Introduction
Human epidermal growth factor receptor 2 (HER2),
also known as erbB2 and HER2/neu, is a proto-oncogene located at
17q12–21.32, playing an important role in the development and
progression of human tumors, most notably breast cancer. This gene
encodes a 185-kDa transmembrane protein with tyrosine kinase
activity known to be involved in signal transduction during cell
growth (1,2). Amplification of the gene has been
identified in approximately 25–30% of human breast tumors (3,4).
Amplification of HER2 and concomitant overexpression of the protein
is considered to be an important biological marker of poor
prognosis, more aggressive disease, and an increased risk of
recurrence (3–5), as well as being a useful indicator of
response to anti-HER2 therapy, for example using trastuzumab. To
assess whether patients are likely to benefit from trastuzumab,
monitoring of HER2 status has become routine practice using two
techniques that are often used together: detection of gene
amplification and protein overepression using fluorescence in
situ hybridization (FISH) and immunohistochemistry (IHC),
respectively.
Most studies have reported that the HER2 positivity
rate in gastric cancer is about 15–25% (6–9), and
that HER2 positivity in gastric cancer is associated with poorer
prognosis, more aggressive disease and shorter survival (7,9–14).
Trastuzumab treatment has been shown to be clearly beneficial for
patients with gastric cancer showing HER2 gene amplification and/or
protein overexpression (15).
Breast cancer was the first malignancy for which HER2 gene
amplification or protein overexpression was detected and
trastuzumab therapy applied, and this was later extended to gastric
cancer. However, no detailed study has yet investigated HER2 gene
amplification and protein overexpression in thyroid cancer.
Telomeres, which are located at the ends of
chromosomes, are composed of a repeated DNA sequence (TTAGGG) and
specific binding proteins. These structures protect chromosome ends
and prevent them from being recognized as DNA double-strand breaks
(16). The telomere repeat
sequence becomes shortened by each cell division, DNA damage due to
oxidative stress or through changes in telomere-associated proteins
(17,18). It has been proposed that telomere
shortening is an important biological factor involved in
carcinogenesis, cell senescence, cell replication, cell immortality
and aging (19–21). Thus, as a biological marker,
telomere length reflects malignant potential, and might also be
associated with genetic instability and the degree of malignancy
risk (22). The enzyme telomerase
is a reverse transcriptase that maintains chromosome ends by
addition of the DNA sequence repeat TTAGGG to the end of a DNA
strand. The enzyme is composed of a telomerase RNA component
(TERC), and a protein component called human telomerase reverse
transcriptase (hTERT), along with specific accessory proteins. The
telomerase synthesizes the telomeric DNA, and counteracts
progressive shortening of the telomere (23). Some cancers, including thyroid
cancer (24), and their precursor
lesions have been reported to show telomere shortening (25–29),
telomerase activation (27) and
expression of hTERT mRNA (30).
The aim of this study was to clarify whether HER2
gene amplification and protein overexpression are detectable in
differentiated thyroid cancer, and to investigate any correlations
between HER2 status and feature of malignancy such as telomere
shortening or hTERT expression. For this purpose, we measured
telomere lengths in thyroid cancer using quantitative fluorescence
in situ hybridization (Q-FISH), which allowed us to estimate
the telomere lengths of individual cells in each section. We
examined the expression of hTERT and overexpression of HER2 protein
by IHC using an anti-hTERT polyclonal antibody and an anti-c-erbB2
monoclonal antibody, respectively. Furthermore, we performed FISH
to detect amplification of the HER2 gene in differentiated thyroid
cancer.
Materials and methods
Tissue specimens
We examined a total of 69 thyroid tumors, including
61 papillary carcinomas and 8 follicular carcinomas. All samples
were collected after obtaining informed consent from the patients,
who underwent thyroidectomy at the Kanaji Thyroid Hospital, Tokyo,
Japan. Tumor and adjacent normal tissues were obtained from each
patient and stored at −80°C until fixation. The tissues were then
fixed for 2 h in 10% buffered formalin solution and embedded in
paraffin according to standard processing procedures. They were
then sliced into sections 4 μm thick for FISH and IHC
analysis.
Tissue Q-FISH
Tissue Q-FISH was performed as described previously
(31,32). In brief, tissue sections were
deparaffinized and treated with 0.2 N HCl, 1 M sodium thiocyanate
at 80°C, 1% pepsin at 37°C, and 10 mg/ml RNase at 37°C. A peptide
nucleic acid (PNA) telomere probe conjugated to Cy3 (telo C Cy3
probe: 5′-CCCTAACCCTAACCCTAA-3′, Fasmac, Kanagawa, Japan) and a PNA
centromere probe conjugated to fluorescein isothiocyanate (FITC)
(Cenp 1 probe: 5′-CTTCGTT GGAAACGGGT-3′, Fasmac) were applied to
each section. The nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis,
MO).
FISH images were recorded by a CCD camera attached
to an epifluorescence microscope (Eclipse 90i, Nikon, Tokyo, Japan)
equipped with a triple band-pass filter set for DAPI/FITC/Cy3
(61000v2m, Chroma Technology Corp., Rockingham, VT) and a ×40
objective lens (Plan Fluor ×40/0.75, Nikon). Microscope control and
image acquisition were performed using Image-Pro Plus software
(version 6.3, Media Cybernetics, Bethesda, MD). The captured images
were analyzed as described previously using our original software
(32), ‘Tissue Telo’, which allows
manual identification of nuclear regions from the composite color
image: DAPI (blue channel), FITC (green) and Cy3 (red).
