Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common tumor worldwide and the third most common cause of mortality
from tumor, causing approximately 600,000 cases of mortality
worldwide each year (1). The high
mortality rate of HCC can in part be attributed to a lack of
diagnostic methods that allow for early detection. It is well known
that early detection and treatment can improve the survival rate of
patients with HCC (2). Several
studies have demonstrated that surveillance for high risk
individuals, such as chronic hepatitis (CH) and liver cirrhosis
(LC) patients, is a vital method to detect HCC earlier and to
provide optimal opportunity for treatment (3), which has been shown to improve
patient survival (4–6). Although α-fetoprotein (AFP) is the
most effective serological marker available to detect HCC, its
sensitivity and specificity are not optimal (7). Therefore, it is imperative to develop
more effective methods, especially at the early stage, for the
diagnosis of HCC.
Previous studies have shown that in the case of HCC,
antecedent LC and CH are common precursor conditions and during
transition to malignancy some patients develop autoantibodies that
were not present during the preceding chronic liver disease phase
(8). These autoantibodies to
tumor-associated antigens (TAAs), which are known as ‘reporters’
from the immune system, identify the antigenic changes in cellular
proteins involved in the transformation process (9). Serological screening of
autoantibodies to TAAs may be used as an effective method to
identify patients with HCC at an early stage (10). With the widespread application of
the technologies, more TAAs have been identified in HCC, and also
anti-TAA autoantibodies have been detected in sera from patients
with HCC. The concern is that the sensitivity and specificity of
autoantibodies to single TAA as a diagnostic marker in HCC are
currently still low and insufficient for the diagnosis of HCC
(10). However, using a mini-array
of multiple TAAs to detect autoantibodies simultaneously may
enhance the sensitivity and specificity, which may be a potential
valuable approach for cancer diagnosis (11). Previous studies in our lab have
demonstrated that the final cumulative prevalence of autoantibodies
to TAAs can reach 66.2% by using an array of 10 TAAs including
c-myc, p53, cyclin B1, p62, Koc, IMP-1, survivin, p16, Sui1 and
RalA, to detect autoantibodies in sera from patients with HCC
(12). In order to improve both
the sensitivity and specificity of anti-TAA autoantibodies as
biomarkers in HCC detection, the major task is to continue
identifying and validating more valuable TAAs in HCC to add in the
mini-array of TAAs which we have created in previous studies, for
optimizing the combination of the mini-array of TAAs in HCC.
Glucose-regulated protein 78 (GRP78), also referred
to as immunoglobulin heavy chain binding protein (BiP), is a
chaperone protein belonging to the HSP70 protein family, which
resides primarily in the lumen of endoplasmic reticulum (ER)
(13,14). GRP78 is a vital functional protein
in the physiological and pathological conditions of ER, which can
facilitate protein folding, assembly, transport, calcium
homeostasis, and can also regulate ER stress signaling under the ER
stress (14,15). Overexpression of GRP78 in certain
types of tumors, such as lung, breast, stomach, prostate and HCC,
has been widely reported (16–20).
Many studies have indicated that the function of GRP78 is closely
related to tumor proliferation, survival, metastasis, apoptosis,
angiogenesis, and chemoresistance (18,21–27).
Importantly, ectopic expression of GRP78 on the cancer cell
surface, but not in normal cells, has been revealed, suggesting
that GRP78 may be a potential target of cancer therapy (28–30).
Furthermore, autoantibodies against GRP78 have been detected at
high levels in the sera from patients with prostate and gastric
cancers (31–33). Whether autoantibodies against GRP78
can also be detected in sera from patients with HCC and whether
autoantibodies against GRP78 can be used as a serological
diagnostic markers in HCC remain to be investigated. This study
determines the prevalence of anti-GRP78 autoantibodies in sera from
patients with HCC, LC and CH, as well as from normal human sera
(NHS), to further validate the diagnostic value of anti-GRP78
autoantibodies in the immunodiagnosis of HCC.
Materials and methods
Sera and general information
All sera used in this study, 76 sera of HCC, 30 sera
of LC, 30 sera of CH, and 86 NHS, were obtained from the serum bank
of Cancer Autoimmunity and Epidemiology Research Laboratory at UTEP
(University of Texas at El Paso), which were originally provided by
our collaborator, Dr X.-X. Peng at Sun Yat-sen University,
Guangzhou, China. This study was approved by the Institutional
Review Board of UTEP and collaborating institutions.
All HCC patients were diagnosed according to the
criteria described in a previous study (34), and had not received treatment with
any chemotherapy or radiotherapy. Patients with CH and LC were
followed up at least 18 months after collecting blood to exclude
individuals with primary biliary cirrhosis and asymptomatic or
clinically undetectable HCC. NHS were assembled from individuals at
the same locality during annual health examinations, who had no
obvious evidence of malignancy. In all 76 HCC patients, general
information for 69 patients was available. Of the 69 HCC patients,
50 (72.5%) were male, and 19 (27.5%) were female. Mean age was
58.1±13.1 years (range, 24–78 years). Of these 69 patients, 51
(73.9%) were positive for HBV, 7 (10.1%) for HCV, and 4 (5.8%) for
both HBV and HCV; 44 (63.8%) patients had a previous history of CH,
13 (18.8%) of LC, and 24 (34.8%) patients had no previous history
of either CH or LC. Based on the Chinese guideline for liver
cancer, 22 (31.9%) patients were in clinical stage I, 13 (18.8%)
patients in stage II, 21 (30.4%) in stage III, 8 (11.6%) in stage
IV, respectively and for 5 (7.2%) patients there was no available
data on clinical stages. Of the 76 HCC sera, 63 had been tested by
α-fetoprotein (AFP) in a previous study.
