Introduction
Despite the existence of excellent screening and
preventive strategies, colorectal carcinoma remains a major public
health problem in western countries. The American Cancer Society
estimates there will be 142,570 new cases diagnosed in 2010 and
51,370 people will die of the disease. In Germany, the incidence
was about 70,404 in 2010 and 27,582 people died of colorectal
cancer (2). Hepatic metastasis of
colorectal cancer is common and by the time of diagnosis, 25% of
colon cancers and 50%–70% of cancers of the rectum will have
extended through the bowel wall metastasising to lymph nodes in
50%–60% (3). The most common site
of extra-lymphatic involvement is the liver, with the lungs as the
most frequently affected extra-abdominal organ. Patients with
metastatic colorectal tumours frequently die of hepatic failure due
to liver metastases. Therefore, treatment of liver metastases may
prolong survival, even in the presence of extra-hepatic
disease.
Treatment options for patients with liver metastatic
colorectal cancer are limited and clinical outcome is generally
poor. Surgical resection in selected patients can achieve 30–70%
5-year survival (4–6). However, only 20–30% of patients with
colorectal liver metastases are suitable for resection at initial
presentation (7,8) and 70–80% of patients will develop
recurrence within 5 years (9).
Untreated colorectal metastases to the liver have a
poor prognosis and are associated with a median survival of 5–10
months (10). Systemic
chemotherapy can palliate symptoms and improve survival.
5-Fluorouracil (5-FU)-based chemotherapy has been the cornerstone
of treatment of unresectable CRC for more than 40 years, and new
drugs such as irinotecan, oxaliplatin, cetuximab and bevacizumab
have recently broadened the options for treatment. With the
introduction of these novel agents, median survival has increased
significantly from 6 months to over 20 months (11).
In addition to systemic chemotherapy, current
therapies of unresectable liver lesions include hepatic arterial
infusion of chemotherapeutic drugs, transarterial
chemoembolisation, radiofrequency ablation, cryotherapy,
laser-induced thermotherapy (LITT) and yttrium-90 radioembolization
(12–16).
Recent reports on the use of irinotecan loaded DC
beads (DEBIRI) in transarterial chemoembolisation (TACE) therapy
for metastatic colorectal cancer show promising results (17,18).
These early reports suggest DEBIRI as a safe and effective
treatment for patients with liver predominant unresectable
metastases from colorectal cancer. This study was the first
clinical evaluation in the development of DEBIRI. Critical features
of this study relate to safety (adverse events), technical
feasibility, PK, profile and efficacy (tumour response, time to
progression and necrosis) which were considered pivotal in the
assessment of DEBIRI as a therapy for unresectable metastases from
colorectal cancer.
Materials and methods
This prospective, pilot, single-arm study, approved
by an independent ethics committee, was conducted in our
institution from January 2006 to April 2008. Thirty patients were
planned to be enrolled, but the study was closed after 11 patients
were included due to the slow recruitment rate.
Colorectal cancer stage IV (Dukes’ D, TNM: TX, NX,
M1) patients were eligible for enrolment if they gave written
informed consent, were at least 18-year-old, of any race or sex,
had their primary tumour(s) successfully removed by surgery, had 1
to 8 measurable unresectable metastase(s) that were confined to the
liver, with a tumour burden of no more than 30% of liver volume. In
addition, patients were required to have an ECOG performance status
score of ≤1, with a life expectancy of >6 months, and adequate
liver, renal and hematologic function. Women of child bearing
potential and fertile men were required to use effective
contraception and prior therapy was permitted (with or without
irinotecan) if the last chemotherapy or prior radiotherapy
treatment was completed at least 4 weeks before enrolment.
Exclusion to therapy included the presence of extrahepatic
metastases, any contraindications to irinotecan, active bacterial,
viral or fungal infection, presence of another concurrent
malignancy, prior malignancy in the last 5 years (except adequately
treated basal or squamous cell skin cancer or carcinoma in
situ of the cervix) and any contraindication for hepatic
embolisation procedures.
All patients had baseline MRI/CT to document the
extent of liver disease prior to therapy. Patients were to receive
TACE treatments with DEBIRI as monotherapy every 3 weeks (up to 4
treatments) and followed up for 6 months. Last chemotherapy or
prior radiotherapy treatment had to be completed at least 4 weeks
before study entry (first treatment).
The primary study endpoint of safety was measured by
adverse events, serious adverse events, and physical and laboratory
assessments. Secondary study endpoints of technical feasibility,
pharmacokinetic profile (irinotecan and SN-38) and efficacy [tumour
response and time to progression (TTP) measured by MRI/CT
assessments according to RECIST at 3 and 6 months following the
first DEBIRI treatment] were also measured. Where feasible, the
percentage necrosis was calculated as total necrotic tumour volume
divided by total treated tumour volume.
Transarterial chemoembolisation with
irinotecan loaded DC bead (DEBIRI)
DC Bead (Biocompatibles, Farnham, UK) comprise a
range of modified polyvinylalcohol hydrogel microspheres that are
biocompatible, hydrophilic, non-resorbable, and precisely
calibrated. DC Bead is a CE marked drug delivery embolisation
system and is indicated for loading irinotecan (DEBIRI) for
embolisation of vessels supplying malignant colorectal cancer
metastasised to the liver (mCRC) and is capable of eluting a local,
controlled, sustained dose of irinotecan to hepatic metastases of
colorectal cancer. DC Beads of 100–300 μm and 300–500 μm in size
were loaded with irinotecan to dose of 50 mg/ml beads according to
the instructions for use. After removing saline from the vials, 5
ml of 20 mg/ml irinotecan solution was added to each vial of DC
Bead. At the time of our study, vials were stored for at least 4 h
for loading; however, the recommended loading time is 2 h. At the
end of the loading period the supernatant was discarded and beads
mixed 50:50 with non-ionic contrast (Omnipaque®).
