Introduction
The HER2 (ErbB2/neu) proto-oncogene encodes a
transmembrane receptor protein of 185 kDa, which is structurally
related to the epidermal growth factor receptor. ErbB2 is usually
expressed at a very low level in adult tissues, but it is
overexpressed in a variety of human tumors, such as breast cancer
(25–30%), ovarian cancer (25–32%), lung cancer (30–35%), and
primary renal cell carcinoma (30–40%) (1–4).
A recombinant humanized monoclonal antibody,
Herceptin (trastuzumab) has shown good response in clinical trials,
in the treatment of HER2-overexpressing metastatic breast cancers
(5–8). The objective response rates of
Herceptin monotherapy were lower than its combination therapies.
Current treatment regimens which combine Herceptin with the taxane,
paclitaxel or docetaxel have increased the response rates, time to
progression, and survival (9,10).
The humanized Herceptin suppressed the growth of
HER2-overexpressing tumor cells by inhibiting HER2-driven
signaling, inhibiting VEGF secretion or activity and mediating
antibody-dependent cellular cytotoxicity (ADCC) and
complement-dependent cytotoxicity (11–14),
but the humanized Herceptin did not induce apoptosis when it is
used alone.
Interleukin-2 (IL-2) is an immunoregulatory protein
with a wide range of immune effects. IL-2 exerts its biological
effects by binding with the IL-2 receptor on the surface of target
cells. The activities of IL-2 include stimulating T cells to
proliferate and become cytotoxic, augment NK cytotoxicity,
enhancing macrophages cytotoxicity, giving rise to
lymphokine-activated killer (LAK) cells (15–20).
In tumor therapy, IL-2 has already shown some efficacy in the
treatment of metastatic melanoma, kidney cancer or non-Hodgkin’s
lymphoma when used alone or combined with other anticancer drugs.
However, the antitumor efficiency of IL-2 is often associated with
unacceptable toxicity. HFI ensures both the prevention of the
systemic side-effects and the locally restricted enrichment
activity to the tumor sites with decreased IL-2 toxicity (21–23).
Despite the success of the Herceptin in clinical
trials for the treatment of ErbB2-expressing metastatic breast
cancer, the fusion of IL-2 to an ErbB2-specific antibody may
enhance its antitumor efficacy. Firstly, the presence of the
antibody variable region should target the tumor cells, allowing
for higher doses of IL-2 at HER2 antigen located in tumor tissue,
where they elicit an immune response and thus destroy tumors.
Secondly, IL-2 is unstable in vivo and it will be quickly
eliminated by the kidney due to limiting quantities of the protein.
The half-life of IL-2 may be extended by MAb-conjugation due to
both the size and stability of the MAb. Thirdly, an antibody-IL2
fusion protein may increase immune access to the antibody’s target
ligand by increasing permeability of blood vessels near the tumor
nidus (24–30). In preliminary studies, it was
observed that the HFI expression of clonal cells strained between
60–80 mg/l; the in vitro experiments proved that the HFI
contained dual activity of antibodies and IL-2; the in vivo
tests showed that the HFI inhibited HER2 expression of ovarian
cancer; in (SKOV3) transgenic mouse tumor growth, the HFI antibody
ADCC activity and IL-2-mediated killing activity of effector cells;
to stimulate effector cells to secrete perforin, and the adhesion
factor. However, many aspects of HFI has not been verified,
including whether the cell lines is stable, whether the HFI
purification technology is mature, or the HFI sample is stable,
whether the HFI inhibits the role of HER2 overexpression in other
cell lines, safety and so on. In this study, the HFI drug ability
for some of these issues were analyzed and discussed.
Materials and methods
Expression and purification of the
HFI
The highest-producing of HFI fusion clone was
harvested after the HFI expression vector had been transfected into
adherent Chinese Hamster Ovary (CHO-K1) cells (ATCC No. CRL-9096),
incubated with the selection medium containing 300 μg/ml
G418 (also known as geneticin) (31,32).
The primary cell bank (PCB) of the HFI engineering cells was built
when the adherent CHO cells was domesticated into suspension cells
in tissue culture with HyClone SFM4CHO (HyClone) or EX-CELL™325
(Sigma-Aldrich) cell culture medium, which is a protein-free
formulation that contained no components of bovine origin. The
master cell bank (MCB) and the working cell bank (WCB) with the
stable expressing HFI were obtained from the PCB in tissue culture
roller bottles with the cell culture medium, as described above.
The supernatant containing fusion protein of HFI was harvested as
the cell culture process of the Cell Bioreactor
(BIOSTAT® Bplus), which was determined through the batch
culture or the continuous flow culture. The fusion protein of HFI
was purified over the following sophisticated purification
processes, including microfiltration, ion-exchange, affinity and
molecular sieve chromatography.
Enzyme-linked immunosorbent assay
(ELISA)
Quantification of the HFI was assayed by ELISA. Goat
anti-human (100 μl) IgG (1:200 dilution) per well was coated
onto 96-well ELISA plates overnight at 4°C. After washing the
plates, serial dilution of the samples, which were maintained in
PBS with 3% bovine serum albumin (BSA) were added to the plates and
incubated at 37°C for 2 h. Then after washing the plates, 100
μl per well of horse-radish peroxidase-conjugated goat
anti-human IgG (1:1,000 dilution) was bound and incubated at 37°C
for 1 h. Color was generated by adding peroxidase substrate and
absorbance was measured on a plate reader at 492 nm. Dilutions of
the HFI, for which the concentration was calibrated by the Kjeldahl
method and the Lowry method, were used to generate a standard curve
to estimate the concentration of unknown samples.
Bioactivity assay
The cytokine activity of the HFI was determined by
IL-2 dependent T cell proliferation assay. Recombinant human IL-2
(rhIL-2) standard was obtained from National Institute for the
control of Pharmaceutical and Biological products. The mouse CTLL-2
cell line expressing high-affinity IL-2 receptor was used to
determine the IL-2 bioactivity by the T cell proliferation assay.
Serially diluted samples and IL-2 standards were incubated with
4×104 cells per well in triplicate in 96-well flat
bottom plates for 24 h at 37°C in a humidified atmosphere of 5%
CO2. MTT (5 mg/ml) reagent was added 4 h before the end
of culture, and then cells were lysed with 10% SDS and 50%
N,N-dimethyl formamide, pH was maintained at 7.2. The
OD values were read at 570 nm. The mouse CTLL-2 cell was provided
by Professor N. Guo (Institute of Basic Medical Sciences,
China).
Antigen binding assay
About 1×106 cells per well were incubated
with varying concentrations of the HFI or the same molar
concentrations of the different proteins in a final volume of 0.2
ml. After incubation on ice for 30 min, cells were washed twice
with PBS-BSA, and a fluorescein isothiocyanate (FITC)-conjugated
goat anti-human IgG antibody (Beijing Dingguo Biotechnology Co.
Ltd.) was added. Incubation was continued for an additional 30 min
on ice. After two washes with PBS-BSA, the cells were analyzed by
flow cytometry (FACSCalibur, Becton Dickinson).
Animal experiments
The three types of mice including female FVB/neu
mice, BALB/c mice and female Balb/C athymic nude mice of 7 to
10-weeks-age, were obtained from Shanghai Laboratory Animal Center
of the Chinese Academy of Sciences. All mice were housed under
specific pathogen-free conditions. Experiments were carried out
according to the National Institute of Healthy Guide for Care and
Use of Laboratory Animals, and were approved by the Institutional
Animal Care and Use Committee at the Shanghai Institute of Materia
Medica, Chinese Academy of Sciences.
Pharmacokinetic analysis
BALB/c mice (48 animals per group) were injected
with 25 or 100 μg of the purified HFI sample in a volume of
0.2 ml in the tail vein using a slow push, respectively. At various
time points, small blood samples (6 animals per time point) were
taken by retro-orbital bleeding and collected in tubes coated with
heparin to prevent clotting. After centrifugation, the plasma was
assayed by ELISA with goat anti-human IgG and goat anti-human IL-2
antibody. The results were normalized to the initial concentration
in the serum of each mouse taken immediately after injection.
In vitro antitumor activity
When the signals of HER2 were blocked on the surface
of the HER2 overexpression cells, the growth of cells was inhibited
or the cells entered apoptosis. The three types of cells including
SKBR3 cells (HER2+++), SKOV3 cells (HER2++)
and MCF7 cells (HER2±) were provided by Professor N. Guo
(Institute of Basic Medical Sciences, China), and were chosen to
evaluate HFI antitumor activity in vitro(33). Firstly, the cells were placed on
96-well cell plates, then, serial dilution of the samples were
added to the plates and incubated at 37°C in a humidified
atmosphere of 5% CO2. Apoptosis of the cells were
assayed for different time points by MTT cell proliferation assay
methods.
