Introduction
Epithelial-mesenchymal transition (EMT) is a dynamic
process that occurs during the tumor progression of various
cancers. It is characterized by the loss of epithelial factors,
including E-cadherin, claudins, and cytokeratins, and the
upregulated expression of mesenchymal markers, including
N-cadherin, vimentin and fibronectin. Cancer cells that have
undergone EMT are thought to acquire a fibroblast-like motile and
an invasive phenotype (1,2).
Transforming growth factor (TGF)-β one of the most
potent inducers of EMT, induces spindle-shaped cell morphology,
inhibits cell proliferation, and promotes tumor cell motility and
invasion (2–4). TGF-β binds to two different
serine/threonine kinase receptors; type I (TGF-βRI) and type II
receptors (TGF-βRII). The formation of hetero-dimers of TGF-βRI and
TGF-βRII leads to the activation of the signaling pathways mediated
by TGF-β (5). Human pancreatic
cancer (PANC-1) cells express both TGF-βRI and TGF-βRII, whereas
MIA PaCa-2 pancreatic cancer cells do not express either of them
(6). Smads are key intracellular
mediators of the transcriptional responses to TGF-β, and Smad4
mutations are known to be present in more than 50% of pancreatic
cancer cases (7,8). In addition, the activation of other
Smads plays important roles in the tumor progression of pancreatic
cancer. The phosphorylation of Smad2 and Smad3 causes them to
translocate from the cytoplasm to the nucleus and associate with
Smad4. In the nucleus, the activated Smad complex binds to target
gene promoters and regulates the transcriptional responses to TGF-β
(5,9,10).
There are several EMT inducing transcription
factors, such as snail, slug, twist and ZEB1 (11–14).
Since there were some reports on the roles of snail in PANC-1, we
selected snail as a marker of positive control during EMT. Snail is
expressed in various tumor cells and regulates the expression of
E-cadherin, claudins, and some mesenchymal markers, which are
involved in tumor invasion, metastasis, and cell motility (1,15,16).
Furthermore, TGF-β directly activates snail transcription through
the Smad3 phosphorylation (17).
We have shown that DEC1 (BHLHE40/Stra13/Sharp2) is
involved in the regulation of apoptosis and the cell cycle in human
breast and oral cancer cells (18–20).
DEC1 is also highly expressed in various tumors (20–24).
However, the role of DEC1 in EMT is still unknown. In this study,
we focused on the role of DEC1 in EMT of pancreatic cancer cells
during TGF-β treatment and demonstrated that DEC1 plays important
roles in EMT of pancreatic cancer cells.
Materials and methods
Cell culture and treatment
Human pancreatic cancer PANC-1 and MIA PaCa-2 cells
were cultured as described previously (25,26).
These cells were incubated with recombinant human TGF-β (R&D
Systems, Minneapolis, MN, USA) or A-83-01 (Takara, Shiga, Japan) at
various concentrations and periods.
Knockdown of DEC1 by RNA
interference
Short interference RNA (siRNA) against DEC1 was
synthesized by Qiagen (Hilden, Germany). The sequences of the sense
and anti-sense DEC1 siRNA and the negative control (scrambled)
siRNA, and the siRNA transfection were performed as described
previously (18).
DEC1 overexpression
DEC1 overexpression was induced using pcDNA vector
as described previously (19).
After transfection, the cells were incubated for 24 h and subjected
to the invasion assay.
Western blotting
Cells were lysed using M-PER lysis buffer (Thermo
Scientific, Rockford, IL, USA) and their protein concentration was
determined using the bicinchoninic acid (BCA) assay. Their lysates
were subjected to SDS-PAGE and detected the protein expression as
described previously (18,19).
Antibodies
The membranes for western blotting were incubated
with antibodies specific to DEC1 (1:10,000; Novus Biologicals Inc.,
Littleton, CO, USA), Smad3 (1:1,000; Epitomics Inc., Burlingame,
CA, USA), pSmad3 (1:6,000; Epitomics), phospho extracellular
signal-related kinases (p-ERK)½ (1:1,000; Epitomics), pSmad2
(1:5,000; Invitrogen, Carlsbad, CA, USA), slug (1:3,000; Cell
Signaling Technology Inc.), snail (1:3,000; Cell Signaling
Technology Inc., Danvers, MA, USA), ERK1/2 (1:30,000; Cell
Signaling Technology Inc.), α-smooth muscle actin (α-SMA)
(1:20,000; Sigma Chemical Co., St. Louis, MO, USA), vimentin
(1:10,000; Epitomics), N-cadherin (1:10,000; ECM Biosciences,
Versailles, KY, USA), E-cadherin (1:1000; Takara), claudin-1
(1:10,000; Invitrogen), claudin-4 (1:20,000; Invitrogen), claudin-7
(1:1,000; Invitrogen), and actin (1:30,000; Sigma), followed by
horseradish peroxidase-conjugated secondary antibody
(Immuno-Biological Laboratories, Fujioka, Japan). Can Get Signal
immunoreaction enhancer solution (Toyobo Co. Ltd., Osaka, Japan) or
Immunoshot immunoreaction enhancer solution (Cosmobio Co. Ltd.,
Tokyo, Japan) was used to dilute the primary antibody.
Real-time polymerase chain reaction
(PCR)
We prepared three independent RNA samples (n=3).
Total-RNA was isolated and first-strand cDNA was synthesized as
described previously (19). The
real-time PCR was performed using SYBR-Green Master Mix (Life
Technologies, Carlsbad, CA, USA). The sequences of the primers and
the sizes of products are shown in Table I.
 | Table ISequences of the primer sets and the
product sizes of real-time PCR. |
Table I
Sequences of the primer sets and the
product sizes of real-time PCR.