Fluorescence intensities of telomere signals (Cy3) and centromere
signals (FITC) for each nucleus were measured, and then the
telomere-centromere ratio (TCR) was calculated, as there is no
guarantee that the entire nucleus will be captured within any given
tissue section.
IHC staining
The expression of hTERT protein was determined by
IHC staining using rabbit antiserum against hTERT prepared by a
colleague (O.Y.). It has been confirmed that telomerase activity is
included in the immune precipitate obtained with this rabbit
antiserum (33). The tissue
sections were pre-treated with Tris-EDTA buffer solution (pH 9.0)
at 95°C. After incubation with the primary antibody for 2 h,
visualization was performed using a polymer IHC detection system
(Envision Kit, Dako Japan, Kyoto, Japan). The expression of HER2
protein was determined using anti-c-erbB2 antibody (Dako, Glostrup,
Denmark) in accordance with the manufacturer’s instructions. The
degree of HER2 staining was scored as 0, 1, 2 or 3 according to the
criteria for gastric cancer (34),
because there are no established criteria for thyroid cancer, and
the staining pattern of thyroid cancer was found to be similar to
that of gastric cancer, as described below. The HER2 score was
judged by a pathologist (M.K.).
HER2 FISH
We demonstrated HER2 FISH in 3 cases with a HER2 IHC
score of 0 or 1, and in all cases with a HER2 IHC score of 2 or 3.
HER2 FISH was performed using the Histra HER2 FISH kit (Jokoh,
Tokyo, Japan), which employs two DNA probes. The probe specific for
the HER2 gene was labeled with rhodamine, and an α satellite probe
targeting the centromere region of chromosome 17 (CEP17) was
labeled with FITC. The assay was performed in accordance with the
manufacturer’s instructions. In brief, specimens were incubated in
pretreatment solution (99°C for 20 min) and then digested with
protease (37°C for 10 min). The DNA probe was applied and
hybridized to each section overnight at 37°C. The slides were then
washed, counterstained with DAPI, and observed by fluorescence
microscopy. In each of the specimens of papillary carcinoma, the
total numbers of HER2 and CEP17 signals were counted in 20 tumor
cell nuclei, and the ratios of HER2 signals to CEP17 signals were
calculated according to the ASCO/CAP criteria (35). Polysomy 17 was defined as the
occurrence of a centromere copy number of three or more for
chromosome 17 per cell.
Statistical analysis
Correlations between HER2 IHC score and FISH ratio
were analyzed using the Spearman’s rank correlation coefficient. We
used an agglomerative hierarchical clustering approach with Ward’s
method to divide the data for the HER2 FISH ratio into two groups.
The significance of differences was examined by TCR with Welch’s
t-test. The significance of differences in age and tumor size was
examined using Student’s t-test. The significance of differences in
sex, TNM classification and hTERT expression was examined using
Fisher’s exact probability test. Differences at P<0.05 were
considered significant.
Results
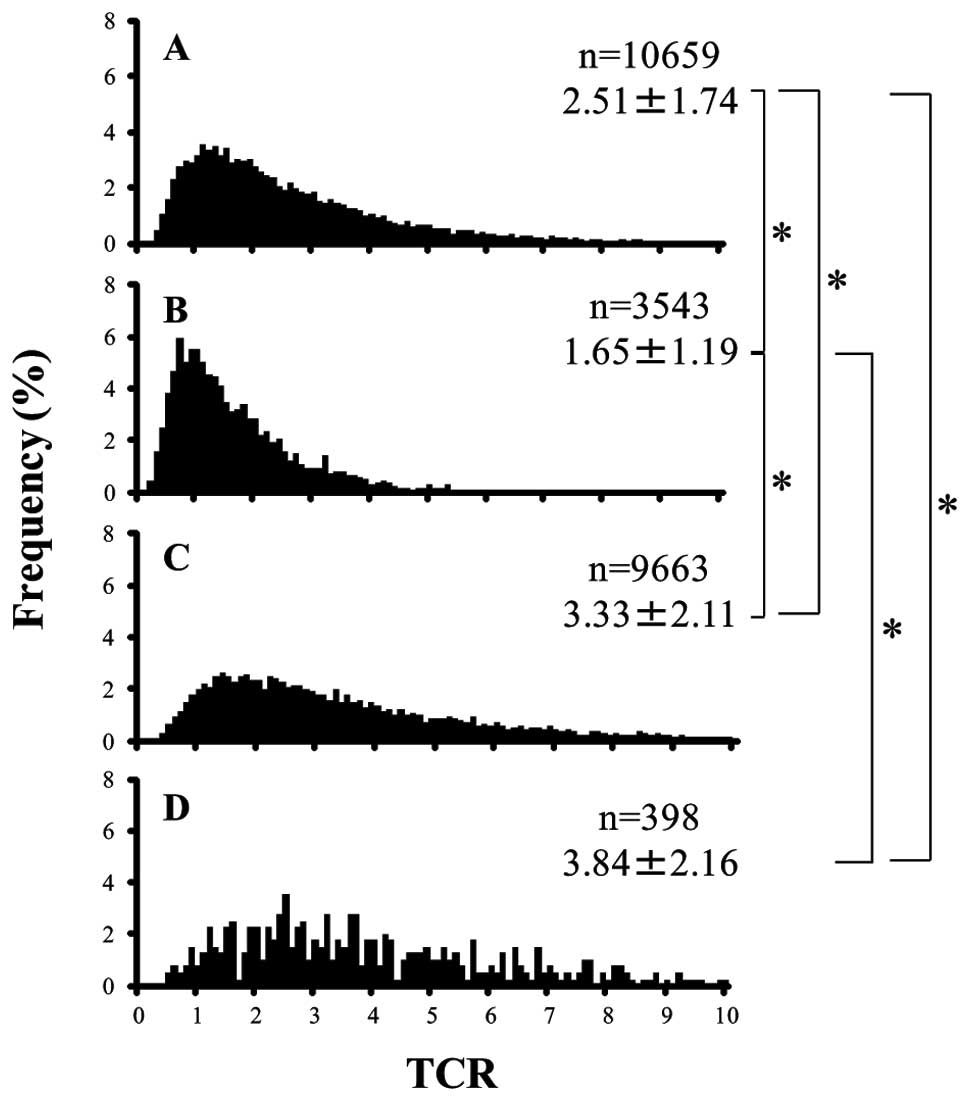
Tissue Q-FISH
Telomere signals (small red spots within nuclei) of
tumor cells in papillary carcinoma (Fig. 1A-2 and -4) and follicular tumors
were weaker than those in adjacent normal follicular epithelial
cells (Fig. 1A-1 and -3). The mean
TCR of papillary carcinoma and follicular carcinoma cells was
significantly less than that of normal follicular epithelial cells
and fibroblasts (Fig. 2,
P<0.05). In addition, the mean TCR of follicular carcinoma cells
was significantly less than that of papillary carcinoma cells
(Fig. 2, P<0.05). The peak TCR
in papillary carcinoma cells was 1 to 2, and that in follicular
carcinoma cells was close to 1. The peak TCR in normal follicular
epithelial cells was 1.5 to 3.5, and that in fibroblasts was 2 or
more, and the peak TCR in normal follicular epithelial cells and
fibroblasts had a very wide distribution (Fig. 2).
