Introduction
Since endometrial carcinoma is the most frequently
diagnosed gynecologic pathology, studies aimed at understanding the
molecular mechanisms that underlie the onset and progression of
endometrial transformation, should be considered of the greatest
importance. Endometrial tissue represents a very highly interactive
system involving different cell types, which relies on a complex
network of intercellular and intracellular signalling in which cell
cycle proteins, estrogens, cytokines and growth factors play
relevant roles (1,2). Therefore, this system appears
particularly suitable for solving the puzzling relationships
between mitosis and timed programmed cell death as suggested by the
very latest scientific observations. The clarification of such
interactions should consider all factors that contribute to a
correct and balanced cell cycle progression. In this regard, the
specific function of hormones, growth factors, adhesion molecules
and apoptotic factors which play important roles in endometrial
biology still needs to be clarified.
Clusterin (CLU) is a glycoprotein with a nearly
ubiquitous tissue distribution and involvement in biological
processes ranging from neurodegeneration in Alzheimer’s disease to
cancer initiation and progression (3,4).
Indeed, CLU has been implicated in numerous physiologic and
pathologic processes important for carcinogenesis and tumor growth,
including apoptotic cell death, cell cycle regulation, DNA repair,
cell adhesion, tissue remodelling, lipid transportation, membrane
recycling and immune system regulation (3,4).
Unravelling the functions of CLU has been an elusive goal: as cited
above, it has been ascribed to many, and sometimes contradictory,
processes. Part of this ambiguity results from the existence of two
functionally divergent isoforms: a glycosylated secreted
heterodimer of approximately 80 kDa, sCLU and a 55 kDa
non-glycosylated nuclear form, nCLU. The sCLU isoform is the
full-length secreted heterodimer with documented anti-apoptotic
function. The shorter nCLU isoform is synthesized from an
alternative transcription start site, does not undergo cleavage or
extensive glycosylation, is reported to translocate from the
cytoplasm to the nucleus following cytotoxic events and postulated
to induce apoptosis (4,5). While accumulating data identify
mature sCLU as an inhibitor of apoptosis, precise site(s) of action
and binding proteins remain poorly defined. Likewise, the mature
sCLU has a cytoprotective function, while under certain conditions
pro-apoptotic signals may induce the expression of the nCLU isoform
(6,7). A third transcript, called isoform
11036, has been recently characterized in colon cancer, but up to
now its functional role remains unclear (8).
Previous studies, not taking into account specific
isoform expression, have shown an increased expression of CLU in
several human tumour tissues such as prostate, breast, renal,
ovarian, colon, cervix, lung and anaplastic large cell lymphoma
(9–16), where either abnormal cell death or
proliferation occur (17).
Upregulation of CLU mRNAs and proteins are widespread phenomena in
developmental and patho-physiological states, suggesting that the
control of its expression levels is of pivotal importance. CLU
expression increases in surviving cells following a variety of
stressors, including cytotoxic chemotherapy, radiation, her2/neu
blockade and androgen or estrogens withdrawal in hormone-dependent
tumours, presumably as a pro-survival response (18). These findings further demonstrate
that CLU is a cell survival gene upregulated by apoptotic triggers,
and confers resistance when overexpressed, thereby identifying CLU
as a therapeutic target for cancer (19). Recent experimental evidence seems
to indicate that a balance between sCLU:nCLU in the cell dictates
its fate and is critical for cancer survival and progression.
Indeed, in preclinical models of prostate cancer, the sCLU
antisense oligonucleotide improved the efficacy of chemotherapy,
radiation and androgen withdrawal by inhibiting expression of CLU
and enhancing the apoptotic response (20). In addition, preclinical activity
has been reported in lung, renal cell, cervical and breast cancers
(9,10,12,15).
On this basis, investigating the CLU expression and function in
human endometrial cancer could provide a novel therapeutic target
for this malignancy.
Even though previous papers demonstrated that CLU is
an estrogen-regulated gene in rat endometrial adenocarcinoma cells
(21), whether a similar
regulation occurs also in human endometrium still remains to be
investigated. In this light, CLU may represent a target gene that
can be used to monitor changes that are responsible for the
progression from normal to malignant endometrial tissue. To
investigate the involvement of CLU in the modulation of cell
proliferation, apoptosis and clinical outcome in endometrial
cancer, we used an in vivo experimental system consisting of
endometrial tissues, already characterized in our laboratory during
recent years (22–36). We established here for the first
time the differential expression of CLU isoforms in both
physiological and pathological, hyperplastic and neoplastic human
endometrial tissues.
A better understanding of the biological mechanisms
responsible for the uncontrolled growth of hormonally regulated
cancers such as endometrial carcinoma, is critical to devise novel
effective diagnostic and/or prognostic biomarkers such as CLU,
which can be potentially used as ‘signatures’ to predict the
clinical transformation of the disease in its different progressive
phases.
Moreover, since the suppression of the sCLU isoform
expression renders human cancer cells sensitive to chemotherapeutic
drug-mediated apoptosis, and it is currently an antisense target in
clinical trials for prostate cancer, the long-term objective of our
studies is to state CLU as a new targeted modulator of endometrial
cell proliferation potentially useful to generate rationally
designed drugs based on tissue specific expression, in order to
control cancer cell proliferation.
Materials and methods
Patients
This study was carried out using 64 endometrial
tissue specimens from patients (Table
I) submitted to laparotomic hysterectomy at the Department of
Gynecology, Obstetrics and Neonatology of the University of Bari
(Italy). Informed consent was obtained for all patients before the
surgery as approved by the Internal Review Board. Samples were
collected immediately after surgical resection and stored at −80°C
until use.
 | Table IClinical features of patients
analyzed. |
Table I
Clinical features of patients
analyzed.
| Patients | Age (years) | Post menopausal age
(years) | Histology | Grade | Stage | Assays |
|---|
| N1 | 41 | - | Normal
proliferative endometrium | - | - | RT-qPCR, IH, |
| N2 | 54 | - | Normal
proliferative endometrium | - | - | NB, RT-qPCR |
| N3 | 47 | - | Normal
proliferative endometrium | - | - | NB, RT-qPCR, IH,
WB |
| N4 | 44 | - | Normal
proliferative endometrium | - | - | NB, RT-qPCR, IH,
WB |
| N5 | 43 | - | Normal
proliferative endometrium | - | - | NB, WB |
| N6 | 44 | - | Normal
proliferative endometrium | - | - | NB, RT-qPCR |
| N7 | 44 | - | Normal
proliferative endometrium | - | - | IH |
| N8 | 43 | - | Normal
proliferative endometrium | - | - | NB, IH |
| N9 | 46 | - | Normal
proliferative endometrium | - | - | NB, IH, R |
| N10 | 47 | - | Normal
proliferative endometrium | - | - | NB, WB, R |
| N11 | 43 | - | Normal
proliferative endometrium | - | - | NB, WB |
| S1 | 50 | - | Secretive
endometrium | - | - | NB, IH, R |
| S2 | - | - | Secretive
endometrium | - | - | NB, RT-qPCR, R |
| S3 | 45 | - | Secretive
endometrium | - | - | NB, RT-qPCR, IH,
WB |
| S4 | - | - | Secretive
endometrium | - | - | NB, RT-qPCR, IH,
R |
| S5 | 50 | - | Secretive
endometrium | - | - | NB, RT-qPCR,
WB |
| S6 | 45 | - | Secretive
endometrium | - | - | NB, IH, WB |
| S7 | 52 | - | Secretive
endometrium | - | - | NB, RT-qPCR |
| S8 | 44 | - | Secretive
endometrium | - | - | NB, IH |
| S9 | 47 | - | Secretive
endometrium | - | - | NB, WB |
| A1 | 59 | 7 | Atrophic
endometrium | - | - | NB, RT-qPCR,
IH |
| A2 | 52 | 3 | Atrophic
endometrium | - | - | NB, IH, WB |
| A3 | 56 | 6 | Atrophic
endometrium | - | - | NB, RT-qPCR, IH,
WB |
| A4 | 51 | 4 | Atrophic
endometrium | - | - | NB, RT-qPCR, IH,
WB |
| A5 | 50 | - | Atrophic
endometrium | - | - | NB, RT-qPCR, IH,
R |
| A6 | 72 | 17 | Atrophic
endometrium | - | - | RT-qPCR, IH, R |
|
| H1 | 50 | - | Simple
hyperplasia | - | - | NB,RT-qPCR, IH |
| H2 | 56 | 1 | Simple
hyperplasia | - | - | NB, RT-qPCR |
| H3 | 62 | 12 | Simple
hyperplasia | - | - | NB, RT-qPCR |
| H4 | 72 | 22 | Simple
hyperplasia | - | - | NB, RT-qPCR |
| H5 | 45 | - | Simple
hyperplasia | - | - | NB, R |
| H6 | 54 | 9 | Simple
hyperplasia | - | - | NB, IH |
| H7 | 51 | - | Simple
hyperplasia | - | - | NB, IH |
| H8 | 43 | - | Simple
hyperplasia | - | - | NB, RT-qPCR |
| H9 | 35 | - | Simple
hyperplasia | - | - | NB, R, WB |
| H10 | 52 | 2 | Simple
hyperplasia | - | - | NB, IH |
| H11 | 50 | - | Simple
hyperplasia | - | - | NB, R |
| H12 | 60 | 4 months | Simple
hyperplasia | - | - | IH, WB |
| H13 | 50 | - | Simple
hyperplasia | - | - | IH |
| AH1 | 49 | - | Atypical
hyperplasia | - | - | NB, IH |
| AH2 | 62 | 12 | Atypical
hyperplasia | - | - | NB, RT-qPCR |
| AH3 | 55 | 2 | Atypical
hyperplasia | - | - | NB, RT-qPCR |
| AH4 | 76 | 26 | Atypical
hyperplasia | - | - | NB, RT-qPCR |
| AH5 | 83 | 33 | Atypical
hyperplasia | - | - | NB, RT-qPCR |
| AH6 | 61 | 4 | Atypical
hyperplasia | - | - | IH |
| AH7 | 71 | 31 | Atypical
hyperplasia | - | - | IH |
| AH8 | 49 | - | Atypical
hyperplasia | - | - | IH |
| K1 | 85 | 34 | Mucinous
adenocarcinoma | 1 | 1b | NB, RT-qPCR |
| K2 | 65 | 11 | Endometrioid
adenocarcinoma | 1 | 1c | NB, RT-qPCR,
WB |
| K3 | 65 | 10 | Endometrioid
adenocarcinoma | 1 | 1c | NB, RT-qPCR, WB,
R |
| K4 | 67 | 18 | Endometrioid
adenocarcinoma | 1 | 1c | NB, RT-qPCR |
| K5 | 73 | 31 | Endometrioid
adenocarcinoma | 1 | 1c | NB, RT-qPCR, IH,
WB |
| K6 | 65 | 25 | Endometrioid
adenocarcinoma | 1 | 4b | NB, RT-qPCR,
WB |
| K7 | 68 | 12 | Endometrioid
adenocarcinoma | 1 | 1b | NB, WB, R |
| K8 | 72 | 22 | Clear cell
carcinoma | 1 | 1b | NB, RT-qPCR,
IH |
| K9 | 50 | - | Endometrioid
adenocarcinoma | 1 | 1b | IH, R |
| K10 | 69 | 17 | Endometrioid
adenocarcinoma | 2 | 1c | NB, IH |
| K11 | 51 | 3 | Endometrioid
adenocarcinoma | 2 | 1c | NB, IH, WB |
| K12 | 74 | 22 | Endometrioid
adenocarcinoma | 2 | 1c | NB, RT-qPCR |
| K13 | 65 | 15 | Endometrioid
adenocarcinoma | 2 | 1b | NB, IH, R |
| K14 | 59 | 6 | Endometrioid
adenocarcinoma | 2 | 3a | IH, WB |
| K15 | 57 | 9 | Serous
adenocarcinoma | 3 | 1c | NB, RT-qPCR,
WB |
| K16 | 76 | 22 | Endometrioid
adenocarcinoma | 3 | 1c | NB, IH |
| K17 | 74 | 14 | Endometrioid
adenocarcinoma | 3 | 2a | NB, IH, WB |
Two subgroups of patients were identified: the first
included 26 patients with normal endometria who underwent
hysterectomy for different pathologic conditions not involving the
endometrium, such as uterine prolapse, ovarian cysts or subserosal
leiomyomata. Based on histological features, these were
characterized as: normal proliferative endometria (N1–N11; median
age at surgery: 45±3 years), secretive endometria (S1–S9; median
age at surgery: 48±3 years) and atrophic endometria (A1–A6; median
age at surgery: 57±8 years).
