Introduction
Anaplastic thyroid carcinoma is an aggressive
malignancy characterized by an extensive local invasion, early
systemic dissemination and marked resistance to chemo- and
radio-therapy, and always has a poor prognosis with a mean survival
of only few months (1). Current
systemic therapy fails to eradicate this cancer or even to stop
tumor progress. It has been hypothesized that this may be explained
by the failure of current drugs to effectively target cancer
stem-like cells (CSCs) (2,3). To date, CSCs have been reported in
various solid tumors and in cancer cell lines (4–9).
However, until recently, there are only very few studies on adult
thyroid stem/progenitor cells and thyroid CSCs (10–12).
We and others have recently described and characterized thyroid
cancer stem cells as a side population (13–17)
that may play a critical role in the progression and recurrence of
cancer and its subsequent metastasis (18).
Epithelial to mesenchymal transition (EMT) is a
vital process for morphogenesis during embryonic development, but
it has also been implicated in the conversion of early stage tumors
into invasive malignancies (19).
More recent studies have further demonstrated that EMT plays a
critical role not only in tumor metastasis but also in tumor
recurrence that is believed to be tightly linked with the biology
of cancer stem-like cells or cancer-initiating cells (20–23).
The relationship between EMT and CSCs has been observed, with the
evidence suggesting that EMT cells acquire stem cell-like traits
and that CSCs exhibit a mesenchymal-like appearance in immortalized
non-tumorigenic mammary epithelial cells and breast cancers
(22). In thyroid cancer, it was
found in our previous study that cancer stem cell content was
associated with EMT-phenotype, e.g. mesenchymal-type cells of HTH74
was detected with a much bigger cancer stem-like cell population
than epithelial-type cells of FTC133. With these findings in mind,
we proposed a plausible hypothesis that EMT program induces the
generation of thyroid cancer stem cells.
To test the hypothesis we performed EMT induction
through HIF-1α cDNA transfection on EMT-negative thyroid cancer
cells, and found that HIF-1α-overexpressed FTC133 cells acquired
EMT phenotype and shared stem-like cell features. Most importantly,
EMT induction was directly associated with increased stem-like cell
population. These data suggest that EMT represent the vital impetus
of cancer stem cell generation and thus prompt tumor progression
and metastasis, and these findings may provide useful perspectives
for anticancer drug exploitation in containment of thyroid CSC
generation.
Materials and methods
Cell culture
The human thyroid carcinoma cell line FTC133 was
kindly provided by Professor M. Derwahl, Humboldt University,
Germany. The FTC133 cell line was derived from the primary tumor of
a 42-year-old male patient with meta-static thyroid follicular
carcinoma (24), and then cultured
in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum and penicillin/streptomycin in a humidified
incubator at 37°C and 5% CO2.
Plasmids and transfections
Recombinant plasmid pcDNA3.1(-)/HIF-1α was kindly
provided by Dr Luo, Affiliated Beijing Anzhen Hospital of Capital
Medical University, China. For EMT induction, EMT-negative
(epithelial-type) FTC133 cells were transfected with
pcDNA3.1(-)/HIF-1α with Lipofectamine 2000 system (Invitrogen).
After 24 h of transient transfection, cells were subsequently
cultured in medium containing 600 μg/ml of G418 for 4 weeks.
HIF-1α positive cell clones were selected and expanded. FTC133
cells stably transfected with HIF-1α were designated as
FTC133/HIF-1α. After transfection, cells were maintained in hypoxic
environment (under 1% oxygen) for further culture and
experiments.
Western blot analysis
Antibodies against HIF-1α (as an EMT inducer),
Glut-1, VEGF (as HIF-1α target proteins), E-cadherin, CK18 (as
epithelial proteins), fibronectin, vimentin (as mesenchymal
markers), β-actin (as an internal control) were used for western
blot analyses as per instructions from manufacturers. Cell lysate
preparation and blotting conditions have been described previously
(25).
Immunofluorescent staining
Cells were fixed in 10% paraformaldehyde for 30 min
and blocked with goat serum for 30 min, respectively. Cells were
then incubated at 37°C for 1 h with rabbit anti-human β-catenin
antibody (Santa Cruz Biotechnology) at a dilution of 1:100. After
washing 3 times with PBS, cells were co-incubated with fluorescence
isothiocyanate (FITC) conjugated goat anti-rabbit antibody at 37°C
for 1 h. DAPI was used for nuclear DNA staining. The fluorescence
staining intensity and intracellular localization were then
examined by Olympus immunofluorescence microscope.
Invasion assays
The cells were cultured in 6-well plates to 90%
confluency and then collected after trypsinization. An analysis
assessing the invasion of cells was performed using 6-well
Transwell inserts with 6.5-mm diameters and 8-μM pores
(Corning). In brief, the filters were precoated for 30 min at 37°C
with 25 μl extracellular matrix (Sigma-Aldrich) gel mixed
with dimethyl sulfoxide (1:1). The trypsinised cells
(7×104) were washed with PBS, resuspended in the
serum-free medium, and placed in the upper chamber, and a medium
containing 10% FBS was used as a chemoattractant in the lower
chamber. Cells were incubated at 37°C in 5% CO2 for 24,
48 and 72 h, respectively, and the number of cells that invaded
across the membranes were fixed and stained with Giemsa. The
non-migratory cells on the upper chamber were removed with cotton
swabs, and the migratory cells present on the lower surface were
counted in 10 random fields and photographed by field at ×100
magnification under an inverted microscope (Olympus).
