Introduction
Resveratrol
(3,5,4′-trihydroxy-trans-stilbene) is a member of the
phytoalexin class of antibiotics and its production can be induced
in a variety of plants in response to fungal infections,
environmental stress, or injury (1). Resveratrol is a well-known component
of the skin of grapes, is present in high concentrations in red
wine, and is also found in other plant foods including blueberries,
mulberries, rhubarb, and cranberries. Jang et al (2) were the first to describe the ability
of resveratrol to inhibit events associated with the initiation,
promotion, and progression of cancer. Subsequent to the report by
Jang et al, numerous in vitro and in vivo
investigations have provided support for the concept that
resveratrol may be efficacious in the prevention of certain
cancers. Resveratrol has been shown to act as a chemopreventive
agent against chemically-induced mammary and esophageal
carcinogenesis in rats (3,4), lung cancer and transplanted liver
tumors in mice (5,6), and has been reported to suppress the
growth of aberrant crypt foci in the colon (7). Resveratrol given to A/J mice at a
daily intraperitoneal (i.p.) dose of 40 mg/kg body weight for 28
days significantly inhibited the growth of subcutaneously
xenografted neuroblastoma cells in mice (8). A 6-fold decrease in tumor volume and
a significantly improved survival rate (70%) were observed in the
mice receiving resveratrol compared to control mice. Resveratrol
has been shown to inhibit proliferation and induce apoptotic cell
death in a number of different types of cancer cells in
vitro. Numerous reports have been published on the potential
mechanisms that might contribute to the anti-cancer activity of
this compound (1,9–11).
Mechanistic studies have revealed that resveratrol can act as an
antioxidant, inhibit transcription factor activation, and inhibit
kinase pathways involved in cell signaling, including those
involved in progression of the cell cycle.
Acute lymphoblastic leukemia (ALL) with chromosomal
translocation t(4;11) is a highly aggressive leukemia found in
60–85% of infants, 2% of children, and 3–6% of adults with ALL
(12,13). The presence of this translocation
is strongly associated with poor responses to conventional
chemotherapeutic agents, relapse, and a poor prognosis for
survival. The mechanisms underlying the malignancy of t(4;11) ALL
are poorly understood. Defective or dysregulated apoptotic pathways
during hematopoiesis may play a role in the generation of these
leukemias. Several cell lines have been established from patients
with ALL carrying the t(4;11) (q21;q23) chromosomal translocation,
and these lines provide useful tools to evaluate the efficacy of
alternative preventive and therapeutic strategies (14–16).
The nonobese diabetic/severe combined
immunodeficient (NOD/SCID) mouse model has been successfully used
to examine chemotherapeutic strategies against hematopoietic
cancers (17–20). It was shown that engraftment of ALL
in these mice mimics the human disease by homing to bone marrow,
spleen, and liver, with significant presentation in peripheral
blood (18,21). We have shown that resveratrol can
effectively induce apoptotic cell death in vitro in cell
lines that were established from patients with ALL that carry the
t(4;11) (q21;q23) chromosomal translocation, as well as other ALL
lines without the translocation (22). We hypothesized that resveratrol
would be efficacious in the treatment of high-risk t(4;11) ALL
in vivo. In the present study, we evaluated the efficacy of
parenterally administered resveratrol in the NOD/SCID mouse model
after engrafting the SEM cell line that carries the t(4;11)
translocation. Resveratrol was compared to a standard
chemotherapeutic agent, vincristine, that is used in clinical
settings to treat t(4;11) ALL (23). Serum levels of resveratrol and
metabolites were measured by liquid chromatography/mass
spectrometry (LC/MS) to determine whether parenteral administration
would provide the levels of resveratrol needed to prevent the
growth of this leukemia.
Materials and methods
Cells and reagents
SEM is an established cell line from a patient
diagnosed with high-risk pre-B ALL containing the chromosomal
translocation t(4;11)(q21;q23) (14). The cells were grown at 37°C, 5%
CO2 in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented
with 10% fetal bovine serum (Sigma, St. Louis, MO), 50 IU/ml
penicillin, 50 μg/ml streptomycin, 0.25 μg/ml amphotericin B, 1 mM
sodium pyruvate, and 2 mM L-glutamine (Invitrogen). For injection
into mice, SEM cells were harvested, washed twice in Dulbecco’s
phosphate-buffered saline (PBS) without Ca2+ or
Mg+ (Sigma), and resuspended at a final concentration of
50×106 cells/ml in PBS.
Vincristine sulfate and resveratrol (>99% pure)
were purchased from Sigma. Vincristine sulfate was dissolved in
PBS. Resveratrol was dissolved in dimethylsulfoxide (DMSO, Sigma).
The solutions were filter sterilized, aliquoted, and frozen at
−20°C until use. Phycoerythrin-cyanin 7 (PE-Cy7)-conjugated
anti-human CD19, allophycocyanin-Cy7 (APC-Cy7) conjugated
anti-mouse CD45 were purchased from Becton-Dickinson (San Jose,
CA). The trans-isomers of resveratrol, tetra-deuterated resveratrol
(d4-resveratrol), resveratrol-3-O-D-glucuronide,
resveratrol-4′-O-D-glucuronide, and resveratrol-3-O-sulfate, and
1-cyclohexyluriedo-3-dodecanoic acid (CUDA) were obtained from
Cayman Chemical Co. (Ann Arbor, MI). Sulfatase from Aerobacter
aerogenes, β-glucuronidase (Type IX-A) from Escherichia
coli, formic acid, glycerol, potassium 4-nitrophenyl sulfate,
and 4-nitrophenyl β-D-glucuronide were purchased from Sigma.
Ammonium hydroxide and LC/MS grades of methanol, acetonitrile, and
water were obtained from Fisher Scientific (Fair Lawn, NJ). Normal
mouse serum was obtained from United States Biological (Swampscott,
MA).
