Introduction
The majority of malignant tumors affecting the
exocrine pancreas are histologically defined as pancreatic ductal
adenocarcinomas. These types of tumor rapidly grow and metastasize
representing one of the leading causes of cancer-related death in
developed countries (1,2). Current therapeutic treatments for
patients with advanced disease that are not suitable for surgical
resection have only modest effects due to low response rates,
substantial toxicity and a median survival of less than six months
(3,4). In this respect, numerous attempts
have been made with several chemotherapeutic drugs, however, with
little success because pancreatic cancer shows an inexplicable drug
resistance towards platinum agents, taxanes and topoisomerase
inhibitors (5). The molecular
mechanisms associated with drug resistance in pancreatic cancer are
subject of intense investigations although loss of p53 function,
deregulated Ras signaling and altered phosphatidylinositol-3-kinase
(PI3K)/AKT pathway may play a major role (6,7).
Currently, it is generally accepted that one of the
major factors contributing to the resistance of pancreatic cancer
cells to treatment with chemotherapeutic agents is represented by
the autocrine epidermal growth factor (EGF)-mediated signaling
which results in stimulation of the PI3K pathway and is required
for transformation of several cell lineages by RAS-family
oncogenes (8,9). Consistent with the existence of the
aforementioned autocrine loop is the notion that pancreatic cancer
cells overexpress EGF-family ligands and receptors (EGFR, HER-2 and
-3) (10,11). The EGFR protein also known as
ErbB1, belongs to a family of transmembrane tyrosine kinase growth
factor receptors whose stimulation by ligand binding results in the
activation of the MAPK-, PI3K/AKT pathways, transcription factors
and signal transducers (1). EGFR
is expressed in 30–89% of pancreatic cancers and its aberrant
expression has been shown to correlate, in some cases, with worse
outcome and more aggressive disease. Interestingly, in xenograft
models of pancreatic cancer, the combination of gemcitabine and
EGFR-targeted therapy significantly inhibited the metastatic
process and resulted in improved overall survival (12,13).
A major partner of EGFR is HER-2 (ErbB2) whose activation is
dependent on dimerization with other members of the aforementioned
family of receptor tyrosine kinases. Aberrant expression of HER-2
in pancreatic cancer has been reported in a number of studies and
associated with resistance to various chemotherapeutic agents
(14,15). Recently, improved understanding of
the molecular mechanisms responsible for the acquired or inherent
resistance of pancreatic cancer cells towards EGFR- or HER-2
targeted therapy suggested that combination therapy based on agents
targeting both receptors and/or multiple pathways supporting
proliferation of cancer cells might represent a more efficacious
treatment approach towards this disease.
A preliminary screening with a panel of compounds
bearing a 2-triazenoazaindole scaffold (16), expected to inhibit EGFR kinase
activity and exert cytotoxic effects towards cells carrying
aberrant expression of EGFR, led to the identification of a novel
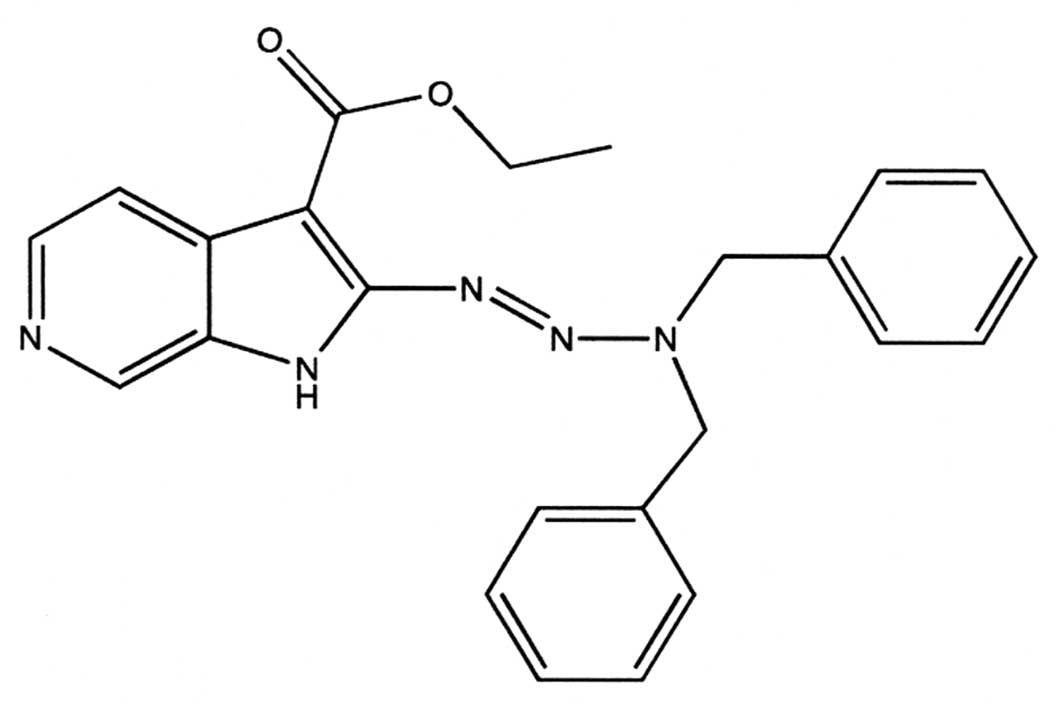
low molecular-weight agent, i.e., ethyl 2-(3,3-dibenzyl
1-triazenyl)-1H-pyrido(2,3-c)pyrrolo-3-carboxylate (AS104), showing
significant anti-proliferative effects (Fig. 1). In the present study, in view of
these preliminary findings we examined the biological and
biochemical effects of AS104 on pancreatic cancer cells notoriously
resistant to gemcitabine treatment (17,18)
and showing aberrant expression of EGFR and HER-2 tyrosine kinases,
respectively. We report for the first time evidence that
simultaneous targeting of these receptors may represent an
effective strategy for overcoming treatment resistance of
pancreatic cancer cells.
Materials and methods
Cell culture and treatments
The pancreatic ductal adenocarcinoma PANC-1, BxPC-3,
Capan-1 and MiaPaCa-2 cell lines and the human osteosarcoma U2OS
cell line were purchased from the American Type Culture Collection
(ATCC), cultured according to the growth conditions recommended by
the supplier and maintained at 37°C in a humidified atmosphere
containing 5% CO2. Cells were treated with DMSO (the
final concentration was 0.2% in all experiments), doxorubicin
(Sigma) or AS104 (16) as
described in the figures legend. Where indicated, cells were
incubated with 100 ng/ml human recombinant epidermal growth factor
(Sigma) for 10 min after 24 h starvation in serum-free medium or 50
μM MG132 (Calbiochem) for 6 h.
Determination of cell viability,
proliferation and clonogenic survival
The WST-1 viability assay (Roche) was performed in
96-well plates. Twenty-four hours after seeding, cells were treated
with DMSO or various concentrations of AS104 for 48 h. WST-1
reagent was added to the cells and cell viability was determined as
previously described (18). Cell
proliferation was determined by the Cell Proliferation Assay
(Calbiochem) following the manufacturer’s instructions and as
reported earlier (18). For the
clonogenic survival assay, cells were treated with various
concentrations of AS104 for 24 h. Afterwards, cells were
trypsinized and seeded in 6-well plates and colonies were allowed
to grow for 14 days. Subsequently, cells were washed in PBS and
stained with a solution containing 0.1% crystal violet in 20%
ethanol and 0.1 M sodium borate and destained with dd water.