MTT assay for cell proliferation
Cells (1×104) were split into each well
of 96-well tissue culture plates. At various time points (1, 2, 3,
4 and 5 days), MTT solution (2.5 mg/ml; 50 μl) was added
into the cell culture plate and incubated with cells for an
additional 4 h. The media were collected separately from each
chamber, and cell-associated MTT crystals were dissolved separately
in dimethyl sulfuroxide (DMSO, 150 μl/well) on a shaker at
room temperature. The color intensity was measured at 570 nm
against the appropriate blank controls (0.1% BSA/RPMI-1640 medium
with MTT solution and 150 μl DMSO) using a Bio-Rad
Technologies Microplate Reader.
Spheroid formation assay
The potential of self-renewal was assessed using
Corning Ultra-Low Attachment Surface (Corning). Cells suspension
was sieved through a 30 μm strainer, centrifuged and
resuspended in growth factor-enriched medium: serum-free DMEM/Ham’s
F-12 (1:1) containing B-27 (1:50), 20 ng/ml EGF (Invitrogen,
Karlsruhe, Germany), and 20 ng/ml bFGF (Invitrogen). Single
cellularity was confirmed under a microscope. Cells were cultured
in 100-mm dishes at 10,000 viable cells/ml at 37°C, in 5%
CO2 culture incubator. Every 2–3 days, B27, bFGF and EGF
were added. After 10 days of culturing, spheres in 10 independent
microscopic fields were enumerated. The percentage of cells that
initiated a sphere was presented as sphere-forming efficiency
(CFE).
Colony-forming assays
Single-cell suspensions (200 cells per dish) were
seeded on 60-mm dishes (Corning) and cultured for 14 days. The
media were changed every 4 days. The colonies were washed twice
with PBS, and counted under a microscope. Only colonies with >32
cells were scored. The percentage of cells that initiated a clone
was presented as colony-forming efficiency (CFE).
SP sorting with FACS
Cells were harvested with Hoechst 33342 staining,
and verapamil (Sigma, St. Louis, MO, USA), an inhibitor of ABCG2
transporter, was used to identify specific cell populations, as
described previously (14). A
350-nm UV laser was used to excite Hoechst 33342. Analysis was
performed using a dual-wavelength analysis (blue, 420 nm; red, 670
nm). The side population was identified by gating on the
characteristic fluorescence emission profile. Equal numbers of SP
and non-SP (NSP) cells were collected for the following
experiments.
RNA isolation and reverse transcription
polymerase chain reaction (RT-PCR)
Total RNA was extracted using the RNeasy Micro kit
(Qiagen) according to the manufacturer’s specifications. RT-PCR was
performed as described previously (14). For PCR amplification, reactions
were carried out at 95°C for 10 min, 25–30 cycles of 95°C for 30
sec, 52–59°C (primer specific) for 30 sec and 72°C for 1 min,
followed by a final extension at 72°C for 10 min and termination at
4°C. RT-PCR cycles varied between primer sets to ensure that
amplification has not reached saturation level.
In all PCR analyses, β-actin was used as an internal
control. Primer sequences, product sizes, and annealing
temperatures are listed in Table
I. The PCR products were separated by electrophoresis on 1.5%
agarose gels stained with ethidium bromide. Signals corresponding
to target gene expression were normalized relative to β-actin for
each sample.
 | Table I.Primer sequences, annealing
temperatures and product sizes for semi-quantitative RT-PCR. |
Table I.
Primer sequences, annealing
temperatures and product sizes for semi-quantitative RT-PCR.
| Target gene | Primer
sequencesa | Annealing
temperature (°C) | Expected size
(bp) |
|---|
| E-cadherin | S:
5′-ctgaagtgactcgtaacgac-3′ | | |
| AS:
5′-catgtcagccagcttcttgaag-3′ | 55 | 300 |
| Vimentin | S:
5′-tggcacgtcttgaccttgaa-3′ | | |
| AS:
5′-ggtcatcgtgatgctgagaa-3′ | 56 | 749 |
| ABCG2 | S:
5′-agttccatggcactggccata-3′ | | |
| AS:
5′-tcaggtaggcaattgtgagg-3′ | 56 | 379 |
| OCT4 | S:
5′-gacaacaatgagaaccttcaggag-3′ | | |
| AS:
5′-ctggcgccggttacagaacca-3′ | 56 | 216 |
| TG | S:
5′-agtcctaagtcccctgatgc-3′ | | |
| AS:
5′-caagggagacgtcgagtgt-3′ | 52 | 280 |
| NIS | S:
5′-tctctcagtcaacgcctct-3′ | | |
| AS:
5′-atccaggatggccacttctt-3′ | 58 | 299 |
| β-actin | S:
5′-cccaggcaccagggcgtgat-3′ | | |
| AS:
5′-tcaaacatgatctgggtcat-3′ | 59 | 280 |
Statistical analysis
All data are represented as mean ± SD from at least
three independent experiments. When two groups were compared, the
Student’s t-test was used, and p<0.05 was considered
significant.
Results
Overexpression of HIF-1α induces EMT and
β-catenin nucleus translocation
HIF-1α has been shown to induce EMT, during which
epithelial cells lose their polarity and convert to a mesenchymal
phenotype (25). In this study, we
transfected FTC133 cells with vector encoding human HIF-1α. Six
weeks later, the stable FTC133/HIF-1α cell clones were obtained.
