Introduction
Vascular endothelial cells (ECs) are known serve an
important in the entire cardiovascular system (1,2),
and they have anticoagulation and anti-adhesive effects. All
stimulating factors can activate vascular ECs and cause cell
injury. The injury of vascular ECs by oxidative stress is a major
initiator and driving factor in the progression of atherosclerosis
(3,4). Therefore, in anti-atherosclerotic
treatment, it is key is to reduce the injury and loss of vascular
ECs. Studies have indicated that the major type of injury from
oxidative damage is apoptosis, which may affect the natural
function and structure of vascular ECs, and result in the loss of
vascular ECs (4).
Autophagy is a lysosome-dependent catabolic process
involving the degradation of long-lived proteins and organelles,
and recycling of cytoplasmic components (5,6).
It is known that autophagy has a crucial role in maintaining
cardiovascular cell functions and structure, and to degrade
long-lived proteins and their own organelles (7). It has been indicated that autophagy
serves a vital role in cardiovascular disease by regulating EC
functions (8).
Salvianolic acid B (Sal B) is the most abundant
water-soluble compound extracted from Danshen and possesses
anti-oxidative and anti-inflammatory effects. Its chemical
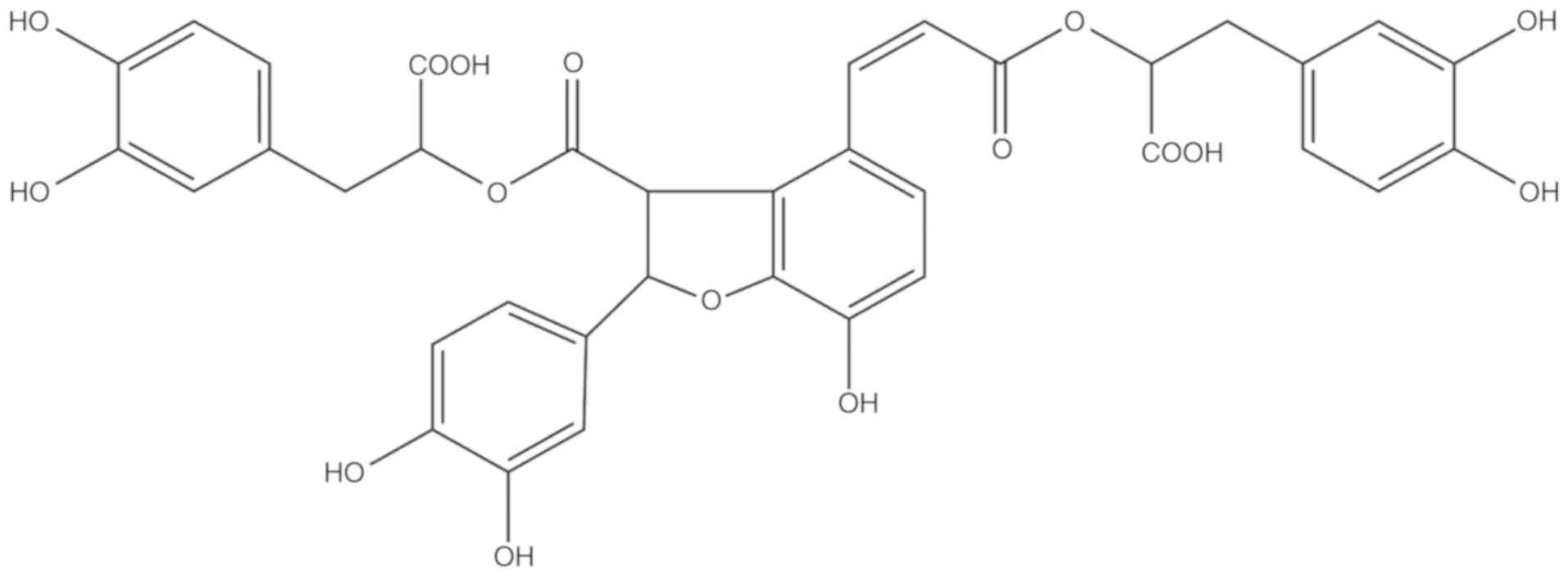
structure is presented in Fig. 1.
The Chinese medicinal formulation 'Shuangdan oral solution' is used
to treat atherosclerosis and one of its major components is Sal B
(9); however, the mechanisms of
action remain to be fully elucidated. Therefore, the present study
further examined the anti-atherosclerotic effects of Sal B and
investigated the underlying mechanisms. Previous studies have
confirmed that Sal B has anti-oxidative effects and eliminates
superoxide anion radicals (O2−), thereby suppressing
hydrogen peroxide (H2O2)-induced apoptosis
(10,11). Another study indicated that Sal B
promotes human umbilical vein endothelial cell (HUVEC)
proliferation (12). However, the
mechanisms, including whether autophagy is involved, remain to be
fully elucidated. Autophagy and apoptosis are crucial mechanisms in
regulating cell survival (13).
The possible association between autophagy and apoptosis in the
effects of Sal B also remains to be determined. Therefore, the
present study aimed to investigate the protective effects of Sal B
against H2O2-induced apoptosis in HUVECs and
the underlying mechanisms in order to provide novel approaches for
the treatment of atherosclerosis.
Materials and methods
Reagents and antibodies
The HUVECs were provided by the Cell Bank of the
Chinese Academy of Sciences. Dulbecco's modified Eagle's medium
(DMEM) was obtained from Gibco/Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The MTT kit was purchased from
Beyotime Institute of Biotechnology. Hoechst 33258, the Caspase 3
Activity Assay kit, adenovirus (Ad)-mCherry-green fluorescence
protein (GFP)-light chain (LC)3B and the bicin-choninic acid (BCA)
Protein Assay kit were all purchased from Beyotime Institute of
Biotechnology. Antibodies against p62 (cat. no. ab109012;
1:10,000), LC3-II (cat. no. ab192890; 1:2,000), AMPK (cat. no.
ab32047; 1:1,000), phosphorylated (p)-AMPK (cat. no. ab131357;
1:500), cytochrome c (cat. no. ab133504; 1:5,000), AKT (cat.
no. ab179463; 1:10,000), p-AKT (cat. no. ab81283; 1:5,000), caspase
3 (cat. no. ab32351; 1:5,000), mammalian target of rapamycin (mTOR;
cat. no. ab32028; 1:1,000) and p-mTOR (cat. no. ab109268; 1:1,000)
were purchased from Abcam (Cambridge, MA, USA), and antibody
against Beclin-1 (cat. no. 3495T; 1:1,000) was obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). β-actin (cat. no.
bs-0061R; 1:5,000) and goat anti-mouse IgG (cat. no. bs-0295GS;
1:2,000) were obtained from Bioss Biotechnology (Beijing, China).
3-Methyladenine (3-MA) (Selleck, Houston, TX, USA) was used to
inhibit autophagy the and mTOR inhibitor, rapamycin (Selleck,
Houston, TX, USA), was used to activate autophagy. Compound C was
purchased from Calbiochem (VWR, Lutterworth, UK).
Cell culture
The HUVECs were grown in DMEM supplemented with 10%
fetal bovine serum (GIBCO/Invitrogen; Thermo Fisher Scientific,
Inc.) in an incubator at 37°C and in a humidified atmosphere with
5% CO2. The HUVECs were pre-treated with Sal B (5, 10 or
20 µg/ml) for 24 h, following which the liquids were removed
and H2O2 (800 µM) was added for 24 h
at 37°C.
Cell viability assay
An MTT assay was used to assess the cell viability,
as described previously (14).