| Gene | Product size
(bp) | Primer
sequences |
|---|
| DEC1 | 76 | F:
5′-GAAAGGATCGGCGCAATTAA-3′
R: 5′-CATCATCCGAAAGCTGCATC-3′ |
| Snail | 73 | F:
5′-CTTCAACTGCAAATACTGCAACAAG-3′
R: 5′-GCGTGTGGCTTCGGATGT-3′ |
|
E-cadherin | 75 | F:
5′-ACAGTCACTGACACCAACGATAATC-3′
R: 5′-ACTGCTGCTTGGCCTCAAA-3′ |
|
Claudin-4 | 64 | F:
5′-TGGGAGGGCCTATGGATGA-3′
R: 5′-TCGTACACCTTGCACTGCATCT-3′ |
|
N-cadherin | 77 | F:
5′-TGATCGAGAAAAAGTGCAACAGTAT-3′
R: 5′-GGCTGTGTTTGAAAGGCCATA-3′ |
| TGF-βRI | 105 | F:
5′-CTGAAGTTCTCGATGATTCCATAAATAT-3′
R: 5′-GAACATCGTCGAGCAATTTCC-3′ |
| 18S
rRNA | 150 | F:
5′-GTAACCCGTTGAACCCCATT-3′
R: 5′-CCATCCAATCGGTAGTAGCG-3′ |
Immunofluorescent staining
Immunofluorescent staining was performed as
described previously (18). The
permeabilized cells were incubated with anti-DEC1 (1:300), pSmad3
(1:300), snail (1:300), N-cadherin (1:300), E-cadherin (1:300),
claudin-1 (1:300), claudin-4 (1:300), or claudin-7 (1:300)
antibodies at 4°C overnight. The cells were then incubated for 1 h
with goat anti-rabbit IgG antibody conjugated to Alexa 488 dye
(Molecular Probes Inc., Tokyo, Japan), while nuclear staining was
performed using 4′,6-diamidino-2-phenylindole (DAPI). These cells
were visualized using confocal laser scanning microscopy LSM 710
(Zeiss, Wetzlar, Germany).
Cell stain was carried out using the CnT-ST-100
stain kit (CellnTEC Advanced Cell Systems AG, Bern, Switzerland),
in accordance with the manufacturer’s instructions.
Invasion assay and migration assay
The invasion assay was performed using a BD BioCoat
Matrigel invasion chamber kit (Becton Dickinson, Franklin Lakes,
NJ, USA). PANC-1 cells were separated using cell dissociation
solution (Sigma) and then (5×104 cells/600 μl) were
added to the top chamber of a cell culture insert in a 24-well
companion plate. After overnight incubation, the cells that had
invaded the lower surface of the membrane were fixed with methanol
and subjected to Giemsa staining. The number of cells that had
migrated was quantified by counting them in ten random distinct
fields using a light microscope.
For the migration assay, PANC-1 cells were seeded in
a 4-chamber slide glass, and an artificial ‘wound’ was carefully
created at 0 h by scratching the confluent cell monolayer with the
tip of a P-200 pipette. Microphotographs were taken at 0, 24 and 48
h.
Human pancreatic tissues
We examined immunohistochemical analyses of
surgically resected pancreatic cancers (n=17), which have been
filed in Hirosaki University Hospital, Japan (Table II). All of the 17 cases examined
were invasive ductal carcinoma of pancreas. Histological specimens
were retrieved from the archives of our hospital according to the
guidelines produced by the Japanese Society of Pathology.
 | Table IIImmunohistochemical expression of
DEC1, pSmad3, and claudin-4 proteins in human pancreatic cancer
tissues. |
Table II
Immunohistochemical expression of
DEC1, pSmad3, and claudin-4 proteins in human pancreatic cancer
tissues.
| | | DEC1
| pSmad3
| claudin-4
|
|---|
| C | A/S | D | T | N | T | N | T | N |
|---|
| 1 | 66/F | Moderately | Strong | Weak | Strong | Weak | Weak | Strong |
| 2 | 62/M | Moderately | Strong | Weak | Strong | Weak | Strong | Weak |
| 3 | 66/F | Moderately | Strong | Weak | Strong | Weak | Weak | Strong |
| 4 | 66/M | Moderately | Strong | Weak | Strong | Weak | Weak | Strong |
| 5 | 67/F | Moderately | Strong | Weak | Strong | Weak | Weak | Strong |
| 6 | 62/F | Moderately | Strong | Weak | Strong | Weak | Weak | Strong |
| 7 | 75/M | Moderately | Strong | Weak | Strong | Strong | Weak | Strong |
| 8 | 58/M | Moderately | Strong | Strong | Strong | Weak | Weak | Strong |
| 9 | 65/M | Moderately | Strong | Weak | Strong | Weak | Weak | Strong |
| 10 | 72/F | Well | Strong | Weak | Strong | Weak | Strong | Weak |
| 11 | 67/M | Poorly | Strong | Weak | Strong | Strong | Weak | Strong |
| 12 | 71/F | Well | Strong | Weak | Strong | Weak | Weak | Weak |
| 13 | 74/M | Moderately | Strong | Strong | Strong | Weak | Weak | Strong |
| 14 | 72/M | Poorly | Strong | Weak | Strong | Weak | Strong | Weak |
| 15 | 55/M | Moderately | Strong | Weak | Strong | Weak | Weak | Weak |
| 16 | 61/F | Moderately | Strong | Strong | Strong | Strong | Weak | Strong |
| 17 | 50/F | Moderately | Strong | Weak | Strong | Weak | Strong | Weak |
Immunohistochemistry
Immunohistochemistry was performed as described
previously (27). The sections
were incubated overnight at 4°C with anti-DEC1 (1:100), pSmad3
(1:200), or claudin-4 (1:400) antibodies diluted in Can Get Signal
Immunostain Solution (Toyobo Co.). Finally, the sections were
counterstained with Mayer’s hematoxylin.
Results
DEC1 expression was induced by TGF-β in
PANC-1 cells
TGF-β treatment induced Smad3 phosphorylation, and
upregulated the expression of DEC1, snail, α-SMA, vimentin and
N-cadherin, whereas it downregulated E-cadherin, claudin-1 and
claudin-4 in PANC-1 cells (Fig. 1A and
B). The highest level of DEC1 expression was observed in the
cells treated with 2 ng/ml of TGF-β for 24 h. TGF-β had little
effect on the Smad2 phosphorylation and the expression of Smad3,
slug and claudin-7 in PANC-1 cells. On the other hand, no apparent
effects were observed on the expression of the aforementioned
molecules after TGF-β treatment in MIA PaCa-2 cells. Next, we
investigated the endogenous mRNA expression of these proteins in
PANC-1 cells treated with TGF-β. The expression of DEC1, snail and
N-cadherin was upregulated by TGF-β, whereas the expression of
E-cadherin and claudin-4 was downregulated (Fig. 1C). A-83-01-an inhibitor of the
TGF-β signaling pathway prevents Smad2/3 phosphorylation. We
therefore investigated whether A-83-01 affects the Smad3
phosphorylation and DEC1 expression induced by TGF-β in PANC-1
cells. A-83-01 suppressed the Smad3 phosphorylation, and the
expression of DEC1 and snail. On the other hand, claudin-4
expression was upregulated by A-83-01 (Fig. 1D).
