal gland and thinner collagen fibers were found in the 2
mg/kg Pani group, and the pathological changes were gradually
improved; compared with the 2 mg/kg Pani group, the diameter of the
bile duct became shorter, the bile duct wall was thickened, the
thickness of the collagen fibers and the degree of inflammation
were reduced in the 4 and 6 mg/kg Pani groups, and the texture was
soft (P<0.05); these trends differed from those in the sham
group (P<0.05). No significant difference in pathology was found
between the 4 mg/kg Pani group and 6 mg/kg Pani group
(P>0.05).
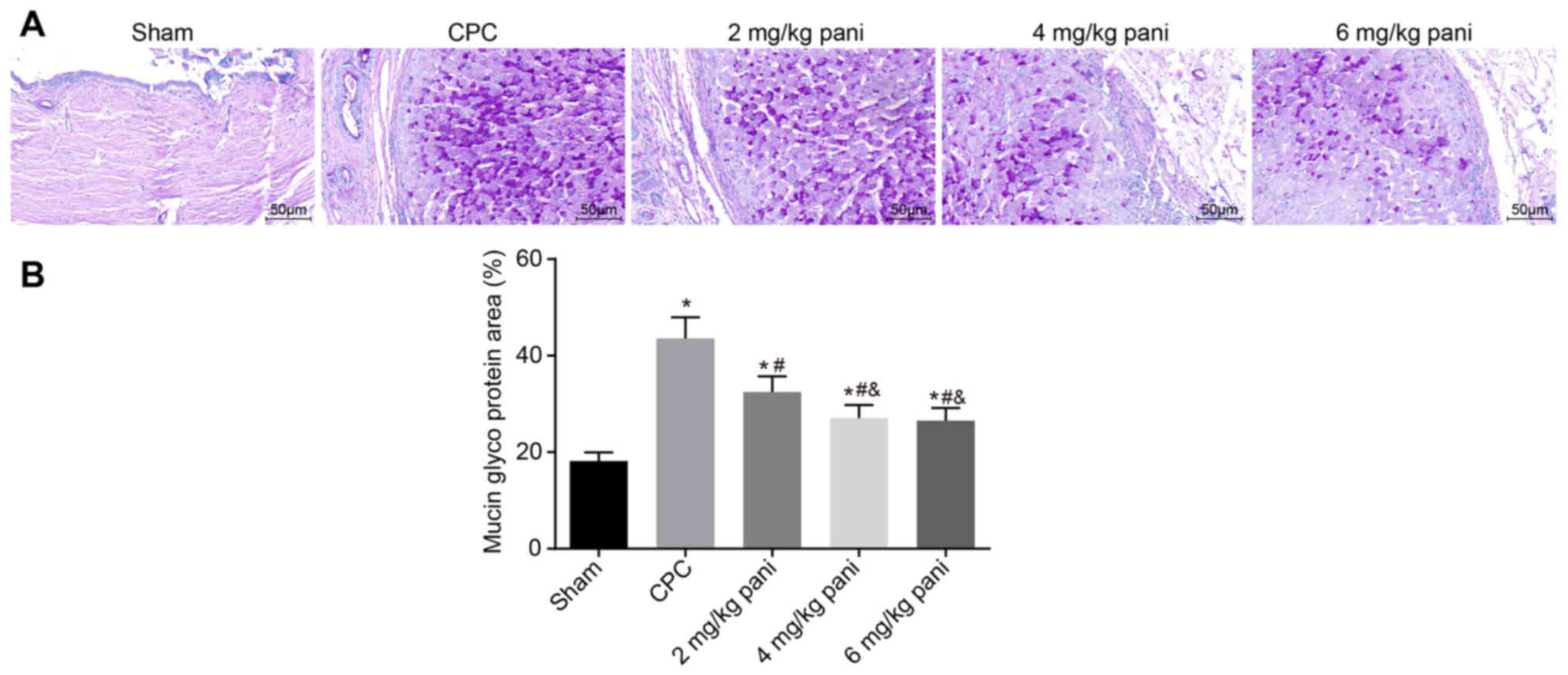
EGFR monoclonal antibody Pani inhibits
the release of mucin-like glycoproteins
Mucin serves an important role in bile viscosity and
the aggregation and deposition of stone-forming elements. In the
present study, PAS staining was used to analyze the differential
levels of mucin-like glycoproteins in the bile duct tissues of
rats. The results are shown in Fig.
2A and B. Mucin can be expressed in the cytoplasm and cell
membrane of the mucosa epithelium and submucosal gland. Mucin-like
glycoprotein was purple red on PAS staining. Compared with the sham
group, PAS staining of the bile duct epithelium and the wall glands
of the CPC group was markedly enhanced, presenting as increased
positive staining in the cytoplasm and membrane of the bile duct
epithelium and submucosal gland. Compared with the sham group, PAS
staining in the 2 mg/kg Pani group was weaker than that in the CPC
group (P<0.05). Additionally, PAS staining in the 4 mg/kg and 6
mg/kg Pani groups was weaker than that in the 2 mg/kg Pani group
(P<0.05) but more marked than that in the sham group
(P<0.05). The results showed that the EGFR monoclonal antibody
Pani inhibited the secretion of mucin-like glycoprotein in the bile
duct wall, thus effectively reducing the bile viscosity,
aggregation and deposition of stone-forming elements.
