Introduction
Angiogenesis is the development of new capillaries
from preexisting vasculature during tumor growth and metastasis
(1,2) and is regulated by the balanced action
of angiogenic activators and inhibitors (3,4).
This process is complex and involves diverse cellular actions, such
as degradation of the extracellular matrix (ECM), proliferation and
migration of endothelial cells, and morphological differentiation
of endothelial cells to form tubes (5).
Angiogenesis and inflammation are important
processes involved in tumor growth and expansion (6–8).
Natural compounds with anti-inflammatory properties can also have
anti-angiogenic activities since these processes share a common
signaling pathway such as NF-κB and Akt (9,10).
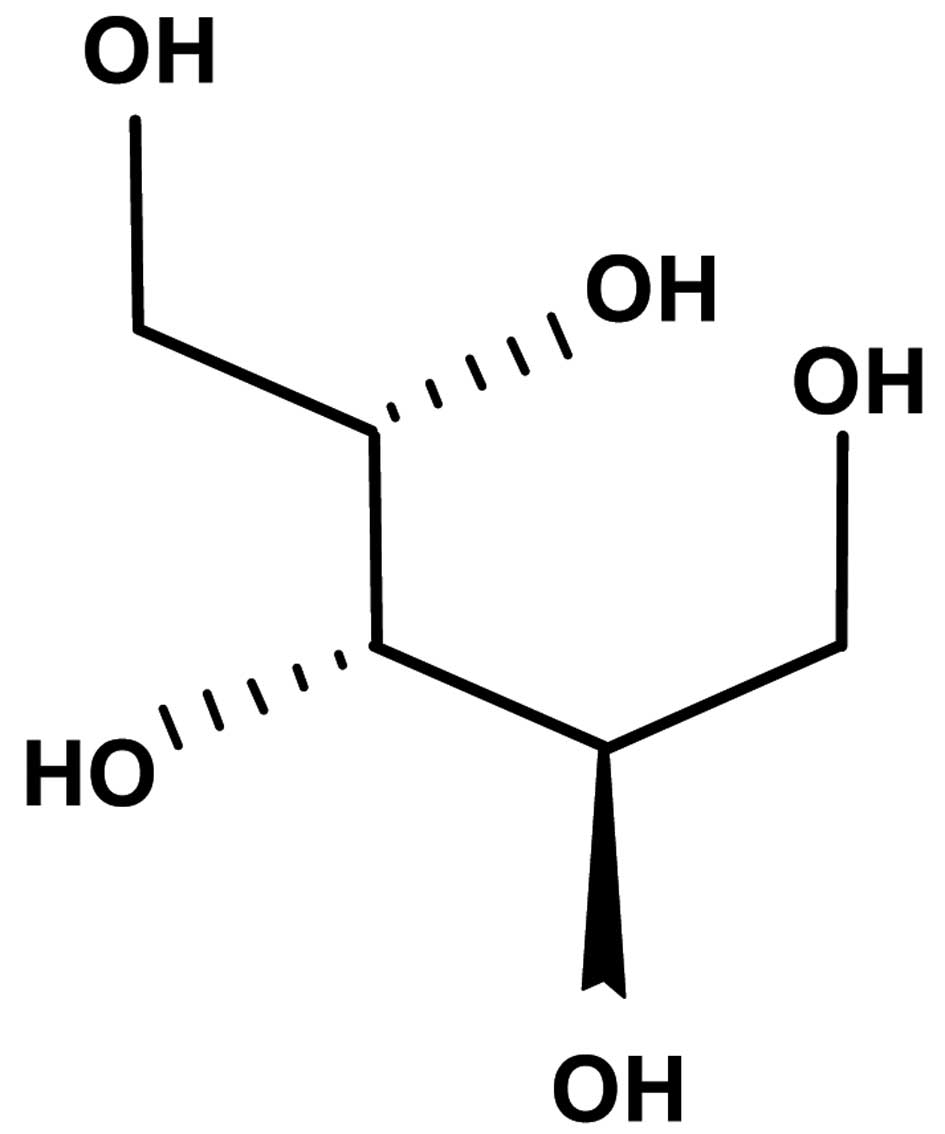
One of these natural compounds is xylitol. Xylitol, first derived
from Birch trees in Finland in the 20th century (11), is a 5-carbon sugar alcohol
(12) (Fig. 1). It has been widely used as a
sugar substitute and is found in the fibers of many fruits and
vegetables (13). Xylitol can also
be used as an artificial sweetener in chewing gums to prevent tooth
decay (14,15).
In a recent study, xylitol was shown to inhibit
lipopolysaccharide (LPS)-induced inflammatory cytokine expression
(16). Because inflammation and
angiogenesis share a common signaling pathway (17,18),
we tested whether xylitol has anti-angiogenic effects by using
in vitro and in vivo angiogenesis assays. We found
that xylitol suppressed tube formation, migration and invasion of
human umbilical vein endothelial cells (HUVECs). Xylitol also
inhibited in vivo angiogenesis in a Matrigel plug assay.
Additionally, xylitol downregulated the mRNA expression of vascular
endothelial growth factor (VEGF), VEGFR-II (KDR), basic fibroblast
growth factor (bFGF), bFGFR-II, matrix metalloproteinase-2 (MMP-2)
and MMP-9 of HUVECs. We also found that xylitol suppressed NF-κB
and Akt activation in HUVECs.
