Introduction
Cancer is a serious public health issue worldwide.
Pancreatic cancer (PC) is the fourth to fifth most common type of
cancer in Western countries and has the highest case-fatality rate
of any of the major cancer types (1). In Korea, the prevalence of PC is
generally increasing by 1.7% per year and in 2008, the mortality
rate from PC was ranked fifth (2).
The only effective therapy is surgical excision, yet only 10 to 15%
of patients have disease localized to the pancreas at the time of
diagnosis (3). The majority of
patients with PC succumb to the disease due to recurrence and
metastatis. However, the etiology of PC is not yet fully
understood. Previous studies have demonstrated that inflammation,
which occurs as a response to most chronic illnesses, may play a
significant role in this form of carcinogenesis (3–5). For
example, pro-inflammatory cytokines induce the activation of NF-κB
via the phosphorylation of the extracellular signal regulated
kinase (ERK) pathway, increase Snail expression and the secretion
of invasion-promorting matrix metalloproteinases (MMPs) (5–8).
Therefore, controlling inflammation is a significant factor in
decreasing the risk of developing human PC.
Protein acetylation influences cellular processes
including transcriptional regulation via the recruitment of
enzymes, histone acetyltransferases (HATs) and histone deacetylases
(HDACs) (9). Acetylation of
specific lysine residues within the amino-terminal tails of
nucleosomal histones is generally linked to chromatin disruption
and the transcriptional activation of genes (10). HDAC inhibitors have been reported
to induce cell growth arrest, apoptosis and differentiation in
tumor cells (10,11). Thus, dysregulation of the balance
between protein acetylation and deacetylation is often associated
with the initiation of tumorigenesis, cancer metastasis and other
diseases.
The antioxidant and/or anti-inflammatory effects of
dietary polyphenols (curcumin and resveratrol) have been shown to
play a role in either controlling chromatin remodelling or NF-κB
activation through the modulation of HDAC activity and
subsequently, the expression of various genes (11,12).
(-)-Epigallocatechin-3-gallate (EGCG), an antioxidant found in
green tea, has been reported to inhibit HAT and/or HDAC activity by
a number of studies (13–18). EGCG decreases the hyperacetylation
of p65 by directly inhibiting the activity of HAT enzymes. This
hypoacetylation of p65 leads to the downregulation of NF-κB
activity by diverse inflammatory signals (13). Although the regulation of NF-κB
activity by histone deacetylation is a potential anti-metastatic
mechanism in various types of cancer, the precise role of EGCG in
human PC cells remains unclear. Thus, we hypothesized that EGCG
inhibits invasive metastaic activity through the suppression of the
NF-κB signaling pathway by inducing RKIP expression and inhibiting
HDAC activity in human PC cells.
RKIP, a 23-kDa protein, was identified as a
physiological inhibitor of the Raf-MEK-ERK pathway (6), it is a member of the
phosphatidylethanolamine-binding protein (PEBP) family (19) and is well known for its metastasis
suppressor function in various types of cancer, including prostate,
breast, colorectal, cervical cancer and malanoma. The loss or
diminution of RKIP expression has been associated with the
increasing number of aggressive cancers (20–24).
Studies have suggested that NF-κB activation by RKIP diminution
negatively interferes with NF-κB signaling (25–27).
Several genes with tumor suppressive functions are thought to be
silenced by aberrant histone modifications (28). Inhibitors of HDAC, such as
trichostatin A (TSA), sodium butyrate (NaBT) and suberoylanilide
hydroxamic acid (SAHA), have displayed anti-cancer activities by
inducing the expression of downregulated genes related to
metastasis, motility and invasiveness (28–31).
First of all, we tested that RKIP expression in PC cell lines was
induced by TSA through the modulation of HDAC activity. And then we
examined whether EGCG can prevent PC metastasis by inhibiting HDAC
activity and inducing RKIP expression, thus inhibiting cell
signaling pathways involved in proliferation, survival and
metastasis.
MMPs, a family of Zinc-dependent endopeptidases, are
collectively capable of cleaving virtually all extracellular matrix
(ECM) substrates, and play an important role in some physiological
and pathological processes (32,33).
MMPs have also been implicated as possible mediators of invasion
and metastasis in breast cancer, colon cancer and melanoma cell
lines (34,35). Since MMPs have many physiological
functions in metastasis, the inhibition of the activity of MMPs
holds great promise for the prevention or inhibition of metastasis.
MMP-2 (gelatinases) is preferentially secreted from fibroblasts and
various epithelial cells, while MMP-9 (gelatinases) is
preferentially expressed by inflammatory cells (36), and both have been frequently
associated with the invasive metastatic potential of tumor cells.
However, the biochemical mechanisms underlying the EGCG-induced
inhibition of metastasis and invasion are not clear in AsPC-1 PC
cells.
Snail was identified as a transcription factor
implicated in the epithelial-mesenchymal transition (EMT) during
embryonic development (37). The
increased expression of Snail in invasive cancer cells accompanied
with the loss of E-cadherin expression has been reported in many
types of human cancer, including melanoma, hepatocarcinoma and
squamous cell carcinoma (38–40).
E-cadherin is a cell-cell adhesion molecule that is specifically
expressed on the membranes of epithelial cells, and its decreased
expression has been reported to play a role in the invasion and
metastasis of cancers (41,42).
The downregulation of E-cadherin expression has been explained by
the DNA hypermethylation of its promoter region in breast and
prostate cancer cells (43).
Therefore, in this study, we investigated the
induction of RKIP expression by EGCG through the modification of
histone deacetlyation. We also examined the effects of RKIP
induction by EGCG on metastasis and invasive parameters, including
MMP activity by gelatin zymography, Matrigel membrane invasion and
metastasis-related proteins by western blot analysis using the
AsPC-1 human adenocarcinoma metastatic cell line.
Materials and methods
Cell culture and cell viability
The human PC cell lines (Table I), MIA PaCa-2, AsPC-1, PANC-1 and
BxPC-3, were obtained from the American Type Culture Collection
(ATCC; (Rockville, MD) and cultured in DMBM and IMDM supplemented
with 10% fetal bovine serum (FBS) and fetal calf serum (FCS) (Gibco
BRL, Grand Island, NY) at 37°C in a humidified atmosphere
containing 5% CO2. For the cell viability assay, the
cells were plated at 1×104 cells/well in 200 μl
medium containing 10 μM EGCG with/without 1 μM TSA
(Sigma-Aldrich, St. Louis, MO) in a 96-well plate (Nunc™, Roskilde,
Denmark). After incubation with concentrations of 0, 5, 10, 15
μM EGCG for 24 h, cell viability was determined using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, which is based on the conversion of MTT to MTT-formazan by
mitochondria. Cells were incubated with 1 mg/ml MTT (Chemicon,
Temecula, CA) in PBS for 4 h at 37°C in 5% CO2.
Isopropanol and hydrochloric acid were then added at final
concentrations of 50% and 20 mM, respectively. The optical density
at 570 nm (OD570) was determined using an ELISA plate
reader (MN 3663 Molecular Devices Co., Sunnyvale, CA) with a
reference wavelength of 630 nm.
 | Table I.Comparisons of pancreatic cancer cell
lines.a |
Table I.