Immunodeficient NOD/SCID mice
All experimental procedures were approved by the
University of California Davis Institutional Animal Care and Use
Committee. Five- to six-weeks-old female
NOD.CB17-Prkdcscid/J mice were purchased from the
Jackson Laboratory (Bar Harbor, ME, common name NOD/SCID). Mice
were housed and handled under pathogen-free conditions at the
University of California, Davis vivarium in a temperature
controlled environment with a 12-h light-dark cycle. Mice were fed
a commercial rodent diet (Diet 7013, Harlan Teklad, Madison, WI)
that was sterilized by gamma irradiation. Mice were given
sterilized food and water ad libitum. Mice were weighed once
per week in a biosafety cabinet to maintain pathogen-free
conditions. At the age of 8 weeks, each mouse was injected with
5×106 SEM cells through the tail vein using a
1-cm3 syringe with a 30-G needle (Becton-Dickinson). The
injection volume was 100 μl.
Detection of leukemia cell
engraftment
Beginning 2 weeks after the tail vein injections of
leukemia cells, ~50 μl of blood was collected from the tail artery
of each mouse once per week to monitor engraftment of the human
leukemia cells. Blood was collected directly into heparinized
Microvette tubes (Sarstedt, Newton, NC) and transferred to 1.5 ml
microfuge tubes, where red blood cells were lysed using PharmLyse
(Becton-Dickinson) according to the manufacturer’s recommendation.
The peripheral blood leukocytes (PBLs) were stained with PE-Cy7
conjugated anti-human CD19 and APC-Cy7 conjugated anti-mouse CD45
at room temperature for 20 min. The cells were washed in PBS
containing 0.1% BSA and 7 mm sodium azide (Sigma) and then fixed in
1% paraformaldehyde (Sigma) before analysis by flow cytometry. The
stained cells were analyzed on a FACSCanto™ fluorescence-activated
cell sorter (FACS) using FACSDiva™ software (Becton-Dickinson).
Each analysis of peripheral blood cells was performed using
appropriate scatter gates to exclude cellular debris and aggregated
cells. PBLs prepared from NOD/SCID mice not injected with leukemia
cells were used as a negative control for engraftment. These cells
were frozen at −80°C in 10% DMSO, 90% fetal bovine serum until use.
As a positive control for CD19+ cells, SEM cells were
added to an aliquot of thawed PBLs from non-engrafted mice. The
negative and positive control cells were stained as described above
and used to set the gates for human CD19+ cells. Thirty
thousand events were collected for each sample. Positive
engraftment was established when the proportion of human
CD19+ cells reached 1% in the murine PBL population
(17,18).
Initial treatment with resveratrol and
toxicity assessment
Once engraftment of leukemia was observed in the
peripheral blood, mice were randomly separated into control,
resveratrol, and vincristine treatment groups (n=13–14 per group).
The mice were treated daily with i.p. injections of DMSO and
resveratrol (40 mg/kg body weight), or once per week with
vincristine (0.5 mg/kg body weight). The 40 mg/kg dose of
resveratrol was chosen because it was reported to be effective
against neuroblastoma in mice (8).
The approximate volume per injection was between 80–120 μl
depending upon the weight of the mouse. During the treatments, the
blood from each mouse was monitored for growth of the leukemia
cells by flow cytometry. Body weights were obtained weekly in order
to adjust the quantity of chemical per animal.
The 40-mg/kg dose of resveratrol proved toxic to the
leukemic NOD/SCID mice, requiring a toxicity analysis. Sixteen mice
were separated into groups of 4 mice each and treated i.p. daily
with DMSO, or doses of resveratrol at 5, 10, or 20 mg/kg body
weight for one week. After one week, the mice were injected with
the same doses once every other day for 3 weeks. The mice were
monitored daily for signs of illness.
Treatment with resveratrol at a lower
concentration
Forty-eight mice (age of 8 weeks) were injected with
SEM leukemia cells as described above. Once engraftment of leukemia
was observed in the peripheral blood by flow cytometry, mice were
randomly separated into control, resveratrol, or vincristine
treatment groups (n=16 per group). The mice were treated every
other day with i.p. injections of DMSO and resveratrol (10 mg/kg
body weight), or three times per week with vincristine (0.5 mg/kg
body weight). The mice were treated for 4 weeks. Body weights and
percent of human CD19+ cells in the mouse PBMC
population were measured weekly. For this experiment, volumes of
DMSO and resveratrol were reduced to 40–60 μl per mouse according
to body weight. Injection volumes of vincristine were between
80–130 μl per mouse.
Analysis of engraftment sites
Blood, spleens, and bone marrow were harvested from
4 mice from the DMSO treatment group following euthanasia to
confirm engraftment sites of the SEM leukemia cells. PBLs were
prepared as described above. Spleens were removed, placed in RPMI
medium, and perfused with medium using a syringe and 25 G needle.
The spleens were then shredded using the end of the needle, the
cells that were released into the medium were collected, and the
red blood cells were lysed with PharmLyse. Both femurs from each
mouse were placed into RPMI medium and the ends of the femurs were
cut. Bone marrow was removed by perfusing the inside of the bone
with medium using a 27-G needle with syringe. PBLs, splenocytes,
and bone marrow cells were stained with PE-Cy7 conjugated
anti-human CD19 and APC-Cy7 conjugated anti-mouse CD45 and analyzed
by flow cytometry as described above.
