Introduction
Human osteosarcoma (OS) is the most commom primary
malignant bone tumor, accounting for approximately 20% of all
primary sarcomas in bone (1).
Well-known for its metastasis and high local recurrence rate
(2,3), osteosarcoma is a type of cancer whose
treatment requires an extensive multimodal approach including
surgery, radiotherapy and chemotherapy. Currently, chemotherapeutic
regimens for human osteosarcoma treatment use the combination of
multiple chemotherapeutic agents including high-dose methotrexate
(HD-MTX) with leucovorin rescue, doxorubicin (adriamycin),
cisplatin and ifosfamide either with or without etoposide (4). Although new therapies consisting of
aggressive adjuvant chemotherapy and wide tumor excision have led
to a significant benefit in terms of patients’ survival, the
frequent acquisition of drug-resistant phenotypes and unwanted
side-effects are often associated with chemotherapy and remain as
serious problems (5). It is
therefore urgent that new therapeutic strategies which can improve
the effect of current chemotherapy be developed.
Chinese herbal medicine, a major modality in
traditional Chinese medicine (TCM) and practiced for thousands of
years in China and other Asian countries, is used for treating
cancers (6–8). Herbal formulations are the common
form of administration in Chinese herbal practice, and herbal
formulas are well documented in ancient and modern literature
(9,10). According to Chinese herbal theory,
interactions among the different herbs in a formula exert a
synergistic effect and neutralize potential toxicity and
side-effects of the individual constituents (11,12).
However, there is as yet a lack of rigorous scientific evaluation
of such formulas.
The classical formula Xiao Jin Wan (XJW),
formerly known as Xiao Jin Dan (XJD), first documented in
the book Wai Ke Zheng Zhi Quan Sheng Ji (13), consists of ten component herbs, She
Xiang (Moschus), Mu Bie Zi (Cochinchina momordica
seed), Zhi Cao Wu (Radic aconiti Kusnezoffii preparata),
Feng Xiang Zhi (Resina liquidambaris), Ru Xiang
(Frankincense), Mo Yao (Myrrh), Dang Gui (Chinese
angelica), Wu Ling Zhi (Trogopterus dung), Di Long
(Pheretima) and Xiang Mo (Pine-soot ink). As a
well-known traditional Chinese folk-medicine, it is used for
eliminating stagnation, removing of blood stasis, promoting of
blood circulation and alleviating pain (14), which is commonly used for treatment
of various types of diseases including cancers, such as breast
cancer. However, the mechanism of XJW’s anticancer activity of
human osteosarcoma, have not yet been reported.
The cell cycle is the series of events that take
place in a cell leading to its division and duplication
(replication), which is monitored and regulated by cell cycle
checkpoints which establish the timing and strength of arrest,
repair and apoptotic responses to a damaging agent (15). Molecules regulating cell division,
such as cyclin-dependent kinases (CDKs) and inhibitors for CDKs,
are also implicated in regulating apoptosis. The tumor suppressor
p53 and its downstream transcriptional target
p21Cip1/Waf1 are also essential to sustain G2/M phase
arrest after DNA damage through the inhibition of cdc2 (16). In addition, recent studies suggest
that caspase-mediated cleavage of p21Cip1/Waf1 is a
critical step in converting cancer cells from growth arrest to
apoptosis (17).
Apoptosis is a genetically mediated mechanism by
which individual cells orchestrate their own deletion in normal and
diseased tissues. It is a complex process which includes signal
transduction (18) and the
degradation of cellular protein and DNA (19). Disturbed regulation of this vital
process represents a major causative factor in the pathogenesis of
cancers including osteosarcoma (20,21).
The Bcl-2 family proteins are important regulators of apoptosis
including both anti-apoptotic members such as Bcl-2 and
pro-apoptotic members such as Bax (22,23).
One possible mechanism by which Bcl-2 family proteins regulate
apoptosis is through their influence on the permeability of
mitochondrial outer membrane (MOM) following homo- or
hetero-association. It has been demonstrated that after activation,
the pro-apoptotic Bax or Bak is sufficient to induce mitochondrial
outer membrane permeabilization (MOMP) (20–23),
releasing apoptogenic proteins such as cytochrome c,
Smac/DIABLO and apoptosis inducing factor (AIF) (24,25).
The released cytochrome c leads to apoptotic
protease-activating factor (Apaf-1)-mediated activation of
initiator caspase-9, which in turn activates effector caspases
(26). Meanwhile, anti-apoptotic
Bcl-2 proteins have been reported to protect cells from many
different apoptotic stimuli and are important for cell survival
(27,28) and may bind to active Bax to prevent
it from damaging the MOM (22,29).
Thus, the balance of active anti- and pro-apoptotic Bcl-2 family
members determines the fate of cells and alteration of the ratio by
aberrant expression of these proteins impairs the normal apoptotic
program contributing to various apoptosis-related diseases
(30). Therefore, promoting cell
apoptosis through regulating the Bcl-2 family proteins has been the
main focus in the development of anticancer therapies. In order to
extend the clinical observations of the potential anticancer effect
of XJW and help to elucidate the mechanism of its anticancer
activity, in this study, we investigated the cellular effect of the
XJW on the proliferation and apoptosis of U-2OS human osteosarcoma
cells. We found that XJW inhibited the growth through arresting in
the G2/M phase of the cell cycle and promoted apoptosis of U-2OS
cells by loss of mitochondrial membrane potential (Δψm), activation
of caspase-9 and caspase-3 and upregulation of Bax to Bcl-2 ratio,
suggesting that inhibition the proliferation via blocking cell
cycle progression at the G2/M phase and promotion of apoptosis via
activating of the mitochondrion-dependent pathway may be one of the
mechanisms by which XJW can be effective in the treatment of
cancer.
Materials and methods
Materials and reagents
Fetal bovine serum (FBS), Dulbecco’s modified
Eagle’s medium (DMEM), and trypsin were purchased from Hyclone
Laboratories Inc. (Logan, UT, USA). A cell cycle assay kit and an
apoptosis assay (Annexin V-FITC Apoptosis Dection Kit II) were
provided by Becton-Dickinson (San Jose, CA, USA). A JC-1
mitochondrial membrane potential detection assay was obtained from
Biotium Inc. (Hayward, CA, USA). Caspase-9 and caspase-3
colorimetric protease assays and Hoechst 33258 were obtained from
Invitrogen Inc. (Grand Island, NY, USA). The Bcl-2, Bax and GAPDH
primers were purchased from Sangon Biotech Co. Ltd. (Shanghai,
China). Bcl-2, Bax antibodies, horseradish peroxidase
(HRP)-conjugated secondary antibodies and antibody against β-actin
were obtained from Cell Signaling Technology Inc. (Danvers, MA,
USA).
Herbal preparation
Moschus, Cochinchina momordica seed, Radic
aconiti Kusnezoffii preparata, Resina liquidambaris,
Frankincense, Myrrh, Chinese angelica,
Trogopterus dung, Pheretima and Pine-soot ink
used in XJW were prepared with traditional methods after harvest
(12) and purchased from the Tong
Ren Tang pharmaceutical company (Beijing, China). The ten herbs
were ground into powder, respectively. XJW was formulated by mixing
herbal powders in relative proportions according to the Chinese
pharmacopoeia (14) (see Table I). Stock solutions of XJW were
prepared by dissolving the XJW powder in water to a concentration
of 30 mg/ml and stored at −20°C. The working concentrations of XJW
were made by diluting the stock solution in the culture medium.
 | Table IComposition of Xiao Jin Wan
(XJW) formula. |
Table I
Composition of Xiao Jin Wan
(XJW) formula.
| Herb name | Relative
proportion |
|---|
| Moschus | 10 |
| Cochinchina
momordica seed | 50 |
| Radic aconiti
Kusnezoffii preparata | 50 |
| Resina
liquidambaris | 50 |
|
Frankincense | 25 |
| Myrrh | 25 |
| Chinese
angelica | 50 |
| Trogopterus
dung | 25 |
|
Pheretima | 50 |
| Pine-soot
ink | 4 |
Cell culture
Human osteosarcoma cell lines U-2OS were obtained
from the American Type Culture Collection (ATCC, Manassas, VA, USA)
and maintained in DMEM supplemented with 10% (v/v) FBS and 100 U/ml
penicillin and 100 μg/ml streptomycin at 37°C in 5%
CO2. The cells were subcultured at 80–90% confluency.
