Introduction
The small cell ovarian carcinoma of the
hypercalcemic type (SCCOHT) is defined as a rare form of an
aggressive ovarian tumor predominantly affecting young women
between ages of 13 to 35 which is mostly associated with
paraendocrine hypercalcemia (1,2).
Following the initial histopathological evaluation of several
clinical cases, the SCCOHT has been classified as a separate
pathological entity (3).
This malignancy with poor prognosis has been
described to be different and clearly distinguishable from other
related cancer types including transitional cell carcinoma of the
ovary, ovarian epithelial tumors and ovarian germ cell tumors
(3,4). A variety of cell lines are available
to study different types of ovarian carcinoma including BG-1
(5), NIH:OVCAR-3 (6), OTN11 (7) and Ov202 (8), however, no cellular model so far
exhibits properties of an SCCOHT. A previous cell line (OS-1)
described to be associated with an ovarian small cell carcinoma of
the hypercalcemic type failed to develop tumors in immunodeficient
nude mice (9). Therefore, no cell
system associated with an appropriate SCCOHT in vivo model
is available up to now.
Based upon initial immunohistochemical analysis the
SCCOHT has been postulated to represent a germ cell-derived tumor
(10). Other evaluations including
electron microscopy of tumor specimen reported SCCOHT as an
epithelial-like originating tumor (2). However, further investigations using
immunohistochemistry, electron microscopy and genetic analysis of
SCCOHT tumor specimen suggested a heterogeneous tumor entity not
confirming a germ cell-derived or an epithelial cell-derived tumor
origin (11–13). Considering these controversial
reports, the histogenesis of SCCOHT and the mechanism of the
development of the hypercalcemia still remain unclear.
Properties of the SCCOHT appear to be heterogeneous
and no defined markers for the characterization of these tumor
cells have been identified. Morphological evaluations of the cells
in SCCOHT tumor biopsies described irregularly clumped nuclear
chromatin with small detectable nucleoli (14). Moreover, some populations stained
positive for epithelial cell markers whereas the intermediate
filament protein vimentin has been described in the majority of
cells in the SCCOHT (11). In
addition, cell cycle analysis of several SCCOHT tumors by flow
cytometry reported a broad distribution with 4.7% to 18% of S phase
cells and 1.5% to 19.5% of G2/M phase cells (15).
The heterogeneity of these data may be explainable
in part due to the limitations of the biopsy material from patients
and the lack of further cell sources, whereby the histogenesis and
properties of the SCCOHT still remain poorly understood.
Consequently, reasonable approaches for the treatment of SCCOHT
patients or a sufficient (chemo) therapeutic management remain
unknown. So far, a multi-modality treatment is suggested which
includes surgery followed by a cisplatin- and etoposide-based or
carboplatin- and taxane-based chemotherapy and the addition of a
sequential or concurrent radiotherapy (16,17)
although the level of tumor relapses remains high. Thus, more than
70% of the SCCOHT patients who had received a tumor-reductive
surgery and subsequent chemotherapy rapidly developed a tumor
relapse and only very few patients survived longer than two years
(18–21). Current recommendations also include
the administration of high dose chemotherapy which may yield longer
term remissions (22).
In the present study, we describe a permanently
growing and transplantable SCCOHT-derived cell population
representing a model for appropriate in vitro and in
vivo tumor studies which may contribute to a more detailed
understanding of this devastating disease.
Materials and methods
Patient biopsy and primary culture of
SCCOHT-derived cells
A 31-year-old woman was diagnosed clinically and
histopathologically with an ovarian small-cell carcinoma of the
hypercalcemic type, FIGO Ia. The German Gynecopathologic Reference
Laboratory in Mannheim confirmed the diagnosis of this rare tumor.
The patient was primarily treated by oophorectomy and refused
chemotherapy secondary to desired pregnancy. Only 11 months later
she presented with hypercalcemia (2.87 mmol/l, normal less than
2.60 mmol/l) and recurrent pelvic mass. An exploratory laparotomy
was performed and revealed large intraabdominal tumor masses. The
patient died 13 months after primary diagnosis. The final diagnosis
rendered was recurrent small cell carcinoma of the hypercalcemic
type.
During surgery of this 31-year-old patient with
recurrent SCCOHT a tumor biopsy was taken and washed several times
in PBS supplemented with 100 U/ml penicillin and 100 μg/ml
streptomycin. Informed written consent was obtained from the
patient for the use of this biopsy material and the study has been
approved by the Institutional Review Board, Project no. 3916 on
June 15th, 2005. Following removal of most erythrocytes, the tumor
tissue was minced with a scalpel into approximately 2
mm3 large tissue pieces, washed again and incubated in
growth medium with 400 μM Ca2+, i.e. RPMI-1640
supplemented with 10% (v/v) fetal calf serum or human AB serum, 100
U/ml L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin. The tissue culture was performed at 37°C in a
humidified atmosphere of 5% (v/v) CO2 and the medium was
changed at intervals of 3 to 4 days to remove remaining blood cells
and small debris. Following the outgrowth of an adherent cell
population, the tumor tissue pieces were removed and the adherent
cell layer could be harvested by gentle scraping using a sterile
rubber policeman to avoid the use of non-specific proteases such as
trypsin. The cells were centrifuged (320 g/6 min) and resuspended
in growth medium and this primary culture continued to grow without
further purification or selection. The proliferative capacity at
various conditions and the population doublings in parallel to the
cell viability during culture were determined in a hemocytometer
using the trypan blue exclusion test.
Scanning electron microscopy (SEM)
Cells grown on coverslips were washed in PBS and
immersed in a fixative solution composed of 2.5% glutaraldehyde, 2%
formaldehyde, freshly prepared from paraformaldehyde, 1.7 mM
CaCl2 and Na-Cacodylate-HCl buffer pH 7.3 for at least 4
h at 4°C. After washing in 0.1 M Na-Cacodylate-HCl buffer to which
0.22 M sucrose was added the cells were postfixed in buffered 2%
OsO4, dehydrated in ascending concentrations of acetone
and subsequently dried in a Balzers CPD 030 critical point dryer
(Bal-Tec AG, Balzers, Liechtenstein). The coverslips were mounted
on aluminium stubs with conductive plates (Plano, Wetzlar,
Germany), sputter coated with gold in a Polaron E 5400 sputter
coater (Polaron Equipment Ltd., Watford, UK) and investigated in a
Philips SEM 505 scanning electron microscope at an acceleration
voltage of 10 kV. Images were recorded using the SEM software
version 2.0 (23).
Transmission electron microscopy
(TEM)
Cells grown in plastic Petri dishes with a diameter
of 3.5 cm were fixed as described for SEM. After washing in 0.1 M
Na-Cacodylate-HCl supplemented with 0.22 M sucrose and
post-fixation in 2% Na-Cacodylate-buffered OsO4 for 90
min at room temperature, the cells were dehydrated in ascending
concentrations of ethanol and finally embedded in epoxy resin
(Serva, Heidelberg, Germany).
Thin sections (about 70 nm thick) were cut with an
ultra-microtome Reichert Ultracut E, collected on formvar-coated
copper slot grids, contrasted with uranylacetate and lead citrate
and observed in a transmission electron microscope Morgagni 268
(FEI, Eindhoven, The Netherlands) at an acceleration voltage of 80
kV. Images were acquired using a Veleta CCD camera (Olympus SIS,
Münster, Germany) controlled by iTEM software Version 5.1 (Olympus
SIS).
Analysis of surface markers by flow
cytometry
Continuously proliferating SCCOHT-derived cells in
logarithmic growth phase were harvested and analysed for cell
surface marker and intermediate filament expression. After blocking
non-specific binding to Fc-receptors by incubation of
106 SCCOHT-derived cells with human IgG (10 mg/ml) for
30 min at 4°C and washing with PBS-BSA, the cells were incubated
with the specific FITC- or PE-conjugated antibodies listed in
Table I, respectively. A parallel
incubation with the appropriately labeled IgG subclass antibody
served as a control. Following antibody incubation, all samples
were washed twice with PBS-BSA and flow cytometry was performed in
a Galaxy FACScan (Partec GmbH, Münster, Germany) using FloMax
analysis software (Partec GmbH).
 | Table ISurface markers and intermediate
filaments in SCCOHT-1 cells. |
Table I
Surface markers and intermediate
filaments in SCCOHT-1 cells.