Quantification of resveratrol and
resveratrol metabolites
Protocols optimizing resveratrol metabolite
deconjugation and resveratrol extraction from mouse sera, and ultra
performance liquid chromatography-tandem mass spectrometry
(UPLC-MS/MS) quantification were developed for this study. Serum
from each mouse was removed from −70°C, thawed on ice, and
separated into three 25-μl aliquots. Each aliquot was spiked with
nitrophenyl glucuronide and nitrophenyl sulfate, each at a final
concentration of 0.5 μM as digestion controls, and d4-resveratrol
at a final concentration of 2 μM as a recovery surrogate. Positive
controls were prepared in each analytical batch from three 25 μl
aliquots of commercially available normal mouse serum prepared as
above, which also received resveratrol, resveratrol-3-sulfate, and
the two resveratrol glucuronides at the final concentrations shown
in Table I. The aliquots from each
triplicate set were then spiked with either 5 μl 0.1 mM ammonium
formate pH 6.9 (mock digest), 5 μl (0.5 kU) β-glucuronidase
reconstituted in formate buffer, or 10 μl sulfatase (0.11 U) as
supplied. Samples were incubated at 37°C for 1 h in an orbital
water bath (Boekel, Feasterville, PA) at 60 Hz protected from
light. Reactions were chilled and quenched with 100 μl cold
acetonitrile using a 5 min 4°C vortex. Samples were further chilled
at −20°C for 10 min to assist protein precipitation, and
centrifuged at 14,000 × g for 10 min at 4°C. Sample supernatants
were removed to a screw capped polypropylene tube containing 5 μl
50% methanolic glycerol, pellets were re-extracted with 100 μl
acetonitrile, and extracts were pooled. The extracts were dried
using a Savant SV110A SpeedVac (Savant Instrument Inc., Holbrook,
NY) and reconstituted in 100 μl 100 nM CUDA in methanol. After
vortexing, the reconstituted extracts were filtered with 0.1 μm
Amicon Ultrafree-MC durapore PVDF filter (Millipore, Billerica, MA)
for 4 min at 4,000 × g and transferred to a glass insert in 2 ml
amber vials, capped, and stored at 4°C for LC-MS/MS analysis. In
addition, the digestion and extraction controls described above,
each extraction batch also contained mock digested, unspiked
aliquots of normal mouse serum samples whose extracts were enriched
with target analytes just before filtration. These samples served
as a ‘matrix normalization solution’ allowing assessment of matrix
effects upon the analyte detection. Methanol normalization
solutions were prepared by spiking 100 μl of 100 nM CUDA with 1 μl
of the spike used for matrix normalization solutions, and were
analyzed as a further control. With each batch of samples, a seven
point standard curve was constructed ranging from 1 to 3,000 nM of
resveratrol, resveratrol sulfate and glucuronides, and nitrophenyl
sulfate and glucuronide in a methanolic solution of 100 nM CUDA and
2 μM d4-resveratrol.
 | Table IAnalyte specific API 4000 QTrap
MS/MSa and control sample
parameters. |
Table I
Analyte specific API 4000 QTrap
MS/MSa and control sample
parameters.
| Analyte | Precursor (m/z) | Product (m/z) | +DCP (V) | +CE (V) | Spiked normal serum
(μM) |
|---|
| Resveratrol | 227.32 | 185.21 | −80 | −30 | 0.60 |
| Resveratrol-d4 | 231.32 | 188.21 | −80 | −30 | 2.0 |
|
Resveratrol-3-O-D-glucuronide | 403.32 | 227.32 | −15 | −37 | 0.20 |
|
Resveratrol-4′-O-D-glucuronide | 403.32 | 227.32 | −15 | −37 | 0.20 |
|
Resveratrol-3-O-sulfate | 307.32 | 227.32 | −40 | −30 | 0.20 |
|
Potassium4-nitrophenyl sulfate | 218.2 | 138.2 | −50 | −30 | 0.50 |
| 4-Nitrophenyl
β-D-glucuronide | 314.2 | 138.2 | −50 | −30 | 0.50 |
| CUDA | 339.36 | 214.2 | −65 | −30 | - |
Chromatographic separation was achieved using a
gradient of acidified water and acetonitrile on a 2.1×150 mm, 1.7
μm Acquity UPLC BEH C18 column on an Acquity ultra
performance liquid chromatograph (UPLC, Waters Corp., Milford, MA).
Sample were held at 10°C and aliquots (10 μl) were injected on to
the 50°C column equilibrated with 90/10 v/v 0.1% formic
acid:acetonitrile. Initial conditions were held for 1 min, ramped
to 65% acetonitrile at 7 min, 95% acetonitrile at 8 min and held
for 2 min. Solvent flow was 0.25 ml/min. Mass spectral analysis of
the UPLC effluent was performed with an API 4000 QTrap tandem mass
spectrometer (AB SCIEX, Foster City, CA) using negative
electrospray ionization (ESI-) in multiresidue mode (MRM).
Optimized analyte parameters are summarized in Table I. Data analysis was performed using
Analyst 1.4.2 (AB SCIEX). All analyte signals were measured as peak
area ratios to the internal standard, CUDA, to correct for
variability in sample volume, injection volume, and instrumental
drift. Resveratrol serum concentrations were determined from 1/x
weighted linear regressions of methanolic standard curves described
above. Reported serum resveratrol concentrations are corrected for
procedural losses by dividing CUDA-linked results by the fractional
recovery of d4-resveratrol in each sample. The limit of detection
of resveratrol was based on visually defined peaks with a signal to
noise ratio >2 (24).
Statistical analysis
For statistical comparisons between treatment
groups, the event-free survival (EFS) was calculated beginning with
the initiation of treatment. An event was defined as overt clinical
illness necessitating euthanasia, which included >20% weight
loss, lethargy, severe weakness, or inability to reach food or
water for 24 h. All statistical analyses were performed with
GraphPad software (GraphPad Software, Inc., San Diego, CA) and data
are displayed as arithmetic means ± standard deviation (SD), unless
otherwise noted. One-way and two-way analysis of variance (ANOVA)
were used to compare body weights, percent engraftment at the
beginning of treatment, and percent CD19+ cells over
time with Bonferroni post-tests. EFS was analyzed by Kaplan-Meier
plots with differences calculated using the log-rank test.
Differences were considered significant for α (p<0.05).
Results
Resveratrol toxicity in leukemic
mice
Engraftment in the mice as determined by flow
cytometry was 100% for both of the engraftment/treatment
experiments. A 40-mg/kg body weight dose of resveratrol was used
successfully to treat subcutaneously xenografted neuroblastoma
cells in mice (8). Therefore, we
used this dose in our initial experiments. However, with daily i.p.
