Introduction
Extrahepatic bile duct cancer (EHBDCA), consisting
of hilar cholangiocarcinoma and distal bile duct adenocarcinoma
(excluding gallbladder cancer), is a rare disease in the United
States with an incidence of 1–2/100,000/year (1). It occurs with great frequency in
Asian countries, and is one of the common causes of cancer death in
Japan, with near to 17,000 deaths annually (2). The 5-year survival rate of EHBDCA,
even after the surgical resection is poor, ranging from 20 to 45%
(3–5). The incidence of EHBDCA is increasing
throughout the world with a high fatality rate; therefore, new
prognostic markers and treatment for EHBDCA patients are urgently
needed.
Mesothelin is expressed on normal mesothelial cells
lining the pleura, pericardium and peritoneum (6,7). In
addition, the overexpression of mesothelin has been found in
several cancer types, including malignant mesothelioma, ovarian
cancer and pancreatic cancer (8–11,12).
The full length of human mesothelin gene codes the primary
product, which is a 71-kDa precursor protein. This protein can be
physiologically cleaved by certain furin-like proteases into a
40-kDa C-terminal fragment that remains membrane-bound and a
31-kDa N-terminal fragment, which is secreted into the blood
(6). The C-terminal 40-kDa
fragment is named mesothelin and is attached to the cell membrane
through a glycosyl-phosphatidylinositol (GPI) anchor (13). The biological functions of
mesothelin are not clearly understood, although recent studies have
suggested that enforced expression of mesothelin increases cell
proliferation and migration (14).
In ovarian cancers, higher mesothelin expression was found to be
associated with chemoresistance and shorter patient survival
(15). In pancreatic cancer,
mesothelin expression was immunohistochemically observed in all
cases, while its absence was noted in non-cancerous pancreatic
ductal epithelium, with or without pancreatitis (8,12,16,17).
We recently found that the expression of mesothelin was related to
an unfavorable patient outcome in pancreatic ductal adenocarcinoma
(12), while the opposite result
was reported in gastric cancer, in which the mesothelin expression
was correlated with prolonged patients’ survival (18). However, our consecutive
investigation for mesothelin expression patterns in gastric cancer
recently discovered that luminal membrane expression, not
cytoplasmic expression of mesothelin is a prominent negative
prognostic factor for gastric cancer (19), suggesting the significance of
expression pattern of mesothelin in clinicopathological analysis of
cancer. In EHBDCA, Zhao et al, who first studied mesothelin
expression in dysplasia and carcinoma of external bile duct,
reported that mesothelin was expressed in 5 of 10 adenocarcinomas
(50%) in cell membranes and cytoplasm (20); however, the detailed
clinicopathological analysis of mesothelin expression in EHBDCA,
especially with large number of the cases, has not yet been
performed.
In this study, we investigated the mesothelin
expression in 61 EHBDCA cases by immunohistochemistry, and its
clinico-pathological significance associated with patients’ outcome
was analyzed. Moreover, we focused on the intracellular
localization of mesothelin, i.e., in luminal membrane and/or
cytoplasm, and its clinicopathological significance associated with
the patients’ outcome.
Materials and methods
Patients’ demography and tumor
specimens
This study was performed with the approval of the
Internal Review Board on Ethical Issues of Hokkaido University
Hospital, Sapporo, Japan. The samples and the patient information
were obtained under a blanket written informed consent. The
subjects of this study were 61 patients who underwent radical
surgery for bile duct adenocarcinoma between the years 2000 and
2008 at Hokkaido University Hospital by the Department of General
Surgery, Hokkaido University, Graduate School of Medicine, Sapporo,
Japan. The clinicopathological characteristics of these cases are
summarized in Table I.
 | Table IClinicopathological characteristics
of 61 patients with EHBDCA in this study. |
Table I
Clinicopathological characteristics
of 61 patients with EHBDCA in this study.
| Parameter | No. of cases |
|---|
| Age (years) | |
| <60 | 11 |
| ≥60 | 50 |
| Mean ± SD | 67.5±9.0 |
| Gender | |
| Male | 47 |
| Female | 14 |
| Location | |
| Hilar | 16 |
| Upper | 17 |
| Middle | 20 |
| Distal | 8 |
| Surgical
procedure | |
|
Pancreatoduodenectomy | 21 |
|
Pylorus-preserving
pancreatoduodenectomy | 5 |
| Extended right or
left hemihepatectomy with bile duct resection | 28 |
| Extrahepatic bile
duct resection | 7 |
| Resection
status | |
| R0 | 39 |
| R1 | 22 |
| T-factor | |
| T1 | 5 |
| T2 | 27 |
| T3 | 19 |
| T4 | 10 |
| N-factor | |
| N0 | 25 |
| N1 | 36 |
| M-factor | |
| M0 | 58 |
| M1 | 3 |
| Stage | |
| IA | 4 |
| IB | 14 |
| IIA | 4 |
| IIB | 28 |
| III | 8 |
| IV | 3 |
| Median survival
(months) | 29.8±3.5 |
Mean age of patients was 67.5 years [±9.0 standard
deviation (SD)]; 47 patients (77.0%) were male and 14 patients
(23.0%) were female. The predominant sites of the cancer were the
hilar bile duct in 16 cases (26.2%), upper bile duct in 17 cases
(27.9%), middle bile duct in 20 cases (32.8%) and distal bile duct
in 8 cases (13.1%). The surgical procedures consisted of the
standard pancreatoduodenectomy in 21 (34.4%) cases, the
pylorus-preserving pancreatoduodenecomy in 5 cases (8.2%), the
extended right or left hemihepatectomy with extrahepatic bile duct
resection in 28 cases (45.9%), and the extrahepatic bile duct
resection in 7 cases (11.5%). Intraoperative diagnosis of the
ductal resection margins was performed using frozen sections. When
a positive margin was found, additional resection of marginal bile
duct was performed to the maximum extent possible. R0 curative
resection was achieved in 39 cases (63.9%), and R1 resection was
achieved in 22 cases (36.1%). T-factor, N-factor, M-factor and
clinical stage were assigned according to the TNM classification of
the Union Internationale Contre le Cancer (UICC) (21). The median survival time of patients
was 29.8 months (±3.5 SD).
Formalin-fixed paraffin-embedded tissue blocks were
prepared from surgical specimens and sections were sliced and
stained with hematoxylin and eosin (H&E) for routine
histopathological examination. All specimens were diagnosed as
EHBDCA.
Immunohistochemical evaluation
Immunohistochemical staining against mesothelin was
performed as described previously (12). In brief, the tissue sections were
incubated with a mouse monoclonal antibody against mesothelin
(clone 5B2 diluted 1:50; Novocastra, Newcastle Upon Tyne, UK) at a
1:50 dilution, and reacted with a dextran polymer reagent combined
with secondary antibodies and peroxidase (Envision/HRP; Dako). All
assessments were made on the tumor region of the specimen (×400).
