Introduction
Reconstruction of bone defects, especially massive
segmental defects, remains a major challenge. Various approaches
have been reported as suitable reconstruction techniques, but all
of them are associated with different disadvantages, which limit
their wide application (1,2).
Although autografts and allografts usually result in satisfactory
outcomes, they also have many shortcomings, including, wound
infection, bone graft exposure and graft failure (3-5).
There has been a recent focus on tissue-engineered bone grafts
(TEBGs) as an alternative to autografts for bone repair and
reconstruction (6,7). Autografts generally result in
satisfactory healing outcomes because of their similar composition
to the bone matrix as a scaffold for bone regeneration, as well as
the presence of living differentiated and progenitor cells, leading
to osteointegration and osteoconduction (8). Notably, the activity of the growth
factors present in autografts has been shown to mediate new bone
formation through signaling and the recruitment of osteoprogenitor
cells (9). The three major
components of TEBGs include scaffolds, cells and cell factors that
promote osteogenesis (10). TEBGs
are constructed based on the composition of autografts. Scaffolds
are made of biomaterials, which have the advantages of unlimited
supplies, safety, biocompatibility, and bioactivity; they are also
capable of inducing the differentiation of stem cells (11). Biphasic calcium phosphates (BCPs),
which are a combination of hydroxyapatite (HA) and β-tricalcium
phosphate (β-TCP), have been shown to be able to achieve a good
outcome in repairing small defects in the clinic (12). However, they cannot be used for
load-bearing areas or massive segmental defects, especially
critical-size defects, since it is difficult to find an optimal
ratio of HA/β-TCP to balance the degradation of the scaffold and
the rate of bone formation, which guarantees the stability of the
tissue-engineered bone while promoting bone formation (13). Overcoming rapid scaffold
degradation therefore involves improving the quantity, quality and
rate of new bone formation during the treatment of critically-sized
skeletal defects by BCPs.
It was previously reported that the addition of
autologic osteoblasts to biomaterials promoted new bone formation,
and increased levels of growth factors such as bone morphogenetic
protein 2 (BMP-2) (14,15). However, the short-lived in
vivo efficacy of BMP-2 and the difficulty of osteoblast
isolation limits their usage (16,17). Recent studies have therefore
focused on the combination of osteogenic gene-modified mesenchymal
stem cells (MSCs) and BCPs for TEBGs, and an important goal is to
identify the most effective osteogenic genes in this process
(18,19).
Osteoblast differentiation is a fundamental step
during bone formation, and is regulated via several signaling
pathways including transforming growth factor-β, BMP, and WNT, as
well as a number of transcription factors that are regulated by
microRNAs (miRNAs/miRs) (20-23). Understanding the signaling
pathways and regulators of osteogenesis is critical for also
understanding the mechanism underlying bone formation, and for
developing novel strategies for bone repair and reconstruction.
miRNAs are endogenous, eukaryotic highly conserved,
noncoding RNAs of 19-25 nucleotides in length, which function by
complementary pairing to the 3'-untranslated region (UTR) of target
mRNAs to inhibit their translation (24). Some miRNAs were recently shown to
be able to regulate the osteogenic differentiation of MSCs in
vitro; these included miRNA-154-5p, miRNA-30, miRNA-29b,
miRNA-133 and miRNA-135 (24-28). However, it is not clear if miRNAs
play a role in osteogenesis in vivo. We recently showed that
down-regulating endogenous miRNA-26a-5p (antimiR-26a-5p) promoted
the osteogenic differentiation of ADSCs (29). These data suggested that
antimiR-26a-5p-modified ADSCs combined with BCP scaffolds may
accelerate new bone formation and compensate for the rapid
degradation of this biomaterial. However, this hypothesis has not
been verified.
The present study analyzed the effects of miR-26a-5p
on bone formation by transplanting rat femoral defect models with
TEBGs constructed usingmiR-26a-5p- and antimiR-26a-5p-modified
ADSCs combined with BCP scaffolds. The molecular mechanism of this
phenomenon was also investigated by evaluating the expression of
key proteins in the Wnt/Ca2+signaling pathway that were
implicated in our previous study (29). The aim of the present study was to
improve the understanding of the role of miR-26a-5p in bone
regeneration, to verify whether the results in vitro
obtained by our previous work corresponded with those of the
present experiments in vivo, and to potentially provide a
new method to optimize the osteogenic ability of BCPs.
Materials and methods
Isolation, culture and identification of
ADSCs
A total of 10 four-week-old female Sprague-Dawley
(SD) rats (weight, 75 g) obtained from the Sichuan University
Animal Experimental Center were used as the source of ADSCs, and
all procedures were approved by the Animal Research Committee of
Sichuan University. Animals were maintained in the same room under
standard conditions including temperature (22-24°C), humidity
(55-65%) and light (12-h light/dark cycle) with free access to food
and tap water. The ADSCs were harvested from the inguinal fat pads
of the rats as previously described (30). Briefly, the excised inguinal fat
pads were soaked in PBS with 1% penicillin/streptomycin, and washed
3 times with PBS. They were then minced into small pieces, and
digested with 0.2% collagenase type I at 37°C in a shaking
incubator for 45 min. After centrifugation at 200 × g for 5 min at
room temperature, the cells were resuspended in 3 ml regular growth
medium consisting of Minimum Essential Medium α-medium (α-MEM;
HyClone; GE Healthcare Life Sciences), 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), penicillin (100
U/ml)/streptomycin (100 µg/ml; Sigma-Aldrich; Merck KGaA),
and cultured in T25 culture flasks (BD Falcon Labware; BD
Biosciences) at 37°C in the presence of 5% CO2. and 95%
humidity. The culture medium was renewed every 2 days. Upon
reaching 80% confluence, the adherent cells were resuspended with
0.25% trypsin and subcultured. After 2 weeks of culture, the
multipotent differentiation of ADSCs (passage 3) was demonstrated
based on alizarin red staining, oil red-O staining and βIII-tubulin
immunofluorescence staining of transdifferentiated cells as
described previously (25,31)
(Fig. 1A-D). Cells from passage 3
were used for all subsequent experiments.
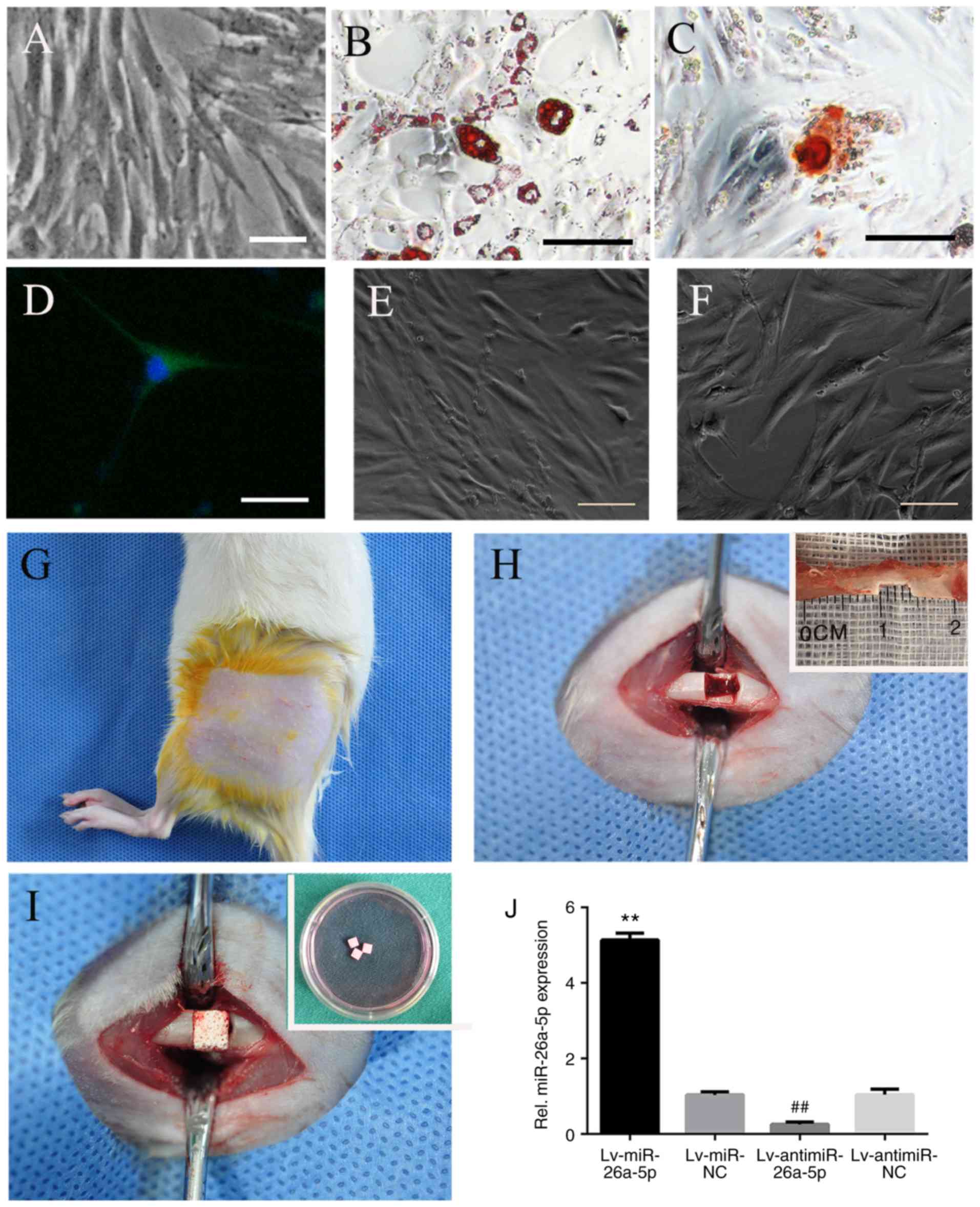 | Figure 1Identification of ADSCs and surgical
procedures. (A) The fusiform shape and adherent growth of ADSCs
(passage 3), (B) Oil red-O staining of adipogenic differentiated
ADSCs, (C) Alizarin red staining of osteogenic differentiated
ADSCs, and (D) βIII-tubulin immunofluorescence identification of
neurogenic differentiated ADSCs. Identification of lentivirus
infection: (E) Morphology of ADSCs before infection and (F)
transfection efficiency of lentivirus was estimated based on green
fluorescent protein expression in ADSCs observed through
fluorescence microscopy. Scale bars, 50 µm. Surgical
procedures: (G) Left thigh incision in Sprague-Dawley rats; (H)
exposure of left femoral diaphysis, and 4 mm long defect made on
the medial third of diaphysis; and (I) implantation of
cell-scaffold complex (biphasic calcium phosphate scaffold and
ADSCs) into the femoral defect. (J) Expression of miR-26a-5p in rat
ADSCs infected with Lv-miR-26a-5p, Lv-miR-NC, Lv-antimiR-26a-5p and
Lv-antimiR-NC were assayed by reverse transcription-quantitative
PCR at 72 h after induction. **P<0.01 vs. miR-NC;
##P<0.01 vs. antimiR-NC. miR, microRNA; NC, negative
control; ADSCs, adipose-derived mesenchymal stem cells; Lv,
lentivirus. |
Preparation of lentivirus vectors and
infection
The construction of the lentiviral vectors
containing miR-26a-5p, miR-negative control (NC), antimiR-26a-5p
and antimiR-NC as well as the sequences were described in our
previous study (29). ADSCs were
seeded in 6-well plates (4×105 cells/well) one day
before infection. Cells were then incubated overnight with
Lv-miR-26a-5p, Lv-miR-NC, Lv-antimiR-26a-5p or Lv-antimiR-NC at
final concentrations of 100 nM with Lipofectamine 2000™
(Invitrogen; Thermo Fisher Scientific, Inc.) in
Opti-MEM® (Gibco; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. At 72 h post-infection,
the expression of green fluorescent protein was measured under
fluorescence microscopy to evaluate the transfection efficiency.
