Introduction
Lung cancer has become the most common malignant
tumor and the major cause of cancer deaths worldwide. The overall
5-years survival rate for lung cancer is only 15% (1). The largest gain in life expectancy in
lung cancer patients has been among those with localized disease
versus those with regional or distant metastasis. However, due to
the asymptomatic nature of the early disease and the lack of
effective screening methods, only 19% of lung cancers are localized
at the time of diagnosis (2).
Although there has been significant progress in diagnostic tools at
clinics including CT (computed tomography) scans, bronchoscopy and
sputum analysis, none of these turns out to be an effective tool in
early diagnosis of lung cancer. How to establish a methodology to
identify the high-risk individuals for lung cancer remains to be
investigated.
Cancer has long been recognized as a multi-step
process which involves not only genetic changes conferring growth
advantage but also factors which disrupt regulation of growth and
differentiation. It is possible that some of these factors could be
identified and their functions evaluated with the aid of
autoantibodies arising during tumorigenesis. The heterogeneity in
the molecular pathogenesis of human cancers must be taken into
account in both the design and interpretation of studies to
identify makers which will be useful for early diagnosis of cancer.
Many studies have demonstrated that the cancer cells can generate a
unique set of antigenic change which can be recognized by the
immune system of patients themselves. These antigens are called
tumor-associated antigens (TAAs), whose abnormal regulation or
excessive expressed are closely related to tumorigenesis (3). The appearance of circulating
autoantibodies against these TAAs is one of the important ways
which are manifested by cancer patient’s immune system (4,5).
These anti-TAA autoantibodies can magnify the signal when the tumor
antigen expression is very low even when it is not able to be
detected, suggesting that these autoantibodies may be reporters
identifying aberrant cellular mechanisms in tumorigenesis and also
served as immunodiagnostic markers for cancer detection, especially
because of the general absence of these antoantibodies in normal
individuals and non-cancer conditions. For example, autoantibodies
to TAAs such as p53 and p16 were detected in 10–20% of most type of
cancer patients (6,7), while autoantibodies to p62 were 21%
positive in patients with hepatocellular carcinoma (8). Due to the fact that these
autoantibodies do not exist or appear with a very low titer in the
serum samples of healthy people, therefore, the autoantibodies may
become potential biomarkers in the diagnosis of certain type of
cancer.
Insulin-like growth factor-binding protein-2
(IGFBP-2) is one member of the insulin-like growth factor family
(IGFs), and recently reported to be a tumor-associated antigen
(9). IGFBP-2 is overexpressed in a
majority of malignant tumors, including glioma (10) and colorectal (11,12),
prostate (13), ovarian (14) and breast cancers (15). Although overexpressed IGFBP-2 is
associated with increased tumor stages and grades (12), relapse, metastasis (14) and prognosis (16) in advanced cancers, it is less
satisfactory in diagnosing early cancers. A recently published
paper (17) first demonstrated
that serum anti-IGFBP-2 antibody was significantly elevated in
gliomas and colorectal carcinoma, suggesting that the detection of
circulating anti-IGFBP-2 antibody may be a potential approach in
diagnosing early cancers. In the current study, we aimed to
evaluate whether serum IGFBP-2 and anti-IGFBP-2 antibody can be
used as biomarkers in detection of lung cancer.
Materials and methods
Patients and samples
Blood samples (n=294) were prospectively collected
from consented individuals under an Institutional Ethics
Committee-approved study from the First Hospital of Xi’an Jiaotong
University for patients with lung cancer (n=190; 55 lung cancers
stage I–II and 135 lung cancers stage III–IV), from 2011 to 2012.
In addition, 9 patients also consented to a follow-up evaluation of
anti-IGFBP-2 antibodies. Sera from six patients with lung cancer
were collected after surgery and chemotherapy, and sera from three
patients were also collected after each cycle of chemotherapy. As
control, sera from patients with benign lung disease such as
phthisis, pneumonia and hamartoma (n=33) were obtained from
individuals from the same hospital who were having annual health
examinations and had no evidence of malignancy (n=71, see Table I).
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| n
(male/female) | Range of the age
(years) | Mean of the
age(years) |
|---|
| Normal
controls | 71 (49/22) | 36–77 | 58.75 |
| Benign lung
disease | 33 (20/13) | 34–88 | 59.94 |
| Lung cancers | 190 (138/52) | 27–82 | 61.38 |
| Lung cancer
I | 21 (15/6) | 42–82 | 65.67 |
| Lung cancer
II | 34 (24/10) | 38–76 | 59.88 |
| Lung cancer
III | 73 (53/20) | 27–82 | 60.52 |
| Lung cancer
IV | 62 (46/16) | 32–78 | 61.61 |
| Total | 294 (207/87) | 27–88 | 60.55 |
The blood was drawn before surgery, chemotherapy or
radiotherapy, blood samples were separated by centrifugation at
2,500 rpm for 8 min and stored at −80°C until further analysis.
Electrochemical luminescence kits (Roche, Mannheim, Germany) were
used to measure serum levels of carcinoembryonic antigen (CEA),
cytokeratin 19 fragments (CYFRA21-1) and neuron-specific enolase
(NSE) in 138 patients (98 lung cancer, 17 lung benign disease and
23 controls, see Table II). Cutoff
values for CEA, CYFRA21-1 and NSE were 3.4, 3.3 and 15.2 ng/ml,
respectively, used by the First Hospital of Xi’an Jiaotong
University. The same sera were also used for IGFBP-2 and
anti-IGFBP-2 antibody measurement. Immunohistochemical analysis
(IHC) of IGFBP-2 expression was performed on tissue specimens,
collected during surgical operation.
 | Table II.Characteristics of patients. |
Table II.