Recombinant proteins and antibodies used
in this study
The full-length recombinant protein GRP78 was
commercially purchased (Abcam, Cambridge, MA). Polyclonal
anti-GRP78 rabbit antibody and monoclonal anti-β-actin mouse
antibody were purchased (Cell Signaling Technology, Inc., Danvers,
MA). Horseradish peroxidase (HRP)-conjugated goat anti-human IgG,
HRP-conjugated goat anti-rabbit IgG, HRP-conjugated goat anti-mouse
IgG and FITC-conjugated goat anti-human IgG were purchased (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA). Anti-rabbit IgG Fab2
(Alexa Fluor 488) was purchased (Life Technologies, Grand Island,
NY).
Cell lines and cell extracts
Nine different tumor cell lines, human epidermoid
carcinoma (Hep2), human hepatocellular carcinoma (HepG2), human
hepatocellular carcinoma (SUN449), human breast cancer (SKBR3),
human ovarian carcinoma (SKOV3), human lung epithelial
adenocarcinoma (A549), human urinary bladder carcinoma (T24), human
acute lymphoblastic leukemia (MOLT-4) and leukemia (KOPN63), were
obtained from the Tumor Cell bank of our Laboratory and cultured
following the specific protocol for each cell line. Cells grown in
monolayers were solubilized directly in Laemmli’s sample buffer
containing protease inhibitors. Solubilized lysates were briefly
sonicated before electrophoresis on SDS-polyacrylamide gels.
Enzyme-linked immunosorbent assay
(ELISA)
Standard protocol for ELISA was used as described in
our previous study (12). In
brief, a 96-well microtiter plate (Thermo Scientific, Waltham, MA)
was coated overnight at 4°C with recombinant GRP78 protein at a
final concentration of 0.5 μg/ml in phosphate-buffered
saline (PBS). The antigen-coated wells were blocked with gelatin
post-coating solution at room temperature for 2 h. Human sera
diluted at 1:100 with serum diluent were incubated for 2 h at room
temperature in the antigen-coated wells, followed by HRP-conjugated
goat anti-human IgG. The substrate
2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS,
Sigma-Aldrich, St. Louis, MO) was used as detecting reagent. The
average optical density (OD) value at a wavelength of 405 nm was
applied as data analysis. The cutoff value designating positive
reaction was the mean OD of 86 NHS + 3SD.
Western blotting
Denatured recombinant GRP78 protein and cancer cell
lysates were electrophoresed on 10% SDS-PAGE and transferred to
nitrocellulose papers, respectively. After blocking in PBS with 5%
nonfat milk and 0.05% Tween-20 for 1 h at room temperature, the
nitrocellulose papers were incubated overnight at 4°C with 1:200
dilution of human sera, 1:1000 dilution of polyclonal anti-GRP78
antibody and 1:500 dilution of monoclonal anti-β-actin mouse
antibody, separately. HRP-conjugated goat anti-human IgG,
HRP-conjugated goat anti-rabbit IgG and HRP-conjugated goat
anti-mouse IgG were applied as secondary antibody at a 1:3000
dilution. The ECL-kit was used to detect immunoreactive bands
according to the manufacturer’s instructions (Thermo Scientific,
Waltham, MA).
Indirect immunofluorescence assay
(IIFA)
HEP-2 Antigen Substrate for IIFA Test System was
incubated with dilution of sera (1:40) and preabsorbed sera for 30
min at room temperature or anti-GRP78 antibody (1:50) overnight at
4°C, separately. FITC-conjugated goat anti-human IgG or anti-rabbit
IgG Fab2 (Alexa Fluor 488) was separately used as secondary
antibody at a 1:100 dilution. Fluorescence microscope (Leica
DM1000, Germany) was used for examination.
Absorption of antibodies with recombinant
protein
The diluted human sera (1:40) were incubated with
recombinant protein (final concentration of recombinant protein in
the diluted human sera was 0.01 μg/μl) overnight at
4°C, then centrifuged at 10,000 × g for 10 min. The supernatant was
used for immunofluorescence assay.
Immunohistochemistry (IHC) with tissue
array slides
Liver cancer tissue array slide with normal tissue
controls (12 cases/24 cores, including clinical stages and
pathology grades) were purchased (US Biomax, Inc., Rockville, MD),
and used to detect the expression of the GRP78 protein. Tissue
array slides were deparaffinized with xylene and dehydrated with
ethanol. Antigen retrieval was performed by microwave-heating
methods in Trilogy™ pretreatment solution for 20 min. Avidin/biotin
blocking solution were used to prevent nonspecific binding of
antibodies. The sections were incubated with polyclonal anti-GRP78
antibody (1:50 dilution) for 1 h at room temperature. HRP Detection
System (HRP streptavidin label and polyvalent biotinylated link)
and DAB Substrate Kit were used as detecting reagents. After
counterstaining with hematoxylin, the sections were dehydrated and
mounted. The slides were observed by light microscopy.
Statistical analysis
The mean OD value of each group of patients’ sera
was compared using the Mann-Whitney U test; the frequency of
autoantibody to TAAs in each group of patients’ sera was compared
using the χ2 test with Fisher’s exact test, and two
significant levels (0.05 and 0.01) were used. Methods for
calculating sensitivity and specificity were used as previously
described (35).
Results
Frequency and titer of autoantibodies
against GRP78 in HCC
The full-length recombinant GRP78 protein was used
as coating antigen in ELISA to screen autoantibodies against GRP78
in sera from patients with HCC, LC and CH, as well as NHS. In
total, 76 sera from patients with HCC, 30 from LC, 30 from CH, and
86 sera from normal human individuals were used in this study. As
shown in Table. I, the prevalence
of autoantibody against GRP78 was 35.5% (27/76) in HCC, which was
significantly higher than that in LC, CH, and NHS (P<0.01).