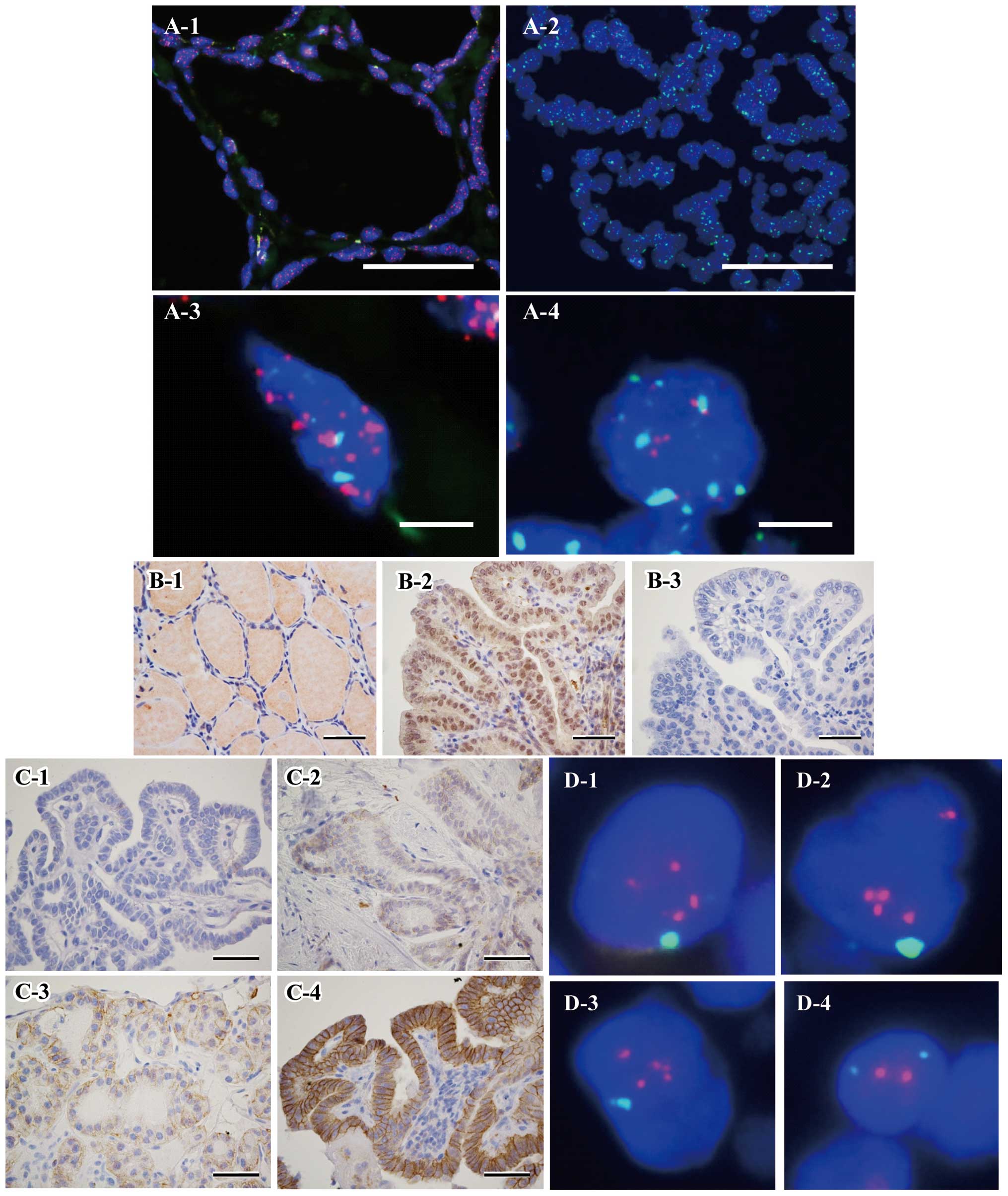 | Figure 1(A) Representative tissue Q-FISH
images of normal follicular epithelial cells (A-1 and -3) and
papillary carcinomas (A-2 and -4). The nuclei are stained
fluorescent blue (DAPI). Red spots (Cy3) and green spots (FITC)
within nuclei indicate telomere and centromere signals,
respectively. The scale bar is 50 and 5 μm in A-1, -2 and
A-3, -4, respectively. Telomere signals of cancer cells are clearly
weaker than those in adjacent normal follicular epithelial cells.
