Introduction
Small cell lung cancer (SCLC), which accounts for
∼15% of all lung cancer cases, is the most aggressive metastatic
form of lung cancer and does not respond well to surgery or
radiotherapy (1). Although up to
90% of small cell lung cancer (SCLC) initially responds to
chemotherapy, patients with SCLC often relapse with multidrug
resistant formation (MDR), leading to 5-year survival <5%
(2,3) indicating that MDR is a major obstacle
for successful small cell lung cancer chemotherapy. ATP-binding
cassette transporter proteins such as ATP-binding cassette (ABC)
transporters P-glycoprotein (P-gp, MDR1), multidrug
resistance-associated protein (MRP) and breast cancer resistance
protein (BCRP/ABCG2) (4,5), transporting a wide variety of
chemical compounds in ATP-dependent manner, have been found to
contribute to MDR formation in a variety of tumors arising from
gastric, renal, endometrium, melanoma and soft tissue (6–12).
However, the role of ABCG2 and MRP2 in lung cancer associated MDR
formation is still uncertain and this is important for dealing with
lung cancer associated multidrug resistance.
Various cellular pathways might be simultaneously
involved in the clinical drug resistance of cancer patients
(13–22). Ataxia telangiectasia mutanted
(ATM), a nuclear serine-threonine kinase involving in DNA double
strand break (DSB) repair, was reported to upregulate MDR
associated genes expression and contribute to multidrug resistance
(14–16). NF-κB, which is involved in
regulating apoptosis, inflammatory response, cell survival and
immune response, was also reported to play roles in tumor growth,
invasion and metastasis (18–22).
Although the phosphorylation of NEMO Ser85 by ATM was
shown in HEK293 cells (23), the
role of ATM-NF-κB pathway in lung cancer associated MDR formation
is still uncertain and needs further exploration.
In the present study, human small cell lung cancer
NCI-H446 cells were used as lung cancer model and the roles of
ATM/NF-κB activation induced by chemotherapeutic drugs in MDR
formation were explored. The results showed that: firstly, the
expressions of ABCG2, MRP2 and Bcl-2 were upregulated in response
to camptothecin and cisplatin treatment; secondly, ATM and NF-κB
pathways were activated by campto thecin or cisplatin treatment;
moreover, NF-κB activation was dependent on ATM phosphorylation in
camptothecin or cisplatin treatment conditions; most importantly,
both ABCG2 and MRP2 upregulation induced by camptothecin could be
impaired by NF-κB and ATM inhibitors. These findings indicated that
chemotherapeutic drugs such as camptothecin and cisplatin could
upregulate ABCG2 and MRP2 expression by activating ATM/NF-κB
pathway in small cell lung cancer chemotherapy, providing ATM as
potential target molecular for overcoming lung cancer
chemotherapy-associated multidrug resistance.
Materials and methods
Reagents
Reagents were purchased from the following
companies: Rhodamine 123 (rho123; Molecular Probes, Eugene, OR,
USA) was the dye used to detect early stage of cell apoptosis.
Calcein-AM (Molecular Probes) is a cell permeate dye that can be
used to determine cell viability. Camptothecin (CPT) and cisplatin
(DDP) were purchased from Calbiochem (San Diego, CA, USA).
Anti-phospho-IκBα, anti-p65, anti-phospho-p65, anti-ATM,
anti-phospho-ATM, anti-ABCG2, anti-MRP2 and anti-Bcl-2 were from
Cell Signaling Technology (Beverly, MA, USA). Anti-histone H3,
anti-β-actin and anti-tubulin were from Santa Cruz Biothenology
(Santa Cruz, CA, USA).
Cell line and treatment condition
Small cell lung cancer (NCI-H446) cells (American
Type Culture Collection, HTB-171, Bethesda, MD, USA) were grown in
RPMI-1640 media containing penicillin/streptomycin (Gibco,
Gaithersburg, MD) and 10% fetal bovine serum (Hyclone, Logan, UT)
at 37°C in 5% CO2. NCI-H446 cells were passaged every 2
days. Cells were synchronized by serum starvation (in RPMI-1640
without serum) for ≥12 h before treated with dose-escalated
camptothecin or cisplatin for 18 h.
Cell apoptosis assay
Cell apoptosis assay was determined by flow
cytometry according to the method described previously (24). Briefly, NCI-H446 cells were treated
with camptothecin or cisplatin for 18 h at indicated final
concentration. Then, cells were removed by trypsinization, rinsed
with PBS and re-suspended in binding buffer containing Annexin
V-FITC and propidium iodide (PI) for 20 min at room temper ature.
The samples were analyzed on FACSCalibur and data were analyzed
with CellQuest software.
Measurement of mitochondrial membrane
potential by flow cytometry
Mitochondrial membrane was monitored using the
fluorescent dye Rhodamine 123, which detected the early stage of
cell apoptosis (25). Briefly,
NCI-H446 cells were treated with camptothecin or cisplatin for 18 h
at indicated final concentration. Then, cells were rinsed with PBS
and Rhodamine 123 was added at a final concentration of 1
μg/ml. After 15 min co-incubation at 37°C, the cells were
collected, washed twice with PBS and then analyzed by flow
cytometry.
Calcein-AM has been used as an excellent tool for
the studies of cell membrane integrity and is a true end-point
assay for cell viability. Calcein-AM (final concentration of 0.5
μM) was added to cells after camptothecin or cisplatin
treatment as described above. After 15-min co-incubation at 37°C,
the cells were collected, washed twice and analyzed by flow
cytometry.
Confocal immunofluorescence assay
The effect of ATM phosphorylation and p65 nuclear
translocation were investigated by immunofluorescence assay as
described previously (26).
Briefly, NCI-H446 cells were serum-starved for ≥12 h followed by
0.5 μg/ml camptothecin stimulation for indicated periods.
Then, cells were fixed and permeabilized in 100% methanol for 15
min, washed with PBS and blocked with 10% non-fat milk in PBS for 3
h. Primary antibodies (phospho-p65 or phospho-ATM) were incubated
in a humid chamber overnight at 4°C. Finally, FITC-conjugated
secondary antibodies were incubated for 1 h at 37°C and DAPI
counterstaining was performed to visualize cell nuclei. The cells
were observed and images were recorded by a confocal fluorescence
microscope at the wavelength of 488 nm. ATM phosphorylation
inhibitor CGK (20 μM) was pre-treated for 1 h before
camptothecin treatment to observe the effect of ATM activation on
p65 phosphorylation and translocation. Cells were washed three
times in each step to remove non-binding substance.