Titer of anti-GRP78 antibodies in human sera are shown in Fig. 1. The titer of anti-GRP78 antibodies
in sera from some of the HCC patients was much higher than that in
other groups. The average titer of autoantibody against GRP78 in
HCC sera was higher than that in HC, CH, and NHS (P<0.01). The
ELISA results were also confirmed by western blot analysis.
Fig. 2 shows that representative
HCC sera with positive reaction to GRP78 in ELISA also have strong
reactivity in western blotting compared to LC, CH, and normal sera,
though weakly reactive bands were shown in some LC and CH sera. Of
the 76 HCC sera, 63 were tested with α-fetoprotein (AFP). In 63 HCC
sera tested with both anti-GRP78 autoantibody and AFP, 37 (58.7%)
had an AFP level >100 ng/ml, 31 (49.2%) had an AFP level >200
ng/ml and 25 (39.7%) were positive with anti-GRP78 autoantibody.
When both anti-GRP78 autoantibody and AFP (either >100 ng/ml or
>200 ng/ml) were simultaneously used as diagnostic markers, 45
(71.4%, AFP >100 ng/ml) and 43 of 63 HCC sera (68.3%, AFP
>200 ng/ml) were positive, respectively.
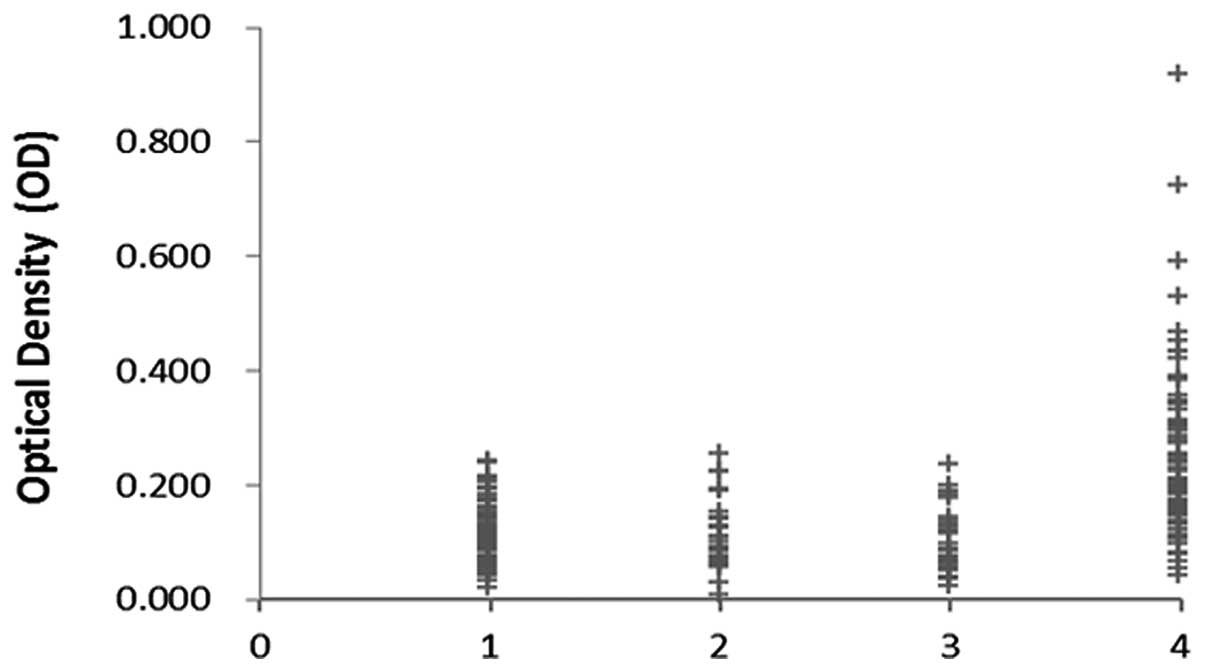 | Figure 1Titer of autoantibody against GRP78
in human sera by ELISA. The range of antibody titers to GRP78 was
expressed as optical density (OD) obtained from ELISA. The mean +
3SD of NHS are shown in relationship to all serum samples. The mean
OD value of NHS, CH, LC, HCC was 0.112, 0.111, 0.100, 0.247,
respectively. Titer of anti-GRP78 in HCC is much higher than that
in other types of sera (P<0.01). Lane 1, NHS; lane 2, CH; lane
3, LC; lane 4, HCC. |
 | Table IFrequency of autoantibody against
GRP78 in human sera by ELISA. |
Table I
Frequency of autoantibody against
GRP78 in human sera by ELISA.