Comparisons of pancreatic cancer cell
lines.a
| Organism | Growth
properties | Disease |
|---|
| MIA PaCa-2 | Homo sapiens
(human) | Adherent, single
cells, loosely attached clusters | Carcinoma |
| BxPC-3 | Homo sapiens
(human) | Adherent | Adenocarcinoma |
| PANC-1 | Homo sapiens
(human) | Adherent | Epithelioid
carcinoma |
| AsPC-1 | Homo sapiens
(human) | Adherent | Adenocarcinoma
(metastatic cells) |
MMP activity using gelatin
zymography
Cell-free supernatant was collected and mixed with
2X sample buffer and then subjected to Novex Zymogram gels
(Invitrogen, Camarillo, CA). After electrophoresis, the gels were
washed twice at room temperature or 30 min in 2.5% Triton X-100,
subsequently washed in buffer containing 50 mM Tris-HCl, 150 mM
NaCl, 5 mM CaCl2, 1 μM ZnCl2, 0.02%
NaN3 at pH 7.5 and incubated in this buffer at 37°C for
24 h. Thereafter, the gels were stained with 0.25% (w/v) Coomassie
brilliant blue G-250 (Bio-Rad Laboratoties, Hercules, CA) and then
lightly destained in water solution containing methanol and acetic
acid for 1 h, respectively. The gelatinolytic activity was
evidenced as clear bands (area of gelatin degradation) against the
blue background of stained gelatin.
Matrigel invasion assay
In order to determine the effects of EGCG on AsPC-1
cell invasivness, the cells were exposed to 10 μM of EGCG
for 6 h and the pre-treated cells were plated onto the apical side
of the Matrigel-coated filters (Sigma-Aldrich) in serum-free medium
containing either EGCG or DMSO. Medium containing 20% FBS was
placed in the basolateral chamber to act as a chemoattractant.
After 72 h, cells on the apical side were wiped off using a Q-tip.
The cells on the bottom of the filter were stained with hematoxylin
and eosin Y and then counted (three fields of each triplicate
filter) using an inverted microscope (Nikon, Tokyo, Japan).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using RNeasy (Qiagen,
Valencia, CA). RNA (1 μg) was reverse-transcribed in a 20
μl reaction mixture using MMLV reverse transcriptase
(Invitrogen). The cDNA was amplified in a 20 μl reaction
mixture. The PCR conditions were as follows: 0.4 μM of each
primer (Table II), 0.2 mM
deoxynucleoside triphosphate mixture (Perkin-Elmer, Norwalk, CT,
USA), 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2,
and 1.0 U of Taq DNA polymerase (Perkin-Elmer). The reaction
mixtures were incubated in a thermal controller (Model PTC-100; MJ
Research, Ramsey, MN) for 35 cycles (denaturation at 94°C for 45
sec, annealing at 55°C for 45 sec, extension at 72°C for 90 sec).
The PCR products were resolved on 1% agarose gels containing
ethidum bromide (EtBr). The intensities of the bands were measured
using an image documentation system (ImageMaster VDS; Pharmacia,
Uppsala, Sweden) with image analysis software (ImageMaste TotalLab;
Pharmacia). The DNA size marker was run in parallel to validate the
predicted sizes of the amplified bands (GeneRuler 1 kb DNA Ladder;
MBI, Amherst, NY). GADPH, MMP-2 and -9, RKIP, Snail, E-cadherin and
ERK primer sequences were designed using Beacon Designer software
(Premier Biosoft, Palo Alto, CA) and synthesized by IDT (Skokie,
IL).
 | Table II.Oligonucleotides used in RT-PCR. |
Table II.
Oligonucleotides used in RT-PCR.
| Gene | Primer
sequence |
|---|
| GAPDH | |
| Sense | 5′-CGG AGT CAA CGG
ATT TGG TCG TAT-3′ |
| Antisense | 5′-AGC CTT CTC CAT
GGT GGT GAA GAC-3′ |
| RKIP | |
| Sense | 5′- CAC AAT GTG ATT
TTA TGG T -3′ |
| Antisense | 5′- TCT TCA TTC AGG
TTT CTA T -3′ |
| E-cadherin | |
| Sense | 5′- GAA CAG CAC GTA
CAC AGC CCT-3′ |
| Antisense | 5′- GCA GAA GTG TCC
CTG TTC CAG-3′ |
| Snail | |
| Sense | 5′- TAT GCT GCC TTC
CCA GGC TTG-3′ |
| Antisense | 5′- ATG TGC ATC TTG
AGG GCA CCC-3′ |
| MMP-2 | |
| Sense | 5′-CAG GCT CTT CTC
CTT TCG CAA C-3′ |
| Antisense | 5′-AAG CCA CGG CTT
GGT TTT CCT C-3′ |
| MMP-9 | |
| Sense | 5′-TGG GCT ACG TGA
CCT ATG ACC AT-3′ |
| Antisense | 5′-GCC CAG CCC ACC
TCC ACT CCT C-3′ |
Immunoblot analysis
Western immunoblots were prepared to analyze the
protein levels. After pre-treatment with the ERK inhibitor, U0126,
for 1 h and treatment with/without EGCG for 24 h, the cells were
harvested and then proteins were extracted with NP-40 protein lysis
buffer compositions; 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM
EDTA, 25 mM NaF, 10 mM sodium pyrophosphate, 25 mM
β-glycerophosphate, 0.2 mM Na3VO4, 10
μg/ml leupeptin, 10 μg/ml aprotinin, protease
inhibitor and 1 mM PMSF. Cytoplasmic and nuclear extracts were
prepared using NE-PER nuclear and cytosolic extraction reagents
(Pierce, Rockford, IL). Quantification of protein concentration was
carried out using the Bradford method (Bio-Rad protein assay
reagent) and total protein was resuspended in Laemmli sample buffer
containing 5% β-mercaptoethanol and heated at 65°C for 10 min.
Aliquots containing ∼20–50 μg of total cell proteins were
resolved on 8–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose
membranes (Amersham, Arlington Heights, IL). Membranes were blocked
in 5% non-fat milk (w/v) in Tris-buffered saline (TBS) containing
0.05% Tween-20 (TBST) for 1 h at room temperature and the membranes
were subjected to immunoblot analysis with the desired antibodies
(Table III). After an overnight
incubation at 4°C, the membranes were washed in TBST and incubated
with the appropriate peroxidase-conjugated secondary antibody
(Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were
developed using chemiluminescence according to the enhanced
chemiluminescence western blotting detection reagent (Pierce).
 | Table III.List of antibodies used in this
study. |
Table III.