injections of 40 mg/kg resveratrol, survival of the leukemic mice
was greatly reduced compared to both the DMSO control and
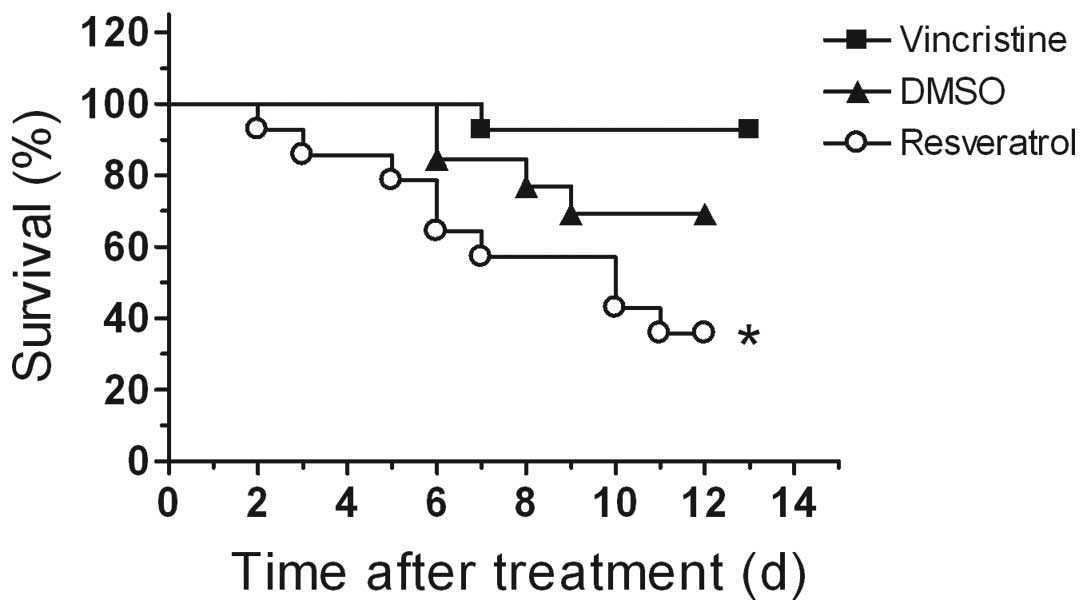
vincristine-treated mice (Fig. 1,
p<0.05, log-rank test). Approximately 36% of mice in the
resveratrol group survived compared to 69% in the DMSO control
group and 93% in the vincristine group by day 12 of treatment. The
experiment was ended on day 13 of treatment and all mice were
euthanized.
A toxicity test with lower doses of resveratrol was
performed to determine the highest acceptable dose for treatment.
Doses of resveratrol at 5, 10, and 20 mg/kg body weight were
evaluated in non-leukemic NOD/SCID mice. Mice were injected i.p.
daily for one week. We found that these mice were intolerant to the
80–120-μl volumes of DMSO, and exhibited temporary weakness in the
hindlimbs after injections. Therefore, after the first week of
daily injections, the treatment was changed to every other day and
was continued for a further 3 weeks. A resveratrol dose of 10 mg/kg
body weight in a 40–60-μl volume of DMSO administered every other
day was determined to be the optimal volume and concentration that
did not show toxicity (data not shown).
Intraperitoneal administration of
resveratrol does not kill t(4;11) ALL
Resveratrol was administered i.p. at a dose of 10
mg/kg body weight to leukemic mice every other day. To reduce the
hindlimb weakness that was observed with the DMSO, the injection
volumes of the DMSO control vehicle and resveratrol were reduced by
half (injection volumes between 40–60 μl) for this study. These
smaller volumes reduced signs of discomfort and weakness in the
mice. The mean percents of human CD19+ cells in the
mouse PBL population at the beginning of treatment were not
different between the groups of mice (DMSO control group was
3.5±3.0%; vincristine group was 1.9±1.1%; resveratrol group was
2.5±2.1%, p>0.05). Treatments began between 3–4 weeks after the
injection of the leukemia cells into the mice (age 11–12 weeks).
After 4 weeks of treatment, survival curves show that resveratrol
was similar in efficacy to the DMSO control (Fig. 2). Treatment with vincristine
increased survival of the mice compared to the control mice
(p<0.05, log-rank test).
Other measured parameters showed the course of
disease in these mice. Weekly body weight measurements showed no
difference between the DMSO and resveratrol treated mice and loss
of body weight began at ~3 weeks after injection of the leukemia
cells in both groups (age 11 weeks, Fig. 3A). Vincristine-treated mice weighed
more than the DMSO control mice at 12 and 13 weeks of age (20.0±1.6
vs 17.8±1.7 g, respectively at 13 weeks of age, p<0.05). The
increasing burden of human CD19+ cells in peripheral
blood of the mice corresponded to a reduction in body weight and
the beginning of clinical illness (Fig. 3B). The percents of human
CD19+ cells in the blood of DMSO and resveratrol treated
mice were similar and were significantly higher than for
vincristine treated mice from 12–14 weeks of age (p<0.05).
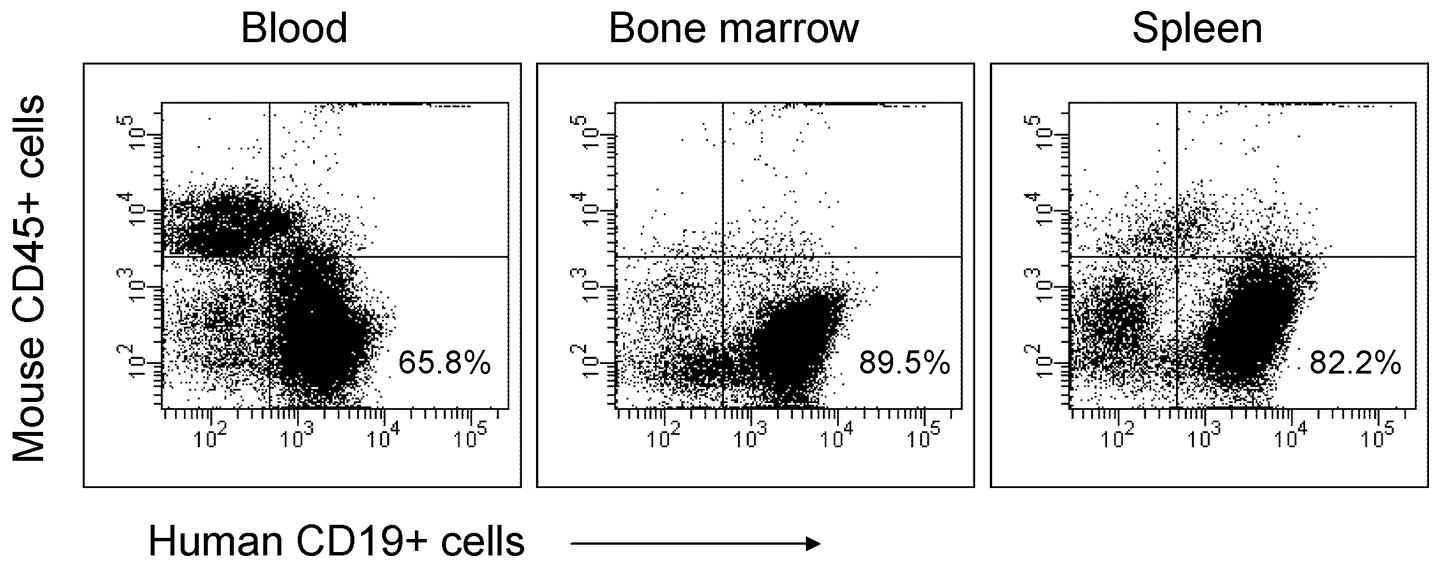
Following euthanasia, engraftment sites of the SEM cells were
evaluated in four mice from the DMSO control group. As expected for
this model, substantial numbers of SEM cells were observed in bone
marrow and spleen (>80% of total leukocytes), as well as the
significant presence of the engrafted cells in the peripheral blood
(Fig. 4).
