Introduction
Adenocarcinoma of the stomach is the leading cause
of gastrointestinal cancer in the world and is the second leading
cause of cancer death worldwide (1). Currently available chemotherapy for
advanced gastric cancer (GC) includes fluoropyrimidine-based
agents. S-1 (TS-1) is an orally active combination of tegafur (a
prodrug that is converted by cells to fluorouracil), gimeracil (an
inhibitor of dihydropyrimidine dehydrogenase, which degrades
fluorouracil) and oteracil (which inhibits the phosphorylation of
fluorouracil in the gastrointestinal tract, thereby reducing the
gastrointestinal toxic effects of fluorouracil) in a molar ratio of
1:0.4:1 (2,3). Results of the large-scale ACTS-GC
(adjuvant chemotherapy trial of S-1 for gastric cancer) trial,
which enrolled patients with locally advanced (stage II or III) GC
who underwent D2 surgery, indicated that S-1 is an effective
adjuvant treatment in this patient population. Other clinical
trials have reported response rates of 30–50% for S-1 in advanced
GC (4–6). Based on these results, S-1 is now
recognized as one of the standard chemotherapeutic agents for this
disease (7,8).
Although advanced GC is treated predominantly by
combination chemotherapy regimens that include fluoropyrimidine
derivatives, overall survival (OS) can still be improved (9,10).
Investigational chemoradiotherapy regimens have also left room for
improvement. In recent years, substantial advances have been made
in the development of molecularly targeted therapies for various
types of cancer. Targeted therapies block the growth of cancer
cells by interfering with specific molecules required for
carcinogenesis and tumor growth (11). Targeted cancer therapies have the
potential to be more effective than current treatments and less
harmful to normal cells.
Overexpression of human epidermal growth factor
receptor 1 (EGFR; ErbB1; HER1 in humans) has been detected in
approximately 30–70% of GC cases and is associated with poor
outcomes and aggressive disease (12,13).
EGFR status was reported to be significantly associated with OS and
relapse-free survival (RFS) in both the surgery alone group and the
S-1 group in the ACTS-GC study (Terashima M, et al, 2011
ASCO Meeting, 4013). Progress in the understanding of the
involvement of the EGFR pathway in GC has recently been made. The
binding of a ligand to the extracellular portion of EGFR results in
phosphorylation of the tyrosine kinase domain located in the
intracellular portion, resulting in activation of intracellular
effectors involved in signaling, such as the G protein K-ras and
the protein kinase RAF [Ras/mitogen-activated protein kinase
pathway (MAPK)], as well as phosphoinositide 3-kinase (PI3K/Akt
pathway). Cetuximab is a chimeric (mouse/human) IgG1monoclonal
antibody directed against EGFR that is administered by intravenous
infusion for treatment of metastatic colorectal cancer and head and
neck cancer. Cetuximab has been proven to be effective in
irinotecan-resistant metastatic colorectal cancer expressing EGFR,
detected by immunohistochemistry (IHC), with response rates ranging
from 8.8% when used as monotherapy to 22.9% when combined with
irinotecan (14,15). Despite recent advances in the
molecular understanding of GC, there is a noticeable lack of
targeted therapies in clinical development for this malignancy.
In this study, we investigated whether cetuximab
alone or in combination with S-1 can be used in the treatment of
EGFR-overexpressing GC in cell culture and xenograft models as an
indication of its potential efficacy for treating patients with
GC.
Materials and methods
Cell culture and reagents
Human GC cell lines (MKN45, KATOIII, MKN74, MKN28
and MKN1) were obtained from the Japanese Cancer Research Resources
Bank (Tokyo, Japan). MKN45 and KATOIII cells were derived from
poorly-differentiated adenocarcinomas. MKN74 and MKN28 cells were
derived from moderately- and well-differentiated adenocarcinomas,
respectively. The MKN1 cell line is a gastric adenocarcinoma cell
line obtained from a metastatic lymph node and has the ability to
differentiate into either adenomatous or squamous cells. The KGC01
line was obtained from a patient with clinically diagnosed GC with
a pathological diagnosis of type 4 carcinoma (pT4N1H0CY1M0/stage
IV); this patient provided informed consent (approved by the ethics
committee of Keio University; no. 17–47). These cell lines were
cultured in RPMI-1640 (Sigma, St. Louis, MO) containing 10% fetal
bovine serum (FBS; Invitrogen, Carlsbad, CA) and
penicillin-streptomycin mixed solution (penicillin 10,000 units/ml,
streptomycin 10,000 μg/ml; Nacalai Tesque, Inc., Kyoto, Japan). In
all experiments, cells were cultured at 37˚C in a humidified 5%
CO2/95% air atmosphere. Cetuximab (Erbitux, Merck, Lyon,
France) was obtained from Bristol-Myers Squibb Co. (New York, NY),
5FU was obtained from Kyowa Hakko Kirin Co., Ltd. (Tokyo, Japan)
and tegafur, gimeracil and oteracil, all of which are components of
S-1, were synthesized by Taiho Pharmaceutical.
Analysis of EGFR expression
To detect expression of EGFR, cells were removed
from the culture dish using trypsin and EDTA, pelleted by
centrifugation, washed with phosphate-buffered saline (PBS) and
resuspended at 37˚C in Hanks' balanced salt solution (HBSS)
containing 2% FBS and 10 mM HEPES (Invitrogen). Cells were
incubated with Blocking One solution (Nacalai Tesque) for 20 min at
room temperature. They were then washed and incubated with
anti-human EGFR antibody conjugated with FITC (BD Pharmingen, San
Jose, CA) for 30 min at 4˚C. Finally, cells were subsequently
counterstained with 1 μg/ml propidium iodide (PI; Sigma) to label
dead cells. Then, 1×106 viable cells were analyzed using
a FACSVantage™ SE (Becton-Dickinson, San Jose, CA). The
distribution of cells was analyzed using FlowJo software (Tomy
Digital Biology, Tokyo, Japan).