The HUVECs were cultured in 96-well plates (5,000 per well) and
incubated at 37°C for 12 h. Following the different treatments, 10
ml MTT solution at a 1:10 dilution was added to each well, followed
by incubation at 37°C for 40 min. The absorbance at 450 nm was
detected with a microplate reader (Bio-Rad 550; Bio-Rad
Laboratories, Inc.). The optical density (OD) was used to determine
the percentage of viable cells via the following formula: Cell
viability (%)=(ODtreatment group-ODblank
group/ODcontrol group-ODblank group)
×100%.
Hoechst 33258 fluorescence staining
The HUVECs received the same treatments as described
above, followed by a wash with PBS and fixing with 0.5 ml 4%
paraformal-dehyde for 15 min. Following rinsing twice with PBS, the
cells were stained with 0.5 ml Hoechst 33258 (5 mg/ml) for 15 min
and examined under a fluorescence microscope. HUVECs exhibited a
normal nuclear size and uniform fluorescence, whereas apoptotic
cells membrane exhibited increased permeability, chromatin
shrinkage and denser apoptotic nuclei.
Flow cytometric analysis
An Annexin V-FITC/propidium iodide (PI) dual
staining detection kit was used to detect apoptosis in compliance
with the manufacturer's protocols. Following treatment, the HUVECs
were washed twice in cold PBS, and the cells were then stained with
Annexin V-FITC and PI in binding buffer for 15 min at room
temperature in the dark. The cells were subsequently examined by
flow cytometry.
Caspase-3 activity assay
The activity of caspase-3 was measured using the
caspase-3 activity kit (Beyotime Institute of Biotechnology).
Cellular extracts (50 µl) were incubated in a 96-well
microtiter plate with 2 mM Ac-DEVD-pNA, a substrate of active
caspase-3, for 2 h at 37°C. Caspase activity was measured by
cleavage of the Ac-DEVD-pNA substrate to pNA (15). The absorbance at 405 nm was
measured by an ELISA reader at room temperature.
mCherry-GFP-LC3B
The cells were transfected with Ad-mCherry-GFP-LC3B
under non-autophagic conditions, following which the
mCherry-GFP-LC3B was present in the cytoplasm, as indicated by
dispersive yellow fluorescence. Under conditions of autophagy,
mCherry-GFP-LC3B is aggregated on the autophagic membrane,
visualized as yellow spots. When the autophagosome is fused with
lysosomes, the fluorescence of GFP is quenched, with the reagent
presents as red spots. The cells were washed twice with PBS,
followed by the addition of 1.5 ml fresh medium and transfected
with Ad-mCherry-GFP-LC3B adenovirus (Beyotime Institute of
Biotechnology), which adopts a mature E1 defective recombinant
adenovirus vector system and expresses the fusion protein of red
fluorescent protein mCherry, GFP and LC3B in target cells after
infection at a MOI of 20 at 37°C for 24 h. Following Sal B and
H2O2 treatment, the variations in LC3B
fluorescence were recorded with a fluorescence microscope.
Western blot analysis
The levels of p62, LC3-II, AMPK and p-AMPK,
cytochrome c, AKT, p-AKT, cleaved caspase-3, mTOR and p-mTOR
in protein extracts from the HUVECs were detected by western blot
analysis. The cells were lysed at 4°C for 30 min with RIPA lysis
buffer (Beyotime Institute of Biotechnology) containing protease
and phosphatase inhibitors, and soluble lysates were harvested via
centrifugation at 168 × g for 20 min at 4°C and boiled, and protein
concentration was determined with the BCA kit. The protein samples
(7 µl per lane) were fractionated by SDS-PAGE, the
percentage of which was decided by the type of protein. LC3 was
used with a 15% separation gel and the other proteins were used
with a 12% separation gel. Transferred onto a polyvinylidene
difluoride membrane (Merck KGaA, Darmstadt, Germany) and then
blocked in 5% skimmed milk powder. The membranes were incubated
with primary antibodies for 24 h and secondary antibody for 1 h at
room temperature. Following washing with tris buffered saline
containing 0.1% Tween-20, the enhanced chemiluminescence western
blotting substrate (Merck KGaA) was added to the membranes, which
were evaluated using a gel imaging system (Bio-Rad Laboratories,
Inc.). The analysis of each protein was performed three times.
Statistical analyses
Data are expressed as the mean ± standard error of
mean. SPSS statistical software (version 17.0; SPSS, Inc., Chicago,
IL, USA.) was used for statistical analyses. One-way ANOVA was used
for comparison between multiple groups, followed by the Bonferroni
post hoc test or unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Sal B protects HUVECs from
H2O2-induced cytotoxicity
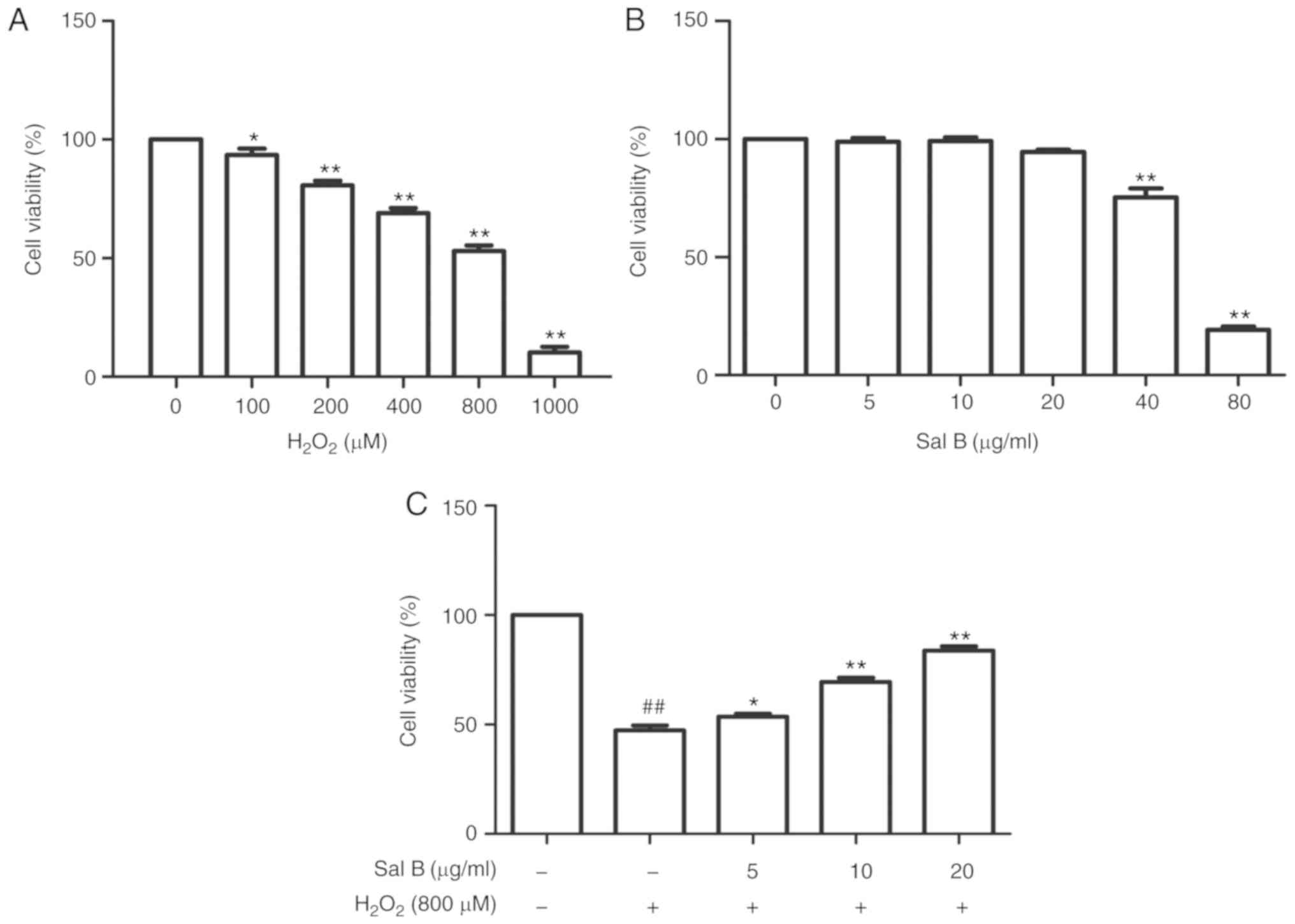
First, the HUVECs were treated with different
concentrations of H2O2 for 24 h to determine
the dose to achieve optimal oxidative stress conditions. The cell
viability in the different groups was detected using an MTT assay.