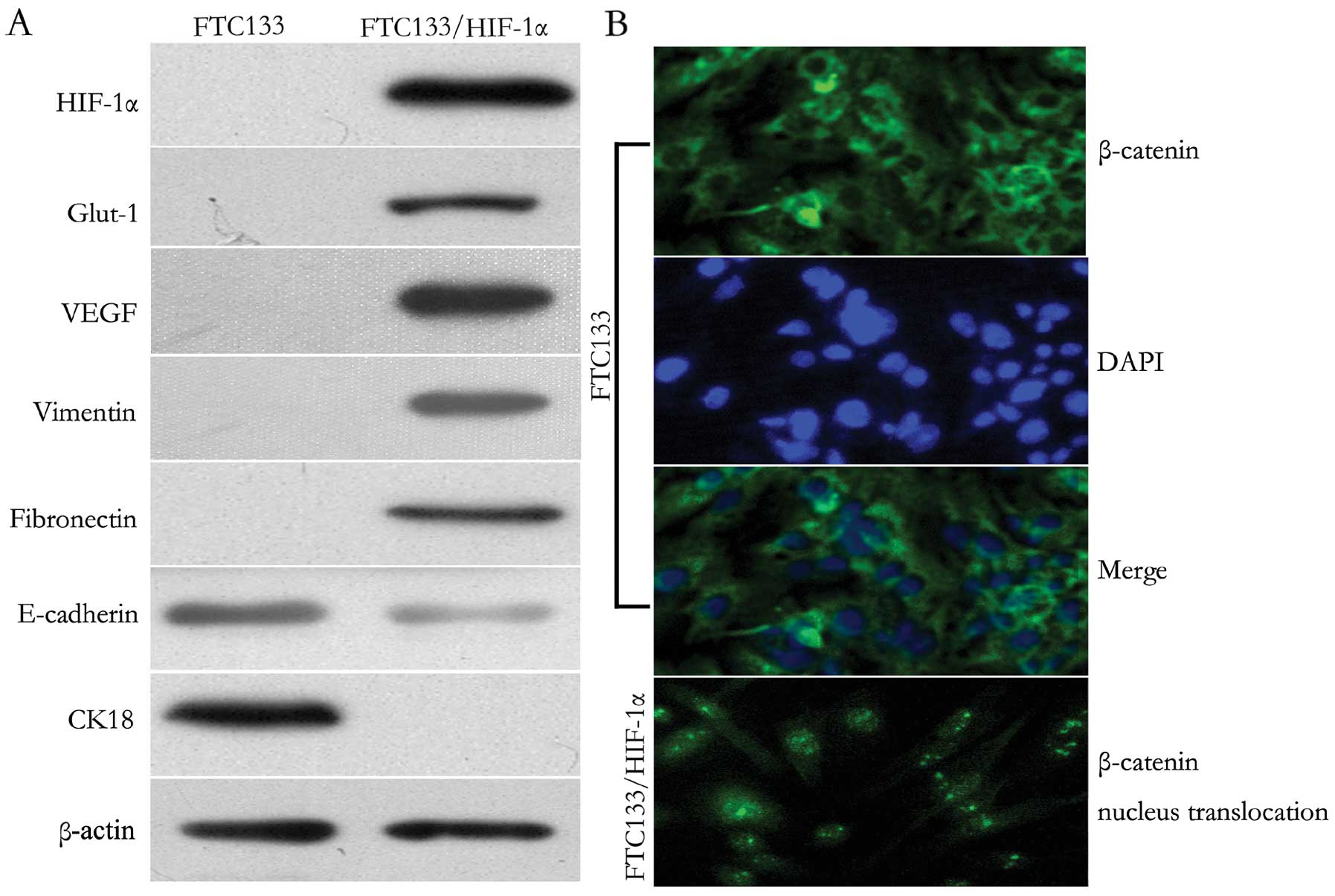
Upon observation by microscopy, the FTC133/HIF-1α cells showed
typical fibroblast-like and spindle-shaped appearance, whereas the
untransfected FTC133 cells showed robust cellular junctions with
typical ‘cobblestone-shaped’ and epithelial-like appearance
(Fig. 1B). To determine if the
pattern of protein expression correlated with EMT, western blot
analysis was performed. Functional expression of HIF-1α was
confirmed since two downstream target proteins, Glut-1 and VEGF,
regulated by HIF-1α were both positively expressed in FTC133/HIF-1α
cells. In addition, the increased expression of HIF-1α resulted in
a decrease of the epithelial markers E-cadherin and CK18, but an
increase of vimentin and fibronectin (Fig. 1A). Moreover, as shown in
immunofluorescent staining (Fig.
1B), HIF-1α induction resulted in specific nucleus
translocation of β-catenin from the cytoplasma. These results
demonstrated that increased HIF-1α expression prompted EMT
induction and Wnt/β-catenin signal activation in FTC133 cells.