Staining of the colonies was then quantified by subsequent
re-solubilization of crystal violet dye in the presence of methanol
and reading of the difference in absorbance at 540 nm
wavelength.
Flow cytometry
Cells treated according to the conditions indicated
in the figure legends were collected by trypsinization, washed with
PBS and fixed for 24 h with 70% ethanol at -20°C. For the
determination of cell death (i.e., the sub-G1 region which
comprises cells with reduced DNA in late apoptosis or necrosis),
cells were incubated with 20 μg/ml propidium iodide (Sigma) and 40
μg/ml RNAse A (Sigma) in PBS for 30 min at room temperature. To
detect the formation of acidic autophagic vacuoles, control or
treated cells were stained with 1 μg/ml acridine orange (Sigma) for
15 min at 37°C, removed from the plates by trypsinization and
immediately afterwards analyzed by flow cytometry. Cells were
analyzed on a FACSCalibur flow cytometer (Becton-Dickinson) and
data were processed using the CellQuest™ Pro software. For each
measurement, 10,000 events were analyzed.
Preparation of cell extract and Western
blot analysis
Cell lysates were prepared as previously described
(19). Proteins were detected by
probing Western blot membranes with the following antibodies: mouse
monoclonal anti-poly (ADPribose)polymerase (PARP), anti-mTOR,
anti-AKT (all from BD Biosciences); rabbit polyclonal
anti-p44/42MAPK, rabbit monoclonal anti-phospho-p44/42MAPK
(T202/Y204), rabbit polyclonal anti-phospho-AKT (T308), mouse
monoclonal anti-phospho-AKT (S473), rabbit polyclonal anti-LC3B,
rabbit polyclonal anti-HER-2, rabbit polyclonal anti-phospho-HER-2
(Y1221/1222), rabbit polyclonal anti-cytochrome c (all from Cell
Signaling Technology); mouse monoclonal anti-Myc, rabbit polyclonal
anti-EGFR, goat polyclonal anti-phospho-EGFR (Y1173), mouse
monoclonal anti-ATP5B (all from Santa Cruz Biotechnology); mouse
monoclonal anti-β-actin (Sigma). Protein-antibody complexes were
visualized by chemiluminescence using CDP-Star (Applied Biosystems)
as a substrate according to the manufacturer’s recommendations.
Isolation of mitochondria from whole cell
lysate
Cells were harvested by trypsinization, washed with
ice-cold PBS, counted and adjusted to 106 cells/sample.
Subsequently, they were re-suspended in lysis buffer (19). Isolation of mitochondria was
carried out by the Mitochondria Magnetic Isolation kit (Miltenyi
Biotech) following the manufacturer’s instructions.
Quantitative real-time PCR
Total RNA from cells treated as indicated in the
figure legends was isolated using the RiboPure™ kit (Ambion). Total
RNA (1 μg) was treated with DNAse I kit (Invitrogen) and used for
reverse transcription using the Cloned AMV FS Synthesis kit
(Invitrogen). cDNAs were then used as a template for the subsequent
PCR. PCR reactions were performed in a 20-μl total volume
consisting of 30 ng template, 1X SYBR® Green JumpStart™
Taq ReadyMix™ (Sigma), forward and reverse primers relative to the
cDNA of the analyzed proteins (200 nM forward and reverse primers
for HER-2 and β-actin; 300 nM forward and 150 nM reverse primers
for EGFR). All samples were prepared in triplicate. The reactions
consisted of a 10-min initial denaturation (95°C) followed by 40
cycles of denaturation (95°C, 15 sec) and annealing/extension
(60°C, 1 min for HER-2 and β-actin; 65°C, 1 min for EGFR).
Measurement of EGFR-, HER-2- and β-actin gene expression levels was
carried out according to the quantification method of the
StepOnePlus Real-Time PCR System (Applied Biosytems). All mRNA
expression values are ratios relative to β-actin. Fold changes in
samples derived from drug-treated cells were determined relative to
DMSO-treated samples. Primer pairs were as follows: for EGFR,
forward primer 5′-GGCACTTTTGAAGATCATTTTCTC-3′ and reverse primer
5′-CTGTGTTGAGGGCAATGAG-3′; for HER-2, forward primer
5′-CCAGGACCTGCTGAACTGGT-3′ and reverse primer
5′-TGTACGAGCCGCACATCC-3′; for β-actin, forward primer
5′-GACAGGATGCAGAAGGAGATTACT-3′ and reverse primer
5′-TGATCCACATCTGCTGGAAGGT-3′ (20,21).
Protein kinase profiling
The activity of protein kinases shown in Table I was tested against 10 μM AS104 by
Reaction Biology Corp. (RBC) using a radioactive-based assay as
described by the manufacturer. The ATP concentration employed by
RBC was 10 μM. Residual kinase activities are expressed as the
percentage of control activity measured in the absence of
compound.
 | Table ISpecificity of AS104 [ethyl
2-(3,3-dibenzyl1-triazenyl)-1H-pyrido(2,3-c)pyrrolo-3-carboxylate]. |
Table I
Specificity of AS104 [ethyl
2-(3,3-dibenzyl1-triazenyl)-1H-pyrido(2,3-c)pyrrolo-3-carboxylate].
| Kinase | Activity (%) |
|---|
| ABL1 | 92.0 |
| ABL2/ARG | 96.6 |
| AMPK
(A1/B1/G1) | 87.8 |
| AMPK
(A1/B1/G1) | 92.5 |
| ASK1/MAP3K5 | 106.6 |
| Aurora A | 100.1 |
| BRAF | 101.0 |
| c-Kit | 94.1 |
| CAMK1a | 72.6 |
| CAMKK1 | 155.2 |
| CAMKK2 | 101.7 |
| CDK1/cyclin A2 | 93.5 |
| CDK2/cyclin A | 96.2 |
| CHK1 | 104.4 |
| CHK2 | 89.1 |
| CK1e | 106.6 |
| CK2a | 104.3 |
| CK2a2 | 103.8 |
| COT1/MAP3K8 | 92.5 |
| DYRK1/DYRK1A | 100.0 |
| EGFR | 103.0 |
| EPHA1 | 94.5 |
| EPHB4 | 87.9 |
| ERBB4/HER4 | 97.5 |
| ERK1 | 117.9 |
| ERK2/MAPK1 | 115.4 |
| FGFR1 | 86.8 |
| FGFR2 | 97.7 |
| FGFR3 | 60.9 |
| FGFR4 | 97.9 |
| FLT1/VEGFR1 | 100.0 |
| FLT3 | 84.8 |
| FLT4/VEGFR3 | 87.9 |
| IGF1R | 91.0 |
| JAK3 | 85.6 |
| JNK2 | 115.0 |
| KDR/VEGFR2 | 78.9 |
| LCK | 88.1 |
| LINK1 | 101.1 |
| LKB1 | 178.6 |
| LYN | 94.7 |
| MEK1 | 101.7 |
| MKK6 | 94.7 |
| MLK1/MAP3K9 | 95.4 |
| MST1/STK4 | 90.8 |
| mTOR/FRAP1 | 97.0 |
| PIM1 | 86.8 |
| PIM2 | 94.4 |
| PIM3 | 76.3 |
| RAF-1 | 101.6 |
Statistical and densitometric
analysis
The statistical significance of differences between
the mean of two sets of data was evaluated by the two-tailed t-test
(Student’s t-test). The levels of significance are indicated in the
figures legend. Quantification of the intensity of protein bands on
Western blots was carried out with the ImageJ software (NIH).