The second subgroup included 21 patients affected by
histologically identified endometrial hyperplasia. Specifically,
there were 13 simple hyperplasias (H1–H13) and 8 complex
hyperplasias with cytological atypia (AH1–AH8). The median age of
patients with simple hyperplastic disease (H1–H13) at surgery was
52±9 years and for the patients affected by hyperplasia with
cytological atypia (AH1–AH8) was 63±12 years.
The remaining patients included histologically
proven endometrial proliferative diseases, whose stage and grade
were assessed according to the International Federation of
Gynecology and Obstetrics (FIGO) (37). Overall, 17 patients were affected
by endometrial adenocarcinoma (K1–K17; median age at surgery: 67±15
years) that were classified as follows: 14 endometrioid
adenocarcinomas (7 well differentiated, G1; 5 moderately, G2; and 2
poorly differentiated, G3), 1 serous carcinoma (G3), 1 mucinous
carcinoma (G1) and 1 clear cell carcinoma (G1). None of the
patients received any treatment (radiotherapy, chemotherapy or
hormone therapy) before surgery.
RNA extraction and northern blot
analysis
Frozen tissue samples were pulverized and cellular
RNA was extracted using the guanidinium isothiocyanate-cesium
chloride procedure as previously described (22). Quantity and purity of nucleic acid
preparations were determined using a NanoDrop ND-1000 UV-VIS
spectrophotometer (Thermo Scientific, Wilmington, DE, USA). RNA
quality was assessed with a BioAnalyzer 2100 (Agilent Technologies,
Böblingen, Germany). Total RNA (25 μg) isolated from the
tissues was electrophoresed through 1% denaturing agarose gel
containing 660 mmol/l formaldehyde, and transferred to a nylon
membrane (Hybond N+; Amersham, Milan, Italy), and
northern blot analysis of CLU mRNA was performed as previously
described (22). Radiolabeled
probes were generated using the Megaprime DNA labeling kit
(Amersham), 100 μCi of α-[32P]-dcTP (3,000
Ci/mmol, Amersham) and 25 ng of double-stranded 1,649 bp specific
fragment encoding for the full-length human CLU cDNA (7) (nucleotides 52–1700 actually
corresponding to NM_001831, GenBank).
The filters were prehybridized overnight at 42°C
with a buffer consisting of 50% formamide, 5X Denhardt’s solution
(1% Ficoll 400, 1% polyvinylpyrrolidone, 1% bovine serum albumin),
5X sodium chloride/sodium phosphate/ethylene-diaminetetraacetic
acid (SSPE) (3 mol/l NaCl, 200 mmol/l
Na2H2PO4, pH 7.0, 19 mmol/l
ethylenediaminetetraacetic acid), 0.5% sodium dodecyl sulphate
(SDS) and 100 mg/ml of sonicated salmon sperm DNA. The filters were
then hybridized for 20 h at 42°C by adding 3×106 cpm of
[32P]-labeled probe/ml to the prehybridization solution.
Subsequently the filters were washed once with 2X SSPE, 0.1% SDS
for 10 min at room temperature, once with 1X SSPE, 0.1% SDS at
42°C, followed by several washes up to 0.1X SSPE, 0.1% SDS at 65°C
and, finally, exposed overnight or longer to Kodak X-Omat AR 5 film
(Kodak, Rochester, NY, USA) at −80°C.
Quantitative analysis was performed by densitometric
scanning of the autoradiographs using a Bio-Rad GS-700 Imaging
Densitometer (Bio-Rad, Richmond, CA, USA); multiple exposures of
the same northern blots in a linear range were performed. mRNA
levels were normalized by using the 28S ribosomal RNA, a
constitutively expressed gene (38). For this purpose, blots were
stripped in 0.1% boiling SDS and re-probed with the radiolabeled
[32P]-28S cDNA probe. The ratio between the CLU mRNA
levels and the 28S rRNA levels was calculated for each sample to
take into account for differences in RNA loading. CLU mRNA levels
in secretive, atrophic, neoplastic and hyperplastic endometria were
calculated as percentage of normal proliferative endometria mRNA
average levels, hybridized on the same filter and settled at 100
(arbitrary unit). The variability of CLU mRNA levels in
proliferative endometrial tissue specimens, measured by comparing
the percentage of mRNA level from different normal endometrial
tissues present on the same filter, was always less than 10% in the
different measurements. For each specimen, the mean value (± SEM)
of results obtained in at least three experiments was
calculated.
HL60 cells and human liver
One sample of histologically proven normal human
liver, obtained during cholecystectomy, was also included in this
study and submitted to RNA extraction to use as positive control in
the northern hybridization experiments (28).
Human promyelocytic leukemia HL60 cells were grown
in RPMI-1640 (Gibco, Life Technologies, Milan, Italy), with 50
mg/ml gentamicin, 2 mmol/l glutamine and 15% inactivated fetal calf
serum, at 37°C in presence of 5% CO2. Total RNA from
differentiated HL60 cells was extracted 24 h after incubation with
160 nmol/l TPA (or PMA phorbol-12-myristate-13-acetate; Sigma) and
used as negative control in the northern hybridization experiments
(29).
RT-qPCR experiments
Total RNA from human endometrial tissues was
extracted as described in the section RNA extraction and northern
blot analysis. Parameters of total RNA were assessed as follows:
quantification of RNA was carried out using NanoDrop ND-1000
(Thermo Scientific) spectrophotometer; the quality of RNA was
evaluated on Agilent 2100 Bioanalyzer (Agilent Technologies, Santa
Clara, CA, USA) using an RNA 6000 Nano chip kit, RNA ladder and
Agilent analysis software (Agilent Technologies).
cDNA synthesis was performed from 1 μg of
total RNA using QuantiTect® Reverse Transcription kit
(Qiagen). Variant-specific PCRs, using the cDNAs as templates, were
performed using the GoTaq® Flexi DNA Polymerase
(Promega). Variant-specific primer pairs were constructed with
Primer3 software, combining specific forward primers located in the
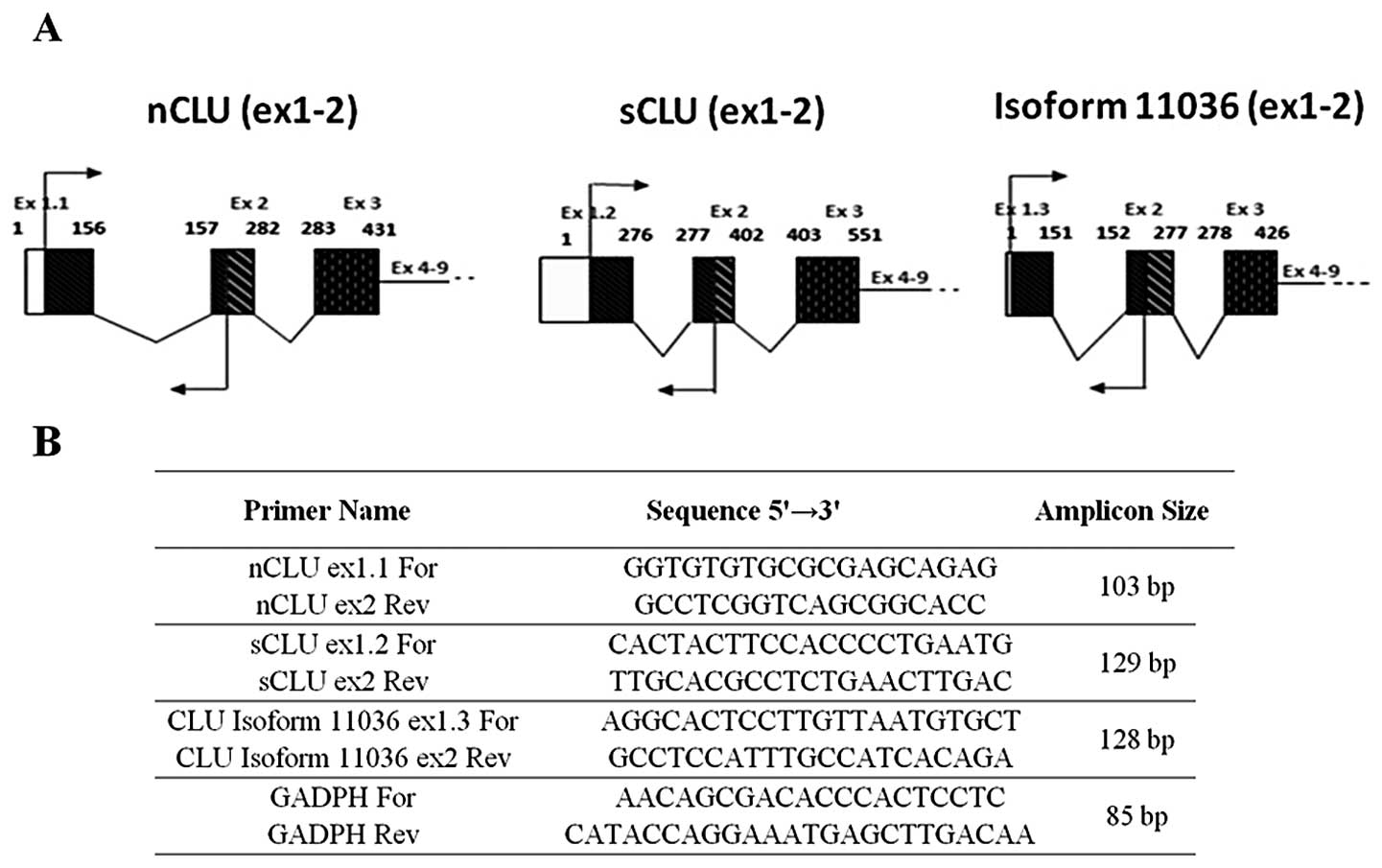
unique exons 1 (1a, 1b or 1c) and the reverse primer located in
exon 2 (Fig. 1A). The sequence of
primers are listed in Fig. 1B.
A total of 1 μl of each cDNA was used as
template in qPCR assays, performed in triplicate on ABI PRISM
7900HT (Applied Biosystems) using the QuantiTect®
SYBR-Green PCR Master mix (Qiagen). Amplification parameters were
as follows: hot start at 95°C for 15 min; 50 cycles of
amplification (94°C for 30 sec, 64°C for 30 sec, 72°C for 40 sec);
dissociation curve step (95°C for 15 sec, 60°C for 15 sec, 95°C for
15 sec). Fluorescence raw data were exported from the SDS 2.2.1
software (Applied Biosystems) and analyzed with the DART-PCR Excel
workbook (39). For each tissue,
the relative expression ratio (rER) of different CLU transcripts
was calculated applying the following formula: [(1 +
E(calibrator))^Ct(calibrator)/(1 +
E(target))^Ct(target)], where E is the
average amplification efficiency calculated by DART-PCR for each
primers pair and Ct is the average Ct obtained for the calibrator
(proliferative endometrial tissues) and for the target (all
endometrial tissue analyzed). Moreover, for each CLU isoform their
relative expression ratio (rER) with respect to each endometrial
tissues analyzed, was calculated by applying the following formula:
[(1 + E(target))^(Ctsample −
Ctcalibrator)target]/[(1 + E(EC))
(Ctsample − Ctcalibrator)EC],
where EC is the endogenous control and the calibrator was arbitrary
the nCLU. The average data from at least two independent
experiments are reported.