Characteristics of patients.
| n
(male/female) | Range of the age
(years) | Mean of the
age(years) |
|---|
| Normal
controls | 23 (16/7) | 36–77 | 58.13 |
| Benign lung
disease | 17 (13/4) | 34–83 | 60.94 |
| Lung cancers | 98 (68/30) | 35–82 | 59.63 |
| Lung cancer
I | 17 (12/5) | 42–82 | 66.06 |
| Lung cancer
II | 21 (14/7) | 38–76 | 58.19 |
| Lung cancer
III | 40 (24/16) | 35–79 | 58.35 |
| Lung cancer
IV | 20 (18/2) | 37–75 | 58.25 |
| Total | 138 (97/41) | 35–83 | 59.54 |
Indirect ELISA for serum IgG antibodies
against IGFBP-2
Serum IgG antibody against IGFBP-2 was assessed by
indirect enzyme-linked immunosorbent assay (ELISA) as previously
described (17). Briefly, Immulon
4HBX microtiter plates (Dynex), were coated overnight (at least for
24 h) with 50 μl of highly purified, human recombinant
IGFBP-2 protein (R&D Systems Inc., Minneapolis, MN) diluted in
50 ml carbonate buffer (Sigma-Aldrich Corp., St Louis, MO) to a
concentration of 1.0 μg/ml or carbonate buffer alone in
alternating columns. The last column of wells was incubated with 50
μl per well of serially diluted, purified human IgG (25,
100, 250, 500, 1,000 and 2,000 ng/ml) (R&D Systems Inc.) to
provide a standard curve.
Plates were blocked with 5% bovine serum albumin
(BSA) in phosphate-buffered saline (PBS), 50 μl per well,
for 2 h at room temperature, washed four times with PBST (0.1%
Tween-20 in PBS), and then incubated with diluted (1/5) serum in 1%
BSA/PBS, 50 μl/well, for 2 h at room temperature with gentle
shaking. Plates were washed 4 times again and 50 μl per well
of goat anti-human IgG-HRP (Invitrogen Ltd, Paisley, UK) diluted
1:30,000 in 1% BSA was added and incubated for 1 h at room
temperature. Following four washes, 100 μl per well of
tetramethylbenzidine (TMB) was added, reaction was stopped by
adding 100 μl 2M sulphuric acid
(H2SO4). Plates were then read at 450 nm. The
optical density (OD) of each serum dilution was calculated as the
OD of the protein-coated wells minus the OD of the buffer-coated
wells. The concentration of serum anti-IGFBP-2 antibody was
calculated based on the standard curve on each plate.
Direct ELISA for serum IGFBP-2
Serum IGFBP-2 was measured with commercially
available IGFBP-2 ELISA kits (RapidBio Laboratory, Calabasas, CA)
following the manufacturer’s instructions. The concentrations of
the IGFBP-2 standards for building a standard curve were 62.5, 125,
250, 500, 1,000 and 2,000 ng/ml. Serum was serially diluted.
IHC analysis
IGFBP-2 immunostaining was carried out as described
(10). The sections of tumor
tissues were immunostained with monoclonal anti-IGFBP-2 antibody
(Cell Signaling Technology, Boston, MA). Five most heavily stained
fields were chosen to determine the percentage of positive cells.
Zero represents no positive, + represents <10%, ++ represents
11%–30%, +++ represents 31%–60%, and ++++ represents >60%
positive staining cells.
Statistical analysis
Statistical analyses were carried out using SPSS
13.0. Demographic data were analyzed with χ2 test. The
differences of anti-IGFBP-2 antibody and serum IGFBP-2 levels
between controls and patients were assessed using a standard
non-parametric Mann-Whitney U test. Receiver operating
characteristic (ROC) curves were constructed to determine the
discriminatory capacity of anti-IGFBP-2 antibodies for diagnosis,
and the area under the curve (AUC) was analyzed by χ2
test. Sensitivity was defined as the number of patients with
elevated anti-IGFBP-2 antibodies in serum divided by the total
number of patients with lung cancer. Specificity was defined as the
number of patients without elevated anti-IGFBP-2 antibodies in
serum divided by the total number of control patients. Correlations
of serum anti-IGFBP-2 antibody levels with tumor tissue IGFBP-2
expression levels were assessed by Pearson’s correlation analysis.
The results were considered to indicate a statistically significant
difference at p-value <0.05.
Results
Elevated serum anti-IGFBP-2 antibody
levels in lung cancer
Serum levels of anti-IGFBP-2 antibodies were
determined by ELISA as described in the section of Materials and
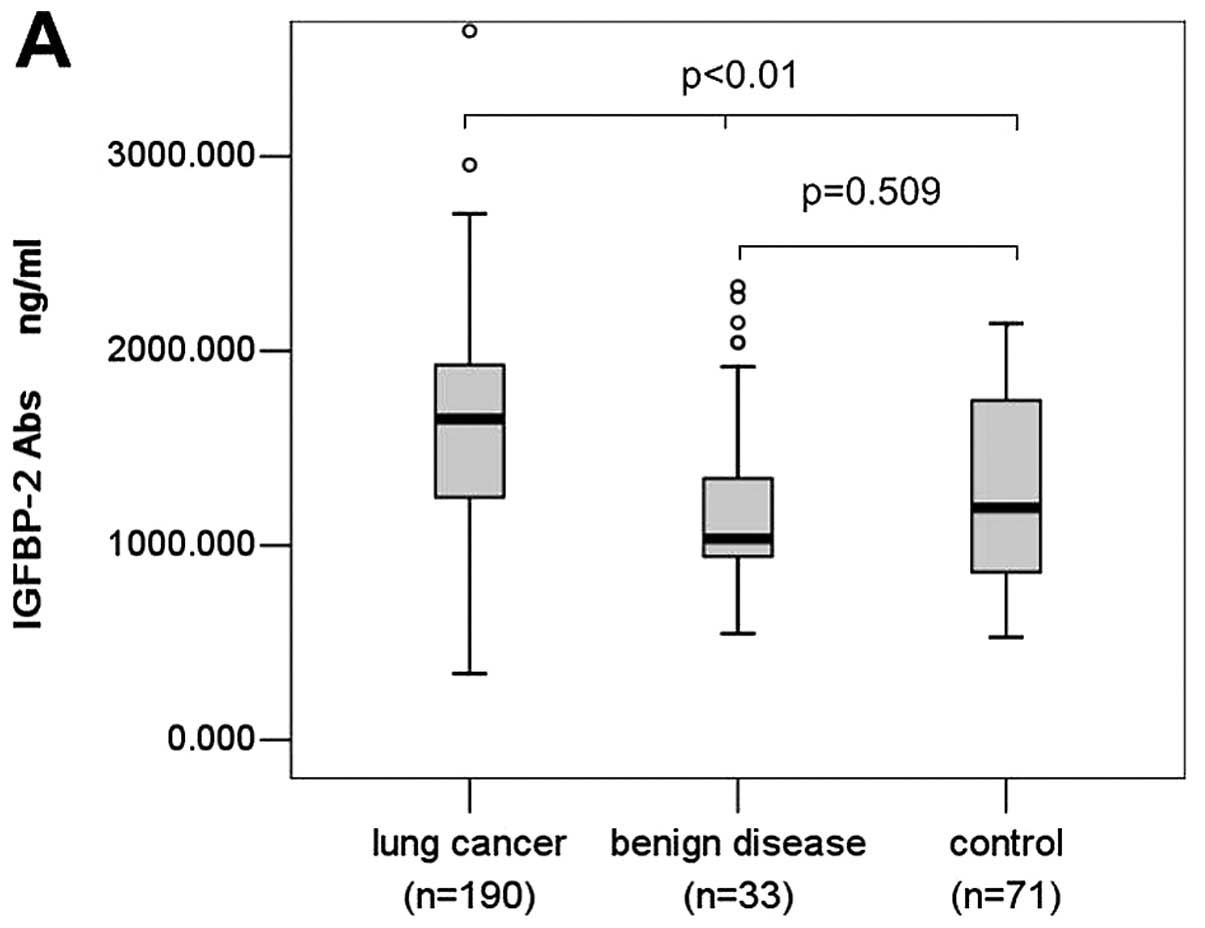
methods, the study population is summarized in Table I. We found serum anti-IGFBP-2
antibody level of lung cancer patients (mean, 1633.318 ng/ml;
median, 1651.462 ng/ml; range, 342.732–4932.582 ng/ml) was
significantly higher than that of patients with benign lung disease
(mean, 1210.139 ng/ml; median, 1035.900 ng/ml; range,
547.596–2331.167 ng/ml) and normal controls (mean, 1303.369 ng/ml;
median, 1194.800 ng/ml; range, 528.200–2140.500 ng/ml) (P<0.001,
Fig. 1A). However, there was no
significant difference between serum anti-IGFBP-2 antibody levels
of patients with lung benign disease and normal controls (P= 0.509,
Fig. 1A).