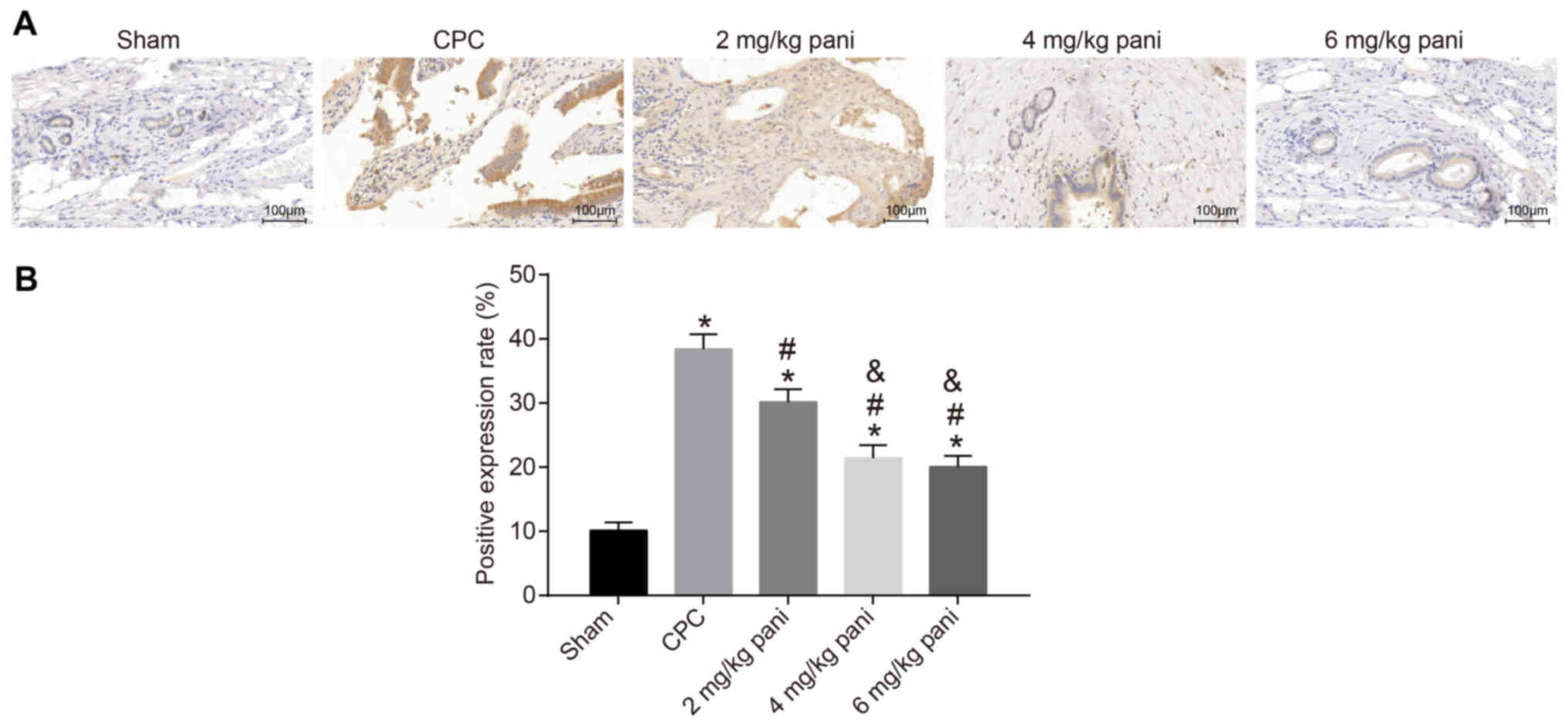
Pani inhibits the positive rate of EGFR
in bile duct tissues
Immunohistochemistry was conducted to measure the
positive rates of EGFR expressed in the bile duct tissues of rats.
The positive staining of EGFR in the bile duct tissues of each
group is shown in Fig. 3A. The
cytoplasm and cell membrane were stained brownish yellow, with
maximum staining in the cytoplasm. Compared with the sham group,
the CPC group showed more EGFR-positive staining in the cytoplasm
and cell membrane of the hyperplastic bile duct epithelium and
submucosal glands. As shown in Fig.
3B, the positive rate was used as a parameter to quantify the
expression of EGFR in each group. The results showed that the
positive rate in the 2 mg/kg Pani group (30.15%) was lower than
that in the CPC group (38.41%). Additionally, the positive rates in
the 4 mg/kg Pani group (21.42%) and the 6 mg/kg Pani group (20.07%)
were lower than that in the 2 mg/kg Pani group. No marked
differences were noted between the 4 mg/kg Pani group and 6 mg/kg
Pani group, however, the rates were higher than that in the sham
group (8.32%).
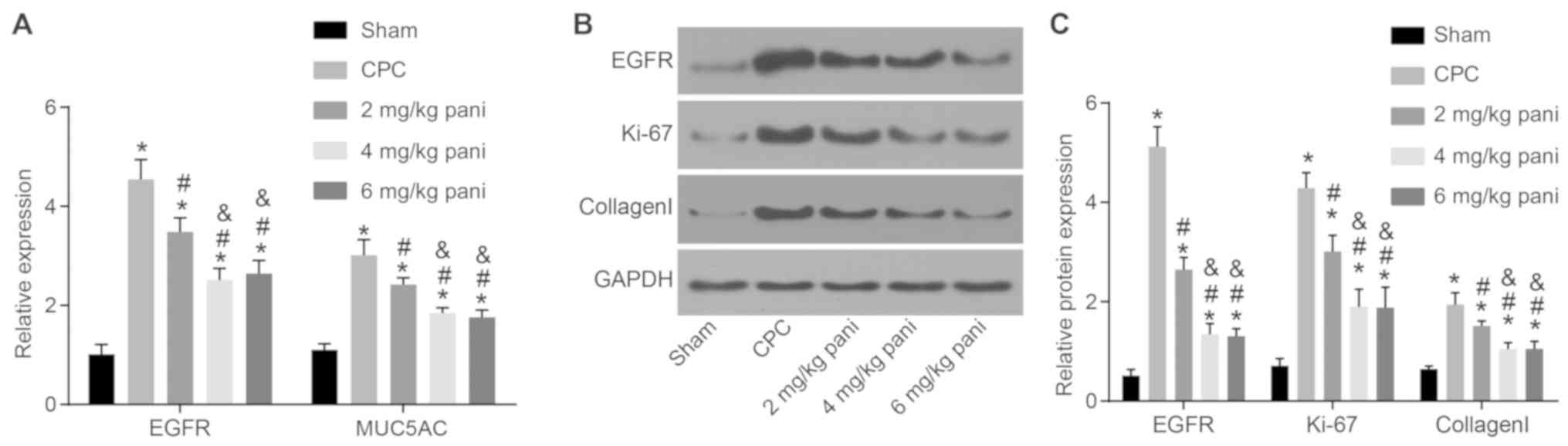
EGFR monoclonal antibody Pani inhibits
CPC by downregulating the expression of EGFR and
proliferation-associated genes in bile duct tissues
The expression of genes that contribute to the
hyperproliferation of collagen fibers in the bile duct wall and
that may cause the formation of bile duct stones, including EGFR,
Ki-67, type I collagen and MUC5AC, exist in bile duct tissues.
