Introduction
Neuroblastoma (NB) is a tumour of the neural
crest-derived sympathetic ervous system and is therefore
predominantly located in the paravertebral sympathetic trunk and in
the adrenal medulla. It is the most frequent extracranial solid
tumour in childhood with a peak of incidence in the second year of
life. Clinically, NB is divided into stages 1, 2, 3, 4 and 4s,
according to the international neuroblastoma staging system (INSS)
(1–3). Roughly they form two distinct groups,
which are characterized as follows. The first group, clinically
referred to as stages 1 and 2, comprises localized and unilateral
tumours with regional lymph node involvement only in stage 2B. The
tumours can differentiate into benign ganglioneuroblastoma or even
may regress spontaneously. The second group, stages 3 and 4,
includes infiltrating and disseminating tumours with affection of
loco-regional or contra-lateral lymph nodes in stage 3 and
metastases to distant lymph nodes, bone marrow, liver and other
organs in stage 4. These lesions aggressively invade surrounding
tissues, respond poorly to chemotherapy or relapse frequently due
to the existence of therapy-resistant cells. While children with
stage 1 and 2 tumours have a favourable prognosis, stage 4 NB often
has an adverse outcome. The ‘special’ stage 4s, which, by
definition, is only diagnosed in children younger than 1 year of
age, bears features from both groups. It is characterised by skin
and liver metastases and weak dissemination to lymph nodes and bone
marrow. Despite the presence of metastases, these children have a
favourable prognosis as their lesions respond well to mild
chemotherapy and tend to have spontaneous differentiation or
complete regression.
To date, the most important prognostic molecule in
NB is the proto-oncogene MYCN. Its amplification strongly
correlates with failure of chemotherapy and unfavourable outcome
(for review see refs. 4–6). We have started a search for molecules
that control the metastatic behaviour of NB and observed regulation
of the extra-cellular matrix protein keratoepithelin, the secreted
hormone-like glycoprotein stanniocalcin-2 and the lymphangiogenesis
inhibitor endogenous soluble vascular endothelial growth factor
receptor-2 (esVEGFR-2) in disseminated stages of NB (7–10).
At least in part, NB metastasizes along the lymphatic vascular
system. In transcriptome micro-array analyses, we and others
observed high expression of Reelin, a neuronal guidance molecule,
in human lymphatic endothelial cells (LECs) as compared to blood
vascular endothelial cells (BECs) (11–13).
This prompted us to investigate the expression and function of
Reelin in NB.
The Reelin gene was identified to harbour the
mutation, which is causative for the phenotype of the so called
‘reeler’ mouse (14). Reelin
encodes a secreted 388 kDa extra-cellular matrix protein, which
tends to form homodimers in vivo and may have
serine-protease activity, although the latter has been called into
question recently (15,16). The spontaneous loss-of-function
mutation of the Reelin gene in the reeler mouse strain causes
inappropriate neuronal migration and lamination in the cerebral
cortex and maldevelopment of the cerebellum, which leads to tremor
and ataxia. Reelin is expressed in numerous tissues outside the
CNS, but its functions there are practically unknown. Reelin
signalling is predominantly transmitted by two trans-membrane
receptors: the very low density lipoprotein receptor (VLDLR) and
the apoprotein E receptor 2 (APOER2/LRP8) (17). Binding of Reelin leads to
clustering of the receptors and subsequent phosphorylation of DAB1
(Drosophila disabled homologue 1), an adapter protein
associated with the intracellular domain of both receptors
(18–20). Further downstream targets are
members of the src-family and the protein kinase B/Akt pathway
(21). Simultaneous knock-out of
the receptors LRP8 (APOER2) and VLDLR, or disruption of DAB1, cause
a phenotype highly similar to that of the Reeler mouse (20,22).
Besides VLDLR and LRP8, Reelin also binds to α3β1-integrin and
thereby inhibits neuronal migration (23). Amyloid precursor protein (APP) is
another potential receptor, located at synaptic membranes. It
induces DAB1 phosphorylation upon Reelin binding and links Reelin
to Alzheimer’s disease (24).
Recently, Yip et al have reported that Reelin and Dab1 are
also expressed in the spinal cord and control the positioning of
the preganglionic sympathetic neuron (25). Evangelisti et al observed
regulation of Reelin by the microRNA-128 (miR-128) in NB (26). Since Reelin is a highly potent
regulator of neuronal migration in the central nervous system, we
hypothesized that it may be involved in the progression of NB. We
show that Reelin induces migration of NB cells in vitro.
Reelin is downregulated in metastatic NB stages, which may render
the cells more susceptible for Reelin from other sources, e.g. the
tumour LECs and BECs. We postulate that the downregulation of
Reelin in tumour cells and its simultaneous upregulation in
endothelial cells is part of a switch that promotes metastasis
formation.
Materials and methods
Cell culture
Initially, neuroblastoma cell lines were cultured in
RPMI-1640 medium containing 10% fetal calf serum (FCS) and 1%
penicillin/streptomycin (Lonza, Cologne, Germany). Cell
supernatants for Reelin western blots were obtained from 48-h
cultures in serum-free RPMI-1640. As we detected considerable
amounts of Reelin in FCS (data not shown), we changed culture
conditions for all subsequent experiments to serum-free PC1 medium
(Lonza) and to serum-free RPMI-1640 for migration assays.
Neuroblastoma cell lines used in this study are: Kelly, Lan 2, NGP,
SH-SY5Y, SK-N-AS and SMS-Kan. Commercial sources and references for
all cell lines were published previously (8).
Supernatants from HEK-293 cells containing a Reelin
expression plasmid or a control vector were obtained from 48-h
cultures of nearly confluent plates with serum-free RPMI-1640
medium (17).
Human umbilical vein endothelial cells (HUVECs) were
freshly prepared from umbilical cords and cultured in endothelial
cell growth medium (Lonza). Lymphatic endothelial cells (LECs) were
cultured in endothelial cell growth medium supplemented with VEGF-C
and were characterized previously, as were the HUVECs (11).
Primary neuroblastoma samples and human
tissues
RNA samples of 50 primary, untreated neuroblastomas,
were provided by the German Neuroblastoma Studies Group (Drs F.
Berthold, B. Hero and J. Theissen, Children’s Hospital University
Cologne, Cologne, Germany). The samples were tested for RNA
integrity with a Bioanalyzer 2100 (Agilent Technologies, Böblingen,
Germany). One sample failed the test and was discarded. The
remaining 49 samples were of the following stages: stage 1 (n=8),
stage 2 (n=6), stage 3 (n=5; 2 were MYCN-amplified), stage 4 (n=20;
10 were MYCN-amplified), stage 4s (n=10; 1 was MYCN-amplified).
Human foreskin was obtained from resection surgery
at the University Medicine Goettingen. The studies were approved by
the university’s ethics committee.
Real-time RT-PCR
Reverse transcription was carried out with 1
μg total RNA and Omniscript reverse transcriptase (Qiagen,
Hilden, Germany) according to the manufacturer’s instructions. For
real-time RT-PCR we used the SYBR-Green JumpStart Taq ReadyMix
(Sigma-Aldrich, Taufkirchen, Germany) and the following primer
pairs (Iba, Göttingen, Germany): β-Actin_fwd
5′-GCATCCCCCAAAGTTCACAA-3′, β-Actin_rev 5′-AGGACTGGGCCATTCTCCTT-3′,
DAB1_fwd 5′-GCCTGGACACATTGACTGAA-3′, DAB1_rev
5′-TCTTGCTGAGTGCAGTGTCC-3′, LRP8_fwd 5′-CTGATGGCTCCGATGAGTC-3′,
LRP8_rev 5′-GGTCCACAGCTCAGCTTCTC-3′, Reelin_fwd
5′-CATGGCTACAGCAACACACC-3′, Reelin_rev 5′-GTGGG TGCACAGTGACATCT-3′,
VLDLR_fwd 5′-GGCAGTGTAATGGTATCCGAGACT-3′, VLDLR_rev
5′-AGGGCCCAAGCACTGATTG-3′.