Resveratrol and metabolite levels in the
sera
To evaluate resveratrol metabolism in the leukemic
NOD/SCID mouse, surviving mice from the resveratrol group that were
showing signs of clinical illness were injected i.p. with
resveratrol, and euthanized at 1 h (n=5), 24 h (n=2), and 48 h
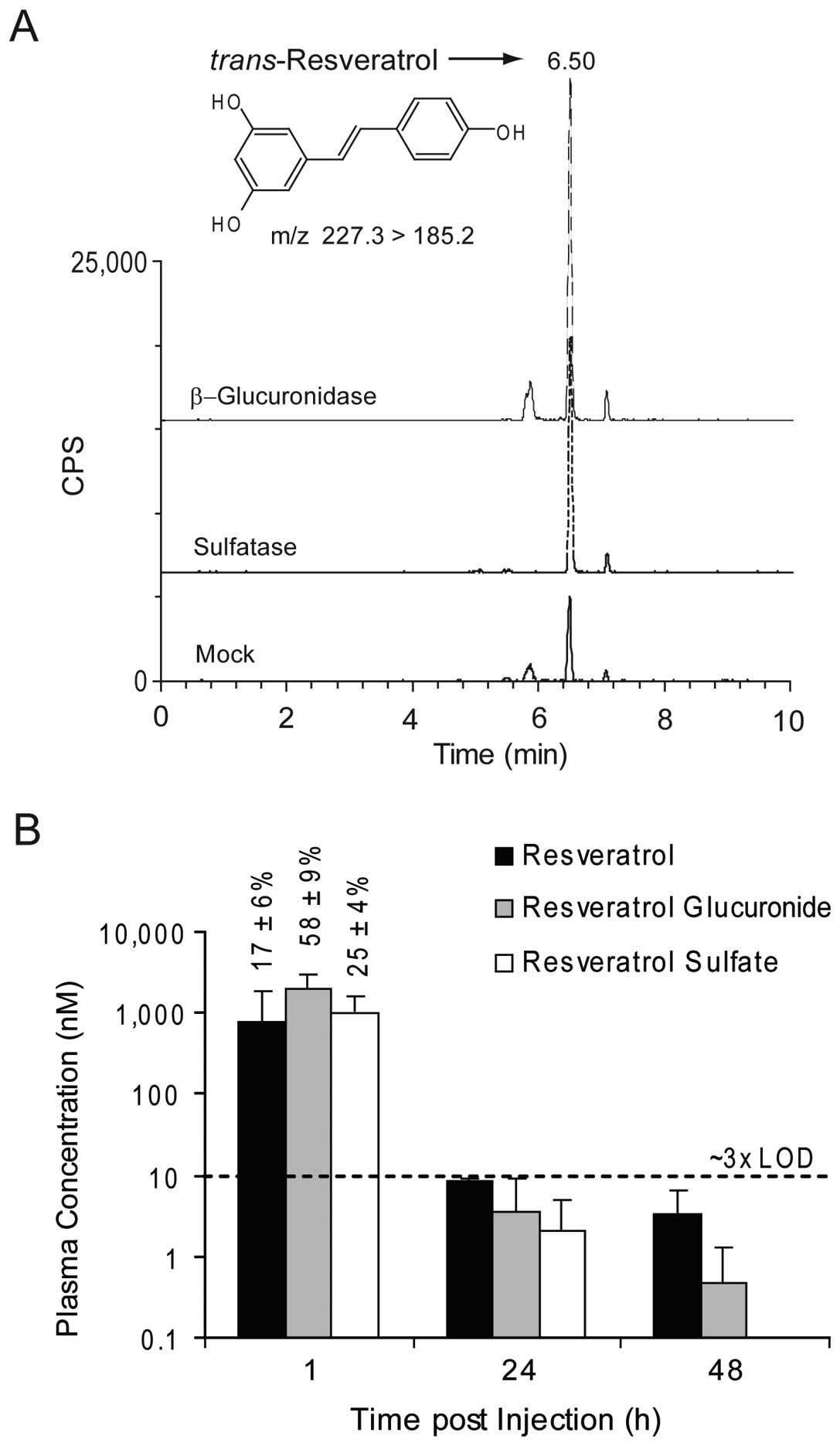
(n=3) post injection. Results are summarized in Fig. 5. Assay performance was routinely
acceptable. The 4-nitrophenyl conjugate controls indicated >99%
deconjugation efficiency, while d4-resveratrol recoveries were
68±6, 68±6 and 86±10% for the mock, β-glucuronidase and sulfatase
digestions, respectively.
As shown in Fig.
5A, sera incubation with either β-glucuronidase or sulfatase
increased extractable resveratrol peak intensities. At 1 h post
injection, serum concentrations of total resveratrol were ~4±2 μM,
roughly distributed in a 1:3:1 ratio of resveratrol:resveratrol
glucuronide:resveratrol sulfate (Fig.
5B). While traces of these metabolites were observed at 24 and
48 h, concentrations were <3x the instrumental detection limit
of 3.7 nM, impacting their quantitative accuracy. Additionally, the
frequency of compound detection decreased over time (1 h = 15/15 or
100%; 24 h = 4/6 or 67%; 48 h 3/9 or 30%). Thus, the leukemic
NOD/SCID mouse definitively retained the ability to metabolize
resveratrol, and at the 10 mg/kg dose, plasma concentrations of
resveratrol were <10 nM within 24 h of i.p. injection.