As presented in Fig. 2A, an
H2O2 dose-dependent increase in cytotoxicity
was observed. The cell viabilities were significantly decreased
following treatment with 1,000 µM
H2O2. Therefore, 800 µM
H2O2 was selected to perform the subsequent
experiments. In order to determine the concentration range of Sal B
to assess its cyto-protective effect, the HUVECs were first treated
with this drug at different concentrations (5, 10, 20, 40 and 80
µg/ml). When the concentration was increased to 80
µg/ml, the cell viability was significantly decreased
(Fig. 2B). Therefore, Sal B was
used at the concentrations of 5, 10 and 20 µg/ml in the
subsequent experiments. To assess the cytoprotective effect of Sal
B, the HUVECs were pre-treated with different concentrations of Sal
B (5, 10 and 20 µg/ml) for 24 h, followed by incubation with
800 µM H2O2 for 24 h. As presented in
Fig. 2C, the cell viability in
the Sal B + H2O2 group was increased compared
with that in the H2O2 group, suggesting that
Sal B protects HUVECs against H2O2-induced
damage.
Sal B inhibits apoptosis in HUVECs
induced by H2O2
To determine whether the effect of Sal B to inhibit
the toxicity of H2O2 to HUVECs was associated
with inhibition of inhibition of cell injury and apoptosis in
vitro, the HUVECs were pre-treated with different
concentrations of Sal B (5, 10 or 20 µg/ml) for 24 h,
followed by H2O2 treatment for 24 h. The
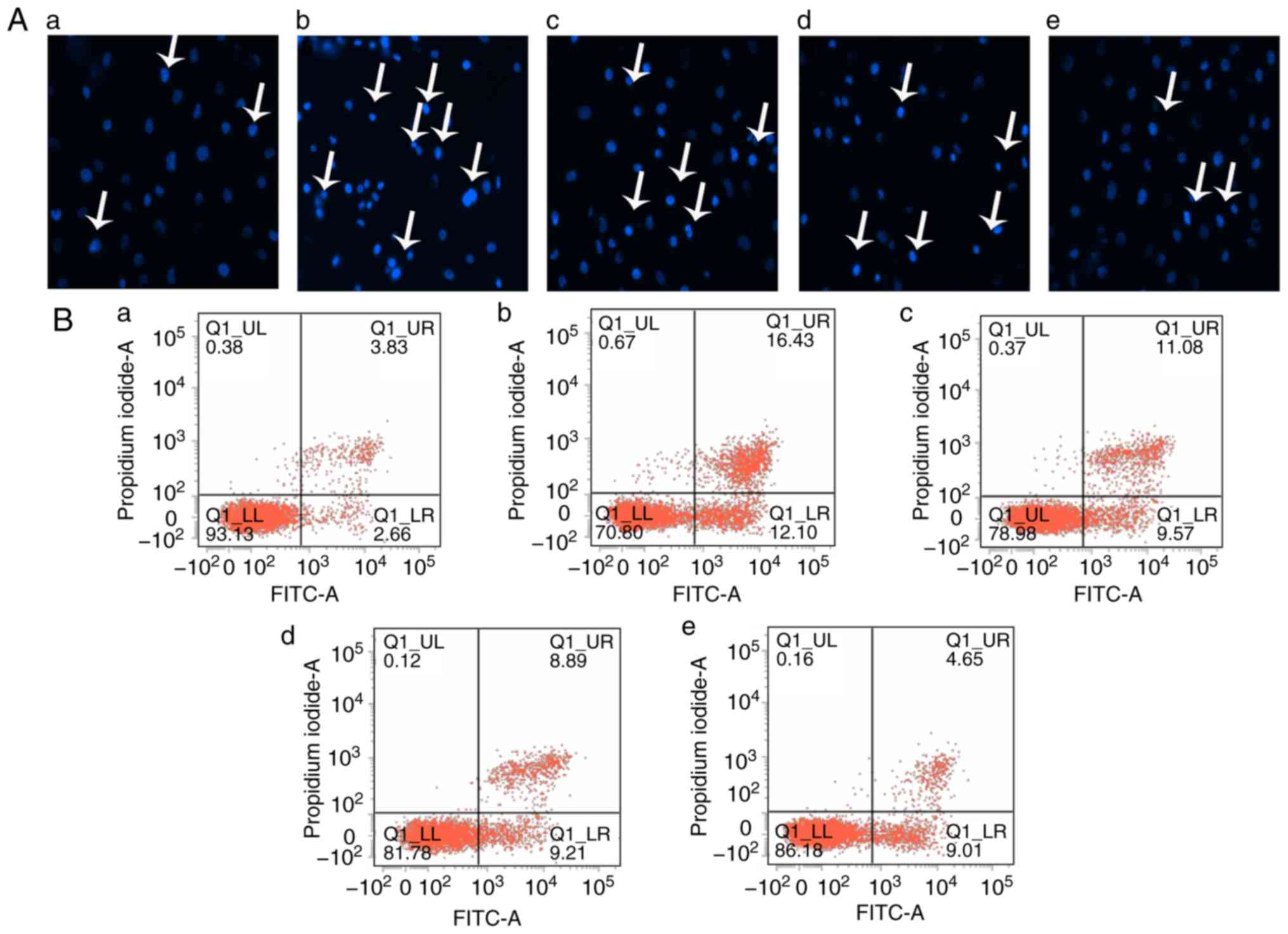
Hoechst staining assay is capable of detecting condensed chromatin
in apoptotic cells (16). The
Hoechst staining results (Fig.
3A) indicated that the proportion of apoptotic cells was
significantly increased in the H2O2 treatment
group compared with that in the control group, but this was
attenuated by pre-treatment with Sal B (Fig. 3C). To further confirm this
observation, flow cytometry with Annexin V-FITC/PI double staining
was performed. In accordance with the results of the Hoechst
staining assay, Annexin V-FITC/PI double staining indicated a
similar increase in cell apoptosis in the
H2O2 group compared with that in the control
group. However, a dose-dependent decrease in the apoptotic rate of
the HUVECs was achieved by pre-treatment with Sal B (Fig. 3C and D). In addition, the activity
of caspase-3, a key enzyme in the apoptotic process, was detected.
The H2O2-induced increase in the activity of
caspase-3 was attenuated by pre-treatment with Sal B (Fig. 3H). Furthermore, the levels of
apoptosis-associated proteins cytochrome c and cleaved caspase-3
were detected by western blotting. The results indicated that
pre-treatment with Sal B decreased the
H2O2-induced cytoplasmic levels of cytochrome
c and cleaved caspase-3 in the HUVECs (Fig. 3E-G). These results all
demonstrated that Sal B protects HUVECs from oxidative
stress-induced apoptosis in vitro.