Western blot analysis
Cell culture supernatant of adherent cells was
harvested and centrifuged for 10 min at 3,000 × g and 4°C. Cells
were counted and the volume of the supernatants was standardized to
1.5×106 cells. Cell lysates were prepared directly from
cultured cells using lysis-buffer (30 mM Tris-Cl, pH 7.5, 150 mM
NaCl, 1 mM EDTA, 1 mM DTT) and sample complete protease inhibitor
cocktail according to the manufacturer’s instructions (Roche,
Grenzach, Germany). Samples were adjusted to equal amounts of total
protein, subjected to SDS-PAGE and subsequent protein blotting to a
PVDF membrane (Roth, Karlsruhe, Germany). Membranes were blocked
using 5% bovine serum albumin in Tris-buffered saline (20 mM
Tris-Cl, 150 mM NaCl, 0.02% Tween-20) for 60 min. Primary and
secondary antibodies were diluted in blocking buffer. Antibodies
used were anti-Reelin (mouse monoclonal; Santa Cruz Biotechnology,
Heidelberg, Germany), anti-VLDLR (mouse, monoclonal; Santa Cruz
Biotechnology) anti-β-actin (mouse, monoclonal; Santa Cruz
Biotechnology), anti-DAB1 (mouse monoclonal clonal; Abnova,
Heidelberg, Germany), anti pY220 DAB1 (rabbit polyclonal; Abnova),
anti-LRP8 (rabbit polyclonal; Abcam, Cambridge, UK). For detection
we used horseradish peroxidase (HRP)-coupled antibodies: goat
anti-mouse HRP or goat anti-rabbit HRP (both Santa Cruz
Biotechnology). Chemiluminescence was achieved using the Amersham
ECL western blotting system (GE Healthcare) and detected and
developed on SuperRX X-ray film (Fujifilm, Düsseldorf,
Germany).
Immunohistology
Formalin-fixed primary NB samples were obtained from
the German Neuroblastoma Studies Group (Children’s Hospital
University Cologne, Germany) and by the Department of Pathology,
University Medicine Goettingen (Head: Professor H.J. Radzun).
Histological grading was evaluated according to (27) and the International Neuroblastoma
Pathology Classification (INPC), established in 1999 and modified
in 2003 (28). Paraffin sections
of 7 μm were prepared for antigen retrieval as described
recently (29). Primary antibodies
used were anti-Reelin (mouse monoclonal; Santa Cruz Biotechnology),
anti-LRP8 (rabbit polyclonal; Abcam), anti-DAB1 (goat polyclonal;
Santa Cruz Biotechnology), anti-neurofilament (mouse monoclonal,
Dako, Hamburg, Germany) and anti-Prox1 (rabbit polyclonal,
Reliatech, Wolfenbüttel, Germany). Secondary antibodies were either
peroxidaseconjugated goat-anti-rabbit IgG, rabbit anti-mouse IgG
(1:100, Sigma-Aldrich) or Alexa 488-conjugated goat anti-mouse IgG,
and Alexa 594-conjugated goat anti-rabbit IgG (both 1:200,
Molecular Probes, Karlsruhe, Germany) for double
immunofluorescence. DAB was used as chromogen for peroxidase
reaction, and slides were counter-stained with nuclear fast red.
For immunofluorescence nuclei were routinely stained with DAPI.
Induction of differentiation
SH-SY5Y (106) were seeded on 10-cm
cell-culture dishes at day 1 in serum-free PC1 medium (Lonza). They
were allowed to adhere overnight. The next day (day 0) we started
treatment with all-trans-retinoic acid (ATRA) dissolved in cell
culture grade DMSO (Sigma). ATRA in DMSO was added to the medium to
a final concentration of 5 and 10 μM. In control samples,
the corresponding amount of DMSO was applied. At days 3, 6 and 9
the medium was renewed and fresh ATRA or DMSO was added. RNA was
isolated at days 0, 5, 9 and 12.
Migration assay
BD-Biocoat culture inserts (BD, Heidelberg, Germany)
without any coating were used for the trans-well migration assays.
Cells (100,000) diluted in 0.5 ml serum-free RPMI-1640 medium were
seeded into the culture insert. The lower chamber was filled with
0.5 ml culture supernatant of HEK-293 cells transfected with a
Reelin expression plasmid (17) or
the control vector. In another set of experiments, cells were
suspended in supernatants of the Reelin transfected HEK-293 cells
and migration towards RPMI-1640 containing 2.5% FCS as an
attractant was studied. Supernatants were concentrated 2-fold using
Vivaspin2 filter units (MWCO: 100 kDa; Sartorius-Stedim,
Goettingen, Germany). After 24 h, cells that migrated through the
membrane towards the attractants were stained using Richardson’s
staining solution for 1 min, mounted for microscopy and counted
manually. Experiments were repeated at least three times. Percent
of migrated cells compared to controls (100%) were calculated.
Calculations and statistics
Molecular weight of LRP8 and VLDLR were calculated
from amino acid sequences provided by the NIH at www.ncbi.nlm.nih.gov using the pI-tool at www.expasy.org. For real-time RT-PCR analyses,
relative expression levels of transcripts were calculated by the
ΔΔCt-method. Statistical analyses were performed using the GraphPad
Prism v 3.0 software (GraphPad Software Inc., La Jolla, CA, USA)
and Microsoft Excel 2008 for Mac (Microsoft Corp., Redmond, WA,
USA). Normality was tested to calculate the variance distribution
and the Mann-Whitney U test or the Unpaired t-test were used to
compare expression levels between stages of localized and
disseminated NB and to compare expression levels in NB with or
without MYCN amplification. Statistical significance is considered
at p<0.05.
Results
Expression of Reelin pathway molecules in
primary NB
To investigate the potential clinical relevance of
Reelin signalling, we measured the mRNA expression of Reelin and
its potential signal transducers LRP8, VLDLR and DAB1 in 49 samples
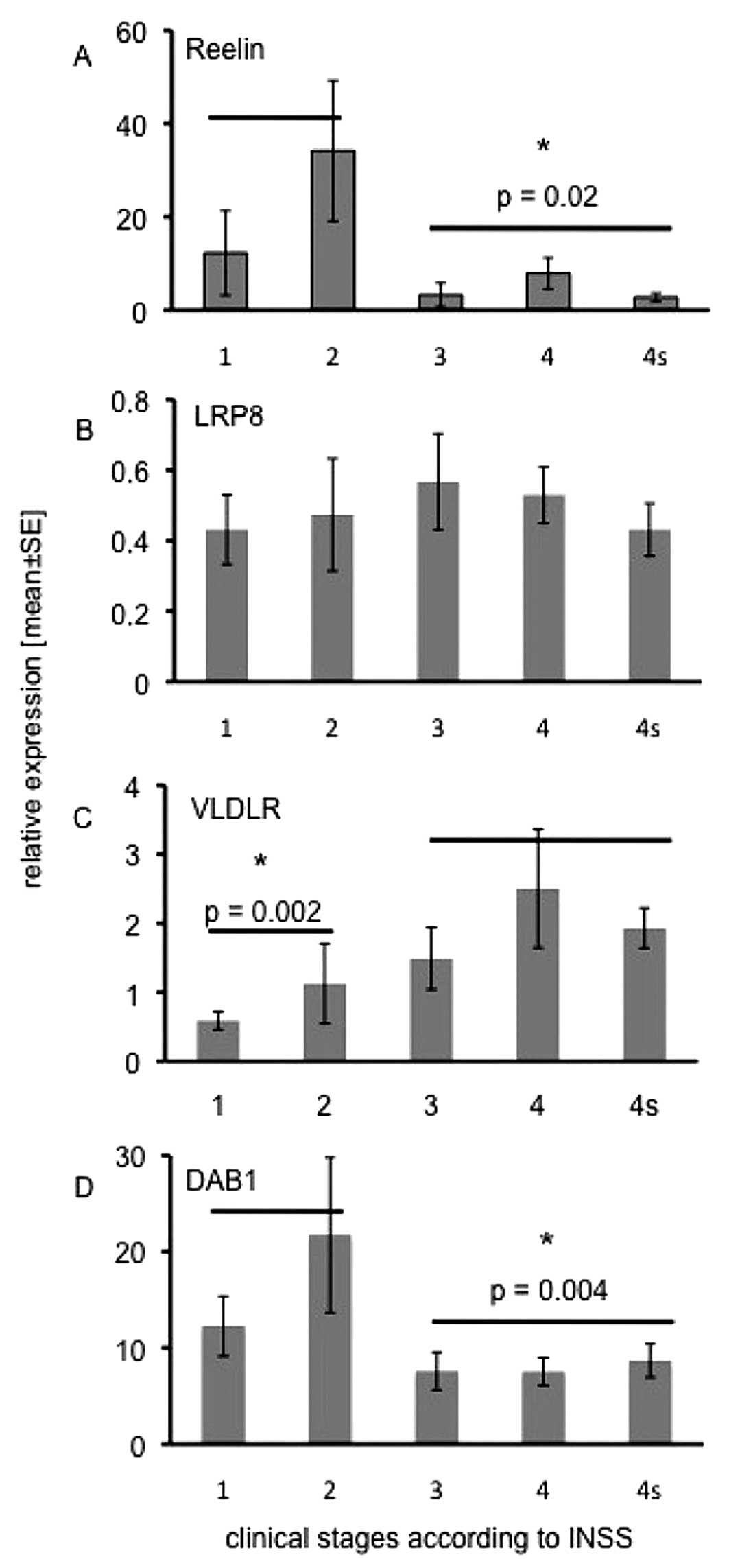
of untreated primary neuroblastoma patients. By real-time RT-PCR we
found that Reelin transcripts were significantly more abundant in
localized stage 1 and stage 2 tumours (n=14) than in the metastatic
stages 3, 4 and 4s (n=35, p=0.02; Fig.
