Introduction
Esophageal cancer is a malignant disease with a
5-year survival after esophagectomy of around 50% according to the
report of the Japan Clinical Oncology Group (1). Although advances in diagnosis and
treatment of esophageal squamous cell carcinoma (ESCC) have been
made in recent years, postoperative survival rates have not
improved in the last decade (2–4).
Therefore, new clinical parameters for prognosis and new approaches
for adjuvant treatment are needed. Cancer-specific immunotherapy is
considered to be a new therapeutic modality. We have been
investigating tumor microenvironments that affect patient survival.
In previous studies, we found that cooperation between
CD4+ and CD8+ T cells appears to improve the
prognosis of ESCC patients (5).
Thus, the host immune response against cancer cells and immune
escape mechanisms by the tumor (6)
seem to play crucial roles in the prevention of disease recurrence
and determine the postoperative prognosis in ESCC.
Human leukocyte antigen (HLA) class I molecules are
critical for the presentation of antigen peptides derived from
tumor cells to cytotoxic T lymphocytes (CTLs). Antigen processing
machinery (APM) is the combination of cellular processes
responsible for the presentation of endogenous peptides by HLA
class I molecules. APM is essential for the successful presentation
of HLA class I antigens. Loss of surface-expressed HLA class I
molecules is particularly important for cancer cell proliferation
and metastasis, because this enables tumor cells to evade
recognition and lysis by CTLs (7–10).
Therefore, down-regulation of APM components may lead to defects in
the expression of HLA class I-peptide complexes and eventually
enable tumor cells to escape from the host immunosurveillance
mediated by CTLs (11–13).
The association between down-regulation of several
APM components and cancer prognosis has been reported in a wide
range of malignancies, including lung, breast, uterine cervix, head
and neck area, larynx, ovary, kidney, skin, and ESCC (14–28).
To the best of our knowledge, there have been few reports that have
comprehensively analyzed the correlations between HLA class I
pathway expression and patient prognosis in ESCC. Here, the
expressions of HLA class I heavy chain (HLA-HC), β2 microglobulin,
and 11 APM components are reported in various esophageal cell
lines. There was a correlation between several APM components and
esophageal cancer prognosis by tissue microarray method.
Materials and methods
Cell lines
The ESCC cell lines TE2, TE4, TE5, TE6, TE8, TE9,
TE10, TE13, TE14, HEC46, and SGF7, and the lung adenocarcinoma cell
line LCD were used. Human squamous cell carcinoma of esophagus cell
line TE series was generously provided by Dr T. Nishihira
(University of Tohoku, Japan). HEC46 was provided by Dr T. Toge
(University of Hiroshima, Japan), and SGF7 was provided by Dr T.
Saito (Toyama Medical and Pharmaceutical University, Japan). LCD
was obtained from the Japanese Cancer Research Resources Bank
(Tokyo, Japan). All cell lines were grown in RPMI-1640
(Sigma-Aldrich Japan, Tokyo, Japan) with 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin, and they were maintained in a
humidified incubator with 5% CO2 in air at 37˚C.
Mice and xenograft models
CB17/severe combined immunodeficiency (SCID) mice
were obtained from Charles River Japan (Yokohama, Japan). All mice
were female, aged 6 to 8 weeks, and maintained under specific
pathogen-free conditions. All animal procedures were conducted in
accordance with the guidelines of the Hokkaido University
Institutional Animal Care and Use Committee. Cultured esophageal
and lung cancer cells (over 5×106) were injected
subcutaneously with a volume of 100 μl of phosphate-buffered saline
(PBS) into the right flank region of each CB17/SCID mouse. When
tumor size exceeded 15 mm in diameter, the mice were euthanized,
and the resected tumors were separated into two blocks: one block
was frozen in liquid nitrogen to extract proteins for Western blot
analysis, and the other was immersed in formalin for
immunohistologic analysis.
Antibodies
The mouse monoclonal antibody (mAb) EMR8-5, which
recognizes the heavy chains of HLA-A, HLA-B, and HLA-C, was
purchased from Hokudo Co., Ltd. (Sapporo, Japan). The β2
microglobulin-specific mAb NAMB-1, tapasin-specific mAb TO-3,
calnexin-specific mAb TO-5, calreticulin-specific mAb TO-11,
ERp57-specific mAb TO-2, TAP-1-specific mAb NOB-1, TAP-2-specific
mAb NOB-2, LMP-7-specific mAb HB-2, LMP-10-specific mAb TO-7,
MB-1-specific mAb SY-5, Delta-specific mAb SJJ-3, and Z-specific
mAb NB-1 were established and characterized at the Department of
Immunology, Rosewell Park Cancer Institute (Buffalo, NY) (29,30).
Anti-β actin mouse monoclonal antibody (MAB1501R) was purchased
from Millipore (Tokyo, Japan). Negative control mouse IgG2b (X0943)
and IgG1 (X0931) were purchased from Dako Japan (Kyoto, Japan).
Peroxidase-conjugated AffiniPure goat anti-mouse IgG
(H+L) was purchased from Jackson ImmunoResearch (West Grove,
PA).
Western blot analysis
Western blotting was performed following the
methodology previously described with minor modifications (30). In brief, lysates from cell lines,
CB17/SCID mouse xenografts, and normal human esophageal/lung tissue
were mixed with SDS-PAGE sample buffer, boiled for 3 min, and then
separated on 10–15% polyacrylamide gels. Separated proteins (20
μg/lane) were transferred to nitrocellulose membranes (Amersham,
Tokyo, Japan) or PVDF membranes (Amersham). After blocking with 1 h
incubation in PBS containing 2% BSA and 5% non-fat dry milk, the
membrane was incubated overnight at 4˚C with antibodies (1:200 in
1% dry milk/TBS-T). The membrane was washed three times for 5 min
each in PBS containing 0.1% Tween 20 and incubated for 1 h at room
temperature with peroxidase-conjugated goat anti-mouse IgG, Fc
fragment antibody in PBS containing 1% non-fat dry milk/TBS-T.
Three additional washings for 5 min each in PBS containing 0.1%
Tween 20 followed. The detection binding antibodies were performed
using the ECL Plus system (Amersham). Lysates from normal human
esophageal mucosa and normal human lung tissue were used as
positive controls.