Each slide was evaluated independently by three pathologists (F.
Kawamata, M. Miyazaki and H. Nishihara) who did not know the
clinical outcomes. Immunostaining for mesothelin was evaluated for
both the proportion and staining intensity of tumor cells in each
case. The proportion of mesothelin expression was assessed
according to the percentage of mesothelin-positive cells as
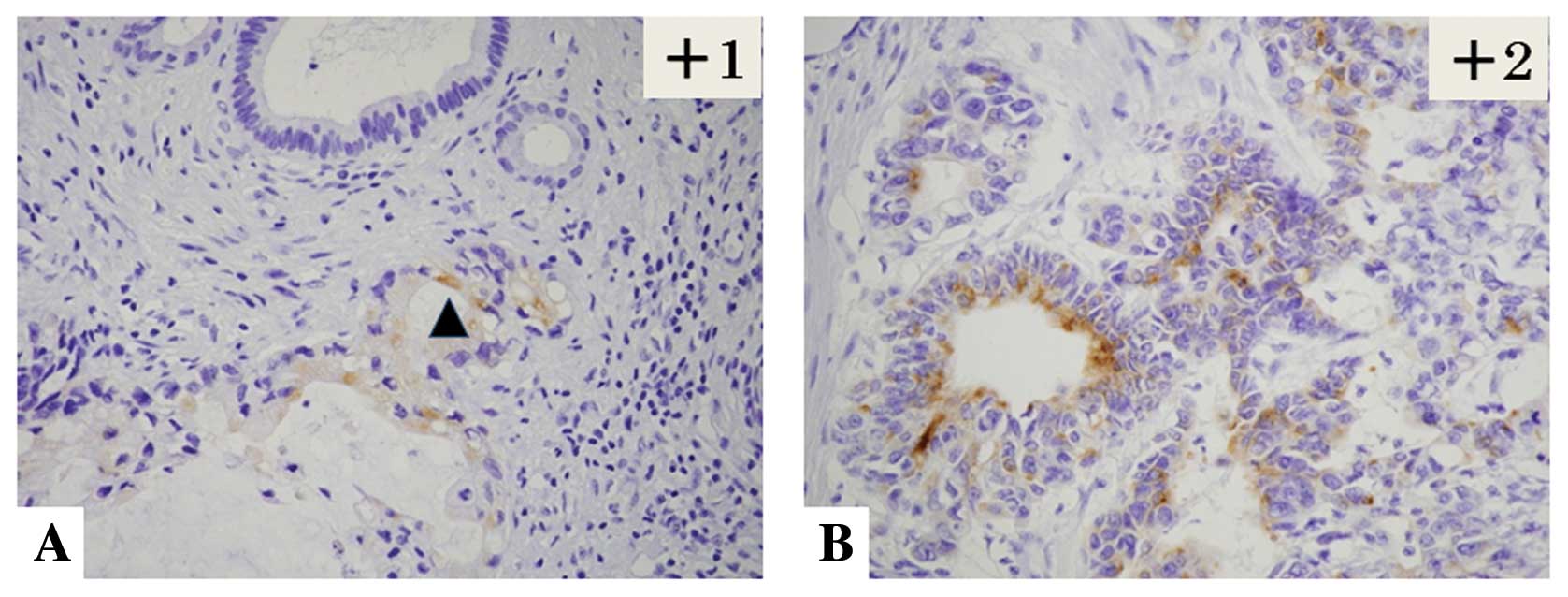
follows: 0, 0%; +1, l<10%; +2, 10–50%; and +3, >50%. The
staining intensity of mesothelin was evaluated as weak (+1) and
moderate to strong (+2) (Table
II). The final evaluation of mesothelin expression was assessed
using the following scoring system: ‘high-level expression’ of
mesothelin was defined as ≥+3 of the proportion score and/or +2 of
the intensity score, while a ‘low-level expression’ of mesothelin
was given when the total score was ≤+3 except in cases when the
proportion score was +1 and the intensity score was +2 (Fig. 1).
 | Table IIImmunohistochemical findings of
mesothelin expression. |
Table II
Immunohistochemical findings of
mesothelin expression.
| Staining intensity
on tumor cells | No. of cases (%)
|
|---|
Percentage of
mesothelin-positive cells
|
|---|
| 0 | 1–10% | 10–50% | >50% |
|---|
| Score 0 | 17 (27.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Score 1 | 0 (0.0) | 13 (21.3) | 2 (3.3) | 1 (1.6) |
| Score 2 | 0 (0.0) | 6 (9.8) | 12
(19.7) | 10
(16.4) |
Furthermore, among the 61 cases of EHBDCA, the
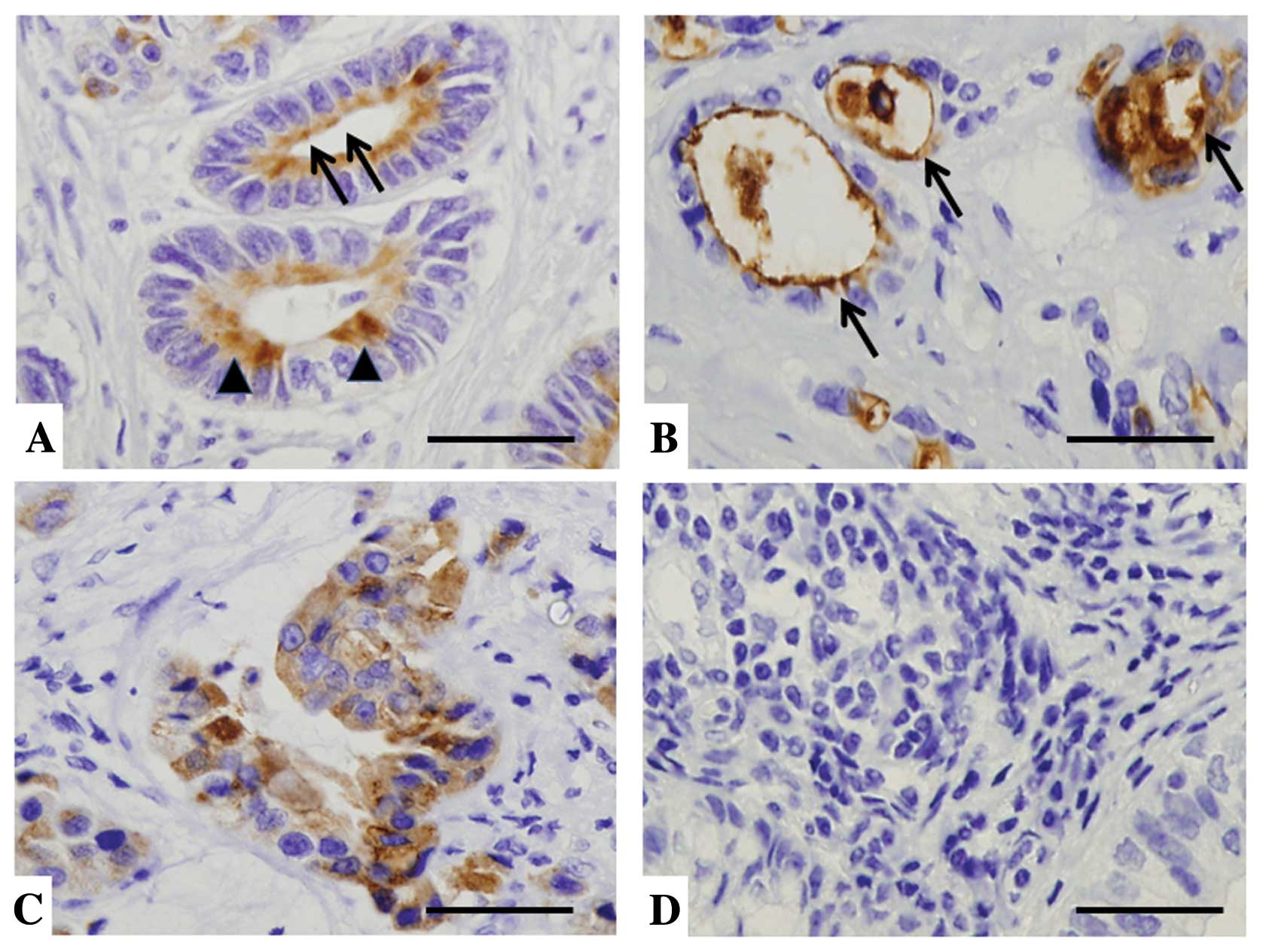
staining localization of mesothelin was evaluated in luminal
membrane or cytoplasm. Cases in which the luminal membrane was
stained even partially or faintly (Fig. 2A), or the entire circumference of
the luminal membrane was explicitly stained (Fig. 2B) were judged as ‘luminal membrane
positive’. In cases with no membrane staining (Fig. 2D) and those in which only
cytoplasmic staining (Fig. 2C) was
observed in any intensity level, the term ‘luminal membrane
negative’ was given.