1A). The Reelin receptor LRP8 (Fig. 1B) was equally expressed in all
stages, whereas the expression of the second Reelin receptor,
VLDLR, continuously increased from stage 1 to stage 4 (Fig. 1C). Statistical analyses revealed
that VLDLR expression levels were significantly lower in stages 1
and 2 vs. stages 3, 4 and 4s (p=0.002). Like Reelin, the adapter
protein DAB1 (Fig. 1D) was
significantly higher in stage 1 and stage 2 tumours as compared to
the disseminated stages 3, 4 and 4s (p=0.004).
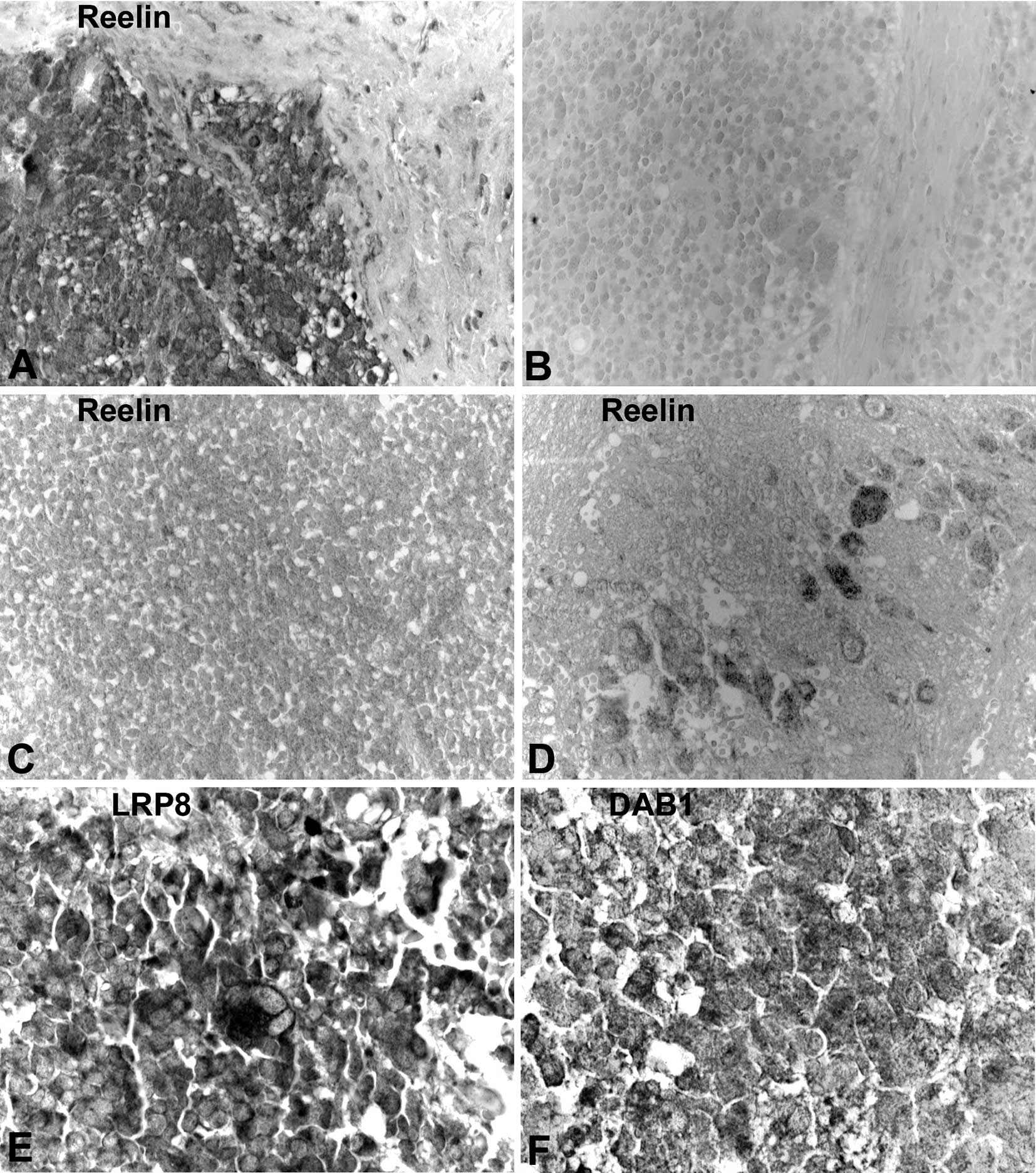
We then studied immunolocalization of Reelin
signalling molecules in primary NB samples. We observed variable
immunoreactivity intensities for Reelin, LRP8 and DAB1 in NB
samples. Thereby, expression of Reelin was higher in low-grade
tumours, where we observed signals in NB cells that showed signs of
differentiation (Fig. 2A, B and
D). In undifferentiated grade 3 NB, Reelin was almost absent
(Fig. 2C). Reelin was also
detectable, though at a lesser extent, in some stroma cells (marked
by an asterisk in Fig. 2A). In
normal tissues LRP8 was located in the cell membrane (data not
shown), whereas in NB it was mainly present in the cytoplasm
(Fig. 2E). For VLDLR we could not
find appropriate antibodies that complied with the
paraffin-embedded specimens. DAB1 was found in the cytoplasm of
differentiating type NB cells (Fig.
2F).
Expression in NB cell lines
For a further evaluation of Reelin signalling
molecules, we screened 24 NB cell lines by real-time RT-PCR and
found varying levels of Reelin, LRP8, VLDLR and DAB1 (data not
shown). We then selected 6 cell lines for western blot analyses of
whole cell lysates and, in case of Reelin, supernatants of these
cell lines. In Reelin-transfected HEK-293 cells we found three
Reelin bands at 400, 250 and 180 kDa (data not shown), as described
previously (17). Reelin was only
present in the supernatant of SK-N-AS (Fig. 3A), which may be due to the fact
that NB cell lines have usually been isolated from progressed NB.
We observed a major band at 250 kDa. For LRP8, which was
consistently found at the RNA level, we could detect a major band
at approximately 35 kDa in Kelly, NGP, SH-SY5Y, Lan 2, SMS-Kan and
SK-N-AS (Fig. 3B). However, in Lan
2 we detected a second band at 60 kDa.
