Contents
Introduction
FAT family
Processing of FAT proteins
Signaling and function of FAT1 and FAT4
Cancer genomics of FAT family genes
Conclusion
Perspectives
Introduction
Drosophila mutants of the fat,
discs large (dlg), lethal giant larvae
(lgl), warts, scribble, salvador and
hippo genes show tissue overgrowth (1–7).
Overgrowth mutants of fat, warts, salvador and
hippo are characterized by hyperplastic tumors mostly
retaining single-layered epithelial structure, whereas those of
scribble, dlg and lgl are characterized by neoplastic
tumors losing epithelial structure (8,9).
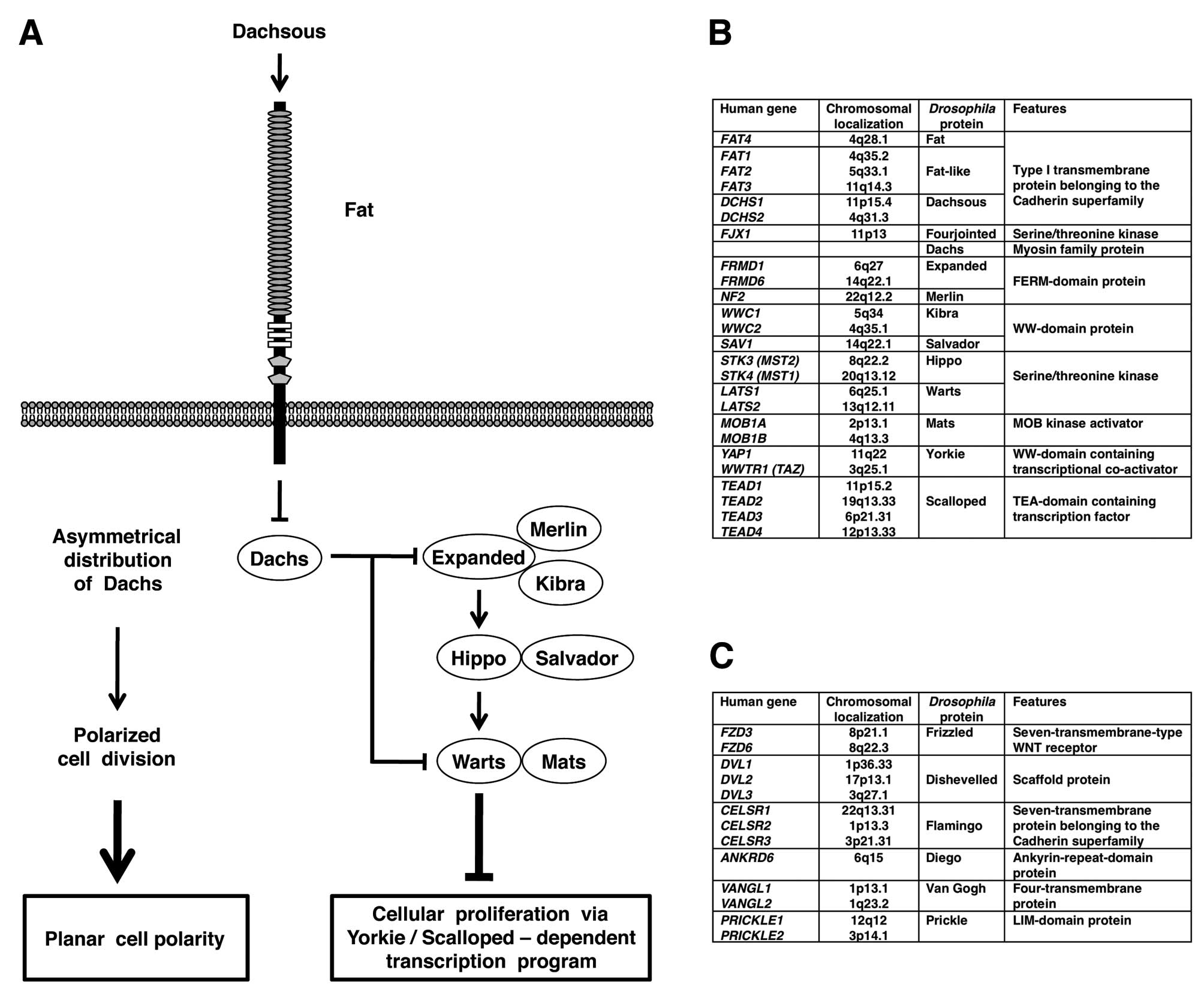
Drosophila fat gene is genetically upstream of the
warts, salvador and hippo genes, which are
involved in the repression of Yokie-Scalloped-dependent
transcription of cyclin E and diap1 genes (10–13).
Because cyclin E and diap1 genes encode cell cycle
accelerator and apoptosis inhibitor, respectively, loss-of-function
mutations of Drosophila fat gene give rise to hyperplastic
tumors through increased cell proliferation and decreased cell
death (Fig. 1A).