Quantitative RT-PCR
otal RNA from cells was extracted using an RNeasy
Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's
protocol. The concentration of total RNA was determined using a
NanoDrop ND-1000 (NanoDrop Technologies, San Diego, CA). Briefly,
purified total RNA was reverse-transcribed to generate
double-stranded cDNA using Eagle Taq Master Mix with ROX (Roche
Applied Science, Indianapolis, IN) and the expression of human EGFR
was analyzed using the Applied Biosystems 7500 Fast Real-Time PCR
System (Applied Biosystems, Foster City, CA). TaqMan gene
expression assay primers and probe mixes were used for GAPDH and
EGFR (assay IDs Hs99999905_m1 and Hs01076078_m1, respectively;
Applied Biosystems). GAPDH was detected using TaqMan primers and
probes and was used as the control gene. The thermal cycling
reaction included incubation at 95˚C for 20 sec and 40 cycles of
95˚C for 3 sec and 60˚C for 30 sec. Data were collected using
analytical software (Applied Biosystems). Using the ΔΔCT
method, the expression level of each gene was determined relative
to the value of the expression of the gene in TMK-1 cells.
DNA extraction and K-ras mutation
analysis
DNA was extracted from GC cell lines using a QIAmp
DNA Mini kit (Qiagen, Duesseldorf, Germany). A NanoDrop ND-1000
(NanoDrop Technologies) was used to evaluate the concentration of
the samples. The reaction mixes contain a single primer set
specific for either the wild-type or mutated sequences in codons 12
and 13 of K-ras. Direct sequencing was done using a Big Dye
Terminator cycle sequencing kit (Applied Biosystems) and analyzed
on an ABI PRISM 310 DNA Analyzer automated sequencer (Applied
Biosystems).
Survival studies with anticancer
agents
Cells were plated in 96-well micro-plates and
cultured for 12 h before exposure to various concentrations of
compounds for 72 h. Cells were quantified using the WST-8 assay.
The optical density (OD) value was detected by Rainbo Sunrise (Wako
Pure Chemical Industries, Ltd., Osaka, Japan). The rate of
inhibition was calculated as follows: % inhibition = (OD of treated
group − blank)/OD of control group × 100. The concentration of
tested drugs resulting in 50% growth inhibition (IC50)
was calculated. Data were analyzed to determine the combination
index (CI), a well-established index of the interaction between two
drugs (16). CI values of <1, 1
and >1 indicate synergistic, additive and antagonistic effects,
respectively.
Phosphorylation activity assay
To evaluate the dependency of cetuximab activity on
EGFR and AKT phosphorylation, cells were exposed to 3.97 μM
cetuximab for 72 h before they were collected. Cells were washed in
PBS and fixed with 2% paraformaldehyde (PFA) in a 37˚C water bath
for 10 min. Then, cells were washed with PBS and pelleted by
centrifugation (800 × g) for 5 min and the supernatant was removed.
Cells were mixed to disrupt the pellet and permeabilized by adding
500 μl of 90% methanol (for 1–10×106 cells) and
incubated on ice for 15 min. After blocking on ice for 10 min,
cells were then washed and incubated with primary antibodies
against EGFR, AKT, phospho-EGFR (Tyr1068) and phospho-AKT (Ser473)
(Cell Signaling Technology, Inc., Danvers, MA) for 60 min at room
temperature. Cells were washed with PBS before incubation for 30
min with Alexa Fluor 488 donkey anti-rabbit IgG antibody
(Invitrogen). Then, each sample was analyzed using a FACSCalibur™
(Becton-Dickinson). Cell distribution was analyzed using FlowJo
software (Tomy Digital Biology).
Apoptosis assay
For apoptosis assays, the supernatant was aspirated
and cells were resuspended in 150 μl binding buffer, and stained
with 5 μl Annexin V-FITC and 5 μl PI at room temperature for 25 min
in the dark. After incubation, cells were processed as directed in
the kit instructions (Annexin V-FITC Apoptosis Detection Kit I, BD
Pharmingen) and analyzed using a FITC signal detector and PI
detector using a flow cytometer (FACSCalibur™) and Cell Quest
software (Becton-Dickinson).
In vivo multiple drug assay
MKN28 cells (1×106) were implanted s.c.
into the axilla of 6-week-old male athymic nude mice. Drug
administration was initiated when tumors in each group achieved an
average volume of 333±2.16 mm3. Mice were randomly
allocated to control and treatment groups. Treatment groups
consisted of control, S-1 alone, cetuximab alone and combination
S-1/cetuximab. Each treatment group included 8 mice. S-1 was
administered at a dose of 6.9 g/kg/day and given by oral gavage
daily for 14 days. Cetuximab (40 mg/kg/day) was given i.p. on Days
1, 4, 8 and 14. Control animals received sterile PBS
administration. Tumor volume was determined from caliper
measurements of tumor length (L) and width (W) according to the
formula LW2/2 and on body weights acquired every 3 days
and on the day of evaluation. Both tumor size and body weight were
measured three times per week. The percentage of tumor growth
inhibition (TGI%) was calculated as follows: TGI (%) = [1 − (tumor
volume of treatment group on evaluation day − tumor volume of
treatment group on Day 1)/(tumor volume of control group at
evaluation day − tumor volume of control group on Day 1)] × 100.
The percentage of body weight change (BWC%) was calculated as
follows: BWC (%) = [(BW on evaluation day) − (BW on Day 1)]/(BW on
Day 1) × 100.
Immunohistochemical analysis
Samples were fixed with 4% PFA for 24 h at RT.
Immunohistochemical staining for EGFR was performed on 4-μm thick
sections placed on pre-coated slides with APS (Matsunami Glass
Ind., Ltd., Osaka, Japan). Briefy, slides were incubated with
blocking reagent-N101 (Wako Pure Chemical Industries, Tokyo, Japan)
for 20 min. After rinsing in PBST, avidin and biotin blocking was
performed for 15 min each. Slides were incubated with anti-human
EGFR monoclonal antibody (Cell Signaling Technology, Inc.).