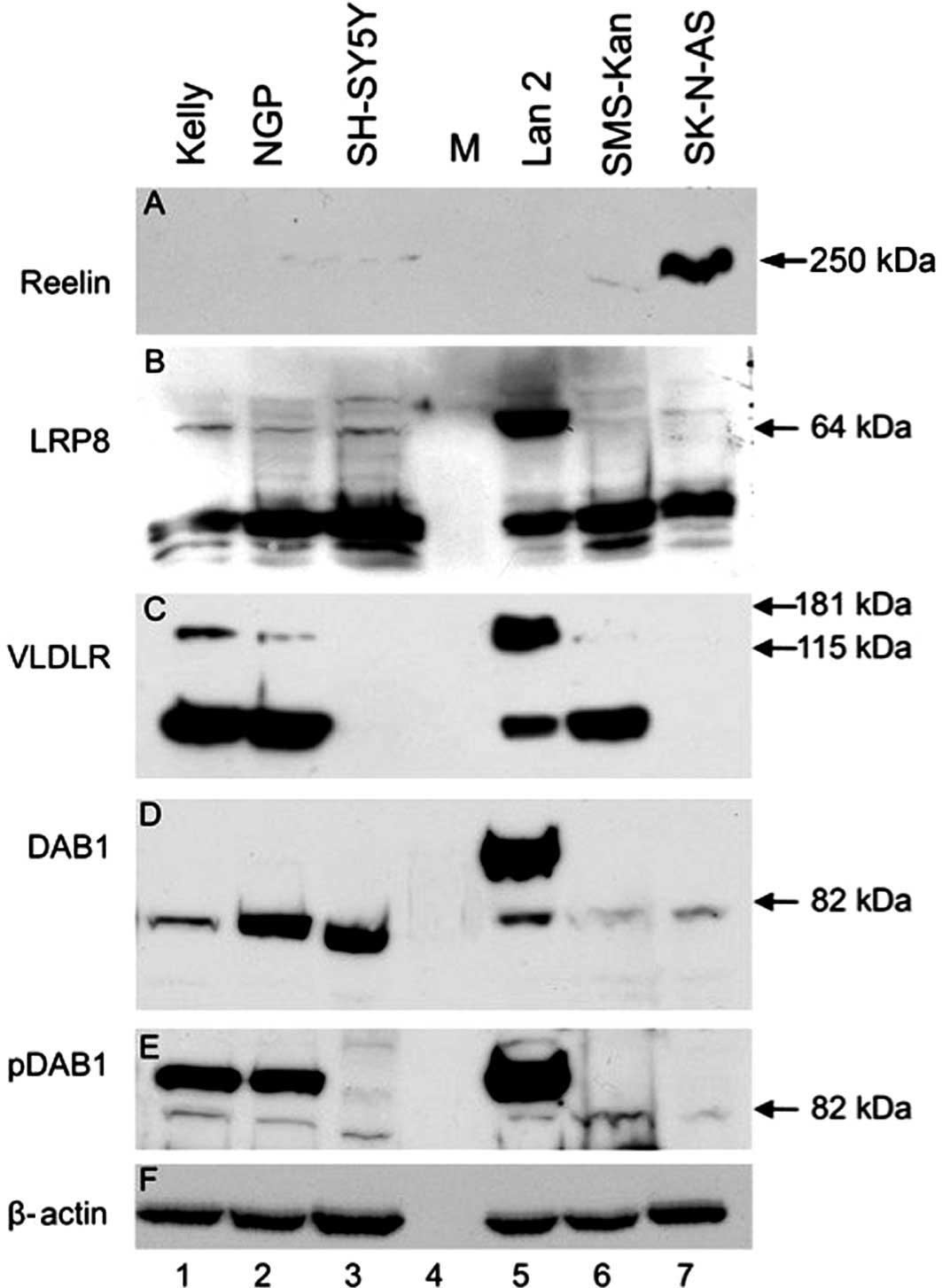 | Figure 3Protein expression of LRP8, VLDLR and
DAB1 in selected cell lines. Western blotting was performed using
cell lysates or culture supernatant of the cell lines Kelly (lane
1), NGP (lane 2), SH-SY5Y (lane 3), Lan 2 (lane 5), SMS-Kan (lane
6) and SK-N-AS (lane 7). Size marker (M) was loaded in lane 4. (A)
Reelin, (B) LRP8, (C) VLDLR, (D) DAB1, (E) phospho tyr220 DAB1, (F)
β-actin immunoreactivity is shown using antibodies as described in
Materials and methods. Note that for each protein the specific
bands appear, but additional bands in some cell lines indicate
altered or pathologic processing. The anti-β-actin was used as
loading control and shows a regular band in all cell lines. |
For VLDLR (Fig. 3C)
a major 64 kDa band was detectable in Kelly, NGP, Lan 2 and
SMS-Kan. In lysates of SH-SY5Y and SK-N-AS no immunoreactivity for
VLDLR was detected. Again, Lan 2 displayed a second major band at
approximately 150 kDa, and unless much weaker, bands of the same
molecular weight were also visible in Kelly and NGP cell
lysates.
For DAB1 (Fig. 3D),
there were two major immunoreactivity bands at approximately 115
and 82 kDa. All cell lines displayed the 82 kDa band, albeit it was
weak for Kelly, Lan 2, SMS-Kan and SK-N-AS. In SH-SY5Y lysates,
however, the corresponding band had a slightly lower molecular
weight. A second band at 115 kDa was only found in Lan 2 cells.
Constitutive DAB1 phosphorylation (pDAB1) was detected in Kelly,
NGP and Lan 2 cells (Fig. 3E). For
these experiments, cells were cultured in standard RPMI-1640 medium
containing 10% FCS. As we noticed later, FCS contains large amounts
of Reelin (data not shown), probably leading to constitutive DAB1
phosphorylation. Migration and differentiation studies performed
later were done with cells cultured under serum-free conditions
using PC1 medium or basal RPMI-1640 medium. All blots were probed
for β-actin to ensure equal loading (Fig. 3F).
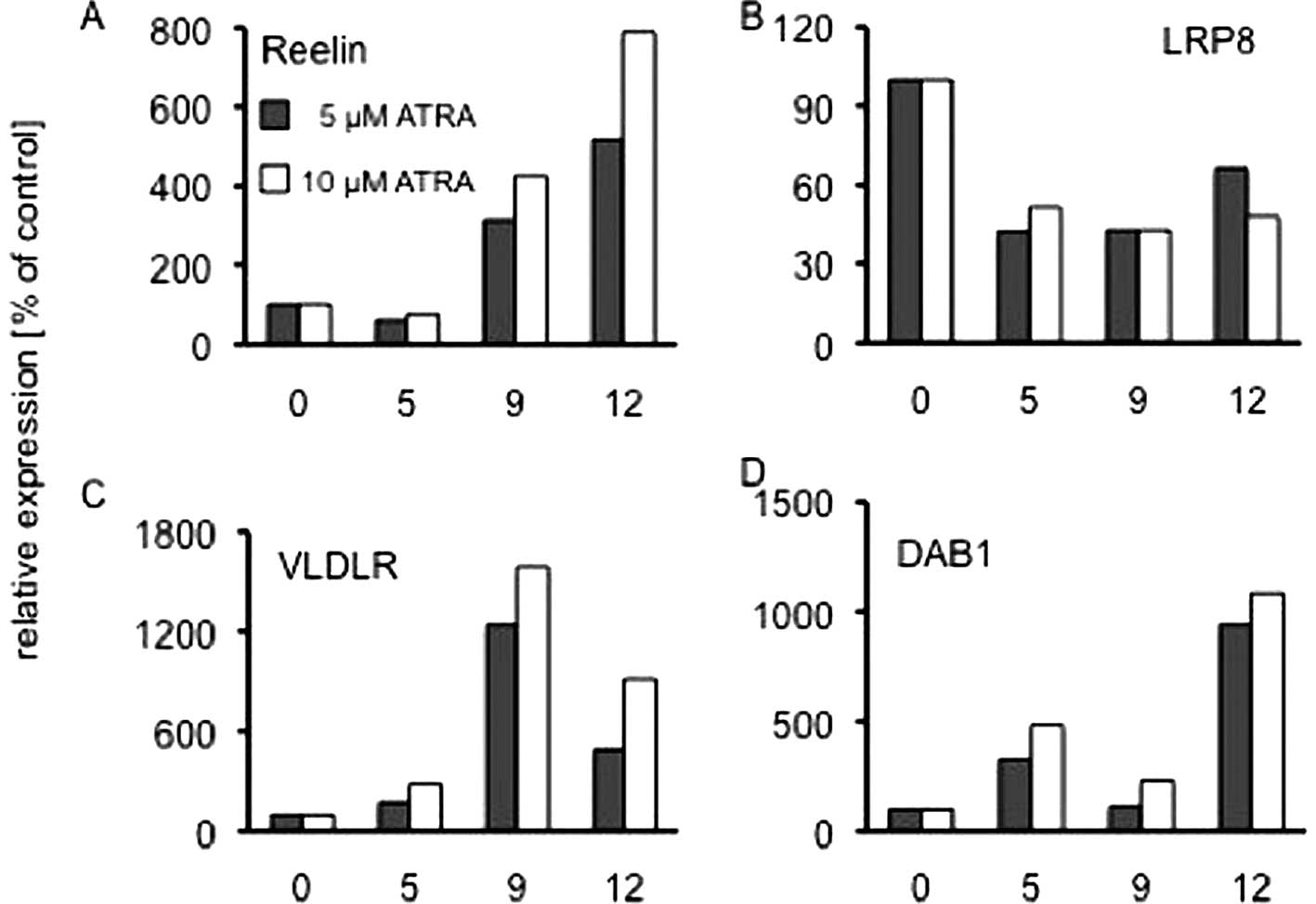
Reelin is induced during differentiation
of the NB cell line SH-SY5Y
The expression of Reelin in the patients’ samples
suggested that Reelin expression might be associated with more
differentiated tumour types and downregulated in undifferentiated
high-grade tumours. It has been reported that Reelin is induced in
the human teratocarcinoma cell line NT2 upon treatment with the
differentiating agent all-trans retinoic acid (ATRA) (30), but downregulated in the NB cell
line SH-SY5Y (26). ATRA is
clinically used in NB treatment regimens, therefore we sought to
test the response to ATRA with regard to Reelin and its signalling
pathway molecules. For this study we also used the NB cell line
SH-SY5Y, which is known to respond well to differentiating agents
(31). By quantitative real-time
RT-PCR we found that during 12 days of treatment with 5 or 10
μM ATRA, Reelin, VLDLR and DAB1 mRNA levels increased
significantly (Fig. 4A, C and D),
whereas LRP8 mRNA expression decreased by 50% during the first 5
days and remained constant thereafter (Fig. 4B). Control experiments were
performed with the solvent DMSO. For Reelin and DAB1 these findings
correlate well with our observation that these molecules are
significantly higher expressed in the NB stages 1 and 2.
