Introduction
Hepatocellular carcinoma (HCC) is the most common
primary malignancy of the liver in adults. It is the fifth most
common solid tumor worldwide and the third leading cause of cancer
mortality with more than 1 million deaths annually.
Historically, surgical resection has been performed
as a curative therapy; more recently, local radiofrequency ablation
(RFA) has been developed. In addition, orthotopic liver
transplantation (OLT) has become a therapeutic option for patients
with HCC. Currently transarterial chemoembolization (TACE) is the
most widely performed treatment for non-resectable HCCs, and
results from randomized controlled trials show a clear survival
benefit after TACE when compared with conservative management
(1,2). Systemic or arterial infusion of
chemotherapeutic drugs is also used to treat non-resectable or
metastatic HCC.
Several factors make it difficult to treat HCC
completely. First, most patients have underlying liver disease
(e.g., liver cirrhosis due to chronic hepatitis C or hepatitis B
virus infection) and impaired liver function; therefore, curative
resection or ablation is often impossible. Second, HCC has a high
rate of recurrence that is caused by intrahepatic metastasis or
multicentric occurrence. Reportedly, the tumor recurrence rate
exceeds 70% at 5 years even after curative resection (3,4).
Similarly, tumor recurrence after OLT is the major obstacle in
preventing successful liver transplantation in patient with HCC
(5). No adjuvant therapy has been
proven to be effective to reduce recurrence rates. Third, HCC is
resistant to conventional cytotoxic chemotherapeutic agents. For
example, tumor response rates for single or multiple agent
chemotherapy regimens are reportedly low and lack durable
remission; these low rates lead to a 1-year survival between 0 to
30% (6). Until recently, level 1
evidence that systemic chemotherapy improves median overall
survival in patients with HCC has been lacking.
Recently, an oral multi-kinase inhibitor, sorafenib,
has become a key drug for treatment of non-resectable HCC. The
results of phase III trials in Europe and Asia showed that
sorafenib increased the overall survival rate in patients with
advanced HCC (7,8). Sorafenib inhibits the
serine/threonine kinase activity of Raf-1 and B-Raf and the
receptor tyrosine kinase activity of vascular endothelial growth
factor receptors (VEGFRs) 1, 2 and 3 and platelet-derived growth
factor receptor-β (PDGFR-β) (9,10),
the cellular signalings of which are implicated in the molecular
pathogenesis of HCC. Despite the encouraging results of sorafenib
for patients with advanced HCC, treatment outcomes are still poor
and necessitate improvement. Several clinical trials have shown
that combination therapies of sorafenib and TACE or infusion of a
cytotoxic chemotherapeutic agent are effective for HCC (11–16);
however, these combinations had only modest survival benefits.
TRAIL selectively induces apoptosis in various
transformed cell lines. Although HCC cells express TRAIL receptors,
these cells are resistant to TRAIL-induced apoptosis (17,18).
However, sorafenib may sensitize HCC cells to TRAIL-induced
apoptosis (19–21).
Given these former results, it is still unclear
whether hypoxia, TRAIL or cytotoxic chemotherapeutic agents is the
better adjuvant to sorafenib in the treatment of HCC; therefore, we
investigated their effects in combination with sorafenib on two HCC
cell lines.
Material and methods
Cell lines and reagents
The human HCC cell lines, HepG2 and Huh 7, were
purchased from the Riken Bioresource Center Cell Bank (Tsukuba,
Japan). Both cell lines were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 1% penicillin/streptomycin (Gibco-BRL, Grand
Island, NY, USA) and 10% heat-inactivated fetal calf serum (FCS)
(Gibco-BRL). Recombinant TRAIL was purchased from R&D Systems
(Minneapolis, MN, USA). Sorafenib was purchased from LC
Laboratories (Woburn, MA, USA). Cisplatin (CDDP), fluorouracil
(5-FU) and doxorubicin were purchased from Sigma-Aldrich.
Cell proliferation and viability
assays
MTT assay
HCC cells were plated at a density of
1×104 cells per well in 96-well microtiter plates
(Corning Glass Works, Corning, NY, USA) and each plate was
incubated for 5 h at 37°C in 5% CO2. Next, 50 μl
of a drug solution was added to each well and the plates were
incubated for 48 h. The live-cell count was assayed using a Cell
Titer 96 Assay kit (Promega, Madison, WI, USA) according to the
manufacturer’s instructions. The absorbance of the contents of each
well was measured at 570 nm with a microtiter plate reader (Bio-Rad
Laboratories, Hercules, CA, USA).
xCELLigence system
Cell proliferation and viability was also assessed
with xCELLigence system (Roche Inc., Basel, Switzerland) according
to manufacturer’s instruction. Briefly, each well of each 16-well
microtiter plate (E-Plate 16) was filled with 100 μl of DMEM
to equilibrate the well membrane, and each plate was incubated for
30 min at 37°C in 5% CO2. HCC cells suspended in 50
μl of growth medium were seeded at a density of
1×104 cells per well. Cells were cultured for 12 to 48
h, with the Real-Time Cell Analyzer (RTCA) single plate (SP).
Instrument placed in a standard incubator at 37°C in 5%
CO2, followed by addition of 50 μl of drug
solution. Then, cell proliferation was monitored by recording Cell
Index (CI) values at 15 min intervals for 48 h.
