Introduction
The incidence of breast cancer continues to rise.
Breast cancer is one of the common carcinoma in women. The
treatment of breast cancer includes routine surgery, radiotherapy,
biotherapy and hormone therapy. These treatments have shortcomings
due to side effects and lack of response due to the different
genetic makeup of the individual and their disposition to breast
cancer. Recently, there has been a move towards an individual
treatment regime. The search therefore continues to find markers
and/or targets for therapy (1,2).
Brain-derived neurotrophic factor, BDNF is a member of the
neurotrophins superfamily composed of 247 amino acids which has
separated and purified from pig brain (3). BDNF activates cellular biological
effects mainly through a cell surface tyrosine kinase receptor,
tropo-myosin-related kinase B (TrkB). It has been reported that
brain-derived neurotrophic factor (BDNF) is aberrantly expressed in
human breast cancer and that a raised level of BDNF is associated
with poor clinical outcome and reduced survival (4,5).
BDNF is secreted by target non-neuronal cells of neuron and has
been implicated in the pathophysiology of the nervous system and is
important in a number of neurological and psychological conditions
(6,7).
Recently, in addition to BDNF involvement in the
nerve system carcinomas, it has also been shown to play a role in
the proliferation, invasion and metastasis of non-neuronal solid
tumours such as breast cancer, myeloma, melanoma, lung cancer,
ovarian cancer, hepatocellular and prostate cancer (5,8–11).
The expression level of BDNF is also of high concern in cancer
research (12).
Nerve growth factor (NGF) and BDNF have similar
primary structures and relative functions which stimulate neuron
cell survival, differentiation and neuroplasticity (6). NGF has been found incorporated to
promote survival and proliferation of breast cancer cells which has
been used in hormone therapy of breast cancer (5). The impact of expression level of BDNF
on breast cancers especially diminishing the expression level would
be helpful to the treatment of the breast cancer.
The present study aimed to investigate the
biological role of BDNF expression in human breast cancer cells. We
found high expression of BDNF in MDA-MB-231, MCF-7 and ZR75-1
breast cancer cells and so anti-BDNF ribozymes were constructed to
knock down the expression of BDNF in MDA-MB-231 and MCF-7 cells.
The expression profile of BDNF was evaluated and screened, then the
biological influence was studied in breast cancer cells.
Materials and methods
Cell lines and culture
Human breast cell lines, MDA-MB-231, MCF-7 and
ZR75-1 were obtained from the European Collection of Animal Cell
Cultures (ECACC, Salisbury, UK). Cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf
serum, 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco BRC,
Paisley, UK) at 37°C and 5% CO2.
RNA preparation and reverse transcription
PCR (RT-PCR)
Total cellular RNA was extacted from the cultured
cells using Total RNA Isolation reagent (ABgene, Epsom, UK). The
concentration of RNA was determined through an ultra-violet
spectrophotometer (WPA UV 1101, Biotech Photometer, Cambridge, UK).
cDNA was obtained from RT-PCR using a transcription kit (Sigma,
Poole, UK). The quality of DNA was verified using GAPDH primers
(forward primer: 5′-AGC TTG TCA TCA ATG GAA AT-3′; reverse primer:
5′-CTT CAC CAC CTT CTT GAT GT-3′). The mRNA level of BDNF were
assessed using the BDNF primers (forward primer: 5′-TTC ATA CTT TGG
TTG CAT GA-3′; reverse primer: 5′-TTC AGT TGG CCT TTT GAT AC-3′).
PCR were running in a GeneAmp PCR System 2400 thermocycler
(Perkin-Elmer). The PCR products were separated by 1% agarose gel
and stained with ethidium bromide then photographed by a digital
camera mounted over a UV transilluminator.
Real-time quantitative polymerase chain
reaction
The mRNA level of gene expression was determined by
the real-time quantitative polymerase chain reaction (QPCR) method
using the prepared cDNA as the template. An additional primer
sequence was added to every QPCR reaction system, known as the Z
sequence (5′-ACT GAA CCT GAC CGT ACA-3′) which is complementary to
the universal Z probe (Intergen Inc., Oxford, UK). The reaction was
carried out on IcyclerIQ™ (Bio-Rad, Hemel Hemstead, UK) in
real-time detection of the 96-well plate. GAPDH expression was used
as an internal control. The reaction condition was: 94°C for 7 min,
80 cycles of 94°C for 15 sec, 55°C for 35 sec (the data capture
step) and 72°C for 20 sec. The levels of the transcripts were
generated from an internal standard that was simultaneously
amplified with the samples.