The transfection efficiency was >95% in all groups (Fig. 1E and F). The transfection
efficiency of Lv-miR-26a-5p, Lv-miR-NC, Lv-antimiR-26a-5p or
Lv-antimiR-NC was verified by reverse transcription-quantitative
PCR as described in our previous study (29) (Fig.
1J).
Preparation of BCP scaffolds and cell
seeding
All of the BCP scaffolds, shaped into 4×4×2
mm3 squares, were obtained from the National Engineering
Research Center for Biomaterials of Sichuan University, and
sterilized by γ-irradiation at 25 kGy. The BCP20/80 used in the
present study was composed of 20% HA and 80% β-TCP. The porosity
was ~74%, and the diameter of the pores was 100-400 µm.
All of the BCP scaffolds were divided randomly and
equally into five groups (miR, miR-NC, antimiR, antimiR-NC and
Control groups) and were conditioned with α-MEM one day before cell
seeding. A total of 9BCP scaffolds from each group were used for
cell adhesion evaluation and cell proliferation assays, and the
remaining 18 scaffolds from each group were used in the animal
experiments. ADSCs infected with Lv-miR-26a-5p, Lv-miR-NC,
Lv-antimiR-26a-5p, or Lv-antimiR-NC, and ADSCs (passage 3) were
harvested with 0.25% trypsin (at 37°C for 1 min), counted with a
hemocytometer, and were loaded onto 5 the corresponding groups of
BCP scaffolds (1×105 cells/scaffold). Cells were
cultured in regular growth medium overnight for cell adhesion. The
miR group consisted of miR-26a-5p-modified ADSCs/BCP; the miR-NC
group consisted of ADSCs infected with Lv-miR-NC/BCP (the negative
control of the miR group); the antimiR group consisted
ofantimiR-26a-5p-modified ADSCs/BCP; the antimiR-NC group consisted
of ADSCs infected with Lv-antimiR-NC/BCP (the negative control of
the antimiR group); and the Control group consisted of ADSCs/BCP
(the empty control).
Evaluation of cell adhesion and
proliferation
After 7 days of incubation, 3 cell-scaffold
structures from each group were fixed separately in 4%
paraformaldehyde solution for 24 hat room temperature, followed by
dehydration with an ethanol concentration series of 30-100% and
dried in a dryer. One scaffold without cells was cultured and
subjected to the same protocol to be used as the blank control. The
structures were then sputter coated with gold and subjected to
scanning electron microscopy (SEM; FEI Quanta 200 ESEM; Thermo
Fisher Scientific, Inc.) to evaluate cell adhesion.
On days 1 and 7 of incubation, a ADSC cell
proliferation assay foreach group was performed using Cell Counting
Kit-8 (CCK8; Dojindo Molecular Technologies, Inc.). Briefly, CCK-8
solution was added to the medium (CCK-8:medium, 1:10). After
incubation for 2 hat 37°C, the supernatant was transferred into a
96 well plate and the absorbance was read at 450 nm with a
microplate reader (BioTek Instruments, Inc.).
Animals and surgical procedures
A total of 90 adult female SD rats (300 g;
10-weeks-old) supplied by the Sichuan University Animal
Experimental Center were used to establish the femoral defect model
in order to study the efficiency of bone regeneration. Animals were
maintained under standard conditions including temperature
(22-24°C), humidity (55-65%) and light (12-h light/dark cycle) with
free access to food and tap water. The surgical procedures and
experimental protocols for animal care were approved by the Animal
Research Committee of Sichuan University. The SD rats were randomly
divided into 5 groups (miR, miR-NC, antimiR, antimiR-NC and Control
groups), with 18 rats in each group, corresponding to the 5 groups
of cell-scaffold structures (Table
I).
 | Table ISubject groups and study design. |
Table I
Subject groups and study design.
| Groups | No. of rats | Treatment |
|---|
| miR | 18 |
miR-26a-5p/ADSCs/BCP |
| miR-NC | 18 |
miR-NC/ADSCs/BCP |
| AntimiR | 18 |
AntimiR-26a-5p/ADSCs/BCP |
| AntimiR-NC | 18 |
AntimiR-NC/ADSCs/BCP |
| Control | 18 | ADSCs/BCP |
Surgery was performed under aseptic conditions.
Prior to surgery, the rats were anesthetized with an
intraperitoneal injection of 10% chloral hydrate (300 mg/kg), the
left thigh was shaved, and the animal received a subcutaneous
injection of 2% lidocaine (4 mg/kg) for local anesthesia (Fig. 1G). The left femur was exposed by a
lateral longitudinal skin incision and by retracting the quadriceps
muscles. The incision was 2.5 cm long in order to expose the medial
part of the femoral diaphysis. After the periosteum was stripped
off the bone, a 4 mm-long ×2 mm-deep defect was made by a pneumatic
fissure bur under sustained saline irrigation (Fig. 1H). Then the cell-scaffold
structure was transplanted into the defect (Fig. 1I). Subsequently, the incision was
closed by suturing. After surgery, the experimental rat received a
penicillin injection (20 U/day) once a day for 3 days and the wound
was cleaned every day for a week to prevent infection. If there was
any sign of local infection, the anti-infection and wound-cleaning
treatment was prolonged.
At 2, 4 or 8 weeks postoperatively, 6 animals from
each group were sacrificed randomly by cervical dislocation after
anesthesia to obtain left femur samples. Prior to euthanasia, the
rats were administered an intraperitoneal injection of 10% chloral
hydrate (300 mg/kg) for general anesthesia. Cervical dislocation
was then conducted by an experienced researcher very quickly and no
rats showed clinical signs of suffering before they died. Only
after the lack of a heartbeat had been confirmed and their bodies
became cold, were samples obtained according to laboratory
standards. In the present study, several signs of suffering were
considered to indicate the requirement of humane endpoints such as
severe infections, uncontrolled body weight loss or apastia over 36
h and bone fracture.
X-ray analysis
The periosteal tissue was stripped out of the femur
diaphysis. Each sample was placed on an occlusal film, then a
standardized anteroposterior radiograph of the femur was performed
with an X-ray unit (CS 2100; Carestream Dental LLC). The exposure
conditions were 70 kV, 8 mA and 0.06 msec.
Micro-computed tomography (CT)
analysis
After X-ray films were taken, all of the samples
were fixed in 4% paraformaldehyde at room temperature. After 24 h,
the samples were dissected to keep the regenerated bone and 4 mm of
the native bone bilaterally beneath the defect, and then samples
were placed in mid-sized sample tubes with 4% paraformaldehyde. The
scans were performed in an axial direction parallel to the long
axis of the samples with a micro-CT machine (µCT-50; SCANCO
Medical) with medium-resolution (500 projections/180°) and a voxel
size of 10 µm. The system was set to 70 kV, 114 mA, and 400
msec integration time. The sagittal images of each sample were
captured and regions of interest (ROIs) were outlined as
regenerated bones, including 300 slices. The threshold of each
image was set as 220-520 to isolate new bone from residual BCP.
Within the ROI, the micro-architecture parameters of the new bone
were evaluated, including the ratio of bone volume to total volume
(BV/TV), bone mineral density (BMD), trabecular number (Tb.N) and
trabecular thickness (Tb. Th) using the recommended software
(NRecon 1.6 and CTAn 1.8; Bruker Corporation). The ratio of the
residual BCP volume to the TV of the ROI was also evaluated using
the software.
Histological analysis
All samples were fixed in 4% paraformaldehyde for 72
hat room temperature, decalcified in 20% ethylenediaminetetraacetic
acid (EDTA) for 8 weeks, and then dehydrated with a concentration
series of alcohol. Samples were embedded in paraffin and sectioned
along the longitudinal axis of the samples into 5 µm thick
slices. Two sections of each sample were stained with hematoxylin
and eosin (H&E) at room temperature for 11 min. DP2-BSW version
2.1 software (Olympus Corporation) coupled with a light microscope
were used to capture the images of each section. The relative
volume of newly formed bone in the defect area was measured with
ImageJ 1.49p software (National Institute of Health).
Immunohistochemical (IHC) analysis
IHC staining was performed to detect the expression
of osteocalcin (OCN), collagen I (COLI), Runt-related transcription
factor 2 (RUNX2), Wnt family member 5A (WNT5A) and
calmodulin-dependent protein kinase II (CaMKII) proteins in the
same 5 µm-thick sections used for H&E staining. The
sections were firstly deparaffinized, and then incubated with 3%
hydrogen peroxide and distilled water at room temperature for 10-20
min to eliminate endogenous peroxidase. Samples were washed three
times with double-distilled water, treated with 10 mM sodium
citrate (pH 6.0) at a high pressure (room temperature) for 3 min
for antigen retrieval and then washed with 0.01 M PBS (pH 7.4)
after allowing to naturally cool. Antigen blocking was conducted
via the dropwise addition of 5-10% normal goat serum (ZSGB-BIO;
OriGene Technologies, Inc.) to the sections at 37°C for 10-30 min.
The serum was then removed, and samples were incubated overnight at
4°C with the appropriate dilutions of primary antibodies specific
to the following biomarkers: OCN (cat. no. ab13418; 1:400; Abcam),
COLI (cat. no. ab34710; 1:200; Abcam), RUNX2 (cat. no. ab76956;
1:200; Abcam), WNT5A (cat. no. Gxp536895-50 ug; 1:200; GenXspan)
and CaMKII (cat. no. Gxp63948-50 ug; 1:200; GenXspan). The sections
were then washed three times with PBS, and biotinylated goat
anti-rabbit secondary antibodies (cat. no. sp9001; 1:400; ZSGB-BIO;
OriGene Technologies, Inc.) were added dropwise on COLI, WNT5A and
CaMKII sections, and incubated for 10-30 min, while biotinylated
goat anti-rat secondary antibodies (cat. no. sp9002; 1:400;
ZSGB-BIO; OriGene Technologies, Inc.) were added dropwise on OCN
and RUNX2 sections and incubated for 10-30 min, all at room
temperature. Samples were washed, and incubated with
streptavidin-horseradish peroxidase conjugate followed by
3,3'-diaminobenzidine treatment. Finally, the nuclei were
counterstained with hematoxylin at room temperature for 1 min
before the sections were dehydrated and placed on cover-slips. The
sections for blank control were incubated with PBS instead of
primary antibody. DP2-BSW version 2.1 Software (Olympus
Corporation) coupled with a light microscope were used to capture
the images of regenerated bone in each section (magnification,
×400). Osteoblasts positive for OCN, COLI, RUNX2, WNT5A and CaMKII
expression were stained yellow or brown and assessed by ImageJ
1.49p software (National Institutes of Health). The mean area of
positive cells was measured and used as the evaluation index.
Statistical analysis
All experiments were performed at least three times.
