Introduction
Tamoxifen is the drug most widely used for breast
cancer treatment. It is used both as adjuvant treatment and in the
treatment of recurrent disease in estrogen receptor (ER)-positive
breast cancer. Unfortunately, almost all patients treated for
advanced disease will eventually develop resistance to treatment
(1). However, several patients
will respond to second- and third-line endocrine therapy.
Fulvestrant is a pure antiestrogen which is approved as second-line
endocrine therapy for advanced breast cancer in postmenopausal
women whose disease have progressed or relapsed on prior
antiestrogen therapy (2), but
development of resistance will inevitably occur. An alternative
treatment option is therefore needed for these patients.
Cisplatin and carboplatin are effective
chemotherapeutic drugs widely used in the treatment of solid
tumors, such as testicular, ovarian, cervix, lung, head and neck,
esophagus and bladder cancer (3).
Cisplatin and carboplatin have been investigated as monotherapies
and in combinations with other chemotherapeutic drugs and with
trastuzumab in metastatic breast cancer (4). The efficacy of the platinum compounds
depends upon which agents they are combined with and whether the
patients have been previously treated with chemotherapy. The
results of three review studies on platinum compounds in the
treatment of advanced breast cancer are controversial. One review
including only studies comparing regimens with platinum compounds
to regimens without platinum compounds showed no benefit for the
use of platinum (5). Another study
concludes that cisplatin and carboplatin appeared to have efficacy
in combination with specific chemotherapeutic agents (6). Likewise, Decatris et al
conclude that platinum-monotherapy and platinum-containing
combination regimens are effective in the treatment of metastatic
breast cancer (7). Furthermore,
preclinical studies have demonstrated a synergistic interaction
between trastuzumab and cisplatin (8,9). The
combination of trastuzumab and cisplatin was effective in
extensively pretreated metastatic breast cancer patients (10), and patients treated with a
combination of adjuvant carboplatin, docetaxel and trastuzumab had
an overall survival equal to patients treated with adjuvant
cyclophosphamide, docetaxel and trastuzumab with lower risk of
cardiotoxicity and leukaemia (11).
Since the platinum compounds are potential
candidates for the treatment of metastatic breast cancer, the
efficacy of cisplatin in antiestrogen-resistant breast cancer cell
lines were tested in a previous work from our laboratory (12). A tamoxifen-resistant cell line and
a panel of six fulvestrant-resistant cell lines were more sensitive
to cisplatin-induced cell death compared to the parental,
ER-positive and antiestrogen-sensitive MCF-7 cell line, indicating
a benefit of using cisplatin in the treatment of
endocrine-resistant breast cancer.
However, cisplatin has severe side effects such as
nefro-, neuro- and ototoxicity as well as major emetic side effect.
The platinum analogue carboplatin with significantly less renal-,
neuro- and ototoxicity and less severe nausea and vomiting is a
useful alternative to cisplatin (3). The major cytotoxic target of
cisplatin and carboplatin is DNA. Their interaction with DNA leads
to DNA adduct formation, primarily intrastrand crosslink adducts,
which induce apoptosis. Thus, the platinum agents kill cells by
triggering their apoptosis program, and downregulation of the
apoptotic signal is an essential characteristic associated with
cisplatin resistance (13).
The Bcl-2 family of proteins is regulators of
apoptosis consisting of both anti-apoptotic cell survival proteins
(i.e. Bcl-2 and Bcl-xL) and pro-apoptotic cell death proteins (i.e.
Bax). The family members form homo- and heterodimers with each
other and the interaction among the different proteins controls the
propensity of a cell to undergo apoptosis (14). Yde et al found that
downregulation of Bcl-2 in MCF-7 cells sensitized the cells to
cisplatin treatment (15). This
was followed by a study of fulvestrant-resistant cell lines and one
tamoxifen-resistant cell line (MCF-7/TAMR-1) where a
decreased level of Bcl-2 in the resistant cell lines compared to
MCF-7, was concurrent with the finding of increased sensitivity to
cisplatin in the resistant cell lines (12). We have previously found that
fulvestrant-resistant cell lines express less Bcl-2 than the
parental, antiestrogen sensitive MCF-7 cell line (16). Bcl-2 is upregulated by
estrogen-mediated activation of ER in MCF-7 breast cancer cells
(17), and the decreased
expression of ER in our antiestrogen-resistant cell model (18–20)
may explain the decreased expression of Bcl-2.
The anti-apoptotic effect of Bcl-2 makes it an
obvious candidate as a predictive marker of response to
chemotherapy. A recent review evaluated the literature on 18 genes
associated with clinical chemosensitivity, and among these, Bcl-2
overexpression was found to be associated with resistance to
first-line chemotherapy (21).
The aim of this study was to explore if
antiestrogen-resistant cell lines have increased sensitivity to
carboplatin similarly to that previously shown with cisplatin.
Moreover, we exploited the role of Bcl-2 on the effect of
carboplatin treatment in tamoxifen-resistant breast cancer, and we
investigated if expression of Bcl-2, Bcl-xL and Bax proteins was
associated with carboplatin sensitivity.
Materials and methods
Cell cultures
The MCF-7 cell line was originally obtained from the
Human Cell Culture Bank, Mason Research Institute (Rockville, MD,
USA) and adapted to grow in low serum concentration (1%) to reduce
the estrogens supplied through the serum to a level resembling the
concentration of circulating estradiol in postmenopausal women
(22). The cells were maintained
in growth medium [Dulbecco's modified Eagle’s medium (DMEM/F12)]
without phenol red (Gibco/Invitrogen, CA, USA), supplemented with
1% heat-inactivated fetal calf serum (FCS) (Gibco), 2 mM glutamax
(Gibco) and 6 ng/ml insulin (Sigma-Aldrich, St. Louis, MO, USA).
Tamoxifen-resistant cell lines, MCF-7/TAMR-1
(TAMR-1), MCF-7/TAMR-4 (TAMR-4),
MCF-7/TAMR-7 (TAMR-7) and
MCF-7/TAMR-8 (TAMR-8) were established from
MCF-7 cells as previously described (18,20,23).
The fulvestrant-resistant cell line MCF-7/182R-6
(182R-6) was established as described (19) and was maintained in growth medium
supplemented with 0.1 μM ICI 182,780 (fulvestrant) (Tocris
Cookson, Bristol, UK), while the tamoxifen-resistant cell lines
TAMR-1, TAMR-4, TAMR-7 and
TAMR-8 were maintained in growth medium supplemented
with 1 μM tamoxifen (Sigma-Aldrich). For experiments, 25
U/ml penicillin and 25 μg/ml streptomycin (Gibco) were added
to the growth medium. All cell lines were maintained at 37°C in
humidified air containing 5% CO2.