| Type of sera | No. tested | Autoantibody to
GRP78 (%) |
|---|
| HCC | 76 | 27
(35.5)** |
| LC | 30 | 0 |
| CH | 30 | 0 |
| NHS | 86 | 0 |
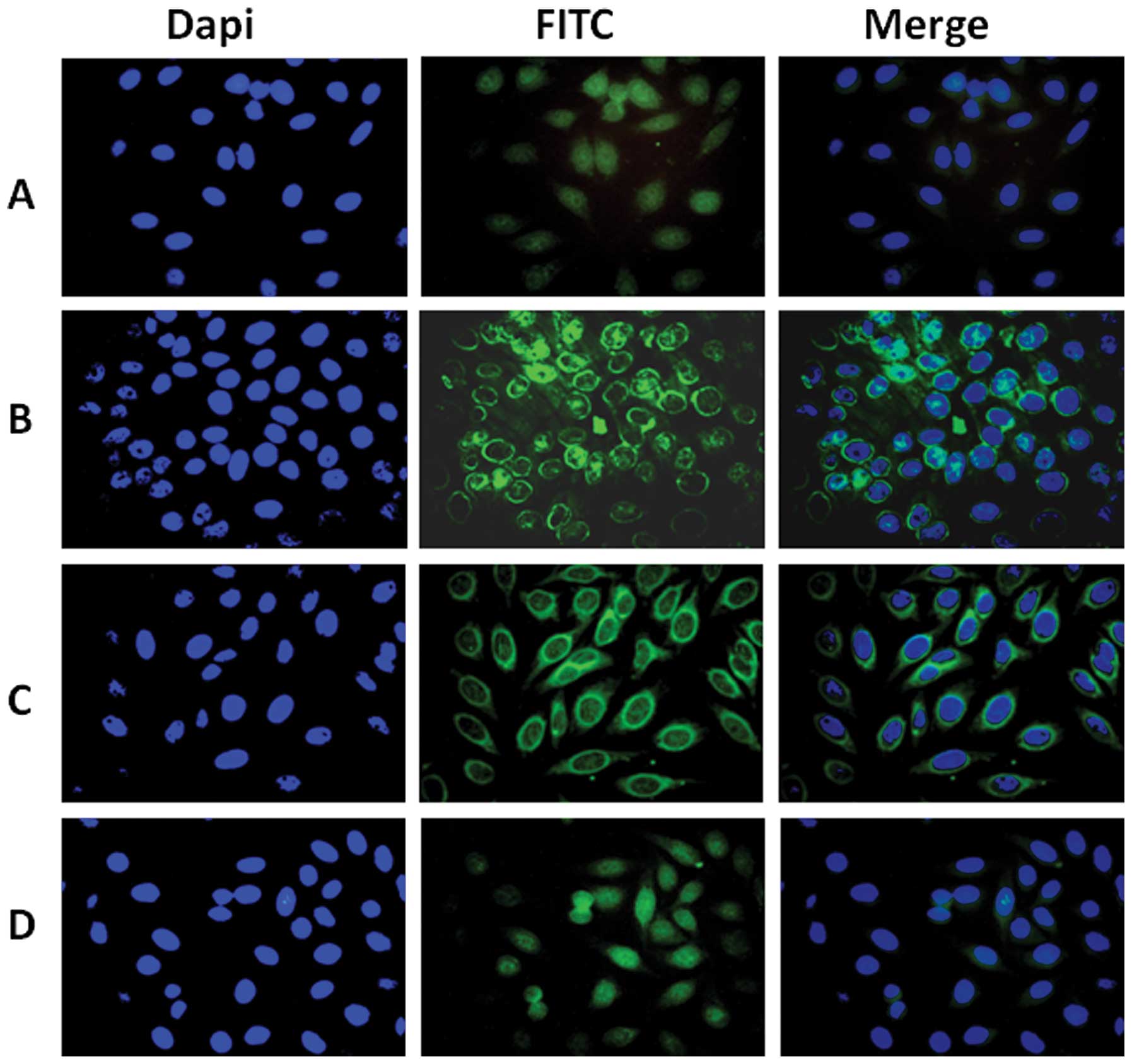
Perinuclear intense staining pattern
showing in HEP-2 cells by indirect immunofluorescence assay with
representative positive HCC sera
To further confirm the reactivity of autoantibody in
HCC sera to GRP78 and the intracellular location of GRP78,
commercially purchased HEP-2 cell slides were used in indirect
immunofluorescence assay to detect HCC sera with anti-GRP78
positive in ELISA. As shown in Fig.
3, a representative anti-GRP78 positive HCC serum had an
intense perinuclear staining pattern, which was similar in pattern
and location to that shown by polyclonal anti-GRP78 antibody. The
fluorescent staining was significantly reduced when the same HCC
serum pre-absorbed with recombinant GRP78 protein.
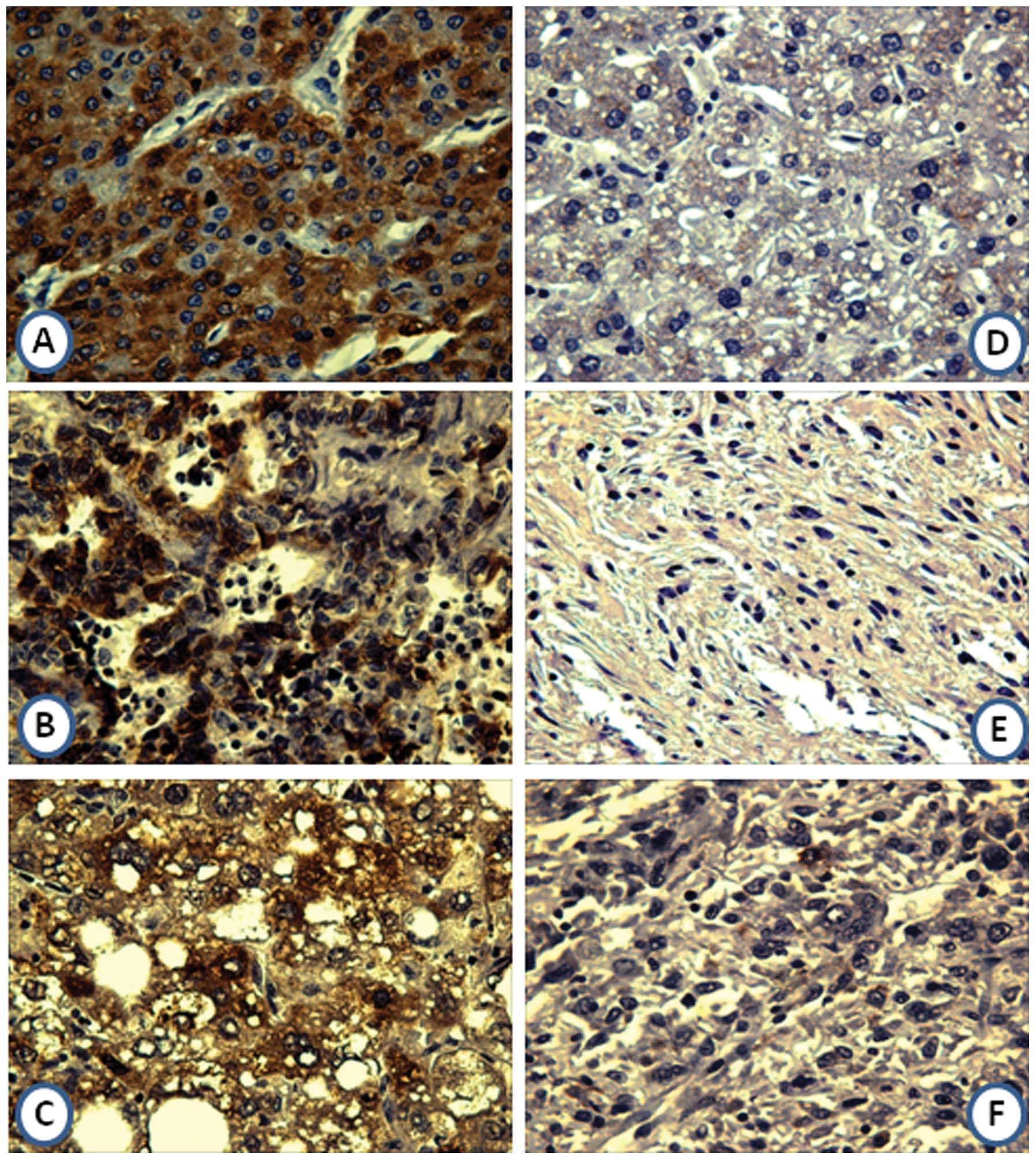
Expression of GRP78 in liver cancer
tissues and normal hepatic tissues by immunohistochemistry
In the current study, the expression profile of
GRP78 in liver cancer tissues and normal liver tissues was examined
by immunohistochemistry with tissue array slides. Tissue array
slides were commercially available for this study, including 5 HCC
tissue specimens, 2 cholangiocellular carcinoma tissue specimens, 1
clear cell carcinoma tissue specimen, 1 malignant fibrohistiocytoma
tissue specimen, 1 angiosarcoma tissue specimen and 2 normal
hepatic tissue specimens. The polyclonal anti-GPR78 antibody was
used as primary antibody to detect the expression of GRP78 in liver
cancer and normal hepatic tissues. As a result, 3 of the 5 HCC
tissues were positively stained (among these 3 positively stained
tissues, 1 was strong positively stained, 1 was positively stained,
and 1 was weak positively stained, respectively); 1 of the 2
cholangiocellular carcinoma tissues was positively stained; the
clear cell carcinoma tissue was positively stained; 2 normal
hepatic tissues were negatively stained; the malignant
fibrohistiocytoma tissue and the angiosarcoma tissue were both
negatively stained. Due to the small sample size of tissues in this