Detection of apoptosis-related
proteins on immunoblots
Expression of survivin, FLIP, XIAP and Bcl-xL in HCC
cell lines were analyzed using immunoblots. Briefly, cells were
harvested after stimulation with sorafenib (0, 2, 5, 10 μM)
for 24 h. Cells were lysed on ice in lysis buffer (50 mM/l Tris-HCl
pH 8.0, 150 mM/l NaCl, 5 mM/l ethylenediaminetetraacetic acid, 1%
NP-40, 1 mM phenylmethylsulfonyl fluoride). Each mixture was
subjected to centrifugation, each supernatant was collected and the
protein content of each sample was measured using the Bio-Rad
protein assay kit (Bio-Rad Laboratories). Aliquots from each sample
containing equal amounts of protein were subjected to 14% sodium
dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and
the separated proteins were transferred onto nitrocellulose
membranes (Toyo Roshi, Tokyo, Japan) using the Bio-Rad
electrotransfer system (Bio-Rad Laboratories). Blots were incubated
in 5% milk with Tris-HCl pH 7.5 and 0.1% Tween-20 for 2 h at room
temperature to block non-specific antibody binding; blots were then
incubated overnight at 4°C with mouse anti-survivin antibody (Santa
Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-FLICE
inhibitory protein (FLIP) monoclonal antibody (Medical &
Biological Laboratories Co., Nagoya, Japan), rabbit anti-B cell
lymphoma-extra large (Bcl-xL) polyclonal antibody (Transduction,
Lexington, KY, USA), or mouse anti-X-linked inhibitor of apoptosis
protein (XIAP) monoclonal antibody (Transduction), diluted 1:1,000
with 5% skim milk in Tris-HCl (pH 7.5) and 0.1% Tween-20. The
immunoblots were then washed and incubated with horseradish
peroxidase conjugated anti-mouse monoclonal IgG or horse-radish
peroxidase-conjugated anti-rabbit IgG (diluted 1:2,000 with 5% milk
in Tris-HCl pH 7.5). Finally, after 3 washes, signals were detected
using an ECL kit (Amersham Pharmacia Biotech, Buckinghamshire,
UK).
Detection of apoptosis
HepG2 cells (1×104 cell/dish) were
cultured in 35-mm dishes for 24 h, then 100 ng/ml of recombinant
human TRAIL, 2 μM of sorafenib or both were added to each
culture. After incubation for 24 h, cell nuclei were stained with
DAPI (Sigma-Aldrich) and examined with a fluorescence microscope
(Zeiss, Gottingen, Germany).
Wnt reporter gene assay
Human HCC cells, HepG2 or Huh7, were incubated in
96-well plates at a density of 1.5×104 cells/well for 24
h at 37°C. The cells were transfected with Cignal TCF/LEF Reporter
luc vector (Qiagen, Tokyo, Japan) using the FuGENE HD Transfection
Reagent (Roche Applied Science, Mannheim, Germany) and incubated
for 24 h at 37°C. Cells were stimulated with 2 μM of
sorafenib for 24 or 48 h. Luciferase activity was determined from
cell extracts using the Dual-Glo luciferase assay system (Promega)
and a 2030 ARVO X luminometer (Perkin-Elmer, Waltham, MA, USA)
according to the manufacturer’s instruction.
Inducing hypoxia
HCC cells were plated at a density of
1×104 cells per well in 96-well microtiter plates, and
each plate was incubated for 24 h at 37°C in 5% CO2. The
media in these plates were replaced with glucose-free and FBS-free
media with or without 2 μM sorafenib; the plates were then
put in a Hypoxia chamber (Veritas Co., Tokyo, Japan), and gas
mixture of 5% CO2 and 95% N2 was flushed
through the chamber at the flow rate of 2 liters per min for 10
min. The chamber was then incubated for 48 h at 37°C in 5%
CO2. The live-cell count was determined using a Cell
Titer 96 Assay kit (Promega) as previously described. In addition,
the TCF/LEF reporter gene assay describe above was also performed
using these hypoxic cells.
Results
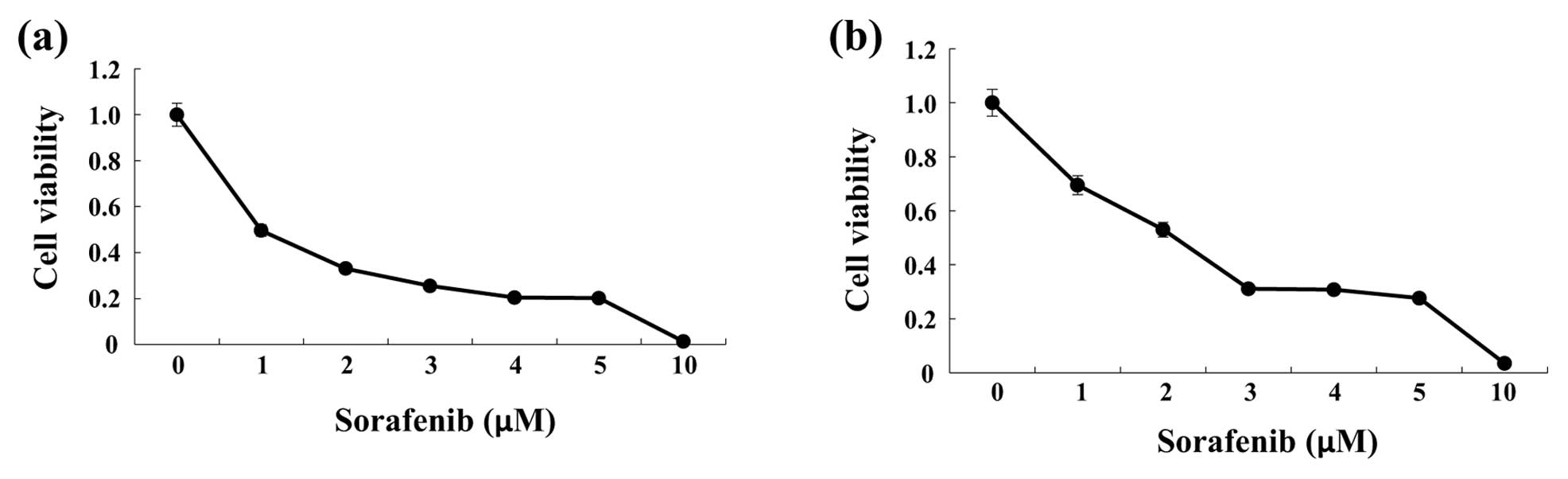
Sorafenib reduced viability of HCC
cells
To investigate changes in cell viability in response
to sorafenib, HCC cells (HepG2 or Huh7) were incubated with various
concentrations of sorafenib for 48 h. Sorafenib decreased cell
viability of HepG2 and Huh7 cells in a concentration-dependent
manner (Fig. 1).