Discussion
In a previous study, we reported that resveratrol
was efficient at killing t(4;11) ALL cells, as well as ALL cells
without the translocation in vitro (22). However, in the current study
resveratrol did not reduce or delay the growth of the t(4;11) ALL
cells in NOD/SCID mice even when it was present at a concentration
20-fold greater than vincristine, a chemotherapeutic agent used in
the clinical treatment of t(4;11) ALL (23). To our knowledge, this is the first
report that i.p. administration of resveratrol does not kill
t(4;11) ALL in vivo. The SEM cell line used for this study
was established from a relapsed patient with ALL containing the
translocation t(4;11) (11). We
recently reported that this leukemia line is sensitive to
vincristine in vitro, but becomes resistant to vincristine
treatment in vivo due in part to the increased expression of
the multi-drug resistant protein P-glycoprotein (25). In this same study, we found that
vincristine was not toxic when administered to mice 3 times per
week at a dose of 0.5 mg/kg body weight rather than once per week
as is done for humans. In the present study, vincristine was able
to impede the growth of the SEM leukemia cells for 7–14 days
compared to the control treatment. Therefore, even though
multi-drug resistant proteins may interfere with efficacy of
therapeutic agents against this cell line over time, resveratrol
was expected to show some ability to inhibit leukemia cell growth
in the NOD/SCID mouse model, especially at a dose that was
considerably higher than vincristine.
Other investigators using different cancer models
have reported efficacy of resveratrol in inhibiting tumor cell
growth. In a study of ovarian cancer using Balb/c nu/nu mice,
resveratrol administered by daily i.p. injection at concentrations
of 50 and 100 mg/kg body weight for 4 weeks reduced the growth of a
subcutaneously xenografted ovarian tumor (26). At a concentration of 20 mg/kg body
weight, daily i.p. administration of resveratrol was reported to
inhibit the growth of subcutaneously xenografted bladder cancer
(27). In the subcutaneous growth
of tumors from Erlich’s ascites, resveratrol at 20 and 40 mg/kg
body weight was able to inhibit growth of the tumor after 20
consecutive days of i.p. injections in mice (28). Neuroblastoma tumor growth, also
subcutaneous, was similarly reported to be reduced when 5 mg
resveratrol (per injection) was injected around the tumor or
directly into the tumor (29).
In the present study, the dose of resveratrol in the
NOD.CB17-Prkdcscid/J mice was limited to 10 mg/kg body
weight due to unexpected toxicity observed at 20 and 40 mg/kg body
weight which resulted in high mortality. It is unclear why the
leukemic mice did not tolerate the higher doses of resveratrol.
This intolerance of leukemic NOD/SCID mice to concentrations of
resveratrol used in other cancer prevention studies may be
reflective of the engraftment sites and systemic illness induced by
this disease compared to subcutaneous xenograft models, and/or the
intolerance may be mouse strain-dependent. Sites of infiltration of
childhood ALL include liver, spleen, kidney, and central nervous
system (30). Oral resveratrol has
shown renal toxicity in rats at a dose of 3 g/kg bodyweight
(31), so it is possible that mice
with leukemic burden in non-hematological organs, such as liver or
kidney, may have an increased sensitivity to resveratrol toxicity
after i.p. injection.
Studies have been performed in both humans and
animals to determine the tissue distribution, excretion rates, and
the general bioavailability of resveratrol. The majority of
bioavailability studies have been performed after oral
administration. For example, 14C-labelled resveratrol at
a single oral dose of 5 mg/kg body weight was detected in the
duodenum, colon, liver, kidney, lung, spleen, heart, brain, and
testis of mice by 3 h (32).
Intraperitoneal injection of resveratrol at a dose of 20 mg/kg body
weight resulted in the predominant presence of both sulfate and
glucuronide conjugates in mouse serum (33). In these experiments, 13 μM
resveratrol sulfate and 5 μM resveratrol glucuronide were detected
in the serum 15 min after i.p. injection, with concentrations
decreasing over the next 2 h. In another study with gerbils, i.p.
injection of resveratrol at a concentration of 30 mg/kg body weight
resulted in approximately 20 μM resveratrol in the serum after 1 h
with a decline to less than 1.5 μM after 24 h, with little
detectable resveratrol present after 48 h (34). The authors reported the resveratrol
was present mainly as the glucuronide form. Our data show a similar
pattern of presentation of resveratrol in the serum of leukemic
mice, in that the resveratrol was rapidly metabolized to
glucuronidated and sulfated conjugates within the first hour after
i.p. injection, with a 1:3:1 distribution in the
aglycone/gluronide/sulfate forms at that time. By 24 h, serum
concentrations of both resveratrol and its metabolites were less
than 10 nM.
The data presented in this study and the
bioavailability studies described above show the importance of the
metabolic products and the biological clearance of these agents
when assessing the potential of phytochemicals as chemopreventive
agents against leukemia. Recent reports on the biological activity
of resveratrol metabolites showed that resveratrol sulfates can
inhibit nitric oxide production, demonstrate radical scavenging
activity, and inhibit cyclooxygenase-1 and -2 activities, whereas
glucuronides had potent antioxidant potential in vitro
(35–37). Resveratrol sulfates were also
cytotoxic to breast cancer cells in vitro, but they were
approximately 3–4-fold less toxic than resveratrol (38). However, the cytotoxicity observed
in the latter study was at non-physiologic concentrations with an
IC50 concentration of resveratrol at more than 60 μM,
questioning the usefulness of these types of experiments.
Parenterally administered resveratrol is metabolized to conjugated
chemical forms that are either not cytotoxic or not at sufficient
concentrations to induce apoptotic cell death in t(4;11) ALL cells
in vivo. Caution should be exercised in future in
vitro and in vivo research with phytochemicals for the
potential prevention of t(4;11) or other ALLs, and researchers will
need to consider the effects of metabolic alterations that occur
in vivo. Finally, we note that while resveratrol by itself
was not efficacious in the current study, it has been argued that
resveratrol may be of value as a complementary agent in the
treatment of select cancers (39).
This is a possibility that clearly merits additional study with
respect to the potential value of resveratrol in treating ALL.