Therefore, the expression of these genes was determined in the
present study to examine the effect of the EGFR monoclonal antibody
Pani on CPC. As shown in Fig.
4A-C, compared with the sham group, the expression levels of
EGFR, Ki-67, type I collagen and MUC5AC in the bile duct tissues of
the other groups were significantly increased (all P<0.05).
Compared with the CPC group, the expression levels of EGFR, Ki-67,
type I collagen and MUC5AC in bile duct tissues in the 2, 4 and 6
mg/kg Pani groups were notably decreased (all P<0.05). Compared
with the 2 mg/kg Pani group, the expression levels of EGFR, Ki-67,
type I collagen and MUC5AC in the bile duct tissues in the 4 and 6
mg/kg Pani groups were significantly reduced (all P<0.05). No
differences were found between the 4 mg/kg Pani group and 6 mg/kg
Pani group in terms of these genes (all P>0.05). The results
showed that the EGFR monoclonal antibody Pani inhibited the
expression of genes associated with cell proliferation and may
cause the formation of bile duct stones, thus inhibiting the
overproliferation and potential of CPC with a receptor-saturation
effect.
EGFR monoclonal antibody Pani inhibits
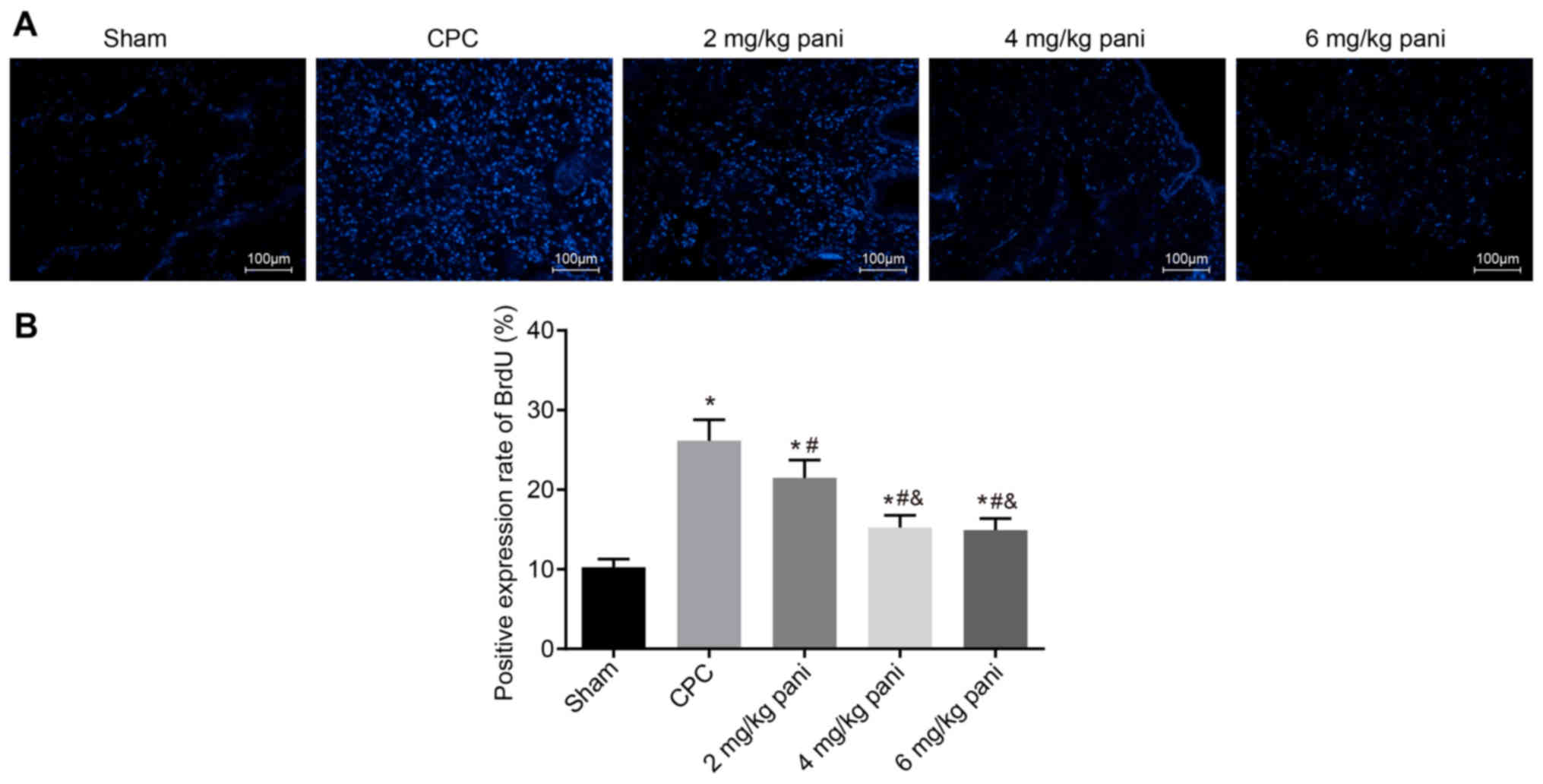
the BrdU-positive rate in bile duct tissues
The immunofluorescence assay was used to determine
the positive rate of BrdU in rat bile duct tissues to examine the
effect of EGFR monoclonal antibody Pani on CPC overproliferation.
Positive-BrdU was demonstrated with the nucleus showing blue
fluorescence. The results (Fig. 5A
and B) indicated that, compared with the sham group, the
positive rate of BrdU in the CPC group was significantly increased
(P<0.05). Compared with the CPC group, the positive rate of BrdU
in the 2 mg/kg Pani group was notably decreased (P<0.05).