List of antibodies used in this
study.
| Antibody | Dilution | Species origin | Company |
|---|
| MMP-2 | 1:500 | Rabbit
polyclonal | Santa Cruz
Biotechnology |
| MMP-9 | 1:500 | Rabbit
polyclonal | Santa Cruz
Biotechnology |
| ERK | 1:1000 | Rabbit
polyclonal | Cell signaling |
| pERK | 1:1000 | Rabbit
polyclonal | Cell signaling |
| NF-κB | 1:1000 | Rabbit
polyclonal | Santa Cruz
Biotechnology |
| IκB | 1:1000 | Rabbit
polyclonal | Santa Cruz
Biotechnology |
| p65 | 1:500 | Mouse
polyclonal | Santa Cruz
Biotechnology |
| p50 | 1:500 | Rabbit
polyclonal | Santa Cruz
Biotechnology |
| Snail | 1:500 | Rabbit
polyclonal | Abcam |
| E-cadherin | 1:500 | Mouse
monoclonal | Santa Cruz
Biotechnology |
| Lamin B | 1:1000 | Rabbit
polyclonal | Santa Cruz
Biotechnology |
| Actin | 1:1000 | Mouse
monoclonal | Sigma |
Statistical analysis
All data are presented as the means ± SD.
Statistical analysis (Student’s t-test) was performed using
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA).
Densitometry was performed using L process V2.01 and Muti Gauge
V2.02 (Fuji Film, Stenford, USA). A value of *p<0.05
was considered to indicate a statistically significant difference.
All the results presented in the figures shown in this study were
obtained from at least three independent experiments.
Results
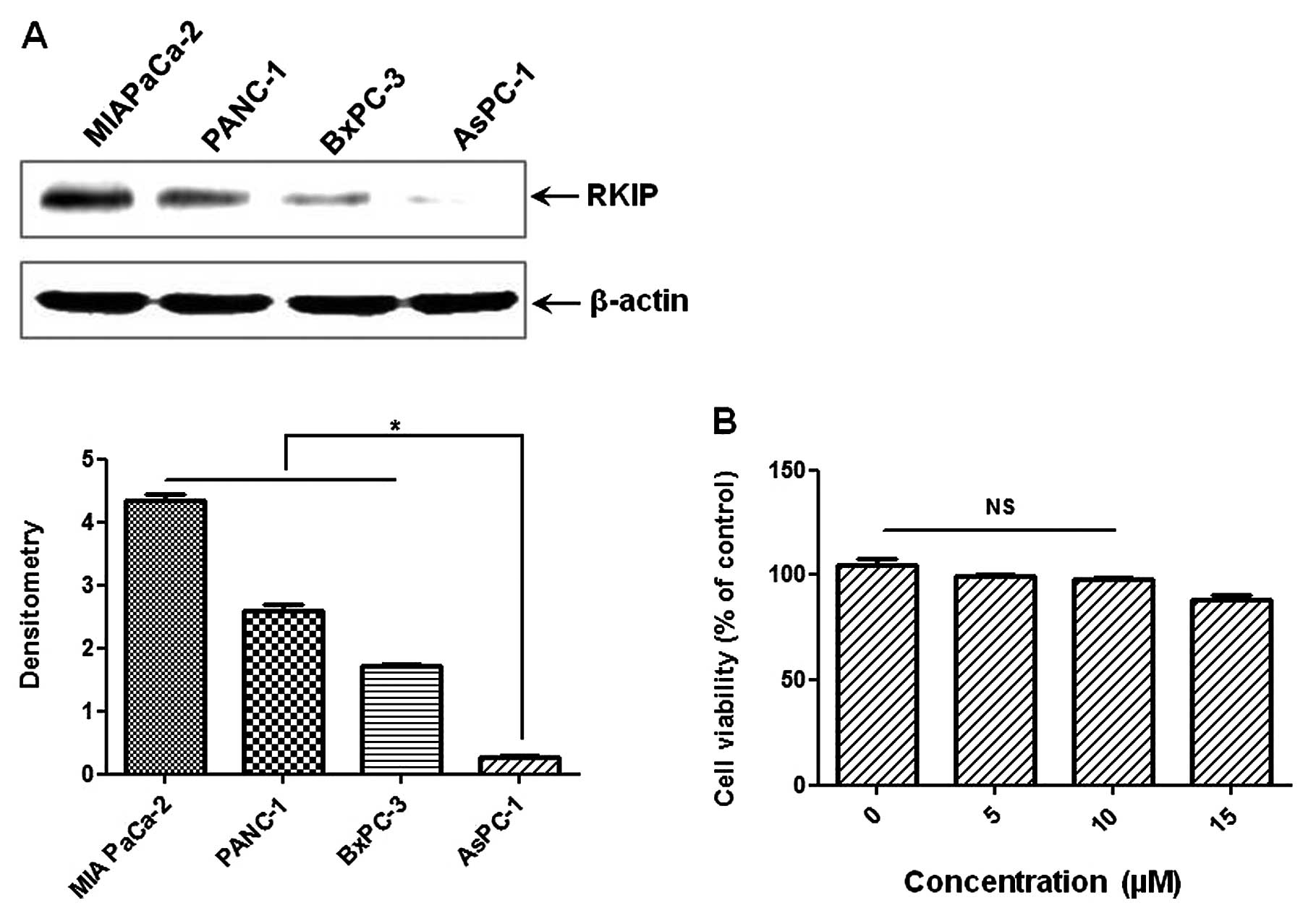
RKIP expression levels differ in human PC
cell lines
The levels of baseline RKIP were investigated in the
PC cell lines, MIA PaCa-2, PANC-1, AsPC-1 and BxPC-3. As shown in
Fig. 1, the order of the basal
levels of RKIP between the cell lines was: MIA PaCa-2 > PANC-1
> BxPC-3 > AsPC-1. To investigate whether RKIP expression was
induced by EGCG treatment, we selected the AsPC-1 cell line, which
had the lowest RKIP level of all the human PC cell lines. For
further investigation, we examined the effect of EGCG on the
viability of AsPC-1 cells by MTT assay. There was no toxicity up to
10 μM EGCG (usual EGCG treatment, 15 μM). The dose of
10 μM EGCG with/without TSA was used for the following
experiment.