Results
Analysis of AS104 effects on viability,
proliferation and survival of human pancreatic cancer cells
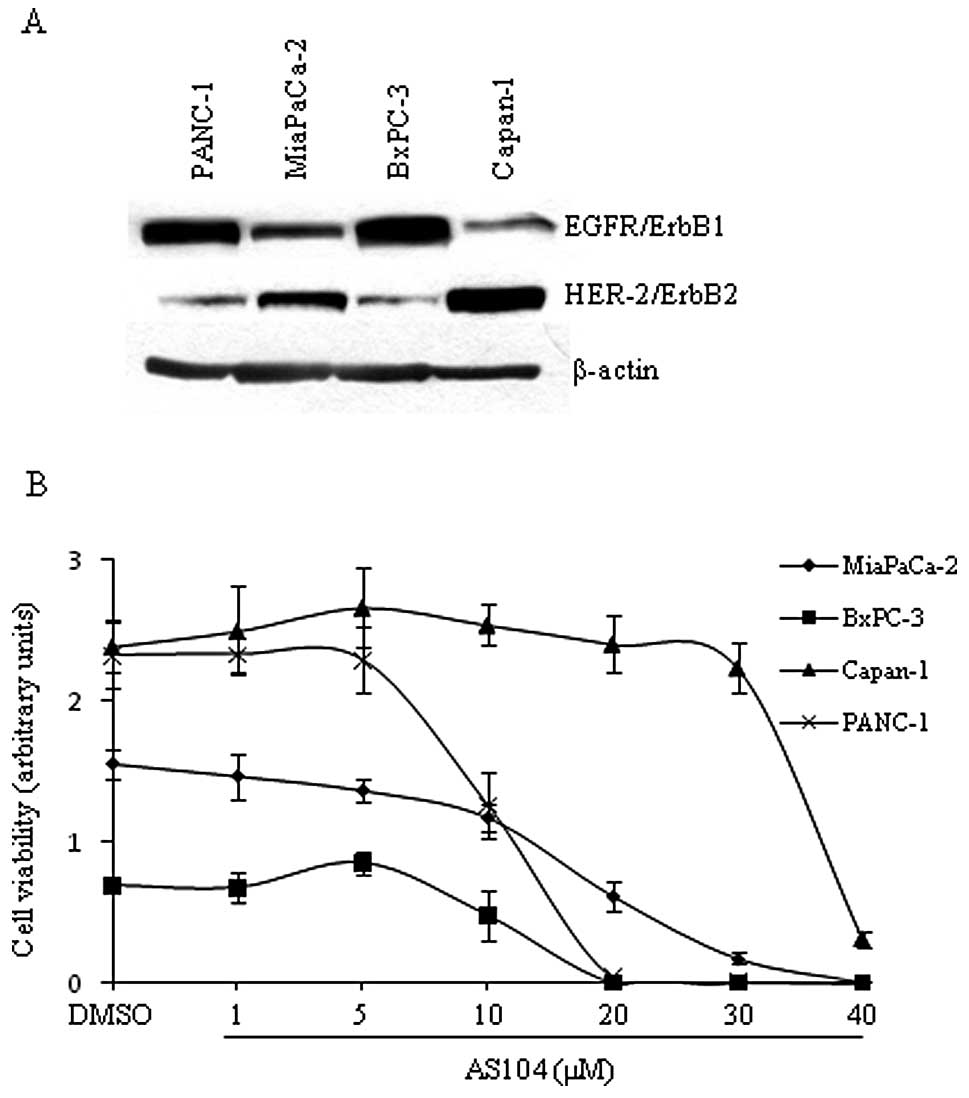
In our initial study, we examined the expression
levels of EGFR as well as HER-2 on whole cell lysates from four
human pancreatic cancer cell lines under basal conditions (Fig. 2A). We found that PANC-1 and BxPC-3
cell lines expressed significant higher levels of EGFR than
MiaPaCa-2 and Capan-1 cell lines which, in contrast, displayed the
highest expression levels of HER-2 in agreement with data reported
earlier (22,23). Next, the four human pancreatic cell
lines were employed for testing the cytotoxicity of AS104. The
amount of metabolically active cells treated in the absence and
presence of AS104, respectively, was determined as shown in
Fig. 2B. Significant cytotoxicity
was observed at concentrations ≥10 μM for three cell lines. Capan-1
cells showed a significant reduction in viability at 40 μM AS104.
Moreover, analysis of the dose-response did not reveal a
correlation between degree of cytotoxicity and level of expression
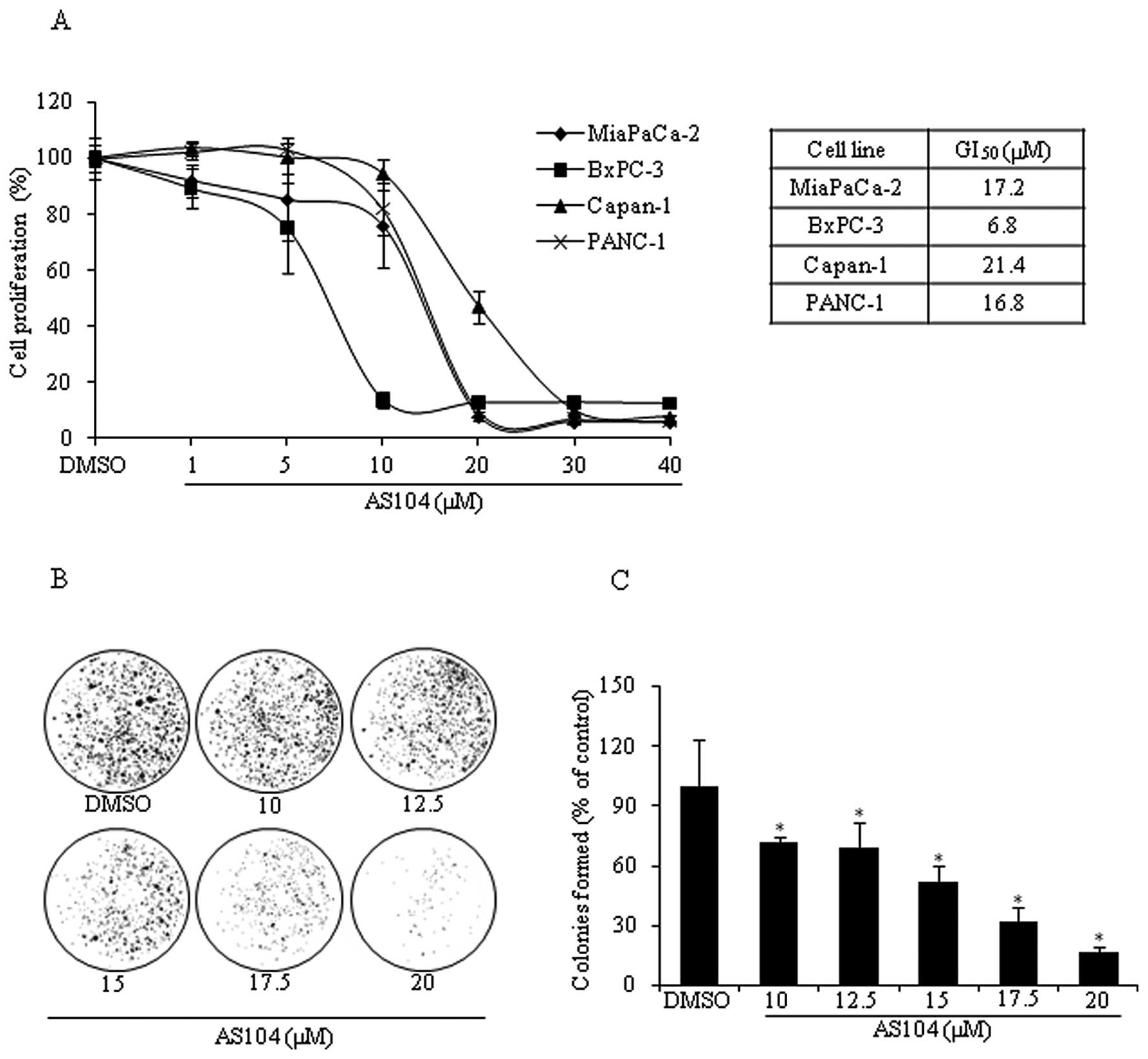
of either EGFR or HER-2. The anti-proliferative effects of AS104
were investigated by measuring BrdU incorporation into newly
synthesized DNA of actively replicating cells (Fig. 3A). AS104 inhibited the