EnVision™+, Rabbit/HRP (Dako, Glostrup, Denmark) was then used as
secondary antibody for 30 min. DAB staining reactions were
conducted for 10 min. Slides were counterstained with haematoxylin.
Finally, slides were cover-slipped with aqueous mounting medium
(Aquatex®, Merck). Specimens were analyzed under a light
microscope, and EGFR positivity was defined as strong membrane and
cytoplasmic staining in at least 50–75% of cells.
Statistical methods
All data were expressed as the mean ± SD.
Statistically significant differences were determined using
two-tailed Student's t-test or χ2 test. P-values
<0.05 were considered statistically significant.
Results
Profile of EGFR amplification in GC
cells
To evaluate EGFR amplification and K-ras mutation
status, six GC cell lines (MKN28, MKN45, MKN74, KATOIII, TMK-1 and
KGC01) were examined for their expression of EGFR protein and mRNA
by flow cytometry and real-time PCR and for mutation analysis by
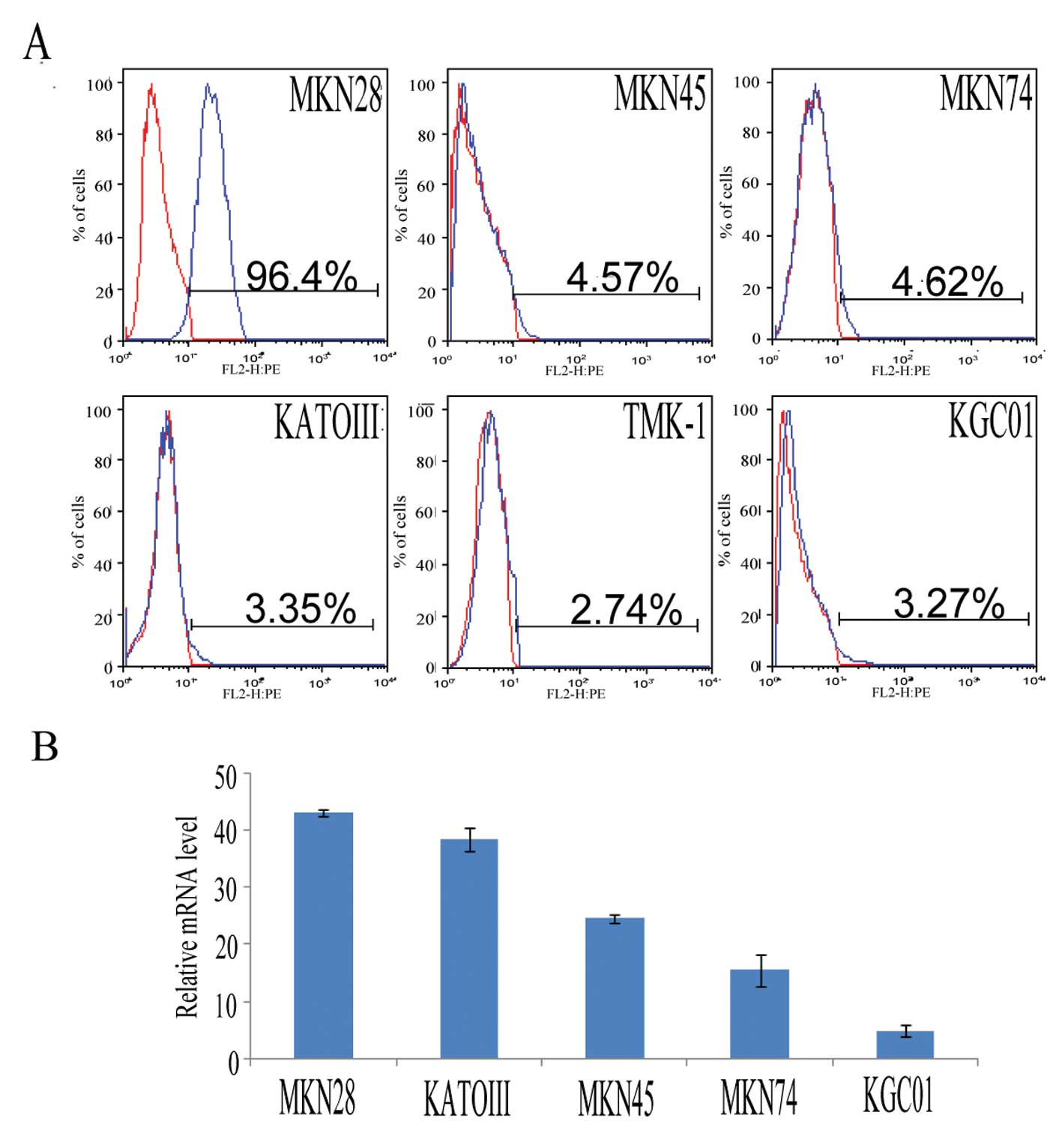
direct sequencing. FACS analysis revealed that EGFR expression was
significantly higher in MKN28 cells compared to other cell lines
(P<0.05) (Fig. 1A). The EGFR
mRNA expression ratio of each cell line was determined relative to
the value of TMK-1. The EGFR mRNA level was 43.08±0.53-fold for
MKN28 cells, 24.57±0.62-fold for MKN45 cells, 15.52±2.79-fold for
MKN74 cells, 38.19±2.07-fold for KATOIII cells and 4.90±1.12-fold
for KGC01 cells (Fig. 1B). K-ras
mutation status for each cell line is shown in Table I. Among the GC cell lines examined,
EGFR protein and mRNA were overexpressed in MKN28 cells, while
KATOIII cells showed amplification of EGFR mRNA, but were negative
for EGFR protein expression.