Cytoplasmic and nuclear extracts
isolation
Cytoplasmic and nuclear extracts from whole cell
extracts were prepared as described previously (27). Briefly, NCI-H446 cells were treated
with camptothecin or cisplatin for 18 h at indicated final
concentration. Then, cells were suspended in ice-cold CER buffer
(cytoplasmic extraction reagent), vortexed for 10 min and ice-cold
CER was added. The cytosolic fraction (supernatant) was separated
by centrifugation (16000 × g, 5 min, 4°C) and the nuclear protein
was separated by incubating insoluble fraction with ice-cold NER
(nuclear extraction reagent) for 40 min and centrifuged at 16000 ×
g for 10 min, 4°C. Protein concentration was estimated using the
Bio-Rad protein assay reagent and an equal amount of proteins per
sample of nuclear extract was further analyzed by Western
blotting.
Western blot analysis
Proteins were obtained in lysis buffer as previously
described (28). To investigate
the effects of camptothecin or cisplatin on MDR-related genes
expression, NCI-H446 cells were treated with camptothecin or
cisplatin at indicated concentration. Protein lysates were
electrophoresed on 8–10% SDS-PAGE gels, then transferred to PVDF
membranes and blotted with primary antibodies. Followed by
appropriate peroxidase-conjugated secondary antibodies and detected
by chemiluminescence ECL. β-actin, tubulin or histone were used as
loading control.
Statistical analysis
Each experiment was repeated at least 3 times and
confirmed that similar data were obtained. All data were expressed
as mean and standard error means. Statistical significance was
tested using one-way ANOVA with post Newman-Keuls test. Statistical
differences were considered to be significant at p<0.05.
Results
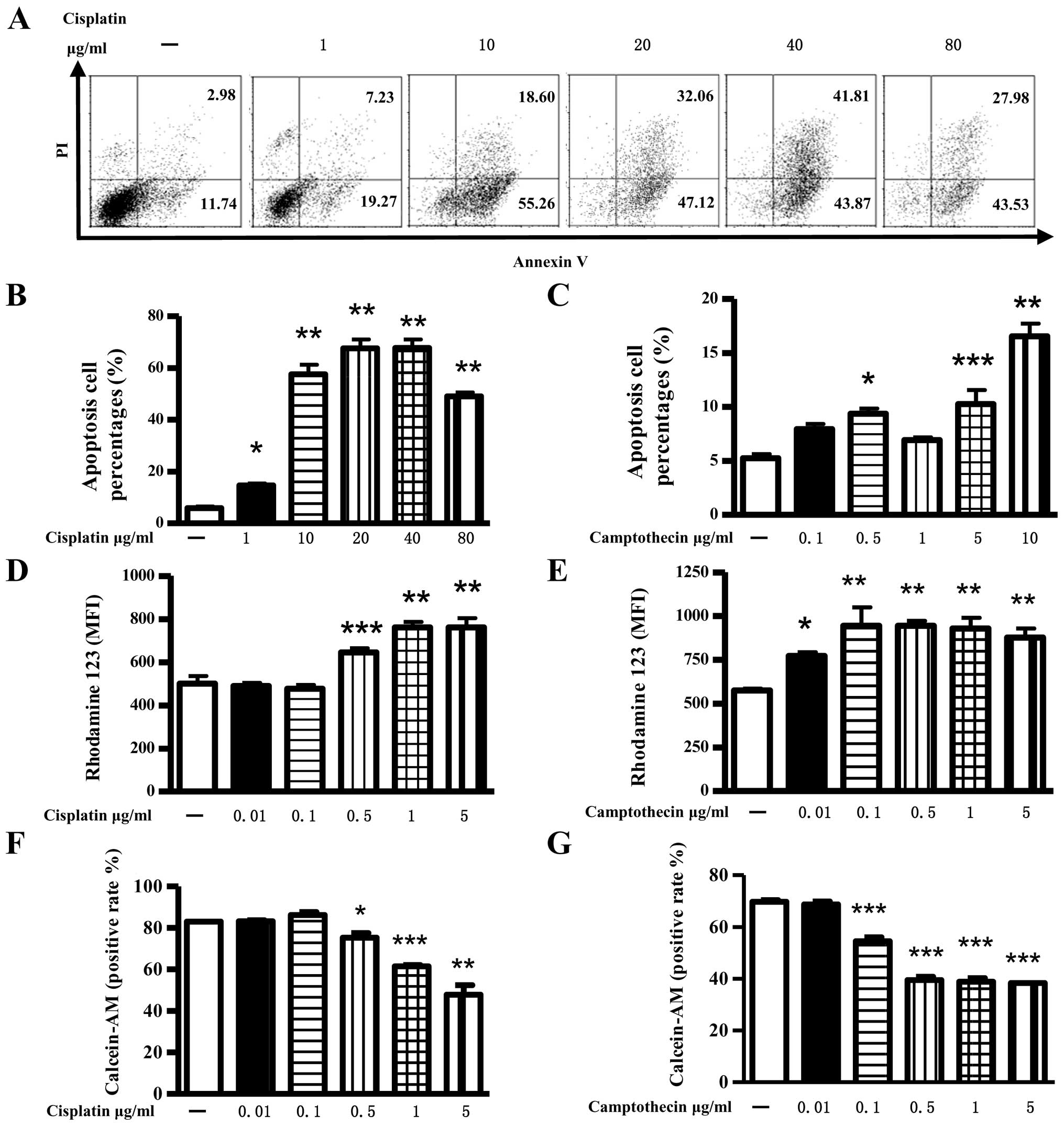
Both cisplatin and camptothecin
independently change mitochondrial membrane potential and induce
cell apoptosis
To investigate the pro-apoptosis effect of
camptothecin and cisplatin, NCI-H446 cells were exposed to
different concentrations of camptothecin or cisplatin for 18 h and
analyzed by flow cytometry. The results showed that: both
camptothecin and cisplatin could effectively induce apoptosis in a
concentration-dependent manner, as both of 0.5 μg/ml
camptothecin and 1 μg/ml cisplatin could induce ∼10%
apoptosis (Fig. 1A–C). Analysis of
early stage cell apoptosis found that camptothecin or cisplatin
efficiently changed the mitochondrial membrane potential, which was
demonstrated by enhanced release of Rhodamine 123 from the
mitochondria and mean of fluorescence index increase in
intracellular fluorescence (Fig. 1D
and E). Cell viability determination of Calcein-AM analysis
showed that 0.1 μg/ml camptothecin or 0.5 μg/ml
cisplatin treatment affected cell viabilities of NCI-H446 cells
(Fig. 1F and G). These data
indicated that >1 μg/ml camptothecin or cisplatin
efficiently induced cell apoptosis, while lower concentration
camptothecin or cisplatin induced early stage cell apoptosis and
had no effect on cell viability.