proliferation of all four cell lines in a dose-dependent manner
irrespective of the levels of EGFR and HER-2. Growth inhibition
(50%) (i.e., GI50) was achieved at concentrations
ranging from 6.8 μM as in the case of BxPC-3 cells to 21.4 μM as
for Capan-1 cells 48 h after treatment. As the analysis of
viability and proliferation showed that all four cell lines were
sensitive to the effects of AS104, subsequent experiments were
carried out with one representative cell line: i.e., PANC-1. The
survival ability of PANC-1 cells was assessed by performing a
clonogenic survival assay (Fig. 3B and
C). Cells incubated for 24 h with increasing concentrations of
AS104 agent were allowed to form colonies for up to 14 days, which
were revealed, afterwards, by crystal violet staining. Results
indicated a survival rate of about 50% for cells incubated with 15
μM AS104 and the percentage of formed colonies rapidly decreased to
~16% when cells were treated with 20 μM AS104.
Treatment of cells with AS104 leads to
simultaneous induction of apoptosis and autophagy
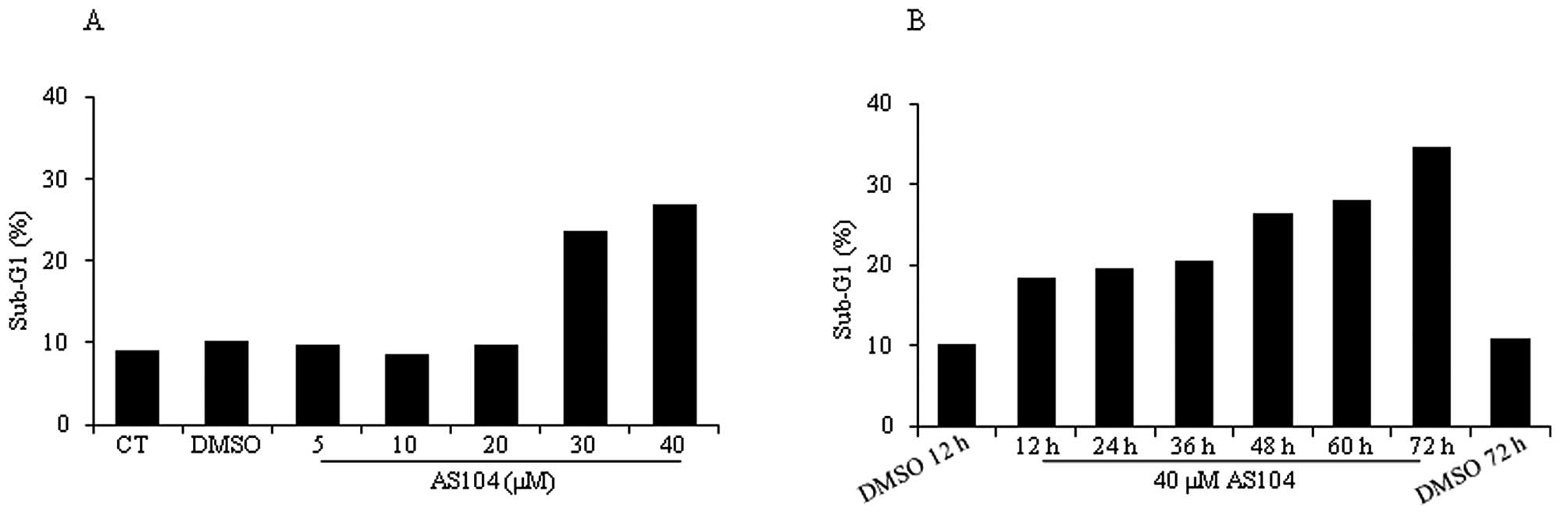
Next, flow cytometry analysis was conducted to
measure induction of cell death in response to treatment with
either increasing concentrations of AS104 for 48 h (Fig. 4A) or 40 μM AS104 and increasing
incubation times (Fig. 4B).