However, it is equally important to note that resveratrol has been
reported to blunt the actions of select chemotherapeutic agents,
such as proteasome inhibitors and paclitaxel (40,41).
The above underscores the need to not only consider the impact of
absorption, distribution, metabolism, and excretion of resveratrol
and its subsequent biological actions, but also the possibility of
resveratrol having deleterious side effects.
Acknowledgements
This study was supported by a grant from National
Institutes of Health, National Cancer Institute, USA, grant no.
1R21CA122117-01. No conflicts of interest are present for any of
the authors. USDA is an equal opportunity provider and
employer.
References
|
1
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.
|
|
2
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CWW, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta
RG, Moon RC and Pezzuto JM: Cancer chemopreventive activity of
resveratrol, a natural product derived from grapes. Science.
275:218–220. 1997.
|
|
3
|
Banerjee S, Bueso-Ramos C and Aggarwal BB:
Suppression of 7,12- dimethylbenz(a)anthracene-induced mammary
carcinogenesis in rats by resveratrol: role of nuclear factor-κB,
cyclooxygenase 2, and matrix metalloproteinase 9. Cancer Res.
62:4945–4954. 2002.
|
|
4
|
Li ZG, Hong T, Shimada Y, Komoto I, Kawabe
A, Ding Y, Kaganoi J, Hashimoto Y and Imamura M: Suppression of
N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis
in F344 rats by resveratrol. Carcinogenesis. 23:1531–1536.
2002.
|
|
5
|
Lee EO, Lee HJ, Hwang HS, Ahn KS, Chae C,
Kang KS, Lu J and Kim SH: Potent inhibition of Lewis lung cancer
growth by heyneanol A from the roots of Vitis amurensis through
apoptotic and anti-angiogenic activities. Carcinogenesis.
27:2059–2069. 2006.
|
|
6
|
Liu HS, Pan CE, Yang W and Liu XM:
Antitumor and immunomodulatory activity of resveratrol on
experimentally implanted tumor of H22 in Balb/c mice. World J
Gastroenterol. 9:1474–1476. 2003.
|
|
7
|
Tessitore L, Davit A, Sarotto I and
Caderni G: Resveratrol depresses the growth of colorectal aberrant
crypt foci by affecting bax and p21(CIP) expression.
Carcinogenesis. 21:1619–1622. 2000.
|
|
8
|
Chen Y, Tseng S-H, Lai H-S and Chen W-J:
Resveratrol-induced cellular apoptosis and cell cycle arrest in
neuroblastoma cells and antitumor effects on neuroblastoma in mice.
Surgery. 136:57–66. 2004.
|
|
9
|
Colin D, Limagne E, Jeanningros S, Jacquel
A, Lizard G, Athias A, Gambert P, Hichami A, Latruffe N, Solary E
and Delmas D: Endocytosis of resveratrol via lipid rafts and
activation of downstream signaling pathways in cancer cells. Cancer
Prev Res. 4:1095–1106. 2011.
|
|
10
|
Li G, He S, Chang L, Lu H, Zhang H and
Chiu J: GADD45α and annexin A1 are involved in the apoptosis of
HL-60 induced by resveratrol. Phytomedicine. 18:704–709. 2011.
|
|
11
|
Kartal M, Saydam G, Sahin F and Baran Y:
Resveratrol triggers apoptosis through ceramide metabolizing genes
in human K562 chronic myeloid leukemia cells. Nutr Cancer.
63:637–644. 2011.
|
|
12
|
Faderl S, Kantarjian HM, Talpaz M and
Estrov Z: Clinical significance of cytogenetic abnormalities in
adult acute lymphoblastic leukemia. Blood. 91:3995–4019. 1998.
|
|
13
|
Greaves MF and Wiemels J: Origins of
chromosome translocations in childhood leukaemia. Nat Rev. 3:1–11.
2003.
|
|
14
|
Greil J, Gramatzki M, Burger R, Marschalek
R, Peltner M, Trautmann U, Hansen-Hagge TE, Bartram CR, Fey GH,
Stehr K and Beck J: The acute lymphoblastic leukaemia cell line SEM
with t(4;11) chromosomal rearrangement is biphenotypic and
responsive to interleukin-7. Br J Hemotol. 86:275–283. 1994.
|
|
15
|
Stong RC, Korsmeyer SJ, Parkin JL, Arthur
DC and Kersey JH: Human acute leukemia cell line with the t(4;11)
chromosomal rearrangement exhibits B lineage and monocytic
characteristics. Blood. 65:21–31. 1985.
|
|
16
|
Lange B, Valtieri M, Santoli D, Caracciolo
D, Mavilio F, Gemperlein I, Griffin C, Emanuel B, Finan J and
Nowell P: Growth factor requirements of childhood acute leukemia:
establishment of GM-CSF-dependent cell lines. Blood. 70:192–199.
1987.
|
|
17
|
Lock RB, Liem N, Farnsworth ML, Milross
CG, Xue C, Tajbakhsh M, Haber M, Norris MD, Marshall GM and Rice
AM: The nonobese diabetic/severe combined immunodeficient
(NOD/SCID) mouse model of childhood acute lymphoblastic leukemia
reveals intrinsic differences in biologic characteristics at
diagnosis and relapse. Blood. 99:4100–4108. 2002.
|
|
18
|
Liem NLM, Papa RA, Milross CG, Schmid MA,
Tajbakhsh M, Choi S, Ramirez CD, Rice AM, Haber M, Norris MD,
MacKenzie KL and Lock RB: Characterization of childhood acute
lymphoblastic leukemia xenograft models for the preclinical
evaluation of new therapies. Blood. 103:3905–3914. 2004.
|
|
19
|
Shultz LD, Ishikawa F and Greiner DL:
Humanized mice in translational biomedical research. Nat Rev
Immunol. 7:118–130. 2007.
|
|
20
|
Lee EM, Bachman PS and Lock RB: Xenograft
models for the preclinical evaluation of new therapies in acute
leukemia. Leuk Lymphoma. 48:659–668. 2007.
|
|
21
|
Borgmann A, Baldy C, von Stackelberg A,
Beyermann B, Fichtner I, Nurnberg P and Henze G: Childhood ALL
blasts retain phenotypic and genotypic characteristics upon
long-term serial passage in NOD/SCID mice. Pediatr Hematol Oncol.