Data were expressed as the mean ± standard derivation. The data
obtained were analyzed using SPSS 20.0 (IBM Corp.). All comparisons
were performed using Student's two-tailed t-test or a one-way
ANOVA. The Student-Newman-Keuls-q test was used as the post hoc
test for multiple comparisons. Statistical values were illustrated
using GraphPad Prism, version 6.05 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
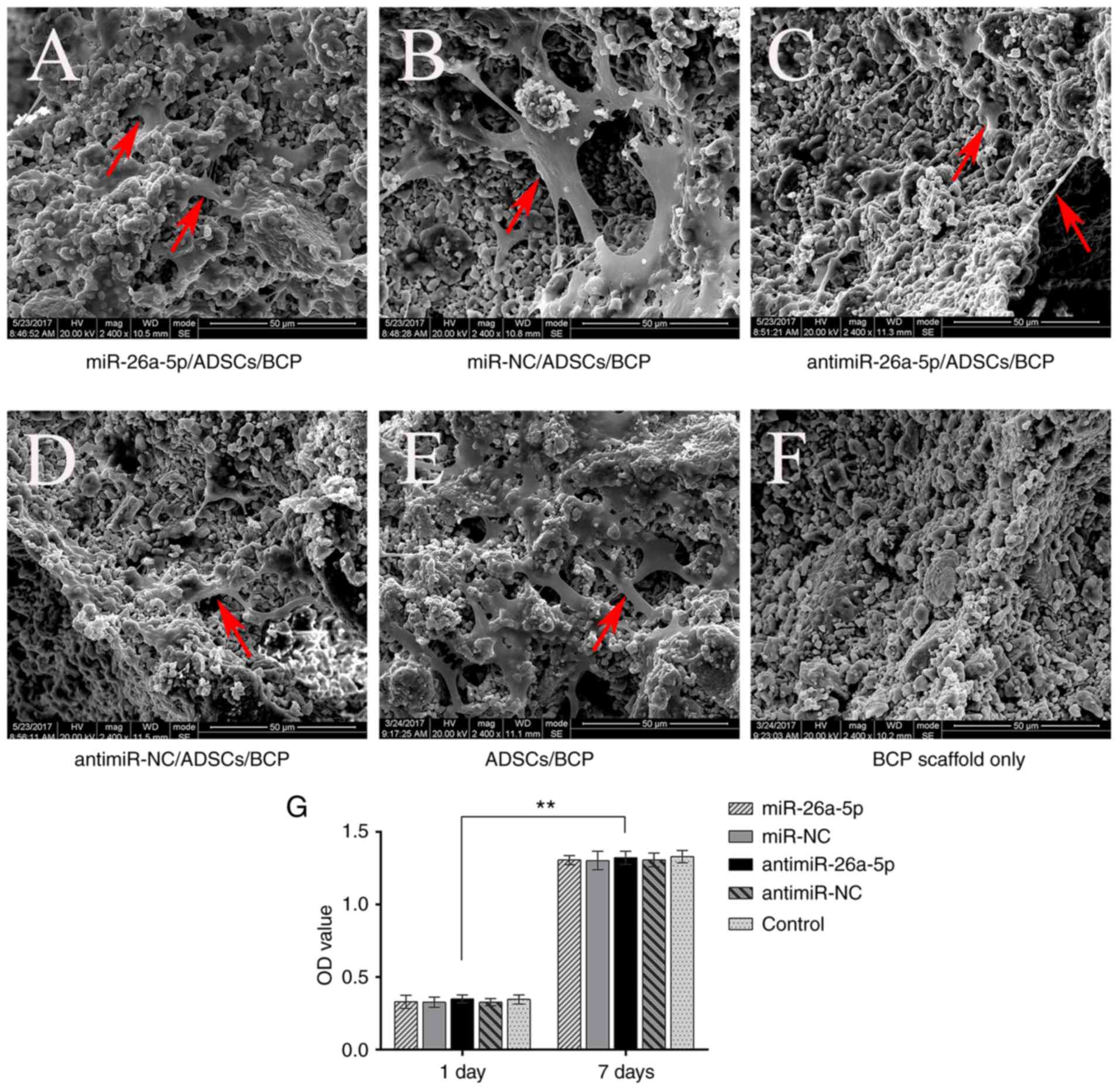
SEM evaluation of cell adhesion and CCK-8
assay evaluation of cell proliferation
The SEM images revealed that the ADSCs successfully
and tightly adhered to the BCP scaffolds. There was an adequate
number of cells on each of the scaffolds in all five groups
(Fig. 2A-F). The ADSCs on the
scaffolds had a fusiform or polygon morphology.
The proliferation of ADSCs on the BCP scaffolds of
each group was confirmed by CCK-8 assay. Following 7 days of
incubation, the number of ADSCs on the BCP scaffolds was
significantly increased compared with 1 day of incubation
(P<0.01); however, no statistically significant differences
between the five groups at the same time point were identified
(Fig. 2G).
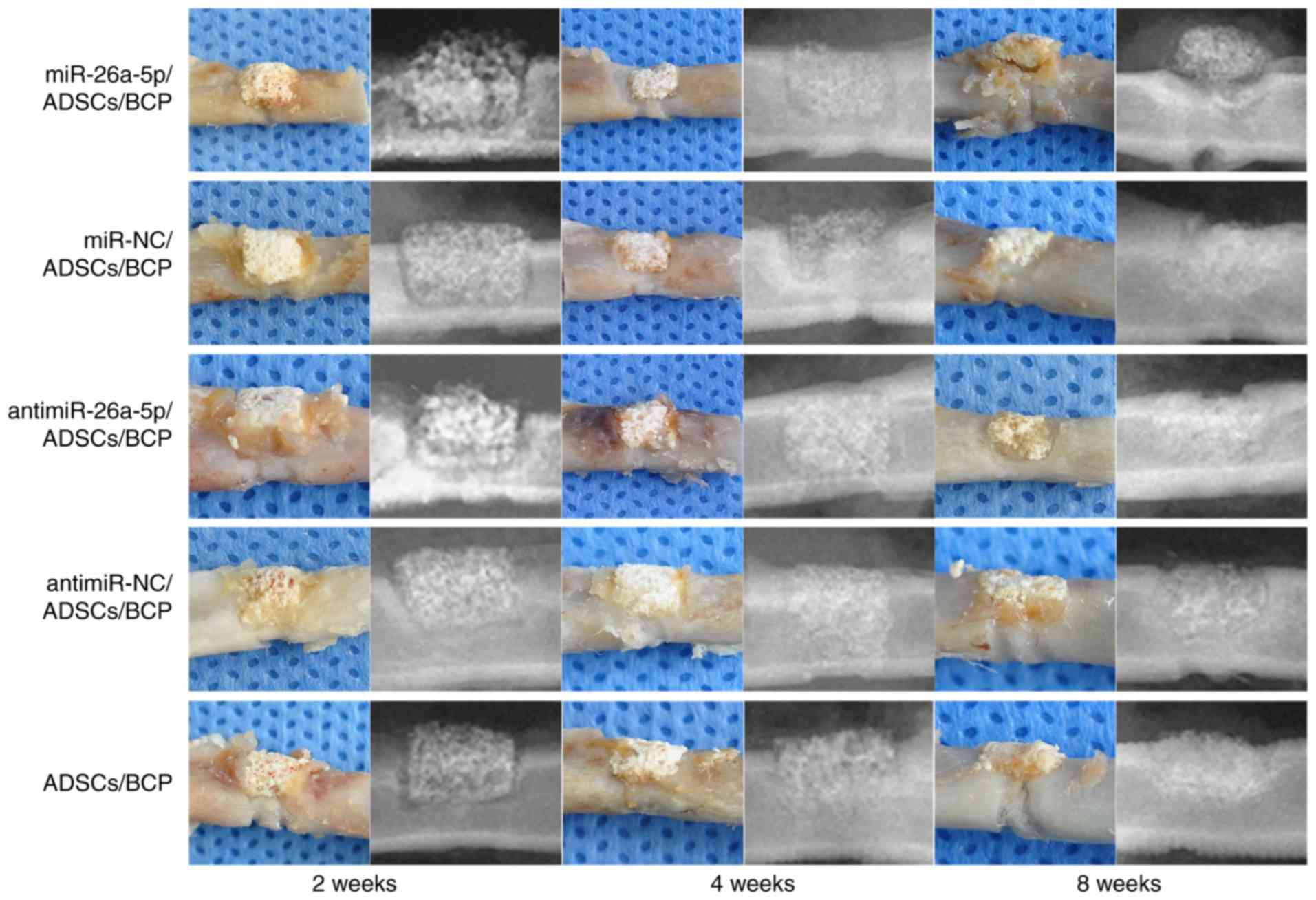
Gross observation and radiographical
analysis of bone regeneration
All experimental animals were healthy and
successfully completed the experiment. No rats presented any signs
of peritonitis following the administration of 10% chloral hydrate
during the study. The maximum percentage of body weight loss
observed in this study was ~8%.
At 2,4 and 8 weeks postoperatively, 6 animals from
each group were chosen randomly and sacrificed by euthanasia. Left
femur samples were harvested for gross observation and X-ray
testing (Fig. 3). All 90
experimental samples were obtained from 90 different rats (30 rats
were sacrificed at each time point; n=6 rats from each group per
time point). The BCP scaffolds were stably connected to the native
bones, and there was some connective tissue surrounding the
scaffolds. Radiographical analysis revealed that although the lines
between BCP and native bones could be clearly identified at 2 weeks
postoperatively, they became indistinct at 4 weeks postoperatively
as the BCP was beginning to be absorbed. At 8 weeks
postoperatively, there was evident new bone formation in the
defects, with the BCP scaffolds becoming smaller when compared with
at 4 weeks. From the radiographical analyses, the boundaries
between BCP and native bones were shown to disappear and the BCP
scaffolds were greatly diminished and fused with the regenerative
bones except the miR group. The residual BCP did not connect
tightly with the newly formed bone in the miR group.
Micro-CT analysis of bone
regeneration
At 2,4 and 8 weeks postoperatively, new bone
formation in the ROI was measured by micro-CT (Fig. 4). Regenerated bone in the defect
increased over time in all 5 groups, starting from surrounding the
BCP scaffolds, gradually filling the macro-pores of the BCPs, and
finally replacing the BCPs. At 2 weeks postoperatively, there was a
significant difference in bone formation between the 5 groups,
including in BV/TV, BMD and Tb.N values, which were the highest in
antimiR group, and lowest in the miR group (P<0.05); this
difference between the groups was retained at 4 and 8 weeks
postoperatively (P<0.05). These data suggested that the antimiR
group had the most newly formed bone and the highest mineralization
rate, while the miR group had the opposite outcome. There was a
time-dependent increase in BV/TV, BMD and Tb.Th, and a
time-dependent decrease in Tb.N (P<0.05). For Tb.Th, at 2 weeks
the antimiR group was the lowest (P<0.01), however, at 4 weeks
the antimiR group was the highest (P<0.01) and the miR group was
the lowest (P<0.05) among the five groups; no significant
differences were found at 8 weeks postoperatively.