(B) Immunohistochemical (IHC) staining of follicular epithelial
cells (B-1) and papillary carcinomas (B-2) using a polyclonal
rabbit antiserum against hTERT. B-3 shows negative control staining
against B-2, using rabbit normal serum. The nuclei and nucleoli of
cancer cells are stained strongly, but the nuclei of follicular
epithelial cells are not stained (scale bars represent 50
μm). (C) HER2 IHC staining of papillary carcinomas. HER2
expression was divided into four depending on staining intensity:
score 0 (C-1), no reaction; score 1 (C-2), barely visible (at high
magnification); score 2 (C-3), weak staining (at low
magnification); score 3 (C-4), strong staining (at low
magnification). Lateral and basolateral epithelial cell membranes
were stained strongly with a score of 3 (scale bars represent 50
μm). (D) Representative HER2 FISH images of cells with (D-1,
-2 and -3) and without (D-4) HER2 gene amplification. Red spots
(rhodamine) and green spots (FITC) within nuclei indicate the HER2
gene and CEP17 signals, respectively. |
hTERT expression
hTERT protein was strongly expressed in the nuclei
and nucleoli of cancer cells (Fig.
1B–2). hTERT expression was confirmed not only in cancer cells
but also lymphocytes, especially those in lymph follicles. hTERT
protein was not expressed in normal follicular epithelial cells
(Fig. 1B-1). More than 10% of
tumor cells were considered positive for hTERT expression (Fig. 1B–2). hTERT expression was detected
in all of the follicular carcinomas and in 40 (66%) of the
papillary carcinomas.
HER2 status
When the degree of HER2 staining was scored as 0, 1,
2 or 3 according to the criteria for gastric cancer, basolateral or
lateral cell membranes of papillary carcinoma were strongly stained
with a score of 3 (Fig. 1C–4).
Seventeen of the papillary carcinomas had an IHC score of 2 and 14
had a score of 3 (Table I). One
case of follicular carcinoma had an IHC score of 2, and 2 cases had
a score of 3 (data not shown). Amplification of the HER2 gene was
confirmed by FISH in cells of differentiated thyroid cancer
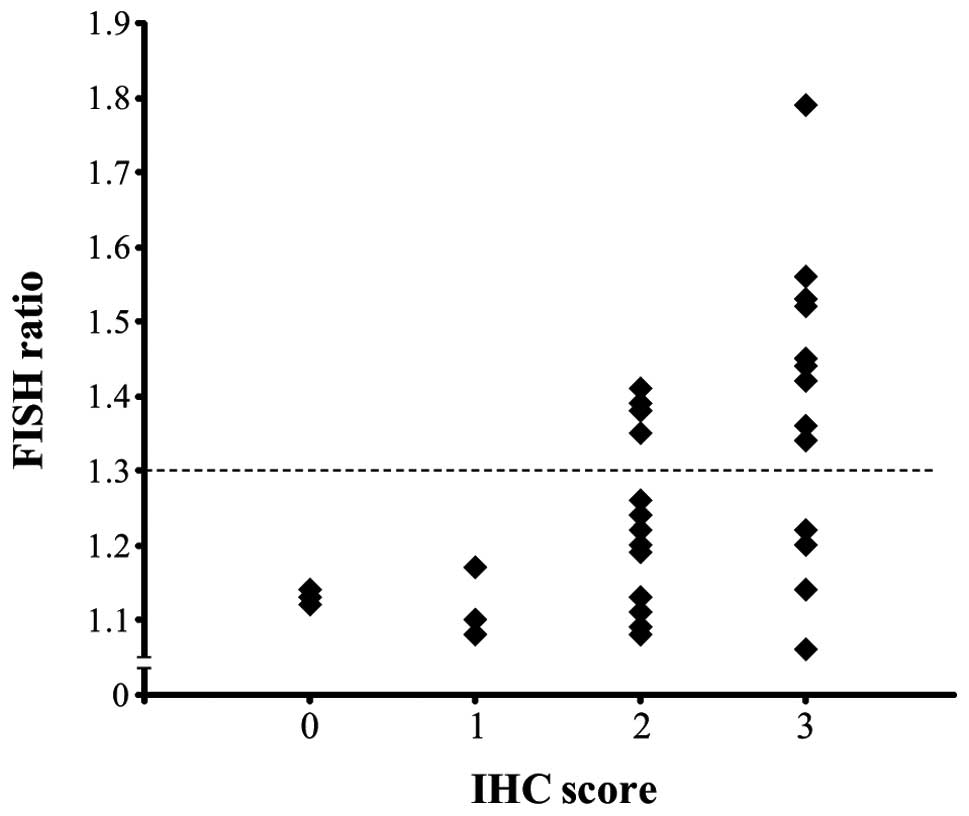
(Fig. 1D-1, -2 and -3); the HER2
FISH ratio was significantly correlated with the IHC score and FISH
ratio (Fig. 3, P<0.001).