17:635–650. 2000.
|
|
22
|
Dörrie J, Gerauer H, Wachter Y and Zunino
SJ: Resveratrol induces extensive apoptosis by depolarizing
mitochondrial membranes and activating caspase-9 in acute
lymphoblastic leukemia cells. Cancer Res. 61:4731–4739. 2001.
|
|
23
|
Nachman JB, Sather HN, Sensel MG, Trigg
ME, Cherlow JM, Lukens JN, Wolff L, Uckun FM and Gaynon PS:
Augmented post-induction therapy for children with high-risk acute
lymphoblastic leukemia and a slow response to initial therapy. N
Engl J Med. 338:1663–1671. 1998.
|
|
24
|
Center for Veterinary Medicine, Food and
Drug Administration. Guidance for industry validation of analytical
procedures for type C medicated feeds #135. United States
Department of Health and Human Services; pp. 1–14. 2005, http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm052530.pdf.
|
|
25
|
Zunino SJ, Storms DH and Ducore JM: Novel
in vivo model of inducible multi-drug resistance in acute
lymphoblastic leukemia with chromosomal translocation t(4;11).
Cancer Lett. 296:49–54. 2010.
|
|
26
|
Lee M-H, Choi BY, Kundu JK, Shin YK, Na
H-K and Surh Y-J: Resveratrol suppresses growth of human ovarian
cancer cells in culture and in a murine xenograft model: eukaryotic
elongation factor 1A2 as a potential target. Cancer Res.
69:7449–7458. 2009.
|
|
27
|
Bai Y, Mao Q-Q, Qin J, Zheng X-Y, Wang
Y-B, Yang K, Shen H-F and Xie L-P: Resveratrol induces apoptosis
and cell cycle arrest of human T24 bladder cancer cells in vitro
and inhibits tumor growth in vivo. Cancer Sci. 101:488–493.
2010.
|
|
28
|
El-Mowafy AM, El-Mesery ME, Salem HA,
Al-Gayyar MM and Darweish MM: Prominent chemopreventive and
chemoenhancing effects for resveratrol: unraveling molecular
targets and the role of C-reactive protein. Chemotherapy. 56:60–65.
2010.
|
|
29
|
Kenealey JD, Subramanian L, Van Ginkel PR,
Darjatmoko S, Lindstrom MJ, Somoza V, Ghosh SK, Song Z, Hsung RP,
Kwon GS, Eliceiri KW, Albert DM and Polans AS: Resveratrol
metabolites do not elicit early pro-apoptotic mechanisms in
neuroblastoma cells. J Agric Food Chem. 59:4979–4986. 2011.
|
|
30
|
Lanzkowsky P, Shende A, Aral I and Saluja
G: Organ irradiation and combination chemotherapy in treatment of
acute lymphocytic leukaemia in children. Arch Dis Child.
50:685–690. 1975.
|
|
31
|
Crowell JA, Korytko PJ, Morrissey RL,
Booth TD and Levine BS: Resveratrol-associated renal toxicity.
Toxicol Sci. 82:614–619. 2004.
|
|
32
|
Vitrac X, Desmouliere A, Brouillaud B,
Krisa S, Deffieux G, Barthe N, Rosenbaum J and Merillon J-M:
Distribution of [14C]-trans-resveratrol, a cancer chemopreventive
polyphenol, in mouse tissues after oral administration. Life Sci.
72:2219–2233. 2003.
|
|
33
|
Yu C, Shin YG, Chow A, Li Y, Kosmeder JW,
Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG and van Breeman RB:
Human, rat, and mouse metabolism of resveratrol. Pharm Res.
19:1907–1914. 2002.
|
|
34
|
Wang Q, Xu J, Rottinghaus GE, Simonyi A,
Lubahn D, Sun GY and Sun AY: Resveratrol protects against global
cerebral ischemic injury in gerbils. Brain Res. 958:439–447.
2002.
|
|
35
|
Calamini B, Ratia K, Malkowski MG, Cuendet
M, Pezzuto JM, Santarsiero BD and Mesecar AD: Pleiotropic
mechanisms facilitated by resveratrol and its metabolites. Biochem
J. 429:273–282. 2010.
|
|
36
|
Hoshino J, Park E-J, Kondratyuk TP, Marler
L, Pezzuto JM, van Breemen RB, Mo S, Li Y and Cushman M: Selective
synthesis and biological evaluation of sulfate-conjugated
resveratrol metabolites. J Med Chem. 53:5033–5043. 2010.
|
|
37
|
Mikulski D and Molski M: Quantitative
structure-antioxidant activity relationship of trans-resveratrol
oligomers, trans-4,40-dihydroxystilbene dimer,
trans-resveratrol-3-O-glucuronide, glucosides: trans-piceid,
cis-piceid, trans-astringin and
trans-resveratrol-40-O-b-D-glucopyranoside. Eur J Med Chem.
45:2366–2380. 2010.
|
|
38
|
Miksits M, Wlcek K, Svoboda M, Kunert O,
Haslinger E, Thalhammer T, Szekeres T and Jäger W: Antitumor
activity of resveratrol and its sulfated metabolites against human
breast cancer cells. Planta Med. 75:1227–1230. 2009.
|
|
39
|
Soto BL, Hank JA, Darjatmoko SR, Polans
AS, Yanke EM, Rakhmilevich AL, Seo S, Kim K, Reisfeld RA, Gillies
SD and Sondel PM: Anti-tumor and immunomodulatory activity of
resveratrol in vitro and its potential for combining with cancer
immunotherapy. Int Immunopharmacol. 11:1877–1886. 2011.
|
|
40
|
Niu XF, Liu BQ, Du ZX, Gao YY, Li C, Li N,
Guan Y and Wang HQ: Resveratrol protects leukemic cells against
cytotoxicity induced by proteasome inhibitors via induction of
FOXO1 and p27Kip1. BMC Cancer. 11:992011.open access. [21418583]
|
|
41
|
Fukui M, Yamabe N and Zhu BT: Resveratrol
attenuates the anticancer efficacy of paclitaxel in human breast
cancer cells in vitro and in vivo. Eur J Cancer. 46:1882–1891.
2010.
|