Li et al(17) demonstrated that anti-IGFBP-2
antibody levels were higher in early cancers than advanced cancers
in gliomas and colorectal cancer. When stratifying the analysis by
American Joint Committee on Cancer stage for lung cancer,
interestingly, a modest difference in anti-IGFBP-2 antibody levels
was observed between early cancers and advanced cancers in lung
cancer. Patients with lung cancer stage I–II (mean, 1549.671 ng/ml;
median, 1651.749 ng/ml) did not have an elevated anti-IGFBP-2
antibodies compared to patients with lung cancer stage III–IV
(1667.397 ng/ml; median, 1648.400 ng/ml) (P= 0.548, Fig. 1B). There was no significant
association of the serum levels of anti-IGFBP-2 antibodies in any
of the collected histological types (P= 0.701) (data not
shown).
Serum anti-IGFBP-2 antibodies as
biomarkers for lung cancer diagnosis
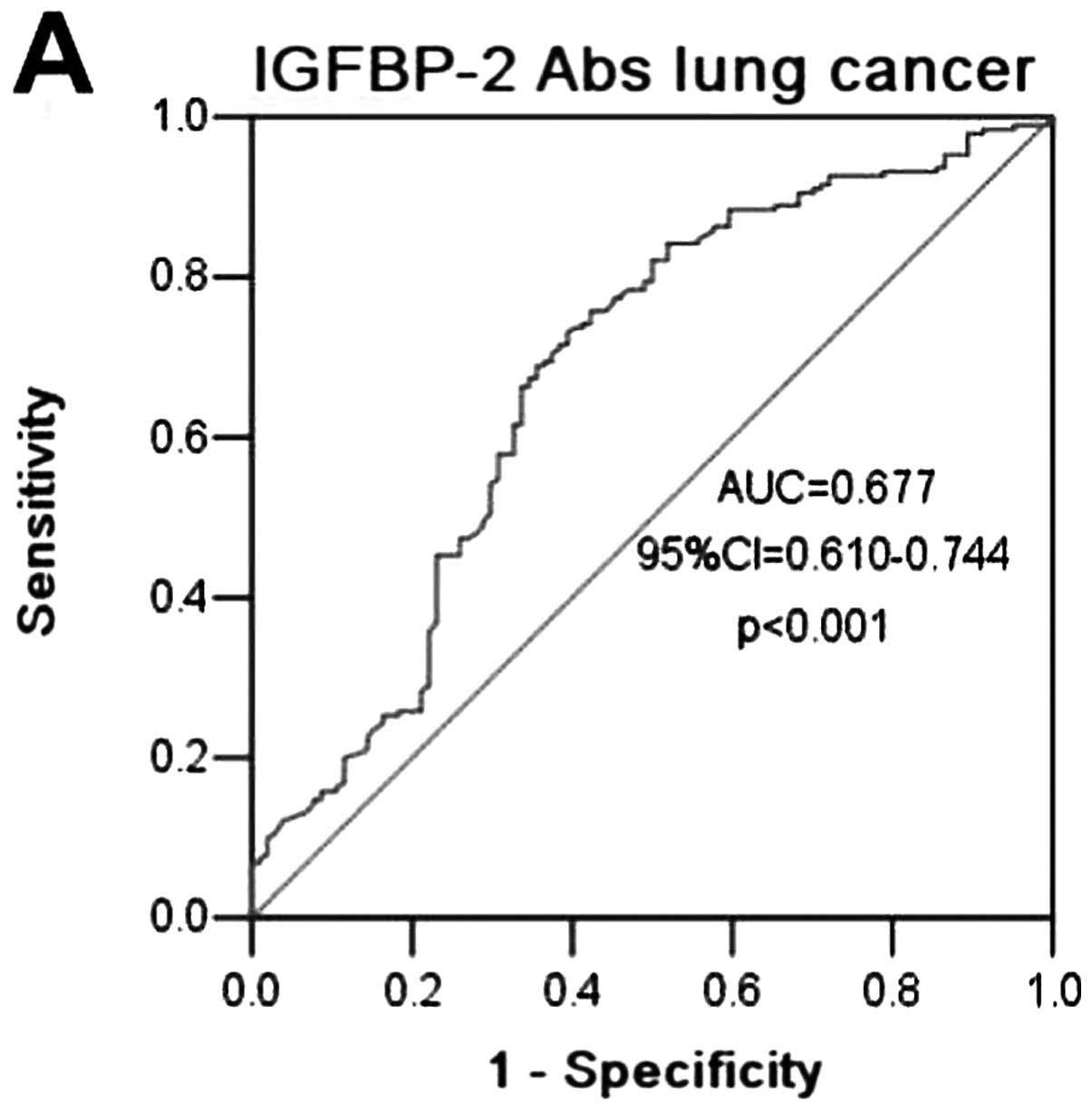
ROC curves were constructed between lung cancer and
benign lung disease and normal controls. The AUC of using
anti-IGFBP-2 antibodies as diagnostic biomarkers for lung cancer
was 0.677 (95% CI=0.610–0.744; P<0.0001) (Fig. 2A), in contrast to an AUC for CEA,
CYFRA21-1 and NSE of 0.770 (95% CI= 0.688–0.853, P<0.0001),
0.794 (95% CI= 0.719–0.864, P<0.0001) and 0.588 (95% CI=
0.490–0.586, P= 0.104), respectively, for cancer in our patient
cohort (Fig. 2A–D). When
stratifying lung cancer by tumor stage, the AUC for lung cancer
I–II and lung cancer III–IV was 0.656 (95% CI=0.568–0.743; P=
0.001) and 0.685 (95% CI= 0.615–0.755; P<0.001) (Fig. 2E and F), respectively, indicating
some ability of anti-IGFBP-2 antibodies to diagnose lung cancer.
