Introduction
Glioblastoma multiforme (GBM) is the most common
type of brain tumor in adults, exhibiting relentless and malignant
progression characterized by widespread invasion throughout the
brain, destruction of normal brain tissue, and resistance to
surgery, radiotherapy or chemotherapy (1). GBM usually recurs at its original
location and within 12 months post-resection (2). Recurrent GBM tumors are usually
comprised of tumor cells that are difficult for radiation and/or
chemotherapy and do not respond adequately to further treatments.
Therefore, the developments of novel agents are urgently needed to
enhance their therapeutic effects for GBM tumors.
Because GBM usually relapses around the original
site and is localized in the central nervous system (CNS), direct
delivery of an oncolytic adenovirus provides the potential to
effectively target these tumors (3). Conditionally replicating adenovirus
(CRAd) propagates in tumor cells but not in normal cells, which
represents a novel approach for cancer treatment. Recent
preclinical research suggested the potential efficacy of these
viruses, and clinical studies confirmed the safety profile of these
agents (4–7). However, the numbers of cancer gene
therapy clinical trials performed to date had fallen short with
respect to initial expectations of demonstrable therapeutic
outcome. A major limitation of these vectors has been the poor
replication and weak transduction in neighboring tumor cells. A
better viral vector can be achieved by the application of
tumor-specific promoters (TSP) to improve tumor-specific
replication of the vector and by the modifications of viral capsids
to enhance viral transduction.
One of the approaches to improve the replicative
specificity of CRAd is based on the tissue- or tumor-specific
transcriptional control of the essential early genes required for
viral replication. For CRAd, the adenovirus genome is usually
genetically modified to replace the E1A promoter region with the
desired tissue- or tumor-specific expression profile. The ideal
tumor specific promoter (TSP) would display an alternatively ‘tumor
on/normal tissue off’ expression profiles. To develop TSP-based
CRAd, one of the commonly used methods is to drive E1 gene
expression with a selected promoter. Survivin, a member of the
inhibitor of apoptosis protein (IAP) family, is highly expressed in
most human tumors and fetal tissues, but rarely detectable in
terminally differentiated cells (8). Eighty percent of GBM tumors
demonstrate abundant survivin expression (9), and a clear correlation is seen
between the histological grade of a glioma and the fraction of
survivin-positive tumor cells (10). Previous studies showed that the
adenoviral E1A driven by the human survivin promoter was
responsible for the enhanced viral replication and improved
oncolytic ability in malignant gliomas (10,11).
Therefore, this promoter is a promising candidate to drive E1
expression in the development of a new CRAd agent.
The wild-type human adenovirus (Ad5) has
demonstrated relatively poor transduction efficiency in malignant
glioma cells because of the low-level expression of the
Coxsackievirus and adenovirus receptor (CAR) on the tumor cells
(12). To circumvent this issue,
the adenovirus fiber protein could be modified by genetic method
for improving the transduction of adenoviral vector in
CAR-deficient cells. One of the approaches is the substitution of
the knob domain of Ad5 with knobs from alternate Ad serotypes, for
example, species B (13). Human
species B adenoviruses can utilize ubiquitously expressed CD46 as a
receptor for entry into host cells (14–16).
Ad11, which belongs to adenovirus species B, was reported to
possess strong ability to bind and infect human CD46 positive cells
(17,18).
Up to now, modified oncolytic adenoviral vectors
have used different methods including introduction of
tumor/tissue-specific promoters, hexon or fiber modification. In
this study, we hypothesized that transcriptional and transductional
control of viral replication would enhance the oncolytic effect of
virus against malignant gliomas. To test this hypothesis, we for
the first time constructed chimeric oncolytic adenoviral vector
CRAd5/11-Sp-eGFP by combinationally using a survivin-driven and
chimeric 5/11 fiber and examined the targeting and oncolytic
efficacy of CRAd5/11-Sp-eGFP in vitro and in vivo.
This novel vector CRAd5/11-Sp-eGFP was found to exhibit enhanced
antitumor activity compared with the wild-type fiber.