Compared with the 2 mg/kg Pani group, the positive rates of BrdU in
the 4 mg/kg Pani and 6 mg/kg Pani groups were markedly reduced
(P<0.05). There were no significant differences between the 4
mg/kg Pani group and 6 mg/kg Pani group (P>0.05). These results
showed that the EGFR monoclonal antibody Pani inhibited the
positive rate of BrdU, thus inhibiting the overproliferation of
CPC, and the efficacy had a receptor-saturation effect.
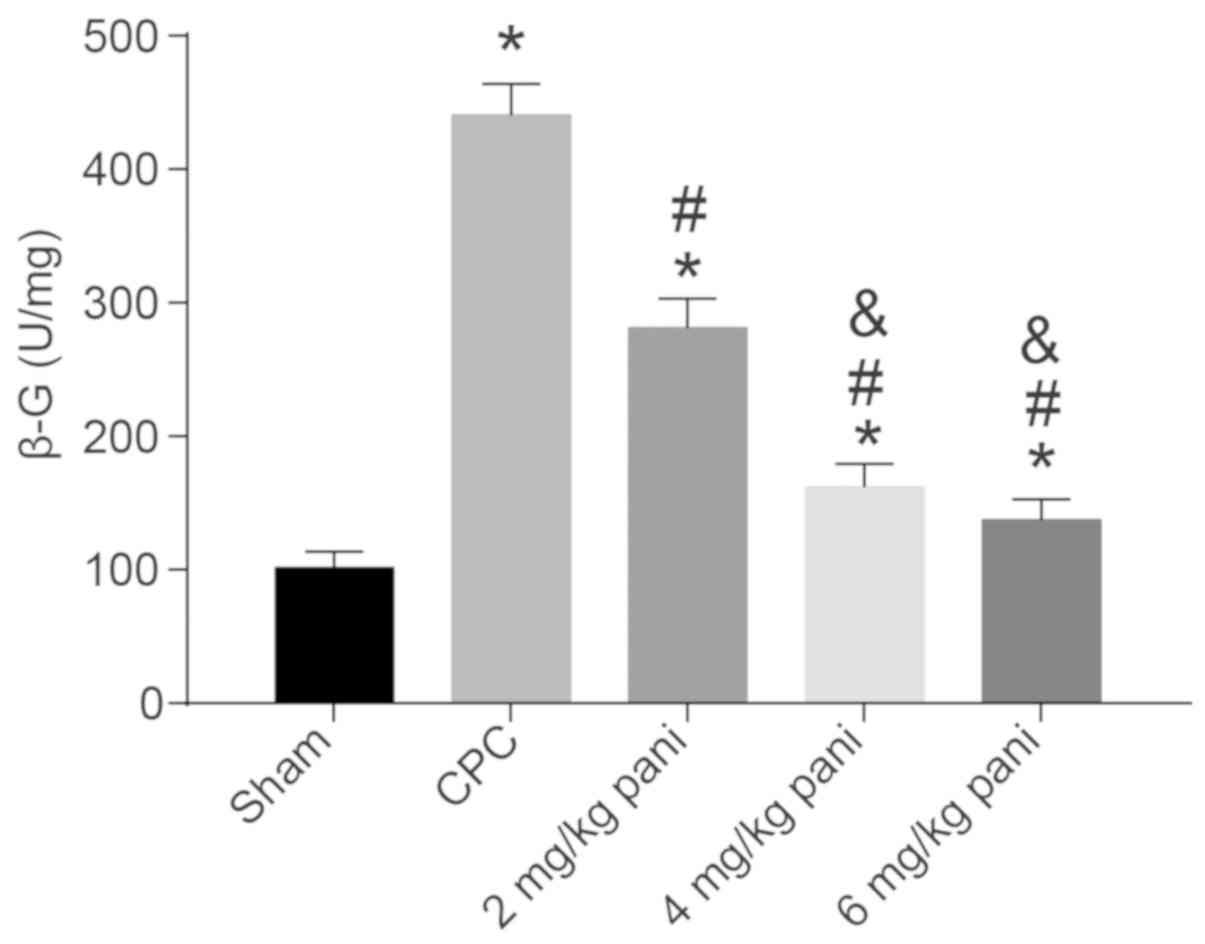
EGFR monoclonal antibody Pani inhibits
endogenous β-G activity
Following biliary tract infection, the endogenous
β-G activity in bile has been shown to be significantly increased
in addition to exogenous β-G, and this endogenous β-G activity in
the bile or liver is significantly increased in patients with bile
pigment stones (18), suggesting
that β-G is important in the formation of intrahepatic bile duct
stones. The present study compared the changes in β-G activity
among the sham, CPC, 2 mg/kg Pani, 4 mg/kg Pani and 6 mg/kg Pani
groups and further analyzed the effect of the EGFR monoclonal
antibody Pani on the stone-formation potential of CPC (Fig. 6). Compared with the sham group,
the activity of β-G in the CPC group was significantly increased
(P<0.05). Compared with the CPC group, the activity of β-G in
the 2 mg/kg Pani group was notably decreased (P<0.05). Compared
with the 2 mg/kg Pani group, the activities of β-G in the 4 and 6
mg/kg Pani groups were markedly reduced (P<0.05). There were no
significant differences between the 4 and 6 mg/kg Pani groups
(P>0.05). These results demonstrated that the EGFR monoclonal
antibody Pani inhibited the activity of β-G, thus inhibiting the
stone-formation potential of CPC, and the efficacy had a
receptor-saturation effect.