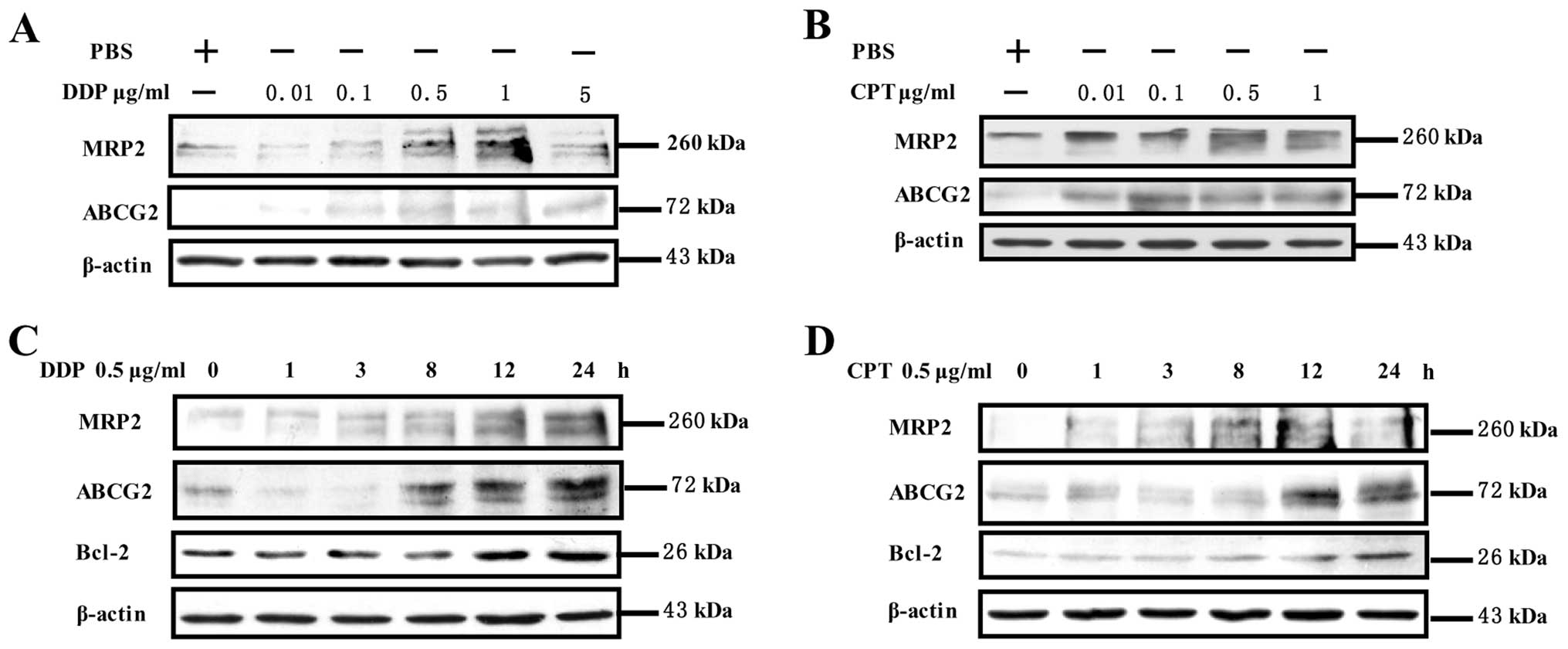
The expression of ABCG2, MRP2 and Bcl-2
was upregulated by camptothecin or cisplatin stimulation in
NCI-H446 cells
To investigate the effects of camptothecin and
cisplatin on expression of ABCG2, MRP2 and Bcl-2, NCI-H446 cells
were treated with camptothecin or cisplatin and the expressions of
ABCG2, MRP2 and Bcl-2 was determined by Western blotting. The
results showed that cisplatin stimulation obviously increased ABCG2
and MRP2 expressions in a concentration- (0.1–5 μg/ml)
(Fig. 2A) and time-dependent
manner (3–24 h) (Fig. 2C), which
reach the maximum at 1 μg/ml and 24 h respectively. Similar
to cisplatin, camptothecin upregulated ABCG2, and MRP2 expression
in a concentration- (0.01–1 μg/ml) (Fig. 2B) and time-dependent manner (1–24
h) (Fig. 2D). Interestingly, not
only ABCG2 and MRP2 but also Bcl-2 was upregulated by cisplatin or
camptothecin stimulation from 3 to 24 h (Fig. 2C and D). Collectively, these data
indicated that camptothecin or cisplatin treatment induced the
expression of multidrug resistance protein and anti-apoptosis
protein, contributing to MDR formation in NCI-H446 cells.
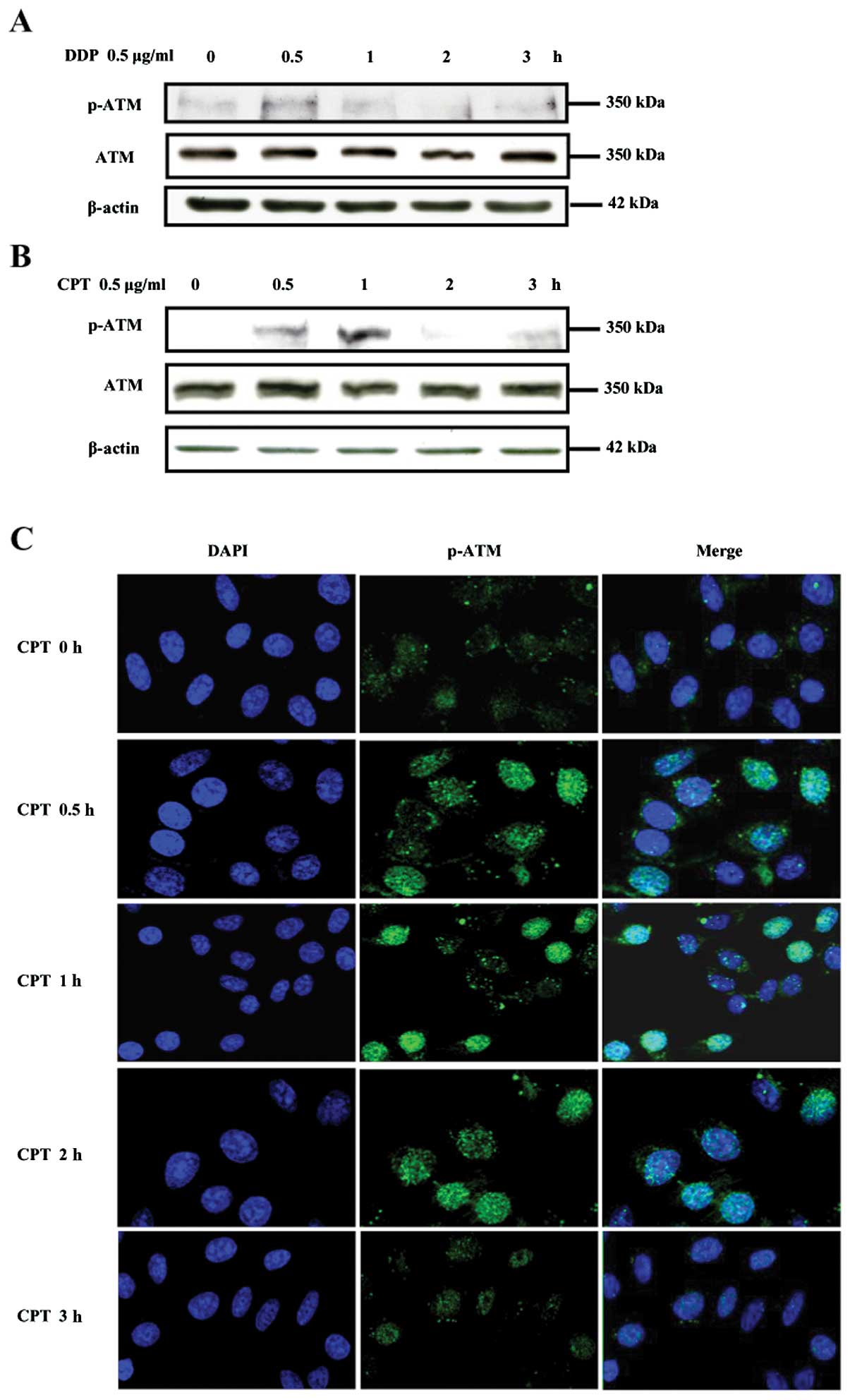
Camptothecin or cisplatin activates ATM