Cells that acquire an EMT phenotype show
high invasiveness and proliferation in vitro
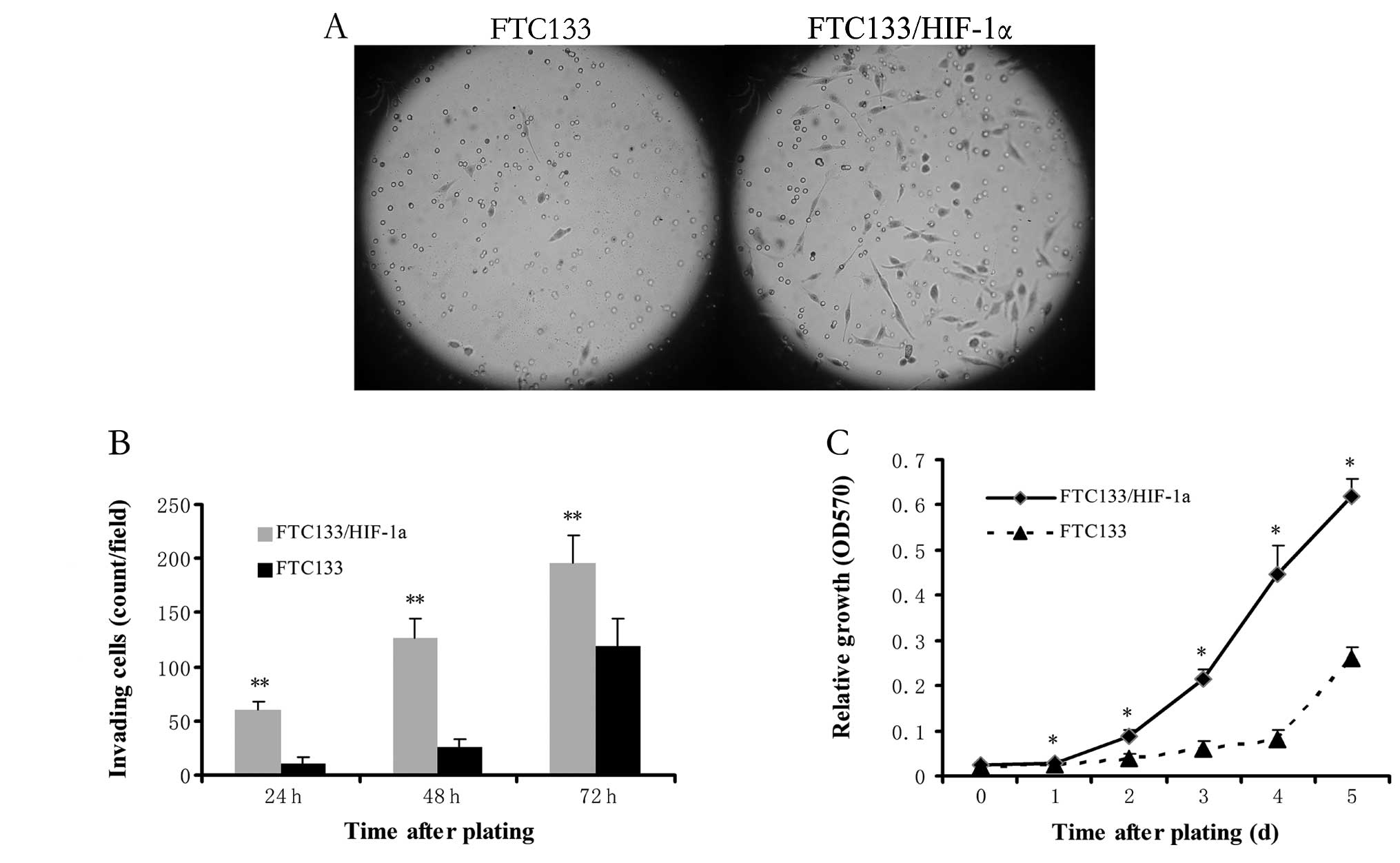
To test whether the cells that have undergone EMT
show changes in invasiveness and metastatic ability, transwell
assay was performed to measure single-cell invasion. After
different time points, the invasive cells were quantified and
photographed at ×100 magnification in 10 randomly chosen fields.
The FTC133/HIF-1α cells were more invasive than untransfected
FTC133 cells and showed a 6-fold increase in gel invasion after
24-h incubation (p<0.01, Fig. 2A
and B), which indicated that enhanced HIF-1α expression
contributes to increased invasiveness. Moreover, MTT proliferation
assay displayed much higher proliferation in FTC133/HIF-1α cells
than untransfected FTC133 cells at each time-point (all p<0.05
except time-point of 0 day, Fig.
2C). Specifically, doubling of FTC133/HIF-1α cells occurs on
day 2, whereas that of FTC133 cells occurs on day 5. This finding
indicates cells that acquire an EMT phenotype were more invasive
and proliferative in vitro.