Immunohistochemistry
Immunohistochemical reactions were carried out using
the universal immunoenzyme polymer method (Nichirei Corp, Tokyo,
Japan). The mouse monoclonal primary antibodies used were EMR8-5,
NAMB-1, TO-3, NOB-1, NOB-2, HB-2, and TO-7. Briefly,
paraffin-embedded tissue sections were deparaffinized with xylene
and rehydrated in a graded series of ethanol solutions. Antigens
were retrieved by pressure cooker in citrate buffer (pH 6.0 or pH
7.0) before staining with mAb. Tissue sections were incubated with
0.3% H2O2 in methanol to block endogenous
peroxidase activity for 10 min. They were saturated with 10% normal
goat serum (Histofine SAB-PO kit; Nichirei Corp) for 30 min at room
temperature and then overnight at 4˚C with the primary antibodies
in the following dilutions: anti-HLA-HC (clone EMR8-5) 1:1000;
anti-β2 microglobulin (clone NAMB-1) 1:100; anti-tapasin (clone
TO-3) 1:100; anti-TAP-1 (clone NOB-1) 1:50; anti-TAP-2 (clone
NOB-2) 1:400; anti-LMP-7 (clone HB-2) 1:100; and anti-LMP-10 (clone
TO-7) 1:200. A biotinylated goat anti-mouse immunoglobulin antibody
(Histofine SAB-PO kit; Nichirei Corp) was applied for 30 min at
room temperature. Staining was visualized using peroxidase
substrate kit 3,3′-diaminobenzidine (Histofine SAB-PO kit; Nichirei
Corp). To improve the sharpness of the staining with mAb NOB-1,
Target Retrieval Solution, high pH was used (Dako, Kyoto, Japan).
Nuclei were lightly counterstained with hematoxylin. TE8 xenografts
were used as positive tissue controls. Normal mucosal tissues were
used in each specimen as internal controls. LCD xenografts were
used as negative tissue controls. Mouse IgG1 was used in place of
the primary antibody for negative controls.
Although this study was performed retrospectively,
the intensity of cancer tissues compared with normal mucosa lesion
in each lesion was evaluated independently by two researchers (K.
Tanaka and M. Miyamoto) who were blinded to patient clinical
information. A pathologist confirmed the results of these
evaluations. The intensity of staining was classified according to
a three-level scale: 1, weak staining compared with normal mucosa
was observed at more than one spot in the cancer; 2, equivalent
staining compared with normal mucosa at all cancer locations; and
3, strong staining compared with normal mucosa at more than one
cancer location. When slides were scored by investigators
differently (ex: score 1, 2 or score 2, 3), the slides were scored
as 2.
Tissue microarray construction
The archival slides for all cases were reviewed. A
slide containing representative tumor was selected, and the total
area of tumor was encircled on the slide. The cases that could be
cored were selected. Using a manual tissue microarrayer (Alphelys,
Plaisir, France), the area needed in the donor block was cored with
a 0.6-mm-diameter needle and transferred to a recipient paraffin
block. The microarray was constructed with multifold redundancy
(four spots in cancer, two spots in normal mucosa for each patient)
to increase accuracy. The finalized array blocks were then sliced
into 4-μm-thick sections and mounted on glass slides.
Patients and esophageal specimens
Surgical specimens of resected ESCC that were
obtained between March 1994 and November 2004 were used in this
study. Patients with primary ESCC underwent radical esophagectomy
at the Department of Surgical Oncology, School of Medicine,
Hokkaido University. A total of 95 ESCC surgical specimens was
examined with the tissue microarray method. Surgical specimens were
obtained from 83 males and 12 females (median age 63, range 46–86
years). The median follow-up period was 95.6 months (range
5.1–183.7 months), and 48 patients (50.5%) died during follow-up.
No distant organ metastases were detected in any patient on
preoperative examinations. Six patients underwent preoperative
chemotherapy and/or radiotherapy. Tumor clinicopathologic stage was
determined according to the tumor-node-metastasis (TNM)
classification system of the International Union Against Cancer
(UICC) (31). One of the patients
was classified as Tis, 44 as T1, 8 as T2, 38 as T3, and 4 as T4.
Overall, 47 patients were classified as N0, and 48 as N1; 75
patients were classified as M0, and 20 as M1. One of the patients
was classified as TNM stage 0, 29 as stage I, 8 as stage IIA, 16 as
stage IIB, 21 as stage III, 2 as stage IV, 6 as stage IVA, and 12
as stage IVB. All informed consent processes for
immunohistochemical staining were conducted in accordance with the
guidelines of the Hokkaido University Institutional Review Board
authorization for this study.
Statistical analysis
The chi-square test and Fisher's exact test were
used as appropriate. Overall patient survival was calculated from
the date of operation to the date of last follow-up or date of
patient death. The Kaplan-Meier method was used to estimate overall
survival, and survival differences were analyzed by the log-rank
test based on APM expression of the cancer lesion compared to
normal tissue. The expression level scores were dichotomized by
combining scores of 1 and 2 or 2 and 3, depending on which had a
more significant relationship with survival on the log-rank test.
Univariate and multivariate analyses were performed using the Cox
proportional hazard regression model. P<0.05 were regarded as
significant in all of the analyses. All analyses were performed
with statistical software (Stat-View J version 5.0; SAS Institute
Inc., Cary, NC).
Results
Expression of HLA class I and APM
components in esophageal cell lines on Western blot analysis
On Western blot analysis with lysates from cultured
cell lines, HLA-HC, β2 microglobulin, tapasin, TAP-1, TAP-2, LMP-7,
and LMP-10 expressions differed among the cancer cell lines. Most
of the cancer cell expressions of these components were
downregulated compared with normal human esophageal mucosa tissues
(NHET). On the other hand, Calnexin, ERp57, MB1, Delta, and Z did
not differ among cultured cell lines. The cancer cell expressions
of ERp57, MB1, Delta, and Z were up-regulated compared with NHET.
HLA-HC, β2 microglobulin, tapasin, and LMP-7 were synchronously
expressed in cell lines such as TE2, TE5, TE9, TE13, HEC46, and
SGF7 (Fig. 1). In subsequent
experiments, we investigated the components of HLA-HC, β2
microglobulin, tapasin, TAP-1, TAP-2, LMP-7, and LMP-10, which
differed among cultured cell lines.