Statistical analysis
We used the χ2 test or Fisher’s exact
test to determine the correlation between mesothelin and
clinico-pathologic data. Survival curves for patients were drawn by
the Kaplan-Meier method. Differences in survival curves were
analyzed by the log-rank test. Prognostic implications of
mesothelin expression and clinicopathologic parameters were
analyzed by Cox univariate and multivariate proportional hazards
models. All differences were considered significant at a P-value of
<0.05. All statistical analyses were performed using the
Ekuseru-Toukei 2010 software for Windows (Social Survey Research
Information Co., Ltd., Tokyo, Japan).
Results
High-level expression of mesothelin was
correlated with liver metastasis and poor patient outcome
The overexpression of mesothelin has been found in
several cancer types, including malignant mesothelioma, ovarian
cancer, and pancreatic cancer (8–11,12);
thus, we first evaluated the comprehensive expression of mesothelin
in EHBDCA. As described in Materials and methods, ‘high-level
expression’ and ‘low-level expression’ of mesothelin was attributed
to all 61 cases of EHBDCA (Fig.
1). As summarized in Table II,
‘high-level expression’ was detected in 29 cases (47.5%), whereas
‘low-level expression’ was detected in 32 cases (52.5%). The
statistical analysis for the clinicopathological parameters such as
histological grade, T-factor and metastasis revealed that
‘high-level expression’ of mesothelin was significantly correlated
with liver metastasis (P=0.013, Table
III). Furthermore, recent studies reported that higher
mesothelin expression was found to be associated with shorter
patient survival; therefore, we examined the correl ation of
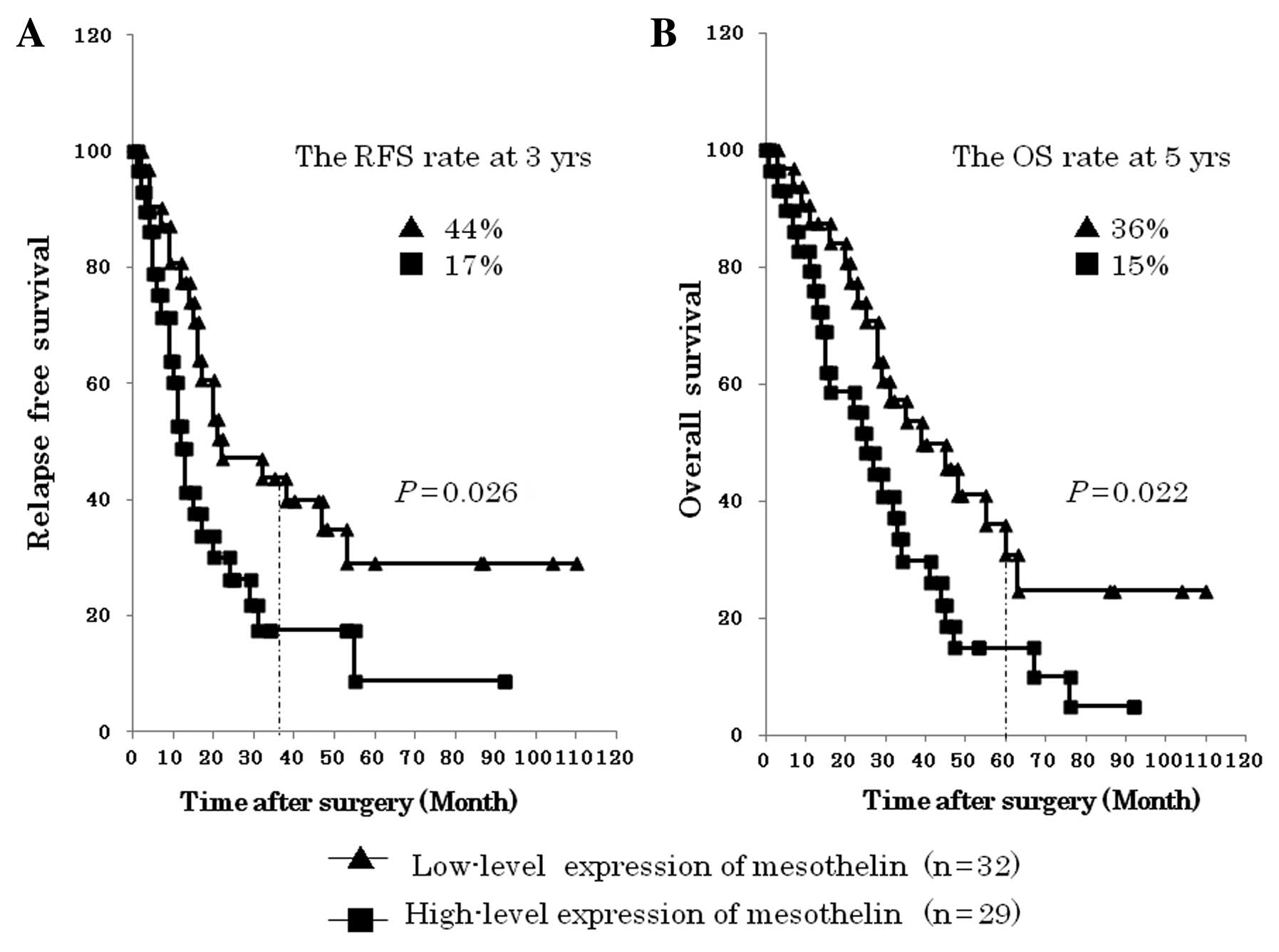
mesothelin overexpression with relapse-free survival (RFS) and
overall survival (OS) in the EHBDCA patients. The group of
‘high-level expression’ of mesothelin had a significantly poorer
RFS than the group of ‘low-level expression‘ of mesothelin
(P=0.026). In addition, the group of ‘high-level expression’ of
mesothelin had a significantly poorer OS than the group of
‘low-level expression’ of mesothelin (P=0.022) (Fig. 3).