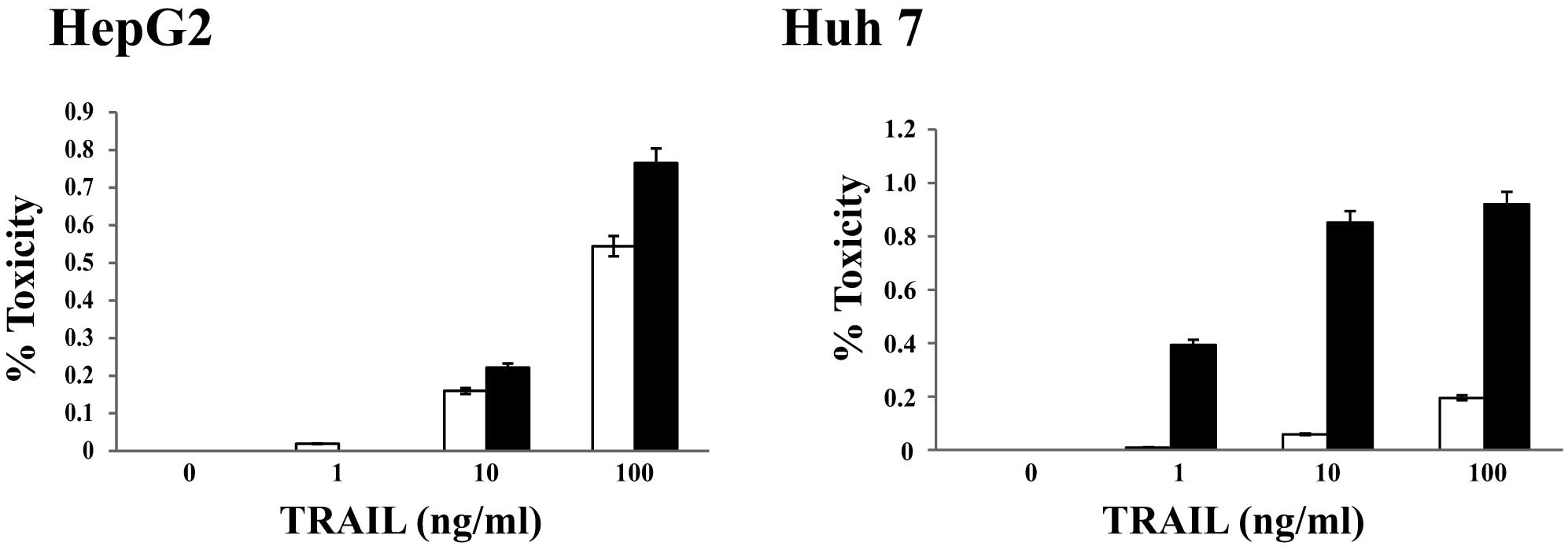
Sorafenib augmented TRAIL-induced
apoptosis in two HCC cell lines
The effects of TRAIL in combination with sorafenib
treatment on HCC cells were examined. We incubated human HCC cells,
with or without 2 μM of sorafenib, with one of four
concentrations (0, 1, 10 or 100 ng/ml) of recombinant human TRAIL;
each concentration is clinically relevant in patients. Cell
viability was assessed after 48 h. Surprisingly, the percentage of
dead cells (HepG2 and Huh7) was higher for cells treated with 2
μM sorafenib and 100 ng/ml TRAIL than for those treated with
only 100 ng/ml TRAIL (Fig. 2). In
fact, the percent lethality for Huh7 cells was approximately 19%
when treated with only 100 ng/ml TRAIL, but it increased to
approximately 92% when cells were treated with 2 μM
sorafenib and 100 ng/ml TRAIL (Fig.
2). The cytotoxicity, as assessed using the xCELLingence
system, was similar to that determined by MTT assay (data not
shown). These results indicated that the combination of TRAIL and
sorafenib has synergistic effects on cytotoxicity for HCC
cells.
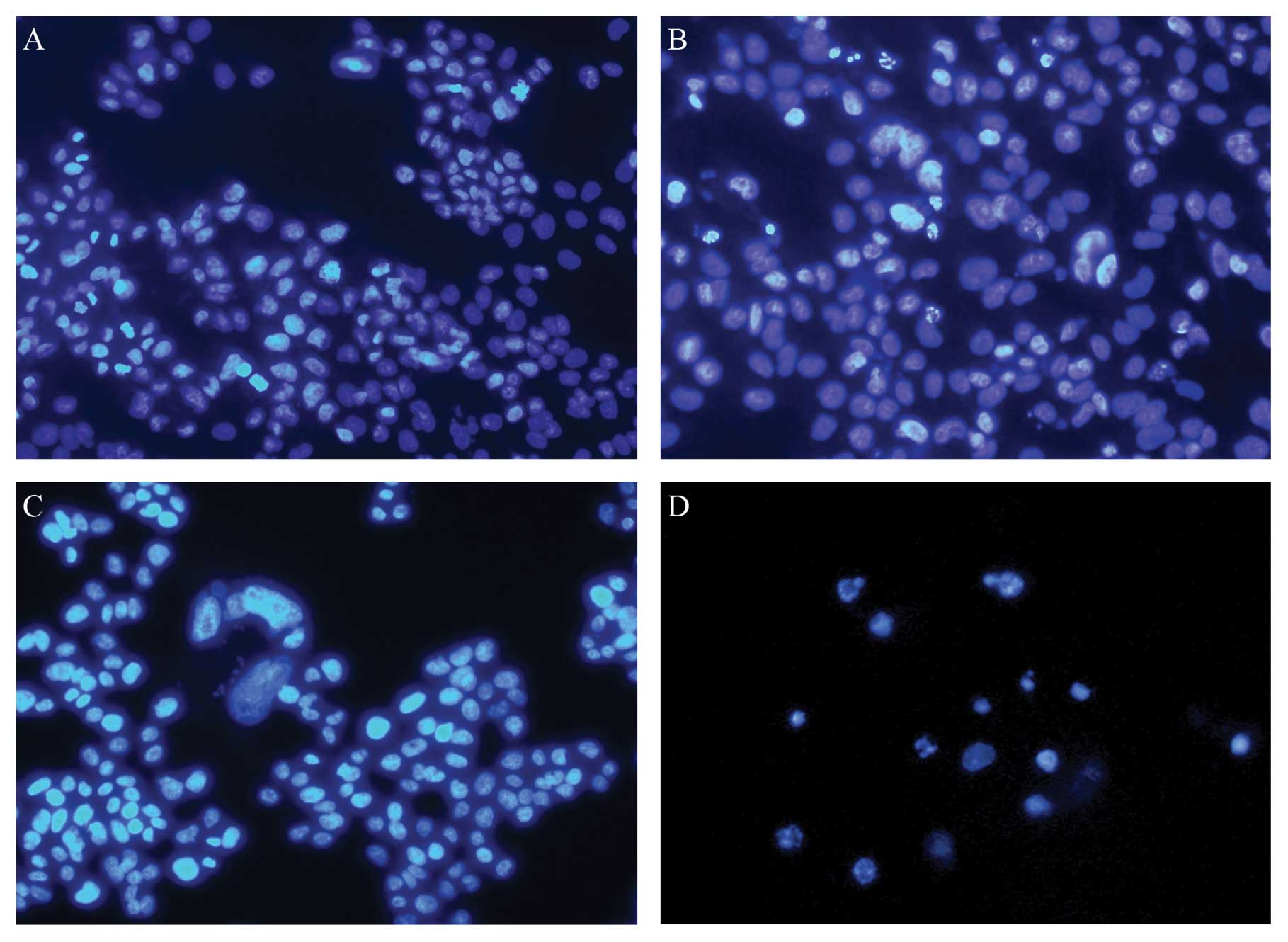
To assess whether sorafenib could induce apoptosis
in HCC cells, we stained HepG2 cells with DAPI 24 h after treatment
with 10 ng/ml recombinant human TRAIL and 2 μM sorafenib.