EMT generates stem-like cells that
express markers associated with both stemness and mesenchymal
properties
To test the hypothesis that EMT program determines
the CSC generation, stem-like side population cells were compared
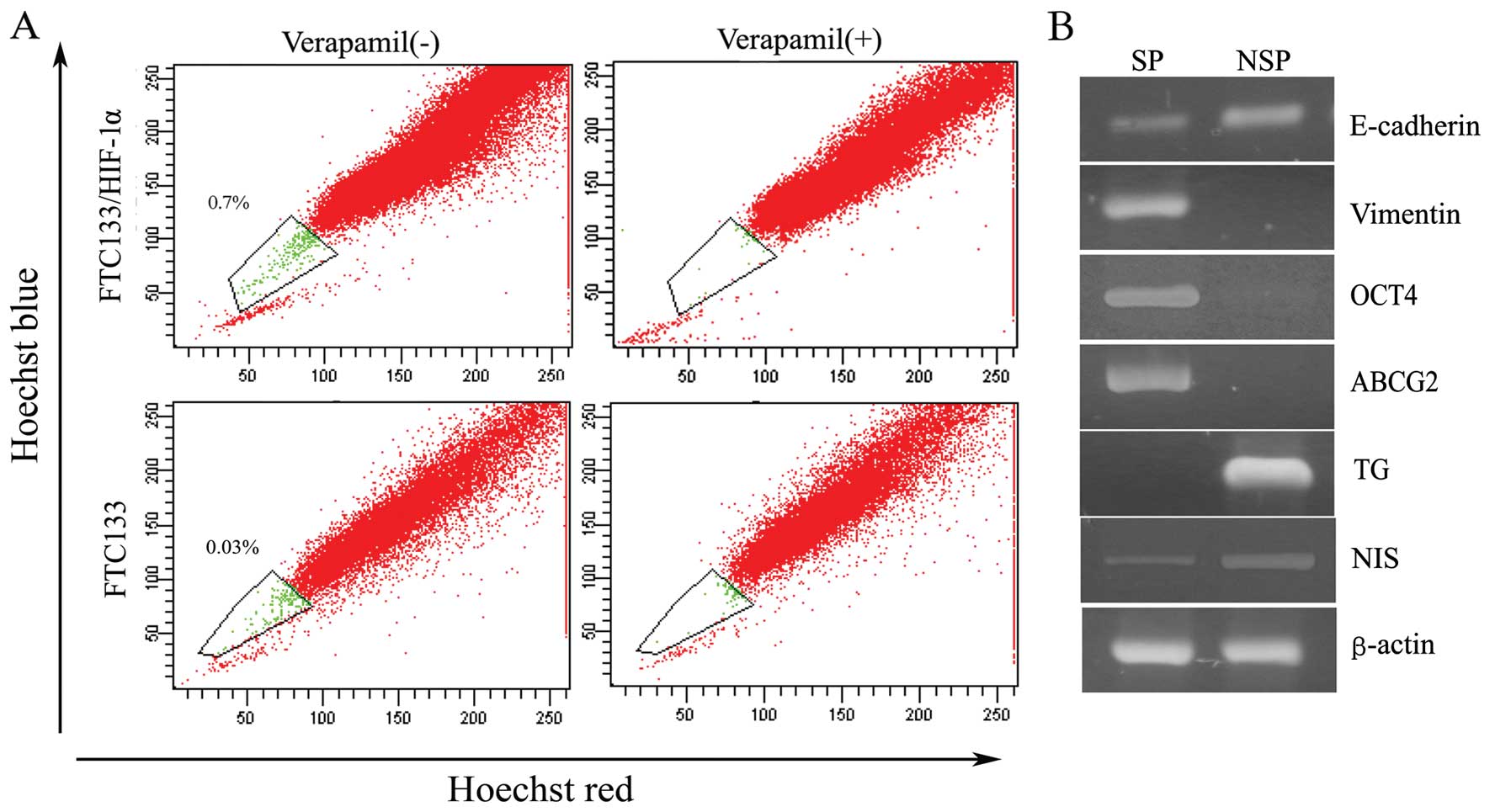
in FTC133 and FTC133/HIF-1α cells. As depicted in Fig. 3A, through HIF-1α
transfection, FTC133 cells acquired a much bigger side population
in contrast to untransfected cells (SP, 0.7 vs. 0.03%). In our
previous study side population cells from either thyroid primary
tissue or anaplastic thyroid cancer cell lines were identified as
primitive stem cells with associated stem cell marker expression
and self-renewal potential (14,16).
Accordingly, in the present study, we used semi-quantitative RT-PCR
to measure the expression of EMT-associated markers as well as
stemness and differentiation-associated genes in SP sorted from
FTC133/HIF-1α cells. Specifically, we found, in contrast to NSP
cells, the side population exhibited a strong reduction in the
expression of E-cadherin and a significantly increased expression
of vimentin (Fig. 3B).
Furthermore, SP cells were characterized with high expression of
stem cell transcription factor OCT4, and ATP-binding cassette
transporter G2 (ABCG2), as well as low expression of thyroid
differentiation factor thyroglobulin (TG) and sodium/iodide
importer (NIS), as shown in Fig.
3B. These measurements provided further indication that the
side population cells exist in a stem-like cell state that
simultaneously closely resembles that of cells that have undergone
EMT.
EMT-generated stem-like cells exhibit
self-renewal and tumorigenic potential in vitro
Our previous studies (14,16)
have demonstrated that the ability to form thyroid-spheres in
vitro depends on the presence of self-renewing stem cells
within the thyroid tissue cell population or thyroid cancer cell
lines. Therefore, a sphere growth culture was performed to test the
self-renewal potential and the colony-forming assay synchronously
served as an in vitro surrogate measure of
tumorigenicity.