study, it is difficult to establish a statistical analysis. The
expression of GRP78 in liver cancer and normal hepatic tissues are
shown in Fig. 4.
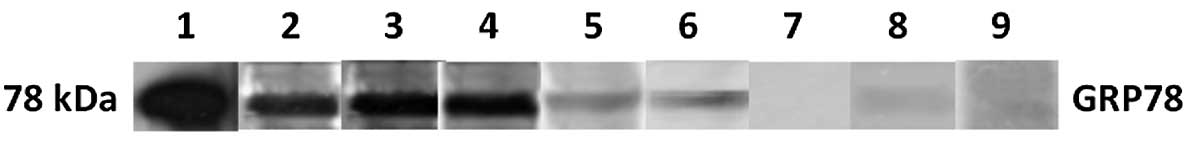
Overexpression of GRP78 in different
cancer cell lines
To confirm the expression of GRP78 in different
tumors, 9 tumor cell lines (Hep2, HepG2, SUN449, SKBR3, SKOV3,
A549, T24, MOLT-4, KOPN63) were analyzed by western blotting. The
polyclonal anti-GPR78 antibody was used as probe for this study. As
shown in Fig. 5, Hep2, HepG2,
SKBR3, SKOV3 cell lines had strong reactivity, and SUN449, A549,
T24, MOLT-4, KOPN63 cell lines had weak reactivity compared to cell
lines which have clear reactive bands.
 | Figure 5Nine types of tumor cell lines were
analyzed by western blotting. The polyclonal anti-GPR78 antibody
was used as probe. Hep2, HepG2, SKBR3, SKOV3 cell lines had strong
reactivity, and SUN449, A549, T24, MOLT-4, KOPN63 cell lines had
weak reactivity compared to cell lines which have clear reactive
bands. 1, Hep2; 2, HepG2; 3, SUN449; 4, SKBR3; 5, SKOV3; 6, A549;
7, T24; 8, MOLT-4; 9, KOPN63. |
Discussion
GRP78 is well recognized as a vital ER molecular
chaperone and regulator in the ER stress signaling pathway
(13–15). Under the conditions or factors of
ER stress-induction, the perturbation of ER homeostasis leads to
the accumulation of misfolded proteins, which triggers the unfolded
protein response (UPR) for cell survival (36). The 3 trans-membrane ER stress
sensors PKR-like ER kinase (PERK), activating transcription factor
(ATF) 6 and inositol requiring (IRE) 1 are dissociated from
molecular chaperone GRP78, which interact with GRP78 in
non-stressed cells in inactive forms, to activate the 3 distinct
UPR signal pathways (15,36). These signaling pathways can reduce
protein synthesis, upregulate the transcription of chaperone genes
to ameliorate ER load and increase capacity of protein folding
(37). GRP78 gene expression can
upregulate similarly in rapidly growing tumors, which attributes to
the tumor microenvironment of ER stress, such as glucose
starvation, acidosis and hypoxia (13,37).