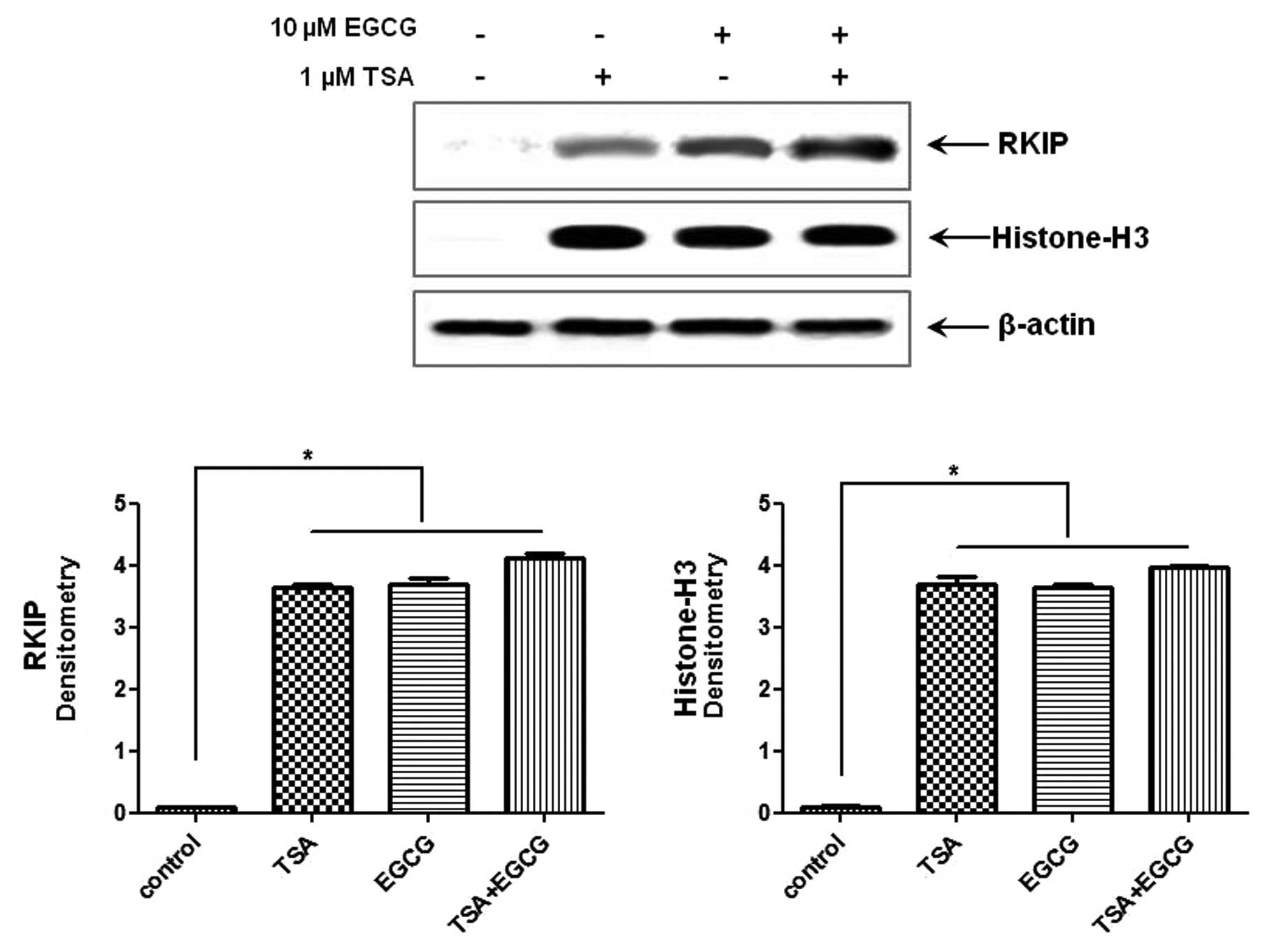
RKIP expression in AsPC-1 cells is
upregulated by EGCG treatment
As shown in Fig. 2,
treatment with 10 μM EGCG, a major polyphenol in green tea,
for 24 h induced RKIP expression in the AsPC-1 cells compared to
the control cells. In order to confirm whether RKIP regulation in
PC is due, in part, to histone modifications we performed the same
experiments while causing RKIP induction with TSA, a known HDAC
inhibitor. TSA induced RKIP expression and the effects were
synergistic to those of EGCG. Histone H3 expression was
significantly increased in the cells in which RKIP expression was
induced compared to the control cells. Therefore, RKIP induction in
AsPC-1 cells treated with EGCG is due, in part, to HDAC
modifications.
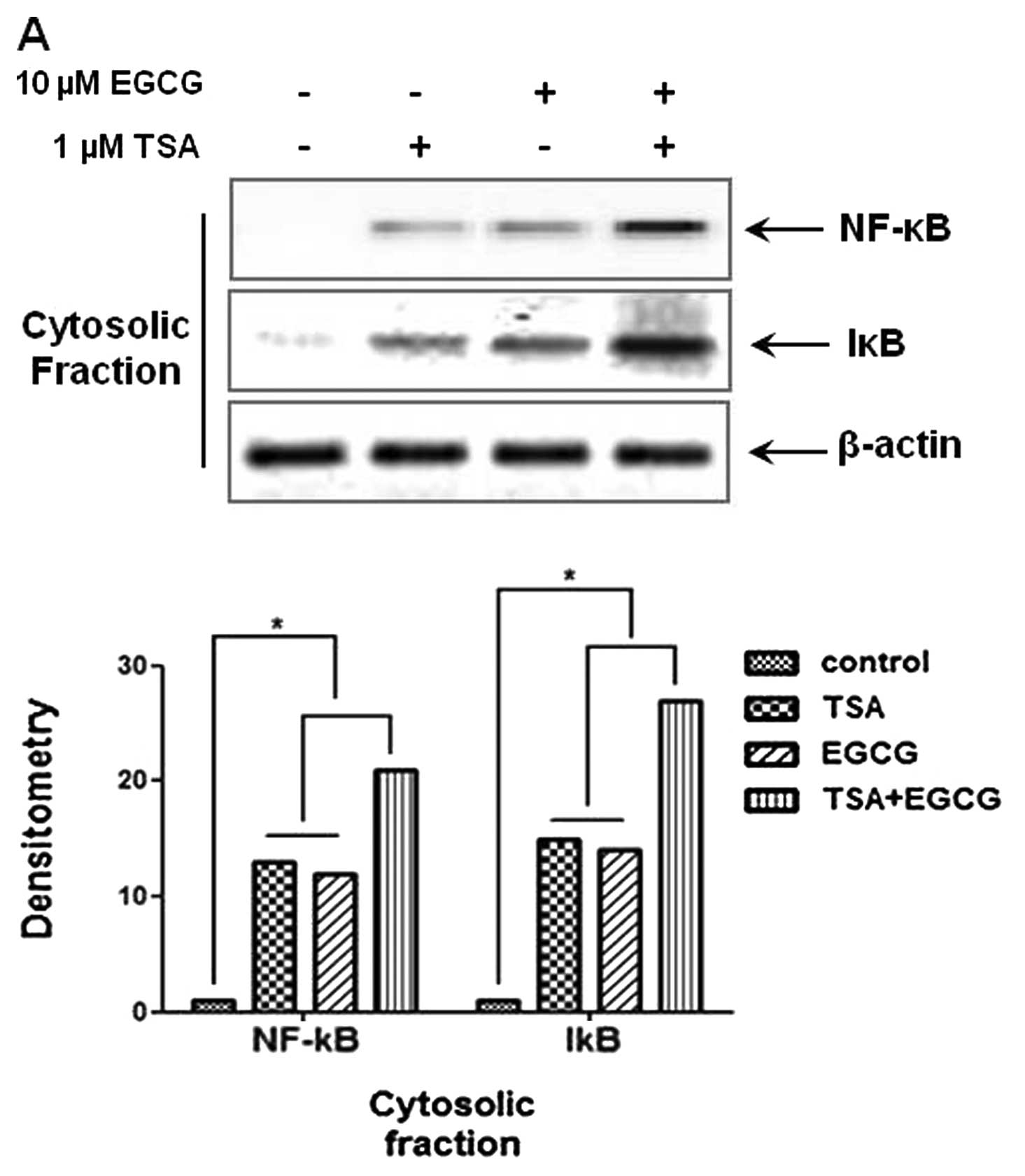
EGCG inhibits translocation of NF-κB into
the nucleus in AsPC-1 cells
The NF-κB signaling pathway is considerably involved
in cancer cell metastasis and invasion. To investigate whether EGCG
inhibits NF-κB translocation in AsPC-1 cells, cytosolic and nuclear
fractions were isolated in AsPC-1 cells. As shown in Fig. 3A and B, treatment with EGCG
decreased the levels of p65 and p50 proteins in the nuclear
fractions and elevated the levels of NF-κB and IκB in the cytosolic
fractions. These results indicte that the metastasis and invasion
of cancer cells are inhibited by the regulation of NF-κB nuclear
translocation. Therefore, we suggest that EGCG exerts
anti-metastatic and anti-invasion activities through the inhibition
of NF-κB nuclear translocation by the induction of RKIP and the
inhibition of HDAC.