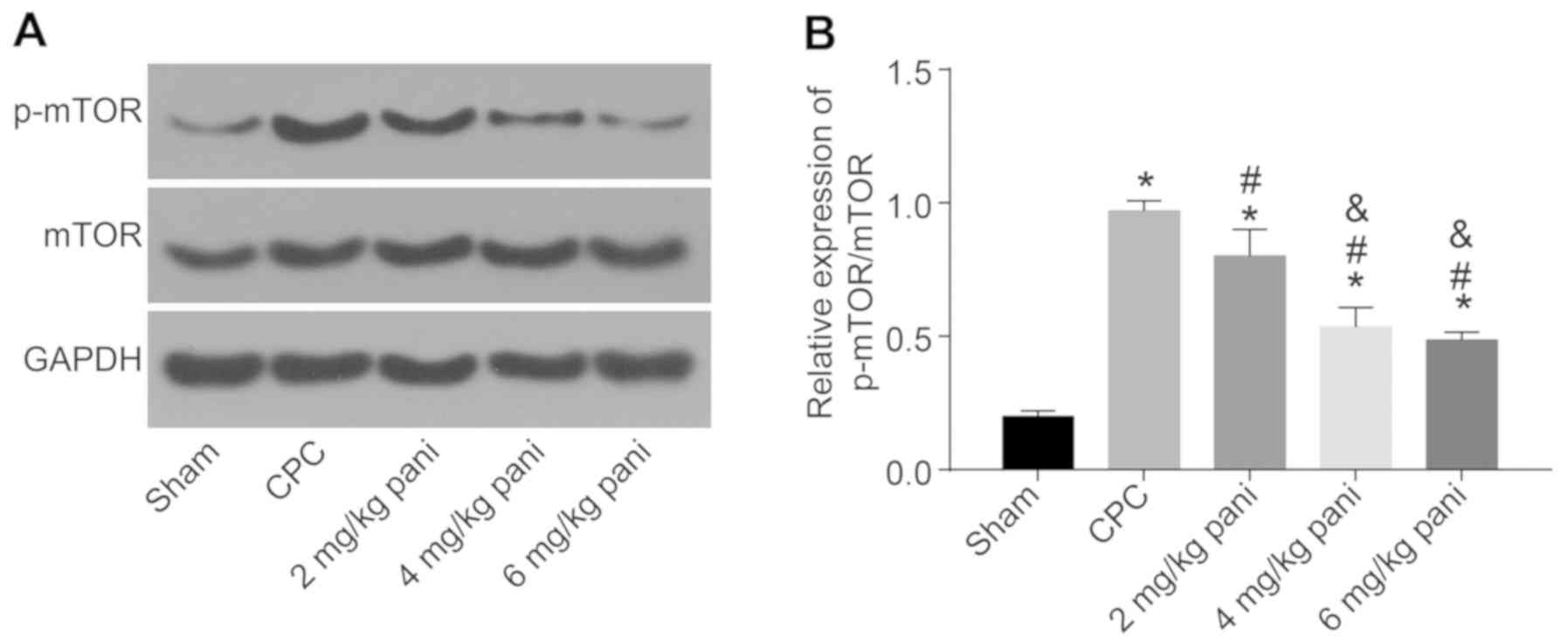
EGFR monoclonal antibody Pani
downregulates mTOR and its phosphorylation by inhibiting expression
of the EGFR gene
The mTOR signaling pathway is important in cell
growth and proliferation. In the present study, the extent of mTOR
phosphorylation in the bile duct tissues of each group was measured
and analyzed. The results (Fig. 7A
and B) revealed that, compared with the sham group, the extent
of mTOR phosphorylation in the CPC group was significantly
increased (P<0.05). Compared with the CPC group, the extent of
mTOR phosphorylation in the 2, 4 and 6 mg/kg Pani groups was
notably decreased (all P<0.05). Compared with the 2 mg/kg Pani
group, the extent of mTOR phosphorylation in the 4 and 6 mg/kg Pani
groups was markedly reduced (both P<0.05). There was no
significant difference between the 4 and 6 mg/kg Pani groups
(P>0.05), and the extent of mTOR phosphorylation was lowest in
the sham group. These results indicate that the EGFR monoclonal
antibody Pani inhibited the phosphorylation of mTOR, thus
inhibiting CPC lesions.
Discussion
High stone recurrence rates and biliary restenosis
rates in patients with hepatolithiasis have been identified to be
closely associated with CPC following surgery, however, effective
strategies have not been found (19). It was confirmed that the EGFR
inhibitor AG-1478 has a potent antiproliferative function on
proliferative cholangitis and is a potential candidate for the
treatment of proliferative cholangitis (14). In addition to antibody therapy,
proliferating cell nuclear antigen short hairpin RNA therapy can
more effectively inhibit the excessive proliferation of collagen
fibers and excessive secretion of mucus in the bile duct of PC
lesions, and may prevent biliary tract restenosis and postoperative
biliary stone recurrence (20).
The present study investigated the role of another EGFR inhibitor,
Pani, in CPC. Consequently, it was found that Pani can inhibit the
excessive proliferation and stone formation ability of bile duct
mucosa in CPC with a receptor-saturation effect. A commercial EGFR
antibody was used to measure its expression, which recognizes the
residue around the Tyr1068 of human EGFR while Pani recognizes the
residue around Ser468.
Primarily, the findings of the present study
revealed that EGFR monoclonal antibody Pani inhibited excessive
proliferation of the bile duct mucosa. CPC can thicken ongoing
fibrosis and result in restenosis of the bile ducts and hyperplasia
of submucosal glands, which may secrete further mucin-like
glycoprotein (5). A previous
study suggested that patients with bile duct carcinoma showed
somatic mutation of EGFR in the tyrosine kinase region, which can
induce signals that maintain cell survival and proliferation
(21). A study conducted by
Miyata et al revealed that ZD1839, a selective EGFR
inhibitor, suppressed cell growth and the radiation-induced
phosphorylation of EGFR in bile duct carcinoma cell lines (22). Pani has been previously reported
to effectively inhibit EGFR wild-type dimerization and activation
(23). Pani inhibits the binding
of EGF and transforming growth factor-α to the receptor, resulting
in the internalization of receptor-antibody complex, which can
inhibit the autophosphorylation of ligand-induced EGFR tyrosine,
thus inhibiting the EGFR pathway (24). Additionally, the results of the
present study showed that the EGFR monoclonal antibody Pani
inhibited the secretion of mucin-like glycoprotein in the bile duct
wall, thus effectively reducing the bile viscosity, aggregation and
deposition of stone-forming elements. Mucin-like glycoprotein, with
the potent lithogenic tendency of bile, together with bile duct
restenosis, elevates the stone recurrence rate (5). Mucin-like glycoproteins, a secretory
product of the gallbladder epithelium, are conducive to the
formation of matrix or nucleus in gallstones and other
biomineralization systems, and certain acidic glycoproteins have
been reported to be involved in bile, gallstones, and lithiasis
(25). In our previous study, it
was found that the activation of EGFR increased mucin expression in
the airway epithelium in vivo and normal human bronchial
epithelial cells in vitro (26).