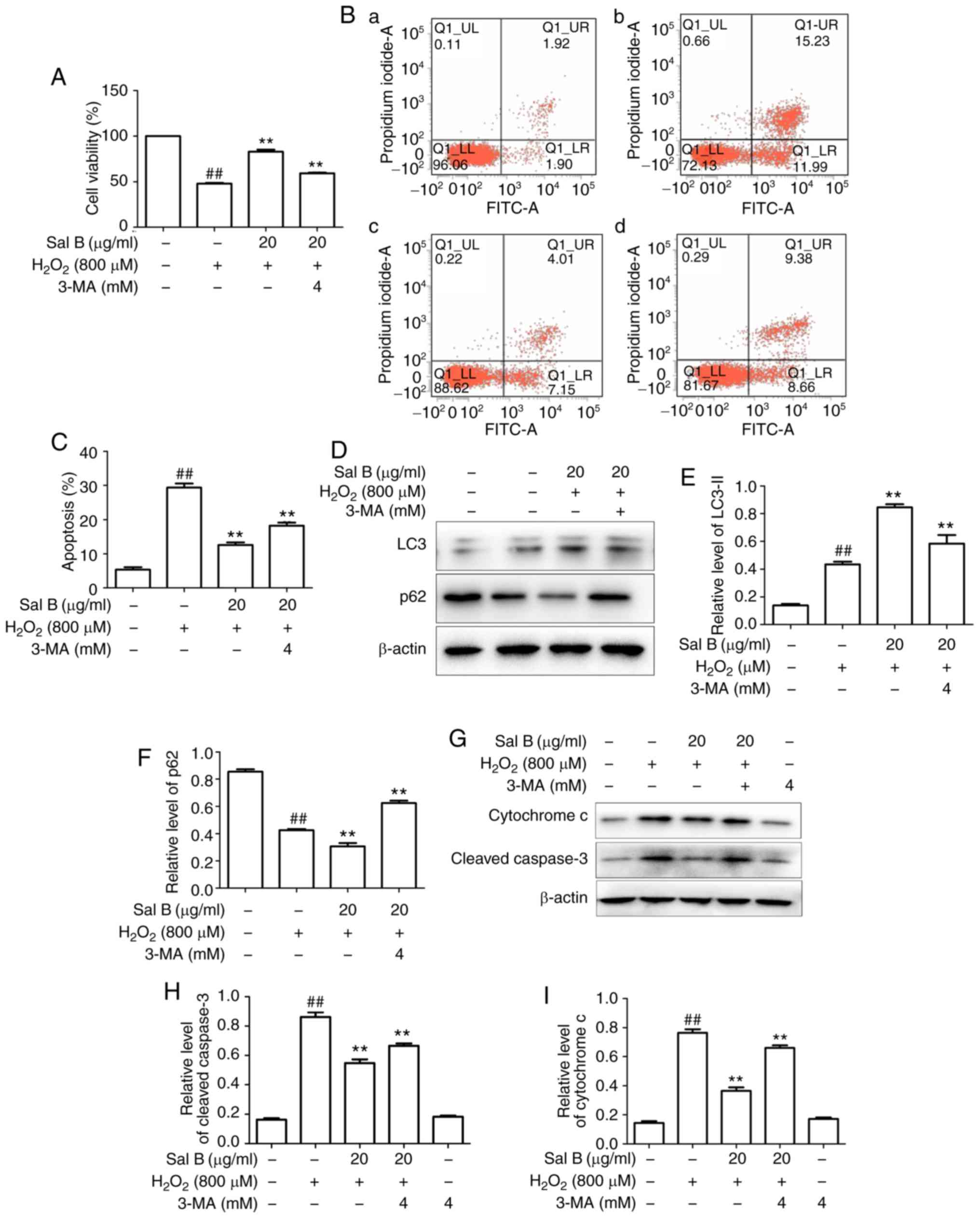
Autophagy protects HUVECs against
H2O2-induced apoptosis
An increasing number of studies have suggested that
an appropriate level of autophagy may protect cells against cell
injury (17). To determine
whether autophagy protects HUVECs from apoptosis under oxidative
stress, the autophagy inhibitor 3-MA and the mTOR inhibitor
rapamycin were used. First, upregulation of LC3-II
(autophagy-associated protein) and downregulation of p62 were
observed following stimulation by H2O2 in
comparison with the control group, and these changes were abrogated
by 3-MA but enhanced by rapamycin (Fig. 4D-F). These results suggest that
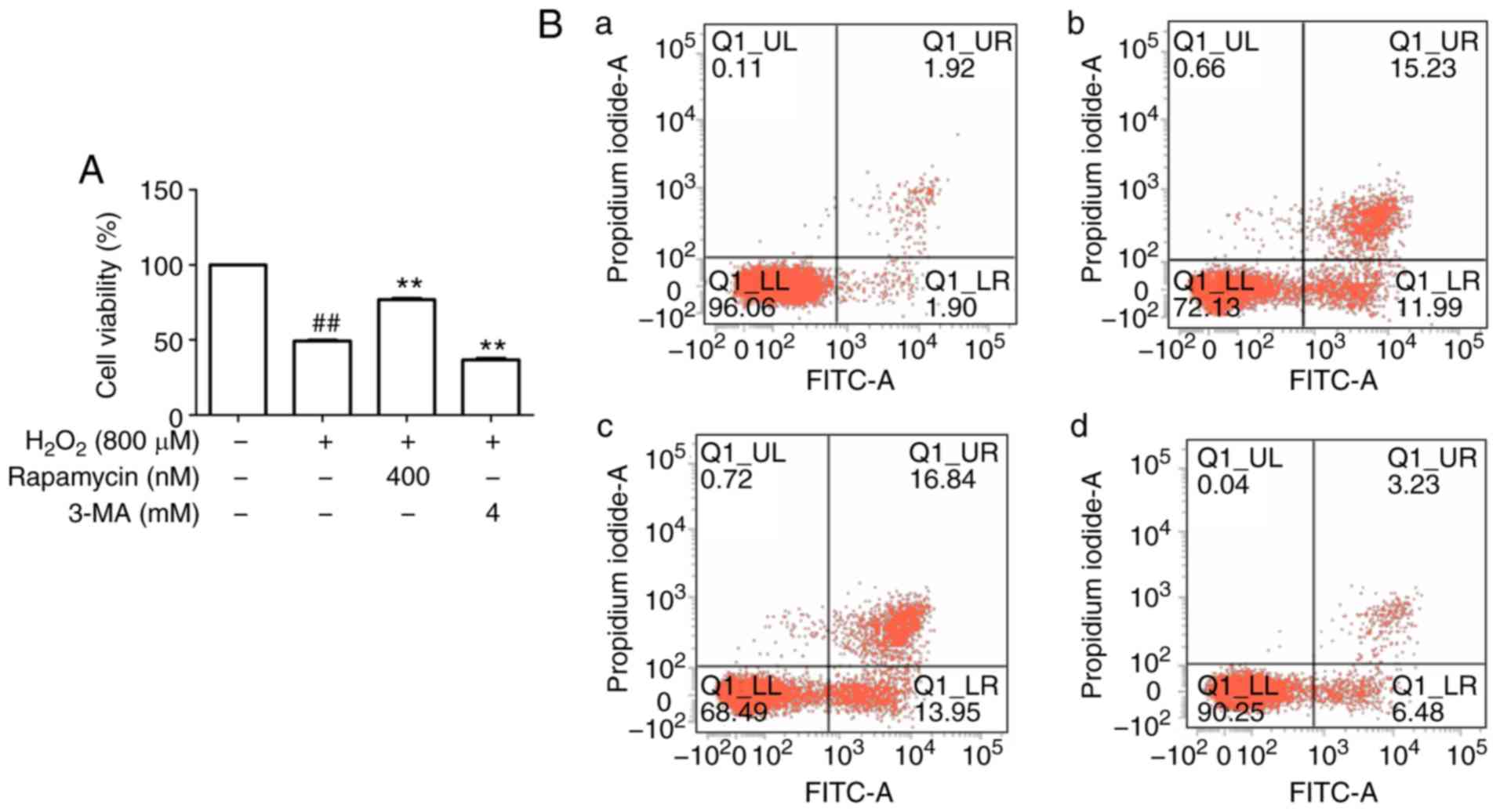
autophagy was induced in this cell model of oxidative stress.