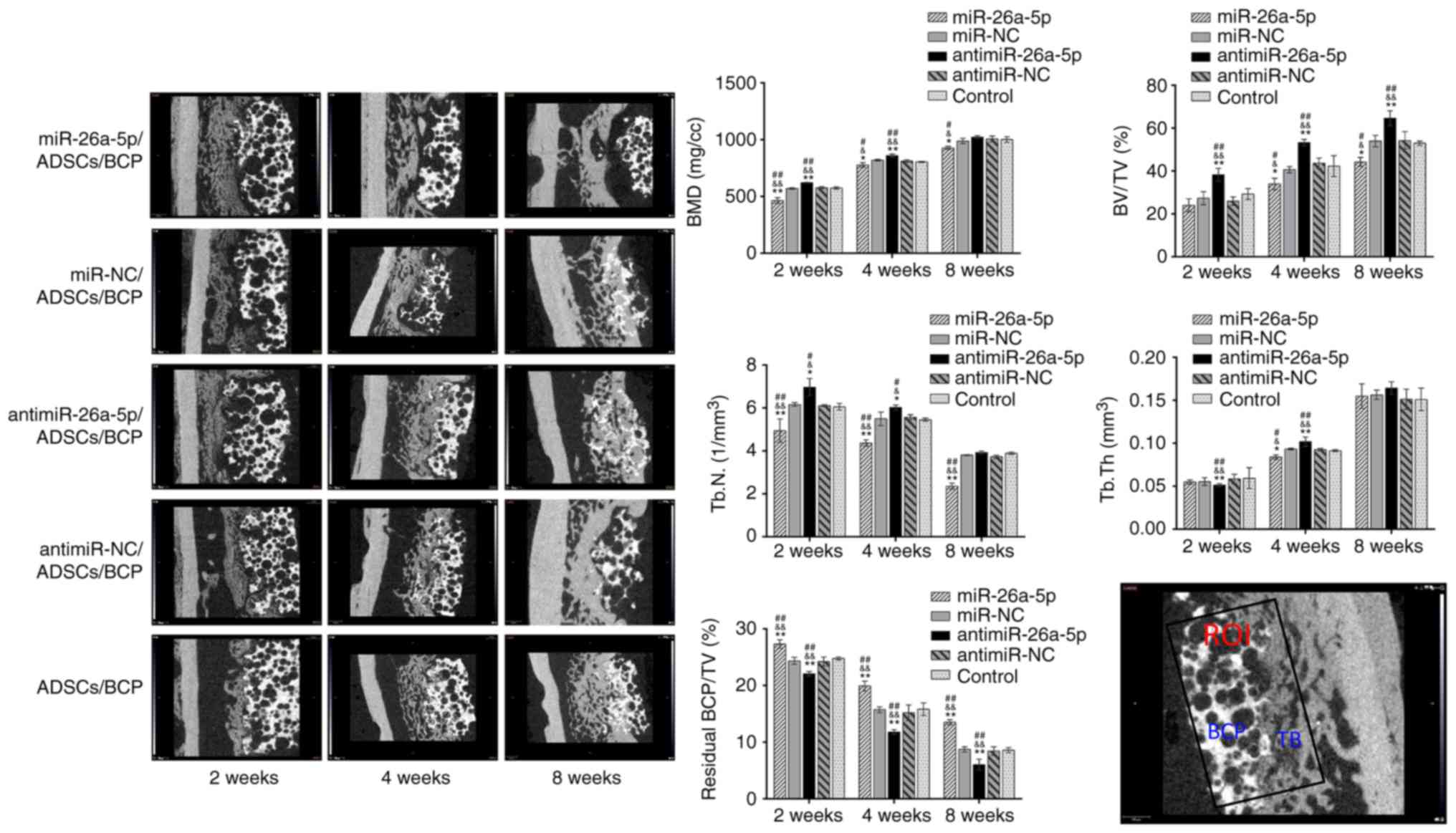 | Figure 4Micro-computed tomography evaluation
of bone regeneration and general trabecular microstructure
parameters of ROI in the miR, miR-NC, antimiR, antimiR-NC and
Control groups. The BMD, BV/TV and Tb.N were significantly lower in
the miR group and higher in the antimiR group compared with the
other three groups. There was no regular change trend in the
pattern of Tb.Th between the five groups at 8 weeks. The percentage
of residual BCP was the lowest in the antimiR group, and the
greatest in the miR group when comparing the five groups.
*P<0.05 and **P<0.01 vs. miR-NC;
&P<0.05 and &&P<0.01 vs.
antimiR-NC; #P<0.05 and ##P<0.01 vs.
control. ROI, region of interest; miR, microRNA; NC, negative
control; ADSCs, adipose-derived mesen-chymal stem cells; BCP,
biphasic calcium phosphate; W, weeks; BV/TV, ratio of bone volume
to total volume; BMD, bone mineral density; Tb.N, trabecular
number; Tb.Th, trabecular thickness; TB, tissue-engineered
bone. |
Evaluation of the relative quantity of residual BCP
scaffolds in the ROI showed a gradual resorption of BCPs with time.
At each time point, the antimiR group had the lowest amount of
residual BCP, while the miR group had the most (P<0.01), which
was the opposite to the results observed for newly formed bone.
Histological evaluation of bone
regeneration
Representative sections of the 5 groups (n>3)
were captured using software coupled with a light microscope. The
red color indicates bone and collagen fibers, and the BCPs formed
blanks filling the entire defect area (Fig. 5). At 2 weeks postoperatively,
there was a thin layer of newly formed bone and collagen fibers
between the BCPs and the adjacent native bones and medullar
cavities. Additionally, there was a large amount of spot-like
collagen fibers, and even new bone in the center, invading into the
macro-pores of the BCPs in the antimiR group. Due to the shape of
the pores in the BCP scaffolds, the newly formed tissue in the
pores manifested as round spots and the blank spaces surrounding
them were occupied by residual BCP. There was no significant tissue
formation in the pores of the scaffolds in the other 4 groups. At 4
weeks postoperatively, regenerated trabecular bone grew thicker and
more abundant compared with at 2 weeks, and had begun
mineralization. The macro-pores of the BCPs began to be filled with
newly formed bone and collagen fibers in all groups except the miR
group. In the antimiR group, the majority of the BCP pores had been
filled by woven bones or collagen fibers. BCP degradation could be
identified based on the decrease in blank spaces filling the
defects. At 8 weeks postoperatively, with the absorption of
scaffolds, the spot-like newly formed bone began to merge together
in all groups. The defects were already filled with newly formed
woven bones, some of which had become mature lamellar bones, and
the rebuilding process was performed with the medullary cavity
starting to form in the defect area of the miR-NC, antimiR and
antimiR-NC groups. The newly formed bones in the miR group did not
completely repair the defect without significant medullary cavity
formation. In addition, reticulation structures from the H&E
sections could be easily identified in the blank area, namely in
the residual BCP scaffolds. This was especially true for the
antimiR group at both 4 and 8 postoperative weeks, where
reticulation structures could be easily identified in the blank
area, namely in the residual BCP scaffolds.
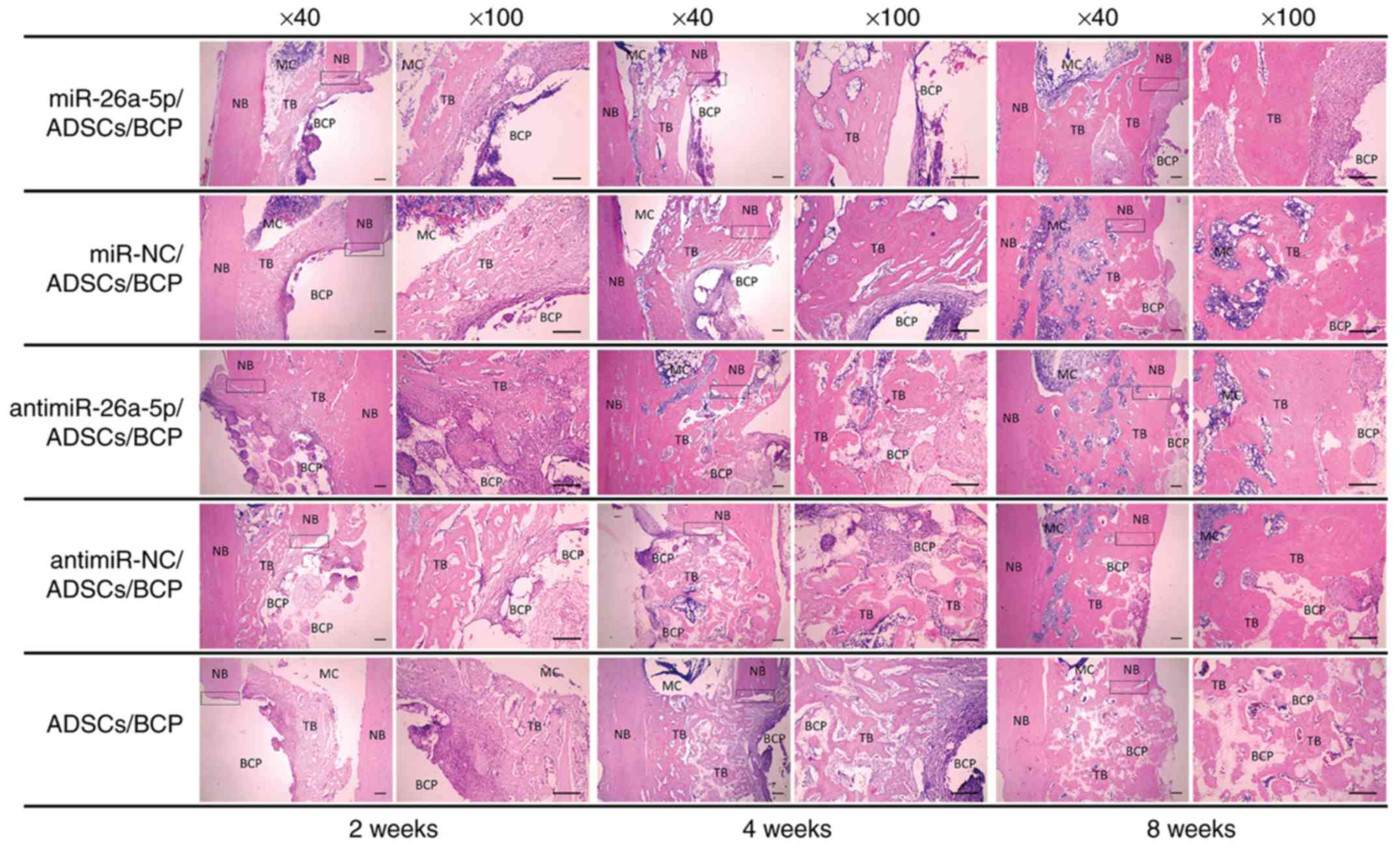 | Figure 5Histological analysis of the
regenerated bone in the miR, miR-NC, antimiR, antimiR-NC and
Control groups at 2,4 and 8 weeks postoperatively (hematoxylin and
eosin staining; magnification, ×40 and ×100 as indicated; Scale
bars, 200 µm). Squares indicate the connection between the
NB and TB. miR, microRNA; NC, negative control; ADSCs,
adipose-derived mesenchymal stem cells; BCP, biphasic calcium
phosphate; MC, medullar cavity; NB, natural bone; TB,
tissue-engineered bone. |
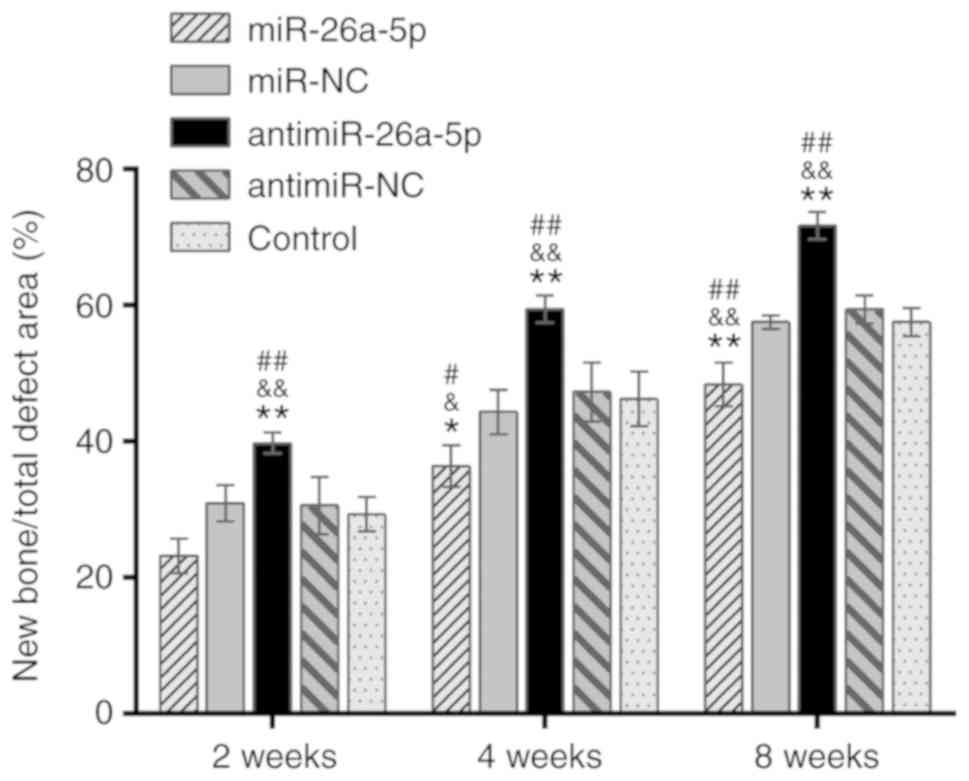
Semi-quantitative analysis of the newly formed bone
in the defect area corresponded with the results of micro-CT
analysis (Fig. 6). Comparison of
the five groups revealed that at each time point, the quantity of
new bone was the greatest in the antimiR group (P<0.01) and the
lowest in the miR group (P<0.05).