Sensitivity, specificity, and all cutoff values of anti-IGFBP-2
antibody levels were determined using ROC analysis. The
anti-IGFBP-2 antibody cutoff values of 1,151.351, 1,261.208,
1,448.053, 1,651.606, 1,922.826, 2,029.312 ng/ml yielded
sensitivities of 80, 73.7, 61.6, 49.5, 25.3 and 16.3%; and
specificities of 50, 59.6, 67.3, 71.2, 83.7 and 89.5%,
respectively. Based on these data, a level of 1,264.306 ng/ml (the
sum of sensitivity and specificity was the highest) was determined
to be the most efficient threshold and we set this level as the
cutoff value. The sensitivity of the assay was 73.2% and the
specificity 60.6%, and the positive and negative predictive values
were 77.2 and 55.3%, respectively. The sensitivity of serum
anti-IGFBP-2 antibodies in the detection of lung cancer was higher
(73.2%) than that of CEA (45.9%), CYFRA21-1 (65.3%), or NSE (50%),
(P<0.001, P=0.166 and P<0.001, respectively) although the
differences were not significant (P<0.001, P=0.166 and
P<0.001, respectively).
Combination of serum IGFBP-2 and
anti-IGFBP-2 antibodies can increase the efficacy of cancer
diagnosis
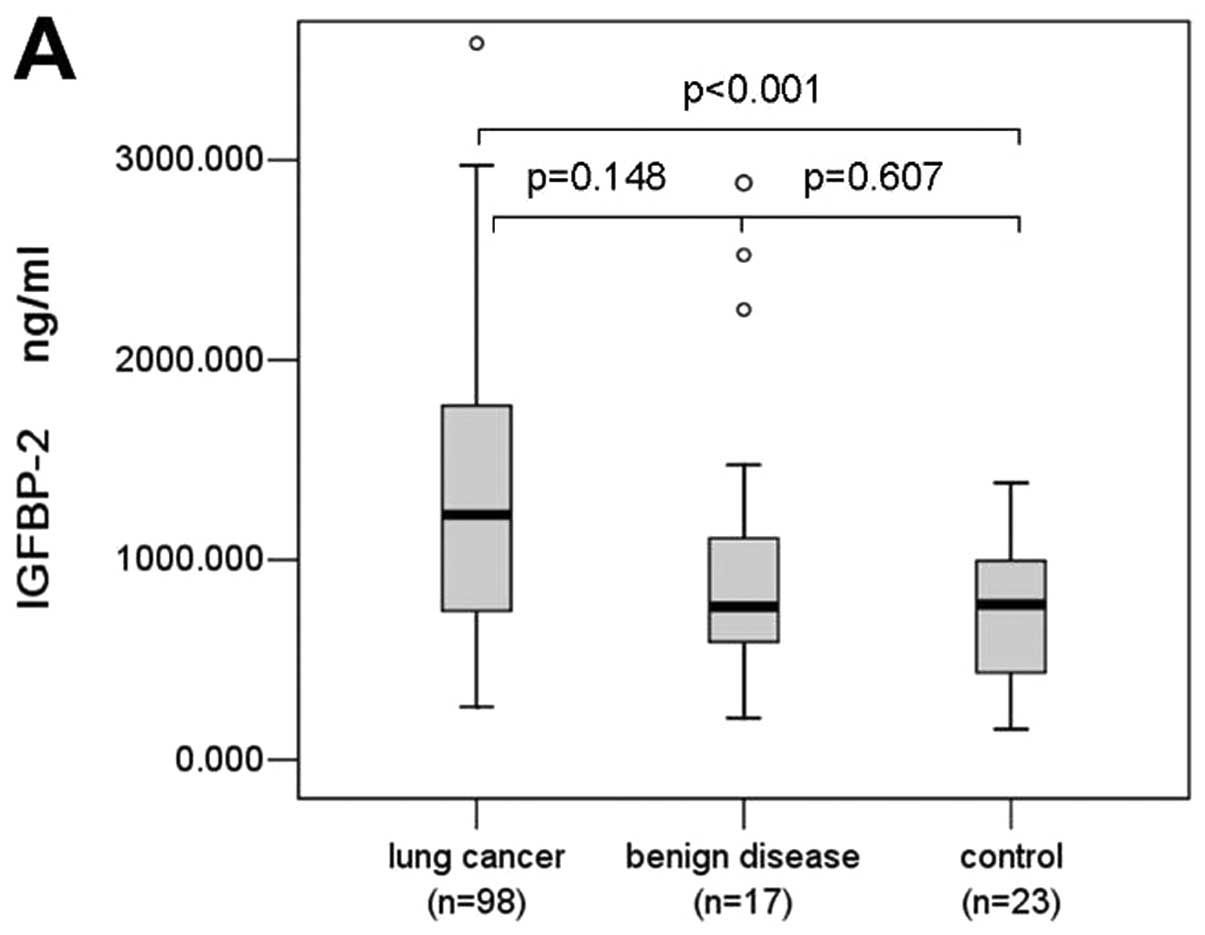
In this study, we also detected serum IGFBP-2 levels
in 98 patients with lung cancer, 17 patients with benign lung
disease, and 23 normal controls, and found that serum IGFBP-2
levels were significantly elevated in lung cancer patients (mean,
1,304.273 ng/ml; median, 1,225.109 ng/ml; range, 265–3,584.674
ng/ml) compared with controls (749.428, 777.228, 51.944–1,384.217
ng/ml) (P<0.0001, Fig. 3A).