 | Table IEGFR status and K-ras mutation in
gastric cancer. |
Table I
EGFR status and K-ras mutation in
gastric cancer.
| Cell line | EGFR protein
expression (%, mean ± SD) | EGFR mRNA
amplification (fold, mean ± SD) | K-ras mutation |
|---|
| MKN28 | 95.90±1.23 | 43.08±0.53 | WT |
| MKN45 | 4.16±0.51 | 24.57±0.62 | WT |
| MKN74 | 4.35±0.26 | 15.52±2.79 | WT |
| TMK-1 | 2.43±0.32 | 1 (control) | WT |
| KATOIII | 3.23±0.12 | 38.19±2.07 | WT |
| KGC01 | 3.09±0.19 | 4.90±1.12 | WT |
Synergistic anti-proliferative effects of
5FU and cetuximab in EGFR-amplified GC cells
The effects of 5FU or cetuximab monotherapy or
combination 5FU/cetuximab therapy on the growth of GC cells with
and without EGFR amplification were evaluated. 5FU was used instead
of S-1 for these in vitro experiments, since tegafur, a
component of S-1, is metabolized to 5FU in the liver. The combined
effect of 5FU and cetuximab was evaluated on the basis of the CI.
5FU monotherapy inhibited the proliferation of GC cells, although
the IC50 values varied significantly between the
individual cell lines (Fig. 2A and
B). On the other hand, EGFR-amplified MKN28 cells showed only
sensitive to cetuximab in a concentration-dependent manner compared
with other GC cells (Fig. 2C). The
combination of 5FU and cetuximab exhibited a synergistic inhibitory
effect on the growth of EGFR-amplified MKN28 cells (C.I. value =
0.92±0.015), but not on cells without EGFR amplification, including
MKN74 and TMK-1 cells (Fig.
2C–F).
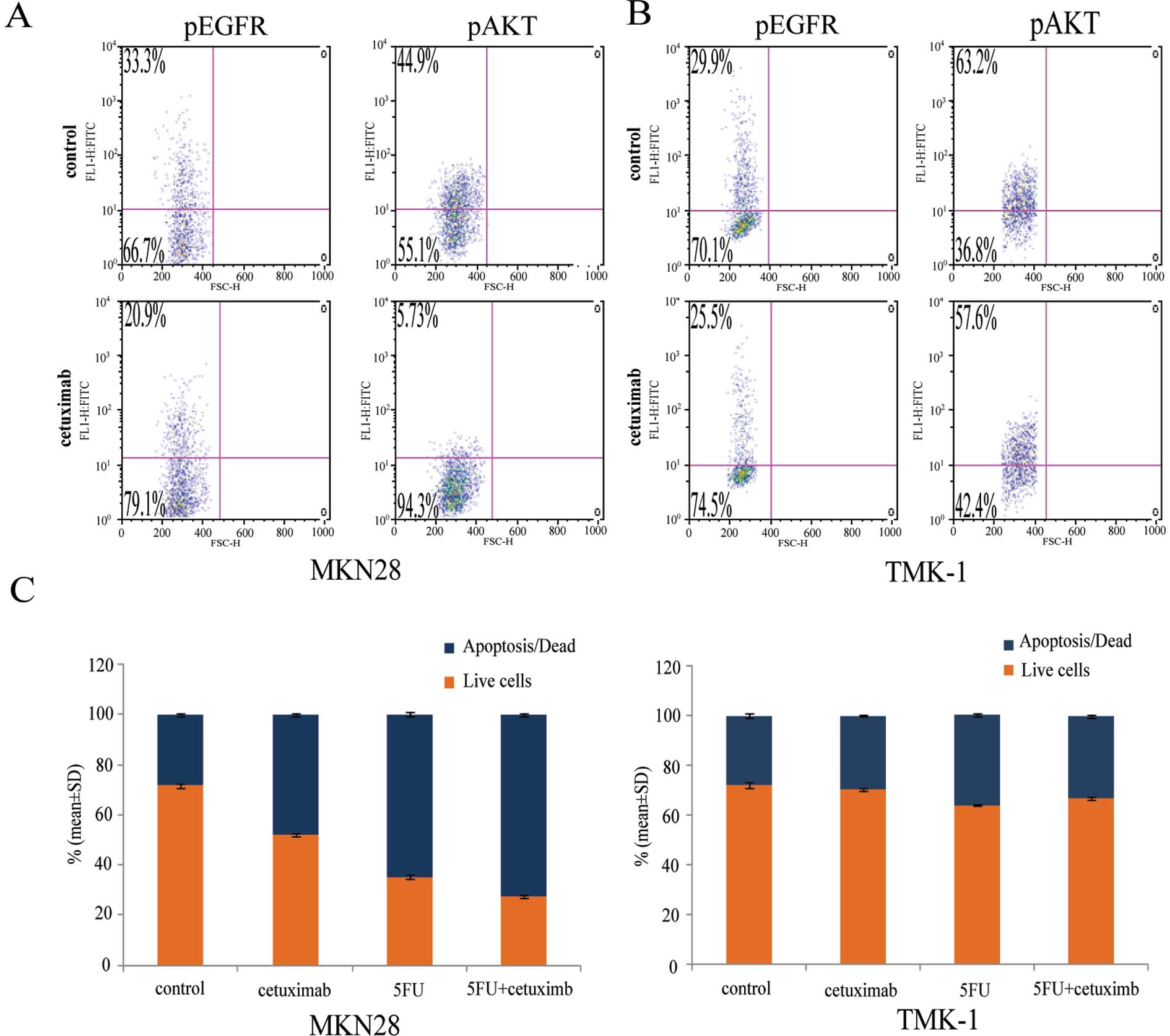
Effect of cetuximab on EGFR and AKT
signaling in GC cells
EGFR can signal through the AKT or MAPK pathways
(17). To explore the
anti-proliferation mechanism of EGFR-targeted agents, we examined
the effects of cetuximab on the EGFR/AKT signaling pathway. MKN28
and TMK-1 cells were treated with cetuximab for 72 h. In the
EGFR-amplified cell line MKN28, cetuximab decreased both EGFR and
AKT phosphorylation when compared with the isotype controls. In
contrast, phosphorylation of EGFR or AKT was not affected by
cetuximab in TMK-1 cells, in which EGFR is not amplified (Fig. 3A). These data indicate that
cetuximab can suppress the activation of key pathways that are
downstream of EGFR.