Overexpression of GRP78 in a variety of cancer tissues compared
with normal tissue has been shown in many studies (16–20).
This study also revealed a high level of expression of GRP78 in the
Hep2, HepG2, SKBR3, SKOV3 cancer cell lines and a relatively weak
expression in the SUN449, A549, T24, MOLT-4, KOPN63 cell lines. Our
study suggested that overexpression of GRP78 was not only in
certain solid tumors, but also in leukemia (MOLT-4, KOPN63) cells,
which further reveals the higher relevance of GRP78 with
malignancy. In tumor cells, expression of GRP78 may play a critical
role in proliferation, survival, anti-apoptosis and chemoresistance
(18,21–27).
Interfering PI3K/Akt signaling was shown as a major mechanism of
GRP78 facilitating tumor growth and resisting apoptosis (38). Recent studies have also shown a
significant correlation between GRP78 and poor cancer prognosis
(18,20,22,23,39).
The expression profile of GRP78 in liver cancer and
normal liver tissues was examined by immunohistochemistry in the
current study. Overexpression of GRP78 in HCC tissues compared with
normal tissues is shown in this study, which is consistent with the
findings of previous reports (20,23,39).
Expression of GRP78 in cholangiocellular carcinoma and clear cell
carcinoma tissues has also been shown, but not in malignant
fibrohistiocytoma and angiosarcoma tissues. It is difficult to
establish a statistical analysis due to the small sample size of
tissues in this study. Upregulation of GRP78 hinting at a poor
prognostic outcome in HCC by promoting invasion and associating
with venous infiltration, vascular invasion and intrahepatic
metastasis has been reported (20,23,39).
However, there is little information about autoantibodies against
GRP78 in sera from HCC patients and a correlation between
anti-GRP78 autoantibodies and HCC, although high levels of
autoantibodies against GRP78 have been detected in the sera from
patients with prostate and gastric cancer (31–33).
In the present study, 35.5% of HCC sera showed immune response to
GRP78 recombinant protein, but not in LC, CH, and NHS. The mean
titer of autoantibody against GRP78 in the sera of HCC was
significantly higher than that in other cohorts. The high frequency
of autoantibodies against GRP78 in HCC sera in one way suggested
that it could be used as a potential serological marker in the
immunodiagnosis of HCC. Notably, when both autoantibody against
GRP78 and AFP are used as diagnostic markers simultaneously,
sensitivity can reach 71.4%, which is much higher than that when
using either anti-GRP78 or AFP as a marker. Taken together, our
data indicate that anti-GRP78 autoantibody might be useful as a
complementary marker in conjunction with AFP in HCC diagnosis.
An important feature of GRP78 is that it can express
on the cancer cell surface, but not on normal cells (28–30).
It reveals a profile of tumor cells different from normal cells.
Cell surface GRP78 serves as a receptor in cell signaling
transduction, survival and anticancer therapeutic targeting
(30,38). For example, α2-macroglobulin (α2M*)
combined with cell surface GRP78 can activate downstream cell
survival signaling in 1-LN prostate cancer cells (40). The combination of Cripto with cell
surface GRP78 can promote tumor growth by suppression of
transforming growth factor-β (TGF-β) signaling (41). Autoantibody against a fragment of
GRP78 in sera from patients with prostate cancer can induce cell
proliferation, which recognizes the same site of GRP78 on the tumor
cell surface as the co-receptor with α2M* (32). The same autoantibody against GRP78
increases tissue factor pro-coagulant activity by binding to cell
surface GRP78 (42). An antibody
against the N-terminus of GRP78 (N-20 antibody) can compete for the
same binding site of GRP78 with Cripto to inhibit Cripto signaling
(43). A commercial polyclonal
antibody directed against the C-terminus of GRP78 can induce
apoptosis in melanoma cells (A375) and prostate cancer cells (1-LN
and DU145), which may lead to the upregulation of p53, inhibition
of NF-κB1 and NF-κB2 activation, and suppression of Ras/MAPK and
PI3K/Akt signaling (44–46). Furthermore, autoantibody against
GRP78 was reported to correlate with aggressive tumor behavior
(14,31). In view of the critical role of
autoantibodies against GRP78 in tumor cell survival, the high
levels of anti-GRP78 autoantibodies detected in HCC sera in this
study implied the close correlation between anti-GRP78
autoantibodies and HCC. Further study is needed to investigate the
role of anti-GRP78 autoantibody in HCC, especially the role of
GRP78 on the tumor cell surface. High titer of autoantibodies
against GRP78 in HCC sera may have potential value for HCC
immunotherapy.
Although the mechanism of emerging autoantibodies
remains unclear, many studies have demonstrated that autoantibody
production is related to abnormal expression of autoantigen
(47). Autoantibodies can be
detected in the sera from patients with autoimmune diseases and
various types of cancer as well as from some healthy individuals
(9,47). In the present study, autoantibody
against GRP78 was also detected in some sera of LC and CH, but the
titer of anti-GRP78 autoantibody was much lower than it was in the
sera of HCC. High titer of autoantibody against GRP78 in HCC sera
may be in accordance with ectopic expression of GRP78, especially
expression on the surface of tumor cells. We propose that the
presence of high titer of autoantibody against GRP78 in sera from
chronic liver diseases could be a signal of malignancy. Monitoring
of the anti-GRP78 autoantibody titer in high risk individuals of
HCC could be valuable for the early detection of malignancy, though
further studies are required.