All patients were adequately pre-medicated according
to standard hospital procedure for chemoembolisation and, where
required, included prophylactic pain management, anti-emetics and
corticoids using 100 mg Pethidin (Dolantin®), 3 mg
Granisetron (Kevatril®) and 20 mg Fortecortin, 10 ml
Mepivacain (Scandicain®) was also used for local
anaesthesia subcutaneously. Additional pain medication and/or
vasodilators were used at the investigator’s discretion.
A diagnostic angiogram was performed to evaluate the
hepatic aterial supply and verify portal patency. The right or left
hepatic artery was selected, depending on the location of the
lesions to be treated. Once the vascular supply of the tumour was
identified, a microcatheter was placed superselectively and distal
to the cystic artery in close proximity to the lesions. Irinotecan
loaded DC Bead was injected slowly until complete stasis of blood
flow was achieved (embolisation endpoint). Up to 8 ml of loaded DC
Bead (400 mg irinotecan) was to be delivered until the embolisation
endpoint was achieved. This dose was considered safe for local
delivery based on two preclinical studies in a porcine liver model
(19) and is less than the
recommended 3-weekly dose of irinotecan monotherapy (350
mg/m2). Infusion of the beads was stopped if the
embolisation endpoint was achieved prior to delivery of the full
dose. No additional embolic agent was used to achieve the
embolisation endpoint. Following the procedure all patients were
observed for vital signs, femoral access site, pain management,
fluid hydration and discharged according to standard hospital
procedure.
Pharmacokinetics
Blood samples were taken prior to and at 5, 10, 15
and 30 min, 1, 2, 4, 6 and 24 h and on day 21 after the first
DEBIRI treatment. Plasma levels of irinotecan and SN-38 were
measured using an HPLC fluorescence method previously validated at
CentraLabs Clinical Research (Huntington, UK). Peak plasma
concentrations (Cmax), area under the curve (AUC) and
plasma half-life (t½) were measured for irinotecan and
its main active metabolite SN-38.
Statistical consideration
This was a pilot phase I/II study intended to assess
feasibility. No well-documented ‘gold-standard’ protocol for
chemoembolisation of liver metastases that could serve as a control
is recognised by the medical community. Furthermore,
placebo-control in patients with metastatic cancer was considered
unethical and ruled out. A single-arm, non-comparative study was
thus the suitable design for this initial safety study. A planned
enrolment of 30 patients was considered adequate for this
assessment. Descriptive and quantitative statistics were used to
summarise categorical variables and continuous variables. When
relevant, the 95% confidence intervals (CI) were also reported.
Spearman rank correlation was used to determine any relationship
between dose and adverse events. Time to progression was calculated
using Kaplan-Meier. Paired t-tests and non-parametric sign tests
were performed on paired data continuous variables. The latter was
used because of the small sample size of this study.
Results
Demographics
A total of 11 patients (8 males, 3 females) with a
mean age of 64 years (range 45–85 years) were enrolled in the
study. All patients had prior surgery to successfully remove their
primary colorectal cancer. Eight patients had further treatment for
their liver metastases including, laser-induced interstitial
thermotherapy, radiofrequency ablation and radiotherapy. Mean sum
of longest diameter in target lesions was 63±25 mm (range 19–102
mm) with percentage liver involvement of 8.2±2.6% (range 4–12%).
Eight patients had information on prior chemotherapy (Table I).
 | Table IPatient demographics and tumour
characteristics. |
Table I
Patient demographics and tumour
characteristics.
| Demographics | n | | % |
|---|
| Male/female | 8/3 | | 73/27 |
| Caucasian/other | 11/0 | | 100/0 |
| Age (mean ± SD,