Cells used in this study were subjected to no more than 20 cell
passages.
Evaluation of cell viability by MTT
assay
Cell viability was assessed by the MTT colorimetric
assay. U-2OS cells were seeded into 96-well plates (Corning Costar
Corporation, Corning, NY, USA) at a density of 1.0×105
cells/ml in 0.1 ml of medium. After 24 h of incubation, the cells
were treated with various concentrations of XJW for 48 h. At the
end of the treatment, 100 μl MTT [0.5 mg/ml in
phosphate-buffered saline (PBS)] was added to each well and the
samples were incubated for an additional 4 h at 37°C. The
purple-blue MTT formazan precipitate was dissolved in 100 μl
DMSO. The absorbance was measured at 570 nm using an Elx808™
absorbance microplate reader (BioTek Instruments Inc., Winooski,
VT, USA). The relative cell viability was expressed as the ratio
(%) of the absorbance in the experimental wells to that of the
control wells (normal culture without treatment). Following this,
the IC50 (cytotoxic concentration for 50% cell death)
was determined from the dose-response curve.
Observation of morphologic changes
U-2OS cells were seeded into 6-well plates at a
density of 2.0×105 cells/well in 2 ml medium. The cells
were treated with various dose of XJW for 48 h. Cell morphology was
observed using a phase-contrast microscope (Olympus, Japan). The
photographs were taken at a magnification, ×100.
Detection of cell cycle by flow cytometry
analysis with propidium iodide (PI) staining
U-2OS cells were digested with 0.25% trypsin and
incubated in 25-cm2 culture flasks at a density of
1×105 cells/ml in 4 ml of medium for 24 h and starved
for 24 h in serum-free DMEM medium and were treated with various
concention of XJW for 48 h. After treatment, the cell cycle of
U-2OS cells were determined by flow cytometric analysis using a
fluorescence-activated cell sorting FACSCalibur cytometer and a
cell cycle assay kit. PI staining was performed according to the
manufacturer’s instructions. The percentage of cells in the
different phases was calculated by the ModFit LT Version 3.0
Software, and the cell numbers in the G0/G1, S and G2/M phases were
obtained.
Assessment of apoptotic morphology by
Hoechst 33258 staining
After treatment with various concentrations of XJW,
trypsinized adherent cells were collected, washed once with
ice-cold PBS, fixed with 1 ml of 4% paraformaldehyde for 20 min,
and washed once with ice-cold PBS. Then, the cells were incubated
in 1 ml PBS containing 10 μmol/l Hoechst 33258 at 37°C for
30 min, washed twice and observed using a fluorescence microscopy
with standard excitation filters (Leica Dmirb) in random
microscopic fields at ×200 magnification.
Detection of apoptosis by flow cytometry
analysis with Annexin V/PI staining
Following incubated with various doses of XJW,
apoptosis of U-2OS cells was determined by flow cytometric (FCM)
analysis using a fluorescence-activated cell sorting (FACS) caliber
(FACSCalibur, Becton-Dickinson) and the Annexin V-FITC Apoptosis
Dection Kit II. Staining was performed according to the
manufacturer’s instructions and as we previously described
(31). The percentage of cells in
early apoptosis was calculated by Annexin V-positivity and
PI-negativity, while the percentage of cells in late apoptosis was
calculated by Annexin V-positivity and PI-positivity.
Measurement of mitochondrial membrane
potential (Δψm) by flow cytometry analysis with JC-1 staining
To evaluate for the loss of mitochondrial membrane
potential, a hallmark of apoptosis, cells were stained with the
fluorescent dye JC-1, which is a cationic dye that exhibits
potential mitochondria-dependent accumulation, indicated by a
fluorescence emission shift from green to red. In this experiment,
1×106 treated U-2OS cells were resuspended after
trypsinization in 0.5 ml of medium and incubated with 10
μg/ml of JC-1 at 37°C, 5% CO2, for 15 min. Both
red and green fluorescence emissions were analyzed by flow
cytometry.
Analysis of caspase activation
The activity of caspase-9 and caspase-3 were
determined with a colorimetric assay using a colorimetric protease
assay, following the manufacturer’s instructions and our previous
description (31). Briefly, after
treated with various dose of XJW for 48 h, U-2OS cells were lysed
with the manufacturer’s provided lysis buffer for 10 min on ice.
The lysed cells were centrifuged at 10,000 x g for 1 min. An
aliquot (150 μg) of the protein was incubated with 50
μl of the colorimetric tetrapeptides, Leu-Glu-His-Asp
(LEHD)-pNA (specific substrate of caspase-9) or Asp-Glue-Val-Asp
(DEVD)-pNA (specific substrate of caspase-3) at 37°C in the dark
for 2 h. Samples were read at 405 nm in an absorbance microplate
reader (Elx808, BioTek Instruments Inc.). The data were normalized
to the activity of the caspases in control cells and represented as
‘fold of control’.
RNA extraction and RT-PCR analysis
U-2OS cells were seeded into 25-cm2
culture flasks at a density of 1×105 cells/ml in 4 ml of
medium and treated with various doses of XJW for 48 h. Total RNA
from U-2OS cells was isolated with TRIzol reagent (Invitrogen).
Oligo(dT)-primed RNA (1 μg) was reverse-transcribed with
SuperScript II reverse transcriptase (Promega) according to the
manufacturer’s instructions. The obtained cDNA was used to
determine the mRNA amount of Bcl-2 or Bax by PCR with Taq DNA
polymerase (Fermentas). GAPDH was used as an internal control. The
primers and the annealing temperature (°C) used for amplification
of Bcl-2, Bax and GAPDH transcripts are as follows: Bcl-2 forward
5′-CAG CTG CAC CTG ACG CCC TT-3, reverse 5′-GCC TCC GTT ATC CTG GAT
CC-3′, 55°C; Bax forward 5′-TGC TTC AGG GTT TCA TCC AGG-3′, reverse
5′-TGG CAA AGT AGA AAA GGG CGA-3′, 55°C; GAPDH forward 5′-GT CAT
CCA TGA CAA CTT TGG-3′, reverse 5′-GA GCT TGA CAA AGT GGT CGT-3′,
60°C.
Western blot analysis
U-2OS cells were seeded into 25-cm2
culture flasks at a density of 1×105 cells/ml in 4 ml of
medium and treated with various doses of XJW for 48 h. The treated
cells were lysed with mammalian cell lysis buffer containing
protease and phosphatase inhibitor cocktails, and the lysates were
separated by 12% SDS-PAGE gel under a reducing condition using 100
V for 1 h. The proteins were then electrophoretically transferred
onto nitrocellulose membranes using the iBlot western detection
stack/iBlot dry blotting system (Invitrogen). Membranes were
blocked for 30 min with agitation at RT in SuperBlock T20 (TBS)
blocking buffer (Thermo Scientific, Rockford, IL, USA). Membranes
were washed in TBS with 0.25% Tween-20 (TBST) and exposed to
primary antibodies against Bcl-2 (1:1,000) or Bax (1:500) overnight
at 4°C with rocking. β-actin (1:1,000) was also measured as an
internal control for protein loading. After membranes were washed
in TBST, secondary horseradish peroxidase (HRP)-conjugated
antibodies (anti-rabbit) were added at 1:2,500 dilution for 1 h at
room temperature and the membranes were washed again in TBST.
Finally, the antibody-bound protein bands were detected with ECL,
and images were taken using a Bio-Rad ChemiDoc XRS+ (Bio-Rad
Laboratories Inc., Hercules, CA, USA). The grayscale value ratio of
the target protein to the internal control was used to measure the
relative amount of Bcl-2 and Bax.
Statistical analysis
Data were analyzed using the statistical software
SPSS13.0. Statistical analysis of the data was performed with
Student’s t-test and one-way analysis of variance (ANOVA). P-values
<0.05 was considered as significant.