Immunoblot analysis
Endometrial frozen tissue samples obtained from
hysterectomy were homogenized in lysis buffer containing 0.1% SDS,
1% Nonidet P-40, 50 mmol/l Tris-HCl pH 7.5, 150 mmol/l NaCl, 200
mmol/l LiCl, 5 mmol/l EDTA, 10% glycerol, 10 μg/ml
aprotinin, 120 μg/ml leupeptin, 170 μg/ml
phenylmethylsulfonyl fluoride. The homogenate was sonicated for 20
sec and then centrifuged for 30 min at 13,000 rpm at 4°C. The
supernatants were centrifuged for 30 min at 13,000 rpm at 4°C and
collected. Tissue extracts (100 μg) were electrophoresed on
10% SDS-polyacrylamide gel (PAGE) under reducing conditions and
immunoblotting was carried out using either anti-CLU α chain
antibody (1 μg/ml) (Upstate Cell Signaling Solutions, Lake
Placid, NJ, USA) or anti-β-tubulin antibody (2 μg/ml) (Zymed
Laboratories). In the first case the blots were incubated for 30
min at 4°C with a blocking solution composed of PBS pH 7.4 (NaCl
137 mM, KCl 3 mM, NaHPO4 10 mM,
KH2PO4) containing 3% non-fat dry milk. Then
the blots were incubated with the CLU α chain antibody for 16 h at
4°C in PBS containing 3% non-fat dry milk. The membrane was then
washed two times in water and incubated with mouse IgG
horse-radish-peroxidase-conjugate goat affinity-purified antibody
(Bio-Rad) 1/3,000 diluted with PBS containing 3% non-fat dry milk
for 90 min at room temperature. After two washes in water, one wash
in PBS-T (PBS-Tween 0.05%) and one wash in water again, the
proteins were visualized using the Amersham enhanced
chemiluminescent system according to the manufacturer’s
instructions. Protein levels were normalized using the
constitutively expressed β-tubulin protein. For this purpose, the
blots were stripped at 55°C for 20 min with stripping buffer [2%
SDS, 10 mmol/l β-mercaptoethanol, 6 mmol/l Tris-HCl (pH 6.8)] and
hybridized, with 2 μg/ml antibody to β-tubulin: the blots
were incubated for 30 min at 4°C with a blocking solution composed
of TBS-T (20 mmol/l Tris, pH 7.5, 150 mmol/l NaCl, 0.2% Tween)
containing 5% non-fat dry milk. Then the blots were incubated for
16 h at 4°C with the β-tubulin antibody, in TBS-T containing 5%
non-fat dry milk. The membrane was then washed for 90 min at room
temperature, in TBS-T and incubated with mouse IgG
horse-radish-peroxidase-conjugate goat affinity-purified antibody
(Bio-Rad), diluted 1/3,000 in TBS-T containing 5% non-fat dry milk.
After two washes in TBS-T, the proteins were visualized using the
Amersham enhanced chemiluminescent system according to the
manufacturer’s instructions.
Densitometric values for immunoreactive bands were
quantified by using a GS-700 Imaging Densitometer (Bio-Rad). CLU
protein levels were calculated as percentage of the control
(proliferative endometrium) taken as 100 in arbitrary units, after
normalization using β-tubulin as control for protein loading. For
each specimen, the mean value (± SEM) of the results obtained in at
least three experiments was calculated.
Nuclear run-on transcription assays
Isolation of nuclei and transcriptional rate assays
were performed as previously described (35). The following linearized recombinant
plasmids were used in the nuclear run-on analysis: the pIREShyg
plasmid containing the 1,649 nt full length CLU cDNA (nucleotides
52–1700 actually corresponding to NM_001831, GenBank) linearized by
Not1 digestion and the pABB plasmid containing the 28S cDNA
(nucleotides 2435 to 2550) linearized by EcoR1 digestion.
After hybridization, the membranes were washed once
in 2X SSPE, 0.1% SDS for 10 min at 42°C, twice in 1X SSPE, 0.1% SDS
for 10 min at 42°C, twice in 0.5X SSPE, 0.1% SDS for 10 min at
42°C, once in 0.1X SSPE, 0.1% SDS for 10 min at 50°C and then
exposed to Kodak X-OMAT AR 5 film (Kodak).
Autoradiographs of the RNA-DNA hybrids obtained
after 7 days exposure at −80°C were analyzed using a GS-700 Imaging
Densitometer (Bio-Rad). All values were standardized according to
the 28S rRNA signal used as internal standard. The average of CLU
transcriptional activity in proliferative endometrial samples
derived from two patients was set at 100 (arbitrary units) and CLU
transcriptional activity in secretive, atrophic, neoplastic and
hyperplastic endometria was calculated as percentage of the
proliferative endometrial transcriptional activity.
Histopathology and
immunohistochemistry
The study material for histopathology included all
surgical samples from which tissue fragments for the molecular
investigations had been taken.
The surgical samples were fixed in 10% neutral
buffered formalin for 12–24 h, embedded in paraffin, cut and
stained with hematoxylin and eosin (H&E). The histological
preparations were reviewed by three pathologists (E. Maiorano, G.
Napoli and A. Napoli) to compare the histological dating with the
clinical dating in normal cycling women, to specify the
histological subtype of endometrial carcinomas and define tumor
grade.
From selected cases (see Table I) for which sufficient and
representative amounts of tissues were available after
morphological analysis, a single paraffin block per case was
selected for immunostaining based on good morphological
preservation. Thick sections (5 μm) were cut, collected on
positively-charged slides, dewaxed and rehydrated. Following
quenching of endogenous peroxidase with 3%
H2O2 for 15 min at room temperature, the
sections were immunostained for CLU and caspase-3 using a
peroxidase-based detection system (En Vision, Dako, Glostrup,
Denmark) with an automated immunostainer (Autostainer, Dako). Prior
to the staining procedure, the sections to be incubated with
anti-clusterin/anti-caspase-3 antibodies were immersed in 0.01 M
citrate buffer, pH 6.0 and incubated at 98°C for 30 min in a water
bath. Mouse monoclonal antibodies against clusterin and caspase-3
(Upstate Cell Signaling Solutions; clone: 41D, dilution 1:200) were
used as primary antibodies with overnight incubations at 4°C.
Control sections for specificity included staining
of positive controls (normal and carcinomatous prostate) and
negative control sections, which were incubated with the
immunoglobulin fraction of normal mouse serum in place of the
specific immunoreagent.
Evaluation of immunoreactivity
In all cases the immunoreactivity was independently
evaluated by two pathologists (E. Maiorano and A. Napoli) by
separately counting the relative number of immunoreactive
superficial and glandular epithelial cells and stromal cells in 10
different microscopic fields, observed at ×400 magnification; the
extent of the immunoreactivity within each cell component
(superficial vs. glandular epithelial cells vs. stromal cells), as
the percentage of immunoreactive cells, was recorded for individual
cases. In the cases of endometrial carcinoma, the immunoreactivity
in superficial epithelial cells and in stromal cells was recorded
to the cells adjacent to the tumor in the surrounding residual
structures, and only the immunoreactivity of glandular epithelial
cells is referred to as neoplastic cells.
Statistical analysis
For each histological endometrial group data are
reported as the mean ± SEM. Statistical analysis was performed
using the ANOVA followed by Duncan’s post hoc test. All experiments
were repeated at least three times.
Results
CLU mRNA expression in the endometrial
tissues
Steady state levels of total CLU mRNA were
quantified by northern hybridization experiments by using total RNA
isolated from 23 physiological endometrial tissues samples (9
proliferative, 9 secretive and 5 atrophic endometrial tissues), 16
neoplastic and 15 hyperplastic endometrial tissues (see Table I). Fig. 2 shows the results of a typical
northern hybridization analysis carried out using the full length
CLU cDNA probe able to hybridize all CLU isoforms (7). Total RNA extracted from HL60 cells
(lane 1) was used as negative control of CLU mRNA expression and
total RNA extracted from human liver (lane 2) was used as positive
control (Fig. 2A). Under our
hybridation and washing conditions (see Materials and methods) a
1.9 kb long transcript was present in all the samples,
corresponding to the specific endometrial CLU mRNA (2), in agreement also with human prostate
studies (13,40). CLU mRNA showed a variable
expression level in the different physiological (lanes 6–12) and
pathological endometrial conditions (lanes 13–20) in comparison to
the normal proliferative endometria (lanes 3–5).
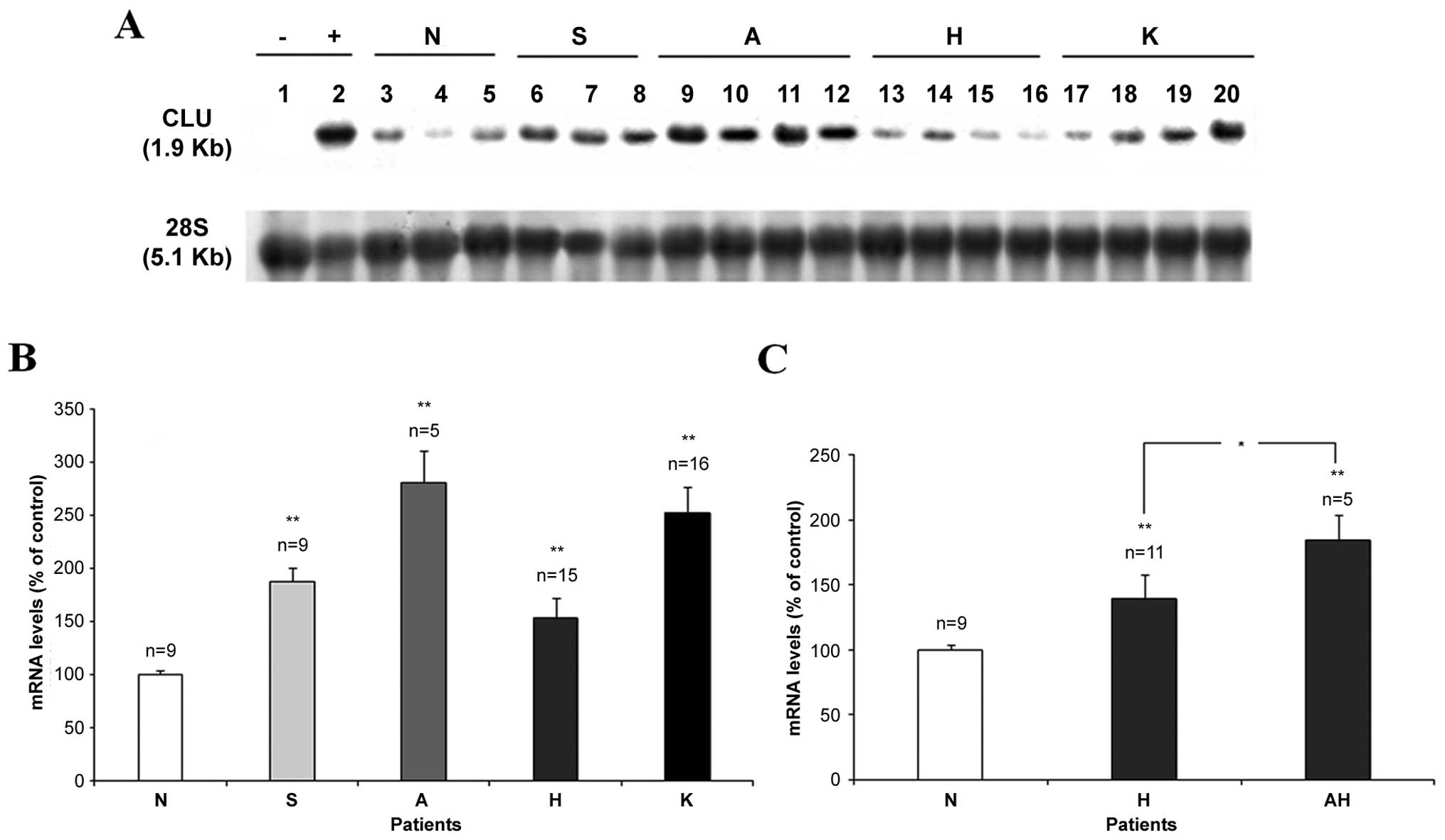 | Figure 2CLU mRNA expression in the
endometrial tissues. (A) Total RNA was isolated from the
endometrial tissue samples indicated in Table I. Total RNA (25 μg) was
used; lane 1, RNA from HL60 cells was used as negative control;
lane 2, RNA from human liver was used as positive control; lanes
3–5, RNA isolated from 3 patients with normal proliferative
endometrial tissues; lanes 6–8, RNA isolated from 3 patients with
secretive endometrial tissues; lanes 9–12, RNA isolated from 4
patients with atrophic endometrial tissues; lanes 13–16, RNA
isolated from 4 patients with hyperplastic endometrial tissues;
lanes 17–20, RNA isolated from 4 patients with neoplastic
endometrial tissues. To normalize the amount of total RNA in each
sample, the blot was stripped and re-hybridized again using a 28S
rRNA cDNA probe. (B) The average ± SEM of CLU mRNA levels in the 9
secretive (S), 5 atrophic (A), 16 hyperplastic (H) and 16
neoplastic (K) endometrial tissue samples were calculated as
percentage of N. (C) The 16 patients with hyperplastic endometria
were divided into two groups: the first included 11 patients
affected by simple hyperplasia (H); the second included 5 patients
affected by atypical hyperplasia (AH) and mRNA levels were
calculated as percentage of N. Mean values ± SEM from at least
three different experiments are illustrated. Error bars indicate
SEM. *P<0.05, **P<0.01. |
To normalize the differences due to the experimental
procedure, such as mRNA loading and transfer, the blots were
de-hybridized and re-hybridized again with the human 28S rRNA cDNA
probe. In this way the possible differences due to the experimental
procedure could be taken into account by calculating for each
sample the ratio between the CLU mRNA band and the 28S rRNA signal.