Materials and methods
Materials and reagents
HUVECs were purchased from InnoPharmaScreen
(Chungnam, Korea). Matrigel was obtained from Collaborative
Biomedical Products (Bedford, MA, USA) for the mouse Matrigel plug
assay. Basic fibroblast growth factor (bFGF) and heparin were
obtained from PeproTech (Gaithersburg, MD, USA). Fetal bovine serum
(FBS), penicillin and streptomycin were purchased from JBI (Daegu,
Korea). Drabkin reagent kit 525 was purchased from Sigma (St.
Louis, MO, USA). The 8-μm pore Transwell filter chambers
were purchased from Corning-Costar (Corning, NY, USA). Antibodies
for NF-κB, phosphor-NF-κB, Akt, and phosphor-Akt were purchased
from Cell Signaling Technology Inc. (Danvers, MA, USA). Xylitol and
gelatin were purchased from Sigma.
HUVECs
HUVECs were grown in M199 supplemented with
heat-inactivated 20% fetal bovine serum (JBI), 20 ng/ml of bFGF, 10
U/ml of heparin, 100 U/ml of penicillin and 100 μg/ml of
streptomycin in a 37°C incubator with a humidified atmosphere
containing 5% CO2.
Animals
Seven-week-old, specific pathogen-free (SPF) male
C57BL/6 mice were supplied by Hyochang Science and Samtako (Daegu
and Kyung-gi, Korea). They were provided with autoclaved tap water
and lab chow ad libitum and were housed at 23±0.5°C, 10%
humidity under a 12-h light-dark cycle. The animal protocol used in
this study was reviewed on the ethical procedures and scientific
care, and approved by the Pusan National University-Institutional
Animal Care and Use Committee (PNU-IACUC).
In vitro tube formation assay
HUVECs (2×104 cells) were seeded on a
layer of previously polymerized Matrigel and treated with or
without xylitol. Matrigel culture was incubated at 37°C. After 6 h,
changes in cell morphology were captured through a phase contrast
microscope and photographed at ×40 magnification. Each sample was
assayed in duplicate and independent experiments were repeated
three times.
In vitro wounding migration assay
HUVECs were seeded onto 24-well culture plates until
confluence and left overnight. Media was aspirated the next day,
and cells were scratched with a 200 μl pipette tip along the
diameter of the well. Cells were washed twice with PBS and
incubated at 37°C and 5% CO2. After wounding, the
cultures were washed with serum-free medium and further incubated
in M199 with 1% serum, 1 mM thymidine and/or xylitol. These culture
conditions minimized proliferation of HUVECs. Wound diameters were
photographed at 18 to 24 h. Wound closure was determined by
measurement with optical microscopy at ×40 magnification. Migration
was quantitated by counting the number of cells that moved beyond
the reference line. Each sample was assayed in duplicate, and
independent experiments were repeated three times.
In vitro invasion assay
Invasiveness of HUVECs was performed in vitro
using a Transwell chambers system (Corning-Costar) with
8.0-μm pore polycarbonate filter inserts. The upper side was
coated with 10 μl of Matrigel (0.5 mg/ml) at room
temperature for 1 h. Cells (2×104 cells) and xylitol in
serum-free medium was placed in the upper part of the filters, and
full medium was treated in the lower parts. Cells were incubated at
37°C for 24 h, fixed with methanol, and then stained with
hematoxylin and eosin. Cells on the upper surface of the membrane
were removed by wiping with a cotton swab. Cell invasion was
determined by counting whole cell numbers in a single filter by
optical microscopy at ×40 magnification. Each sample was assayed in
duplicate and independent experiments were repeated three
times.
In vivo mouse Matrigel plug assay
C57BL/6 mice (7 weeks of age) were injected
subcutaneously into 500 μl of Matrigel (Collaborative
Biomedical Products, Bedford, MA, USA) containing bFGF (100 ng/ml)
and heparin (50 U/ml) without or with xylitol. After injection, the
Matrigel rapidly formed a plug. After 7 days, skin of the mouse was
pulled back to expose the Matrigel plug, which remained intact.
After quantitative differences were noted and photographed,
hemoglobin content was measured using the Drabkin reagent kit 525
(Sigma) for quantification of blood vessel formation. The amount of
hemoglobin was calculated from a known amount of hemoglobin assayed
in parallel. Independent experiments were repeated twice and at
least five mice in each experiment were used.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from HUVECs was isolated using TRIzol
reagent (Invitrogen Corporation, Carlsbad, CA, USA) according to
the manufacturer’s instructions. First-stranded cDNA was
synthesized by M-MLV reverse transcriptase (Promega Corporation,
Madison, WI, USA) with 2 μg each of DNA-free total RNA
sample and oligo (dT)15 (Life Technologies, Grand Island, NY, USA)
according to the manufacturer’s instructions. Equal amounts of cDNA
were subsequently amplified by PCR in a 20 μl reaction
volume containing 1X PCR buffer, dNTP mixture, 10 μM of each
specific primer and i-Taq™ DNA polymerase (iNtRON Biotechnology,
Sungnam, Korea). Amplification products were electrophoresed on 1%
agarose gels and visualized by GelRed (Biotium Inc., Hayward, CA,
USA) staining under ultraviolet trans-illumination.
Western blot analysis
HUVECs were treated with or without xylitol for 24 h
in medium. Total cell lysates were prepared by addition of PRO-PREP
Protein Extraction Solution (iNtRON Biotechnology), including 1 mM
sodium orthovanadate. Equal amounts (30 μg) of samples were
resolved by electrophoresis on a 10% SDS-polyacrylamide gel,
transferred to a membrane and sequentially probed with antibody.
The following primary antibodies were used at the indicated
dilutions: total NF-κB and anti-phospho-NF-κB, total Akt and
anti-phospho-Akt, 1:1,000 in 5% BSA in TBS-T.