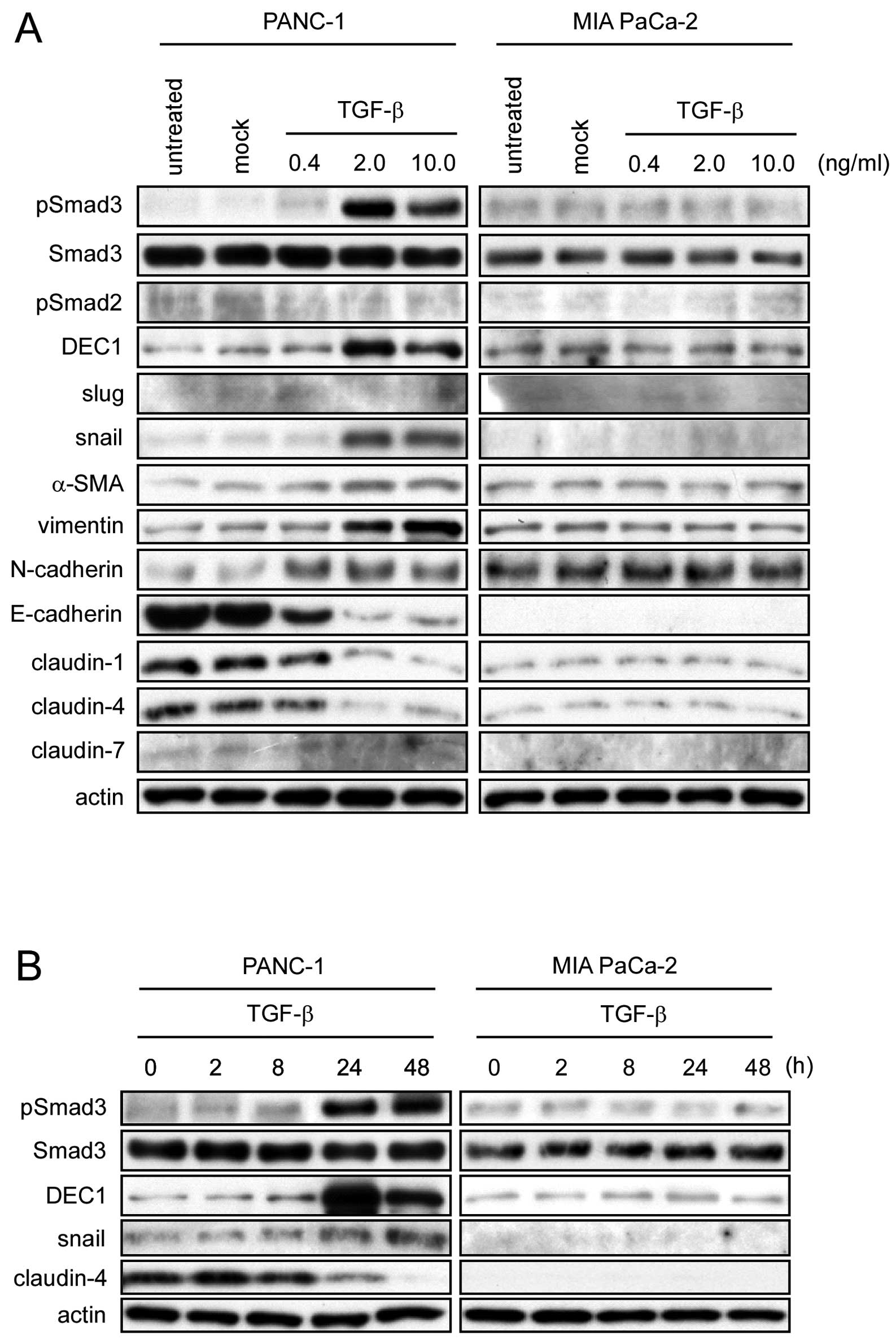 | Figure 1DEC1 expression is upregulated by
TGF-β in human pancreatic cancer PANC-1 cells. (A) PANC-1 and MIA
PaCa-2 cells were treated with various concentrations of TGF-β for
24 h. The control cells (mock) were treated with TGF-β-diluted
buffer. These cells were then lysed and the lysates were subjected
to western blot analyses of pSmad3, Smad3, pSmad2, DEC1, slug,
snail, α-SMA, vimentin, N-cadherin, E-cadherin, claudin-1,
claudin-4, claudin-7 and actin. One representative of at least
three independent experiments with similar results is shown. (B)
PANC-1 and MIA PaCa-2 cells were treated with TGF-β (2 ng/ml) for
various periods. The cells were lysed, and the lysates were
subjected to western blot analyses of pSmad3, Smad3, DEC1, snail,
claudin-4 and actin. One representative of at least three
independent experiments with similar results is shown. (C) PANC-1
cells were treated as above, and total-RNA was prepared and
subjected to real-time PCR of DEC1, snail, E-cadherin, claudin-4
and N-cadherin. Each value represents the mean ± SE (bars) of three
independent experiments *p<0.05, according to the
t-test. (D) PANC-1 cells were treated with the TGF-β inhibitor
A-83-01 (1 or 10 µM) for 90 min before being treated with or
without TGF-β (2 ng/ml) for 24 h, and the lysates were subjected to
western blot analyses of pSmad3, Smad3, DEC1, snail, claudin-4 and
actin. One representative of at least two independent experiments
with similar results is shown. |
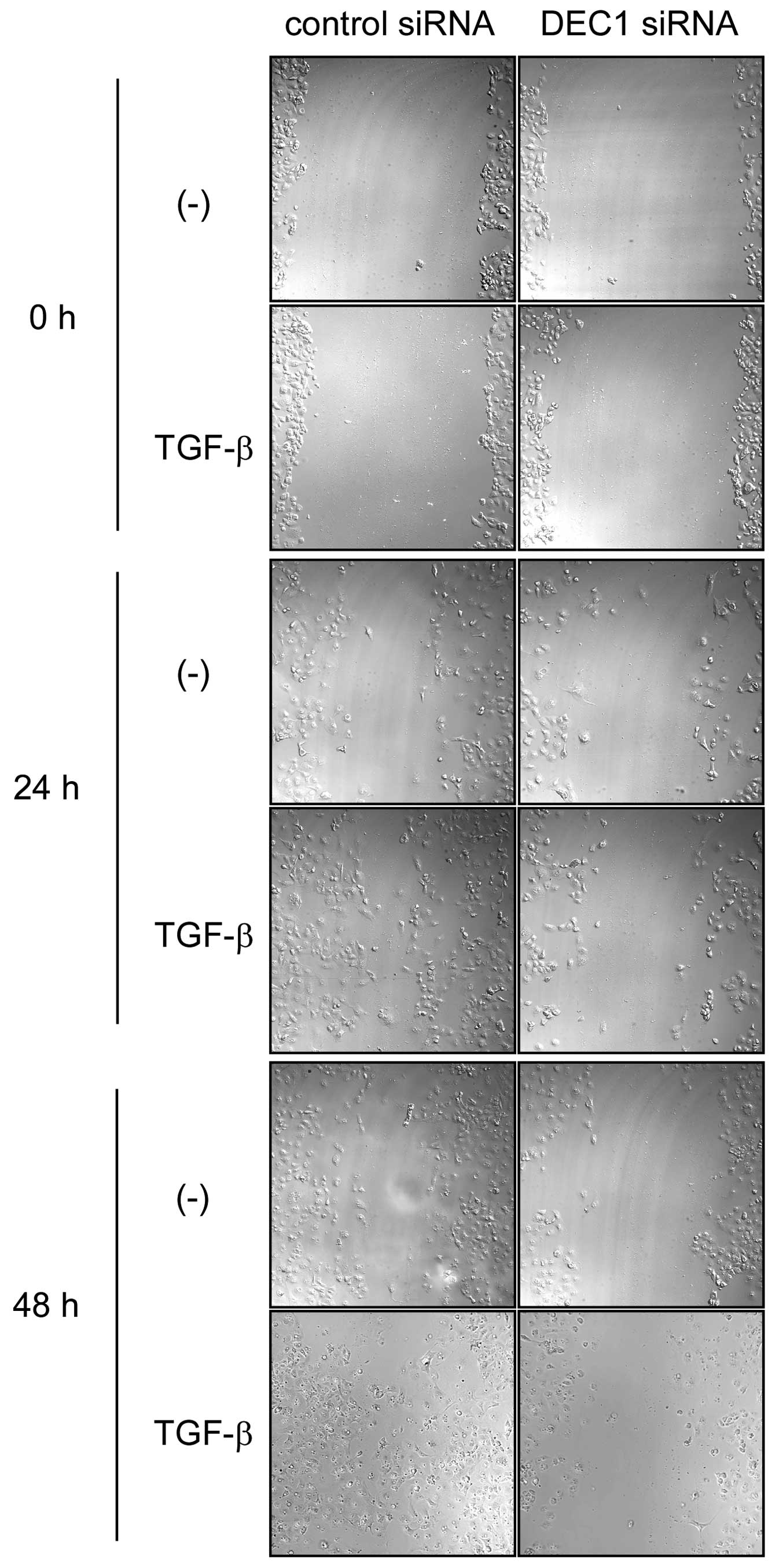
DEC1 knockdown suppressed EMT induced by
TGF-β
The cells that were transfected with the control
siRNA showed a spindle-shaped morphology after 24 h treatment with