Enhanced induction of apoptosis by
combined 5FU and cetuximab in EGFR-amplified GC cells
To investigate the mechanism underlying the
synergistic growth inhibition induced by combination of 5FU and
cetuximab, we examined the effects of each agent alone and in
combination on apoptosis in GC cells. An assay based on the binding
of Annexin V to the cell surface revealed that the frequency of
apoptosis was markedly greater in EGFR-amplified cells treated with
both 5FU and cetuximab than in cells treated with either agent
alone (Fig. 3B). No such effect
was observed in cells negative for EGFR amplification. These data
indicate that the combination of 5FU and cetuximab exhibits an
enhanced apoptotic effect in EGFR-amplified GC cells, but not in
those without EGFR amplification.
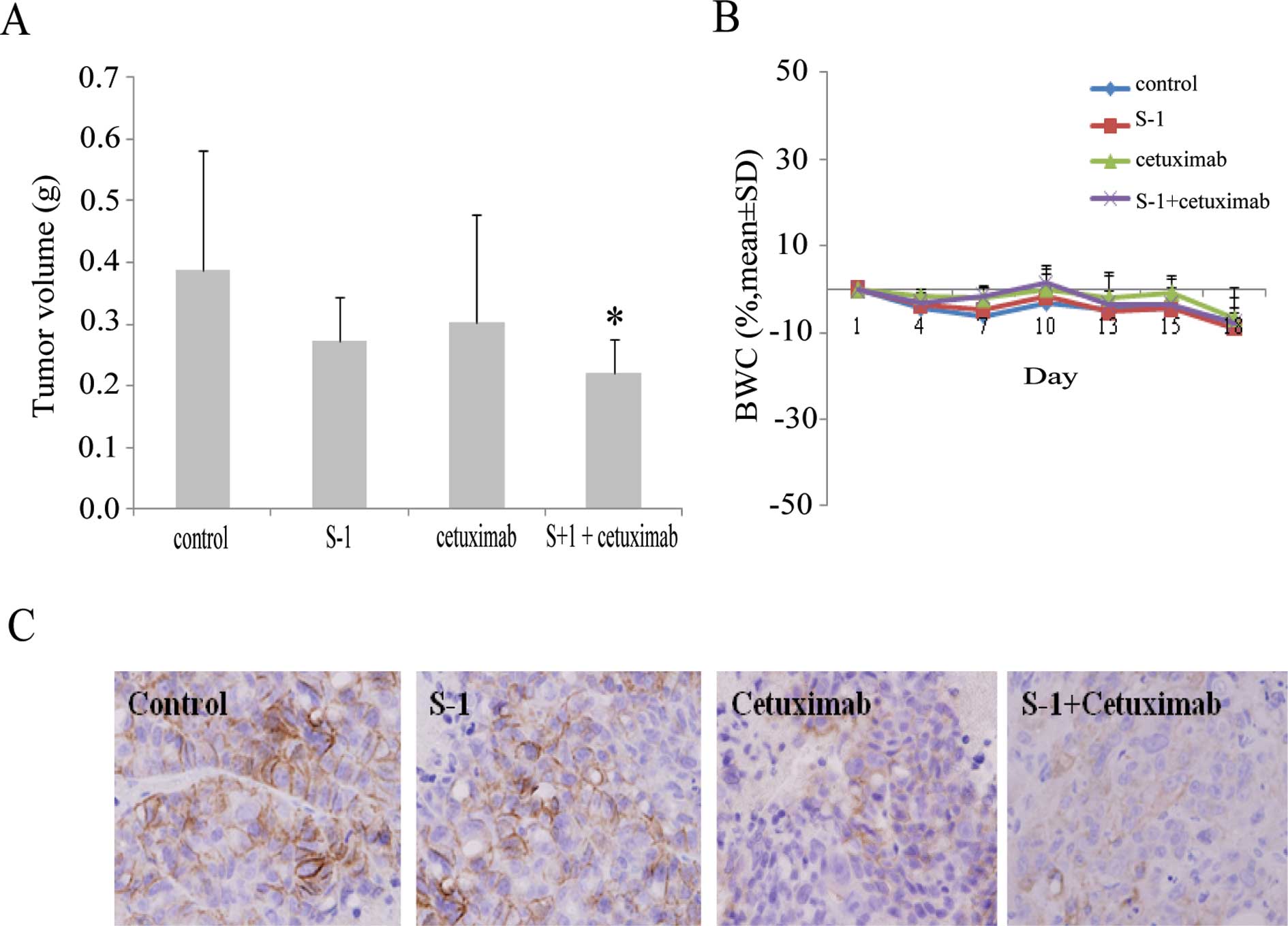
Effects of combination cetuximab and S-1
therapy on EGFR-overexpressing human GC xenograft models
The antitumor activities of cetuximab combined with
chemotherapy were examined in an EGFR-overexpressing human GC
xenograft model. Mice with tumors derived from MKN28 cells were
divided into groups for treatment with vehicle, S-1, cetuximab, or
combined S-1/cetuximab for 14 days. Tumor volume (TV) was evaluated
between groups at the end of the experiment. The TV (g) for
combined S-1/cetuximab was 0.22±0.05 g, whereas for control, S-1
and cetuximab alone was 20.0±1.96 g, 0.27±0.07 g and 0.30±0.17 g,
respectively. Additionally, the TGI % for cetuximab combined with
S-1 was 43.2%, while that for S-1 and cetuximab alone was 29.8 and
22.4%, respectively. Combination S-1/cetuximab therapy inhibited
the growth of tumors formed by EGFR-amplified MKN28 cells compared
to treatment with either agent alone (P<0.05) (Fig. 4A). All treatments were well
tolerated by the mice, with no signs of toxicity or weight loss
during therapy (Fig. 4B).