Knockdown of BDNF expression using
ribozyme and screen of stable transfected cell lines
Targeting human BDNF hammer-head ribozymes were
designed based on the secondary structure of the gene generated
using the Zuker RNA mFold program. The ribozymes were accordingly
synthesized and then cloned into pEF6/V5-his-Topo T/A vector
(Invitrogen, Paisley, UK). Then transfected to MDA-MB-231 and MCF-7
cells respectively using an Easyjet Plus electroporator (EquiBio,
Kent, UK). After selection with culture medium containing 5 μg/ml
blasticidin, the verified transfectants were cultured in medium
containing 0.5 μg/ml blasticidin. Primer sequences of the anti-BDNF
ribozymes were: forward primer: 5′-CTG CAG TTG GCC TTT TGA TAC AGG
GAC CTT TTC AAG GAC TGT CTG ATG AGT CCG TGA GGA-3′; reverse primer:
5′-CTG CAG TTG GCC TTT TGA TAC AGG GAC CTT TTC AAG GAC TGT CTG ATG
AGT CCG TGA GGA-3′.
Western blotting experiment
Human breast cancer cells were collected and lysed
in lysate buffer. The protein concentration was quantified using DC
Protein Assay kit (Bio-Rad) and an ELx800 spectrophotometer
(Bio-Tek). Lysates were detected by SDS-PAGE and western blot
analysis. The transferred membranes were incubated with the primary
antiby anti-BDNF and anti-TrkB antibody (Santa Cruz
Biotechnologies, Santa Cruz, CA, USA). GAPDH expression was used as
an internal control (Santa Cruz Biotechnologies). Then incubated
with the peroxidase-conjugated secondary antibody (Sigma-Aldrich).
Western blotting results were detected by chemiluminescence and
visualized using a Supersignal West Dura system (Pierce
Biotechnology, Inc.) and photographed using a UVI-Tech imager
(Uvitech, Cambridge, UK). Protein level were quantified and
analyzed with NIH Image 1.62.
Cell growth assay
Breast cancer cell growth rates were assessed using
an in vitro growth assay. Cells were planted in sextuplicate
into 96-well plates at a density of 2,000 cells per well. Plates
were then incubated for 24, 48, 72 and 120 h before being fixed in
4% formaldehyde (v/v) and stained with 0.5% (w/v) crystal violet.
The crystal violet stain was then dissolved using 10% acetic acid
(v/v) and cell density was determined by measuring the absorbance
of this solution at 540 nm using a Bio-Tek ELx800 multi-plate
reader (Bio-Tek Instruments Inc., Winooski, VT).
Flow cytometric analysis of
apoptosis
All cells including those floating in the culture
medium were harvested after incubation. Cells were washed in cold
BSS and resuspended in 1X annexin V-binding buffer at a density of
1×106 cells/ml after centrifugation. FITC annexin V (5
μl) and 1 μl of the PI working solution (100 μg/ml) (Molecular
Probes, OR, USA) were added to 100 μl the cell suspension. After a
15-min incubation at room temperature, 400 μl of 1X annexin
V-binding buffer was added, mixed gently and the samples were
immediately placed on ice. The stained cells were analyzed using
the flow cytometer and FlowMax software.
Statistical analysis
Statistical analysis was performed using SPSS.
P<0.05 was considered statistically significant.
Results
mRNA expression of BDNF in human breast
cancer cell lines
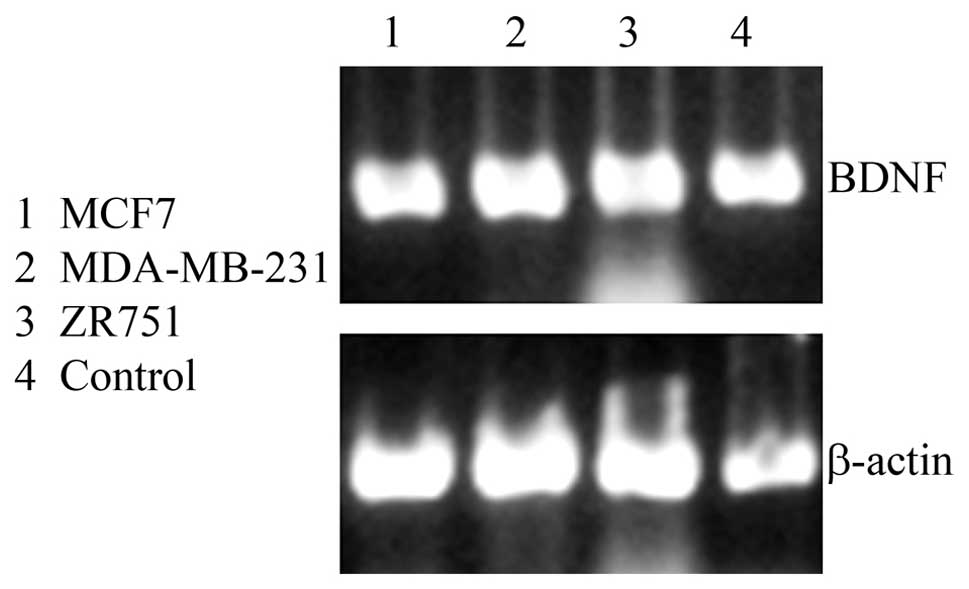
Human cancer cell lines MDA-MB-231, MCF-7 and ZR-751
were examined for the presence of BDNF using RT-PCR (Fig. 1). BDNF was strongly expressed in
all three cell lines. Fetal kidney tissue was used as a positive
control. The negative control had no DNA template (data not
shown).