| Tested antibody on
SCCOHT-1 | IgG subclass | Labeling | Source | SCCOHT-1 signal
(%) |
|---|
| CD11b | IgG1 | PE | DAKO | 0 |
| CD15 | IgG1 | PE | Miltenyi | 15.6 |
| CD24 | IgG2a | FITC | BD Bioscience | 0 |
| CD29 | IgG1 | PE | BD Bioscience | 77.4 |
| CD31 | IgG1 | FITC | DAKO | 0 |
| CD44 | IgG2a | FITC | BD Bioscience | 8.3 |
| CD45 | IgG1 | FITC | DAKO | 0 |
| CD49b | IgG1 | FITC | BD Bioscience | 0 |
| CD49d | IgG1 | PE | BD Bioscience | 0 |
| CD49f | IgG2a | FITC | BD Bioscience | 0 |
| CD54 | IgG2b | PE | BD Bioscience | 0 |
| CD66 | IgG1 | FITC | BD Bioscience | 0 |
| CD73 | IgG1 | PE | BD Bioscience | 0 |
| CD90 | IgG1 | PE | DAKO | 91.0 |
| CD103 | IgG2a | FITC | BD Bioscience | 0 |
| CD105 | IgG1 | FITC | BioLegend | 0 |
| CD133 | IgG2b | PE | Miltenyi | 0 |
| CD138 | IgG1 | PE | BD Bioscience | 0 |
| CD147 | IgG1 | PE | BD Bioscience | 0 |
| CD227 | IgG1 | PE | BD Bioscience | 0 |
| CD271 | IgG1 | PE | Miltenyi | 0 |
| MSCA | IgG1 | PE | Miltenyi | 0 |
| Pan
cytokeratin | IgG1 | FITC | DAKO | 28.0 |
| Vimentin | IgG1 | PE | DAKO | 99.2 |
| Negative
control | IgG1 | FITC/PE | DAKO | 0 |
| Negative
control | IgG2a | FITC | DAKO | 0 |
| Negative
control | IgG2b | PE | Miltenyi | 0 |
Centrifugal counterflow elutriation
(CCE)
The CCE was performed using the Beckmann J6-MC with
the JE-5.0 rotor and the appropriate 5 ml-standard elutriation
chamber (Beckman Coulter GmbH, Krefeld, Germany). Approximately
2×108 SCCOHT-derived cells in an exponential growth
phase were harvested, resuspended in PBS and applied to the
standard chamber (1,600 rpm at 24°C) using a digital flow
controller (Cole-Palmer Instruments Inc., Chicago, IL, USA).
Subsequent fractions of 100 ml aliquots of the elutriated samples
were collected upon progressive increase of the pump speed.
Elutriated cell fractions were examined for viability, cell number
and cell size distribution in a Vi-CELL Series Cell Viability
Analyzer (Beckman Coulter GmbH).
Cell cycle analysis
The cell cycle analysis was performed as described
previously (24). Briefly,
5×105 SCCOHT-derived cells were fixed in 70% (v/v)
ice-cold ethanol at 4°C for 24 h. Thereafter, the fixed cells were
stained with CyStain DNA 2 step kit (Partec GmbH) and filtered
through a 50 μm filter. The samples were then analyzed in a
Galaxy flow cytometer (Partec GmbH) using the MultiCycle cell cycle
software (Phoenix Flow Systems Inc., San Diego, CA, USA).
Cytogenetic investigations
Chromosome preparation and fluorescence-R banding
were performed as previously described (25). Twenty-five metaphases were analysed
in the SCCOHT-derived cells. Karyotypes were described according to
the International System for Human Cytogenetic Nomenclature
(26).
Fluorescence in situ hybridization
(FISH)
FISH was performed according to standard procedures
(probes supplied by Abbott, Wiesbaden, Germany) (27). For each FISH analysis, at least 200
interphase nuclei were analyzed.
Multicolor fluorescence in-situ
hybridization (mFISH)
mFISH analysis was carried out using an mFISH kit
(Meta-Systems, Altlussheim, Germany). The mFISH procedure was
performed according to the manufacturer’s instructions. Metaphase
spreads that had been used for karyotyping were destained in a
decreasing ethanol series (99%, 95%, 70%, 50%) and hybridized with
the SpectraVysion™ 24 chromosome painting kit (Abbott). The mFISH
procedure was performed according to the manufacturer’s
instructions. Fluorochromes were sequentially captured using
specific single-band pass filters in a Zeiss Axioplan 2 microscope
(Zeiss, Jena, Germany). mFISH ISIS software (MetaSystems,
Altlussheim, Germany) was used for image analysis.
DNA extraction
Cell pellet obtained by centrifugation (800 g, 5
min) was resuspended in 400 μl of STE buffer (50 mM
Tris-HCl, 1 mM EDTA, 100 mM NaCl, pH 7.5) containing Proteinase K
(final concentration 3.75 mg ml−1) and 0.5% (v/w) SDS
(sodium dodecyl sulphate). The samples were incubated at 56°C over
night. Then 400 μl of phenol-chloroform-isoamylalcahol
(25:24:1) (saturated with 10 mM Tris-HCl, pH 8.0) were added to
each mixture. Samples were incubated 10 min on ice and centrifuged
(13,200 rpm, 10 min, 4°C). The aqueous phase was recovered and a
second time mixed with 400 μl of
phenol-chloroform-isoamylalcohol, incubated for 10 min on ice and
centrifugated (13,200 rpm, 10 min, 4°C). Then the aqueous phase was
recovered with 400 μl chloroform, resolved carefully,
incubated 10 min on ice and centrifuged (13,200 rpm, 10 min, 4°C).
Nucleic acids were precipitated with 0.1 volume NaAcetate (3 M) and
2.5 volume ethanol (100%) and incubated for 30 min on ice. The
precipitate was then washed with 70% ethanol and resuspended in 50
μl of deionized water.
Array-CGH
Array-CGH was performed using the Agilent Human
Genome Microarray Kit 400A (Agilent Technologies, Santa Clara, CA,
USA), a high resolution 60-mer oligonucleotide based microarray
with median overall probe spacing of about 5 kb. Labeling and
hybridization of genomic DNA was performed according to the
protocol provided by Agilent. Briefly, 1 μg of test DNA and
reference DNA each were labeled by random priming using the Agilent
Genomic DNA Labeling Kit Plus, test DNA with Cy3-dUTP and reference
DNA with Cy5-dUTP. Labeled products were purified by amicon ultra
0.5 ml 30K centrifugal filters (Millipore, Billerica, MA, USA),
combined and then mixed with human Cot-1 DNA (50 μg),
Agilent 10X blocking Agent, and Agilent 2X Hybridization Buffer.
This solution was hybridized to Agilent’s 400K Human Genome CGH
microarray at 65°C with 20 rpm rotation for 40 h. Washing steps
were performed according to the Agilent protocol. Microarray slides
were scanned immediately using an Agilent microarray scanner. For
image analysis, default CGH settings of Feature Extraction Software
(Agilent Technologies, Waldbronn, Germany) were applied. Output
files from Feature Extraction were subsequently imported into
Agilent’s CGH data analysis software, DNA-Workbench. The Aberration
Algorithm ADM2 was applied and Aberration Filters were set to: at
least 50 probes with mean Log2Ratio=0.3.