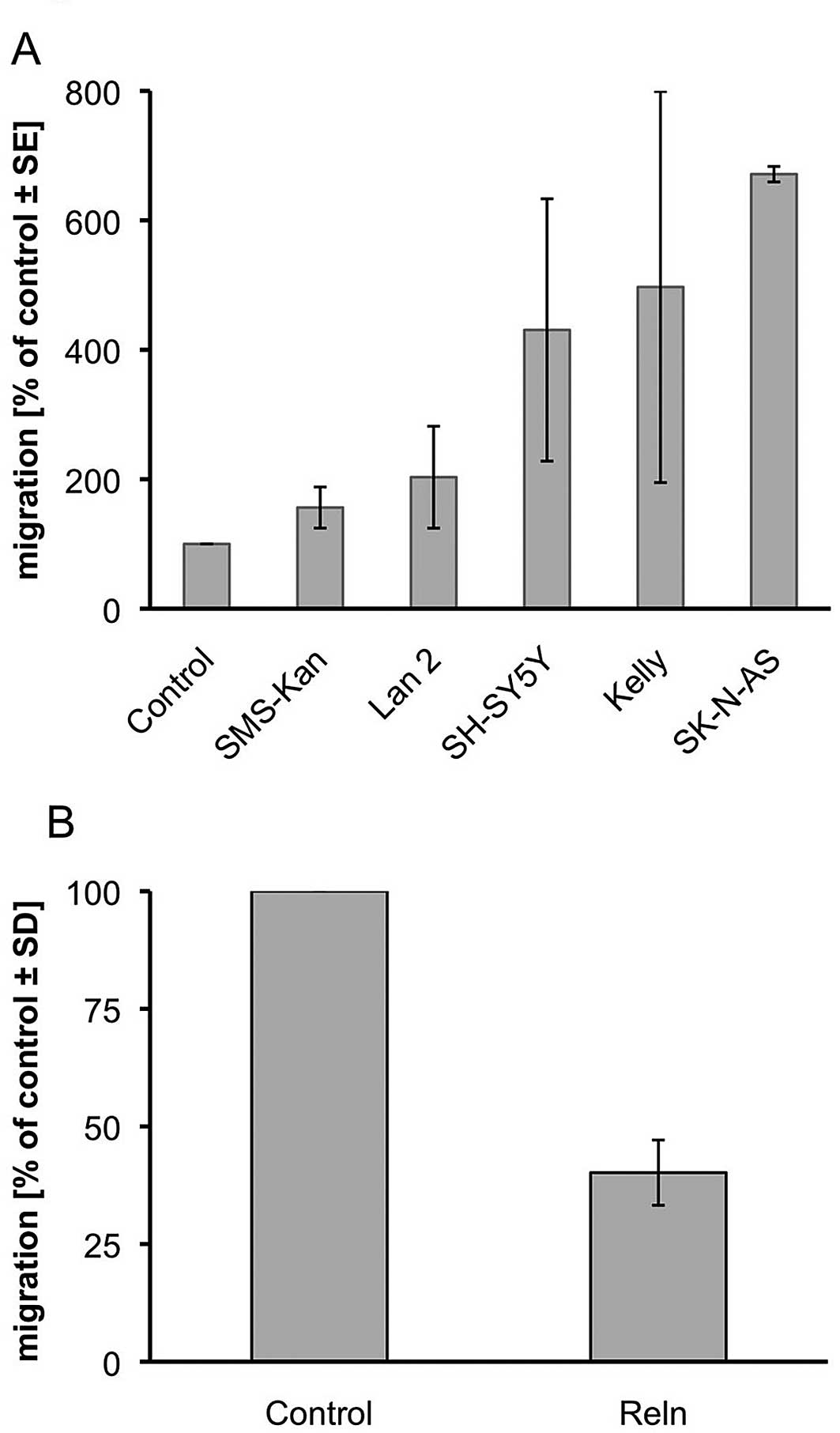
Reelin acts as a chemo-attractant on NB
cells in vitro
Reelin is a key regulator in brain development and
influences neuronal migration and patterning during cortex
lamination. We tested whether Reelin also influences NB cell
migration and subjected the NB cell lines SMS-Kan, LAN 2, SH-SY5Y,
Kelly and SK-N-AS to trans-well migration assays (Fig. 5A). We used modified Boyden chambers
and the supernatant of Reelin-expressing or control
plasmid-expressing HEK-293 cells as an attractant. All tested cell
lines showed increased migration towards the Reelin-containing
medium. After 24 h the number of migrated cells increased by 2-fold
for LAN 2 to nearly 7-fold for SK-N-AS as compared to the
respective controls. We then tested if this migratory effect can be
inhibited by Reelin. We incubated SK-N-AS cells in the upper
chamber in 2-fold concentrated supernatant of the HEK-293 cells
(Reelin-expressing vs. control) and observed a reduction of
migration into the serum-containing lower chamber by 60% (Fig. 5B).