Materials and methods
Cells and culture conditions
Human malignant glioma cell lines U87, A172, U251
and human embryonic kidney cell line HEK-293 were purchased from
American Type Culture Collection (ATCC, Manassas, VA, USA). All
cell lines were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% heat-inactivated fetal calf serum, 100
U/ml penicillin, 100 μg/ml streptomycin, and L-glutamine
(200 μg/ml) and incubated in a humidified 37°C atmosphere of
95% air and 5% CO2.
Construction of recombinant
adenovirus
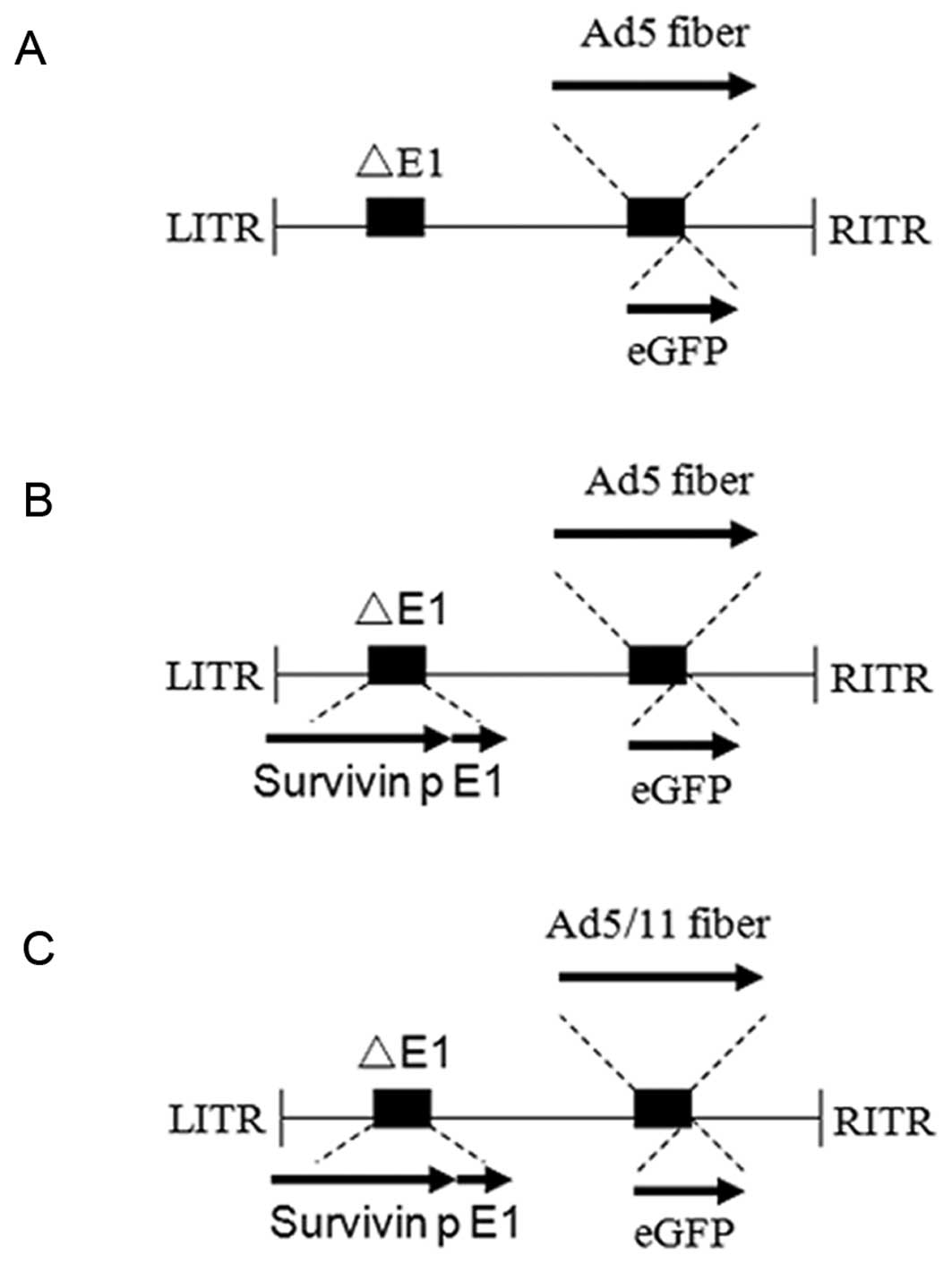
CRAd5/11-Sp-eGFP was constructed as follows: i) The
generation of the shuttle vector pAd5-E1-Sp which the E1 gene was
under the control of survivin promoter. The survivin promoter
fragment corresponding to the region from nt2532 to nt2764 in the
DNA sequence (GenBank Access no: U75285) was amplified from human
genomic DNA by PCR using forward primer,
5′-AGGATCCGCACGCGTTCTTTGAAAGC-3′, and reverse primer,
5′-TAAGCTTCCACCTCTGCCAACGGGTC-3′ (19). The amplified survivin promoter was
subcloned into the plasmid pshuttle-Ad5-E1 by BamHI and
HindIII restriction sites. A SV40 poly-A (PA) fragment was
then cut with XbaI/BamHI from a pGL3B vector
(Invitrogen, Carlsbad, CA, USA) and inserted into the
pshuttle-Ad5-E1 by use of the same restriction sites. The resultant
plasmid was named pAd5-E1-Sp. ii) The construction of pCRAd5-Sp,
which was achieved by co-transforming ScaI linearized
pAd5-E1-Sp and ClaI digested pTG3602/SwaI into E.
coli BJ5183 (20). iii) The
construction of shuttle plasmid pBS/Ad5/11-eGFP which includes the
chimeric fiber Ad5/11 and eGFP. First, we synthesized a gene which
includes Ad5 tail (522–608 bp), Ad11 shaft and knob sequence in
pcDNA 3.1(+) (Sangon, Shanghai, China). The plasmid was named pcDNA
3.1(+)/Ad5/11 which included a unique NdeI and SpeI
restriction introduced separately in its 5′ and 3′ end. The pcDNA
3.1(+)/Ad5/11 digested by NdeI and SpeI was ligated
with an NdeI/SpeI-linearized pBS shuttle vector at
4:1 (v/v) ratio (20). The
positive clones were screened by enzyme digestion and DNA
sequencing and named pBS/Ad5/11. Second, we constructed an
expression cassette that the CMV promotes eGFP expression. This
cassette digested by BamHI and SfuI was inserted into
the region between E4 and the fiber located in the pBS/Ad5/11 to
acquire the shuttle vector pBS/Ad5/11-eGFP. iv) For the generation
of CRAd5/11-Sp-eGFP vector, the pCRAd5-Sp digested with SwaI
and the pBS/Ad5/11-eGFP digested with XhoI was
co-transformed into E. coli BJ5183 cells. The resulting
plasmid was designated pCRAd5/11-Sp-eGFP. PacI linearized
pCRAd5/11-Sp-eGFP was transfected into HEK-293 cells. Then
recombinant adenovirus CRAd5/11-Sp-eGFP was propagated in HEK-293
cells and purified by cesium chloride gradient methods. The titers
were detected by spectrophotometry at an absorbance (A) of 260 nm.
Using similar strategy, the recombinant adenoviruses CRAd5-Sp-eGFP
was produced.
Viral oncolytic potency in human glioma
cells
For determination of virus-mediated cell killing
ability, 5×104 glioma cells (U87, A172 and U251) were
seeded in 24-well plates and infected with adenoviruses at various
multiplicity of infection (MOI) or phosphate-buffered saline (PBS)
for 4 h followed by replacement of infection media with growth
media. Seven days after infection, in order to visualize cell
killing, cells were fixed and stained with 2% crystal violet in 70%
ethanol for 20 min followed by washing with distilled water to
remove excess dye. The plates were dried and images were captured
with an Alpha Innotech FluroChem (Santa Clara, CA, USA).
Cell viability assay
U87, A172 and U251 were cultured in 96-well plates
at 5×103 cells per well. Twenty-four hours later,
viruses at 5 MOI were administered into cells. The plates were
incubated at 37°C and supplemented with 5% CO2. PBS was
used as a control. The medium was removed every 24 h and fresh
medium containing 20 μl of
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 1
μg/ml) solution was added to each well. Four hours later,
MTT was discarded and 150 μl of DMSO was loaded. The
spectrophotometric absorbance of the samples was measured with a
Multiskan MK3 (Thermo Electron Corporation, Waltham, MA, USA) at
570 nm. The percentage of cell viability was calculated using the
following formula: cell viability = absorbance value of infected
cells/absorbance value of uninfected control cells. Six duplicate
wells were measured and the experiments were done at least three
times.