In vivo antitumor activity
i) Six to seven-week-old female Balb/C athymic nude
mice were subcutaneously injected 1×107 Calu-3 cells
(human non-small cell lung cancer) into the right flank. The mice
were treated with PBS (iv: 0.2 ml per mice) (control) or HFI (iv:
1.0, 0.5 and 0.25 mg/kg mice weight, respectively). Seven or eight
mice were used in each group. ii) The antitumor activity of HFI was
evaluated in FVB/neu transgenic mouse model, which expressed the
ErbB2/neu proto-oncogene and closely recapitulated the ontogeny and
progression of human breast cancer. When tumors reached a size of
more than 300 mm3, the mice were grouped (n=5) and PBS
or HFI (1.0 mg/kg mice weight) or Herceptin (3.0 mg/kg mice weight)
in a volume of 0.2 ml, was injected i.v. into the lateral tail
vein. Dosage regimen: the first week for five continuous days, then
once every other day. The mice weight were monitored daily and
tumor volume was measured with a caliper once per two days, using
the formula volume = length × width2/2.
Splenocyte culture
Splenocytes were harvested on day 20 after HFI
administration and were cultured in triplicate
(5×105/well) in 96-well culture plates (Corning) for 24
h with 0.5 μg/ml of concanavalin A (Con A) or 1 μg/ml
of lipopolysaccharide (LPS). The cells were pulsed with 0.5
μCi/well of [3H]thymidine for 8 h and harvested
onto glass fiber filters. The incorporated radioactivity was then
counted using a Beta Scintillation Counter (MicroBeta Trilux,
PerkinElmer Life Sciences, Boston, MA).
ELISA detection of cytokines
Splenocytes were cultured in 24-wells culture plates
(Corning) for 24 and 48 h with 0.5 μg/ml of Con A or 1
μg/ml of LPS. Culture supernatants were harvested and
submitted to assays for the determination of cytokine
concentrations. Cytokines in culture supernatant was assayed using
mouse IFN-γ ELISA kit (BD Pharmingen), according to the
manufacturer’s instructions.
Cytotoxicity assays
Murine YAC-1 cells were used as targets in the NK
assay. Target tumor cells were labeled with 250 kBq
[3H]thymidine by incubating overnight in a culture dish
(Corning) containing 10 ml of culture medium. Targeted cells
(2×106) were cultured in triplicate with different
numbers of effector cells (effector: Target ratio = 100:1, 50:1 or
12.5:1) for 4, 8 and 12 h in 96-wells flat-bottomed plates. Cells
were harvested onto glass fiber filters. The incorporated
radioactivity was then counted using a Beta Scintillation Counter
(MicroBeta Trilux, PerkinElmer Life Sciences, Boston, MA).
Cytotoxic activity was calculated and expressed as the mean of
percent cytotoxicity. Percent cytotoxicity = (cpm in effector-free
wells − cpm in effector positive wells)/(cpm in effector-free
wells) ×100.
Flow cytometric analysis
A single-cell suspension of spleen cells harvested
on day 20 after HFI administration were blocked with
anti-mCD16/CD32 (2.4G2, purified inhouse), stained with fluorescein
isothiocyanate (FITC)-conjugated, phycoerythrin (PE)-conjugated,
(PerCPCy5.5)-conjugated, (APC)-conjugated, and analyzed using a
FACSCalibur system with Cell Quest software (Becton Dickinson).
(FITC), (PE), (PerCPCy5.5) or (APC)-conjugated mAb against CD3
(145-2c11), CD4 (Gk1.5), CD8 (Ly-2) (53-6.7), CD1 (1d3), TCR-γ/δ
(GL3) were purchased from BD Pharmingen. PerCPCy5.5-conjugated
anti-mouse/rat FoxP3 (FJK-16s), PerCPCy5.5-conjugated rat IgG2a and
the FoxP3 staining buffer set were purchased from eBioscience.
Statistics
The statistical significance of the time course data
was determined by two-way analysis of variance testing and
Student’s t-test was used to compare two treatment groups.
Statistical significance was set at p<0.05.
Results
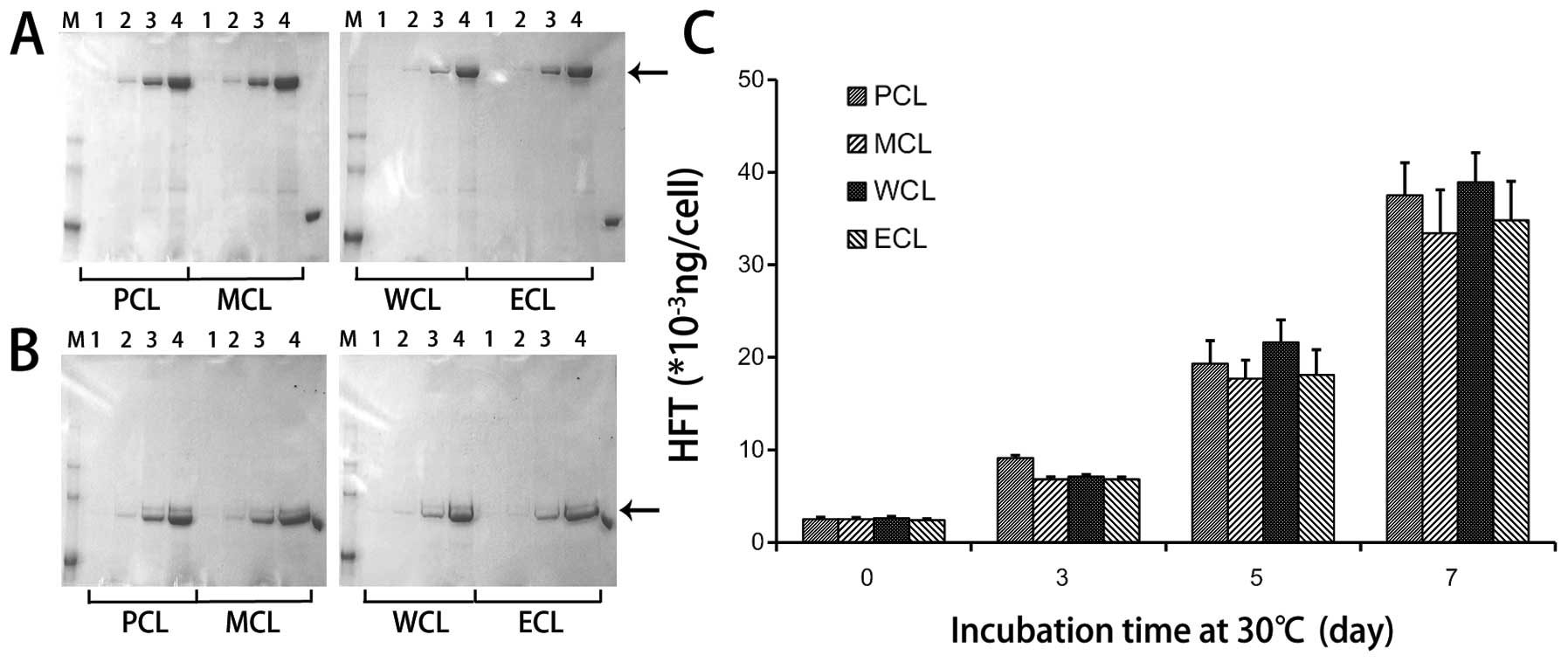
HFI protein expression and its
characterization
The stable HFI expression was first assayed in the
differential course of cell passage by using a pair of goat
anti-human IgG antibodies in a ‘sandwich ELISA’, SDS-PAGE and IL-2
dependent T cell proliferation. The fusion proteins of HFI were
stably expressed in different cell lines, such as the primary cell
line (PCL), the master cell line (MCL), the working cell line (WCL)
and the production of end-stage cell line (ECL) in 1,000 ml tissue
culture roller bottles with a culture volume of 200–300 ml and at
(2–4)×106 cells/ml density at 30°C
for 7 days. The culture supernatants were harvested following 1,500
rpm for 5 min to 8,000 rpm for 30 min. The molecular weight of HFI
was found in a bivalent form of 140 kDa (Fig. 1A) and a monovalent form of 70 kDa
(Fig. 1B) on 8% SDS-PAGE stained
with Coomassie Brilliant Blue. The HFI expression of the four kinds
of cells cultured for 7 days was examined at 100–150 mg/l by ELISA
(Fig. 1C). The IL-2 bioactivity of
HFI was assessed at (2–2.5) ×106 IU/mg by the standard
IL-2 dependent T cell proliferation assay and ELISA calibration
concentration (data not shown). Fig.