Reelin is expressed in tumour endothelial
cells
The down-regulation of Reelin in advanced NB stages
and the attractive potential of Reelin on NB cell lines suggests
that additional Reelin sources may influence the metastatic
behaviour of NB cells. Previously, the mRNA expression of Reelin in
normal lymphatic endothelial cells (LEC) has been found in
micro-array studies (11–13). At protein level, we detected the
250 kDa form of Reelin as the dominant form in LECs, but no
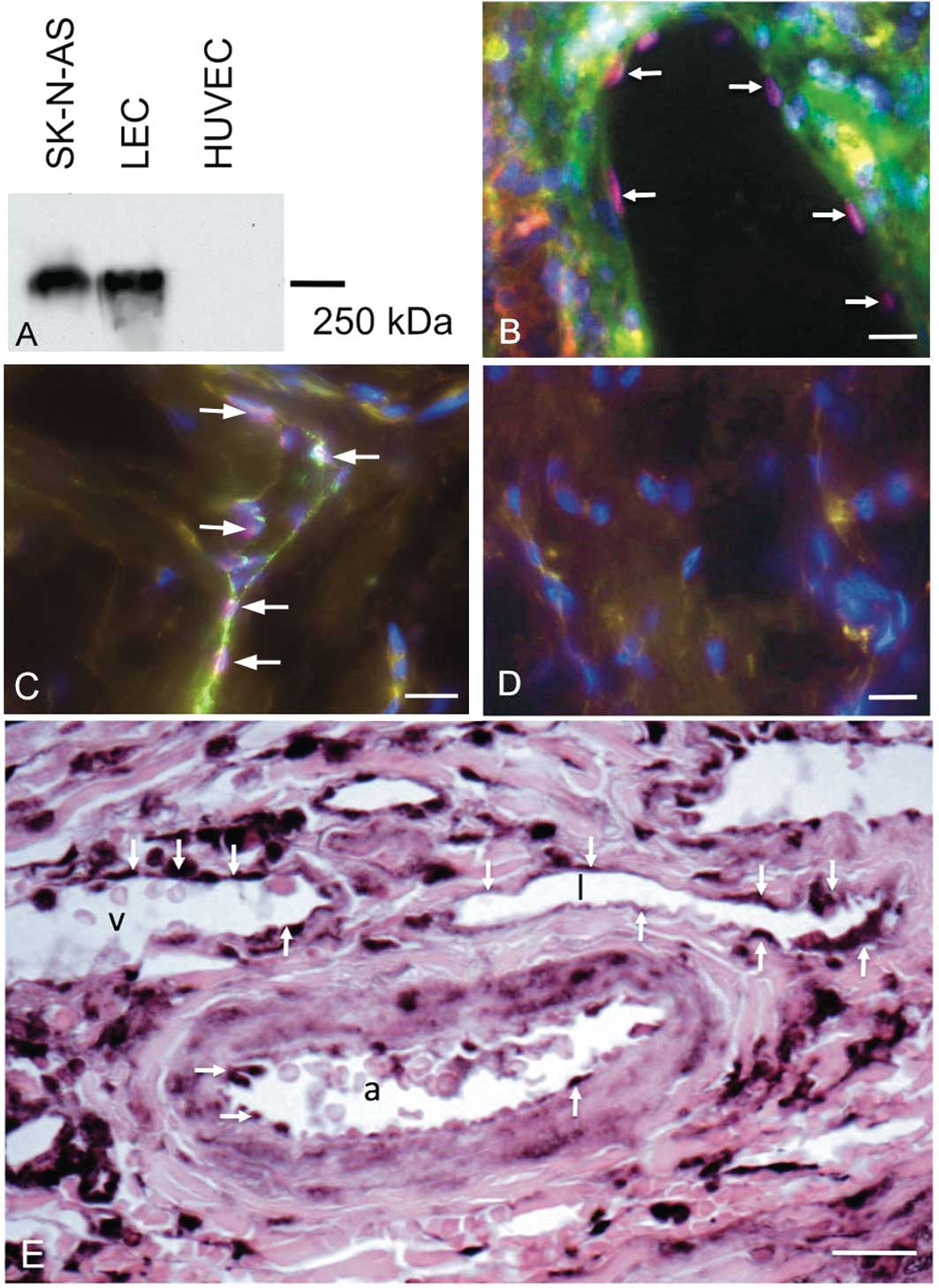
expression in HUVECs (Fig. 6A). By
immunostaining with antibodies against the LEC marker Prox1
(32), we could demonstrate the
presence of Prox1-positive lymphatics in primary NB (Fig. 6B), in accordance with previous
studies (33). Immunoreactivity
for Reelin was found in normal Prox1-positive initial lymphatics of
human foreskin (Fig. 6C and D),
and in lymphatic collectors (data not shown). In primary NB samples
we found Reelin not only in lymphatics, but also in arteries and
veins (Fig. 6E). Since Reelin
expression is hardly detectable in normal BECs (11–13),
this indicates upregulation of Reelin in tumour BECs.
Discussion
Reelin in primary neuroblastoma
Since the first description of the Reeler mouse some
60 years ago, the functions of Reelin have been studied intensively
in the central nervous system (34). Abnormal lamination of the cerebral
cortex, hypoplasia of the cerebellum and abnormal positioning of
sympathetic neurons in the spinal cord have been attributed to
impaired Reelin signalling (25,35).
In humans, malfunctions of Reelin are associated with neurological
disorders such as schizophrenia, bipolar disorders, major
depression, autism and lissencephaly (35), as well as lymphedema (36). Functions for Reelin in the
peripheral nervous system (PNS), chromaffine cells and in tumours
derived from the PNS, such as neuroblastoma (NB), have been studied
very rarely (26,37–39).
This encouraged us to investigate the expression patterns of
Reelin, its receptors LRP8 and VLDLR, as well as DAB1, one of its
most prominent downstream signalling molecules, in primary NB. Our
real-time RT-PCR analyses of 49 primary samples of untreated
patients reveal that the amount of Reelin and DAB1 transcripts are
significantly lower in the advanced NB stages 3 and 4, and in 4s
NB. While LRP8 is not altered throughout the five stages, VLDLR
expression is, in contrast, higher in the advanced stages, and in
stage 4s. By immunohistochemistry we were able to confirm that
Reelin, LRP8 and DAB1 are expressed by NB cells of primary tumours.
Thereby, Reelin is mainly found in differentiating neuroblasts of
low-grade tumours. Reelin is also detectable in the vascularised
stroma of the tumours. Both tumour LECs and BECs express Reelin,
whereas in normal tissues and endothelial cell lines we found
Reelin protein in LECs but not in BECs, which is in line with
transcriptome analyses performed in several labs (11–13).
The results show that two key regulators of the Reelin signalling
pathway, Reelin and Dab1, are significantly lower in progressed NB.
Reelin is obviously a marker for differentiating and differentiated
NB cells. It is almost absent in high-grade NB cells, but expressed
in tumour LECs and upregulated in tumour BECs. Our results indicate
that there is a switch from an autocrine function of Reelin in
low-grade NB to a paracrine function in high-grade NB.
Reelin pathway molecules in NB cell
lines
We screened 24 human NB cell lines for the mRNA
expression of the Reelin pathway molecules. The complete list of
cells can be found in (8). Using
real-time RT-PCR we were able to detect transcripts of all four
major molecules of Reelin signalling: Reelin, LRP8, VLDLR and DAB1.
However, expression levels vary considerably between cell lines
(data not shown). We chose 6 cell lines for western blot analyses
and found differential expression of all four molecules, as well as
the activated, phosphorylated form of DAB1, pDAB1. The pattern of
bands appears to be more complex than in non-tumour cells. Secreted
Reelin is only detectable in SK-N-AS supernatants. The other cell
lines are negative, which may reflect the downregulation of Reelin
in the advanced tumour stages that have been used for the isolation
of the cell lines.
For LRP8, Kim et al have reported a molecular
weight (MW) of 105 kDa. As estimated by sequence analysis, its MW
should be in the range of 80–100 kDa (40). However, none of our cell lines
displayed a band for LRP8 in this range, but a strong signal at
approximately 30 kDa. In murine neuronal cultures, the presence of
a 30 kDa band has been described recently, and the authors show
that this represents a soluble splice variant of LRP8, which binds
Reelin and prevents DAB1 phosphorylation (41). This suggests that in our NB cell
lines there is no transmembrane LRP8, but rather an inhibitory
soluble LRP8 variant. This may account for the observed
immunolocalisation in the cytoplasm. A second band at 65 kDa, only
present in Lan 2 cells, may also be due to alternative splicing, as
it is known that several species- and tissue-specific splice
variants of LRP8 do exist (42–44).
The second receptor for Reelin, VLDLR, is absent in
SH-SY5Y and SK-N-AS. The other cell lines display a major band at
70 kDa. A second band at 150 kDa is prominent in Lan 2, faintly
detectable in Kelly and NGP, but absent in SMS-Kan. As the
calculated size of VLDLR is 96 kDa these bands may also be splice
variants or isoforms of the gene. However, the signals we obtained
are highly reproducible and must therefore be regarded as
specific.
The activity of the adapter protein DAB1 is
regulated by phosphorylation, and transcriptional quantities may
not necessarily reflect its importance in the signalling pathway.