Results
Xylitol inhibits vascular network
formation in HUVECs
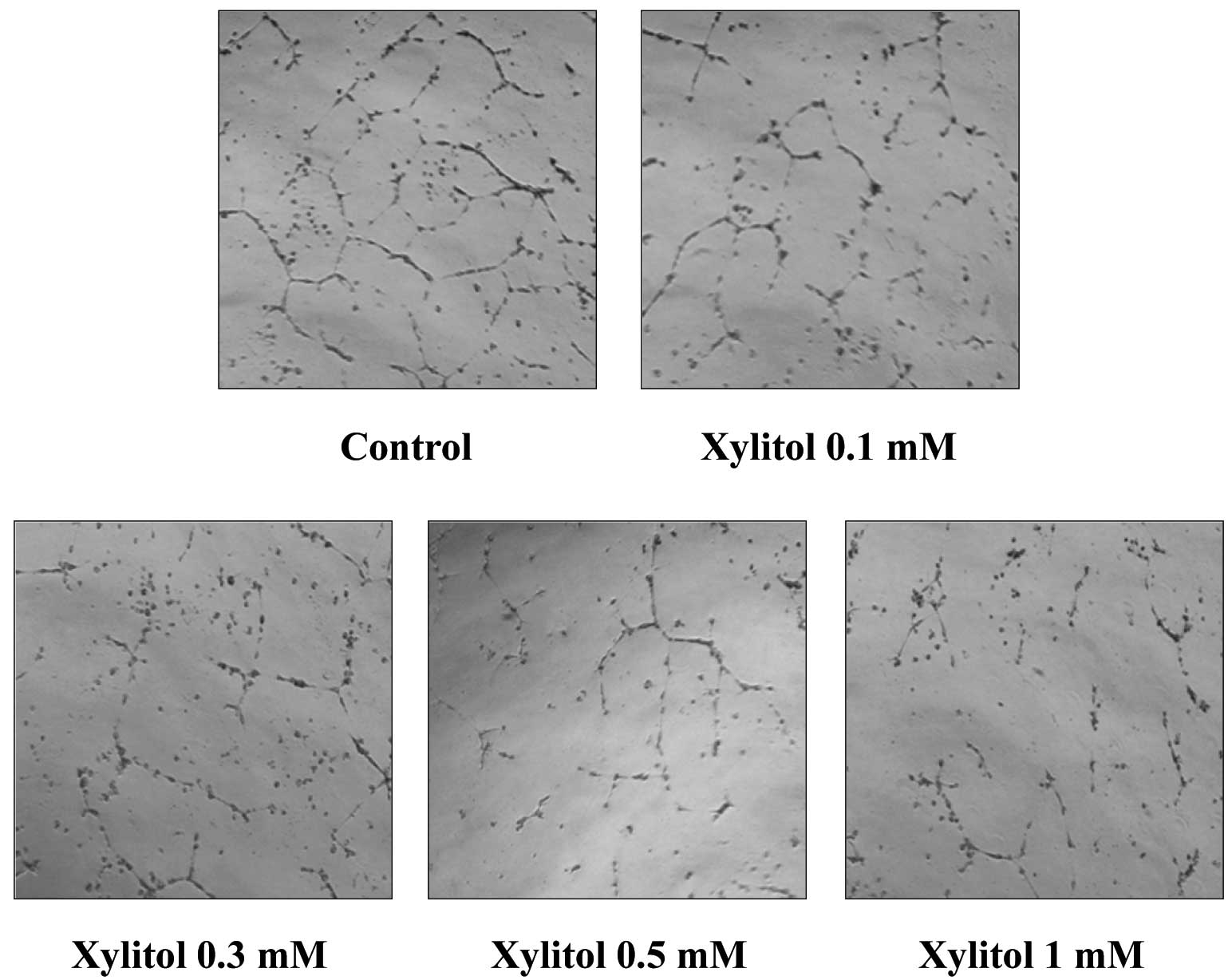
Because differentiation of endothelial cells into a
capillary-like network is important for the process of
angiogenesis, we tested the effect of xylitol on the morphological
differentiation of endothelial cells in vitro. HUVECs were
placed on a Matrigel-coated plate and incubated. Endothelial cells
formed weak capillaries on Matrigel beds, and these tubes became
stronger and more robust with elongated networks over 6–24 h.
HUVECs on Matrigel formed a blood vessel-like network in the
absence of xylitol (Fig. 2),
whereas treatment with xylitol for 18 h resulted in broken,
shortened and narrow tube networks. This result shows that xylitol
inhibits tube formation of HUVECs.