TGF-β, while the cells transfected with DEC1 siRNA displayed no
morphological changes after the same treatment (Fig. 2A). As shown in Fig. 2B, in the absence of TGF-β, DEC1
siRNA upregulated the expression of claudin-4, claudin-7 and
E-cadherin, and downregulated the expression of N-cadherin, whereas
it had little effect on the Smad3 phosphorylation, and the
expression of snail and claudin-1. In the presence of TGF-β, DEC1
siRNA decreased the Smad3 phosphorylation, and the expression of
snail and N-cadherin, whereas it upregulated the expression of
claudin-1, claudin-4, claudin-7, and E-cadherin. In the presence or
absence of TGF-β, DEC1 siRNA had little effect on the ERK1/2
phosphorylation, and the expression of total-ERK1/2, α-SMA and
vimentin. Another DEC1 siRNA oligonucleotide yielded similar
results (data not shown). In order to examine whether DEC1
regulates EMT-related factors at the transcriptional level, we
performed real-time PCR analysis. The altered mRNA expression
patterns of snail, E-cadherin, claudin-4 and N-cadherin were
compatible with the above protein results (Fig. 2C). In the presence of TGF-β, DEC1
siRNA also significantly decreased the expression of TGF-βRI,
although DEC1 siRNA without TGF-β slightly decreased the expression
of TGF-βRI. DEC1 siRNA regardless of TGF-β had little effect on the
expression of TGF-βRII (data not shown).
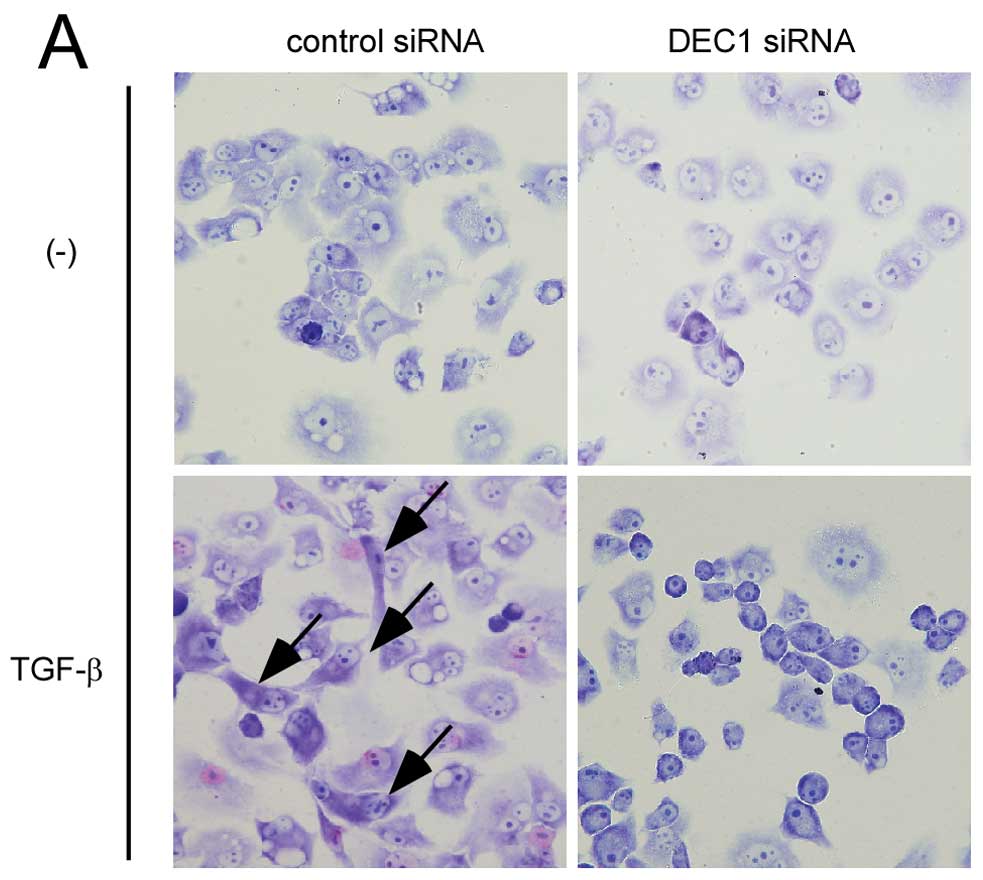 | Figure 2DEC1 knockdown inhibits TGF-β-induced
EMT in PANC-1 cells. (A) PANC-1 cells were transfected with control
siRNA or siRNA against DEC1. At 24 h post-transfection, the cells
were treated with or without TGF-β (2 ng/ml) and incubated for 24
h. The cells were then fixed, stained and observed in bright field.