in NCI-H446 cells
ATM, a serine/threonine protein kinase, could be
activated by ionizing radiation or other agents such as etoposide
which induces DNA double strand breaks (29). To determine the effect of
camptothecin and cisplatin on ATM activation in lung cancer cells,
NCI-H446 cells were treated with camptothecin or cisplatin and ATM
phosphorylation was observed by Western blotting and laser confocal
microscope respectively. The results showed that 0.5 μg/ml
camptothecin or cisplatin treatments obviously increased ATM
phosphorylation in NCI-H446 cells in time-dependent manner which
reach the maximum at 1 h after the exposure to camptothecin or
cisplatin (Fig. 3A and B). The
immunofluorescence observation found that camptothecin increased
the phosphorylation of ATM from 0.5 to 3 h, which reach the maximum
at 1 h after camptothecin stimulation (Fig. 3C).
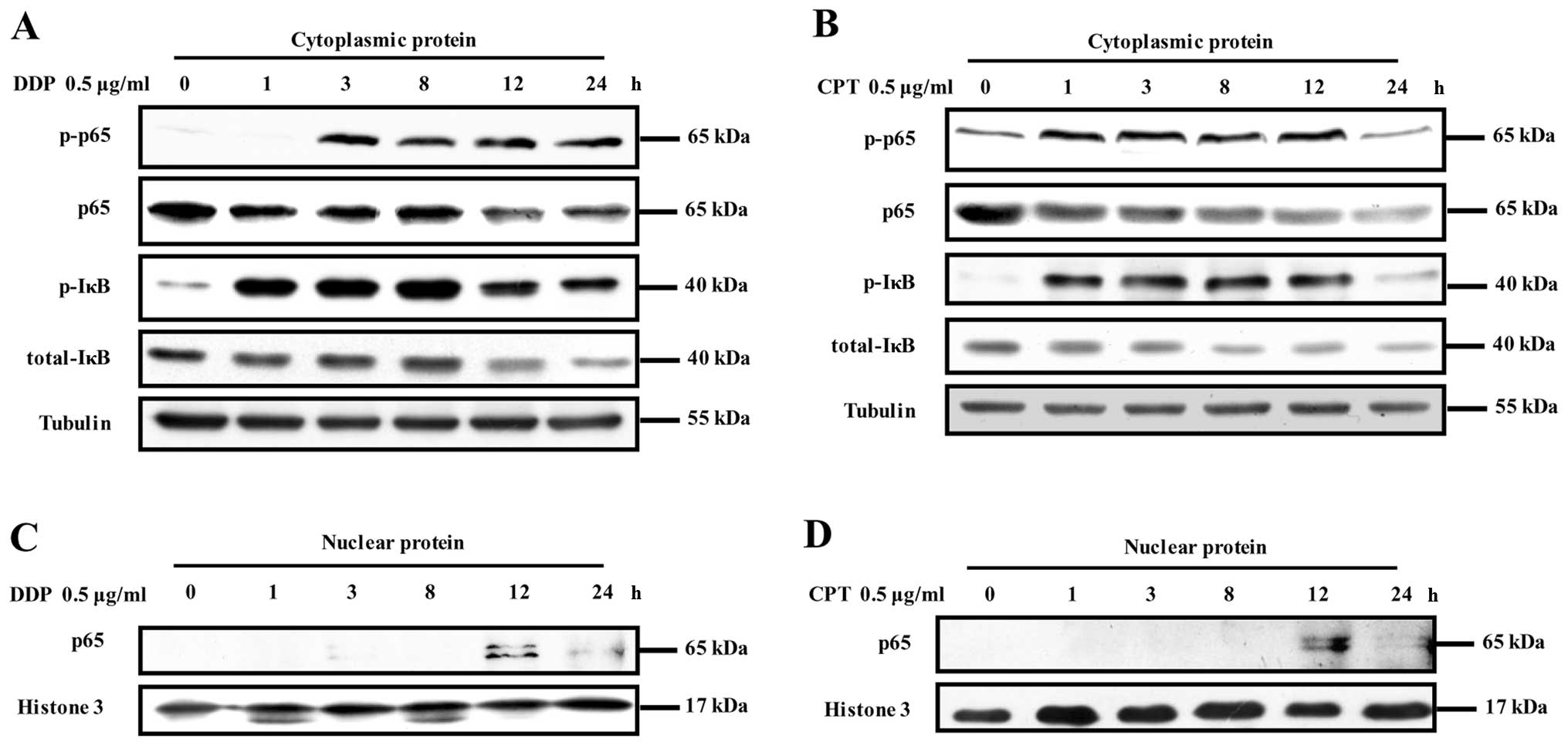
Camptothecin or cisplatin can activate
the NF-κB pathway in NCI-H446 cells
Phosphorylation and degradation of IκBα, the natural
blocker of NF-κB and a direct downstream protein activated by
phosphorylated IKK, is well known as an important prerequisite for
the activation of NF-κB (16,26).