Incubation of cells with AS104 at concentrations ≥30 μM led to a
significant accumulation of cells in sub-G1 (i.e., >20%)
indicative of late-stage apoptosis or necrosis. Similarly, a time
course experiment revealed significant cell death after 12 h of
incubation with the drug (i.e., ~20%) and the percentage of dead
cells increased to up to ~35% after 72 h. Treatment of cells with
DMSO by itself led to a percentage of cell death comparable to the
one deriving from untreated control cells (CT). To study whether
cell treatment with AS104 would lead to an apoptotic type of cell
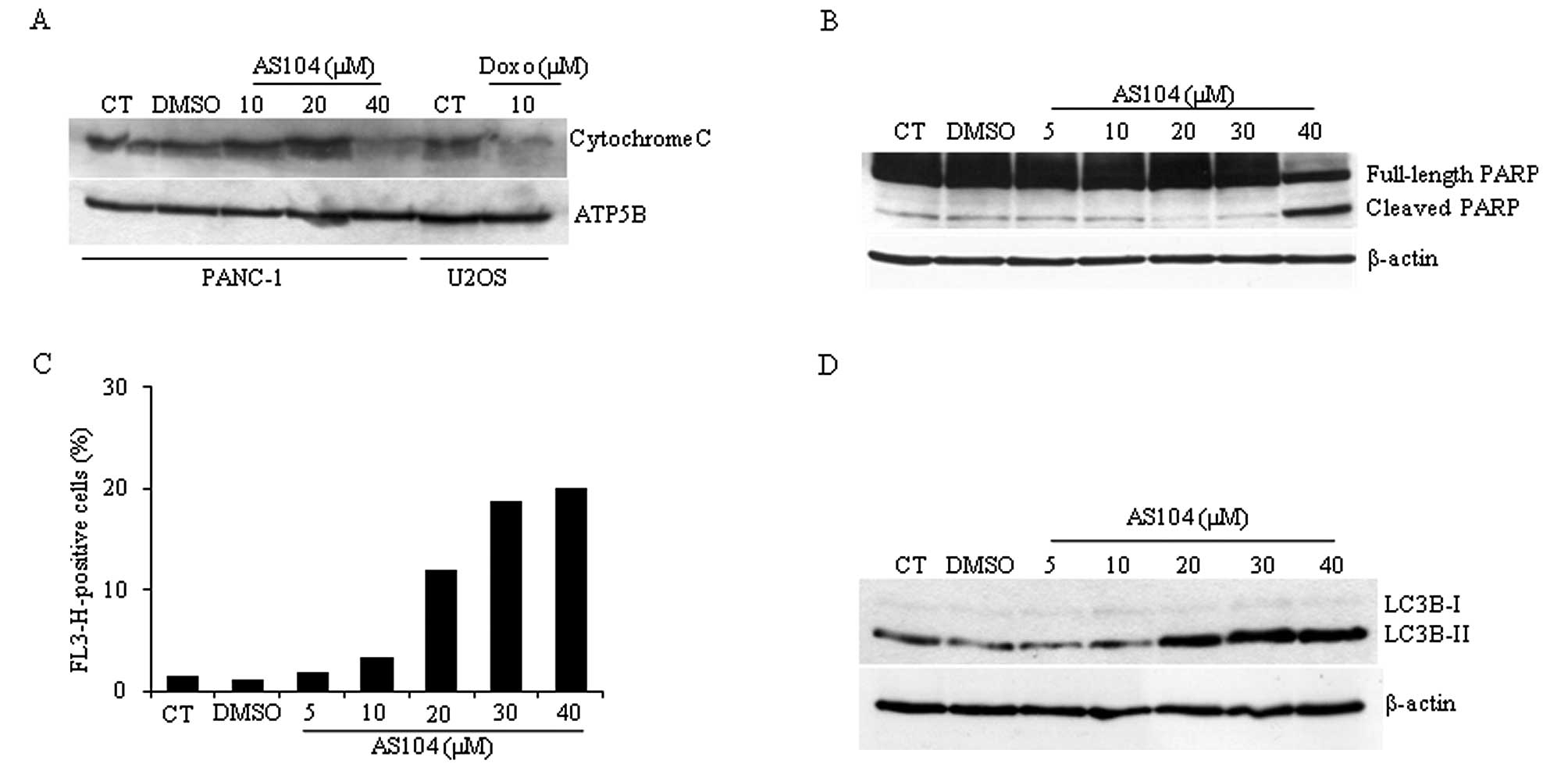
death, cells were analyzed for the release of mitochondrial
cytochrome c (Fig. 5A). A control
experiment was carried out with the human osteosarcoma U2OS cell
line incubated with 10 μM doxorubicin (Doxo) for 24 h as this
compound has been shown to induce caspase-mediated apoptosis in
this cell line (24,25). Detection of cytochrome c content
from isolated mitochondria revealed that treatment with up to 40 μM
AS104 for 48 h resulted in its decreased detection suggesting
release of cytochrome c into the cytosol and hence caspase-mediated
activation of apoptosis. Similar experiments were carried out
investigating the caspase-3/caspase-7-mediated cleavage of PARP
protein. The highest concentration of AS104 (40 μM) resulted in
PARP cleavage in PANC-1 cells (Fig.
5B) supporting the notion that AS014 stimulates cell death
through caspase activation, an early event during induction of
apoptosis. Experiments conducted with the irreversible pan-caspase
inhibitor z-VAD-fmk partially failed to counteract AS104-mediated
cell death suggesting potential involvement of different types of
cell death (data not shown). Therefore, we investigated whether
treatment of cells with AS104 would stimulate autophagy. To detect
and quantify the formation of autophagic vacuoles, cells were
stained with acridine orange and subjected to flow cytometry
analysis (Fig. 5C). AS104
increased red fluorescence up to 40 μM concentration with respect
to control experiments, where an increase of up to ~20% positive
signal was detected. Data obtained by flow cytometry were
supplemented with results obtained by Western blot analysis of
whole cell lysates (Fig. 5D).
Conversion of LC3B-I into LC3B-II confirmed the induction of this
catabolic process.
Cell treatment with AS104 results in
transcriptional down-regulation of the EGFR and HER-2 genes
Next, it was of interest to examine whether the
effects described above were associated with inhibition of EGFR
and/or HER-2 activation as a number of studies suggest that
aberrant expression of EGFR and HER-2 might be associated with
multiple drug resistance in human pancreatic cancer cells (5,6).
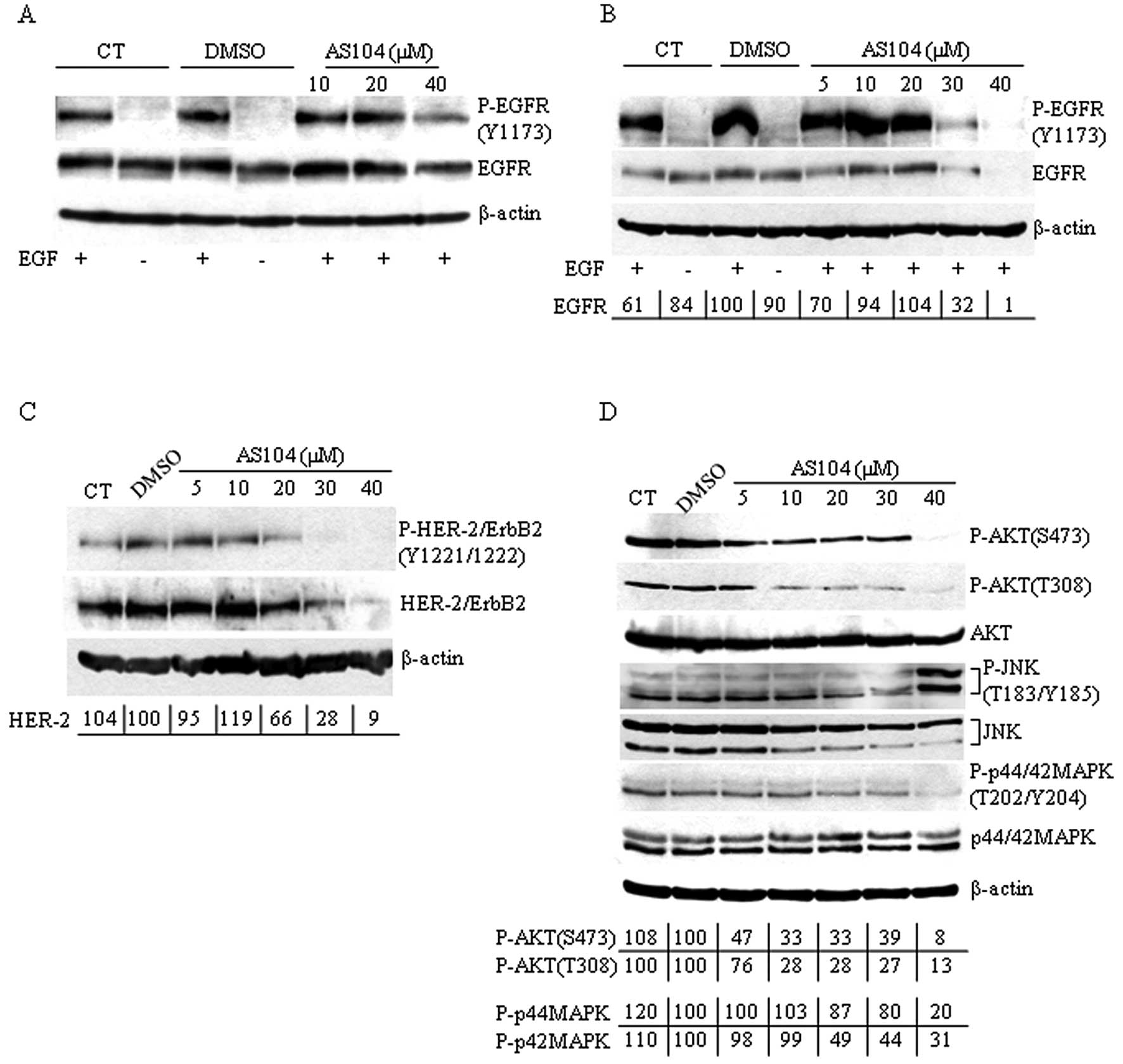
PANC-1 cells were cultured in serum-free medium for 24 h and then
incubated with various concentrations of AS104 for 3 h before
adding epidermal growth factor (100 ng/ml) for 10 min prior
harvesting (Fig. 6A). Extracts
were then analyzed by Western blotting using antibodies directed
against phosphorylated EGFR. We found that cell treatment with
AS104 did not affect the kinase activity of EGFR induced by
incubation with EGF confirming observations reported earlier
(16). The specificity of AS104
was also tested in vitro on a panel of 50 recombinant
protein kinases including EGFR. As shown in Table I, AS104 did not exert any