1 shows that the HFI gene copy number and HFI expression were
relatively stable in the differential course of cell passage.
Since the expression of HFI in the cell lines was
stable, the purification and stability of HFI was crucial to the
ability of this fusion protein. The supernatant of HFI reaction
liquid was sampled at 30°C and assayed for one month by the Cell
Bioreactor under a continuous flow mode. In the HFI purification
process, insoluble cell debris and other irrelevant substances were
first removed from the HFI supernatant by 0.2 or 0.45 μm
microfiltration membrane bag (Millipore), then the microfiltration
sample was concentrated with the 30–50 kD nitrocellulose membrane
bag (Millipore) to a proper volume. This concentration step was
critical for HFI purification, as it could not only remove a large
number of cellular components, but also deplete certain host
proteins and small molecules in culture medium. In order to protect
the affinity chromatography media from erosion, the
ultra-filtration sample was purified by DEAE ion exchange in
0.2–0.4 M NaCl, 40 mM phosphate-citrate buffer and pH 7.2 was
maintained, this process was mainly to remove endotoxin, nucleic
acids and other substances. Purity of antibody protein reached 97%
or higher after affinity chromatography purification. Therefore,
the HFI was purified over Mabselect Sure (GE Healthcare) affinity
chromatography, eluting with acidic conditions. The purified
solution of the HFI was obtained by Superdex 200 (GE Healthcare)
chromatography media to remove HFI monomer and polymer (Fig. 2). After the protein purification
process, the HFI yield was measured by a microplate
spectrophotometer at 490 nm, and the final HFI sample was more than
40% yield, the HFI purity was examined by reducing and non-reducing
SDS-PAGE (Fig. 2A), the quantity
of HFI monomers and polymers were less than 5% of HFI dimers
(Fig. 2A; lanes 7 and 8), more
than 98% purity of the HFI sample was determined by HPLC (Fig. 2B), fluorescent PCR, and host
protein ELISA. Endotoxin was monitored by LAL agglutination. The
HFI bioactivity was consistent with IL-2 activity, measured by a
standard IL-2-dependent T cell proliferation assay (Fig. 2C). Meanwhile, the stability of the
purified solution of the HFI was also analyzed at 4°C for 2 months.
These results suggested that the fusion protein of the HFI was
relatively stable, and could be suitable for further drug
development.
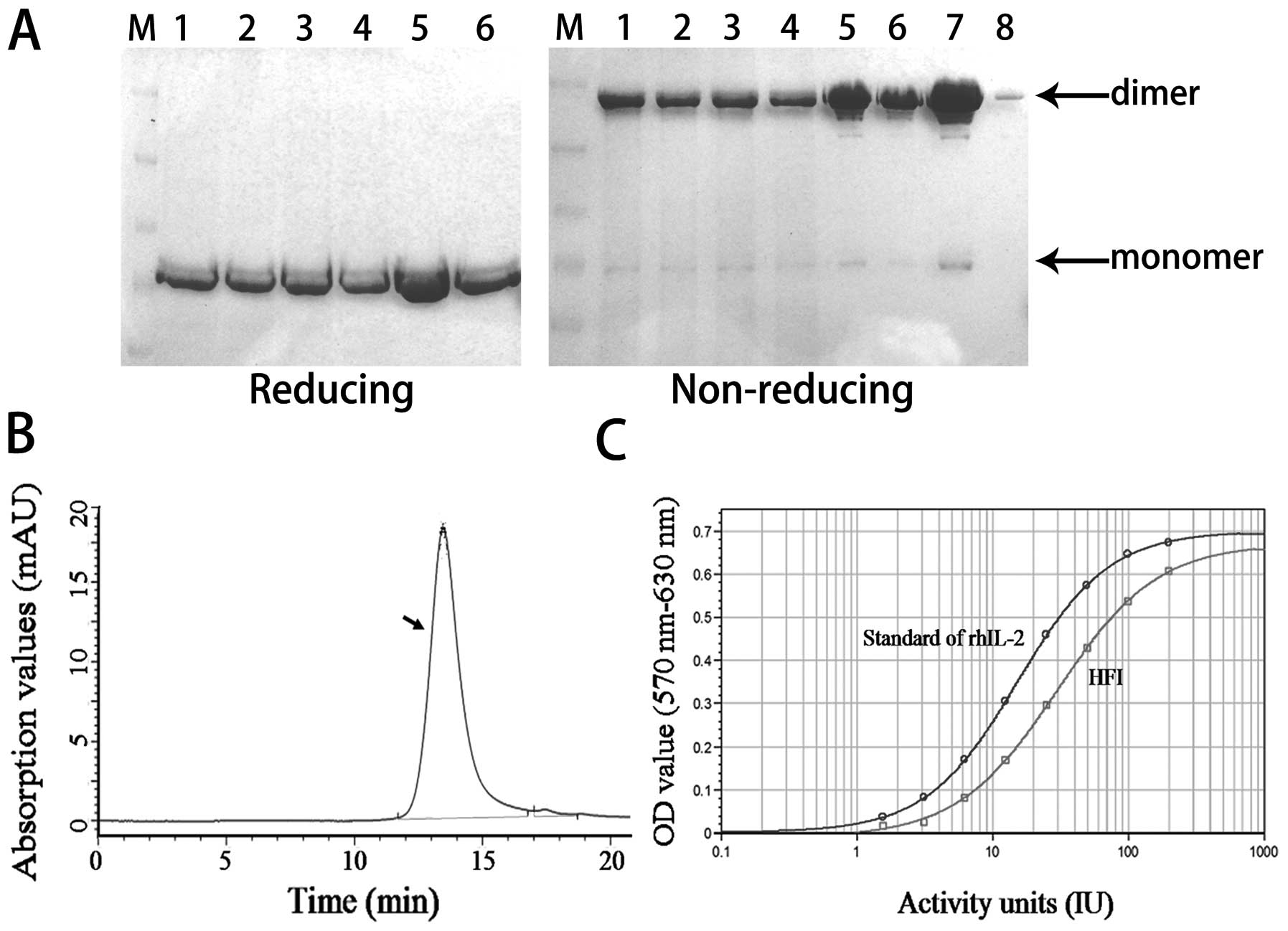 | Figure 2.Quality and activity of purified
protein was analyzed. (A) SDS-PAGE. Lane M is the protein molecular
weight marker; lanes 1, 2, 3, 4, 5 are the HFI cell supernatant,
microfiltration sample, ultrafiltration sample, DEAE ion-exchange
sample, MabSelect Sure chromatography sample, respectively; lanes
6, 7, 8 are the HFI Superdex 200 chromatography sample, among them,
the HFI in well 8 is the amount of 5% of well 7. Above and below
the arrows represent the position of HFI dimer and monomer. (B)
HPLC. HFI purity of the sample was detected by high performance
liquid chromatography G3000SWXL (TSK) on the Agilent
1100 (Agilent). Arrow refers to the HFI peak at 280 nm, shows more
than 98% purity HFI. (C) Bioassay. HFI biological activity,
proliferation of CTLL-2 cells dependent on IL-2 by MTT assay on the
SpectraMax M2/M2e (Molecular Devices). |
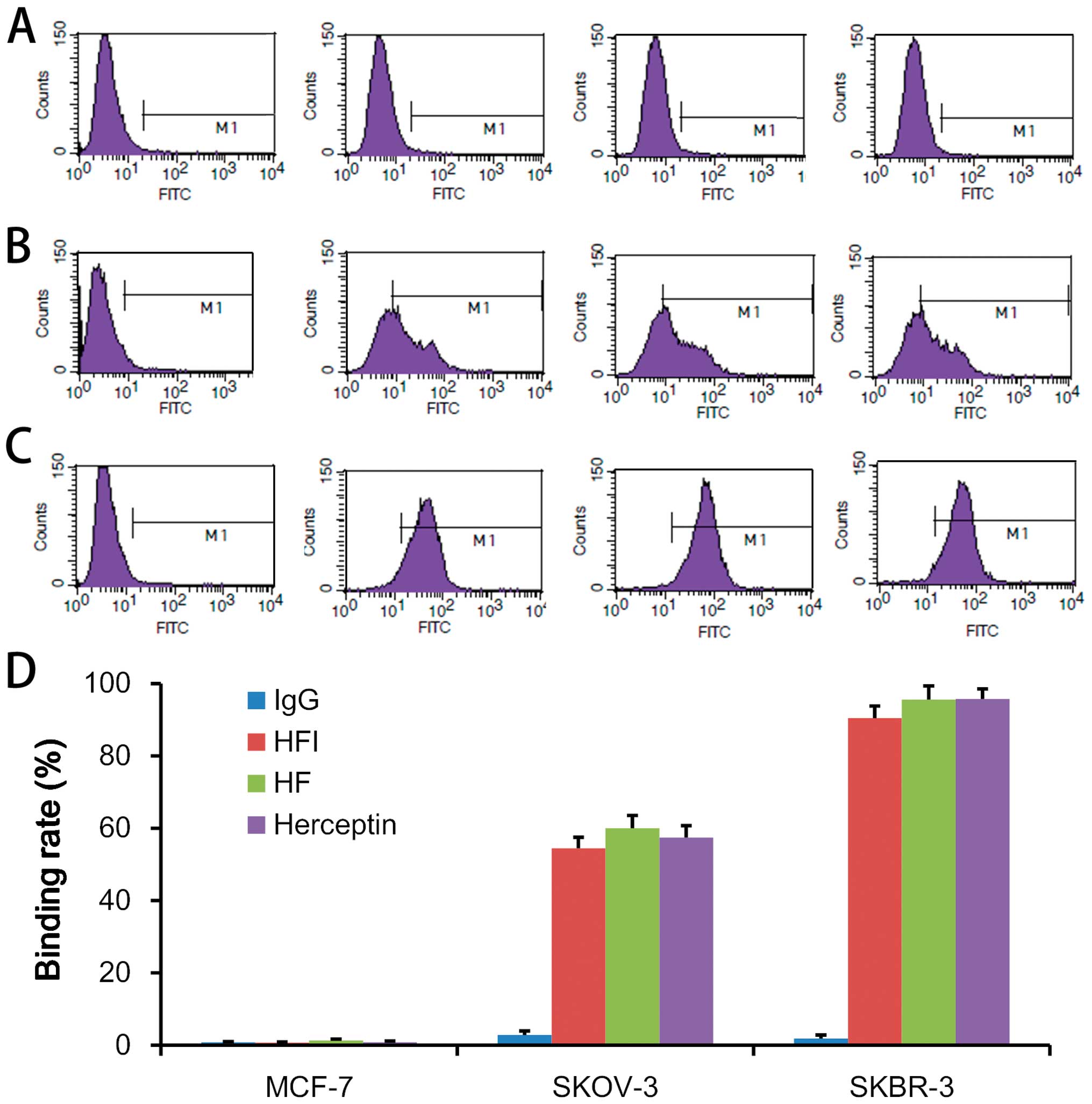
HFI binds specifically to HER2
The HFI built targeting was based on the binding
properties of the ant-ErbB2 antibody and interleukin-2 bioactivity.