The results of the present study also demonstrated
that Pani inhibited the positive expression of EGFR, and the
expression of Ki-67, type I collagen and MUC5AC, and these proteins
can cause the formation of bile duct stones, thus inhibiting the
overproliferation and potential of CPC. The efficacy also had a
receptor-saturation effect. MUC5AC is the most important factor
secreted by surface epithelial cells (27). MUC5AC is a prominent component of
airway mucus, and its hypersecretion is a main sign in patients
with chronic inflammatory airway diseases (28). Kim et al found that the
addition of EGFR markedly elevated the level of MUC5AC in cultured
human airway epithelial cells (26). Ki-67, as a proliferation marker,
has been previously found to be exclusively expressed in
proliferating cells (29). It is
associated with various clinicopathological indicators, including
tumor subtype, tumor size, tumor invasiveness and recurrence rate
in pituitary neoplasia (30).
Ki-67 is expressed at high levels in hepatolithiasis and bile duct
carcinoma tissues, reflecting a high metabolic proliferation
activity and correlating with bile duct epithelial and secondary
hyperplasia in hepatolithiasis and cholangitis (31). Collagen, a main constituent of the
extracellular matrix, is found at high levels in several types of
cancer, and affects the tumor microenvironment by enhancing the
number of macrophages and endothelial cells (32). The level of type I collagen
increases significantly with the progression of fibrosis staging in
patients with chronic hepatitis C virus (33). It has also been reported that the
transactivation of EGFR regulates the high glucose-induced
upregulation of type I collagen via the phosphoinositide
3-kinase-protein kinase Cb1-Akt signaling pathway in mesangial
cells (34).
Furthermore, Pani was shown to inhibit the
BrdU-positive rate and activity of β-G. β-G is a potent tumor
marker for cancer diagnosis and pro-drug therapies, and its
elevated level in tumors is considered to be due to tumor
overexpression and release from tumor tissues or tumor-infiltrating
immune cells (35). Bacterial
β-G, extensively present in intestinal microflora, is also involved
in metabolism and detoxification in mammals, and the decreased
activity of β-G induced by Pani monoclonal antibody in the present
study may refer to relieved CPC (36). The Pani monoclonal antibody
reduces the extent of mTOR phosphorylation by inhibiting the gene
expression of EGFR, thus inhibiting CPC lesions. mTOR, as an
important regulator in cell-signaling pathways that are commonly
deregulated in cancer, regulates cell growth and survival by
connecting with nutrient and hormonal signals (37,38). AKT, activated by EGFR, can
regulate the activation of mTOR, and EGFR is positively associated
with the expression of p-mTOR (39).
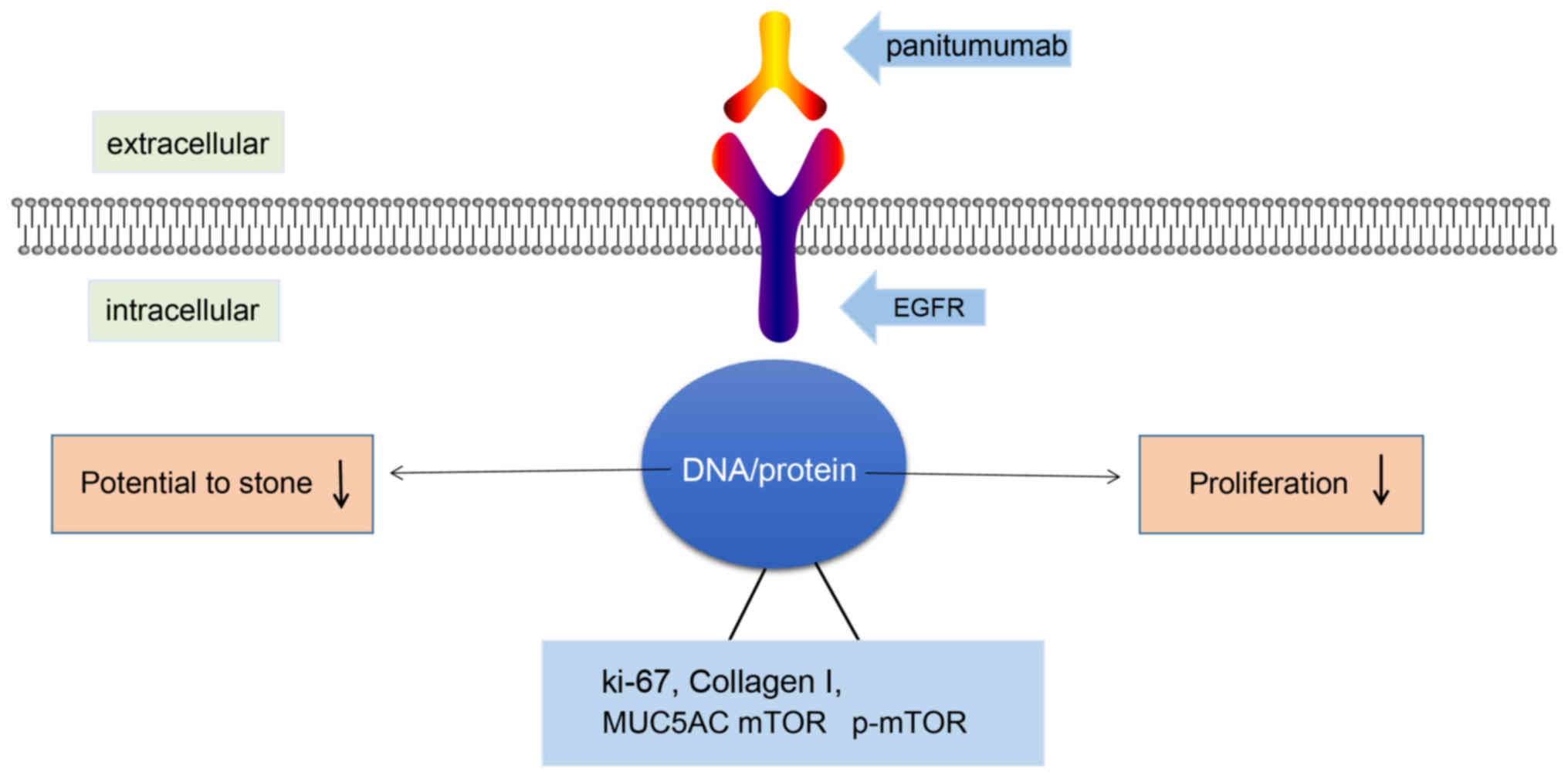
In conclusion, the data obtained in the present
study demonstrated that the EGFR monoclonal antibody Pani can
effectively suppress the excessive proliferation and
stone-formation potential of the bile duct mucosa in CPC with a
receptor saturation effect (Fig.