Results
XJW inhibits the growth of U-2OS
cells
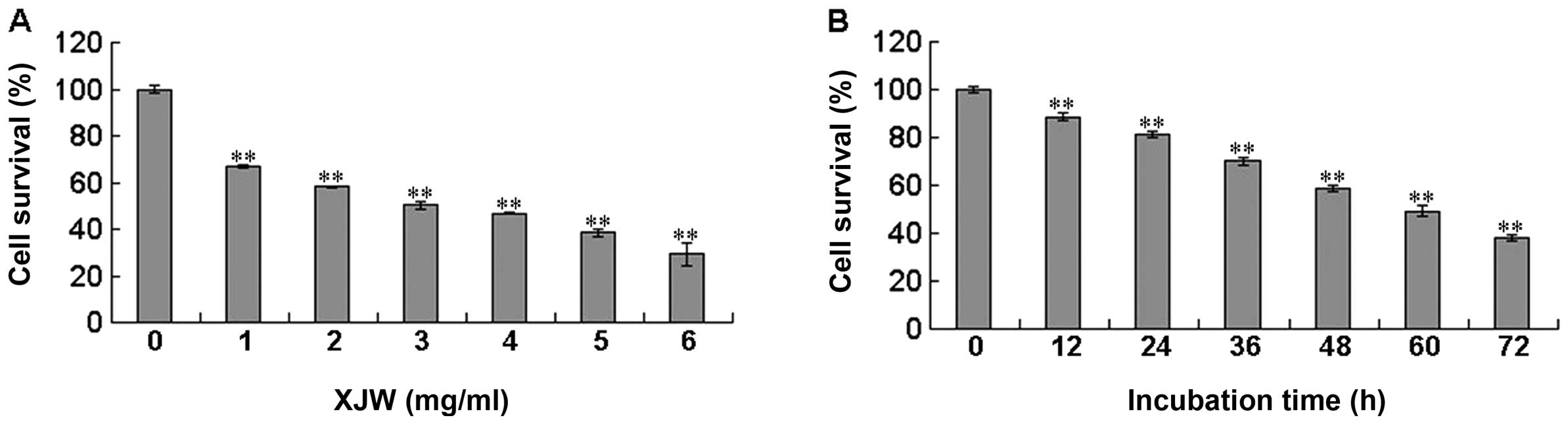
The effect of XJW on the viability of U-2OS cells
was determined by MTT assay. As shown in Fig. 1A, treatment with 1–6 mg/ml of XJW
for 48 h dose-dependently reduced cell viability by 33.24–70.71%
compared to untreated control cells (P<0.01), with an estimated
half-maximal inhibitory concentration (IC50) value of
2.75 mg/ml. The cell viability was decreased to 29.29% at the
highest concentration of XJW (6 mg/ml) in this study. We also
evaluated the effect of 2.75 mg/ml of XJW on cell viability with
incubation for different periods of time. As shown in Fig. 1B, treatment with 2.75 mg/ml of XJW
led to a gradual decrease in cell viability with the increase of
exposure time. These results suggest that XJW inhibits U-2OS cell
growth and viability in a dose- and time-dependent manner. To
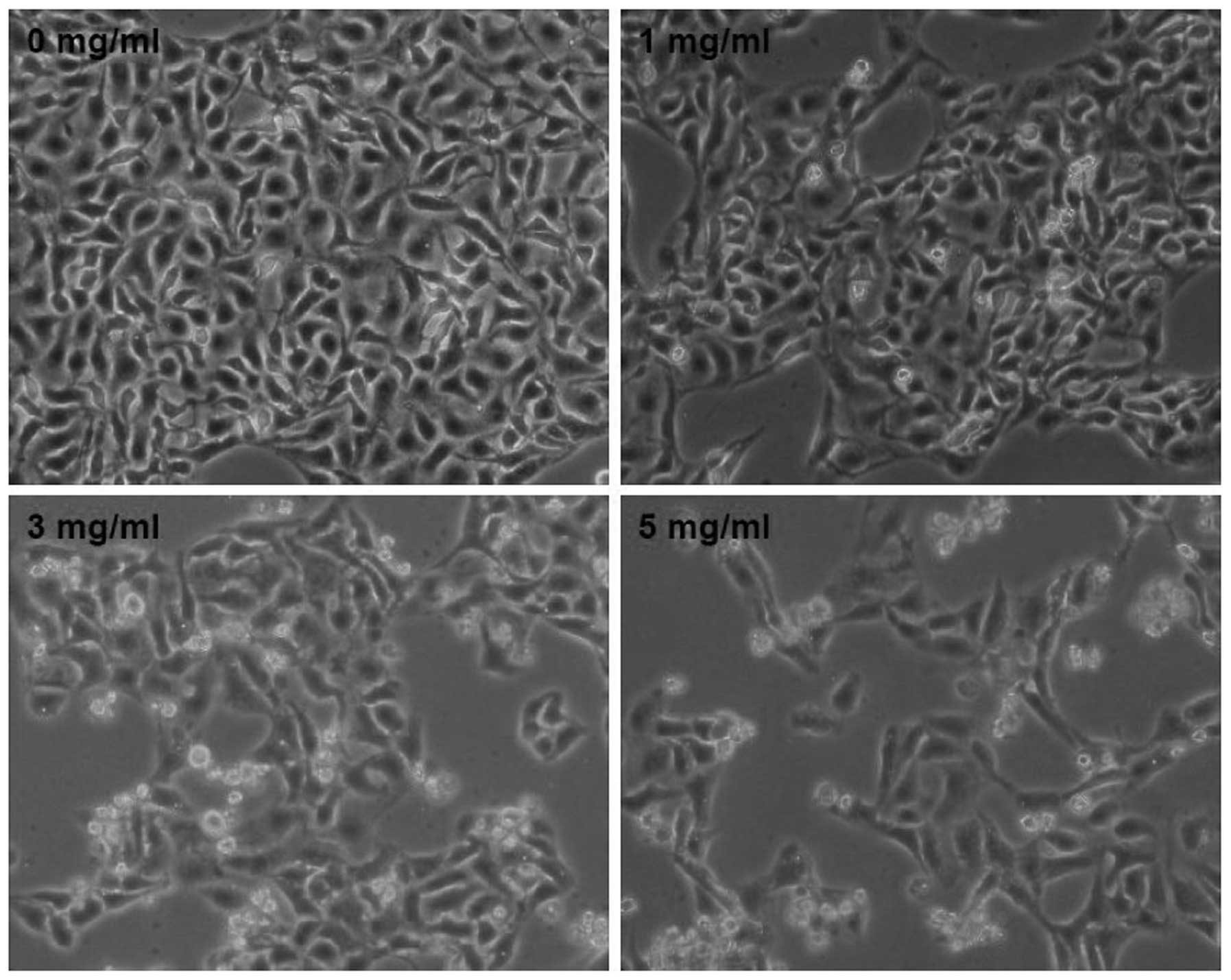
further verify these results, we evaluated the effect of XJW on
U-2OS cell morphology via phase-contrast microscopy, since cell
morphology in culture is indicative of the healthy status of the
cells. As shown in Fig. 2,
untreated U-2OS cells appeared as cobblestone, whereas after
treatment with various doses of XJW for 48 h many of the cells
became bright and shrunken, and detached from each other or floated
in the medium. The phenomenon was much more obviously in the higher
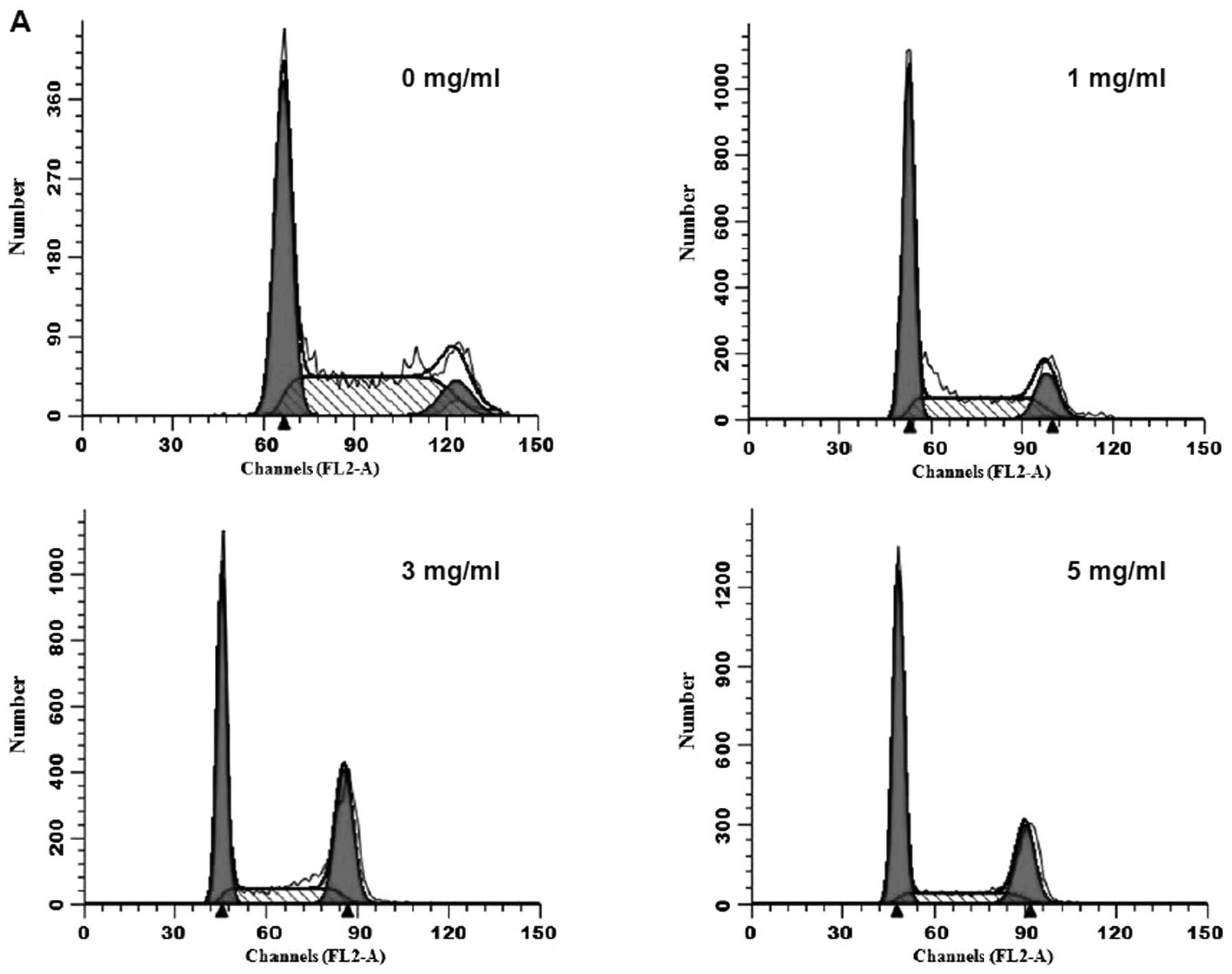
concentration of XJW. In addition, we evaluated the effect of XJW
on the cell cycle of U-2OS cells, since cell cycle plays an
important role in a cell leading to its division and duplication,
which is monitored and regulated by cell cycle checkpoints which
establish the timing and strength of arrest, repair and apoptotic
responses to a damaging agent (15). The G2/M transition is one of the
two main checkpoints used by the cell to regulate the progression
of the cell cycle. As shown in Fig. 3A
and B, the percentage of G2 phase cells following treatment
with 1, 3 and 5 mg/ml of XJW, was 13.76±0.41, 22.64±1.34 and
32.14±1.02%, all of which were significantly higher than that of
untreated cells (8.59±0.26%; P<0.01). Consistently, the
percentage of G1-phase cells showed the opposite trend after XJW
treatment, suggesting that XJW treatment can inhibit cell cycle of
U-2OS cells by inhibiting the G2 to M transition. Taken together,
these data demonstrate that MW inhibits the proliferation of U-2OS
cells.
XJW induces apoptosis in U-2OS cells
To determine whether XJW inhibits the growth of