Cluster analysis of the FISH ratio separated the cancers into two
groups (showing relative strong and weak amplification), with a
borderline of 1.3 (Fig. 3, dotted
line). Fourteen of the 32 papillary carcinomas with an IHC score of
2 or 3 showed a FISH ratio of ≥1.3 (Table I). One of the 3 follicular
carcinomas with an IHC score of 2 or 3 showed a FISH ratio of ≥1.3
(data not shown). All of the cases with an IHC score of 0 or 1
showed a FISH ratio of <1.3 (Fig.
3, Table I). When the cases
positive and negative for HER2 amplification were compared, a
significant difference in mean TCR was observed between them
(Table II). Telomeres in the group
positive for HER2 amplification were significantly shorter than
those in the negative group (Table
II). No significant differences in HER2 amplification were
observed in terms of age, sex, stage, tumor size and expression of
hTERT (Table II).
 | Table ICorrelation between HER2 expression
and HER2 FISH. |
Table I
Correlation between HER2 expression
and HER2 FISH.
| FISH ratio
|
|---|
| HER2 (protein
expression) | <1.3 (negative,
n=23) | ≥1.3 (positive,
n=14) |
|---|
| 0 | 3 | 0 |
| 1 | 3 | 0 |
| 2 | 13 | 4 |
| 3 | 4 | 10 |
 | Table IICharacteristics of the patients with
papillary thyroid carcinoma. |
Table II
Characteristics of the patients with
papillary thyroid carcinoma.
| HER2 IHC 0-1 or
FISH negative | FISH positive | P-value |
|---|
| Age (average ±
SD) | 51.2±15.0 | 47.4±16.3 | 0.42a |
| Sex | | | 0.83b |
| Male | 10 | 4 | |
| Female | 37 | 10 | |
| TNM stage | | | 0.55b |
| I–III | 32 | 10 | |
| IVA | 15 | 4 | |
| Tumor size (average
± SD) | 21.3±10.2 | 22.8±11.6 | 0.26a |
| TCR (average ±
SD) | 2.6±1.8 | 2.3±1.7 |
9.20×10−10c |
| hTERT
expression | | | 0.06b |
| Negative | 19 | 2 | |
| Positive | 28 | 12 | |
Discussion
Papillary carcinoma is the most common form of
thyroid malignancy. Although it generally exhibits indolent
characteristics and is associated with a favorable prognosis, cases
with certain clinicopathological features can be progressive and
may have a poor outcome (36).
Jonklaas et al reported that the 15-year disease-specific
survival of 3572 patients with papillary thyroid cancer was 91% for
males and 96% for females (37).
In a series of investigated 53,856 patients with thyroid cancer,
Hundahl et al reported that the 10-year overall survival of
those with papillary carcinoma and follicular carcinoma was 93 and
85%, respectively. Thus, follicular carcinoma had a slightly poorer
prognosis (38). In the present
study, therefore, we examined telomere length, hTERT protein
expression and HER2 amplification status in papillary carcinomas
and follicular carcinomas of the thyroid.
Southern blot analysis, which has been widely
employed in previous telomere studies has certain problems:
although it can measure telomere length in whole cancer tissues
including lymphocytes and stem cells, it cannot exclude the
subtelomere components (32). In
the present study, therefore, we employed tissue Q-FISH method for
determination of telomere length. We have developed a much more
accurate Q-FISH-based method that can effectively estimate telomere
length in different cell types using separate PNA probes for
telomeres and centromeres. Tissue Q-FISH is able to measure the
telomere length of cancer cells precisely, and here we obtained the
telomere-centromere ratio (TCR) as a parameter representative of
the telomere length. The centromere is an accurate internal control
parameter, and the TCR has been shown in many previous studies to
accurately reflect telomere length (31,32,39).
Using Southern blot analysis, we have already shown that the
telomere length of differentiated thyroid cancer cells is shorter
than that in adjacent normal tissue (27). In the present study, the mean TCR
of cancer cells was significantly less than that of normal
follicular cells. In addition, the peak frequency of TCR for
papillary carcinomas and follicular carcinomas was 1 to 2 and near
1, respectively. The TCR of normal cells had a wide distribution.
Using tissue Q-FISH, Kammori et al(32) and Kurabayashi et al(39) previously investigated telomere
length in esophageal and breast cancers, respectively. They found
that the TCR distribution was very similar to that in our present
study, the peak TCR frequency being <1 for cancer cells and
>1 for adjacent normal cells, and the TCR for normal cells
showing a wide distribution. The telomeres of follicular carcinoma,
which has poorest prognosis among differentiated thyroid cancers,
were shorter than those of papillary carcinoma. Therefore,
measurement of telomere length using tissue Q-FISH appears to be
useful for assessment of malignant potential. To our knowledge,
this is the first report to have documented the use of tissue
Q-FISH for analysis of telomere length in thyroid cancers.
For detection of hTERT protein, strong staining of
nuclei and nucleoli was demonstrated by IHC. In addition, hTERT
protein was recognized in lymphocytes as well as thyroid cancer
cells. Using in situ hybridization (ISH), Kammori et
al examined the expression of hTERT mRNA in colorectal
carcinomas and follicular thyroid carcinomas, and found a pattern
similar to that in the present study (24,30).
hTERT protein was detected in 66% and 100% of papillary and
follicular carcinomas, respectively. In a previous study, we
examined the telomerase activity of thyroid cancer using TRAP, and
found that papillary carcinomas and follicular carcinomas were
positive in 87.5 and 100% of cases, respectively (27). These results suggested that the
expression of hTERT protein was related to the malignant potential
of thyroid cancers.