In addition to tumor suppression, Drosophila
fat gene is involved in planar call polarity (PCP) (Fig. 1A). PCP is the cell polarity within
the plane of epithelial tissues orthogonal to the apical-basal axis
(14–17). PCP is established as a result of
the asymmetrical localization of the
Flamingo-Frizzled-Dishevelled-Diego complex and the
Flamingo-Strabismus-Prickle complex of adjacent cells via
homophilic interaction of extracellular cadherin-repeat region of
Flamingo. Drosophila frizzled, dishevelled, diego, flamingo
(starry night), strabismus (van Gogh) and prickle genes
encode the core PCP components (18–20),
while Drosophila fat, dachsous, four jointed, discs
overgrown and dachs genes encode the additional or
complementary PCP components (21–24).
Drosophila fat gene encodes a large
transmembrane protein with 34 Cadherin repeats, 4 EGF-like domains
and 2 Laminin G-like domains in the extracellular region (25). Fat protein belongs to the Cadherin
superfamily, which is classified into the classical cadherin
family, Flamingo/Celsr family, Fat/Dachsous family and others
(26,27). Extracellular regions of Fat and
Dachsous cadherins on adjacent cells are reported to preferentially
interact in a heterophilic manner (14,15).
Four jointed and Discs overgrown are serine/threonine kinases that
phosphorylate extracellular domain of Fat in the Golgi and
intracellular domain of Fat in the cytoplasm, respectively, to
promote Fat signaling (21–24).
Heterophilic interaction of Fat and Dachsous cadherins leads to
asymmetrical localization of Dachs myosin; depletion of Dachs in
the Fat side and accumulation of Dachs in the Dachsous side.
Asymmetrical Dachs localization induces PCP through tension
anisotrophy-oriented cell rearrangement as well as tumor
suppression though Hippo-Salvador-Warts signaling-mediated Yorkie
repression (Fig. 1A).
Drosophila components of Fat-Hippo and
Fat-PCP signaling cascades are well conserved in mammals,
especially in human (Fig. 1B and
C). Although precise mechanisms of the Fat-Hippo and Fat-PCP
signaling cascades are not completely elucidated, growing pieces of
evidence indicate the involvement of the mammalian FAT signaling
cascades in embryogenesis and carcinogenesis. In this report,
function and cancer genomics of the human FAT family members are
reviewed.
FAT family
The human FAT gene family consists of the
FAT1, FAT2, FAT3 and FAT4 genes
(28–31). Dunne et al reported complete
coding sequence of FAT1 in 1995. Wu and Maniatis reported
complete coding sequence of FAT2 in 2000. Höng et al
reported partial coding sequence of FAT3 in 2004. We
reported complete coding sequence of FAT3 and FAT4 in