Furthermore, tumors in each treatment group were examined for
expression of EGFR protein by IHC. EGFR expression was decreased in
the cetuximab alone and the S-1/cetuximab groups compared to the
control and S-1 alone groups (Fig.
4C). Thus, the combination S-1/cetuximab therapy appears to
result in an enhanced antitumor effect in EGFR-amplified GC
xenografts, consistent with the results obtained in
vitro.
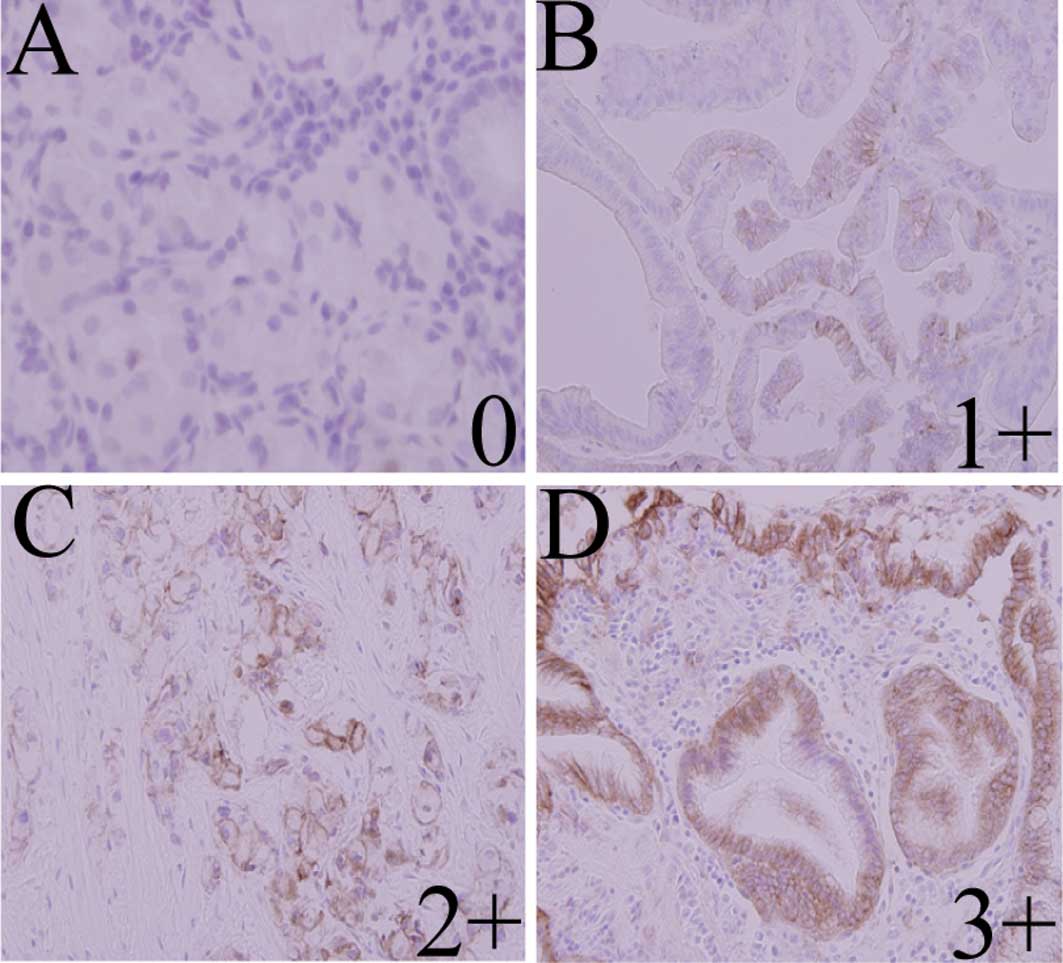
EGFR expression in patients with GC
Among the 40 specimens, the median age of patients
was 67 years (range, 32–94 years); 20 patients had differentiated
carcinoma and the other half had undifferentiated carcinoma.
Formalin-fixed paraffin-embedded specimens from all 40 patients
were examined for EGFR by IHC (Fig.
5). Analysis of EGFR protein expression by microscopic
observation revealed a score of 0 in24 cases (60%), 1+ in 5 cases
(12.5%), 2+ in 6 cases (15%) and 3+ in 5 cases (12.5%). EGFR
reactivity was not correlated with gender, age, differentiation,
lymph node metastasis or distant metastasis. Significantly more
clinicopathological stage III/IV cases had EGFR-positive disease
(30%) compared to stage I/II cases (10%, P=0.0088) (Table II). These results indicate that
EGFR positivity is associated with advanced disease in GC.
 | Table IIAssociation between EGFR status and
clinicopathological characteristics of patients including gender,
age, stage, differentiation and metastasis. |
Table II
Association between EGFR status and
clinicopathological characteristics of patients including gender,
age, stage, differentiation and metastasis.