As described above, the findings in this study
suggest that autoantibodies against GRP78 may play a vital role in
tumor cell survival. This study provides further information on
autoantibodies against GRP78 in the sera of HCC, LC, CH, and
healthy individuals, suggesting that anti-GRP78 autoantibody may be
a potential diagnostic marker for HCC, especially in conjunction
with AFP. Whether the monitoring of the autoantibody titer in high
risk individual of HCC is necessary, remains to be confirmed.
Acknowledgements
The authors thank Dr Eng M. Tan (The
Scripps Research Institute) for his support. This work was
supported by grants (1SC1CA166016-10, 5G12RR08124) from the
National Institutes of Health (NIH), and by a grant from the
National Natural Science Foundation of China (30872962,
81172086).
References
|
1
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: Epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kudo M: Early detection and curative
treatment of early-stage hepatocellular carcinoma. Clin
Gastroenterol Hepatol. 3:S144–S148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar
|
|
4
|
Lencioni R: Surveillance and early
diagnosis of hepatocellular carcinoma. Dig Liver Dis. 42:S223–S227.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang BH, Yang BH and Tang ZY: Randomized
controlled trial of screening for hepatocellular carcinoma. J
Cancer Res Clin Oncol. 130:417–422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong LL, Limm WM, Severino R and Wong LM:
Improved survival with screening for hepatocellular carcinoma.
Liver Transpl. 6:320–325. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daniele B, Bencivenga A, Megna AS and
Tinessa V: α-fetoprotein and ultrasonography screening for
hepatocellular carcinoma. Gastroenterology. 127:S108–S112.
2004.
|
|
8
|
Tan EM: Autoantibodies as reporters
identifying aberrant cellular mechanisms in tumorigenesis. J Clin
Invest. 108:1411–1415. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan EM and Zhang JY: Autoantibodies to
tumor-associated antigens: reporters from the immune system.
Immunol Rev. 222:328–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JY and Tan EM: Autoantibodies to
tumor-associated antigens as diagnostic biomarkers in
hepatocellular carcinoma and other solid tumors. Expert Rev Mol
Diagn. 10:321–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang JY: Mini-array of multiple
tumor-associated antigens to enhance autoantibody detection for
immunodiagnosis of hepatocellular carcinoma. Autoimmun Rev.
6:143–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Zhou YS, Qiu SM, Wang KJ, Liu SW,
Peng XX, Li JF, et al: Autoantibodies to tumor-associated antigens
combined with abnormal alpha-fetoprotein enhance immunodiagnosis of
hepatocellular carcinoma. Cancer Lett. 289:32–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee AS: The glucose-regulated proteins:
stress induction and clinical applications. Trends Biochem Sci.
26:504–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee AS: GRP78 induction in cancer:
therapeutic and prognostic implications. Cancer Res. 67:3496–3499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hendershot LM: The ER function BiP is a
master regulator of ER function. Mt Sinai J Med. 71:289–297.
2004.PubMed/NCBI
|
|
16
|
Uramoto H, Sugio K, Oyama T, Nakata S, Ono
K, Yoshimastu T, Morita M, et al: Expression of endoplasmic
reticulum molecular chaperone Grp78 in human lung cancer and its
clinical significance. Lung Cancer. 49:55–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fernandez PM, Tabbara SO, Jacobs LK,
Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA, et al:
Overexpression of the glucose-regulated stress gene GRP78 in
malignant but not benign human breast lesions. Breast Cancer Res
Treat. 59:15–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng HC, Takahashi H, Li XH, Hara T,
Masuda S, Guan YF and Takano Y: Overexpression of GRP78 and GRP94
are markers for aggressive behavior and poor prognosis in gastric
carcinomas. Hum Pathol. 39:1042–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pootrakul L, Datar RH, Shi SR, Cai J,
Hawes D, Groshen SG, Lee AS, et al: Expression of stress response
protein GRP78 is associated with the development of
castration-resistant prostate cancer. Clin Cancer Res.
12:5987–5993. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luk JM, Lam CT, Siu AFM, Lam BY, Ng IOL,
Hu MY, Che CM, et al: Proteomic profiling of hepatocellular
carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27,
Hsp70, GRP78) up-regulation and their associated prognostic values.
Proteomics. 6:1049–1057. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong DZ, Ni M, Li JZ, Xiong SG, Ye W,
Virrey JJ, Mao CH, et al: Critical role of the stress chaperone
GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis
in transgene-induced mammary tumor development. Cancer Res.
68:498–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Daneshmand S, Quek ML, Lin E, Lee C, Cote
RJ, Hawes D, Cai J, et al: Glucose-regulated protein GRP78 is
up-regulated in prostate cancer and correlates with recurrence and
survival. Hum Pathol. 38:1547–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su RJ, Li Z, Li HD, Song HJ, Bao CF, Wei J
and Cheng LF: Grp78 promotes the invasion of hepatocellular
carcinoma. BMC Cancer. 10:202010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong DZ, Stapleton C, Luo BQ, Xiong SG, Ye
W, Zhang Y, Jhaveri N, et al: A critical role for GRP78/BiP in the
tumor microenvironment for neovascularization during tumor growth
and metastasis. Cancer Res. 71:2848–2857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katanasaka Y, Ishii T, Asai T, Naitou H,
Maeda N, Koizumi F, Miyagawa S, et al: Cancer antineovascular
therapy with liposome drug delivery systems targeted to BiP/GRP78.