Regarding the effect of autophagy on cell viability, it was
observed that the viability in the H2O2 group
was decreased compared with that in the control group, whereas
rapamycin increased cell viability and 3-MA decreased cell
viability compared with that in the H2O2
group (Fig. 4A). Flow cytometric
analysis was then performed for further confirmation, and the
results indicated that apoptosis in the rapamycin group was
decreased, whereas that in the 3-MA group was increased, compared
with that in the H2O2 group (Fig. 4B and C), further suggesting that
autophagy may inhibit apoptosis.
In addition, apoptosis-associated proteins were
determined for further confirmation, and the results indicated
that, compared with those in the H2O2 group,
rapamycin decreased the protein levels of cytochrome c and
cleaved caspase-3, whereas 3-MA enhanced their levels (Fig. 4G-I). These results suggest that
autophagy is a protective mechanism against
H2O2-induced HUVEC apoptosis.
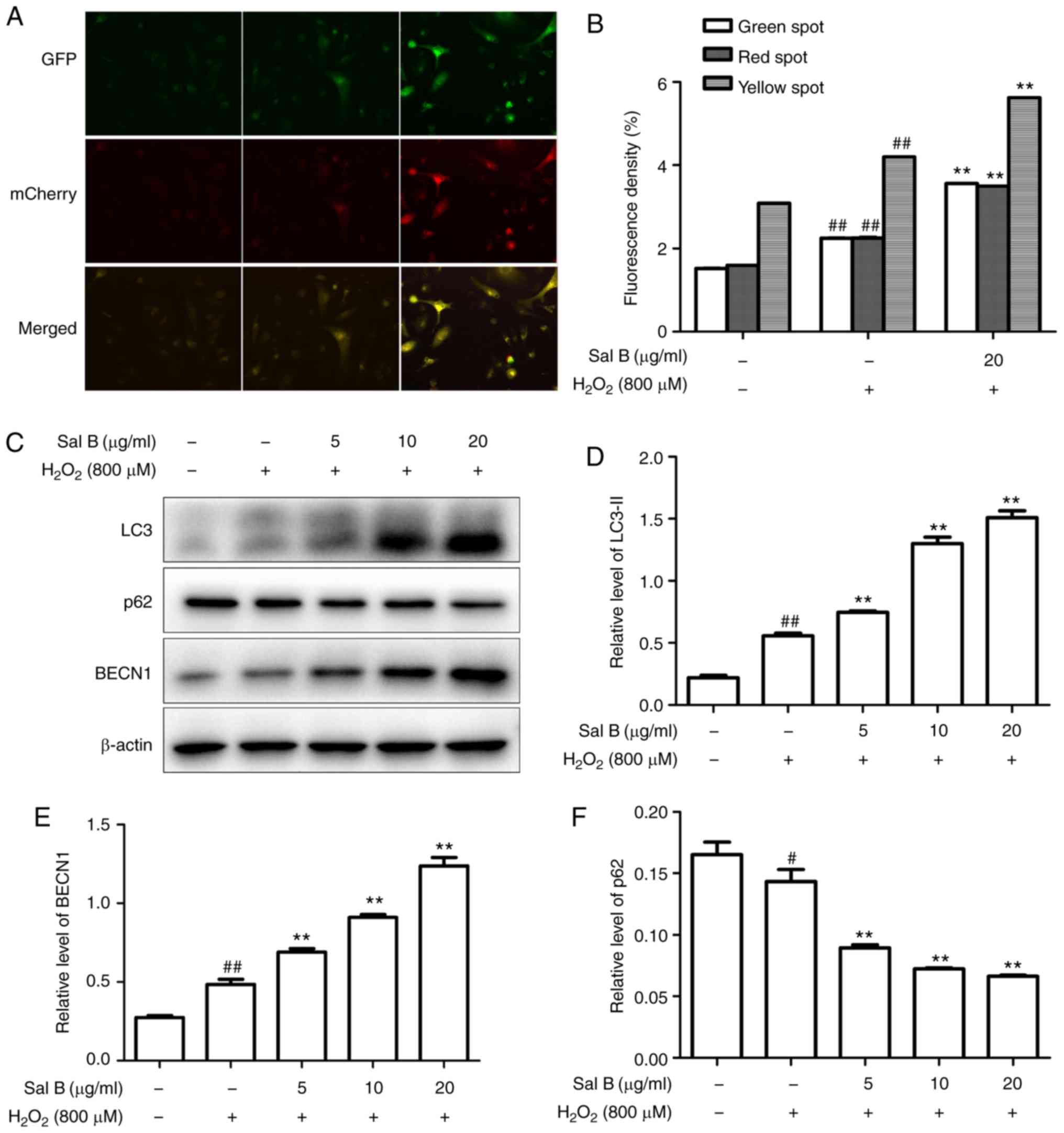
Sal B promotes autophagy in HUVECs under
oxidative stress
The HUVECs were transfected with Ad-mCherry-GFP-LC3B
followed by challenge with H2O2 with
pretreatment of 20 µg/ml Sal B. The numbers of green, red
and yellow spots were increased in the Sal B +
H2O2 group compared with those in the
H2O2 group (Fig. 5A and B). These results suggested
that Sal B promotes autophagy flux. To further confirm whether Sal
B promotes autophagy in HUVECs under oxidative stress, the HUVECs
were pre-treated with different concentrations of Sal B (5, 10 and
20 µg/ml), followed by incubation with 800 µM
H2O2 for 24 h, and the expression levels of
LC3-II, Beclin-1 and p62 were detected. The results indicated that
LC3-II and Beclin-1 were increased, and p62 was decreased by
H2O2 treatment, and these changes were
enhanced by Sal B in a concentration-dependent manner (Fig. 5C-F). Taken together, these results
indicate that Sal B enhances autophagy in HUVECs under oxidative
stress.
Sal B promotes autophagy to decrease
H2O2-induced apoptosis in HUVECs
The HUVECs were pre-treated with different
concentrations of Sal B (5, 10 and 20 µg/ml), followed by
incubation with 800 µM H2O2 and
optionally with 4 mM 3-MA for 24 h. As presented in Fig. 6A-C, Sal B increased the cell
viability and decreased the apoptosis of HUVECs compared with those
in the H2O2 group. However, these effects of
Sal B were attenuated by 3-MA, suggesting that Sal B increased
autophagy to decrease apoptosis. To confirm this, the
apoptosis-associated proteins cytochrome c and
cleaved-caspase 3, and the autophagy-associated proteins LC3-II and
p62 were detected in the above treatment groups. The results
indicated that the addition of 3-MA significantly decreased the
expression of LC3-II and increased that of p62 in the HUVECs
compared with those in the 20 µg/ml Sal B +
H2O2 group (Fig. 6D-F). Furthermore, 3-MA decreased
the expression levels of cytochrome c and cleaved caspase-3
compared with those in the 20 µg/ml Sal B +
H2O2 group (Fig. 6G-I). These results confirmed that
the increased autophagic activity induced by Sal B has a protective
effect against H2O2-induced apoptosis in
HUVECs.