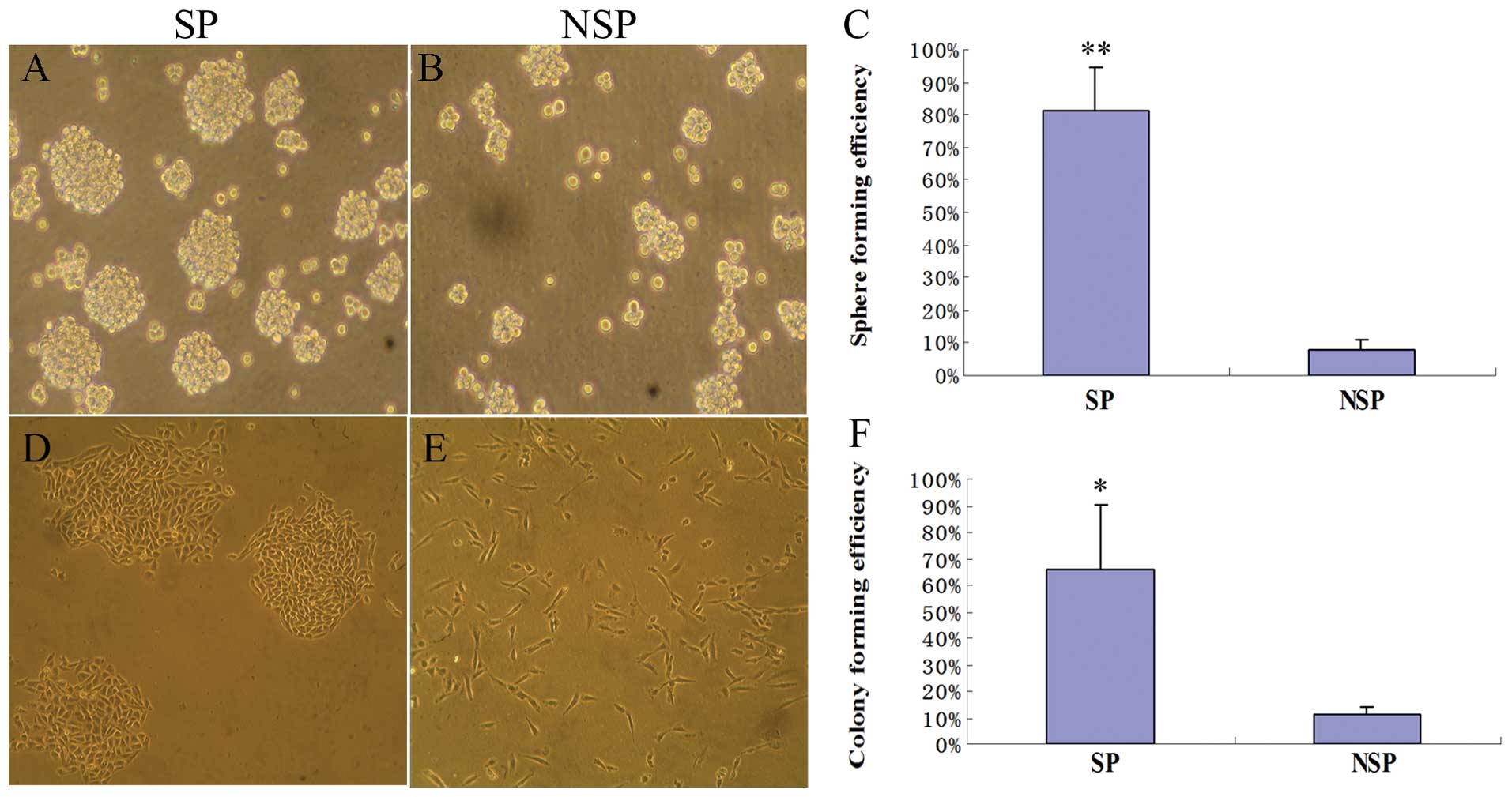
As anticipated, we observed that SP cells from
FTC133/HIF-1α cells formed at least 10-fold more tumor spheres than
the NSP cells (p<0.01, Fig.
4A–C). Moreover, most SP cells could sustain a clone growth and
form characteristic compact round colonies when plated in a very
low density, whereas NSP cells always grew as more elongated or
flattened cells in a scattered pattern with much fewer clones
formed (Fig. 4D–E). As shown in
the colony forming assay, SP cells formed 6-fold more colonies in
soft agar, compared with the NSP cells (p<0.05, Fig. 4F). Based on these functional
assays, we concluded that the cells generated by EMT acquired
further two attributes of thyroid cancer stem cells.
Discussion
Thyroid cancer is the most prevalent endocrine
neoplasia and accounts for approximately 1% of all carcinomas
(26). Among these, anaplastic
thyroid carcinoma is considered to be one of the most rapidly
growing lethal neoplasms in humans. Emerging evidence suggest that
the acquisition of EMT is strongly associated with cancer cell
invasion and tumor metastasis. Recently, studies have shown that
cells with EMT phenotype share characteristics that are consistent
with the signatures of cancer stem-like cells, which are associated
with tumor recurrence and drug resistant phenotype and contribute
to the demise of patients diagnosed with cancer (22,27,28).
Understanding the molecular and cellular changes may generate novel
targets that can overcome the aforementioned issues and reduce
mortality.
In the current study, we made three novel
observations. Firstly, we found that forced-expression of HIF-1α in
epithelial-type thyroid cancer cells induced cellular morphological
changes that were consistent with the acquisition of EMT phenotype
as characterized by the loss of expression of epithelial markers
and the gain or increased expression of mesenchymal markers.
Furthermore, these EMT-induced cells exhibited specific mesenchymal
properties and were highly invasive and proliferative. Most
importantly, the induction of EMT conferred the cells an increased
clonogenic and sphere-forming capacity, the characteristics that
are known to be associated with cancer stem-like cell
characteristics.
EMT-inducers, such as transforming growth factor-β
(TGF-β) or hypoxia, trigger changes in gene expression by complex
signaling pathways. A basic mechanism involved in progression of
EMT is upregulation of the mesenchymal marker vimentin and
downregulation of the epithelial marker E-cadherin, the main
transmembrane adhesion molecule responsible for cell-to-cell
interactions and tissue organization in epithelial cells (29,30).