The black arrows show spindle-shaped cancer cells. One
representative of at least two independent experiments with similar
results is shown. (B) PANC-1 cells were treated with control siRNA
or DEC1 siRNA in the presence or absence of TGF-β (2 ng/ml) for 24
h, and the lysates were subjected to western blot analyses of DEC1,
pSmad3, Smad3, snail, claudin-1, claudin-4, claudin-7, E-cadherin,
N-cadherin, α-SMA, vimentin, pERK1/2, total-ERK1/2 and actin. One
representative of at least three independent experiments with
similar results is shown. (C) PANC-1 cells were treated as above,
and total-RNA was prepared and subjected to real-time PCR of DEC1,
snail, E-cadherin, claudin-4, N-cadherin, and TGF-βRI. Each value
represents the mean ± SE (bars) of three independent experiments
*p<0.05, according to the t-test. (D) PANC-1 cells
were transfected with control siRNA or siRNA against DEC1. At 24 h
post-transfection, the cells were treated with or without TGF-β (2
ng/ml) and incubated for 24 h. The cells were then fixed, incubated
with anti-pSmad3, snail, N-cadherin, E-cadherin, claudin-1,
claudin-4, or claudin-7 antibodies, and visualized using
Alexa488-conjugated secondary antibody (green). The cells were also
counterstained with DAPI (blue) in order to detect their nucleus. A
merged image that is representative of at least two independent
experiments with similar results is shown. |
Effects of DEC1 knockdown on the amounts
of nuclear/cytoplasmic EMT-related factors
As shown in Fig.
2D, DEC1 siRNA without TGF-β decreased the amount of N-cadherin
in the cell membrane, while it increased the cell membrane levels
of E-cadherin, claudin-4 and claudin-7. On the other hand, DEC1
siRNA without TGF-β had little effect on the amounts of
nuclear/cytoplasmic pSmad3, snail and claudin-1. In the presence of
TGF-β, control siRNA increased the levels of pSmad3 and snail in
the nucleus compared with the absence of TGF-β, and slightly
increased the level of N-cadherin in the cell membrane, whereas it
decreased the amounts of E-cadherin and claudin-1 in the cell
membrane, and decreased the amount of claudin-4 in the cytoplasm.
However, it had little effect on the amount of claudin-7 in the
cytoplasm or membrane. In the presence of TGF-β, DEC1 siRNA
decreased the levels of pSmad3 and snail in the nucleus compared
with control siRNA, and it also decreased the amount of N-cadherin
in the cell membrane. On the other hand, a combination treatment of
DEC1 siRNA with TGF-β significantly increased the levels of
E-cadherin, claudin-1 and claudin-4 in the cell membrane compared
with control siRNA. A combination treatment of DEC1 siRNA with
TGF-β also slightly increased the amount of claudin-7 in the cell
membrane. These findings demonstrated that DEC1 has inducible
effects on EMT in PANC-1 cells during TGF-β treatment, which
involved alterations in the cellular amounts of EMT-related
factors.
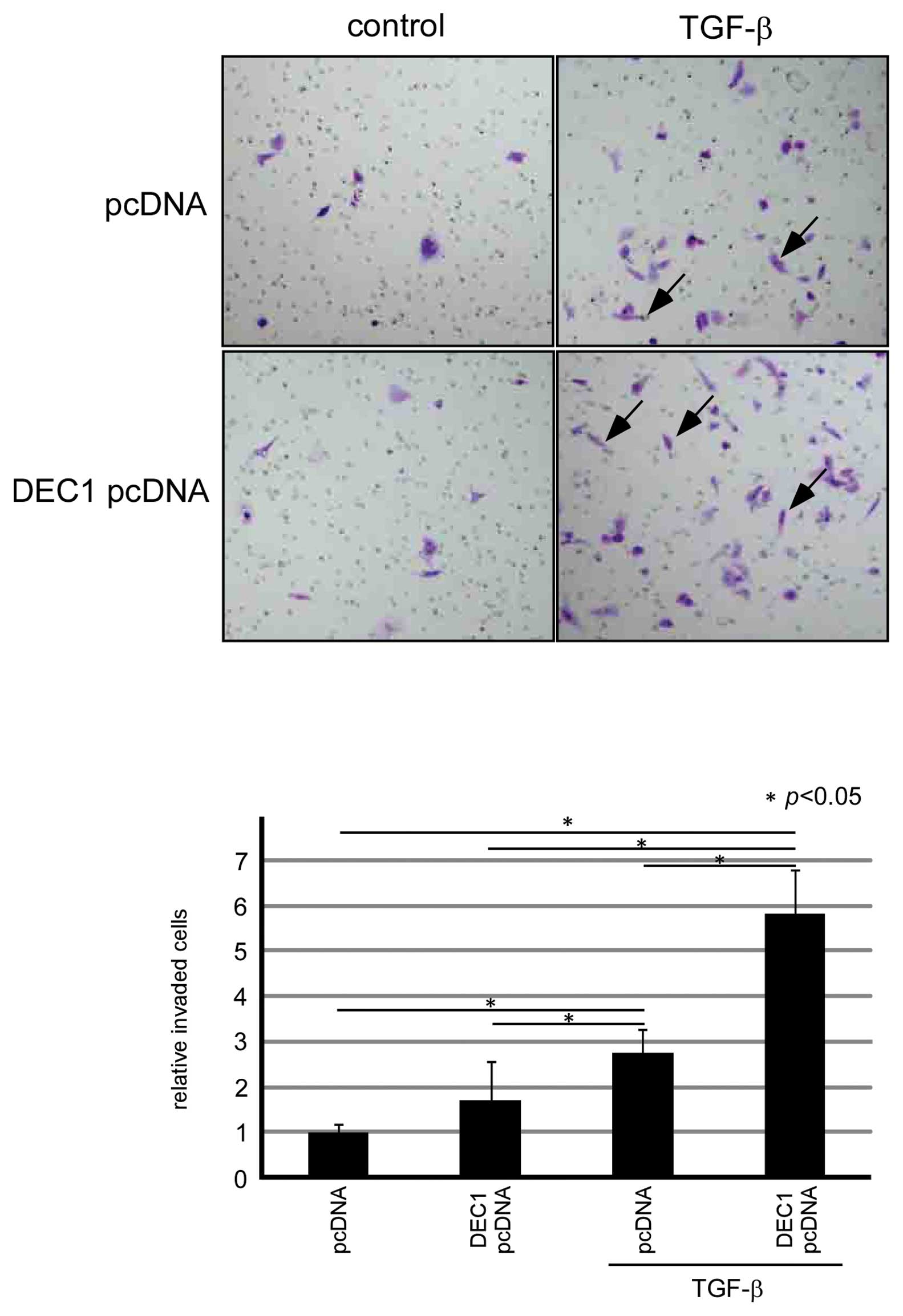
In the presence of TGF-β, DEC1 expression
was closely involved in the migration and invasion
We examined whether DEC1 expression was involved in
the migration and invasion of PANC-1 cells. In the presence of
TGF-β, DEC1 siRNA delayed cell migration for 24–48 h in comparison
with those transfected with the control siRNA (Fig. 3). We performed an invasion assay in
which we transiently transfected the cells with a DEC1 expressing
plasmid. As a result, the number of invasive PANC-1 cells with a
spindle-shaped morphology was increased in the presence of TGF-β
(Fig. 4). The invasion assay also
demonstrated that there were significantly more invasive
spindle-shaped cells among the cells transfected with DEC1 than
among those transfected with the control pcDNA vector.