significant inhibitory effect on either EGFR or any other tested
protein kinase except for CAMK1, FGFR3, VEGFR2 and PIM3 where the
activity slightly decreased by 27.4, 39.1, 21.1 and 23.7%,
respectively, with respect to control assays. However, experiments
with cells incubated for 48 h with increasing concentrations of
AS104 and stimulated with EGF before harvesting, showed a marked
decrease in EGFR phosphorylation accompanied by a concomitant
significant decrease in the total cellular levels of EGFR protein
in cells incubated with 30 and 40 μM AS104, respectively (Fig. 6B). We extended the analysis to the
expression and phosphorylation levels of HER-2 (Fig. 6C). As in the case of EGFR,
incubation of cells with up to 40 μM AS104 markedly decreased the
phosphorylation and protein expression levels of HER-2 with respect
to DMSO-treated cells.
The mitogen-activated protein kinases (MAPKs) ERK1
and ERK2 belong to a protein kinase cascade downstream of the EGF
receptor family-signaling and play a critical role in the
regulation of cell proliferation, growth and survival (26–28).
Another pathway associated with activation of members of the
EGF-receptor family is the PI3K/AKT signaling cascade through which
EGFR and HER-2 proteins provide survival signals to cells (28–30).
The effects of EGFR- and HER-2 alteration were examined on basal
MAPK and AKT activation in PANC-1 cells (Fig. 6D). Cells incubated with increasing
amounts of compound showed reduced basal levels of phosphorylated
MAPK to ~20% (p44MAPK) and ~31% (p42MAPK), respectively, at 40 μM
AS104. MAPK protein levels did not vary with respect to untreated
or DMSO-treated cells. The activity of AKT was analyzed under the
same experimental conditions using phospho-specific AKT antibodies
directed against phosphorylated Ser473 and Thr308 amino acids,
respectively. Here, AS104 treatment led to reduction in the levels
of phosphorylated AKT starting at 5 μM AS104. Interestingly, when
we analyzed the phosphorylation status of Jun-amino-terminal kinase
(JNK) whose role in cell death is well established (reviewed in
ref. 31), we observed JNK
activation/phosphorylation in cells incubated with 40 μM AS104 for
48 h suggesting that the JNK pathway might contribute to cell
killing in pancreatic cancer cells treated with AS104. Next, to
evaluate whether down-regulation of EGFR/HER-2 protein expression
levels resulted from impaired transcriptional activity and/or
accelerated degradation of the EGF receptor family members, EGFR-
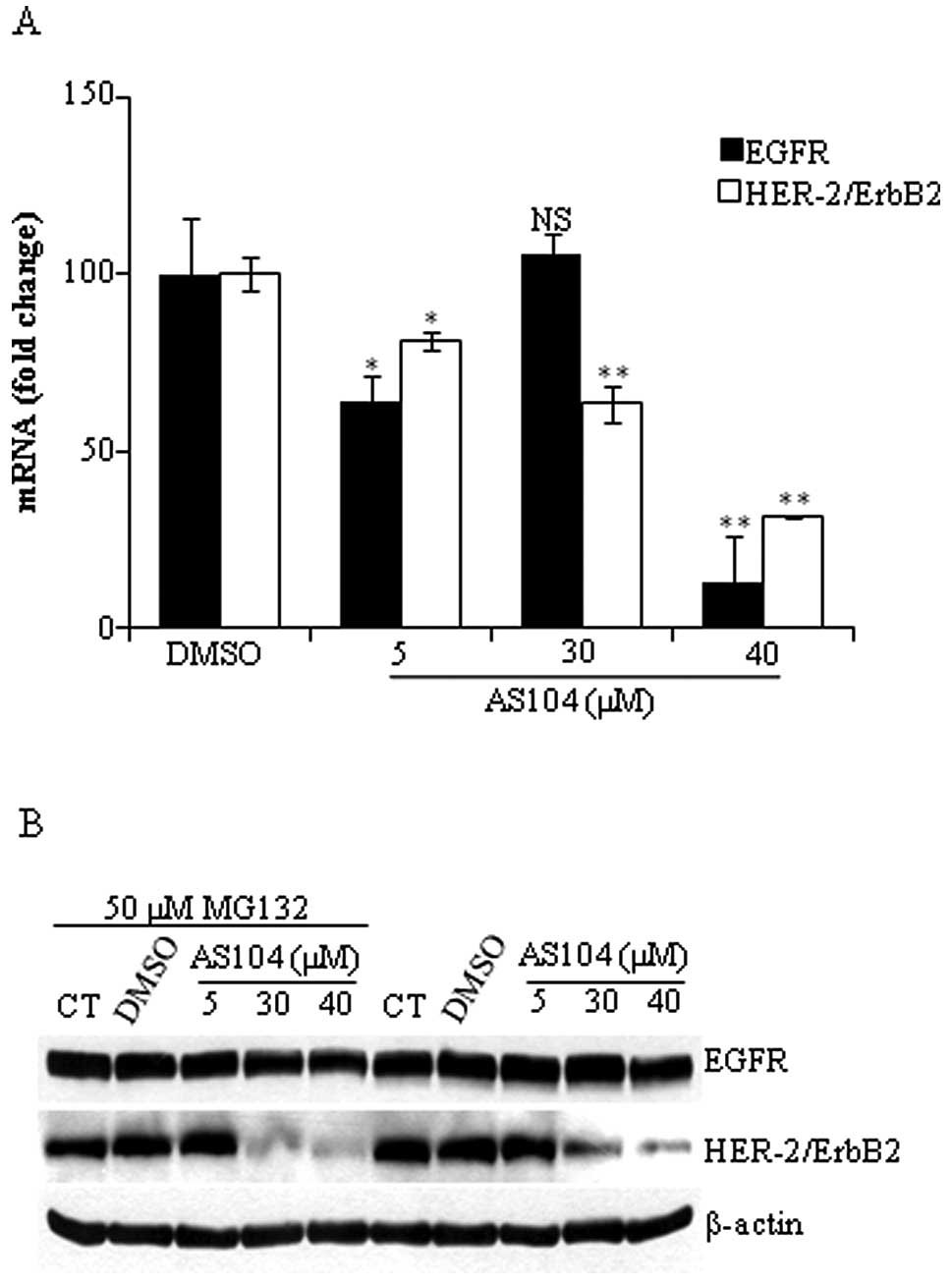
and HER-2-mRNA isolated from cells treated with increasing
concentrations of AS104 for 48 h was subjected to quantitative
RT-PCR analysis as described in Materials and methods. As shown in
Fig. 7A, treatment of cells with
AS104 markedly decreased EGFR and HER-2-mRNA levels in a
dose-dependent manner. Cell incubation with 40 μM AS104 led to an
87% reduction in EGFR-mRNA level while in the case of HER-2 there
was a 69% decrease in the mRNA level with respect to control
experiment. In order to determine whether changes in the expression
levels of both receptors were due to accelerated protein
degradation, cells were incubated for 24 h with increasing
concentrations of AS104 with or without a specific proteasome
inhibitor (i.e., MG132) added to the incubation medium 6 h before
harvesting the cells. As shown in Fig.