Treatments and determination of cell
number and cell death
All cell lines were seeded in 24-well plates (2
cm2 wells) in growth medium 2 days before treatment. At
the onset of treatment, growth medium was changed to medium
containing 50, 100, 200, 300 or 400 μM carboplatin
(Sigma-Aldrich). After 48 h treatment, cell number was determined
by a crystal violet colorimetric assay as described previously
(24). For each cell line at least
four independent dose response growth experiments with carboplatin
were done. Each concentration was tested in quadruplicate.
For cell death determination, MCF-7 and
182R-6 cells treated for 24 and 48 h with carboplatin
were incubated for 15 min with 0.5 μM SYTOX-Green nucleic
acid stain (Invitrogen), harvested by trypsination, and combined
with floating cells from the medium. Cells were sedimented by
centrifugation and resuspended in phosphate-buffered saline (PBS)
containing 1% FCS. The fraction of SYTOX-Green-positive cells
(representing dead cells as SYTOX-Green stains the nucleic acids in
cells with disrupted plasma membrane) was measured using a
FACSCalibur (Becton Dickinson) flow cytometer. Cells (10,000) were
analyzed using the FL-1 filter for determination of the fraction of
SYTOX-positive cells. The data were analyzed using the Cell Quest
Pro software.
Western blot analysis
All cell lines were cultured in T25 flasks (Nunc,
Roskilde, Denmark) until 70-80% confluent. For analyses of ERα,
Bcl-2, Bax and Bcl-xL, antiestrogen-resistant cell lines were grown
in standard medium containing the respective antiestrogen. Medium
was renewed every second or third day. After 6-7 days, the cells
were washed with PBS and harvested in radioimmunoprecipitation
(RIPA) buffer (100 mM NaCl, 20 mM Tris-HCl, 1% Triton X-100, 0.5%
sodium-deoxycholate, 0.1% SDS and 1 mM EDTA, with the addition of 1
mM DTT, 1 mM NaF, 10 mM β-glycerolphosphate, 100 μM
Na3VO4, 150 μM PMSF, and one tablet/10
ml complete mini protease inhibitor cocktail (Roche, Hvidovre,
Denmark)). Determination of protein concentration was done using
the Bio-Rad protein assay kit (Bio-Rad Laboratories, Munich,
Germany) with bovine serum albumin as standard. Proteins were
separated by 15% or 4–15% SDS-PAGE gels and transferred to an
Immobilon-P membrane (Millipore, Bedford, MA, USA) by semi-dry
electroblotting. Membranes were blocked with Tris-buffered saline
(TBS) containing 5% nonfat dry-milk, 5% FCS and 0.2% Tween-20
(Merck, Hohenbrunn, Germany). Immunostaining was performed with
primary antibodies directed against ERα (SP1, 1:5,000, Thermo
Fisher Scientific, Fremont, CA, USA), Bcl-2 (clone 124, 1:2,000,
Dako, Glostrup, Denmark), Bcl-xL (54H6, 1:1,000, Cell Signaling
Technology, Beverly, MA, USA), Bax (2772, 1:1,000, Cell Signaling
Technology) and β-actin (AC-15, 1:100,000, Sigma-Aldrich).
Secondary goat anti-rabbit and rabbit anti-mouse horseradish
peroxidase-conjugated antibodies (P0448 and P0260 respectively;
1:2,000, Dako) were used. The ECL Plus Western Blotting Detection
System (Amersham, GE Healthcare, Buckinghamshire, UK) was used for
visualization of the proteins according to the manufacturer’s
instructions. Western blot analyses were repeated at least three
times on independent cell lysates with reproducible results.
Transfections
Bcl-2 overexpressing MCF-7/TAMR-1 cell
lines were established by transfection of cells with pCEP4-BCL-2
(kindly provided by Dr Marja Jaättelä, Danish Cancer Society,
Copenhagen, Denmark). Control cells were transfected with pCEP4
(empty vector). Transfection was performed using a Nucleofector
electroporation system (Amaxa Biosystems, Cologne, Germany). Stable
Bcl-2 expressing cell clones were selected and maintained in growth
medium containing 50 μg/ml hygromycin B and 1 μM
tamoxifen.
Statistics
For all cell growth assays at least four independent
experiments with quadruplicate measures were performed and the data
pooled for statistical analysis. OD-measurements from crystal
violet staining were converted to a rate of the respective
untreated control and evaluated on the log scale. The assumptions
of normally distributed data on the log scale were assessed using
residuals and variance homogeneity was tested using Levine’s test.
Analysis was done using mixed modeling where treatment and cell
line were regarded as fixed variables and the individual
experiments were regarded as random effects in the model.
The back transformed estimates based on the mixed
model are plotted as Forest Plots, illustrating the ratios between
number of resistant cells and number of the control cells, at each
concentration. A ratio equal to 1.0 means that the two cell lines
were equally reduced compared to their control. Less than 1.0 means
that the resistant cell line was reduced more than MCF-7, analogous
to increased sensitivity to carboplatin. The 95% confidence
intervals for the estimate are also plotted, showing the precision
of the results. Growth experiments with the fulvestrant-resistant
cell line 182R-6 and the transfected cell lines are
illustrated as cell number in % of the untreated control from a
representative experiment. For all experiments, results were
considered significant at P<0.05. Calculations were performed
using SAS, version 9.2 (SAS Institute, Cary, NC, USA).