U-2OS cells also by inducing apoptosis, the morphologic
characteristics of apoptosis was observed. Cells were stained with
Hoechst 33258 after treated with XJW for 48 h and detected by
fluorescence microscopy. We found that control cells showed
distribution of the stain and round homogeneous nuclei, while
apoptotic cells increased gradually in a dose-dependent manner and
displayed typical changes including condensed and fragmented
nucleus (Fig. 4A). For a further
assessment of apoptosis induced by XJW, we examined the exposure of
phosphatidylserine on the cell surface by Annexin V/PI staining
followed by FACS analysis. In this assay, Annexin V/PI
double-negative population (labeled as LL in the FACS diagram)
indicates viable cells; Annexin V-positive/PI-negative or Annexin
V/PI double-positive population (labeled as LR or UR in the FACS
diagram) represents cells undergoing early or late apoptosis,
respectively. As shown in Fig. 4B and
C, the percent of cells undergoing apoptosis following
treatment with 0, 1, 3 and 5 mg/ml of XJW (including the early and
late apoptotic cells) was 4.16±0.902, 9.38±0.866, 15.06±1.553 and
29.45±8.178%, respectively (P<0.01 or 0.05 vs. untreated control
cells). This indicates that XJW treatment induces U-2OS cell
apoptosis in a dose-dependent manner.
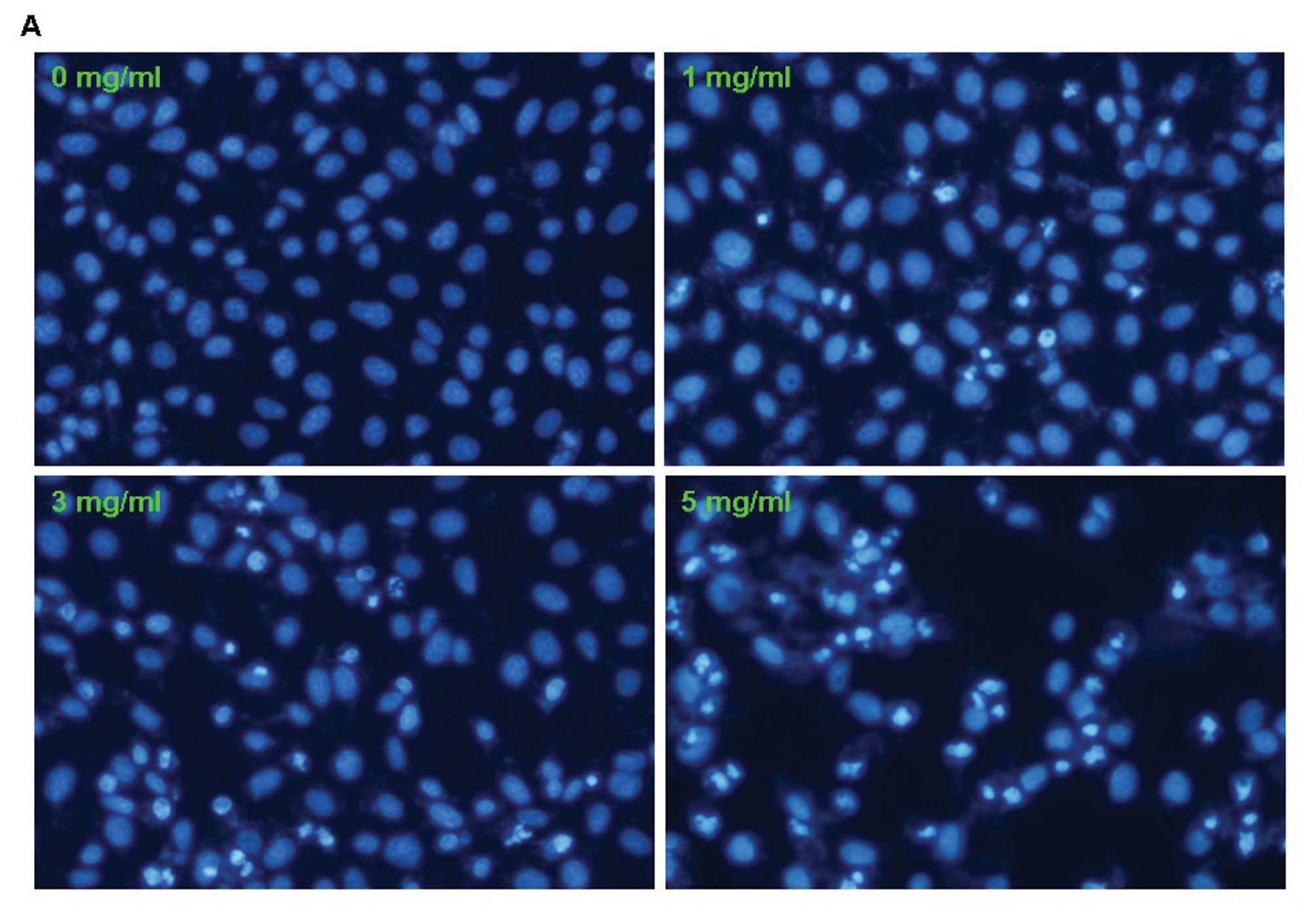 | Figure 4Effect of XJW on the apoptosis of
U-2OS cells. After treatment with the indicated concentrations of
XJW for 48 h, U-2OS cells were collected and stained with Hoechst
33258 staining and observed under a fluorescence microscope and
Annexin V/PI followed by FCM analysis. (A) XJW-mediated cell
apoptosis morphologic changes were examined by Hoechst 33258
staining and observed under a fluorescence microscope at ×200
magnification. The apoptotic cells detected by the fluorescence
microscopy displayed condensed and fragmented nuclei, shrinkage of
cell volume in a concentration-dependent manner. (B) Apoptosis
analysis in U-2OS cells was assessed by Annexin V/PI double
staining. After cells were exposed to four desired concentrations
of XJW for 48 h, respectively, the attached and detached cells were
collected. Following staining with Annexin V and PI, cells were
subjected to flow cytometry analysis. Representative FCM analysis
scatter-grams of Annexin V/PI staining display four different cell
populations labeled as: double-negative stained cells (LL, lower
left) representing the live cell population; Annexin
V-positive/PI-negative stained cells (LR, lower right) and Annexin