Quantitative real-time PCR for the Ad5 E4
gene
U87, A172 and U251 cells were seeded in 6-well
plates (5×105 cell/well) and infected with Ad5-eGFP,
CRAd5-Sp-eGFP and CRAd5/11-Sp-eGFP at a MOI of 5. At each 24 h
post-infection, the cells were washed with PBS and harvested. The
DNA was then purified with a TIANamp blood DNA kit (Tiangen,
China). Quantitative polymerase chain reaction (PCR) was performed
with primers (Ad5 E4 for, 5′-CATGCGCCGCTGCCCTGATA-3′; and Ad5 E4
reverse, 5′-TTCCCGCTCCTCCCGTGTGT-3′) against a 99 base pair region
of the E4 gene. The amplified Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) based on specific primers (GAPDH for,
5′-TGGTGAAGACGCCAGTGGA-3′; and GAPDH reverse,
5′-GCACCGTCAAGGCTGAGAAC-3′) was used for an internal control. All
reactions, performed in triplicate in a total reaction volume of 20
μl, using SYBR Premix Ex Taq™ II (Takara, Japan), were
carried out in a Bio-Rad iQ5 instrument (Bio-Rad, Hercules, CA,
USA). The parameters were used for amplification and melting curve
analysis was described by Li et al (21). For quantitative analysis, viral
genomes were purified from each group using the same quantity of
viral particles (assessed by spectrophotometrically) and serially
diluted to generate a standard curve.
Adenovirus transduction analysis
U87, A172 and U251 cells were plated at a density of
5×105/well in a 60-mm dish culture plate for 24 h prior
to infection, then Ad5-eGFP, CRAd5-Sp-eGFP and CRAd5/11-Sp-eGFP
were applied to the U87, A172 and U251 cells at the dosage of 500
viral particle/cell. For quantification of eGFP expression level,
photographs were taken at 24 h post-infection with 10 × objective
(3-sec exposure time). The infection efficiency was quantified by
counting eGFP-positive cells.
Studies of xenograft tumors in nude
mice
U87 tumor xenografts were established by
subcutaneously inoculating 2×106 cells into the right
flanks of 4- to 6-week-old female BALB/c nude mice (Animal Research
Committee of the Institute of Biochemistry and Cell Biology,
Shanghai, China). When tumors reached between 70 to 100
mm3, 28 mice were randomly assigned to PBS treated
group, Ad5-eGFP treated group, CRAd5-Sp-eGFP treated group and
CRAd5/11-Sp-eGFP treated group, respectively. The established
tumors were injected with 50 μl of PBS or 5×108
plaque-forming units (PFU) of virus. The injections were repeated
four times, every other day. Tumor growth was monitored by periodic
measurements with calipers and tumor volume was calculated using
the following formula: tumor volume (mm3) = length (mm)
× width2 (mm2)/2. The tumor volumes of the 7
mice in each group were measured every 2 days for 1 month.
Statistical analysis
Statistical analysis for all experimental conditions
was performed using the Statistical Package for the Social Sciences
(SPSS) software (version 13, Chicago, IL, USA). The difference
between each control and experimental groups was analyzed by using
one-way analysis of variance between groups (ANOVA/LSD). In all
cases, a p-value <0.05 was considered statistically
significant.
Results
Chimeric fiber 5/11 modified oncolytic
adenovirus CRAd5/11-Sp-eGFP for improved cell killing ability in
glioma cells in vitro
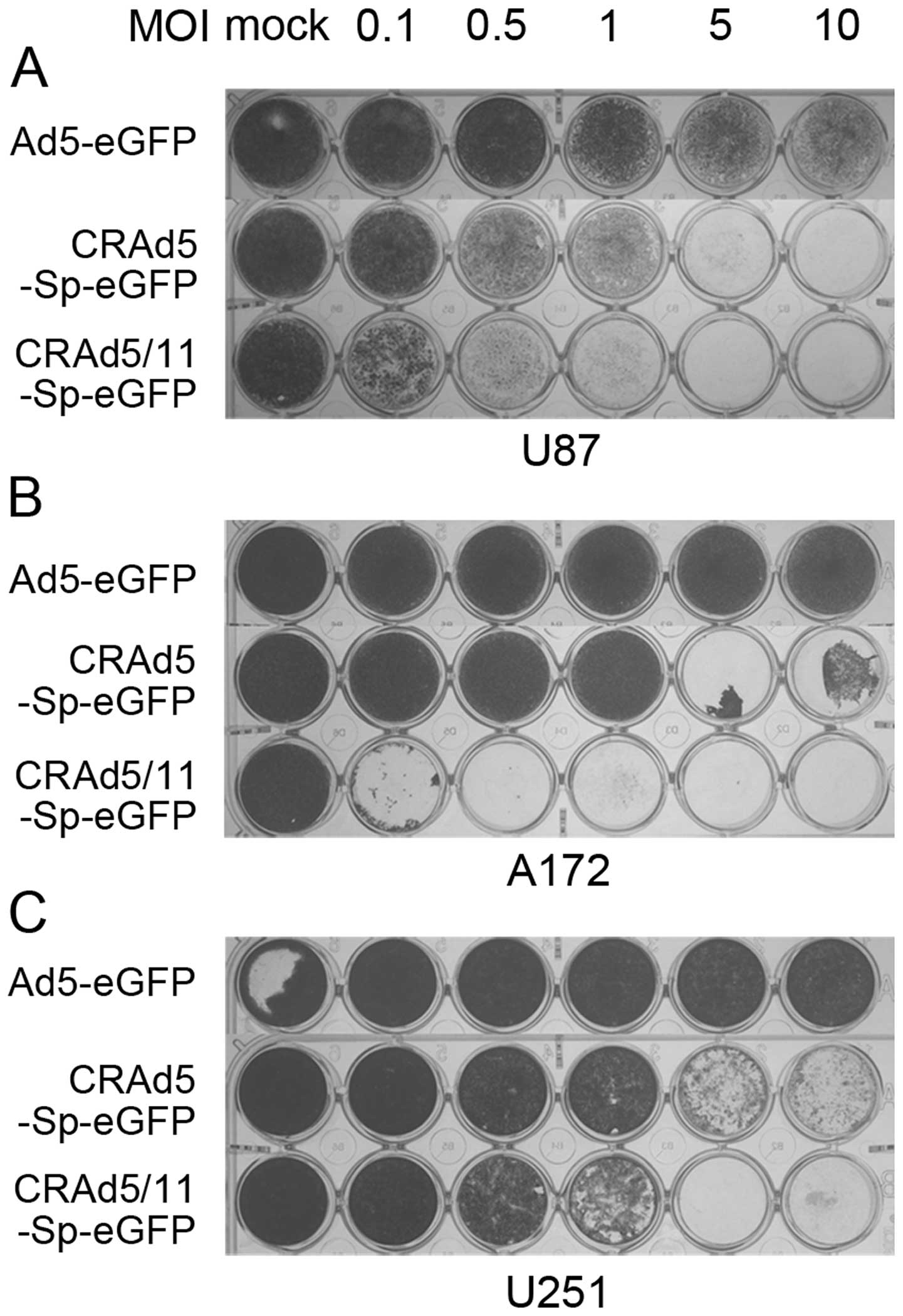
In order to assay biological activity of the
oncolytic adenoviruses (Fig. 1),
glioma cell lines of U87, A172 and U251 were infected with
CRAd5-Sp-eGFP, CRAd5/11-Sp-eGFP or replication-deficient Ad5-eGFP
virus at the dose of 0, 0.1, 0.5, 1, 5 and 10 MOI, respectively.