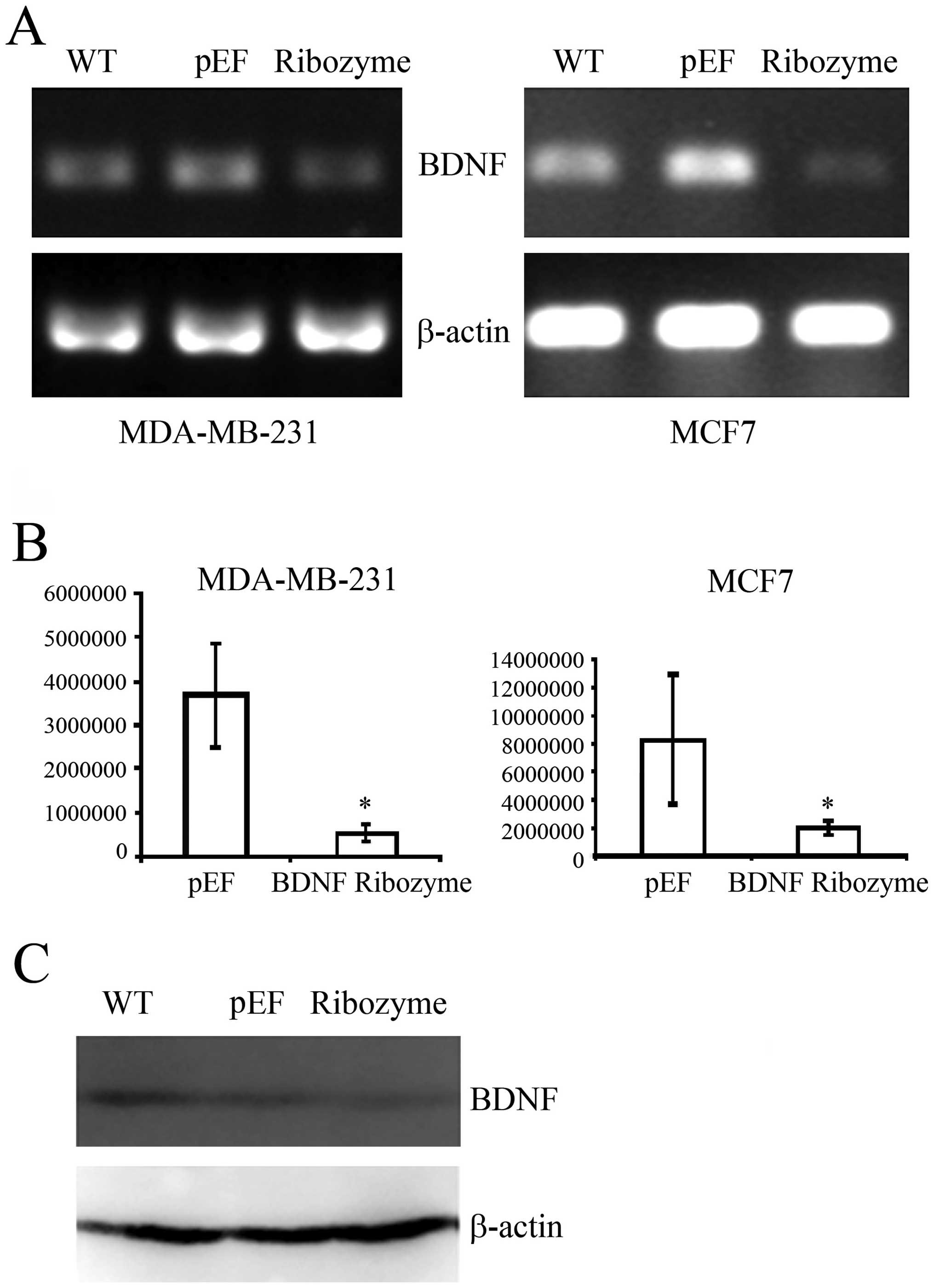
BDNF knockdown and establishment of
stable cell lines
The ribozymes targeting BDNF were cloned into
pEF6/V5-his-Topo T/A vector. MDA-MB-231 and MCF-7 wild-type cells
were subjected to transfection using plasmids containing ribozymes
targeting BDNF or an empty vector control, respectively, followed
by the establishment of BDNF knockdown sub-lines and empty vector