V/PI double-positive stained cells (UR, upper right) representing
early apoptosis and late apoptosis, respectively; Annexin
V-negative and PI-positive stained cells (UL, upper left)
representing dead cells. (C) FCM results are expressed as mean ± SD
of three independent experiments. *P<0.05,
**P<0.01, compared with the control group. |
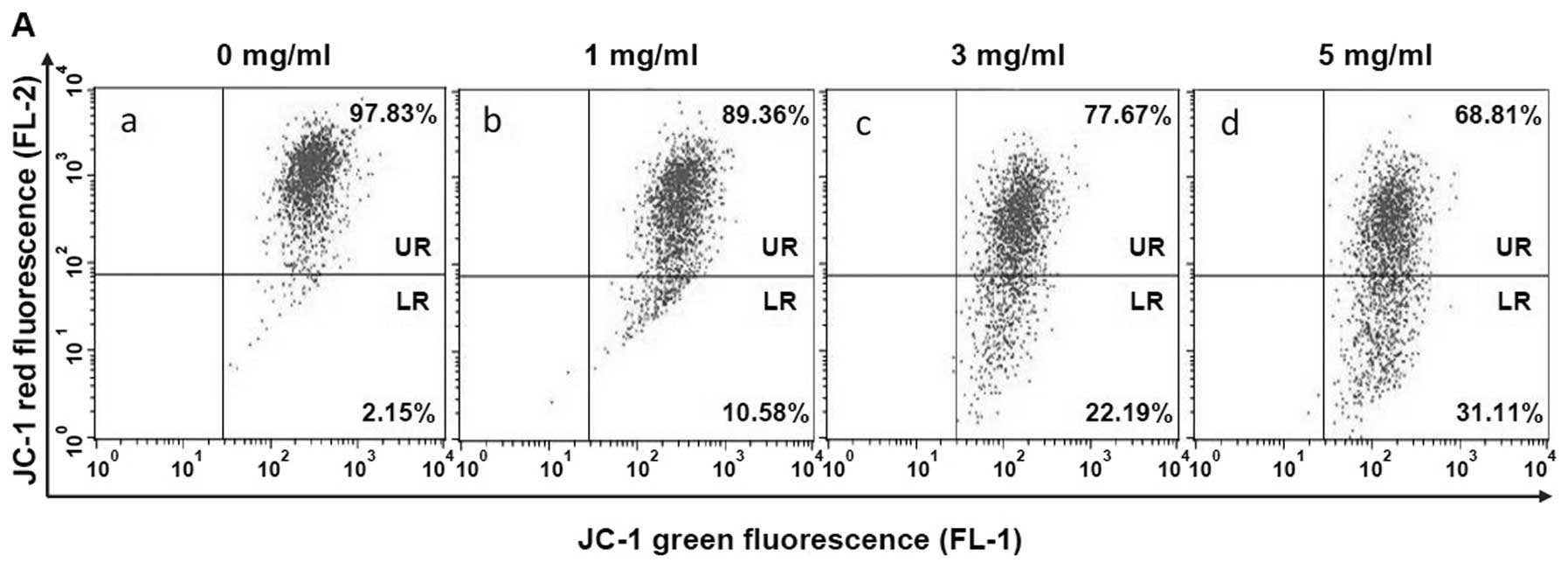
Effect of XJW on the loss of
mitochondrial potential (Δψm) and the activation of caspase-9 and
caspase-3
The mitochondrion-dependent pathway is the most
common apoptotic pathway in vertebrate animal cells. The
mitochondrial membrane permeabilization, accompanied by the
collapse of electrochemical gradient across the mitochondrial
membrane, is one of the key events during cellular apoptosis
(32). This results in the release
of numerous apoptogenic proteins, such as cytochrome c, from
the mitochondria triggering the activation of caspases-9 and -3,
and eventually inducing apoptosis. To investigate the mechanism of
XJW’s inducing U-2OS cell apoptosis, we used FACS analysis with
JC-1 staining to examine the change in mitochondrial membrane
potential after XJW treatment. JC-1 is a lipophilic, cationic dye
that selectively enters into mitochondria. In healthy cells with
high mitochondrial potential, JC-1 forms J-aggregates with intense
red fluorescence (590 nm, FL-2), whereas under apoptotic condition,
the mitochondrial membrane potential collapses, so that JC-1 does
not accumulate within the mitochondria but remains in the cytoplasm
in monomeric form showing green fluorescence (529 nm, FL-1). These
fluorescence differences can be detected by FACS analysis using
JC-1 green and red channels. As shown in Fig. 5A and B, JC-1 fluorescence was
shifted from a JC-1-green-bright/JC-1-red-bright signal in
untreated U-2OS cells to a JC-1-green-bright/JC-1-red-dim signal in
cells treated with XJW in a dose-dependent fashion, indicating
XJW-induced loss of mitochondrial membrane potential in U-2OS
cells. To identify the downstream effectors in the apoptotic
signaling pathway, the activation of caspases-9 and caspases-3 were
examined by a colorimetric assay using specific chromophores,
LEHD-pNA (specific substrate of caspase-9) and DEVD-pNA (specific
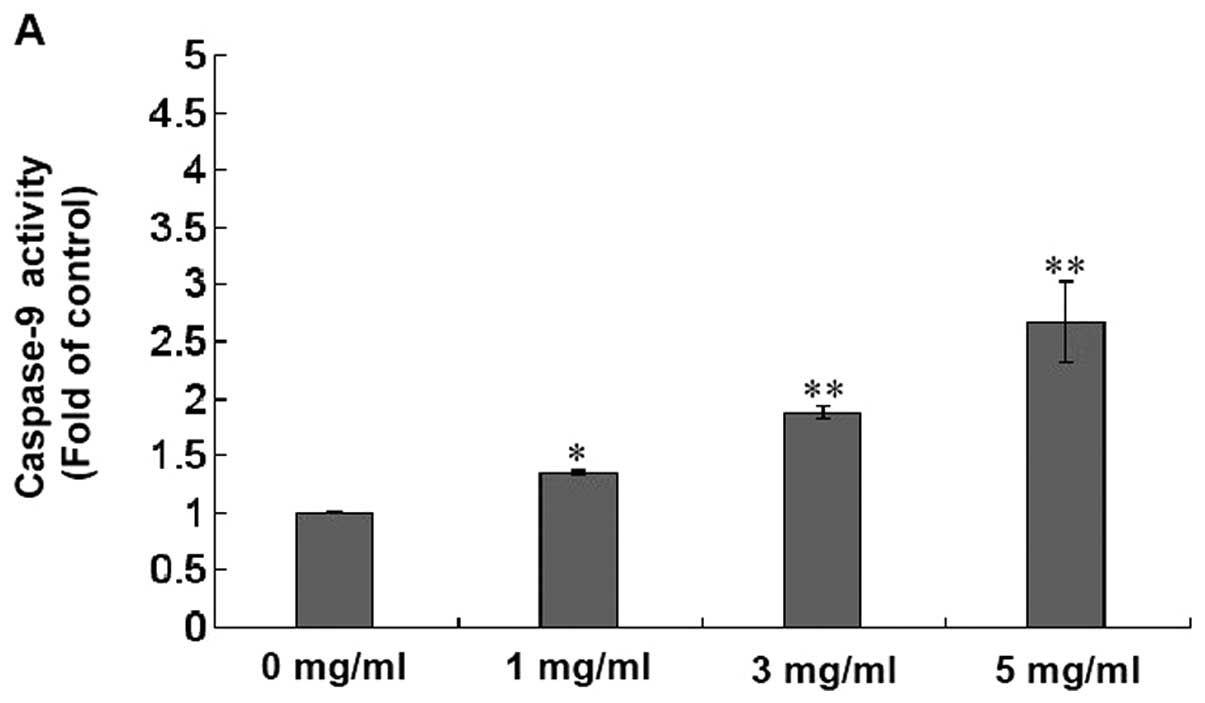
substrate of caspase-3). As shown in Fig. 6A and B, XJW treatment significantly
and dose-dependently induced activation of both caspase-9 and
caspase-3 in U-2OS (P<0.01 or 0.05 vs. untreated control cells).