 | Table IIICorrelation between mesothelin
expression levels and clinicopathological features. |
Table III
Correlation between mesothelin
expression levels and clinicopathological features.
| | Mesothelin
| Luminal membrane
expression
|
|---|
| Parameter | Total | High-level
(n=29) | Low-level
(n=32) | P-value | Positive
(n=32) | Negative
(n=29) | P-value |
|---|
| Histopahological
grade | | | | | | | |
| 1 or 2 | 54 | 26 | 28 | 1.000 | 28 | 26 | 1.000 |
| 3 | 7 | 3 | 4 | | 4 | 3 | |
| pT-factor | | | | | | | |
| pT1–2 | 32 | 13 | 19 | 0.310 | 19 | 13 | 0.310 |
| pT3–4 | 29 | 16 | 13 | | 13 | 16 | |
| pN-factor | | | | | | | |
| Negative | 25 | 11 | 14 | 0.795 | 16 | 9 | 0.193 |
| Positive | 36 | 18 | 18 | | 16 | 20 | |
| pStage | | | | | | | |
| I–IIB | 50 | 24 | 26 | 1.000 | 26 | 24 | 1.000 |
| III–IV | 11 | 5 | 6 | | 6 | 5 | |
| Lymphatic
permeation | | | | | | | |
| Negative | 23 | 10 | 13 | 0.792 | 12 | 11 | 1.000 |
| Positive | 38 | 19 | 19 | | 20 | 18 | |
| Blood vessel
permeation | | | | | | | |
| Negative | 26 | 11 | 15 | 0.606 | 11 | 15 | 0.200 |
| Positive | 35 | 18 | 17 | | 21 | 14 | |
| Perineural
invasion | | | | | | | |
| Negative | 9 | 3 | 6 | 0.478 | 3 | 6 | 0.287 |
| Positive | 52 | 26 | 26 | | 29 | 23 | |
| Resection
margin | | | | | | | |
| pR0 | 39 | 20 | 19 | 0.594 | 24 | 15 | 0.069 |
| pR1 | 22 | 9 | 13 | | 8 | 14 | |
| Recurrence | | | | | | | |
| No | 18 | 6 | 12 | 0.172 | 6 | 12 | 0.090 |
| Yes | 43 | 23 | 20 | | 26 | 17 | |
| Liver
metastasis | | | | | | | |
| No | 47 | 18 | 29 | 0.013 | 20 | 27 | 0.006 |
| Yes | 14 | 11 | 3 | | 12 | 2 | |
| Local
recurrence | | | | | | | |
| No | 46 | 22 | 24 | 1.000 | 25 | 21 | 0.767 |
| Yes | 15 | 7 | 8 | | 7 | 8 | |
| Peritoneal
metastasis | | | | | | | |
| No | 49 | 20 | 29 | 0.052 | 22 | 27 | 0.024 |
| Yes | 12 | 9 | 3 | | 10 | 2 | |
Luminal membrane expression of mesothelin
is a prominent negative prognostic factor for the patients with
EHBDCA
During our previous studies on pancreatic
adenocarcinoma and gastric adenocarcinoma, we already noted that
expression of mesothelin was found in the luminal membrane as well
as in the cytoplasm (19).
Mesothelin was reported to attach to the cell membrane through a
glycosyl-phosphatidylinositol (GPI) anchor after being
physiologically cleaved by some furin-like proteases (22), which are involved in the
translocation of mesothelin, although the biological functions of
mesothelin associated with its intracellular localization are not
fully understood. Thus, we analyzed the intracellular localization
of mesothelin by immunostaining to explore the clinicopathological
significance of its translocation.
As shown in Table
III, the group ‘luminal membrane positive’, which consisted of
the cases with luminal membrane staining even partially, was 32
(52.5%) cases, while the group ‘luminal membrane negative’, which
contained 17 cases which were completely mesothelin negative was
comprised of 29 (47.5%) cases. The statistical analysis revealed
that the incidence of luminal membrane positivity was significantly
correlated with peritoneal metastasis (P=0.024) in addition to
liver metastasis (P=0.006) (Table
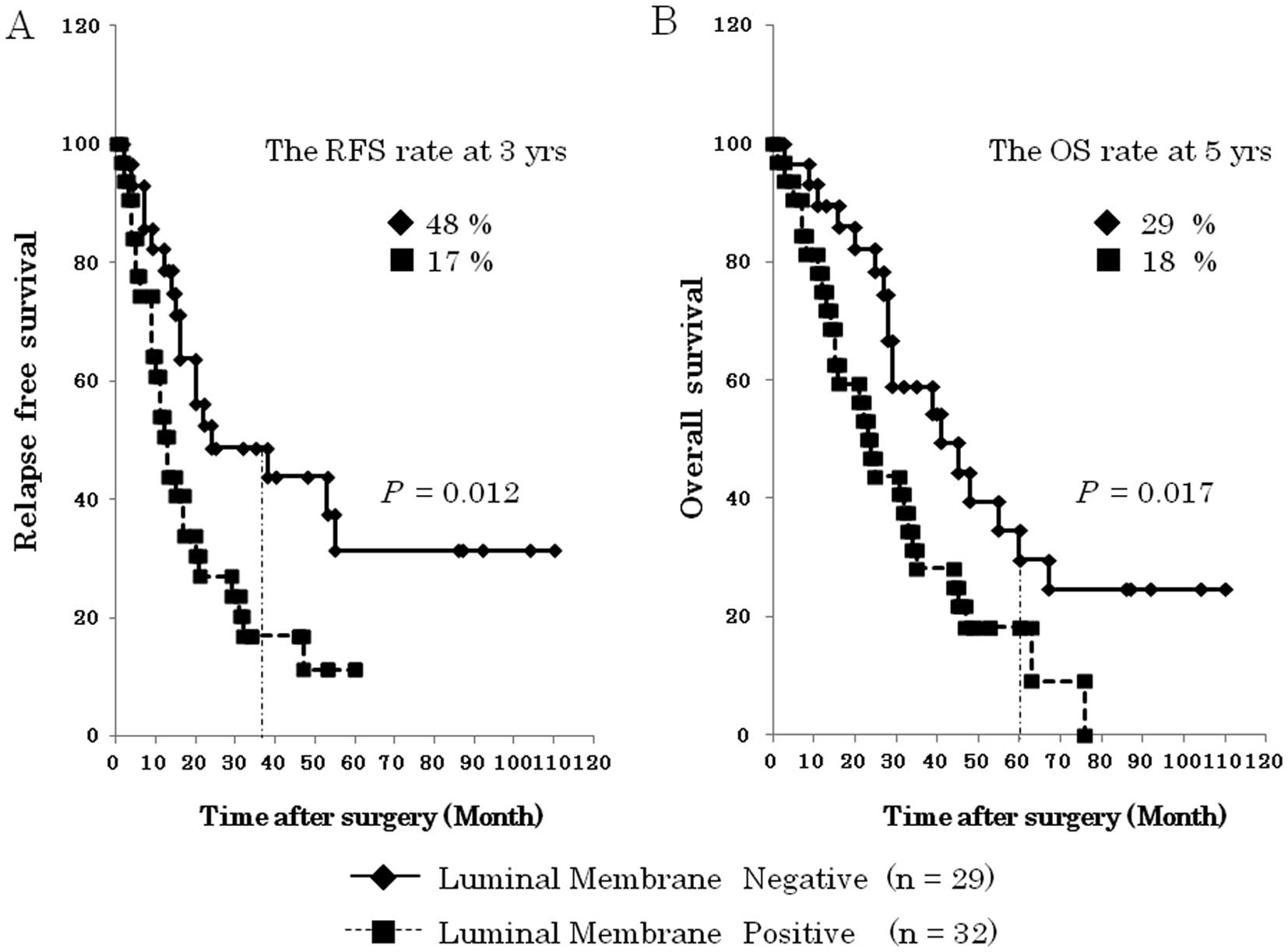
III). The analysis of the patients’ overall survival showed
that ‘luminal membrane positive’ of mesothelin indicated a
significantly unfavorable RFS (P=0.012) and OS (P=0.017) compared
to ‘luminal membrane negative’ of mesothelin (Fig. 4).
To clarify the mesothelin expression as an