Xylitol inhibits migration and invasion
activity in HUVECs
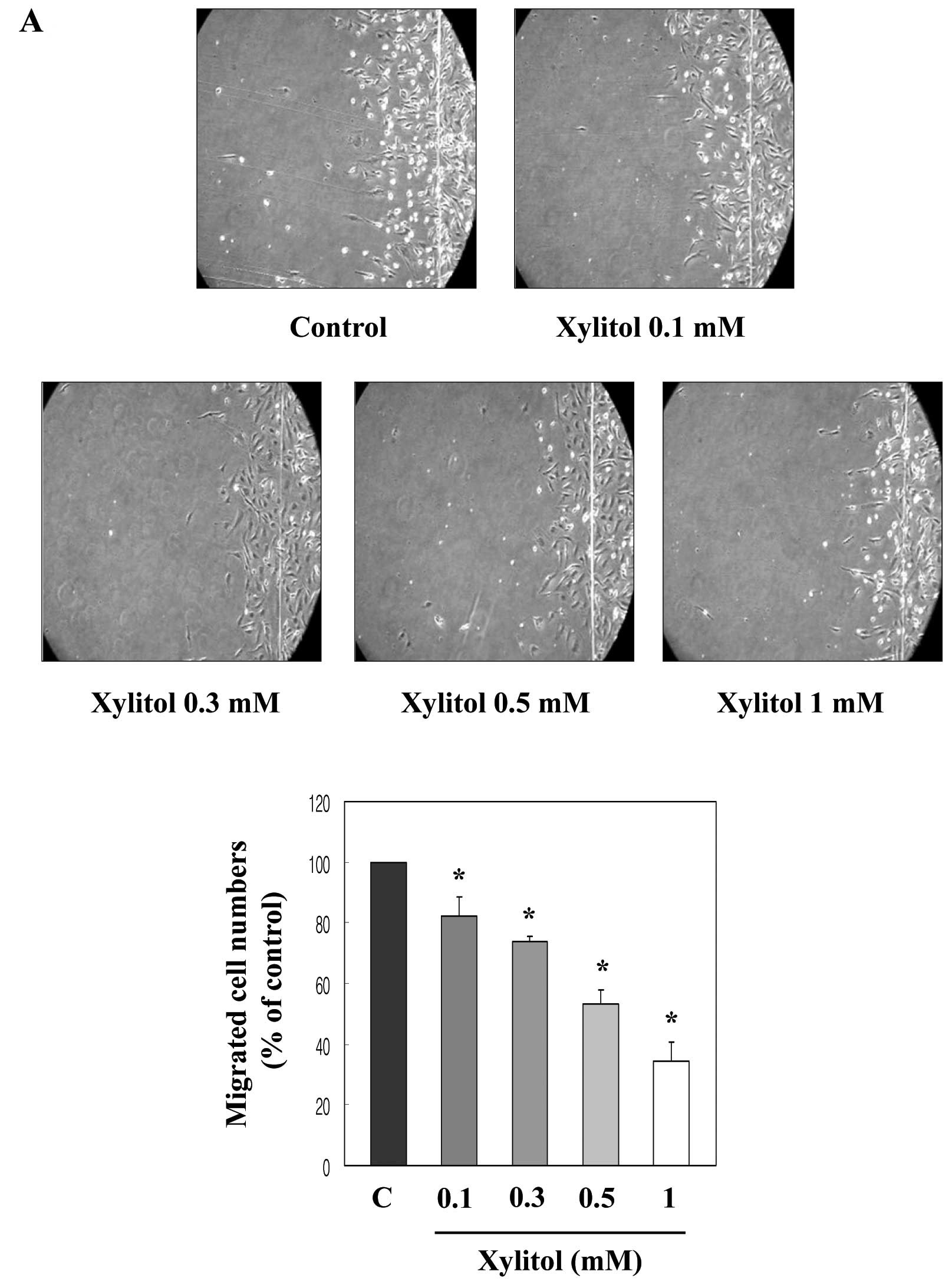
Migration and invasion in endothelial cells is a
critical feature in forming new blood vessels and repairing injured
vessels (19,20). Thus, we examined the effect of
xylitol on the movement of HUVECs from a wounded edge to the open
area by using a wound migration assay. Exposure to xylitol for 20 h
significantly decreased HUVEC migration compared with that of
control cells in a dose-dependent manner (Fig. 3A). To examine the effect of xylitol
on HUVEC invasiveness, we performed an invasion assay by using a
Transwell system. As shown in Fig.
3B, xylitol suppressed HUVEC invasiveness compared with that of
control cells in a dose-dependent manner after 24 h of incubation.
These inhibitory activities of xylitol on the migration and
invasion of HUVECs indicate that xylitol suppresses in vitro
angiogenesis.
Xylitol inhibits in vivo
angiogenesis
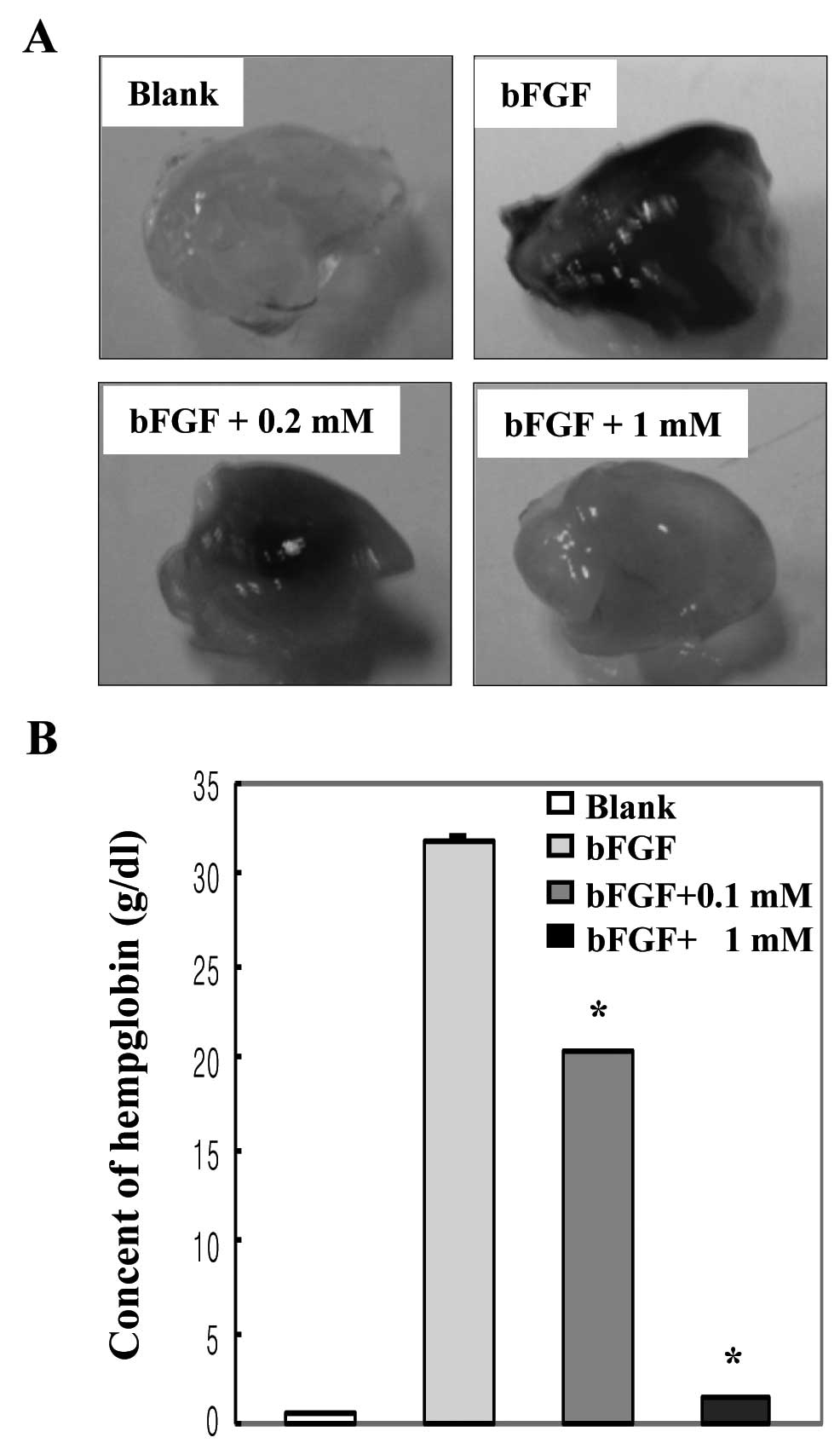
To examine the effect of xylitol on in vivo
angiogenesis, we performed a mouse Matrigel plug assay, an