Results
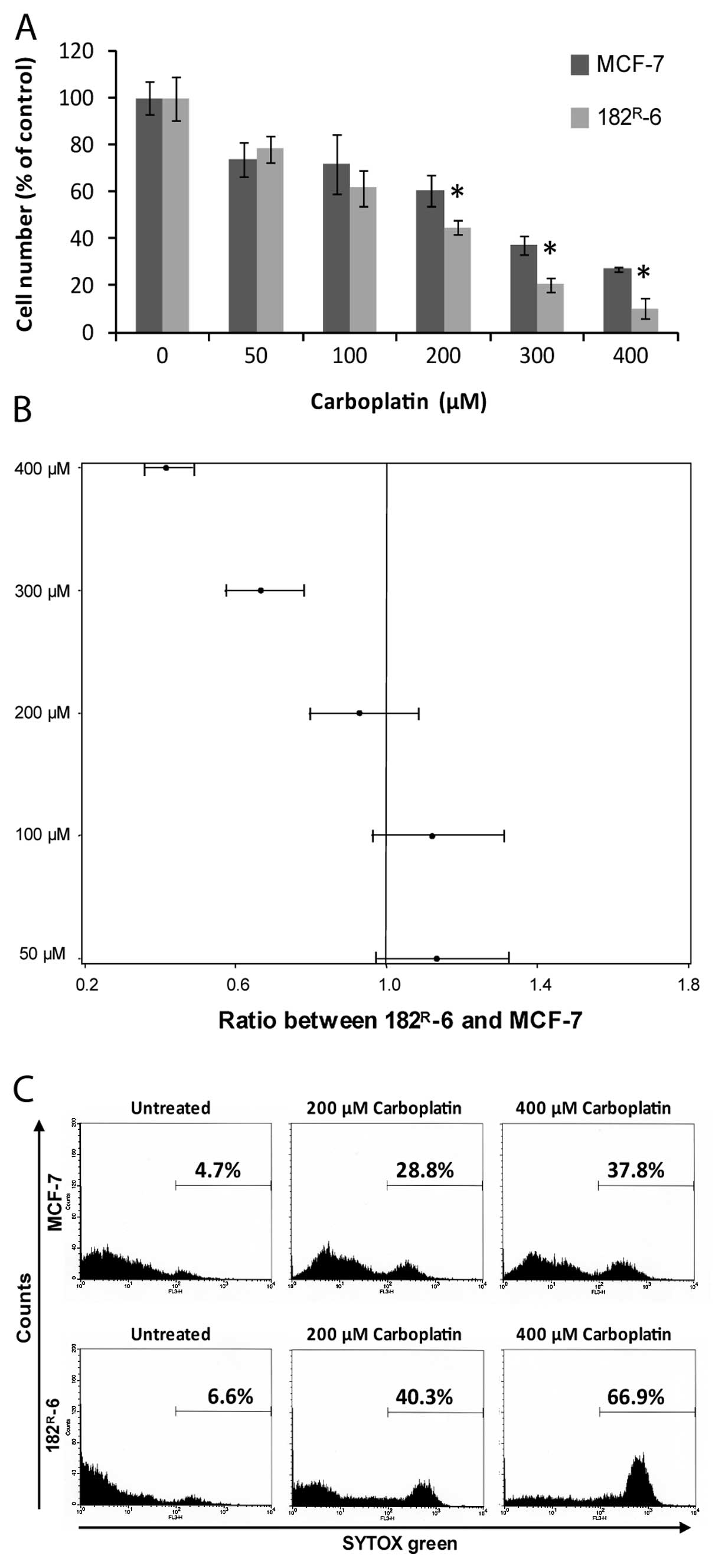
Fulvestrant-resistant cells were more
sensitive to carboplatin than parental MCF-7 cells
The fulvestrant-resistant cell line
182R-6, which had earlier been shown to have increased
sensitivity towards cisplatin-induced cell killing compared to
MCF-7, was chosen for the experiments to test if
fulvestrant-resistant cells were also more sensitive than MCF-7 to
carboplatin. Dose-response experiments were performed after 48 h
treatment with carboplatin. The response was expressed as percent
of the cell number in the untreated control. The result from a
representative experiment with quadruplicate samples at each tested
carboplatin concentration is shown in Fig. 1A. At 200, 300 and 400 μM
carboplatin, 182R-6 cells were statistically
significantly more sensitive than MCF-7 cells. Fig. 1B shows a Forest plot with pooled
data from four experiments, each with quadruplicate samples of the
tested carboplatin concentrations. The ratios between cell number
of 182R-6 and MCF-7 at different concentrations of
carboplatin are shown. This illustrates that 182R-6
cells were significantly more sensitive towards carboplatin at
concentrations of 300 μM and 400 μM with a ratio
between 182R-6 and MCF-7 of 0.67 (95% CI: 0.57–0.78) and
0.42 (95% CI: 0.36–0.49), respectively.
Cell death determinations were performed to disclose
whether the effect of carboplatin was a cytotoxic effect leading to
cell death as previously shown for cisplatin (12). Fig.
1C shows the fraction of dead cells in untreated MCF-7 and
182R-6 cultures and in cultures treated for 48 h with
200 μM and 400 μM carboplatin, respectively. The
fraction of dead cells is low in untreated cultures. Carboplatin
kills both MCF-7 and 182R-6 cells, but at 400 μM
concentration, 182R-6 cells are much more sensitive to
carboplatin-mediated cell killing than MCF-7 cells. The death
fractions indicated in the figure are average values from three
independent experiments.
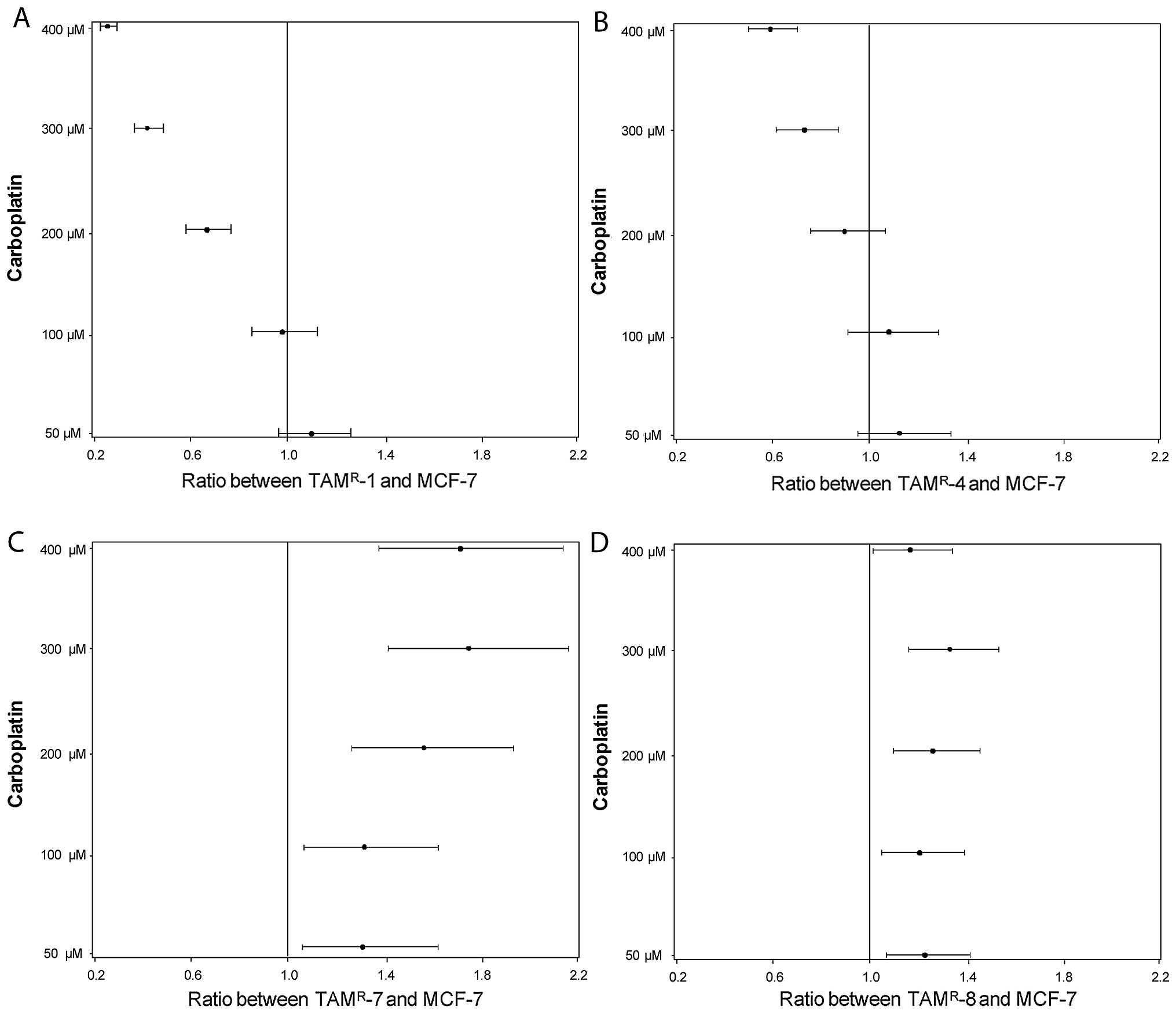
Two out of four tamoxifen-resistant cell
lines were more sensitive to carboplatin
Tamoxifen is a drug that is used far more often than
fulvestrant and we therefore explored the relevance of carboplatin
treatment in a model system of tamoxifen-resistant cell lines,
established by long-term treatment of MCF-7 with tamoxifen.