2006. The FAT1 and FAT3 genes adjoin the
MTNR1A and MTNR1B genes, respectively. FAT1 is
most homologous to FAT3, while MTNR1A is most
homologous to MTNR1B. These facts clearly indicate that the
FAT1-MTNR1A locus on human chromosome 4q35.2 and the
FAT3-MTNR1B locus on human chromosome 11q14.3 are
paralogous regions within the human genome (31).
Human FAT family genes as well as
Drosophila Fat family genes encode large proteins with
extracellular Cadherin repeats, EGF-like domains, and Laminin
G-like domain(s). Codon 275–352 of FAT2 is homologous to the third
Cadherin repeat of FAT1; however, this region of FAT2 was not
predicted as the Cadherin repeat using the conserved domain search
(CDS) program of NCBI. Codon 3790–3828 of FAT1 and codon 3799–3834
of FAT3 are distantly related to the EGF-like domain; however,
these regions were not predicted as the EGF-like domain using the
CDS program. Because Cadherin repeat and EGF-like domain are
defined in a low-stringent manner, it is ambiguous at present
whether regions distantly related to Cadherin repeat and EGF-like
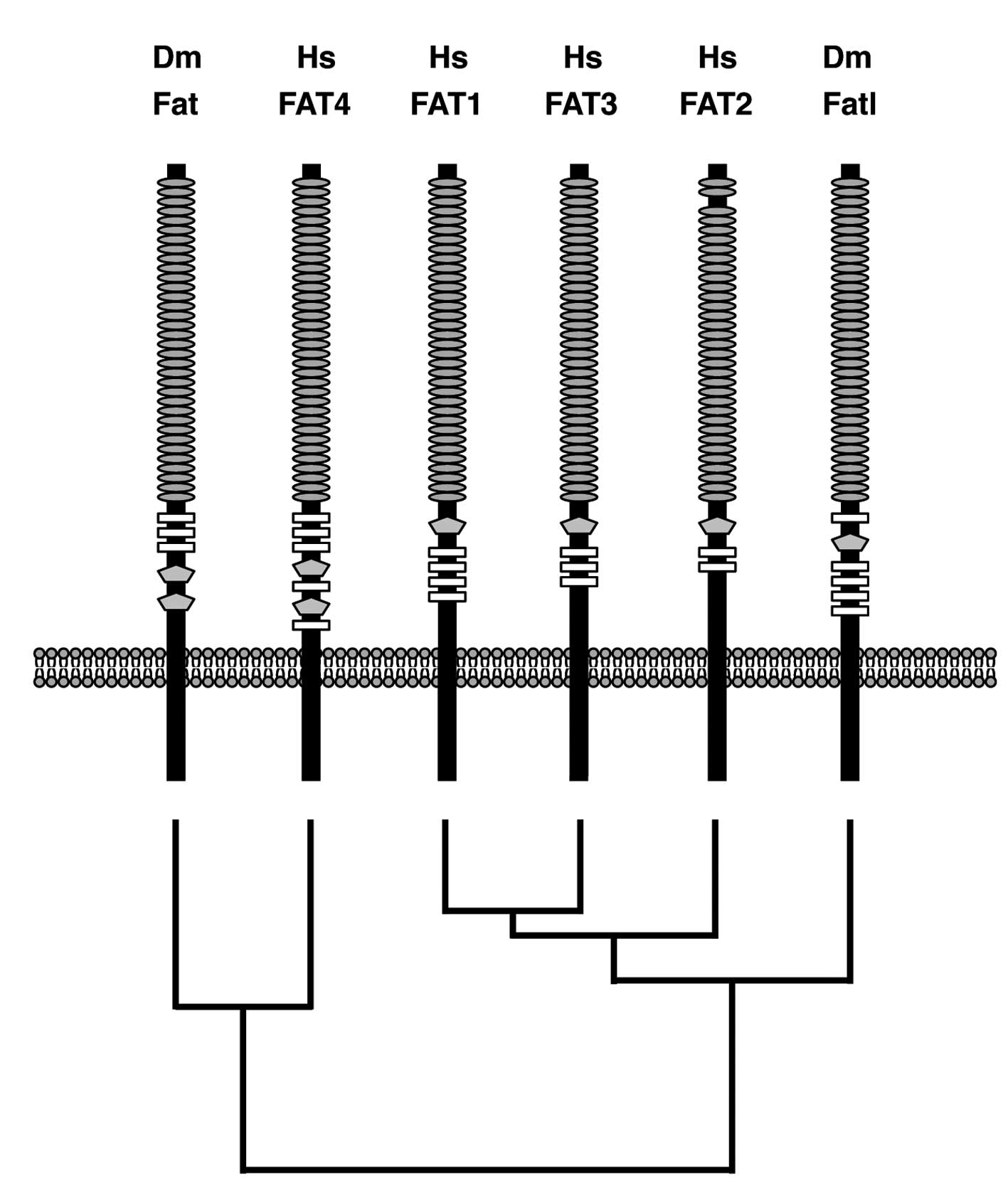
domain are functional or not. Domain architectures of human FAT1,
FAT2 FAT3, FAT4 and Drosophila Fat and Fat-like (Fatl) were
illustrated based on the results of the CDS program using each
RefSeq as a query sequence. Domain-architecture topologies of the
region between Cadherin repeats and the transmembrane domain of
human FAT1, FAT2, FAT3 and Drosophila Fatl are a
Laminin-G-like domain followed by multiple EGF-like domains,
whereas those of human FAT4 and Drosophila Fat are multiple
EGF-like domains followed by two Laminin-G-like domains (Fig. 2). Phylogenetic analyses on human
and Drosophila FAT family proteins revealed that only FAT4
is located within the same branch as Drosophila Fat
(Fig. 2). Together, these facts
indicate that human FAT1, FAT2 and FAT3 are orthologs of
Drosophila Fatl, and that human FAT4 is the ortholog of
Drosophila Fat.
Processing of FAT proteins
FAT1 and FAT4 undergo the first proteolytic cleavage
in the extracellular region by Furin during their maturation step,
which gives rise to non-covalent heteodimer consisting of a larger
subunit corresponding to the most part of the extracellular region
and a smaller subunit containing the transmembrane and cytoplasmic
regions (22,32). Artificial FAT proteins undergo the
second proteolytic cleavage by γ-secretase and the release of
intracellular region, which is similar to the ligand-dependent
processing of NOTCH receptors (33). However, evidence of the
ligand-dependent second cleavage of endogenous FAT proteins remains
unclear.