IHC evaluation of bone regeneration
IHC staining demonstrated that osteoblasts from all
5 groups stained positive for 3 osteogenic-related proteins (RUNX2,
OCN and COLI) and two pathway proteins (WNT5A and CaMKII) at 2,4
and 8 weeks postoperatively. The histograms revealed that the time
courses of expression were similar, with the highest expression at
2 weeks postoperatively, and a decreasing trend over time (Figs. 7-9). The role of miR-26a-5p and
antimiR-26a-5p in new bone regeneration was investigated by
comparing the relative expression levels of RUNX2, OCN and COLI in
the miR and antimiR groups with those in the other groups.
Evaluating the proportion of RUNX2-positive cells at ×400
magnification revealed that the miR group had a significantly lower
proportion of RUNX2-postive osteoblasts compared with the miR-NC
group (P<0.05), while the antimiR group had a significantly
higher number of RUNX2-positive cells compared with the antimiR-NC
group (P<0.05; Fig. 7). There
was no significant difference between the miR-NC, antimiR-NC and
Control groups. The expression of OCN and COLI was similar to that
ofRUNX2 (Fig. 8). These data
indicated that modified ADSCs seeded onto the BCP scaffolds
participated in new bone formation. Furthermore, the expression of
WNT5A- and CaMKII-positive cells was increased in the antimiR group
and decreased in the miR group at all time points (P<0.05;
Fig. 9). Taken together, the
results indicated that miR-26a-5p regulated in vivo bone
formation by ADSCs by inhibiting WNT5A and CaMKII expression.
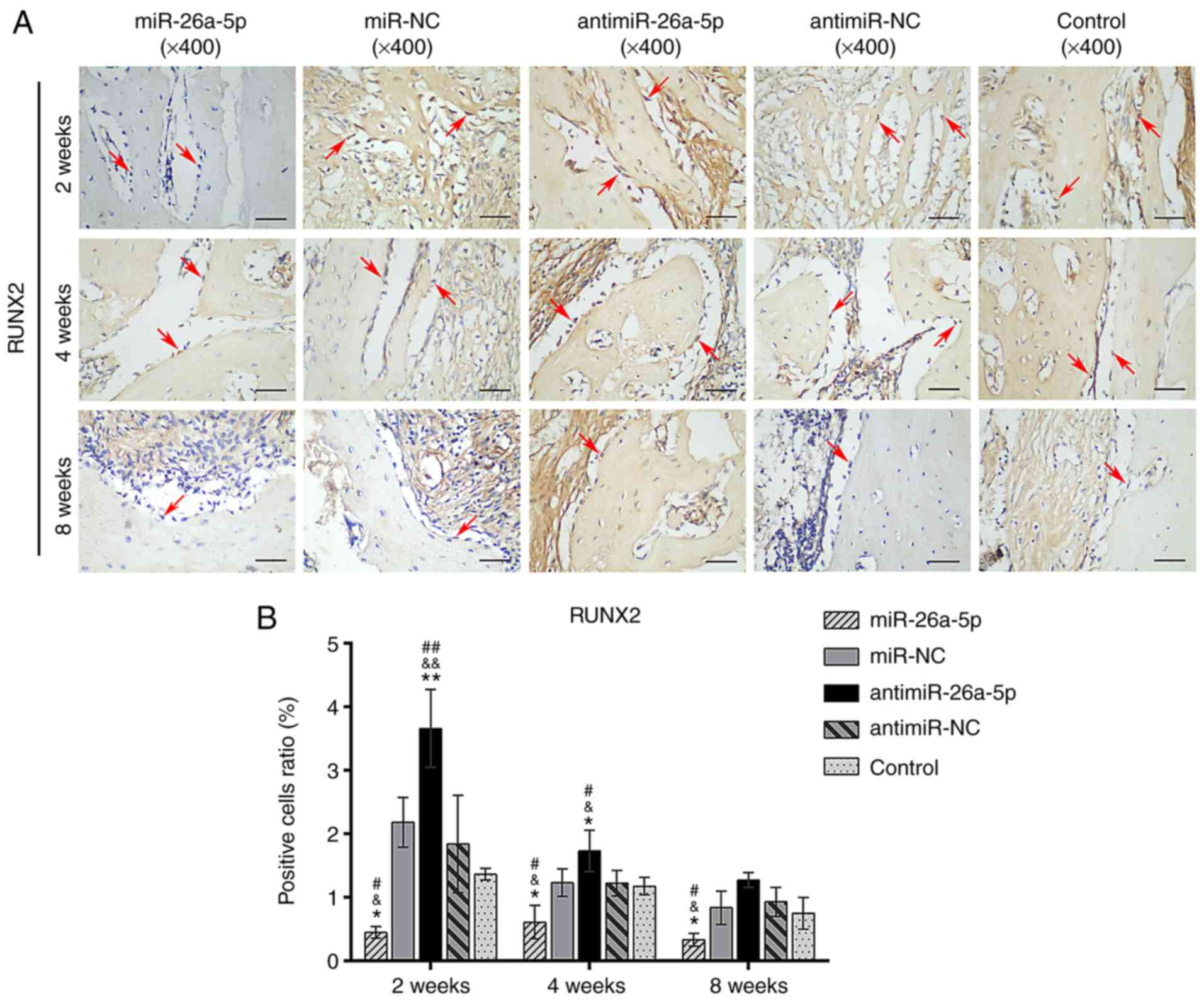 | Figure 7Immunohistochemical analysis specific
for RUNX2 in the miR, miR-NC, antimiR, antimiR-NC and Control
groups at 2,4 and 8 weeks, postoperatively. (A) The cytoplasm of
RUNX2-positive osteoblasts stained brown along the surface of new
bone (magnification, ×400; scale bars, 50 µm). (B) Relative
area of RUNX2-positive osteoblasts to the total area of the images.
Red arrows indicate the positively stained osteoblasts.
*P<0.05 and **P<0.01 vs. miR-NC;
&P<0.05 and &&P<0.01 vs.
antimiR-NC; #P<0.05 and ##P<0.01 vs.