Hereafter, CLU mRNA levels in the endometrial tissues were
calculated as the percentage of proliferative endometrial CLU mRNA
levels, present on the same filter. The mean values of at least
three separate measurements for each patient were recorded and the
average values of the various specimens belonging to the same
histological endometrial tissues group ± SEM, were calculated
(Fig. 2B). The semi-quantitative
analysis of CLU mRNA expression shows an increase in both normal
and diseased endometrial tissues.
With regard to normal endometrial tissues the
steady-state CLU mRNA level was upregulated in 86% of secretive and
in 100% of atrophic endometrial samples, when compared to the
normal proliferative endometria. In fact, the CLU mRNA expression
in secretive tissue samples ranged from 119±16% to 298±7%, with an
average value of 188±13%, while CLU mRNA levels in the atrophic
samples ranged between 187±13% and 408±20%, with a mean percentage
value of 281±30%. This upregulation resulted statistically
significant (p<0.01) both in secretive and atrophic endometrial
tissues in comparison to the normal proliferative tissues.
Considering the neoplastic endometrial tissues, our
analysis showed an increased CLU mRNA level in 94% of endometrial
carcinoma (K) samples. The expression of CLU mRNA in cancer tissues
ranged from 98±21% to 474±8% with a percentage average value of
252±25% and this increase was statistically significant (p<0.01)
in comparison to the normal control. An increased steady state CLU
mRNA level (153±19%) was detected also in 62% of total endometrial
hyperplastic samples and this increase was statistically
significant (p<0.01) in comparison to the normal endometrial
tissues. Moreover, we evaluated the CLU mRNA expression in the
different types of endometrial hyperplasia based on
hystopathological examination (Fig.
2C): 11 hyperplasia without cytological atypia (H) and 5
hyperplasia with atypia (AH). The expression of CLU mRNA in the
hyperplasia without cytological atypia (H) ranged between 98±7% and
243±30% with a percentage average value of 139±19%. In the 5
hyperplasia with atypia (AH) the CLU mRNA levels ranged from 99±12%
up to 252±19% with an average value of 184±20%. The increase of the
CLU mRNA for both these categories was statistically significant
(p<0.01) in comparison to the normal endometrial tissues. The
CLU increase observed in endometrial hyperplasia with atypia (AH)
was statistically (p<0.05) higher than the increase measured in
the simple hyperplasia. This result seems to attest a benign
proliferative origin for the hyperpastic disease even in the
presence of atypia, whereas it was demonstrated that 30% of cases
of hyperplasia with atypia evolve in endometrial carcinoma
(41). In conclusion, total CLU
mRNA expression appears to progressively increase in both normal
differentiated atrophic and secretive tissues, and proliferative
disease such as hyperplasia and carcinoma, in comparison to the
normal endometrial tissue.
CLU expression and tumor progression
Since gene expression profiling of CLU in prostate
cancer, performed with conventional techniques such as northern
hybridization, provides reliable prognostic prediction of prostate
cancer when used in combination with standard clinical information,
such as histological and tumor stage (13), we decided to investigate the
correlation between CLU mRNA expression and endometrial carcinoma
clinical progression (Fig. 3). For
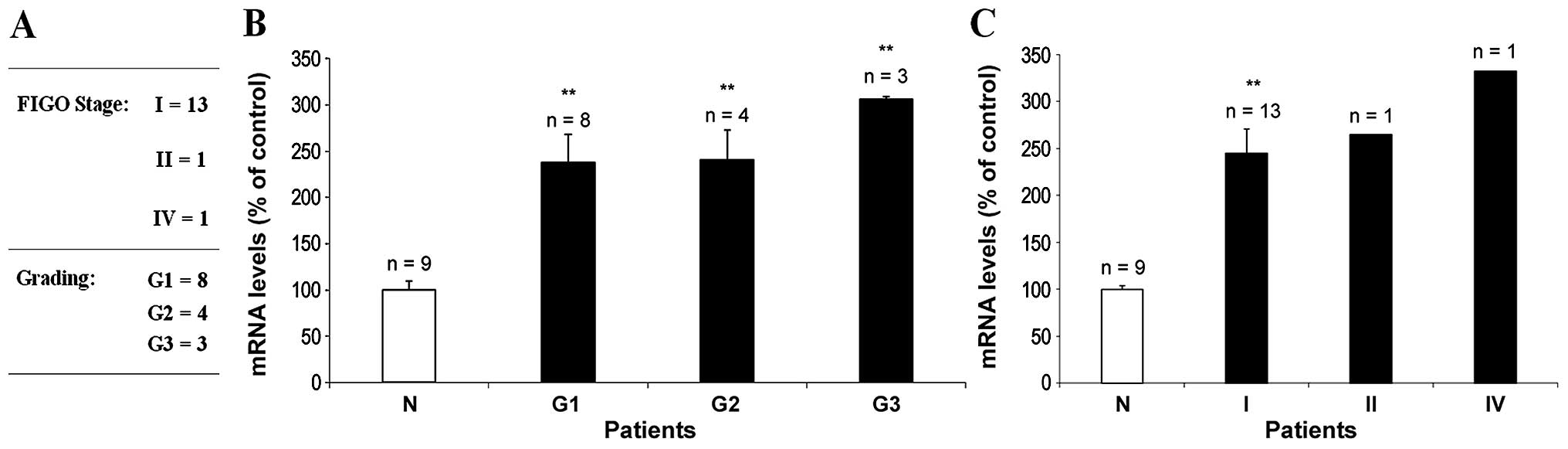
this purpose we evaluated CLU mRNA levels in neoplastic endometrial
samples classified by histological tumor grade and pathological
stage, based on the FIGO system (37) (Fig.
3A). Fig. 3B illustrates CLU
mRNA levels in endometrial neoplastic tissues, classified according
to G grade. A statistically significantly (p<0.01) increase
(306±3%) of CLU mRNA levels was detected in the 3 patients affected
by poorly-differentiated endometrial carcinoma (G3), suggesting a
direct correlation between the CLU mRNA level and the tumour
progression. This trend seems to be confirmed by the analysis
between CLU mRNA expression and the tumor stage (Fig. 3C) whereas, in consideration of the
relatively low number of stage II and stage IV samples (n=1) and
the absence of stage III cases, a statistical evaluation was not
feasible.
In conclusion, since the statistical analysis were
not powerful enough, due to the limited number of cases, we could
not demonstrate a clear association between CLU expression and
tumor grade and/or stage in endometrial carcinoma.
Analysis of CLU mRNA variants
Since it was suggested that sCLU:nCLU cellular
balance is critical for cancer establishment and progression
(4) we decided to characterize the
different CLU mRNA variants present in the endometrial tissues with
the aim of correlating these isoforms to the different
physio-pathological stage of the endometrial tissue.
To identify all possible novel CLU variants, we
first inspected the CLU entries in the ASPicDB, the Alternative
Splicing Prediction Data Base (http://t.caspur.it/ASPicDB/index.php) (42,43).
These inspections revealed multiple possible CLU mRNA variants,
three of which (identified by the Signature ID (44)c7175b345e:9,
1057fea355:9, 8031cb1ea1:9, and corresponding to
nCLU, sCLU and isoform 11036, respectively) stood out by having
substantially more sequence support than the rest. These three
variants all contain nine exons, and each of them presents a unique
exon 1 and shares the remaining 2–9 exons (Fig. 1A). By RT-PCR analysis followed by
sequencing experiments, we have shown for the first time that all
the three CLU mRNA variants are expressed in the endometrial
tissues in vivo. Moreover, by using the specific CLU primers
shown in the Fig. 1B, we did not
observe any additional bands except the three variant-specific
RT-PCRs.
To measure the quantitative variation of CLU isoform
expression in the different physio-pathological endometrial stages
for possible correlation of the expression of each CLU isoform to
the specific condition, we carried out CLU isoform mRNA expression
analyses by RT-qPCR. The primers, firstly validated by PCR, were
used to assess the CLU isoform expression level in the different
biopsy specimens obtained from the human endometrial tissue: normal
proliferative phase (N1–N4, N6), normal secretive (S2–S5, S7),
atrophic (A1, A3–A6), hyperplastic (H1–H4,H8, AH2–AH5), neoplastic
(K1–K6, K8, K12,K15) tissues (see Table I). The normal follicular tissues
(N1) was selected as calibrator of the RT-qPCR experiments, by
means of a preliminary experiment, with GAPDH used for
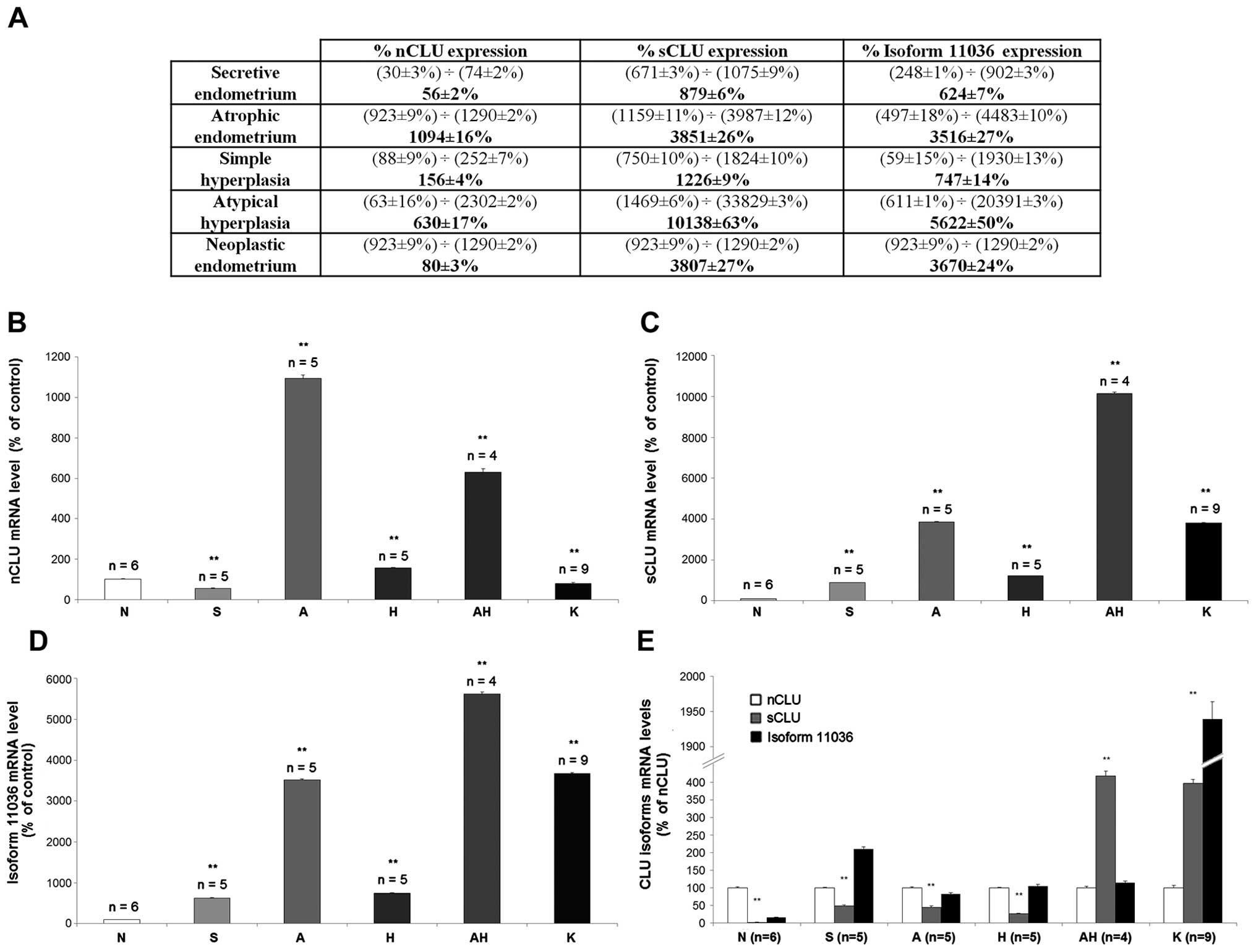
normalization. Fig. 4A shows the
range and the mean value of expression for each CLU isoform in the
different endometrial tissues. The nCLU mRNA expression (Fig. 4B) in the secretive phase (S) was
lower (56±2%) than the normal follicular tissue (N). On the
contrary, the average percentage of nCLU mRNA expression was 10
times higher (1,094±16%) in the atrophic endometrium (A) than the
normal follicular phase (N). In both types of hyperplastic
endometrial tissue samples (H and AH), the percentage of nCLU mRNA
expression was higher (156±4% and 630±17%, respectively) in
comparison to the normal follicular endometria (N).