Tamoxifen-resistant cell lines and MCF-7 cells were treated with
carboplatin in doses of 50, 100, 200, 300 and 400 μM for 48
h and cell numbers were determined. All cell lines, including
MCF-7, responded to carboplatin with a decrease in the cell number.
The ratios between the cell number of the TAMR-1 and
MCF-7 are shown in Fig. 2A,
TAMR-4 and MCF-7 in Fig.
2B, TAMR-7 and MCF-7 in Fig. 2C and TAMR-8 and MCF-7 in
Fig. 2D. Two of the four resistant
cell lines (TAMR-1 and TAMR-4) were
significantly more sensitive towards carboplatin than MCF-7 at 200,
300 and 400 μM (except at 200 μM in the case of
TAMR-4). The other two cell lines, TAMR-7 and
TAMR-8, were less sensitive at all concentrations of
carboplatin, compared to MCF-7 cells.
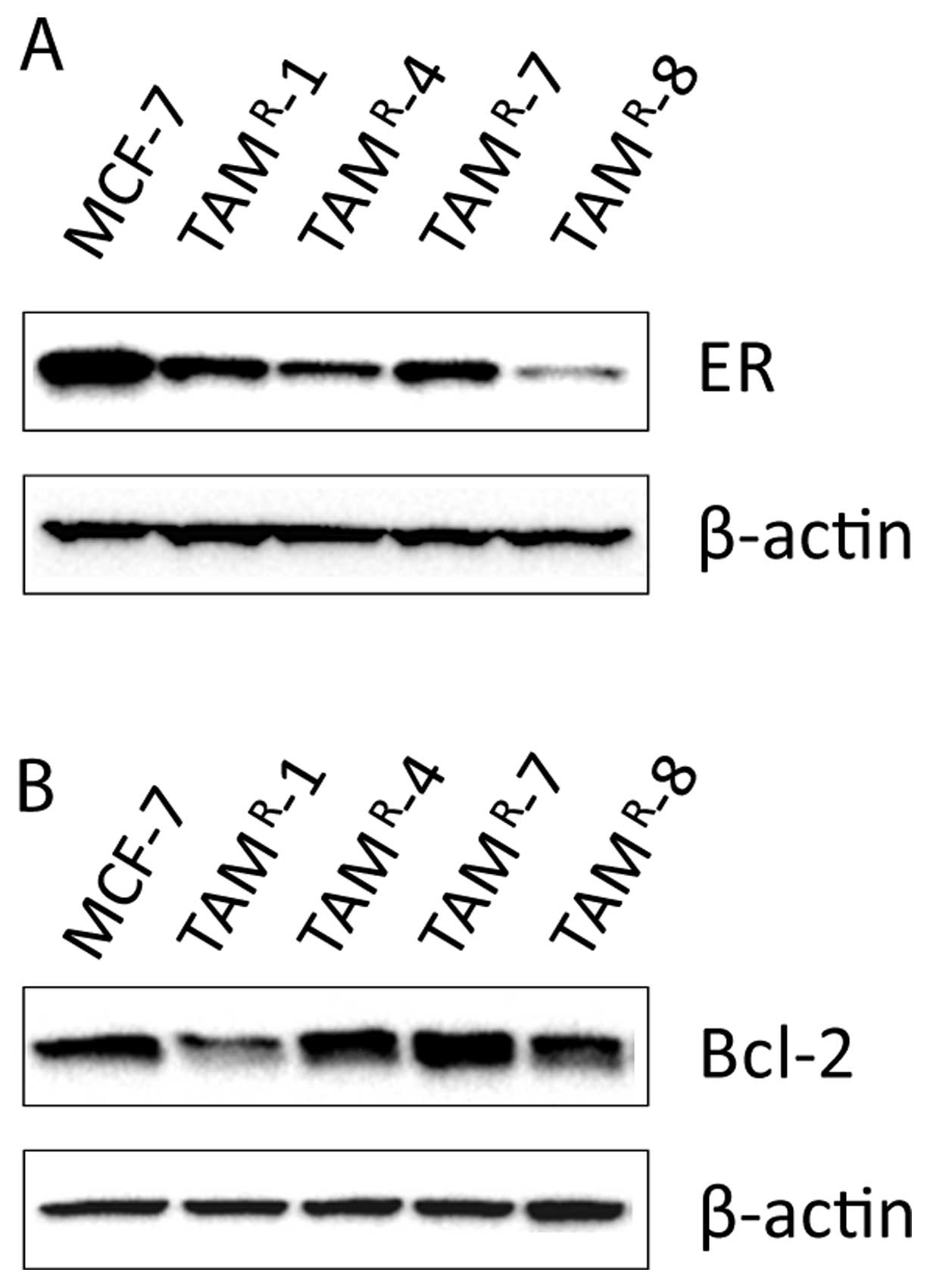
Bcl-2 expression is not associated with
the growth response to carboplatin treatment
Yde et al found in the fulvestrant-resistant
cell lines that the low level of Bcl-2 expression can at least
partly explain the increased sensitivity towards cisplatin
(12). Previous studies have found
low ER level in TAMR-1 and the fulvestrant-resistant
cell lines (18,19). Low Bcl-2 level is expected as a
consequence of the low ER level. Therefore, Western blot analyses
were performed for detection of ER and Bcl-2 protein levels
(Fig. 3), and it was found that
all tamoxifen-resistant cell lines had reduced levels of ER
compared to MCF-7 (Fig. 3A). The
expression of Bcl-2 was lower in TAMR-1 and
TAMR-8 compared to MCF-7 cells, whereas Bcl-2 levels in
TAMR-4 and TAMR-7 were similar to MCF-7
(Fig. 3B). Thus, TAMR-1
was the only cell line in which increased sensitivity towards
carboplatin was associated with low level of Bcl-2. Noteworthy, the
level of Bcl-2 was low in TAMR-8 which had a reduced
carboplatin sensitivity; and the level of Bcl-2 was similar in
TAMR-4, TAMR-7 and MCF-7, contrary to
expectation, since TAMR-4 was more sensitive and
TAMR-7 less sensitive than MCF-7.