range) | | 64±12, 46–85 | |
| ECOG 0/1 | 11/0 | | 100/0 |
| No of lesions | | | |
| 1 | 5 | | 46 |
| 2 | 4 | | 36 |
| 3 | 1 | | 9 |
| >3 | 1 | | 9 |
| Sum of longest
diameter, mm (mean ± SD, range) | | 63±25, 19–102 | |
| Liver involvement, %
(mean ± SD, range) | | 8.2±2.6, 4–12 | |
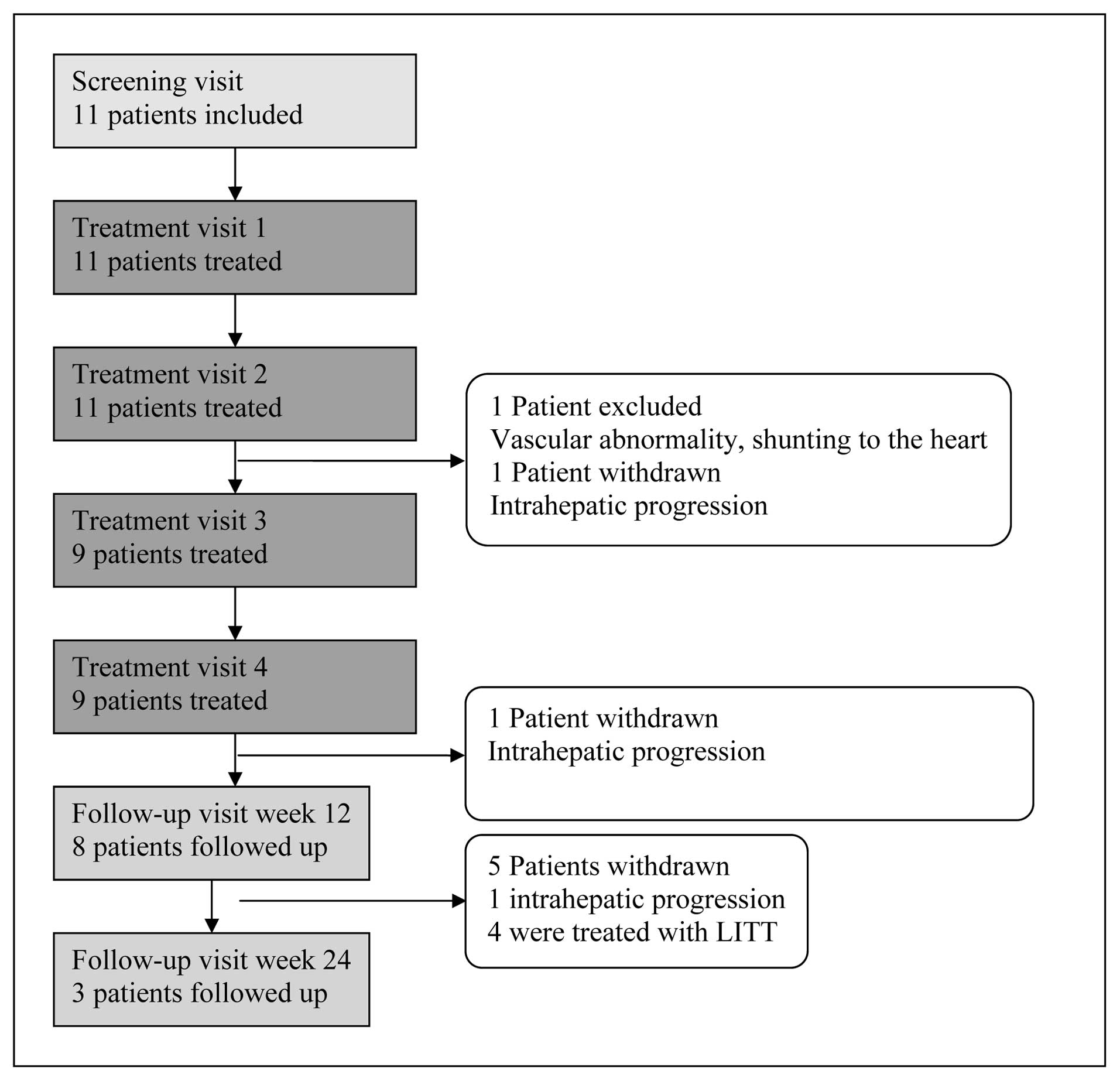
Of the 11 patients enrolled, 9 patients successfully
received 4 TACE treatments with DEBIRI. Two patients were withdrawn
after the second DEBIRI treatment, one due to a vascular
abnormality with shunting to the heart, the other due to
intrahepatic progression (Fig. 1).
The median follow-up period for all the patients was 83 days
(range, 24–176 days). Three patients withdrew from the study due to
intrahepatic progression whilst 4 patients were considered eligible
for laser-induced interstitial thermotherapy (LITT) following
DEBIRI (Fig. 1).
Treatment
The pre-planned dose of irinotecan loaded DC Bead
was up to 400 mg (8 ml beads) in each treatment. Final delivered
dose varied in the patients and was dependant upon volume of beads
delivered prior to the embolisation endpoint.
In all cases, the embolisation endpoint (stasis of
the subsegmented artery) was achieved with less than 4 ml (200 mg
irinotecan). In the 40 DEBIRI treatments performed, between 21–143
mg irinotecan was delivered in each treatment session and an
average total dose of 293 mg (range 124–440 mg) per patient was
achieved across all their individual treatments. Bead size of
100–300 μm and 300–500 μm were used throughout the treatments.
Safety
Ten patients retained an ECOG status of ≤1 during
the study. Nine of the 11 patients (81.8%) experienced a total of
41 adverse events during the study. Each adverse event (AE) was
coded according Medical Dictionary for Regulatory Activities,
MedDRA version 10.0 (MSSO 2007) (Table
II). Gastrointestinal disorders were the most common AEs (63%)
predominantly due to abdominal pain, nausea and vomiting. These are
consistent with the expected post-embolisation syndrome observed in
patients undergoing chemo-embolisation of the liver. The majority
of AEs were mild (61%) with the remainder graded as moderate (39%).
No irinotecan related toxicities such as delayed diarrhoea or
neutropenia were observed.
 | Table IIAdverse events. |
Table II
Adverse events.
| System organ
class | Preferred term | Events | Patients |
|---|
| Cardiac
disorders | Total | 1 | 2.4% | 1 | 9.1% |
| Palpitations | 1 | 2.4% | 1 | 9.1% |
| Gastrointestinal
disorders | Total | 26 | 63.4% | 9 | 81.8% |
| Abdominal
discomfort | 2 | 4.9% | 1 | 9.1% |
| Abdominal
pain | 6 | 14.6% | 5 | 45.5% |
| Constipation | 1 | 2.4% | 1 | 9.1% |
| Nausea | 9 | 22.0% | 6 | 54.5% |
| Vomiting | 8 | 19.5% | 7 | 63.6% |
| General disorders
and administration site conditions | Total | 3 | 7.3% | 3 | 27.3% |
| Asthenia | 1 | 2.4% | 1 | 9.1% |
| Pain | 2 | 4.9% | 2 | 18.1% |
| Infections and
infestations | Total | 1 | 2.4% | 1 | 9.1% |
| Device related
infection | 1 | 2.4% | 1 | 9.1% |
| Investigations | Total | 7 | 17.1% | 2 | 18.1% |
| Blood pressure
increased | 4 | 9.8% | 1 | 9.1% |
| White blood cell
count increased | 3 | 7.3% | 1 | 9.1% |
| Musculoskeletal and
connective tissue disorders | Total | 1 | 2.4% | 1 | 9.1% |
| Musculoskeletal
chest pain | 1 | 2.4% | 1 | 9.1% |
| Respiratory,
thoracic and mediastinal disorders | Total | 2 | 4.9% | 2 | 18.1% |
| Dyspnoea | 2 | 4.9% | 2 | 18.1% |
| Total | | 41 | | 11 | |
No serious adverse events (SAEs) were reported
during the study and no clinically significant changes from
baseline values were observed in the laboratory findings. The
Spearman’s rank correlation coefficient was calculated to examine
whether an association existed between dose delivered and number of
adverse events. The coefficients (rs =0.289 and 0.187 for total
dose and mean dose, respectively) showed no evidence of an
association between dose and total number of adverse events
experienced.