Signaling and function of FAT1 and FAT4
Dachsous1 (DCHS1) and Dachsous2 (DCHS2) are
mammalian orthologs of Drosophila Dachsous (Fig. 1B); however, heterophilic
interaction between extracellular regions of FAT1 and Dachsous1/2
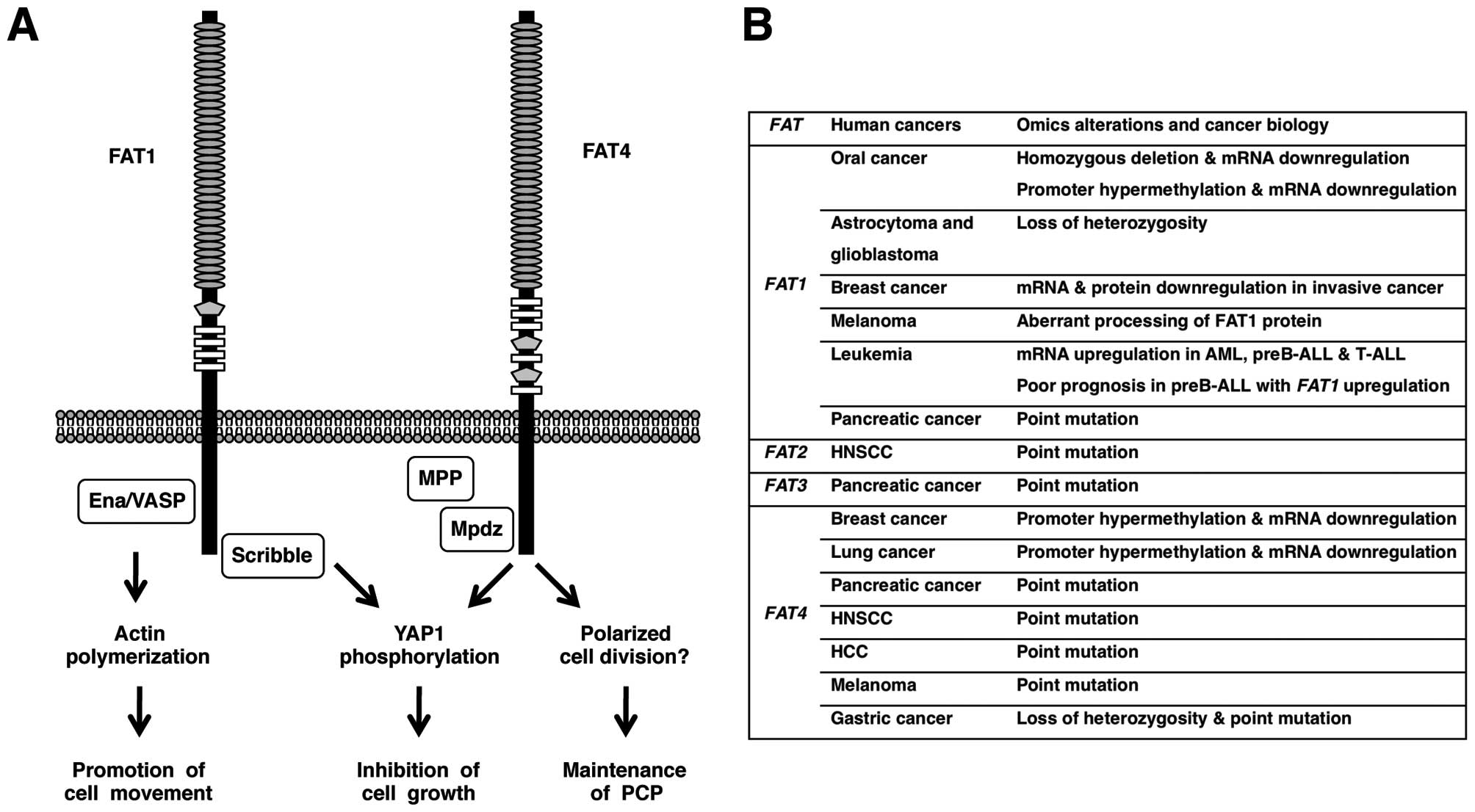
remains unknown. On the other hand, intracellular region of FAT1
directly interacts with Ena/VASP, HOMER, KIF5C and Scribble
proteins (34–37). Ena/VASP and HOMER are EVH1-domain
proteins binding to the cytoplasmic FPPPPEDF motif of Fat1 in a
mutually competitive manner. Because Ena/VASP proteins inhibit
actin capping and induce actin polymerization, Fat1-mediated
recruitment of Ena/VAPS proteins to the leading edge of
lamellipodia and the tip of filopodia results in the promotion of
cell migration (34,35). Scribble proteins are scaffold
proteins with multiple PDZ domains binding to the C-terminal HTEV
motif of Fat1. Fat1 and Scribble are synergistically involved in
the suppression of cystogenesis phenotype through the inhibition of
Yap1 signaling (37). Fat1
knockdown in vascular smooth muscle cells results in decreased
migration and enhanced proliferation (38). FAT1 is involved in promotion of
actin-mediated cell migration as well as inhibition of
YAP1-mediated cell proliferation (Fig.
3A).
Fat4 heterophilically interacts with Dachsous1 at
the apical portion of cell-cell boundaries of neural progenitor
cells, where intracellular region of Fat4 directly interacts with
Mpdz/Mupp1-Mpp5/Pals1 complex (39). Mpp1, Mpp2, Mpp3, Mpp4, Mpp5,
Mpp6/Pals2 and Mpp7 are membrane-associated guanylate kinase
(MAGUK) homologs of Drosophila Stardust (Sdt), which is
involved in the maintenance of apicobasal polarity in epithelial
tissues (40). Fat4
knockout mice die at birth, which are manifested by stereocilia
disorientation in the inner ear, loop tail, broader neural tube and
renal cysts (41). Disorientation
of cochlear hair cells is the typical phenotype of the mammalian
PCP defect in Vangl2, Celsr1 or Dvl1/Dvl2
mutant mice (42). Loop tail and
neural tube abnormalities are also observed in Vangl2 mutant
mice (43) and renal cystogenesis
is synergistically enhanced in
Fat4−/−Vangl2−/+ mice (42). Fat4 knockdown in neural tube
results in an increase of a subset of neural progenitors and
differentiated Lim1+/Lim2+ neurons via
downregulation of Yap1 phosphorylation (44). FAT4 is involved in the maintenance
of PCP as well as inhibition of YAP1-mediated cell proliferation
(Fig. 3A).