These data suggest that XJW promotes U-2OS cell apoptosis via the
mitochondrion-dependent pathway.
XJW regulates the expression of
anti-apoptotic Bcl-2 and pro-apoptotic Bax
Bcl-2 family proteins are key regulators of
mitochondrion-mediated apoptosis, including anti-apoptotic members
such as Bcl-2 and pro-apoptotic members such as Bax. Tissue
homeostasis is maintained by controlling the ratio of active anti-
and pro-apoptotic Bcl-2 family proteins. Higher Bcl-2-to-Bax ratio
by aberrant expression of the proteins is found commonly in various
cancers. To further study the mechanism of XJW inducing apoptosis
activity, we performed RT-PCR and western blot analysis to examine
the mRNA and protein expression of Bcl-2 and Bax in XJW-treated
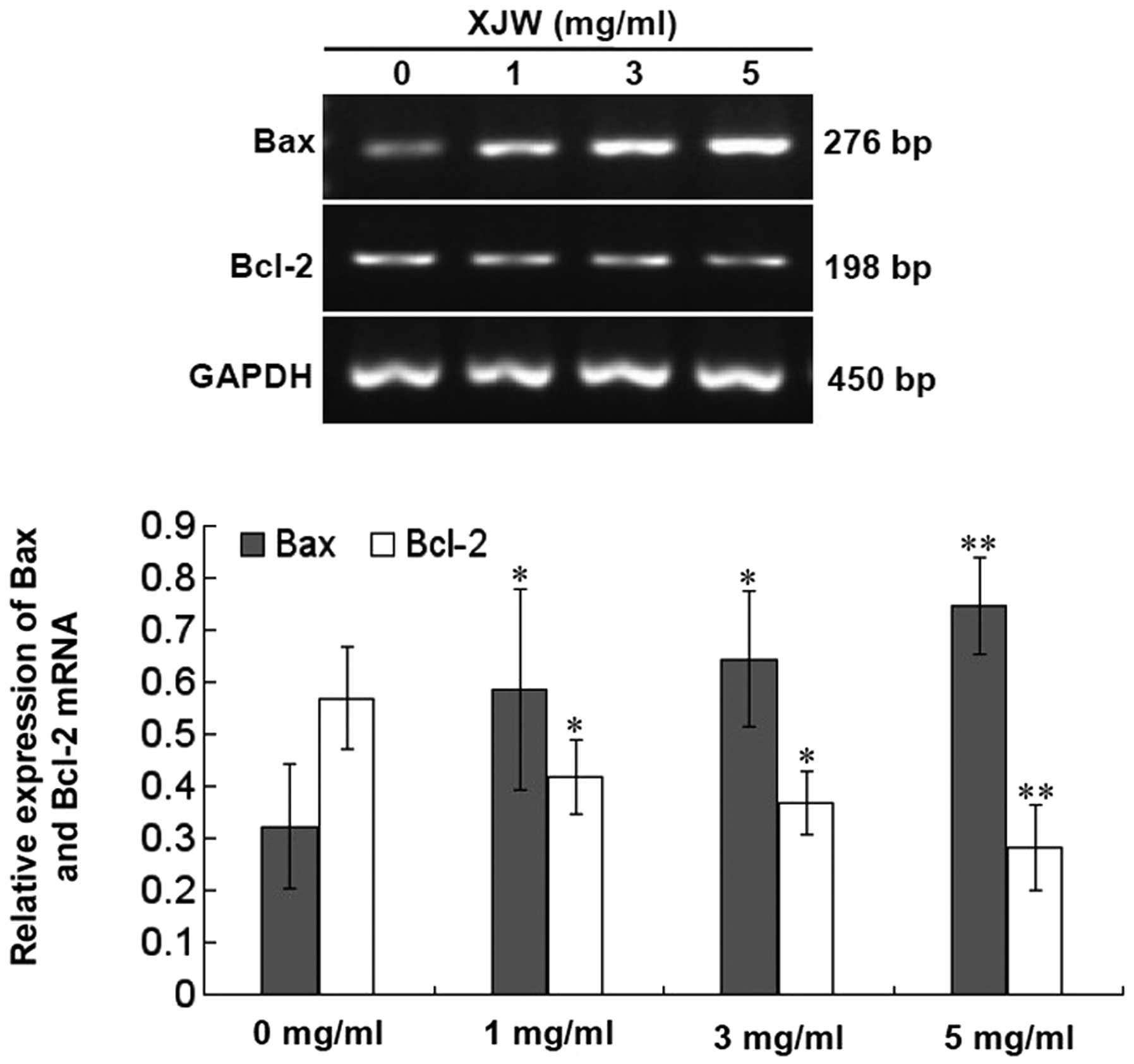
U-2OS cells. The results of the RT-PCR assay showed that XJW
treatment profoundly increased Bax and reduced Bcl-2 mRNA
expression in U-2OS cells (Fig. 7;
P<0.01 or 0.05 vs. untreated control cells); and the pattern of
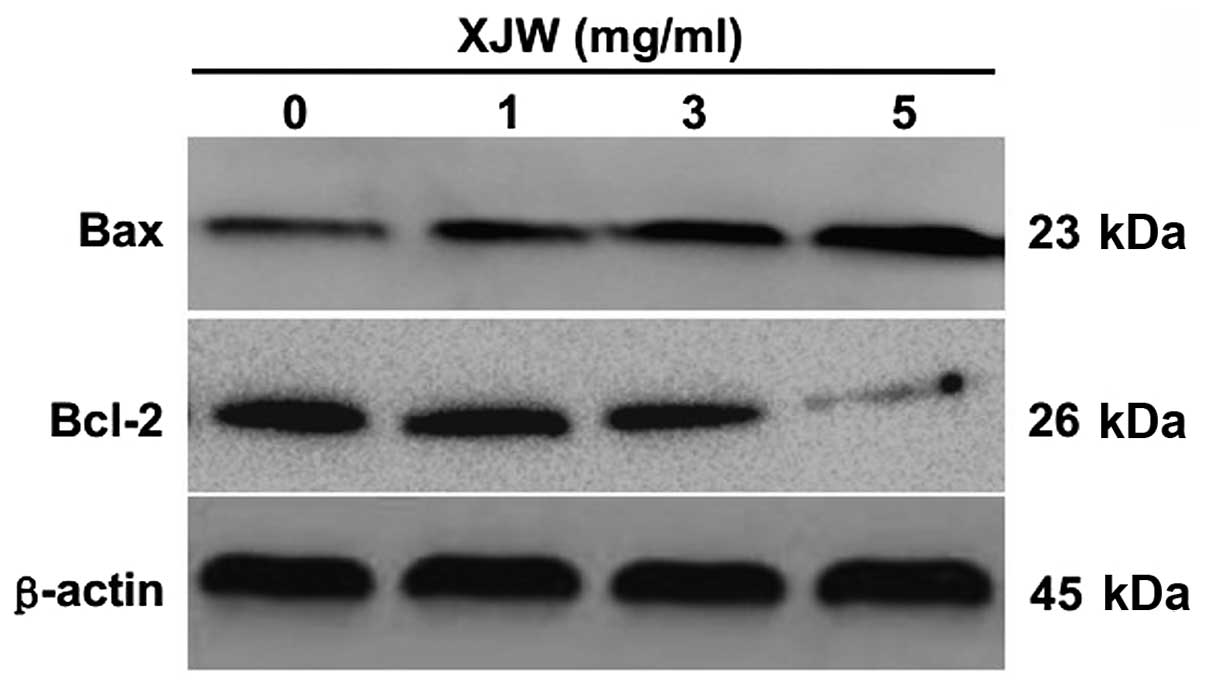
protein expression of Bax and Bcl-2 was similar to their respective
mRNA levels (Fig. 8) suggesting
that XJW induces mitochondrion-dependent apoptosis in U-2OS cells
through the regulation of expression of Bcl-2 family proteins.