Technical feasibility
Forty DEBIRI treatments were successfully performed
in these 11 patients. No technical complications were reported
during the study demonstrating feasibility of DEBIRI in the
treatment of patients with liver metastases from colorectal
cancer.
Pharmacokinetics (PK)
PK analyses were completed for 10 patients. Average
dose of irinotecan delivered in PK patients was 86 mg. Peak plasma
concentrations (Cmax), area under the curve (AUC) and
plasma half-life (t½) results for irinotecan and its
main active metabolite SN-38 are shown in Table III. Cmax for irinotecan
and SN-38 were 194 ng/ml and 16.7 ng/ml, respectively, following
administration of DEBIRI. Average AUC values were 1,680 ng•h/ml and
281 ng•h/ml, respectively. t½ ranged from 1.6–7.2 h
(mean 4.6 h) for irinotecan and 7.6–8.5 h (mean 12.4 h) for SN-38.
Results for AUC and t½ should be treated with caution as
data from the majority of patients did not meet the data acceptance
criteria to calculate the slope of λz requiring a
minimum of 3 terminal data points randomly distributed on a
straight line with a regression coefficient of ≥0.95, the fraction
of the variance accounted for was ≥0.90 and an interval at least
2-fold greater than t½.
 | Table IIIAverage pharmacokinetic parameters
for irinotecan and SN-38. |
Table III
Average pharmacokinetic parameters
for irinotecan and SN-38.
| Cmax
(ng/ml) | Tmax
(h) | AUCt
(ng•h/ml) | AUC24
(ng•h/ml) | AUC (ng•h/ml) | λz
(1/h) | t1/2
(h) |
|---|
| Irinotecan | | | | | | | |
| Mean | 194 | 2a | 1,510 | 1,520 | 1,680 | 0.1502 | 4.6b |
| SD | 124 | | 1,050 | 1,040 | 1,200 | 0.1346 | |
| SN-38 | | | | | | | |
| Mean | 16.7 | 1a | 147 | 147 | 281 | 0.0559 | 12.4b |
| SD | 11.3 | | 99 | 99 | 352 | 0.0238 | |
Efficacy
Tumour response was assessed according to Response
Evaluation Criteria in Solid Tumours (RECIST) (20). Up to 5 target lesions in the liver
were followed and the sum of longest diameters used to assess
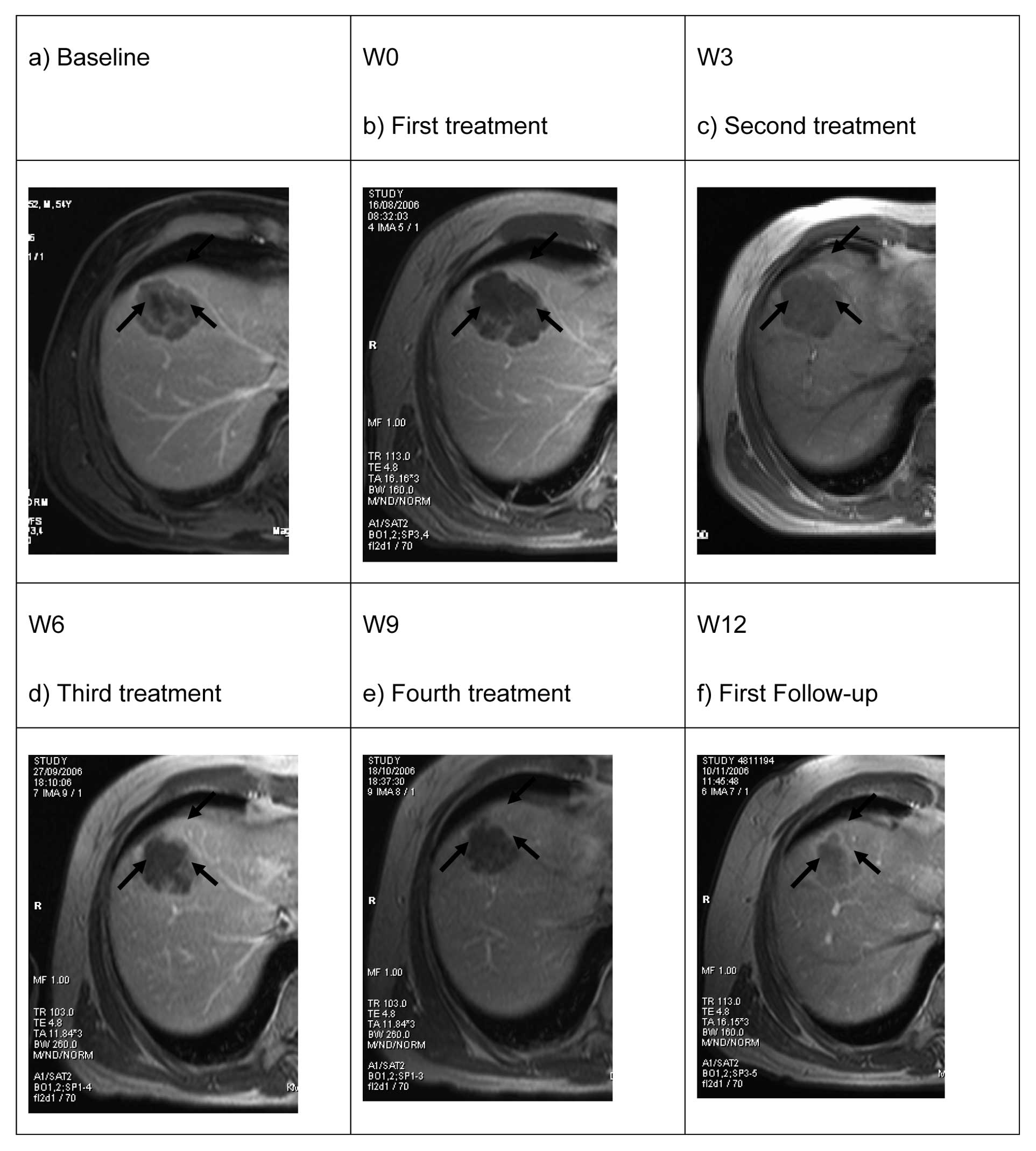
tumour response. Stable disease was reported in 9 (81%) of patients
after the first DEBIRI treatment and 7 (64%) remained stable by the
third treatment (week 9) with one achieving partial response
(Fig. 2).