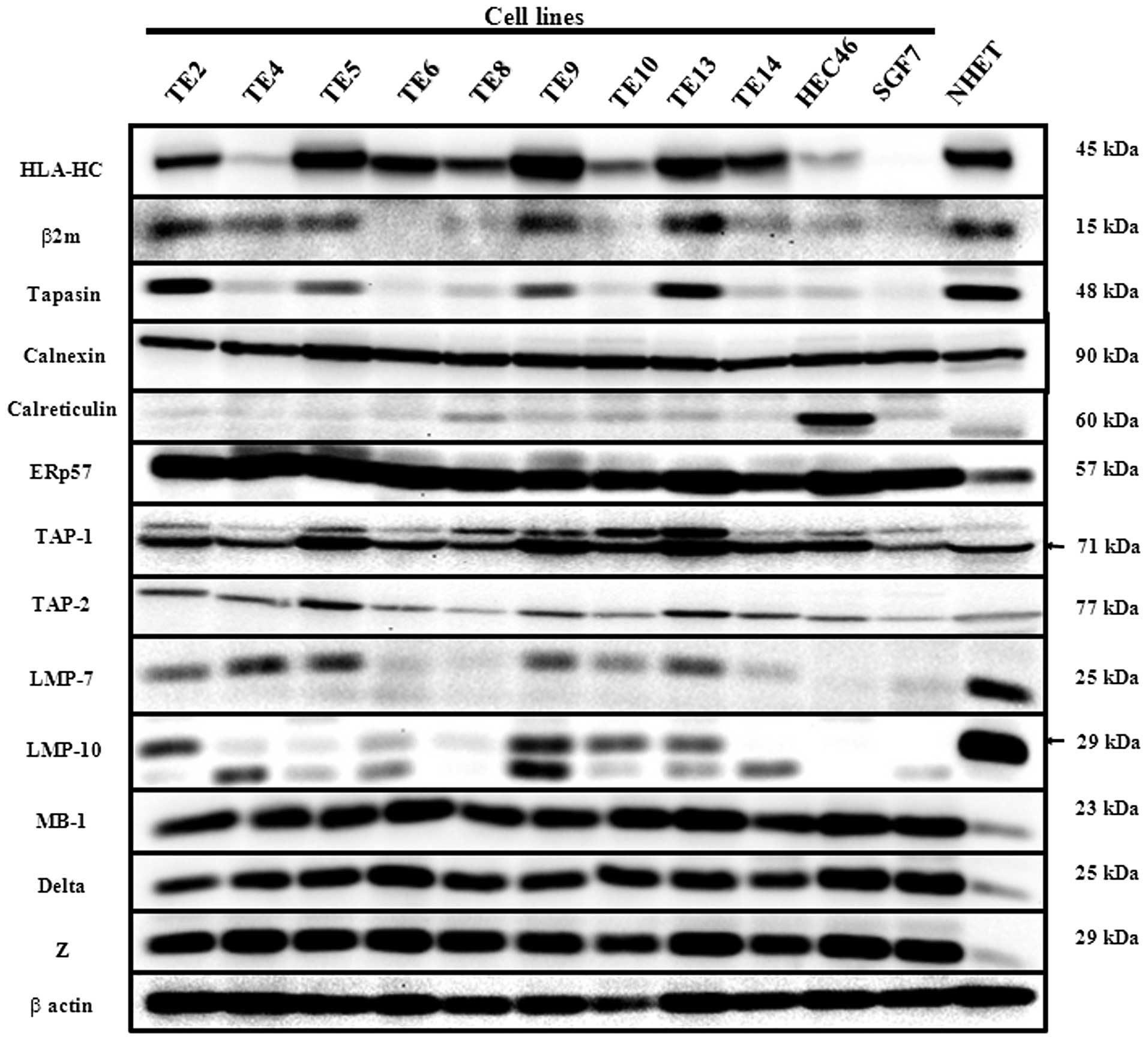 | Figure 1Western blot analysis for HLA class I
antigen processing machinery (APM): HLA class I heavy chain
(HLA-HC), β2 microglobulin, Tapasin, Calnexin, Calreticulin, ERp57,
TAP-1, TAP-2, LMP-7, LMP-10, MB-1, Delta, and Z. Lysates of the
cultured human esophageal cancer cell lines (TE2, 4, 5, 6, 8, 9,
10, 13, 14, HEC46, and SGF7) and homogenized normal human
esophageal mucosa tissue (NHET) were subjected to Western blot
analysis. Lysates of NHET were used as a positive control for APM
component. The difference of expression among cell lines was found
in seven APM molecules (HLA-HC, β2 microglobulin, Tapasin, TAP-1,
TAP-2, LMP-7, LMP-10). |
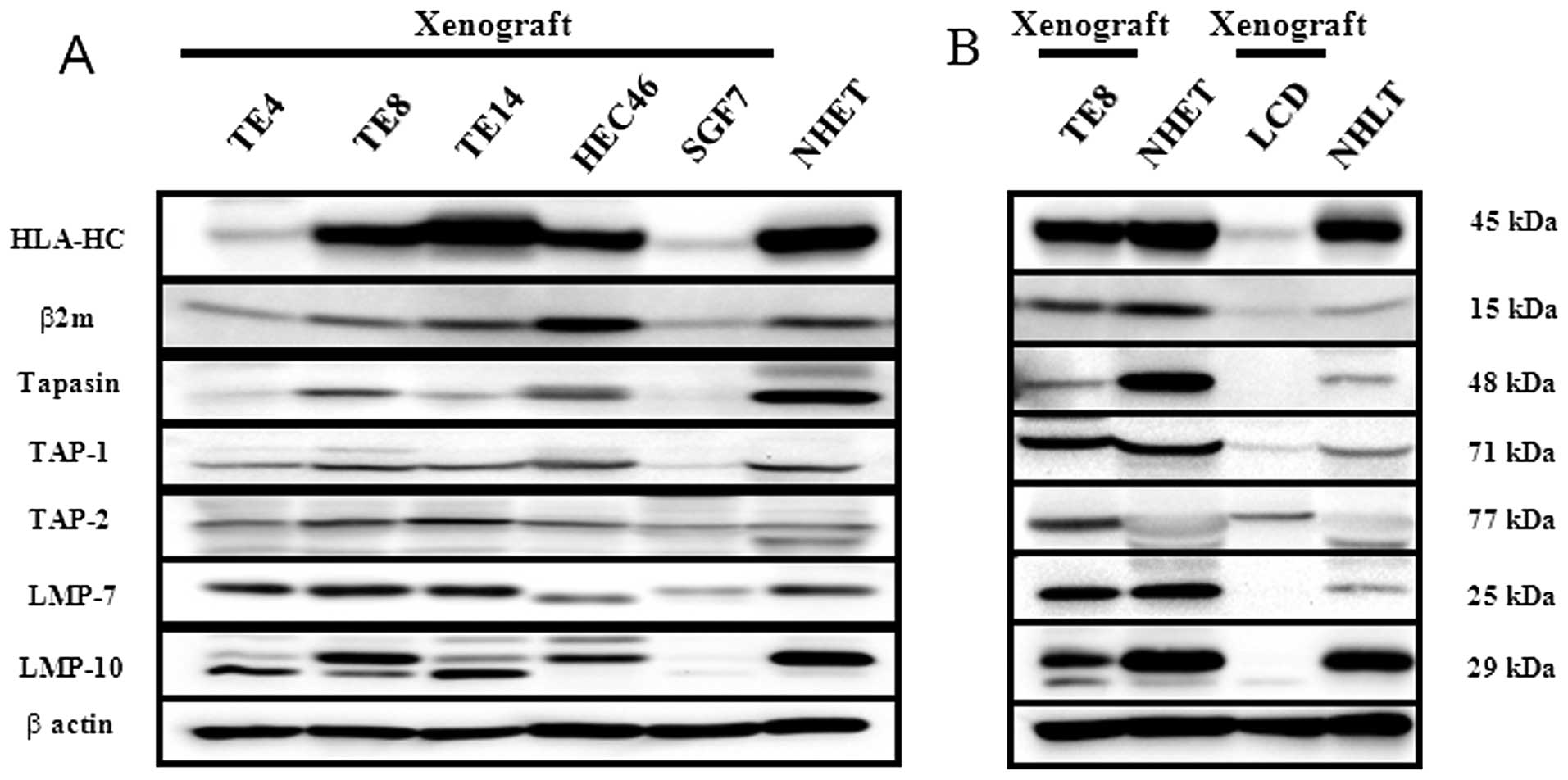
The lysates from CB17/SCID xenografts were subjected
to Western blot analysis because paraffin blocks of CB17/SCID
xenografts were used as positive and negative tissue controls for
IHC. Only five cultured esophageal cell lines (TE4, TE8, TE14,
HEC46, and SGF7) could be implanted in CB17/SCID mice. Each
component's expression in lysates from CB17/SCID xenografts was
very similar to that in cultured cell lines. There were few
CB17/SCID xenografts of the esophagus with expressions that were
negative for every component (Fig.