Sal B activates cell autophagy through
the AMPK/mTOR pathway under oxidative stress
As autophagy may be induced via several different
pathways, the present study aimed to determine which of them is
promoted by Sal B via western blot analysis of AKT, p-AKT, AMPK,
p-AMPK, mTOR and p-mTOR. As is presented in Fig. 7A-D, treatment with Sal B (5, 10
and 20 µg/ml) followed by incubation with 800 µM
H2O2 decreased the levels of p-AKT and p-mTOR
and increased the levels of p-AMPK in a dose-dependent manner
compared with those in the control group. In the group pre-treated
with Sal B, the expression of p-AMPK was increased and that of
p-mTOR was decreased, whereas p-AKT was not significantly affected,
compared with the levels in the H2O2 group.
These results indicate that Sal B may promote autophagy via the
AMPK/mTOR signaling pathway, rather than the PI3K/AKT/mTOR pathway.
To confirm the mechanism of autophagy promoted by Sal B, the AMPK
inhibitor compound C was used to determine the resulting effect on
the level of p-AMPK. In the group that was co-treated with compound
C, the level of p-mTOR was increased and that of p-AMPK was
decreased compared with levels in the Sal B +
H2O2 group (Fig. 7E-G), which confirmed AMPK/mTOR
signaling as at least part of the underlying molecular
mechanism.
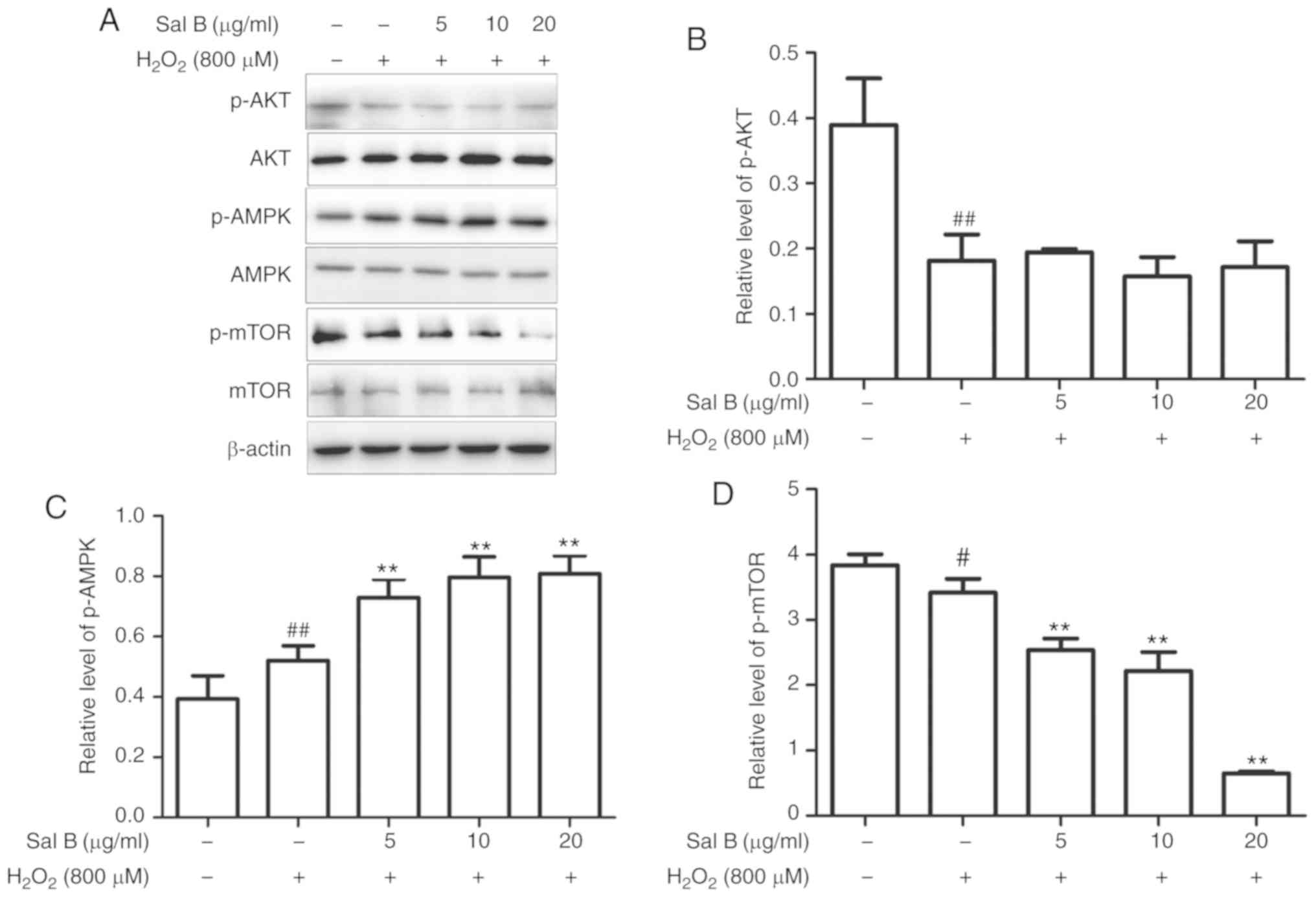 | Figure 7Sal B increases the protein
expression of p-AMPK and decreases that of p-mTOR, resulting in a
decreased rate of apoptosis, whereas compound C decreases the
levels of p-AMPK and increases the rate of apoptosis. (A) Sal B
mediated the protein expression of p-AMPK and p-mTOR, but did not
affect p-AKT. Quantification of (B) p-AKT, (C) p-AMPK and (D)
p-mTOR are expressed as the mean ± SEM of three independent
experiments; #P<0.05 and ##P<0.01
compared with the control group; **P<0.01 compared
with the H2O2 group. (E) Sal B increased the
protein expression of p-AMPK and decreased that of p-mTOR, which
was partly attenuated by compound C attenuated it partly.
Quantification of (F) p-AMPK and (G) p-mTOR are presented as the
mean ± SEM of three independent experiments. #P<0.05
and ##P<0.01 compared with the control group;
*P<0.05 and **P<0.01 compared with the
H2O2 group. Sal B, salvianolic acid B; AMPK,
AMP kinase; mTOR, mammalian target of rapamycin; p-,
phosphorylated; H2O2, hydrogen peroxide. |
Discussion
One of the factors causing atherosclerosis is
vascular EC apoptosis, which may be triggered by free radicals,
oxidized low-density lipoprotein (ox-LDL) and the activation of
blood platelets. Previous studies have indicated that the number of
vascular ECs is decreased by apoptosis, and the prevention of
endothelial activation and reduction of endothelial injury are key
to the prevention and treatment of AS (18-20). Therefore, the present study
investigated a strategy to inhibit vascular EC apoptosis and
thereby prevent atherosclerosis. Numerous studies have confirmed
that Sal B has an anti-atherosclerotic effect due to its
anti-oxidative and anti-inflammatory actions, and its suppression
of the presence of foam cells (21-25). Therefore, Sal B exerts a dual
effect, namely the protection of vascular ECs and a decrease in the
formation of a fibrous cap that stabilizes a plaque, which renders
Sal B suitable for treating atherosclerosis, for example, through
resolving recurrence by stabilizing plaques or as a co-treatment
for enhancing the efficiency of other anti-atherosclerotic
treatments. The results of the present study indicate that Sal B
inhibited the apoptosis of vascular ECs under oxidative stress. In
addition, it was indicated that the induction of autophagy serves
an important role in the anti-apoptotic effects of Sal B under
oxidative stress. Further mechanistic investigation indicated that
Sal B induced autophagy in vascular ECs under oxidative stress by
activating the AMPK/mTOR pathway. In in vitro experiments,
H2O2 is used as cell injury inducer (26). In the present study, 800 µM
H2O2 led to the apoptosis of vascular ECs, as
the survival rate decreased to 53.02±6.07%. Of note, Sal B
suppressed H2O2-induced apoptosis in HUVECs,
suggesting that Sal B has the potential to treat atherosclerosis by
preventing vascular EC apoptosis under oxidative stress conditions.