HepG2 cells treated with TRAIL and sorafenib showed features
typical of apoptosis (Fig. 3).
Based on these findings, we concluded that the reduced viability of
these cells was due to augmented apoptosis.
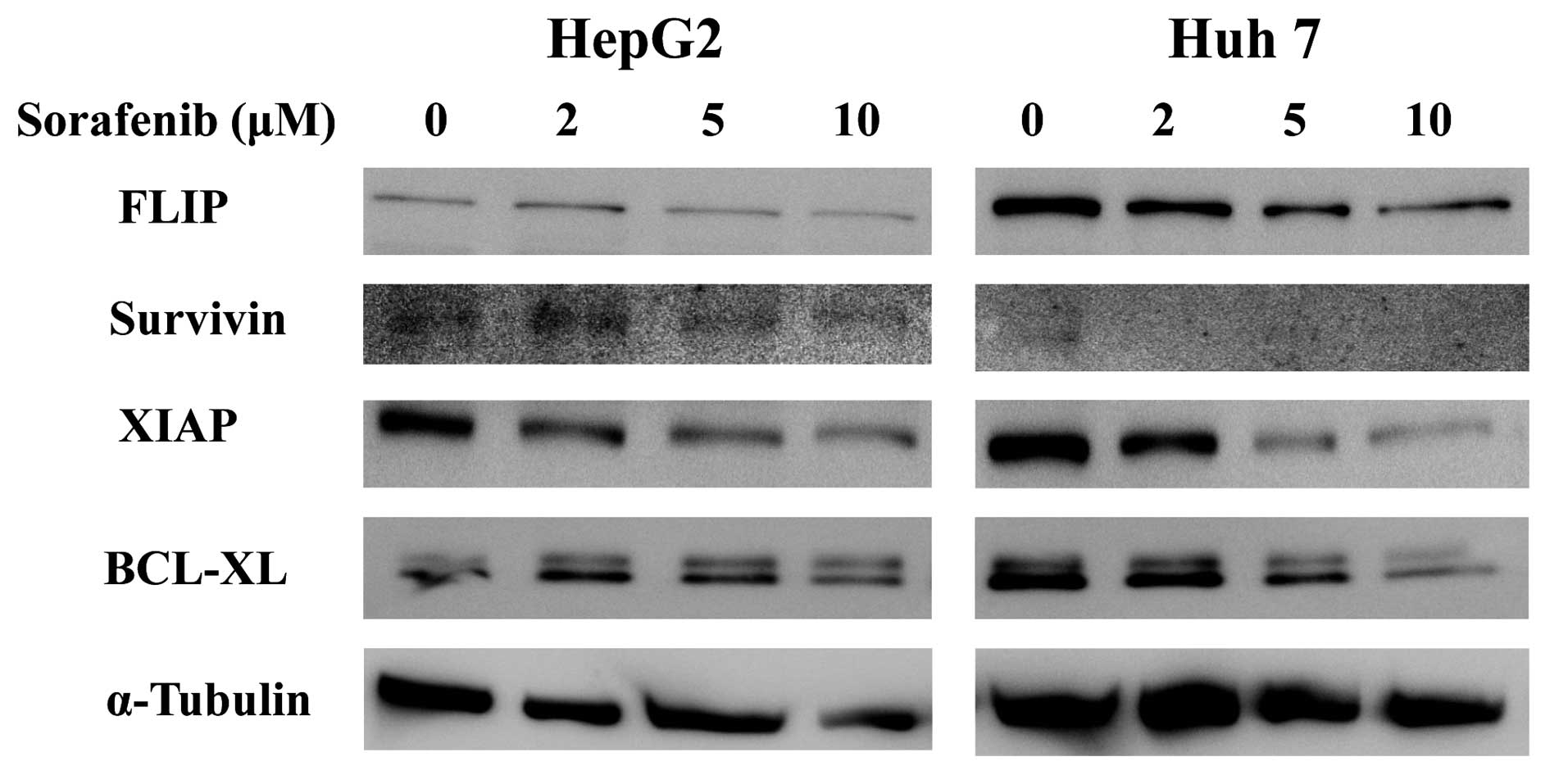
Sorafenib suppressed expression of
apoptosis-related proteins
Next, we used immunoblots to investigate the effects
of sorafenib on intracellular levels of survivin, FLIP, XIAP and
Bcl-xL because these proteins play a major role in controlling
apoptotic pathways (22–25). In both HepG2 and Huh7, levels of
survivin, XIAP and Bcl-xL were markedly reduced in response to
sorafenib treatment in a concentration-dependent manner (Fig. 4).