control. miR, microRNA; NC, negative control; RUNX2, Runt-related
transcription factor 2. |
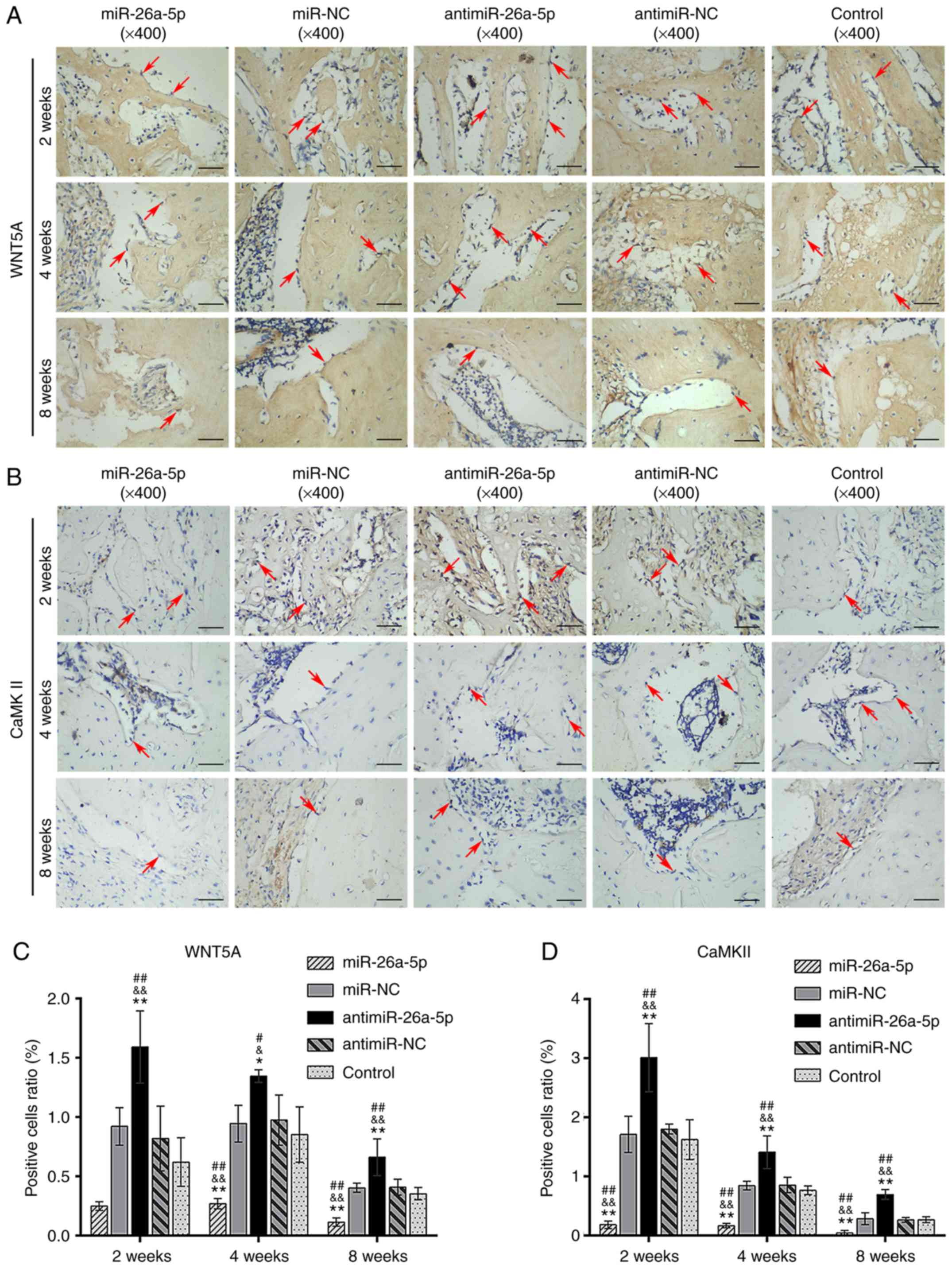 | Figure 9Immunohistochemical analysis specific
for WNT5A and CaMKII in the miR, miR-NC, antimiR, antimiR-NC and
Control groups at 2,4 and 8 weeks, postoperatively. (A)
Immunohistochemical staining specific for WNT5A. The cytoplasm of
WNT5A-positive osteoblasts was stained brown (magnification, ×400;
scale bars, 50 µm). (B) Immunohistochemical staining
specific for CaMKII. The cytoplasm of CaMKII-positive osteoblasts
was stained brown or yellow (magnification, ×400; scale bars, 50
µm). (C) Relative area of WNT5A-positive osteoblasts to the
total area of images. (D) Relative area of CaMKII-positive
osteoblasts to the total area of images. Red arrows indicate the
positively stained osteoblasts. *P<0.05 and
**P<0.01 vs. miR-NC; &P<0.05 and
&&P<0.01 vs. antimiR-NC;
#P<0.05 and ##P<0.01 vs. control. miR,
microRNA; NC, negative control; CaMKII, calmodulin-dependent
protein kinase II; WNT5A, Wnt family member 5A. |
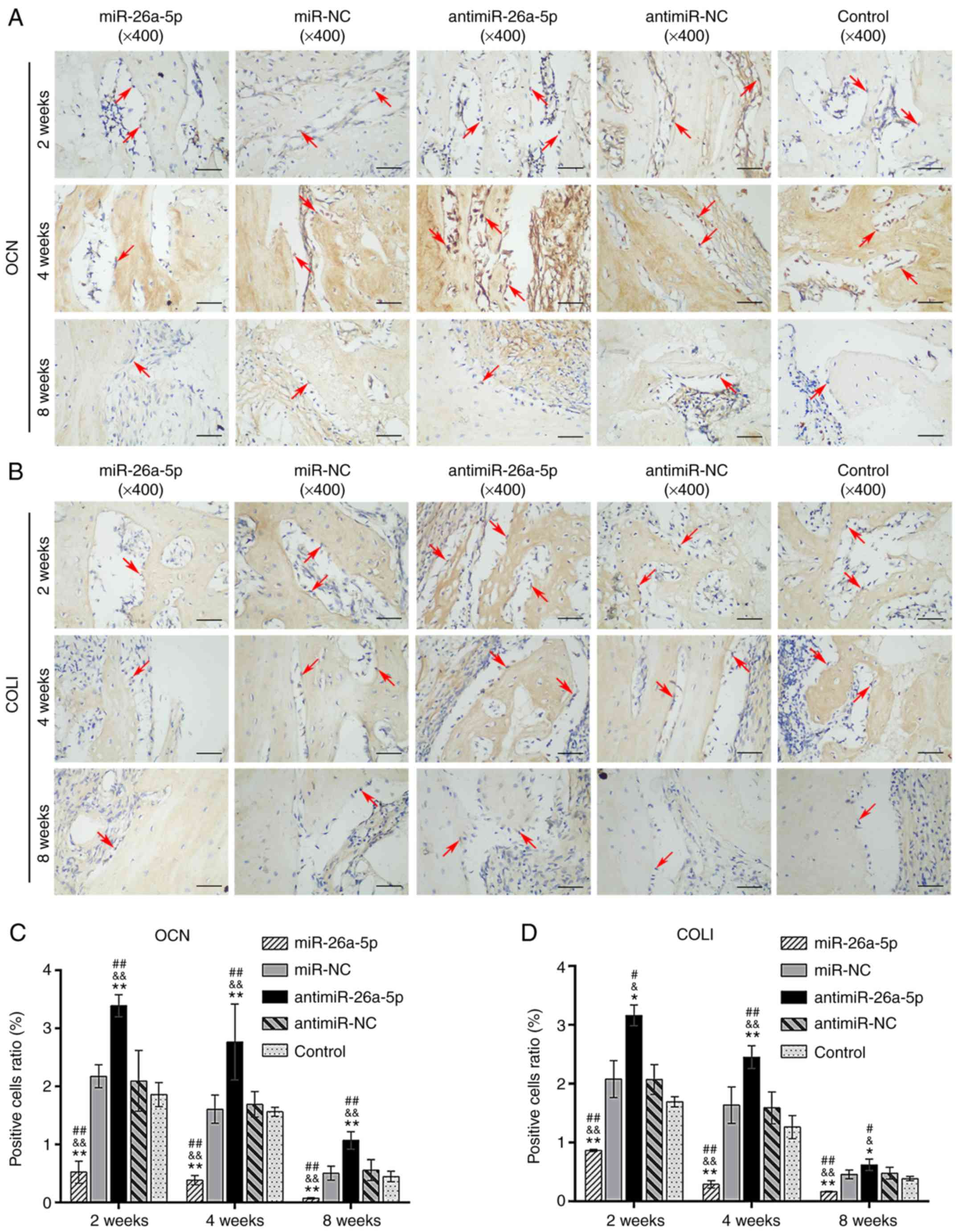 | Figure 8Immunohistochemical analysis specific
for OCN and COLI in the miR, miR-NC, antimiR, antimiR-NC and
Control groups at 2,4 and 8 weeks, postoperatively. (A) The
cytoplasm of OCN-positive osteoblasts was stained brown
(magnification, ×400; scale bars, 50 µm). (B) COLI-positive
osteoblasts were stained brown along the surface of new bones
(magnification, ×400; scale bars, 50 µm). (C) Relative area
of OCN-positive osteoblasts to the total area of images. (D)
Relative area of COLI-positive osteoblasts to the total area of
images. Red arrows indicated the positively-stained osteoblasts.
*P<0.05 and **P<0.01 vs. miR-NC;
&P<0.05 and &&P<0.01 vs.
antimiR-NC; #P<0.05 and ##P<0.01 vs.
control. miR, microRNA; NC, negative control; OCN, osteocalcin;
COLI, collagen I. |
Discussion
The primary aim of the present study was to
construct a novel and effective TEBG using antimiR-26a-5p-modified
ADSCs combined with a BCP scaffold. The cell-material complexes
were initially constructed successfully in vitro. BCPs were
previously shown to have a good ability for cell attachment and
proliferation (32,33), and this was consistent with the
SEM and CCK8 assay results of the present study. The SEM results
demonstrated that the ADSCs adhered and grew on the BCP scaffolds
after 7 days of co-culture, with a fusiform or polygon morphology.
The results of the CCK-8 assay indicated that the proliferation of
ADSCs on the BCPs of the 5 groups was not different, which meant
that the cell vitality was satisfactory in the 5 groups. These
cell-scaffold complexes could be implanted to repair bone
defects.
The present study investigated the role of
miR-26a-5p and antimiR-26a-5p on bone regeneration in a rat femoral
defect model. Quantitative analysis of micro-CT showed that the
quantity and quality of newly formed bone were the lowest in the
miR group and highest in the antimiR group when compared with the
miR-NC, antimiR-NC and Control groups based on the BMD, BV/TV and
Tb.N data (P<0.05). H&E sections from the 5 groups indicated
that the amount of newly generated bone was also the highest in the
antimiR group and the lowest in the miR group (P<0.05). In
addition, the relative expressions of RUNX2, OCN and COLI proteins
were the lowest in the miR group and the highest in the antimiR
group compared with the other 3 groups (P<0.05). There were no
significant differences in the expression of these proteins between
the miR-NC, antimiR-NC and Control groups. Taken together, the
results from the gross observations, X-ray and micro-CT
evaluations, and H&E and IHC examinations all suggested that
miR-26a-5p-modified ADSCs combined with BCP scaffolds attenuated
the bone formation process. By contrast, antimiR-26a-5p-modified
ADSCs combined with BCP scaffolds promoted the process compared
with the unmodified ADSCs. Combined with the results of our
previous study (29), the present
data suggested that miR-26a-5p functions as a negative regulator of
the osteogenic differentiation of ADSCs and osteogenesis, while
antimiR-26a-5p effectively promotes osteogenic differentiation and
osteogenesis. antimiR-26a-5p-modified ADSCs combined with BCP
scaffolds should therefore be able to promote bone regeneration
effectively, providing potential for bone repair and reconstruction
in the load-bearing area.
Although a number of studies have investigated
techniques to promote MSC osteogenic differentiation through miRNA
modifications (34-36), the molecular mechanisms underlying
osteogenesis are not completely understood. We previously showed
that miR-26a-5p inhibited the translation of Wnt5a by directly
binding to the 3'-UTR of Wnt5a (29). In the present study, IHC staining
was conducted to analyze the expression of WNT5A in osteoblasts.
The results showed that the ratio of WNT5a-positive cells to
negative cells was the lowest in the miR group and the highest in
the antimiR group compared with the other groups (P<0.05),
suggesting that miR-26a-5p negatively regulated the expression of
WNT5A and antimiR-26a-5p promoted the expression of WNT5A. WNT5A
has been identified by many studies as a noncanonical Wnt ligand
and activates two noncanonical Wnt pathways, one of which is the
Wnt/Ca2+ signaling pathway (37,38). Additionally, our previous study
proved that miR-26a-5p inhibits the osteogenic differentiation of
ADSCs by downregulating the Wnt5a/Ca2+ signaling pathway
(29). Therefore, the present
study used IHC staining to demonstrate that the expression of
CaMKII in Wnt/Ca2+, a downstream mediator of WNT5A via
the Wnt/Ca2+ pathway (29), exhibited a similar pattern to
WNT5A. Taken together, the results indicated that miR-26a-5p
inhibited the expression of WNT5A, leading to low expression of
CaMKII, finally resulting in attenuation of bone regeneration. The
present study demonstrated that the Wnt/Ca2+signaling
pathway regulated osteogenesis by miR-26a-5p, and these results
were consistent with the conclusions from our previous study
(29).