Discussion
Cancer cells are characterized by an unregulated
increase in cell proliferation and/or a reduction in cell apoptosis
(16). In addition, disrupted
apoptosis contributes to drug resistance of tumor cells, which has
become a significant obstacle for the successful management of
patients with malignant tumors including osteosarcoma (5). Moreover, many currently used
anticancer agents contain intrinsic and potent cytotoxicity to
normal cells, which limits their long-term use and their
therapeutic effectiveness (33).
These problems highlight the urgent need for the development of
novel cancer chemotherapies. Since natural products, such as
traditional Chinese herbal medicines, have relatively fewer
side-effects as compared to modern chemotherapeutics and have long
been used clinically to treat various types of diseases including
cancer (34–36), discovering naturally occurring
agents with antiproliferative activity is a promising approach for
anti-cancer treatment.
XJW is a well-known traditional Chinese
folk-medicine used for eliminating stagnation, removing of blood
stasis, promoting of blood circulation and alleviating pain
(14), which is commonly used for
treatment of various types of diseases including cancers, such as
breast cancer. However, the mode of action for its antitumor is
still largely unknown. Therefore, before XJW can be further
developed as an anticancer agent, its antitumor activity and
underlying molecular mechanism should be elucidated.
Cell cycle plays an important role in U-2OS cells
leading to its division and duplication. Moreover, the G2/M
transition is one of the two main checkpoints used by the cell to
regulate the progression of the cell cycle. Once the checkpoint
late in G2 phase is passed, further progression through the cell
cycle occurs with little or no interference from extracellular
stimuli followed by the decision to continue cell division. To
determine the mechanism of the inhibition of XJW, we examined its
effect on the G2 to M transition in U-2OS cells via PI staining
followed by FACS analysis. We demonstrated that XJW treatment
dose-dependently increased the percentage of G2 phase in U-2OS
cells after treated with 1, 3 and 5 mg/ml of XJW (Fig. 3B; P<0.01 vs. untreated control
cells). The percentage of G1-phase cells showed the opposite trend
after XJW treatment, suggesting that XJW treatment can inhibit cell
cycle of U-2OS cells by blocking the G2 to M transition. Taken
together, these data demonstrate that XJW inhibits the growth of
U-2OS cells.
Apoptosis is activated through two major pathways.
For the intrinsic pathway, death signals are integrated at the
level of the mitochondria. For the extrinsic pathway, death signals
are mediated through cell surface receptors. Both pathways
eventually lead to the activation of caspases and nucleases,
resulting in the destruction of the cell (16). Our experimental results showed that
apoptotic cells induced by XJW displayed condensed and fragmented
nuclei by Hoechst 33258 staining (Fig.
4A). For the loss of plasma membrane asymmetry is one of the
morphologic characteristics of the apoptotic program. In apoptotic
cells, the membrane phospholipid phosphatidylserine (PS) is
translocated from the inner to the outer leaflet of the plasma
membrane, thereby exposing PS to the external cell environment.
Annexin V is a 35–36 kDa Ca2+-dependent
phospholipid-binding protein that has a high affinity for PS.
Annexin V binds to cells with exposed PS. Therefore, flow cytometry
with Annexin V staining was used to further confirmed the results
of Hoechst 33258 staining by showing that the important membrane
alterations relating to apoptosis in U-2OS cells and the percent
apoptosis increased in dose-corresponding manner (Fig. 4B and C). Taken together, these
results suggested that XJW indeed induced apoptosis in U-2OS cells.
The loss of mitochondrial membrane potential is a hallmark of
apoptosis. It is an early event preceding phosphatidylserine
externalization and coincides with caspase activation (32,37).
In healthy cells, the JC-1 dye stains the mitochondria fluorescent
red (38). The negative charge
established by the intact mitochondrial membrane potential allows
this lipophilic dye, bearing a delocalized positive charge, to
enter the mitochondrial matrix where it accumulates. When the
critical concentration is exceeded, the J-aggregates are form.
These aggregates are fluorescent red (590 nm). In apoptotic cells,
the mitochondrial membrane potential collapses, and JC-1 cannot
accumulate within the mitochondria. In these cells, JC-1 remains in
the cytoplasm in the green fluorescent monomeric form. JC-1-stained
apoptotic cells, having primarily green fluorescence (530 nm), are
easily differentiated from healthy cells that have red and green
fluorescence (39). Using FCM,
healthy cells with red JC-1 aggregates are detected in the FL-2
channel and apoptotic cells with green JC-1 monomers are detected
in the FL-1 channel. Thus, JC-1-stained cells that fluoresce in the
FL-2 and FL-1 channels (UR quadrant) carry mitochondria with a
polarized Δψ, whreas JC-1-stained cells that fluoresce in the FL-1
channel and not in the FL-2 channel (LR quadrant) carry
mitochondria with a depolarized Δψ. Therefore, JC-1 dye-based assay
was used to evaluate mitochondrial membrane potential in the study.
Our data clearly showed that treatment with XJW leads to a collapse
of mitochondrial membrane potential (Fig. 5A and B).
The mitochondrion-dependent pathway is the most
common apoptotic pathway in vertebrate animal cells. Mitochondrial
outer membrane permeabilization (MOMP) accompanied by the collapse
of electrochemical gradient across the mitochondrial membrane is a
key commitment step in the induction of mitochondrion-dependent
apoptosis. This is the point of convergence for a large variety of
intracellular apoptotic signaling pathways that eventually lead to
the release of pro-apoptotic proteins from the mitochondrial
inter-membrane space, including cytochrome c, Smac/DIABLO,
and Omi/HtrA2. Released cytochrome c activates APAF-1, which
oligomerizes to form an apoptosome. This structure, in turn,
recruits and activates caspase-9. Activated caspase-9 cleaves and
activates executioner caspases, such as caspase-3, and eventually
results in apoptosis (32,37,40).
Therefore, to evaluate the effect of XJW on the
mitochondrion-dependent apoptosis pathway, we evaluated the
activation of caspase-9 and caspase-3. In this study, we found that
XJW activated both caspase-9 and caspase-3 in U-2OS cells in a
dose-dependent manner (Fig. 6A and
B). Thus, XJW-induced U-2OS cell death is accompanied by the
activities of caspases-9 and caspase-3, which then stimulates the
molecular cascade for apoptosis.
Occurrence of mitochondrial-dependent apoptosis is
typically governed by contradicting the Bcl-2 family (41). Bcl-2 is a well-known anti-apoptotic
protein that can prevent cytochrome c release whereas Bax is
a pro-apoptotic protein that enhance cytochrome c release
from mitochondria into cytosol (42), which is responsible for activating
caspase-9, caspase-3 and facilitates apoptosis (43). Therefore, the ratio of Bax to Bcl-2
is a critical for determining the fate of cells. In this study, we
demonstrated that XJW treatment dose-dependently enhances Bax mRNA
expression and reduces Bcl-2 mRNA expression in U-2OS cells
(Fig. 7; P<0.01 or 0.05 vs.
untreated control cells). This indicates that XJW induces apoptosis
by affecting the ratio of Bax/Bcl-2 at transcriptional level. We
further studied the role of XJW on the expression of proteins
involved in the mitochondrial pathway. The results showed that XJW
treatment upregulates Bax protein expression and downregulates
Bcl-2 protein expression (Fig. 8),
which is in accordance with the pattern of their mRNA expression
after XJW treatment.
In conclusion, our data for the first time
demonstrate that XJW inhibits U-2OS cell proliferation via cell
cycle G2/M arrest and promotes apoptosis via the
mitochondrion-dependent pathway. This study may provide a
mechanistic background for the introduction of this new type of
promising therapeutic agent in the study of cancer
chemotherapy.