Cancer genomics of FAT family
genes
The human FAT1 gene is homozygously deleted
in 23% of oral cancer cell lines and in 80% of primary oral cancer
cases and FAT1 mRNA expression is repressed in oral cancer
cell lines due to homozygous deletion and/or promoter CpG
hypermethylation (45). Loss of
heterozygosity (LOH) of the FAT1 gene occurs in 42% of low
grade diffuse astrocytoma and 63% of glioblastoma multiforme
(46). FAT1 mRNA level in
ductal carcinoma in situ is significantly higher than that
in invasive breast cancer and FAT1 knockdown promotes
progression from ductal carcinoma in situ to invasive breast
cancer (47). FAT1 mRNA
expression is upregulated in 11% of acute myeloid leukemia (AML),
29% of preB acute lymphoblastic leukemia (ALL) and 63% of T-ALL,
and FAT1 upregulation in preB-ALL is associated with shorter
relapse-free survival as well as shorter overall survival (48). FAT1 immunoreactivity is strong in
29% of cholangiocarcinoma (49).
The mouse Fat3 mRNA is significantly
downregulated in lung adenocarcinoma occurred in transgenic mice
expressing wild-type Raf1 transgene under the control of the
human SP-C (surfactant protein C) promoter (50).
The mouse Fat4 gene is inactivated owing to
LOH and promoter CpG hypermethylation in subcutaneous tumor induced
by Cre/LoxP-mediated random chromosomal deletion (51). Tumor growth is inhibited by
re-introduction of Fat4 gene into cells derived from the
cutaneous tumor. Relative YAP1 activity is significantly
upregulated as a result of Fat4 repression.
The human FAT4 mRNA expression is repressed
in 3 out of 6 breast cancer cell lines and in 3 out of 5 cases of
primary breast cancers, partially due to promoter CpG
hypermethylation (51).
FAT4 promoter is hypermethylated in 7 out of 18 cases of
lung adenocarcinoma (stage I) and FAT4 mRNA is downregulated
in 18 out of 23 cases of non-small cell lung tumors (stage I or II)
(52).
Using the whole-exome sequencing approach,
non-synonymous mutations of human FAT1, FAT3 and
FAT4 genes are detected in 1 each, and 2 out of 24
pancreatic cancer samples, respectively (53). Non-synonymous mutations of human
FAT2 and FAT4 genes are detected in 1 and 2 out of 32
cases of head and neck squamous cell carcinoma (HNSCC),
respectively (54). Non-synonymous
FAT4 mutation is detected in 1 out of 10 cases of
hepatocellular carcinoma using the whole-exome sequencing approach
(55). Non-synonymous FAT4
mutations are also detected in 4 out of 6 cases of melanomas using
the whole-exome sequencing approach and in 2 out of additional 9
cases of melanomas using the candidate-exons sequencing approach
(56). Non-synonymous FAT4
mutations are detected in 2 out of 15 cases of gastric cancers
using the whole-exome approach and in 4 out of additional 95 cases
of gastric cancers using the candidate-exon approach (57). Among the human FAT gene
family, FAT4 gene is recurrently mutated in several types of
human cancers, such as melanoma (40%), pancreatic cancer (8%),
HNSCC (6%) and gastric cancer (5%).
Conclusion
FAT1 is downregulated in oral cancer and
invasive breast cancer due to deletion and/or epigenetic silencing,
whereas FAT1 is upregulated in leukemia and prognosis of
preB-ALL with FAT1 upregulation is poor. FAT4 is
mutated in several types of human cancer, such as melanoma,
pancreatic cancer and gastric cancer (Fig. 3B). FAT1 and FAT4 suppress tumor
growth through Hippo signaling activation, while FAT1 promotes
tumor migration through actin polymerization at lamellipodia and
filopodia. Together, these facts indicate that FAT1 is tumor
suppressive or oncogenic in a context-dependent manner and that
FAT4 is preferentially tumor suppressive.
Perspectives
Drosophila Fat is involved in the tumor
suppression via phosphorylation-mediated functional inhibition of
Yorkie through indirect activation of the Expanded-Hippo-Warts
signaling cascade (Fig. 1A).
Expanded interacts with Merlin and Kibra to activate the Hippo
signaling cascade, while Salvador and Mats are involved in the
regulation of Hippo and Warts kinases, respectively (Fig. 1A). FRMD1 and FRMD6 are human
orthologs of Drosophila Expanded; NF2 is the human ortholog
of Drosophila Merlin; WWC1 and WWC2 are human orthologs of
Drosophila Kibra; SAV1 is the human ortholog of
Drosophila Salvador; STK3 and STK4 are human orthologs of
Drosophila Hippo; MOB1A and MOB1B are human orthologs of
Drosophila Mats; LATS1 and LATS2 are human orthologs of
Drosophila Warts; YAP1 and WWTR1 (TAZ) are human orthologs
of Drosophila Yorkie (Fig.