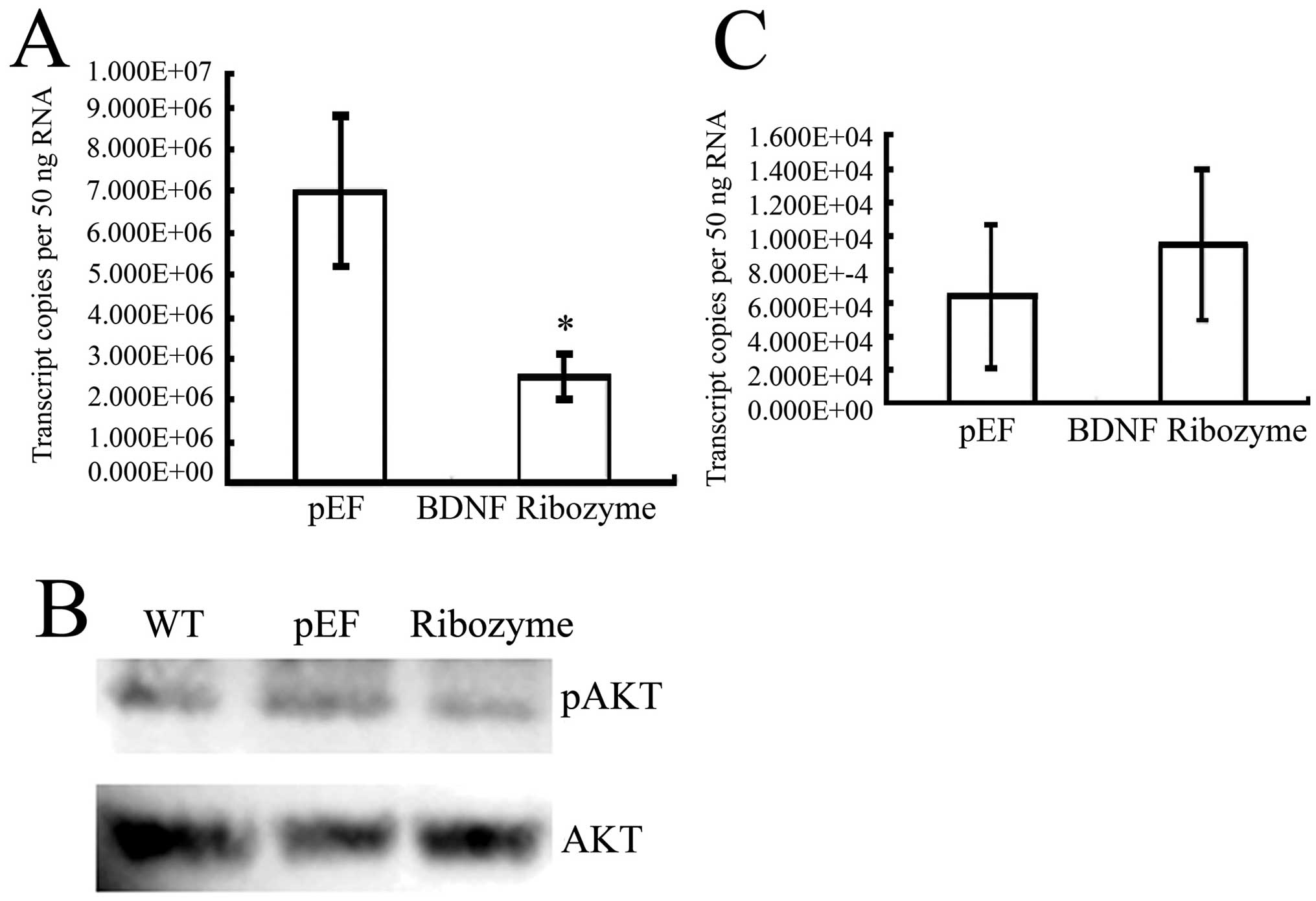
(pEF) control cells. The expression of BDNF at the mRNA level was
reduced in both BDNF knocked down MDA-MB-231 and MCF-7 cells using
RT-PCR and QPCR. (Fig. 2A and B).
The protein levels were also decreased in BDNF knocked down
MDA-MB-231 cells using western blotting (Fig. 2C). We then characterized the effect
of BDNF knockdown in these cells through a series of in
vitro studies.