8). Pani offers promise as a treatment for the prevention and
cure of intrahepatic choledocholithiasis caused by CPC. However, a
positive therapeutic control was not designed in the present study,
as no other drug with the same mechanism as Pani has been found to
date. Therefore, only a blank control was selected. In the future,
large-scale experiments with a positive therapeutic control are
required to further verify the results obtained in the present
study and to identify more effective treatments for CPC.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHL and XFC designed the study. ZBX and JZ collated
the data, designed and developedthe database XFC and ZBX carried
out data analyses and produced the initial draft of the manuscript.
All authors have read and approved the final submitted
manuscript.
Ethics approval and consent to
participate
All animal experiments performed conformed to the
management of local laboratory animals guidelines and Medical
Ethics Committee of Nanfang Hospital (Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Jiang L, Jiang LS, Yan LN, Li FY, Wang W,
Cheng NS and Wen TF: Effects of epidermal growth factor receptor
inhibitor genistein on proliferative cholangitis in rats. J Surg
Res. 162:59–67. 2010. View Article : Google Scholar
|
|
2
|
Li FY, Cheng NS, Cheng JQ, Mao H, Zhou Y,
Jiang LS and Li N: Proliferating cell nuclear antigen shRNA
treatment attenuates chronic proliferative cholangitis in rats. J
Gastroenterol Hepatol. 24:920–926. 2009. View Article : Google Scholar
|
|
3
|
Li FY, Cheng NS, Mao H, Jiang LS, Cheng
JQ, Li QS and Munireddy S: Significance of controlling chronic
proliferative cholangitis in the treatment of hepatolithiasis.
World J Surg. 33:2155–2160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asano T, Shoda J, Ueda T, Maruyama T,
Kawamoto T, Sugimoto Y, Ichikawa A and Tanaka N: Involvement of
prostaglandin E2 and its specific receptor subtype EP4 in chronic
proliferative cholangitis in the bile ducts of patients with
hepatolithiasis. Gastroenterology. 124:A246. 2003. View Article : Google Scholar
|
|
5
|
Koh YX, Chiow AK, Chok AY, Lee LS, Tan SS
and Ibrahim S: Recurrent pyogenic cholangitis: Disease
characteristics and patterns of recurrence. ISRN Surg.
2013:5360812013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li F, Cheng J, He S, Li N, Zhang M, Dong
J, Jiang L, Cheng N and Xiong X: The practical value of applying
chemical biliary duct embolization to chemical hepatectomy for
treatment of hepatolithiasis. J Surg Res. 127:131–138. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okines AF, Ashley SE, Cunningham D, Oates
J, Turner A, Webb J, Saffery C, Chua YJ and Chau I: Epirubicin,
oxaliplatin, and capecitabine with or without panitumumab for
advanced esophagogastric cancer: Dose-finding study for the
prospective multicenter, randomized, phase II/III REAL-3 trial. J
Clin Oncol. 28:3945–3950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Nicolantonio F, Martini M, Molinari F,
Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L,
Frattini M, Siena S and Bardelli A: Wild-type BRAF is required for
response to panitumumab or cetuximab in metastatic colorectal
cancer. J Clin Oncol. 26:5705–5712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peeters M, Price TJ, Cervantes A, Sobrero
AF, Ducreux M, Hotko Y, Andre T, Chan E, Lordick F, Punt CJ, et al:
Randomized phase III study of panitumumab with fluorouracil,
leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as
second-line treatment in patients with metastatic colorectal
cancer. J Clin Oncol. 28:4706–4713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohenuram M and Saif MW: Panitumumab the
first fully human monoclonal antibody: From the bench to the
clinic. Anticancer Drugs. 18:7–15. 2007. View Article : Google Scholar
|
|
11
|
Keating GM: Panitumumab: A review of its
use in metastatic colorectal cancer. Drugs. 70:1059–1078. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lacouture ME, Mitchell EP, Piperdi B,
Pillai MV, Shearer H, Iannotti N, Xu F and Yassine M: Skin toxicity
evaluation protocol with panitumumab (STEPP), a phase II,
open-label, randomized trial evaluating the impact of a pre-Emptive
Skin treatment regimen on skin toxicities and quality of life in
patients with metastatic colorectal cancer. J Clin Oncol.
28:1351–1357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hecht JR, Patnaik A, Berlin J, Venook A,
Malik I, Tchekmedyian S, Navale L, Amado RG and Meropol NJ:
Panitumumab monotherapy in patients with previously treated
metastatic colorectal cancer. Cancer. 110:980–988. 2007. View Article : Google Scholar
|
|
14
|
Li F, Zhou Y, Cheng N, Mao H, Jiang L, Li
N, Li Q, de Jong MC and Pawlik TM: Epidermal growth factor receptor
as a target for anti-proliferative treatment of proliferative
cholangitis in hepatolithiasis. J Surg Res. 166:87–94. 2011.
View Article : Google Scholar
|
|
15
|
Ji YY, Wang ZD, Wang SF, Wang BT, Yang ZA,
Zhou XR, Lei NN and Yue WN: Ischemic preconditioning ameliorates
intestinal injury induced by ischemia-reperfusion in rats. World J
Gastroenterol. 21:8081–8088. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Li N, Xiao LJ, Chen SW, Li L, Xiao BL,
Chen WB, Gao XK and Gu SJ: Mucus histochemical study of bilirubin
cholangiolithiasis in rabbit model. Hua Xi Yi Ke Da Xue Xue Bao.