| Positive patients
(%) | Negative patients
(%) | P-value |
|---|
| Total | 16 (40.0) | 24 (60.0) | |
| Gender | | | |
| Male | 14 (35.0) | 14 (35.0) | 0.078 |
| Female | 2 (5.0) | 10 (25.0) | |
| Age | | | |
| <70 | 9 (22.5) | 7 (17.5) | 0.109 |
| ≥70 | 7 (17.5) | 17 (42.5) | |
| Stage | | | |
| IA/IB/II | 4 (10.0) | 17 (42.5) | 0.008 |
|
IIIA/IIIB/IIIC/IV | 12 (30.0) | 7 (17.5) | |
|
Differentiation | | | |
|
Differentiated | 9 (22.5) | 11 (27.5) | 0.747 |
|
Undifferentiated | 7 (17.5) | 13 (32.5) | |
| Lymph node
metastasis | | | |
| Absent | 3 (7.5) | 12 (30.0) | 0.100 |
| Present | 12 (30.0) | 13 (32.5) | |
| Distant
metastasis | | | |
| Absent | 1 (2.5) | 2 (5.0) | 0.999 |
| Present | 15 (37.5) | 22 (55.0) | |
Discussion
EGFR amplification has been suggested to be
associated with prognosis in gastrointestinal carcinoma. Cetuximab
is a chimeric anti-EGFR monoclonal antibody that inhibits signaling
pathways affecting cellular growth, differentiation and
proliferation (18). Cetuximab is
widely used as a standard therapy for EGFR-positive colorectal and
head and neck cancer, and shows clinical efficacy both alone and in
combination with chemotherapeutic agents (19–23).
However, only limited evaluation of EGFR-targeted agents has been
conducted in GC models and most such studies have been restricted
to EGFR-amplified cells. Furthermore, the mechanisms of action of
EGFR-targeted agents in combination with cytotoxic agents have
remained unclear. In the present study, we have shown that the
combination of S-1 (or 5FU) and EGFR-targeted therapy results in a
synergistic antitumor effect in EGFR-amplified GC cells, but not in
those lacking EGFR amplification.
To explore the efficacy of cetuximab alone or in
combination with S-1 in EGFR-overexpressed GC, we evaluated the
effect of cetuximab in a panel of molecularly characterized human
GC cell lines. The level of EGFR protein expression was determined
for a panel of six human GC cancer cell lines, and mRNA status was
assessed by real-time PCR. Dose-response curves were generated to
determine sensitivity to 5FU or cetuximab. Cetuximab had
concentration-dependent anti-proliferative activity across the
panel, with the greatest effects in EGFR-amplified MKN28 cells. In
contrast, the EGFR low-expressing cell lines MKN74 and TMK-1 were
less sensitive to cetuximab. Moreover, Fig. 2 shows that the combination of 5FU
and cetuximab was highly synergistic in inhibiting cell growth,
with a CI of <1 in MKN28. Cetuximab inhibited phosphorylation of
EGFR (pEGFR) and AKT (pAKR) in EGFR-amplified MKN28 cells, but not
in cells without EGFR amplification. Down-regulation of pEGFR and
pAKR by cetuximab treatment may therefore enhance 5FU-induced
apoptosis. Previous studies reported that activation of EGFR leads
to downstream signaling that activates mitogenic and survival
pathways, such as the MAPK and PI3-K/AKT pathways (24). Inhibition of these pathways by EGFR
antagonists, such as cetuximab, can lead to induction of apoptosis
and anti-proliferative effects (25). These results suggest that
combination therapy may block the signaling pathways downstream of
EGFR. Moreover, the efficacy of cetuximab monotherapy, S-1
monotherapy or combination S-1/cetuximab was examined in an
EGFR-amplified xenograft model. In the MKN28 xenograft, combination
S-1/cetuximab induced a near complete tumor regression in all
treated mice. In the cetuximab monotherapy and combination
S-1/cetuximab treatment groups, EGFR expression was down-regulated
compared to that of the control and S-1 monotherapy groups,
suggested that cetuximab may be blocking combination of ligand and
EGFR-driven signaling. The combination therapy effects were more
pronounced than either cetuximab or S-1 alone. EGFR expression was
required to obtain a positive response to combination
S-1/cetuximab, which is consistent with the results of numerous
studies demonstrating that the antitumor activity of several
anticancer agents increases when combined with cetuximab (26–34).
Clinical specimens from 40 patients with GC were
examined for EGFR by IHC. EGFR expression was detected in 40%
(16/40) of clinical specimens. Overall, 30% of clinicopathological
stage III/IV patients demonstrated EGFR positivity (P=0.0088).
These data indicate that EGFR expression is associated with
advanced disease in GC. EGFR appears to be a potential target
molecule for the treatment of GC. A recently reported phase II
clinical trial showed a significant gain in OS for EGFR-positive
patients with advanced GC who received combined treatment with
cetuximab and FOLFOX6 (35). The
present observations provide a rationale for clinical evaluation of
combination chemotherapy with S-1 and EGFR-targeted agents
according to EGFR amplification status.
In conclusion, the present results demonstrate that
the combination cetuximab/S-1 can enhance S-1 antitumor activity.
EGFR amplification is the best predictive marker for the
anti-proliferative effects of combination chemotherapy with S-1 and
cetuximab. The combination of cetuximab and S-1 may be a promising
therapeutic strategy for some patients with EGFR-amplified GC.
Acknowledgements
We thank Taiho Pharmacy Co., Ltd. for provision of
compounds and for helpful discussions and we are thankful to Yumi
Yoshimura for technical assistance in the experiments.
Abbreviations:
|
GC
|
gastric cancer
|
|
EGFR
|
epidermal growth factor receptor
|
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Mari E, Floriani I, Tinassi A, et al:
Efficacy of adjuvant chemotherapy after curative resection for
gastric cancer: a meta-analysis of published randomized trials. Ann
Oncol. 11:837–843. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Panzini I, Gianni L, Fattori PP, et al:
Adjuvant chemotherapy in gastric cancer: a meta-analysis of
randomized trials and a comparison with previous meta-analyses.
Tumori. 88:21–27. 2002.PubMed/NCBI
|
|
4
|
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1
M otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koizumi W, Kurihara M, Nakano S and
Hasegawa K: Phase II study of S-1, a novel oral derivative of
5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative
Gastric Cancer Study Group. Oncology. 58:191–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1 an oral
fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boku N: Chemotherapy for metastatic
disease: review from JCOG trials. Int J Clin Oncol. 13:196–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar
|
|
10
|
Wesolowski R, Lee C and Kim R: Is there a
role for second-line chemotherapy in advanced gastric cancer?