7B, the presence of MG132 did not result in the accumulation of
EGFR and HER-2 suggesting that lower expression of EGFR/HER-2 was
not due to accelerated protein degradation.
Discussion
To date, pancreatic cancer is a malignancy with poor
prognosis refractory to conventional therapy. Resistance towards
chemotherapeutic treatment is due to alteration of multiple
signaling pathways which are mitogenic, anti-apoptotic,
pro-angiogenic and -invasion. Members of the ErbB family of
transmembrane tyrosine kinase growth factor receptors, which
includes EGFR and HER-2, are frequently overexpressed or
constitutively activated in pancreatic adenocarcinoma and their
expression has been shown to correlate with worse outcome and
multiple drug resistance (32,33).
In recent years, a number of strategies targeting these receptors
have been developed with variable success (1,5).
Multiple mechanisms that can account for the acquired or inherent
resistance of pancreatic cancer have been reported supporting the
notion that for a clinically relevant effect to be achieved,
treatment strategies should consider targeting of multiple
signaling pathways or multiple levels of a major pathway that
sustain pancreatic cancer progression.
In the present study, we tested the cytotoxicity and
growth inhibitory effects of a newly synthesized
2-triazenoazaindole compound, AS104, on a panel of four human
pancreatic cancer cell lines. Incubation of cells with various
concentrations of AS104 led to the finding that AS104 is able to
induce significant cell death in a dose-dependent manner. We report
evidence that AS104 leads to induction of both apoptosis and
autophagy indicated by the fact that release of mitochondrial
cytochrome c, cleavage of full length PARP and accumulation of
LC3B-II proteins were observed under the applied experimental
conditions. Autophagy and apoptosis may act independently, in
parallel or influence one another (34). At present, the interplay between
apoptosis and autophagy is elusive. Induction of autophagy may be
necessary for apoptotic cell death. Alternatively, it may represent
a protective mechanism activated in response to AS104 treatment.
Although AS104 represents a novel triazene class of compounds with
an azaindole moiety that was designed to inhibit EGFR tyrosine
kinase activity, this compound failed to meet this initial
expectation (16). Analysis of
lysates from cells overexpressing EGFR with short-term treatment of
AS104 revealed no changes in the phosphorylation levels of
endogenous EGFR and hence its tyrosine kinase activity. Similar
results were also obtained by measuring the activity of recombinant
EGFR in vitro. However, unexpectedly, cell treatment with
AS104 resulted in significant down-regulation of EGFR- and
HER-2-mRNA levels (Fig. 7A).
Transcriptional inhibition of EGFR and HER-2 receptor was
accompanied by down-regulation of their protein expression levels,
respectively, and decreased levels of activated MAPK and AKT
indicated by reduction in their phosphorylation following treatment
with AS104 (Fig. 6).
Interestingly, phosphorylation of JNK was found up-regulated. JNK
isoforms were initially described biochemically to be
stress-induced protein kinases. Hence, increased phosphorylation of
JNK observed following AS104 treatment might represent a stress
response necessary for initiating the cell death signaling
(31). Treatment of pancreatic
cancer cells with AS104 leads to significant transcriptional
down-regulation of EGFR and HER-2 genes. Although
treatment of cells with 30 μM AS104 results in enhanced EGFR-mRNA
levels, an effect that remains to be addressed in future studies,
it is likely that down-regulation of EGFR- and HER-2-mRNA levels
might be accompanied by decreased expression of transcription
factors controlling the synthesis of these receptors. This has been
shown previously in bladder cancer cells where incubation with
betulinic acid and curcumin down-regulated specificity protein (Sp)
transcription factors and this was accompanied by decreased
expression of EGFR mRNA and protein levels (35). Additionally, one cannot exclude the
regulatory contribution of nuclear factor-κB (NF-κB). Activated
NF-κB translocates to the nucleus resulting in the transcription of
several genes, among which are cyclooxygenase COX-2 and EGFR
(36,37). Inhibition of NF-κB activation by
erlotinib and celecoxib combination treatment has been reported to
result in significant apoptotic cell death and down-regulation of
the transcription of EGFR and COX-2 enzyme (22).
Additional studies are required to determine the
precise molecular mechanisms responsible for the anti-proliferative
effects observed following cell treatment with AS104 as well as the
mode by which AS104 induces decreased EGFR and HER-2 expression by
blocking transcription of the corresponding genes. However, the
observed biochemical selectivity of AS104 for both receptors
together with its potent anti-proliferative effects makes this
class of compounds attractive novel chemotherapeutic agents for the
treatment of patients with pancreatic cancer.
Acknowledgements
This study was supported by grants from the Danish
Cancer Society (DP08152) and the Danish Natural Science Research
Council (272-07-0258) to B.G.
References
|
1
|
Wong HH and Lemoine NR: Pancreatic cancer:
molecular pathogenesis and new therapeutic targets. Nature.
6:412–422. 2009.PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. Cancer J Clin. 60:277–300. 2010.
|
|
3
|
Ahrendt SA and Pitt HA: Surgical
management of pancreatic cancer. Oncology. 16:725–734.
2002.PubMed/NCBI
|
|
4
|
Burris HA III: Recent updates on the role
of chemotherapy in pancreatic cancer. Semin Oncol. 32:S1–S3. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mackenzie RP and McCollum AD: Novel agents
for the treatment of adenocarcinoma of the pancreas. Expert Rev
Anticancer Ther. 9:1473–1485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bardeesy N and DePinho RA: Pancreatic
cancer biology and genetics. Nature. 2:897–909. 2002.
|
|
7
|
Buchholz M and Gress TM: Molecular changes
in pancreatic cancer. Expert Rev Anticancer Ther. 9:1487–1497.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korc M, Chandrasekar B, Yamanaka Y, Friess
H, Buchier M and Beger HG: Overexpression of the epidermal growth
factor receptor in human pancreatic cancer is associated with
concomitant increases in the levels of epidermal growth factor and
transforming growth factor alpha. J Clin Invest. 90:1352–1360.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe M, Nobuta A, Tanaka J and Asaka
M: An effect of K-ras gene mutation on epidermal growth factor
receptor signal transduction in PANC-1 pancreatic carcinoma cells.