2A). The lung cell line with negative expression for all
components was implanted in CB17/SCID mice. The lysates from
implanted tumors derived from TE8, LCD, NHET, and normal human lung
tissue (NHLT) were subjected to Western blot analysis (Fig. 2B). There was a human lung cancer
cell line (LCD) with negative expression for every component except
for TAP2; expression of TE8 was increased in TAP2 compared with
LCD.
Expression of HLA class I and APM
components in esophageal cancer patients
On the basis of the results from Western blot
analysis, the positive and negative tissue controls for
immunostaining were confirmed using CB17/SCID xenografts. As
positive controls, normal esophageal mucosa and TE8 xenograft were
used. As a negative control, LCD xenograft was used. The conditions
of immunohistochemistry that corresponded with the results of
Western blotting were identified. Representative
immunohistochemical staining patterns for HLA-HC and various APM
components are shown for TE8 xenograft, LCD xenograft, carcinoma
lesion, and normal esophageal mucosa in Fig. 3. The basement membrane in normal
esophageal mucosa was stained with each antibody. HLA-HC, β2
microglobulin, tapasin, TAP-1, and TAP-2 were detected on cell
membranes and cytoplasm in both cancer cells and normal mucosa.
LMP-7 and LMP-10 were detected in the nucleus and cytoplasm in both
cancer cells and normal mucosa (Fig.
3).
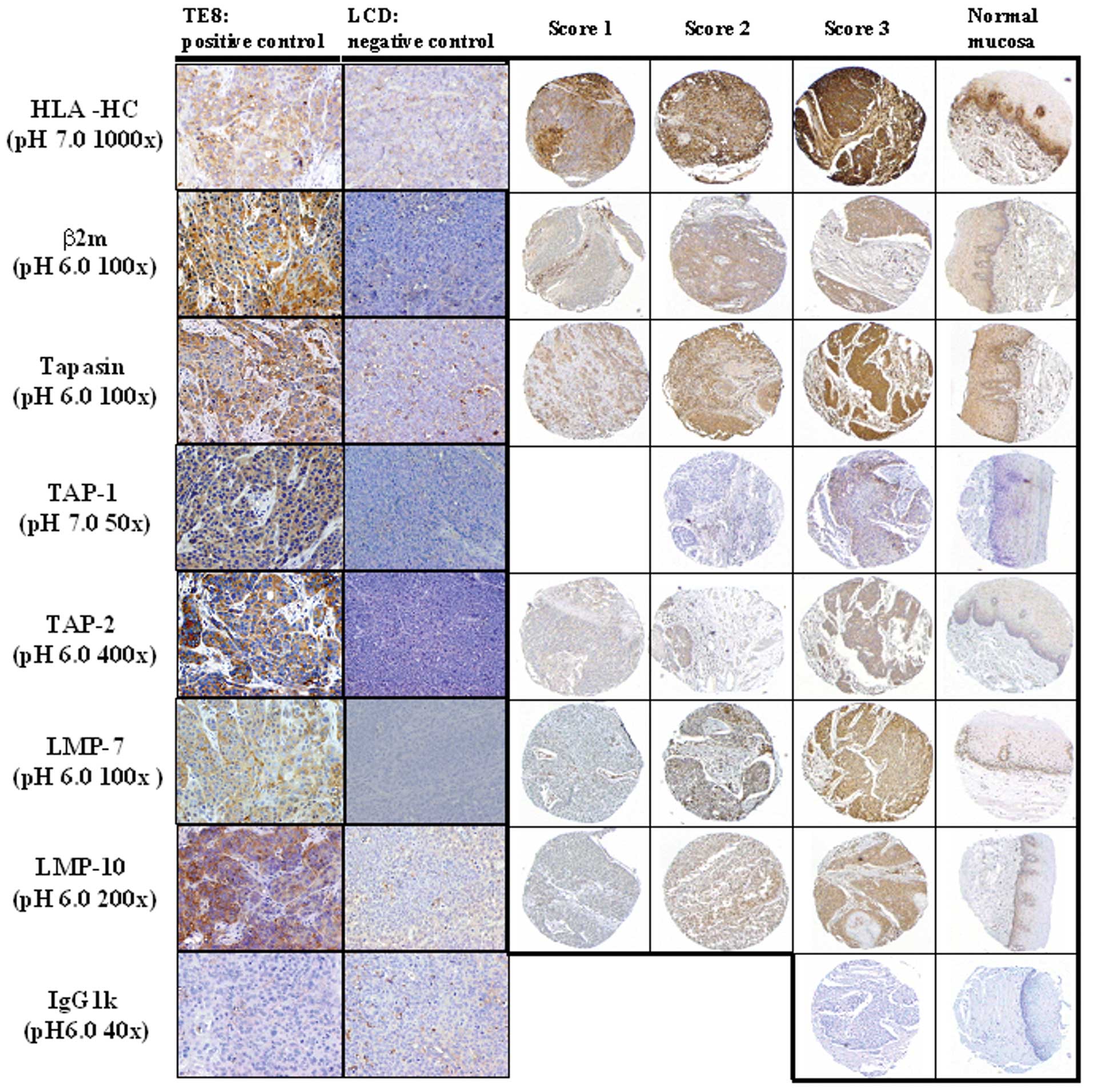 | Figure 3Representative immunohistochemical
staining pattern for APM components in xenografts and human
esophagus. TE8 xenograft and LCD xenograft, which were positive and
negative, respectively, by Western blotting, were stained with mAb
(HLA class I heavy chain (HLA-HC), β2 microglobulin, Tapasin,
TAP-1, TAP-2, LMP-7, LMP-10). TE8 xenograft was used as a positive
control for APM components. LCD xenograft was used as a negative
control. The intensity of staining was scored as 1, 2, or 3
indicating weak, clear, or strong expression respectively. Original
magnification, ×200 (xenograft), ×100 (carcinoma lesion and normal
esophageal mucosa). |
Of 95 ESCC specimens, 31 patients (32.6%) were
scored as 1, 52 (54.7%) as 2, 12 (12.6%) as 3 for HLA-HC. For β2
microglobulin, 3 patients (3.2%) were scored as 1, 71 (74.7%) as 2,
and 21 (22.1%) as 3. For tapasin, 1 patient (1.1%) was scored as 1,
46 (48.4.%) as 2, and 48 (50.5%) as 3. For TAP-1, 73 patients
(76.8%) were scored as 2, and 22 (23.2%) as 3. For TAP-2, 4
patients (4.2%) were scored as 1, 73 (76.8.%) as 2, and 18 (18.9%)
as 3. For LMP-7, 16 patients (16.8%) were scored as 1, 73 (76.8.%)
as 2, and 5 (5.3%) as 3 for LMP-7. For LMP-10, 4 patients (4.2%)
were scored as 1, 44 (46.3%) as 2, and 47 (49.5%) as 3.