In our study, FTC133 cells were identified as epithelial-type cells
with specific protein expression. When overexpressed with HIF-1α,
these cells (FTC133/HIF-1α) adopted mesenchymal cell properties,
and presented fibroblastoid phenotypes, had a more extended and
spindle-like shape. These significant morphological changes
combined with specific protein analysis of a number of canonical
EMT markers demonstrated that the epithelial tumor cells underwent
EMT, in which high invasiveness and proliferation were identified
with functional analysis as invasion and MTT assays. These findings
in FTC133 cells are consistent with our prior study that showed
HIF-1α has a crucial role in EMT and is associated with an
increased invasive ability in human prostate cancers (25). Previous mounting evidence suggests
hypoxia as an important driving force and master regulator for the
multistep process of metastasis (31,32).
In addition, the activated HIFs may induce the expression of
numerous gene products such as induced pluripotency-associated
transcription factors (Oct-3/4, Nanog and Sox-2), glycolysis- and
epithelial-mesenchymal transition (EMT) programme-associated
molecules, including CXC chemokine receptor 4 (CXCR4), snail and
twist, microRNAs and angiogenic factors such as vascular
endothelial growth factor (VEGF). Moreover, several studies have
suggested that Wnt/β-catenin signaling pathway plays an important
role in epithelial-mesenchymal transition (33–36).
Also in the current study, we found that the HIF-1α inducted EMT
program is accompanied by activation of Wnt/β-catenin signal
pathway and nucleus translocation of the adherens junction
component β-catenin. All these gene products in turn can play
critical roles for high self-renewal ability, survival, altered
energy metabolism, invasion and metastases of cancer cells,
angiogenic switch and treatment resistance. Consequently, the
targeting of hypoxic inducible factor signaling network and altered
metabolic pathways in cancer- and metastasis-initiating cells and
their progenies as well as their supporting host cells represents
new promising strategies to simultaneously eradicate the total mass
of cancer cells and improve the efficacy of current therapies
against aggressive and metastatic cancers and prevent disease
relapse.
Self-renewal potential, which is one of the
hallmarks of CSCs, has been demonstrated in CSCs through their
ability to form colonies or spheres (37,38).
In this study, we have shown that the induction of EMT in human
thyroid cancer cells yields cells with much higher invasiveness and
proliferation. We have confirmed, that such cells are greatly
enriched in tumor-initiating cells with markedly high CFE and
sphere-formation capability, as well as specific stemness
signatures of OCT4 and ABCG2. Therefore, our findings suggest a
close correlation between EMT program and the emergence of CSC-like
phenotype in thyroid cancer cells.
In previous studies, some scholars have described a
‘parent-child relationship’ between EMT and CSC based on
experiments that revealed some common molecular mechanisms shared
by the EMT development and CSC generation. Using a mammary tumor
progression model, Morel et al showed that acquisition of
stemness characteristics in epithelial cells can be driven by EMT
induction following the activation of the Ras-MAPK pathway
(39). Mani et al also
reported that the breast cancer cells that had undergone EMT
triggered by various factors are rich source of stem-like cancer
cells and the induction of EMT in differentiated immortalized human
mammary epithelial cells led to the acquisition of
CD44+/CD24− stem cell phenotype (20). These data further support our
findings that EMT program represents the driving force for the
generation of cancer stem-like cells in thyroid cancer.
Collectively, our previous and current studies
revealed, follicular thyroid cancer cells that acquired EMT
phenotype as anaplastic thyroid cancer cells, shared cellular and
molecular characteristics of stem cells or cancer stem-like cells,
most importantly, we firstly reported the increased cancer stem
cell content directly correlates with EMT induction, and concluded
that, the emergence of CSC-like phenotype occurs as a result of EMT
in epithelial thyroid cancer cells. Activated Wnt/β-catenin signals
by hypoxic stimulus played a critical role in linking EMT phenotype
with stem cell signatures by activating EMT program. Therefore, in
future studies we may emphasize elimination of stem-like cells by
undermining the hypoxia microenviroment or hypoxic inducible factor
signaling network, or further reversing EMT phenotypic cells to MET
phenotype in anaplastic thyroid cancer. We firmly believe that
strategies by which one could either reverse the EMT to MET
phenotype or could selectively kill EMT-phenotypic cells or cancer
stem-like cells that are ‘Root cause’ of tumor development and
recurrence, could become a novel approach for the prevention of
tumor progression and/or treatment of anaplastic thyroid cancer and
its metastasis for which newer therapies are urgently needed.
Acknowledgements
The authors are grateful to Professor
Michael Derwahl (Berlin, Germany) for the gift of of FTC133 cell
line and for his excellent technical guidance. This study was
supported by National Natural Science Foundation of China (fund
nos. 30901725, 30700968 and 30800416).
References
|
1.
|
Are C and Shaha AR: Anaplastic thyroid
carcinoma: biology, pathogenesis, prognostic factors and treatment
approaches. Ann Surg Oncol. 13:453–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ward RJ and Dirks PB: Cancer stem cells:
at the headwaters of tumor development. Annu Rev Pathol. 2:175–189.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Nuchtern JG, Jax TW, Gobel U, Goodell MA and Brenner MK: A distinct
‘side population’ of cells with high drug efflux capacity in human
tumor cells. Proc Natl Acad Sci USA. 101:14228–14233. 2004.
|
|
6.
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Re. 63:5821–5828.
2003.PubMed/NCBI
|
|
7.
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ignatova TN, Kukekov VG, Laywell ED,
Suslov ON, Vrionis FD and Steindler DA: Human cortical glial tumors
contain neural stem-like cells expressing astroglial and neuronal
markers in vitro. Glia. 39:193–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Friedman S, Lu M, Schultz A, Thomas D and
Lin RY: CD133+ anaplastic thyroid cancer cells initiate
tumors in immunodeficient mice and are regulated by thyrotropin.