The biological activity of IL-2 of the HFI was based on the ability
of the HFI to support IL-2 dependent T cell proliferation (Fig. 2C). Human CD3+ and
CD16+ cells were detected from the peripheral blood of
mice at several doses of the HFI treated groups (33). The binding characteristics of the
HFI was identified, and then three HER2 expressing cells, SKBR3
(HER2+++), SKOV3 (HER2++) and MCF7 cells
(HER2±) were used to test HFI binding activity in
different tumor cell lines. In the same molar concentration, HFI,
Herceptin (Trastuzumab Injection, Roche Pharma Ltd.) and HF
(antibody part of HFI, self-made sample) showed the same binding
ability (Fig. 3). The HFI binding
capacity was not affected by IL-2 fusion with HFI as compared to HF
(Fig. 3A–D). This result
demonstrated that the HFI binding ErbB2 protein characteristics
depended on the expression of the ErbB2 protein (Fig. 3A–D). HFI showed a similar
characteristic of binding HER2 with the Herceptin (Fig. 3A–D).
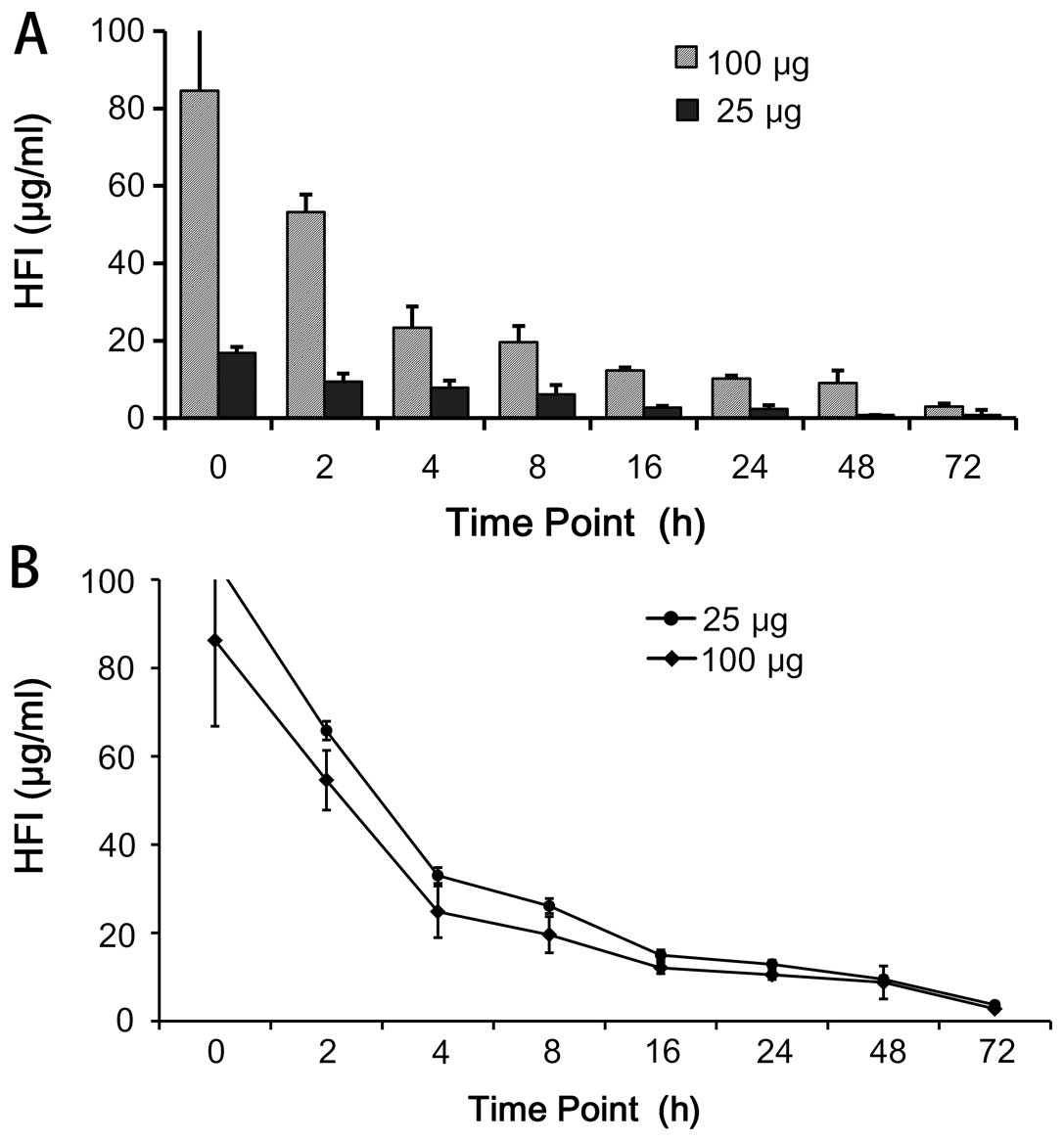
Pharmacokinetic properties
The pharmacokinetic properties of immunocytokines
were reported to be dependent on their uptake by the FcR-bearing
cells and the intracellular proteolysis (28). Half-life of drugs was related to
their molecular size. The structure of HFI was determined by the
in vivo stability, and properties of IL-2 and anti-ErbB2
protein. The half-life of HFI was detected in the mouse serum at
different time points by ELISA. The results showed that the
concentration of HFI decreased quickly during the first 4 h, and
showed a relatively stable plateau following 64 h (Fig. 4). At 100 μg HFI maintained
relatively higher levels than 25 μg HFI, demonstrating that
the higher levels were maintained with higher concentrations
(Fig. 4). The results indicated
that the HFI structural design was conducive to recruitment around
the cells, and also extended the half-life of IL-2.
In vitro antitumor activity
The HFI has a combination of activities, i.e.,
binding properties of HER2 receptor and interleukin-2 bioactivity.