independent prognostic factor, we performed a univariate analysis
of the 61 EHBDCA using the Cox proportional hazards model, the
result indicated that resection margin, ‘high-level expression’ and
‘luminal membrane positive’ of mesothelin were significantly
correlated with risks of cancer mortality. Multivariate analysis
also confirmed that resection margin (RR 3.361, 95% CI,
1.670–6.763, P=0.0007) and ‘luminal membrane positive’ of
mesothelin (RR 2.964, 95% CI, 1.401–6.296, P=0.0045) were
independent predictors of the overall patient survival (Table IV).
 | Table IVUnivariate and multivariate analysis
of patients’ survival in EHBDCA. |
Table IV
Univariate and multivariate analysis
of patients’ survival in EHBDCA.
| | Univariate analysis
| Multivariate
analysis
|
|---|
| Factor | n=61 | P-value | RR (95% CI) | RR (95% CI) | Hazard ratio | P-value |
|---|
| Histopahological
grade | | | | | | |
| 1 or 2 | 54 | 0.3931 | 1 | | NC | |
| 3 | 7 | | 1.508
(0.588–3.871) | | | |
| pT-factor | | | | | | |
| pT1–2 | 32 | 0.4264 | 1 | | NC | |
| pT3–4 | 29 | | 1.266
(0.708–2.262) | | | |
| pN-factor | | | | | | |
| Negative | 25 | 0.3639 | 1 | | NC | |
| Positive | 36 | | 1.314
(0.729–2.368) | | | |
| pStage | | | | | | |
| I–IIB | 50 | 0.2026 | 1 | | NC | |
| III–IV | 11 | | 1.608
(0.774–3.339) | | | |
| Lymphatic
permeation | | | | | | |
| Negative | 23 | 0.1908 | 1 | | NC | |
| Positive | 38 | | 1.537
(0.807–2.924) | | | |
| Blood vessel
permeation | | | | | | |
| Negative | 26 | 0.2999 | 1 | | NC | |
| Positive | 35 | | 1.370
(0.756–2.482) | | | |
| Perineural
invasion | | | | | | |
| Negative | 9 | 0.4733 | 1 | | NC | |
| Positive | 52 | | 0.728
(0.306–1.732) | | | |
| Resection
margin | | | | | | |
| pR0 | 39 | 0.0398 | 1 | 1.670–6.763 | 1 | 0.0007 |
| pR1 | 22 | | 1.859
(1.029–3.356) | | 3.361 | |
| Mesothelin
expression | | | | | | |
| Low-level | 32 | 0.0236 | 1 | 0.864–3.067 | 1 | 0.1317 |
| High-level | 29 | | 1.968
(1.095–3.538) | | 1.621 | |
| Luminal membrane
expressionof mesothelin | | | | | | |
| Negative | 29 | 0.0175 | 1 | 1.401–6.296 | 1 | 0.0045 |
| Positive | 32 | | 2.078
(1.137–3.798) | | 2.964 | |
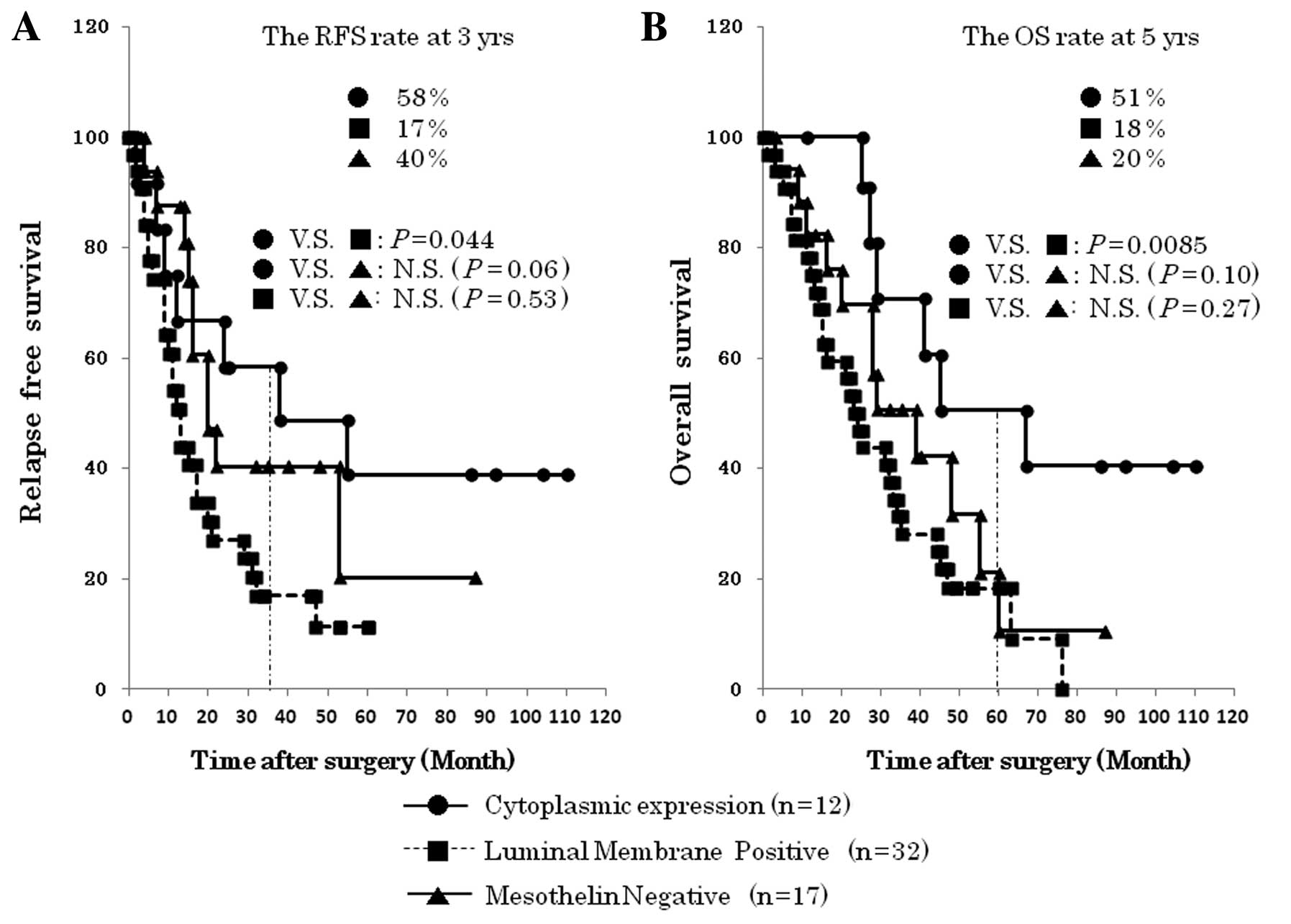
Isolation of ‘cytoplasmic expression’ of
mesothelin potentiates more exquisite prediction of prognosis in
EHBDCA
To explore the clinicopathological value of the
cytoplasmic expression of mesothelin, we performed a sub-analysis
in ‘luminal membrane negative’, dividing the group into 17 cases of
‘mesothelin negative’ and 12 cases of ‘cytoplasmic expression’. The
P-value (OS, P=0.0085) between ‘luminal membrane positive’ and
‘cytoplasmic expression’ was minimum in these survival analyses,
suggesting the clinical benefit of isolation of ‘cytoplasmic
expression’ of mesothelin (Fig.
5). Interestingly, ‘cytoplasmic expression’ of mesothelin
represented relatively favorable patients’ prognosis compared to
‘mesothelin negative’, although it was statistically not
significant (RFS, P=0.06; OS, P=0.10).
Discussion
In this study, we confirmed that mesothelin
expression is a prominent prognostic factor for EHBDCA patients as
well as for other tumors such as pancreatic cancer and ovarian
carcinoma described previously (12,15,23).
Furthermore, we revealed that the expression pattern of mesothelin,
in luminal membrane or cytoplasm, could be a more evident
prediction factor for these patients. These results evidently
support our recent report of mesothelin expression patterns in