Wu et al (27) reported
that Sal B protects ECs against oxidative stress-induced cell
injury through upregulating glucose-regulated protein 78.
Furthermore, Chen et al (28) demonstrated the protective effect
of Sal B against ox-LDL-induced HUVEC injury and apoptosis. In line
with this, another study confirmed that Sal B protects vascular ECs
against injury induced by oxidative stress (29). These previous studies further
support the present observations, and based on these studies, the
underlying molecular mechanisms and pathways were then further
investigated.
The association between autophagy and apoptosis is a
focus of research. Autophagy is an intracellular catabolic process
in which long-lived proteins are recycled and damaged organelles
are eliminated, and under certain conditions, it also promotes
apoptosis (30). Autophagy has
been confirmed to induce apoptosis under high stress conditions
(31). However, autophagy may be
tuned to maintain cellular homeostasis under different stresses
through the inhibition of apoptosis (32). Certain studies have indicated that
autophagy may have two different effects to either prevent or
induce apoptosis, depending on cell function, disease stage,
therapeutic schedule and cell micro-environment (33). It is well-known that autophagy has
a vital role in protecting the cardiovascular system (34). A previous study demonstrated the
cardioprotective effect of Sal B on acute myocardial infarction by
promoting autophagy and neovascularization and inhibiting apoptosis
(35). It has been shown that
autophagy serves to eliminate injured mitochondria when a large
number of apoptotic factors are released into the cytoplasm.
Therefore, promotion of the autophagy of injured cells may provide
a novel anti-atherosclerotic strategy. In the present study, the
autophagy inhibitor, 3-MA, and the autophagy inducer, rapamycin,
were used to confirm the roles of autophagy in HUVECs. Compared
with the control group, treatment of the HUVECs with 3-MA increased
apoptosis, decreased autophagy marker LC3-II, and increased the
levels of p62 and the apoptosis-associated cytochrome c and
cleaved-caspase 3 proteins. However, the autophagy inducer
rapamycin produced the opposite effect, namely decreasing
apoptosis, increasing LC3-II and decreasing the levels of p62,
cytochrome c and cleaved caspase-3. This indicated that autophagy
has an important role in regulating apoptosis to protect cells.
As the results of the present study indicated that
Sal B protects HUVECs against H2O2-induced
cell injury, loss- and gain-of-function experiments were then used
to assess the possible involvement of autophagy as an underlying
mechanism. Pre-treatment with Sal B followed by incubation with 800
µM H2O2 resulted in the upregulation
of LC3-II and Beclin-1 and the downregulation of p62, in addition
to the upregulation of autophagic influx, indicating that autophagy
was promoted to protect the cell. However, simultaneous treatment
with 3-MA partly eliminated this protective effect by Sal B.
Therefore, the promotion of autophagy may be one of the mechanisms
by which Sal B prevents apoptosis under oxidative stress. Autophagy
is regulated by numerous complex signaling pathways, including
AMPK, mTOR and Bcl-2/Beclin-2 (36). The mTOR signaling pathway serves a
crucial role in autophagy (37);
in order to adapt to energy metabolism under stress, AMPK is
activated and then inhibits mTOR, inducing the activation of
autophagy (38). mTOR is an
energy sensor that is contrary to AMPK (39), which is suppressed when energy is
poor and is activated when energy is abundant. It is situated
downstream of pro-growth factors and pro-synthetic metabolic
factors (40). PI3K/Akt/mTOR and
AMPK/mTOR signaling have been investigated in numerous studies as
autophagy signaling pathways. AKT phosphorylated mTOR to generate
activated p-mTOR and triggers downstream signaling to inhibit
autophagy. When a cell is under oxidative stress, serine/threonine
kinase 11 may lead to the phosphorylation of AMPK. p-AMPK
negatively regulates the mTOR signaling pathway to promote
autophagy (36). In the present
study, pre-treatment with Sal B significantly increased the level
of p-AMPK and decreased the level of p-mTOR, but had no effect on
the level of p-AKT. Of note, the AMPK inhibitor, compound C,
decreased p-mTOR and increased p-AMPK. In addition, as presented in
the chemical structure of Sal B in Fig. 1, the molecule bears hydroxyl
groups on a phenol ring that exert potent anti-oxidative effects
which is unlike the effect of other activators of autophagy,
including rapamycin, which directly combines with mTOR. From the
present results, it is evident that Sal B protects HUVECs from
oxidative stress via the AMPK/mTOR pathway. Regarding the
mechanisms of other autophagy-induced antioxidants, resveratrol
acts via the AMPK/Sirtuin 1/autophagy pathway (41), and curcumin activates autophagy
via the PI3K/AKT/mTOR pathway (42). The results of the present study
revealed that Sal B has similarities with and differences from
certain other autophagy-inducing antioxidants.
In conclusion, the results of the present study
indicate that Sal B has the ability to protect HUVECs from
apoptosis by promoting autophagy under oxidative stress, and the
promotion of autophagy induced by Sal B is mediated via the
upregulated phosphorylation of AMPK and the downregulation of mTOR
signaling.
Acknowledgments
The authors would like to thank the Affiliated
Hospital of Southwest Medical University and The Drug and Food
Function Research Center of Southwest Medical University for
providing laboratories in which to perform experiments.
Funding
The present study was supported by grants from the
technology bureau of Luzhou city (grant no. 2015LZCYD-S03), the
Technology Bureau of Luzhou City-Southwest Medical University
(grant no. 2018LZXNYD-PT02).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SG designed the experiments, wrote the manuscript
and performed the MTT assay, western blotting and Hoechst 33258
fluorescence staining. SL designed the experiments, wrote the
manuscript and performed flow cytometric analysis, the caspase-3
activity assay and mCherry-GFP-LC3B transfection. QL, FZ, MS and ZW
assisted with experimental design and analyzed the data. SW
assisted with experimental design and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sitia S, Tomasoni L, Atzeni F, Ambrosio G,
Cordiano G, Catapano A, Tramontana S, Perticone F, Naccarato P,
Camici P, et al: From endothelial dysfunction to atherosclerosis.
Autoimmun Rev. 9:830–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang C: The role of inflammatory
cytokines in endothelial dysfunction. Basic Res Cardiol.
103:398–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vanhoutte PM: Endothelial dysfunction: The
first step toward coronary arteriosclerosis. Circ J. 73:595–601.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vita JA: Endothelial function.
Circulation. 124:e906–e912. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubinsztein DC: The roles of intracellular
protein-degradation pathways in neurodegeneration. Nature.
443:780–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimomura H, Terasaki F, Hayashi T,
Kitaura Y, Isomura T and Suma H: Autophagic degeneration as a
possible mechanism of myocardial cell death in dilated
cardiomyopathy. Jpn Circ J. 65:965–968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Starke RD, Ferraro F, Paschalaki KE,
Dryden NH, McKinnon TA, Sutton RE, Payne EM, Haskard DO, Hughes AD,
Cutler DF, et al: Endothelial von Willebrand factor regulates
angiogenesis. Blood. 117:1071–1080. 2011. View Article : Google Scholar :
|
|
9
|
Zhang QW, Zhang Y, Li JP and Ma H:
Determination of salvianolic acid B in the radix of Salvia
miltiorrhiza Bge by HPLC. Zhongguo Zhong Yao Za Zhi. 26:848–849.