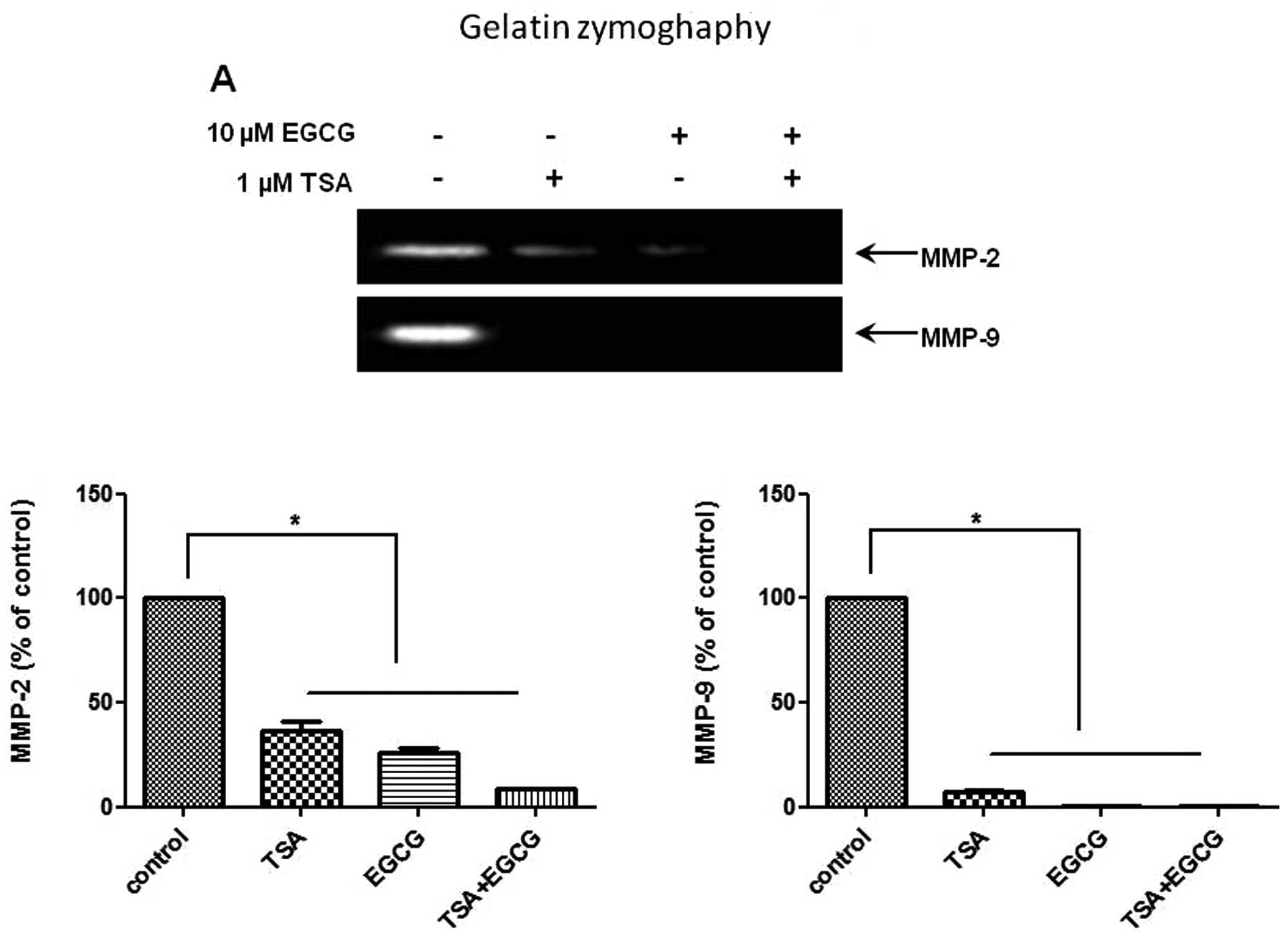
EGCG inhibits MMP activity and invasion
in AsPC-1 cells
To investigate whether EGCG suppresses the activity
of MMP-2 and -9, gelatin zymography was performed on the AsPC-1
cells treated with EGCG. The activities of MMP-2 and -9 were
decreased in the EGCG-treated cells compared to the control cells
(Fig. 4A). EGCG had a greater
inhibitory effect on MMP-9 activity than on MMP-2 activity. MMP
activity is considered to be critically involved in cancer cell
invasion. We then assessed the effects of EGCG on the invasiveness
of AsPC-1 cells using Matrigel chamber invasion assay. The results
of the Matrigel invasion assay (Fig.
4B) showed that the invasive capability of the AsPC-1 cells
treated with EGCG was decreased compared to that of the control
cells. These results indicate that EGCG inhibits the metastasis and
invasion of human PC cells through HDAC modulation and the
repression of MMP activity.