The ability of the HFI binding HER2 receptor depends on the HER2
overexpression on the cell surface. In this study, whether the HFI
inhibited the cell proliferation when the HFI was bound to HER2
receptor was evaluated. The three cell types, breast cancer
(SKBR-3, HER2+++), breast cancer (MCF-7,
HER2±) and ovarian cancer cells (SKOV-3,
HER2++) were incubated with the different concentrations
of HFI for 72 h. The results showed that the HFI did not inhibit
cells proliferation, and proved that the HFI did not have chemical
toxicity. In vitro, the role of HFI was to inhibit tumor
cell growth by activating the effector cells.
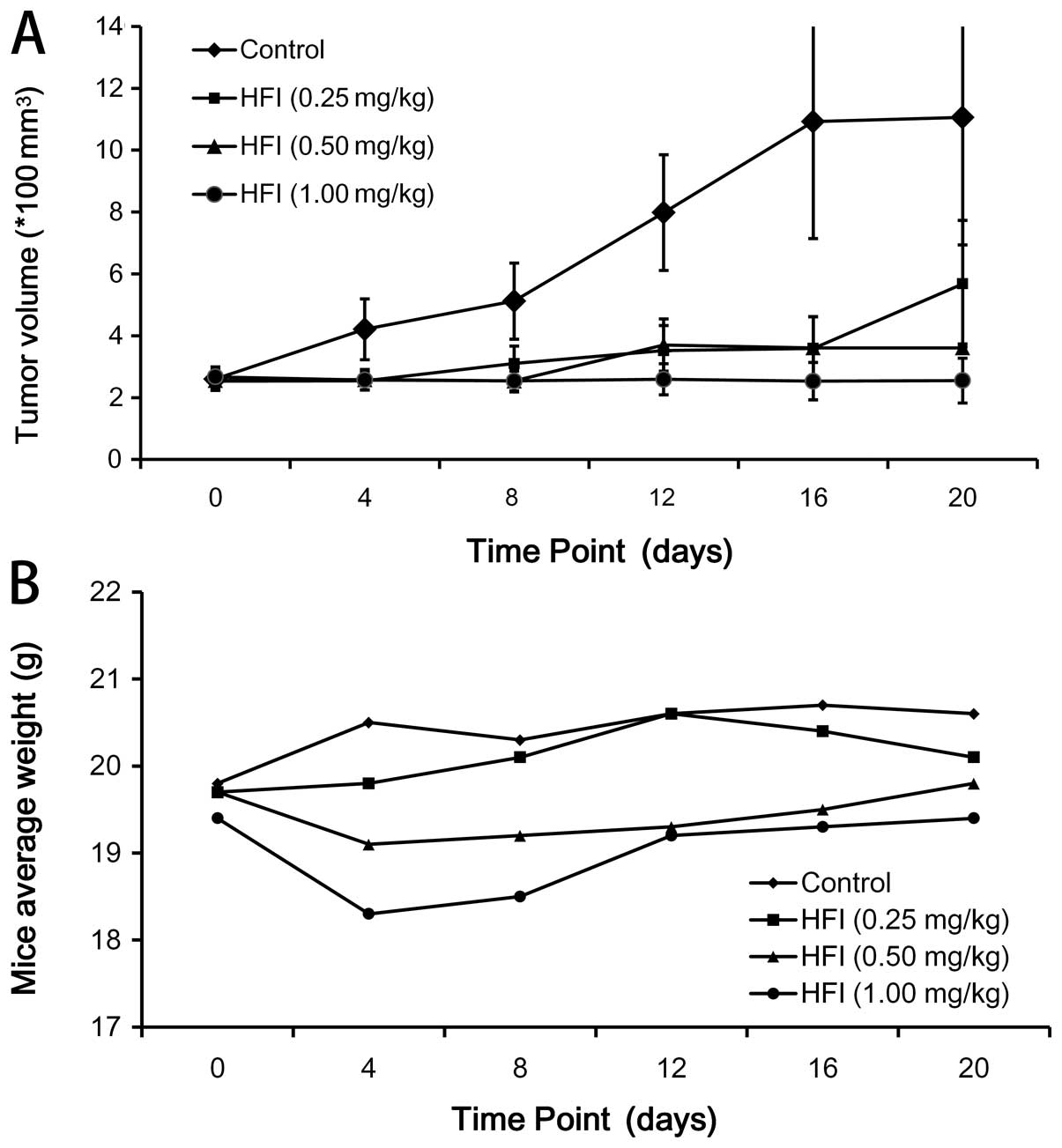
The in vivo antitumor activity was
investigated using a Calu-3 xenograft model. Calu-3 is known to be
a HER2-overpressing tumor. A total of 1×107 Calu-3 cells
suspended in 0.15 ml PBS were injected subcutaneously into the
right flank of the mice. Normally, 9–12 days after tumor cell
implantation, when tumors were clearly palpable and reached a size
of 200 to 350 mm3, the mice were grouped (n=7) and PBS
or the HFI was administered intravenously into the lateral tail
vein, respectively (Fig. 5). To
determine the antitumor activity of HFI at the different doses and
the effect of treatment, therapy was started in groups of mice
treated with PBS or the HFI (1.0, 0.5 or 0.25 mg/kg mice weight),
respectively. The HFI was administered intravenously once daily for
the first 5 days (0–4 day). At day 20 of the treatment, the
therapeutic effect observed in the mice treated with the 1.0 mg/kg
dose was considerably better than that observed with the 0.5 mg/kg
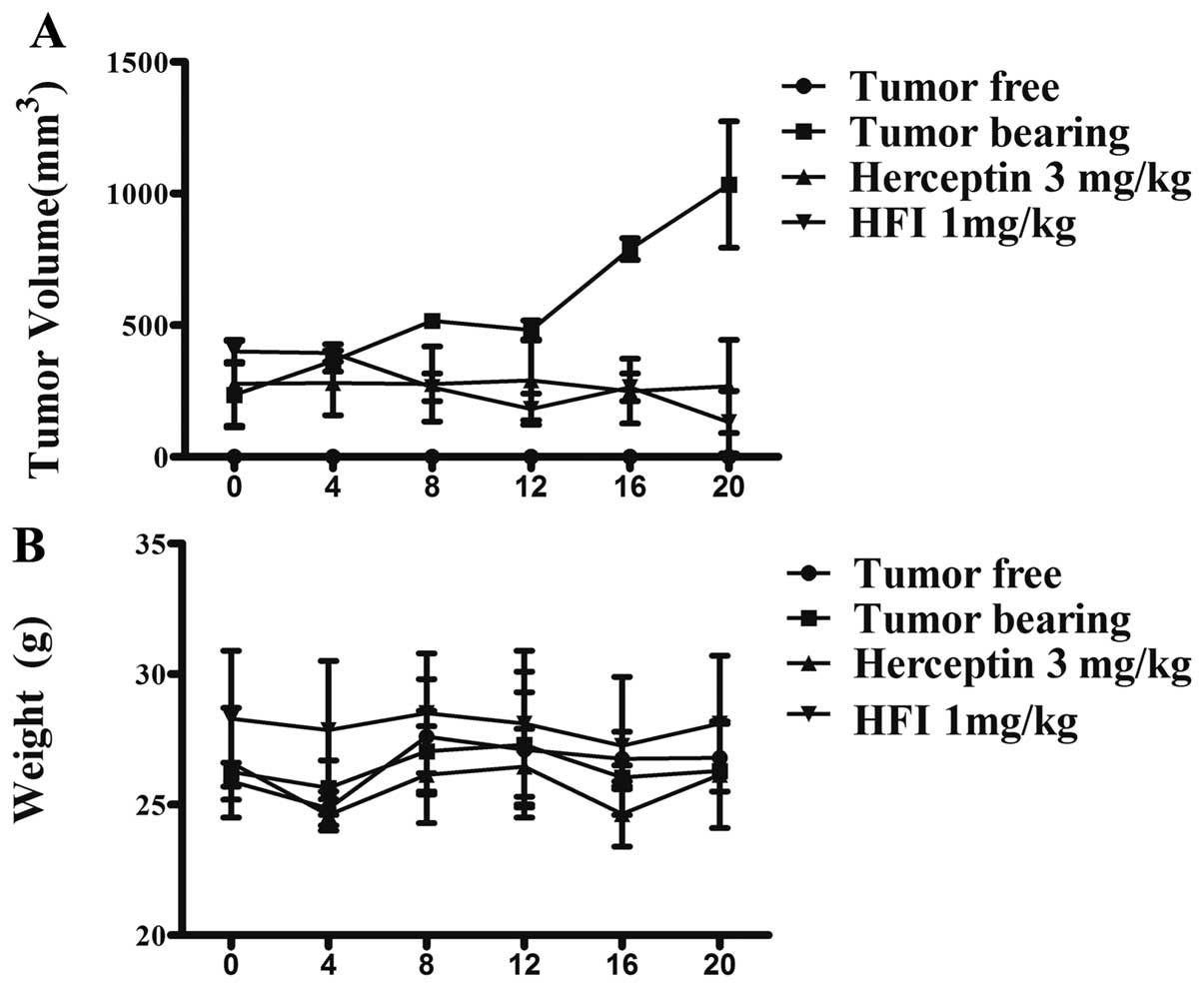
dose (P<0.01), and the 0.25 mg/kg dose (P<0.01). Fig. 6 demonstrates that the HFI had a
significant antitumor activity on models expressing high levels of
HER2 in vivo.