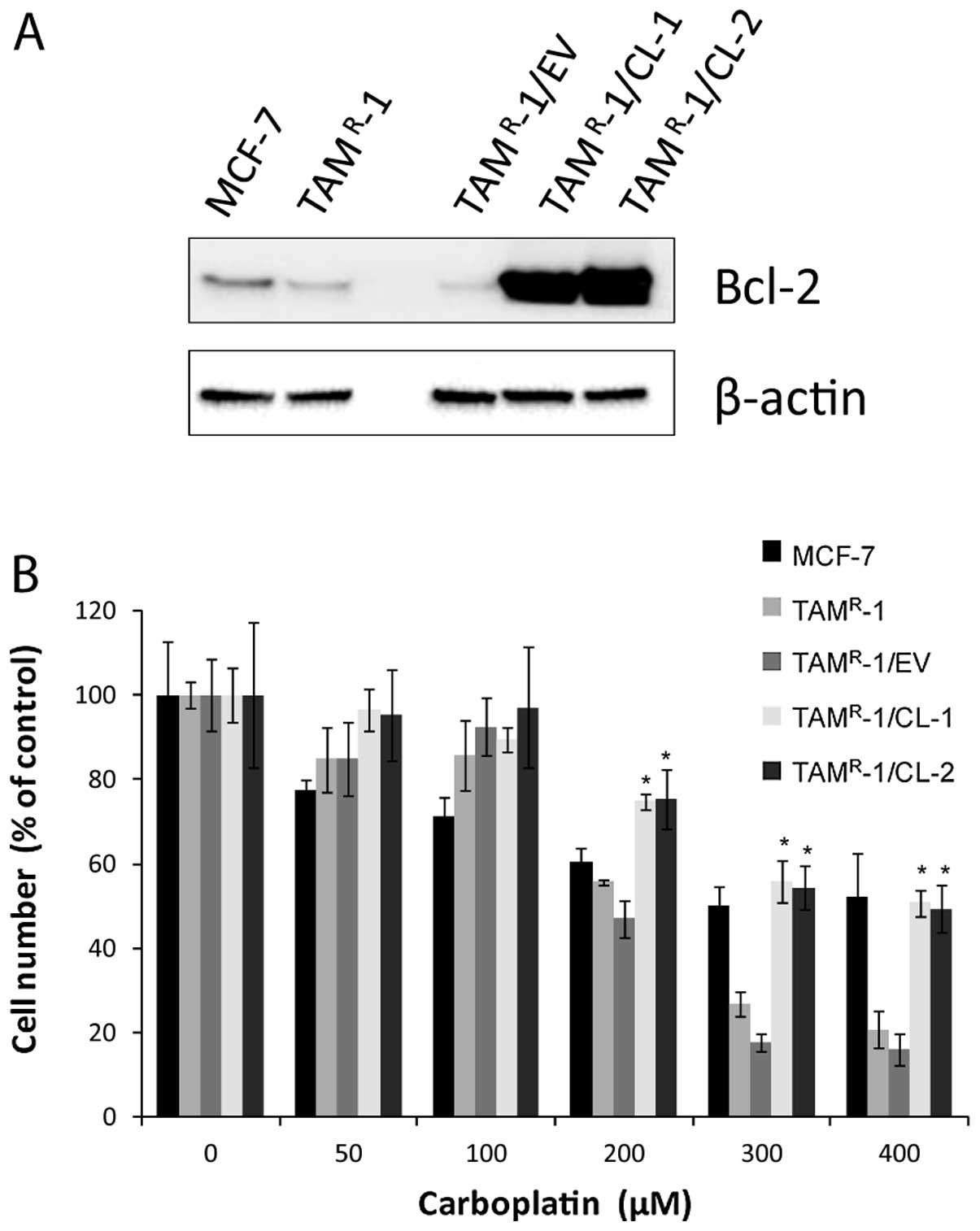
Resistant cells with stable
overexpression of Bcl-2 have decreased sensitivity towards
carboplatin
Since TAMR-1 was the only
tamoxifen-resistant cell line in which reduced sensitivity to
carboplatin treatment was associated with reduced Bcl-2 expression,
we investigated whether stable overexpression of Bcl-2 protein by
transfection of the Bcl-2 gene could reduce the effect of
carboplatin. We used a plasmid without the Bcl-2 insert (empty
vector) as a control. Expression of the Bcl-2 constructs in
TAMR-1 was verified by western blot analysis (Fig. 4A). TAMR-1 cells
transfected with the empty vector (TAMR-1/EV) had a Bcl-2
expression, which was similar to the Bcl-2 expression in
TAMR-1; both of them were low compared to MCF-7 as
expected. The two independently established clones,
TAMR-1/Bcl-2/clone1 (CL-1) and TAMR-1/
Bcl-2/clone2 (CL-2) with a high, stable expression of Bcl-2, were
tested for sensitivity to carboplatin treatment. The two clones
were significantly less sensitive than the empty vector and
TAMR-1 at concentrations of 200–400 μM
carboplatin, Fig. 4B.