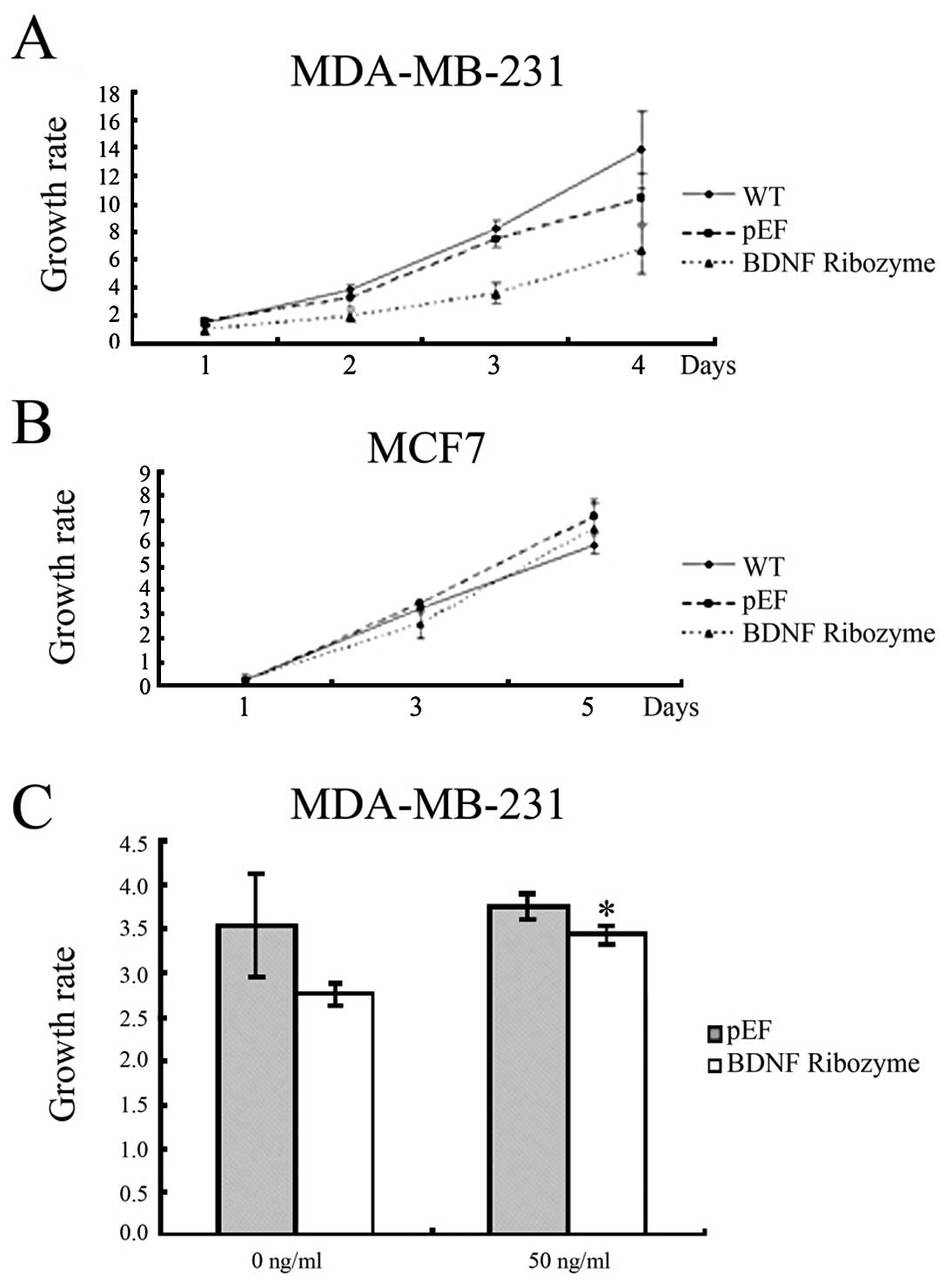
Effects of BDNF knockdown on the growth
of human breast cancer cells
In the in vitro growth assay, knockdown of
BDNF in MDA-MB-231 cells resulted in a reduction of cell growth
rate (growth rate in BDNF knocked down MDA-MB-231 cells by day 3
was 3.55±0.69, compared with 7.47±0.65 in pEF, p<0.001). Loss of
BDNF in MCF-7 cells resulted in reduction of cell growth rate
(growth rate in BDNF knocked-down MCF-7 cells by day 3 was
2.59±0.58, compared with 3.45±0.07 in pEF, p=0.012) (Fig. 3A). These data demonstrate that BDNF
may increase breast cancer cell growth.
To confirm this effect, a rescue assay was performed
with the addition of BDNF protein to the cell culture medium (50
ng/ml) (Abcam, Cambridge, UK). As a result, the reduced growth rate
in the knockdown cells was attenuated (Fig. 3B). These data indicated that BDNF
is involved in increased cell growth.