Cell killing effect was then assessed via crystal violet staining.
Of the tested vectors, CRAd5/11-Sp-eGFP demonstrated a
dose-dependent cell killing effect in all the human glioma cell
lines (Fig. 2) with a dose as low
as 0.1 MOI in A172 cells and at 1 MOI in U87 and U251 cells. Of
note, the oncolytic effect of CRAd5/11-Sp-eGFP was about 100 times
superior to that of CRAd5-Sp-eGFP in the A172 cell lines. Less or
no cell killing effect was observed with the control, the
replication-defective Ad5-eGFP vector. Cell viability was
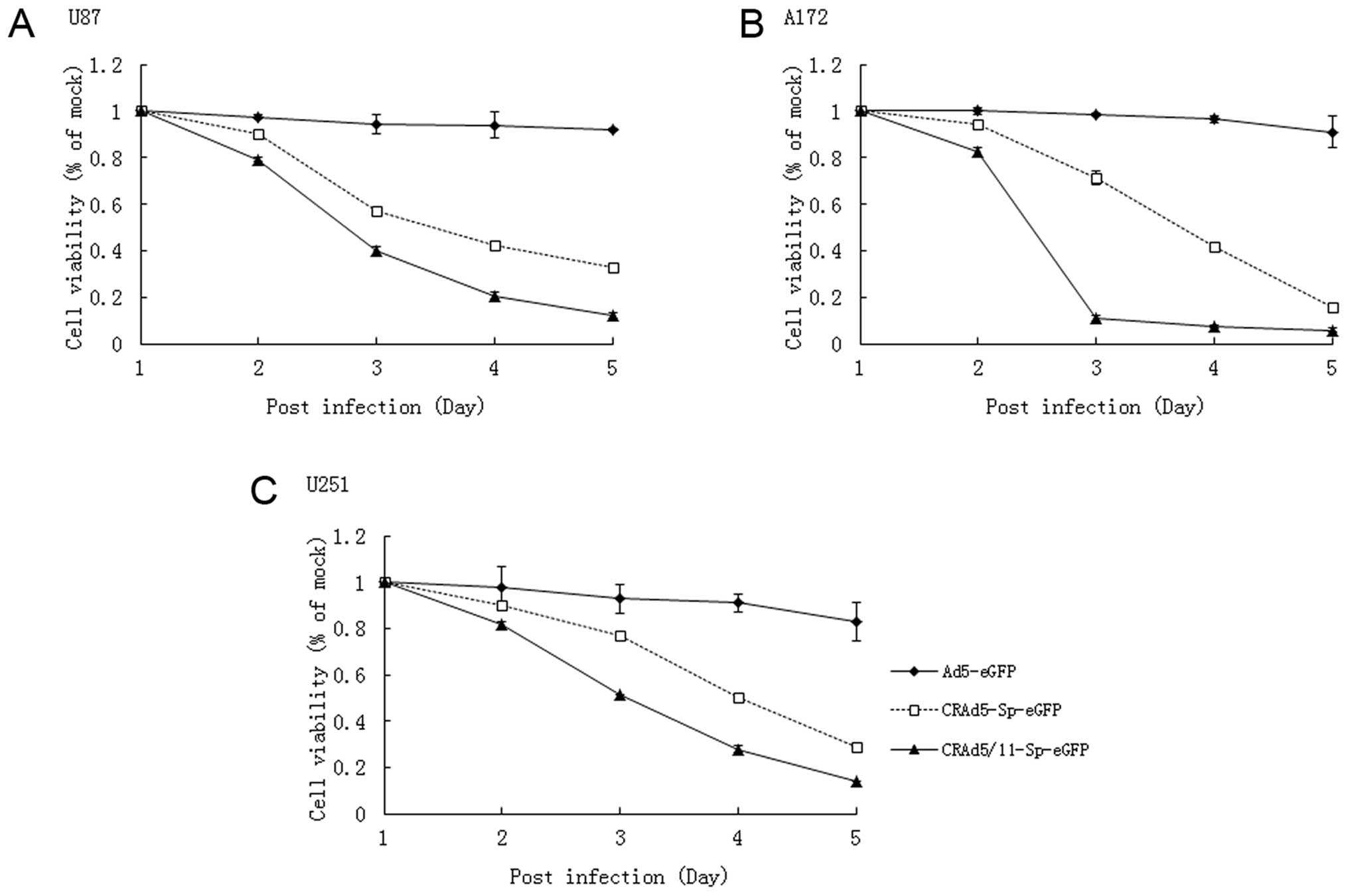
quantified by MTT assay. Tumor cell lines (U87, A172 and U251) were
infected with CRAd5-Sp-eGFP, CRAd5/11-Sp-eGFP or
replication-deficient adenovirus Ad5-eGFP at MOI of 5. As shown in
Fig. 3, CRAd5-Sp-eGFP and
CRAd5/11-Sp-eGFP was able to inhibit growth of glioma cells in a
time-dependent manner. In addition, CRAd5/11-Sp-eGFP significantly
inhibited the growth of all three tumor cell lines compared with
CRAd5-Sp-eGFP. The results indicated that the fiber chimeric
oncolytic virus constructed by us significantly enhanced cell
killing effect against glioma cells compared with fiber unmodified
oncolytic adenovirus.