Anticancer drugs did not have
synergistic effects with sorafenib on viability of HCC cells
We next investigated the effects of sorafenib in
combination with chemotherapy (CDDP, 5-FU or doxorubicin). We
cultured human HCC cells with recombinant human CDDP (0, 10, 100,
500, 1,000 μg/ml), 5-FU (0, 1, 10, 100, 500 μg/ml),
or doxorubicin (0, 0.01, 0.1, 1, 10 mg/ml), which are all
clinically relevant doses in patients, in the presence or absence
of 2 μM sorafenib and cell viability was examined after 48
h. At the highest respective doses, each anticancer agent increased
the percentage of dead cells (HepG2 and Huh7), but sorafenib had
only a slight cytotoxic effect on the cells. In fact, the percent
lethality of 100 μg/ml CDDP alone versus 100 μg/ml
CDDP and sorafenib were approximately 25 versus 45%, respectively,
in HepG2 cells and approximately 13 versus 36%, respectively, in
Huh7 cells (Fig. 5). Similar
results were obtained with using 5-FU or doxorubicin (data not
shown).
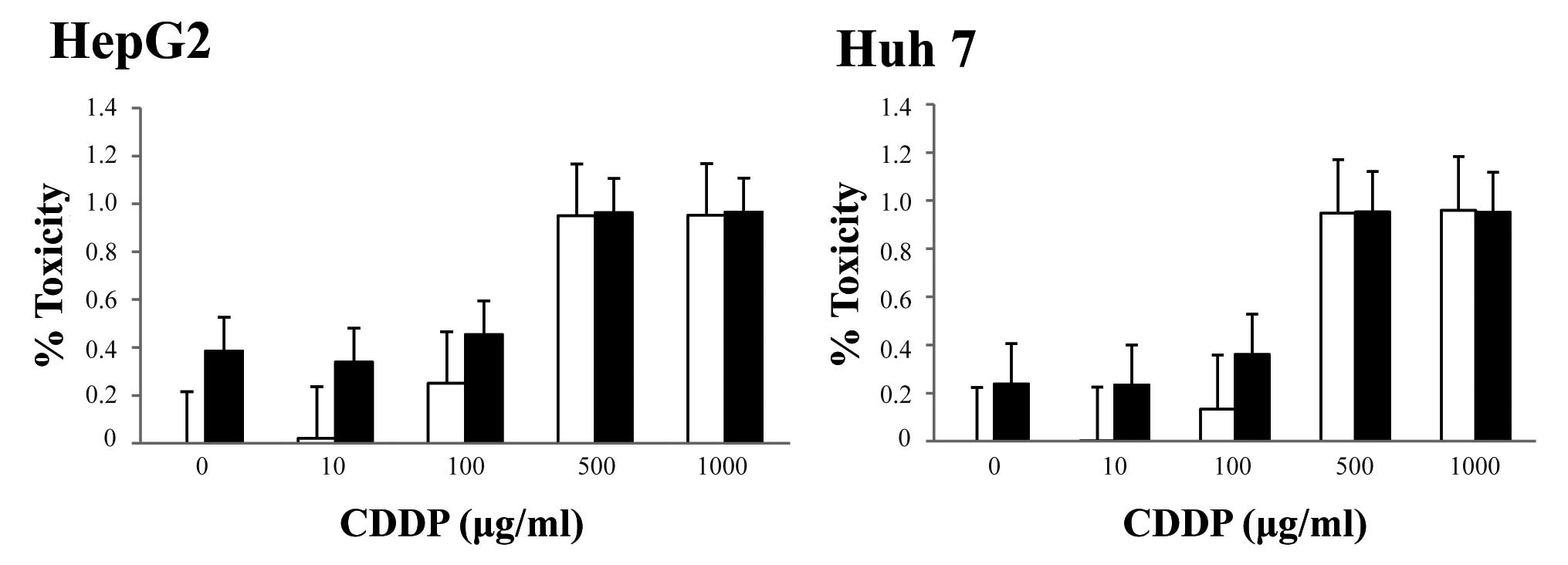 | Figure 5.Effect of the combination of
sorafenib and CDDP on HCC cells. HCC cells were incubated with
various concentrations of CDDP with/without sorafenib for 48 h.
Cell viability was assessed using the MTT assay. Percent lethality
in HCC cells incubated with 0, 10, 100, 500, or 1,000 μg/ml
CDDP alone (□), or with 0, 10, 100, 500 or 1,000 μg/ml CDDP
and 2 μM sorafenib (▪) was determined relative to control
cells. Data are expressed as the mean ± SD of six independent
experiments. |
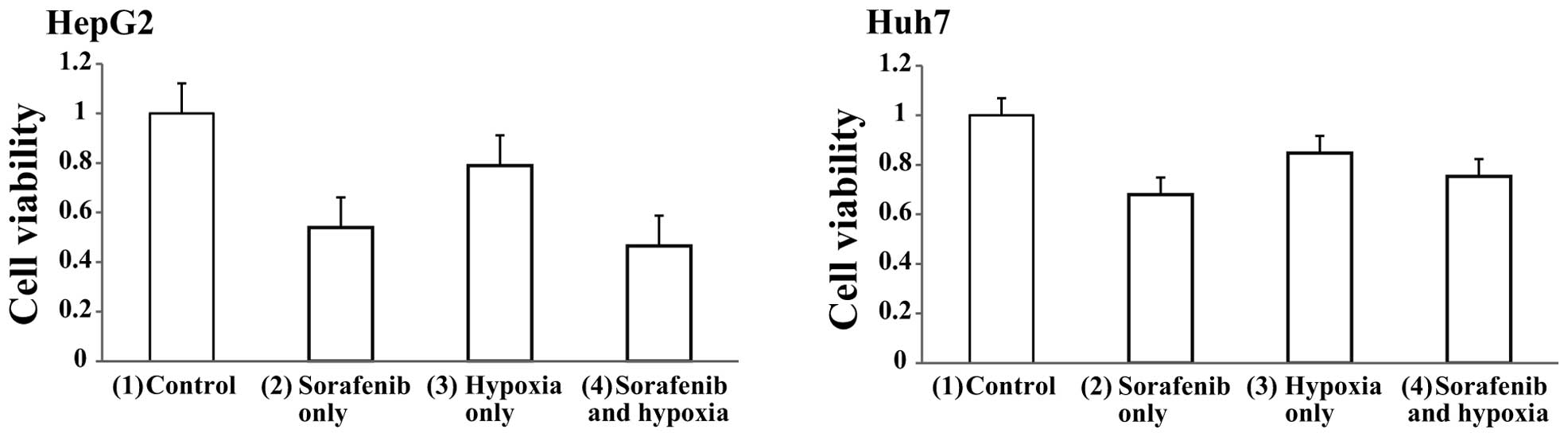
Apoptotic activity in of sorafenib is
not enhanced in hypoxic HCC cells
Next, we examined the cytotoxic effects of the
combination of hypoxia and sorafenib on HCC cells. Both HepG2 and
Huh7 cells were divided into four groups; (1) control (sorafenib−, hypoxia−),
(2) sorafenib only (2 μM
sorafenib+, hypoxia−), (3) hypoxia
only (sorafenib−, hypoxia+), (4)
sorafenib and hypoxia (2 μM sorafenib+, hypoxia+) and cell
viabilities were determined after 48 h of treatment using the MTT
assay. Cell viabilities in HepG2 cells were approximately (2) 55, (3) 80 and (4) 50% of control cells (Fig. 6A). Similarly, cell viabilities in
Huh7 cells were (2) 70, (3) 85 and (4) 80% of control cells (Fig. 6B). These results indicated that
hypoxic treatment did not enhance the effect of sorafenib.