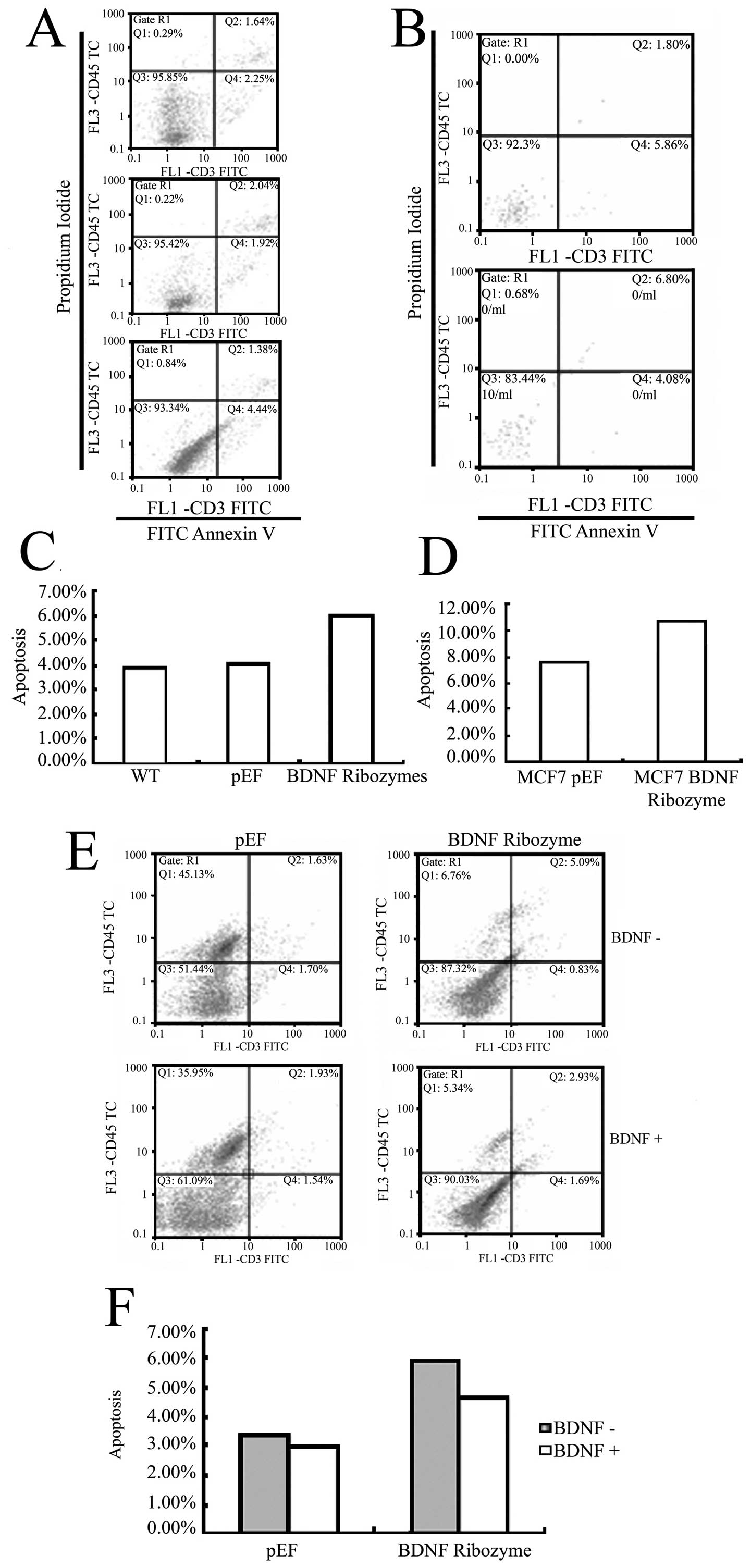
Effects of BDNF knocked-down on cell
apoptosis
To investigate whether apoptosis is involved in the
effect of BDNF knocked down in MDA-MB-231 and MCF-7 cells, we
determined the proportion of apoptotic cells. As shown in Fig. 4A–D, there was an increase in cell
population towards apoptosis in the BDNF knocked down MDA-MB-231
cells, which was 6.01% in the BDNF knocked down cells, compared
with 4.05% in the pEF control. There was also an increase of
apoptosis in the BDNF knocked down MCF-7 cells, which was 10.88% in
the BDNF knocked down cells, compared with 7.66% in the pEF
control. This result suggests that BDNF decreases apoptosis in
these cells.
Moreover, we confirmed whether or not this effect
was specific to BDNF knockdown by rescue experiments. BDNF protein
was added in the cell culture medium (50 ng/ml) and resulted in
negative effect of BDNF knockdown compared to the pEF controls
(Fig. 4E and F).
Effects of BDNF knockdown on cellular
signal pathways
We screened the cells at the mRNA transcript level
for p53 and NFκB in BDNF knocked down MDA-MB-231 cells using QPCR.
The results showed that the level of p53 was decreased in BDNF
knock down MDA-MB-231 and MCF-7 cells, indicating that BDNF
stimulates the message for p53. mRNA levels of the NFκB was
increased in BDNF knock down MDA-MB-231 and MCF-7 cells, indicating
that BDNF affects the level of NFκB. From these results, we can
infer that BDNF increases apoptosis through decreasing the
anti-apoptosis factor NFκB and increase p53 (Fig. 5A and C).
Further, we examined the serine phosphorylation
status of AKT in MDA-MB-231 knocked down cells. We found that the
phosphorylation level of AKT was weak in BDNF knocked-down cells
(Fig. 5B).