Efficient replication of chimeric fiber
5/11 modified CRAd in glioma cells
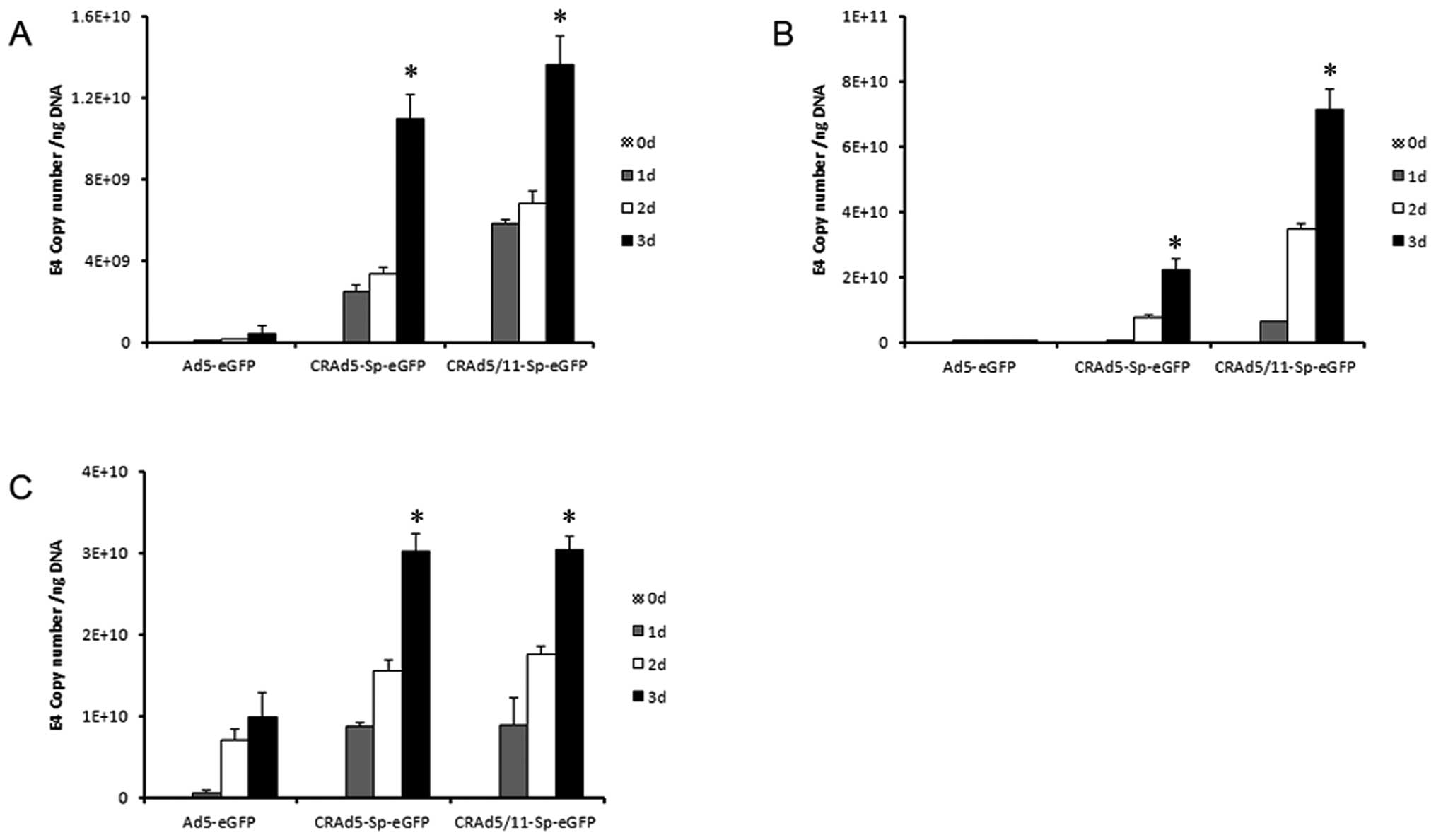
Efficient viral replication can greatly contribute
to the antitumor capacity of CRAd. Here, in order to investigate
the replicative ability of the modified CRAds in glioma cell, U87,
A172 and U251 cells were infected at 5 MOI of Ad5-eGFP,
CRAd5-Sp-eGFP and CRAd5/11-Sp-eGFP and harvested in triplicate at
0, 1, 2 and 3 days after infection. Total DNA was isolated from the
cells and viral replication was measured by qPCR using primers for
the Ad5 E4 gene. As seen in Fig.
4, CRAd5-Sp-eGFP and CRAd5/11-Sp-eGFP all demonstrated
significantly enhanced replication of viral genome compared with
Ad5-eGFP (p<0.001). This result indicated that U87, A172 and
U251 cells maintained high level of survivin promoter activity,
which therefore resulted in specific replication of CRAd5-Sp-eGFP
and CRAd5/11-Sp-eGFP in these three glioma cell lines. The result
also suggested that the eGFP expression cassette located between
the fiber and E4 region did not influence the replication
capacity.
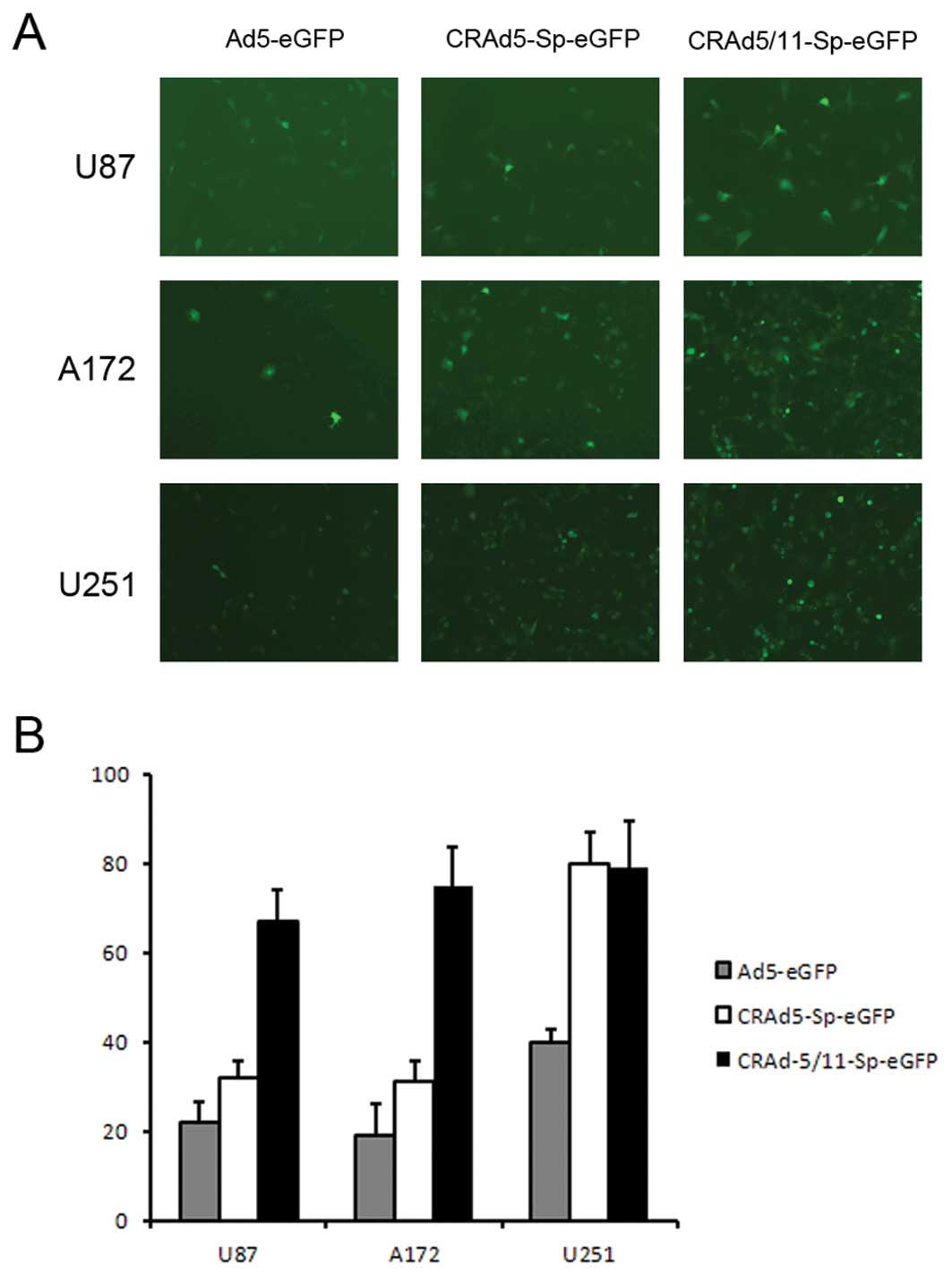
Enhanced transduction efficiency of fiber
modified adenovirus in target cells
To confirm that the improved cytotoxic effect of
Ad5/11 fiber chimeric CRAd in glioma cells, the transduction
efficiency of the chimeric fiber adenovirus was evaluated.