For DAB1, we observed a major band at approximately 82 kDa in all
cell lines. A second band at 115 kDa was present only in Lan 2.
When isolated from mouse brain, a band for Dab1 has been detected
at 80 kDa (45). We have not
further analysed the 115 kDa band in Lan 2, however it seems
possible that this is caused by genetic instability. DAB1 is
located on chromosome 1p, where genetic mutations, translocations
and amplifications occur frequently in NB (6). We observed a 85 kDa band of the
activated pDAB1 only in Kelly, NGP, and Lan 2. We suggest that
activation is due to the presence of Reelin in FCS, which we
observed in immunoblot assays (data not shown). In order to prevent
unwanted Reelin effects, we changed the culture conditions for our
functional experiments and used serum-free media.
The variable patterns of Reelin signalling molecules
in NB cell lines obviously reflect the origin of the cells, namely
aggressive tumours and metastatic lesions (46). Roughly, low stage NB and more
differentiated lesions have a better prognosis than advanced NB,
consisting predominantly of undifferentiated ‘small round’ cells.
We found that high Reelin expression correlates with both low
staging and grading of the tumours. We therefore investigated
Reelin expression after treatment with ATRA, a well-known
differentiating agent in NB that is routinely used in clinical
regimens. In accordance with earlier studies (30), we found that Reelin is induced upon
this treatment. Thereby, the upregulation of Reelin takes time and
can only be observed after 9 and 12 days of treatment, not after 5
days. In a comparable study, Evangelisti et al (26) found downregulation of Reelin after
6 days of ATRA treatment of SH-SY5Y. They did not study later time
points. They used fetal bovine serum, which according to our
experience contains Reelin. Unfortunately, they do not comment on
the expression of Reelin in their 28 primary NB samples. Our data
suggest that the expression of Reelin is a feature of late maturing
sympathetic cells. However, expression of Reelin during development
of the peripheral sympathetic nervous system and the adrenal
medulla have not been studied in detail.
Guidance functions of Reelin
There is an ongoing debate whether Reelin acts as a
stop-signal or as an attractant for neurons during patterning of
the cerebral cortex (47). The
high Reelin expression in low stage NB suggested a metastases
inhibiting function, and we speculated it might act as a stop
signal for NB cells. This speculation is strengthened by our
results, showing that preincubation of SK-N-AS cells with
Reelin-containing cell supernatant reduces the migration towards a
serum gradient. However, we also demonstrate here that Reelin has
migration-promoting abilities in all cell lines tested when it is
used as an attractant. As discussed below, it appears that in
vivo external sources provide attractive Reelin in a paracrine
manner. The attraction of neurons by Reelin from Cajal-Retzius
neurons has been shown earlier (48), but still it remains to be studied,
how the signal is transmitted. Especially, SH-SY5Y and SK-N-AS,
which do respond to Reelin, do not seem to possess functional
receptors. However, very recently Ephrin-B family members have been
identified to bind Reelin, and their importance for proper
signalling has been demonstrated in the mouse brain (49). We detected high mRNA expression of
EphrinB1, EphrinB2 and EphrinB3 in both primary NB and NB cell
lines (data not shown), which suggests that additional receptors
may be relevant for Reelin signalling in NB. Earlier studies also
showed binding of Reelin to α3β1-integrin and amyloid precursor
protein (23,24,50).
Taken together, it is very likely that VLDLR and APOER2/LRP8 are
not the only functional receptors and additional molecules
influence Reelin signalling in NB.
Reelin in neuroblastoma metastases
Our data suggest that Reelin has a dual function in
NB. First, Reelin may act as an inhibitor of migration, when
differentiating NB cells produce high amounts of the protein. In
this case Reelin may act in an autocrine manner. However, when
endogenous Reelin is low, as observed in the majority of NB cell
lines and in the advanced tumour stages and high-grade NB cells,
the cells may migrate towards a Reelin gradient formed by external
sources. To our understanding there are at least two possible
sources for Reelin acting in a paracrine mode: firstly, we found
Reelin in FCS (data not shown), and it is likely that children may
also have high levels of Reelin in their blood, facilitating
hematogenic metastases of NB. Secondly, we found Reelin in both
tumour BECs and LECs. As we have shown, expression of Reelin in
LECs is also found in normal healthy tissues, however, expression
in BECs appears to be tumour-specific. Reelin in BECs may promote
hematogenic metastases, in LECs it may promote nodal metastases.
Both types of metastases can be observed in the clinics of NB.
Therefore, loss of Reelin in NB cells may promote both hematogenic
and lymphogenic metastases, as tumour cells with low endogenous
Reelin expression tend to migrate towards Reelin from external
sources. This is supported by studies of Sato et al who
found that knock-down of Reelin in pancreatic cancer increases cell
motility, invasiveness and substrate-independent colony formation
of tumour cells (51). They also
found that Reelin is downregulated in pancreatic cancer, as
compared to healthy pancreatic tissue. Together, these findings
indicate that reduced Reelin expression is characteristic for less
differentiated, more malignant tumours. However, conflicting data
showing that Reelin is a marker for aggressiveness in prostate
cancer, retinoblastoma and oesophageal cancer also exist and
indicate a tissue-specific role of Reelin (52–54).
However, the exact localization of Reelin in these tumours remains
to be studied.
Acknowledgements
We thank Mrs. S. Schwoch, Mrs. Ch.
Zelent, Mr. B. Manshausen and Mr. F. Ludewig for their excellent
technical assistance and Drs Frank Berthold, Barbara Hero and
Jessica Theissen of the German Neuroblastoma Studies Group
(Children’s Hospital University Cologne, Cologne, Germany), for
providing tumour samples and data. We are grateful to Professors
Tom Curran (CHOP, Philadelphia, PA) and E. Förster (UKE, Hamburg,
Germany) for providing Reelin plasmid constructs, Reelin-expressing
HEK-293 cells and helpful discussions.
References
|
1
|
Castleberry RP: Neuroblastoma. Eur J
Cancer. 33:1430–1438. 1997. View Article : Google Scholar
|
|
2
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weinstein JL, Katzenstein HM and Cohn SL:
Advances in the diagnosis and treatment of neuroblastoma.
Oncologist. 8:278–292. 2003. View Article : Google Scholar
|
|
6
|
Westermann F and Schwab M: Genetic
parameters of neuroblastomas. Cancer Lett. 184:127–147. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becker J, Erdlenbruch B, Noskova I, et al:
Keratoepithelin suppresses the progression of experimental human
neuroblastomas. Cancer Res. 66:5314–5321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Becker J, Pavlakovic H, Ludewig F, et al:
Neuroblastoma progression correlates with downregulation of the
lymphangiogenesis inhibitor sVEGFR-2. Clin Cancer Res.
16:1431–1441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Becker J, Volland S, Noskova I, Schramm A,
Schweigerer LL and Wilting J: Keratoepithelin reverts the
suppression of tissue factor pathway inhibitor 2 by MYCN in human
neuroblastoma: a mechanism to inhibit invasion. Int J Oncol.
32:235–240. 2008.PubMed/NCBI
|
|
10
|
Volland S, Kugler W, Schweigerer L,
Wilting J and Becker J: Stanniocalcin 2 promotes invasion and is
associated with metastatic stages in neuroblastoma. Int J Cancer.
125:2049–2057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Norgall S, Papoutsi M, Rossler J,
Schweigerer L, Wilting J and Weich HA: Elevated expression of
VEGFR-3 in lymphatic endothelial cells from lymphangiomas. BMC
Cancer. 7:1052007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petrova TV, Makinen T, Makela TP, et al:
Lymphatic endothelial reprogramming of vascular endothelial cells
by the Prox-1 homeobox transcription factor. EMBO J. 21:4593–4599.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Podgrabinska S, Braun P, Velasco P, Kloos
B, Pepper MS and Skobe M: Molecular characterization of lymphatic
endothelial cells. Proc Natl Acad Sci USA. 99:16069–16074. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
D’Arcangelo G, Miao GG, Chen SC, Soares
HD, Morgan JI and Curran T: A protein related to extracellular
matrix proteins deleted in the mouse mutant reeler. Nature.