In the neoplastic endometrial tissue samples (K) the
average percentage of nCLU mRNA expression was lower (80±3%) than
the normal follicular tissues (N). The sCLU mRNA expression
(Fig. 4C) in the secretive
endometrium (S) amounted to 879% (±6) in comparison to the normal
follicular tissues; in the atrophic endometrium (A) the sCLU mRNA
expression was higher (3,851±26%) than the normal follicular
tissues. sCLU mRNA expression in the endometrial hyperplasia was
higher in both hyperplastic tissues without (H) and with atypia
(AH) (1,226±9% and 10,138±63%, respectively) than the normal
follicular endometrium (N). In the neoplastic endometrial tissue
samples (K) the sCLU mRNA expression increased up to 3,807±27%
respect to normal endometrium (N). The isoform 11036 CLU mRNA level
(Fig. 4D) resulted higher (624±7%)
in the secretive endometrium (S) than the normal follicular
tissues; in the atrophic endometrium (A) it amounted to 3,516±27%.
In both types of hyperplastic endometrial tissue (without atypia,
H; or with atypia, AH) the isoform 11036 CLU mRNA was higher
(747±14% and 5,622±50%, respectively) than the normal follicular
endometria (N). In the neoplastic samples (K) isoform 11036 CLU
mRNA was higher (3,670±24%) than the normal follicular endometria
(N).
To provide data on the amount of the variation of
expression for each CLU isoform in the different physiological and
pathological stage, we analyzed the balance of the three isoforms
in the different endometrial tissues. The identification of the
specific relative ratio of expression of these three isoforms in
the endometrial tissues may allow to identify a possible role of
one of these isoforms during the different states of
differentiation (follicular < secretive < atrophic) and/or
its involvement in the tissue remodelling and/or block of cell
progression. Moreover, the analysis of the ratio of the three
isoforms may help to identify a possible specific correlation with
the physio-pathological stage or during the transition from the
normal to the neoplastic transformation, possibly allowing us to
use CLU as a biomarker for the diagnosis and/or prognosis of the
endometrial proliferative disease. Fig. 4E shows the sCLU and isoform 11036
mRNA expression compared to the nCLU mRNA, taken as calibrator and
arbitrarily set as 100%. In the normal follicular phase (N) both
the sCLU and isoform 11036 mRNA percentage of expression were lower
(2.5±1% and 16±1%, respectively) than the nCLU mRNA expression. In
the secretive endometrial tissue (S) the sCLU mRNA expression level
was lower (49±2%) than the nCLU, while the isoform 11036 mRNA
expression was higher (210±6%) than the nCLU. In the atrophic
endometrial tissue (A) both the sCLU and isoform 11036 mRNA
expression were lower (45±4% and 85±4%, respectively) than the
nCLU. In the hyperplastic endometrial tissue without cytological
atypia (H) the sCLU mRNA was lower (26±2%) than the nCLU; on the
contrary in the hyperplastic endometrial tissue with cytological
atypia (AH) the sCLU expression was higher (413±13%) than nCLU. In
both tissues (H and AH) the isoform 11036 was comparable to nCLU
(104±5% and 115±4%, respectively). In the neoplastic endometrial
tissues (K) both the sCLU and isoform 11036 mRNA were higher
(396±11% and 1,939±25%) when compared to the nCLU.
These results indicate a shift in the CLU mRNA
expression from the pro-apoptotic nCLU to the
cytoprotective-oncogenic sCLU isoform during the transition from
normal to malignant cell in the neoplastic transformation process.
Concerning isoform 11036, our results show for the first time that
it is expressed in all endometrial phases, whereas further
experiments are necessary to clarify its role, its expression is
differentially regulated in the physio-pathological endometrial
stages.
CLU protein expression in endometrial
tissues
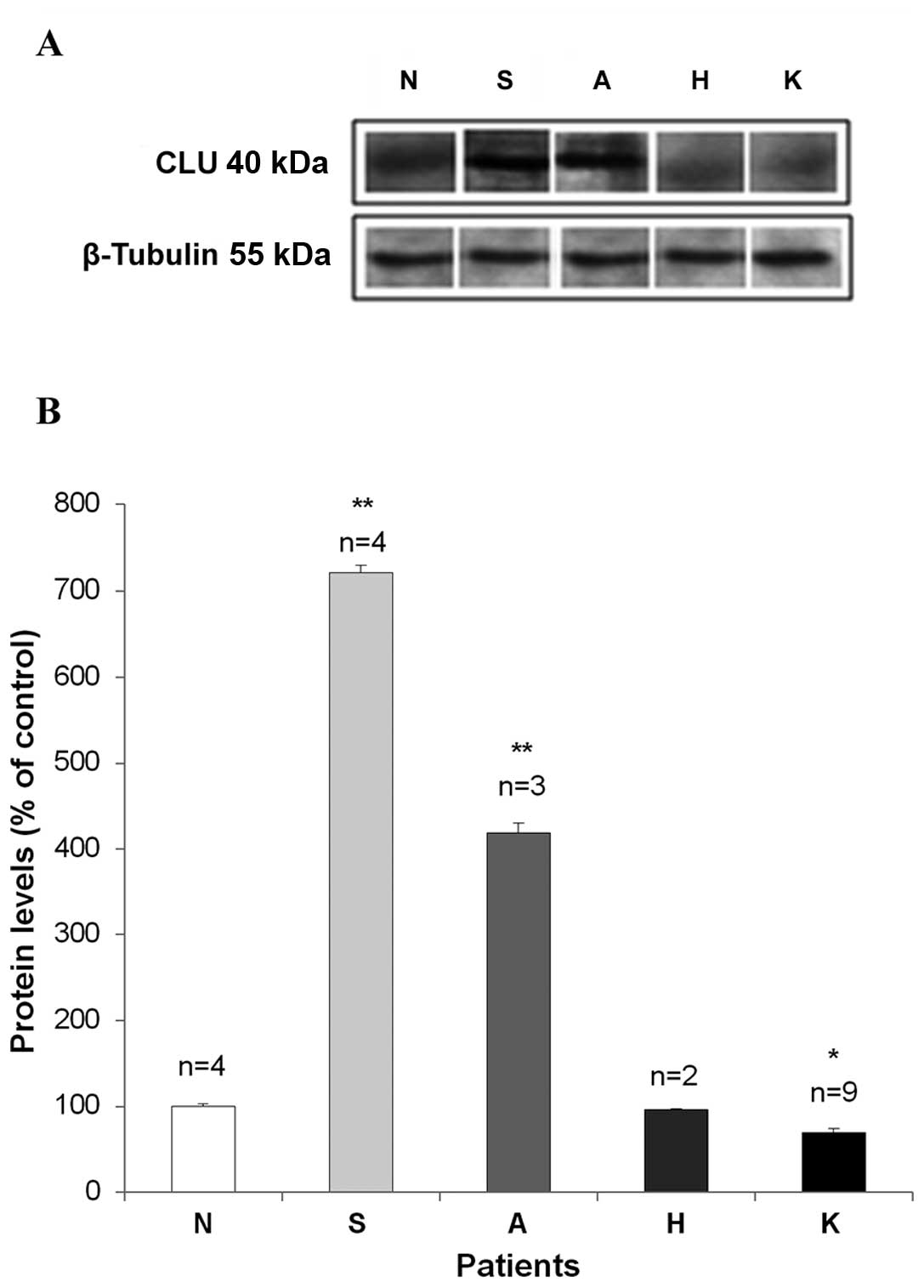
To clarify the role of CLU during the neoplastic
cell transformation, we measured its protein level by western blot
analysis (Fig. 5). Since specific
antibodies for each isoforms are not available we could not
evaluate the different protein CLU variants but the α subunit of
the CLU heterodimer was recognized by the (clone 41D) antibody that
is a monoclonal anti-human CLU (Upstate Cell Signaling Solutions).
After normalization with β-tubulin protein expression, the mean
value of at least three separate measurements for each patient were
recorded and the CLU protein level (average ± SEM) was calculated
as percentage of normal proliferative endometrium. Upregulated CLU
protein levels were detected in secretive (721±1%) and atrophic
(410±1%) endometrial samples, in comparison to the proli ferative
endometria. In the secretive tissues (S) CLU expression ranged
between 601±1% and 928±1%; in the atrophic tissues (A) the CLU
expression ranged between 132±1% and 808±1%. These increases
resulted statistically significant (p<0.01) in comparison with
control.
In the neoplastic tissue (K) a statistically
significant (p<0.05) decrease of CLU protein level (70±1%) was
measured in comparison to the proliferative endometrium, ranging
between 29±1% and 109±1%. No alteration was found in the
hyperplastic tissues (H): CLU protein ranged between 91±1% and
101±1%. We observed a direct correlation (r=0.97; p=0.035) between
protein and mRNA CLU levels in the secretive and atrophic
endometrial tissue suggesting a possible transcriptional mechanism
of regulation. The indirect correlation (r=−0.93; p=0.005) between
CLU protein and mRNA level in both carcinoma and hyperplasia,
suggests a post-transcriptional regulation, possibly involving mRNA
turn-over and/or protein synthesis.
Transcriptional regulation of CLU
expression
To investigate the possible molecular mechanisms
implicated in the regulation of CLU expression, we measured the
transcriptional activity of the CLU gene by nuclear run-on assays.