2001.In Chinese.
|
|
10
|
Song Q, Han X, Xue Y, Song T, Chu X, Zhang
X, Zhang Y, Zhang Y, Zhang J and Chu L: Effects of salvianolic acid
B on L-type calcium channels and myocardial contractility in
isolated rat ventricular myocytes and hERG K+ channels
expressed in HEK293 cells. Naunyn Schmiedebergs Arch Pharmacol.
390:791–799. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang JY, Zhang B, Wang M, Wang W, Liao P,
Sun GB and Sun XB: Calcium homeostasis and endoplasmic reticulum
stress are involved in Salvianolic acid B-offered protection
against cardiac toxicity of arsenic trioxide. Oncotarget.
8:97384–97393. 2017.PubMed/NCBI
|
|
12
|
Chang TM, Shi GY, Wu HL, Wu CH, Su YD,
Wang HL, Wen HY and Huang HC: Effects of salvianolic Acid B on
protein expression in human umbilical vein endothelial cells. Evid
Based Complement Alternat Med. 2011:2130502011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryter SW, Mizumura K and Choi AM: The
impact of autophagy on cell death modalities. Int J Cell Biol.
2014:5026762014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao J, Yang G, Pi R, Li R, Wang P, Zhang
H, Le K, Chen S and Liu P: Tanshinone IIA protects neonatal rat
cardiomyocytes from adriamycin-induced apoptosis. Transl Res.
151:79–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belmokhtar CA, Hillion J and
Ségal-Bendirdjian E: Staurosporine induces apoptosis through both
caspase-dependent and caspase-independent mechanisms. Oncogene.
20:3354–3362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frey T: Nucleic acid dyes for detection of
apoptosis in live cells. Cytometry. 21:265–274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Meyer GR and Martinet W: Autophagy in
the cardiovascular system. Biochim Biophys Acta. 1793:1485–1495.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun GB, Qin M, Luo Y, Pan RL, Meng XB,
Wang M, Zou YH and Sun XB: Protect effects and the underlying
mechanisms of myricitrin against vascular endothelial cells
apoptosis induced by oxidative stress. Yao Xue Xue Bao. 48:615–620.
2013.In Chinese. PubMed/NCBI
|
|
19
|
Su J, Guo WL, Li X, Kang Y and Gao WZ:
Protective effects of dioscin in vascular endothelial cells
apoptosis induced by oxidized low-density lipoprotein. J Tianjin
Med Univ. 2:175–178. 2012.
|
|
20
|
Liu KX, Chen GP, Lin PL, Huang JC, Lin X,
Qi JC and Lin QC: Detection and analysis of apoptosis- and
autophagy-related miRNAs of mouse vascular endothelial cells in
chronic intermittent hypoxia model. Life Sci. 193:194–199. 2018.
View Article : Google Scholar
|
|
21
|
Wang J, Zhang Y, Guo LL, Wu GJ and Liu RH:
Salvianolic acid B inhibits the TLR4-NF-κB-TNFα pathway and
attenuates neonatal rat cardiomyocyte injury induced by
lipopolysaccha-ride. Chin J lntegr Med. 17:775–779. 2011.
View Article : Google Scholar
|
|
22
|
Bao Y, Wang L, Xu Y, Yang Y, Wang L, Si S,
Cho S and Hong B: Salvianolic acid B inhibits macrophage uptake of
modified low density lipoprotein (mLDL) in a scavenger receptor
CD36-dependent manner. Atherosclerosis. 223:152–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng CC, Yang SP, Lin WS, Ho LJ, Lai JH,
Cheng SM and Lin WY: Magnesium lithospermate B mediates
anti-inflammation targeting activator protein-1 and nuclear
factor-kappa B signaling pathways in human peripheral T
lymphocytes. Int Immunopharmacol. 13:354–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu M, Ye J, Gao S, Fang W, Li H, Geng B,
Zou J, Chen X, Chen S, Zhang L, et al: Salvianolic acid B protects
cardiomyocytes from angiotensin II-induced hypertrophy via
inhibition of PARP-1. Biochem Biophys Res Commun. 444:346–353.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Quan W, Yin Y, Xi M, Zhou D, Zhu Y, Guan
Y, Guo C, Wang Y, Duan J and Wen A: Antioxidant properties of
magnesium lithospermate B contribute to the cardioprotection
against myocardial ischemia/reperfusion injury in vivo and in
vitro. J Tradit Chin Med. 33:85–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue L, Wu Z, Ji XP, Gao XQ and Guo YH:
Effect and mechanism of salvianolic acid B on the myocardial
ischemia-reperfusion injury in rats. Asian Pac J Trop Med.
7:280–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu HL, Li YH, Lin YH, Wang R, Li YB, Tie
L, Song QL, Guo DA, Yu HM and Li XJ: Salvianolic acid B protects
human endothelial cells from oxidative stress damage: A possible
protective role of glucose-regulated protein 78 induction.
Cardiovasc Res. 81:148–158. 2009. View Article : Google Scholar
|
|
28
|
Chen HM, Luo H, Zeng WB, Liu B, Huang JC,
Liu M, Zeng YJ, Zheng Q, Li JQ, Sun XG and Zhou YC: Salvianolic
acid B attenuates oxidized low-density lipoprotein-induced
endothelial cell apoptosis through inhibition of oxidative stress,
p53, and caspase-3 pathways. Chin J Integr Med. 2017. View Article : Google Scholar
|
|
29
|
Chen YH, Lin SJ, Chen YL, Liu PL and Chen
JW: Anti-inflammatory effects of different drugs/agents with
antioxidant property on endothelial expression of adhesion
molecules. Cardiovasc Hematol Disord Drug Targets. 6:279–304. 2006.
View Article : Google Scholar
|
|
30
|
Lv XC and Zhou HY: Resveratrol protects
H9c2 embryonic rat heart derived cells from oxidative stress by
inducing autophagy: Role of p38 mitogen-activated protein kinase.
Can J Physiol Pharmacol. 90:655–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valentim L, Laurence KM, Townsend PA,
Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS and
Stephanou A: Urocortin inhibits Beclin1-mediated autophagic cell
death in cardiac myocytes exposed to ischaemia/reperfusion injury.
J Mol Cell Cardiol. 40:846–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baehrecke EH: Autophagy: Dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ahn J and Kim J: Nutritional status and
cardiac autophagy. Diabetes Metab J. 37:30–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin C, Liu Z, Lu Y, Yao Y, Zhang Y, Ma Z,
Kuai M, Sun X, Sun S, Jing Y, et al: Cardioprotective effect of
Salvianolic acid B on acute myocardial infarction by promoting
autophagy and neovascularization and inhibiting apoptosis. J Pharm
Pharmacol. 68:941–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar :
|
|
37
|
Pyo JO, Nah J and Jung YK: Molecules and
their functions in autophagy. Exp Mol Med. 44:73–80. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Alers S, Löffler AS, Wesselborg S and
Stork B: Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy:
Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 32:2–11. 2012.
View Article : Google Scholar :
|
|
39
|
Inoki K, Kim J and Guan KL: AMPK and mTOR
in cellular energy homeostasis and drug targets. Annu Rev Pharmacol
Toxicol. 52:381–400. 2012. View Article : Google Scholar
|
|
40
|
Dazert E and Hall MN: mTOR signaling in
disease. Curr Opin Cell Biol. 23:744–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo H, Chen Y, Liao L and Wu W:
Resveratrol protects HUVECs from oxidized-LDL induced oxidative
damage by autophagy upregulation via the AMPK/SIRT1 pathway.
Cardiovasc Drugs Ther. 27:189–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W,
Yang D, Yang A and Yu Y: Antitumor activity of curcumin by
modulation of apoptosis and autophagy in human lung cancer A549
cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep.
39:1523–1531. 2018.PubMed/NCBI
|