EGCG regulates expression levels of
metastasis-related genes in AsPC-1 cells
To elucidate the mechanism by which EGCG inhibits
metastasis and invasion, we examined the levels of
metastasis-related genes, such as MMP-2 and -9, Snail and
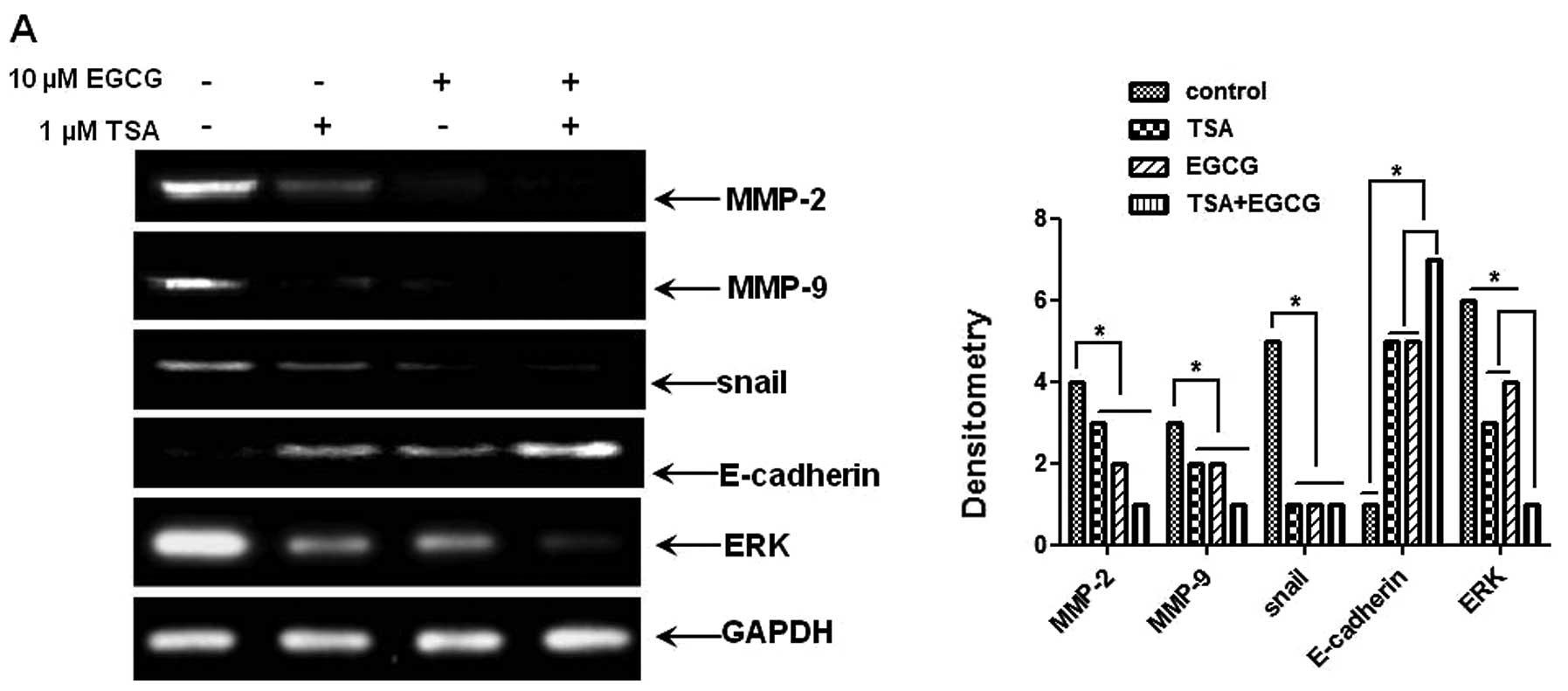
E-cadherin, using RT-PCR and western blot analysis. As shown in
Fig. 5A and B, mRNA and protein
levels of MMP-2 and -9, pERK and Snail were significantly
downregulated in the EGCG-treated AsPC-1 cells compared to those of
the control cells. The level of E-cadherin, a Snail nuclear
translocation inhibitor, was markedly increased by EGCG treatment.
As RKIP regulates the activation of NF-κB via the MEK/ERK signaling
pathway (44), we investigated
whether treatment with EGCG inhibits the phosphorylation of ERK. As
shown in Fig. 5C, the treatment of
cells with EGCG suppressed the phosphorylation of ERK compared to
the control cells and increased RKIP expression. These results
prove that the induction of RKIP expression by EGCG inhibits the
phosphorylation of ERK and NF-κB activation and decreases Snail
expression. Therefore, EGCG as a HDAC inhibitor, can prevent the
invasive metastasis of human PC cells.
Discussion
PC is the most severe gastrointestinal malignancy
due to its propensity for local invasion and early metastasis
(45,46). Modulation of histone deacetylation
by a HDAC inhibitor has been associated with transcriptional
repression, an important epigenetic event that plays a critical
role in inflammation (47).
Inhibitors of HDACs, such as TSA, have been shown to display
anti-cancer activities by inducing the expression of otherwise
silenced genes (28). In this
study, our objective was to treat PC cells with EGCG and
investigate its effects on RKIP levels within the cells and
determine whether an association exists between RKIP levels and
cell metastasis.
RKIP was initially identified as a PEBP in the
bovine brain. It was later identified as a protein that inhibits
the Raf kinase activation of MEK (47). RKIP has a multi-functional role,
exerting an inhibitory effect on pathways involved in cell
proliferation, survival and metastasis (47–49).
Beach et al, as well as others have reported that the
expression levels of RKIP proteins progressively decrease in breast
and prostate cancer cell lines of increasing metastatic potential
(48,50). A decrease in RKIP expression can
promote invasion in other types of cancer and the induction of RKIP
expression with HDAC inhibitors exerts anti-cancer effects in the
majority of cancer cells by suppressing the activity of
metastasis-related genes (26,49).
In this study, treatment with EGCG caused the induction of RKIP
expression in PC cells. Our results confirmed that the regulation
of RKIP expression in PC cells was due, in part, to histone
modifications following treatment with HDAC inhibitors, such as
TSA. EGCG suppressed the invasive and metastatic abilities of the
PC cells. These effects correlated with RKIP expression. In
accordance with our results, Fujimori et al also reported
that the loss of RKIP expression was associated with tumor
progression and poor survival. Negative RKIP expression combined
with positive p-ERK expression is an independent predictor of poor
outcomes in patients with gastric cancer (51). Evidence has emerged that RKIP can
function as a suppressor of cancer metastasis.
EGCG, a green tea-derived polyphenol, has been
reported to exert an inhibitory effect on NF-κB activity in various
human malignancies (13–16). However, the mechanisms behind the
EGCG regulation of NF-κB-dependent activation in PC cells remain
unclear (whether EGCG acts by regulating the stability or
inhibiting the activity of invasive metastatic proteins). In this
study, to determine whether RKIP is repressed by HDAC in PC cells,
we investigated the effects of EGCG on RKIP expression levels in
AsPC-1 cells. EGCG treatment led to the induction of RKIP
expression within 24 h and inhibited NF-κB nuclear translocation by
suppressing ERK phosphorylation in the cells. The same experiments
performed using TSA in the cells showed similar results at 1
μM TSA concentration. These results confirm that EGCG acts
as a HDAC inhibitor to suppress pro-inflammatory events, such as
NF-κB activation. Tang et al and Yeung et al reported
that RKIP negatively regulates the Raf/MEK/ERK and NF-κB pathways
(5,6). Li et al reported that EGCG, in
combination with the HDAC inhibitor, TSA, led to the synergistic
epigenetic reactivation of estrogen receptor-α (ERα) in
ERα-negative breast cancer cells (16).
MMPs are considered important proteolytic enzymes
during organ development and tissue regeneration. However, they
also play important roles in cancer invasion and metastasis. In
particular, MMP-2 and -9 play important roles in tumor invasion and
angiogenesis; therefore, tumor metastasis may be inhibited by
blocking MMP synthesis and activity (32,33).
Hazgui et al(52) reported
that the anti-metastatic activity of a polyphenolic constituent of
green tea, EGCG, was associated with a reduction in MMP-2 activity.
In a study by Shankar et al(53), it was reported that EGCG-treated
mice with PC had a significantly reduced ERK activity, invasion,
metastasis and angiogenesis. Takada et al(54) demonstrated that indole-3-carbinol,
a phytochemical agent, inhibited the cell proliferation and
invasion of leukemic cells by inhibiting MMP-9 activity.