1B). Copy number aberration, translocation and point mutation
of human FAT1, FAT2, FAT3, FAT4, FRMD1, FRMD6, NF2, WWC1, WWC2,
SAV1, STK3, STK4, MOB1A, MOB1B, LATS1, LATS2, YAP1 and
WWTR1 genes should be comprehensively investigated in
various types of human cancers using high-throughput sequencing
technology to elucidate the mutation landscape of the FAT-Hippo
signaling cascades.
YAP1 and WWTR1 directly interact with β-catenin and
Hippo signaling-induced phosphorylation of YAP1 results in the
inhibition of the canonical WNT signaling cascade (58). WNT signaling cascades crosstalk
with FGF, Notch, Hedgehog and TGFβ/BMP signaling cascades to
constitute the stem-cell signaling network (59). Because Hippo-YAP1/WWTR1 signaling
cascade is located at the crossroads of adhesion signaling,
G-protein-coupled receptor (GPCR) signaling, receptor tyrosine
kinase (RTK) signaling and stem cell biology (12,60–62),
cancer genomics of the FAT signaling cascades could be applied for
diagnostics, prognostics and therapeutics in the era of
personalized medicine.
Acknowledgements
This study was supported in part by
National Cancer Center Research and Development Fund.
References
|
1.
|
PJ BryantB HuettnerLI Held JrJ RyerseJ
SzidonyaMutations at the fat locus interfere with cell
proliferation control and epithelial morphogenesis in
DrosophilaDev Biol1295415541988
|
|
2.
|
DF WoodsPJ BryantThe discs-large
tumor suppressor gene of Drosophila encodes a guanylate
kinase homolog localized at septate junctionsCell664514641991
|
|
3.
|
D StrandI RaskaBM MechlerThe Drosophila
lethal(2) giant larvae tumor suppressor protein is a component
of the cytoskeletonJ Cell Biol127134513601994
|
|
4.
|
RW JusticeO ZilianDF WoodsM NollPJ
BryantThe Drosophila tumor suppressor gene warts
encodes a homolog of human myotonic dystrophy kinase and is
required for the control of cell shape and proliferationGenes
Dev95345461995
|
|
5.
|
D BilderN PerrimonLocalization of apical
epithelial determinants by the basolateral PDZ protein
ScribbleNature403676680200010.1038/3500110810688207
|
|
6.
|
N TaponKF HarveyDW Bellsalvador
promotes both cell cycle exit and apoptosis in Drosophila
and is mutated in human cancer cell
linesCell110467478200210.1016/S0092-8674(02)00824-3
|
|
7.
|
S PantalacciN TaponP LéopoldThe Salvador
partner Hippo promotes apoptosis and cell-cycle exit in
DrosophilaNat Cell
Biol5921927200310.1038/ncb105114502295
|
|
8.
|
PJ BryantKL WatsonRW JusticeDF WoodsTumor
suppressor genes encoding proteins required for cell interactions
and signal transduction in DrosophilaDev
Suppl23924919938049479
|
|
9.
|
IK HariharanD BilderRegulation of imaginal
disc growth by tumor-suppressor genes in DrosophilaAnnu Rev
Genet40335361200610.1146/annurev.genet.39.073003.10073816872256
|
|
10.
|
LJ SaucedoBA EdgarFilling out the Hippo
pathwayNat Rev Mol Cell Biol8613621200710.1038/nrm222117622252
|
|
11.
|
BV ReddyKD IrvineThe Fat and Warts
signaling pathways: new insights into their regulation, mechanism
and
conservationDevelopment13528272838200810.1242/dev.02097418697904
|
|
12.
|
D PanThe hippo signaling pathway in
development and cancerDev
Cell19491505201010.1016/j.devcel.2010.09.01120951342
|
|
13.
|
A GenevetN TaponThe Hippo pathway and
apico-basal cell polarityBiochem
J436213224201110.1042/BJ2011021721568941
|
|
14.
|
CH YangJD AxelrodMA SimonRegulation of
Frizzled by fat-like cadherins during planar polarity signaling in
the Drosophila compound
eyeCell108675688200210.1016/S0092-8674(02)00658-X11893338
|
|
15.
|
H StruttD StruttNonautonomous planar
polarity patterning in Drosophila: dishevelled-independent
functions of frizzledDev
Cell3851863200210.1016/S1534-5807(02)00363-512479810
|
|
16.
|
JD AxelrodProgress and challenges in
understanding planar cell polarity signalingSemin Cell Dev
Biol20964971200910.1016/j.semcdb.2009.08.00119665570
|
|
17.
|
A DjianeM MlodzikThe Drosophila
GIPC homologue can modulate myosin based processes and planar cell
polarity but is not essential for developmentPLoS
One5e112282010
|
|
18.