established in vivo angiogenesis model. As shown in Fig. 4A, Matrigel plugs containing bFGF
were abundantly filled with intact red blood cells, indicating
formation of a functional vasculature inside the Matrigel, whereas
vessels were not observed in the Matrigel alone (blank). Matrigel
plugs containing xylitol produced fewer vessels compared with plugs
containing bFGF, indicating that xylitol inhibits formation of
bFGF-induced neo-microvessels. We then measured the hemoglobin
content inside the Matrigel plugs to quantify the anti-angiogenic
effect of xylitol. The amount of hemoglobin indicates the degree of
formation of a functional vasculature inside the Matrigel. The
hemoglobin content of bFGF-treated plugs was 31.8 g/dl, whereas
that of 1 mM xylitol-treated plugs was profoundly lowered to
approximately 1.4 g/dl (Fig. 4B).
These results indicate that xylitol has strong anti-angiogenic
activity in vivo.
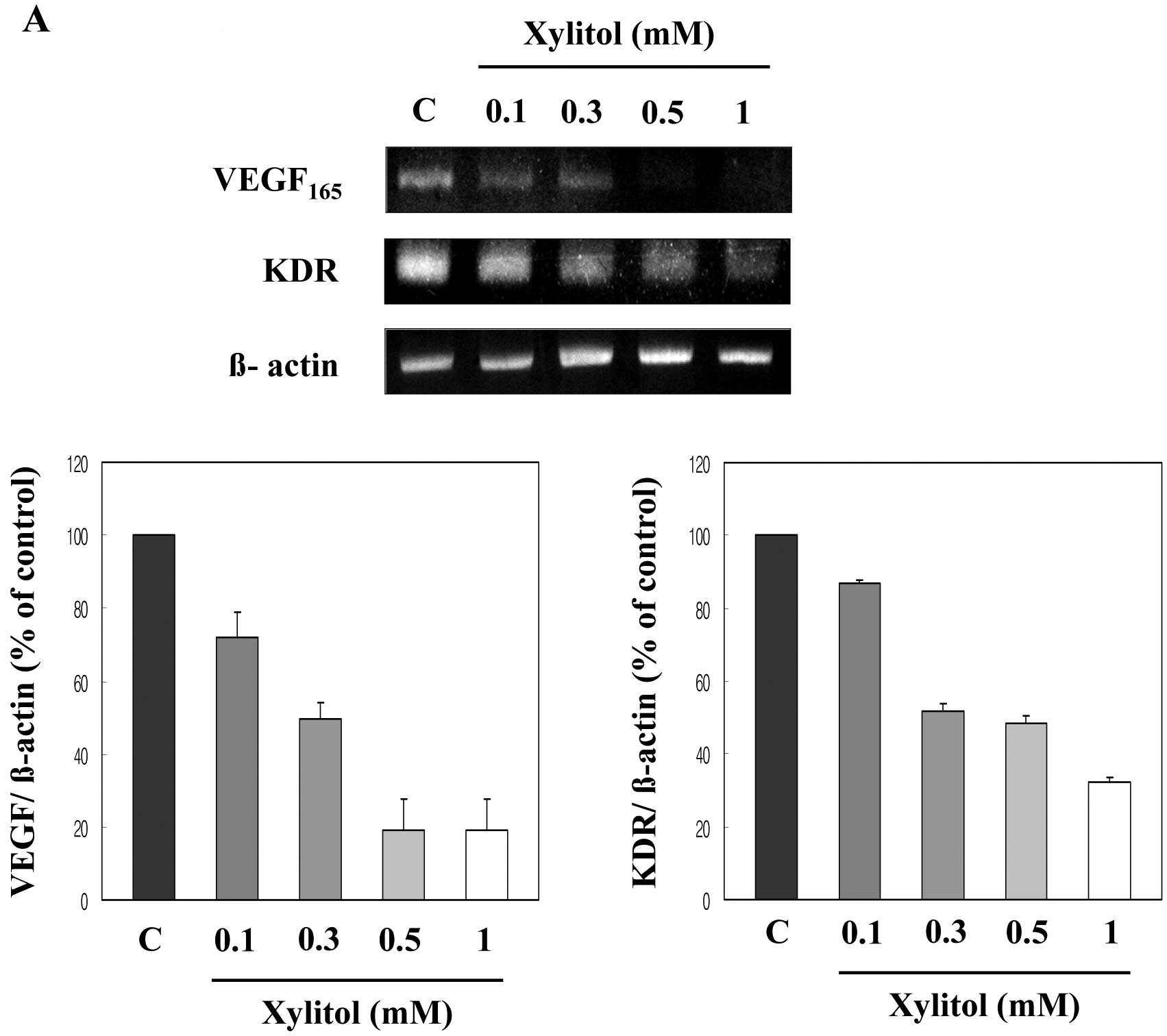
Xylitol downregulates mRNA expression of
angiogenesis-related genes
To determine which molecules are involved in the
anti-angiogenic activity of xylitol, we examined mRNA expression of