Bcl-xL and Bax did not give supplementary
information on the sensitivity of the cell lines
To see if other proteins from the Bcl-2 family might
explain the differences in carboplatin sensitivity, we investigated
the level of two other proteins from the Bcl-2 family: the
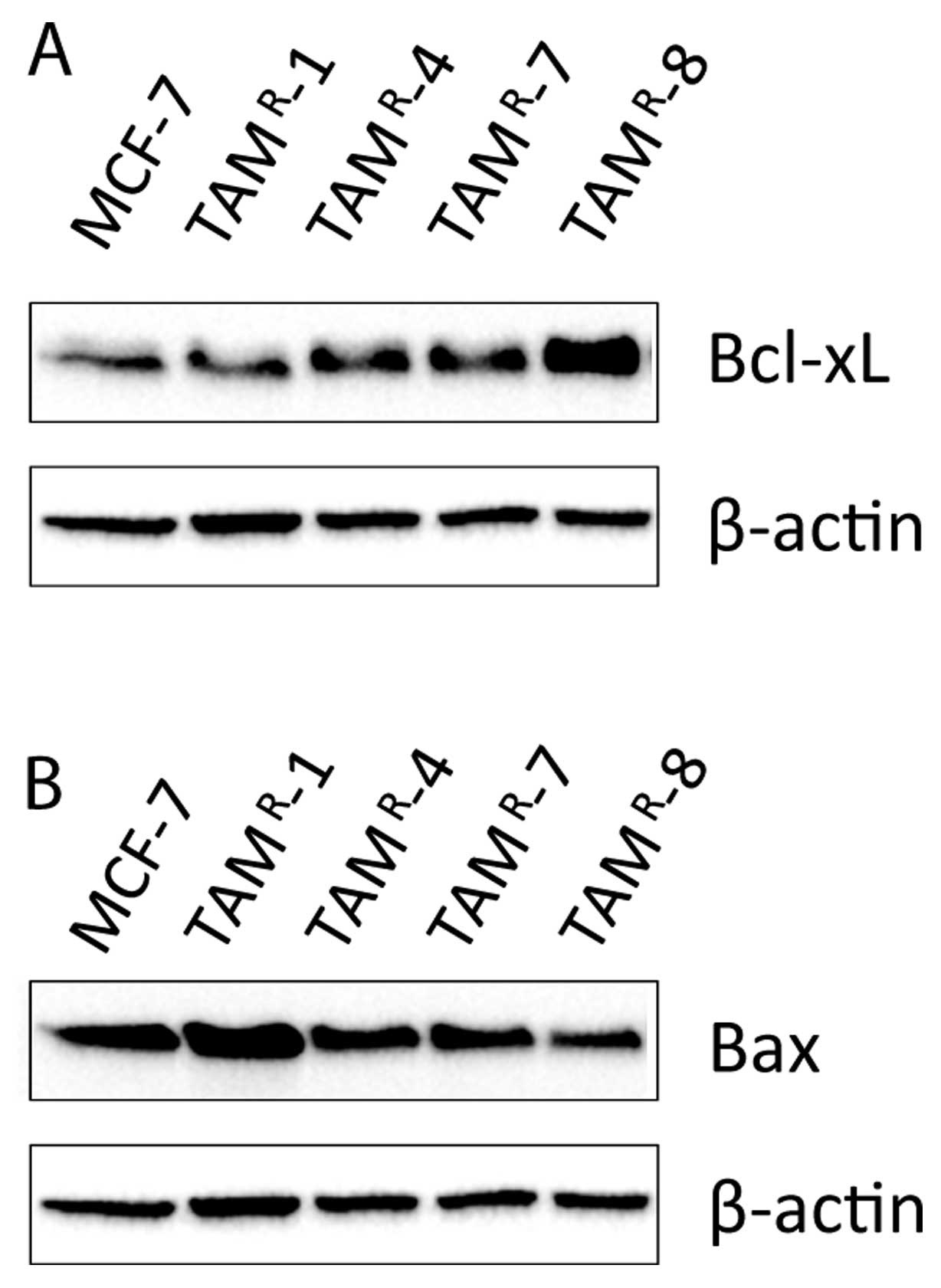
anti-apoptotic Bcl-xL and the pro-apoptotic Bax proteins. As seen
in Fig. 5A, the level of Bcl-xL
was similar in MCF-7, TAMR-1, TAMR-4, and
TAMR-7. Only TAMR-8 had an increased level of
Bcl-xL compared to MCF-7. The level of Bax was increased in
TAMR-1 and decreased in TAMR-8 compared to
MCF-7 whereas TAMR-4 and TAMR-7 displayed
similar level as MCF-7 cells, Fig.
5B.
Discussion
Based on previous results showing increased
sensitivity to cisplatin in the tamoxifen-resistant cell line,
TAMR-1, and a panel of fulvestrant-resistant cell lines
(12), we investigated whether
antiestrogen-resistant cell lines have increased sensitivity to
carboplatin. Furthermore, based on the extensive use of tamoxifen
as first-line endocrine therapy for premenopausal breast cancer
patients and also for many postmenopausal breast cancer patients,
we wanted to test a panel of tamoxifen-resistant cell lines for
response to carboplatin treatment to unravel whether it is a
general phenomenon that both fulvestrant- and tamoxifen-resistant
cell lines are sensitized to treatment with platinum compounds.
We found that carboplatin, a drug with less severe
side effects than cisplatin, had the same advantage as cisplatin in
exerting more severe cytotoxic effect on the fulvestrant-resistant
182R-6 cell line than MCF-7 cells. Regarding the effect
of carboplatin in tamoxifen-resistant cell lines, two out of four
cell lines were more sensitive to carboplatin compared to MCF-7,
whereas the other two were less sensitive.
A possible explanation for the increased sensitivity
of the fulvestrant-resistant cell lines is a lower expression level
of the anti-apototic Bcl-2 protein due to the lower level of ER in
fulvestrant-resistant cells compared to MCF-7. Therefore, the ER
level was determined in the tamoxifen-resistant cell lines and all
four cell lines displayed reduced ER level compared to MCF-7. In
contrast to fulvestrant-resistant cell lines, only two of the four
tamoxifen-resistant cell lines expressed reduced Bcl-2 level, and
in only one cell line, TAMR-1, the reduced Bcl-2 level
was associated with increased sensitivity to carboplatin treatment.
We then tested the effect of Bcl-2 overexpression in
TAMR-1 by transfection of a plasmid containing the Bcl-2
gene, and this confirmed that Bcl-2 can protect TAMR-1
cells from the toxic effect of carboplatin.
The finding that TAMR-8 cells, which also
had reduced Bcl-2 level, were less sensitive to carboplatin
treatment than MCF-7 cells, clearly demonstrated that Bcl-2 is not
the only factor influencing the response to carboplatin treatment.