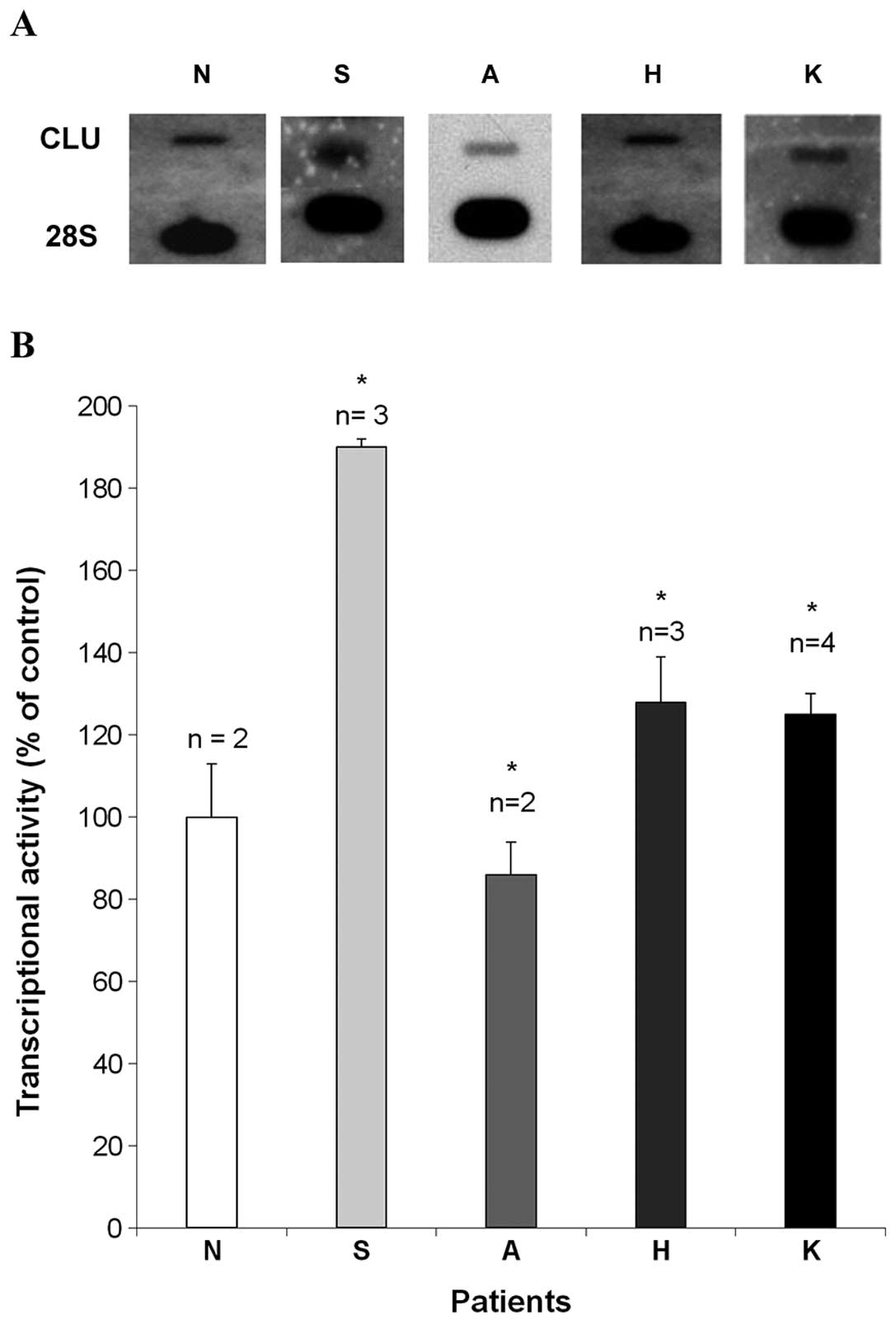
The autoradiographic signals obtained in a typical run-on
experiment carried out using one representative normal
proliferative endometrium (N), one secretive (S), one atrophic (A),
one neoplastic (K) and one hyperplastic (H) sample, are shown in
Fig. 6A. The semi-quantitative
analysis of the autoradiographic signals (Fig. 6B) indicated an increase (190±2%) of
CLU transcriptional activity in normal secretive endometrial
tissues (S), in comparison to the normal proliferative tissues (N).
Although these results need to be verified in a larger population,
they seem to indicate that the CLU gene transcriptional activity
may possibly be responsible for the CLU mRNA upregulation.
On the contrary, the gene transcriptional activity
is not responsible by itself for CLU mRNA increase measured in the
atrophic tissues (A) since CLU transcriptional activity was
downregulated (86±8%) in comparison to the normal proliferative
tissues (N). In the neoplastic (K) endometrial tissues CLU
transcriptional activity ranged between 104±2% and 144±8%, showing
a statistically significant (p<0.05) increase (125±5%) when
compared to the proliferative endometrial tissues. Moreover, we
demonstrated a statistically significant (p<0.05) increase of
CLU transcriptional activity (128±13%) in the endometrial
hyperplasia (H) in comparison to the proliferative endometrial
tissue (N).
The above results seem to indicate that CLU
transcriptional activity may be responsible for mRNA increase in
both carcinoma and hyperplasia. Since the CLU protein levels do not
correlate to the CLU mRNA level, the results from run-on
experiments suggest the existence of a fine regulatory mechanism of
CLU protein expression in the neoplastic disease possibly acting at
post-transcriptional level.
CLU immunohistochemistry
CLU immunoreactivity, carried out using the antibody
described above, was evaluated in 7 proliferative, 5 secretive, 6
atrophic endometria, 6 simple and 4 hyperplasias with atypia and in
8 endometrial carcinomas (see Table
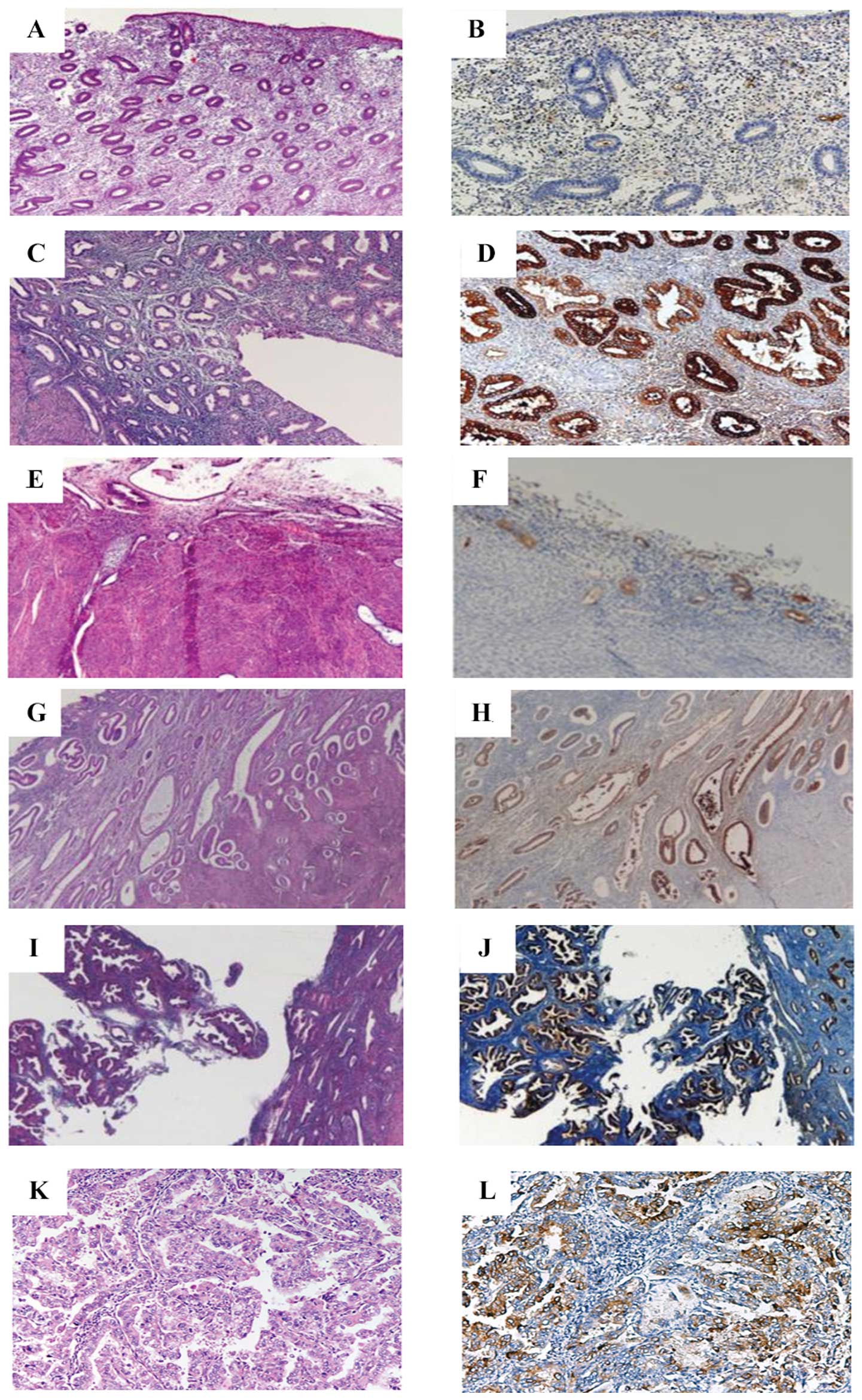
I). Fig. 7 shows the
hematoxylin and eosin (A, C, E, G, I, K) and the corresponding ABC
immonohistochemical (B, D, F, H, J, L) microscopical features of
the different endometrial conditions. CLU immunoreactivity was more
intense in the glandular basal cells, in comparison to the
superficial epithelial and stromal cells. Moreover, anti-CLU
antibody largely shows positive staining in the cytoplasm of all
sections, as reported in previous studies documenting the CLU
localization in the cytoplasm of breast, prostate and ovarian
tissues (7,9,45).
Table II reports the descriptive
analysis of CLU immunoreactivity in the different cellular
compartments of the various endometrial tissue sections. The number
of immunoreactive cells was higher in the glandular basal cells
than the superficial epithelial and stromal cells of the normal (F,
proliferative; S, secretive; and A, atrophic) proliferative
endometrium. Moreover, comparing normal and neoplastic endometrial
tissue we observed prevalent CLU expression in the glandular
epithelial compartment. The CLU expression in the neoplastic tissue
was downregulated in both epithelial (glandular and superficial)
and stromal cells in comparison to the physiological conditions
(proliferative, secretive and atrophic endometrial tissue). CLU
immunoreactivity in the hyperplastic endometrial tissues does not
show a significant difference between hyperplastic endometrial
tissue without cytological atypia (H) and with cytological atypia
(AH), confirming the protein results obtained by western blot
analysis, suggesting a benign proliferative origin for the
hyperplastic disease.
 | Table IICLU immunoreactivity in the
endometrial tissues. |
Table II
CLU immunoreactivity in the
endometrial tissues.
| Tissue type (no. of
patients) | Range of IR
superficial epithelial cells (%) | Median of IR
superficial epithelial cells (%) | Range of IR
glandular epithelial cells (%) | Median of IR
glandular epithelial cells (%) | Range of IR stromal
cells (%) | Median of IR
stromal cells (%) |
|---|
| Normal
proliferative endometrium (n=7) | 0–10 | 0 | 0–90 | 0 | 0–40 | 20 |
| Secretive
endometrium (n=5) | 0–50 | 2 | 5–50 | 35 | 4–10 | 5 |
| Atrophic
endometrium (n=6) | 0–20 | 5 | 10–95 | 40 | 0–20 | 0 |
| Simple hyperplasia
(n=6) | 0–30 | 0 | 0–75 | 30 | 0–25 | 2 |
| Atypical
hyperplasia (n=4) | 0–30 | 4 | 0–75 | 35 | 0–25 | 5 |
| Endometrioid
adenocarcinoma (n=9) | 0–20 | 2 | 0–90 | 20 | 0 (in all sections
analyzed) | 0 |
As the statistical analysis were not powerful
enough, due to the limited number of investigated cases (G1=3,
G2=4, G3=2 and stage I=7, stage II=1, stage III=1), we could not
demonstrate any correlation between CLU expression and tumor grade
and stage in endometrial carcinomas. Since, the monoclonal antibody
(clone 41D) recognizes the α subunit of the CLU heterodimer, we can
suggest a key role for the sCLU isoform in endometrial
carcinogenesis probably acting by cytoprotective and anti-apoptotic
pathways. To confirm this hypothesis we examined epithelial cell
apoptosis using anti-caspase-3 antibody. Immunohistochemical
analysis demonstrated that caspase-3 protein expression is absent
within the nuclei of all the epithelial endometrial cells of our
samples (data not shown) thus confirming the anti-apoptotic role of
CLU.
Discussion
Although several molecules may be associated with
endometrial carcinogenesis, their role in malignant transformation
has not yet been well established. We have previously focused our
attention on the role of growth factors and adhesion factors in the
aetiopathogenesis of human proliferative diseases providing the
first evidence of a transcriptional and post-transcriptional in
vivo regulation of the expression of these factors during
cellular transformation leading to the malignant phenotype
(22–36). Among these, CLU is an enigmatic
glycoprotein with a nearly ubiquitous tissue distribution and an
apparent involvement in biological processes ranging from
neurodegeneration in Alzheimer’s disease to cancer initiation and
progression. It exists in at least two protein variants, secreted
and truncated forms. The secreted form of CLU is implicated in a
variety of activities such as programmed cell death, regulation of
complement mediated cell lysis, membrane recycling, cell-cell
adhesion and transformation; the nuclear isoform acts as a
pro-death signal, inhibiting cell survival. It has been previously
shown that CLU plays a key role in the cell transformation process,
and it has been reported to be overexpressed in several human
tumour tissues such as prostate, breast, renal, ovarian, colon,
cervix, lung and anaplastic large cell lymphoma (9–16).
Upregulation of CLU mRNA and protein are widespread phenomena in
developmental and patho-physiological states, suggesting that
control of expression levels is important. Increased expression of
CLU has been established in diseases where either abnormal cell
death or proliferation is occurring (18). Despite the growing evidence for a
role of CLU in the aetiology of cancer in various tissues, the
regulation of its expression and function in human endometrial
carcinoma is not well established.