Int J Cancer. 127:2685–2698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugawara S, Takeda K, Lee A and Dennert G:
Suppression of stress protein GRP78 induction in tumor B/C10ME
eliminates resistance to cell mediated cytotoxicity. Cancer Res.
53:6001–6005. 1993.PubMed/NCBI
|
|
27
|
Chiou JF, Tai CJ, Huang MT, Wei PL, Wang
YH, BS JA, Wu CH, et al: Glucose-regulated protein 78 is a novel
contributor to acquisition of resistance to sorafenib in
hepatocellular carcinoma. Ann Surg Oncol. 17:603–612. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berger CL, Dong ZM, Hanlon D, Bisaccia E
and Edelson RL: A lymphocyte cell surface heat shock protein
homologous to the endoplasmic reticulum chaperone, immunoglobulin
heavy chain binding protein BIP. Int J Cancer. 71:1077–1085. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin BK, Wang H, Yim AM, Naour FL,
Brichory F, Jang JH, Zhao R, et al: Global profiling of the cell
surface proteome of cancer cells uncovers an abundance of proteins
with chaperone function. J Biol Chem. 278:7607–7616. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arap MA, Lahdenranta J, Mintz PJ, Hajitou
A, Sarkis AS, Arap W and Pasqualini R: Cell surface expression of
the stress response chaperone GRP78 enables tumor targeting by
circulating ligands. Cancer Cell. 6:275–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mintz PJ, Kim J, Do KA, Wang XM, Zinner
RG, Cristofanilli M, Arap MA, et al: Fingerprinting the circulating
repertoire of antibodies from cancer patients. Nat Biotechnol.
21:57–63. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gonzalez-Gronow M, Cuchacovich M, Llanos
C, Urzua C, Gawdi G and Pizzo SV: Prostate cancer cell
proliferation in vitro is modulated by antibodies against
glucose-regulated protein 78 isolated from patient serum. Cancer
Res. 66:11424–11431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsunemi S, Nakanishi T, Fujita Y, Bouras
G, Miyamoto Y, MiyamotoI A, Nomura E, et al: Proteomics-based
identification of a tumor-associated antigen and its corresponding
autoantibody in gastric cancer. Oncol Rep. 23:949–956.
2010.PubMed/NCBI
|
|
34
|
Johnson PJ, Leung N, Cheng P, Welby C,
Leung WT, Lau WY, Yu S and Ho S: ‘Hepatoma-specific’
alphafetoprotein may permit preclinical diagnosis of malignant
change in patients with chronic liver disease. Br J Cancer.
75:236–240. 1997.
|
|
35
|
Gordis L: Assessing the validity and
reliability of diagnostic and screening tests. Epidemiology. 2nd
edition. W.B. Saunders Company; Philadelphia, PA: pp. 63–81.
2000
|
|
36
|
Kaufman RJ: Orchestrating the unfolded
protein response in health and disease. J Clin Invest.
110:1389–1398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ni M and Lee AS: ER chaperones in
mammalian development and human diseases. FEBS Lett. 581:3641–3651.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pfaffenbach KT and Lee AS: The critical
role of GRP78 in physiologic and pathologic stress. Curr Opin Cell
Biol. 23:150–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lim SO, Park SG, Yoo JH, Park YM, Kim HJ,
Jang KT, Cho JW, et al: Expression of heat shock proteins (HSP27,
HSP60, HSP70, HSP90, GRP78, GRP94) in hepatitis B virus-related
hepatocellular carcinomas and dysplastic nodules. World J
Gastroenterol. 11:2072–2079. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Misra UK, Deedwania R and Pizzo SV:
Binding of activated α2-macroglobulin to its cell surface receptor
GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent
activation of LIMK. J Biol Chem. 280:26278–26286. 2005.
|
|
41
|
Shani G, Fischer WH, Justice NJ, Kelber
JA, Vale W and Gray PC: GRP78 and Cripto form a complex at the cell
surface and collaborate to inhibit transforming growth factor β
signaling and enhance cell growth. Mol Cell Biol. 28:666–677.
2008.PubMed/NCBI
|
|
42
|
Al-Hashimi AA, Caldwell J, Gonzalez-Gronow
M, Pizzo SV, Aboumrad D, Pozza L, Al-Bayati H, et al: Binding of
anti-GRP78 autoantibodies to cell surface GRP78 increases tissue
factor procoagulant activity via the release of calcium from
endoplasmic reticulum stores. J Biol Chem. 285:28912–28923. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kelber JA, Panopoulos AD, Shani G, Booker
EC, Belmonte JC, Vale WW and Gray PC: Blockade of Cripto binding to
cell surface GRP78 inhibits oncogenic Cripto signaling via
MAPK/PI3K and Smad2/3 pathways. Oncogene. 28:2324–2336. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ni M, Zhang Y and Lee AS: Beyond the
endoplasmic reticulum: atypical GRP78 in cell viability, signalling
and therapeutic targeting. Biochem J. 434:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Misra UK and Pizzo AV: Ligation of cell
surface GRP78 with antibody directed against the COOH-terminal
domain of GRP78 suppresses Ras/MAPK and PI3-kinase/AKT signaling
while promoting caspase activation in human prostate cancer cells.
Cancer Biol Ther. 9:1–11. 2010. View Article : Google Scholar
|
|
46
|
Misra UK, Mowery Y, Kaczowka S and Pizzo
SV: Ligation of cancer cell surface GRP78 with antibodies directed
against its COOH-terminal domain up-regulates p53 activity and
promotes apoptosis. Mol Cancer Ther. 8:1350–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Backes C, Ludwig N, Leidinger P, Harz C,
Hoffmann J, Keller A, Meese E, et al: Immunogenicity of
autoantigens. BMC Genomics. 12:3402011. View Article : Google Scholar : PubMed/NCBI
|