The fusion proteins (HFI) retained the activities of
both HER2 binding and IL-2. The role of IL-2 was mainly to activate
lymphocytes to release cytokines and other cells which indirectly
killed tumor cells. Thus, nude mice could not fully reflect the HFI
antitumor activity in vivo. In order to verify the integrity
of antitumor activity of HFI, the high expression of HER2
transgenic mouse model (immunocompetent) of spontaneous tumor
formation was chosen, and the mice were treated with HFI (Fig. 6). After 4-day treatment, HFI (1.0
mg/kg mouse weight) caused a dramatic suppression of FVB/neu in the
transgenic mouse model. Moreover, after treatment for 15 days, the
tumor volume which was initially <400 mm3, was
undetectable.
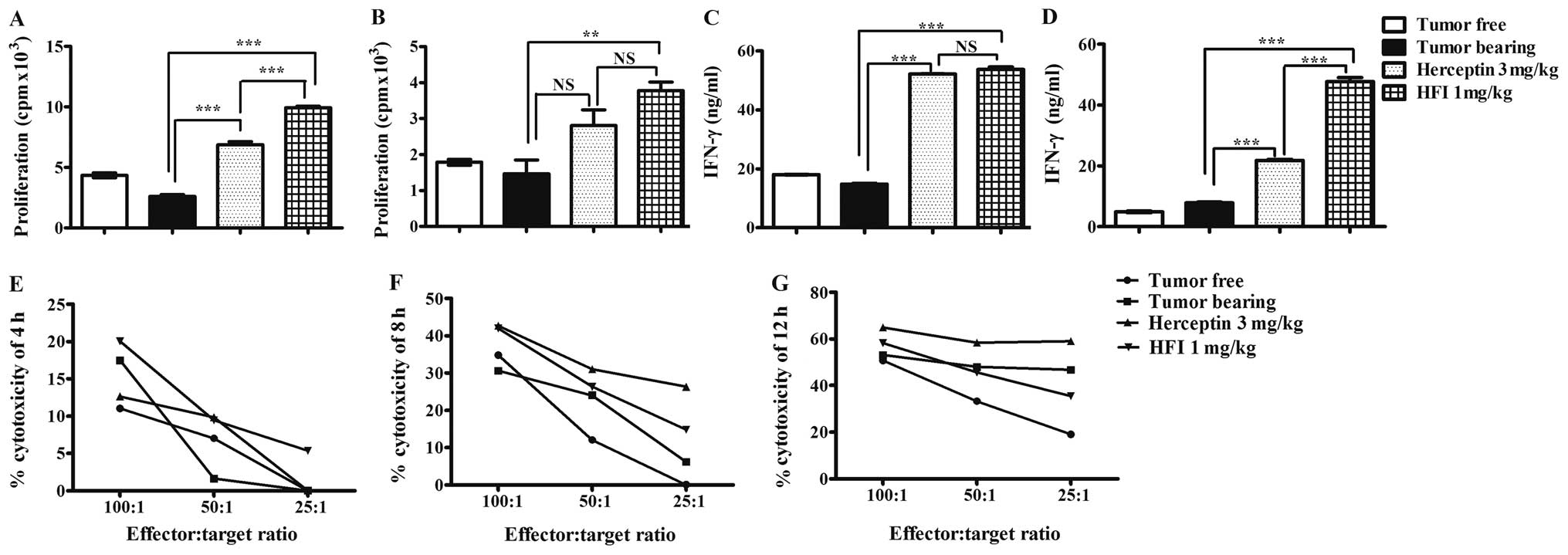
HFI promoted mitogen-induced splenocyte
proliferation and natural killer (NK) cell-mediated
cytotoxicity
The ex vivo immunoenhancement of HFI was
evaluated on splenocyte proliferation induced by Con A and LPS. The
results showed that the splenocytes of FVB/neu tumor bearing mice
treated with 1 mg/kg HFI for 3 weeks had significant proliferation
activity compared to those treated with PBS induced by LPS
(Fig. 7A) or Con A (Fig. 7B). The production of IFN-γ was
induced by FVB/neu in mice treated with HFI (Fig. 7C) or Herceptin (Fig. 7D). HFI also increased the NK
cell-mediated cytotoxicity (Fig.
7E–G).
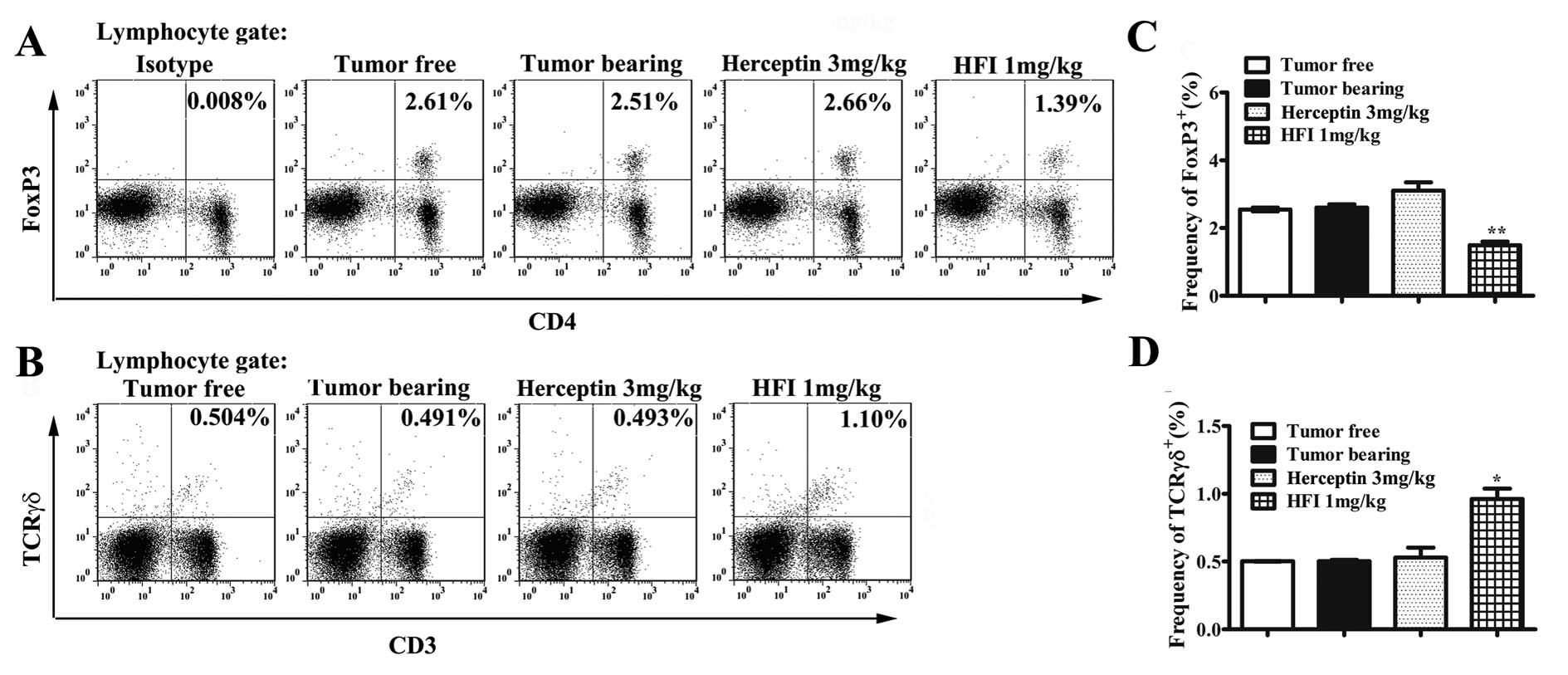
Effect of HFI treatment on regulatory T
cells and T cell percentage
The therapeutic mechanisms of HFI were investigated
ex vivo to determine whether its effects were attributable
to influenced FoxP3+ Treg cells, and T cells.
Intracellular staining of transcription factors showed that HFI
treatment significantly reduced the expression of FoxP3 (Fig. 8A and C). HFI also increased the T
cell population (Fig. 8B and
D).