Therefore, Bcl-2 alone is not a suitable marker for evaluation of
the effect of carboplatin. As Bcl-2 is a member of a large family
of proteins regulating apoptosis, we explored the possibility that
other members of the family could counterbalance the effect of the
Bcl-2 level. We tested the association of sensitivity with two
other apoptosis regulating proteins, the anti-apoptotic protein
Bcl-xL and the pro-apoptotic protein Bax. The Bcl-xL level was
increased and Bax level was decreased in TAMR-8 compared
to MCF-7. Considering that the Bcl-2 level was not as severely
decreased in TAMR-8 as in TAMR-1 cells, the
increase in the anti-apoptotic Bcl-xL and the decrease in the
pro-apoptotic Bax level may explain the reduced sensitivity in
TAMR-8 compared to MCF-7 cells. The level of Bax was
increased in TAMR-1, which fits with the increased
sensitivity of these cells. However, in TAMR-4 and
TAMR-7 cells, neither Bcl-xL nor Bax could explain the
observed carboplatin sensitivity.
Altogether, the individual tamoxifen-resistant cell
lines appear to be very different. Compared to the
fulvestrant-resistant cell lines, all showing increased sensitivity
to platinum treatment, the tamoxifen-resistant cell lines
TAMR-1, 4, 7 and 8 were very different concerning their
sensitivity to carboplatin treatment and the expression of
apoptosis-related proteins. This may reflect that many different
mechanisms contribute to the occurrence of tamoxifen resistance,
whereas fulvestrant resistance appears to occur from a shift from
ER to HER signaling (25). In
tamoxifen-resistant cells, ER may still be functional and tamoxifen
can even act as an agonist; while in fulvestrant-resistant cells,
ER is completely blocked. Although the Bcl-2 level was reduced
compared to MCF-7 in two tamoxifen-resistant cell lines, the Bcl-2
level was even lower in fulvestrant-resistant cells (16). This leaves the possibility that
other apoptosis-related proteins could easily overrule the effect
of Bcl-2 in the tamoxifen-resistant cells.
These data show that the determination of Bcl-2
status alone is not sufficient in assessing the impact on apoptosis
of the Bcl-2 death pathway; the assessment of levels of the other
members of the Bcl-2 family may better determine the extent to
which apoptosis may occur. A clinical study reported a correlation
between reduced Bax and shorter overall survival, faster time to
progression, and failure to respond to chemotherapy in patients
with metastatic breast cancer (26). We found that Bax and Bcl-xL could
not explain differences in carboplatin sensitivity of
TAMR-4 and TAMR-7 compared to MCF-7,
indicating that other pathways regulating apoptosis are more
important. Although Bcl-2 was found to predict response to
chemotherapy in some studies (21), contradictory results of no
relationship between Bcl-2 expression and clinical response to
chemotherapy were reported by others (27). On the other hand, the latter study
found mutated p53 to be a significant predictor of poor clinical
response rate. This again points to the possibility that assessment
of other apoptosis pathways are required to evaluate the propensity
of a cell to undergo apoptosis as a response to chemotherapy.
Apoptosis depends on the expression of a specific set of genes,
among them are wild-type p53 that can induce apoptosis, and Akt
that has anti-apoptotic activity. The wild-type p53
transcriptionally downregulates the expression of the Bcl-2 gene
and activates the expression of the Bax gene (28). In human breast carcinoma, an
inverse correlation between p53 immunostaining, a surrogate
end-marker of mutant p53 protein and Bcl-2 expression has been
reported (29). Akt has the
ability to inactivate the pro-apoptotic factor Bad (30). A previous study from our laboratory
with four fulvestrant-resistant cell lines and TAMR-1
revealed a higher level of phosphorylated Akt in three of the
fulvestrant-resistant cell lines and in TAMR-1 (31). These are examples of possible
contributors to a complex regulation of apoptosis related to the
response to chemotherapy.
In conclusion, this study shows that carboplatin is
more efficient for treatment of fulvestrant-resistant
182R-6 cells than MCF-7 cells in agreement with the
previous finding of increased sensitivity to cisplatin in
fulvestrant-resistant cell lines (12). However, this did not apply to all
tamoxifen-resistant cell lines, indicating that platinum compounds
would be a candidate for treatment of breast cancer relapse after
fulvestrant treatment but not necessarily after tamoxifen
treatment.
Our previous study indicated that low Bcl-2 level is
a potential predictive marker for sensitivity to cisplatin
treatment (12). If carboplatin
should be used in the treatment of breast cancer patients relapsing
on tamoxifen treatment, a marker to predict the most sensitive
tumors would be needed. Our study on four tamoxifen-resistant cell
lines did not support the use of low Bcl-2 level as single marker
for carboplatin response. Determination of the level of the
anti-apoptotic Bcl-xL protein and the pro-apoptotic Bax protein was
neither sufficient to explain the observed responses to carboplatin
treatment, demonstrating that a complex network of factors is
involved in the response of tamoxifen-resistant breast cancer cells
to carboplatin treatment.
Acknowledgements
We thank Inger Heiberg for excellent
technical assistance. The study was financially supported by Danish
Cancer Society (grant no. DP08013) and Danish Agency for Science
Technology and Innovation (grant no. 09-063068 and 09-063623).
References
|
1.
|
Ali S and Coombes RC: Endocrine-responsive
breast cancer and strategies for combating resistance. Nat Rev
Cancer. 2:101–112. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Howell A, DeFriend DJ, Robertson JF,
Blamey RW, Anderson L, Anderson E, Sutcliffe FA and Walton P:
Pharmacokinetics, pharmacological and anti-tumour effects of the
specific anti-oestrogen ICI 182780 in women with advanced breast
cancer. Br J Cancer. 74:300–308. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Go RS and Adjei AA: Review of the
comparative pharmacology and clinical activity of cisplatin and
carboplatin. J Clin Oncol. 17:409–422. 1999.PubMed/NCBI
|
|
4.
|
Perez EA: Carboplatin in combination
therapy for metastatic breast cancer. Oncologist. 9:518–527. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Carrick S, Ghersi D, Wilcken N and Simes
J: Platinum containing regimens for metastatic breast cancer.