The ideal properties for scaffolds of
tissue-engineering bone include optimal biocompatibility,
biodegradability, osteoconduction, osteoinduction, plasticity,
mechanical strength, and the ability to be easily sterilized and
preserved (11,39). BCPs are composed of calcium
phosphate, the same inorganic material which comprises the natural
skeleton (12,13,15). The present study suggested that
tissue-engineered bone constructed with BCP scaffolds could
successfully repair the bone defect, and BCP could be absorbed
in vivo (40,41). These results validated earlier
studies that reported that BCP is the ideal choice of scaffolds for
tissue-engineered bone (12,13,15). HA, with a calcium to phosphorus
ratio (Ca/P) of 1.67, is considered to be the stable phase and is
hardly absorbed, which may be an obstacle for its use in bone
regeneration (42,43). By contrast, β-TCP, with a Ca/P
ratio of 1.5, was easily absorbed (43). BCPs are combinations of HA and
β-TCP, and have the advantages of both; the combinations compensate
for the individual disadvantages of each material (44,45). The biological properties of BCPs,
including the biocompatibility, bioactivity, biodegradation,
osteoconduction, osteoinduction and cell adhesion are influenced by
the physicochemical properties, the most important of which is the
HA/β-TCP ratio (39,46,47). Porous BCPs with a lower HA/β-TCP
ratio have been shown to have better degradation and
osteoinduction, which correlates with better osteogenesis (48). By contrast, a high ratio of β-TCP
was shown to correlate with poor mechanical strength and a rapid
degradation rate, leading to cracks between BCPs and the newly
formed bone, and failure to regenerate bone (49). Previous results revealed that a
porous BCP with a HA/β-TCP ratio of 20/80 had good
biocompatibility, bioactivity and a fast degradation rate (18,19). The micro-CT and histological
analysis in the present study showed that the residual BCP
particles were packed by newly formed bone without cracks in the
antimiR group, indicating that the rate of bone formation could
compensate for the rate of BCP degradation. In the miR group,
however, the residual BCP scaffold was repelled, resulting in no
support for further bone formation to fill the defect. These
results suggested that an increased bone formation rate may lead to
successful bone reconstruction with BCP scaffolds, covering the
shortages of BCP20/80.
Additionally, micro-CT was used to evaluate the
degradation rates of BCP scaffolds in the 5 groups. The present
results revealed an accelerated degradation rate in the antimiR
group, and decelerated the degradation rate in the miR group
compared with the miR-NC, antimiR-NC and Control groups.
Simultaneously, the antimiR group exhibited the fastest bone
formation rate while the miR group had the slowest bone formation
rate. These data suggested that the differences in BCP degradation
rates may be related to the application of miR-26a-5p and
antimiR-26a-5p. It was previously reported that the
microenvironment of bone formation and extracellular matrix
facilitated osteoclast adhesion, which is important for material
degradation (50). In addition,
osteoblasts provide a microenvironment for osteoclastogenesis, and
WNT5A secreted by osteoblasts is a new co-stimulatory cytokine for
osteoclastogenesis; furthermore, receptor tyrosine kinase-like
orphan receptor 2 acts as the functional receptor of Wnt5a in
osteoclast precursors (51).
Therefore, increased expression of osteoblasts and WNT5A in the
antimiR group activated the function of osteoclasts and accelerated
the degradation of BCP, a process which requires further
investigation.
There were 3 key osteogenic-related proteins that
had similar expression profiles among the 5 groups during the time
course of the present study. Since OCN, COLI and RUNX2 are markers
of osteoblasts and are mainly secreted by them, the ratio of
positive cells represents the quantity of osteoblasts and their
viability, which further indicates better osteogenesis in
vivo. Combined with the results of H&E and micro-CT, IHC
analysis may prove the regulatory function of miR-26a-5p in bone
formation at the molecular level. The expression of RUNX2, OCN and
COLI reached a peak at 2 postoperative weeks, and had slowly
declined by 4 postoperative weeks; they reached very low levels at
8 postoperative weeks. A previous study reported the same
expression pattern of OCN and RUNX2 as the present study, and that
OCN and RUNX2 reached a peak at an early stage (52). However, OCN and COLI are
considered to be the late-stage osteogenic markers (53-55). Since the present data indicated
that miR-26a-5p and antimiR-26a-5p had the opposite effects on
osteogenesis in the miR and antimiR groups, we hypothesized that
the early-stage high expression of OCN and COLI may be associated
with the calcium and phosphate ions released into the
microenvironment by the BCP scaffolds. Previous studies have
reported that, with a degradation rate determined by the
composition of BCPs, suitable concentrations of ions are released
into the microenvironment of tissue-engineered bone, triggering an
early and high expression of osteogenic-related genes and proteins
through the BMP signaling pathway (56). Elevated Ca2+
concentrations were also shown to promote the recruitment of bone
marrow progenitor cells in vivo, regulate the expression of
bone morphogenetic protein and COLI in osteoblasts, and modulate
the osteoinduction of MSCs (57,58). It is therefore possible that the
rapid degradation of BCP20/80 may lead to increased extracellular
Ca2+ concentrations, resulting in the early-stage
elevated expression of OCN and COLI in osteoblasts. Further
investigations are necessary to examine the concentration of ions
in the extracellular matrix to verify this hypothesis.
In conclusion, the results of the present study
revealed that miR-26a-5p was a negative regulator of osteogenic
differentiation of ADSCs. miR-26a-5p-modified ADSCs combined with
BCP scaffolds significantly attenuated bone regeneration, while
antimiR-26a-5p-modified ADSCs enhanced bone formation. Based on the
variation in the expression of WNT5A and its downstream protein,
CaMKII, as well as that of osteogenesis-related proteins, it is
likely that miR-26a-5p regulated the osteogenic differentiation of
ADSCs and bone formation via the Wnt/Ca2+ signaling
pathway. The present results indicated a potential regulatory role
for miR-26a-5p in bone regeneration. It was also suggested that the
combination of antimiR-26a-5p-modified ADSCs and the BCP scaffold
could provide a promising and effective strategy for bone repair
and reconstruction. The present results provide important
preclinical data to support the application of miR-26a-5p in
bone-related diseases.
Funding
The present study was supported by grants from the
National Nature Science Foundation of China (grant nos. 31570950,
10502037 and 31070833) and the Science and Technology Foundation of
Sichuan Province (grant. nos. 2017SZ0032, 2010GZ0225, 2011GZ0335
and 2009SZ0139).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XY conceived and designed the study, collected,
analyzed and interpreted the data, and wrote the manuscript. LH
conceived and designed the study, and collection and presented the
data. HL analyzed and interpreted the data, and wrote the
manuscript. ZG analyzed and interpreted the data. YH conceived and
designed the study, and collected the data. SL performed the
statistical analysis. TL was involved in the animal experiments.
WTa was involved in cell culture and gene transfection. WTi
conceived and designed the study. JL conceived and designed the
study, wrote the manuscript, and gave final approval of the
manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
approved by the Animal Research Committee of Sichuan
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lee JE, Kim MB, Han DH, Pyo SH and Lee YH:
One-barrel microsurgical fibula flap for reconstruction of large
defects of the femur. Ann Plast Surg. 80:373–378. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohseni M, Jahandideh A, Abedi G,
Akbarzadeh A and Hesaraki S: Assessment of tricalcium
phosphate/collagen (TCP/collagene) nanocomposite scaffold compared
with hydroxyapatite (HA) on healing of segmental femur bone defect
in rabbits. Artif Cells Nanomed Biotechnol. 46:242–249. 2018.
View Article : Google Scholar
|
|
3
|
Sakkas A, Schramm A, Winter K and Wilde F:
Risk factors for post-operative complications after procedures for
autologous bone augmentation from different donor sites. J
Craniomaxillofac Surg. 46:312–322. 2018. View Article : Google Scholar
|
|
4
|
Burk T, Del Valle J, Finn RA and Phillips
C: Maximum quantity of bone available for harvest from the anterior
iliac crest, posterior iliac crest, and proximal tibia using a
standardized surgical approach: A cadaveric study. J Oral
Maxillofac Surg. 74:2532–2548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aponte-Tinao LA, Albergo JI, Ayerza MA,
Muscolo DL, Ing FM and Farfalli GL: What Are the complications of
allograft reconstructions for sarcoma resection in children younger
than 10 years at long-term followup? Clin Orthop Relat Res.
476:548–555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khodakaram-Tafti A, Mehrabani D,
Shaterzadeh-Yazdi H, Zamiri B and Omidi M: Tissue engineering in
maxillary bone defects. World J Plast Surg. 7:3–11. 2018.PubMed/NCBI
|
|
7
|
Diomede F, Gugliandolo A, Cardelli P,
Merciaro I, Ettorre V, Traini T, Bedini R, Scionti D, Bramanti A,
Nanci A, et al: Three-dimensional printed PLA scaffold and human
gingival stem cell-derived extracellular vesicles: A new tool for
bone defect repair. Stem Cell Res Ther. 9:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reichert JC, Saifzadeh S, Wullschleger ME,
Epari DR, Schütz MA, Duda GN, Schell H, van Griensven M, Redl H and
Hutmacher DW: The challenge of establishing preclinical models for
segmental bone defect research. Biomaterials. 30:2149–2163. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le BQ, Nurcombe V, Cool SM, van
Blitterswijk CA, de Boer J and LaPointe VLS: The components of bone
and what they can teach us about regeneration. Materials (Basel).
11. pp. E142017, View Article : Google Scholar
|
|
10
|
Horch RE, Beier JP, Kneser U and Arkudas
A: Successful human long-term application of in situ bone tissue
engineering. J Cell Mol Med. 18:1478–1485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pennesi G, Scaglione S, Giannoni P and
Quarto R: Regulatory influence of scaffolds on cell behavior: How
cells decode bioma-terials. Curr Pharm Biotechnol. 12:151–159.
2011. View Article : Google Scholar
|
|
12
|
Uzeda MJ, de Brito Resende RF, Sartoretto
SC, Alves ATNN, Granjeiro JM and Calasans-Maia MD: Randomized
clinical trial for the biological evaluation of two nanostructured
biphasic calcium phosphate biomaterials as a bone substitute. Clin
Implant Dent Relat Res. 19:802–811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yun PY, Kim YK, Jeong KI, Park JC and Choi
YJ: Influence of bone morphogenetic protein and proportion of
hydroxyapatite on new bone formation in biphasic calcium phosphate
graft: Two pilot studies in animal bony defect model. J
Craniomaxillofac Surg. 42:1909–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Zhang Z, Zhao J, Zhang X, Sun X,
Xia L, Chang Q, Ye D and Jiang X: Vertical alveolar ridge
augmentation with beta-tricalcium phosphate and autologous
osteoblasts in canine mandible. Biomaterials. 30:2489–2498. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shuang Y, Yizhen L, Zhang Y,
Fujioka-Kobayashi M, Sculean A and Miron RJ: In vitro
characterization of an osteoinductive biphasic calcium phosphate in
combination with recombinant BMP2. BMC Oral Health. 17:352016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dragonas P, Palin C, Khan S, Gajendrareddy
PK and Weiner WD: Complications associated with the use of
recombinant human bone morphogenic protein-2 in ridge augmentation:
A case report. J Oral Implantol. 43:351–359. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uludag H, D'Augusta D, Palmer R, Timony G
and Wozney J: Characterization ofrhBMP-2 pharmacokinetics implanted
with biomaterial carriers in the rat ectopic model. J Biomed Mater
Res. 46:193–202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arinzeh TL, Tran T, Mcalary J and Daculsi
G: A comparative study of biphasic calcium phosphate ceramics for
human mesenchymal stem-cell-induced bone formation. Biomaterials.