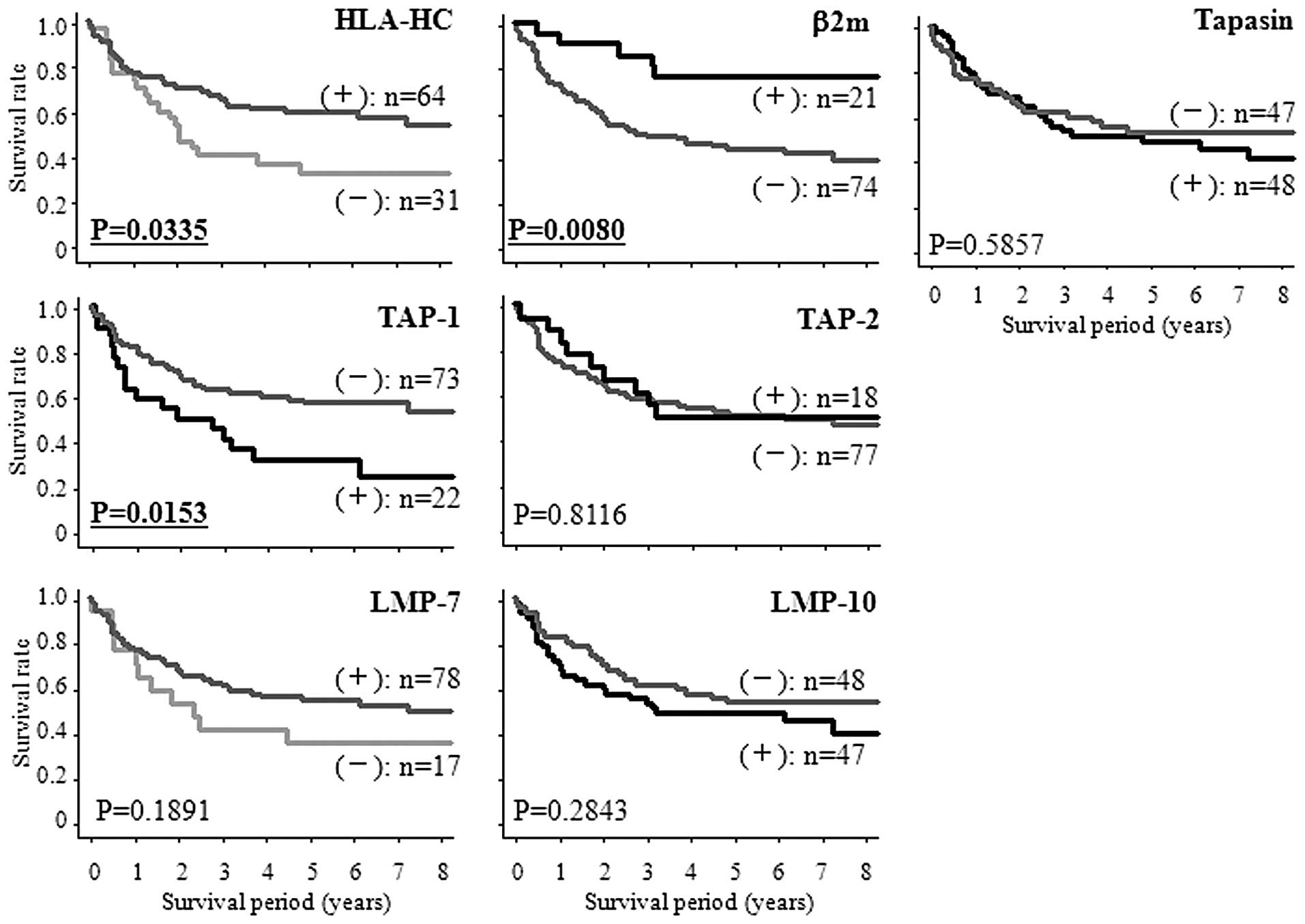
Kaplan-Meier survival analysis of HLA
class I and APM component expressions
For β2 microglobulin, tapasin, TAP-1, TAP-2, and
LMP-10, the patients were divided into two groups according to
combining score 1 and score 2 vs. score 3. Scores 1 and 2 were
defined as (−), score 3 was defined as (+). For HLA-HC and LMP-7,
the patients were divided into two groups according to score 1 vs.
combining score 2 and score 3. Score 1 was defined as (−), scores 2
and 3 were defined as (+). The survival rates were significantly
lower for patients with down-regulation of HLA-HC than for those
without (P=0.0335). The survival rates were significantly lower for
patients with up-regulation of TAP-1 than for those without
(P=0.0153). The survival rates were significantly higher for
patients with up-regulation of β2 microglobulin than for those
without (P=0.0080) (Fig. 4).