To investigate the effects of camptothecin and cisplatin on NF-κB
pathway activation, NCI-H446 cells were treated with camptothecin
or cisplatin and phosphorylation of IκBα, p65 and p65 translocation
were determinated by Western blotting. The results showed that both
IκBα and p65 phosphorylation in cytoplasm was increased at 3 h and
remained elevated at 8 h after camptothecin or cisplatin treatment.
In contrast, total IκBα and total p65 in cytoplasm decreased
accordingly in a time-dependent manner (Fig. 4A and B). Consistent with
phosphorylation of IκBα and p65 in cytoplasm, the translocation of
p65 from cytoplasm to neuclei was observed at 3 h and reached the
maximum at 12 h, which remained elevated at 24 h after camptothecin
or cisplatin treatment (Fig. 4C and
D), indicating that camptothecin or cisplatin stimulation
induced NF-κB pathway activation in NCI-H446 cells.
ATM phosphorylation is involved in
camptothecin or cisplatin-induced NF-κB activation in NCI-H446
cells
Two independent studies have reported an essential
role of ATM in DSB-induced NF-κB activation (14,30).
To explore the role of ATM phosphorylation in camptothecin and
cisplatin-induced NF-κB activation, ATM phosphorylation inhibitor
CGK was used prior to camptothecin or cisplatin treatment and NF-κB
activation, the translocation was determined by Western blotting
and confocal microscrope observation, respectively. The results
showed that camptothecin stimulation effectively induced both p65
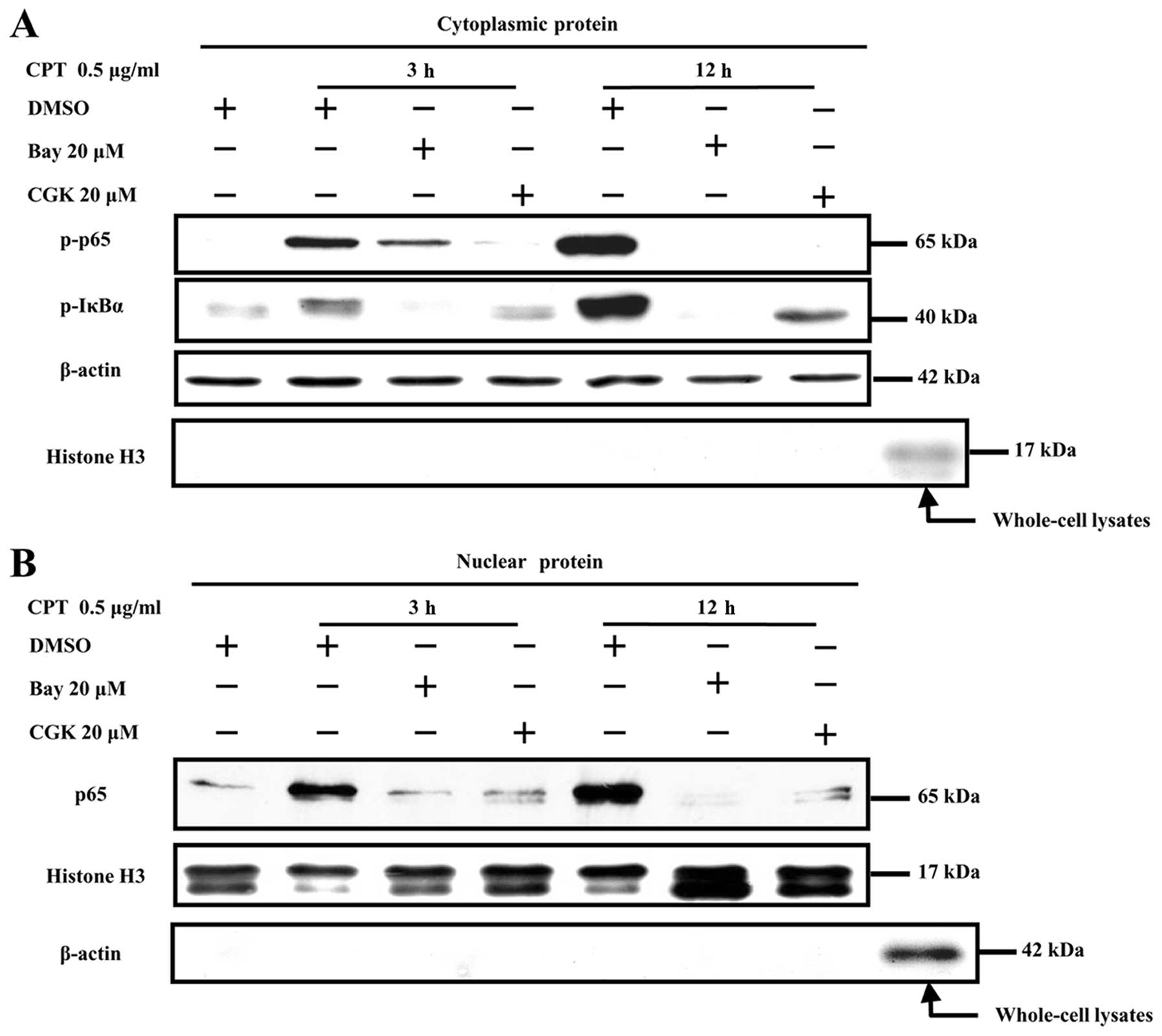
and IκBα phosphorylation from 3 to 12 h (Fig. 5A) and enhanced p65 translocation
from cytoplasm to nucleus (Fig.
5B), CGK (20 μM) pre-treatment decreased IκBα and p65
phosphorylation (Fig. 5A) and
blocked p65 translocation (Fig.
5B). As BAY, an NF-κB activation inhibitor, could effectively
inhibit p65 phosphorylation (Fig.
5A) and translocation (Fig.
5B), the reduction of NF-κB activation derived by the usage of
CGK indicated that camptothecin induced NF-κB activation was ATM
phosphorylation dependent in NCI-H446 cells.