26:3631–3638. 2005. View Article : Google Scholar
|
|
19
|
van Esterik FA, Zandieh-Doulabi B,
Kleverlaan CJ and Klein-Nulend J: Enhanced Osteogenic and
Vasculogenic differentiation potential of human adipose stem cells
on biphasic calcium phosphate scaffolds in fibrin gels. Stem Cells
Int. 2016:19342702016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lian JB, Stein GS, van Wijnen AJ, Stein
JL, Hassan MQ, Gaur T and Zhang Y: MicroRNA control of bone
formation and homeostasis. Nat Rev Endocrinol. 8:212–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zuo B, Zhu J, Li J, Wang C, Zhao X, Cai G,
Li Z, Peng J, Wang P, Shen C, et al: MicroRNA-103a functions as a
mechanosensitive microRNA to inhibit bone formation through
targeting Runx2. J Bone Miner Res. 30:330–345. 2015. View Article : Google Scholar
|
|
22
|
Hupkes M, Sotoca AM, Hendriks JM, van
Zoelen EJ and Dechering KJ: Micro-RNA miR-378 promotes BMP2-induced
osteogenic differentiation of mesenchymal progenitor cells. BMC Mol
Biol. 15:12014. View Article : Google Scholar
|
|
23
|
Wang Q, Cai J, Cai XH and Chen L: MiR-346
regulates osteogenic differentiation of human bone marrow-derived
mesenchymal stem cells by targeting the Wnt/β-catenin pathway. PLoS
One. 8:pp. e722662013, View Article : Google Scholar
|
|
24
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li JW, Hu C, Han L, Liu L, Jing W, Tang W,
Tian WD and Long J: MiR-154-5p regulates osteogenic differentiation
of adipose-derived mesenchymal stem cells under tensile stress
through the Wnt/PCP pathway by targeting Wnt11. Bone. 78:130–141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu T, Zhou H, Hong Y, Li J, Jiang X and
Huang H: MiR-30 family members negatively regulate osteoblast
differentiation. J Biol Chem. 287:7503–7511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Hassan MQ, Volinia S, van Wijnen AJ,
Stein JL, Croce CM, Lian JB and Stein GS: A microRNA signature for
a BMP2-induced osteoblast lineage commitment program. Proc Natl
Acad Sci USA. 105:13906–13911. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suh JS, Lee JY, Choi YS, Chong PC and Park
YJ: Peptide-mediated intracellular delivery of miRNA-29b for
osteogenic stem cell differentiation. Biomaterials. 34:4347–4359.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Hu C, Li J, Liu L, Jing W, Tang W,
Tian W and Long J: Effect of miR-26a-5p on the Wnt/Ca(2+) Pathway
and Osteogenic differentiation of mouse Adipose-Derived mesenchymal
stem cells. Calcif Tissue Int. 99:174–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choudhery MS, Badowski M, Muise A and
Harris DT: Comparison of human mesenchymal stem cells derived from
adipose and cord tissue. Cytotherapy. 15:330–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ansari S, Diniz IM, Chen C, Sarrion P,
Tamayol A, Wu BM and Moshaverinia A: Human periodontal ligament-
and gingiva-derived mesenchymal stem cells promote nerve
regeneration when encapsulated in alginate/hyaluronic acid 3D
scaffold. Adv Healthc Mater. Oct 27–2017, Epub ahead of print.
PubMed/NCBI
|
|
32
|
Chen Y, Wang J, Zhu XD, Tang ZR, Yang X,
Tan YF, Fan YJ and Zhang XD: Enhanced effect of β-tricalcium
phosphate phase on neovascularizationof porous calcium phosphate
ceramics: In vitro and in vivo evidence. Acta Biomater. 11:435–448.
2015. View Article : Google Scholar
|
|
33
|
Huang L, Zhou B, Wu H, Zheng L and Zhao
JM: Effect of apatite formation of Biphasic calcium phosphate (BCP)
on the osteoblastogenesis using simulated body fluid with or
without bovine serum albumin. Mater Sci Eng C Mater Biol Appl.
70:955–961. 2017. View Article : Google Scholar
|
|
34
|
Meng YB, Li X, Li ZY, Zhao J, Yuan XB, Ren
Y, Cui ZD, Liu YD and Yang XJ: MicroRNA-21 promotes osteogenic
differentiation of mesenchymal stem cells by the PI3K/β-catenin
pathway. J Orthop Res. 33:957–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liao YH, Chang YH, Sung LY, Li KC, Yeh CL,
Yen TC, Hwang SM, Lin KJ and Hu YC: Osteogenic differentiation of
adipose-derived stem cells and calvarial defect repair using
baculovirus-mediated co-expression of BMP-2 and miR-148b.
Biomaterials. 35:4901–4910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang S, Wang S, Bian C, Yang Z, Zhou H,
Zeng Y, Li H, Han Q and Zhao RC: Upregulation of miR-22 promotes
osteogenic differentiation and inhibits adipogenic differentiation
of human adipose tissue-derived mesenchymal stem cells by
repressing HDAC6 protein expression. Stem Cells Dev. 21:2531–2540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci (Landmark Ed). 19:379–407.
2014. View Article : Google Scholar
|
|
38
|
Martineau X, Abed É, Martel-Pelletier J,
Pelletier JP and Lajeunesse D: Alteration of Wnt5a expression and
of the non-canonical Wnt/PCP and Wnt/PKC-Ca2+ pathways
in human osteoarthritis osteoblasts. PLoS One. 12:pp. e01807112017,
View Article : Google Scholar
|
|
39
|
Lacroix D, Chateau A, Ginebra MP and
Planell JA: Micro-finite element models of bone tissue-engineering
scaffolds. Biomaterials. 27:5326–5334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bouler JM, Pilet P, Gauthier O and Verron
E: Biphasic calcium phosphate ceramics for bone reconstruction: A
reviewof biological response. Acta Biomater. 53:1–12. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang XH, Mao LX, Liu JQ, Yang Z, Zhang W,
Shu MJ, Hu NT, Jiang LY and Fang B: Fabrication, characterization
and cellular biocompatibility of porous biphasic calcium phosphate
bioceramic scaffolds with different pore sizes. Ceram Int.
42:15311–15318. 2016. View Article : Google Scholar
|
|
42
|
Wu Y, Xia L, Zhou Y, Ma W, Zhang N, Chang
J, Lin KL, Xu YJ and Jiang XQ: Evaluation of osteogenesis and
angiogenesis of icariin loaded on micro/nanohybrid structured
hydroxyapatite granules as a local drug delivery system forfemoral
defect repair. J Mater Chem B. 3:4871–4883. 2015. View Article : Google Scholar
|
|
43
|
Ebrahimi M, Botelho MG and Dorozhkin SV:
Biphasic calcium phosphates bioceramics (HA/TCP): Concept,
physicochemical properties and the impact of standardization of
study protocols in biomaterials research. Mater Sci Eng C Mater
Biol Appl. 71:1293–1312. 2017. View Article : Google Scholar
|
|
44
|
Jensen SS, Bornstein MM, Dard M, Bosshardt
DD and Buser D: Comparative study of biphasic calcium phosphates
with different HA/TCP ratios in mandibular bone defects. Along-term
histo-morphometric study in minipigs. J Biomed Mater Res B Appl
Biomater. 90:171–181. 2009.
|
|
45
|
Zhu Y, Zhang K, Zhao R, Ye X, Chen X, Xiao
Z, Yang X, Zhu X, Zhang K, Fan Y and Zhang X: Bone regeneration
with micro/nano hybrid-structured biphasic calcium phosphate
bioceramics at segmental bone defect and the induced
immunoregulation of MSCs. Biomaterials. 147:133–144. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ng AM, Tan KK, Phang MY, Aziyati O, Tan
GH, Isa MR, Aminuddin BS, Naseem M, Fauziah O and Ruszymah BH:
Differential osteogenic activity of osteoprogenitor cells on HA and
TCP/HA scaffold of tissue engineered bone. J Biomed Mater Res A.
85:301–312. 2008. View Article : Google Scholar
|
|
47
|
Ebrahimian-Hosseinabadi M, Etemadifar M
and Ashrafizadeh F: Effects of nanobiphasic calcium phosphate
composite on bioactivity and osteoblast cell behavior in tissue
engineering applications. J Med Signals Sens. 6:237–242.
2016.PubMed/NCBI
|
|
48
|
Huang J, Ten E, Liu G, Finzen M, Yu W, Lee
JS, Saiz E and Tomsia AP: Biocomposites of pHEMA with HA/beta-TCP
(60/40) for bone tissue engineering: Swelling, hydrolytic
degradation, and in vitro behavior. Polymer (Guildf). 54:1197–1207.
2013. View Article : Google Scholar
|
|
49
|
Daculsi G, Bouler JM and LeGeros RZ:
Adaptive crystal formation in normal and pathological
calcifications in synthetic calcium phosphate and related
biomaterials. Int Rev Cytol. 172:129–191. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Monchau F, Lefevre A, Descamps M,
Belquinmyrdycz A, Laffargue P and Hildebrand HF: In vitro studies
of human and rat osteoclast activity on hydroxyapatite,
beta-tricalcium phosphate, calcium carbonate. Biomol Eng.
19:143–152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Maeda K, Kobayashi Y, Udagawa N, Uehara S,
Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, et
al: Wnt5a-Ror2 signaling between osteoblast-lineage cells and
osteoclast precursors enhances osteoclastogenesis. Nat Med.
18:405–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang X, Li Y, Chen YE, Chen J and Ma PX:
Cell-free 3D scaffold with two-stage delivery of miRNA-26a to
regenerate critical-sized bone defects. Nat Commun. 7:103762016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun LY, Wu L, Bao CY, Fu CH, Wang XL, Yao
JF, Zhang XD and van Blitterswijk CA: Gene expressions of collagen
type I, ALP and BMP-4 in osteoinductive BCP implants show similar
pattern to that of natural healing bones. Mater Sci Eng C.
29:1829–1834. 2009. View Article : Google Scholar
|
|
54
|
Wang J, Chen Y, Zhu X, Yuan T, Tan Y, Fan
Y and Zhang X: Effect of phase composition on protein adsorption
and osteoinduction of porous calcium phosphate ceramics in mice. J
Biomed Mater Res A. 102:4234–4243. 2014.PubMed/NCBI
|
|
55
|
Yi T, Jun CM, Kim SJ and Yun JH:
Evaluation of in vivo osteogenic potential of bone morphogenetic
Protein 2-Overexpressing human periodontal ligament stem cells
combined with biphasic calcium phosphate block scaffolds in a
Critical-Size bone defect model. Tissue Eng Part A. 22:501–512.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Viti F, Landini M, Mezzelani A, Petecchia
L, Milanesi L and Scaglione S: Osteogenic differentiation of MSC
through calcium signaling activation: Transcriptomics and
functional analysis. PLoS One. 11:pp. e01481732016, View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tang Z, Tan Y, Ni Y, Wang J, Zhu X, Fan Y,
Chen X, Yang X and Zhang X: Comparison of ectopic bone formation
process induced by four calcium phosphate ceramics in mice. Mater
Sci Eng C Mater Biol Appl. 70:1000–1010. 2017. View Article : Google Scholar
|
|
58
|
González-Vázquez A, Planell JA and Engel
E: Extracellular calcium and CaSR drive osteoinduction in
mesenchymal stromal cells. Acta Biomater. 10:2824–2833. 2014.
View Article : Google Scholar : PubMed/NCBI
|