Ad5-eGFP, CRAd5-Sp-eGFP and CRAd5/11-Sp-eGFP were applied to U87,
A172 and U251 cells that express a high level of CD46, the receptor
of Ad11. The results showed that the percentage of eGFP positive
cells in CRAd5/11-Sp-eGFP group was higher than that in the control
groups Ad5-eGFP and CRAd5-Sp-eGFP in U87 and A172 cells (Fig. 5). This indicated that the tropism
efficiently enhanced U87 and A172 target cells and it was mainly
attributed to the modified fiber.
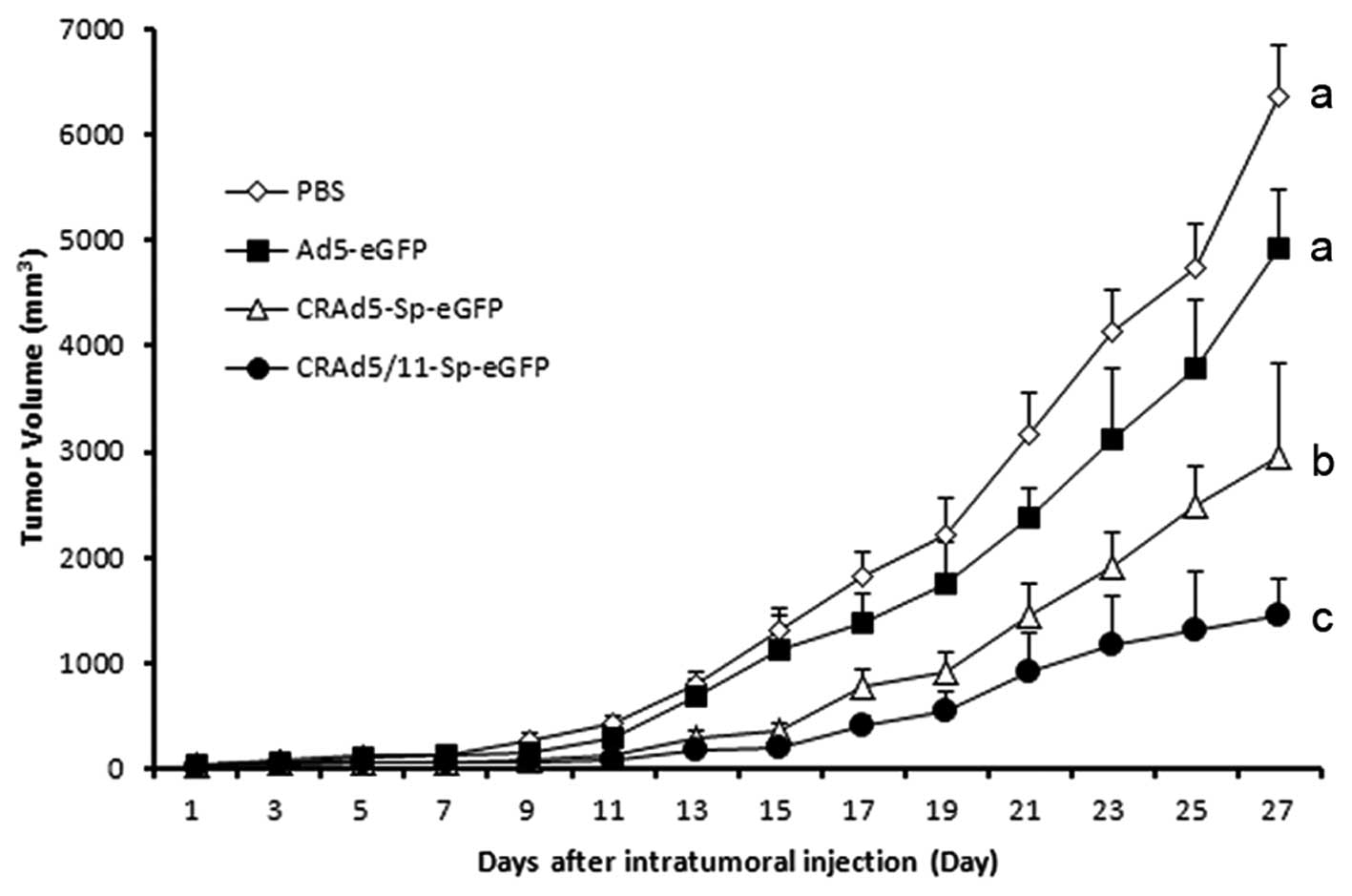
CRAd5/11-Sp-eGFP for improved antitumor
activity in nude mice
In order to evaluate antitumor activity of
CRAd5/11-Sp-eGFP in vivo, U87 human glioma xenograft model
was established in nude mice. When the tumors reached 70-100
mm3, 50 μl of Ad5-eGFP, CRAd5-Sp-eGFP,
CRAd5/11-Sp-eGFP (5×108 PFU each) or PBS as the control
was injected intratumorally every other day four times. The results
indicated that animals treated with CRAd5/11-Sp-eGFP exhibited
significant suppression of tumor growth compared with those treated
with PBS (p<0.05) or Ad5-eGFP (p<0.05).