|
JD AxelrodJR MillerJM ShulmanRT MoonN
PerrimonDifferential recruitment of Dishevelled provides signaling
specificity in the planar cell polarity and Wingless signaling
pathwaysGenes Dev1226102622199810.1101/gad.12.16.26109716412
|
|
19.
|
M MlodzikPlanar cell polarization: do the
same mechanisms regulate Drosophila tissue polarity and
vertebrate gastrulation?Trends
Genet18564571200210.1016/S0168-9525(02)02770-112414186
|
|
20.
|
M KatohWNT/PCP signaling pathway and human
cancer (Review)Oncol Rep1415831588200516273260
|
|
21.
|
HO IshikawaH TakeuchiRS HaltiwangerKD
IrvineFour-jointed is a Golgi kinase that phosphorylates a subset
of cadherin
domainsScience321401404200810.1126/science.115815918635802
|
|
22.
|
R SopkoH McNeillThe skinny on Fat: an
enormous cadherin that regulates cell adhesion, tissue growth, and
planar cell polarityCurr Opin Cell
Biol21717723200910.1016/j.ceb.2009.07.00119679459
|
|
23.
|
C ThomasD StruttThe roles of the cadherins
Fat and Dachsous in planar polarity specification in
DrosophilaDev Dyn2412739201210.1002/dvdy.2273621919123
|
|
24.
|
F BosveldI BonnetB GuiraoMechanical
control of morphogenesis by Fat/Dachsous/Four-jointed planar cell
polarity
pathwayScience336724727201210.1126/science.122107122499807
|
|
25.
|
PA MahoneyU WeberP OnofrechukH BiessmannPJ
BryantCS GoodmanThe fat tumor suppressor gene in
Drosophila encodes a novel member of the cadherin gene
superfamilyCell678538681991
|
|
26.
|
T TanoueM TakeichiNew insights into Fat
cadherinsJ Cell Sci11823472353200510.1242/jcs.0239815923647
|
|
27.
|
P HulpiauF van RoyMolecular evolution of
the cadherin superfamilyInt J Biochem Cell
Biol41349369200910.1016/j.biocel.2008.09.027
|
|
28.
|
J DunneAM HanbyR PoulsomMolecular cloning
and tissue expression of FAT, the human homologue of the
Drosophila fat gene that is located on chromosome 4q34-q35
and encodes a putative adhesion moleculeGenomics302072231995
|
|
29.
|
Q WuT ManiatisLarge exons encoding
multiple ectodomains are a characteristic feature of protocadherin
genesProc Natl Acad Sci
USA9731243129200010.1073/pnas.97.7.312410716726
|
|
30.
|
JC HöngNV IvanovP HodorIdentification of
new human cadherin genes using a combination of protein motif
search and gene finding methodsJ Mol Biol337307317200415003449
|
|
31.
|
Y KatohM KatohComparative integromics on
FAT1, FAT2, FAT3 and FAT4Int J Mol
Med185235282006
|
|
32.
|
E SadeqzadehCE de BockXD ZhangDual
processing of FAT1 cadherin protein by human melanoma cells
generates distinct protein productsJ Biol
Chem2862818128191201110.1074/jbc.M111.23441921680732
|
|
33.
|
T MaggD SchreinerGP SolisEG BadeHW
HoferProcessing of the human protocadherin Fat1 and translocation
of its cytoplasmic domain to the nucleusExp Cell
Res307100108200510.1016/j.yexcr.2005.03.00615922730
|
|
34.
|
T TanoueM TakeichiMammalian Fat1 cadherin
regulates actin dynamics and cell-cell contactJ Cell
Biol165517528200410.1083/jcb.20040300615148305
|
|
35.
|
MJ MoellerA SoofiGS BraunProtocadherin
FAT1 binds Ena/VASP proteins and is necessary for actin dynamics
and cell polarizationEMBO
J2337693779200410.1038/sj.emboj.760038015343270
|
|
36.
|
D SchreinerK MüllerHW HoferThe
intracellular domain of the human protocadherin hFat1 interacts
with Homer signalling scaffolding proteinsFEBS
Lett58052955300200610.1016/j.febslet.2006.08.07916979624
|
|
37.
|
K SkouloudakiM PuetzM SimonsScribble
participates in Hippo signaling and is required for normal
zebrafish pronephros developmentProc Natl Acad Sci
USA10685798584200910.1073/pnas.081169110619439659
|
|
38.
|
R HouL LiuS AneesS HiroyasuNE SibingaThe
Fat1 cadherin integrates vascular smooth muscle cell growth and
migration signalsJ Cell
Biol173417429200610.1083/jcb.20050812116682528
|
|
39.
|
T IshiuchiK MisakiS YonemuraM TakeichiT
TanoueMammalian Fat and Dachsous cadherins regulate apical membrane
organization in the embryonic cerebral cortexJ Cell
Biol185959967200910.1083/jcb.20081103019506035
|
|
40.
|
M KatohM KatohIdentification and
characterization of human MPP7 gene and mouse Mpp7
gene in silicoInt J Mol Med133333382004
|
|
41.