Camptothecin upregulates the expressions
of ABCG2, MRP2 and Bcl-2 by activating the ATM/NF-κB pathway
To explore the role of ATM/NF-κB activation in
camptothecin-induced upregulation of ABCG2, MRP2 and Bcl-2 in
NCI-H446 cells, ATM phosphorylation inhibitor CGK and NF-κB
inhibitor BAY were used prior to camptothecin stimulation and the
expressions of ABCG2, MRP2 and Bcl-2 was determined by Western
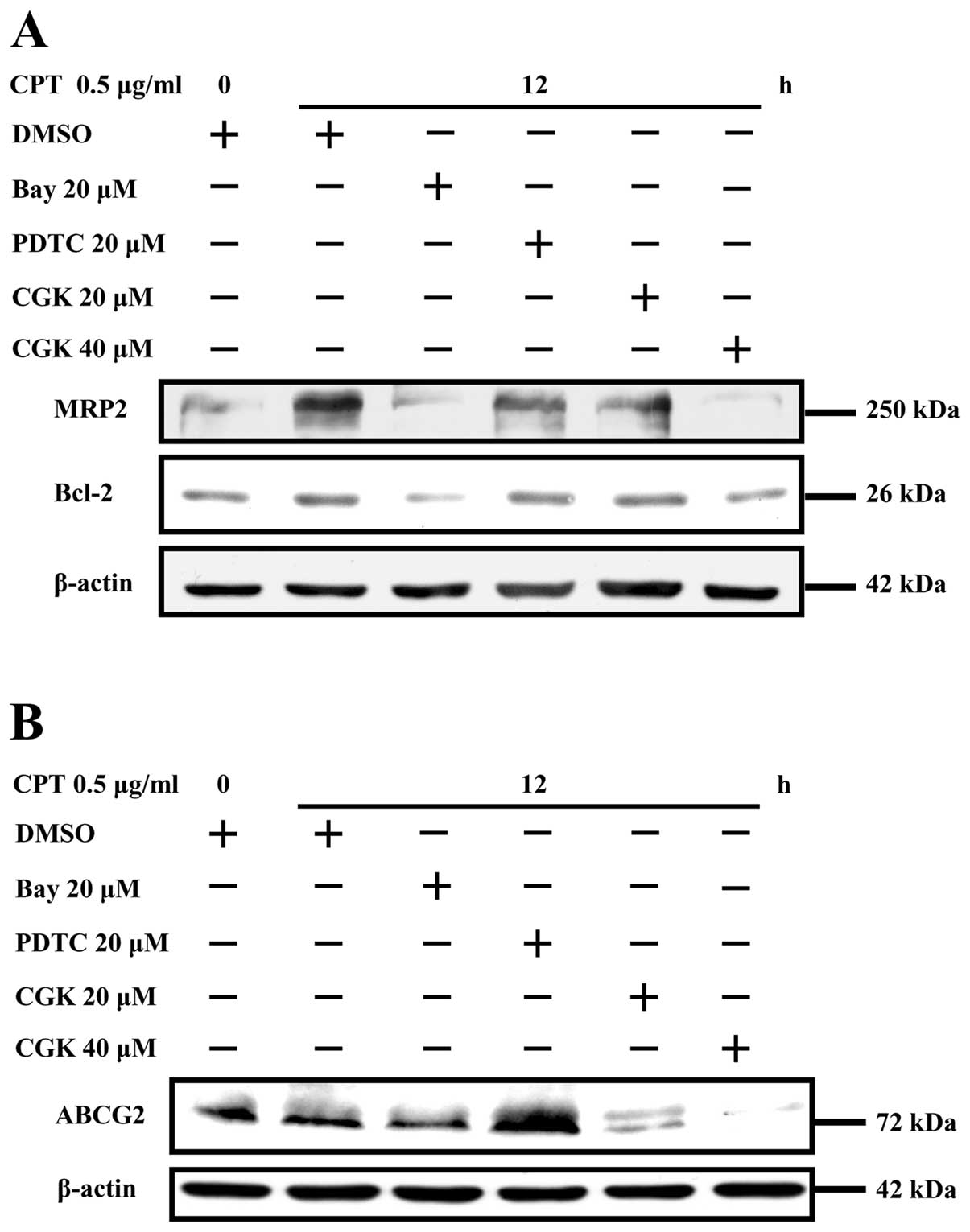
blotting. The results showed that campto thecin stimulation
obviously enhanced MRP2, Bcl-2 (Fig.
6A) and ABCG2 (Fig. 6B)
expressions, the usage of ATM phosphorylation inhibitor CGK and
effectively eliminated the upregulation effect of camptothecin on
MRP2, Bcl-2 (Fig. 6A) and ABCG2
(Fig. 6B) expression in a CGK
concentration-dependent manner. NF-κB activation inhibitor BAY was
also found to block MRP2, Bcl-2 and ABCG2 upregulation induced by
camptothecin. However, the inhibitory effect of PDTC on MRP2, Bcl-2
and ABCG2 upregulation was not clear, which might be due to PDTC
that did not inhibit camptothecin- induced NF-κB activation, as
Togashi et al showed that PDTC did not inhibit tumor
necrosis factor-α-induced NF-κB activation in astrocytes (31). As CGK (20 μM) and BAY (20
μM) were capable of sufficiently inhibiting NF-κB
activation, the inhibition effect of CGK and BAY on ABCG2, MRP2,
Bcl-2 expressions indicated that camptothecin upregulated ABCG2,
MRP2 and Bcl-2 expression, respectively, by activating ATM/NF-κB
pathway in NCI-H446 cells.
Discussion
Although doxorubicin has been reported to induce
MRP1 expression by activating the c-jun kinase pathway in human
small cell lung cancer cell lines (1), a definitive conclusion with regard to
the impact of drug resistance factors can not be derived due to the
heterogeneity of the present study. Thus, MDR associated genes and
mechanism need further exploration for dealing with
chemotherapeutic multidrug resistance. In the present study, ABCG2
and MRP2 were used as representative multidrug resistance proteins
and the roles of ATM-NF-κB activation in lung cancer-associated MDR
formation were investigated in camptothecin or cisplatin treatment
conditions. Our results showed that: ATM and NF-κB activation,
resulting in cell survival, play an essential role in MDR
development in camptothecin or cisplatin treated NCI-H446 cells;
importantly, camptothecin or cisplatin was able to upregulate
ABCG2, MRP2 expression by activating the ATM-NF-κB pathway, which
was demonstrated by ATM and NF-κB inhibitors abrogating
camptothecin-increased ABCG2 and MRP2 expression, indicating that
ATM inhibitor might be useful for overcoming multidrug resistance
in lung cancer chemotherapy.
Mitochondrial membrane potential is a key indicator
of cellular viability, which reflects the pumping of hydrogen ions
across the inner membrane during the process of electron transport
and oxidative phosphorylation. In the present study, mitochondrial
membrane was monitored by Rhodamine 123 and Calcein-AM flow
cytometry. As Rhodamine 123 preferentially partition into active
mitochondria based on depolarization of mitochondrial membrane
potential when early stage cell apoptosis results in the release of
Rhodamine 123 from the mitochondria and an increase in
intracellular fluorescence (25).
Calcein-AM is a widely used green fluorescent cell marker which is
membrane permeant and can be introduced into cells via incubation.
Once inside the cells, calcein-AM, a non-fluorescent molecule
itself, is hydrolyzed by endogenous esterase into the highly
negatively charged green fluorescent calcein and retained in the
cytoplasm in live cells (32).