Association of HLA class I and APM
components with various clinicopathological features
Associations of HLA-HC, β2 microglobulin, and TAP-1
with clinicopathologic features are summarized in Table I. HLA-HC, β2 microglobulin, and
TAP-1 expressions were not significantly associated with
clinicopathological parameters, such as age, gender, and TNM
classification (Table I).
 | Table ICorrelation between
clinicopathological features of the 95 ESCC patients and expression
of APM components. |
Table I
Correlation between
clinicopathological features of the 95 ESCC patients and expression
of APM components.
| HLA class I Heavy
Chain | β2-microglobulin | TAP-1 |
|---|
|
|
|
|
|---|
| (−) n=31 | (+) n=64 | P-value | (−) n=74 | (+) n=21 | P-value | (−) n=73 | (+) n=22 | P-value |
|---|
| Age (years) |
| ≥63 | 20 | 31 | 0.141 | 40 | 11 | 0.892 | 43 | 8 | 0.063 |
| <63 | 11 | 33 | | 34 | 10 | | 30 | 14 | |
| Gender |
| Male | 28 | 55 | 0.745a | 67 | 16 | 0.129a | 63 | 20 | 0.727a |
| Female | 3 | 9 | | 7 | 5 | | 10 | 2 | |
| pT
classification |
| T1 | 15 | 29 | 0.890 | 32 | 12 | 0.131 | 35 | 9 | 0.489 |
| T2/T3/T4 | 16 | 34 | | 42 | 8 | | 37 | 13 | |
| pN
classification |
| Negative | 18 | 29 | 0.244 | 33 | 14 | 0.074 | 39 | 8 | 0.161 |
| Positive | 13 | 35 | | 41 | 7 | | 34 | 14 | |
| pM
classification |
| M0 | 26 | 49 | 0.413 | 59 | 16 | 0.765a | 58 | 17 | 0.775a |
| M1 | 5 | 15 | | 15 | 5 | | 15 | 5 | |
| P-stage |
| 0/I/II | 18 | 36 | 0.867 | 40 | 14 | 0.3030 | 43 | 11 | 0.460 |
| III/IV | 13 | 28 | | 34 | 7 | | 30 | 11 | |
Univariate and multivariate analyses of
HLA class I and APM component expressions and clinicopathologic
variables
Univariate analysis for overall survival using a Cox
regression model identified HLA-HC (P=0.0081), β2 microglobulin
(P=0.0123), TAP-1 (P=0.0177), T classification (P=0.0015), and N
classification (P=0.0022) as significant prognostic predictors.
Moreover, multivariate analysis of the same set of patients was
performed using the significant factors on univariate analysis. The
results identified that down-regulation of HLA-HC and up-regulation
of TAP-1 were independent unfavorable prognostic factors (hazard
ratio, 2.361; P=0.0141 and hazard ratio, 2.297; P=0.0145,
respectively). pT classification also had independent prognostic
value, with a hazard ratio of 2.341 (P=0.0102) (Table II).
 | Table IIUnivariate and multivariate analyses
of patients. |
Table II
Univariate and multivariate analyses
of patients.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| HR | 95% CI | P | HR | 95% CI | P |
|---|
| HLA-HC |
| −/+ | 1.855 | 1.040–3.309 | 0.0081 | 2.361 | 1.189–4.686 | 0.0141 |
| β2m |
| −/+ | 3.271 | 1.294–8.269 | 0.0123 | 2.006 | 0.750–5.361 | 0.1652 |
| Tapasin |
| +/− | 1.171 | 0.663–2.067 | 0.5861 | | | |
| TAP-1 |
| +/− | 2.073 | 1.135–3.786 | 0.0177 | 2.297 | 1.179–4.476 | 0.0145 |
| TAP-2 |
| +/− | 1.092 | 0.529–2.256 | 0.8117 | | | |
| LMP-7 |
| −/+ | 1.566 | 0.797–3.077 | 0.1928 | | | |
| LMP-10 |
| +/− | 1.362 | 0.722–2.405 | 0.2862 | | | |
| Age (years) |
| ≥63/<63 | 1.307 | 0.736–2.321 | 0.3600 | | | |
| Gender |
| Male/Female | 1.871 | 0.671–5.214 | 0.2311 | | | |
| pT
classification |
| T2T3T4/T1 | 2.661 | 1.456–4.868 | 0.0015 | 2.341 | 1.224–4.479 | 0.0102 |
| pN
classification |
| 1/0 | 2.441 | 1.087–3.471 | 0.0022 | 1.393 | 0.723–2.685 | 0.3214 |
| pM
classification |
| M1/M0 | 1.587 | 0.824–3.056 | 0.1672 | | | |
| P-Stage |
| III IV/I II | 2.907 | 1.628–5.189 | 0.0003 | | | |
Analysis of HLA class I and APM component
expressions in stage I/II or stage III/IV esophageal cancer
patients
Among stage I and II esophageal cancer patients,
there were no significant prognostic factors using a Cox regression
model. Among stage III and IV esophageal cancer patients,
univariate analysis for overall survival using a Cox regression
model identified only down-regulation of HLA-HC as a significant
prognostic factor (hazard ratio, 2.361; P=0.0138) (Table III).
 | Table IIIUnivariate analyses of patients in
stage I/II and stage III/IV. |
Table III
Univariate analyses of patients in
stage I/II and stage III/IV.
| Stage I/II | Stage III/IV |
|---|
|
|
|
|---|
| HR | 95% CI | P | HR | 95% CI | P |
|---|
| HLA-HC |
| −/+ | 1.521 | 0.619–3.739 | 0.3609 | 2.751 | 1.230–6.154 | 0.0138 |
| β2m |
| −/+ | 2.317 | 0.678–7.913 | 0.1799 | 4.004 | 0.946–16.943 | 0.0594 |
| Tapasin |
| −/+ | 0.604 | 0.250–1.461 | 0.2635 | 1.567 | 0.742–3.309 | 0.2389 |
| TAP-1 |
| +/− | 1.986 | 0.761–5.183 | 0.1607 | 1.987 | 0.915–4.316 | 0.0828 |
| TAP-2 |
| −/+ | 0.710 | 0.257–1.958 | 0.5081 | 1.565 | 0.542–4.522 | 0.4081 |
| LMP-7 |
| −/+ | 1.581 | 0.573–4.366 | 0.3763 | 1.723 | 0.681–4.356 | 0.2504 |
| LMP-10 |
| +/− | 1.068 | 0.442–2.580 | 0.8832 | 1.367 | 0.639–2.926 | 0.4200 |
| Age (years) |
| ≥63/<63 | 1.254 | 0.519–3.029 | 0.6143 | 1.164 | 0.544–2.490 | 0.6949 |
| Gender |
| Male/Female | 3.548 | 0.473–26.596 | 0.2179 | 1.244 | 0.375–4.131 | 0.7213 |
| pT
classification |
| 2,3,4/1 | 2.117 | 0.842–5.319 | 0.1107 | - | - | - |
| 4/1,2,3 | - | - | - | 1.702 | 0.403–7.184 | 0.4689 |
| pN
classification |
| 1/0 | 1.345 | 0.536–3.376 | 0.5272 | 1.185 | 0.480–2.928 | 0.7123 |
| pM
classification |
| 0/1 | - | - | - | 1.473 | 0.695–3.123 | 0.3121 |
| pStage |
| 2/1 | 1.358 | 0.565–3.268 | 0.4940 | - | - | - |
| 4/3 | - | - | - | 1.473 | 0.695–3.123 | 0.3121 |
Discussion
This study has three major findings. First,
expressions of HLA-HC, β2 microglobulin, and five APM components
(tapasin, TAP1/2, LMP7/10) were reduced in esophageal cancer cell
lines compared with normal tissue, and their components had
different expression levels among the esophageal cancer cell lines
on Western blot analysis. Second, down-regulation of HLA-HC and
up-regulation of TAP-1 were prognostic factors associated with a
poor prognosis in patients with esophageal cancer. Overexpression
of β2 microglobulin was associated with a good prognosis in
patients with esophageal cancer. Third, among stage III and IV
cancer patients, HLA-HC down-regulation was an unfavorable
prognostic factor, although there was no significant difference
between stages I and II.