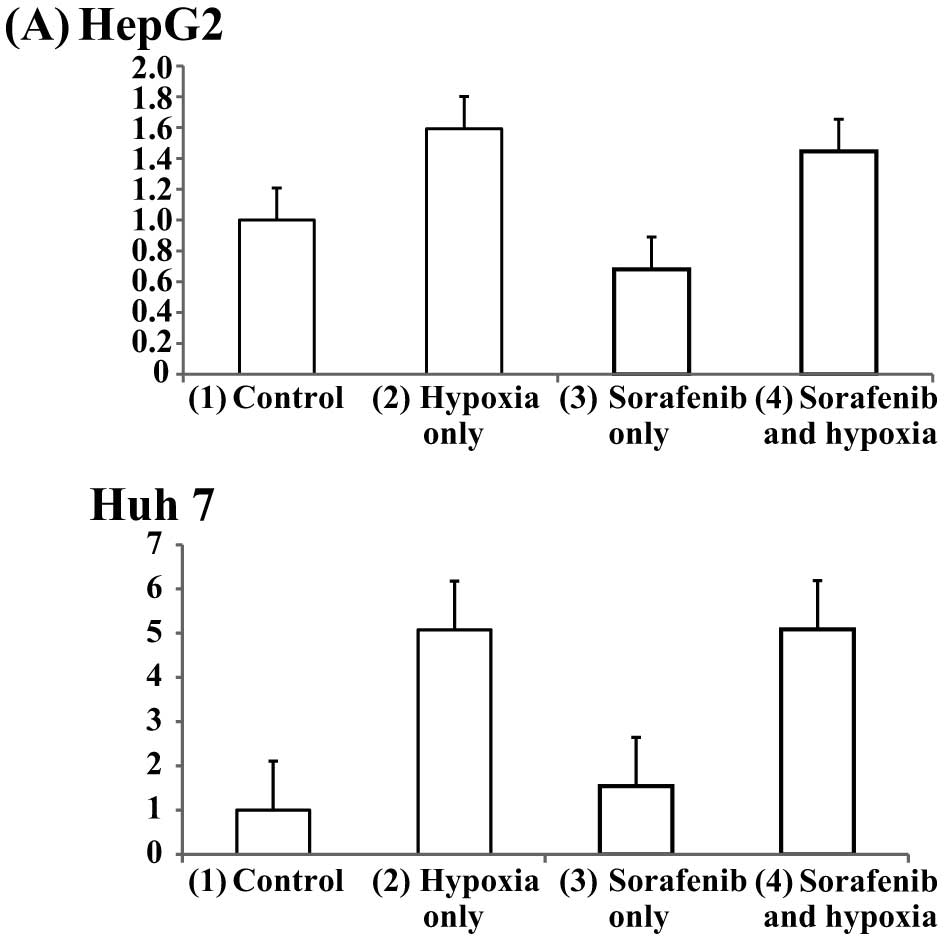
Wnt/β-catenin signal are activated by
hypoxia in HCC cell lines
Next, to examine whether sorafenib and hypoxia
affected the Wnt/β-catenin pathway, HCC cells were plated at a
density of 1.5×104 cells per well in 96-well microtiter
plates and incubated for 5 h at 37°C. The cells were transfected
with the TCF/LEF Reporter using the FuGENE HD Transfection Reagent.
After 24 h, the cells were stimulated with 2 μM of sorafenib
or with nothing and subjected to a normal oxygen concentration or
hypoxia for 12 or 24 h. Wnt/β-catenin activities were assessed
using a luciferase assay as previously described. Both HepG2 and
Huh7 cells were divided into four groups; (1) control (sorafenib−, hypoxia−),
(2) hypoxia only (sorafenib−,
hypoxia+), (3) sorafenib only (2
μM sorafenib+, hypoxia−), (4) sorafenib and hypoxia (2 μM
sorafenib+, hypoxia+). The signal activity ratio of cells in each
group relative to control cells after 12 h were (2) 1.6, (3) 0.7, (4) 1.4 for HepG2 cells, and (2) 5.0, (3) 1.5, (4) 5.0 for Huh7 cells (Fig. 7A). Similarly, the signal activity
ratio of cells in each group after 24 h was (2) 4.0, (3) 1.3, (4)
2.4 for HepG2 cells and (2) 1.4, (3) 1.4, (4) 1.9 for Huh7 cells
(Fig. 7B). These results indicated
that Wnt/β-catenin signaling was activated significantly by hypoxia
in HCC cells in the presence or absence of sorafenib and that
sorafenib alone did not affect Wnt/β-catenin signaling.
Discussion
We examined the effects of sorafenib on TRAIL
signaling, on cytotoxic chemotherapeutic agents, and on hypoxia to
determine which adjuvant could potentiate a sorafenib-based
treatment for HCC. The results demonstrated that TRAIL and
sorafenib was the best combination among the treatments examined in
this study.
In a phase III trial performed in Europe (SHARP
study), sorafenib prolonged the median overall survival of patients
with HCC by three months (10.7 months in the sorafenib group and
7.9 months in the placebo group) (7). The sorafenib group also showed
prolonged median overall survival compared to the placebo group in
a trial in the Asia-Pacific region (6.5 months vs. 4.2 months)
(8). Additionally, Kim et
al reported that sorafenib resulted in superior survival in
patients with HCC with extra-hepatic spread and
massive/infiltrative tumors compared to other therapies such as
TACE, systemic cytotoxic chemotherapy or radiotherapy (26). The most important characteristic of
sorafenib is that it has many points of action. However, despite
the advantage over other drugs, sorafenib still does not control
the progression of HCC completely, and it does not change prognosis
drastically. One reason for the limited efficacy of sorafenib is
that HCCs are complex and heterogeneous tumors each with several
genomic alternations. Therefore, many key signal transduction
pathways are implicated in the pathogenesis of HCC (27,28).