Function-deficient mutations of ATM has been
reported in ataxia telangiectasia, which account for autosomal
recessive disorder of cerebella ataxia, oculocutaneous
telangiectasia, immunodeficiency, radiation sensitivity, growth
retardation, premature aging and cancer predisposition (33). ATM was also implicated recently in
metabolic pathways seemingly unrelated to DNA damage (34). However, as DNA damage sensor
(14), ATM is a nuclear
serine-threonine kinase involved in DNA double strand break (DSB)
repair and plays an important role in chemotherapeutic drug induced
MDR formation. Although the present study showed that ATM could be
activated by camptothecin and cisplatin and ATM phosphorylation was
crucial for the upregulation of ABCG2 and MRP2, the mutation of ATM
induced by chemotherapeutic drug and the relationship of ATM
mutation and MDR formation could be very important for lung cancer
chemotherapy and needs further clarification.
Korita et al(35) reported that MRP2 expression
determines the efficacy of cisplatin-based chemotherapy in patients
with hepatocellular carcinoma. The MRP1 upregulation in tumors
after chemotherapy attributed to selection of pre-existing MDR
cells was also reported (12,36–38).
Several studies have been performed to investigate potential
correlation between ABCG2 expression and clinical outcomes. Given
the specific tissue localizations, the role of ABCG2 in healthy
tissue may be to protect an organism or tissue from potentially
harmful toxins. Here we showed that significant increases of ABCG2,
MRP2 and Bcl-2 were observed within 12 h of exposure of NCI-H446
cells to camptothecin or cisplatin (Fig. 2).
IKK or NF-κB activation is ATM
phosphorylation-dependent in transformed cells (39–41).
In the present study, CGK, the ATM inhibitor, not only inhibited
the phosphorylation of IκBα and p65 but also blocked p65
translocation from cytoplasm to nucleus (Fig. 5), indicating that ATM
phosphorylation is necessary for IκBα phosphorylation in
camptothecin or cisplatin-induced NF-κB activation. As IKKγ/NEMO
was reported as NF-κB essential modulator (39) and PI3K/AKT activation in response
to IL-1 stimulation leads to NF-κB activation (42), both IKKγ/NEMO and PI3K/AKT might
induce NF-κB activation. Although ATM phosphorylation was necessary
for camptothecin-induced NF-κB activation and MDR formation, the
mechanisms of phosphorylated ATM inducing IκBα phosphorylation by
interacting with NEMO or AKT are still unknown and need further
investigation.
NF-κB is involved in apoptotic response of cells
exposed to chemotherapeutic agents (20–23).
Activated NF-κB binds to specific DNA sequences of target genes and
regulates gene transcription involved in chemoresistance and
radioresistance, including COX-2, cyclin D1, Bcl-2, Bcl-xl,
survivin and XIAP (43). In the
present study, we have characterized the role of ATM and p65
activation in camptothecin or cisplatin induced ABCG2, MRP2 and
Bcl-2 upregulation in NCI-H446 cells (Fig. 5A). Whether other NF-κB components
also regulate ABCG2, MRP2 and Bcl-2 expression is still
uncertain.
Collectively, our study demonstrated that
camptothecin or cisplatin treatment increased expression of ABCG2,
MRP2 and Bcl-2 by activating the ATM/NF-κB pathway in human
NCI-H446 cells. This study may explain one of the key mechanisms of
MDR development following lung cancer chemotherapy. Furthermore,
the present research suggests that combined treatment of ATM and
NF-κB inhibitors might prevent the development of MDR under
clinical conditions.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (no. 30901738
and 81273203), and the Natural Science Foundation of Xiamen (no.
3502Z20104002).
References
|
1
|
Tan XL, Moyer AM, Fridley BL, et al:
Genetic variation predicting cisplatin cytotoxicity associated with
overall survival in lung cancer patients receiving platinum-based
chemotherapy. Clin Cancer Res. 17:5801–5811. 2011. View Article : Google Scholar
|
|
2
|
Shinoda C, Maruyama M, Fujishita T, et al:
Doxorubicin induces expression of multidrug resistance-associated
protein 1 in human small cell lung cancer cell lines by the c-jun
N-terminal kinase pathway. Int J Cancer. 117:21–31. 2005.
View Article : Google Scholar
|
|
3
|
Spiro SG, Tanner NT, Silvestri GA, et al:
Lung cancer: progress in diagnosis, staging and therapy.
Respirology. 15:44–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamazaki R, Nishiyama Y, Furuta T, et al:
Novel acrylonitrile derivatives, YHO-13177 and YHO-13351, reverse
BCRP/ABCG2-mediated drug resistance in vitro and in vivo. Mol
Cancer Ther. 10:1252–1263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DH, Sriharsha L, Xu W, et al: Clinical
relevance of a pharmacogenetic approach using multiple candidate
genes to predict response and resistance to imatinib therapy in
chronic myeloid leukemia. Clin Cancer Res. 15:4750–4758. 2009.
View Article : Google Scholar
|
|
6
|
Doyle LA and Ross DD: Multidrug resistance
mediated by the breast cancer resistance protein BCRP (ABCG2).
Oncogene. 22:7340–7358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robey RW, Medina-Pérez WY, Nishiyama K, et
al: Over-expression of the ATP-binding cassette half-transporter,
ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast
cancer cells. Clin Cancer Res. 7:145–152. 2001.PubMed/NCBI
|
|
8
|
Candeil L, Gourdier I, Peyron D, et al:
ABCG2 overexpression in colon cancer cells resistant to SN38 and in
irinotecan-treated metastases. Int J Cancer. 109:848–854. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diestra JE, Scheffer GL, Catala I, et al:
Frequent expression of the multi-drug resistance-associated protein
BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21
monoclonal antibody in paraffin-embedded material. J Pathol.
198:213–219. 2002. View Article : Google Scholar
|
|
10
|
Turne JG, Gump JL, Zhang C, et al: ABCG2
expression, function and promoter methylation in human multiple
myeloma. Blood. 108:3881–3889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamasaki M, Makino T, Masuzawa T, et al:
Role of multidrug resistance protein 2 (MRP2) in chemoresistance
and clinical outcome in oesophageal squamous cell carcinoma. Br J
Cancer. 104:707–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen ZS and Tiwari AK: Multidrug
resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic
diseases. FEBS J. 278:3226–3245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spanswick VJ, Lowe HL, Newton C, et al:
Evidence for different mechanisms of ‘unhooking’ for melphalan and
cisplatin-induced DNA interstrand cross-links in vitro and in
clinical acquired resistant tumour samples. BMC Cancer.