Discussion
BDNF has been described as a cancer related factor
involved in breast cancer cell growth with a correlation to
survival relevance in patients with breast cancer. However, the
biological function of BDNF and the many cellular molecular
pathways induced by BDNF are unknown (4).
The expression of BDNF and TrkB mRNA has been found
to be higher in human cancer cell lines than in normal tissues
(8). Our results also show mRNA
expression level of BDNF in human breast cancer to be elevated and
so we utilised RNA knockdown to study the influence of BDNF
expression on cellular function and possible molecular mechanisms.
In the present study we obtained stable knockdown of BDNF in human
breast cancer cell lines using BDNF ribozymes.
When BDNF was stably knocked down in MDA-MB-231 and
MCF-7 cell lines, the growth decreased compared with the vector
control, suggesting that reduced BDNF gene expression could inhibit
cellular proliferation. Cell proliferation was restored when
stimulated by BDNF protein. BDNF is therefore involved in
proliferation regulation of human breast cancer cell
proliferation.
The apoptosis experiments demonstrated that in those
MDA-MB-231 and MCF-7 cell lines in which BDNF was stably knocked
down, apoptosis increased compared with the vector control. This
suggests that BDNF is a regulator of apoptosis in these cells. It
can be concluded that BDNF can maintain breast cancer cell survival
and proliferation.
The influence of BDNF on cellular biological
function is induced mainly by its receptor TrkB. When BDNF binds to
TrkB the tyrosine kinase activity of the receptor is activated via
phosphorylation of tyrosine residues in the cytoplasmic region of
the receptor which in turn induces cellular signaling (13). The PI3K-AKT pathway is highly
related to cell survival and PI3K has been shown to play a key role
in anti-apoptotic survival and proliferation (14–16).
We have found that phosphorylation of the AKT signal was weak in
BDNF knocked down cells compared with vector control when treatment
with BDNF. This result show that BDNF activates the AKT pathway in
order to maintain cell survival (10). The MAPK pathway is known to be
involved in proliferation but we did not find obvious changes in
ERK1/2 phosphorylation in BDNF knocked down cells.
In this study, we also investigated the expression
of downstream molecules related to the AKT pathway. The nuclear
transcription factor, NFκB was found to be downregulation in the
BDNF knocked down MDA-MB-231 cells but Bcl-2 demostrated no obvious
changes (data not shown) compared with vector control. Therefore,
the NFκB expression inhibition induced by BDNF downregulation may
inhibit nucleolar transcription as a trigger for cell apoptosis
(17). Accordingly, BDNF can
facilitate NFκB expression to induce cell proliferation.
We found that the mRNA transcription level of p53
was elevated in BDNF knocked down MDA-MB-231 cells compared with
vector control. p53 is well-known to be a pro-apoptotic protein
related to cell survival. p53 trancription increase induced by
lower BDNF expression may increase cell apoptosis. BDNF has been
found to mediate protection from apoptosis by p53 activation
(18). High expression of BDNF and
its receptor TrkB have been found in cancers related to metastasis
and poor prognosis, in addition p53 is related in the late process
of tumor progression and predict of a poor prognosis in squamous
cell carcinoma of the uterine cervix (4,19).
The BDNF downregulation induced abolishment of
protection from apoptosis is affected by triggering downstream
interplay between NFκB activation and p53 inhibition. This
abolishment is not effected by upregulation of the BDNF-Akt-Bcl2
anti-apoptotic signaling pathway (15).
In conclusion, our study showed that BDNF
facilitated cell proliferation and inhibited cell apoptosis in
human breast cancer cells. Reduced BDNF expression induces changes
in downstream signaling molecules, which are related to cell
survival and apoptosis. BDNF is therefore a potential therapeutic
target in breast cancer and its effect in human breast cancer
requires further investigation.
Abbreviations:
|
BDNF
|
brain derived neurotrophic factor;
|
|
NGF
|
nerve growth factor;
|
|
QPCR
|
quantitative polymerase chain
reaction;
|
|
NFκB
|
nuclear factor κB;
|
|
TrkB
|
tyrosine kinase or BDNF/NT-3 growth
factors receptor;
|
|
PI3K
|
phosphatidylinositol 3-kinase;
|
|
AKT
|
protein kinase B (PKB);
|
|
MAPK
|
mitogen-activated protein (MAP)
kinases;
|
|
ERK
|
extracellular signal-regulated
kinase;
|
|
ECACC
|
European Collection of Cell
Cultures;
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium;
|
|
FITC
|
fluorescein isothiocyanate
|
Acknowledgements
We wish to thank Breast Cancer Hope
Foundation and Cancer Research Wales for their support. Dr Yang is
a recipient of the China Medical Scholarship of Cardiff University
and sponsorship from the Albert Hung Foundation.