By the end of the study, intrahepatic progression of
the disease according to RECIST was reported in 7 patients (63%), a
partial response to treatment in 2 patients (18%) and stable
disease reported in 2 patients (18%) (Table IV). These four patients received
the LITT treatment in the follow-up. Best overall response was
defined as the best response from start of treatment over all
follow up visits or until disease progression/recurrence. Best
overall response during the study showed disease control in 9
patients (2 patients with partial response and 7 with stable
disease).
 | Table IVTumour response according to
RECIST. |
Table IV
Tumour response according to
RECIST.
| Patient | Post-treatment 1
(week 3) | Post-treatment 2
(week 6) | Post-treatment 3
(week 9) | Post-treatment 4
(week 12) | Follow-up (week
24) |
|---|
| 001 | Stable | Stable | Stable | Progression | - |
| 002 | Stable | Stable | Stable | Stable | Progression |
| 003 | Stable | Stable | Progression | - | - |
| 004 | Stable | Stable | Stable | Stable | - |
| 005 | Stable | Stable | Stable | Stable | Progression |
| 006 | Stable | Stable | Partial
response | Partial
response | - |
| 007 | Progression | Progression | - | - | - |
| 008 | Stable | Stable | Stable | Stable | - |
| 009 | Stable | Stable | Stable | Stable | Progression |
| 010 | Stable | Stable | Stable | Partial
response | - |
| 011 | Progression | - | - | - | - |
It was planned to measure the extent of tumour
necrosis following DEBIRI, however, we found that tumour necrosis
was difficult to assess and only possible in 3 patients. No
conclusions could be drawn from this assessment.
Tumour markers
Carcinoembryonic antigen (CEA) and
cancer/carbohydrate antigen 19-9 (CA 19-9) (21) were measured pre- and post-DEBIRI
treatment. Nine (82%) patients had clinically significant levels of
CEA at baseline that remained throughout the study. Three patients
(27%) saw an average decrease of 71% (range 61 to 79%) in their CEA
levels. When compared to overall treatment outcome (RECIST), an
increase in CEA level from baseline to last measurement was
associated with disease progression in 6 of the 7 (86%) patients.
In the 3 patients with a decrease in CEA level compared to baseline
values, a stable disease or partial responses was observed. A
similar trend was also found in the CA 19-9 results.
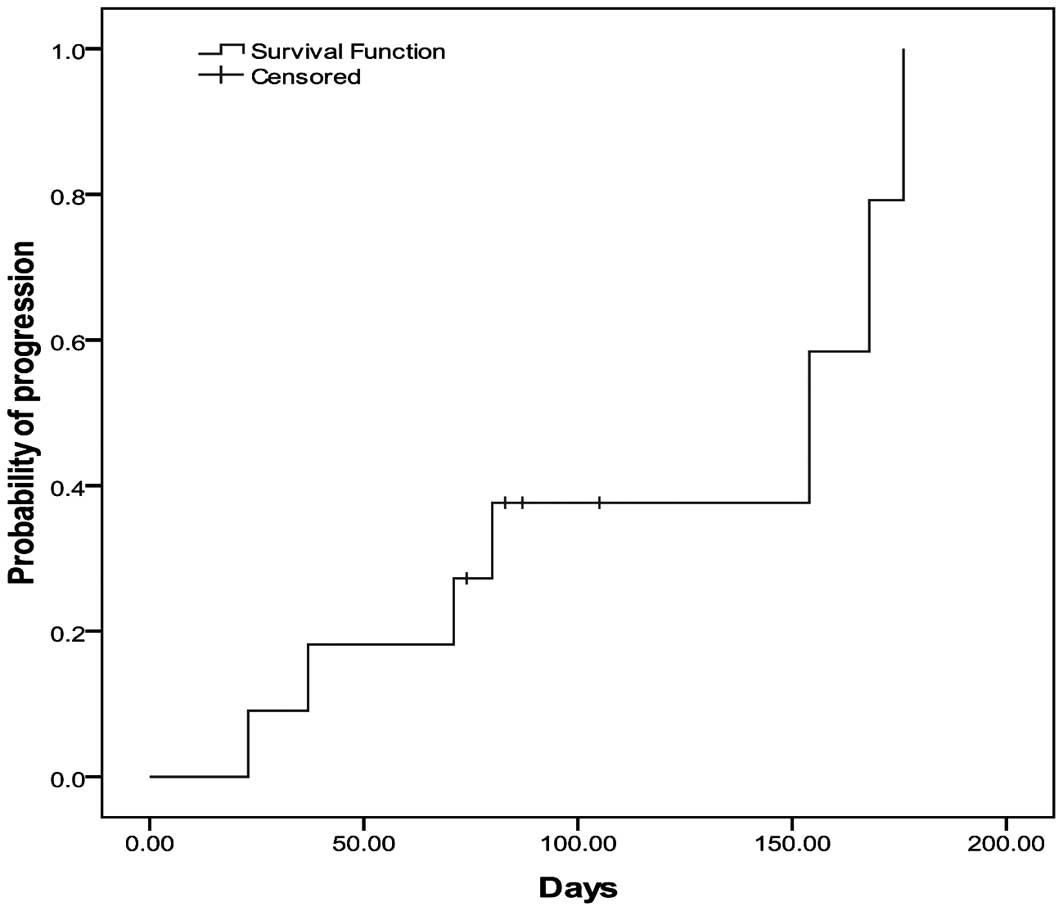
Time to progression (TTP)
Probability of disease progression was determined
using the Kaplan-Meier method. Based on responses assessed at the
treatment and follow-up visits, median TTP from first treatment was
154 days (95% CI, 17–291 days) (Fig.