Additionally, Jin et al(55) reported that Snail plays a critical
role in tumor growth and metastasis of ovarian carcinoma through
the regulation of MMP activity. These results may explain why the
expression of MMP-2 and -9 is elevated in parametrial invasion. Our
results indicated a marked inhibition of MMP-2 and -9 mRNA, protein
levels and activities following treatment with EGCG with/without
TSA. Snail and E-cadherin expression has also been related to
cancer metastasis and MMP activity. In accordance with our results,
Miyoshi et al suggested that Snail, a zinc-finger
transcription factor, represses E-cadherin expression and induces
MMP expression, which causes vascular invasion and intrahepatic
metastasis in primary hepatocellular carcinoma tumors (56). Batlle et al reported that
Snail regulates other genes required for the scattering process, in
conjunction with E-cadherin downregulation (38).
Collectively, these data demonstrate that the green
tea-derived polyphenol, EGCG, regulates RKIP expression by
modulating histone deacetylation. The results from the present
study demonstrate that EGCG treatment leads to the inactivation of
NF-κB and downstream target genes, such as Snail, as well as to the
inactivation of MMPs, resulting in the inhibition of the invasive
metastatic ability of PC cells. Therefore, we suggest that EGCG can
potentially be a used as a therapeutic agent for PC. Further
studies are required in order to gain a deeper understanding of the
efficacy of EGCG in PC prevention. Future studies should focus on
elucidating the molecular mechanisms involved by using in
vivo methods with proper animal models of PC.
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Korea Ministry of Health and Welfare:
Annual report of cancer statistics in Korea in 2008. http://mw.go.kr.
2011
|
|
3.
|
Warshaw AL, Gu ZY, Wittenberg J and
Waltman AC: Preoperative staging and assessment of resectability of
pancreatic cancer. Arch Surg. 125:230–233. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Whitcomb DC: Inflammation and Cancer V.
Chronic pancreatitis and pancreatic cancer. Am J Physiol
Gastrointest Liver Physiol. 287:G315–G319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tang H, Park S, Sun SC, Trumbly R, Ren G,
Tsung E and Yeung KC: RKIP inhibits NF-kappaB in cancer cells by
regulating upstream signaling components of the IkappaB kinase
complex. FEBS Lett. 4:662–668. 2009.PubMed/NCBI
|
|
6.
|
Yeung K, Janosch P, McFerran B, Rose DW,
Mischak H, Sedivy JM and Kolch W: Mechanism of suppression of the
Raf/MEK/extracellular signal-regulated kinase pathway by the raf
kinase inhibitor protein. Mol Cell Biol. 20:3079–3085. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Barbera MJ, Puig I, Dominguez D,
Julien-Grille S, Guaita-Esteruelas S, Peiró S, Baulida J, Francí C,
Dedhar S, Larue L and García de Herreros A: Regulation of Snail
transcription during epithelial to mesenchymal transition of tumor
cells. Oncogene. 23:7345–7354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Munshi HG and Stack MS: Reciprocal
interactions between adhesion receptor signaling and MMP
regulation. Cancer Metastasis Rev. 25:45–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gregory PD, Wagner K and Horz W: Histone
acetylation and chromatin remodeling. Exp Cell Res. 265:195–202.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Struhl K: Histone acetylation and
transcriptional regulatory mechanisms. Genes Dev. 12:599–606. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gray SG and Teh BT: Histone
acetylation/deacetylation and cancer: an ‘open’ and ‘shut’ case?
Curr Mol Med. 1:401–429. 2001.
|
|
12.
|
Hayden MS and Ghosh S: Signaling to NF-κB.
Genes Dev. 18:2195–2224. 2004.
|
|
13.
|
Choi KC, Jung MG, Lee YH, Yoon JC, Kwon
SH, Kang HB, Kim MJ, Cha JH, Kim YJ, Jun WJ, Lee JM and Yoon HG:
Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor,
inhibits EBV-induced B lymphocyte transformation via suppression of
RelA acetylation. Cancer Res. 69:583–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Thakur VS, Gupta K and Gupta S: Green tea
polyphenols increase p53 transcriptional activity and acetylation
by suppressing class I histone deacetylases. Int J Oncol.
41:353–361. 2012.PubMed/NCBI
|
|
15.
|
Choudhury SR, Balasubramanian S, Chew YC,
Han B, Marquez VE and Eckert RL: (-)-Epigallocatechin-3-gallate and
DZNep reduce polycomb protein level via a proteasome-dependent
mechanism in skin cancer cells. Carcinogenesis. 32:1525–1532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Li Y, Yuan YY, Meeran SM and Tollefsbol
TO: Synergistic epigenetic reactivation of estrogen receptor-α
(ERα) by combined green tea polyphenol and histone deacetylase
inhibitor in ERα-negative breast cancer cells. Mol Cancer.
9:274–286. 2010.PubMed/NCBI
|
|
17.
|
Yun JM, Jialal I and Devaraj S: Effects of
epigallocatechin gallate on regulatory T cell number and function
in obese v. lean volunteers. Br J Nutr. 103:1771–1777. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Fang M, Chen D and Yang CS: Dietary
polyphenols may affect DNA methylation. J Nutr. 137:223S–228S.
2007.PubMed/NCBI
|
|
19.
|
Yeung K, Seitz T, Li S, Janosch P,
McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H,
Sedivy JM and Kolch W: Suppression of Raf-1 kinase activity and MAP
kinase signalling by RKIP. Nature. 401:173–177. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ren G, Baritaki S, Marathe H, Feng J, Park
S, Beach S, Bazeley PS, Beshir AB, Fenteany G, Mehra R, Daignault
S, Al-Mulla F, Keller E, Bonavida B, de la Serna I and Yeung KC:
Polycomb protein EZH2 regulates tumor invasion via the
transcriptional repression of the metastasis suppressor RKIP in
breast and prostate cancer. Cancer Res. 72:3091–3104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Minn AJ, Bevilacqua E, Yun J and Rosner
MR: Identification of novel metastasis suppressor signaling
pathways for breast cancer. Cell Cycle. 11:2452–2457. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Karamitopoulou E, Zlobec I, Panayiotides
I, Patsouris ES, Peros G, Rallis G, Lapas C, Karakitsos P,
Terracciano LM and Lugli A: Systematic analysis of proteins from
different signaling pathways in the tumor center and the invasive
front of colorectal cancer. Hum Pathol. 42:1888–1896. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hu CJ, Zhou L, Zhang J, Huang C and Zhang
GM: Immunohistochemical detection of Raf kinase inhibitor protein
in normal cervical tissue and cervical cancer tissue. J Int Med
Res. 39:229–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zebisch A, Wölfler A, Fried I, Wolf O,
Lind K, Bodner C, Haller M, Drasche A, Pirkebner D, Matallanas D,
Rath O, Blyth K, Delwel R, Taskesen E, Quehenberger F, Kolch W,
Troppmair J and Sill H: Frequent loss of RAF kinase inhibitor
protein expression in acute myeloid leukemia. Leukemia.
26:1842–1849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wu K and Bonavida B: The activated
NF-kappaB-Snail-RKIP circuitry in cancer regulates both the
metastatic cascade and resistance to apoptosis by cytotoxic drugs.