References
|
1
|
Foretova L, Petrakova K, Palacova M,
Kalabova R, Svoboda M, Navratilova M, Schneiderova M, Bolcak K,
Krejci E, Drazan L, et al: Genetic testing and prevention of
hereditary cancer at the MMCI - over 10 years of experience. Klin
Oncol. 23:388–400. 2010.PubMed/NCBI
|
|
2
|
Gluck S and Mamounas T: Improving outcomes
in early-stage breast cancer. Oncology. 24:1–15. 2011.
|
|
3
|
Barde YA, Edgar D and Thoenen H:
Purification of a new neurotrophic factor from mammalian brain.
EMBO J. 1:549–553. 1982.PubMed/NCBI
|
|
4
|
Patani N, Jiang WG and Mokbel K:
Brain-derived neurotrophic factor expression predicts adverse
pathological and clinical outcomes in human breast cancer. Cancer
Cell Int. 11:232011. View Article : Google Scholar
|
|
5
|
Vanhecke E, Adriaenssens E, Verbeke S,
Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X and
Hondermarck H: Brain-derived neurotrophic factor and
neurotrophin-4/5 are expressed in breast cancer and can be targeted
to inhibit tumor cell survival. Clin Cancer Res. 17:1741–1752.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshii A and Constantine-Paton M:
Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity,
and disease. Dev Neurobiol. 70:304–322. 2010.PubMed/NCBI
|
|
7
|
Cowansage KK, LeDoux JE and Monfils MH:
Brain-derived neurotrophic factor: a dynamic gatekeeper of neural
plasticity. Curr Mol Pharmacol. 3:12–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo D, Hou X, Zhang H, Sun W, Zhu L, Liang
J and Jiang X: More expressions of BDNF and TrkB in multiple
hepatocellular carcinoma and anti-BDNF or K252a induced apoptosis,
supressed invasion of HepG2 and HCCLM3 cells. J Exp Clin Cancer
Res. 30:972011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harada T, Yatabe Y, Takeshita M, Koga T,
Yano T, Wang Y and Giaccone G: Role and relevance of TrkB mutations
and expression in non-small cell lung cancer. Clin Cancer Res.
17:2638–2645. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu X, Liu L, Cai B, He Y and Wan X:
Suppression of anoikis by the neurotrophic receptor TrkB in human
ovarian cancer. Cancer Sci. 99:543–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Festuccia C, Gravina GL, Millimaggi D,
Muzi P, Speca S, Ricevuto E, Vicentini C and Bologna M: Uncoupling
of the epidermal growth factor receptor from downstream signal
transduction molecules guides the acquired resistance to gefitinib
in prostate cancer cells. Oncol Rep. 18:503–511. 2007.
|
|
12
|
Brunetto de Farias C, Rosemberg DB, Heinen
TE, Koehler-Santos P, Abujamra AL, Kapczinski F, Brunetto AL,
Ashton-Prolla P, Meurer L, Reis Bogo M, et al: BDNF/TrkB content
and interaction with gastrin-releasing peptide receptor blockade in
colorectal cancer. Oncology. 79:430–439. 2011.PubMed/NCBI
|
|
13
|
Kawamura N, Kawamura K, Manabe M and
Tanaka T: Inhibition of brain-derived neurotrophic factor/tyrosine
kinase B signaling suppresses choriocarcinoma cell growth.
Endocrinology. 151:3006–3014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z and Thiele CJ: Targeting Akt to
increase the sensitivity of neuroblastoma to chemotherapy: lessons
learned from the brain-derived neurotrophic factor/TrkB signal
transduction pathway. Expert Opin Ther Targets. 11:1611–1621. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheikh AM, Malik M, Wen G, Chauhan A,
Chauhan V, Gong CX, Liu F, Brown WT and Li X: BDNF-Akt-Bcl2
antiapoptotic signaling pathway is compromised in the brain of
autistic subjects. J Neurosci Res. 88:2641–2647. 2010.PubMed/NCBI
|
|
16
|
Sun CY, Hu Y, Huang J, Chu ZB, Zhang L,
She XM and Chen L: Brain-derived neurotrophic factor induces
proliferation, migration, and VEGF secretion in human multiple
myeloma cells via activation of MEK-ERK and PI3K/AKT signaling.
Tumour Biol. 31:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
D’Intino G, Vaccari F, Sivilia S,
Scagliarini A, Gandini G, Giardino L and Calza L: A molecular study
of hippocampus in dogs with convulsion during canine distemper
virus encephalitis. Brain Res. 1098:186–195. 2006.PubMed/NCBI
|
|
18
|
Kalita K, Makonchuk D, Gomes C, Zheng JJ
and Hetman M: Inhibition of nucleolar transcription as a trigger
for neuronal apoptosis. J Neurochem. 105:2286–2299. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moon A, Won KY, Lee JY, Kang I, Lee SK and
Lee J: Expression of BDNF, TrkB, and p53 in early-stage squamous
cell carcinoma of the uterine cervix. Pathology. 43:453–458. 2011.
View Article : Google Scholar : PubMed/NCBI
|