3).
Discussion
The management of metastatic colorectal cancer is
becoming far more complex and requires a multidisciplinary and
collaborative approach in selecting the optimal treatment for
patients with liver metastases of colorectal cancer. Despite
advances in the development of new cytotoxics and targeted
biologics for the treatment of hepatic metastases from colorectal
cancer, there is still interest in liver directed, locoregional
therapy to improve treatment response and potentially improve
survival. Prognosis in these patients remains poor with 1- and
3-year median survival rates of 31% and 2% (5,22–24).
With response rates in second-line systemic chemotherapeutic
treatments between 4–22%, a more precise chemotherapeutic delivery
system to maximize response rates and reduce side effects is a
potential option in the treatment algorithm for hepatic metastases
from colorectal cancer (25).
Transarterial chemoembolisation (TACE) is a
locoregional therapy that is most widely used for the treatment of
unresectable HCC (26–28) and involves the periodic injection
of a chemotherapeutic agent, mixed with an embolic material, into
selected branches of the hepatic arteries feeding a liver tumour.
The expected advantage of TACE is that higher concentrations of the
drug can be delivered to the tumour with decreased systemic
exposure compared with systemic chemotherapy. We have learnt from
hepatic artery infusion (HAI), that even tumours unresponsive to
systemic therapy may benefit from the higher doses afforded by
local delivery (29).
Early reports on the use of conventional TACE in the
treatment of hepatic metastases from colorectal cancer were
encouraging (30) and data from
several reports have now emerged on effectiveness, response rates,
side effects and overall survival. One of the largest series
published evaluated the efficacy of TACE with respect to local
control and survival (31).
Patients (n=463) with liver metastases from colorectal cancer were
treated with repeated TACE at 4-week intervals. In total, 2,441
chemoembolisations were performed with a mean of 5.3 sessions per
patient. The chemotherapy consisted of mitomycin C with/without
gemcitabin or mitomycin C with irinotecan and embolisation was
performed with lipiodol and starch microspheres for vessel
occlusion. Tumour response according to RECIST 3 months after the
three TACE administrations was evaluated by MRI. Partial response
was observed in 12% of patients, stable disease in 51% and
progressive disease in 37%. The 1-year survival rate was 62%, but
dropped to 38% by 2 years. Median survival time from first TACE
treatment was 1.34 years (31).
In recent decades, many efforts have been made to
improve the outcome of TACE by integrating new chemotherapeutic
agents and providing a more accurate dosage of drug delivery to the
liver for a more prolonged period. DC Bead is capable of loading
irinotecan and release the drug in a sustained way in the liver
after injection in the hepatic artery. Fiorentini et
al(17) evaluated irinotecan
loaded DC Beads (DEBIRI) in 20 patients with liver metastases from
colorectal cancer in a palliative setting. A relevant response was
observed in 16 out of 20 patients, with significant reduction of
lesional contrast enhancement in all responding patients. The
procedure was well tolerated by most patients with a median
duration of hospitalization of 3 days (range 1–10 days). The most
important adverse effect was abdominal pain, especially during
injection of irinotecan loaded DC beads.
A randomised, phase III study (1), compared DEBIRI vs FOLFIRI in patients
who failed 2/3 lines of systemic chemotherapy. At a median
follow-up of 24 months, median overall survival was improved by 43%
in the DEBIRI arm (690 days) compared with the FOLFIRI arm (482
days) with significantly better response and PFS in favour of
DEBIRI (68%, 225 days vs 18%, 94 days, respectively). Acute
toxicity (G3 pain, nausea, fever) was greater with DEBIRI and was
observed in the context of post-embolisation syndrome, and were
controlled with intravenous hydration, morphine and antibiotics.
Late toxicity was significantly higher with FOLFIRI. Diarrhoea,
asthenia, leucopenia, anaemia and G3 fever were substantially less
with DEBIRI (2–20% vs 35–50%). This is the first study to report a
clear survival benefit of DEBIRI over systemic chemotherapy with a
reduction in overall cost. Martin et al published data from
an open-label multi-center study where 55 patients with
unresectable liver metastases from CRC underwent 99 DEBIRI
treatments (32). Median length of
hospital stay was 24 h. Median disease-free and overall survival
from the time of first treatment was 247 days and 343 days,
respectively. Six patients (10%) were downstaged from their
original disease status.
In our pilot study, 11 patients with up to 8 hepatic
lesions from CRC were treated with DEBIRI. This was the first
clinical evaluation in the development of DEBIRI and aimed to
evaluate the safety, technical feasibility, PK profile and efficacy
of this new treatment.
The treatment showed an excellent safety profile
with no serious adverse events reported. No clinically significant
change in blood biochemistry or dose limiting toxicity for
irinotecan was observed. These results are of interest since
anaemia has been reported in approximately 58.7% of patients
receiving intravenous irinotecan monotherapy at the recommended
dose and neutropenia and delayed diarrhoea are known to cause
dose-limiting toxicity (33).
These results confirm reports by Fiorentini et al(17) and Martin et al(32).
We report a relatively high incidence of
post-procedural pain which is expected following hepatic arterial
injection of irinotecan. Use of intravenous opioids and
intra-arterial injection of 1% lidocaine has now become standard in
the pain management protocols of patients undergoing DEBIRI
treatment.