angiogenic factors and their receptors in HUVECs following xylitol
treatment by using RT-PCR. As shown in Fig. 5A, VEGF mRNA expression was
significantly reduced in the presence of xylitol. VEGFR-II (KDR)
mRNA expression was also downregulated following treatment with
xylitol in a dose-dependent manner. mRNA expression of other
angiogenic molecules, including bFGF and bFGR-II, was remarkably
reduced by xylitol (Fig. 5B).
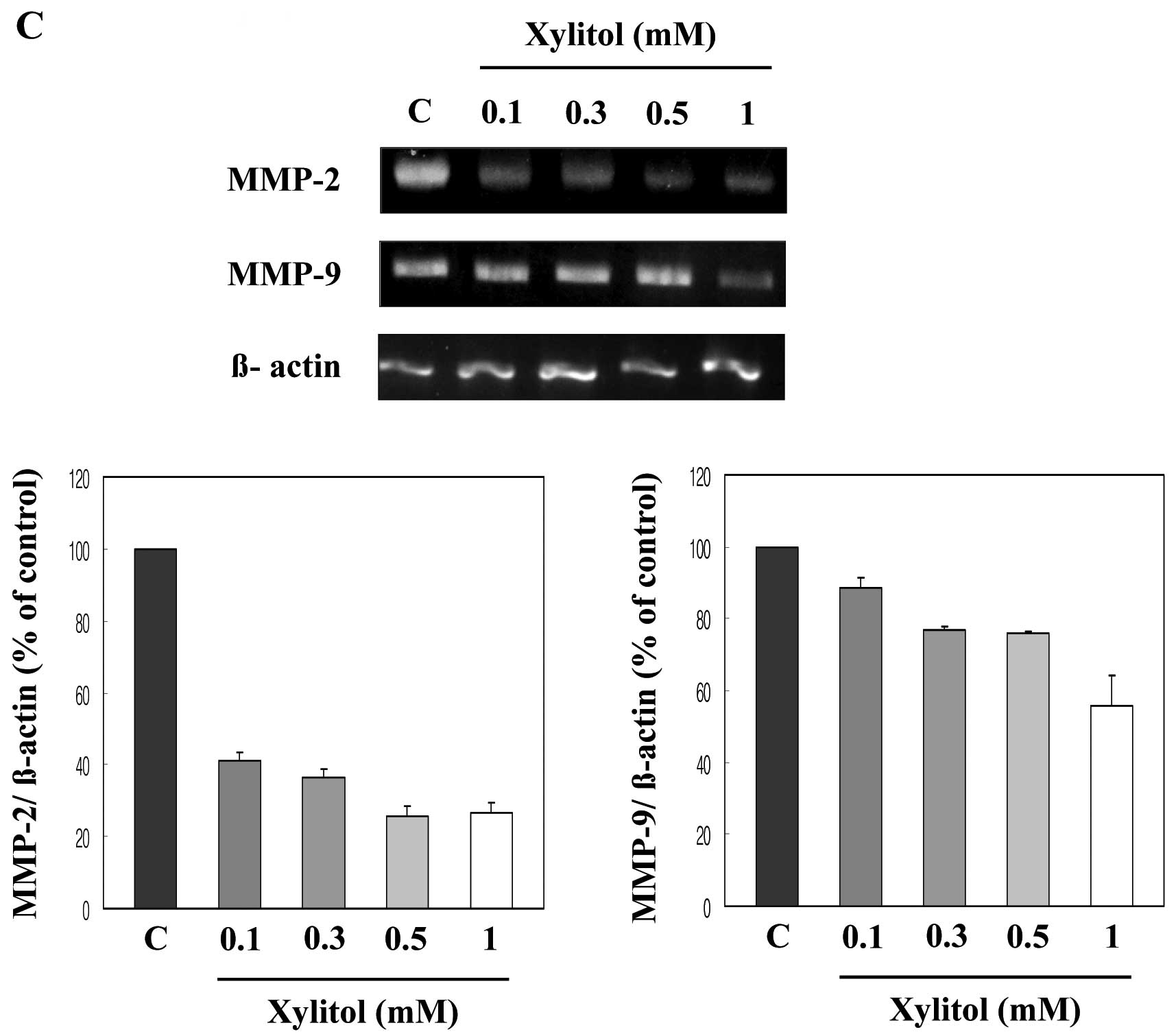
Regulation of extracellular proteolytic activity is
important for endothelial cell migration and invasion, and MMP-2
and MMP-9 are major functional molecules involved in the
degradation of the extracellular membrane (21). Thus, we examined the effect of
xylitol on the mRNA expression of MMP-2 and MMP-9. Expression of
both molecules was markedly downregulated following treatment with
xylitol in a dose-dependent manner (Fig. 5C). These data suggest that
downregulation of VEGF, KDR, bFGF, bFGFR-II, MMP-2 and MMP-9 mRNA
expression may be responsible for the anti-angiogenic action
observed in HUVECs treated with xylitol.