Serum IGFBP-2 levels in patients with lung cancer were higher than
that of benign lung disease (1,036.015, 766.304, 209.185–2,885.543
ng/ml), but the difference was not significant (P=0.148, Fig. 3A). There was no significant
difference between lung cancer I–II and lung cancer III–IV either
(P= 0.236, Fig. 3B). We conducted
ROC curve analyses using serum IGFBP-2 levels in discriminating
between patients and controls (Fig.
3C). The AUC of using IGFBP-2 as diagnostic biomarker for lung
cancer was 0.608 (95% CI=0.504–0.712, p=0.047), the sensitivity and
specificity were 54.1 and 72.5%, respectively, with the cutoff
value of 1,173.033 ng/ml. We found that the combination of serum
IGFBP-2 and anti-IGFBP-2 antibodies can augment the discriminative
power for lung cancer with the sensitivity of 85.7% and the
specificity of 57.5%.
Relationship of serum anti-IGFBP-2
antibodies and tumor IGFBP-2 expression in lung cancer
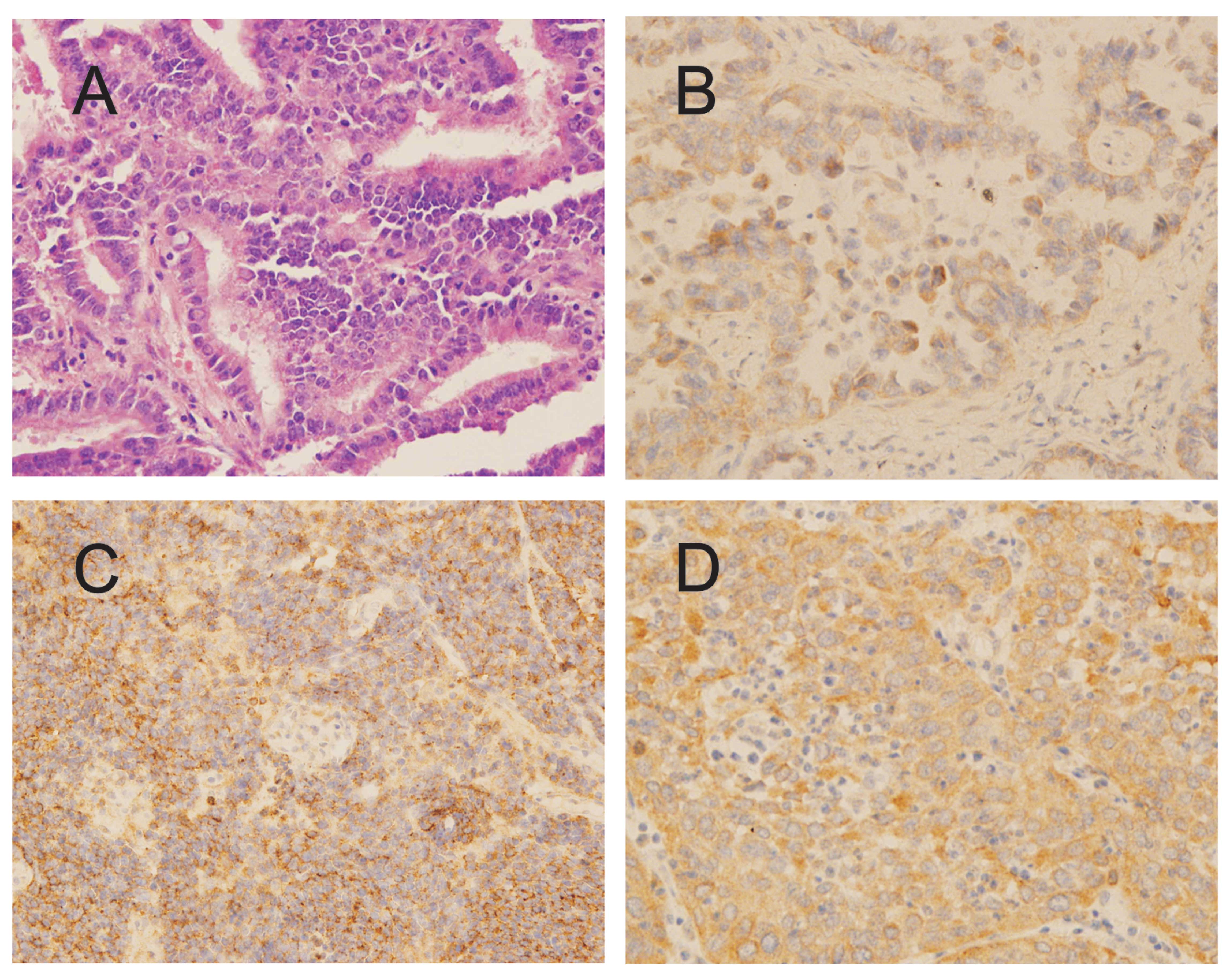
In this study, we also examined IGFBP-2 expression
in 23 lung cancer tissue sections (Fig. 4), and found 69.6% (16/23) of the
lung cancer specimens had low (43.5%) to high (26.1%) IGFBP-2
expression, and 82.6% (19/23) of the tissue specimens had
anti-IGFBP-2 antibody levels above the cut-off value of 1,264.306
ng/ml (data were not shown). Interestingly, we did not find a
correlation between serum anti-IGFBP-2 antibody level and tumor
IGFBP-2 expression level in lung cancer.
Follow-up of serum levels of anti-IGFBP-2
antibodies in lung cancer
In order to determine whether concentration of
anti-IGFBP-2 antibodies would change with the clinical treatment, a
follow-up study has been performed. In the current study, 6
patients were observed, and serum samples were collected from these
patients at 3–4 weeks after tumor resection, and for some patients
samples were also collected after 1–2 cycles of postoperative
chemotherapy. Data from three patients with chemotherapy instead of
surgical resection were also analyzed (Table III).
 | Table III.Characteristics of patients for
follow-up. |
Table III.