Sorafenib does not seem to be able to block all of these signaling
pathways, and it is possible that when one pathway is blocked by
sorafenib, another pathway is activated to compensate for the loss.
Therefore, we believe adjuvant treatment to sorafenib is necessary
to improve the outcomes of treatments for HCC.
Here, we found that sorafenib significantly enhanced
the cytotoxic effects of TRAIL signaling on HCC cells. Moreover,
DAPI staining showed nuclear fragmentation, indicating increased
apoptosis. However, similar effects were not observed with Fas
ligation (data not shown). TRAIL triggers apoptosis by binding to
DR4 or DR5 receptors, and the death domains (DD) of these receptors
recruits Fas associated DD-containing protein (FADD), which in turn
binds pro-caspase 8. Pro-caspase 8 is then activated by
autoproteolytic cleavage, and this activation results in the
initiation of apoptotic signaling. TRAIL-based therapies are
currently undergoing phase I/II clinical evaluation in cancer
patients (29,30). However, many types of cancer cells
including HCC are resistant to TRAIL-signaling. Inhibitor of
apoptosis (IAPs) are overexpressed on HCC cells and confer tumor
cell survival and proliferation mainly by inhibiting the caspase
cascade (22–24). Here, we also found that sorafenib
downregulated several IAPs (FLIP, Survivin, and XIAP) and Bcl-xL
(Fig. 4). Overexpression of these
anti-apoptotic proteins is one of the main factors that neutralizes
TRAIL-related signaling and causes HCC cells to be resistant to
TRAIL. Our results indicated that sorafenib sensitized HCC cells to
TRAIL signaling by downregulating these anti-apoptotic proteins.
Reportedly, sorafenib inhibits phosphorylation of STAT 3, which
regulates expression of numerous apoptosis-related proteins
including Bcl-xL, Mcl-1 and survivin in pancreas cancer cells and
HCC cells (21,31); moreover, Llobet et al have
also reported that sorafenib reduces both Mcl-1 and FLIP levels in
endometrial cancer cells (32).
Our results are consistent with these previous findings; however,
all data, including ours, were obtained using cultured cancer
cells, and we think further evaluation using in vivo
experiments is needed.
We also examined the effect of sorafenib in
combination with hypoxia and each of three cytotoxic
chemotherapeutic agents (CDDP, 5-FU, or doxorubicin), all of which
are routinely used in systemic chemotherapy or TACE for treatment
of HCC. Several clinical studies have previously suggested that
sorafenib is effective in combination with 5-FU or doxorubicin in
the treatment of advanced HCC (12,13,16).
However, the effect of these chemotherapeutic drugs in combination
with sorafenib cannot be evaluated because the sorafenib-alone
groups were not used as the controls in these studies. Our result
showed that sorafenib did not add to the effects of any of the
chemotherapeutic drugs examined. Though the reasons for this
ineffectiveness were not examined in our study, Heim et al
showed that sorafenib significantly reduced uptake of platinum
compounds by colorectal cancer cell lines (33) and it may be that a similar
phenomenon occurred in HCC cells as well.
In addition, a phase III clinical study comparing
TACE plus sorafenib versus TACE plus placebo in patients with HCC
is ongoing (11). However, our
data demonstrated that hypoxia did not enhance the cytotoxic effect
of sorafenib, that sorafenib did not increase the effect of hypoxia
on HCC cells and that hypoxic treatment activated Wnt/β-catenin
signaling in HCC cells. In the canonical Wnt signaling pathway,
β-catenin plays an important role in cell survival, proliferation
and differentiation (34). In the
absence of Wnt ligand, β-catenin is phosphorylated by a cytosolic
multiprotein complex that includes axin, adenomatous polyposis coli
(APC) and glycogen synthase kinase 3 (GSK-3); then β-catenin is
degraded by the ubiquitin/proteasome system. When Wnt binds the
Frizzled trans-membrane receptors, Disheveled (Dsh) inhibits GSK-3
and stabilizes β-catenin. Accumulated β-catenin then binds to T
cell-specific transcription factor/lymphoid enhancer-binding factor
1 (TCF/LEF) to transactivate proliferation-related genes. Several
studies have indicated that Wnt signaling is modulated by hypoxia.
Reportedly, hypoxia-inducible factor-1α (HIF-α) suppresses Wnt
signaling by directly binding β-catenin and causing the
β-catenin/TCF/LEF complex to dissociate (35) or by binding arrest defective 1
(ARD1) and inhibiting acetylation of β-catenin (36). However, other studies have shown
that HIF-α enhances β-catenin activation and expression of TCF/LEF
(37) and that, under hypoxic
conditions, Wnt signaling promotes hepatocyte survival by involving
β-catenin as a transcriptional coactivator of HIF-α (38). In light of our finding that hypoxia
induced activation of β-catenin-TCF/LEF signaling in HCC cells, it
may be that this activation of β-catenin is, in part, responsible
for the reduced efficacy of TACE to HCC.
In conclusion, our data demonstrated that the
TRAIL-sorafenib combination has a synergistic cytokilling effect on
HCC cells and that this effect derives from the downregulation of
anti-apoptotic proteins by sorafenib. We believe that among
currently available procedures, TRAIL is the most promising
adjuvant to sorafenib for the treatment of HCC; however, we
acknowledge that clinical data are still lacking. In this regard,
further investigation with clinical trial is necessary.
References
|
1.
|
Lo CM, Ngan H, Tso WK, et al: Randomized
controlled trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology. 35:1164–1171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar
|
|
3.
|
Poon RT, Fan ST, Lo CM, Liu CL and Wong J:
Intrahepatic recurrence after curative resection of hepatocellular
carcinoma: long-term results of treatment and prognostic factors.
Ann Surg. 229:216–222. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Minagawa M, Makuuchi M, Takayama T and
Kokudo N: Selection criteria for repeat hepatectomy in patients
with recurrent hepatocellular carcinoma. Ann Surg. 238:703–710.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Takada Y and Uemoto S: Liver
transplantation for hepatocellular carcinoma: the Kyoto experience.