Xylitol suppresses NF-κB and Akt
activation of HUVECs
NF-κB and Akt are the main signaling pathways
involved in tumor progression and angiogenesis (22,23).
Therefore, we examined the effect of xylitol on NF-κB and Akt
activation in HUVECs. In this experiment, total proteins were
prepared to detect NF-κB and Akt phosphorylation and then analyzed
by western blot analysis. As shown in Fig. 6A, NF-κB phosphorylation was
suppressed by xylitol in a dose-dependent manner. Because NF-κB
activation is known to be mediated by the Akt signal pathway, we
then examined Akt activation (17,24).
Akt phosphorylation was inhibited in the presence of xylitol in a
dose-dependent manner (Fig. 6B).
These results indicate that xylitol interferes with NF-κB and Akt
phosphorylation in HUVECs.
Discussion
Angiogenesis is required for tumor growth and
expansion. Angiogenesis is the formation of new blood vessels from
existing vessels; these processes involve several cascades
(19,20,25).
Inflammation is also known to be a central process in many cancers
during tumorigenesis (26).
Therefore, signal transduction of angiogenesis and inflammation can
occur simultaneously (27).
Xylitol was first derived from Birch trees in
Finland, and it is used as a safe sweetener for diabetic patients
(11). Xylitol is obtained from
natural sources, including plums, strawberries, raspberries, and
rowanberries (12,28,29).
It is used in various food products such as chewing gum, candy and
soft drinks (30). Xylitol has
also been used to prevent tooth decay since it inhibits the growth
of Streptococcus mutans (31,32).
Recently, xylitol was reported to have an inhibitory
effect on LPS-induced inflammatory cytokine expression (16). Since inflammation and angiogenesis
share a common signaling pathway (17,18),
molecules that show anti-inflammatory activity also show
anti-angiogenic activity (8,27).
Therefore, we examined whether xylitol has anti-angiogenic effects
by using in vitro and in vivo angiogenesis assays. In
this study, we found that xylitol inhibited in vitro and
in vivo angiogenesis. Xylitol strongly inhibited tube
formation (Fig. 2), migration
(Fig. 3A), and invasion (Fig. 3B) of HUVECs. Xylitol prominently
inhibited the formation of neo-microvessels in the Matrigel assay
(Fig. 4A), and reduced the
hemoglobin content in the Matrigel plug (Fig. 4B). These results suggest that
xylitol suppresses angiogenesis both in vitro and in
vivo.
Tumors produce various angiogenic molecules during
the angiogenesis process. VEGF, bFGF and their receptors are well
known as the main stimuli for angiogenesis (33–35).
Therefore, we examined the involvement of xylitol in the expression
of major angiogenic factors and their receptors. Xylitol decreased
the mRNA expression of key angiogenic molecules and their receptors
(VEGF, KDR, bFGF and bFGFR-II) in a dose-dependent manner (Fig. 5).
MMPs are secreted as proenzymes and they regulate
extracellular matrix degradation. Regulation of extracellular
proteolytic activity is important for cell migration and invasion
(21). Thus, we tested the effect
of xylitol on mRNA expression of MMP-2 and MMP-9 by using RT-PCR.
As shown in Fig. 5C, xylitol
decreased the mRNA expression of both MMP-2 and MMP-9.
NF-κB and Akt comprise a multiunit transcription
factor that plays a central role in tumorigenesis and is involved
in tumor cell invasion and metastasis (24,36).
We examined the effect of xylitol on NF-κB and Akt activation by
western blot analysis. Protein expression of NF-κB and Akt did not
change following treatment with xylitol; however, NF-κB and Akt
phosphorylation was suppressed in a dose-dependent manner (Fig. 6).
In summary, we found that xylitol inhibited in
vitro and in vivo angiogenesis. These effects of xylitol
are linked with the mRNA expression of VEGF, KDR, bFGF, bFGFR-II,
MMP-2 and MMP-9. Xylitol inhibited angiogenesis by inhibiting the
NF-κB and Akt signal pathway. Furthermore, xylitol is widely used
as a sugar substitute as well as an artificial sweetener in chewing
gums to prevent tooth decay. Therefore, xylitol may be a promising
candidate as an inhibitor of angiogenesis-related diseases.
Acknowledgements
This study was financially supported
by the 2012 Postdoc. Development Program of Pusan National
University and was supported by the Basic Science Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Education, Science and Technology
(2011-0024330).
References
|
1.
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.
|
|
2.
|
Siddiqui FA, Desai H, Siddiqui TF and
Francis JL: Hemoglobin induces the expression and secretion of
vascular endothelial growth factor from human malignant cells.