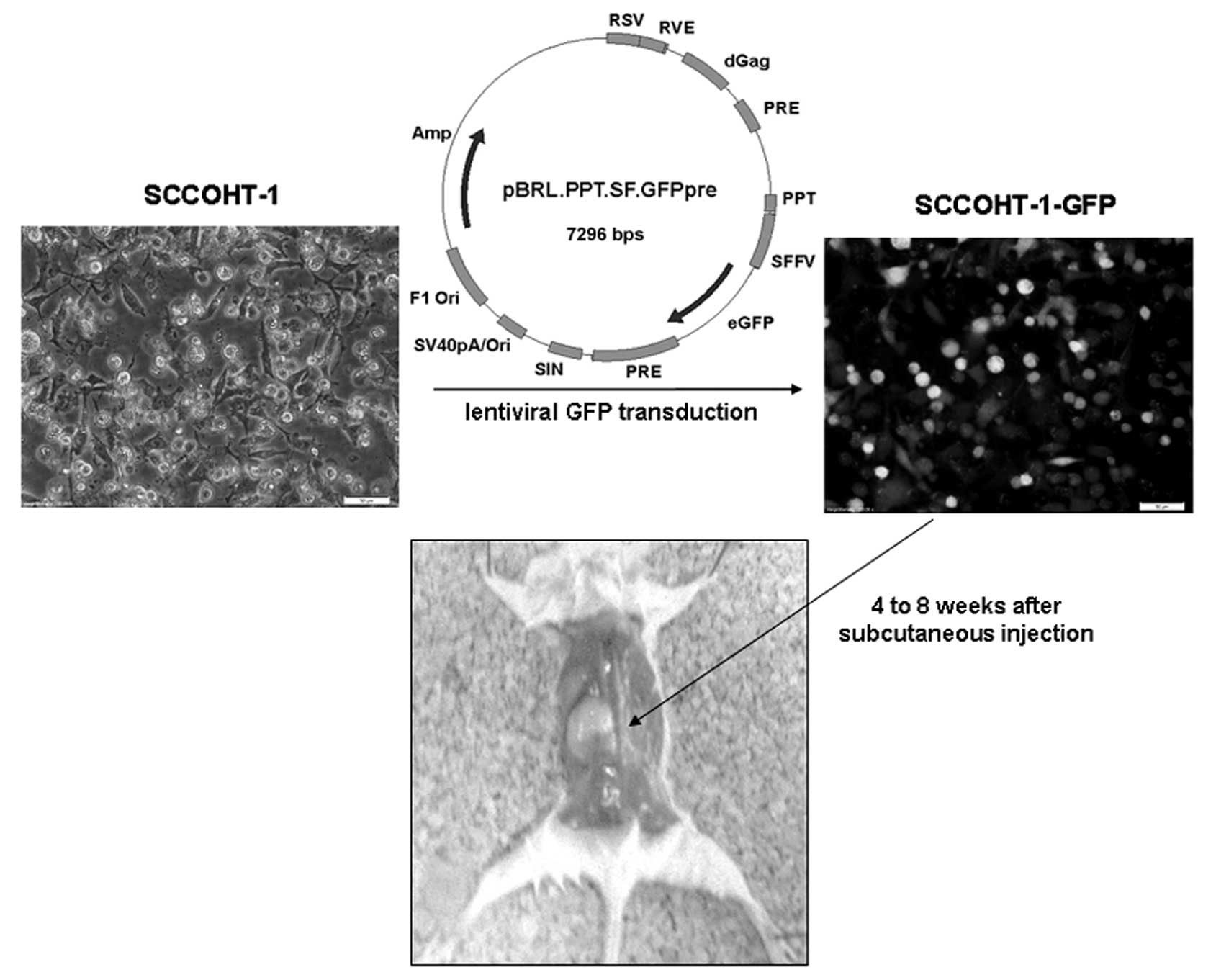
Lentiviral transduction of SCCOHT-1 with
the GFP gene
The construction, testing and production of
lentiviral vectors for gene transfer have been described previously
(28). In brief, a 3rd generation
lentiviral vector encoding the green fluorescent protein (GFP)
driven by an internal spleen-focus forming SFFV U3 promoter
(pRRL.PPT.SF.GFPpre) was co-transfected together with HIV-1
gag/pol, RSV-Rev and VSV glycoprotein expression plasmids. The
infectious lentiviral vector particles were harvested 48 to 72 h
post transfection and subsequently stored at −80°C until usage.
Next, 1×105 SCCOHT-derived cells/well were incubated in
culture medium in a 6-well plate and lentiviral transduction was
performed. Following addition of the virus at an MOI5 in the
presence of protamine sulphate, the plates were immediately
centrifuged (2,000 rpm/30 min/4°C) followed by an incubation at
37°C/5% CO2 in a humidified atmosphere for 24 h.
Thereafter, the medium containing the virus particles was removed
and the tranduced cells were cultured further on with new medium.
The successful transduction of the GFP gene was examined under a
fluorescence microscope.
In vivo experiments
Animal research using NOD/scid mice was carried out
by following internationally recognized guidelines on animal
welfare and has been approved by the institutional licensing
committee ref. no. 33.9-42502-04-06/1178 on Sept. 22th, 2010. About
1×106 GFP-labeled SCCOHT-derived cells of the
elutriation fractions were injected subcutaneously into 5 to 6
weeks-old female NOD/scid mice. After 4 to 8 weeks of SCCOHT-1
injection, the mice had developed large subcutaneous tumors and
were sacrificed by CO2 anesthesia and cervical
dislocation. Following UV light examination for the detection of
GFP positive tissue, the tumors were dissected, weighed and washed
in PBS supplemented with 100 U/ml penicillin and 100 μg/ml
streptomycin. Thereafter, the tumor tissue was minced with a
scalpel into approximately 2 mm3 large tissue pieces,
washed again and incubated in growth culture medium to obtain
primary cultures of the NOD/scid mouse tumors. In parallel, parts
of the mouse tumor tissue were fixed in 4% glutardialdehyde
solution for histopathological evaluations.
For calcium measurements retroorbital blood was
taken from NOD/scid mice which had developed an SCCOHT-1
cell-derived subcutaneous tumor and serum was prepared and analyzed
for (Ca2+) concentration using the Arsenazo Reagent
(Beckman Coulter GmbH) in an AU400 Chemistry System (Beckman
Coulter GmbH). According to the manufacturer’s manual, the
procedure is based on (Ca2+) reacting with Arsenazo III
[2,2′-(1,8-Dihydroxy-3,6-disulphonaphthylene-2,7-bisazo)-bisbenzenear-sonic
acid] to form an intense purple-colored complex. Magnesium does not
significantly interfere in calcium determination using Arsenazo
III. In this method the absorbance of the
(Ca2+)-Arsenazo III complex is measured bichromatically
at 660/700 nm. The resulting increase in absorbance of the reaction
mixture is directly proportional to the calcium concentration in
the sample.
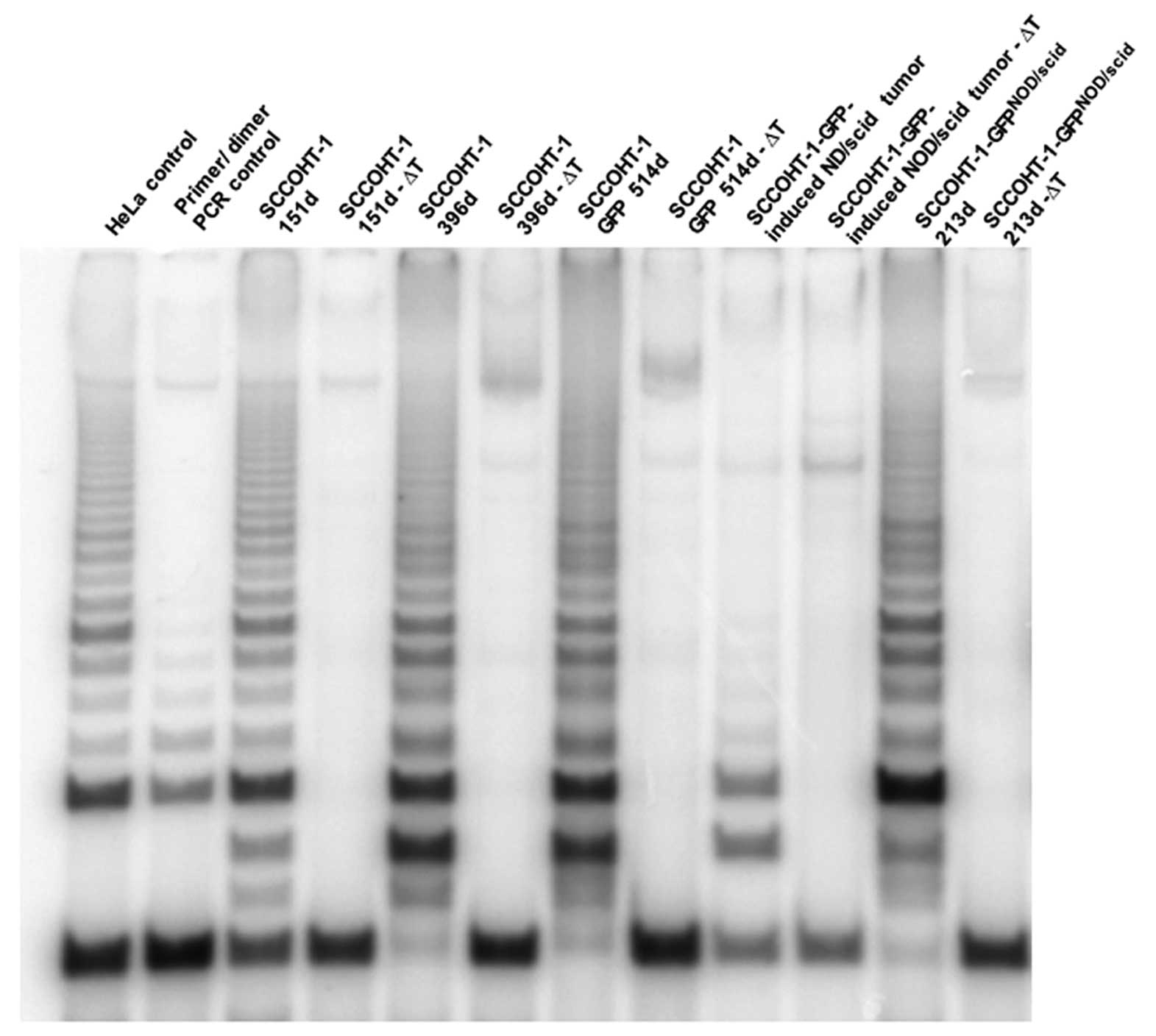
Telomerase assay
The activity of this nuclear enzyme in SCCOHT-1
cells was detected by TRAPeze telomerase detection kit (Millipore,
Beverly, MA, USA) in a radioactive assay. Briefly, homogenates of
SCCOHT-1 cells and tumor tissue from NOD/scid mice was resuspended
in CHAPS lysis buffer and combined with the reaction mixture
including a [γ-32P] ATP radiolabeled TS primer which has
been previously labelled with T4-polynucleotide kinase (New England
BioLabs, Beverly, MA, USA). Evaluation and adjustment of equal
protein was performed using the Bradford method (Bio-Rad Inc.,
Richmond, CA, USA). The different SCCOHT samples were subjected to
PCR amplification according to the manufacturer’s instructions
using Taq DNA polymerase (New England BioLabs). Thereafter, loading
dye was added to the amplified DNA and the samples were loaded onto
a 10% non-denaturing polyacrylamide gel. Following electrophoresis,
the gel was dried and the radioactive bands were visualized in a
PhosphorImager (Storm 820, Amersham Biosciences, Piscataway, NJ,
USA).