gastric cancer in which luminal membrane expression, not
cytoplasmic expression of mesothelin is a prominent negative
prognostic factor for gastric cancer (19).
The mechanism for the membranous localization of
mesothelin should be explained as follows: the full length of the
human mesothelin gene encodes a 71-kDa precursor protein
that is proteolytically cleaved by some furin-like proteases into
an N-terminal secreted form and a C-terminal
fragment, the 40-kDa mesothelin, which is a
glycosyl-phosphatidylinositol (GPI)-linked glycoprotein (6,13,15).
Many researchers have investigated the role of the mesothelin
expression in tumor biology and demonstrated the importance of
mesothelin expression for tumor progression in vitro
(14,24–26)
and in vivo (27,28); however, the clinicopathological
significance of the membrane localization of mesothelin has not
been clarified. The 5B2 anti-mesothelin antibody, which we employed
here for IHC, can detect both the 71-kDa precursor protein and the
40-kDa C-terminal fragment, but not the 30-kDa
N-terminal fragment. According to the reported molecular
processing mechanism of mesothelin and specificity of antibody,
luminal membrane staining probably indicates the 40-kDa
membrane-bound form of mesothelin, while cytoplasmic staining would
mean the 71-kDa precursor form of mesothelin. Our results support
the idea that the 40-kDa membrane-bound form of mesothelin is an
active form and promotes the aggressive features including
increased cell motility, invasion or migration capabilities and
growth of metastatic tumors (24,25,29).
The fact that ‘cytoplasmic expression’ of mesothelin
paradoxically resulted in better OS than mesothelin with
‘mesothelin negative’ took us by surprise (Fig. 5B). The RFS rate at 3 years (58 and
40%, respectively) and OS at 5 years (51 and 20%, respectively)
were demonstrably better in ‘cytoplasmic expression’ compared to
‘mesothelin negative’, although the final RFS and OS were not
statistically significant (RFS, P=0.06; OS, P=0.10). As indicated
above, the majority of mesothelin in cytoplasm must be the 71-kDa
precursor form and might behave like a dominant negative form of
mesothelin as a tumor suppressor. The conflicting results in some
previous reports in which mesothelin expression was correlated with
prolonged patient survival in gastric cancer (18) and in ovarian serous carcinoma
(30), may be explained by
confusing the luminal membrane and cytoplasmic expression of
mesothelin. Isolation of ‘mesothelin negative’ might give us
another disease entity, mesothelin-independent EHBDCA. The tumor
cells in such a type of EHBDCA would obtain invasive ability
without the association of mesothelin; therefore, this could
indicate an alternative gene expression profiling. In fact,
additional sub-analysis for clinicopathological parameters among
the three groups showed interesting results. Frequent perineural
invasion was observed in ‘mesothelin negative’ rather than in
mesothelin positive cases even in luminal membrane or cytoplasm
(P=0.049 and 0.028, respectively), while liver metastasis was
abundantly found in ‘luminal membrane positive’ (Table V). Such conflicting results may
suggest the distinct oncogenic process between
mesothelin-associated and mesothelin-independent EHBDCA.
 | Table VSub-analysis among three groups
according to the intracellular expression pattern of
mesothelin. |
Table V
Sub-analysis among three groups
according to the intracellular expression pattern of
mesothelin.