There are two established techniques for monitoring
HER2 status: gene amplification detected by fluorescence in
situ hybridization (FISH) and protein overepression detected by
IHC. Early studies indicated that there was a close correlation
between the data obtained by these two methods. In a series of 2279
patients with breast cancer, Lal et al found that FISH data
were correlated with an IHC score of 0, 1 or 3, whereas only 25% of
cases with an IHC score of 2 demonstrated gene amplification by
FISH (40). On the other hand,
Kammori et al have reported that detection of HER2 protein
expression using IHC was not satisfactory for evaluation of HER2
status in breast cancer, because a number of cases showing
discrepancy between IHC and FISH data were found (41). The present study revealed a
significant correlation between IHC score and the FISH ratio. As
the IHC staining characteristics of thyroid cancer were very
similar to those of gastric cancer (lateral and basolateral
epithelial cell membranes being strongly stained), we used the IHC
scoring criteria for gastric cancer in this study. In cases of
breast and gastric cancer, HER2 amplification is considered
positive when FISH demonstrated a ratio of ≥2.0, but none of our
present specimens of thyroid cancer attained this value. However,
FISH would theoretically show a ratio of 1.0 for normal cells
showing no HER2 gene amplification. Despite the low frequency of
HER2 gene amplification, it is thought to be certainly present in
thyroid cancer cells. When we performed cluster analysis of the
HER2 FISH ratio, we were able to divide the cases into two groups,
one showing strong amplification (FISH ratio ≥1.3) and the other
showing weak amplification (FISH ratio <1.3); 23% of papillary
carcinomas showed strong amplification. The HER2 positivity rate
has been reported to be around 25% in breast cancers and 15–25% in
gastric cancers (3,4,6–9), and
that for thyroid cancer was considered to be proportional to FISH
ratio of 1.3. HER2-positive cases of breast and gastric cancer are
known to have a poor prognosis (3–5,7,9–14).
Positivity for HER2 gene amplification was considered to be an
indicator of poor prognosis in thyroid cancer, because papillary
carcinomas with a HER2:FISH ratio of ≥1.3 had short telomeres and
high malignant potential. Although some studies have tried to
detect gene amplification and/or protein overexpression of HRE2 in
thyroid cancer, they did not involve detailed examination of the
FISH ratio (42–46). To our knowledge, this is the first
reported study to have examined the HER2 status of thyroid cancer
in detail.
Thyroid cancer generally exhibits indolent behavior
and has a good prognosis (36–39).
Many previous studies have suggested that short telomeres in cancer
tissues are a negative prognostic indicator (47–52).
In the present study, papillary carcinomas, which have a relatively
good prognosis, maintained their telomere lengths and had a low
rate of positivity for hTERT protein in comparison with follicular
carcinomas, suggesting that measurement of telomere length by
tissue Q-FISH and expression of hTERT protein using IHC would be
useful for indicating the malignant potential of thyroid cancer. It
was also suggested that HER2 status might have an important
prognostic impact in thyroid cancer, as is the case for breast
cancer and gastric cancer, because in the present series, cancers
with marked HER2 gene amplification had short telomeres. When we
applied the HER2 amplification criteria for breast and gastric
cancer strictly to thyroid cancer, all of cases examined were
judged as negative. However, the low frequency of HER2 gene
amplification may not be surprising when considering that thyroid
cancer exhibits indolent characteristics and very slow growth.
Currently, there is no effective chemotherapy for
differentiated thyroid cancer, and radiation therapy using 131I is
common in a postoperative adjuvant setting. If HER2 positivity can
be detected in thyroid cancer, it may be possible to try
alternative therapies such as trastuzumab for patients with
intractable thyroid cancer that does not respond to radiation
therapy.
In summary, the present study has identified
amplification of the HER2 gene and/or overexpression of HER2
protein in approximately 23% of human papillary thyroid carcinomas,
and shown that the telomeres of HER2-positive cancers are shorter
than those of negative cancers. These results suggest that highly
malignant thyroid cancer can be detected by monitoring HER2 status
and telomere length, and that trastuzumab therapy may be effective
for patients with advanced thyroid cancer.
Acknowledgements
We would like to thank Dr Mitsuyoshi
Hirokawa (Kuma Hospital) for pathological diagnosis, and Miyoko
Matsumoto (Kanaji Hospital) for assistance with preparation of the
manuscript.
References
|
1.
|
Hynes NE and Stern DF: The biology of
erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta.
1198:165–184. 1994.PubMed/NCBI
|
|
2.
|
Akiyama T, Sudo C, Ogawara H, Toyoshima K
and Yamamoto T: The product of the human c-erbB-2 gene: a
185-kilodalton glycoprotein with tyrosine kinase activity. Science.
232:1644–1646. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Salmon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Salmon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A and
Press MF: Studies of the HER-2/neu proto-oncogene in human breast
and ovarian cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ross JS and Fletcher JA: The HER-2/neu
oncogene in breast cancer: prognostic factor, predictive factor,
and target for therapy. Oncologist. 3:237–252. 1998.
|
|
6.
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Park DI, Yun JW, Park JH, Oh SJ, Kim HJ,
Cho YK, Shon CI, Jeon WK, Kim BI, Yoo CH, Son BH, Cho EY, Chae SW,
Kim EJ, Shon JH, Ryu SH and Sepulveda AR: HER-2/neu amplification
is an independent prognostic factor in gastric cancer. Dig Dis Sci.