Results
Morphological evaluation of SCCOHT-1
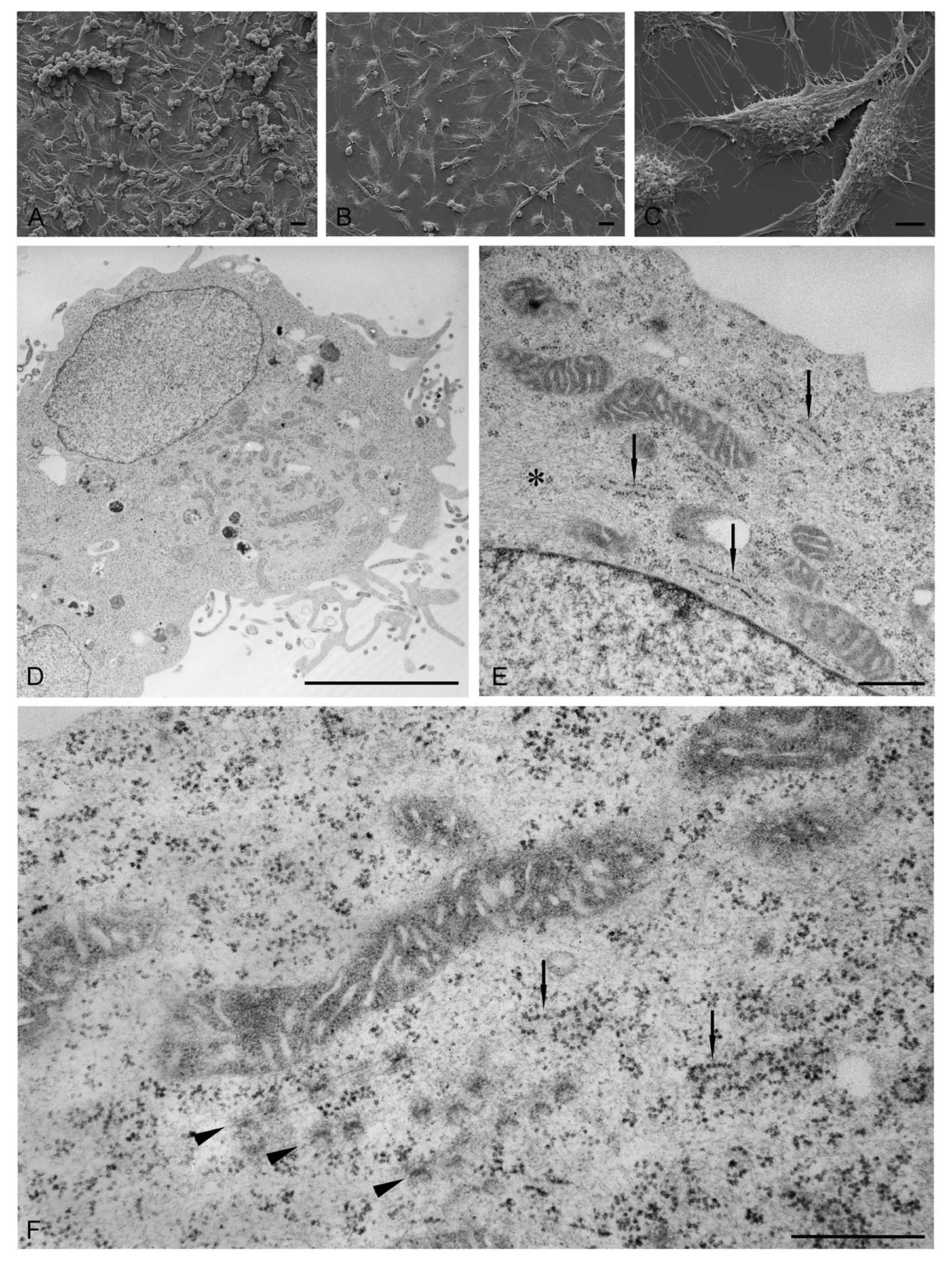
SEM (Fig. 1A–C)
revealed predominantly spindle-shaped adherent cells and some
spherical cells. When grown to high confluency the more spherical
cells occasionally formed wall-like three-dimensional clusters
(Fig. 1A). Although spherical
cells were present at sub-confluent densities as well no formation
of three-dimensional clusters could be observed (Fig. 1B). Especially the abundant
spindle-shaped cells showed numerous filopodial extensions spread
on the support. In addition the cells were rich in membranous
protrusions at their apical surface (Fig. 1C).
Thin section electron microscopy showed flat cells
attached to the bottom of the Petri dish and cells with a more
round profile. In sections cut perpendicular to the dish surface,
the latter cells were often located in a higher position compared
to the flat cells. All cells showed numerous filopodia extending
from their circumference (Fig.
1D). The sectioned nuclei frequently contained nucleoli and the
chromatin was mostly in an extended (euchromatic) state (Fig. 1D and E). Within the cells abundant
and often elongated mitochondria were conspicuous (Fig. 1D–F). A large number of ribosomes
occurred free within the cytoplasm. However, some were associated
to short cisternae or tubules of the endoplasmic reticulum
(Fig. 1E and F). Occasionally,
annulate lamellae could be detected (Fig. 1F). Both, filamentous and tubular
components of the cytoskeleton were regularly observed.
Cell surface marker analysis and
intermediate filaments
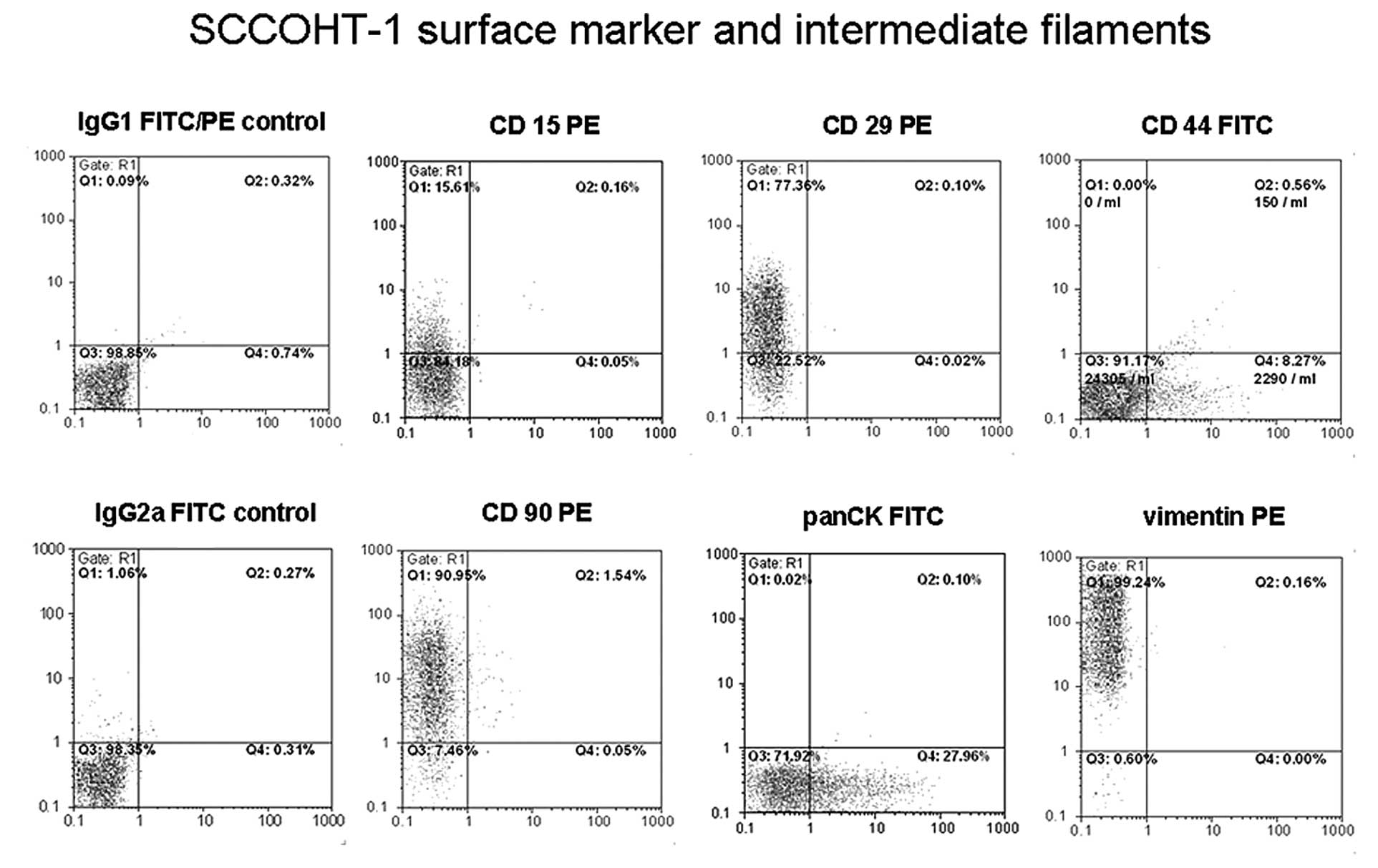
Flow cytometric analysis of 22 different cell
surface proteins and 2 types of intermediate filaments was
performed according to the antibody specificities indicated in
Table I and revealed only 6
partially positive reactions. Thus, about 15.6% of the SCCOHT-1
cells expressed the epitope of CD15 representing a carbohydrate
adhesion molecule (3-fucosyl-N-acetyl-lactosamine) which can be
associated with surface glycoproteins and/or glycolipids (Fig. 2). Moreover, 77.4% of SCCOHT-1 cells
expressed CD29. Expression of the CD44 antigen was detectable on
8.3% of the population and the CD90 marker was represented by 91%
of the SCCOHT-1 cells (Fig. 2).