Characteristics of patients for
follow-up.
| Patients | Age/sex | Stage | Pathology | Therapy |
|---|
| 40 | 62/M | II B | Squamous | Radical
procedure |
| 74 | 45/F | II A | Adeno | Radical
procedure |
| 79 | 55/M | III A | Adeno | Radical
procedure |
| 161 | 41/F | II A | Adeno | Radical
procedure |
| 181 | 62/M | III A | Adeno | Radical
procedure |
| 299 | 48/M | III B | Adeno | Radical
procedure |
| 41 | 56/M | III A | Squamous | Chemotherapy
(NP) |
| 43 | 62/F | III B | Adeno | Chemotherapy
(TP) |
| 298 | 41/M | IV | Adeno | Chemotherapy
(TP) |
Anti-IGFBP-2 antibody level before operation was
elevated in all of the 6 patients (100%). Interestingly, antibody
level was decreased in 4 patients (66.7%) after 3–4 week treatment,
and only one patient (16.7%) has an elevated antibody level, and
one patient’s antibody level was not changed. We also found that
anti-IGFBP-2 antibody levels in all patients were reduced with the
time progression (Fig. 5B).
In the follow-up observation of three patients who
underwent chemotherapy, levels of anti-IGFBP-2 antibodies were
elevated in all of the 3 patients (100%) at diagnosis, levels
decreased in 2 patients (66.7%) after 1 cycle chemotherapy, and
slightly elevated in one patient whose disease progressed at that
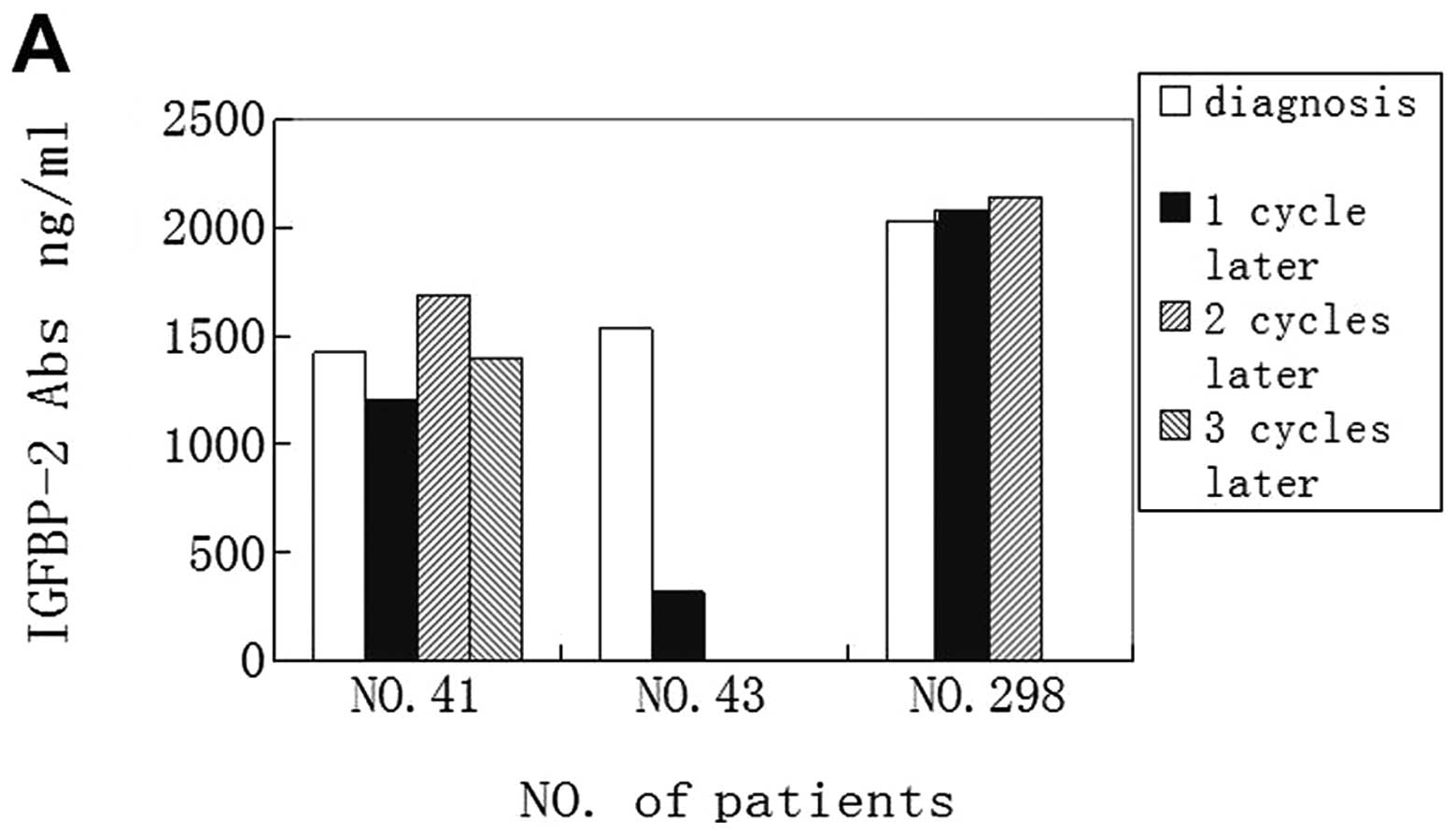
time. For patient no. 41, who developed a liver metastasis after 2
cycles of chemotherapy of vinorelbine and cisplatin, the antibody
levels were also decreased when the disease progressed (Fig. 5A).
Discussion
Previous studies have indicated that IGFBPs are
modulators of IGF signaling through sequestration of IGFs. Soluble
and membrane-associated IGFBP-2 directly binds to proteoglycans and
integrins (18,19), suggesting that IGFBP-2 may be a
negative or positive regulator of cell adhesion, migration, and
invasion in an IGF-independent manner (20–24).
In the same way, IGFBP-2 positively or negatively regulates cell
growth and survival in certain types of cancers in both of in
vitro and in vivo studies (20–22,25,26).
Thus, increased IGFBP-2 confers advantage or disadvantage for tumor
growth, depending on cell type and physiological conditions.
Despite these two opposite effects of IGFBP-2 on the
biological behavior of cancers, studies on biochemistry and
molecular pathology have demonstrated that IGFBP-2 is
over-expressed in a wide variety of human malignancies, including
lung cancer, glioma, prostate cancer, colorectal cancer, ovarian
cancer, adrenocortical tumor, breast cancer and leukemia.