The average tumor volume of the CRAd5/11-Sp-eGFP
group was about 1,400 mm3 at 29 days after virus
treatment, whereas the tumor volume of PBS- or Ad5-eGFP-treated
mice was 6,351±496.8 or 4,933.4±542.6 mm3, respectively.
In addition, the CRAd5-Sp-eGFP-treated group showed notable
antitumor efficacy compared with the PBS- or Ad5-eGFP-treated group
(p<0.05) (Fig. 6). These data
indicated that survivin-driven fiber chimeric oncolytic adenovirus,
CRAd5/11-Sp-eGFP exerted strong antitumor activity.
Discussion
In the present study, we constructed a
survivin-driven, chimeric 5/11 fiber-modified conditionally
replicating adenovirus, CRAd5/11-Sp-eGFP. In previous studies, the
exogenous gene expression cassettes usually were located in the E1
region or E3 region (22–26). In this study, the exogenous gene
expression cassette was inserted into the region between E4 and the
fiber by using a novel shuttle vector which contained the modified
fiber and an expression cassette. This method provided us a new
option for expressing an exogenous gene in oncolytic adenovirus
except for conventional E1 or E3 region. In addition, the
modification of the fiber protein and the insertion of an exogenous
gene expression cassette into the region between the fiber and E4
could be conveniently done via one step homologous recombination by
using the novel shuttle vector. The results showed that this novel
oncolytic adenoviral vector exhibited enhanced tumor targeting and
antiglioma activity both in vitro and in vivo.
The replicative specificity of CRAd is based on
tumor-specific transcriptional control of the essential early genes
required for replication. The ideal tumor specific promoter (TSP)
would exhibit accurate expression profiles in the specific cancer
tissue. Survivin is an important member of the IAP family that
serves a dual role in the inhibition of apoptosis and regulation of
cell division. One of the most important features of survivin is
the differential expression of this protein in cancer versus normal
tissue. Dramatic overexpression of survivin has been demonstrated
in brain tumor, including malignant glioma (27,28).
Zhu et al showed that the survivin promoter is upregulated
in brain tumors (27). Moreover, a
study from Van Houdt et al demonstrated that survivin
promoter-based CRAd could be efficiently replicated within and kill
a variety of established glioma tumor cells and significantly
inhibit the growth of glioma xenografts in vivo (10). Similarly, in our study the CRAd
that uses the survivin promoter revealed improved replication
activity and oncolytic effect in glioma cells compared with the
controls.
CRAd-mediated gene therapy in the context of
malignant brain tumors is hindered by lack of a sufficient number
of Ad receptors or CAR, on the glioma cell surface (29). Fiber chimeric adenovirus vectors
from species B adenovirus have been reported (3,13,30,31),
but 5/11 fiber with a replication-competent vector to improve virus
transduction in gliomas has not been identified. There is a lack of
5/11 chimeric fiber adenovirus vector that have the high
replication efficiency in gliomas and still carry a quantifiable
marker gene. A precise evaluation of the oncolytic vectors with
5/11 chimeric fiber in different glioma cells remains to be
addressed. In this study, the viral entry into glioma cells is
significantly enhanced when the Ad5 fiber knob is replaced with the
Ad11 knob, the latter of which recognizes the CD46 receptor that is
widely expressed on tumor cells including gliomas (13,18).
In addition, the Ad11 belongs to species B group 3 which
preferentially interacts with CD46, but also uses receptor
desmoglein 2 (32). The Ad3 shares
the receptor desmoglein 2 with Ad11 and Ad35 nearly exclusively
uses CD46 as its receptor. Thus, we hypothesis Ad11 have a more
wide-range to target tumor cells and the results need further
study. In the present study, we have shown that the virus of
CRAd5/11-Sp-eGFP efficiently infected the U87 and A172 cell lines,
as demonstrated by fluorescent microscopy. Therefore, infection of
CRAd5/11-Sp-eGFP significantly inhibited U87 and A172 cell growth.
Nevertheless, in U251 cell, the adenovirus of CRAd5-Sp-eGFP and
CRAd5/11-Sp-eGFP had little difference in transduction efficiency.
Allen et al reported U251 expresses the high level of CAR
compared with other gliomas, such as U87, U118 and TE671 (33). Mäenpää et al found that U251
was moderately positive for CD46 expression (34). These results illustrated that
CRAd5-Sp-eGFP and CRAd5/11-Sp-eGFP possessed the same level of
eGFP-positive cells.
Compared with CRAd5-Sp-eGFP in vitro,
CRAd5/11-Sp-eGFP exhibited higher infection efficiency and stronger
oncolytic ability in glioma cells, and enhanced antitumor efficacy
in human glioma U87 xenograft models. In summary, survivin-driven
and chimeric 5/11 fiber-modified CRAds vector CRAd5/11-Sp-eGFP is a
promising candidate in the treatment of malignant glioma.
Acknowledgements
This study was supported by the
research grants to H.X. from National Natural Science Foundation of
China (no. 30872993 and no. 31070137).
References
|
1.
|
Furnari FB, Fenton T, Bachoo RM, et al:
Malignant astrocytic glioma: genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Grossman SA, Ye X, Piantadosi S, et al:
Survival of patients with newly diagnosed glioblastoma treated with
radiation and temozolomide in research studies in the United
States. Clin Cancer Res. 16:2443–2449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ulasov IV, Zhu ZB, Tyler MA, et al:
Survivin-driven and fiber-modified oncolytic adenovirus exhibits
potent antitumor activity in established intracranial glioma. Hum
Gene Ther. 18:589–602. 2007. View Article : Google Scholar
|
|
4.
|
Chiocca EA, Abbed KM, Tatter S, et al: A
phase I open label, dose-escalation, multi-institutional trial of
injection with an E1B-attenuated adenovirus, ONYX-015, into the
peritumoral region of recurrent malignant gliomas, in the adjuvant
setting. Mol Ther. 10:958–966. 2004. View Article : Google Scholar
|
|
5.
|
Yazaki T, Manz HJ, Rabkin SD and Martuza
EL: Treatment of human malignant meningiomas by G207, a
replication-competent multimutated herpes simplex virus 1. Cancer
Res. 55:4752–4756. 1995.PubMed/NCBI
|
|
6.