With respect to intermediate filaments, the pan-cytokeratin
antibody tested positive in 28.2% of the cells and vimentin was
present in all SCCOHT-1 cells (99.2%) (Fig. 2). These expression levels on
SCCOHT-1 cells did not alter during long-term culture and revealed
similar results in populations cultured for 136, 230 and 382 days,
respectively. Likewise, culture of SCCOHT-1 cells under hypoxic (5%
O2) conditions had little if any effect on the
expression patterns as compared to the normoxic (21% O2)
environment (data not shown).
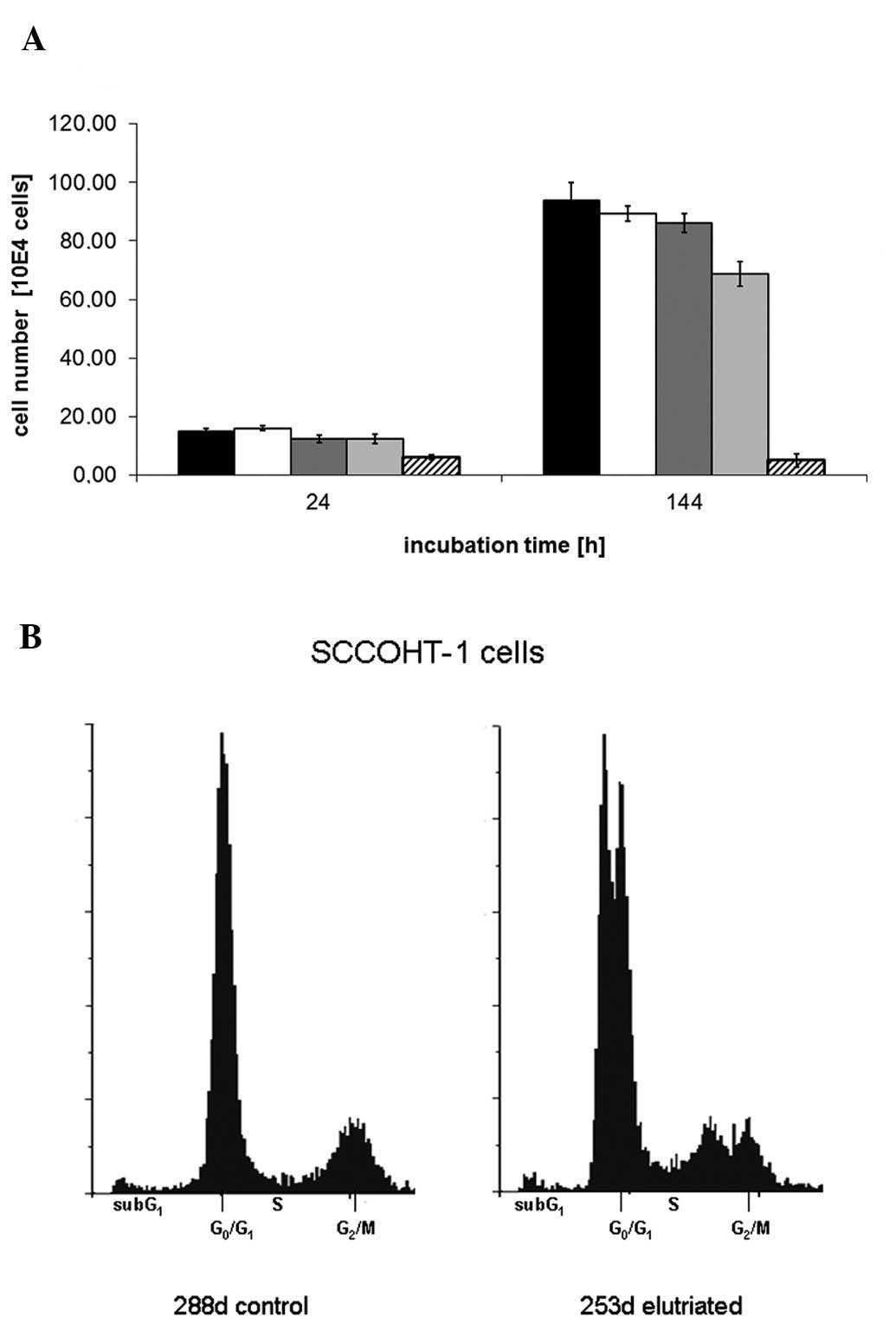
Proliferation
The proliferative capacity of SCCOHT-1 was initially
tested under different conditions. A comparison of culture medium
supplemented with either human AB serum or fetal calf serum
demonstrated the requirement of serum addition for optimal growth
conditions, however, no significant difference was detectable in
the use of the xeno-free (Fig.
3A). Therefore, 10% FCS was used as standard culture condition
for SCCOHT-1 cells and revealed an average population doubling of
38.1 h (n=4). An optimization of the cell density revealed a cell
concentration of about 1×105 cells/ml. The culture of
SCCOHT-1 cells in a hypoxic microenvironment (5% O2) as
compared to the normoxic culture conditions (21% O2) for
more than 7 days demonstrated little if any differences in the
proliferative capacity (data not shown).
Flow cytometric cell cycle analysis of
logarithmically-growing SCCOHT-1 cells revealed a distribution of
continuously proliferating cells with about 67% in
G0/G1 phase, 13% in S phase and 20% in the
mitotic G2/M phase as evaluated by the MultiCycle cell
cycle software (Fig. 3B).
Following separation and cell size enrichment of SCCOHT-1 by
centrifugal counterflow elutriation (CCE), however, subsequent
analysis of the cell cycle phases could distinguish two separate
peaks in both, the G0/G1 and G2/M
phase, respectively, suggesting that SCCOHT-1 cells represent a
mixture of at least two subpopulations (Fig. 3B).