Importantly, IGFBP-2 is frequently overexpressed in advanced
cancers, suggesting that it may be involved in the metastatic
process. Serum IGFBP-2 can be used for prediction of chemotherapy
response and prognosis in ovarian cancer and acute lymphoblastic
leukemia (27). Some other studies
have also demonstrated that IGFBP-2 can be considered as a
regulator of phosphatidylinositol 3-kinase (PI3K)/Akt/PTEN to
promote tumor progression (19,21,28)
and also as a p53 target (29).
Grimberg et al(29) have
reported that loss of IGFBP-2 can inhibit the ability of p53 to
further activate extracellular signal-regulated kinase (ERK)1 by
IGF-I. Migita et al(30)
also found that intracellular IGFBP-2 regulates caspase-3
expression and contributes to the inhibitory effect on apoptosis
independent of IGF in lung adenocarcinoma. Therefore, IGFBP-2 may
offer a novel therapeutic target and serve as an anti-apoptotic
biomarker for lung adenocarcinoma.
As mentioned above, the autoantibodies against TAAs
can be used as reporters in identifying aberrant cellular
mechanisms in tumorigenesis and also served as immunodiagnostic
markers for cancer detection. Our results provide the first
evidence that the serum levels of anti-IGFBP-2 antibodies in
patients with lung cancer are higher than that of patients with
benign lung diseases and normal controls. The majority of patients
with lung cancer present with advanced disease because there are no
symptoms at the early stage. Although CEA, CYFRA21-1 and NSE are
commonly used markers in lung cancer diagnosis, none of these
markers is optimal. The finding in this study may provide a
potential marker of anti-IGFBP-2 antibody in diagnosing lung
cancer. The sensitivity of the assay was 73.2% and the specificity
60.6% with the cutoff value of 1,264.306 ng/ml, which is better
than CEA, CYFRA21-1 and NSE in lung cancer detection.
A recently published paper demonstrated that serum
anti-IGFBP-2 antibody levels were significantly elevated in early
cancer compared to advanced cancers in gliomas and colorectal
carcinoma (17). Interestingly,
our study suggested different results in lung cancer, indicating a
different immunogenicity of IGFBP-2 in patients with lung cancer
compared to patients with gliomas and colorectal carcinoma. It may
be related to the microenvironment of the various tumors (31) and immunosuppressive mechanisms
induced by tumor cells (32) and
also a different role of TAAs in tumor development.
Due to the high specificity of the autoantibodies to
TAAs in cancer, anti-TAA antibodies have been generally considered
as reliable biomarkers in cancer. At the cut-offs of 1,264.306 and
2,029.312 ng/ml of anti-IGFBP-2 antibodies, the specificity for
lung cancer were 60.6 and 89.5%, respectively. It is well known
that if only one anti-TAA antibody is used as tumor marker, the
sensitivity is about 10–20%. As described above, IGFBP-2 is
frequently overexpressed in advanced cancers and can be used for
prediction of chemotherapy response and prognosis in some
malignancy. When serum IGFBP-2 and anti-IGFBP-2 antibodies were
detected simultaneously, the sensitivity of the assay was raised to
85.7%, and the specificity was 57.5% indicating that use of both
serum IGFBP2 and anti-IGFBP-2 antibody can increase the diagnostic
efficacy in lung cancer.
Our study also found that most of patient serum
levels of anti-IGFBP-2 antibodies (66.7%) were decreased after
surgical operation and chemotherapy. When the tumor size was
increasing or the patient was developing metastasis, the serum
levels of anti-IGFBP-2 antibodies were increased, suggesting a role
for anti-IGFBP-2 antibodies in assessing response to therapy in
lung cancer.
The weakness of the study is that the healthy
controls did not receive a bronchoscopy, possibly leading to
misclassification of the study subjects, and there was also the
relatively small sample size, especially for patients with benign
lung diseases. Further studies with larger sample size from
different type of cancer will be performed to confirm and validate
whether anti-IGFBP-2 antibodies can be also used as a diagnostic
marker in other type of cancer.
Abbreviations:
|
IGFBP-2
|
insulin-like growth factor-binding
protein-2
|
|
CT
|
computed tomography
|
|
TAAs
|
tumor-associated antigens
|
|
IGFs
|
insulin-like growth factor family
|
|
CEA
|
carcinoembryonic antigen
|
|
CYFRA21-1
|
cytokeratin 19 fragments
|
|
NSE
|
neuron-specific enolase
|
|
IHC
|
immunohistochemical analysis
|
|
ELISA
|
enzymelinked immunosorbent assay
|
|
IgG
|
intravenous gamma globulin
|
|
BSA
|
bovine serum albumin
|
|
PBS
|
phosphate-buffered saline
|
|
HRP
|
horse radish peroxidase
|
|
TMB
|
tetramethylbenzidine
|
|
H2SO4
|
|
OD
|
optical density
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curve
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Akt
|
serine-threonine kinase
|
|
PTEN
|
phosphatase and tensin homolog deleted
on chromosome ten
|
|
ERK
|
extracellular signal-regulated
kinase
|
Acknowledgements
We thank Huixun Ren at Xi’an Jiaotong
University School of Medicine for his help. The study was supported
by the Clinical Innovation Funds of The First Affiliated Hospital
of XJTU (grant no. 11ZD05).
References
|
1.
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar
|
|
2.
|
Woodward RM, Brown ML, Stewart ST, Cronin
KA and Cutler DM: The value of medical interventions for lung
cancer in the elderly: results from SEER-CMHSF. Cancer.
110:2511–2518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ernst A, Anantham D, Eberhardt R, Krasnik
M and Herth FJ: Diagnosis of mediastinal adenopathy-real-time
endobronchial ultrasound guided needle aspiration versus
mediastinoscopy. J Thorac Oncol. 3:577–582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nakashima A, Murakami Y, Uemura K, et al:
Usefulness of human telomerase reverse transcriptase in pancreatic
juice as a biomarker of pancreatic malignancy. Pancreas.
38:527–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ghaneh P, Greenhalf W, Humphreys M, et al:
Adenovirus-mediated transfer of p53 and p16 (INK4a) results in
pancreatic cancer regression in vitro and in vivo. Gene Ther.
8:199–208. 2007. View Article : Google Scholar
|
|
6.
|
Soussi T: p53 antibodies in the sera of
patients with various types of cancer. Cancer Res. 60:1777–1788.