20:417–420. 1989.In Chinese. PubMed/NCBI
|
|
18
|
Ho KJ, Lin XZ, Yu SC, Chen JS and Wu CZ:
Cholelithiasis in Taiwan. Gallstone characteristics, surgical
incidence, bile lipid composition, and role of beta-glucuronidase.
Dig Dis Sci. 40:1963–1973. 1995. View Article : Google Scholar
|
|
19
|
Li FY, Cheng NS, Cheng JQ, Mao H, Jiang
LS, Li QS and Zhou Y: Practical value of applying cdc2 kinase shRNA
to chronic proliferative cholangitis in treatment of
hepatolithiasis. Hepatogastroenterology. 56:1477–1482. 2009.
|
|
20
|
Li FY, Cheng NS, Cheng JQ, Mao H, Jiang
LS, Li N and He S: Treatment of chronic proliferative cholangitis
with c-myc shRNA. World J Gastroenterol. 15:95–101. 2009.
View Article : Google Scholar :
|
|
21
|
Leone F, Cavalloni G, Pignochino Y,
Sarotto I, Ferraris R, Piacibello W, Venesio T, Capussotti L, Risio
M and Aglietta M: Somatic mutations of epidermal growth factor
receptor in bile duct and gallbladder carcinoma. Clin Cancer Res.
12:1680–1685. 2006. View Article : Google Scholar
|
|
22
|
Miyata H, Sasaki T, Kuwahara K, Serikawa M
and Chayama K: The effects of ZD1839 (Iressa), a highly selective
EGFR tyrosine kinase inhibitor, as a radiosensitiser in bile duct
carcinoma cell lines. Int J Oncol. 28:915–921. 2006.
|
|
23
|
Gajadhar AS, Bogdanovic E, Munoz DM and
Guha A: In situ analysis of mutant EGFRs prevalent in glioblastoma
multiforme reveals aberrant dimerization, activation, and
differential response to anti-EGFR targeted therapy. Mol Cancer
Res. 10:428–440. 2012. View Article : Google Scholar
|
|
24
|
Peeters M, Balfour J and Arnold D: Review
article: Panitumumab-a fully human anti-EGFR monoclonal antibody
for treatment of metastatic colorectal cancer. Aliment Pharmacol
Ther. 28:269–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imano M, Satou T, Itoh T, Takeyama Y,
Yasuda A, Peng YF, Shinkai M, Haji S, Yasuda C, Nakai T, et al: An
immunohisto-chemical study of osteopontin in pigment gallstone
formation. Am Surg. 76:91–95. 2010.PubMed/NCBI
|
|
26
|
Kim S, Schein AJ and Nadel JA: E-cadherin
promotes EGFR-mediated cell differentiation and MUC5AC mucin
expression in cultured human airway epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 289:L1049–L1060. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barbier D, Garcia-Verdugo I, Pothlichet J,
Khazen R, Descamps D, Rousseau K, Thornton D, Si-Tahar M, Touqui L,
Chignard M and Sallenave JM: Influenza A induces the major secreted
airway mucin MUC5AC in a protease-EGFR-extracellular regulated
kinase-Sp1-dependent pathway. Am J Respir Cell Mol Biol.
47:149–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shao MX and Nadel JA: Dual oxidase
1-dependent MUC5AC mucin expression in cultured human airway
epithelial cells. Proc Natl Acad Sci USA. 102:767–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bullwinkel J, Baron-Luhr B, Ludemann A,
Wohlenberg C, Gerdes J and Scholzen T: Ki-67 protein is associated
with ribosomal RNA transcription in quiescent and proliferating
cells. J Cell Physiol. 206:624–635. 2006. View Article : Google Scholar
|
|
30
|
Salehi F, Agur A, Scheithauer BW, Kovacs
K, Lloyd RV and Cusimano M: Ki-67 in pituitary neoplasms: A
review-part I. Neurosurgery. 65:429–437. 2009. View Article : Google Scholar
|
|
31
|
Wang P, He Y, Ma X, Sun B, Huang B, Zhu C
and Liu Y: Expression and significance of COX-2 and Ki-67 in
hepatolithiasis with bile duct carcinoma. Med Sci Monit.
21:2943–2949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang H, Li X, Chen B, Wang B, Zhao Y,
Zhuang Y, Shen H, Zhang Z and Dai J: A collagen-binding EGFR
single-chain Fv antibody fragment for the targeted cancer therapy.
J Control Release. 209:101–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Attallah AM, Mosa TE, Omran MM, Abo-Zeid
MM, El-Dosoky I and Shaker YM: Immunodetection of collagen types I,
II, III, and IV for differentiation of liver fibrosis stages in
patients with chronic HCV. J Immunoassay Immunochem. 28:155–168.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu D, Peng F, Zhang B, Ingram AJ, Kelly
DJ, Gilbert RE, Gao B, Kumar S and Krepinsky JC: EGFR-PLCgamma1
signaling mediates high glucose-induced PKCbeta1-Akt activation and
collagen I upregulation in mesangial cells. Am J Physiol Renal
Physiol. 297:F822–F834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Su YC, Cheng TC, Leu YL, Roffler SR, Wang
JY, Chuang CH, Kao CH, Chen KC, Wang HE and Cheng TL: PET imaging
of beta-glucuronidase activity by an activity-based 124I-trapping
probe for the personalized glucuronide prodrug targeted therapy.
Mol Cancer Ther. 13:2852–2863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen M, Cheng KW, Chen YJ, Wang CH, Cheng
TC, Chang KC, Kao AP and Chuang KH: Real-time imaging of intestinal
bacterial β-glucuronidase activity by hydrolysis of a fluorescent
probe. Sci Rep. 7:31422017. View Article : Google Scholar
|
|
37
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feldman ME, Apsel B, Uotila A, Loewith R,
Knight ZA, Ruggero D and Shokat KM: Active-site inhibitors of mTOR
target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol.
7:e382009. View Article : Google Scholar
|
|
39
|
Schmid K, Bago-Horvath Z, Berger W, Haitel
A, Cejka D, Werzowa J, Filipits M, Herberger B, Hayden H and
Sieghart W: Dual inhibition of EGFR and mTOR pathways in small cell
lung cancer. Br J Cancer. 103:622–628. 2010. View Article : Google Scholar
|