|
Markert JM, Medlock MD, Rabkin SD, et al:
Conditionally replicating herpes simplex virus mutant, G207 for the
treatment of malignant glioma: results of a phase I trial. Gene
Ther. 7:867–874. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sonabend AM, Ulasov IV and Lesniak MS:
Gene therapy trials for the treatment of high-grade gliomas. Gene
Ther Mol Biol. 11:79–92. 2007.PubMed/NCBI
|
|
8.
|
Sah NK, Khan Z, Khan GJ and Bisen PS:
Structural, functional and therapeutic biology of survivin. Cancer
Lett. 244:164–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chakravarti A, Noll E, Black PM,
Finkelstein DF, Finkelstein DM, Dyson NJ and Loeffler JS:
Quantitatively determined survivin expression levels are of
prognostic value in human gliomas. J Clin Oncol. 20:1063–1068.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Van Houdt WJ, Haviv YS, Lu B, et al: The
human survivin promoter: a novel transcriptional targeting strategy
for treatment of glioma. J Neurosurg. 104:583–592. 2006.PubMed/NCBI
|
|
11.
|
Ulasov IV, Rivera AA, Sonabend AM, Rivera
LB, Wang M, Zhu ZB and Lesniak MS: Comparative evaluation of
survivin, midkine, and CXCR4 promoters for transcriptional
targeting of glioma gene therapy. Cancer Biol Ther. 6:679–685.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Asaoka K, Tada M, Sawamura Y, Ikeda J and
Abe H: Dependence of efficient adenoviral gene delivery in
malignant glioma cells on the expression levels of the
Coxsackievirus and adenovirus receptor. J Neurosurg. 92:1002–1008.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nandi S, Ulasov IV, Rolle CE, Han Y and
Lesniak MS: A chimeric adenovirus with an Ad 3 fiber knob
modification augments glioma virotherapy. J Gene Med. 11:1005–1011.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Segerman A, Atkinson JP, Marttila M,
Dennerquist V, Wadell G and Arnberg N: Adenovirus type 11 uses CD46
as a cellular receptor. J Virol. 77:9183–9191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gaggar A, Shayakhmetov DM and Lieber A:
CD46 is a cellular receptor for group B adenoviruses. Nat Med.
9:1408–1412. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sirena D, Lilienfeld B, Eisenhut M, et al:
The human membrane cofactor CD46 is a receptor for species B
adenovirus serotype 3. J Virol. 78:4454–4462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Fleischli C, Sirena D, Lesage G, Havenga
MJ, Cattaneo R, Greber UF and Hemmi S: Species B adenovirus
serotypes 3, 7, 11 and 35 share similar binding sites on the
membrane cofactor protein CD46 receptor. J Gen Virol. 88:2925–2934.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Gustafsson DJ, Andersson EK, Hu YL,
Marttila M, Lindman K and Strand M: Adenovirus 11p downregulates
CD46 early in infection. Virology. 405:474–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zhu ZB, Makhija SK, Lu B, et al:
Incorporating the survivin promoter in an infectivity enhanced
CRAd-analysis of oncolysis and antitumor effects in vitro
and in vivo. Int J Oncol. 27:237–246. 2005.PubMed/NCBI
|
|
20.
|
Xia H, Anderson B, Mao Q and Davidson BL:
Recombinant human adenovirus: targeting to the human transferrin
receptor improves gene transfer to brain microcapillary
endothelium. J Virol. 74:11359–11366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Li X, Liu S, Wang D, Chen H and Xia H:
Adenoviral delivered eGFP-intron splicing system for multiple gene
RNAi. Biotechnol Lett. 33:1723–1728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Zhang KJ, Wang YG, Cao X, et al: Potent
antitumor effect of interleukin-24 gene in the survivin promoter
and retinoblastoma double-regulated oncolytic adenovirus. Hum Gene
Ther. 20:818–830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Chen W, Wu Y, Liu W, et al: Enhanced
antitumor efficacy of a novel fiber chimeric oncolytic adenovirus
expressing p53 on hepatocellular carcinoma. Cancer Lett.
307:93–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Xiao LL, Wu YM, Qian J, et al: The
antitumor efficacy of IL-24 mediated by E1A and E1B triple
regulated oncolytic adenovirus. Cancer Biol Ther. 10:242–250. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Bortolanza S, Bunuales M, Otano I, et al:
Treatment of pancreatic cancer with an oncolytic adenovirus
expressing interleukin-12 in Syrian hamsters. Mol Ther. 17:614–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wohlfahrt ME, Beard BC, Lieber A and Kiem
HP: A capsid-modified, conditionally replicating oncolytic
adenovirus vector expressing TRAIL leads to enhanced cancer cell
killing in human glioblastoma models. Cancer Res. 67:8783–8790.
2007. View Article : Google Scholar
|
|
27.
|
Zhu ZB, Makhija SK, Lu B, et al:
Transcriptional targeting of tumors with a novel tumor-specific
survivin promoter. Cancer Gene Ther. 11:256–262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ulasov IV, Tyler MA, Zhu ZB, Han Y, He TC
and Lesniak MS: Oncolytic adenoviral vectors which employ the
survivin promoter induce glioma oncolysis via a process of
beclin-dependent autophagy. Int J Oncol. 34:729–742.
2009.PubMed/NCBI
|
|
29.
|
Fuxe J, Liu L, Malin S, Philipson L,
Collins VP and Pettersson RF: Expression of the coxsackie and
adenovirus receptor in human astrocytic tumors and xenografts. Int
J Cancer. 103:723–729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Silver J and Mei YF: Transduction and
oncolytic profile of a potent replication-competent adenovirus 11p
vector (RCAd11pGFP) in colon carcinoma cells. Plos One.
6:e175322011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Brouwer E, Havenga MJ, Ophorst O, et al:
Human adenovirus type 35 vector for gene therapy of brain cancer:
improved transduction and bypass of pre-existing anti-vector
immunity in cancer patients. Cancer Gene Ther. 14:211–219. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wang H, Li ZY, Liu Y, et al: Desmoglein 2
is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med.
17:96–104. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Allen C, McDonald C, Giannini C, Peng KW,
Rosales G, Russell SJ and Galanis E: Adenoviral vectors expressing
fusogenic membrane glycoproteins activated via matrix
metalloproteinase cleavable linkers have significant antitumor
potential in the gene therapy of gliomas. J Gene Med. 6:1216–1227.
2004. View
Article : Google Scholar
|
|
34.
|
Mäenpää A, Junnikkala S, Hakulinen J,
Timonen T and Meri S: Expression of complement membrane regulators
membrane cofactor protein (CD46), decay accelerating factor (CD55),
and protectin (CD59) in human malignant gliomas. Am J Pathol.
148:1139–1152. 1996.
|