Lancet Oncol. 10:903–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iqbal S, Goldman B, Fenoglio-Preiser CM,
Lenz HJ, Zhang W, Danenberg KD, Shibata SI and Blanke CD: Southwest
Oncology Group study S0413: a phase II trial of lapatinib
(GW572016) as first-line therapy in patients with advanced or
metastatic gastric cancer. Ann Oncol. May 20–2011.(Epub ahead of
print).
|
|
13
|
Cappetta A, Lonardi S, Pastorelli D,
Bergamo F, Lombardi G and Zagonel V: Advanced gastric cancer (GC)
and cancer of the gastro-oesophageal junction (GEJ): focus on
targeted therapies. Crit Rev Oncol Hematol. Jan 19–2011.(Epub ahead
of print).
|
|
14
|
Cunningham D, Humblet Y, Siena S, et al:
Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saltz LB, Meropol NJ, Loehrer PJ Sr,
Needle MN, Kopit J and Mayer RJ: Phase II trial of cetuximabin
patients with refractory colorectal cancer that expresses the
epidermal growth factor receptor. J Clin Oncol. 22:1201–1208. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carpenter G and Cohen S: Epidermal growth
factor. J Biol Chem. 265:7709–7712. 1999.
|
|
19
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vermorken JB, Mesia R, Rivera F, et al:
Platinum-based chemotherapy plus cetuximab in head and neck cancer.
N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hanna N, Lilenbaum R, Ansari R, et al:
Phase II trial of cetuximab in patients with previously treated
non-small cell lung cancer. J Clin Oncol. 24:5253–5258. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JC, Wang ST, Chow NH and Yang HB:
Investigation of the prognostic value of coexpressed erbB family
members for the survival of colorectal cancer patients after
curative surgery. Eur J Cancer. 38:1065–1071. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Porebska I, Harlozinska A and Bojarowski
T: Expression of the tyrosine kinase activity growth factor
receptors (EGFR, ERBB2, ERBB3) in colorectal adenocarcinomas and
adenomas. Tumor Biol. 21:105–115. 2002.PubMed/NCBI
|
|
24
|
Bancroft CC, Chen Z, Yeh J, et al: Effects
of pharmacologic antagonists of epidermal growth factor receptor,
PI3K and MEK signal kinases on NF-κB and AP-1 activation and IL-8
and VEGF expression in human head and neck squamous cell carcinoma
lines. Int J Cancer. 99:538–548. 2002.
|
|
25
|
Raymond E, Fauvre S and Armand JP:
Epidermal growth factor receptor tyrosine kinase as a target for
anticancer therapy. Drugs. 60(Suppl 1): S15–S23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baselga J, Norton L, Masui H, Pandiella A,
Coplan K, Miller WH and Mendelsohn J: Antitumor effects of
doxorubicin in combination with anti-epidermal growth factor
receptor monoclonal antibodies. J Natl Cancer Inst. 85:1327–1333.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruns CJ, Harbison MT, Davis DW, et al:
Epidermal growth factor receptor blockade with cetuximab plus
gemcitabine results in regression of human pancreatic carcinoma
growing orthotopically in nude mice by antiangiogenic mechanisms.
Clin Cancer Res. 6:1936–1948. 2006.
|
|
28
|
Ciardiello F, Bianco R, Damiano V, et al:
Antitumor activity of sequential treatment with topotecan and
anti-epidermal growth factor receptor monoclonal antibody C225.
Clin Cancer Res. 4:909–916. 1999.PubMed/NCBI
|
|
29
|
Fan Z, Baselga J, Masui H and Mendelsohn
J: Antitumor effect of anti-EGF receptor monoclonal antibodies plus
cis-diamminedichloroplatinum (cis-ddp) on well established A431
cell xenografts. Cancer Res. 53:4637–4642. 1993.PubMed/NCBI
|
|
30
|
Huang SM, Bock JM and Harari PM: Epidermal
growth factor receptor blockade with C225 modulates proliferation,
apoptosis, and radiosensitivity in squamous cell carcinomas of the
head and neck. Cancer Res. 59:1935–1940. 1999.PubMed/NCBI
|
|
31
|
Huang SM and Harari PM: Modulation of
radiation response after epidermal growth factor receptor blockade
in squamous cell carcinomas: inhibition of damage repair, cell
cycle kinetics, and tumor angiogenesis. Clin Cancer Res.
6:2166–2174. 2000.
|
|
32
|
Inoue K, Slaton JW, Perrotte P, et al:
Paclitaxel enhances the effects of the anti-epidermal growth factor
receptor monoclonal antibody ImClone C225 in mice with metastatic
human bladder transitional cell carcinoma. Clin Cancer Res.
6:4874–4884. 2000.
|
|
33
|
Prewett MC, Hooper AT, Bassi R, Ellis LM,
Waksal HW and Hicklin DJ: Enhanced antitumor activity of
anti-epidermal growth factor receptor monoclonal antibody IMCC225
in combination with irinotecan (CPT-11) against human colorectal
tumor xenografts. Clin Cancer Res. 8:994–1003. 2002.
|
|
34
|
Shin DM, Donato NJ, Perez-Soler R, et al:
Epidermal growth factor receptor-targeted therapy with C225 and
cisplatin in patients with head and neck cancer. Clin Cancer Res.
7:1204–1213. 2001.PubMed/NCBI
|
|
35
|
Han SW, Oh DY, Im SA, Park SR, Lee KW,
Bang YJ and Kim TY: Phase II study and biomarker analysis of
cetuximab combined with modified FOLFOX6 in advanced gastric
cancer. Br J Cancer. 100:298–304. 2009. View Article : Google Scholar : PubMed/NCBI
|