|
S SaburiI HesterE FischerLoss of
Fat4 disrupts PCP signaling and oriented cell division and
leads to cystic kidney diseaseNat Genet40101010152008
|
|
42.
|
C JonesP ChenPlanar cell polarity
signaling in
vertebratesBioessays29120132200710.1002/bies.2052617226800
|
|
43.
|
Z KibarKJ VoganN GroulxMJ JusticeDA
UnderhillP GrosLtap, a mammalian homolog of Drosophila
Strabismus/Van Gogh, is altered in the mouse neural tube mutant
Loop-tailNat Genet28251255200110.1038/90081
|
|
44.
|
NJ van HaterenRM DasGM HautbergueAG
BoryckiM PlaczekSA WilsonFatJ acts via the Hippo mediator Yap1 to
restrict the size of neural progenitor cell
poolsDevelopment13818931902201121521736
|
|
45.
|
K NakayaHD YamagataN AritaIdentification
of homozygous deletions of tumor suppressor gene FAT in oral
cancer using
CGH-arrayOncogene2653005308200710.1038/sj.onc.121033017325662
|
|
46.
|
K ChosdolA MisraS PuriFrequent loss of
heterozygosity and altered expression of the candidate tumor
suppressor gene ‘FAT’ in human astrocytic tumorsBMC
Cancer95200910.1186/1471-2407-9-519126244
|
|
47.
|
S LeeS StewartI NagtegaalDifferentially
expressed genes regulating the progression of ductal carcinoma in
situ to invasive breast cancerCancer
Res7245744586201210.1158/0008-5472.CAN-12-063622751464
|
|
48.
|
CE de BockA ArdjmandTJ MolloyThe Fat1
cadherin is overexpressed and an independent prognostic factor for
survival in paired diagnosis-relapse samples of precursor B-cell
acute lymphoblastic leukemiaLeukemia26918926201222116550
|
|
49.
|
J SettakornN KaewpilaGF BurnsAS LeongFAT,
E-cadherin, β-catenin, HER 2/neu, Ki67 immuno-expression, and
histological grade in intrahepatic cholangiocarcinomaJ Clin
Pathol58124912542005
|
|
50.
|
A RohrbeckJ BorlakCancer genomics
identifies regulatory gene networks associated with the transition
from dysplasia to advanced lung adenocarcinomas induced by
c-Raf-1PLoS One4e7315200910.1371/journal.pone.000731519812696
|
|
51.
|
C QiYT ZhuL HuYJ ZhuIdentification of
Fat4 as a candidate tumor suppressor gene in breast
cancersInt J Cancer1247937982009
|
|
52.
|
TA RauchZ WangX WuKH KernstineAD RiggsGP
PfeiferDNA methylation biomarkers for lung cancerTumour
Biol33287296201210.1007/s13277-011-0282-222143938
|
|
53.
|
S JonesX ZhangDW ParsonsCore signaling
pathways in human pancreatic cancers revealed by global genomic
analysesScience32118011806200810.1126/science.116436818772397
|
|
54.
|
N AgrawalMJ FrederickCR PickeringExome
sequencing of head and neck squamous cell carcinoma reveals
inactivating mutations in
NOTCH1Science33311541157201110.1126/science.120692321798897
|
|
55.
|
M LiH ZhaoX ZhangInactivating mutations of
the chromatin remodeling gene ARID2 in hepatocellular
carcinomaNat Genet43828829201110.1038/ng.903
|
|
56.
|
SI NikolaevD RimoldiC IseliExome
sequencing identifies recurrent somatic MAP2K1 and
MAP2K2 mutations in melanomaNat
Genet44133139201110.1038/ng.1026
|
|
57.
|
ZJ ZangI CutcutacheSL PoonExome sequencing
of gastric adenocarcinoma identifies recurrent somatic mutations in
cell adhesion and chromatin remodeling genesNat
Genet44570574201210.1038/ng.224622484628
|
|
58.
|
M ImajoK MiyatakeA IimuraA MiyamotoE
NishidaA molecular mechanism that links Hippo signalling to the
inhibition of Wnt/β-catenin signallingEMBO
J3111091122201222234184
|
|
59.
|
M KatohM KatohWNT signaling pathway and
stem cell signaling networkClin Cancer
Res1340424045200710.1158/1078-0432.CCR-06-231617634527
|
|
60.
|
FX YuB ZhaoN PanupinthuRegulation of the
Hippo-YAP pathway by G-Protein-coupled receptor
signalingCell150780791201210.1016/j.cell.2012.06.03722863277
|
|
61.
|
W HuangX LvC LiuThe N-terminal
phosphodegron targets TAZ/WWTR1 protein for SCF β-TrCP-dependent
degradation in response to phosphatidylinositol 3-kinase
inhibitionJ Biol Chem2872624526253201222692215
|
|
62.
|
M CordenonsiF ZanconatoL AzzolinThe Hippo
transducer TAZ confers cancer stem cell-related traits on breast
cancer cellsCell147759772201110.1016/j.cell.2011.09.04822078877
|