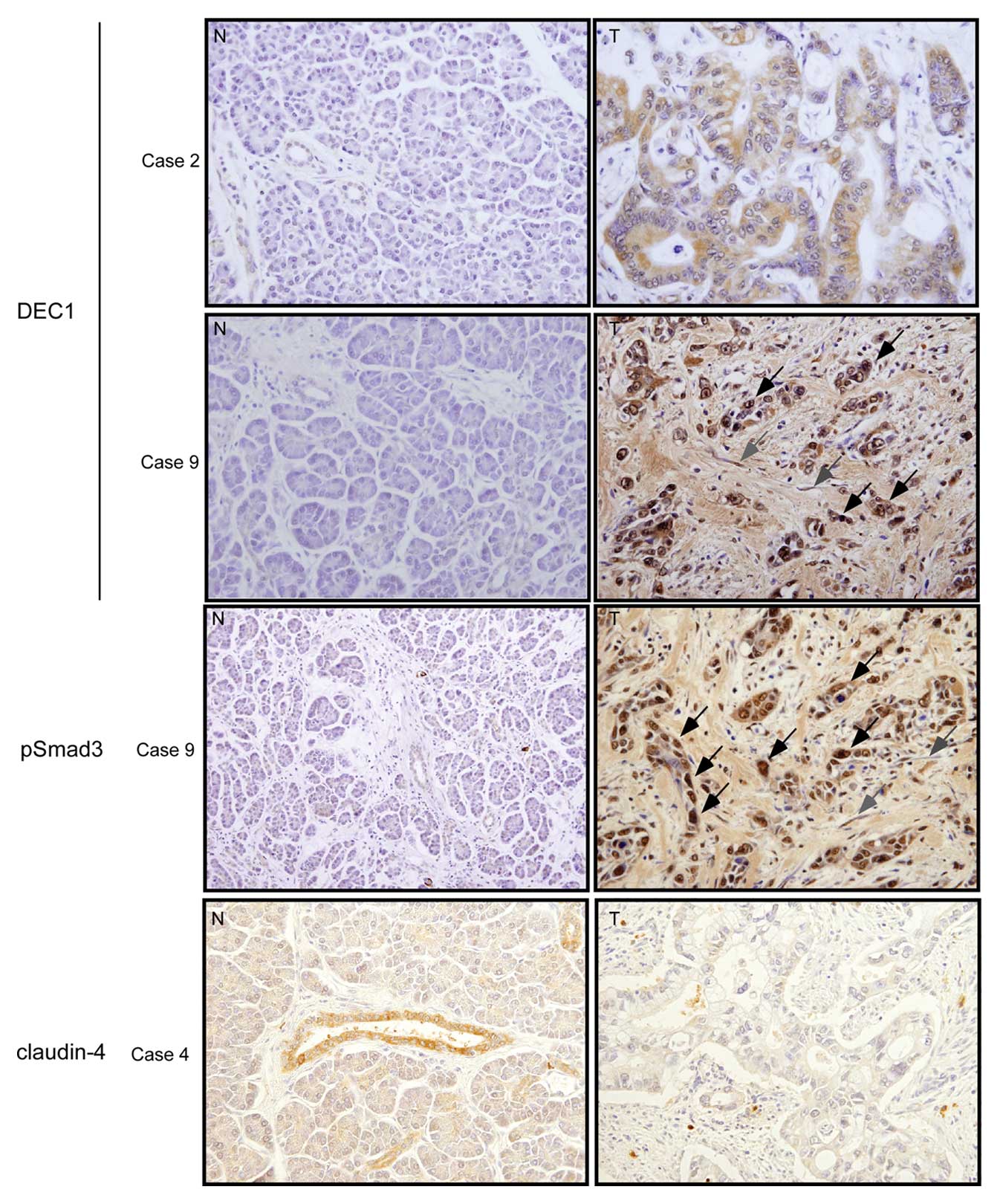
DEC1, pSmad3 and claudin-4 protein
expression in human pancreatic cancer tissues and the adjacent
non-cancerous pancreatic tissues
We examined the immunohistochemical expression of
DEC1, pSmad3 and claudin-4 in human pancreatic cancer tissues.
Photographs of DEC1, pSmad3 and claudin-4 expression in
representative cases are shown in Fig.
5. Significant DEC1 immunoreactivity was detected in the cancer
tissues (100%, 17/17 cases) compared with the adjacent
non-cancerous pancreatic tissues, and it was predominantly located
within the cytoplasm of the cancer cells, although very weak DEC1
immunoreactivity was found in the non-cancerous pancreatic ductal
cells of all cases. DEC1 immunoreactivity was detected in parts of
the adjacent non-cancerous tissues (17%, 3/17 cases).
It is often difficult to distinguish between
spindle-shaped cells of the cancer invasive front and fibroblasts
in stroma. We distinguish them by dyskaryosis and size. Firstly,
dyskaryosis was shown in spindle-shaped cells, whereas it was not
shown in fibroblasts in stroma. Secondary, the sizes of
spindle-shaped cells were larger than fibroblasts. The
spindle-shaped cells of the cancer invasive front showed strong
DEC1 immunoreactivity similar to that seen in the other cancer
regions (case 9, right panel). Marked claudin-4 immunoreactivity
was detected in the membrane and/or cytoplasm of non-cancerous
pancreatic ductal cells, whereas that in the cancer cells was weak,
except for 4 cases in which strong claudin-4 expression was
detected in cancer cells. Significant pSmad3 expression was found
in the nucleus of spindle-shaped cancer cells, whereas it was
detected in non-cancerous ductal cells in 3 cases. The changes in
the immunohistochemical expression of DEC1, pSmad3, and claudin-4
were found to be independent of the cancer grade and the patient’s
age and gender.