12:4362012.
|
|
14
|
Gaudio E, Spizzo R, Paduano F, et al: Tcl1
interacts with ATM and enhances NF-κB activation in hematologic
malignancies. Blood. 119:180–187. 2012.PubMed/NCBI
|
|
15
|
Svirnovski AI, Serhiyenka TF, Kustanovich
AM, Khlebko PV, Fedosenko VV, Taras IB and Bakun AV: DNA-PK, ATM
and MDR proteins inhibitors in overcoming fludarabine resistance in
CLL cells. Exp Oncol. 32:258–262. 2010.PubMed/NCBI
|
|
16
|
Bae JB, Mukhopadhyay SS, Liu L, et al:
Snm1B/Apollo mediates replication fork collapse and S Phase
checkpoint activation in response to DNA interstrand cross-links.
Oncogene. 27:5045–5056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bakkenist CJ and Kastan MB: DNA damage
activates ATM through intermolecular autophosphorylation and dimer
dissociation. Nature. 421:499–506. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karin M and Greten FR: NF-kappaB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin Y, Bai L, Chen W and Xu S: The
NF-kappaB activation pathways, emerging molecular targets for
cancer prevention and therapy. Expert Opin Ther Targets. 14:45–55.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campbell KJ, O’Shea JM and Perkins ND:
Differential regulation of NF-kappaB activation and function by
topoisomerase II inhibitors. BMC Cancer. 6:1012006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Melisi D and Chiao PJ: NF-kappa B as a
target for cancer therapy. Expert Opin Ther Targets. 11:133–144.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konstantinopoulos PA, Fountzilas E, Pillay
K, et al: Carboplatin-induced gene expression changes in vitro are
prognostic of survival in epithelial ovarian cancer. BMC Med
Genomics. 1:592008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu ZH, Shi Y, Tibbetts RS and Miyamoto S:
Molecular linkage between the kinase ATM and NF-kappaB signaling in
response to genotoxic stimuli. Science. 311:1141–1146. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu SX, Sui HX, Jin HJ, et al:
Lipopolysaccharide and dose of nicotine determine the effects of
nicotine on murine bone marrow-derived dendritic cells. Mol Med
Rep. 5:1005–1010. 2012.PubMed/NCBI
|
|
25
|
Zhang L and Yu H: Neuroprotective effects
of salidroside against beta-amyloid-induced oxidative stress in
SH-SY5Y human neuroblastoma cells. Neurochem Int. 57:547–555. 2010.
View Article : Google Scholar
|
|
26
|
Buhrmann C, Mobasheri A, Busch F, Aldinger
C, Stahlmann R, Montaseri A and Shakibaei M: Curcumin modulates
nuclear factor kappaB (NF-kappaB)-mediated inflammation in human
tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt
pathway. J Biol Chem. 286:28556–28566. 2011. View Article : Google Scholar
|
|
27
|
Dioum EM, Osborne JK, Goetsch S, Russell
J, Schneider JW and Cobb MH: A small molecule differentiation
inducer increases insulin production by pancreatic β cells. Proc
Natl Acad Sci USA. 108:20713–20718. 2011.PubMed/NCBI
|
|
28
|
Jin HJ, Li HT, Sui HX, Xue MQ, Wang YN,
Wang JX and Gao FG: Nicotine stimulated bone marrow-derived
dendritic cells could augment HBV specific CTL priming by
activating PI3K-Akt pathway. Immunol Lett. 146:40–49. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heo JI, Oh SJ, Kho, et al: ATM mediates
interdependent activation of p53 and ERK through formation of a
ternary complex with p-p53 and p-ERK in response to DNA damage. Mol
Biol Rep. 39:8007–8014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ai L, Skehan RR, Saydi J, Lin T and Brown
KD: Ataxia-telangiectasia, mutated (ATM)/nuclear factor κ light
chain enhancer of activated B cells (NF-κB) signaling controls
basal and DNA damage-induced transglutaminase 2 expression. J Biol
Chem. 287:18330–18341. 2012.
|
|
31
|
Togashi H, Sasaki M, Frohman E, et al:
Neuronal (type I) nitric oxide synthase regulates nuclear factor κB
activity and immunologic (type II) nitric oxide synthase
expression. Proc Natl Acad Sci USA. 94:2676–2680. 1997.
|
|
32
|
Tenopoulou M, Kurz T, Doulias PT, Galaris
D and Brunk UT: Does the calcein-AM method assay the total cellular
‘labile iron pool’ or only a fraction of it? Biochem J.
403:261–266. 2007.
|
|
33
|
Zolner AE, Abdou I, Ye R, et al:
Phosphorylation of polynucleotide kinase/phosphatase by
DNA-dependent protein kinase and ataxia-telangiectasia mutated
regulates its association with sites of DNA damage. Nucleic Acids
Res. 39:9224–9237. 2011. View Article : Google Scholar
|
|
34
|
Alexande A, Cai SL, Kim J, et al: ATM
signals to TSC2 in the cytoplasm to regulate mTORC1 in response to
ROS. Proc Natl Acad Sci USA. 107:4153–4158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Korita PV, Wakai T, Shirai Y, et al:
Multidrug resistance associated protein 2 determines the efficacy
of cisplatin in patients with hepatocellular carcinoma. Oncol Rep.
23:965–972. 2010.PubMed/NCBI
|
|
36
|
Yasui K, Mihara S, Zhao C, et al:
Alteration in copy numbers of genes as a mechanism for acquired
drug resistance. Cancer Res. 64:1403–1410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho S, Lu M, He X, Ee PL, Bhat U,
Schneider E, Miele L and Beck WT: Notch1 regulates the expression
of the multidrug resistance gene ABCC1/MRP1 in cultured
cancer cells. Proc Natl Acad Sci USA. 108:20778–20783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bram EE, Stark M, Raz S and Assaraf YG:
Chemotherapeutic drug-induced ABCG2 promoter demethylation as a
novel mechanism of acquired multidrug resistance. Neoplasia.
11:1359–1370. 2009.PubMed/NCBI
|
|
39
|
Yang Y, Xia F, Hermance N, et al: A
cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the
NF-kappaB and p38 mitogen-activated protein kinase
(MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol Cell
Biol. 31:2774–2786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miyamoto S: Nuclear initiated NF-κB
signaling: NEMO and ATM take center stage. Cell Res. 21:116–130.
2011.
|
|
41
|
Wu ZH and Miyamoto S: Induction of a
pro-apoptotic ATM-NF-κB pathway and its repression by ATR in
response to replication stress. EMBO J. 27:1963–1973. 2008.
|
|
42
|
Ling J, Kang Y, Zhao R, et al:
KrasG12D-induced IKK2/β/NF-ϰB activation by IL-1α and
p62 feedforward loops is required for development of pancreatic
ductal adenocarcinoma. Cancer Cell. 21:105–120. 2012.
|
|
43
|
Li F and Sethi G: Targeting transcription
factor NF-κB to overcome chemoresistance and radioresistance in
cancer therapy. Biochim Biophys Acta. 1805:167–180. 2010.
|