We have demonstrated in this paper for the first
time that CLU mRNA expression is upregulated in human endometrial
carcinoma in vivo, when compared with normal proliferative
endometrial tissues. Our results are in agreement with the data
from other laboratories showing increased CLU mRNA expression in
hormonally regulated cancers such as human prostate (7) and breast carcinoma (9). In both cases the upregulation of CLU
mRNA was associated with increased cancer cell survival, suggesting
that CLU overexpression may contribute to the increase of tumour
cell survival. Moreover, to investigate the possible use of CLU as
prognostic marker to predict the clinical progression of
endometrial cancer disease, we investigated the possible
involvement of CLU in the clinical progression of endometrial
proliferative diseases. Even if our preliminary results need to be
verified in a larger and more representative population, we
observed a progressive general CLU mRNA increase with increasing
tumour grade, reaching its peak in poorly differentiated (G3)
endometrial cancer, suggesting a possible direct correlation
between CLU mRNA expression and tumour grade.
We also investigated the role of CLU during the
physiological changes of uterine tissues throughout the menstrual
cycle. The semi-quantitative analysis of northern hybridization
experiments showed an increased steady-state CLU mRNA levels in
both secretive and atrophic endometrial samples when compared to
the proliferative tissue.
Our results are in agreement with previous data
(46), showing that CLU is
expressed in human endometrium during the secretive phase of
menstrual cycle, probably playing a cytoprotective role. Other
experimental data showed CLU overexpression in normal cells, such
as human prostate epithelial cells (47) and quiescent normal skin fibroblasts
(48), suggesting that CLU gene
expression may play a key role in the regulation of cell cycle
progression. Apparently, the results of immunohistochemical stains
do not recapitulate those obtained with different technical
procedures. It should be emphasized that immunohistochemistry is
not intended as a quantitative method and that, even though an
attempt was done to semi-quantitatively evaluate the number of
immunoreactive cells in different cell compartments of the
endometrium, the results of such attempts should be primarily
evaluated to assess the cell types involved in CLU production and
accumulation (i.e., glandular epithelial cells vs. superficial
epithelial cells and stromal cells). In fact, both the intensity of
staining and the number of immunoreactive cells do not adequately
recapitulate the functional activities of the cells and should not
be used as a direct support to the results of the many other
molecular techniques used in this study. Furthermore, we
demonstrated here for the first time the CLU mRNA upregulation in
the hyperplastic endometrial diseases and this increase was higher
in the hyperplasia with atypia than in the simple hyperplasia,
suggesting that CLU may protect cells from death also in
non-malignant proliferative lesions and this anti-apoptotic
function is higher in the less benign proliferation state of
disease.
Our data demonstrate that CLU mRNA expression is
upregulated in human endometrial hyperplasia and carcinoma in
comparison to the normal tissue, thus providing the first
circumstantial evidence for the possible use of CLU as a new
potential biomarker for endometrial proliferative diseases.
Since up to now experimental evidence seems to
indicate that a balance of sCLU:nCLU ratio in the cell dictates its
fate and is critical for cancer survival and progression, we
analyzed the balance between the CLU isoforms in the different
physio-pathological conditions to possibly identify a specific
association among CLU isoforms and different endometrial
conditions. RT-qPCR experiments demonstrated a differential
expression of the nCLU, sCLU and isoform 11036 in the different
endometrial tissues and a comparison among physio logical and
pathological conditions was carried out. Changes of nCLU mRNA
expression were observed in the physiological endometrial tissues,
probably depending on the hormonal regulation during the uterine
cycle. The nCLU mRNA expression is downregulated in the secretive
tissue, in comparison to the follicular tissue. nCLU is known to be
downregulated during the anchoring of cells to the extracellular
matrix (ECM) (49,50). Since the secretive endometrial
tissue is a well differentiated tissue, with relatively little
tendency to proliferate and strong interactions with the ECM, the
downregulation of the nCLU, which inhibits the formation of
cross-linked actin filaments by binding the α-actinin, prevents the
disassembly of the cytoskeleton, peculiar of a cell with a low
differentiation degree that meet the rapid cycles of division and
death.
Furthermore, we demonstrated an upregulation of nCLU
mRNA in the atrophic endometrial tissues, which is typical
physiological involution of post-menopausal stage caused by the
estrogens decrease, presenting a thin epithelium with the glandular
component layer absent. Normal cells require adhesion to the ECM to
survive, and inadequate interaction can lead to anoikis (51). An increase of nCLU mRNA would
determine the formation of specific ties with the α-actinin,
resulting in cytoskeletal disruption, non-adhesion of cells to ECM
and low proliferative capacity, induction of cell cycle arrest and
a tendency to death, characteristics of a typical atrophic
endometrial tissue. The downregulation of nCLU mRNA observed in the
endometrial cancer tissues agrees with most studies reported in the
literature, which show loss of expression of this isoform in
advanced cancer (52). As already
shown in other human cancers, endometrial cancer cells downregulate
the nCLU expression, which leads to altered cell death pathway and
does not imply nCLU/Ku70–Ku80 complex formation, the latter being
necessary to eliminate cancer cells (53). The upregulation of nCLU mRNA
measured in the hyperplastic endometrial tissue samples suggests an
hormonal dependence of its expression, confirmed by the detection
of increased nCLU mRNA levels during the transition from
hyperplasia without atypia to hyperplasia with cytologic atypia.
This result seems to attest a benign proliferative origin for the
hyperplastic disease even in the presence of atypia, whereas it was
demonstrated (41) that 30% of
cases of hyperplasia with atypia evolve in endometrial carcinoma.
The low level of sCLU mRNA expression observed in the secretive
phase suggests a reduction of the pro-proliferative isoform during
the transition to a more differentiated cell state. Indeed, during
the secretive phase, endometrium continues to grow thick while
ceasing proliferation of epithelial cells due to the cytostatic
effect of progesterone.
Our RT-qPCR experiments show a sCLU increase in
atrophic endometrial tissue, a non-proliferative and quiescent
tissue (G0 phase of the cell cycle) confirming the essential role
of this isoform in the cell cycle regulation (4). This result is in agreement with the
accumulation of sCLU in the G0 phase of cell cycle reported in the
human and murine fibroblasts under conditions of quiescence and
senescence (54–56). Transient overexpression of CLU in
human prostate cells immortalized with SV40, caused blockage of
G0/G1 progression with a reduction of DNA synthesis (6,57,58).
We demonstrated the upregulation of the sCLU mRNA
expression in the neoplastic endometrium, thus confirming its role
as oncogene, consistent with data reported for other tumours such
as prostate (7,53–55),
breast (9), lung (12), colon (8–11),
ovary (14) cancers. The 11036
isoform was recently characterized in the colon cancer (8), however, its functional role remains
unclear. Our RT-qPCR experiments indicate its presence in the
different endometrial tissues, further studies will be necessary to
clarify its role in the endometrial physiopathology. To provide
data on the variation of expression of each CLU isoforms in the
different physiological and pathological endometrial tissue, it was
analyzed in these three isoforms in the different endometrial
conditions. The measure of this ratio may allow us to identify a
specific role of one of the CLU variants in the transition from the
physiological to the pathological stage.
The results of our experiments suggest a trend
toward the prevalence of the sCLU in the endometrial carcinoma with
respect to the nCLU suggesting that the cytoprotective sCLU isoform
is probably preferentially retained in the cytosol of cancer cells,
promoting cell survival. The synthesis of the two sCLU and nCLU
isoforms may therefore play a crucial role in controlling the
balance between proliferation and cell death, strongly influencing
the cell life. The neoplastic transformation is featured by the
alteration of this mechanism with a dominance of the sCLU respect
to the nCLU, thus promoting survival of transformed cells. This
hypothesis is in agreement with previous studies, suggesting that
the survival of cancer cells is related to an overexpression of
sCLU and a downregulation of nCLU (40). To better clarify the role of CLU
during the neoplastic cell transformation, we measured CLU protein
level in the endometrial tissues, even if it is not possible to
evaluate the different CLU variants as specific antibodies for the
different isoforms are still not available. The CLU protein
expression measured by western blot analysis in the secretive and
atrophic tissue samples was upregulated in comparison to the normal
follicular tissue. On the contrary, CLU protein expression level in
hyperplastic and neoplastic tissue was downregulated in comparison
to the normal tissue. Whereas our results on CLU protein expression
in neoplastic endometrial tissues appear to differ from those
reported by Ahn et al(21),
the differences could be explained by the heterogeneity of the
cases.
To investigate the molecular mechanisms responsible
for the regulation of CLU expression, we carried out in vivo
transcriptional assays by means of run-on experiments. Even if
these results need to be verified in a larger and more
representative population, we concluded that CLU transcriptional
activity may be partially responsible for increased CLU mRNA levels
in the normal secretive and atrophic endometrial tissue. The
results of run-on analysis in both neoplastic and hyperplastic
tissues show the existence of a post-transcriptional regulation of
CLU expression possibly involving the mRNA turn-over and/or protein
synthesis. Since in previous studies CLU was localized mostly in
the cytoplasm of breast, prostate and ovarian normal and neoplastic
tissues (7,9,14),
we decided to investigate the CLU protein expression and its
sub-cellular localization by immunohistochemical analysis. These
results are in agreement with other studies conducted in ovarian
cancer (45). Our results show a
positive CLU immunoreactivity in the glandular cells of all
endometrial tissue samples in comparison to the epithelial and
stromal cells where the CLU immunoreactivity was lower or absent.
The prevalent CLU expression in the epithelial compartment, in
agreement with data from others laboratories, suggests that the CLU
expression would play a cytoprotective role in a large variety of
epithelia other than endometria, such as esophagus, urethra and
rete testis epithelial cells (4).
In agreement with this hypothesis, we observed absence of the
caspase-3 expression, suggesting that the sCLU variant probably
contributes to the ability of malignant cells to evade apoptosis.
Since caspase-3 has been identified as being a key mediator of
mammalian cell apoptosis and loss of its expression is thought to
contribute to the capability of malignant cells to evade apoptosis,
the CLU-mediated endometrial proliferation induction probably
proceeds via caspase-3 inactivation, such as in prostate cancer
(17,18). Our findings indicate that CLU is a
cell survival gene upregulated by apoptotic triggers, and confers
resistance when overexpressed, thereby suggesting the potential use
of CLU as a therapeutic target for cancer (18).
Our results provide important new insights into the
timing and molecular nature of the critical events involved in
endometrial carcinogenesis and may turn out useful in the design of
improved surveillance strategies for endometrial carcinoma in the
future. Moreover, the shift of sCLU:nCLU towards sCLU is critical
for endometrial cancer survival and progression, providing a novel
biomarker for this malignancy. Since the suppression of the sCLU
isoform expression renders human cancer cells sensitive to
chemotherapeutic drug-mediated apoptosis, and it is currently an
antisense target in clinical trials for prostate cancer, the
long-term objective of our studies is to state CLU as a new
targeted modulator of endometrial cell proliferation potentially
useful to generate rationally designed drugs based on tissue
specific expression, in order to control cancer cell
proliferation.
Acknowledgements
This study was supported by the
FIRB-MERIT RBNE08YFN3_005 and PON01_01297 R&C 2007–2013,
VIRTUALAB by MIUR. The authors wish to thank Giuseppe Cananzi for
technical assistance.
References
|
1
|
Oehler MK, Brand A and Wain GV: Molecular
genetics and endometrial cancer. J Br Menopause Soc. 9:27–31. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wunsche W, Tenniswood MP, Schneider MR and
Vollmer G: Estrogenic regulation of clusterin mRNA in normal and
malignant endometrial tissue. Int J Cancer. 76:684–688. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenberg ME and Silkensen J: Clusterin:
physiologic and pathophysiologic considerations. Int J Biochem Cell
Biol. 27:633–645. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shannan B, Seifert M, Leskov K, Willis J,
Boothman D, Tilgen W and Reichrath J: Challenge and promise: roles
for clusterin in pathogenesis, progression and therapy of cancer.