Discussion
DEC1 is expressed in various tumors and regulates
the responses to hypoxia, apoptosis and the cell cycle (19,20,23,24,28).
Recent studies have shown that the expression of DEC1 is related to
apoptosis resistance in pancreatic cancer cells (29). However, the significance of DEC1 in
pancreatic cancer is poorly understood. In the present study, we
showed that DEC1 was upregulated by TGF-β in PANC-1 cells. DEC1
expression was highest in the cells cultured in the presence of
TGF-β for 24 h, whereas snail expression was highest in the cells
cultured in the presence of TGF-β for 48 h. PANC-1 cells showed the
highest level of Smad3 phosphorylation when cultured in the
presence of TGF-β for 24–48 h, which also induced a spindle-shaped
morphology and enhanced migration and invasiveness. These findings
indicate that EMT of PANC-1 is induced by 24 h TGF-β treatment and
that TGF-β-induced DEC1 expression is closely related to EMT
phenomena. Previous studies have reported that TGF-β affects
circadian phase shifts in rat fibroblasts, as well as the
immediate-early induction of DEC1, and Smad binding sites (SBE)
were presented in the DEC1 promoter (30). The differences in DEC1 induction
time between the previous report and our present study are probably
related to cell type. It was reported that TGF-β increased the
protein expression of Smad4 in PANC-1 cells (31). We performed a chip assay whether
Smad3 or Smad4 bound to the DEC1 promoter in PANC-1 cells, and
found that in the presence of TGF-β, Smad3 or Smad4 bound to the
SBE in the DEC1 promoter (data not shown). Thus, we thought, at
least, Smad3 or Smad4 might have activities for binding to the DEC1
promoter in PANC-1 cells.
We demonstrated that a combination treatment of DEC1
siRNA with TGF-β downregulated the Smad3 phosphorylation, and the
expression of TGF-βRI and snail, and it also decreased the amounts
of pSmad3 and snail in the nucleus. These findings suggest that
DEC1 is an upstream factor of these genes. It has been reported
that DEC1 upregulates and downregulates target genes by binding to
sp1 and E-box sites, respectively (21,32,33).
The promoter region of TGF-βRI contains sp1 sites (34). Therefore, we speculate that DEC1
binds to sp1 sites of the TGF-βRI promoter and regulates
EMT-related factors through pSmad3/snail signaling. TGF-β also
affects the ERK1/2 phosphorylation independent of Smad pathway
(35). However, DEC1 siRNA
regardless of TGF-β had little effect on the ERK1/2
phosphorylation. These results suggest that DEC1 specifically
regulates the pSmad3/snail pathway induced by TGF-β. In MIA PaCa-2
cells lacking the TGF-β receptor, TGF-β treatment had little effect
on the expression of DEC1 and snail, and the Smad3 phosphorylation,
and cell morphology. These findings suggest that DEC1 regulates
pSmad3 by positive and negative feedback systems during EMT of
pancreatic cancer.
Claudin-4 expression has been shown to decrease the
invasiveness and metastatic potential of pancreatic cancer
(36). Claudin and E-cadherin
expression were found to be down-regulated in breast, esophageal,
and head and neck cancer tissue (37–39).
The promoter regions of claudins and E-cadherin contain E-boxes
(15,40,41).
We showed that DEC1 had effect on the expression and the amounts of
claudin-4, claudin-7, and E-cadherin in the cell membrane, while
DEC1 and claudin-4 were immunohistochemically detected in cancer
tissues and non-cancerous ducts, respectively. Based on the above
findings, DEC1 is considered to negatively regulate the expression
of E-cadherin and claudins.
Our study is the first report to demonstrate marked
pSmad3 immunoreactivity in pancreatic cancer cells compared with
that in the adjacent non-cancerous pancreatic tissues. In
particular, strong pSmad3 immunoreactivity was found in the nucleus
of the spindle-shaped cancer cells, which were located at the
cancer invasive front. A recent study reported that Smad3, but not
Smad2, increased the expression of matrix metalloproteinase-9 in
lung cancer cells and contributed to EMT through TGF-β (42) which suggests that pSmad3 expression
is closely involved in EMT of cancer cells.
In the present study, we demonstrated that DEC1 has
inducible effects on EMT, which are mediated through the Smad3
phosphorylation, and plays an important role in EMT of pancreatic
cancer; i.e., it alters the expression of EMT-related factors and
affects the morphology, migration, and invasion of cancer
cells.
Abbreviations:
|
DEC1
|
differentiated embryonic chondrocyte
gene 1
|
|
TGF-β
|
transforming growth factor-β
|
|
EMT
|
epithelial-mesenchymal transition
|
|
PANC-1
|
human pancreatic cancer
|
Acknowledgements
This study was supported by
Grants-in-Aid for Science from the Ministry of Education, Culture,
Sports, Science, and Technology of Japan; a Grant for Hirosaki
University Institutional Research; and the Fund for the Promotion
of International Scientific Research.
References
|
1
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miettinen PJ, Ebner R, Lopez AR and
Derynck R: TGF-beta induced transdifferentiation of mammary
epithelial cells to mesenchymal cells: involvement of type I
receptors. J Cell Biol. 127:2021–2036. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyazawa K, Shinozaki M, Hara T, et al:
Two major Smad pathways in TGF-beta superfamily signalling. Genes
Cells. 7:1191–1204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giehl K, Seidel B, Gierschik P, et al:
TGFbeta1 represses proliferation of pancreatic carcinoma cells
which correlates with Smad4-independent inhibition of ERK
activation. Oncogene. 19:4531–4541. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hahn SA, Schutte M, Hoque AT, et al: DPC4,
a candidate tumor suppressor gene at human chromosome 18q21.1.
Science. 271:350–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartsch D, Hahn SA, Danichevski KD, et al:
Mutations of the DPC4/Smad4 gene in neuroendocrine pancreatic
tumors. Oncogene. 18:2367–2371. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Massagué J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000.PubMed/NCBI
|
|
10
|
Zawel L, Dai JL, Buckhaults P, et al:
Human Smad3 and Smad4 are sequence-specific transcription
activators. Mol Cell. 1:611–617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohashi S, Natsuizaka M, Naganuma S, et al:
A NOTCH3-mediated squamous cell differentiation program limits
expansion of EMT-competent cells that express the ZEB transcription
factors. Cancer Res. 71:6836–6847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dave N, Guaita-Esteruelas S, Gutarra S, et
al: Functional cooperation between Snail1 and twist in the
regulation of ZEB1 expression during epithelial to mesenchymal
transition. J Biol Chem. 286:12024–12032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gregory PA, Bracken CP, Smith E, et al: An
autocrine TGF-beta/ ZEB/miR-200 signaling network regulates
establishment and maintenance of epithelial-mesenchymal transition.