Cochrane Database Syst Rev. 3:CD0033742004.
|
|
6.
|
Martin M: Platinum compounds in the
treatment of advanced breast cancer. Clin Breast Cancer. 2:190–208.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Decatris MP, Sundar S and O’Byrne KJ:
Platinum-based chemotherapy in metastatic breast cancer: current
status. Cancer Treat Rev. 30:53–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Colbern GT, Hiller AJ, Musterer RS,
Working PK and Henderson IC: Antitumor activity of Herceptin in
combination with STEALTH liposomal cisplatin or nonliposomal
cisplatin in a HER2 positive human breast cancer model. J Inorg
Biochem. 77:117–120. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pegram M, Hsu S, Lewis G, Pietras R, Beryt
M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F and Slamon D:
Inhibitory effects of combinations of HER-2/neu antibody and
chemo-therapeutic agents used for treatment of human breast
cancers. Oncogene. 18:2241–2251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Pegram MD, Lipton A, Hayes DF, Weber BL,
Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA
and Slamon DJ: Phase II study of receptor-enhanced chemosensitivity
using recombinant humanized anti-p185HER2/neu monoclonal antibody
plus cisplatin in patients with HER2/neu-overex-pressing metastatic
breast cancer refractory to chemotherapy treatment. J Clin Oncol.
16:2659–2671. 1998.
|
|
11.
|
Slamon D, Eiermann W, Robert N, Pienkowski
T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M,
Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee
V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A and
Crown J; Breast Cancer International Research Group: Adjuvant
trastuzumab in HER2-positive breast cancer. N Engl J Med.
365:1273–1283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yde CW, Gyrd-Hansen M, Lykkesfeldt AE,
Issinger OG and Stenvang J: Breast cancer cells with acquired
antiestrogen resistance are sensitized to cisplatin-induced cell
death. Mol Cancer Ther. 6:1869–1876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Siddik ZH: Cisplatin: mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Schorr K, Li M, Krajewski S, Reed JC and
Furth PA: Bcl-2 gene family and related proteins in mammary gland
involution and breast cancer. J Mammary Gland Biol Neoplasia.
4:153–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yde CW and Issinger OG: Enhancing
cisplatin sensitivity in MCF-7 human breast cancer cells by
down-regulation of Bcl-2 and cyclin D1. Int J Oncol. 29:1397–1404.
2006.PubMed/NCBI
|
|
16.
|
Christensen GL, Jepsen JS, Fog CK,
Christensen IJ and Lykkesfeldt AE: Sequential versus combined
treatment of human breast cancer cells with antiestrogens and the
vitamin D analogue EB1089 and evaluation of predictive markers for
vitamin D treatment. Breast Cancer Res Treat. 85:53–63. 2004.
View Article : Google Scholar
|
|
17.
|
Larsen SS, Heiberg I and Lykkesfeldt AE:
Anti-oestrogen resistant human breast cancer cell lines are more
sensitive towards treatment with the vitamin D analogue EB1089 than
parent MCF-7 cells. Br J Cancer. 84:686–690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lykkesfeldt AE, Madsen MW and Briand P:
Altered expression of estrogen-regulated genes in a
tamoxifen-resistant and ICI 164,384 and ICI 182,780 sensitive human
breast cancer cell line, MCF-7/TAMR-1. Cancer Res. 54:1587–1595.
1994.PubMed/NCBI
|
|
19.
|
Lykkesfeldt AE, Larsen SS and Briand P:
Human breast cancer cell lines resistant to pure anti-estrogens are
sensitive to tamoxifen treatment. Int J Cancer. 61:529–534. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Madsen MW, Reiter BE, Larsen SS, Briand P
and Lykkesfeldt AE: Estrogen receptor messenger RNA splice variants
are not involved in antiestrogen resistance in sublines of MCF-7
human breast cancer cells. Cancer Res. 57:585–589. 1997.PubMed/NCBI
|
|
21.
|
Sekine I, Shimizu C, Nishio K, Saijo N and
Tamura T: A literature review of molecular markers predictive of
clinical response to cytotoxic chemotherapy in patients with breast
cancer. Int J Clin Oncol. 14:112–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lykkesfeldt AE and Sorensen EK: Effect of
estrogen and antiestrogens on cell proliferation and synthesis of
secreted proteins in the human breast cancer cell line MCF-7 and a
tamoxifen resistant variant subline, AL-1. Acta Oncol. 31:131–138.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lykkesfeldt AE and Briand P: Indirect
mechanism of oestradiol stimulation of cell proliferation of human
breast cancer cell lines. Br J Cancer. 53:29–35. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lundholt BK, Briand P and Lykkesfeldt AE:
Growth inhibition and growth stimulation by estradiol of estrogen
receptor transfected human breast epithelial cell lines involve
different pathways. Breast Cancer Res Treat. 67:199–214. 2001.
View Article : Google Scholar
|
|
25.
|
Frogne T, Benjaminsen RV, Sonne-Hansen K,
Sorensen BS, Nexo E, Laenkholm AV, Rasmussen LM, Riese DJ II, de
Cremoux P, Stenvang P and Lykkesfeldt AE: Activation of ErbB3, EGFR
and Erk is essential for growth of human breast cancer cell lines
with acquired resistance to fulvestrant. Breast Cancer Res Treat.
114:263–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Krajewski S, Blomqvist C, Franssila K,
Krajewska M, Wasenius VM, Niskanen E, Nordling S and Reed JC:
Reduced expression of proapoptotic gene BAX is associated with poor
response rates to combination chemotherapy and shorter survival in
women with metastatic breast adenocarcinoma. Cancer Res.
55:4471–4478. 1995.
|
|
27.
|
Bottini A, Berruti A, Bersiga A, Brizzi
MP, Brunelli A, Gorzegno G, DiMarco B, Aguggini S, Bolsi G, Cirillo
F, Filippini L, Betri E, Bertoli G, Alquati P and Dogliotti L: p53
but not bcl-2 immunostaining is predictive of poor clinical
complete response to primary chemotherapy in breast cancer
patients. Clin Cancer Res. 6:2751–2758. 2000.PubMed/NCBI
|
|
28.
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
29.
|
Silvestrini R, Veneroni S, Daidone MG,
Benini E, Boracchi P, Mezzetti M, Di Fronzo G, Rilke F and Veronesi
U: The Bcl-2 protein: a prognostic indicator strongly related to
p53 protein in lymph node-negative breast cancer patients. J Natl
Cancer Inst. 86:499–504. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Benbrook DM and Masamha CP: The
pro-survival function of Akt kinase can be overridden or altered to
contribute to induction of apoptosis. Curr Cancer Drug Targets.
11:586–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Frogne T, Jepsen JS, Larsen SS, Fog CK,
Brockdorff BL and Lykkesfeldt AE: Antiestrogen-resistant human
breast cancer cells require activated protein kinase B/Akt for
growth. Endocr Relat Cancer. 12:599–614. 2005. View Article : Google Scholar : PubMed/NCBI
|