Western blot analysis showed that most of the
components were downregulated, and the expression patterns differed
among cell lines. Similar expression patterns of HLA-HC, β2
microglobulin, tapasin, TAP-1, TAP-2, and LMP-7 were observed in
TE2, TE5, TE9, TE13, and NHET. These data suggest that the
expressions of these components correlate with each other. The
factor with a different expression pattern among the cell lines may
cause a difference in cell character. By examining these factors
and their correlations with clinico-pathologic factors, it might be
possible to deduce the role of the factor. Moreover,
immunohistochemical staining for these factors would be objective,
because both tissue positive controls and negative tissue controls
can be easily obtained using these cell lines. These components
that were screened by Western blot analysis have been previously
reported as prognostic factors in many studies separately (16,22–27).
Western blot analysis with cancer cell lines was a useful way to
identify candidate prognostic factors.
One of the major characteristics of the present
study is the use of CB17/SCID xenografts as positive and negative
tissue controls in immunohistochemistry. Based on the expression on
Western blotting of CB17/SCID xenografts, the optimal staining
conditions were decided to conform the immunohistochemical staining
intensity of CB17/SCID xenografts with each antibody (32). By adopting the approach of strict
condition setting, the performance of antibodies without
nonspecific staining was confirmed. We confirmed that patient
specimens could be finely stained under these staining conditions.
In our view, making tissue controls is absolutely necessary for
immunohistochemical research using antibodies. This is the first
report to use the strict approach for choosing the
immunohistochemical conditions.
The present study showed that there was a
correlation between poor prognosis and HLA-HC down-regulation in
esophageal cancer patients. This result was consistent with other
reports (16,26). In one of these reports, HLA-HC
expression was investigated in ESCC patients using the same
antibody (EMR8-5). The frequency of HLA-HC down-regulation and the
correlation between prognosis and HLA-HC found in the present study
are similar to those described earlier (26). Furthermore, the present results
showed that patients with up-regulation of β2 microglobulin had a
good prognosis. Many reports showed that down-regulation of β2
microglobulin was associated with a poor prognosis (24,27,33).
These reports adopted different immunohistochemistry evaluation
criteria from those used in the present evaluation. The other
reports adopted two major evaluation methods: one was the
percentage of stained tumor cells in an entire lesion (20,24,27),
and the other was a combination of percentage and intensity of
stained tumor (25,33). There was no category of
up-regulation in these two evaluations. The present results also
showed that down-regulation of β2 microglobulin was a significant
factor related to poor prognosis compared to up-regulation (data
not shown).
Surprisingly, in stage III and IV esophageal cancer
patients, down-regulation of HLA-HC was a negative prognostic
factor. These results support the conjecture that, after radical
surgery for advanced cancer, micrometastases of the residual cancer
cells expressing HLA class I were eradicated by the immune system.
We have already reported that the cooperation between
CD4+ and CD8+ T cells drastically improves
the prognosis of patients with ESCC (5). Our results support that the host
immune response against cancer cells and the status of HLA class I
molecules on cancer cells are important factors for postoperative
prognosis. If the cancer cells in surgical specimens express
HLA-HC, the patients would undergo adjuvant immunotherapy to
eradicate residual tumor. It may be possible to change the
postoperative adjuvant therapy based on HLA-HC expression.
In conclusion, the current results suggest that
three HLA class I APMs (HLA-HC, β2 microglobulin, and TAP-1) are
correlated with postoperative survival in esophageal cancer, and
that the down-regulation of HLA-HC in tumors is associated with a
poor prognosis among stages III and IV esophageal cancer patients.
Thus, the present study suggests that immunohistochemical staining
for HLA-HC is useful as a prognostic marker and for selection of
adjuvant therapy in stage III and IV esophageal cancer
patients.
Acknowledgements
The authors appreciate the contributions of Hiraku
Shida with respect to technical support in immunohistochemistry.
The authors are especially grateful to Naomi Saitoh for help in
preparing the manuscript. Finally, the authors are grateful to the
Pathology department for tissue collection, and for the advice and
expertise of Katsuji Marukawa and Jun Moriya.
References
|
1
|
Ando N, Iizuka T, Ide H, Ishida K, Shinoda
M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, Makuuchi
H, Tanaka O, Yamana H, Ikeuchi S, Kabuto T, Nagai K, Shimada Y,
Kinjo Y and Fukuda H: Surgery plus chemotherapy compared with
surgery alone for localized squamous cell carcinoma of the thoracic
esophagus: a Japan Clinical Oncology Group Study - JCOG9204. J Clin
Oncol. 21:4592–4596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adham M, Baulieux J, Mornex F, de La Roche
de Bransat E, Ducerf C, Souquet JC and Gerard JP: Combined
chemotherapy and radiotherapy followed by surgery in the treatment
of patients with squamous cell carcinoma of the esophagus. Cancer.
89:946–954. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y
and Kitajima M: Improvement in the results of surgical treatment of
advanced squamous esophageal carcinoma during 15 consecutive years.
Ann Surg. 232:225–232. 2000.PubMed/NCBI
|
|
4
|
Morita M, Yoshida R, Ikeda K, Egashira A,
Oki E, Sadanaga N, Kakeji Y, Yamanaka T and Maehara Y: Advances in
esophageal cancer surgery in Japan: an analysis of 1000 consecutive
patients treated at a single institute. Surgery. 143:499–508. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho Y, Miyamoto M, Kato K, Fukunaga A,
Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki
M, Nakakubo Y, Hiraoka K, Murakami S, Shinohara T, Itoh T, Okushiba
S, Kondo S and Katoh H: CD4+ and CD8+ T cells
cooperate to improve prognosis of patients with esophageal squamous
cell carcinoma. Cancer Res. 63:1555–1559. 2003.
|
|
6
|
Kim R, Emi M and Tanabe K: Cancer
immunoediting from immune surveillance to immune escape.
Immunology. 121:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garrido F, Ruiz-Cabello F, Cabrera T,
Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M and Stern PL:
Implications for immunosurveillance of altered HLA class I
phenotypes in human tumours. Immunol Today. 18:89–95. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seliger B, Maeurer MJ and Ferrone S:
Antigen-processing machinery breakdown and tumor growth. Immunol
Today. 21:455–464. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seliger B, Cabrera T, Garrido F and
Ferrone S: HLA class I antigen abnormalities and immune escape by
malignant cells. Semin Cancer Biol. 12:3–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruiz-Cabello F, Cabrera T, Lopez-Nevot MA
and Garrido F: Impaired surface antigen presentation in tumors:
implications for T cell-based immunotherapy. Semin Cancer Biol.