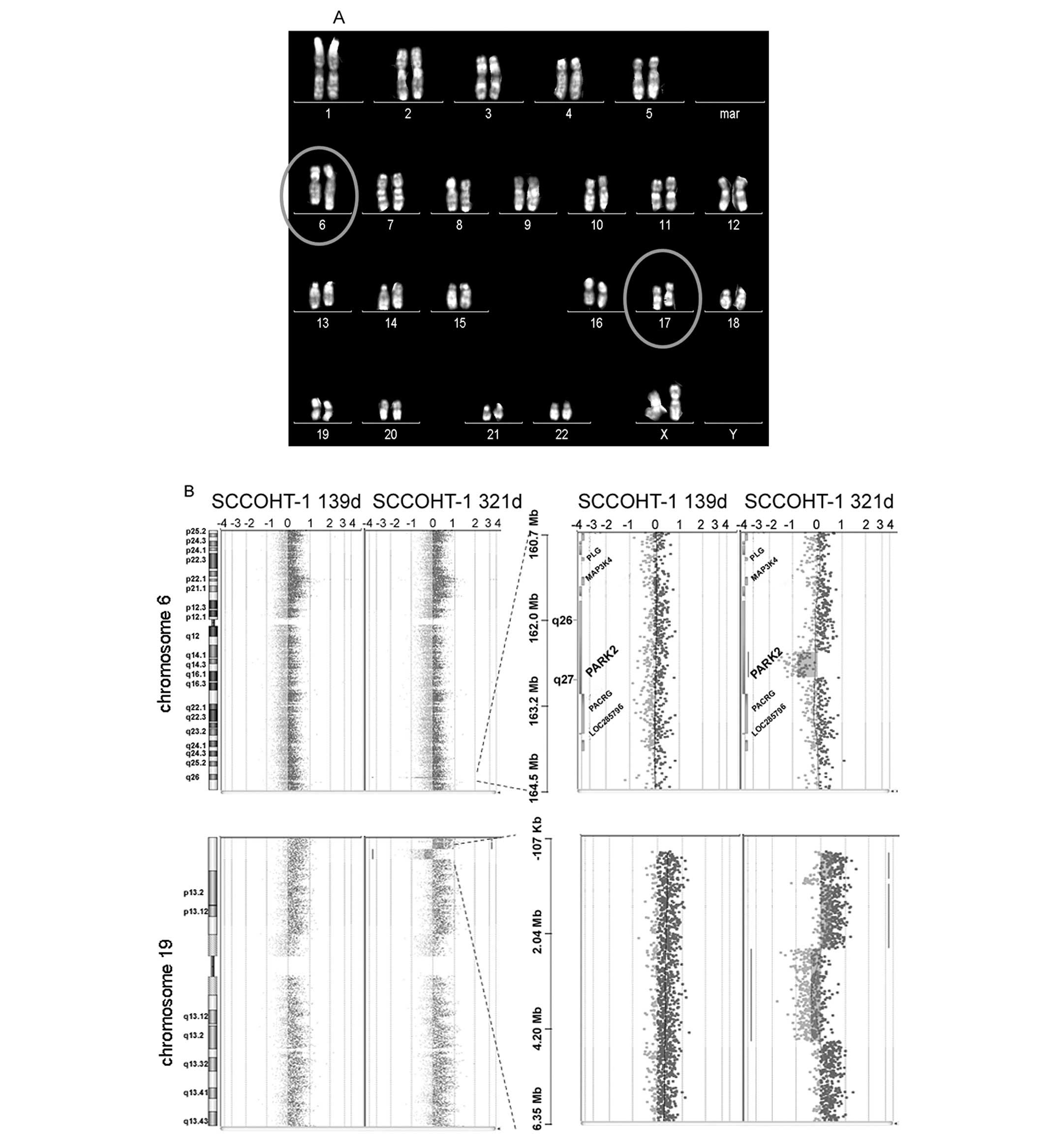
Classical cytogenetic analysis, mFISH and
array-CGH analysis
According to the distinguishable subpopulations in
the cell cycle analysis, the question was addressed as to whether
potential chromosomal aberrations may be associated with the
SCCOHT-1 cells. Thus, classical cytogenetic analysis and mFISH of
the SCCOHT-1 metaphase chromosomes was performed and revealed an
unbalanced translocation between chromosome 6 and chromosomes 17
der(6)t(6;17)(q26;q22) in 3 out of
21 metaphases (Fig. 4A). Eighteen
metaphases showed a normal karyotype.
The genetic stability was further investigated in a
more detailed analysis between 139 and 321 days-old SCCOHT-1
populations using a DNA array CGH analysis (Fig. 4B). Under the given filter criteria
8 focal genomic alterations were detected in the 139-day-old and 21
alterations in the 321-day-old culture population. Among them were
a deletion in 6q26 (162.528 Mb-162.902 Mb) including the PARK2
gene, a deletion in 8p23.2 (3.502 Mb-3.678 Mb) including the CSMD1
gene and a deletion of 12p13.1 (14.016 Mb-14.546 Mb) including the
genes GRIN2B and ATF7IP. These alterations were observed in the
321-day-old, but not in the early SCCOHT-1 population.
Additional alterations were observed during culture,
among them a duplication 19p13.3 (0.280 Mb-2.445 Mb) adjacent to a
deletion in 19p13.3 (2.461 Mb-4.546 Mb) (Fig. 4B). A 3 Mb duplication of the region
11p15.5-15.4 was present in both populations. To validate the
results of the aCGH an additional FISH on interphases was perfomed.
A submicroscopic deletion of the subtelomeric region 19p could be
detected in 14% and a submicroscopic trisomy and tetrasomy of the
subtelomeric region of 11p could be detected in 10% and 88% of the
inter-phase nuclei, respectively.
SCCOHT-1-induced NOD/scid mouse
tumors
In order to investigate the tumorigenic potential of
SCCOHT-1, 1×106 cells of a GFP-labeled population after
lentiviral transduction was injected subcutaneously into NOD/scid
mice. Following injection of 7 mice with the SCCOHT-1 cells and 6
elutriated fractions, all 7 mice developed a significant
subcutaneous tumor within 4 to 8 weeks (Fig. 5). The tumor weight in relation to
the mouse weight varied between 0.43% and 8.92% in the different
elutriated cell fractions as compared to 1.98% in the original
SCCOHT-1 control cells (Table II).
Fluorescence scanning of the organs revealed at least one mouse
(elutriated-4) carrying liver metastasis. Blood serum analysis for
calcium concentrations revealed 3.36 mmol/l (Ca2+) in a
SCCOHT-1 tumor carrying mouse whereby normal control NOD/scid mice
exhibit levels of 2.75±0.15 mmol/l (Ca2+). The mice were
sacrificed and the mouse tumors were dissected for both,
immunohistochemical analysis and tissue culture, respectively.
After re-cultivation of the minced NOD/scid mice-derived tumors
using SCCOHT-1 culture medium in an explant culture, continuously
proliferating primary cultures from all tumors were obtained,
termed SCCOHT-1-GFPNOD/scid. These cells from
re-cultivated NOD/scid mice-derived tumors were cultured so far for
at least 213 days and demonstrated no significant differences as
compared to the original SCCOHT-1 cells and one representative
population was subjected in a telomerase assay to test the
continuous growth activities in the cells.
 | Table IITumor weight in NOD/scid mice. |
Table II
Tumor weight in NOD/scid mice.
| Injected
population | Mouse weight
(g) | Tumor weight
(g) | Relation tumor
weight/mouse weight (%) |
|---|
| SCCOHT-1
control | 18.92 | 0.374 | 1.98 |
| SCCOHT-1
elutriated-1 | 23.37 | 0.1 | 0.43 |
| SCCOHT-1
elutriated-3 | 22.68 | 0.136 | 0.6 |
| SCCOHT-1
elutriated-4 | 17.82 | 1.59 | 8.92 |
| SCCOHT-1
elutriated-5 | 11.06 | 0.601 | 5.43 |
| SCCOHT-1
elutriated-6 | 23.4 | 0.095 | 0.41 |
| SCCOHT-1
elutriated-7 | 17.82 | 0.24 | 1.35 |
Telomerase assay
SCCOHT-1 cells cultured for 151 and 396 days
demonstrated a strong telomerase activity which sustained in 514
days-old SCCOHT-1 cells carrying the GFP gene after lentiviral
transduction (Fig. 6). This
SCCOHT-1 telomerase measurement revealed similar activity levels
according to the positive HeLa assay control and each sample
included an appropriate heat-sensitive control (ΔT) (Fig. 6). Moreover, lysates from NOD/scid
mouse-dissected SCCOHT-1-derived tumor tissue also represented
telomerase activity although at markedly reduced levels. In
addition, re-cultured cells from explant cultures of NOD/scid
mouse-derived SCCOHT-1 tumors (SCCOHT-1-GFPNOD/scid)
revealed a similarly high telomerase activity as compared to the
original SCCOHT-1 cells suggesting a constantly remaining and
stable proliferative capacity also after xenograft (Fig. 6).