TACE is well established in the treatment algorithm
for hepatocellular carcinoma and is becoming more accepted in the
treatment of liver metastases from colorectal cancer. DEBIRI was
found to be technically feasible in our study and since that time
has gained increasing evidence as a viable treatment option for
these patients. With respect to PK, average Cmax, AUC
and t½ results for irinotecan in this study were 194
ng/ml, 1,680 ng•h/ml and 4.6 h, respectively. These results are
considerably lower than reported values following i.v.
administration of irinotecan (34)
demonstrating the low bioavailability following DEBIRI treatment.
In contrast, higher than expected levels of the main active
metabolite SN-38 was observed. Average Cmax, AUC and
t½ for SN-38 were 16.7 ng/ml, 281 ng•h/ml and 12.4 h,
respectively. It is too early to conclude whether DEBIRI has any
potential to increase the availability of SN-38 and further
research is required to confirm this hypothesis.
Response rates in the current study showed
stabilisation of the disease in 64% and a partial response in 18%
of patients at 9 weeks after the first treatment. These response
rates are in line with those reported in the second-line
(refractory to 5-fluorouracil) phase II studies that formed the
basis of approval for irinotecan monotherapy where response rates
of 13.3–21.7% were achieved (35),
however 9 weeks is a relatively short follow-up in this study and
longer term data has now been published on the efficacy of DEBIRI.
The time to progression of 154 days was shorter than previously
reported but may reflect the patient population in which many had
prior treatments for their colorectal disease. Following DEBIRI
treatment, four patients were found to be eligible for
laser-induced thermotherapy with the potential for better
prognosis.
Our results for safety are consistent with recent
publications on DEBIRI, but our efficacy results differ from recent
reports. As this was the first clinical study of DEBIRI in mCRC we
adopted the same technical approach for TACE in the treatment of
hepatocellular carcinoma using a superselective stasis endpoint.
Liver metastases from colorectal cancer are generally more
hypovascular than HCC and may explain the lower than planned volume
of DEBIRI delivered during the study. Since the time of our study,
more experience has been gained on the optimised technique to use
with DEBIRI. For patients with enough liver preserve, a more
proximal catheter position and a lobar approach is now recommended
to ensure delivery of DEBIRI to entire tumour area. The catheter
should be positioned in lobar arteries past any extrahepatic vessel
(e.g. cystic, pancreatic, gastroduodenal arteries). From there
DEBIRI should be injected slowly (∼1 ml/min) until an endpoint of
stasis in the intratumoral vessels whilst maintaining flow in the
afferent vessels is achieved. It is not recommended to achieve
stasis up to the catheter position. Our superselective approach
with stasis endpoint may have missed some non-visible lesions
causing them to be undertreated. This may explain the differences
in efficacy results reported in our study compared to more recent
publications.
Results from the present study are encouraging but
are limited due to the small sample size and the relatively short
follow-up. We demonstrated that DEBIRI therapy is a safe
alternative treatment in the management of patients with hepatic
metastases from colorectal cancer with an optimised pharmacokinetic
profile as compared to systemic chemotherapy. Patients tolerated
the treatment well at the doses and schedules used in this study
which could allow this therapy to be considered in combination with
systemic chemotherapy including irinotecan, oxaliplatin, as well as
bevacizumab and cetuximab and other new biologic agents.
RECIST is a well established criteria for tumour
response assessment in oncology trials, however, it is not
appropriate for locoregional treatments such as DEBIRI. Assessment
of tumour viability and tumour necrosis such as the mRECIST
criteria in HCC (36) may provide
a better assessment of treatment response. In our patients we were
unable to measure tumour necrosis accurately. In addition, our
technical approach was different to that reported in more recent
publications and may explain the higher rate of progressions seen
in our study. We are now participating in a German, multicentre,
randomised study of DEBIRI + cetuximab (DEBIRITUX) vs irinotecan +
cetuximab using the recommended DEBIRI technique (proximal
approach) to assess progression-free survival of this combined
treatment.
In conclusion, our study shows that
chemoembolisation with irinotecan loaded DC Beads (DEBIRI) is safe,
technically feasible and effective with a good PK profile. These
positive and promising results have now been further investigated
and confirmed in a larger patient population. Studies are underway
to investigate this technology in all lines of therapy and in
combination with existing systemic treatments for liver dominant
disease from colorectal cancer.
References
|
1
|
Fiorentini G AC, Montagnani F, Tilli M,
Mambrini A and Benea G: Trans-arterial chemoembolisation of
metastatic colorectal carcinoma (mCRC) to the liver adopting
polyvinylalcohol microspheres loaded with irinotecan (DEBIRI)
compared to FOLFIRI (CT): evaluation at two years of a phase III
clinical trial. Ann Oncol. 21:2010.
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pazdur R, Coia L, Hoskins WJ and Wagman
LD: Cancer management: a multidisciplinary approach. Medical,
Surgical and Radiation Oncology. 7th edition. CMP Healthcare Media;
New York, NY: 2004
|
|
4
|
Fong Y, Cohen AM, Fortner JG, et al: Liver
resection for colorectal metastases. J Clin Oncol. 15:938–946.
1997.PubMed/NCBI
|
|
5
|
Adam R: Chemotherapy and surgery: new
perspectives on the treatment of unresectable liver metastases. Ann
Oncol. 14(Suppl 2): ii13–ii16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adam R, Delvart V, Pascal G, et al: Rescue
surgery for unresectable colorectal liver metastases downstaged by
chemotherapy: a model to predict long-term survival. Ann Surg.
240:644–658. 2004.PubMed/NCBI
|
|
7
|
Stangl R, Altendorf-Hofmann A, Charnley RM
and Scheele J: Factors influencing the natural history of
colorectal liver metastases. Lancet. 343:1405–1410. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scheele J, Stang R, Altendorf-Hofmann A
and Paul M: Resection of colorectal liver metastases. World J Surg.