| Parameter | Total (n=44) | Luminal membrane
positive (n=32) | Cytoplasmic
expression (n=12) | P-value | Total (n=49) | Luminal membrane
positive (n=32) | Negative expression
(n=17) | P-value | Total (n=29) | Cytoplasmic
expression (n=12) | Negative expression
(n=17) | P-value |
|---|
| Histopahological
grade | | | | | | | | | | | | |
| 1 or 2 | 39 | 28 | 11 | 1.000 | 43 | 28 | 15 | 1.000 | 26 | 11 | 15 | 1.000 |
| 3 | 5 | 4 | 1 | | 6 | 4 | 2 | | 3 | 1 | 2 | |
| pT-factor | | | | | | | | | | | | |
| pT1–2 | 23 | 19 | 4 | 0.179 | 28 | 19 | 9 | 0.765 | 13 | 4 | 9 | 0.452 |
| pT3–4 | 21 | 13 | 8 | | 21 | 13 | 8 | | 16 | 8 | 8 | |
| pN-factor | | | | | | | | | | | | |
| Negative | 18 | 16 | 2 | 0.083 | 23 | 16 | 7 | 0.764 | 9 | 2 | 7 | 0.234 |
| Positive | 26 | 16 | 10 | | 26 | 16 | 10 | | 20 | 10 | 10 | |
| pStage | | | | | | | | | | | | |
| I–IIB | 37 | 26 | 11 | 0.653 | 39 | 26 | 13 | 0.722 | 24 | 11 | 13 | 0.370 |
| III–IV | 7 | 6 | 1 | | 10 | 6 | 4 | | 5 | 1 | 4 | |
| Lymphatic
permeation | | | | | | | | | | | | |
| Negative | 14 | 12 | 2 | 0.282 | 21 | 12 | 9 | 0.370 | 11 | 2 | 9 | 0.064 |
| Positive | 30 | 20 | 10 | | 28 | 20 | 8 | | 18 | 10 | 8 | |
| Blood vessel
permeation | | | | | | | | | | | | |
| Negative | 16 | 11 | 5 | 0.732 | 21 | 11 | 10 | 0.134 | 15 | 5 | 10 | 0.462 |
| Positive | 28 | 21 | 7 | | 28 | 21 | 7 | | 14 | 7 | 7 | |
| Perineural
invasion | | | | | | | | | | | | |
| Negative | 3 | 3 | 0 | 0.551 | 9 | 3 | 6 | 0.049 | 6 | 0 | 6 | 0.028 |
| Positive | 41 | 29 | 12 | | 40 | 29 | 11 | | 23 | 12 | 11 | |
| Resection
margin | | | | | | | | | | | | |
| pR0 | 30 | 24 | 6 | 0.152 | 32 | 24 | 8 | 0.065 | 14 | 6 | 8 | 1.000 |
| pR1 | 14 | 8 | 6 | | 17 | 8 | 9 | | 15 | 6 | 9 | |
| Recurrence | | | | | | | | | | | | |
| No | 11 | 6 | 5 | 0.139 | 13 | 6 | 7 | 0.172 | 12 | 5 | 7 | 1.000 |
| Yes | 33 | 26 | 7 | | 36 | 26 | 10 | | 17 | 7 | 10 | |
| Liver
metastasis | | | | | | | | | | | | |
| No | 30 | 20 | 10 | 0.282 | 36 | 20 | 16 | 0.020 | 26 | 10 | 16 | 0.553 |
| Yes | 14 | 12 | 2 | | 13 | 12 | 1 | | 3 | 2 | 1 | |
| Local
recurrence | | | | | | | | | | | | |
| No | 34 | 25 | 9 | 1.000 | 37 | 25 | 12 | 0.729 | 21 | 9 | 12 | 1.000 |
| Yes | 10 | 7 | 3 | | 12 | 7 | 5 | | 8 | 3 | 5 | |
| Peritoneal
metastasis | | | | | | | | | | | | |
| No | 34 | 22 | 12 | 0.041 | 37 | 22 | 15 | 0.175 | 27 | 12 | 15 | 0.498 |
| Yes | 10 | 10 | 0 | | 12 | 10 | 2 | | 2 | 0 | 2 | |
In terms of discovering the clinicopathological
parameters, there are many previous studies demonstrating the
prognostic significance of various molecules, such as epidermal
growth factor receptor (EFGR) and c-erbB-2 (HER-2) in colorectal,
breast and lung cancer (31).
There are some other case reports describing a series of promising
results targeting EGFR in patients with advanced biliary tract
cancer (32–34); however, identification of useful
prognostic markers for EHBDCA still needs investigation. In
addition, lack of effective adjuvant therapy against advanced
EHBDCA requires establishing new therapeutic methods based on
reliable molecular targeting markers; thus, mesothelin could be one
of the potential targets for cancer molecular targeting therapy.
Recombinant anti-mesothelin immunotoxin SS1P (CAT-5001) and a high
affinity chimeric anti-mesothelin monoclonal antibody MORAb-009
recently entered phase II clinical trials (35,36).
To evaluate the therapeutic effect of such antibody-based medicine,
pathological verification of membranous expression of the target
molecule must be performed, because antibody-based drugs can
usually access the molecules located on the cell membrane. We
believe that luminal membrane expression of mesothelin in EHBDCA
would be of clinical benefit not only as a prognostic factor but
also as a predictive factor for the eligibility to
mesothelin-targeting therapies (13,14,27,37,38).
In conclusion, we demonstrated the
clinicopathological significance of the mesothelin expression as an
independent prognostic factor. Moreover, identification of luminal
membrane or cytoplasmic expression of mesothelin could be a
reliable prognostic factor for EHBDCA and might offer a novel
therapeutic strategy for patients with EHBDCA, including
immunotherapy using peptide vaccine or monoclonal antibody
therapy.
Acknowledgements
This research was supported by a
Grant-in-Aid for Scientific Research (KAKENHI). The study sponsors
had no involvement in the study design, in the collection, analysis
and interpretation of data, in the writing of the manuscript, or in
the decision to submit the manuscript for publication.
References
|
1.
|
Ito K, Ito H, Allen PJ, et al: Adequate
lymph node assessment for extrahepatic bile duct adenocarcinoma.
Ann Surg. 251:675–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Ohashi M, Kusumi T, Sato F, et al:
Expression of syndecan-1 and E-cadherin is inversely correlated
with poor patient’s prognosis and recurrent status of extrahepatic
bile duct carcinoma. Biomed Res. 30:79–86. 2009.PubMed/NCBI
|
|
3.
|
Jarnagin WR, Fong Y, DeMatteo RP, et al:
Staging, resectability, and outcome in 225 patients with hilar
cholangiocarcinoma. Ann Surg. 234:507–519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Akoad M and Jenkins R: Proximal biliary
malignancy. Surg Clin North Am. 88:1409–1428. x–xi. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Veillette G and Castillo CF: Distal
biliary malignancy. Surg Clin North Am. 88:1429–1447. xi2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Chang K and Pastan I: Molecular cloning of
mesothelin, a differentiation antigen present on mesothelium,
mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA.