12:15–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolfel T, Klehmann E, Muller C, Schutt KH,
Meyer zum Buschenfelde KH and Knuth A: Lysis of human melanoma
cells by autologous cytolytic T cell clones. Identification of
human histocompatibility leukocyte antigen A2 as a restriction
element for three different antigens. J Exp Med. 170:797–810. 1989.
View Article : Google Scholar
|
|
12
|
Crowley NJ, Darrow TL, Quinn-Allen MA and
Seigler HF: MHC-restricted recognition of autologous melanoma by
tumor-specific cytotoxic T cells. Evidence for restriction by a
dominant HLA-A allele. J Immunol. 146:1692–1699. 1991.PubMed/NCBI
|
|
13
|
Marincola FM, Jaffee EM, Hicklin DJ and
Ferrone S: Escape of human solid tumors from T-cell recognition:
molecular mechanisms and functional significance. Adv Immunol.
74:181–273. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rockett JC, Darnton SJ, Crocker J,
Matthews HR and Morris AG: Expression of HLA-ABC, HLA-DR and
intercellular adhesion molecule-1 in oesophageal carcinoma. J Clin
Pathol. 48:539–544. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rockett JC, Darnton SJ, Crocker J,
Matthews HR and Morris AG: Lymphocyte infiltration in oesophageal
carcinoma: lack of correlation with MHC antigens, ICAM-1, and
tumour stage and grade. J Clin Pathol. 49:264–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosch SB, Izbicki JR, Pichlmeier U,
Stoecklein N, Niendorf A, Knoefel WT, Broelsch CE and Pantel K:
Expression and prognostic significance of immunoregulatory
molecules in esophageal cancer. Int J Cancer. 74:582–587. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vitale M, Rezzani R, Rodella L, Zauli G,
Grigolato P, Cadei M, Hicklin DJ and Ferrone S: HLA class I antigen
and transporter associated with antigen processing (TAP1 and TAP2)
down-regulation in high-grade primary breast carcinoma lesions.
Cancer Res. 58:737–742. 1998.PubMed/NCBI
|
|
18
|
Kageshita T, Hirai S, Ono T, Hicklin DJ
and Ferrone S: Down-regulation of HLA class I antigen-processing
molecules in malignant melanoma: association with disease
progression. Am J Pathol. 154:745–754. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nie Y, Yang G, Song Y, Zhao X, So C, Liao
J, Wang LD and Yang CS: DNA hypermethylation is a mechanism for
loss of expression of the HLA class I genes in human esophageal
squamous cell carcinomas. Carcinogenesis. 22:1615–1623. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seliger B, Atkins D, Bock M, Ritz U,
Ferrone S, Huber C and Storkel S: Characterization of human
lymphocyte antigen class I antigen-processing machinery defects in
renal cell carcinoma lesions with special emphasis on
transporter-associated with antigen-processing down-regulation.
Clin Cancer Res. 9:1721–1727. 2003.
|
|
21
|
Atkins D, Ferrone S, Schmahl GE, Storkel S
and Seliger B: Down-regulation of HLA class I antigen processing
molecules: an immune escape mechanism of renal cell carcinoma? J
Urol. 171:885–889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meissner M, Reichert TE, Kunkel M, Gooding
W, Whiteside TL, Ferrone S and Seliger B: Defects in the human
leukocyte antigen class I antigen processing machinery in head and
neck squamous cell carcinoma: association with clinical outcome.
Clin Cancer Res. 11:2552–2560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramnath N, Tan D, Li Q, Hylander BL,
Bogner P, Ryes L and Ferrone S: Is downregulation of MHC class I
antigen expression in human non-small cell lung cancer associated
with prolonged survival? Cancer Immunol Immunother. 55:891–899.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogino T, Shigyo H, Ishii H, Katayama A,
Miyokawa N, Harabuchi Y and Ferrone S: HLA class I antigen
down-regulation in primary laryngeal squamous cell carcinoma
lesions as a poor prognostic marker. Cancer Res. 66:9281–9289.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mehta AM, Jordanova ES, Kenter GG, Ferrone
S and Fleuren GJ: Association of antigen processing machinery and
HLA class I defects with clinicopathological outcome in cervical
carcinoma. Cancer Immunol Immunother. 57:197–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizukami Y, Kono K, Maruyama T, Watanabe
M, Kawaguchi Y, Kamimura K and Fujii H: Downregulation of HLA Class
I molecules in the tumour is associated with a poor prognosis in
patients with oesophageal squamous cell carcinoma. Br J Cancer.
99:1462–1467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han LY, Fletcher MS, Urbauer DL, Mueller
P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers
MT, De Geest K, Gershenson DM, Lutgendorf SK, Ferrone S and Sood
AK: HLA class I antigen processing machinery component expression
and intratumoral T-Cell infiltrate as independent prognostic
markers in ovarian carcinoma. Clin Cancer Res. 14:3372–3379. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Q, Hao C, Su P and Shi J:
Down-regulation of HLA class I antigen-processing machinery
components in esophageal squamous cell carcinomas: association with
disease progression. Scand J Gastroenterol. 44:960–969. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogino T, Wang X, Kato S, Miyokawa N,
Harabuchi Y and Ferrone S: Endoplasmic reticulum chaperone-specific
monoclonal antibodies for flow cytometry and immunohistochemical
staining. Tissue Antigens. 62:385–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bandoh N, Ogino T, Cho HS, Hur SY, Shen J,
Wang X, Kato S, Miyokawa N, Harabuchi Y and Ferrone S: Development
and characterization of human constitutive proteasome and
immunoproteasome subunit-specific monoclonal antibodies. Tissue
Antigens. 66:185–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumours. 6th edit. John Wiley and Sons;
New York: pp. 60–64. 2002
|
|
32
|
Ishikawa K, Miyamoto M, Yoshioka T, Kato
T, Kaji M, Ohbuchi T, Hirano S, Itoh T, Dosaka-Akita H and Kondo S:
Up-regulation of CD40 with juxtacrine activity in human nonsmall
lung cancer cells correlates with poor prognosis. Cancer.
113:530–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leffers N, Gooden MJ, Mokhova AA, Kast WM,
Boezen HM, Ten Hoor KA, Hollema H, Daemen T, van der Zee AG and
Nijman HW: Down-regulation of proteasomal subunit MB1 is an
independent predictor of improved survival in ovarian cancer.
Gynecol Oncol. 113:256–263. 2009. View Article : Google Scholar : PubMed/NCBI
|