51:1371–1379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yano T, Doi T, Ohtsu A, Boku N, Hashizume
K, Nakanishi M and Ochiai A: Comparison of HER2 gene amplification
assessed by fluorescence in situ hybridization and HER2
protein expression assessed by immunohistochemistry in gastric
cancer. Oncol Rep. 15:65–71. 2006.PubMed/NCBI
|
|
9.
|
Zhag XL, Yang YS, Xu DP, Qu JH, Guo MZ,
Gong Y and Huang J: Comparative study on overexpression of her2/neu
and her3 in gastric cancer. World J Surg. 33:2112–2118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Uchino S, Tsuda H, Maruyama K, Kinoshita
T, Sasako M, Saito T, Kobayashi M and Hirohashi S: Overexpression
of c-erbB-2 protein in gastric cancer. Its correlation with
long-term survival of patients. Cancer. 72:3179–3184. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nakajima M, Sawada H, Yamada Y, Watanabe
A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirano T and
Nakano H: The prognostic significance of amplification and
overexpression of c-met and c-erb B-2 in human gastric carcinomas.
Cancer. 85:1894–1902. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Allgayer H, Babic R, Gruetzner KU,
Tarabichi A, Schildberg FW and Heiss MM: c-erbB-2 is of independent
prognostic relevance in gastric cancer and is associated with the
expression of tumor-associated protease systems. J Clin Oncol.
18:2201–2209. 2000.PubMed/NCBI
|
|
13.
|
Garcia I, Vizoso F, Martin A, Sanz L,
Abdel-Lah O, Raigoso P and García-Muñiz JL: Clinical significance
of the epidermal growth factor receptor and HER2 receptor in
resectable gastric cancer. Ann Surg Oncol. 10:234–241. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tanner M, Hollmén M, Junttila TT, Kapanen
AI, Tommola S, Soni Y, Helin H, Salo J, Joensuu H, Sihvo E, Elenius
K and Isola J: Amplification of HER-2 in gastric carcinoma:
association with topoisomerase IIalpha gene amplification,
intestinal type, poor prognosis and sensitivity to trastuzumab. Ann
Oncol. 16:273–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J and Kang YK; ToGA
Trial Investigators: Trastuzumab in combination with chemotherapy
versus chemotherapy alone for treatment of HER2-positive advanced
gastric or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
16.
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Von Zglinicki T: Oxidative stress shortens
telomeres. Trends Biochem Sci. 27:339–344. 2002.PubMed/NCBI
|
|
19.
|
Harly CB and Villeponteau B: Telomeres and
telomerase in aging and cancer. Curr Opin Genet Dev. 5:249–255.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
De Lange T: Telomeres and senescence:
ending the debate. Science. 279:334–335. 1998.PubMed/NCBI
|
|
21.
|
DePinho RA: The age of cancer. Nature.
408:248–254. 2000. View
Article : Google Scholar
|
|
22.
|
Svenson U and Roos G: Telomere length as a
biological marker in malignancy. Biochim Biophys Acta.
1792:317–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wyatt HD, West SC and Beattie TL:
InTERTpreting telomerase structure and function. Nucleic Acids Res.
38:5609–5622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kammori M, Nakamura K, Hashimoto M, Ogawa
T, Kaminishi M and Takubo K: Clinical application of human
telomerase reverse transcriptase gene expression in thyroid
follicular tumors by fine-needle aspirations using in situ
hybridization. Int J Oncol. 22:985–991. 2003.PubMed/NCBI
|
|
25.
|
Furugori E, Hirayama R, Nakamura K,
Kammori M, Esaki Y and Takubo K: Telomere shortening in gastric
carcinoma with aging despite telomerase activation. J Cancer Res
Clin Oncol. 126:481–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Nakamura K, Furugori E, Esaki Y, Arai T,
Sawabe M, Okayasu I, Fujiwara M, Kammori M, Mafune K, Kato M,
Oshimura M, Sasajima K and Takubo K: Correlation of telomere
lengths in normal and cancers tissue in the large bowel. Cancer
Lett. 158:179–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kammori M, Takubo K, Nakamura K, Furugouri
E, Endo H, Kanauchi H, Mimura Y and Kaminishi M: Telomerase
activity and telomere length in benign and malignant human thyroid
tissues. Cancer Lett. 159:175–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
O’Sullivan JN, Bronner MP, Brentnall TA,
Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA, Moskovitz AH,
Crispin DA, Potter JD and Rabinovitch PS: Chromosomal instability
in ulcerative colitis is related to telomere shortening. Nat Genet.
32:280–284. 2002.PubMed/NCBI
|
|
29.
|
Meeker AK, Gage WR, Hicks JL, Simon I,
Coffman JR, Platz EA, March GE and De Marzo AM: Telomere length
assessment in human archival tissues: combined telomere
fluorescence in situ hybridization and immunostaining. Am J Pathol.
160:1259–1268. 2002. View Article : Google Scholar
|
|
30.
|
Kammori M, Kanauchi H, Nakamura K,
Kawahara M, Weber TK, Mafune K, Kaminishi M and Takubo K:
Demonstration of human telomerase reverse transcriptase in human
colorectal carcinomas by in situ hybridization. Int J Oncol.
20:15–21. 2002.PubMed/NCBI
|
|
31.
|
Aida J, Izumiyama-Shimomura N, Nakamura K,
Ishii A, Ishikawa N, Honma N, Kurabayashi R, Kammori M, Poon SS,
Arai T and Takubo K: Telomere length variations in 6 mucosal cell
types of gastric tissue observed using a novel quantitative
fluorescence in situ hybridization method. Hum Pathol.
38:1192–1200. 2007. View Article : Google Scholar
|
|
32.
|
Kammori M, Izumiyama N, Nakamura K,
Kurabayashi R, Kashio M, Aida J, Poon SS and Kaminishi M: Telomere
metabolism and diagnostic demonstration of telomere measurement in
the human esophagus for distinguishing benign from malignant tissue
by tissue quantitative fluorescence in situ hybridization.