93:136–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chang K, Pastan I and Willingham MC:
Isolation and characterization of a monoclonal antibody, K1,
reactive with ovarian cancers and normal mesothelium. Int J Cancer.
50:373–381. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Argani P, Iacobuzio-Donahue C, Ryu B, et
al: Mesothelin is overexpressed in the vast majority of ductal
adenocarcinomas of the pancreas: identification of a new pancreatic
cancer marker by serial analysis of gene expression (SAGE). Clin
Cancer Res. 7:3862–3868. 2001.PubMed/NCBI
|
|
9.
|
Hassan R, Kreitman RJ, Pastan I and
Willingham MC: Localization of mesothelin in epithelial ovarian
cancer. Appl Immunohistochem Mol Morphol. 13:243–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ordonez NG: Value of mesothelin
immunostaining in the diagnosis of mesothelioma. Mod Pathol.
16:192–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ordonez NG: Application of mesothelin
immunostaining in tumor diagnosis. Am J Surg Pathol. 27:1418–1428.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Einama T, Kamachi H, Nishihara H, et al:
Co-Expression of mesothelin and CA125 correlates with unfavorable
patient outcome in pancreatic ductal adenocarcinoma. Pancreas.
40:1276–1282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hassan R, Bera T and Pastan I: Mesothelin:
a new target for immunotherapy. Clin Cancer Res. 10:3937–3942.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Li M, Bharadwaj U, Zhang R, et al:
Mesothelin is a malignant factor and therapeutic vaccine target for
pancreatic cancer. Mol Cancer Ther. 7:286–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cheng WF, Huang CY, Chang MC, et al: High
mesothelin correlates with chemoresistance and poor survival in
epithelial ovarian carcinoma. Br J Cancer. 100:1144–1153. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hassan R, Laszik ZG, Lerner M, Raffeld M,
Postier R and Brackett D: Mesothelin is overexpressed in
pancreaticobiliary adenocarcinomas but not in normal pancreas and
chronic pancreatitis. Am J Clin Pathol. 124:838–845. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Swierczynski SL, Maitra A, Abraham SC, et
al: Analysis of novel tumor markers in pancreatic and biliary
carcinomas using tissue microarrays. Hum Pathol. 35:357–366. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Baba K, Ishigami S, Arigami T, et al:
Mesothelin expression correlates with prolonged patient survival in
gastric cancer. J Surg Oncol. 105:195–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Einama T, Homma S, Kamachi H, et al:
Luminal membrane expression of mesothelin is a prominent poor
prognostic factor for gastric cancer. Br J Cancer. 107:137–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhao H, Davydova L, Mandich D, Cartun RW
and Ligato S: S100A4 protein and mesothelin expression in dysplasia
and carcinoma of the extrahepatic bile duct. Am J Clin Pathol.
127:374–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sobin LH and Wittekind CW: TNM
Classification of Malignant Tumors. 6th edition. Wiley-Liss; New
York: 2002
|
|
22.
|
Inami K, Kajino K, Abe M, et al: Secretion
of N-ERC/mesothelin and expression of C-ERC/mesothelin in human
pancreatic ductal carcinoma. Oncol Rep. 20:1375–1380.
2008.PubMed/NCBI
|
|
23.
|
Shimizu A, Hirono S, Tani M, et al:
Coexpression of MUC16 and mesothelin is related to the invasion
process in pancreatic ductal adenocarcinoma. Cancer Sci.
103:739–746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Bharadwaj U, Marin-Muller C, Li M, Chen C
and Yao Q: Mesothelin overexpression promotes autocrine IL-6/sIL-6R
trans-signaling to stimulate pancreatic cancer cell proliferation.
Carcinogenesis. 32:1013–1024. 2011. View Article : Google Scholar
|
|
25.
|
Bharadwaj U, Marin-Muller C, Li M, Chen C
and Yao Q: Mesothelin confers pancreatic cancer cell resistance to
TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and
IL-6/Mcl-1 overexpression. Mol Cancer. 10:1062011.PubMed/NCBI
|
|
26.
|
Chang MC, Chen CA, Hsieh CY, et al:
Mesothelin inhibits paclitaxel-induced apoptosis through the PI3K
pathway. Biochem J. 424:449–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hassan R, Schweizer C, Lu KF, et al:
Inhibition of mesothelin-CA-125 interaction in patients with
mesothelioma by the anti-mesothelin monoclonal antibody MORAb-009:
implications for cancer therapy. Lung Cancer. 68:455–459. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bharadwaj U, Li M, Chen C and Yao Q:
Mesothelin-induced pancreatic cancer cell proliferation involves
alteration of cyclin E via activation of signal transducer and
activator of transcription protein 3. Mol Cancer Res. 6:1755–1765.
2008. View Article : Google Scholar
|
|
29.
|
Inami K, Abe M, Takeda K, et al: Antitumor
activity of anti-CERC/mesothelin monoclonal antibody in vivo.
Cancer Sci. 101:969–974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yen MJ, Hsu CY, Mao TL, et al: Diffuse
mesothelin expression correlates with prolonged patient survival in
ovarian serous carcinoma. Clin Cancer Res. 12:827–831. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Hudis CA: Trastuzumab - mechanism of
action and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Huang TW, Wang CH and Hsieh CB: Effects of
the anti-epidermal growth factor receptor antibody cetuximab on
cholangiocarcinoma of the liver. Onkologie. 30:129–131. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Sprinzl MF, Schimanski CC, Moehler M,
Schadmand-Fischer S, Galle PR and Kanzler S: Gemcitabine in
combination with EGF-Receptor antibody (Cetuximab) as a treatment
of cholangiocarcinoma: a case report. BMC Cancer. 6:1902006.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Bralet MP, Bellin MF, Guettier C, Adam R
and Paule B: Response to cetuximab and gemcitabine-oxaliplatin in
an advanced case of intrahepatic cholangiocarcinoma. Clin Oncol (R
Coll Radiol). 18:4262006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Kreitman RJ, Hassan R, Fitzgerald DJ and
Pastan I: Phase I trial of continuous infusion anti-mesothelin
recombinant immunotoxin SS1P. Clin Cancer Res. 15:5274–5279. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hassan R, Cohen SJ, Phillips M, et al:
Phase I clinical trial of the Chimeric anti-mesothelin monoclonal
antibody MORAb-009 in patients with mesothelin expressing cancers.
Clin Cancer Res. 16:6132–6138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Hassan R, Bullock S, Premkumar A, et al:
Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin
given as a bolus I.V. infusion to patients with
mesothelin-expressing mesothelioma, ovarian, and pancreatic
cancers. Clin Cancer Res. 13:5144–5149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Hassan R, Ebel W, Routhier EL, et al:
Preclinical evaluation of MORAb-009, a chimeric antibody targeting
tumor-associated mesothelin. Cancer Immun. 7:202007.PubMed/NCBI
|