374:719–723. 1995.
|
|
15
|
Kohno T and Hattori M: Re-evaluation of
protease activity of reelin. Biol Pharm Bull. 33:1047–1049. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quattrocchi CC, Wannenes F, Persico AM, et
al: Reelin is a serine protease of the extracellular matrix. J Biol
Chem. 277:303–309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
D’Arcangelo G, Homayouni R, Keshvara L,
Rice DS, Sheldon M and Curran T: Reelin is a ligand for lipoprotein
receptors. Neuron. 24:471–479. 1999.
|
|
18
|
Benhayon D, Magdaleno S and Curran T:
Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine
phosphorylation of Disabled-1. Brain Res Mol Brain Res. 112:33–45.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hiesberger T, Trommsdorff M, Howell BW, et
al: Direct binding of Reelin to VLDL receptor and ApoE receptor 2
induces tyrosine phosphorylation of disabled-1 and modulates tau
phosphorylation. Neuron. 24:481–489. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Howell BW, Hawkes R, Soriano P and Cooper
JA: Neuronal position in the developing brain is regulated by mouse
disabled-1. Nature. 389:733–737. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Strasser V, Fasching D, Hauser C, et al:
Receptor clustering is involved in Reelin signaling. Mol Cell Biol.
24:1378–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trommsdorff M, Gotthardt M, Hiesberger T,
et al: Reeler/Disabled-like disruption of neuronal migration in
knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell.
97:689–701. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dulabon L, Olson EC, Taglienti MG, et al:
Reelin binds alpha-3beta1 integrin and inhibits neuronal migration.
Neuron. 27:33–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hoe HS, Lee KJ, Carney RS, et al:
Interaction of reelin with amyloid precursor protein promotes
neurite outgrowth. J Neurosci. 29:7459–7473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yip YP, Kronstadt-O’Brien P, Capriotti C,
Cooper JA and Yip JW: Migration of sympathetic preganglionic
neurons in the spinal cord is regulated by Reelin-dependent Dab1
tyrosine phosphorylation and CrkL. J Comp Neurol. 502:635–643.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Evangelisti C, Florian MC, Massimi I, et
al: MiR-128 up-regulation inhibits Reelin and DCX expression and
reduces neuroblastoma cell motility and invasiveness. FASEB J.
23:4276–4287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hughes M, Marsden HB and Palmer MK:
Histologic patterns of neuroblastoma related to prognosis and
clinical staging. Cancer. 34:1706–1711. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peuchmaur M, d’Amore ES, Joshi VV, et al:
Revision of the International Neuroblastoma Pathology
Classification: confirmation of favorable and unfavorable
prognostic subsets in ganglioneuroblastoma, nodular. Cancer.
98:2274–2281. 2003. View Article : Google Scholar
|
|
29
|
Becker J, Wang B, Pavlakovic H, Buttler K
and Wilting J: Homeobox transcription factor Prox1 in sympathetic
ganglia of vertebrate embryos: correlation with human stage 4s
neuroblastoma. Pediatr Res. 68:112–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, Kundakovic M, Agis-Balboa RC,
Pinna G and Grayson DR: Induction of the reelin promoter by
retinoic acid is mediated by Sp1. J Neurochem. 103:650–665. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pahlman S, Hoehner JC, Nanberg E, et al:
Differentiation and survival influences of growth factors in human
neuroblastoma. Eur J Cancer. 31A:453–458. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilting J, Papoutsi M, Christ B, et al:
The transcription factor Prox1 is a marker for lymphatic
endothelial cells in normal and diseased human tissues. FASEB J.
16:1271–1273. 2002.PubMed/NCBI
|
|
33
|
Lagodny J, Juttner E, Kayser G, Niemeyer
CM and Rossler J: Lymphangiogenesis and its regulation in human
neuroblastoma. Biochem Biophys Res Commun. 352:571–577. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Falconer DS and Sierts-Roth U: Dreher, a
new gene of the waltzer-shaker group in the house mouse. Z Indukt
Abstamm Vererbungsl. 84:71–73. 1951.(In undetermined language).
|
|
35
|
Fatemi SH: Reelin mutations in mouse and
man: from reeler mouse to schizophrenia, mood disorders, autism and
lissencephaly. Mol Psychiatry. 6:129–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hourihane JO, Bennett CP, Chaudhuri R,
Robb SA and Martin ND: A sibship with a neuronal migration defect,
cerebellar hypoplasia and congenital lymphedema. Neuropediatrics.
24:43–46. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lorenzetto E, Panteri R, Marino R, Keller
F and Buffelli M: Impaired nerve regeneration in reeler mice after
peripheral nerve injury. Eur J Neurosci. 27:12–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Panteri R, Mey J, Zhelyaznik N, et al:
Reelin is transiently expressed in the peripheral nerve during
development and is upregulated following nerve crush. Mol Cell
Neurosci. 32:133–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smalheiser NR, Costa E, Guidotti A, et al:
Expression of reelin in adult mammalian blood, liver, pituitary
pars intermedia, and adrenal chromaffin cells. Proc Natl Acad Sci
USA. 97:1281–1286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim DH, Iijima H, Goto K, et al: Human
apolipoprotein E receptor 2. A novel lipoprotein receptor of the
low density lipoprotein receptor family predominantly expressed in
brain. J Biol Chem. 271:8373–8380. 1996.PubMed/NCBI
|
|
41
|
Koch S, Strasser V, Hauser C, et al: A
secreted soluble form of ApoE receptor 2 acts as a
dominant-negative receptor and inhibits Reelin signaling. EMBO J.
21:5996–6004. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brandes AA, Palmisano V, Pasetto LM, Basso
U and Monfardini S: High-dose chemotherapy with bone marrow rescue
for high-grade gliomas in adults. Cancer Invest. 19:41–48. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brandes C, Novak S, Stockinger W, Herz J,
Schneider WJ and Nimpf J: Avian and murine LR8B and human
apolipoprotein E receptor 2: differentially spliced products from
corresponding genes. Genomics. 42:185–191. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim DH, Magoori K, Inoue TR, et al:
Exon/intron organization, chromosome localization, alternative
splicing, and transcription units of the human apolipoprotein E
receptor 2 gene. J Biol Chem. 272:8498–8504. 1997. View Article : Google Scholar
|
|
45
|
Bock HH, Jossin Y, Liu P, et al:
Phosphatidylinositol 3-kinase interacts with the adaptor protein
Dab1 in response to Reelin signaling and is required for normal
cortical lamination. J Biol Chem. 278:38772–38779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Thiele C: Neuroblastoma cell lines. Human
Cell Culture. 1. Masters J: Kluwer Academic Publishers; Lancaster:
pp. 21–53. 1998, View Article : Google Scholar
|
|
47
|
Zhao S and Frotscher M: Go or stop?
Divergent roles of Reelin in radial neuronal migration.
Neuroscientist. 16:421–434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meyer G and Goffinet AM: Prenatal
development of reelin-immunoreactive neurons in the human
neocortex. J Comp Neurol. 397:29–40. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Senturk A, Pfennig S, Weiss A, Burk K and
Acker-Palmer A: Ephrin Bs are essential components of the Reelin
pathway to regulate neuronal migration. Nature. 472:356–360. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schmid RS, Jo R, Shelton S, Kreidberg JA
and Anton ES: Reelin, integrin and DAB1 interactions during
embryonic cerebral cortical development. Cereb Cortex.
15:1632–1636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sato N, Fukushima N, Chang R, Matsubayashi
H and Goggins M: Differential and epigenetic gene expression
profiling identifies frequent disruption of the RELN pathway in
pancreatic cancers. Gastroenterology. 130:548–565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Perrone G, Vincenzi B, Zagami M, et al:
Reelin expression in human prostate cancer: a marker of tumor
aggressiveness based on correlation with grade. Mod Pathol.
20:344–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Seigel GM, Hackam AS, Ganguly A, Mandell
LM and Gonzalez-Fernandez F: Human embryonic and neuronal stem cell
markers in retinoblastoma. Mol Vis. 13:823–832. 2007.PubMed/NCBI
|
|
54
|
Wang Q, Lu J, Yang C, et al: CASK and its
target gene Reelin were co-upregulated in human esophageal
carcinoma. Cancer Lett. 179:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|