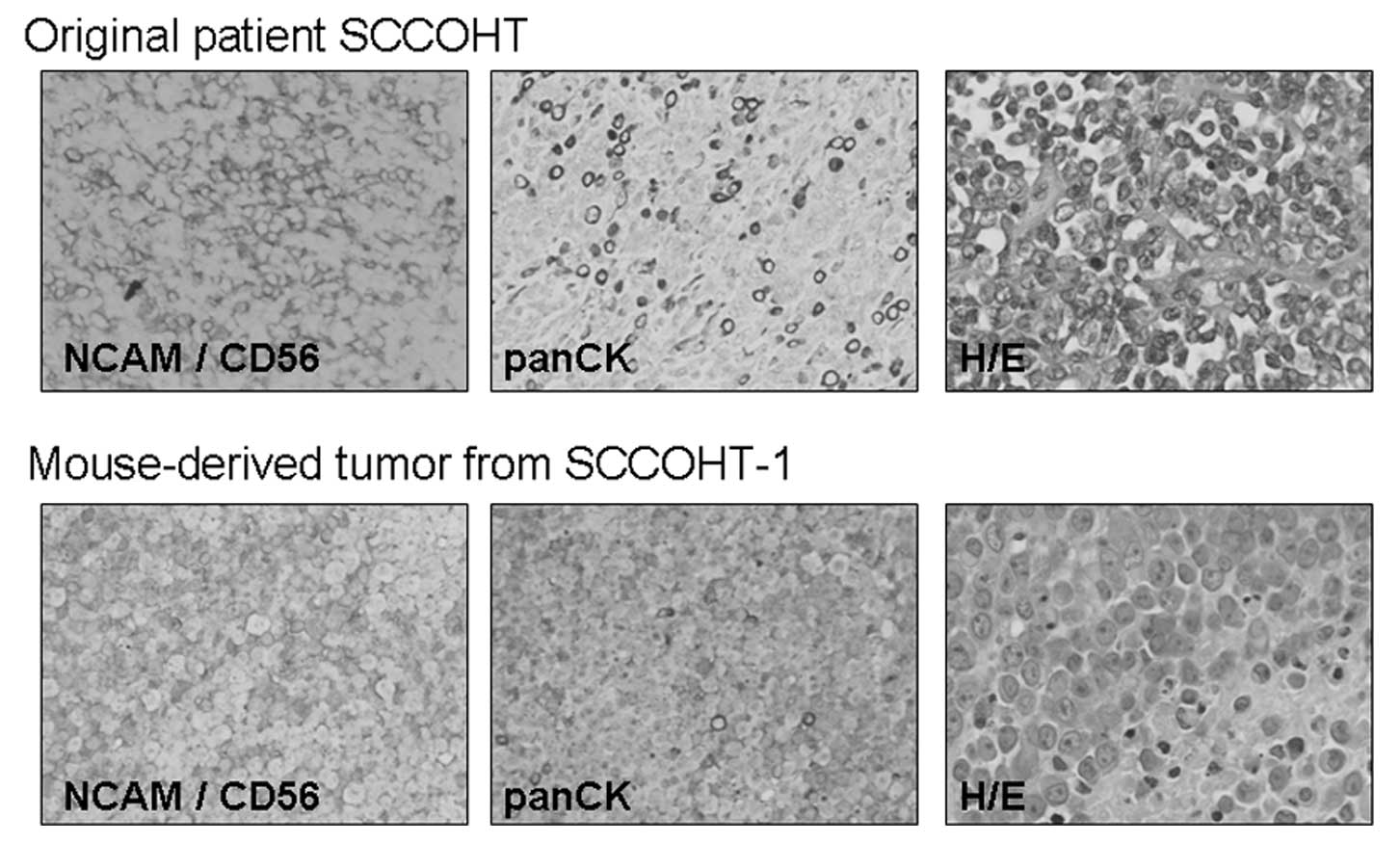
Immunohistochemical analysis
Tumor sections of the NOD/scid mouse-derived
SCCOHT-1-GFP-induced tumors were compared to appropriate sections
of the original SCCOHT patient tumor by immunohistopathological
evaluation (Fig. 7). The tumor
sections of both sources were stained with a neuroendocrine marker
(NCAM/CD56) and with an anti-cytokeratin antibody (pan-CK) and
revealed similar expression patterns. Moreover, the cells within
the tumor tissue were stained with hematoxylin and eosin (H&E)
and revealed a comparable morphology and distribution in the
mouse-derived tumors as compared to the original tumor tissue of
the patient (Fig. 7).
Discussion
SCCOHT represents an aggressive female tumor with
poor prognosis, and the present study introduces and characterises
the first cellular model for this devastating disease. With respect
to morphology the large number of rounded cells shown by both SEM
and TEM is concomitant with rapidly and frequently dividing cells,
a well-known feature of malignant tumor cells. This is further
supported by the observation of annulate lamellae which tend to
occur in rapidly dividing cells and at early stages of
differentiation. The predominantly euchromatic state of the
chromatin in conjunction with the large amount of ribosomes
indicate high activity in protein synthesis. However, the
prevalence of free ribosomes suggests synthesis of structural
proteins needed for cell growth rather than proteins destined for
secretion. Together, the ultrastructural features displayed by the
cells are concomitant with rapid division, early stages of
differentiation and active synthesis of structural proteins. These
morphological findings are also supported by the functional data
demonstrating a continuous proliferative capacity by permanent cell
cycle traverse and a persisting telomerase activity whereby the
SCCOHT-1 cells displayed a stable karyotype.
Moreover, the morphological characterization also
correlates with the expression of surface markers of a more
undifferentiated phenotype. Indeed, SCCOHT-1 cells demonstrated a
scarce expression pattern of the cell surface markers and filaments
tested in this study. Thus, only a minor population expressed
cytokeratins, the adhesion molecule CD15 and the cellular
communication hyaluronic acid receptor CD44. The majority of
SCCOHT-1 cells exhibited the β-1 integrin subunit CD29 which also
functions in cell-cell communication, i.e. as the α3β1 integrin,
and may play a role during metastatic diffusion of certain tumor
cells. The appearance of the neuroendocrine marker CD56 in the
SCCOHT specimen has also been discussed as one of the markers in
other types of ovarian cancers including ovarian granulose cell
tumors and ovarian sex cord-stromal tumors (29,30).
Nearly all SCCOHT-1 cells expressed the intermediate
filament vimentin and the CD90 antigen which represents a
glycophosphatidylinositol-anchored membrane protein in the outer
leaflet of lipid rafts, suggesting intense cell-cell and
cell-matrix interactions. A variety of immune competent cells
express CD90 such as precursor T cells (thymocytes), T cells and
some NK cell populations. Moreover, CD90 can be expressed by
hematopoietic stem cells and it is predominantly present on
mesenchymal stem cells in a normoxic and hypoxic microenvironment
(31,32). Using a tumor model during induced
epithelial to mesenchymal transition of non-small cell lung cancer
cells, a reduction in cytokeratins and E-cadherin has been detected
concomitant with a progressively increased expression of CD90 and
vimentin (33) indicating that the
SCCOHT-1 cells may represent an intermediate state between the
epithelial and mesenchymal phenotype. However, no genetic origin of
SCCOHT has been identified to date and accordingly, little is known
about potential mutations in SCCOHT and the corresponding
patients.
Certain genetic alterations including BRCA gene
mutations which play a major role in hereditary breast cancer
development are also discussed for some ovarian cancers. Some cases
were reported about familial SCCOHT, however, no simultaneous BRCA
mutations were detectable (34)
suggesting significant differences between ovarian cancer-types and
SCCOHT. Moreover, the absence of 12p aberrations would rule out a
germ cell tumor-derived neoplasm since 12p gain represents a
pathognomonic marker of malignant germ cell tumors. The chromosomal
imbalances observed particularly in long-term cultured SCCOHT-1
cells in the present study, included deletions of the CSMD1,
GRIN2b, ATF7IP and the PARK2 gene,
respectively. The PARK2 gene product represents the E3
ubiquitin protein ligase (parkin) involved in the regulation of
proteasomal protein turnover. In SCCOHT-1 cells, the breakpoint
6q26 including parkin is associated with the translocation t(6;17)
(q26:q22) as determined by metaphase cytogenetics. Previous work
suggested that PARK2 which is located within a fragile area
of chromosome 6, may exhibit certain tumor suppressor function as
it is also deleted in a large amount of ovarian and lung cancer
patients (35). Likewise, the
CSMD1 gene product has been attributed with
tumor-suppressive properties since deletion of this gene in
invasive ductal breast cancer patients has been reported with high
malignancy and poor survival (36). GRIN2b among other genes
(i.e. HOXA1, MT1G and SFRP4) represents a
methylation marker during progression of breast cancer (37). The ATF7IP gene encodes for
ATFa-associated modulator (AM) and activating transcription factor
7-interacting protein, a regulator of telomerase reverse
transcriptase expression and previous work has demonstrated that
variants near ATF7IP are accompanied with testicular germ
cell cancer (38).
Besides these characterized chromosomal imbalances
SCCOHT-1 cells exhibit a stable phenotype which is also determined
by metaphase cytogenetics. The stability of these progressively
proliferating tumor cells is further verified by the in vivo
experiments demonstrating high tumorigenicity in all NOD/scid mice
and a histopathology of the mouse tumors which corresponded to the
original patient tumor biopsy. Moreover, induction of
SCCOHT-1-induced tumors in NOD/scid mice was associated with
hypercalcemia which likewise represents a property in human SCCOHT.
Furthermore, re-culture of NOD/scid mice-derived tumor cells were
indistinguishable from the GFP-transduced primary SCCOHT-1 cells
further supporting a stable phenotype derived from the original
patient tumor.