J Hepatobiliary Pancreat Sci. 17:527–532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Thomas MB, Jaffe D, Choti MM, et al:
Hepatocellular carcinoma: consensus recommendations of the National
Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol.
28:3994–4005. 2010. View Article : Google Scholar
|
|
7.
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chang YS, Adnane J, Trail PA, et al:
Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization
and induces tumor apoptosis and hypoxia in RCC xenograft models.
Cancer Chemother Pharmacol. 59:561–574. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hoffmann K, Glimm H, Radeleff B, et al:
Prospective, randomized, double-blind, multi-center, phase III
clinical study on transarterial chemoembolization (TACE) combined
with Sorafenib versus TACE plus placebo in patients with
hepatocellular cancer before liver transplantation - HeiLivCa
[ISRCTN24081794]. BMC Cancer. 8:3492008.PubMed/NCBI
|
|
12.
|
Abou-Alfa GK, Johnson P, Knox JJ, et al:
Doxorubicin plus sorafenib vs doxorubicin alone in patients with
advanced hepatocellular carcinoma: a randomized trial. JAMA.
304:2154–2160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hsu CH, Shen YC, Lin ZZ, et al: Phase II
study of combining sorafenib with metronomic tegafur/uracil for
advanced hepatocellular carcinoma. J Hepatol. 53:126–131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR,
Bhagat N and Geschwind JF: Phase II trial of sorafenib combined
with concurrent transarterial chemoembolization with drug-eluting
beads for hepatocellular carcinoma. J Clin Oncol. 29:3960–3967.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cabrera R, Pannu DS, Caridi J, et al: The
combination of sorafenib with transarterial chemoembolisation for
hepatocellular carcinoma. Aliment Pharmacol Ther. 34:205–213. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Petrini I, Lencioni M, Ricasoli M, et al:
Phase II trial of sorafenib in combination with 5-fluorouracil
infusion in advanced hepatocellular carcinoma. Cancer Chemother
Pharmacol. 69:773–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yamanaka T, Shiraki K, Sugimoto K, et al:
Chemotherapeutic agents augment TRAIL-induced apoptosis in human
hepatocellular carcinoma cell lines. Hepatology. 32:482–490. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Shin EC, Seong YR, Kim CH, et al: Human
hepatocellular carcinoma cells resist to TRAIL-induced apoptosis,
and the resistance is abolished by cisplatin. Exp Mol Med.
34:114–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ricci MS, Kim SH, Ogi K, et al: Reduction
of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes
resistant human cancer cells to TRAIL-induced death. Cancer Cell.
12:66–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Koehler BC, Urbanik T, Vick B, et al:
TRAIL-induced apoptosis of hepatocellular carcinoma cells is
augmented by targeted therapies. World J Gastroenterol.
15:5924–5935. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Chen KF, Tai WT, Liu TH, et al: Sorafenib
overcomes TRAIL resistance of hepatocellular carcinoma cells
through the inhibition of STAT3. Clin Cancer Res. 16:5189–5199.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ito T, Shiraki K, Sugimoto K, et al:
Survivin promotes cell proliferation in human hepatocellular
carcinoma. Hepatology. 31:1080–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Okano H, Shiraki K, Inoue H, et al:
Cellular FLICE/caspase-8-inhibitory protein as a principal
regulator of cell death and survival in human hepatocellular
carcinoma. Lab Invest. 83:1033–1043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Shiraki K, Sugimoto K, Yamanaka Y, et al:
Overexpression of X-linked inhibitor of apoptosis in human
hepatocellular carcinoma. Int J Mol Med. 12:705–708.
2003.PubMed/NCBI
|
|
25.
|
Yamaguchi Y, Shiraki K, Fuke H, et al:
Targeting of X-linked inhibitor of apoptosis protein or survivin by
short interfering RNAs sensitize hepatoma cells to TNF-related
apoptosis-inducing ligand- and chemotherapeutic agent-induced cell
death. Oncol Rep. 14:1311–1316. 2005.
|
|
26.
|
Kim JW, Lee JO, Han SW, et al: Clinical
outcomes of sorafenib treatment in patients with metastatic
hepatocellular carcinoma who had been previously treated with
fluoropyrimidine plus platinum-based chemotherapy. Am J Clin Oncol.
34:125–129. 2010.
|
|
27.
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Koschny R, Walczak H and Ganten TM: The
promise of TRAIL - potential and risks of a novel anticancer
therapy. J Mol Med (Berl). 85:923–935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Huang S and Sinicrope FA: Sorafenib
inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in
human pancreatic cancer cells. Mol Cancer Ther. 9:742–750. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Llobet D, Eritja N, Yeramian A, et al: The
multikinase inhibitor Sorafenib induces apoptosis and sensitises
endometrial cancer cells to TRAIL by different mechanisms. Eur J
Cancer. 46:836–850. 2010. View Article : Google Scholar
|
|
33.
|
Heim M, Scharifi M, Zisowsky J, et al: The
Raf kinase inhibitor BAY 43-9006 reduces cellular uptake of
platinum compounds and cytotoxicity in human colorectal carcinoma
cell lines. Anticancer Drugs. 16:129–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Willert K and Jones KA: Wnt signaling: is
the party in the nucleus? Genes Dev. 20:1394–1404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Kaidi A, Williams AC and Paraskeva C:
Interaction between beta-catenin and HIF-1 promotes cellular
adaptation to hypoxia. Nat Cell Biol. 9:210–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Lim JH, Chun YS and Park JW:
Hypoxia-inducible factor-1alpha obstructs a Wnt signaling pathway
by inhibiting the hARD1-mediated activation of beta-catenin. Cancer
Res. 68:5177–5184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Mazumdar J, O’Brien WT, Johnson RS, et al:
O2 regulates stem cells through Wnt/beta-catenin signalling. Nat
Cell Biol. 12:1007–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Lehwald N, Tao GZ, Jang KY, Sorkin M,
Knoefel WT and Sylvester KG: Wnt-beta-catenin signaling protects
against hepatic ischemia and reperfusion injury in mice.
Gastroenterology. 141:707–718. 2011. View Article : Google Scholar : PubMed/NCBI
|