19:59–71. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fong Y: Hepatic colorectal metastasis:
current surgical therapy, selection criteria for hepatectomy, and
role for adjuvant therapy. Adv Surg. 34:351–381. 2000.PubMed/NCBI
|
|
10
|
Fong Y and Blumgart LH: Hepatic colorectal
metastasis: current status of surgical therapy. Oncology (Williston
Park). 12:1489–1503. 1998.PubMed/NCBI
|
|
11
|
Meyerhardt JA and Mayer RJ: Systemic
therapy for colorectal cancer. N Engl J Med. 352:476–487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kennedy A, Nag S, Salem R, et al:
Recommendations for radioembolization of hepatic malignancies using
yttrium-90 microsphere brachytherapy: a consensus panel report from
the radioembolization brachytherapy oncology consortium. Int J
Radiat Oncol Biol Phys. 68:13–23. 2007. View Article : Google Scholar
|
|
13
|
Germer CT, Buhr HJ and Isbert C:
Nonoperative ablation for liver metastases. Possibilities and
limitations as a curative treatment. Chirurg. 76:552–563. 2005.(In
German).
|
|
14
|
Brown DB, Geschwind JF, Soulen MC,
Millward SF and Sacks D: Society of Interventional Radiology
position statement on chemoembolization of hepatic malignancies. J
Vasc Interv Radiol. 17:217–223. 2006. View Article : Google Scholar
|
|
15
|
Bavisotto LM, Patel NH, Althaus SJ, et al:
Hepatic transcatheter arterial chemoembolization alternating with
systemic protracted continuous infusion 5-fluorouracil for
gastrointestinal malignancies metastatic to liver: a phase II trial
of the Puget Sound Oncology Consortium (PSOC 1104). Clin Cancer
Res. 5:95–109. 1999.
|
|
16
|
Vogl TJ, Straub R, Eichler K, Sollner O
and Mack MG: Colorectal carcinoma metastases in liver:
laser-induced interstitial thermotherapy - local tumor control rate
and survival data. Radiology. 230:450–458. 2004. View Article : Google Scholar
|
|
17
|
Fiorentini G, Aliberti C, Turrisi G, et
al: Intraarterial hepatic chemoembolization of liver metastases
from colorectal cancer adopting irinotecan-eluting beads: results
of a phase II clinical study. In Vivo. 21:1085–1091. 2007.
|
|
18
|
Aliberti C, Tilli M, Benea G and
Fiorentini G: Trans-arterial chemoembolization (TACE) of liver
metastases from colorectal cancer using irinotecan-eluting beads:
preliminary results. Anticancer Res. 26:3793–3795. 2006.
|
|
19
|
Rofe APS: A preclinical study to evaluate
the plasma levels of irinotecan after hepatic embolisation using
drug loaded in a porcine model. Institute of Medical and Veterinary
Science; Adelaide: 2006
|
|
20
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
21
|
Locker GY, Hamilton S, Harris J, et al:
ASCO 2006 update of recommendations for the use of tumor markers in
gastrointestinal cancer. J Clin Oncol. 24:5313–5327. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chong G and Cunningham D: Improving
long-term outcomes for patients with liver metastases from
colorectal cancer. J Clin Oncol. 23:9063–9066. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen AD and Kemeny NE: An update on
hepatic arterial infusion chemotherapy for colorectal cancer.
Oncologist. 8:553–566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saltz LB, Cox JV, Blanke C, et al:
Irinotecan plus fluorouracil and leucovorin for metastatic
colorectal cancer. Irinotecan Study Group N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leonard GD, Brenner B and Kemeny NE:
Neoadjuvant chemotherapy before liver resection for patients with
unresectable liver metastases from colorectal carcinoma. J Clin
Oncol. 23:2038–2048. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Llovet JM and Beaugrand M: Hepatocellular
carcinoma: present status and future prospects. J Hepatol. 38(Suppl
1): S136–S149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lo CM, Ngan H, Tso WK, et al: Randomized
controlled trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology. 35:1164–1171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar
|
|
29
|
Barber FD, Mavligit G and Kurzrock R:
Hepatic arterial infusion chemotherapy for metastatic colorectal
cancer: a concise overview. Cancer Treat Rev. 30:425–436. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wallace S, Carrasco CH, Charnsangavej C,
Richli WR, Wright K and Gianturco C: Hepatic artery infusion and
chemoembolization in the management of liver metastases. Cardiovasc
Intervent Radiol. 13:153–160. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vogl TJ, Gruber T, Balzer JO, Eichler K,
Hammerstingl R and Zangos S: Repeated transarterial
chemoembolization in the treatment of liver metastases of
colorectal cancer: prospective study. Radiology. 250:281–289. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin RC, Robbins K, Tomalty D, et al:
Transarterial chemoembolisation (TACE) using irinotecan-loaded
beads for the treatment of unresectable metastases to the liver in
patients with colorectal cancer: an interim report. World J Surg
Oncol. 7:802009. View Article : Google Scholar
|
|
33
|
Pfizer: Camptosar® - irinotecan
hydrochloride injection. Prescribing information (US), 2008.
|
|
34
|
de Forni M, Bugat R, Chabot GG, et al:
Phase I and pharmacokinetic study of the camptothecin derivative
irinotecan, administered on a weekly schedule in cancer patients.
Cancer Res. 54:4347–4354. 1994.PubMed/NCBI
|
|
35
|
Pazdur R: Irinotecan: toward clinical end
points in drug development. Oncology (Williston Park). 12:13–21.
1998.
|
|
36
|
Lencioni R, Malagari K, Vogl T, et al: A
randomized phase II trial of a drug eluting bead in the treatment
of hepatocellular carcinoma by transcatheter arterial
chemoembolization. In: Proc ASCO Annual Meeting. ASCO GI;
Alexandria, VA. 2009
|