Together, these findings suggested the successful
isolation and characterization of a spontaneous permanently growing
SCCOHT-derived cell population. This culture provides the first
cellular model to further investigate this malignancy particularly
with respect to cell biological properties and signaling pathways
related to the paralleled hypercalcemia. Moreover, the reproducible
generation of in vivo tumors in NOD/scid mice may serve as
an appropriately corresponding in vivo tumor model, which
also for the first time enables a disease-focused drug screening to
search and examine effective and sufficient therapeutic strategies
for this rather unknown type of cancer.
Acknowledgements
The technical support by Marianne
Thren is appreciated. The authors are grateful for the help of Dr
Silke Glage (Hannover Medical School) with the calcium
measurements. This study was supported by a grant from the
Niedersächsische Krebsgesellschaft e.V. to R.H.
References
|
1
|
Dickersin GR, Kline IW and Scully RE:
Small cell carcinoma of the ovary with hypercalcemia: a report of
eleven cases. Cancer. 49:188–197. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young RH, Oliva E and Scully RE: Small
cell carcinoma of the hypercalcemic type in the ovary. Gynecol
Oncol. 57:7–8. 1995.PubMed/NCBI
|
|
3
|
Scully RE: Atlas of tumor pathology:
tumors of the ovary and maldeveloped gonads. Armed Forces Institute
of Pathology; Washington DC: 1979
|
|
4
|
Eichhorn JH and Young RH: Transitional
cell carcinoma of the ovary: a morphologic study of 100 cases with
emphasis on differential diagnosis. Am J Surg Pathol. 28:453–463.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geisinger KR, Kute TE, Pettenati MJ, et
al: Characterization of a human ovarian carcinoma cell line with
estrogen and progesterone receptors. Cancer. 63:280–288. 1989.
View Article : Google Scholar
|
|
6
|
Hamilton TC, Young RC, McKoy WM, et al:
Characterization of a human ovarian carcinoma cell line
(NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res.
43:5379–5389. 1983.PubMed/NCBI
|
|
7
|
Poels LG, Jap PH, Ramaekers FF, et al:
Characterization of a hormone-producing ovarian carcinoma cell
line. Gynecol Oncol. 32:203–214. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bible KC, Boerner SA, Kirkland K, et al:
Characterization of an ovarian carcinoma cell line resistant to
cisplatin and flavopiridol. Clin Cancer Res. 6:661–670.
2000.PubMed/NCBI
|
|
9
|
Ohi S, Niimi S, Okada N, et al:
Establishment and characterization of a human ovarian small cell
carcinoma, hypercalcemic type, cell line (OS-1) secreting PTH,
PthrP and ACTH--special reference to the susceptibility of
anti-cancer drugs. Hum Cell. 17:203–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ulbright TM, Roth LM, Stehman FB, Talerman
A and Senekjian EK: Poorly differentiated (small cell) carcinoma of
the ovary in young women: evidence supporting a germ cell origin.
Hum Pathol. 18:175–184. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aguirre P, Thor AD and Scully RE: Ovarian
small cell carcinoma. Histogenetic considerations based on
immunohistochemical and other findings. Am J Clin Pathol.
92:140–149. 1989.PubMed/NCBI
|
|
12
|
Walt H, Hornung R, Fink D, et al:
Hypercalcemic-type of small cell carcinoma of the ovary:
characterization of a new tumor line. Anticancer Res. 21:3253–3259.
2001.PubMed/NCBI
|
|
13
|
McCluggage WG, Oliva E, Connolly LE,
McBride HA and Young RH: An immunohistochemical analysis of ovarian
small cell carcinoma of hypercalcemic type. Int J Gynecol Pathol.
23:330–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scully RE: Small cell carcinoma of
hypercalcemic type. Int J Gynecol Pathol. 12:148–152. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eichhorn JH, Bell DA, Young RH, et al: DNA
content and proliferative activity in ovarian small cell carcinomas
of the hypercalcemic type. Implications for diagnosis, prognosis,
and histogenesis. Am J Clin Pathol. 98:579–586. 1992.PubMed/NCBI
|
|
16
|
Shrimali RK, Correa PD and Reed NS:
Dose-dense and dose-intense chemotherapy for small cell ovarian
cancer: 2 cases and review of literature. Med Oncol. 28:766–770.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harrison ML, Hoskins P, du Bois A, et al:
Small cell of the ovary, hypercalcemic type - analysis of combined
experience and recommendation for management. A GCIG study. Gynecol
Oncol. 100:233–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benrubi GI, Pitel P and Lammert N: Small
cell carcinoma of the ovary with hypercalcemia responsive to
sequencing chemotherapy. South Med J. 86:247–248. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reed WC: Small cell carcinoma of the ovary
with hypercalcemia: report of a case of survival without recurrence
5 years after surgery and chemotherapy. Gynecol Oncol. 56:452–455.
1995.PubMed/NCBI
|
|
20
|
Barondeau J, Rodgers M, Braun L, Azarow K,
Forouhar M and Faucette K: Small cell ovarian carcinoma: a rare,
aggressive tumor masquerading as constipation in a teenager with a
fatal outcome. J Pediatr Hematol Oncol. 32:e139–e141. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dykgraaf RH, de Jong D, van Veen M,
Ewing-Graham PC, Helmerhorst TJ and van der Burg ME: Clinical
management of ovarian small-cell carcinoma of the hypercalcemic
type: a proposal for conservative surgery in an advanced stage of
disease. Int J Gynecol Cancer. 19:348–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Distelmaier F, Calaminus G, Harms D, et
al: Ovarian small cell carcinoma of the hypercalcemic type in
children and adolescents: a prognostically unfavorable but curable
disease. Cancer. 107:2298–2306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gebert A and Preiss G: A simple method for
the acquisition of high-quality digital images from analog scanning
electron microscopes. J Microsc. 191:297–302. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bertram C and Hass R: Cellular senescence
of human mammary epithelial cells (HMEC) is associated with an
altered MMP-7/HB-EGF signaling and increased formation of
elastin-like structures. Mech Ageing Dev. 130:657–669. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schlegelberger B, Metzke S, Harder S,
Zühlke-Jenisch R, Zhang Y and Siebert R: Classical and Molecular
Cytogenetics of Tumor Cells. Springer Verlag; Heidelberg: 1999
|
|
26
|
Shaffer L, Slovak M and Champbell L: ISCN
- An International System for Human Cytogenetic Nomenclature. S.
Karger AG; Basel: 2009
|
|
27
|
Gohring G, Hanke C, Kratz C, et al:
Fluorescence in situ hybridization using the subtelomeric 11q probe
as a diagnostic tool for congenital thrombocytopenia. Ann Hematol.
85:883–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schambach A, Galla M, Modlich U, Will E,
Chandra S, Reeves L, Colbert M, Williams DA, von Kalle C and Baum
C: Lentiviral vectors pseudotyped with murine ecotropic envelope:
increased biosafety and convenience in preclinical research. Exp
Hematol. 34:588–592. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McCluggage WG, McKenna M and McBride HA:
CD56 is a sensitive and diagnostically useful immunohistochemical
marker of ovarian sex cord-stromal tumors. Int J Gynecol Pathol.
26:322–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohishi Y, Kaku T, Oya M, Kobayashi H, Wake
N and Tsuneyoshi M: CD56 expression in ovarian granulosa cell
tumors, and its diagnostic utility and pitfalls. Gynecol Oncol.
107:30–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Majore I, Moretti P, Hass R and Kasper C:
Identification of subpopulations in mesenchymal stem cell-like
cultures from human umbilical cord. Cell Commun Signal. 7:62009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lavrentieva A, Majore I, Kasper C and Hass
R: Effects of hypoxic culture conditions on umbilical cord-derived
human mesenchymal stem cells. Cell Commun Signal. 8:182010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pirozzi G, Tirion V, Camerlingo R, et al:
Epithelial to mesenchymal transition by TGFbeta-1 induction
increases stemness characteristics in primary non small cell lung
cancer cell line. PLoS One. 6:e215482011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martinez-Borges AR, Petty JK, Hurt G,
Stribling JT, Press JZ and Castellino SM: Familial small cell
carcinoma of the ovary. Pediatr Blood Cancer. 53:1334–1336. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Denison SR, Callahan G, Becker NA,
Phillips LA and Smith DI: Characterization of FRA6E and its
potential role in autosomal recessive juvenile parkinsonism and
ovarian cancer. Genes Chromosomes Cancer. 38:40–52. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kamal M, Shaaban AM, Zhang L, et al: Loss
of CSMD1 expression is associated with high tumour grade and poor
survival in invasive ductal breast carcinoma. Breast Cancer Res
Treat. 121:555–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park SY, Kwon HJ, Lee HE, et al: Promoter
CpG island hyper-methylation during breast cancer progression.
Virchows Arch. 458:73–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Turnbull C, Rapley EA, Seal S, et al:
Variants near DMRT1, TERT and ATF7IP are associated with testicular
germ cell cancer. Nat Genet. 42:604–607. 2010. View Article : Google Scholar : PubMed/NCBI
|