2010.PubMed/NCBI
|
|
7.
|
Looi K, Megliorino R, Shi FD, Peng XX,
Chen Y and Zhang JY: Humoral immune response to p16, a
cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep.
16:1105–1110. 2006.PubMed/NCBI
|
|
8.
|
Zhang JY, Chan EK, Peng XX and Tan EM: A
novel cytoplasmic protein with RNA-binding motifs is an autoantigen
in human hepatocellular carcinoma. J Exp Med. 189:1101–1110. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Park KH, Gad E, Goodell V, et al:
Insulin-like growth factor-binding protein-2 is a target for the
immunomodulation of breast cancer. Cancer Res. 68:8400–8409. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lin Y, Jiang T, Zhou K, et al: Plasma
IGFBP-2 levels predict clinical outcomes of patients with
high-grade gliomas. Neuro Oncol. 11:468–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Miraki-Moud F, Jenkins PJ, Fairclough PD,
et al: Increased levels of insulin-like growth factor binding
protein-2 in sera and tumours from patients with colonic neoplasia
with and without acromegaly. Clin Endocrinol (Oxf). 54:499–508.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Liou JM, Shun CT, Liang JT, et al: Plasma
insulin-like growth factor-binding protein-2 levels as diagnostic
and prognostic biomarker of colorectal cancer. J Clin Endocrinol
Metab. 95:1717–1725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shariat SF, Lamb DJ, Kattan MW, et al:
Association of preoperative plasma levels of insulin-like growth
factor I and insulin-like growth factor binding proteins-2 and -3
with prostate cancer invasion, progression, and metastasis. J Clin
Oncol. 20:833–841. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wang H, Rosen DG, Wang H, Fuller GN, Zhang
W and Liu J: Insulin-like growth factor-binding protein 2 and 5 are
differentially regulated in ovarian cancer of different histologic
types. Mod Pathol. 19:1149–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Busund LT, Richardsen E, Busund R, Ukkonen
T, Bjørnsen T, Busch C and Stalsberg H: Significant expression of
IGFBP2 in breast cancer compared with benign lesions. J Clin
Pathol. 58:361–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Baron-Hay S, Boyle F, Ferrier A and Scott
C: Elevated serum insulin-like growth factor binding protein-2 as a
prognostic marker in patients with ovarian cancer. Clin Cancer Res.
10:1796–1806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Li Y, Jiang T, Zhang J, et al: Elevated
serum antibodies against insulin-like growth factor-binding
protein-2 allow detecting early-stage cancers: evidences from
glioma and colorectal carcinoma studies. Ann Oncol. 23:2415–2422.
2012. View Article : Google Scholar
|
|
18.
|
Pereira JJ, Meyer T, Docherty SE, Reid HH,
et al: Bimolecular interaction of insulin-like growth factor (IGF)
binding protein-2 with alphavbeta3 negatively modulates
IGF-I-mediated migration and tumor growth. Cancer Res. 64:977–984.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wang GK, Hu L, Fuller GN and Zhang W: An
interaction between insulin-like growth factor-binding protein 2
(IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell
mobility. J Biol Chem. 281:14085–14091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hoeflich A, Reisinger R, Lahm H, et al:
Insulin-like growth factor-binding protein 2 in tumorigenesis:
protector or promoter. Cancer Res. 61:8601–8610. 2001.PubMed/NCBI
|
|
21.
|
Lee EJ, Mircean C, Shmulevich I, et al:
Insulin-like growth factor binding protein 2 promotes ovarian
cancer cell invasion. Mol Cancer. 4:72005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Miyake H, Hara I, Yamanaka K, Muramaki M,
Gleave M and Eto H: Introduction of insulin-like growth factor
binding protein-2 gene into human bladder cancer cells enhances
their metastatic potential. Oncol Rep. 13:341–345. 2005.
|
|
23.
|
Schütt BS, Langkamp M, Rauschnabel U,
Ranke MB and Elmlinger MW: Integrin-mediated action of insulin-like
growth factor binding protein-2 in tumor cells. J Mol Endocrinol.
32:859–868. 2004.PubMed/NCBI
|
|
24.
|
Hoeflich A, Reisinger R, Schuett BS, et
al: Peri/nuclear localization of intact insulin-like growth factor
binding protein-2 and a distinct carboxyl-terminal IGFBP-2 fragment
in vivo. Biochem Biophys Res Commun. 324:705–710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hoeflich A, Fettscher O, Lahm H, et al:
Overexpression of insulin-like growth factor-binding protein-2
results in increased tumorigenic potential in Y-1 adrenocortical
tumor cells. Cancer Res. 60:834–838. 2000.PubMed/NCBI
|
|
26.
|
Chatterjee S, Park ES and Soloff MS:
Proliferation of DU145 prostate cancer cells is inhibited by
suppressing insulin-like growth factor binding protein-2. Int J
Urol. 11:876–884. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Vorwerk P, Mohnike K, Wex H, Röhl FW,
Zimmermann M, Blum WF and Mittler U: Insulin-like growth factor
binding protein-2 at diagnosis of childhood acute lymphoblastic
leukemia and the prediction of relapse risk. J Clin Endocrinol
Metab. 90:3022–3027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mehrian-Shai R, Chen CD, Shi T, et al:
Insulin growth factor-binding protein 2 is a candidate biomarker
for PTEN status and PI3K/Akt pathway activation in glioblastoma and
prostate cancer. Proc Natl Acad Sci USA. 104:5563–5568. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Grimberg A, Coleman CM, Shi Z, Burns TF,
MacLachlan TK, Wang W and El-Deiry WS: Insulin-like growth factor
factor binding protein-2 is a novel mediator of p53 inhibition of
insulin-like growth factor signaling. Cancer Biol Ther.
5:1408–1414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Migita T, Narita T, Asaka R, et al: Role
of insulin-like growth factor binding protein 2 in lung
adenocarcinoma: IGF-independent antiapoptotic effect via caspase-3.
Am J Pathol. 176:1756–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lubin R, Schlichtholz B, Teillaud JL,
Garay E, Bussel A and Wild CP: p53 antibodies in patients with
various types of cancer assay, identification, and
characterization. Clin Cancer Res. 1:1463–1469. 1995.PubMed/NCBI
|
|
32.
|
Comtesse N, Zippel A, Walle S, et al:
Complex humoral immune response against a benign tumor: frequent
antibody response against specific antigens as diagnostic targets.
Proc Natl Acad Sci USA. 102:9601–9606. 2005. View Article : Google Scholar
|