Int J Cancer. 67:264–268. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobrin MS, Funatomi H, Friess H, Buchler
MW, Stathis P and Korc M: Induction and expression of
heparin-binding EGF-like growth factor in human pancreatic cancer.
Biochem Biophys Res Commun. 202:1705–1709. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamanaka Y, Friess H, Kobrin MS, Buchler
M, Beger HG and Korc M: Coexpression of epidermal growth factor
receptor and ligands in human pancreatic cancer is associated with
enhanced tumor aggressiveness. Anticancer Res. 13:565–569.
1993.PubMed/NCBI
|
|
12
|
Jimeno A, Tan AC, Coffa J, Rajeshkumar NV,
Kulesza P, Rubio-Viqueira B, Wheelhouse J, Diosdado B, Messersmith
WA, Iacobuzio-Donahue C, Maitra A, Varella-Garcia M, Hirsch FR,
Meijer GA and Hidalgo M: Coordinated epidermal growth factor
receptor pathway gene overexpression predicts epidermal growth
factor receptor inhibitor sensitivity in pancreatic cancer. Cancer
Res. 68:2841–2849. 2008. View Article : Google Scholar
|
|
13
|
Kim IY, Kang YS, Lee DS, Park HJ, Choi EK,
Oh YK, Son HJ and Kim JS: Antitumor activity of EGFR targeted
pH-sensitive immunoliposomes encapsulating gemcitabine in A549
xenograft nude mice. J Control Release. 140:55–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
te Velde EA, Franke AC, van Hillegersberg
R, Elshof SM, de Weger RW, Borel Rinkes IH and van Diest PJ:
HER-familiy gene amplification and expression in resected
pancreatic cancer. Eur J Surg Oncol. 35:1098–1104. 2009.PubMed/NCBI
|
|
15
|
Yu D and Hung MC: Overexpression of ErbB2
in cancer and ErbB2-targeting strategies. Oncogene. 19:6115–6121.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diana P, Stagno A, Barraja P, Carbone A,
Parrino B, Dall’Acqua F, Vedaldi F, Salvador A, Brun P,
Castagliuolo I, Issinger OG and Cirrincione G: Synthesis of
trazenoazaindoles: a new class of triazenes with antitumor
activity. Chem Med Chem. 6:1291–1299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pham NA, Tsao MS, Cao P and Hedley DW:
Dissociation of gemcitabine sensitivity and protein kinase B
signaling in pancreatic ductal adenocarcinoma models. Pancreas.
35:16–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kreutzer JN, Ruzzene M and Guerra B:
Enhancing chemosensitivity to gemcitabine via RNA interference
targeting the catalytic subunits of protein kinase CK2 in human
pancreatic cancer cells. BMC Cancer. 10:4402010. View Article : Google Scholar
|
|
19
|
Yde CW, Olsen BB, Meek D, Watanabe N and
Guerra B: The regulatory beta subunit of protein kinase CK2
regulates cell-cycle progression at the onset of mitosis. Oncogene.
27:4986–4997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen G, Kronenberger P, Teugels E and De
Grève J: Influence of RT-qPCR primer postion on EGFR interference
efficacy in lung cancer cells. Biol Proced Online.
11:132010.PubMed/NCBI
|
|
21
|
Walch A, Specht K, Bink K, Zitzelsberger
H, Braselmann H, Bauer M, Aubele M, Stein H, Siewert JR, Höfler H
and Werner M: Her-2/neu gene amplification, elevated mRNA
expression, and protein overexpression in the
metaplasia-dysplasia-adenocarcinoma sequence of Barrett’s
esophagus. Lab Invest. 81:791–801. 2001.PubMed/NCBI
|
|
22
|
Ali S, El-Rayes BF, Sarkar FH and Philip
PA: Simultaneous targeting of the epidermal growth factor receptor
and cyclooxygenase-2 pathways for pancreatic cancer therapy. Mol
Cancer Ther. 4:1943–1951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schlieman MG, Fahy BN, Ramsamooj R,
Beckett L and Bold RJ: Incidence, mechanism and prognostic value of
activated AKT in pancreas cancer. Br J Cancer. 89:2110–2115. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sadasivam S, Gupta S, Radha V, Batta K,
Kundu TK and Swarup G: Caspase-1 activator Ipaf is a p53-inducible
gene involved in apoptosis. Oncogene. 24:627–636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orosco A, Fromigué O, Bazille C,
Entz-Werle N, Levillain P, Marie PJ and Modrowski D: Syndecan-2
affects the basal and chemotherapy-induced apoptosis in
osteosarcoma. Cancer Res. 67:3708–3715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rennefhart U, Janakiraman M, Ollinger R
and Troppmair J: Stress kinase signaling in cancer: fact or
fiction? Cancer Lett. 217:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marshall J: Clinical implications of the
mechanisms of epidermal growth factor receptor inhibitors. Cancer.
107:1207–1218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonder VM, Sweeney-Gotsch B, Andreeff M,
Mills GB and McConkey DJ: Inhibition of the phosphatidyl inositol
3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma
cells in vitro and in vivo. Mol Cancer Ther. 1:989–997. 2002.
|
|
30
|
Altomare DA, Tanno S, De Rienzo A,
Klein-Szanto AJ, Tanno S, Skele KL, Hoffman JP and Testa JR:
Frequent activation of AKT2 kinase in human pancreatic carcinomas.
J Cell Biochem. 87:470–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dent P, Yacoub A, Fisher PB, Hagan MP and
Grant S: MAPK pathways in radiation responses. Oncogene.
22:5885–5896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lurje G and Lenz H: EGFR signaling and
drug discovery. Oncology. 77:400–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujita H, Ohuchida K, Mizumoto K, Itaba S,
Ito T, Nakata K, Yu J, Kayashima T, Hayashi A, Souzaki R, Tajiri T,
Onimaru M, Manabe T, Ohtsuka T and Tanaka M: High EGFR mRNA
expression is a prognostic factor for reduced survival in
pancreatic cancer after gemcitabine-based adjuvant chemotherapy.
Int J Oncol. 38:629–641. 2011.PubMed/NCBI
|
|
34
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: apoptosis, autophagy and the
cross-talk between them. Cell Death Diff. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chadalapaka G, Jutooru I, Burghardt R and
Safe F: Drugs that target specificity proteins downregulate
epidermal growth factor receptor in bladder cancer cells. Mol
Cancer Res. 8:739–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishi H, Neta G, Nishi KH, Akers LM,
Rikiyama T, Proctor KN, Murphy BA and Johnson AC: Analysis of the
epidermal growth factor receptor promoter: the effect of nuclear
factor-κB. Int J Mol Med. 11:49–55. 2003.
|
|
37
|
Schmedtje JF, Ji YS, Liu WL, DuBois RN and
Runge MS: Hypoxia induces cyclooxigenase-2 via the NF-κB p65
transcription factor in human vascular endothelial cells. J Biol
Chem. 272:601–608. 1997.PubMed/NCBI
|