PLoS One. 4:e53952009.
|
|
11.
|
Zito G, Richiusa P, Bommarito A, Carissimi
E, Russo L, Coppola A, Zerilli M, Rodolico V, Criscimanna A, Amato
M, Pizzolanti G, Galluzzo A and Giordano C: In vitro identification
and characterization of CD133(pos) cancer stem-like cells in
anaplastic thyroid carcinoma cell lines. PLoS One. 3:e35442008.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Thomas D, Friedman S and Lin RY: Thyroid
stem cells: lessons from normal development and thyroid cancer.
Endocr Relat Cancer. 15:51–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Thomas T, Nowka K, Lan L and Derwahl M:
Expression of endoderm stem cell markers: evidence for the presence
of adult stem cells in human thyroid glands. Thyroid. 16:537–544.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lan L, Cui D, Nowka K and Derwahl M: Stem
cells derived from goiters in adults form spheres in response to
intense growth stimulation and require thyrotropin for
differentiation into thyrocytes. J Clin Endocrinol Metab.
92:3681–3688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fierabracci A, Puglisi MA, Giuliani L,
Mattarocci S and Gallinella-Muzi M: Identification of an adult
stem/progenitor cell-like population in the human thyroid. J
Endocrinol. 198:471–487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zheng XQ, Cui D, Xu SH, Brabant G and
Dereahl M: Doxorubicin fails to eradicate cancer stem cells derived
from anaplastic thyroid carcinoma cells: characterization of
resistant cells. Int J Oncol. 37:307–315. 2010.PubMed/NCBI
|
|
17.
|
Mitsutake N, Iwao A, Nagai K, Namba H,
Ohtsuru A, Saenko V and Yamashita S: Characterization of side
population in thyroid cancer cell lines: cancer stem-like cells are
enriched partly but not exclusively. Endocrinology. 148:1797–1803.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Takano T and Amino N: Fetal cell
carcinogenesis: a new hypothesis for better understanding of
thyroid carcinoma. Thyroid. 15:432–438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell
LL, Polyak K, Brisken C, Yang J and Weinberg RA: The epithelial
mesenchymal transition generates cells with properties of stem
cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Raimondi C, Gianni W, Cortesi E and
Gazzaniga P: Cancer stem cells and epithelial-mesenchymal
transition: revisiting minimal residual disease. Current Cancer
Drug Targets. 10:496–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hollier BG, Evans K and Mani SA: The
epithelial-to-mesenchymal transition and cancer stem cells: a
coalition against cancer therapies. J Mammary Gland Biol Neoplasia.
14:29–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hayashida T, Jinno H, Kitagawa Y and
Kitajima M: Cooperation of cancer stem cell properties and
epithelial-mesenchymal transition in the establishment of breast
cancer metastasis. J Oncol. 2011:5914272011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Goretzki PE, Frilling A, Simon D and
Roeher HD: Growth regulation of normal thyroids and thyroid tumors
in man. Recent Results Cancer Res. 118:48–63. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Luo Y, He DL, Ning L, Shen SL, Li L, Li X,
Zhau HE and Chung LW: Over-expression of hypoxia-inducible
factor-1alpha increases the invasive potency of LNCaP cells in
vitro. BJU Int. 98:1315–1319. 2006. View Article : Google Scholar
|
|
26.
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995. Cancer.
83:2638–2648. 1998.PubMed/NCBI
|
|
27.
|
Kong D, Banerjee S, Ahmad A, Li Y, Wang Z,
Sethi S and Sarkar FH: Epithelial to mesenchymal transition is
mechanistically linked with stem cell signatures in prostate cancer
cells. PLoS One. 5:e124452010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Klarmann GJ, Hurt EM, Mathews LA, Zhang X,
Duhagon MA, Mistree T, Thomas SB and Farrar WL: Invasive prostate
cancer cells are tumor initiating cells that have a stem cell-like
genomic signature. Clin Exp Metastasis. 26:433–446. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Salnikov AV, Liu L, Platen M, Gladkich J,
Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J,
Schemmer P, Buechler MW and Herr I: Hypoxia induces EMT in low and
highly aggressive pancreatic tumor cells but only cells with cancer
stem cell characteristics acquire pronounced migratory potential.
PLoS One. 7:e463912012. View Article : Google Scholar
|
|
31.
|
Lu X and Kang YB: Hypoxia and
hypoxia-inducible factors: master regulators of metastasis. Clin
Cancer Res. 16:5928–5935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 17:30–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kim K, Lu Z and Hay ED: Direct evidence
for a role of beta-catenin/ LEF-1 signaling pathway in induction of
EMT. Cell Biol Int. 26:463–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Medici D, Hay ED and Olsen BR: Snail and
Slug promote epithelialmesenchymal transition through
beta-catenin-T-cell factor-4-dependent expression of transforming
growth factor-beta3. Mol Biol Cell. 19:4875–4887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Yang L, Lin C and Liu ZR: P68 RNA helicase
mediates PDGF-induced epithelial mesenchymal transition by
displacing Axin from beta-catenin. Cell. 127:139–155. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar
|
|
38.
|
Hurt EM, Chan K, Serrat MA, Thomas SB,
Veenstra TD and Farrar WL: Identification of vitronectin as an
extrinsic inducer of cancer stem celldifferentiation and tumor
formation. Stem Cell. 28:390–398. 2010.PubMed/NCBI
|
|
39.
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|