Cell Death Differ. 13:12–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trougakos IP, Lourda M, Agiostratidou G,
Kletsas D and Gonos ES: Differential effects of
clusterin/apolipoprotein J on cellular growth and survival. Free
Radic Biol Med. 38:436–449. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scaltriti M, Santamaria A, Paciucci R and
Bettuzzi S: Intracellular clusterin induces G2-M phase arrest and
cell death in PC-3 prostate cancer cells. Cancer Res. 64:6174–6182.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scaltriti M, Bettuzzi S, Sharrard RM,
Caporali A, Caccamo AE and Maitland NJ: Clusterin overexpression in
both malignant and nonmalignant prostate epithelial cells induces
cell cycle arrest and apoptosis. Br J Cancer. 91:1842–1850. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andersen CL, Schepeler T, Thorsen K, et
al: Clusterin expression in normal mucosa and colorectal cancer.
Mol Cell Proteomics. 6:1039–1048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Redondo M, Villar E, Torres-Munoz J,
Tellez T, Morell M and Petito CK: Overexpression of clusterin in
human breast carcinoma. Am J Pathol. 157:393–399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurahashi T, Muramaki M, Yamanaka K, Hara
I and Miyake H: Expression of the secreted form of clusterin
protein in renal cell carcinoma as a predictor of disease
extension. BJU Int. 96:895–899. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pucci S, Bonanno E, Pichiorri F, Angeloni
C and Spagnoli LG: Modulation of different clusterin isoforms in
human colon tumorigenesis. Oncogene. 23:2298–2304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
July LV, Beraldi E, So A, Fazli L, Evans
K, English JC and Gleave ME: Nucleotide-based therapies targeting
clusterin chemosensitize human lung adenocarcinoma cells both in
vitro and in vivo. Mol Cancer Ther. 3:223–232. 2004.PubMed/NCBI
|
|
13
|
Scaltriti M, Brausi M, Amorosi A, et al:
Clusterin (SGP-2, ApoJ) expression is downregulated in low- and
high-grade human prostate cancer. Int J Cancer. 108:23–30. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassan MK, Watari H, Han Y, et al:
Clusterin is a potential molecular predictor for ovarian cancer
patient’s survival: targeting Clusterin improves response to
paclitaxel. J Exp Clin Cancer Res. 113:1–14. 2011.PubMed/NCBI
|
|
15
|
Watari H, Ohta Y, Hassan MK, Xiong Y,
Tanaka S and Sakuragi N: Clusterin expression predicts survival of
invasive cervical cancer patients treated with radical hysterectomy
and systematic lymphadenectomy. Gynecol Oncol. 108:527–532. 2008.
View Article : Google Scholar
|
|
16
|
Wellmann A, Thieblemont C, Pittaluga S,
Sakai A, Jaffe ES, Siebert P and Raffeld M: Detection of
differentially expressed genes in lymphomas using cDNA arrays:
identification of clusterin as a new diagnostic marker for
anaplastic large-cell lymphomas. Blood. 96:398–404. 2000.PubMed/NCBI
|
|
17
|
Moretti RM, Marelli MM, Mai S, Cariboni A,
Scaltriti M, Bettuzzi S and Limonta P: Clusterin isoforms
differentially affect growth and motility of prostate cells:
possible implications in prostate tumorigenesis. Cancer Res.
67:10325–10333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gleave M and Chi KN: Knock-down of the
cytoprotective gene, clusterin, to enhance hormone and
chemosensitivity in prostate and other cancers. Ann NY Acad Sci.
1058:1–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seol MB, Bong JJ and Baik M: Expression
profiles of apoptosis genes in mammary epithelial cells. Mol Cells.
20:97–104. 2005.PubMed/NCBI
|
|
20
|
Miyake H, Hara I, Fujisawa M and Gleave
ME: The potential of clusterin inhibiting antisense
oligodeoxynucleotide therapy for prostate cancer. Expert Opin
Investig Drugs. 15:507–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahn HJ, Bae J, Lee S, Ko JE, Yoon S, Kim
SJ and Sakuragi N: Differential expression of clusterin according
to histological type of endometrial carcinoma. Gynecol Oncol.
110:222–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perlino E, Lovecchio M, Vacca RA, et al:
Regulation of mRNA and protein levels of beta1 integrin variants in
human prostate carcinoma. Am J Pathol. 157:1727–1734. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moro L, Greco M, Ditonno P, Battaglia M,
Marra E and Perlino E: Transcriptional regulation of the β1C
integrin splice variant in human prostate adenocarcinoma. Int J
Oncol. 23:1601–1606. 2003.
|
|
24
|
Perlino E, Tommasi S, Moro L, Bellizzi A,
Marra E, Casavola V and Reshkin SJ: TGF-β1 and IGF-1 expression are
differently regulated by serum in metastatic and non-metastatic
human breast cancer cells. Int J Oncol. 16:155–160. 2000.
|
|
25
|
Maiorano E, Ciampolillo A, Viale G, et al:
Insulin-like growth factor 1 expression in thyroid tumors. Appl
Immunohistochem Mol Morphol. 8:110–119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perlino E, Moro L, Loverro G, Maiorano E,
Selvaggi L and Marra E: Role of the growth factors IGF-1 and
TGF-beta 1 in the endometrial adenocarcinoma. Int J Med Biol Env.
29:193–200. 2001.
|
|
27
|
Vacca RA, Marra E, Loverro G, et al:
Differential expression of beta 1c integrin messenger ribonucleic
acid and protein levels in human endometrium and decidua during the
menstrual cycle and pregnancy. J Clin Endocrinol Metab. 88:720–729.
2003. View Article : Google Scholar
|
|
28
|
Lovecchio M, Maiorano E, Vacca RA, et al:
Beta 1C integrin expression in human endometrial proliferative
diseases. Am J Pathol. 163:2543–2553. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tommasi S, Fedele V, Lacalamita R,
Crapolicchio A, Perlino E, Bellizzi A and Paradiso A: Molecular and
functional characteristics of erbB2 in normal and cancer breast
cells. Cancer Lett. 209:215–222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vacca RA, Moro L, Maiorano E, Selvaggi L,
Marra E and Perlino E: Alternatively spliced variants of b1
integrin are involved in the modulation of human endometrial
transformation in different physiological/pathological conditions.
Recent Res Devel Proteins. 2:25–47. 2004.
|
|
31
|
Ciampolillo A, De Tullio C, Perlino E and
Maiorano E: The IGF-I axis in thyroid carcinoma. Curr Pharm Des.
13:729–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moro L, Perlino E, Marra E, Languino LR
and Greco M: Regulation of beta1C and beta1A integrin expression in
prostate carcinoma cells. J Biol Chem. 279:1692–1702. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moro L, Greco M, Maiorano E, Selvaggi L,
Marra E and Perlino E: Transcriptional regulation of β1 integrin
expression in the physio/pathological states of human endometrial
tissues. Int J Oncol. 26:457–465. 2005.
|
|
34
|
Fuzio P, Lucarelli G, Perlino E, Battaglia
M, Bettocchi C, Selvaggi FP and Ditonno P: Androgen deprivation
therapy regulation of β1C integrin expression in prostate cancer.
Oncol Rep. 22:327–335. 2009.
|
|
35
|
Fuzio P, Ditonno P, Lucarelli G, Battaglia
M, Trabucco S and Perlino E: Androgen deprivation therapy affects
BCL-2 expression in human prostate cancer. Int J Oncol.
39:1233–1242. 2011.PubMed/NCBI
|
|
36
|
Fuzio P, Ditonno P, Rutigliano M, et al:
Regulation of TGF-β1 expression by androgen deprivation therapy of
prostate cancer. Cancer Lett. 318:135–144. 2012.
|
|
37
|
Benedet JL, Bender H, Jones H, Ngan HY and
Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO Committee
on Gynecologic Oncology. Int J Gynaecol Obstet. 70:209–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bhatia P, Taylor WR, Greenberg AH and
Wright JA: Comparison of glyceraldehyde-3-phosphate dehydrogenase
and 28S-ribosomal RNA gene expression as RNA loading controls for
northern blot analysis of cell lines of varying malignant
potential. Anal Biochem. 216:223–226. 1994. View Article : Google Scholar
|
|
39
|
Peirson SN, Butler JN and Foster RG:
Experimental validation of novel and conventional approaches to
quantitative real-time PCR data analysis. Nucleic Acids Res.
31:e732003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rizzi F and Bettuzzi F: The clusterin
paradigm in prostate and breast carcinogenesis. Endocr Relat
Cancer. 17:R1–R17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nieminen TT, Gylling A, Abdel-Rahman WM,
et al: Molecular analysis of endometrial tumorigenesis: importance
of complex hyperplasia regardless of atypia. Clin Cancer Res.
15:5772–5783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Castrignanò T, D’Antonio M, Anselmo A, et
al: ASPicDB: a database resource for alternative splicing analysis.
Bioinformatics. 24:1300–1304. 2008.
|
|
43
|
Martelli PL, D’Antonio M, Bonizzoni P, et
al: ASPicDB: a database of annotated transcript and protein
variants generated by alternative splicing. Nucleic Acids Res.
39:D80–D85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Riva A and Pesole G: A unique, consistent
identifier for alternatively spliced transcript variants. PLoS One.
4:e76312009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie D, Lau SH, Sham JS, et al:
Up-regulated expression of cytoplasmic clusterin in human ovarian
carcinoma. Cancer. 103:277–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brown TL, Moulton BC, Baker VV, Mira J and
Harmony JA: Expression of apolipoprotein J in the uterus is
associated with tissue remodeling. Biol Reprod. 52:1038–1049. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bettuzzi S, Scorcioni F, Astancolle S,
Davalli P, Scaltriti M and Corti A: Clusterin (SGP-2) transient
overexpression decreases proliferation rate of SV40-immortalized
human prostate epithelial cells by slowing down cell cycle
progression. Oncogene. 21:4328–4334. 2002. View Article : Google Scholar
|
|
48
|
Bettuzzi S, Astancolle S, Guidetti G,
Moretti M, Tiozzo R and Corti A: Clusterin (SGP-2) gene expression
is cell cycle dependent in normal human dermal fibroblasts. FEBS
Lett. 448:297–300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Goldberg GS, Jin Z, Ichikawa H, Naito A,
Ohki M, El-Deiry WS and Tsuda H: Global effects of anchorage on
gene expression during mammary carcinoma cell growth reveal role of
tumor necrosis factor-related apoptosis-inducing ligand in anoikis.
Cancer Res. 61:1334–1337. 2001.
|
|
50
|
Caccamo AE, Desenzani S, Belloni L,
Borghetti AF and Bettuzzi S: Nuclear clusterin accumulation during
heat shock response: implications for cell survival and
thermotolerance induction in immortalized and prostate cancer
cells. J Cell Physiol. 207:208–219. 2006. View Article : Google Scholar
|
|
51
|
Frisch SM and Screaton RA: Anoikis
mechanisms. Curr Opin Cell Biol. 13:555–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Trougakos IP, Djeu JY, Gonos ES and
Boothman DA: Advances and challenges in basic and translational
research on clusterin. Cancer Res. 69:403–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang CR, Leskov K, Hosley-Eberlein K,
Criswell T, Pink JJ, Kinsella TJ and Boothman DA: Nuclear
clusterin/XIP8, an x-ray-induced Ku70-binding protein that signals
cell death. Proc Natl Acad Sci USA. 97:5907–5912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Petropoulou C, Trougakos IP, Kolettas E,
Toussaint O and Gonos ES: Clusterin/apolipoprotein J is a novel
biomarker of cellular senescence that does not affect the
proliferative capacity of human diploid fibroblasto. FEBS Lett.
509:287–297. 2001. View Article : Google Scholar
|
|
55
|
Gonos ES, Derventzi A, Kveiborg M,
Agiostratidou G, Kassem M, Clark BF, Jat PS and Rattan SI: Cloning
and identification of genes that associate with mammalian
replicative senescence. Exp Cell Res. 240:66–74. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Miyake H, Hara I, Kamidono S, Gleave ME
and Eto H: Resistance to cytotoxic chemotherapy-induced apoptosis
in human prostate cancer cells is associated with intracellular
clusterin expression. Oncol Rep. 10:469–473. 2003.
|
|
57
|
Miyake H, Hara I, Gleave ME and Eto H:
Protection of androgen-dependent human prostate cancer cells from
oxidative stress-induced DNA damage by overexpression of clusterin
and its modulation by androgen. Prostate. 61:318–323. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zellweger T, Kiyama S, Chi K, Miyake H,
Adomat H, Skov K and Gleave ME: Overexpression of the
cytoprotective protein clusterin decreases radiosensitivity in the
human LNCaP prostate tumour model. BJU Int. 92:463–469. 2003.
View Article : Google Scholar : PubMed/NCBI
|