Crit Rev Immunol. 29:241–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Yeung KC, Rose DW, Dhillon AS, Yaros D,
Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W and Sedivy
JM: Raf kinase inhibitor protein interacts with NF-kappaB-inducing
kinase and TAK1 and inhibits NF-kappaB activation. Mol Cell Biol.
21:7207–7217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000.PubMed/NCBI
|
|
28.
|
Emonds E, Fitzner B and Jaster R:
Molecular determinants of the antitumor effects of trichostatin A
in pancreatic cancer cells. World J Gastroenterol. 16:1970–1978.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Farrow B, Rychahou P, O’Connor KL and
Evers BM: Butyrate inhibits pancreatic cancer invasion. J
Gastrointest Surg. 7:864–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hardtner C, Multhoff G, Falk W and Radons
J: (-)-Epigallocatechin-3-gallate, a green tea-derived catechin,
synergizes with celecoxib to inhibit IL-1-induced tumorigenic
mediators by human pancreatic adenocarcinoma cells Colo357. Eur J
Pharmacol. 684:36–43. 2012. View Article : Google Scholar
|
|
31.
|
Choi KC, Lee YH, Jung MG, Kwon SH, Kim MJ,
Jun WJ, Lee J, Lee JM and Yoon HG: Gallic acid suppresses
lipopolysaccharide-induced nuclear factor-kappaB signaling by
preventing RelA acetylation in A549 lung cancer cells. Mol Cancer
Res. 7:2011–2021. 2009. View Article : Google Scholar
|
|
32.
|
Zucker S, Cao J and Chen WT: Critical
appraisal of the use of matrix metalloproteinase inhibitors in
cancer treatment. Oncogene. 19:6642–6650. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
34.
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O’Higgins N: Metalloproteinases: role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Vihinen P, Ala-aho R and Kähäri VM: Matrix
metalloproteinases as therapeutic targets in cancer. Curr Cancer
Drug Targets. 5:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Gibbs DF, Warner RL, Weiss SJ, Johnson KJ
and Varani J: Characterization of matrix metalloproteinases
produced by rat alveolar macrophages. Am J Respir Cell Mol Biol.
20:1136–1144. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Yokoyama K, Kamata N, Hayashi E, Hoteiya
T, Ueda N, Fujimoto R and Nagayama M: Reverse correlation of
E-cadherin and snail expression in oral squamous cell carcinoma
cells in vitro. Oral Oncol. 37:65–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor Snail is a repressor of E-cadherin gene expression in
epithelial tumor cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Jiao W, Miyazaki K and Kitajima Y: Inverse
correlation of E-cadherin and Snail expression in hepatocellular
cell lines in vitro and in vivo. Br J Cancer. 86:98–101. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Poser I, Domínguez D, de Herreros AG,
Varnai A, Buettner R and Bosserhoff AK: Loss of E-cadherin
expression in melanoma cells involves up-regulation of the
transcriptional repression Snail. J Biol Chem. 276:24661–24666.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Luo J, Lubaroff DM and Hendrix MJ:
Suppression of prostate cancer invasive potential and matrix
metalloproteinase activity by E-cadherin transfection. Cancer Res.
59:3552–3556. 1999.PubMed/NCBI
|
|
42.
|
Oka H, Shiozaki H, Kobayashi K, Inoue M,
Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S,
Takeichi M and Mori T: Expression of E-cadherin cell adhesion
molecules in human breast cancer tissues and its relationship to
metastasis. Cancer Res. 53:1696–1701. 1993.PubMed/NCBI
|
|
43.
|
Graff JR, Herman JG, Lapidus RG, Chopra H,
Xu R, Jarrard DF, Isaacs WB, Pitha PM, Davidson NE and Baylin SB:
E-cadherin expression is silenced by DNA hypermethylation in human
breast and prostate carcinomas. Cancer Res. 55:5195–5199.
1995.PubMed/NCBI
|
|
44.
|
Odabaei G, Chatterjee D, Jazirehi AR,
Goodglick L, Yeung K and Bonavida B: Raf-1 kinase inhibitor
protein: structure, function, regulation of cell signaling, and
pivotal role in apoptosis. Adv Cancer Res. 91:169–200. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Eckel F, Schneider G and Schmid RM:
Pancreatic cancer: a review of recent advances. Expert Opin
Investig Drugs. 15:1395–1410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Schneider G, Siveke JT, Eckel F and Schmid
RM: Pancreatic cancer: basic and clinical aspects.
Gastroenterology. 128:1606–1625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Escara-Wilke J, Yeung K and Keller ET: Raf
kinase inhibitor protein (RKIP) in cancer. Cancer Metastasis Rev.
Jun 9–2012.(Epub ahead of print).
|
|
48.
|
Klysik J, Theroux SJ, Sedivy JM, Moffit JS
and Boekelheide K: Signaling crossroads: the function of Raf kinase
inhibitory protein in cancer, the central nervous system and
reproduction. Cell Signal. 20:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Zeng L, Imamoto A and Rosner MR: Raf
kinase inhibitory protein (RKIP): a physiological regulator and
future therapeutic target. Expert Opin Ther Targets. 12:1275–1287.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Beach S, Tang H, Park S, Dhillon AS,
Keller ET, Kolch W and Yeung KC: Snail is a repressor of RKIP
transcription in metastatic prostate cancer cells. Oncogene.
27:2243–2248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Fujimori Y, Inokuchi M, Takagi Y, Kato K,
Kojima K and Sugihara K: Prognostic value of RKIP and p-ERK in
gastric cancer. J Exp Clin Cancer Res. 31:30–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Hazgui S, Bonnomet A, Nawrocki-Raby B,
Milliot M, Terryn C, Cutrona J, Polette M, Birembaut P and Zahm JM:
Epigallocatechin-3-gallate (EGCG) inhibits the migratory behavior
of tumor bronchial epithelial cells. Respir Res. 9:33–46. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Shankar S, Ganapathy S, Hingorani SR and
Srivastava RK: EGCG inhibits growth, invasion, angiogenesis and
metastasis of pancreatic cancer. Front Biosci. 13:440–452. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Takada Y, Andreeff M and Aggarwal BB:
Indole-3-carbinol suppresses NF-κB and IκBα kinase activation,
causing inhibition of expression of NF-κB-regulated antiapoptotic
and metastatic gene products and enhancement of apoptosis in
myeloid and leukemia cells. Blood. 106:641–649. 2005.
|
|
55.
|
Jin H, Yu Y, Zhang T, Zhou X, Zhou J, Jia
L, Wu Y, Zhou BP and Feng Y: Snail is critical for tumor growth and
metastasis of ovarian carcinoma. Int J Cancer. 126:2102–2111.
2010.PubMed/NCBI
|
|
56.
|
Miyoshi A, Kitajima Y, Kido S, Shimonishi
T, Matsuyama S, Kitahara K and Miyazaki K: Snail accelerates cancer
invasion by upregulating MMP expression and is associated with poor
prognosis of hepatocellular carcinoma. Br J Cancer. 92:252–258.
2005.PubMed/NCBI
|