Oncology. 71:430–436. 2006. View Article : Google Scholar
|
|
33.
|
Kawauchi K, Ihjima K and Yamada O: IL-2
increases human telomerase reverse transcriptase activity
transcriptionally and posttranslationally through
phosphatidylinositol 3′-kinase/Akt, heat shock protein 90, and
mammalian target of rapamycin in transformed NK cells. J Immunol.
174:5261–5269. 2005.PubMed/NCBI
|
|
34.
|
Rüschoff J, Dietel M, Baretton G, Arbogast
S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I,
Schlake W, Höfler H and Kreipe HH: HER2 diagnostics in gastric
cancer-guideline validation and development of standardized
immunohistochemical testing. Vichows Arch. 457:299–307.
2010.PubMed/NCBI
|
|
35.
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A,
Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler
TM and Hayes DF: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal
growth factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
36.
|
Ito Y, Fukushima M, Kihara M, Takamura Y,
Kobayashi K, Miya A and Miyauchi A: Investigation of the prognosis
of patients with papillary thyroid carcinoma by tumor size. Endocr
J. 59:457–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Jonklaas J, Nogueras-Gonzalez G, Munsell
M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR,
Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL,
Maxon HR and Sherman SI: The impact of age and gender on papillary
thyroid cancer survival. J Clin Endocrinol Metab. 97:E878–E887.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A national cancer data base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995. Cancer.
83:2638–2648. 1998.PubMed/NCBI
|
|
39.
|
Kurabayashi R, Takubo K, Aida J, Honma N,
Poon SS, Kammori M, Izumiyama-Shimomura N, Nakamura K, Tsuji E,
Matsuura M, Ogawa T and Kaminishi M: Luminal and cancer cells in
the breast show more rapid telomere shortening than myoepithelial
cells and fibroblasts. Hum Pathol. 39:1647–1655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Lal P, Salazar PA, Hudis CA, Ladanyi M and
Chen B: HER-2 testing in breast cancer using immunohistochemical
analysis and fluorescence in situ hybridization: a
single-institution experience of 2,279 cases and comparison of
dual-color and single-color scoring. Am J Clin Pathol. 121:631–636.
2004. View Article : Google Scholar
|
|
41.
|
Kammori M, Kurabayashi R, Kashio M,
Sakamoto A, Yoshimoto M, Amano S, Kaminishi M, Yamada T and Takubo
K: Prognostic utility of fluorescence in situ hybridization
for determining HER2 gene amplification in breast cancer. Oncol
Rep. 19:651–656. 2008.
|
|
42.
|
Kremser R, Obrist P, Spizzo G, Erler H,
Kendler D, Kemmler G, Mikuz G and Ensinger C: Her2/neu
overexpression in differentiated thyroid carcinomas predicts
metastatic disease. Virchows Arch. 442:322–328. 2003.PubMed/NCBI
|
|
43.
|
Mondi MM, Rich R, Ituarte P, Wong M,
Bergman S, Clark OH and Perrier ND: HER2 expression in thyroid
tumors. Am Surg. 69:1100–1103. 2003.PubMed/NCBI
|
|
44.
|
Ensinger C, Prommegger R, Kendler D,
Gabriel M, Spizzo G, Mikuz G and Kremser R: Her2/neu expression in
poorly-differentiated and anaplastic thyroid carcinomas. Anticancer
Res. 23:2349–2354. 2003.PubMed/NCBI
|
|
45.
|
Freudenberg LS, Sheu S, Görges R, Mann K,
Bokler S, Frilling A, Schmid KW, Bockisch A and Otterbach F:
Prognostic value of c-erbB-2 expression in papillary thyroid
carcinoma. Nuklearmedizin. 44:179–184. 2005.PubMed/NCBI
|
|
46.
|
Mitteldorf CA, Leite KR, Meirelles MI,
Gattas GJ and Camara-Lopes LH: Overexpression of HER2/neu
oncoprotein in cytologic specimens. Acta Cytol. 48:199–206. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Griffith JK, Bryant JE, Fordyce CA,
Gilliland FD, Joste NE and Moyzis RK: Reduced telomere DNA content
is correlated with genomic instability and metastasis in invasive
human breast carcinoma. Breast Cancer Res Treat. 54:59–64. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Fordyce CA, Heaphy CM, Bisoffi M, Wyaco
JL, Joste NE, Mangalik A, Baumgartner KB, Baumgartner RN, Hunt WC
and Griffith JK: Telomere content correlates with stage and
prognosis in breast cancer. Breast Cancer Res Treat. 99:193–202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Heaphy CM, Baumgartner KB, Bisoffi M,
Baumgartner RN and Griffith JK: Telomere DNA content predicts
breast cancer-free survival interval. Clin Cancer Res.
13:7037–7043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Donaldson L, Fordyce C, Gilliland F, Smith
A, Feddersen R, Joste N, Moyzis R and Griffith J: Association
between outcome and telomere DNA content in prostate cancer. J
Urol. 162:1788–1792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Fordyce CA, Heaphy CM, Joste NE, Smith AY,
Hunt WC and Griffith JK: Association between cancer-free survival
and telomere DNA content in prostate tumors. J Urol. 173:610–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Frías C, García-Aranda C, De Juan C, Morán
A, Ortega P, Gómez A, Hernando F, López-Asenjo JA, Torres AJ,
Benito M and Iniesta P: Telomere shortening is associated with poor
prognosis and telomerase activity correlates with DNA repair
impairment in non-small cell lung cancer. Lung Cancer. 60:416–425.
2008.PubMed/NCBI
|