Abbreviations:
|
OS
|
osteosarcoma;
|
|
XJW
|
Xiao Jin Wan;
|
|
FBS
|
fetal bovine serum;
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium;
|
|
DMSO
|
dimethyl sulfoxide;
|
|
MTT
|
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
Acknowledgements
This study was supported by the
Developmental Fund of Chen Keji Integrative Medicine (CKJ2010023)
and the Youth Science Foundation of Fujian Provincial Health
Department (2010-2-65).
References
|
1
|
Longhi A, Errani C and De Paolis M:
Primary bone osteosarcoma in the pediatric age: state of the art.
Cancer Treat Rev. 32:423–436. 2006.PubMed/NCBI
|
|
2
|
Kim SJ, Choi JA, Lee SH, Choi JY, Hong SH,
Chung HW and Kang HS: Imaging findings of extrapulmonary metastases
of osteosarcoma. Clin Imaging. 28:291–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kager L, Zoubek A, Potschger U, Kastner U,
Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M,
Winkelmann W, Jundt G, Kabisch H, Reichardt P, Jurgens H, Gadner H
and Bielack SS: Primary metastatic osteosarcoma: presentation and
outcome of patients treated on neoadjuvant Cooperative Osteosarcoma
Study Group protocols. J Clin Oncol. 21:2011–2018. 2003. View Article : Google Scholar
|
|
4
|
Federman N, Bernthal N, Eilber F and Tap
W: The multidisciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009.
|
|
5
|
De Saint Aubain Somerhausen N and Fletcher
CD: Soft-tissue sarcomas: an update. Eur J Surg Oncol. 25:215–220.
1999.
|
|
6
|
Lin W, Zheng LP, Zhao JY, Zhuang QC, Hong
ZF, Xu W, Chen YQ, Sferra TJ and Peng J: Anti-angiogenic effect of
Spica prunellae extract in vivo and in vitro. Afr J Pharm
Pharmacol. 24:2647–2654. 2012.
|
|
7
|
Wei LH, Chen YQ, Lin JM, Zhao JY, Chen XZ,
Xu W, Liu XX, Sferra TJ and Peng J: Scutellaria barbata D.
Don induces apoptosis of human colon carcinoma cell through
activation of the mitochondrion-dependent pathway. J Med Plant Res.
10:1962–1970. 2011.
|
|
8
|
Wei LH, Lin JM, Xu W, Hong ZF, Liu XX and
Peng J: Inhibition of tumor angiogenesis by Scutellaria
barbata D. Don via suppressing proliferation, migration and
tube formation of endothelial cells and downregulation of the
expression of VEGF-A in cancer cells. J Med Plant Res.
14:3260–3268. 2011.
|
|
9
|
Peng HR: Grand Dictionary of Chinese
Medicinal Formula (Zhong Yi Fang Ji Da Ci Dian). 1st edition. 1.
People’s Health Press; Beijing: pp. 11131993
|
|
10
|
Sun SM: Thousand Ducat Prescriptions (Qian
Jin Fang). 1st edition. Jilin Publishing Group Co Ltd; 2011
|
|
11
|
Scheid V, Bensky D, Ellis A and Barolet R:
Chinese Herbal Medicine: Formulas and Strategies. 2nd edition.
Eastland Press; Seattle, WA: 2009
|
|
12
|
Bensky D, Gamble A and Stöger E: Chinese
Herbal Medicine: Materia Medica. 3rd edition. Eastland Press;
Seattle, WA: 2004
|
|
13
|
Wang WD: Wai Ke Zheng Zhi Quan Sheng Ji.
1st edition. Hu X.F.: People’s Medical Publishing House; pp.
322006
|
|
14
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the Peoples Republic of China. 1. Chinese Medical
Science and Technology Press; pp. 506–507. 2010
|
|
15
|
Elledge SJ: Cell cycle checkpoints:
preventing an identity crisis. Science. 274:1664–1672. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:18032001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Fujita N and Tsuruo T:
Caspase-mediated cleavage of p21Waf1/Cip1 converts
cancer cells from growth arrest to undergoing apoptosis. Oncogene.
18:11311999.PubMed/NCBI
|
|
18
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thornberry NA and Lazebnik Y: Caspases:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cory S and Adams JM: The Bcl-2 family:
regulators of the cellular life-of-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gross A, McDonnell JM and Korsmeyer SJ:
Bcl-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolter KG, Hsu YT, Smith CL, Nechushtan A,
Xi XG and Youle RJ: Movement of Bax from the cytosol to
mitochondria. J Cell Biol. 139:1281–1292. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Antonsson B, Montessuit S, Lauper S, Eskes
R and Martinou JC: Bax oligomerization is required for
channel-forming activity in liposomes and to trigger cytochrome c
release from mitochondria. Biochem J. 345:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou H, Li Y, Liu X and Wang X: An
APAF1-cytochrome c multimeric complex is a functional apoptosome
that activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Opferman JT, Letai A, Beard C, Sorcinelli
MD, Ong CC and Korsmeyer SJ: Development and maintenance of B and T
lymphocytes requires antiapoptotic MCL-1. Nature. 426:671–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Veis DJ, Sorenson CM, Shutter JR and
Korsmeyer SJ: Bcl-2-deficient mice demonstrate fulminant lymphoid
apoptosis, polycystic kidneys, and hypopigmented hair. Cell.
75:229–240. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomenius MJ, Wang NS, Reineks EZ, Wang Z
and Distelhorst CW: Bcl-2 on the endoplasmic reticulum regulates
Bax activity by binding to BH3-only proteins. J Biol Chem.
278:6243–6250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu GW, Sferra TJ, Chen XZ, Chen YQ, Wu MX,
Xu HF, Peng J and Liu XX: Millimeter wave treatment inhibits the
mitochondrion-dependent apoptosis pathway in chondrocytes. Mol Med
Rep. 4:1001–1006. 2011.PubMed/NCBI
|
|
32
|
Korper S, Nolte F, Rojewski MT, Thiel E
and Schrezenmeier H: The K+ channel openers diazoxide and NS1619
induce depolarization of mitochondria and have differential effects
on cell Ca2+ in CD34+ cell line KG-1. Exp
Hematol. 31:815–823. 2003.
|
|
33
|
Boose G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Louis Jeune MA, Kumi-Diaka J and Brown J:
Anticancer activities of pomegranate extracts and genistein in
human breast cancer cells. J Med Food. 8:469–475. 2005.PubMed/NCBI
|
|
36
|
Won HJ, Han CH, Kim YH, Kwon HJ, Kim BW,
Choi JS and Kim KH: Induction of apoptosis in human acute leukemia
Jurkat T cells by Albizia julibrissin extract is mediated
via mitochondria-dependent caspase-3 activation. J Ethnopharmacol.
106:383–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mantymaa P, Siitonen T, Guttorm T, et al:
Induction of mitochondrial manganese superoxide dismutase confers
resistance to apoptosis in acute myeloblastic leukaemia cells
exposed to etoposide. Br J Haematol. 108:574–581. 2000. View Article : Google Scholar
|
|
38
|
Cossarizza A, Baccarani-Contri M,
Kalashnikova G and Franceschi C: A new method for the
cytofluorimetric analysis of mitochondrial membrane potential using
the J-aggregate forming lipophilic cation
5,5′,6,6′-tetrachloro-1,1′,3,3′
tetraethylbenzimidazolylcarbocyanine iodide (JC-1). Biochem Biophys
Res Commun. 197:40–45. 1993.PubMed/NCBI
|
|
39
|
Smiley ST, Reers M, Mottola-Hartshorn C,
et al: Intracellular heterogeneity in mitochondrial membrane
potentials revealed by a J-aggregate forming lipophilic cation
JC-1. Proc Natl Acad Sci USA. 88:3671–3675. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eskes R, Desagher S, Antonsson B and
Martinou JC: Bid induces the ligomerization and insertion of bax
into the outer mitochondrial membrane. Mol Cell Biol. 20:929–935.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adams JM and Cory S: Life-or-death
decisions by the Bcl-2 protein family. Trends Biochem Sci.
26:61–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujio Y, Nguyen T, Wencker D, Kitsis RN
and Walsh K: Akt promotes survival of cardiomyocytes in vitro and
protects against ischemia-reperfusion injury in mouse heart.
Circulation. 101:660–667. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brown GC and Borutaite V: Nitric oxide,
cytochrome c and mitochondria. Biochem Soc Symp. 66:17–25.
1999.PubMed/NCBI
|