Mol Biol Cell. 22:1686–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong J, Zhou J, Fu J, et al:
Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1
protein and promotes breast cancer cell invasiveness. Cancer Res.
71:3980–3990. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci.
116:1959–1967. 2003. View Article : Google Scholar
|
|
16
|
Ohkubo T and Ozawa M: The transcription
factor Snail downregulates the tight junction components
independently of E-cadherin downregulation. J Cell Sci.
117:1675–1685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zavadil J, Cermak L, Soto-Nieves N, et al:
Integration of TGF-beta/Smad and Jagged1/Notch signalling in
epithelial-to-mesenchymal transition. EMBO J. 23:1155–1165. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Sato F, Bhawal UK, et al: Basic
helix-loop-helix transcription factors DEC1 and DEC2 regulate the
paclitaxel-induced apoptotic pathway of MCF-7 human breast cancer
cells. Int J Mol Med. 27:491–495. 2011.PubMed/NCBI
|
|
19
|
Liu Y, Sato F, Kawamoto T, et al:
Anti-apoptotic effect of the basic helix-loop-helix (bHLH)
transcription factor DEC2 in human breast cancer cells. Genes
Cells. 15:315–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhawal UK, Sato F, Arakawa Y, et al: Basic
helix-loop-helix transcription factor DEC1 negatively regulates
cyclin D1. J Pathol. 224:420–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Xie M, Yang J, et al: The expression
of antiapoptotic protein survivin is transcriptionally upregulated
by DEC1 primarily through multiple sp1 binding sites in the
proximal promoter. Oncogene. 25:3296–3306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turley H, Wykoff CC, Troup S, et al: The
hypoxia-regulated transcription factor DEC1 (Stra13, SHARP-2) and
its expression in human tissues and tumours. J Pathol. 203:808–813.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chakrabarti J, Turley H, Campo L, et al:
The transcription factor DEC1 (stra13, SHARP2) is associated with
the hypoxic response and high tumour grade in human breast cancers.
Br J Cancer. 91:954–958. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, et al: DEC1 (STRA13) protein expression relates to hypoxia-
inducible factor 1-alpha and carbonic anhydrase-9 overexpression in
non-small cell lung cancer. J Pathol. 200:222–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kondo J, Sato F, Kusumi T, et al:
Claudin-1 expression is induced by tumor necrosis factor-α in human
pancreatic cancer cells. Int J Mol Med. 22:645–649. 2008.
|
|
26
|
Suzuki T, Sato F, Kondo J, et al: Period
is involved in the proliferation of human pancreatic MIA-PaCa2
cancer cells by TNF-alpha. Biomed Res. 29:99–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato F, Wu Y, Bhawal UK, Liu Y, et al:
PERIOD1 (PER1) has anti-apoptotic effects, and PER3 has
pro-apoptotic effects during cisplatin (CDDP) treatment in human
gingival cancer CA9-22 cells. Eur J Cancer. 47:1747–1758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Zhang H, Xie M, et al: Abundant
expression of Dec1/stra13/sharp2 in colon carcinoma: its
antagonizing role in serum deprivation-induced apoptosis and
selective inhibition of procaspase activation. Biochem J.
367:413–422. 2002. View Article : Google Scholar
|
|
29
|
Wang W, Reiser-Erkan C, Michalski CW, et
al: Hypoxia inducible BHLHB2 is a novel and independent prognostic
marker in pancreatic ductal adenocarcinoma. Biochem Biophys Res
Commun. 401:422–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kon N, Hirota T, Kawamoto T, et al:
Activation of TGF-beta/ activin signalling resets the circadian
clock through rapid induction of Dec1 transcripts. Nat Cell Biol.
10:1463–1469. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nicolás FJ and Hill CS: Attenuation of the
TGF-beta-Smad signaling pathway in pancreatic tumor cells confers
resistance to TGF-beta-induced growth arrest. Oncogene.
22:3698–3711. 2003.PubMed/NCBI
|
|
32
|
Sato F, Kawamoto T, Fujimoto K, et al:
Functional analysis of the basic helix-loop-helix transcription
factor DEC1 in circadian regulation. Interaction with BMAL1. Eur J
Biochem. 271:4409–4419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawamoto T, Noshiro M, Sato F, et al: A
novel autofeedback loop of Dec1 transcription involved in circadian
rhythm regulation. Biochem Biophys Res Commun. 313:117–124. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bloom BB, Humphries DE, Kuang PP, et al:
Structure and expression of the promoter for the R4/ALK5 human type
I transforming growth factor-beta receptor: regulation by TGF-beta.
Biochim Biophys Acta. 1312:243–248. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mulder KM: Role of ras and Mapks in
TGF-beta signaling. Cytokine Growth Factor Rev. 11:23–35. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Michl P, Barth C, Buchholz M, et al:
Claudin-4 expression decreases invasiveness and metastatic
potential of pancreatic cancer. Cancer Res. 63:6265–6271.
2003.PubMed/NCBI
|
|
37
|
Turksen K and Troy TC: Junctions gone bad:
Claudins and loss of the barrier in cancer. Biochim Biophys Acta.
1816:73–79. 2011.PubMed/NCBI
|
|
38
|
Berx G and Van Roy F: The
E-cadherin/catenin complex: an important gatekeeper in breast
cancer tumorigenesis and malignant progression. Breast Cancer Res.
3:289–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wells A, Yates C and Shepard CR:
E-cadherin as an indicator of mesenchymal to epithelial reverting
transitions during the metastatic seeding of disseminated
carcinomas. Clin Exp Metastasis. 25:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martínez-Estrada OM, Cullerés A, Soriano
FX, et al: The transcription factors Slug and Snail act as
repressors of Claudin-1 expression in epithelial cells. Biochem J.
394:449–457. 2006.PubMed/NCBI
|
|
41
|
Batlle E, Sancho E, Francí C, et al: The
transcription factor snail is a repressor of E-cadherin gene
expression in epithelial tumour cells. Nat Cell Biol. 2:84–89.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reka AK, Kurapati H, Narala VR, et al:
Peroxisome proliferator-activated receptor-gamma activation
inhibits tumor metastasis by antagonizing Smad3-mediated
epithelial-mesenchymal transition. Mol Cancer Ther. 9:3221–3232.
2010. View Article : Google Scholar
|