Discussion
Proteins are marginally stable and readily denatured
by various stresses encountered in solution or in the frozen or
dried states. Various additives are known to minimize the damage
and to enhance the stability of proteins (34). The recombinant anti-ErbB2
scFv-Fc-IL-2 fusion gene was transfected into CHO dhfr-cells and
selected for the dhfr-phenotype (31,32,35).
The CHO cells stably expressing the fusion proteins (HFI) were
cultured in serum-free medium, so that HFI stability was guaranteed
when the protein purification process was downstream. HFI purified
liquid was stable at 4°C for two months (Fig. 2), indicating that HFI can be
produced in big batches. Stability data of freeze-dried HFI
products were obtained after one year’s experiment, including
quality analysis and activity detection, there was no stability
data of the HFI available for more than three years, yet the
current process and the data showed that the stability was not a
problem for HFI, in drug development.
IL-2 is an important pleiotrophic cytokine and
exhibits a wide variety of biological activities, including the
stimulation of antitumor effector cells (18,36,37).
IL-2 treatment can augment the activation of pre-existing
antigen-specific T cells, enhance their recognition and destruction
of neoplastic tissue, and activate NK cells (33,38,39).
However, systemic cytokine therapy frequently caused severe
problems with toxicity, and made it impossible to achieve an
effective dose at the tumor sites. The recombinant human IL-2 gene
is fused with the anti-ErbB2 scFv-Fc gene at the C-terminal
(31,32,35).
The biological activities of both antibody and IL-2 were
maintained. The specific local activation of IL-2 at tumor sites
provided more effective tumor destruction (33,40,41).
HFI not only extended IL-2 half-life in vivo (Fig. 4), but also reduced the retention
time of IL-2 in the blood because of HFI targeted activity
(Fig. 3), and thus reduced the
systemic toxicity of IL-2.
Patients with ErbB2-overexpressing tumors have shown
a significantly lower overall survival rate and shorter relapse
time than those with ErbB2-negative tumors (33,42,43).
In therapy, ErbB2 overexpression is a significant negative
prognostic indicator for a variety of therapies (33,44).
In the pre-clinical trials using murine models, antibody-IL-2
fusion proteins have shown to be very effective antitumor agents
(11,45–48).
HFI was effective in killing SKBR3 and SKOV3 cells, which expressed
ErbB2 at higher levels, than MCF7 cells (33,49),
suggesting that the killing effect mediated by HFI was dependent on
the expression level of ErbB2 molecules on the target cells. To
further determine the efficacy of HFI in vivo, the xenograft
models of human non-small cell lung cancer (Calu-3) cells
(HER2+++) in nude mice were treated with different
concentrations of HFI (Fig. 5).
The 1.0 mg/kg dose of HFI showed that the HFI had more than 60% of
antitumor activity in vivo. These data suggested that HFI
had significant antitumor activity on HER2-overexpressing tumor
cells. Moreover, HFI could also prevent the progression of tumor
development. In order to verify whether the HFI could inhibit tumor
growth, FVB/neu transgenic mouse model (50) was used. In Fig. 6, results indicate that HFI
significantly inhibited tumor growth activity in vivo. In
ex vivo, HFI increased mitogen-induced splenocyte
proliferation, IFN-γ production and cytotoxic responses to tumor
cells (Fig. 7). Moreover, with
early or post-operative treatment, the tumor could be completely
suppressed or treated. IL-2 is critical for Treg cell growth,
survival, and activity. Treg cells are inhibitors of antitumor
immunity and considered as immunotherapy target (51). It is reported that daily low-dose
of IL-2 increased numbers of Treg cells (52). Interestingly, in this study, the
CD4+ population expressed relatively low FoxP3 levels in
FVB/neu mice treated with HFI for 21 days, but the number of T
cells increased. NK cells, NKT cells, T cells along with cytokines
such as IFN-γ have been implicated in the processes of elimination
and immuno-editing during cancer immunosurveillance (53). In this study, significant changes
of the population of NK cells or NKT cells were not observed.
In this study, it was observed that HFI had a
similar binding capacity with HER2 by equimolar concentration of
Herceptin (Fig. 3). Moreover, the
capacity of HFI binding HER2 did not receive its structural
effects. HFI retained IL-2 biological activity (Fig. 2A), with efficient stimulation by
targeted IL-2, and lymphocytes elicited an antitumor response in
patients who are deficient in lymphocytes following high-dose
chemotherapy.
In conclusion, HFI purified solution was stable, and
could be prepared in mass for drug development. Fusion protein of
the HFI retained ErbB2 specificity and IL-2 biological activity.
Pharmacokinetics of HFI was not only associated with its molecular
weight, but also positively correlated with HFI concentration.
In vivo, HFI had significant activity on inhibiting
HER2-overexpressing tumor growth. HFI demonstrated potency of
initiating a cytotoxic activity on unstimulated PBMC against human
breast and ovarian cancer cells at low effector-to-target ratios
in vitro experiments. In this study, it was observed that
HFI promoted apoptosis had no impact on HER2 expression, indicating
HFI had potential toxicity. There are many issues to be resolved
during the process of HFI drug development, including the long-term
stability, mechanism of action, toxicological effects as well as
the immunogenic effects.
Acknowledgements
This study was supported by ‘The Key
New Drug Creation and Manufacturing’ (2009ZX09103-695) of the
Eleventh Five-Year Plan. We thank Professor Ning Guo and Dr Ming
Shi for excellent technical assistance.
References
|
1.
|
Jallal B, Schlessinger J and Ullrich A:
Tyrosine phosphatase inhibition permits analysis of signal
transduction complexes in p185HER2/neu-overexpressing human tumor
cells. J Biol Chem. 267:4357–4363. 1992.
|
|
2.
|
Rusnak DW, Lackey K, Affleck K, et al: The
effects of the novel, reversible epidermal growth factor
receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of
human normal and tumor-derived cell lines in vitro and in vivo. Mol
Cancer Ther. 1:85–94. 2001.
|
|
3.
|
Agus DB, Akita RW, Fox WD, et al:
Targeting ligand-activated ErbB2 signaling inhibits breast and
prostate tumor growth. Cancer Cell. 2:127–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Konopleva M, Zhang W, Shi YX, et al:
Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic
acid induces growth arrest in HER2-overexpressing breast cancer
cells. Mol Cancer Ther. 5:317–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bergman I, Barmada MA, Griffin JA and
Slamon DJ: Treatment of meningeal breast cancer xenografts in the
rat using an anti-p185/HER2 antibody. Clin Cancer Res. 7:2050–2056.
2001.PubMed/NCBI
|
|
6.
|
Spiridon CI, Ghetie MA, Uhr J, et al:
Targeting multiple Her-2 epitopes with monoclonal antibodies
results in improved anti-growth activity of a human breast cancer
cell line in vitro and in vivo. Clin Cancer Res. 8:1720–1730.
2002.PubMed/NCBI
|
|
7.
|
Meng S, Tripathy D, Shete S, et al: HER-2
gene amplification can be acquired as breast cancer progresses.
Proc Natl Acad Sci USA. 101:9393–9398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jerome L, Alami N, Belanger S, et al:
Recombinant human insulin-like growth factor binding protein 3
inhibits growth of human epidermal growth factor
receptor-2-overexpressing breast tumors and potentiates herceptin
activity in vivo. Cancer Res. 66:7245–7252. 2006. View Article : Google Scholar
|
|
9.
|
Merlin JL, Barberi-Heyob M and Bachmann N:
In vitro comparative evaluation of trastuzumab (Herceptin) combined
with paclitaxel (Taxol) or docetaxel (Taxotere) in HER2-expressing
human breast cancer cell lines. Ann Oncol. 13:1743–1748. 2002.
View Article : Google Scholar
|
|
10.
|
Nahta R, Hung MC and Esteva FJ: The
HER-2-targeting antibodies trastuzumab and pertuzumab
synergistically inhibit the survival of breast cancer cells. Cancer
Res. 64:2343–2346. 2004. View Article : Google Scholar
|
|
11.
|
Moulder SL, Yakes FM, Muthuswamy SK,
Bianco R, Simpson JF and Arteaga CL: Epidermal growth factor
receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits
HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in
vivo. Cancer Res. 61:8887–8895. 2001.PubMed/NCBI
|
|
12.
|
Liang K, Lu Y, Jin W, Ang KK, Milas L and
Fan Z: Sensitization of breast cancer cells to radiation by
trastuzumab. Mol Cancer Ther. 2:1113–1120. 2003.PubMed/NCBI
|
|
13.
|
Tseng PH, Wang YC, Weng SC, et al:
Overcoming trastuzumab resistance in HER2-overexpressing breast
cancer cells by using a novel celecoxib-derived
phosphoinositide-dependent kinase-1 inhibitor. Mol Pharmacol.