Hematol J. 3:264–270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ferrara N and Kerbel RS: Angiogenesis as
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar
|
|
5.
|
Isner JM and Asahara T: Angiogenesis and
vasculogenesis as therapeutics strategies for postnatal
neovascularization. J Clin Invest. 103:1231–1236. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Folkman J: Tumor angiogenesis; therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Szekanecz Z and Koch AE: Endothelial cells
in inflammation and angiogenesis. Curr Drug Targets Inflam Allergy.
4:319–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ono M: Molecular links between tumor
angiogenesis and inflammation: inflammatory stimuli of macrophages
and cancer cells as targets for therapeutic strategy. Cancer Sci.
99:1501–1506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Karin M and Greteo FR: NF-κB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005.
|
|
10.
|
Albini A, Tosetti F, Benelli R and Noonan
DM: Tumor inflammatory angiogenesis and its chemoprevention. Cancer
Res. 65:10639–10641. 2005. View Article : Google Scholar
|
|
11.
|
Tanzer JM: Xylitol chewing gum and dental
caries. Int Dent J. 45:65–76. 1995.PubMed/NCBI
|
|
12.
|
Bradshaw DJ and Marsh PD: Effect of sugar
alcohols on the composition and metabolism of a mixed culture of
oral bacteria grown in a chemostat. Caries Res. 28:251–256. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Makinen KK and Soderling E: A quantitative
study of mannitol, sorbitol, xylitol, and xylose in wild berries
and commercial fruits. J Food Sci. 45:367–371. 1980. View Article : Google Scholar
|
|
14.
|
Makinen KK, Bennett CA, Hujoel PP, et al:
Xylitol chewing gums and caries rates: a 40-month cohort study. J
Dent Res. 74:1904–1913. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Söderling E, Mäkinen KK, Chen CY, et al:
Effect of sorbitol, xylitol, and xylitol/sorbitol chewing gums on
dental plaque. Caries Res. 23:378–384. 1989.PubMed/NCBI
|
|
16.
|
Han SJ, Jeong SY, Nam YJ, et al: Xylitol
inhibits inflammatory cytokine expression induced by
lipopolysaccharide from Porphyromonas gingivalis. Clin Diagn
Lab Immunol. 12:1285–1291. 2005.PubMed/NCBI
|
|
17.
|
Bisacchi D, Benelli R, Venzetto C, et al:
Anti-angiognesis and angioprevention: mechanisms, problems and
perspectives. Cancer Detect Prev. 27:229–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yoshimura A: Signal transduction of
inflammatory cytokines and tumor development. Cancer Sci.
97:439–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Pang R and Poon RT: Angiogenesis and
antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett.
242:151–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Femke H and Arjan WG: Tumor
vascularization: sprouting angiogenesis and beyond. Cancer
Metastasis Rev. 26:489–502. 2007. View Article : Google Scholar
|
|
21.
|
Bergers G, Brekken R, McMahon G, et al:
Matrix matalloproteinases-9 triggers the angiogenic switch during
angiogenesis. Nature Cell Biol. 2:737–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Francesco P and Antonio L: NF-κB solid
tumors. Biochem Pharmacol. 1142–1152. 2006.
|
|
23.
|
Lin YG, Kunnumarkkara AB, Nair A, et al:
Curcumin inhibits growth and angiogenesis in ovarian carcinoma by
targeting the nuclear factor-κB pathway. Clin Cancer Res.
13:3423–3430. 2007.PubMed/NCBI
|
|
24.
|
Karin M: Nuclear factor-κB in cancer
development and progression. Nature. 441:431–436. 2006.
|
|
25.
|
Pawel D, Kathryn H, Susan JB and Hong C:
Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist.
Cancer Res. 64:910–919. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Sporn MB and Suh N: Chemoprevention of
cancer. Carcinogenesis. 21:525–530. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Jones KM, Wang H, Peskar BM, et al:
Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs:
insight into mechanism and implications for cancer growth ulcer
healing. Nat Med. 5:1418–1423. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Renko M, Valkonen P, Tapiainen T, et al:
Xylitol-supplemented nutrition enhances bacterial killing and
prolongs survival of rats in experimental pneumococcal sepsis. BMC
Microbiol. 8:1–7. 2008. View Article : Google Scholar
|
|
29.
|
Georgieff M, Moldawer LL, Bistrian BR, et
al: Xylitol, an energy source for intravenous nutrition after
trauma. J Parenter Enteral Nutr. 9:199–209. 1985.PubMed/NCBI
|
|
30.
|
Panagiotou G, Christakopoulos P, Grotkjaer
T, et al: Engineering of the redox imbalance of Fusarium
oxysporum enables anaerobic growth on xylose. Metab Eng.
8:474–482. 2006.PubMed/NCBI
|
|
31.
|
Loesche WJ, Grossman NS, Earnest R, et al:
The effect of chewing xylitol gum on the plaque and saliva levels
of Streptococcus mutans. J Am Dent Assoc. 108:587–592. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Miyasawa H, Iwami Y, Mayanagi H, et al:
Xylitol inhibition of anaerobic acid production by Streptococcus
mutans at various pH levels. Oral Microbiol Immunol.
18:215–219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Meadows KN, Bryant P and Pumiglia K:
Vascular endothelial growth factor induction of the angiogenic
phenotype requires ras activation. J Bio Chem. 276:49289–49298.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Leung DW, Cachianes G, Kuang WJ, et al:
Vascular endothelial growth factor is a secreted angiogenic
mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Asahara T, Bauters C, Zheng LP, et al:
Synergistic effect of vascular endothelial growth factor and basic
fibroblast growth factor on angiogenesis in vivo. Circulation.
92:11365–11371. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Gilmore TD: The Rel/NF-κB/IκB signal
transduction pathway and cancer. Cancer Treat Res. 115:241–265.
2003.
|