70:1534–1541. 2006. View Article : Google Scholar
|
|
14.
|
Arpino G, Gutierrez C, Weiss H, et al:
Treatment of human epidermal growth factor receptor
2-overexpressing breast cancer xenografts with multiagent
HER-targeted therapy. J Natl Cancer Inst. 99:694–705. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Talmadge JE, Phillips H, Schindler J,
Tribble H and Pennington R: Systematic preclinical study on the
therapeutic properties of recombinant human interleukin 2 for the
treatment of metastatic disease. Cancer Res. 47:5725–5732.
1987.PubMed/NCBI
|
|
16.
|
Kovacs JA, Vogel S, Albert JM, et al:
Controlled trial of interleukin-2 infusions in patients infected
with the human immunodeficiency virus. N Engl J Med. 335:1350–1356.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kozlowski T, Sablinski T, Basker M, et al:
Decreased graft-versus-host disease after haplotype mismatched bone
marrow allografts in miniature swine following interleukin-2
treatment. Bone Marrow Transplant. 25:47–52. 2000. View Article : Google Scholar
|
|
18.
|
Gaffen SL and Liu KD: Overview of
interleukin-2 function, production and clinical applications.
Cytokine. 28:109–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Malek TR and Bayer AL: Tolerance, not
immunity, crucially depends on IL-2. Nat Rev Immunol. 4:665–674.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cesana GC, DeRaffele G, Cohen S, et al:
Characterization of CD4+CD25+ regulatory T
cells in patients treated with high-dose interleukin-2 for
metastatic melanoma or renal cell carcinoma. J Clin Oncol.
24:1169–1177. 2006.PubMed/NCBI
|
|
21.
|
Goldsmith MA, Lai SY, Xu W, et al: Growth
signal transduction by the human interleukin-2 receptor requires
cytoplasmic tyrosines of the beta chain and non-tyrosine residues
of the gamma c chain. J Biol Chem. 270:21729–21737. 1995.
View Article : Google Scholar
|
|
22.
|
Hughes-Fulford M, Sugano E, Schopper T, Li
CF, Boonyaratanakornkit JB and Cogoli A: Early immune response and
regulation of IL-2 receptor subunits. Cell Signal. 17:1111–1124.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Dybkaer K, Iqbal J, Zhou G, et al: Genome
wide transcriptional analysis of resting and IL2 activated human
natural killer cells: gene expression signatures indicative of
novel molecular signaling pathways. BMC Genomics. 8:2302007.
View Article : Google Scholar
|
|
24.
|
Harvill ET and Morrison SL: An IgG3-IL2
fusion protein activates complement, binds Fc gamma RI, generates
LAK activity and shows enhanced binding to the high affinity IL-2R.
Immunotechnology. 1:95–105. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Budagian V, Nanni P, Lollini PL, et al:
Enhanced inhibition of tumour growth and metastasis, and induction
of antitumour immunity by IL-2-IgG2b fusion protein. Scand J
Immunol. 55:484–492. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Heuser C, Ganser M, Hombach A, et al: An
anti-MUC1-antibody-interleukin-2 fusion protein that activates
resting NK cells to lysis of MUC1-positive tumour cells. Br J
Cancer. 89:1130–1139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Neal ZC, Yang JC, Rakhmilevich AL, et al:
Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2
neuroblastoma when combined with interleukin 2 therapy. Clin Cancer
Res. 10:4839–4847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Gillies SD, Lan Y, Williams S, et al: An
anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse
model of established human B lymphoma. Blood. 105:3972–3978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wagner K, Schulz P, Scholz A, Wiedenmann B
and Menrad A: The targeted immunocytokine L19-IL2 efficiently
inhibits the growth of orthotopic pancreatic cancer. Clin Cancer
Res. 14:4951–4960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Marlind J, Kaspar M, Trachsel E, et al:
Antibody-mediated delivery of interleukin-2 to the stroma of breast
cancer strongly enhances the potency of chemotherapy. Clin Cancer
Res. 14:6515–6524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Shi M, Xie Z, Feng J, et al: A recombinant
anti-erbB2, scFv-Fc-IL-2 fusion protein retains antigen specificity
and cytokine function. Biotechnol Lett. 25:815–819. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Shi M, Xie Z, Yu M, Shen B and Guo N:
Controlled growth of Chinese hamster ovary cells and high
expression of antibody-IL-2 fusion proteins by temperature
manipulation. Biotechnol Lett. 27:1879–1884. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Shi M, Zhang L, Gu HT, et al: Efficient
growth inhibition of ErbB2-overexpressing tumor cells by anti-ErbB2
ScFv-Fc-IL-2 fusion protein in vitro and in vivo. Acta Pharmacol
Sin. 28:1611–1620. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Arakawa T, Prestrelski SJ, Kenney WC and
Carpenter JF: Factors affecting short-term and long-term
stabilities of proteins. Adv Drug Deliv Rev. 46:307–326. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Chen L, Xie Z, Teng Y, et al: Highly
efficient selection of the stable clones expressing antibody-IL-2
fusion protein by a dicistronic expression vector containing a
mutant neo gene. J Immunol Methods. 295:49–56. 2004. View Article : Google Scholar
|
|
36.
|
Lin JX and Leonard WJ: Signaling from the
IL-2 receptor to the nucleus. Cytokine Growth Factor Rev.
8:313–332. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Lacosta S, Merali Z and Anisman H: Central
monoamine activity following acute and repeated systemic
interleukin-2 administration. Neuroimmunomodulation. 8:83–90. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sereti I, Anthony KB, Martinez-Wilson H,
et al: IL-2-induced CD4+ T-cell expansion in
HIV-infected patients is associated with long-term decreases in
T-cell proliferation. Blood. 104:775–780. 2004.PubMed/NCBI
|
|
39.
|
Jo D, Liu D, Yao S, Collins RD and Hawiger
J: Intracellular protein therapy with SOCS3 inhibits inflammation
and apoptosis. Nat Med. 11:892–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Lewis GD, Figari I, Fendly B, et al:
Differential responses of human tumor cell lines to anti-p185HER2
monoclonal antibodies. Cancer Immunol Immunother. 37:255–263. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Smith KA and Griffin JD: Following the
cytokine signaling pathway to leukemogenesis: a chronology. J Clin
Invest. 118:3564–3573. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Friess T, Scheuer W and Hasmann M:
Combination treatment with erlotinib and pertuzumab against human
tumor xenografts is superior to monotherapy. Clin Cancer Res.
11:5300–5309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Johnson BE and Janne PA: Rationale for a
phase II trial of pertuzumab, a HER-2 dimerization inhibitor, in
patients with non-small cell lung cancer. Clin Cancer Res.
12:4436s–4440s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Konecny GE, Pegram MD, Venkatesan N, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
King DM, Albertini MR, Schalch H, et al:
Phase I clinical trial of the immunocytokine EMD 273063 in melanoma
patients. J Clin Oncol. 22:4463–4473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Zhang X, Feng J, Ye X, Yao Y, Zhou P and
Chen X: Development of an immunocytokine, IL-2-183B2scFv, for
targeted immunotherapy of ovarian cancer. Gynecol Oncol.
103:848–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Johnson EE, Lum HD, Rakhmilevich AL, et
al: Intratumoral immunocytokine treatment results in enhanced
antitumor effects. Cancer Immunol Immunother. 57:1891–1902. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Singh H, Serrano LM, Pfeiffer T, et al:
Combining adoptive cellular and immunocytokine therapies to improve
treatment of B-lineage malignancy. Cancer Res. 67:2872–2880. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Spiridon CI, Guinn S and Vitetta ES: A
comparison of the in vitro and in vivo activities of IgG and
F(ab’)2 fragments of a mixture of three monoclonal anti-Her-2
antibodies. Clin Cancer Res. 10:3542–3551. 2004.PubMed/NCBI
|
|
50.
|
Xie H, Lin L, Tong L, et al: AST1306, a
novel irreversible inhibitor of the epidermal growth factor
receptor 1 and 2, exhibits antitumor activity both in vitro and in
vivo. PLoS One. 6:e214872011. View Article : Google Scholar
|
|
51.
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Koreth J, Matsuoka K, Kim HT, et al:
Interleukin-2 and regulatory T cells in graft-versus-host disease.
N Engl J Med. 365:2055–2066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Vesely MD, Kershaw MH, Schreiber RD and
Smyth MJ: Natural innate and adaptive immunity to cancer. Annu Rev
Immunol. 29:235–271. 2011. View Article : Google Scholar : PubMed/NCBI
|